Abstract
Clinical management of many chronic ophthalmological disorders requires direct delivery of drugs into the vitreous. There is an important need to investigate novel needle-less alternatives to deliver drugs to the vitreous. The purpose of this study is to assess the effects of a needle-less system using ultrasound to enhance vitreal delivery of small molecules through the sclera in an ex vivo model and to evaluate whether changes in permeability are mainly due to the heat generated by sonication. An eye cup containing 1 mL of sodium fluorescein 0.1% was placed on top of the sclera of cadaveric rabbit eyes. Treated eyes were sonicated for 10 minutes, and left in contact with the fluorescein solution for an additional 50 minutes. Control eyes received the same exposure to fluorescein solution (60 minutes) in the eye cup without ultrasound treatment. Vitreous humor was collected and analyzed using a fluorescence spectrophotometer to calculate the concentration of fluorescein that diffused into the vitreous humor. An additional set of eyes was treated using a heating probe to evaluate whether changes in permeability were mainly due to heat. Vitreous samples from ultrasound-treated eyes showed a 44.6% higher concentration of fluorescein compared to control eyes. The concentration of fluorescein in the vitreous of heat-treated eyes did not show a significant difference when compared to control eyes. Thus, phonophoresis is a promising needle-less method for vitreal drug delivery, and local heating conducted to the surface of the sclera should be mitigated because it does not enhance the efficacy of the method.
Keywords: Ultrasonic, sclera, drug delivery systems, vitreous, intravitreal
Introduction
Current clinical management of many chronic ophthalmological disorders such as age-related macular degeneration (AMD) and diabetic retinopathy (DR) requires local delivery of steroids, anti-vascular endothelial growth factors and/or other therapeutic compounds inside the vitreous [1-4]. Intravitreal injection (IVI) is performed by inserting a needle through the sclera and is the most common procedure used to deliver drugs into the vitreous, with an estimated total of 5.9 million IVIs performed in the US in 2016 [5]. The percent risk of complications related to IVI may be considered low, but due to the large number of procedures performed, in absolute terms there are many cases of retinal detachment, cataract, and vision threatening endophthalmitis that have been reported in the literature [6,7]. In addition to that, IVI can be a stressful procedure for the patient because it is not painless and multiple injections are often required to treat chronic retinal disorders due to the short half-life of the injected drugs [8,9]. Surgically implanted slow-release devices are being developed as an alternative to monthly or bimonthly IVIs, but despite lasting 5 to 10 times longer than IVIs, they require a more complex procedure and have a higher risk of complications [10-13]. Therefore, there is an important clinical need to investigate novel alternatives to deliver drugs to the vitreous.
The use of ultrasound waves to enhance the movement of drugs through intact skin and cornea has been reported since the 1950s in Europe [14-16]. The mechanisms by which ultrasound enhances drug delivery are not fully understood and include acoustic radiation forces, acoustic streaming, and acoustic cavitation [17-19]. We recently reported the successful use of ultrasound energy, applied with an 880 kHz ultrasound transducer, as a means to increase the delivery of riboflavin into the cornea [20]. The vitreous humor comprises a gel-like collagenous solution that fills the posterior segment of the eye, between the lens and the retina. The most direct route of access to the vitreous is through the sclera, the white, fibrous, outer protective coating of the eye. Differences in collagen types and their distribution make the optical and biomechanical properties of the sclera very different from those of the cornea [21,22].
The purpose of this study is to assess the effects of ultrasound treatment with an 880 kHz transducer on vitreal delivery of a small molecule through the sclera and to investigate if changes in permeability are mainly due to the heat generated by sonication.
Materials and methods
Fresh cadaveric rabbit eyes (Pel-Freez, Rogers, AR, USA) were shipped in saline solution on ice, and used between 24 to 30 hours after enucleation. Eyes were cleared of surface connective tissues and conjunctiva and were placed in saline solution at 35-37°C (temperature-controlled water bath) for at least 30 minutes prior to use, to equilibrate at approximate physiological temperature conditions. Temperature was held in this range for the duration of the experiment. Twenty-two eyes were randomly assigned to either ultrasound treatment (n = 12) or non-treatment as controls (n = 10).
Sodium fluorescein 0.1% (Sigma, St. Louis, MI, USA) in phosphate buffered solution (PBS) was chosen to represent the therapeutic topical solution used during treatment. The solution was protected using aluminum foil to prevent light degradation and warmed up to 35-37°C before treatment. A customized eye cup (surface area 133 mm2) was placed over each eye and lightly taped to the sclera to form a seal at approximately 4 mm posterior to the limbus (Figure 1). Care was taken not to add additional pressure to the globe. The eye cup was filled with 1 mL of sodium fluorescein 0.1% solution, and the ultrasound transducer was submerged in the center of the eye cup. The distance between the tip of the transducer and the target area of the eyes was kept between 2-2.5 mm due to the custom fit of the eye cup. Ultrasound was delivered using a UZT-1.040 Power Generator, with a 10 mm diameter planar transducer operated at 880 kHz. Ultrasound intensity output was set for ISATA = 1 W/cm2, under continuous wave operation. Treated eyes were sonicated for 10 minutes, and were left in contact with the fluorescein solution for an additional 50 minutes (n = 12). Control eyes received the same exposure to the sodium fluorescein solution (60 minutes) in the eye cup without ultrasound treatment (n = 10).
Figure 1.

Schematic of ultrasound method setup. The ultrasound transducer was placed inside a custom eye cup filled with 0.1% sodium fluorescein solution, positioned over the rabbit eye’s sclera with a contact surface area of 133 mm2. In the treated group, ultrasound waves (880 kHz, ISATA = 1 W/cm2, continuous wave mode) were applied for 10 minutes, after which the eye was left in contact with the fluorescein solution for an additional 50 minutes. Control eyes received the same exposure to the sodium fluorescein solution (60 minutes) in the eye cup without ultrasound treatment.
In order to avoid structural damage and increase in permeation results, an additional set of eyes was used to measure the variation in temperature of the vitreous underneath the treated area of the sclera and of the fluorescein solution before and after 10 minutes of the ultrasonic treatment (n = 7). Temperature readings were taken using a single junction 30-gauge needle microprobe thermocouple sensor (Physitemp, Clifton, NJ, USA) and meter (HH509R Thermometer, Omega, Stamford, CT, USA).
To investigate if changes in permeability were mainly due to the heat generated by sonication, a third group of eyes (n = 3) was treated using a heating probe of similar size and diameter to the ultrasound probe (A748 Elite Submersible Heater, Hagen, Baie d’Urfé, QC, Canada). Eyes were heat-treated for 10 minutes and then left in contact with the fluorescein solution for an additional 50 minutes. The heating probe was controlled to maintain a temperature range similar to ultrasound treated group during the 10 minutes (up to 46°C).
Vitreous humor was collected using an automated vitrectomy system (Accurus, Alcon Labs, Fort Worth, TX, USA). For direct access to the vitreous, a semi-circular incision was created 2 mm posterior to the limbus, on the opposite side of the globe from the treated area, which was stained by contact with the solution. This was done to prevent fluorescence contamination of the vitreous sample. Each sample was collected using a 25-gauge vitrectomy probe. Approximately 1 mL of vitreous humor was aspirated per eye.
Vitreous samples were light-protected and mixed well prior to analysis using a fluorescence spectrophotometer (Spectramax, Molecular Devices, Sunnyvale, ca, USA) with 492 nm excitation and 520 nm emission wavelengths. Measurements were given as Relative Fluorescence Units (RFU) and compared to standard curves of known sodium fluorescein dilutions to calculate the concentration diffused into the vitreous humor (mcg/mL).
To investigate whether the increase in fluid temperature due to self-heating of the ultrasound transducer could be mitigated with external cooling of the ultrasound hand piece, we designed a custom hydraulic cooling system (Figure 2). The same eye cup was placed on top of the ultrasound transducer, fixed in place with Parafilm® (Pechiney Plastic Packaging, Menasha, WI, USA) and filled with 1 mL of sodium fluorescein 0.1% solution at room temperature (22 to 24°C). Two microprobe thermocouple sensors were inserted: one inside the eye cup and one into the vitreous, through the sclera on the opposite side of the treated area of the globe, to measure the temperature in the center of the vitreous chamber. The eye was treated for 10 minutes with ultrasound (1 W/cm2) under continuous wave operation. Infrared thermographic temperature images of the applicator and eye, with and without the cooling system, were taken every 2.5 minutes for 10 minutes (Seek Compact Pro, Seek Thermal, Santa Barbara, CA, USA). A sheet of metal was placed in the background of the image to serve as control for the room temperature. A suture (A.C.S. Nylon 8-0, Alcon Labs, Forth Worth, TX, USA) was placed on the superior area of sclera, to help lift the eye at the end of the treatment. After 10 minutes of treatment, the human eye was lifted, quickly blotted dry, and a thermal picture was taken to show the temperature on the scleral surface. The procedure was repeated 3 times for each condition (cooling on and cooling off) using the same human eye after the temperatures of the system and vitreous had returned to and stabilized at room temperature.
Figure 2.

Ultrasound transducer with cooling system set up. A water-based cooling system was placed around the ultrasound transducer (a) and the eye cup (b) was fixed on top of the transducer with Parafilm®. Thermocouples (c) were inserted inside the eye cup and in the posterior segment of the eye (vitreous). A piece of metal was placed 10 cm away from the transducer (d) to represent the background room temperature. A nylon suture (e) was placed on the superior region of the sclera to facilitate lifting the eye after the ultrasound treatment.
Statistical analysis
Each sample of rabbit vitreous humor was measured in quadruplicate. Data is presented as mean ± SEM. Graph Pad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA) was used to plot graphs and to perform statistical analyses. Unpaired Student’s t-test was used for comparison of vitreous fluorescence values. A two-way repeated-measures ANOVA followed by Bonferroni post hoc test was used to compare the temperature values at 0, 2.5, 5, 7.5, 10 minutes in ultrasound treatment groups (with and without cooling) An alpha level of P less than or equal to 0.05 was chosen as the criterion of statistical significance.
Results
Treatment efficacy
Vitreous samples from ultrasound treated eyes showed a 44.6% higher concentration of fluorescein (mean = 4.12 ± 0.53 mcg/mL, n = 12) compared to control eyes (mean = 2.85 ± 0.37 mcg/mL, n = 10) (Figure 3). The difference was found to be statistically significant (p value = 0.036) indicating an enhancement with transscleral fluorescein delivery to the vitreous mediated by ultrasound treatment.
Figure 3.
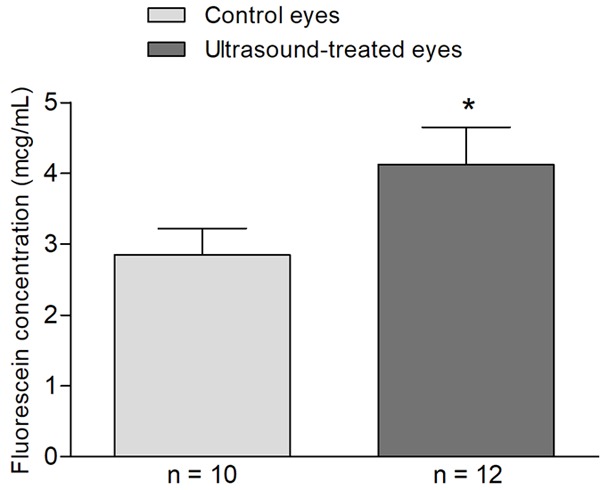
Ultrasound-enhanced intravitreal delivery of fluorescein. Rabbit’s eye cup was filled with 1 mL of sodium fluorescein 0.1% solution, and the ultrasound transducer was submerged in the center of the eye cup. Ultrasound was delivered using a UZT-1.040 Power Generator, with a 10 mm diameter planar transducer operated at 880 kHz. Ultrasound intensity output was set for ISATA = 1 W/cm2, under continuous wave operation. Treated eyes were sonicated for 10 minutes and were left in contact with the fluorescein solution for an additional 50 minutes (n = 12). Control eyes received the same exposure to the sodium fluorescein solution (60 minutes) in the eye cup without ultrasound treatment (n = 10). Immediately after 60 minutes of exposure to fluorescein, vitreous humor was collected using a 25-gauge vitrectomy probe, inserted through a semi-circular incision created 2 mm posterior to the limbus, on the opposite side of the treated area of the rabbit’s eye globe. We observed that fluorescein concentration in the vitreous of ultrasound-treated eyes (mean = 4.12 ± 0.53 mcg/mL, n = 12) is increased (*P = 0.036; unpaired Student’s t test) when compared to control eyes (mean = 2.85 ± 0.37 mcg/mL, n = 10), demonstrating the efficacy of the ultrasound method.
Significance of temperature increase on treatment efficacy
The mean temperature of the rabbit eye vitreous increased from 33.4 ± 0.1°C to 39.6 ± 0.1°C, while the temperature readings in the fluorescein solution inside the eye cup increased from 35.5 ± 0.1°C to 42.7 ± 0.8°C after 10 minutes of ultrasonic treatment (Figure 4). In order to investigate the influence of the heat alone without ultrasound, a second group of eyes were exposed to higher temperature fluid. The concentration of fluorescein in the vitreous of heat-alone treated eyes did not show a significant difference from the control eyes (p value = 0.34) (Figure 5).
Figure 4.

Temperature increase following 10 minutes of ultrasonic treatment. Temperature readings of the vitreous underneath the treated area of the sclera and of the fluorescein solution were taken using a 30-gauge needle microprobe thermocouple sensor before (T0) and after 10 minutes (T10) of the ultrasonic treatment (1 W/cm2, under continuous wave operation).
Figure 5.

Thermal effects on scleral permeability to fluorescein. An eye cup was filled with 1 mL of sodium fluorescein 0.1% solution (warmed up to 35-37°C) before treatment and a heating probe of similar size and diameter to the ultrasound probe was submerged in the center of the eye cup. In the heat-treated group, the heating probe was controlled to maintain a temperature range up to 46°C for 10 minutes and then eyes were left in contact with the fluorescein solution for an additional 50 minutes. Control eyes were left in contact with fluorescein solution for 60 minutes. Immediately after 60 minutes of exposure to fluorescein, vitreous humor was collected using a 25-gauge vitrectomy probe, inserted through a semi-circular incision created 2 mm posterior to the limbus, on the opposite side of the treated area of the rabbit’s eye globe. We observed that fluorescein concentration in the vitreous of heat-treated eyes (mean 2.55 mcg/mL ± 0.36 mcg/mL, n = 3) was not significantly different (P = 0.34; unpaired Student’s t test) when compared to control eyes (mean = 2.85 ± 0.37 mcg/mL, n = 10). This result supports the suggestion that the effects observed of increased permeation with ultrasound treatment are probably not attributable to the elevation in temperature of the treated area.
Mitigation of conductive heating
A significant decrease (P < 0.0001) in the final temperature of the solution inside the eye cup is observed when the ultrasound treatment is performed with the cooling system on (25.7 ± 0°C) compared to the treatment with the cooling system off (32.4 ± 0.6°C). The thermal images illustrate a relative increase in the surface temperature of the sclera on the treated area near the ultrasound transducer. The increase in temperature within the fluid and the eye is mitigated when the treatment is performed with the transducer’s cooling system on (Figure 6).
Figure 6.

Thermal changes during ultrasound treatment with and without cooling. Thermal images of a human globe being treated with ultrasound (1 W/cm2, continuous wave) for 10 minutes with the transducer’s cooling system off (row A) and on (row B). Color scale illustrates the relative temperatures of the surface obtained with a thermal camera with a set range of 30°C (min. = 14°C; max. = 44°C). Points with surface temperatures below 14°C appear as black pixels in the image. Values in the table represent the average temperatures measured with a thermocouple in the solution (S) and in the center of the vitreous (V) at each time point (0, 2.5, 5, 7.5, and 10 minutes). Mean values are presented in °C ± SEM, n = 3. In this experiment, we found that temperature of the solution in the group with cooling system on was reduced when compared to the group with cooling system off (F (1, 4) = 418.4, ****P < 0.0001, when compared at 2.5, 5, 7.5, and 10 minutes after application of ultrasound; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). However, when the temperature was measured in the center of the vitreous, there was no significant difference observed between the groups with cooling system on or off (F (1, 4) = 0.1432, P = 0.7243, compared at 0, 2.5, 5, 7.5, and 10 minutes after application of ultrasound; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). This result supports the suggestion that temperature increase in the eye surface is mostly due to self-heating of the ultrasound transducer being thermally conducted into the small volume of solution in the eye cup. The increase in fluid temperature is mitigated with external cooling of the ultrasound hand piece. Of note, the last image of each row (at 10 minutes) shows the human eye being suspended right after the end of the treatment. The experiment was performed at room temperature and the differences from the background color suggest that there was a gradient in eye’s surface temperature, with higher temperatures closer to the ultrasound transducer.
The temperature measured with the thermocouple sensor in the center of the human vitreous was not significantly different when comparing ultrasound treatment groups (with or without cooling).
Discussion
In this study we evaluated the applicability of the same transducer, successfully used by our group to increase penetration of riboflavin into the cornea [20], on the sclera. Considering the increased stiffness of the sclera, the length of the ultrasound treatment was increased to 10 minutes and the eyes were left in contact with fluorescein solution for an extra 50 minutes.
The ultrasound treatment facilitated the entry of topical fluorescein through the sclera, in the treated-eyes group, showing on average a 1.44-fold increase in fluorescein permeation into the vitreous humor, compared to the untreated-eyes group. The result was statistically significant and confirms the efficacy of the method, suggesting that the application of low frequency ultrasound has the potential to increase the permeation of small hydrophilic molecules into the vitreous humor. These findings are very promising and provide motivation for exploration of different ultrasound intensities, frequencies, and pulse modulations that can be applied to make the treatment delivery more practicable for in vivo experiments and to translate the method to the clinical setting. We have previously reported an increase of 3.6 times in the penetration of riboflavin into cornea using an 880 kHz transducer for 6 minutes, and in the literature there are reports using a similar method to increase the corneal penetration of hydrocortisone, papain, and hypotensive agents [14-16]. Due to anatomical and biomechanical differences between cornea and sclera, it is unlikely that a single device would work on both with the same efficacy. A transducer with a smaller surface area and less heat generation is desirable for transscleral delivery. In addition, riboflavin and fluorescein are both examples of small hydrophilic molecules with similar molecular weights (376 Da and 332 Da, respectively). Currently, retinal treatments often require the use of large biologics, such as many commercially available anti-VEGF therapeutics, and therefore the phonophoretic parameters may need to be optimized for delivering large compounds [23].
Ultrasound may increase the penetration of drugs into tissues by thermal and mechanical mechanisms [17,24]. The mechanical mechanisms are acoustic streaming, representing the development of unidirectional flow currents in presence of sound waves and cavitation. This cavitation is a formation of gaseous cavities that can repetitively oscillate and generate streaming, local changes to tissue structure, or collapse and cause structural alteration in the surrounding tissue.
Our findings in untreated eyes, compared to heat-only treated eyes, did not show a significant difference from each other, supporting the suggestion that the effects observed of increased permeation with ultrasound treatment are probably not attributable to the elevation in temperature of the treated area. Previous studies on the thermal effect on human retinal pigment epithelium cells have shown that they can tolerate temperatures of 39.5-40°C for one hour, but marked morphological changes are observed when heat exposure hits 45.5-46°C for one hour [25]. Based upon our results (Figure 4), we observed a moderate increase in temperature of the rabbit vitreous to approximately 40°C during the ultrasound treatment, which is higher than the recommended levels proposed by the American Institute of Ultrasound in Medicine for diagnostic ultrasound (thermal index of 1 degree Celsius) [26]. However, in consideration of therapeutic applications with ultrasound and other energy sources, higher temperatures may be acceptable [27,28].
It is also important to mention that according to our data, increases in temperature of the solution up to 46°C (heat alone without ultrasound) did not significantly affect the transscleral delivery results, meaning that smaller increases within the clinically tolerable temperature range will not likely boost the ultrasound-related mechanical effects.
Further, our thermal experiment with the human eye (Figure 6) shows that the temperature increase observed in and near the treated area of the sclera is mainly due to the power transfer efficiency of the transducer, with self-heating of the ultrasound applicator being thermally conducted into the small fluid volume of the eye cup. We show that it is possible to mitigate the increase in fluid temperature with external cooling of the ultrasound hand piece. The thermal images show that when the cooling system is on, the final temperature of the eye is more even across the globe surface, and it is close to background room temperature (purple color). In this setting, temperature elevation within the sclera and vitreous are reduced considerably.
At the beginning of each experiment (time 0), the temperature at the surface of the human eye was stabilized at about one degree less than room temperature. We hypothesize that this happens mainly because of evaporation of liquids on the eye surface.
The differences in the temperature measured with the thermocouple sensor in the rabbit and human set ups are probably because of four main factors: difference in eye size and vitreous volume, addition of Parafilm® to fix the eye cup in the human set up, position of the thermocouple, and initial temperature of the eye and solution. We decided to perform the human experiment at room temperature to allow better visualization of the temperature gradient over the globe surface using a thermal camera. Moreover, in the human set up, the thermocouple was inserted away from the treatment zone, pointing toward the center of the vitreous as oppose to the rabbit experiments, where the thermocouple was inserted through the treated scleral area to measure the temperature of the vitreous close to it. Using both technologies allowed us to present a more comprehensive report and better understand the mechanisms responsible for change in temperature in different areas.
Previous in vitro studies have suggested the potential of phonophoresis to promote drug delivery through the sclera. Huang and colleagues reported a two-fold increase in the penetration of bovine serum albumin labeled with fluorescein isothyacianate (FITC-BSA, MW 66 KDa) using 1 MHz 0.5 W/cm2 continuous mode for 5 minutes [29]. Also, Razavi and colleagues reported a 10-fold increase in the scleral permeability to fluorescein using 1.1 MHz pulsed ultrasound, with up to 5.4 W time-averaged acoustic powers [30]. Both findings used rabbit sclera in their in vitro models [29,30]. In vitro experiments are usually performed by resecting and placing a flattened piece of sclera in between two chambers (donor and receiver), followed by application of ultrasound on the donor side and measurement of the amount of the tested compound on the receiver side. These in vitro models have limitations, as manipulation of the scleral sample may affect the structural properties of the sclera and have an impact on the permeability results. In fact, Razavi and colleagues performed transmission electron microscopy in the scleral samples treated in vitro and found similar evidence of damage to fibroblasts and collagen bundle disorganization, in both sham and ultrasound-treated samples [30].
Our ex vivo model is a step closer to real-life circumstances in the process of investigating the translational potential of using ultrasound to deliver drugs into a patient’s vitreous. However, it also has limitations considering the absence of the choroidal blood flow and that the eyes were removed from the animal’s head, possibly affecting the absorption and reflection of the ultrasound waves leaving the eye. In addition to that, conjunctiva and connective tissues were removed, and the solution containing fluorescein was directly applied on the bare sclera. However, the use of ex vivo eyes is a much more cost-efficient model and probably the best to use for optimization of the method and selection of a few lead treatment settings for future in vivo testing. There are very few studies investigating the use of ultrasound over the sclera in vivo, and the reported methods were neither safe nor practical for prompt clinical translation. Suen and colleagues reported a significant increase in dextran penetration through the sclera of live rabbits using phonophoresis. However, they used a very large transducer (1591 mm2 surface area) operating at 40 kHz, and to achieve best results, they had to treat the eyes for a relatively long session of 19.5 minutes (90 seconds of ultrasound followed by 5 minutes in contact with the solution, repeated for a total of three cycles) [31]. They also reported a 14-day period of “healing” of the scleral barrier, after which the permeation to dextran returned to baseline levels in ultrasound-treated eyes [31], which raises a question about future clinical safety. In our study, we show promising results using a smaller transducer at a higher frequency, but we did not investigate how long after the application the sclera remains permeable, nor did we examine histology of samples to show retinal and scleral integrity. Future studies should aim to develop even smaller transducers capable of delivering drugs across the pars plana and reducing the scleral and RPE cell exposure to the ultrasound.
In order to better understand the impact of phonophoresis upon delivery of small molecules through the sclera, we did not add any enhancers to the chemical solution in this study. However, we do believe that the association of phonophoresis with solutions containing chemical enhancers or microbubbles might be the answer to reducing the time and energy necessary to deliver drugs to the vitreous. The gas in microbubbles lowers the cavitation threshold, and there are reports of successful association of ultrasound and microbubbles to deliver drugs and genes to the cornea [32,33].
In conclusion, we observed an increase in the vitreous concentration of fluorescein, suggesting that phonophoresis with an 880 kHz transducer can be an effective needle-less method to enhance the penetration of small molecules into the vitreous. We also show that the observed ranges of increase in temperature are not a significant component in the phonophoresis’ mechanism of action. Lastly, we show that it is possible to reduce excessive increases in scleral surface heating by adding a cooling system to the ultrasound hand piece. There is a clear need to develop alternatives to intravitreal injections, and ultrasound based methods are promising.
Acknowledgements
Supported by grants from: That Man May See, Inc.; NIH-NEI 1R01EY024004-01A1; NIH-NEI EY002162 - Core Grant for Vision Research and by the Research to Prevent Blindness Unrestricted Grant.
Disclosure of conflict of interest
None.
References
- 1.Braithwaite T, Nanji AA, Lindsley K, Greenberg PB. Anti-vascular endothelial growth factor for macular oedema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2014:CD007325. doi: 10.1002/14651858.CD007325.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagan XJ, Al-Qureshi S. Intravitreal injections: a review of the evidence for best practice. Clin Exp Ophthalmol. 2013;41:500–507. doi: 10.1111/ceo.12026. [DOI] [PubMed] [Google Scholar]
- 3.Osaadon P, Fagan XJ, Lifshitz T, Levy J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond) 2014;28:510–520. doi: 10.1038/eye.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014:CD005139. doi: 10.1002/14651858.CD005139.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams GA. Intravitreal injections: health policy implications. Rev Ophthalmol. 2014;21:62–64. [Google Scholar]
- 6.van der Reis MI, La Heij EC, De Jong-Hesse Y, Ringens PJ, Hendrikse F, Schouten JS. A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011;31:1449–1469. doi: 10.1097/IAE.0b013e3182278ab4. [DOI] [PubMed] [Google Scholar]
- 7.Vodencarevic AN, Terzic S, Terzic A. Endophthalmitis after intravitreal injections: incidence, presentation, management, and visual outcome. Am J Ophthalmol. 2015;160:843–844. doi: 10.1016/j.ajo.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Rifkin L, Schaal S. Factors affecting patients’ pain intensity during in office intravitreal injection procedure. Retina. 2012;32:696–700. doi: 10.1097/IAE.0b013e3182252ad3. [DOI] [PubMed] [Google Scholar]
- 9.Shiroma HF, Takaschima AK, Farah ME, Hofling-Lima AL, de Luca Canto G, Benedetti RH, Rodrigues EB. Patient pain during intravitreal injections under topical anesthesia: a systematic review. Int J Retina Vitreous. 2017;3:23. doi: 10.1186/s40942-017-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid GA, Sahota DS, Sarhan M. Observed complications from dexamethasone intravitreal implant for the treatment of macular edema in retinal vein occlusion over 3 treatment rounds. Retina. 2015;35:1647–1655. doi: 10.1097/IAE.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 11.Georgalas I, Koutsandrea C, Papaconstantinou D, Mpouritis D, Petrou P. Scleral melt following Retisert intravitreal fluocinolone implant. Drug Des Devel Ther. 2014;8:2373–2375. doi: 10.2147/DDDT.S66634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khurana RN, Appa SN, McCannel CA, Elman MJ, Wittenberg SE, Parks DJ, Ahmad S, Yeh S. Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology. 2014;121:67–71. doi: 10.1016/j.ophtha.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Stewart MW. Extended release anti-VEGF systems: a strategy whose time has come? Or already gone? Expert Rev Ophthalmol. 2016;11:167–169. [Google Scholar]
- 14.Cherkasov IS, Marmur RK, Radkovskaia A, Loskova LM. [Phonophoresis of hypotensive agents in the treatment of simple glaucoma] . Oftalmol Zh. 1974;29:114–118. [PubMed] [Google Scholar]
- 15.Fellinger K, Schmid J. Klinik und therapie des chronischen gelenkrheumatismus. Austria, Maudrich, Vienna: 1954. pp. 549–552. [Google Scholar]
- 16.Zobina LV, Proskurova GI. [Phonophoresis of hydrocortisone through the cornea] . Oftalmol Zh. 1970;25:502–506. [PubMed] [Google Scholar]
- 17.Lavon I, Kost J. Ultrasound and transdermal drug delivery. Drug Discov Today. 2004;9:670–676. doi: 10.1016/S1359-6446(04)03170-8. [DOI] [PubMed] [Google Scholar]
- 18.Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery--a general review. Expert Opin Drug Deliv. 2004;1:37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zderic V, Clark JI, Martin RW, Vaezy S. Ultrasound-enhanced transcorneal drug delivery. Cornea. 2004;23:804–811. doi: 10.1097/01.ico.0000134189.33549.cc. [DOI] [PubMed] [Google Scholar]
- 20.Lamy R, Chan E, Zhang H, Salgaonkar VA, Good SD, Porco TC, Diederich CJ, Stewart JM. Ultrasound-enhanced penetration of topical riboflavin into the corneal stroma. Invest Ophthalmol Vis Sci. 2013;54:5908–5912. doi: 10.1167/iovs.13-12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakraborty N, Wang M, Solocinski J, Kim W, Argento A. Imaging of scleral collagen deformation using combined confocal raman microspectroscopy and polarized light microscopy techniques. PLoS One. 2016;11:e0165520. doi: 10.1371/journal.pone.0165520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meek KM, Fullwood NJ. Corneal and scleral collagens--a microscopist’s perspective. Micron. 2001;32:261–272. doi: 10.1016/s0968-4328(00)00041-x. [DOI] [PubMed] [Google Scholar]
- 23.Ambati J, Canakis CS, Miller JW, Gragoudas ES, Edwards A, Weissgold DJ, Kim I, Delori FC, Adamis AP. Diffusion of high molecular weight compounds through sclera. Invest Ophthalmol Vis Sci. 2000;41:1181–1185. [PubMed] [Google Scholar]
- 24.Cheung AC, Yu Y, Tay D, Wong HS, Ellis-Behnke R, Chau Y. Ultrasound-enhanced intrascleral delivery of protein. Int J Pharm. 2010;401:16–24. doi: 10.1016/j.ijpharm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Wakakura M, Foulds WS. Heat shock response and thermal resistance in cultured human retinal pigment epithelium. Exp Eye Res. 1993;56:17–24. doi: 10.1006/exer.1993.1004. [DOI] [PubMed] [Google Scholar]
- 26.Bigelow TA, Church CC, Sandstrom K, Abbott JG, Ziskin MC, Edmonds PD, Herman B, Thomenius KE, Teo TJ. The thermal index: its strengths, weaknesses, and proposed improvements. J Ultrasound Med. 2011;30:714–734. doi: 10.7863/jum.2011.30.5.714. [DOI] [PubMed] [Google Scholar]
- 27.Aptel F, Lafon C. Therapeutic applications of ultrasound in ophthalmology. Int J Hyperthermia. 2012;28:405–418. doi: 10.3109/02656736.2012.665566. [DOI] [PubMed] [Google Scholar]
- 28.Trujillo M, Ribera V, Quesada R, Berjano E. Applicator for RF thermokeratoplasty: feasibility study using theoretical modeling and ex vivo experiments. Ann Biomed Eng. 2012;40:1182–1191. doi: 10.1007/s10439-011-0492-1. [DOI] [PubMed] [Google Scholar]
- 29.Huang D, Wang L, Dong Y, Pan X, Li G, Wu C. A novel technology using transscleral ultrasound to deliver protein loaded nanoparticles. Eur J Pharm Biopharm. 2014;88:104–115. doi: 10.1016/j.ejpb.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Razavi A, Clement D, Fowler RA, Birer A, Chavrier F, Mestas JL, Romano F, Chapelon JY, Begle A, Lafon C. Contribution of inertial cavitation in the enhancement of in vitro transscleral drug delivery. Ultrasound Med Biol. 2014;40:1216–1227. doi: 10.1016/j.ultrasmedbio.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Suen WL, Wong HS, Yu Y, Lau LC, Lo AC, Chau Y. Ultrasound-mediated transscleral delivery of macromolecules to the posterior segment of rabbit eye in vivo. Invest Ophthalmol Vis Sci. 2013;54:4358–4365. doi: 10.1167/iovs.13-11978. [DOI] [PubMed] [Google Scholar]
- 32.Nabili M, Shenoy A, Chawla S, Mahesh S, Liu J, Geist C, Zderic V. Ultrasound-enhanced ocular delivery of dexamethasone sodium phosphate: an in vivo study. J Ther Ultrasound. 2014;2:6. doi: 10.1186/2050-5736-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonoda S, Tachibana K, Uchino E, Okubo A, Yamamoto M, Sakoda K, Hisatomi T, Sonoda KH, Negishi Y, Izumi Y, Takao S, Sakamoto T. Gene transfer to corneal epithelium and keratocytes mediated by ultrasound with microbubbles. Invest Ophthalmol Vis Sci. 2006;47:558–564. doi: 10.1167/iovs.05-0889. [DOI] [PubMed] [Google Scholar]


