Abstract
There are a wide variety of extracellular matrices that can be used for regenerative purposes. Placental tissue‐based matrices are quickly becoming an attractive option given the availability of the tissue source and the wide variety of bioactive molecules knows to exist in unprocessed placental tissues. As fresh placental tissues are seldom an option at the point of care, we examined both the composition and bioactivity of a commercially packaged flowable placental connective tissue matrix (FPTM) (BioECM®, Skye Biologics, Inc.) that was preserved by the proprietary HydraTek® process. The FPTM contained significant amounts of collagen and various growth factors such as bFGF, EGF, PDGF, KGF, and PIGF. In addition, it contained high levels of tissue inhibitors of metalloproteinases (TIMP‐1 and 2) and molecules known to modulate the immune response including TGF‐β and IL‐4. In terms of its bioactivity, the FPTM displayed the ability (1) to suppress INF‐γ secretion in activated T‐cells nearly fourfold over control media, (2) to inhibit methicillin resistant Staphylococcus aureus (MRSA) and Staphylococcus saprophyticus proliferation, (3) to increase the migration of adipose‐derived stem cells (ASCs) nearly threefold over control media and (4) to adhere to ASCs in culture. When ASCs were exposed to FPTM in culture, the cells maintained healthy morphology and showed no significant changes in the expression of five genes involved in tissue growth and repair as compared to culture in standard growth media. © 2018 The Authors Journal of Biomedical Materials Research Part B: Applied Biomaterials Published by Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 106B: 2731–2740, 2018.
Keywords: placenta, extracellular matrix, growth factors, connective tissue, adipose‐derived stromal/stem cells
INTRODUCTION
Extracellular matrices (ECMs) are widely used to augment wound healing and tissue repair for a variety of injuries in both surgical and nonsurgical applications. In addition to providing a substrate for tissue reconstruction, many ECM scaffolds can sequester and release growth factors1, 2 that can promote tissue regeneration through (1) the modulation of the immune response3 and (2) the recruitment and stimulation of stem/progenitor cells and fibroblasts.4
While synthetic ECM options can serve as scaffolds, ECMs derived from placental tissues have many additional beneficial properties largely due to the complexity of the natural tissue. In particular, placental derived membranes provide a more suitable scaffolding for tissue remodeling as they are rich in various types of collagens and other structural proteins such as laminins, fibronectins, and proteoglycans.5 These placental tissues also contain a variety of growth factors known to play a role in the normal healing process such as transforming growth factor‐β (TGF‐β), epidermal growth factor (EGF), platelet derived growth factor (PDGF), and vascular endothelial growth factor (VEGF).5, 6, 7, 8 In addition, placental membranes have been shown to regulate inflammation, prevent fibrosis, and limit microbial growth, all while eliciting little or no immune response in the recipient.9, 10, 11, 12
As a result, fresh human placenta tissue has been used to treat a variety of injuries including cutaneous lesions and corneal defects.12, 13, 14 It has also been used successfully in animal models of cartilage and tendon repair, cardiac ischemia, and liver fibrosis.15, 16, 17, 18, 19 Given the difficulties inherent in using fresh placental tissues at the point of care such as limited tissue availability, lack of sterility, and the need for rapid pathogen screening, a variety of processes have been developed to preserve placental membranes for subsequent therapeutic use.5, 7, 20, 21
While these processes serve to make placental ECMs more readily available, damage to the tissue during the preservation process can not only disrupt the structural and bioactive components of the ECM, but it can also inhibit the natural degradation of the ECM and the subsequent release of key growth factors.7, 22, 23, 24 Therefore, when using a commercially processed placental tissue, it is essential to determine that the product retains structural and bioactive molecules and the ability to modulate the healing process through the recruitment and regulation of stem/progenitor cells, immune cells, and fibroblasts.
In this study, we evaluated the bioactivity and composition of a BioECM® flowable placental derived complete connective tissue matrix (FPTM) product in which the extracted placental tissue is processed by a proprietary HydraTek® process (Human Regenerative Technologies, LLC, El Segundo, CA). While processing leaves the placental matrix devoid of live cells, it produces a flowable connective tissue scaffolding matrix that is stable at room temperature. In this study, we examined the levels of structural and bioactive components that are present in the processed FPTM. In addition, we examined the ability of the FPTM to modulate immune cell function, to suppress bacterial growth, and to stimulate migration and adhesion of mesenchymal stem cells in vitro.
METHODS
Flowable placental tissue matrix allograft
The flowable placental tissue matrix allograft (FPTM: ActiveMatrix®) is a flowable placental tissue matrix harvested from the connective tissue of human placentas sourced from selectively screened Cesarean section deliveries and processed with proprietary HydraTek® Technology; (A BioECM® product processed exclusively for Skye Biologics). FPTM is created by a proprietary cleansing, processing, and preservation process that utilizes connective tissue from various portions of the placental organ. Once extracted, the HydraTek BioAware preservation system cleanses the source tissues using inert, nontoxic, pH‐balanced solutions and agents. The process then uses a hydrodynamic system to stabilize the tissues, which maintains a precise level of moisture in the fresh‐preserved flowable product forms. This system avoids a heat or freeze‐drying process and eliminates the use of common industry additives such as formaldehyde, glutaraldehyde, or other chemical cross‐linking agents that can adversely affect the structural organization and bioactivity of the tissue.
Collagen measurement
Total collagen content was measured in six FPTM samples from six different lots using the QuickZyme Total Collagen Assay Kit. Briefly, the extracted connective tissue in the FPTM was pelleted via centrifugation at 20,000g for 10 min. The tissue pellet was subjected to acid hydrolysis to liberate the hydroxyproline residues from the collagen in the tissue. The hydrolyzed samples were diluted and then the hydroxyproline residues were oxidized according to the manufacturer's instructions. The oxidized hydroxyproline levels in the samples were then measured using a colormetric assay on a Tecan Infinite 200Pro plate reader. The values were compared to a collagen standard curve to determine the collagen concentrations of the samples.
Growth factor, cytokine, and TIMP quantification via multiplex ELISA array
Levels of various growth factors, cytokines, and metalloproteinase inhibitors were measured in the FPTM. Eleven FPTM samples, each from a different lot, were tested using a multiplex ELISA (RayBiotech). The FPTM was centrifuged at 20,000g for 10 min to pellet the extracted connective tissue and separate the fluid component. The fluid was stored at −80°C until analysis. To extract factors from the connective tissue pellet, it was incubated in tissue protein extraction buffer (Thermo‐Fisher) supplemented with Halt Protease Inhibitor Cocktail (Thermo‐Fisher) for 24 h at 4°C. Following the incubation, the extracted connective tissue was then homogenized manually and then centrifuged at 10,000g for 10 min to pellet the membrane fragments. The extract supernatant was removed and stored at −80°C until analysis. For analysis, a custom Quantibody ELISA array (RayBiotech) was used to determine the levels of the following twelve proteins in the samples: platelet derived growth factor‐AA (PDGF‐AA), platelet derived growth factor‐BB (PDGF‐BB), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), keratinocyte growth factor (KGF), placental growth factor (PIGF), interleukin‐4 (IL‐4), transforming growth factor‐beta1 (TGF‐β1), transforming growth factor‐beta3 (TGF‐β3), vascular endothelial growth factor (VEGF), tissue inhibitor of metalloproteinase‐1 (TIMP‐1), and tissue inhibitor of metalloproteinase‐2 (TIMP‐2). The arrays were processed following the manufacturer's instructions and were read using a standard microplate reader. The raw data was then analyzed using custom software (RayBiotech) to determine the level of the various factors in the samples. Because of the high limit of detection for TGF‐β1 on the multiplex array, the TGF‐β1 levels in the extracts were determined using an ELISA test kit manufactured by RayBiotech.
Isolation and establishment of ASC cell lines
Primary ASC lines were established by isolating cells from excess liposuction aspirates harvested from subcutaneous adipose tissue of subjects that were undergoing elective orthopedic procedures at the Trinity Sports Medicine and Performance Center Clinic. The ASC isolation and culturing procedure was performed as described previously.25 (The use of human tissue samples was approved by the Franciscan University of Steubenville's Institutional Review Board.) The effect of the FPTM was assessed on ASC cells lines that were established from different individuals. For example, in the case of the gene expression assay, ASC cell lines were established from four different individuals.
In vitro effects of FPTM on ASCs: Cell adhesion and gene expression
Passage 2 ASCs were plated in six‐well dishes at a concentration of 5000 cells/cm2. The cells were incubated for 48 h in DMEM/F12 media supplemented with 10% fetal bovine serum (FBS) and antibiotics (standard growth media). After 48 h, the media was changed and cells were incubated in standard growth media supplemented with 10% FPTM (FPTM media) and allowed to grow for another 48 h.
To examine cell adhesion of the ASCs to the FPTM connective tissue, the FPTM media was removed and the cells were gently washed 4× with HBSS to remove any nonadherent extracted connective tissue. Pictures were taken of the cells along with any adherent FPTM connective tissue bound to the cells. Four pictures were taken at 100× magnification, one from each quadrant of the well, using a Leica inverted scope with image modulation contrast (IMC). The morphology was examined and the percentage of cells with adhered FPTM connective tissue was scored for all four pictures.
Separate wells were used to study the effect of the FPTM on gene expression. Briefly, after 48 h incubation in the FPTM media, the cells were washed with HBSS and lysed with lysis buffer (BioRad). Total RNA was extracted using the BioRad total Arum RNA prep kit with on‐column DNase digestion to eliminate genomic DNA. Quantity and quality of the total RNA for each preparation was determined using a Nanoquant plate on a Tecan Infinite 200Pro plate reader. RNA from each sample (∼0.1 μg) was reverse transcribed using iScript Reverse Transcription Supermix (Bio‐Rad). Gene expression levels were then evaluated by performing real‐time PCR reactions using a SYBR green PCR kit (BioRad) and a MJ Mini thermal cycler (BioRad). All reactions were carried out using validated primer pairs purchased from http://www.realtimeprimers.com.
The five genes that were examined were bone morphogenetic protein‐2 (BMP‐2), vascular endothelial growth factor‐B (VEGF‐B), insulin‐like growth factor‐1 (IGF‐1), transforming growth factor‐beta1 (TGF‐β1), and platelet‐derived growth factor‐B (PDGF‐B). The follow primers (http://www.realtimeprimers.com) were used: BMP‐2, Forward 5′‐ACC AGG TTG GTG AAT CAG AA‐3′, Reverse 5′‐CAA TGG CCT TAT CTG TGA CC‐3′; VEGF‐B, Forward 5′‐AGA CTC AGC AGG GTG ACT TG‐3′, Reverse 5′‐CTG GTA TGT GAC CCC TCT TG‐3′; IGF‐1, Forward 5′‐CCC CAC TCA CCA ACT CAT AG‐3′, Reverse 5′‐GGT ATT TGG GGC CTT TAT GT‐3′; TGF‐ β1, Forward 5′‐CGT GGA GCT GTA CCA GAA ATA‐3′, Reverse 5′‐TCC GGT GAC ATC AAA AGA TAA‐3′; PDGF‐B, Forward 5′‐TTC CTC CCC ATA CTC CAC TC‐3′, Reverse 5′‐CCC TGG CCT CTA GTC TTC TG‐3′. All real‐time PCR reactions were carried out in duplicate and control reactions were performed using templates that had been mock reverse‐transcribed (no reverse transcriptase). The control reactions confirmed that genomic DNA had been efficiently removed. Relative gene expression levels were calculated using a ΔΔCq method with GAPDH and RPL13A expression serving as reference genes. The control primer sequences were as follows: GAPDH, Forward 5′‐GAG TCA ACG GAT TTG GTC GT‐3′, Reverse 5′‐TTG ATT TTGGAG GGA TCT CG‐3′; RPL13A, Forward 5′‐CCT GGA GGA GAA GAG GAA AGA GA, Reverse 5′‐TTG AGG ACC TCT GTG TAT TTG TCA A‐3′. Previous work identified these reference genes as being stably expressed in ASCs following different culture conditions.26
In vitro suppression of T cell activation
Lymphocyte separation medium (Lonza) was used to purify lymphocytes from fresh human blood obtained from healthy donors using density gradient centrifugation. (The use of human tissue samples was approved by the Franciscan University of Steubenville's Institutional Review Board.) The lymphocytes were plated in 96‐well plates at a concentration of 500,000 cells/ml with a total volume of 200 μl per well. To test the FPTM, some of the wells were supplemented with 20% (40 μl) of FPTM. (20% FPTM was used as it was the lowest concentration of FPTM tested that displayed a consistent ability to suppress INF‐γ secretion across lots.) Five different vials of FPTM were tested, each from different lots (A–E), and all vials were tested in duplicate.
The T cells were activated by adding 2uls of CD3/CD28 T‐activator Dynabeads™ to each well per the manufacturer's recommendations. The negative control well did not receive beads while the positive control well did not receive FPTM. The cells were incubated in the presence of the Dynabeads™ for 48 h. After 48 h, the contents of each well were transferred to Eppendorf tubes and spun in a microcentrifuge at maximum speed for 5 min. The supernatant was removed and frozen at −80°C for further analysis. After thawing, the level of INF‐γ in the supernatant was then measured using an INF‐γ ELISA test manufactured by RayBiotech.
Human ASC cell migration assay
Cell migration was analyzed in vitro using an 8 μm Cytoselect™ transwell migration assay (Cell Biolabs Inc.). Passage 2 ASCs were starved in serum‐free DMEM/F12 media for 24 h prior to experimentation. Prior to adding the cells, 500 μl DMEM/F12 media (negative control) or DMEM/F12 media supplemented with 20% FPTM was added below the membrane to the bottom well. (20% FPTM was used as it was the lowest concentration of FPTM tested that displayed a consistent ability to attract migrating ASCs across lots.) Passage 2 ASCs suspended in 300 μl of serum‐free DMEM/F12 media were then added above the transwell membrane at a concentration of 0.5 × 106/ml. The cells were incubated in the transwell for 8 h under standard mammalian cell culture conditions (37°C and 5% CO2).
After 8 h, the migratory cells were detached from the underside of the transwell membrane by incubating it for 30 min in cell detachment solution (Cell Biolabs Inc.). The detached cells were then lysed and incubated with CyQuant® GR dye and the lysates were read on a fluorescence plate reader (Tecan Infinite 200Pro) at 480 nm/520 nm. To quantify the cells that had migrated, a standard curve using passage 2 ASCs was generated. These cells were counted using a Nexcelom Cellometer® Vision cell counter and known amounts were lysed, incubated with CyQuant® GR dye, and read on the plate reader. This assay was performed using FPTM samples (S1‐S4) from four different lots.
Antibacterial assay
The ability of FPTM to suppress the growth of Methicillin resistant Staphylococcus aureus (ATCC stock #BAA 1761) Staphylococcus saprophyticus (ATCC stock #15305) was examined using a derivation of a previously published resazurin‐based assay.27, 28 Samples from six different lots were tested. A serial dilution of the FPTM was made in standard nutrient broth media such that the wells had 100 μl of growth media/FPTM at FPTM concentrations of 50%, 25%, 12.5%, and 6.25%. From bacterial stock solutions that contained 1 × 108 CFU/ml, 5 μl of the stock was added to each well. After 10 h of incubation at 37°C, 10 μl of 0.657% resazurin solution was added to each well. The plates were incubated at 37°C for an additional 2 h, and then fluorescence was measured using a Tecan Infinite 200Pro plate reader (520 nm/590 nm). Positive controls containing media and bacteria and blanks containing only broth were also analyzed. The ability of the FPTM to inhibit bacterial growth is presented as a percent survival as compared to the growth seen on media alone according to the following formula29:
RESULTS
Collagen content of extracted connective tissue in FPTM
Six samples of the FPTM were analyzed for collagen content. Each vial contained ∼300 mg of extracted connective tissue by wet weight, which corresponds to roughly 45 mg of extracted placental connective tissue by dry weight. The collagen content of the samples averaged 9.2 ± 2.2 mg per vial, which indicates that 20% of the dry weight of the extracted placenta connective tissue is collagen in the final product. This percentage is similar to values previously published for unprocessed placental membranes30 indicating that the majority of the collagen content is retained in the FPTM.
Growth factor, cytokine, and TIMP levels in FPTM
Using a multiplex ELISA array, the levels of specific growth factors, cytokines, and TIMPs known to be associated with tissue growth and repair were examined in 11 different FPTM samples. Of the nine growth factors analyzed, eight of them were detected in the FPTM samples (Table 1). Of these nine, transforming growth factor‐β1 (TGF‐ β1), transforming growth factor‐β3 (TGF‐ β3), epidermal growth factor (EGF), and basic fibroblast growth factor (bFGF) were found at levels >100 pg/mL. Keratinocyte growth factor (KGF), placental growth factor (PIGF), platelet‐derived growth factor‐BB (PDGFB), and platelet‐derived growth factor‐AA (PDGFA) were all found at levels <100 pg/ml. Only vascular endothelial growth factor (VEGF) was not present in levels that could be reliably detected by the assay as only one of the eleven samples contained detectable levels.
Table 1.
The Levels of 12 Bioactive Molecules in the FPTM
| Bioactive Factor | Concentration (pg/mL) |
|---|---|
| PDGF‐AA | 71.4 ± 21.4 |
| PDGF‐BB | 45.2 ± 14.5 |
| bFGF | 165.5 ± 53.2 |
| EGF | 298.8 ± 108.0 |
| KGF | 9.16 ± 3.00 |
| PIGF | 12.0 ± 2.81 |
| IL‐4 | 230.7 ± 77.6 |
| TGF‐β1 | 897.7 ± 446.1 |
| TGF‐β3 | 506.4 ± 95.8 |
| VEGF | N.D. |
| TIMP‐1 | 7663 ± 2869 |
| TIMP‐2 | 7188 ± 1342 |
The level of each factor is reported as pg/mL of FPTM (n = 11).
The cytokine IL‐4, which has anti‐inflammatory properties, was also examined and was found to be present in relatively high amount in the FPTM samples, >200 pg/mL. Finally, the levels of two tissue inhibitors of metalloproteinases (TIMPs) were measured. Both TIMP1 and TIMP2 were detected in the FPTM at levels >7,000 pg/ml. The levels for all of the molecules presented in Table 1 represent the sum of amounts found in both the fluid and extracted connective tissue portions of the FPTM product.
Suppression of T cell activation by FPTM
Upon activation, T cells secrete a variety of factors including interferon‐γ. To assess the ability of the FPTM to modulate the immune response in vitro, the amount of INF‐γ produced by activated human T cells was measured after 48 h in the presence or absence of FPTM. T cells incubated without Dynabeads™ (neg control) had no detectable levels of INF‐γ secretion while the T cells incubated with the Dynabeads™ (pos control) displayed high levels of INF‐γ secretion, 3335 ± 382 pg/ml (Figure 1). The addition of 20% of the flowable FPTM significantly inhibited T cell secretion of INF‐γ as the secretion dropped to 833 ± 238 pg/ml.
Figure 1.
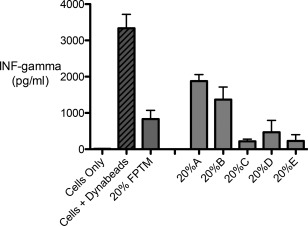
INF‐gamma secretion following Dynabead T cell activation in the presence or absence of 20% FPTM. INF‐gamma secretion was significantly inhibited by the addition of the placental product. INF‐gamma secretion was consistently reduced across five different lots of FPTM (A–E). The 20% FPTM bar refers to the mean value of the five different lots tested.
Five samples of flowable FPTM (A–E) from different lots were tested in this experiment. All five samples exhibited the ability to inhibit INF‐γ secretion although the level of inhibition varied between samples. Sample C had the greatest level of inhibition as INF‐γ secretion dropped to 220 ± 59 pg/ml while sample A had the lowest level as INF‐γ secretion dropped to 1880 ± 178 pg/ml (Figure 1). Despite this variability, the FPTM consistently displayed the ability to inhibit T cell production of INF‐γ.
Response of ASCs to FPTM in vitro
To examine the response of stem cells to FPTM, adipose‐derived stem cells (ASCs) were cultured in the presence of FPTM. Four different passage 2 ASC cell lines were cultured for 48 h in standard 10% FBS growth media supplemented with 10% FPTM. There were no obvious changes in cell morphology compared to control media (Figure 2) and the ASCs exhibited normal morphology in all cases. However, the ASCs did adhere tightly to the extracted connective tissue contained in the FPTM. Over 95% of the ASCs in the culture dish bound to the extracted connective tissue in the 10% FPTM. The tissue remained attached to the cells even after repeated washing of the cells to remove any loosely bound or unbound tissue (Figure 2). In addition, the tissue did not display nonspecific binding to the culture dish (Figure 2) as unbound tissue was easily removed by the wash steps. Even lower concentrations of FPTM (1%) displayed tissue binding to the cells, although at a significantly lower level as only between 10% and 20% of the cells bound tissue under these conditions.
Figure 2.
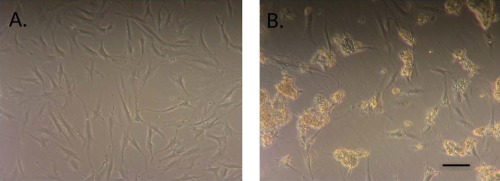
ASCs in culture bind to the placental connective tissue in FPTM product. (a) ASCs in control media. (b) ASCs in media supplemented with 10% FPTM. Scale bar = 100 μm.
To further assess the effect that co‐culture with FPTM had on ASCs, we examined changes in the expression of a select number of genes involved in healing, growth, and repair of damaged connective tissue. A total of five genes were examined in the four different primary ASC cell lines using FPTM from four different lots; bone morphogenetic protein‐2 (BMP‐2), transforming growth factor‐beta (TGF‐beta), platelet‐derived growth factor (PDGF), insulin growth factor‐1 (IGF‐1), and vascular endothelial growth factor B (VEGF‐B).
The expression of four of these genes was increased but not significantly by the addition of the FPTM (Figure 3); BMP‐2 (1.29 ± 0.37 relative to control media), IGF‐1 (1.60 ± 1.27), TGF‐B (1.20 ± 0.32), and VEGF‐B (2.12 ± 2.0). PDGF‐B showed a slight decrease in expression (0.696 ± 0.47) that was not statistically significant. There was some variability in gene expression seen in different cells lines; one cell line displayed a 3.3‐fold increase in IGF‐1 expression in the presence of FPTM, while another cell line had a decrease in expression; 0.44 compared to the control. This level of variability is not surprising given the different genetic make‐up and patient specific history of each primary ASC cell line used. Previous research has shown that there exists a good deal of heterogeneity between ASCs derived from different individuals and even from different regions within the same individual.31, 32 Overall though, these results indicate that the FPTM does not have a negative effect on ASC gene expression and cell behavior in vitro.
Figure 3.
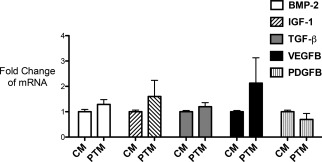
Changes in gene expression in ASCs cultured in control media versus 10% FPTM supplemented media. The addition of FPTM did not significantly alter the expression of any of the genes tested although the majority of the genes displayed a slight increase in expression levels.
Stimulation of ASCs migration in vitro by FPTM
In addition to examining the effect that FPTM had on the gene expression of ASCs, we tested the ability of the FPTM to stimulate ASC migration in vitro using a transwell assay. When the DMEM/F12 growth media supplemented with 20% of FPTM was used in the bottom of the transwell, migration of ASCs through the membrane to the bottom of the transwell increased roughly three‐fold over the use of DMEM/F12 control media alone. During the 8 h incubation period, an average of 15,662 ± 1153 cells migrated in response to the 20% FPTM as opposed to the average of 6,075 ± 263 cells that migrated in response to the control media (Figure 4).
Figure 4.
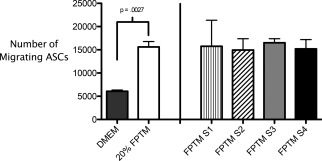
The migratory capacity of ASCs was up‐regulated significantly by the addition of 20% FPTM. The addition of FPTM samples from four different lots (S1–S4) consistently upregulated cell migration nearly threefold over baseline. The 20% FPTM bar refers to the mean value of the four different lots tested.
To assess the variability in the ability of FPTM samples to stimulate the migration of ASCs, we tested four different FPTM samples (S1–S4), all of which had been prepared from different lots. All four of the samples increased ASC migration over control media and all four showed a very similar ability to stimulation ASC migration. The average number of migratory cells was 15,765 ± 5606 for sample one, 14,950 ± 2426 for sample two, 16,509 ± 871 for sample three and 15,189 ± 2004 for sample four. While sample one showed more intrasample variability than the other three samples, there was very little variability between samples (range of 14,950–16,509 migratory cells between the samples) (Figure 4).
Antimicrobial properties of FPTM
The effects of FPTM on the growth of two medically relevant bacterial strains—methicillin‐resistant S. aureus (MRSA) and S. saprophyticus—were also examined. Six different FPTM samples all displayed the ability to suppress the growth of both strains (Figure 5). The inhibition of S. saprophyticus showed a dose‐dependent response with a reduction in inhibition associated with a reduction of the concentration of FPTM added. Growth of S. saprophyticus was reduced 84.1% in 50% FPTM while it was only reduced 67.8% in 6.25% FPTM, a difference that was statistically significant (p = 0.0267).
Figure 5.
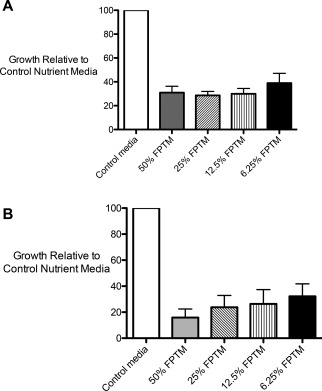
(a) Effect of FPTM on the growth of MRSA. (b) Effect of FPTM on the growth of Sa.ccharomyces saprophyticus. The relative growth was determined following 10 h at 37°C in nutrient media or nutrient media supplemented with various percentages of FPTM. The FPTM inhibits the growth of both bacterial strains.
The FPTM also strongly suppressed MRSA growth; however, the magnitude of suppression was not as large as that seen with S. saprophyticus; 69.0% suppression versus 84.1% suppression in 50% PTM and 61.1% suppression versus 67.8% in 6.25% FPTM (Figure 5.). While the lowest concentration of FPTM used displayed the least amount of growth suppression (6.25%), there was no significant difference between the levels of suppression of MRSA at the different concentrations used.
DISCUSSION
Natural biologic connective tissue ECMs have particular utility in wound healing and the repair of musculoskeletal injuries, soft tissue damage, and other defects.33, 34 While there are a wide variety of biologic options, placental‐based connective tissue ECMs hold particular promise as their complex bioactive matrix contains structural proteins, growth factors, and cytokines known to promote tissue repair, modulate the inflammation response and recruit cells to the site of injury.35 In fact, placental‐based tissue ECMs have been used successfully to treat a range of connective tissue defects.36, 37, 38, 39, 40
With the recent adoption and quick growth of placental and amniotic biologics on the market, validation of the contents and bioactivity of many products has yet to be done. In addition, some biologic processing systems can alter the tissue in ways that diminish the effectiveness of the biologic.7, 24, 41 Here we examined a flowable placenta tissue derived connective tissue matrix processed, stabilized, and preserved by the HydraTek® process to determine if this commercially available biologic retains the components and bioactivity associated with fresh placenta tissue.
After processing, roughly 20% of the dry weight of the extracted placenta connective tissue is collagen. This percentage is similar to values previously published for unprocessed placental membranes, 37% for amnion and 17% for chorion, indicating that the majority of the collagen content is maintained in the FPTM.30
We also found that significant levels of a variety of growth factors known to aid tissue healing and repair were retained in the processed FPTM. The levels found here were generally higher than those seen in extracts from unprocessed placental tissues.6, 7, 8 Direct comparisons are difficult though due to the fact that different placenta tissues were involved in previous studies and different extraction techniques were used. For example, a study that examined growth factor content in whole placenta acid extracts found concentrations that were about 10‐fold lower than that seen here for EGF, FGF, and TGF‐β.6 Studies looking at levels of growth factors in unprocessed amniotic membrane also reported levels lower than found here.7, 8 Of these previous studies, the one reporting the highest levels indicated that the amniotic membranes contained to 14.1 pg of EGF, 24.8 pg of FGF, and 9.18 pg of KGF per gram of fresh tissue.7 Given that roughly 300 mg of tissue is present per ml of FPTM, the levels per gram of fresh tissue in this study corresponded to 993.3 pg for EGF, 551.7 pg for FGF, and 30.5 pg for KGF. These differences are likely explained by two factors. First, the FPTM studied here contains a selective combination of placental tissues as opposed to the entire placenta including the decidua6 or merely the amnion.7, 8 Second, different extraction techniques were used as studies examining growth factors in the amnion used a saline solution rather than a tissue extraction reagent as used here.7, 8
The FPTM retained a number of growth factors including FGF, KGF, and EGF, which are known to increase cell proliferation during the healing process and aid in tissue regeneration.42, 43 In addition, it retained PDGF which can stimulate fibroblast proliferation and TGF‐β which can stimulate macrophages to increase production of FGF and PDGF further increasing the levels of these growth factors during tissue repair.44
Tissue turnover and remodeling is a critical factor in tissue repair and regeneration. Often, excess levels of matrix metalloproteinase (MMP) activity can inhibit the regeneration phase and lead to a chronic nonhealing wound.45 The FPTM contained high amounts of TIMPs, molecules which can facilitate regeneration and prevent chronic tissue damage through their inhibition of various MMPs.46, 47
The only factor examined not found in the FPTM at significant levels was VEGF. Given the fact that high levels of VEGF are associated with cartilage inflammation and damage, the lack of significant amounts of VEGF in the FPTM may be advantageous in using this flowable product to treat joint injuries.48
Unprocessed placental tissue has been shown to suppress excessive inflammatory responses that tend to inhibit tissue regeneration.9, 10, 11, 12 The FPTM retained significant amounts of TGF‐β and IL‐4, both of which have anti‐inflammatory properties and promote wound healing.49, 50, 51 In addition, the FPTM product examined here retained the ability to inhibit T cell activation in vitro as measured by levels of T‐cell INF‐γ secretion. This result is similar to what has been seen previously using another placental‐derived tissue: frozen amnion membrane.10 There was significant variability in this assay as seen in Figure 1. This is most likely due to the variability in the levels of cytokines in the FPTM. For example, the levels of IL‐4 varied from 40 to 349 pg/ml in the samples tested. Despite this variability, there was consistent suppression of INF‐γ secretion by all of the FPTM lots tested in this assay.
The reduction in INF‐γ secretion is likely an important factor in suppressing aberrant levels of inflammation as INF‐γ is a proinflammatory cytokine known to increase leukocyte migration and adhesion, promote T cell maturation and upregulate macrophage and natural killer cell activity.52, 53 In addition, INF‐γ is associated with the proinflammatory Th1 response, a response that is known to be associated with tissue graft rejection.54, 55 These immunosuppressive properties are of particular interest in the treatment of disorders such as rheumatoid arthritis, where the therapeutic success of using a tissue matrix will be limited by the ongoing autoimmune response.52, 56
It is well known that fresh placental membranes have the ability to suppress bacterial growth, particularly when used for wound coverings.23, 57 Even dried and irradiated placental membranes have been shown to limit bacterial growth.58 We found that the FPTM could suppress the growth of two medically relevant strains of Staphylococcus. Both MRSA and S. saprophyticus were suppressed even when low levels of FPTM were used. Given the risk of MRSA infections in surgical settings and the delayed healing associated with infection,59, 60 the ability of the FPTM to suppress MRSA represents an important clinical benefit. Whether this robust inhibitory effect extends beyond Staphylococcus strains, will require further studies.
The recruitment of cells by the FPTM is also of particular importance given that cells are the only component of the regenerative triad that the processed FPTM does not provide directly. Our data demonstrate that the FPTM acts as a chemoattractant in vitro for the migration of ASCs as the addition of the FPTM increased the migratory ability of the ASCs threefold over control media. This level of stimulation was consistent across four different samples and is similar to results from previous studies looking at placental membrane extracts. While previous research has shown the ability of extracts of other placenta tissue membrane products to stimulate migration of human umbilical vein endothelial cells,61 ASCs,62 and progenitor cells,63 this is the first demonstration of this ability in a flowable placental tissue product.
It is known that progenitor and adult stem cells have the ability to hone to sites of injury guided by the secretion of signaling factors from the injured tissue.64, 65, 66 A variety of studies have shown that mesenchymal stem cells migrate toward a number of growth factors including IGF‐1, HGF, PDGF, and TGF‐β, all of which are liberated and released from the damaged tissue site.67, 68, 69 The FPTM product tested here contains a number of these factors, including PDGF and TGF‐β.
In addition to the ability to attract native progenitor and stem cells, the growth factors in the FPTM may also attract neutrophils, macrophages, and fibroblasts. These cells, which would participate in the remodeling of the damaged tissue and the proper integration of the FPTM graph,35 are known to be attracted toward PDGF and TGF‐β, both of which are found in high amounts in this FPTM.35
Not only does the FPTM attract stem cells in vitro, but ASCs also bind readily to the matrix components of the FPTM product. Cell binding to the ECM is an important aspect of tissue regeneration as it promotes cell proliferation and survival and sequesters cells at the site of the injury. There are a variety of benefits associated with this in terms of tissue regeneration as it would promote the retention of the FPTM at the site of injection allowing the growth factors and cytokines contained in the tissues to diffuse out over time. In addition, it would provide migratory cells or coinjected stem cells with a site for adhesion, thereby assisting in the retention of these cells at the site of injury.
Upon adhering to the FPTM, cells may undergo metabolic changes due to the activation of various cell adhesion‐based signaling pathways.70, 71 However, in this study, we found that for plastic adherent ASCs, the additional binding of FPTM was associated with no significant changes in the expression of five genes involved in growth and tissue repair; IGF‐1, TGF‐β, PDGF, VEGF‐B, and BMP‐2. The fact that the cells also maintained a normal morphology indicates that the cells do not display gross changes in vitro when exposed to FPTM. Further studies looking at gene and protein expression on a global level may be able to determine subtle changes in behavior once ASCs are exposed to FPTM.
While the in vitro studies performed here may not directly transfer to the complex in vivo environment of the injured tissue, the FPTM has been associated with positive outcomes in human studies including tendon repairs39 and skin lesions.40 While there are a number of ongoing clinical trials using processed placenta tissue, further clinical work is needed to better identify degenerative conditions, injuries, and wounds that will respond optimally to the FPTM product described here.
CONCLUSIONS
The bioactivity and composition of the FPTM identified in this study demonstrate that the HydraTek® process effectively retains bioactive molecules that are found in unprocessed placental tissue. In addition, the FPTM displayed the ability to bind to ASCs in vitro, to act as a chemoattractant for ASC migration, to suppress the inflammatory response of activated T cells, and to inhibit the growth of strains of Staphylococcus. These data make it an attractive option for further clinical studies in the treatment and repair of a variety of connective tissue injuries and defects.
ACKNOWLEDGMENTS
The authors wish to thank Denise Lombard and Dr Kyle McKenna for their assistance with the research presented here and the Franciscan Institute for Science and Health, which provided financial support for this project. This study was funded in part by Human Regenerative Technologies, LLC, which produces the placental tissue derived product described in the article. DK serves as a paid research consultant for HRT. None of the other authors has any financial interest in HRT or any of the products mentioned in this article.
How to cite this article: Irvin J, Danchik C, Rall J, Babcock A, Pine M, Barnaby D, Pathakamuri J, Kuebler D. 2018. Bioactivity and composition of a preserved connective tissue matrix derived from human placental tissue. J Biomed Mater Res Part B 2018:106B:2731–2740.
REFERENCES
- 1. Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015;84:25–34. [DOI] [PubMed] [Google Scholar]
- 2. Reing JE, Zhang L, Myers‐Irvin J, Cordero KE, Freytes DO, Heber‐Katz E, Bedelbaeva K, McIntosh D, Dewilde A, Braunhut SJ, Badylak SF. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng A 2009;15(3):605–614. [DOI] [PubMed] [Google Scholar]
- 3. Sicari BM, Dziki JL, Siu BF, Medberry CJ, Dearth CL, Badylak SF. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials 2014;35(30):8605–8512. [DOI] [PubMed] [Google Scholar]
- 4. Zantop T, Gilbert TW, Yoder MC, Badylak SF. Extracellular matrix scaffolds are repopulated by bone marrow‐derived cells in a mouse model of achilles tendon reconstruction. J Orthop Res 2006;24(6):1299–1309. [DOI] [PubMed] [Google Scholar]
- 5. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 2008;15:88–99. [DOI] [PubMed] [Google Scholar]
- 6. Uhlrich S, Tiollier J, Chirouze V, Tardy M, Tayot J‐L. Biochemical and biological characterization of a crude growth factor extract (EAP) from human term‐placental tissue. Trophoblast Res 1992;6:19–37. [Google Scholar]
- 7. Russo A, Bonci P, Bonci P. The effects of different preservation processes on the total protein and growth factor content in a new biological product developed from human amniotic membrane. Cell Tissue Bank 2012;13(2):353–361. [DOI] [PubMed] [Google Scholar]
- 8. Lopez‐Valladares MJ, Teresa Rodriguez‐Ares M, Tourino R, Gude F, Teresa Silva M, Couceiro J. Donor age and gestational age influence on growth factor levels in human amniotic membrane. Acta Ophthalmol 2010;88(6):e211–e216. [DOI] [PubMed] [Google Scholar]
- 9. Li W, He H, Kawakita T, Espana EM, Tseng SC. Amniotic membrane induces apoptosis of interferon‐gamma activated macrophages in vitro. Exp Eye Res 2006;82(2):282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ueta M, Kweon M‐N, Sano Y, Sotozono C, Yamada J, Koizumi N, Kiyono H, Kinoshita S. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol 2002;129(3):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hori J, Wang M, Kamiya K, Takahashi H, Sakuragawa N. Immunological characteristics of amniotic epithelium. Cornea 2006;25:S53–S58. [DOI] [PubMed] [Google Scholar]
- 12. Faulk WP, Matthews R, Stevens PJ, Bennett JP, Burgos H, Hsi BL. Human amnion as an adjunct in wound healing. Lancet 1980;1(8179):1156–1158. [DOI] [PubMed] [Google Scholar]
- 13. Gomes JA, Romano A, Santos MS, Dua HS. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol 2005;16(4):233–240. [DOI] [PubMed] [Google Scholar]
- 14. Mermet I, Pottier N, Sainthillier JM, Malugani C, Cairey‐Remonnay S, Maddens S, Riethmuller D, Tiberghien P, Humbert P, Aubin F. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen 2007;15(4):459–464. [DOI] [PubMed] [Google Scholar]
- 15. Demirkan F, Colakoglu N, Herek O, Erkula G. The use of amniotic membrane in flexor tendon repair: An experimental model. Arch Orthop Trauma Surg 2002;122(7):396–399. [DOI] [PubMed] [Google Scholar]
- 16. Jin CZ, Park SR, Choi BH, Lee KY, Kang CK, Min BH. Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng 2007;13(4):693–702. [DOI] [PubMed] [Google Scholar]
- 17. Cargnoni A, Di Marcello M, Campagnol M, Nassuato C, Albertini A, Parolini O. Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplant 2009;18:1147–1159. [DOI] [PubMed] [Google Scholar]
- 18. Sant'Anna LB, Cargnoni A, Ressel L, Vanosi G, Parolini O. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant 2011;20(3):441–453. [DOI] [PubMed] [Google Scholar]
- 19. Manuelpillai U, Moodley Y, Borlongan CV, Parolini O. Amniotic membrane and amniotic cells: Potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta 2011;32(Suppl 4):S320–S325. [DOI] [PubMed] [Google Scholar]
- 20. Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, Li WW, Gurtner G. Biological properties of dehydrated human amnion/chorion composite graft: Implications for chronic wound healing. Int Wound J 2013;10(5):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koob TJ, Lim JJ, Zabek N, Massee M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J Biomed Mater Res B Appl Biomater 2015;103(5):1133–1140. [DOI] [PubMed] [Google Scholar]
- 22. Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater 2009;5(1):1–13. [DOI] [PubMed] [Google Scholar]
- 23. Kesting MR, Wolff K, Hohlweg‐Majert B, Steinstraesser L. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res 2008;29:907–916. [DOI] [PubMed] [Google Scholar]
- 24. Branski LK, Kulp G, Jeschke MG, Norbury WB, Herndon DN. Amniotic membrane as wound coverage: The effects of irradiation and different processing methods on growth factor content. J Srug Res 2007;137:339. [Google Scholar]
- 25. McLaughlin M, Gagnet P, Cunningham E, Yeager R, D'Amico M, Guski K, Scarpone M, Kuebler D. Allogeneic platelet releasate preparations derived via a novel rapid thrombin activation process promote growth and increased BMP‐2 and BMP‐4 expression in human adipose‐derived stem cells. Stem Cells Int 2016;2016:7183734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amable PR, Teixeira MVT, Carias RBV, Granjeiro JM, Borojevic R. Identification of appropriate reference genes for human mesenchymal cells during expansion and differentiation. PLoS One;8:e73792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarker SD, Nahar L, Kumarasamy Y, Microtitre plate‐based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007;42:321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mariscal A, Lopez‐Gigosos RM, Carnero‐Varo M, Fernandez‐Crehuet J. Fluorescent assay based on resazurin for detection of activity of disinfectants against bacterial biofilm. Appl Microbiol Biotechnol 2009;82:773–783. [DOI] [PubMed] [Google Scholar]
- 29. Campbell J. High‐throughput assessment of bacterial growth inhibition by optical density measurements. Curr Protoc Chem Biol 2011;3:100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halaburt JT, Uldbjerg N, Helmig R, Ohlsson K. The concentration of collagen and collagenolytic activity in the amnion and the chorion. Eur J Obstet Gynecol Reprod Biol 1989;31:75–82. [DOI] [PubMed] [Google Scholar]
- 31. Cleal L, Aldea T, Chau YY. Fifty shades of white: Understanding heterogeneity in white adipose stem cells. Adipocyte 2017;6(3):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Harmelen V, Skurk T, Röhrig K, Lee Y‐M, Halbleib M, Aprath‐Husmann I, Hauner H. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord 2003;27:889–895. [DOI] [PubMed] [Google Scholar]
- 33. Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol 2002;13(5):377–383. [DOI] [PubMed] [Google Scholar]
- 34. Kugelberg E. Biological scaffolds modulate immune cells. Nat Rev Immunol 2016;16:276–277. [DOI] [PubMed] [Google Scholar]
- 35. Diegelmann RF, Evans MC. Wound healing: An overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–289. [DOI] [PubMed] [Google Scholar]
- 36. Werber B, Martin E. A prospective study of 20 foot and ankle wounds treated with cryopreserved amniotic membrane and fluid allograft. J Foot Ankle Surg 2013;52(5):615–621. [DOI] [PubMed] [Google Scholar]
- 37. Riordan NH, George BA, Chandler TB, McKenna RW. Case report of non‐healing surgical wound treated with dehydrated human amniotic membrane. J Transl Med 2015;13:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zelen CM, Poka A, Andrews J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis–a feasibility study. Foot Ankle Int 2013;34(10):1332–1339. [DOI] [PubMed] [Google Scholar]
- 39. Lullove E. A flowable placental tissue matrix allograft in lower extremity injuries: A pilot study. Cureus 2015;7(6):e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lullove EJ. Use of a dehydrated amniotic membrane allograft in the treatment of lower extremity wounds: A retrospective cohort study. Wounds 2017;29(11):346–351. [PubMed] [Google Scholar]
- 41. Tejwani SG, Chen J, Funahashi TT, Love R, Maletis GB. Revision risk after allograft anterior cruciate ligament reconstruction: Association with graft processing techniques, patient characteristics, and graft type. Am J Sports Med 2015;43:2696–2705. [DOI] [PubMed] [Google Scholar]
- 42. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–870. [DOI] [PubMed] [Google Scholar]
- 43. Li X, Su G, Wang J, Zhou Z, Li L, Liu L, Guan M, Zhang Q, Wang H. Exogenous bFGF promotes articular cartilage repair via up‐regulation of multiple growth factors. Osteoarthr Cartil 2013;21(10):1567–1575. [DOI] [PubMed] [Google Scholar]
- 44. Kim WJ, Gittes GK, Longaker MT. Signal transduction in wound healing. Arch Pharm Res 1998;21(5):487–495. [DOI] [PubMed] [Google Scholar]
- 45. Caley MP, Martins VL, O'Toole EA. Metalloproteinases and wound healing. Adv Wound Care (New Rochelle) 2015;4(4):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davis ME, Gumucio JP, Sugg KB, Bedi A, Mendias CL. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J Appl Physiol (1985) 2013;115(6):884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: The ratio of MMP‐1 to TIMP‐1 is a predictor of wound healing. Diabet Med 2008;25(4):419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murata M, Yudoh K, Masuko K. The potential role of vascular endothelial growth factor (VEGF) in cartilage: How the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthr Cartil 2008;16(3):279–286. [DOI] [PubMed] [Google Scholar]
- 49. Salmon‐Ehr V, Ramont L, Godeau G, Birembaut P, Guenounou M, Bernard P, Maquart FX. Implication of interleukin‐4 in wound healing. Lab Invest 2000;80:1337–1343. [DOI] [PubMed] [Google Scholar]
- 50. Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti‐inflammatory and pro‐inflammatory roles of TGF‐beta, IL‐10, and IL‐22 in immunity and autoimmunity. Curr Opin Pharmacol 2009;9(4):447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF‐β family in wound healing, burns and scarring: A review. Int J Burns Trauma 2012;2(1):18–28. [PMC free article] [PubMed] [Google Scholar]
- 52. Lin F‐C, Young HA. The talented interferon‐gamma. Adv Biosci Biotechnol 2013;4(7):6–13. [Google Scholar]
- 53. Larkin J, 3rd , Ahmed CM, Wilson TD, Johnson HM. Regulation of interferon gamma signaling by suppressors of cytokine signaling and regulatory T cells. Front Immunol 2013;4:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mariotti J, Foley J, Ryan K, Buxhoeveden N, Kapoor V, Amarnath S, Fowler DH. Graft rejection as a Th1‐type process amenable to regulation by donor Th2‐type cells through an interleukin‐4/STAT6 pathway. Blood 2008;112(12):4765–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bach FH, Ferran C, Candinas D, Miyatake T, Koyamada N, Mark W, Hechenleitner P, Hancock WW. Accommodation of xenografts: Expression of “protective genes” in endothelial and smooth muscle cells. Transplant Proc 1997;29:56–58. [DOI] [PubMed] [Google Scholar]
- 56. Clements JN. Treatment of rheumatoid arthritis: A review of recommendations and emerging therapy. Formulary 2011;46:532–545. [Google Scholar]
- 57. Maral T, Borman H, Arslan H, Demirhan B, Akinbingol G, Haberal M. Effectiveness of human amnion preserved long‐term in glycerol as a temporary biological dressing. Burns 1999;25:625–635. [DOI] [PubMed] [Google Scholar]
- 58. Singh R, Chacharkar MP. Dried gamma‐irradiated amniotic membrane as dressing in burn wound care. J Tissue Viability 2011;20:49–54. [DOI] [PubMed] [Google Scholar]
- 59. Chen AF, Wessel CB, Rao N. Staphylococcus aureus screening and decolonization in orthopaedic surgery and reduction of surgical site infections. Clin Orthop Relat Res 2013;471:2383–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kelley M, Weber DJ, Dooley KE, Rutala WA. Healthcare associated methicillin resistant Staphylococcus aureus . Semin Infect Control 2001;1:157–171. [Google Scholar]
- 61. Koob TJ, Lim JJ, Massee M, Zabek N, Rennert R, Gurtner G, Li WW. Angiogenic properties of dehydrated human amnion/chorion allografts: Therapeutic potential for soft tissue repair and regeneration. Vasc Cell 2014;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Massee M, Chinn K, Lim JJ, Godwin L, Young CS, Koob TJ. Type I and II diabetic adipose‐derived stem cells respond in vitro to dehydrated human amnion/chorion membrane allograft treatment by increasing proliferation, migration, and altering cytokine secretion. Adv Wound Care (New Rochelle) 2016;5(2):43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maan ZN, Rennert RC, Koob TJ, Januszyk M, Li WW, Gurtner GC. Cell recruitment by amnion chorion grafts promotes neovascularization. J Surg Res 2015;193(2):953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rustad KC, Gurtner GC. Mesenchymal stem cells home to sites of injury and inflammation. Adv Wound Care 2012;1:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maxson S, Lopez EA, Yoo D, Danilkovitch‐Miagkova A, LeRoux MA. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008;180(4):2581–2587. [DOI] [PubMed] [Google Scholar]
- 67. Wan M, Li C, Zhen G, Jiao K, He W, Jia X, Wang W, Shi C, Xing Q, Chen Y‐F, Jan De Beur S, Yu B, Cao X. Injury‐activated transforming growth factor beta controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells 2012;30(11):2498–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res 2008;26:1407–1412. [DOI] [PubMed] [Google Scholar]
- 69. Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007;25(7):1737–1745. [DOI] [PubMed] [Google Scholar]
- 70. Loeser RF. Integrins and chondrocyte–matrix interactions in articular cartilage. Matrix Biol 2014;39:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Docheva D, Popov C, Alberton P, Aszodi A. Integrin signaling in skeletal development and function. Birth Defects Res C 2014;102:13–36. [DOI] [PubMed] [Google Scholar]


