Abstract
Objectives.
Clinical insomnia is known to affect pain, but mechanisms are unclear. Insomnia can dysregulate inflammatory pathways, and inflammation plays a mediating role in pain. It is unclear whether insomnia-related alterations in inflammation can be modified with insomnia improvement, and if such alterations parallel improvement in pain. The current study objective was to provide proof of concept for the role of insomnia in inflammation and pain by testing whether improving insomnia would reduce pain and related physical function, and, concurrently, modulate inflammatory responses.
Methods.
Thirty adults with osteoarthritis (OA) knee pain and insomnia (Insomnia Severity Index (ISI) above 10) provided baseline measures of clinical OA and laboratory pain, and serial blood samples for inflammatory biomarkers, interleukin (IL)-6 and tumor necrosis factor (TNF)-α, before and after pain testing. To manipulate insomnia, participants were randomly assigned to 6-week cognitive behavioral therapy for insomnia (n = 16); or wait-list control (n = 14). At 8-weeks (Time 2), all measures were repeated. To directly test insomnia improvement effects, participants were grouped by insomnia status at Time 2 after confirming baseline equivalency on all outcomes.
Results.
Compared to those maintaining insomnia at Time 2 (ISI ≥ 8; n = 18), those whose insomnia improved at Time 2 (n = 12) had significantly improved physical functioning, decline in knee pain during transfer activities, and attenuated increase in IL-6 and less decrease in TNF-α across the pain testing session.
Discussion.
These findings suggest further exploration of inflammatory pathways linking clinical insomnia, and its improvement, to chronic pain.
Keywords: Insomnia, pain, inflammation, cytokines, knee osteoarthritis
1. Introduction
Sleep problems, including insomnia, are a significant complaint among adults with chronic pain, with prevalence rates up to 81% reported in older adults with osteoarthritis (OA) 1,2. Both subjective and objective sleep disturbance can aggravate pain 3–6. Conversely, improving sleep quality among adults with co-morbid insomnia and OA can improve OA-related pain 7, although results are equivocal 8,9.
Given the role of inflammation in pain 10,11, particularly arthritis 12, improving insomnia may improve pain by modulating inflammatory pathways. Emerging evidence suggests that sleep disturbances, including insomnia, can sensitize or dysregulate inflammatory pathways 13. Both clinically identified insomnia (that is, via validated self-report measures) and subjectively poor sleep quality are associated with altered circulating levels of inflammatory cytokines at rest 14,15 and in response to stress 16–18. Clinical evidence to support linkages among poor sleep, inflammatory cytokine levels, and pain comes primarily from cross-sectional studies. For example, poorer sleep quality, higher IL-6 levels, and greater pain were associated among otherwise healthy adults with chronic low back pain, but not among those without chronic pain 19. Recently, laboratory pain-evoked IL-6 responses, but not subjective pain reports, were found to be higher among participants with insomnia disorder than participants without insomnia or knee OA 17. Lending mechanistic support for a sleep to inflammation to pain pathway, healthy individuals who underwent 4 hours of sleep restriction per night for almost 2 weeks showed higher levels of circulating IL-6 and bodily pain across a subsequent week, compared to those who were assigned to sleep 8 hours during that period 20.
In the current pilot study, we tested whether insomnia improvement led to improved pain, and altered inflammatory cytokine response to pain. To manipulate insomnia, we randomly assigned adults 50 years of age and older, who met diagnostic criteria for insomnia disorder and OA, to either receive cognitive behavioral therapy for insomnia (CBT-I), or no treatment. We evaluated their morning IL-6 and TNF-α levels at rest and in response to pain, and their clinical OA and laboratory pain, before and after insomnia treatment (or no treatment). As our primary aim was to conduct a proof of concept study to experimentally test the role of insomnia improvement in altering inflammation and pain (rather than an efficacy study to determine CBT-I’s effects on outcomes), we compared pain-related outcomes and inflammatory responses among those whose insomnia had or had not improved at follow-up. We tested the hypothesis that, relative to individuals without insomnia improvement, those with improved insomnia would evidence OA-pain improvement, less subjective pain, and lower IL-6 levels, in response to laboratory pain testing. TNF-α can increase in circulation in response to acute laboratory stressors 21, but its release is also observed to be down-regulated by IL-6 22 and stress-related adrenergic pathways 23. Therefore, we tested a non-directional hypothesis that individuals with insomnia improvement would show altered TNF-α responses to pain testing compared to those without insomnia improvement.
2. Materials and Methods
2.1. Participants
Forty-eight participants, 50 to 75 years of age, were recruited through university medical center orthopedics clinics and through advertisements in local newspapers and primary care clinics following university Institutional Review Board approval. Participants were eligible if they had radiographic evidence of knee osteoarthritis or physician confirmation of diagnosis, reported knee pain and related disability on most days for at least 6 months, met research diagnostic criteria for Insomnia Disorder 24, Insomnia Severity Index 25 score ≥10, and reported either two or more awakenings per night of >15 minutes or had wake time after sleep onset >30 minutes. Participants were excluded for: immune- and psychiatric-related health conditions (e.g., rheumatoid arthritis, heart or blood vessel disease, uncontrolled diabetes, untreated depression, history of bipolar disorder); regular use of systemic corticosteroids; contraindications for nociceptive flexion reflex (NFR) and/or cold pressor test; prior bilateral hip or knee replacement or prosthetics; BMI > 39 kg/m2; dementia or cognitive impairment diagnosis or Mini Mental Examination score ≥ 24; active substance dependence (from MINI International Neuropsychiatric Exam 26); untreated hypertension; use of antipsychotics, mood stabilizers, sedative hypnotics, opiate analgesics, or unwilling to discontinue their use, with a two-week washout period prior to study start; an Apnea Hypopnea Index ≥ 10 (controlled apnea via positive airway pressure treatment was not exclusionary) or Periodic Limb Movement Index with arousals ≥ 15. Women were postmenopausal. Following an overnight laboratory sleep screening (with one eligible participant withdrawing prior to sleep screening), 33 participants remained eligible for the study; 30 completed the study. All participants provided informed consent for participation.
2.2. Procedures
Objective sleep screenings and data scoring were conducted via overnight, laboratory-based, polysomnography (PSG) using Embla N7000 (Embla Systems Inc., Broomfield, CO), according to established guidelines 27. Bedtime was set between 9:30–11:00 p.m. and matched participant’s average self-reported bedtime. Eligible participants were invited back the next evening to complete a separate baseline PSG assessment.
The morning after 8-hour baseline PSG recording, subjects were escorted to the Clinical Research Center (CRC) at 8:00 a.m. To ensure minimal influence of dietary consumption on inflammatory pathways, a CRC bionutritionist designed a standardized breakfast for participants that contained foods choices with low inflammatory impact (e.g., oatmeal, whole grain toast, fruit, low cholesterol egg and vegetable omelet). To avoid physiological stress due to caffeine withdrawal, caffeinated coffee or tea was allowed but limited to 6 oz (if selected) and was consumed approximately 2 hours prior to baseline blood draw (caffeine consumption was unrelated to study outcomes). Following the standardized breakfast, a research nurse attached an indwelling venous cannula to the subjects’ non-dominant arm for unobtrusive blood sampling. Subjects completed self-report instruments and sat quietly for a 30-minute adaptation period. Subjects then were prepped for pain testing (electrodes attached) and participated in laboratory pain testing, comprising multiple trials of electrical stimulation to the ankle (nociceptive flexion reflex (NFR) threshold testing; detailed in 28) followed by a cold pressor test (CPT). For CPT, we followed procedures previously used to assess IL-6 responses to pain.29 Specifically, subjects immersed their hand, or foot (participants’ choice), in a cold water bath maintained at 4º C for 30 sec for 4 repeated immersions with 2 minutes in between immersions. For a fifth immersion, participants were asked to keep their hand (or foot) in the water for as long as tolerated, but no more than 3 minutes. Following pain testing, subjects sat quietly for 90 minutes, and completed self-report instruments and had blood sampled.
At the end of the baseline assessment, subjects were randomized to either behavioral sleep intervention or no intervention. Subjects assigned to sleep intervention participated in a 6-week manualized behavioral sleep therapy, Cognitive Behavioral Therapy for Insomnia (CBT-I), an efficacious intervention for insomnia in patients with comorbid insomnia and chronic pain 30. Subjects assigned to the no intervention condition received 6 weekly “check-in” calls. At weeks 4 and 7 following baseline, all subjects were interviewed by phone to provide self-reports on insomnia severity, pain, and potential changes to study-relevant medications (e.g., hypnotics, anti-depressants). Within 2 weeks following the 6-week intervention period, subjects returned for post-intervention assessment, at which time all assessments conducted at baseline were repeated.
2.3. Assessments
The Insomnia Severity Index (ISI; 25) was assessed at baseline and post-intervention. Clinical OA pain was measured by the Western Ontario and McMaster University OA (WOMAC) Index 31, providing a total scale score as well as subscale scores for pain, stiffness, and physical function. The Knee Pain Scale (KPS; 32) also indexed clinical OA pain, with two subscales measuring pain with transfer activities (e.g., getting out of bed) and ambulation or climbing (e.g., stairs). Subjective pain was verbally reported after each CPT water immersion (every 15 seconds) on a scale of 0 (no pain), 50 (pain), and 100 (excruciating pain). The amount of time participants kept their hand (or foot) immersed in the cold water bath provided a measure of pain tolerance.
2.4. Serum Cytokine Measurement
Blood samples for analysis were drawn into serum tubes from the indwelling cannula immediately centrifuged and frozen at −80º C. Samples were drawn at the end of the adaptation period (post-adaptation), after NFR electrode prep (immediately pre-pain testing), and at 5 time points following pain testing (immediately following and at 20-, 40-, 60-, and 90- minutes). IL-6 and TNF-α were assayed in duplicate (CVs < .10) using Quantikine high sensitivity (HS) ELISA kits and standards (R&D Systems, Minneapolis, MN), with mean minimum detectable limits of 0.039 pg/mL (IL-6) and 0.106 pg/mL (TNF).
2.5. Statistical Analysis
A manipulation check was conducted with ANOVA testing insomnia severity differences (ISI scores) at follow-up by CBT-I versus control, controlling for gender and baseline insomnia severity. Insomnia improvement was indexed by ISI severity < 8 (yes) or ≥ 8 (no). Although we used a higher ISI cutoff for study eligibility, we used the more conservative validated cutoff of 8 to distinguish between those who had improved to the point of having no insomnia (ISI range of 0–7) and those with some remaining level of insomnia (ISI of 8 or greater). In validation work, a cutoff of 8 identified 98.2% of individuals without an insomnia diagnosis in a clinical sample and 98.3% in a community sample 33. IL-6 levels were skewed (Kolmogorov-Smirnov tests, p < 0.05) and, therefore, log transformed; TNF-α values were not skewed and absolute values were used in analyses. Two participants had outlying cytokine values that were trimmed to within 3 mean standard deviations.
Effects of insomnia improvement on clinical and cold pressor pain at follow-up were tested with repeated measures analysis of covariance, adjusting for age, gender, and insomnia severity at baseline. To test effects of insomnia improvement on inflammatory cytokine responses to pain testing, mixed effects models with random intercepts were fit to IL-6 and TNF-α at follow-up, and accounted for correlation in measurements from each subject across time. Independent variables assessed in each model were insomnia improvement at follow-up (yes versus no) and time (immediately, 20-min, 40-min, 60-min, and 90-min post pain testing), as well as their interactions; age, gender, baseline insomnia severity, and baseline level of the dependent variable (IL-6 or TNF-α) were included as covariates. Models for IL-6 included the quadratic term after visual inspection of IL-6 change over the visits. Hand or foot immersion during CPT was considered a potential covariate; however, only 1 participant chose to immerse her foot in the cold water bath rather than her hand. Excluding this participant from analyses did not alter outcomes. Control for medication changes were also planned a priori; however, there were no reported changes to medications across the 10 week study period in this sample.
3. Results
3.1. Sample characteristics
Demographics and medication use for the total sample, and by insomnia status at follow-up, can be found in Table 1. The mean age of the sample was 61 (SD = 6.64), and the majority were female (60%). The sample comprised predominantly White, Non-Hispanic (87%) participants, followed by African American (7%) and White, Hispanic (3%) participants.
Baseline equivalency tests and manipulation check for CBT-I effects on insomnia severity
There were no baseline differences in insomnia severity between the CBT-I group (n = 16; M = 15.19, SD = 2.99) and controls (n = 14; M = 15.65, SD = 3.86; t(31) = .38, p = .71). Men (M = 16.33, SD = 2.71) reported higher insomnia severity than women (M = 14.17, SD = 2.73; t(1) = 2.14, p = .04). At the 8-week follow-up, insomnia severity scores in the CBT-I group (Madj = 6.32, SE = 1.14) were significantly lower than in the control group (Madj = 13.07, SE = 1.23), adjusting for gender and baseline insomnia severity (F(1, 29) = 15.09, p = .001). The effect size for CBT-I on insomnia severity score (d = 1.04) was comparable to our prior work examining efficacy of CBT-I on insomnia severity in individuals with chronic, non-malignant pain 34.
3.2. Operationalizing clinical insomnia severity improvement for hypothesis testing
Of the 16 individuals assigned to the CBT-I group, 6 (37.5%) maintained ISI severity score ≥ 8 at follow-up. Further, two of the 14 (16.8%) participants assigned to the control group showed ISI severity < 8 at follow-up, indicating improved insomnia. Gender distribution was not different across insomnia improvement categories at follow-up (χ2 = .52, p = .54). Final groups used for hypothesis testing included participants with (n = 12, female, 67%) vs. without (n = 18; female, 56%) insomnia improvement. Insomnia severity scores did not differ between the insomnia severity improvement (yes/no) groups (p = .24).
To ensure baseline equivalence on pain and inflammatory marker outcomes, those with and without insomnia improvement at follow-up were compared on WOMAC, KPS, cold pressor test pain and tolerance, and IL-6 and TNF-α response across the baseline laboratory session. As shown in Table 2, there was no evidence of baseline differences on the physical function or pain measures. Figure 1A and1C show IL-6 and TNF-α trajectories across the baseline pain testing session. Preliminary analysis of the IL-6 data across the session indicated a curvilinear (quadratic) change across time. A mixed model testing change in IL-6 across the baseline session by insomnia improvement, controlling for age, gender, and baseline value, indicated significant change across time (quadratic; F(1,136) = 6.56, p < .001), but no significant group effect (insomnia improvement: F(1, 45.27) = .78, p = .38), or interaction of insomnia improvement x time (quadratic) (F(1, 136) = .70, p = .40). At 90-min post pain testing at the baseline session, mean IL-6 levels adjusted for age and gender (Madj = .42, SE = .06) were higher compared to post-adaptation period levels across the groups (Madj = .11, SE = .07; p < .001), with no observed group differences (p=.70). Findings for TNF at the baseline sessions were similar, with no group effect of insomnia improvement (F(1, 24.97) = 1.40, p = .25), and no group differences across time (Baseline session: time (F(1, 26) = 25.66, p < .001), insomnia improvement x time (F(1, 26) = .16, p = .69), after controlling for age and gender. In contrast to IL-6, however, participants had significantly lower TNF levels at 90-minutes post pain testing compared to baseline, after adjustment for age and gender (post-adaptation: Madj = .89, SE = .07, 90-min post pain testing: Madj = .76, SE = .07; p < .001).
Figure 1. Log transformed IL-6 and TNF at baseline (A, C) and follow-up (B, D) by insomnia improvement (improver versus non-improver) at follow-up.
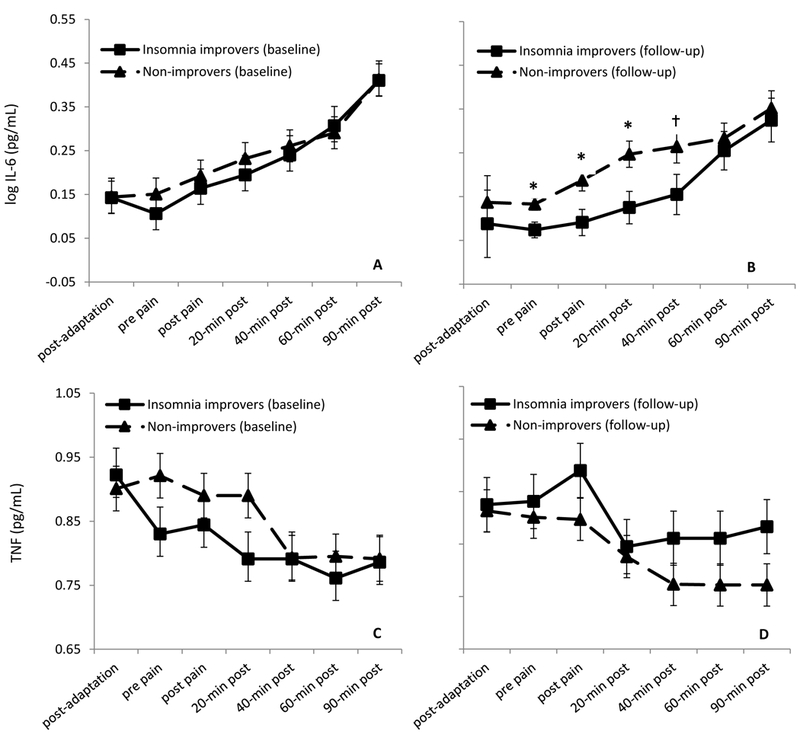
Values are marginal means (with standard error bars) from models adjusted for age, gender, and baseline cytokine value. * Indicates adjusted mean differences between insomnia improvers versus non-improvers significant at p < .05; ✝indicates p < .10).
Baseline insomnia severity was greater, though not significantly, among participants who did not evidence improved insomnia at follow-up (M = 15.61, SD = 3.01), relative to those with insomnia improvement (M = 14.17, SD = 2.55; t(1) = −1.36, p = .18). As such, baseline insomnia severity was included as a covariate in hypothesis testing.
3.3. Insomnia improvement effects on clinical pain and acute pain
Table 2 displays adjusted mean scores from the WOMAC and KPS, and subjective pain ratings from CPT at baseline and follow-up by insomnia improvement at follow-up. For clinical pain measures, participants with insomnia improvement showed a larger decline in WOMAC total scores compared to those without insomnia improvement after controlling for baseline insomnia severity, gender, age, and baseline WOMAC total score (insomnia improvement x visit: F(1, 24) = 5.64, p = .03). Notably, the difference in average WOMAC scores between the groups at follow-up was 18.25 points, suggesting a clinically important difference in overall functioning 35. In addition, WOMAC scores declined on average for the insomnia improvement group by 9.06 points, further suggesting a clinically important change in participants with insomnia improvement 36. When examining WOMAC subscales, significantly greater change was evident on the physical function subscale for those with insomnia improvement compared to those without (insomnia improvement x visit: F(1, 24) = 4.76, p = .04). No significant differences were observed on the pain subscale (insomnia improvement x visit: F(1, 25) = 2.85, p = .10) or the stiffness subscale (insomnia improvement x visit: F(1, 25) = 2.35, p = .14).
With respect to the Knee Pain Scale, reported pain intensity on the transfer activities subscale (i.e., getting in/out of a bed, chair, or car) showed a significantly greater decline at follow-up among participants with insomnia improvement as compared to those without insomnia improvement (insomnia improvement x visit: F(1, 25) = 9.25, p = .01). In contrast, there was no difference between the groups over time on the ambulation/climbing intensity subscale, suggesting similar pain intensity while walking and climbing up or down stairs (insomnia improvement x visit: F(1, 25) = 2.17, p = .15).
When examining acute pain ratings during the cold pressor test, there were no significant differences between those with and without insomnia improvement for mean change in pain ratings from baseline to follow-up (insomnia improvement x visit: F(1, 28) = 1.52, p = .23), nor for pain tolerance (insomnia improvement x visit: F(1, 24) = 1.15, p = .30), controlling for gender, age, and baseline insomnia severity. Further, at follow-up there was no significant difference between the groups for mean pain ratings across the 4 trials at 15 seconds (F(1, 24) = 0.28, p = .60) or 30 seconds (F(1, 24) = 1.16, p = .29) into the cold pressor test, when controlling for gender, age, and baseline insomnia severity.
3.4. Insomnia improvement effects on inflammatory cytokine responses
When assessing IL-6 response at the follow-up session, The insomnia improvement x time (quadratic) interaction was significant (F(1, 138)=11.47, p = .001); Participants who had insomnia improvement at follow-up showed significantly lower IL-6 levels immediately prior to and following pain testing (ps < .05), reaching similar levels of non-improvers by 60-minutes post pain testing (Figure 1B). Based on the group term, insomnia improvers’ overall IL-6 levels were lower than non-improvers after adjustment for baseline, although this difference was not significant (insomnia improvement: F(1, 127.64)=3.81, p = .053).
For the full model testing TNF, there was a main effect of time (F(1, 27) = 15.80, p < .001), but the insomnia improvement x time interaction was not significant (F(1, 27) = 1.87, p = .18), nor was there a significant group effect (F(1, 25.21) = .11, p = .74). Visual data examination (Figure 1D), however, suggested a potential difference between the groups. In a post-hoc, exploratory analysis stratified by group, after controlling for age and gender, participants with insomnia improvement no longer showed significant change in TNF-α across the session as they did at baseline (Baseline session: time (F (1, 10) = 13.97, p = .004); Follow-up session (F (1, 10) = 3.44, p = .09), whereas those with insomnia at follow-up showed the same significant decline observed at baseline session (Baseline session: time (F(1,16) = 15.98, p = .001); Follow-up session (F(1, 17) = 16.80, p =.001), with significantly lower TNF at 90-minutes post pain testing compared to their post-adaptation period level, adjusted for age and gender (post-adaptation: Madj = .93, SE = .08, 90-min post pain testing: Madj = .79, SE = .07; p < .03).
3.5. Exploratory Analysis of Correlations among IL-6, Pain, and Insomnia Severity Changes
Our pilot study was designed a priori to examine whether insomnia improvement affected both pain and inflammatory cytokines outcomes, providing proof of concept for a path model (and a larger powered clinical trial) whereby insomnia improvement leads to changes in inflammation and, in turn, improvements in pain. To provide further proof of concept for links between inflammatory cytokine changes and pain improvement in older adults with knee osteoarthritis, we explored whether changes from baseline to follow-up visit in mean IL-6 levels (averaging values from pre- to 40-min post pain testing and creating change scores (mean IL-6 level at follow-up minus mean IL-6 level at baseline)) correlated with changes in WOMAC scores and KPS transfer intensity scores, across the sample. From partial correlation analysis controlling for age and gender, larger declines in mean IL-6 levels from baseline to follow-up were associated with larger decreases in WOMAC total scores (i.e., WOMAC score at follow-up minus score at baseline), representing greater improvements in clinical pain over time (rp = .40, p = .04); IL-6 change over time was not associated with KPS transfer intensity scores.
Finally, we determined if the degree of change in insomnia severity from baseline to follow-up was associated with changes in IL-6 levels and clinical pain across the sample. After controlling for age and gender, larger declines in ISI scores were associated with larger declines in KPS transfer intensity scores (rp = .45, p = .02); insomnia severity change showed a small but non-significant correlation with change in WOMAC scores (rp = .22, p = .28). ISI scores were not associated with IL-6 mean level change ((rp = −.03, p = .89).
4. Discussion
In this pilot study of older adults with comorbid insomnia and knee osteoarthritis, improvement in insomnia symptoms was related to altered inflammatory cytokine responses to pain testing, enhancement of physical function, and reduction in knee pain during transfer activities. Notably, insomnia improvers showed clinically meaningful improvements in their physical function. Altogether, these findings suggest that insomnia improvement holds promise as a means to modulate inflammatory pathways in adults with chronic pain. Given evidence that inflammation can contribute to pain sensitivity, and that chronic pain may increase mortality risk from inflammatory-related disease 37, further elaboration of inflammatory mechanisms linking insomnia to clinical pain could support translation to more targeted interventions for pain.
In a prior cross-sectional study 17, insomnia was associated with larger IL-6 responses to pain testing in older adults with or without knee osteoarthritis or insomnia. We hypothesized that insomnia improvement would be associated with lower IL-6 response to laboratory pain testing, which was partially supported. Relative to older adults without insomnia improvement, those with insomnia improvement showed an attenuation in the earlier rise in circulating IL-6 levels that was observed in the baseline laboratory pain testing session. Further, at the follow-up visit, insomnia improvers showed significantly lower IL-6 levels prior to the start of pain testing compared to non-improvers. At 60-minutes post-cold pressor test, insomnia improvers’ IL-6 levels had increased to a level comparable to non-improvers. IL-6 increases are typically evident at 40–90 minutes post-stressor or pain provocation 21,38. This pattern of results may reflect potential effects of insomnia improvement on adaptation to the pain testing situation, via multiple pathways, including better emotion and physiological regulation. Subjectively poor sleep is associated with greater negative affect and IL-6 responses to acute stressors in older adults 16, and insomnia is associated with dysregulation of physiological stress systems 39,40. Thus, the delayed rise in IL-6 across the pain testing session among insomnia improvers may reflect better emotion regulation in anticipation of noxious stimulation. Given the integral overlap among somatosensory and physiological stress systems, further study is needed to elaborate the neurobiological and likely bi-directional pathways through which inflammation may link chronic pain and poor sleep.
While our findings of increased circulating IL-6 in response to laboratory pain testing are in line with previous studies 17,29, less is known about pain-evoked TNF-α response, although TNF-α is responsive to acute stressors 21. In the current study, TNF-α showed an overall decline in response to the laboratory pain testing at the baseline testing session. Overall, TNF-α was modestly affected by insomnia improvement, with findings from the exploratory analysis suggesting that insomnia improvement was associated with more stable levels of TNF-α in response to laboratory pain testing. Existing research on the interplay between IL-6 and TNF-α may be relevant to this preliminary observation. Although we did not observe any consistent associations between IL-6 and TNF-α in our small pilot study (data not shown), it is known that IL-6 has inhibitory effects on TNF-α 22, presumably via sympathetic nervous system modulation 23. As insomnia is characterized by increased sympathetic activation 41,42, if insomnia improvement attenuates sympathetic arousal and associated pathways, TNF production could be affected. Investigations among the interplay among sympathetic activation and inflammation in insomnia, and implications for pain, may shed light on these integrative pathways.
The current study expands on previous research by examining sleep and pain-associated inflammation and inflammatory responses longitudinally, rather than just cross-sectionally 16,17,19,29. Further, our design afforded comparison of adults with chronic knee pain who demonstrated improvements in insomnia versus those who did not. This prospective design provides novel proof of concept for the role of insomnia improvement in altering inflammatory responses stimulated by an acute stressor. Chronic pain itself is associated with dysregulated stress responses, including inflammatory responses 43. Specific mechanisms of insomnia-mediated inflammatory alterations, and their causal role in pain improvement, remain to be identified by future trials.
As noted previously, while some prior trials of CBT-I have observed significant or clinically meaningful effects of CBT-I 7, or insomnia and sleep improvement 8,44, on clinical pain outcomes in older adults with insomnia, some have not 9. Although not a primary aim of the study, for comparison to these existing larger-scale clinical trials, we note that the CBT-I intervention here showed statistically significant effects on self-reported OA pain from the WOMAC (Cohen’s d = .45; data not shown). Also for comparison to other trials, our sample had a mean WOMAC pain subscale score of 10.6 at baseline, which is comparable or even higher than baseline scores in knee osteoarthritis trials 45. Nevertheless, our sample was highly selective given the stringent inclusion criteria, reducing heterogeneity due to factors such as depression, obesity, and inflammatory conditions. This selection was intended to improve signal to noise ratio to observe treatment effects. As a result, our findings may have reduced generalizability, given that these factors are among common comorbidities of chronic pain.
The observation that insomnia improvement had differential effects on clinical versus experimental pain suggests that sleep benefits may differ as a function of nociceptive fiber involvement. Cytokines contribute to persistent arthritic pain primarily via sensitization of nociceptive C-fibers, and to a lesser extent via sensitization of nociceptive A-delta fibers 46. In contrast, the experimental pain induced by the cold pressor test is mediated primarily by A-delta fibers 47. Hence, insomnia improvement may help to reduce the dull, aching sensations that are characteristic of C-fiber activation and osteoarthritis pain, but have little or no effect on A-delta mediated sharp, cold pressor pain sensations.
The small sample size of this pilot study is an obvious limitation, as larger studies that take comorbidities into explicit consideration are needed. Although we found an association between the magnitude of insomnia improvement and improvement in transfer-related knee pain, larger trials are needed to discern the degree to which insomnia severity must improve to observe clinically meaningful changes in pain and inflammation. Given our small sample, we were also unable to examine how medication and supplement use, such as use of COX-II inhibitors or herbal supplements, may moderate or mediate insomnia improvement effects on outcomes. In addition, although we did not observe changes in important study-relevant medications across the study period for any participant, medication use was self-reported, which is a limitation. Despite these limitations, the current findings suggest that improving insomnia among older adults with chronic knee osteoarthritis pain can alter acute inflammatory responses to pain stimulation and diminish clinical pain complaints. These preliminary findings underscore inflammatory pathways as a possible mechanism linking insomnia treatment to reduced clinical pain. These findings also suggest further study of CBT-I and its potential efficacy for altering neurobiological pathways in pain.
Supplementary Material
Footnotes
Conflicts of interest and source of funding: No conflicts of interest. This work was supported by a National Institutes of Health grant R21AG041942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Allen KD, Renner JB, Devellis B, Helmick CG, Jordan JM. Osteoarthritis and sleep: the Johnston County Osteoarthritis Project. J. Rheumatol June 2008;35(6):1102–1107. [PMC free article] [PubMed] [Google Scholar]
- 2.Wilcox S, Brenes GA, Levine D, Sevick MA, Shumaker SA, Craven T. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J. Am. Geriatr. Soc October 2000;48(10):1241–1251. [DOI] [PubMed] [Google Scholar]
- 3.Chiu YH, Silman AJ, Macfarlane GJ, et al. Poor sleep and depression are independently associated with a reduced pain threshold. Results of a population based study. Pain June 2005;115(3):316–321. [DOI] [PubMed] [Google Scholar]
- 4.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev October 2006;10(5):357–369. [DOI] [PubMed] [Google Scholar]
- 5.Moldofsky H. Sleep and pain. Sleep Med Rev October 2001;5(5):385–396. [DOI] [PubMed] [Google Scholar]
- 6.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. April 2004;8(2):119–132. [DOI] [PubMed] [Google Scholar]
- 7.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med August 15 2009;5(4):355–362. [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MT, Finan PH, Buenaver LF, et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: a randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol. May 2015;67(5):1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J. Am. Geriatr. Soc June 2013;61(6):947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev November 2007;56(1):148–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv. Exp. Med. Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 12.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann. Rheum. Dis. April 2013;72(4):535–540. [DOI] [PubMed] [Google Scholar]
- 13.Irwin MR, Olmstead R, Carroll JE: Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biological Psychiatry. 2016, 80:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgos I, Richter L, Klein T, et al. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain. Behav. Immun May 2006;20(3):246–253. [DOI] [PubMed] [Google Scholar]
- 15.Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism July 2002;51(7):887–892. [DOI] [PubMed] [Google Scholar]
- 16.Heffner KL, Ng HM, Suhr JA, et al. Sleep disturbance and older adults’ inflammatory responses to acute stress. Am. J. Geriatr. Psychiatry. September 2012;20(9):744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quartana PJ, Finan PH, Page GG, Smith MT. Effects of insomnia disorder and knee osteoarthritis on resting and pain-evoked inflammatory markers. Brain. Behav. Immun July 2015;47:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prather AA, Puterman E, Epel ES, Dhabhar FS. Poor sleep quality potentiates stress-induced cytokine reactivity in postmenopausal women with high visceral abdominal adiposity. Brain. Behav. Immun. Jan 2014;35:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heffner KL, France CR, Trost Z, Ng HM, Pigeon WR. Chronic low back pain, sleep disturbance, and interleukin-6. Clin. J. Pain. January 2011;27(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep September 1 2007;30(9):1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain. Behav. Immun August 2017;64:208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. January 01 1990;75(1):40–47. [PubMed] [Google Scholar]
- 23.Goebel MU, Mills PJ, Irwin MR, Ziegler MG. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom. Med. Jul-Aug 2000;62(4):591–598. [DOI] [PubMed] [Google Scholar]
- 24.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. December 15 2004;27(8):1567–1596. [DOI] [PubMed] [Google Scholar]
- 25.Morin CM. Insomnia: Psychological Assessment and Management. New York: Guilford Press; 1993. [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59 Suppl 20:22–33;quiz 34–57. [PubMed] [Google Scholar]
- 27.Iber C, Ancoli-Israel S, Chesson A, Quan S Jr. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 28.France CR, Keefe FJ, Emery CF, et al. Laboratory pain perception and clinical pain in post-menopausal women and age-matched men with osteoarthritis: relationship to pain coping and hormonal status. Pain. Dec 2004;112(3):274–281. [DOI] [PubMed] [Google Scholar]
- 29.Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain November 15 2008;140(1):135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finan PH, Buenaver LF, Coryell VT, Smith MT. Cognitive-Behavioral Therapy for Comorbid Insomnia and Chronic Pain. Sleep Med Clin June 01 2014;9(2):261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum October 2001;45(5):453–461. [DOI] [PubMed] [Google Scholar]
- 32.Rejeski WJ, Ettinger WH, Schumaker S Jr., James P, Burns R, Elam JT. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthritis Cartilage September 1995;3(3):157–167. [DOI] [PubMed] [Google Scholar]
- 33.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. May 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jungquist CR, O’Brien C, Matteson-Rusby S, et al. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. Mar 2010;11(3):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams VJ, Piva SR, Irrgang JJ, Crossley C, Fitzgerald GK. Comparison of reliability and responsiveness of patient-reported clinical outcome measures in knee osteoarthritis rehabilitation. J. Orthop. Sports Phys. Ther. Aug 2012;42(8):716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann. Rheum. Dis. January 2005;64(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith D, Wilkie R, Uthman O, Jordan JL, McBeth J. Chronic pain and mortality: a systematic review. PLoS One. 2014;9(6):e99048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain. Behav. Immun October 2007;21(7):901–912. [DOI] [PubMed] [Google Scholar]
- 39.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom. Med Sep-Oct 1998;60(5):610–615. [DOI] [PubMed] [Google Scholar]
- 40.Floam S, Simpson N, Nemeth E, Scott-Sutherland J, Gautam S, Haack M. Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. J. Sleep Res. June 2015;24(3):296–304. [DOI] [PubMed] [Google Scholar]
- 41.Covassin N, de Zambotti M, Sarlo M, De Min Tona G, Sarasso S, Stegagno L. Cognitive performance and cardiovascular markers of hyperarousal in primary insomnia. Int. J. Psychophysiol. April 2011;80(1):79–86. [DOI] [PubMed] [Google Scholar]
- 42.de Zambotti M, Covassin N, De Min Tona G, Sarlo M, Stegagno L. Sleep onset and cardiovascular activity in primary insomnia. J. Sleep Res. June 2011;20(2):318–325. [DOI] [PubMed] [Google Scholar]
- 43.Woda A, Picard P, Dutheil F. Dysfunctional stress responses in chronic pain. Psychoneuroendocrinology September 2016;71:127–135. [DOI] [PubMed] [Google Scholar]
- 44.Vitiello MV, McCurry SM, Shortreed SM, et al. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain. August 2014;155(8):1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koog YH, Wi H, Jung WY. Eligibility criteria in knee osteoarthritis clinical trials: systematic review. Clin. Rheumatol. November 2013;32(11):1569–1574. [DOI] [PubMed] [Google Scholar]
- 46.Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther. 2014;16(5):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis KD. Cold-induced pain and prickle in the glabrous and hairy skin. Pain March 1998;75(1):47–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


