Abstract
Preglomerular arteriopathy (PA) induced by hyperuricemia contributes to the progression of chronic kidney disease (CKD). Green tea polyphenols (GTPs) are antioxidant ingredients thought to assist in preventing hyperuricemia. However, the underlying mechanism by which GTPs affect renal function remains unclear. Both normal and remnant kidney (RK) rats were administrated oxonic acid (OX) to induce hyperuricemia. The hyperuricemia RK rats were concomitantly treated with GTPs. Hematoxlyin-eosin (H&E) and periodic acid-Schiff (PAS) staining methods were used to examine renal function and arterial morphology. The expression of proteins in the Jagged1/Notch1 pathway was assessed via immunohistochemistry, in situ hybridization, the quantitative polymerase chain reaction (qPCR), and western blotting techniques. Our results showed that an RK rat model with preglomerular vascular disease had been successfully established. Treatment of the RK rats with GTPs effectively alleviated the damage due to preglomerular arteriopathy, significantly alleviated pathological symptoms, and reduced the levels of proteinuria, serum UA, BUN, and creatinine. Our results also suggested involvement of the Jagged1/Notch1 pathway in the preglomerular vascular lesions. The levels of Jagged1, Notch1-ICD, Hes5, and p-STAT3 were significantly decreased in RK + OA-treated rats when compared with those in RK rats. Treatment with GTPs upregulated the levels of Jagged1, Notch1, Hes5, p-STAT3, and MnSOD2, and downregulated xanthine oxidase (XO) expression in rats with preglomerular arteriopathy. However, the beneficial effects of GTPs were lost when the Jagged1/Notch1-STAT3 pathway was inactivated by siRNA. In conclusion, GTPs exert a therapeutic effect on perglomerular arteriopathy. Our results also revealed a novel mechanism that mediates preglomerular arteriopathy, and suggest GTPs as effective novel renal protective agents.
Keywords: Green tea polyphenols, hyperuricemia, preglomerular arteriopathy, jagged1/notch1 signaling pathway
Introduction
Hyperuricemia, defined as a serum uric acid (UA) concentration > 408 μmol/L (6·8 mg/dL), is associated with chronic kidney diseases (CKDs), chronic cardiovascular diseases, and gout [1]. It has long been accepted that hyperuricemia is a consequence of impaired renal function, and new evidence suggests that hyperuricemia can induce endothelial dysfunction and alter vascular architecture [2-4]. The vascular changes that occur, such as the thickening of vessel walls, can result in ischemia to the postglomerular circulation and glomerular hypertension. A recent clinical study confirmed that high serum UA levels were related to renal arteriolar damage in patients with CKD [5]. Therefore, the preglomerular arteriopathy caused by hyperuricemia is critical for the progression of renal disease.
The Notch signaling pathway participates in regulating cell differentiation, proliferation, and apoptosis [6]. The binding of a Notch ligand (e.g., Delta-like1, 3, 4 or Jagged1, 2) to its receptor (Notch1-4) mediates the transcription of downstream target genes such as Hes1, Hes5, Hey1, and Hey2. During the biological response to a vascular injury, the expression of Jagged/Notch pathway proteins (e.g., Jagged1, Notch1, Hey1, Hey2) is induced in the endothelial or vascular smooth muscle cells [7,8]. Genetic alternations of Jagged1, Notch1, or Hey2 lead to defects in the vasculature and its response to arterial injury [9,10]. Recent studies have shown that Notch-1 is essential for UA-induced oxidative stress [11]. The link between oxidative stress and renal vascular lesions has been documented [12]; however, it remains unknown whether the Jagged/Notch pathway participates in formation of the preglomerular vascular lesions found in cases of CKD accompanied by hyperuricemia. It has recently been reported that many ingredients found in foods thought to have antioxidant effects help to protect against various chronic diseases [13]. For instance, green tea is one of the most popular beverages worldwide, and contains a high levels of polyphenolic constituents [14]. Green tea polyphenols (GTPs) have been demonstrated to prevent the formation of and scavenge free radical species by acting as metal chelators or antioxidant enzyme modulators [15]. In addition, GTPs also exert antioxidant, anti-inflammatory, and anticancer effects by activating the Akt/GSK-3β/caveolin and phosphatidylinositol (PI) 3-kinase/Akt pathways, as well as other signaling pathways [15,16]. A recent study has showed that GTPs reduce UA levels by regulating xanthine oxidase (XO) activity in the liver and renal urate transporters in hyperuricemic conditions [17]. Nevertheless, the effects of GTPs on renal function in patients with both CKD and hyperuricemia remain uncertain. In this study, we employed an experimental model of chronic renal disease to determine whether GTPs are effective in preventing preglomerular arteriopathy, and subsequently alleviating progressive renal disease accompanied by hyperuricemia.
Materials and methods
Experimental animals
The protocols for all animal studies were approved by The Animal Care Committee of the Central South University of China. Female Sprague-Dawley rats (180-220 g) were used for all the experiments. Each rat was anaesthetized with amobarbital sodium (30 mg/kg); after which, a 5/6 nephrectomy was performed by removing the right kidney and surgically resecting the upper and lower thirds of the left kidney. To induce hyperuricemia, the rats were fed a normal-salt diet containing 2% OA (Ji’nan branch of a chemical company located in Jinan, Shandong, China) starting three days after the renal ablation. To prevent hyperuricemia, GTPs (Hua Cheng Bio, Changsha, Hunan, China) were administered at a dose of either 750 mg/kg or 350 mg/kg by gastric gavage on a daily basis; after which, a chromatographic analysis was performed (Figure S3). The rats were assigned to three groups: RK (n = 8); RK + OA (n = 8); RK + OA + GTP (n = 8). Four weeks after the operation, the rats were sacrificed for further examination.
Uric acid, proteinuria, and renal function
Blood samples were collected from the rats and centrifuged at 4000 g for 10 min to obtain serum. Next the levels of serum UA, BUN, creatinine, and reactive oxygen species (ROS) were determined using commercial assay kits. Urine samples were collected from the rats and used for proteinuria analysis. All assays were performed according to instructions provided with the assay kit.
Cell culture and transfection
HK-1 cells were purchased from ATCC (Manassas, VA, USA) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum (FBS) and 1% streptomycin-penicillin (Gibco, Waltham, MA, USA) in a 37°C chamber containing 5% CO2. Cells in their log-growth phase were used for further experiments. HK-1 cells were cultured in vitro and divided into four groups: blank group; hyperuricemia group; hyperuricemia + GTP group (siRNA NC); hyperuricemia + GTP + siRNA group (siRNA-jagged1). The cells were transfected for 72 hours and then collected for use in subsequent assays. The nucleotide fragments used for transfection were: siRNA-jagged1 sense: 5’-ACACUUUGAAGUAUGUGUCAC-3’; si-NC sense: 5’-ACACUUUGAAGUAUGUGUCAC-3’ (Ruibo Bio, Beijing, China).
Renal histology
Specimens of rat renal tissue were fixed in 4% paraformaldehyde for 24 h and then embedded in paraffin. Hematoxylin-eosin (H&E), periodic acid Schiff (PAS), and Masson’s trichrome staining were performed on 4 μm tissue sections. All analyses of tissue samples were performed in blinded manner.
Glomerulosclerosis (GS) index scores and tubulointerstitial (TI) index scores were based on semi-quantitative scoring systems. The score assessments were made by viewing periodic-acid Schiff (PAS)-stained tissue sections at a magnification of ×200 [18]. The GS index was assessed in 20 glomeruli per animal and graded on a scale of 0 to 4 according to the percentage of glomeruli displaying obliteration or hyalinosis: grade 0 = no sclerosis; grade 1 = 0-25%; grade 2 = 25-50%; grade 3 = 50-75%; grade 4 = 75-100%. The TI index was assessed in 20 fields per kidney section, and the lesions were graded on a scale of 0 to 5 according to the amount of tissue area that displayed tubule interstitial changes (tubular atrophy, casts, interstitial inflammation, and fibrosis): grade 0 = normal; grade 1 = 0-10%; grade 2 = 10-25%; grade 3 = 25-50%; grade 4 = 50-75%; grade 5 = 75-100%. The final index score for each rat was determined by calculating the mean value of all the obtained scores.
Images of arcuate (×200), interlobular (×200), and afferent (×400) arteries were analyzed as previously described [4], and the results were used to evaluate the wall thickening of preglomerular arteries. Briefly, an outline of the vessel and its internal lumen (excluding the endothelium) were studied with a Motic Med 6.0 digital medical image analysis system (Motic Group Co., Xiamen, Fujian, China). The total medial area was calculated by subtracting the lumen area from the outline area. Next, the M/L ratio was calculated in 10 arteries of each type to obtain the mean value for each rat.
Immunohistochemistry (IHC) staining
Paraffin embedded sections (4 μm) were deparaffinized and blocked for 10 min with 0.3% hydrogen peroxide at room temperature, followed by antigen retrieval. After being rinsed with PBS, the sections were incubated overnight at 4°C with rabbit polyclonal antibodies against Jagged1 (1:100, Abcam, Cambridge, MA, USA), Notch1-ICD (1:100, Anbobio, San Francisco, CA, USA), MnSOD2 (1:500, Abcam), XO (1:100, Abcam, Cambridge, MA, USA), rabbit monoclonal antibodies against Hes5 (1:200, Abcam, Cambridge, MA, USA), and mouse monoclonal antibodies against p-STAT3 (1:400, Cell Signaling Technology, Beverly, MA, USA). Negative control tissue sections were incubated without the primary antibody. Next, the sections were stained using a two-step EnVision System (Dako Cytomation, Santa Clara, CA, USA) according to manufacturer’s instructions. Each tissue section was viewed under a microscope, and ten arteries of each type (arcuate, interlobular and afferent) were analyzed using the Motic Med 6.0 digital medical image analysis system. The amount of signal-positive area relative to the vascular area was calculated, and the mean values were used to evaluate gene expression.
In situ hybridization (ISH)
Paraffin embedded sections (4 μm) were deparaffinized and treated with pepsin (Boster, Beijing, China) for 15 min at 37°C. After post-fixation in 4% paraformaldehyde (PFA), the sections were hybridized with digoxigenin (DIG)-labeled nucleic acid probes (40 ng/μL) at 42°C overnight. The oligonucleotide sequence used for Jagged1 was 5’-CGGATGTCTTCGGCAGAAATGGC-3’, and was synthesized by Sangon Bio (Shanghai, China). Negative control hybridizations were performed with the omission of probes. The slides were sequentially washed in 2×SSC, 0.5×SSC, and 0.2×SSC at 37°C and then sequentially incubated with the blocking buffer for 30 min at 37°C, with anti-DIG/biotin conjugate antibodies (Boster, Beijing, China) for 60 min at 37°C, and with streptavidin-biotin complex (SABC) for 60 min at 37°C. Finally, the slides were stained with 0.05% DAB (Boster, Beijing, China). Each section was examined under a microscope, and ten arteries of each type (arcuate, interlobular, and afferent) were analyzed using the Motic Med 6.0 digital medical image analysis system. The amount of Jagged1 positive area relative to the vascular area was calculated, and the mean values were used to evaluate Jagged1 expression.
qPCR
Tissue samples were pulverized in liquid nitrogen and the dispersed cells were collected by centrifugation at 10,000 g for 1 min. The cells were then treated with Trizol reagent (100 mg tissue: 1 mL Trizol, cells: 600 μL Trizol) (Invitrogen, Carlsbad, CA, USA). The cell solution was transferred to an Ep tube and 200 μL of chloroform was added. After vibration for 15 sec, 500 μL of isopropanol was added to the upper aqueous phase and the 2-phase solution was let sit for 10 min. The solution was then centrifuged at 12000 g for 10 min, and 1 mL of 75% ethanol was added to the precipitate. The precipitate was then pelleted by centrifugation at 7500 g for 5 min at 4°C; after which, the supernatant was removed and the tube was dried for 10 min. Next, the RNA-containing pellet was dissolved in DEPC water, and its RNA content and purity were determined by ultraviolet spectroscopy. A reaction solution containing the following ingredients was prepared according to instructions provided with the reaction kit: 2 μg total RNA, 1 μL oligo primer (50 μM), 1 μL dNTP mix (10 μM), and ddH2O. The solution was pre-incubated for 5 min at 65°C. Next, a cDNA first chain synthesis reaction system containing the following ingredients was prepared: 2 μL 10×RT buffer, 4 μL MgCl2 (25 μM), 2 μL DTT (0.1 M), l μL RNAase OUT (40 U/μL), 1 μL SuperScrip III RT (200 U/μL), and ddH2O (Promega, USA). The reaction conditions were as follows: 50°C for 50 min followed by 85°C for 5 min. Real-time quantitative PCR was then performed using a SYBR Premix Ex Taq GC kit (Takara, Japan), a LightCycler 480 PCR machine (Roche Diagnostics, Indianapolis, IN, USA), and the following conditions: denaturation at 94°C for 30 sec, followed by 40 cycles of denaturation at 94°C for 5 sec and annealing at 60°C for 30 sec. The primer sequences used and amplification lengths are shown in Table 1. GAPDH was used as an internal control. The relative levels of gene expression were semiquantitatively analyzed using the 2-ΔΔCt method; where 2-ΔΔCt = gene copy number in the test group/gene copy number in the control. All the experiments were conducted in triplicate.
Table 1.
Primer sequences used in the qPCR assay
| ID | Sequence (5’-3’) | Product (bp) |
|---|---|---|
| GAPDH F | CCTCGTCTCATAGACAAGATGGT | 169 |
| GAPDH R | GGGTAGAGTCATACTGGAACATG | |
| jagged1 F | CAAAGTGTGCCTCAAGGAGT | 147 |
| jagged1 R | AAAGGCAGTACGATGCGATT | |
| notch1-cd F | CGTGGACAAGATCAACGAGT | 235 |
| notch1-cd R | ACCATCCTTGCACAAACCAT | |
| hes5 F | CCCAACTCCAAACTGGAGAA | 128 |
| hes5 R | CACGAGTAACCCTCGCTGTA |
F, Forward primer; R, Reverse primer.
Western blotting assay
Total cellular proteins were extracted using reagents in a radio immunoprecipitation assay (RIPA) kit, and the protein extracts were centrifuged at 10000 g for 30 min. The bicinchoninic acid (BCA) assay was used to quantify the amount of total protein in each extract. Next, a 50 μg sample of protein from each extract was separated by electrophoresis on an 8% sodium dodecyl sulfate polyacrylamide gel for 3 h, and the separated protein bands were transferred onto a polyvinylidene fluoride (PVDF) membrane by electrophoresis at 200 mA for 100 min. After being blocked with 5% skimmed milk at room temperature for 60 min, the membrane was incubated with the following primary antibodies (anti-notch1, anti-jagged1, anti-hes5, anti-p-STAT3, anti-MnSOD, anti-XO, and anti-GAPDH; Abcam, USA) at 4°C overnight. Following incubation, the membrane was washed with phosphate buffered saline Tween 20 (PBST) solution and then incubated with a HRP-labeled secondary antibody (Abcam, USA) at room temperature for 60 min. The membrane was then treated with electrochemiluminescence (ECL) reagent and developed.
Hoechst staining
Cells were treated with berberine (50 μM) for 48 h and then harvested by trypsinization. After being washed twice with phosphate buffered saline (PBS) solution, the cells were resuspended in binding buffer and cultured at 37°C for 24 h; after which, they were stained with 0.1 μg/mL Hoechst 33342 (Sigma-Aldrich, Temecula, CA, USA). A fluorescence microscope (Olympus IX71; Olympus Corporation, Tokyo, Japan) equipped with a filter for Hoechst 33342 (365 nm) was used to detect any changes in nuclear morphology.
Reactive oxygen species (ROS)
Cells were digested and incubated for 20 min at 37°C with a 0.1% 2’,7’-Dichlorodihydrofluorescein diacetate (DCFH-DA) probe (Beyotime, Beijing, China) that had been diluted with serum-free medium to a final concentration of 10 mM, as recommended in the manufacturer’s instructions The cells were then washed three times with PBS and tested with a Beckman Coulter CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, USA).
Statistical analysis
All statistical analyses were performed using SPSS Statistics for Windows, Version 17.0 (SPSS Inc., Chicago, IL, USA). Statistical results are presented as the mean ± SD. Differences between groups were evaluated by one-way ANOVA, followed by the Bonferroni (data with homogeneity of variance) or Game-Howell (data with uneven variance) post-hoc comparison test. Correlations between variables were assessed by Pearson’s correlation analysis. Statistical significance was defined as a P-value < 0.05.
Results
Hyperuricemia caused preglomerular vascular disease in the RK model
The 5/6 nephrectomies were performed to induce CKD in the rats, and oxonic acid (OA) was given to induce hyperuricemia. The successful establishment of a CKD model was confirmed by the significant increases in proteinuria, BUN, creatinine levels, glomerulosclerosis, and interstitial fibrosis (Table 2). An OA concentration of 750 mg/kg was selected for use in this study based on the body weights, proteinuria levels, and serum UA levels of the rats. Administration of OA to the RK rats accelerated their renal disease progression (Figure 1A). The morphology of preglomerular arteries was analyzed by PAS staining. Histological examinations revealed that extensive renal ablation had induced prominent periarterial adventitial fibrosis and thickening of the preglomerular artery (arcuate artery, interlobular artery, and afferent artery) wall (Figure 1B). OA administration caused hypertrophy of the preglomerular vessels, as indicated by their higher media to lumen ratios (M/L) when compared with those of RK rats (Figure 1C).
Table 2.
The rat body weights and levels of proteinuria, serum UA, BUN, and creatinine before and after treatment with GTPs
| Group | 4 w | 8 w | 12 w | |
|---|---|---|---|---|
| Body weight | Sham | 50.00 ± 10.00 | 73.57 ± 20.14 | 101.67 ± 18.92 |
| RK | 35.71 ± 6.34a | 49.28 ± 8.38a | 71.00 ± 11.40a | |
| RK + OA | 24.00 ± 6.48a,b | 25.71 ± 7.86a,b | 48.57 ± 16.51a,b | |
| RK + OA + Tea low dose | 25.00 ± 5.77a,b,c | 41.00 ± 7.07a,c | 66.00 ± 5.70a,c | |
| RK + OA + Tea high dose | 28.00 ± 6.89a,b,c | 52.87 ± 9.35a,c | 69.34 ± 3.67a,c | |
| RK + OA + AP | 16.25 ± 6.29a,b,c | 39.33 ± 8.75a,c | 65.00 ± 10.36a,c | |
| Proteinuria | Sham | 3.12 ± 1.06 | 3.10 ± 1.00 | 3.16 ± 0.95 |
| RK | 5.31 ± 1.72a | 7.13 ± 1.21a | 7.64 ± 1.61a | |
| RK + OA | 8.40 ± 2.05a,b | 9.49 ± 1.55a,b | 5.58 ± 1.29a,b | |
| RK + OA + Tea low dose | 5.42 ± 1.49a,c,d | 4.26 ± 1.04b,c | 5.13 ± 1.44a,b | |
| RK + OA + Tea high dose | 5.25 ± 1.34a,c,d | 4.15 ± 0.87b,c | 4.64 ± 1.05a,b | |
| RK + OA + AP | 7.77 ± 2.26a,b | 5.72 ± 1.57a,c | 5.59 ± 1.79a | |
| Serum UA | Sham | 87.10 ± 23.06 | 87.60 ± 21.30 | 86.93 ± 20.63 |
| RK | 87.88 ± 28.49 | 102.55 ± 22.52 | 77.66 ± 20.75 | |
| RK + OA | 206.10 ± 59.15a,b | 226.48 ± 36.89a,b | 195.88 ± 51.67a,b | |
| RK + OA + Tea low dose | 180.82 ± 38.04a,b | 153.96 ± 31.52a,b,c | 143.38 ± 36.68a,b,c | |
| RK + OA + Tea high dose | 175.35 ± 34.16a,b | 137.58 ± 28.18a,b,c | 130.74 ± 40.34a,b,c | |
| RK + OA + AP | 154.24 ± 28.55a,b,c | 147.95 ± 25.27a,b,c | 135.05 ± 33.80a,b,c | |
| BUN | Sham | 5.34 ± 1.40 | 5.31 ± 1.32 | 5.27 ± 1.46 |
| RK | 8.77 ± 1.25a | 10.74 ± 1.08a | 12.13 ± 1.48a | |
| RK + OA | 11.66 ± 0.91a,b | 13.06 ± 2.72a,b | 15.72 ± 4.43a,b | |
| RK + OA + Tea | 12.64 ± 2.23a,b | 9.44 ± 1.38a,c,d | 9.54 ± 1.53a,c | |
| RK + OA + AP | 12.28 ± 3.77a,b | 13.37 ± 1.58a,b | 11.18 ± 2.15a,c | |
| Creatinine | Sham | 52.36 ± 11.75 | 52.36 ± 10.77 | 57.85 ± 9.81 |
| RK | 68.03 ± 12.56a | 70.05 ± 9.07a | 78.20 ± 13.73a | |
| RK + OA | 88.10 ± 15.15a,b | 93.15 ± 16.42a,b | 102.30 ± 20.15a,b | |
| RK + OA + Tea | 87.32 ± 10.74a,b | 73.25 ± 10.93a,c | 66.13 ± 9.62c,d | |
| RK + OA + AP | 97.64 ± 7.33a,b | 75.33 ± 12.81a,c | 83.48 ± 10.59a,c |
P < 0.05 vs. Sham;
P < 0.05 vs. RK;
P < 0.05 vs. RK + OA;
P < 0.05 vs. RK + OA + AP.
AP: allopurinol.
Figure 1.
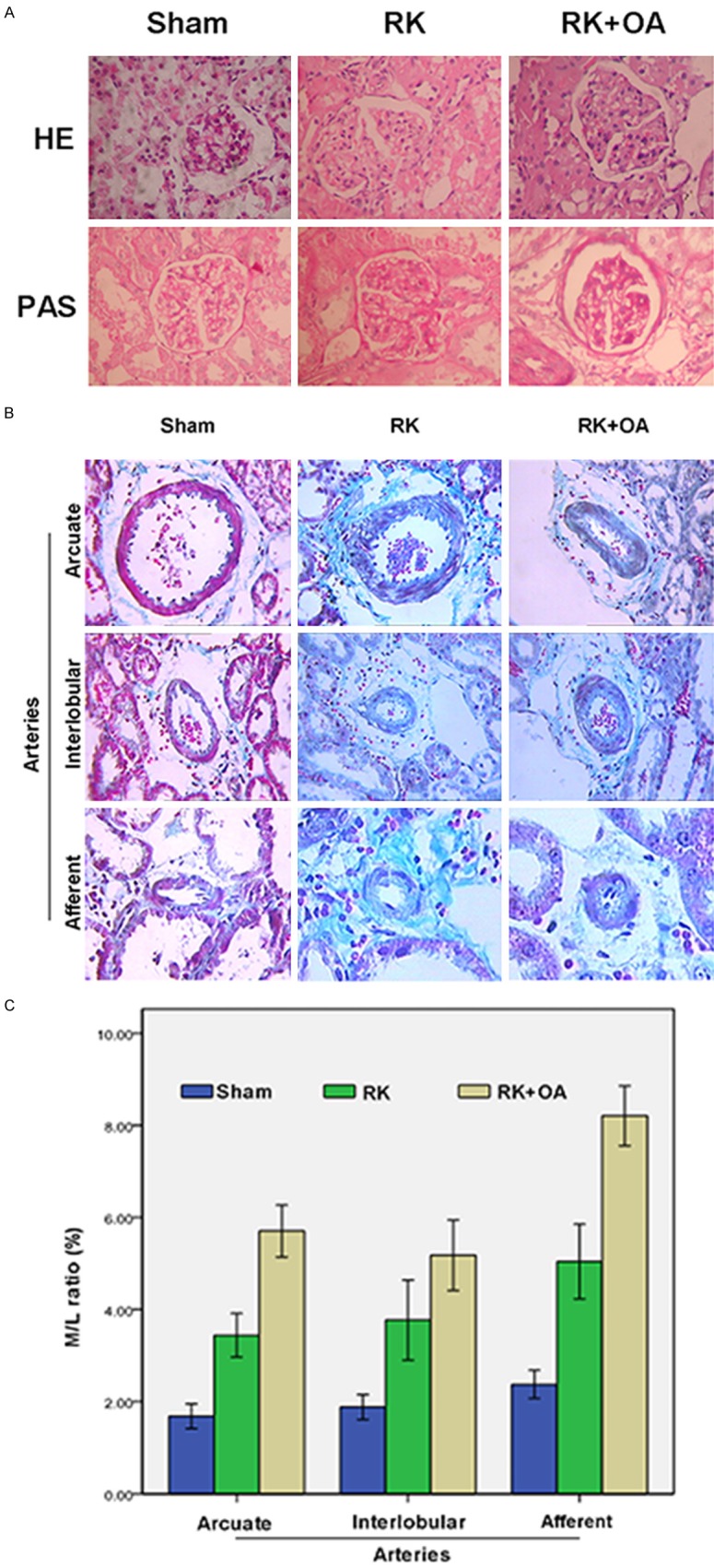
The morphology of glomeruli and preglomerular arteries. A: Histologic examinations were performed after H&E staining (upper panel) and PAS staining (lower panel) to observe the morphology of glomeruli in the Sham, RK, and RK + OA groups. OA aggravated renal damage in RK rats. B: Representative images of arcuate arteries (×200), interlobular arteries (×200), and afferent arteries (×400). C: The wall thickness of preglomerular arteries was evaluated by calculating the M/L ratio. OA promoted periarterial adventitial fibrosis and thickening of the preglomerular artery in RK rats
.
Effects of GTPs on preglomerular arteriopathy
Because GTPs can function as antioxidants, we investigated the effect of GTPs on preglomerular arteriopathy. The results showed that when compared with PA symptoms in the RK + OA group, the pathological symptoms of PA were remarkably alleviated following the treatment with GTPs (Figure 2A). Furthermore, the mean weight of rats in RK + OA + GTP group was significantly higher than that in the RK + OA group, and the levels of proteinuria, serum UA, BUN, and creatinine in the RK + OA + GTP group all decreased in a GTP-dose dependent manner (Table 2). Data concerning the M/L ratio of the preglomerular artery indicated that the values for the arcuate, interlobular, and afferent arteries of rats in the RK + OA + GTP group were significantly decreased when compared to those values in the RK + OA group (Table 3), and this was further confirmed by H&E and PAS staining data (Figures S1 and S2). This implied that GTP treatment could effectively prevent the damage caused by preglomerular vascular disease in the hyperuricemic CKD model.
Figure 2.
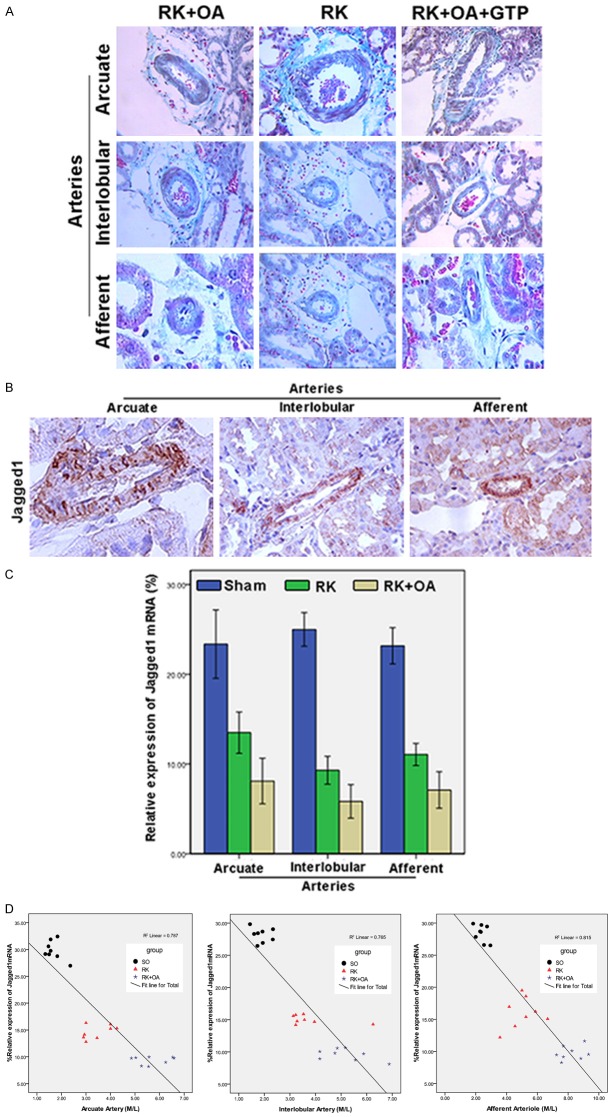
Effect of GTPs and Jagged1 on preglomerular arteriopathy. A: GTPs relieved the pathological symptoms caused by OA in RK rats. Representative images of arcuate arteries (×200), interlobular arteries (×200), and afferent arteries (×400). B: Expression of Jagged1 mRNA on the preglomerular arteries of rats in the RK + OA group was analyzed by ISH (representative images). C: The levels of Jagged1 mRNA on the preglomerular arteries in the Sham, RK, and RK + OA groups. D: The M/L ratios of the preglomerular arteries were determined by Pearson’s correlation analysis.
Table 3.
The media to lumen ratio of preglomerular artery
| Group | N | Arcuate artery | Interlobular artery | Afferent artery |
|---|---|---|---|---|
| Sham | 8 | 1.68±0.32 | 1.88±0.32 | 2.38±0.36 |
| RK | 8 | 3.44±0.56** | 3.77±1.04** | 5.05±0.97** |
| RK+OA | 8 | 5.71±0.68**,‡ | 5.18±0.91**,† | 8.21±0.78**,‡ |
| RK+OA+GTP | 8 | 2.49±0.43**,‡,Δ | 2.69±0.40*,‡,Δ | 3.52±0.55**,‡,Δ |
P < 0.05 vs. Sham;
P < 0.01 vs. Sham;
P < 0.05 vs. RK;
P < 0.01 vs. RK;
P < 0.01 vs. RK+OA.
Involvement of the jagged1/notch1 pathway in preglomerular vascular lesions
To investigate the mechanism underlying preglomerular arteriopathy, the expression of Jagged1 was examined using ISH (Figure 2B). In the normal rats, most of the Jagged1 mRNA was found on the arterial intima and media, and only small amounts were found on the arterial adventitia. Rats in the RK and RK + OA groups displayed significantly decreased levels of Jagged1 expression in the arcuate (Sham vs. RK vs. RK + OA, 23.36 ± 4.56 vs. 13.49 ± 2.74 vs. 8.10 ± 3.04, P < 0.01), interlobular (Sham vs. RK vs. RK + OA, 24.99 ± 2.24 vs. 9.29 ± 1.86 vs. 5.83 ± 2.23, P < 0.01), and afferent arteries (Sham vs. RK vs. RK + OA, 23.17 ± 2.43 vs. 11.06 ± 1.48 vs. 7.10 ± 2.43, P < 0.01) (Figure 2C). Moreover, the levels of Jagged1 mRNA were significantly correlated with the M/L ratios of the preglomerular arteries (Figure 2D).
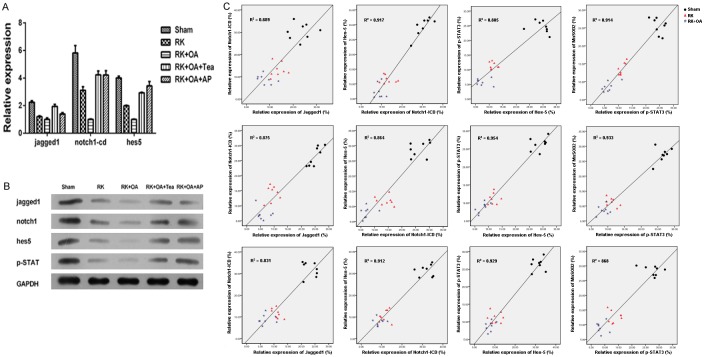
Jagged1/Notch1 signaling is critical for vascular remodeling [19]. Accordingly, we determined the levels of Jagged1, Notch1-ICD, and Hes5 on the preglomerular vessels (Figure 3A). Our results showed that the levels of Jagged1, Notch1-CID, and Hes5 were significantly lower in the RK rats, and were further decreased in the hyperuricemic RK rats when compared to their levels in the control rats (Figure 3B), suggesting that Notch1 signaling is suppressed during the formation preglomerular vascular lesions. Similarly, the preglomerular vascular levels of MnSOD2, a ROS scavenger, and also p-STAT3 expression, were decreased in the RK group and were lowest in the RK + OA group (Figure 3C and 3D). In contrast, XO, which promotes ROS production, exhibited higher levels in the RK group and reached its highest level in the RK + OA group (Figure 3C and 3D). qPCR and western blot studies indicated that the levels of Jagged1, Notch1-ICD, Hes5, and p-STAT3 were significantly decreased in RK + OA group when compared to those in the RK group (Figure 4A and 4B). Importantly, a quantitative analysis revealed positive correlations among the levels of Notch1-ICD, Jagged1, Notch1-ICD, Hes5, p-STAT3, and MnSOD2 in the RK + OA group, (Figure 4C), suggesting that Jagged1/Notch1-STAT3-MnSOD2 signal transduction plays a role in the progression of preglomerular vascular injuries.
Figure 3.
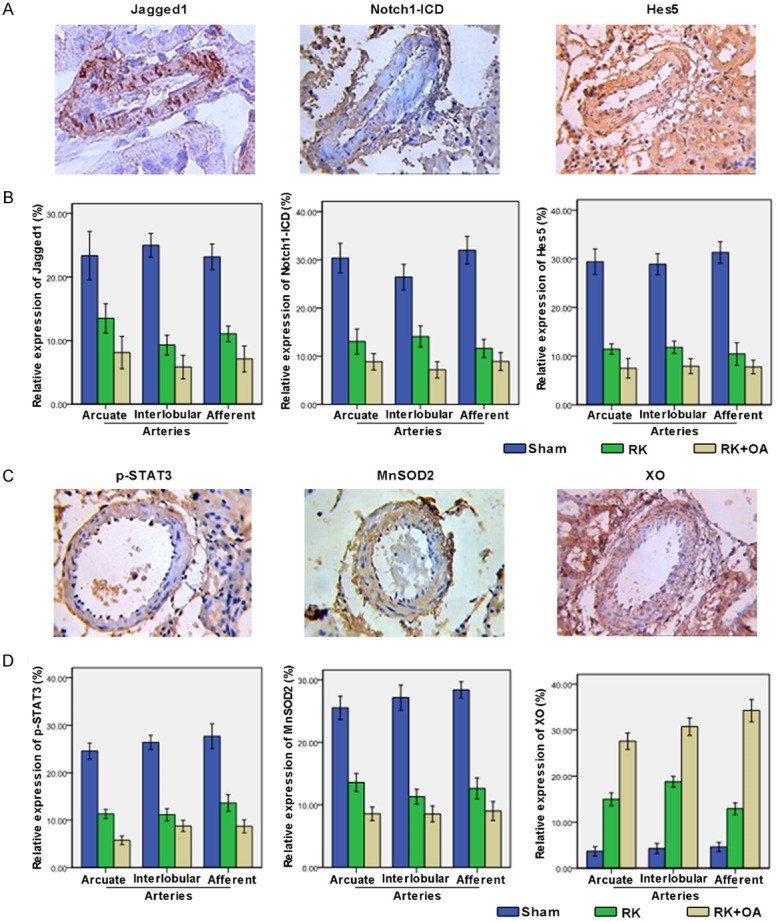
Expression of Jagged1, Notch1-ICD, Hes5, p-STAT3, MnSOD2, and XO on the preglomerular arteries. A: Expression of Jagged1, Notch1-ICD, and Hes5 on the preglomerular arteries was analyzed by IHC, and representative images are shown. B: Quantitative analysis of the Jagged1, Notch1-ICD, and Hes5 levels. C: Representative images of p-STAT3, MnSOD2, and XO staining in the Sham group. D: The levels of p-STAT3, MnSOD2, and XO on the preglomerular arteries in each group were quantified.
Figure 4.
Expression of Jagged1, Notch1-ICD, Hes5, and p-STAT3 and the correlations among the Sham, RK, RK + OA, RK + OA + Tea, and RK + OA + Tea + AP groups. A: mRNA levels were detected by qPCR. The levels of Jagged1, Notch1-ICD, and Hes5 were remarkably decreased in the RK rats and RK rats treated with OA. The administration of Tea or AP reversed the effect of OA. B: Protein levels were measured by western blot methods. Jagged1, Notch1-ICD, Hes5, and p-STAT3 expression was reduced in the RK rats and RK rats treated with OA. The administration of Tea or AP reversed the effect of OA. C: Correlations between the levels of Jagged1, Notch1-ICD, Hes5, and p-STAT3, A Pearson’s correlation analysis showed positive correlations among the levels of Notch1-ICD, Jagged1, Notch1-ICD, Hes5, p-STAT3, and MnSOD2.
Effect of GTPs on preglomerular arteriopathy via the Jagged1/Notch1-STAT3 pathway
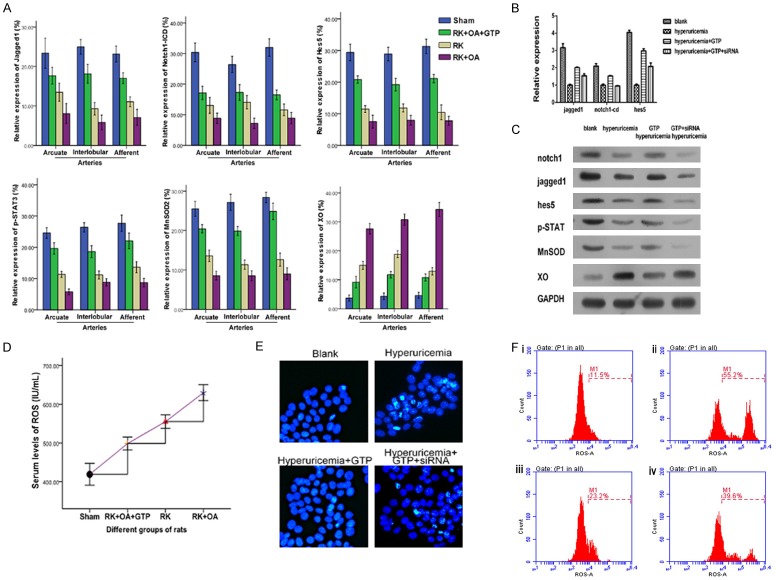
When compared with the RK + OA group, treatment with GTPs increased the expressions of Jagged1, Notch1-ICD, Hes5, and p-STAT3 to levels similar to those in the positive control group (RK + OA + AP (allopurinol)) (Figure 4). We also investigated whether the regulatory role played by GTPs in preglomerular arteriopathy might be mediated via the Jagged1/Notch1-STAT3 pathway. Treatment with GTPs significantly restored Jagged1, Notch1-ICD, Hes5, p-STAT3, and MnSOD2 expression on preglomerular vessels. In contrast, the preglomerular vascular levels of XO in the RK + OA + GTP group were significantly lower than those in the RK + OA group (Figure 5A). Additionally, GTPs significantly reduced ROS levels in the hyperuricemic RK rats (RK + OA + GTP vs. RK + OA = 498.65 ± 20.19 vs. 629.79 ± 24.48, P < 0.01). Collectively, GTPs increased activity of the Jagged1/Notch1-STAT3 signaling pathway via their ability to inhibit ROS formation (Figure 5D) and reduce UA levels (Table 1). Our in vitro data indicated that the ability of GTPs to induce Jagged1, Notch1 Hes5, p-STAT3, and MnSOD2 expression, and reduce XO, ROS and cell apoptosis levels, was lost when Jagged1/Notch1 signaling was inactivated. This suggests that GTPs alleviate preglomerular arteriopathy by affecting the Jagged1/Notch1-STAT3 pathway (Figure 5B, 5C, 5E, 5F).
Figure 5.
Effects of GTP on preglomerular arteriopathy via the Jagged1/Notch1-STAT3 pathway. A: The levels of Jagged1, Notch1-ICD, Hes5, p-STAT3, MnSOD2, and XO on preglomerular arteries were analyzed by IHC and quantified. GTPs alleviated the damage caused by OA in RK rats by increasing the levels of Jagged1, Notch1-ICD, Hes5, p-STAT3, and MnSOD2 and reducing XO expression. B: The levels of mRNA for Jagged1, Notch1-ICD, and Hes5 were detected by qPCR. GTPs inhibited the impact of OA by elevating Jagged1, Notch1-ICD, and Hes5 expression, while GTP siRNA abolished the effect of GTPs. C: Western blot detection methods showed that the levels of Jagged1, Notch1-ICD, Hes5, p-STAT3, and MnSOD proteins were increased in the GTP group, but were negatively regulated by OA and GPT siRNA. D: GTPs reduced the ROS content in RK rats treated with OA. E: The effect of GTPs on HK-1 cell apoptosis was examined by Hoechst staining. GTPs suppressed the cell apoptosis that was previously induced by hyperuricemia. F: GTPs impeded the ROS production caused by hyperuricemia. i, BLANK; ii, Hyperuricemia; iii, Hyperuricemia + GTP; iv, Hyperuricemia + GTP + siRNA.
Discussion
Renal arteriopathy is responsible for arterial hypertension that occurs during the progression various renal diseases [4,20]. Prevention of preglomerular arteriopathy may be an attractive approach for treating CKD. Clinical and animal studies have demonstrated that high levels of UA can result in preglomerular vascular damage [2-5,12,21,22]. Recent reports have suggested that the aberrant alterations found in preglomerular vessels may result from oxidative stress [12,21] and abnormal regulation of the angiotensin system [2,3,22]. Herein, we propose that green tea polyphenol compounds may help to alleviate hyperuricemia-induced preglomerular arteriopathy. Accumulating evidence indicates that GTPs have potent anti-oxidative properties that may account for their beneficial effects in preventing the oxidative stress associated with ischemia injuries and renal damage [23,24]. Consistent with those findings, our results indicate that treatment with GTPs prevented damage to the preglomerular vascular in a hyperuricemic CKD model.
The Notch pathway plays important roles in angiogenesis and vasculature maintenance under both physiological and pathological conditions [25]. On the other hand, the roles played by ROS and redox events in regulating vascular morphogenesis have only recently been discovered [26]. Interestingly, several studies have suggested a correlation between Notch pathway activity and oxidative stress [8,27-29]. Because GTPs help regulate anti-oxidative stress, we speculate that Notch1 signaling may be involved in the ability GTPs to protect against preglomerular arteriopathy. Our in vivo results showed that Jagged1/Notch1 pathway activity was associated with the severity of preglomerular vascular diseases in hyperuricemic RK rats. Moreover, the expression pattern of Jagged1/Notch1 signaling on preglomerular vessels was consistent with that of activated STAT3 and MnSOD2, but displayed a trend opposite to that of XOD and ROS. It was noteworthy that restoration of the Jagged1/Notch1-STAT3 pathway by GTPs significantly impaired ROS production and thereby protected hyperuricemic RK rats against preglomerular vascular damage.
In HUVEC cells, Notch1 signaling was shown to modulate ROS generation by suppressing Nox4 expression [27]. In myocardial infarction, Jagged1 facilitated the activation of Notch1 by increasing Mfn2 expression and suppressing ROS production, which subsequently attenuated the injuries due to myocardial infarction [28]. In a hepatic I/R or burn-induced myocardial injury model, Notch1 increased MnSOD expression by activating STAT3, and thereby promoted the scavenging role of ROS [8,29]. Consistent with those findings, our in vivo results further verified the antioxidant role played by Notch1 and the underlying mechanism mediated by Notch1-STAT3 signal transduction in our model of CKD with hyperuricemia.
XO inhibitors such as allopurinol and topiroxostat are clinically approved for use in the treatment of hyperuricemia, and have been demonstrated to prevent the thickening of preglomerular artery walls in hyperuricemic RK rats [22,30,31]. Accumulating evidence has shown that polyphenol compounds extracted from natural products can suppress the expression or activity of XO [32]. It was observed that GTP administration decreased the serum levels of uric acid in PO-induced hyperuricemic mice, partly by reducing XO expression [17]. Our results further confirmed the efficacy of GTPs in treating hyperuricemia, even though we used a different model. Moreover, GTP administration was found to increase the levels of ROS and MnSOD. When the Jagged/Notch1-STAT3 pathway was inactivated by siRNA, the effect of GTPs was significantly impeded. Future studies are required to determine how the Jagged/Notch1-STAT3 pathway specifically affects the behavior of endothelial cells and/or smooth muscle cells isolated from preglomerular arteries.
Conclusion
The present study showed that GTP compounds helped to protect against CKD progression by activating the Jagged1/Notch1-STAT3 pathway. Furthermore, GTPs also alleviated preglomerular vascular lesions and reduced apoptosis and ROS levels. Our results highlight the clinical benefits of using GTPs in treatment of CKD, as well as the importance of regulating Notch1 pathway activity.
Acknowledgements
This research was supported by National Natural Science Foundation of China (No. 30500546).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039–2052. doi: 10.1016/S0140-6736(16)00346-9. [DOI] [PubMed] [Google Scholar]
- 2.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 3.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Lozada LG, Tapia E, Santamaria J, Avila-Casado C, Soto V, Nepomuceno T, Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67:237–247. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 5.Kohagura K, Kochi M, Miyagi T, Kinjyo T, Maehara Y, Nagahama K, Sakima A, Iseki K, Ohya Y. An association between uric acid levels and renal arteriolopathy in chronic kidney disease: a biopsy-based study. Hypertens Res. 2013;36:43–49. doi: 10.1038/hr.2012.135. [DOI] [PubMed] [Google Scholar]
- 6.Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13:654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindner V, Booth C, Prudovsky I, Small D, Maciag T, Liaw L. Members of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interaction. Am J Pathol. 2001;159:875–883. doi: 10.1016/S0002-9440(10)61763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai W, Yang X, Han S, Guo H, Zheng Z, Wang H, Guan H, Jia Y, Gao J, Yang T, Zhu X, Hu D. Notch1 pathway protects against burn-induced myocardial injury by repressing reactive oxygen species production through JAK2/STAT3 signaling. Oxid Med Cell Longev. 2016;2016:5638943. doi: 10.1155/2016/5638943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, Fong GH, Sakmar TP, Rafii S, Ding BS. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med. 2016;22:154–162. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Takeshita K, Liu PY, Satoh M, Oyama N, Mukai Y, Chin MT, Krebs L, Kotlikoff MI, Radtke F, Gridley T, Liao JK. Smooth muscle Notch1 mediates neointimal formation after vascular injury. Circulation. 2009;119:2686–2692. doi: 10.1161/CIRCULATIONAHA.108.790485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie H, Sun J, Chen Y, Zong M, Li S, Wang Y. EGCG attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the NOTCH pathway. Oxid Med Cell Longev. 2015;2015:214836. doi: 10.1155/2015/214836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, Franco M, Rodriguez-Iturbe B, Johnson RJ. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol. 2008;295:F1134–1141. doi: 10.1152/ajprenal.00104.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abuajah CI, Ogbonna AC, Osuji CM. Functional components and medicinal properties of food: a review. J Food Sci Technol. 2015;52:2522–2529. doi: 10.1007/s13197-014-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Afzal M, Safer AM, Menon M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology. 2015;23:151–161. doi: 10.1007/s10787-015-0236-1. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh SR, Hsu CS, Lu CH, Chen WC, Chiu CH, Liou YM. Epigallocatechin-3-gallate-mediated cardioprotection by Akt/GSK-3beta/caveolin signalling in H9c2 rat cardiomyoblasts. J Biomed Sci. 2013;20:86. doi: 10.1186/1423-0127-20-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Tan ML, Li KK, Leung PC, Ko CH. Green tea polyphenols decreases uric acid level through xanthine oxidase and renal urate transporters in hyperuricemic mice. J Ethnopharmacol. 2015;175:14–20. doi: 10.1016/j.jep.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 18.Gadola L, Noboa O, Marquez MN, Rodriguez MJ, Nin N, Boggia J, Ferreiro A, Garcia S, Ortega V, Musto ML, Ponte P, Sesser P, Pizarrosa C, Ravaglio S, Vallega A. Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int. 2004;65:1224–1230. doi: 10.1111/j.1523-1755.2004.00496.x. [DOI] [PubMed] [Google Scholar]
- 19.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill GS, Heudes D, Jacquot C, Gauthier E, Bariety J. Morphometric evidence for impairment of renal autoregulation in advanced essential hypertension. Kidney Int. 2006;69:823–831. doi: 10.1038/sj.ki.5000163. [DOI] [PubMed] [Google Scholar]
- 21.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 22.Berni A, Boddi M, Fattori EB, Cecioni I, Berardino S, Montuschi F, Chiostri M, Poggesi L. Serum uric acid levels and renal damage in hyperuricemic hypertensive patients treated with renin-angiotensin system blockers. Am J Hypertens. 2010;23:675–680. doi: 10.1038/ajh.2010.33. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh SR, Cheng WC, Su YM, Chiu CH, Liou YM. Molecular targets for anti-oxidative protection of green tea polyphenols against myocardial ischemic injury. Biomedicine (Taipei) 2014;4:23. doi: 10.7603/s40681-014-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokozawa T, Noh JS, Park CH. Green tea polyphenols for the protection against renal damage caused by oxidative stress. Evid Based Complement Alternat Med. 2012;2012:845917. doi: 10.1155/2012/845917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Sewduth R, Santoro MM. “Decoding” angiogenesis: new facets controlling endothelial cell behavior. Front Physiol. 2016;7:306. doi: 10.3389/fphys.2016.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai WX, Liang L, Wang L, Han JT, Zhu XX, Han H, Hu DH, Zhang P. Inhibition of Notch signaling leads to increased intracellular ROS by up-regulating Nox4 expression in primary HUVECs. Cell Immunol. 2014;287:129–135. doi: 10.1016/j.cellimm.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Pei H, Du J, Song X, He L, Zhang Y, Li X, Qiu C, Zhang Y, Hou J, Feng J, Gao E, Li D, Yang Y. Melatonin prevents adverse myocardial infarction remodeling via Notch1/Mfn2 pathway. Free Radic Biol Med. 2016;97:408–417. doi: 10.1016/j.freeradbiomed.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Yu HC, Qin HY, He F, Wang L, Fu W, Liu D, Guo FC, Liang L, Dou KF, Han H. Canonical notch pathway protects hepatocytes from ischemia/reperfusion injury in mice by repressing reactive oxygen species production through JAK2/STAT3 signaling. Hepatology. 2011;54:979–988. doi: 10.1002/hep.24469. [DOI] [PubMed] [Google Scholar]
- 30.Burns CM, Wortmann RL. Gout therapeutics: new drugs for an old disease. Lancet. 2011;377:165–177. doi: 10.1016/S0140-6736(10)60665-4. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka A, Nakamura T, Sato E, Node K. Clinical effects of topiroxostat on renal and endothelial function in a patient with chronic kidney disease and hyperuricemic arteriolopathy: a case report. Drugs R D. 2017;17:97–101. doi: 10.1007/s40268-016-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suganya N, Bhakkiyalakshmi E, Sarada DV, Ramkumar KM. Reversibility of endothelial dysfunction in diabetes: role of polyphenols. Br J Nutr. 2016;116:223–246. doi: 10.1017/S0007114516001884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.