Abstract
Specific mate recognition relies on the chemical senses in most animals, and especially in nocturnal insects. Two signal types mediate premating olfactory communication in terrestrial habitats: sex pheromones, which blend into an atmosphere of plant odorants. We show that host plant volatiles affect the perception of sex pheromone in males of the African cotton leafworm Spodoptera littoralis and that pheromone and plant volatiles are not perceived as independent messages. In clean air, S. littoralis males are attracted to single synthetic pheromone components or even the pheromone of a sibling species, oriental cotton leafworm S. litura. Presence of host plant volatiles, however, reduces the male response to deficient or heterospecific pheromone signals. That plant cues enhance discrimination of sex pheromone quality confirms the idea that specific mate recognition in noctuid moths has evolved in concert with adaptation to host plants. Shifts in either female host preference or sex pheromone biosynthesis give rise to new communication channels that have the potential to initiate or contribute to reproductive isolation.
Keywords: Ecological speciation, premating sexual communication, reproductive isolation, specific mate recognition
Specific mate communication and recognition, which is shaped during adaptation to natural habitats, involves both sex signals and environmental or habitat sensory cues and is under both sexual and natural selection (Paterson 1978, 1980; Endler 1992; Blows 2002; Boughman 2002; Scordato et al. 2014; Rosenthal 2017). In nature, females of phytophagous insects release sex pheromone into an atmosphere that is filled with plant volatiles. The effect of plant volatiles on the male moth behavioral response to sex pheromone has long been investigated (Landolt and Phillips 1997; Reddy and Guerrero 2004). Perception of sex and plant volatiles typically involves discrete peripheral input channels, and two different types of insect olfactory receptors, pheromone, and general odorant receptors, respectively (Krieger et al. 2004; Sakurai et al. 2004; Zhang and Löfstedt 2015). Integration of pheromone and plant volatile stimuli occurs in olfactory sensory neurons on the antennae in some species (Rouyar et al. 2015; Lebreton et al. 2017) and otherwise in the antennal lobe, the primary olfactory center in the insect brain (Namiki et al. 2008; Trona et al. 2010, 2013; Chaffiol et al. 2012, 2014; Hatano et al. 2015; Ian et al. 2017).
Curiously, the behavioral consequences of blending plant volatiles with sex pheromones differ among species and the plant chemicals investigated: plant volatiles can both synergize and antagonize the male response to sex pheromone (Dickens et al. 1990; Light et al. 1993; Yang et al. 2004; Schmidt‐Büsser et al. 2009; Trona et al. 2013; Badeke et al. 2016). A tentative explanation for this is that plant volatiles serve diverse behavioral roles, they signal plants for adult feeding, for mating or egglaying, or plants that that are not suitable as adult or larval food. Different messages conveyed by plant volatiles would account for different behavioral responses when blended with sex pheromone. This is evidenced by a response modulation according to internal physiological state in males and females of African cotton leafworm, Spodoptera littoralis (Lepidoptera, Noctuidae): unmated female moths are attracted to floral odorants for adult feeding, and soon after mating to cotton leaf volatiles for egg‐laying (Saveer et al. 2012). Male moths respond to host leaf volatiles only prior to mating, since these probably signal rendez‐vous sites (Kromann et al. 2015). Cotton leafworm moths further discriminate between volatiles of preferred and nonpreferred larval food plants (Thöming et al. 2013; Proffit et al. 2015) and between volatiles from healthy and damaged cotton plants (Zakir et al. 2013a,b; Hatano et al. 2015).
Inadequate plant stimuli, signaling damaged plants or nonhost plants that are unsuitable for oviposition, antagonize the male response to pheromone (Hatano et al. 2015; Badeke et al. 2016; Wang et al. 2016). This poses the question whether perception of host plant volatiles interacts with inadequate, heterospecific pheromone stimuli. Geographic distributions of the African and Oriental cotton leafworms, S. littoralis and S. litura, overlap in the Middle East, where cotton is a main larval host plant (Pogue 2002; Kergoat et al. 2012). The sex pheromones of the sibling species S. littoralis and S. litura are different, yet they are similar in composition and share several pheromone components: in a no‐choice situation in the laboratory, as many S. littoralis males are attracted to conspecific females and to S. litura females (Saveer et al. 2014). Since matings are prevented by incompatible genital morphology, we asked whether presence of the host plant cotton has an effect on male attraction to heterospecific pheromone.
Experiments with synthetic plant volatiles and pheromones, as well as live plants and pheromone‐releasing females show that males of S. littoralis best respond to a mixture of conspecific, complete sex pheromone, and volatiles of the larval food plant cotton. Attraction to heterospecific pheromone of the sibling species S. litura was much reduced in the presence of cotton volatiles, which demonstrates that mate recognition in cotton leafworm is mediated by a combination of plant volatiles and sex pheromone. This finding contributes to our understanding of olfactory‐mediated premating communication and reproductive isolation in insect herbivores.
Materials and Methods
Laboratory colonies of African cotton leafworm Spodoptera littoralis and Oriental cotton leafworm S. litura (Lepidoptera, Noctuidea) were established with insects collected near Alexandria, Egypt and Chiba, Japan, respectively. Insect colonies were maintained with ca. 50 ovipositing females per generation. Every year, the S. littoralis lab population was separated by sex and interbred with 25–50 field‐collected males and females from Alexandria, Egypt. Insects were raised on a semisynthetic agar‐based diet (modified from Hinks and Byers 1976) under a 16L:8D photoperiod, at 24°C and 50–60% RH. Males and females were separated as pupae into 30 × 30 × 30 cm Plexiglas cages. Three‐day‐old unmated male moths were used in all bioassays.
Cotton seedlings, Gossypium hirsutum (cv. Delprim DPL 491), were grown singly in pots at 25°C and 70% RH in a greenhouse, under daylight and an added, artificial light source (metal halide, 400 W). Cotton plants used in wind tunnel assays had 8–12 fully developed true leaves and were 7–9 weeks old. Damaged plants were obtained by letting four, 24‐hour starved, fifth‐instar larvae feed on the plant for 4 hours prior to experiments. Larvae were removed before wind tunnel experiments. These growth conditions are similar to those used for cotton headspace analysis and behavioral tests reported in Saveer et al. (2012) and Borrero‐Echeverry et al. (2015).
CHEMICALS
Volatiles released from cotton, which elicit either an antennal or a behavioral response in S. littoralis (Borrero‐Echeverry et al. 2015) were tested as single compounds: β‐myrcene (97% chemical purity; CAS #123‐35‐3; Fluka), (R)‐(+)‐limonene (95% chemical purity; CAS # 5989‐27‐5; Aldrich), (E)‐β‐ocimene (91% chemical purity; CAS #13877‐91‐3; Fluka), 4,8‐dimethyl‐1,3(E),7‐nonatriene (DMNT) (95% chemical purity; CAS #019945‐61‐0; a gift from Wittko Francke, Hamburg), (Z)‐3‐hexenyl acetate (99% chemical purity; CAS #3681‐71‐8; Aldrich), (R)‐(‐)‐linalool (95% chemical purity; CAS #126‐90‐9; Firmenich), (S)‐(+)‐linalool (95% chemical purity; CAS #126‐90‐9; Firmenich), and nonanal (90% chemical purity; CAS #124‐19‐6; Fluka). Two further compounds identified from maize headspace, another S. littoralis host plant (Bengtsson et al. 2006; Thöming et al. 2013) were included in the experiments with single plant volatiles: α‐farnesene (>90% chemical purity; CAS #502‐61‐4; Bedoukian) and β‐farnesene (>90% chemical purity; CAS #18794‐84‐8; Bedoukian).
The S. littoralis pheromone components, (Z,E)‐9,11‐tetradecadienyl acetate (Z9,E11‐14Ac) (main pheromone compound), (Z)‐9‐tetradecenyl acetate (Z9‐14Ac), (Z,E)‐9,12‐tetradecadienyl acetate (Z9,E12‐14Ac), were purchased from Pherobank and (E,E)‐10,12‐tetradecadienyl acetate (E10,E12‐14Ac) was provided by David Hall (Greenwich, UK). Isomeric purity was >96.3% for the dienic compounds, and >99.1% for Z9‐14Ac. These four components were consistently found in pheromone gland extracts (Saveer et al. 2014; El‐Sayed 2017). The solvent used for diluting synthetic compounds was redistilled ethanol (100% pure, Labscan, Malmö, Sweden).
WIND TUNNEL BIOASSAY
Wind tunnel experiments were performed in a Plexiglas wind tunnel (180 × 90 × 60 cm) following the protocol of Borrero‐Echeverry et al. (2015). Briefly, males and females were kept in separate rooms to avoid pre‐exposure to pheromone before experiments. One hour before experiments, moths were transferred individually to 2.5 × 12.5 cm glass tubes closed with gauze. Tests were carried out between 1 and 4 hours after the onset of scotophase. The wind tunnel was illuminated from above and the side (6 lux), moths were flown at a wind speed of 30 cm/s, at 24 ± 2°C air temperature and 60 ± 10% RH. Incoming and outgoing air was filtered with active charcoal. Moths for every treatment (N = 50) were released individually from glass tubes at the downwind end of the tunnel. Males were scored for anemotactic upwind flight (Carde and Baker 1984) over 150 cm, from the release tube to the odor source.
Synthetic odor blends were delivered from the center of the upwind end of the wind tunnel from a piezo‐electric sprayer (El‐Sayed et al. 1999; Becher et al. 2010). Samples were loaded into a 1‐mL glass syringe operated by a microinjection pump (CMA Microdialysis AB, Solna, Sweden) that delivered test solutions at a constant rate of 10 μL/min through Teflon tubing into a glass capillary with a narrow, elongated tip. The capillary was attached to a piezo‐ceramic disk, which produced an aerosol that was carried downwind. A glass cylinder (95 mm diameter × 100 mm height), covered by a fine metal mesh (pore size 2 mm) was placed in front of the capillary as landing platform. Live plants were placed at the upwind end of the wind tunnel. In experiments with calling, pheromone‐releasing females, three calling females were placed downwind from plants in glass tubes covered at both ends with a mesh.
We tested the main pheromone compound, Z9,E11‐14Ac and a 4‐component synthetic pheromone blend of Z9,E11‐14Ac, Z9‐14Ac, E10,E12‐14Ac, and Z9,E12‐14Ac, in a 100:30:20:4 proportion. The release rate of the main compound Z9,E11‐14Ac was 100 pg/min, corresponding to the amount of pheromone emitted by calling females (Saveer et al. 2014). Males were further tested with pheromone‐releasing S. littoralis and S. litura females. Single plant compounds were released at a rate of 10 ng/min, and a 4‐component plant volatile blend containing nonanal, (R)‐(+)‐limonene, (Z)‐3‐hexenyl acetate, (E)‐β‐ocimene in a 33:12:33:23 proportion (Borrero‐Echeverry et al. 2015) was also released at 10 ng/min. A 5‐component plant volatile blend, mimicking herbivore damage, was formulated by adding DMNT at 10 ng/min to the 4‐component blend (Hatano et al. 2015). Males were further tested with undamaged cotton plants and plants on which 5th‐instar larvae of S. littoralis had been feeding during 4 h. Males were flown to single sources of plant volatiles and pheromones, and to combinations of both.
STATISTICAL ANALYSIS
Generalized linear models (GLM) with a binomial distribution were used to analyze the number males attracted to different stimuli. Upwind flight was used as the target effect. Post‐hoc Wald pairwise comparison tests were used to identify differences between treatments. All statistical analysis was carried out using R (R Core Team 2013), using the NLME package (Pinheiro et al. 2018).
Results
When cotton volatiles were tested one by one, only α‐farnesene elicited significant upwind flight attraction in S. littoralis males (z = 2.07; P = 0.039) (Table 1). All of these plant volatiles significantly reduced male attraction, when added to the main pheromone compound Z9,E11‐14Ac. In stark contrast, seven of these plant volatiles did not affect male attraction when mixed with the complete, four‐component synthetic sex pheromone. Only three volatiles reduced attraction when mixed with the four‐component sex pheromone blend: DMNT was the strongest antagonist (z = 4.60; P < 0.001), followed by (E)‐β‐ocimene (z = 2.38; P = 0.017) and (Z)‐3 hexenyl acetate (z = 1.80; P = 0.072) (Table 1). Larval feeding on cotton leaves strongly increases release of DMNT, which has been shown to interfere with perception of the main pheromone compound Z9,E11‐14Ac (Hatano et al. 2015).
Table 1.
Male cotton leafworm S. littoralis upwind flight attraction to synthetic cotton volatiles (Loughrin et al. 1995; Saveer et al. 2012; Yang et al. 2013) and sex pheromone compounds (Saveer et al. 2014; El‐Sayed 2017)
| Male upwind flight attraction [%] | |||
|---|---|---|---|
| Pheromone added | None | Main pheromone compounda | 4‐Component sex pheromone blendb |
| 48 | 64 | ||
| Plant compoundsc | |||
| α‐Farnesene | 14* | 24* | 76 |
| Nonanal | 0 | 18* | 72 |
| (R)‐(+)‐Limonene | 6 | 18* | 64 |
| (S)‐(+)‐Linalool | 8 | 14* | 62 |
| β‐Farnesene | 2 | 20* | 58 |
| (R)‐(+)‐Linalool | 0 | 0* | 54 |
| β‐Myrcene | 8 | 14* | 50 |
| (Z)‐3‐Hexenylacetate | 0 | 8* | 46* |
| (E)‐β‐Ocimene | 4 | 22* | 40* |
| DMNTd | 0 | 2* | 16* |
aZ9,E11‐14Ac, release rate 100 pg/min.
b100:30:20:4‐blend of Z9,E11‐14Ac, Z9‐14Ac, E10,E12‐14Ac and Z9,E12‐14Ac, release rate 100 pg/min.
crelease rate 10 ng/min.
d4,8‐dimethyl‐1,3(E),7‐nonatriene.
Single cotton volatiles were tested alone, in mixtures with the S. littoralis main pheromone compound, and with an optimized, four‐component synthetic sex pheromone blend. Asterisks show significant differences between attraction to pheromone alone and pheromone blended with single cotton volatile compounds; α‐farnesene was the only cotton volatile to elicit significant attraction by itself (binomial GLM and post‐hoc Wald pairwise comparison; n = 50).
This differential effect of complete versus incomplete pheromone on male attraction, when mixed with plant compounds, was confirmed by experiments using a 4‐component cotton volatile blend, instead of single cotton volatiles. Attraction to a combination of this cotton blend and the main pheromone compound was significantly reduced, compared with attraction to pheromone alone (z = 3.73; P < 0.001), while a combination of the same cotton blend with four‐component pheromone did not reduce attraction (Fig. 1A). An undamaged cotton plant produced the same result: male attraction was significantly reduced to the plant in combination with the main pheromone compound (z = 2.21; P = 0.027), and not with the complete pheromone blend (z = 0.21; P = 0.834; Fig. 1B).
Figure 1.
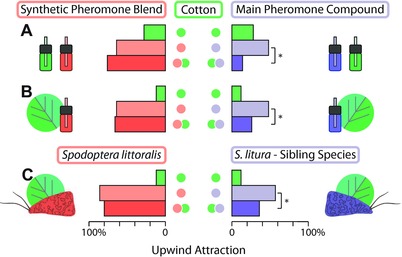
Male S. littoralis upwind flight attraction toward pheromone and cotton volatiles. The top two bars of each subplot show attraction to single plant and pheromone stimuli, respectively, while the third bar shows attraction to the combination of the respective plant and pheromone stimulus. The stimuli are main pheromone compound alone, an optimized four‐component S. littoralis synthetic sex pheromone blend (A, B) or pheromone‐reasing S. littoralis or S. litura females (C), in combination with a synthetic cotton volatile blend (A) or a live cotton plant (B, C). Bars with asterisks are significantly different from attraction to pheromone control (binomial GLM and post‐hoc Wald pairwise comparisons; n = 50).
We next replaced synthetic with authentic sex pheromone, released by live calling conspecific females or by females of the sibling species S. litura. Both species share main pheromone components, which explains S. littoralis male attraction to pheromone‐releasing S. litura females in clean air (Fig. 1C; Saveer et al. 2014). However, these two pheromone blends differ in composition and males are capable of discriminating conspecific from heterospecific pheromone, since they prefer conspecific over heterospecific S. litura females in choice tests (Saveer et al. 2014). Tests with pheromone‐releasing females on cotton plants confirm the results obtained with synthetic pheromone: adding cotton to S. litura females significantly reduced male upwind flights, compared to calling S. litura females alone (z = 1.99; P = 0.046) (Fig. 1C).
Lastly, we examined the effect of volatiles from cotton challenged by larval feeding on male sex pheromone attraction. We used a synthetic cotton blend and cotton plants on which S. littoralis larvae had been feeding. The synthetic blend mimicking damaged cotton and a cotton plant damaged by feeding larvae both significantly reduced attraction to the 4‐component synthetic pheromone, respectively (z = 2.21; P = 0.027 and z = 2.95; P = 0.003; z = 3.90). Damaged cotton plants even reduced attraction to calling S. littoralis females (z = 3.992; P < 0.001) (Table 2).
Table 2.
Male S. littoralis upwind flight attraction towards blends of S. littoralis pheromone and volatiles of cotton plants damaged by larval feeding
| Male upwind flight attraction [%] | |||
|---|---|---|---|
| Pheromone stimulus | Main pheromone compounda | 4‐Component sex pheromone blendb | Pheromone‐releasing S. littoralis female |
| 48* | 64* | 88* | |
| Plant stimulus added | |||
| Damaged cotton volatiles c | 2 | 34 | —d |
| Damaged cotton plant | 10 | 24 | 48 |
aZ9,E11‐14Ac, release rate 100 pg/min.
b100:30:20:4‐blend of Z9,E11‐14Ac, Z9‐14Ac, E10,E12‐14Ac and Z9,E12‐14Ac, release rate 100 pg/min.
c33:12:33:23‐blend of nonanal, (R)‐(+)‐limonene, (Z)‐3‐hexenyl acetate, (E)‐β‐ocimene (release rate 10 ng/min), and 4,8‐dimethyl‐1,3(E),7‐nonatriene (DMNT; release rate 10 ng/min).
dnot tested.
Males were flown to the main pheromone compound, a four‐component sex pheromone blend or a pheromone‐releasing S. littoralis female, together with a synthetic blend of herbivore‐damaged cotton volatiles, or a live cotton plant damaged by S. littoralis larval feeding. Asterisks show significant differences between attraction to pheromone and to pheromone and plant stimulus (binomial GLM and post‐hoc Wald pairwise comparisons; n = 50).
Discussion
That mate finding is elicited by an ensemble of sexual and environmental odorants, which mutually affect each other, provides a new perspective of premating communication in moths. The behavioral role of plant volatiles in male moth sexual behavior has not been entirely resolved. It has been proposed that host plant volatiles mediate male attraction to mating sites either by themselves, before the onset of pheromone release by females, or by synergizing the response to sex pheromone (Landolt and Phillips 1997; Reddy and Guerrero 2004; Beyaert and Hilker 2014). In some species, host plant volatiles increase male attraction toward sex pheromone (Dickens et al. 1993; Light et al. 1993; Yang et al. 2004; Schmidt‐Büsser et al. 2009; Varela et al. 2011; von Arx et al. 2012), whereas they produce an antagonistic effect in other species (Pregitzer et al. 2012; Jung et al. 2013; Party et al. 2013; Rouyar et al. 2015). It is conceivable that volatiles from nonhost plants (Wang et al. 2016), volatiles from damaged plants, such as DMNT (Fig. 1C; Hatano et al. 2015), or floral odorants, such as β‐ocimene, which signal adult food sources (Fig. 1C; Kroman et al. 2015; Zakir et al. 2017) do not synergize pheromone attraction. It seems counter‐intuitive, on the other hand, that volatiles of larval food plants would inhibit male attraction to conspecific female sex pheromone, since many moths mate on their respective host plants, where females oviposit.
Our results offer an explanation for this conundrum. Host plant volatiles reduce male attraction to heterospecific or incomplete synthetic pheromone (Table 1, Fig. 1). This compares to findings in grapevine moth Lobesia botrana, where host plant volatiles increased male attraction to an optimized pheromone blend, but decreased attraction to a single pheromone component (Sans et al. 2016). On the other hand, it has already been shown that volatiles from non‐host plants or less preferred host plants reduce attraction to conspecific pheromone in S. littoralis and fall armyworm S. frugiperda (Anderson et al. 2013; Binyameen et al. 2013; Thöming et al. 2013; Unbehend et al. 2013).
Cotton is a common plant host for the sibling species S. littoralis and S. litura, which are distributed in Africa and the West Palearctic and in Asia, respectively; their distributions overlap in the Middle East (Pogue 2002; Kergoat et al. 2012). Males of the African cotton leafworm S. littoralis are attracted to females of both species, but prefer conspecific females in choice tests; heterospecific matings with S.litura females are prevented by genital morphology (Saveer et al. 2014). Presence of the plant host accentuates differences between conspecific and heterospecific sex pheromones (Fig. 1B,C). This interaction between plant cues and sex signals is consequential, since it facilitates specific mate finding and recognition in closely related species, which frequently use pheromone blends that share compounds and partially overlap in composition (El‐Sayed 2017). Sex pheromones typically consist of a blend of several compounds that have been shown to function as a unit (Linn et al. 1986). Our findings demonstrate that this laboratory‐derived concept must be updated to accommodate the role of host plant volatiles in pheromone communication: it is the ensemble of social signals and environmental cues that mediates mate finding and recognition in natural environments.
Changes in female pheromone production and the corresponding shift in male preference are driven by sexual selection. Divergence of sex pheromone blends has been documented in populations of the same species (Cardé et al. 1978; Malausa et al. 2005; Groot et al. 2008; Velasquez‐Velez et al. 2011) or in sibling species (Lofstedt and van der Pers 1985; Bengtsson et al. 2014; Saveer et al. 2014). According to the “asymmetric tracking” hypothesis, males track changes of the female pheromone composition and rather quickly develop a preference for new pheromone blends (Phelan 1992; Heckel 2010; Droney et al. 2012). Such changes are believed to enable sympatric speciation in moths through premating behavioral isolation (Smadja and Butlin 2009; M'Gonigle et al. 2012).
Host plant shifts in females, on the other hand, are driven by natural selection. If shifts in female host plant preference lead to disruptive selection on host use, they may lead to speciation with little or no changes in pheromone composition (Lofstedt and van der Pers 1985; Witzgall et al. 1991; Drès and Mallet 2002; Bengtsson et al. 2006; Leppik and Frérot 2012). Matsubayashi et al. (2010) suggest that changes in host plant preference may lead to premating isolation based solely on a reduced probability of encounters between populations associated with different hosts. In polyphagous species where populations have generalized diets, individuals may have preferences for a particular host plant and may be subject to selective pressures that may lead to diversification (Bolnick et al. 2003; Rueffler et al. 2006). The recent finding that host plant choice in S. littoralis is modified by larval experience or adult learning (Proffit et al. 2015) supports a scenario where individual preference could lead to host plant shifts and initiate divergence.
If host plant volatiles affect pheromone perception, it follows that male moth pheromone detection and mate finding is under combined sexual and natural selection. Traits combining local adaptation and mating decisions have been termed “magic traits” since they facilitate phylogenetic divergence, especially in insect herbivores (Gavrilets 2004; Pfennig and Pfennig 2009; Smadja and Butlin 2009; Servedio et al. 2011; Safran et al. 2013; Thibert‐Plante and Gavrilets 2013; Rebar and Rodríguez 2015). Under sympatric conditions, natural selection alone is unlikely to lead to speciation due to random mating, however, if selection acts on both habitat and mate preference simultaneously, speciation is far more likely to occur.
Our system demonstrates a mechanism where the behavioral consequences of shifts in sex pheromone biosynthesis are reinforced by host plant volatiles. New pheromone communication channels may give rise to reproductive isolation, especially in populations diverging onto new host plants. Mate finding mediated by a combination of pheromones and host plant volatile signatures will reinforce premating barriers when a population undergoes disruptive selection (Ritchie 2007; Butlin et al. 2012; Boughman and Svanbäck 2016). This adds further support to the view that sympatric speciation has contributed to shaping the tremendous diversity of phytophagous insects (Tauber and Tauber 1989; Berlocher and Feder 2002; Drès and Mallet 2002; Forbes et al. 2017).
DATA ACCESSIBILITY
Data available at the Dryad Digital Repository https://doi.org/10.5061/dryad.v54v8.
Associate Editor: G. Rosenthal
Handling Editor: P. Tiffin
Supporting information
Treatment
Single host plant volatiles
Main pheromone component (MPC) + single host plant volatiles
4 component pheromone (4CP) + single host plant volatiles
Main pheromone component (MPC) + plant blends
4 component pheromone (4CP) + plant blends
S. littoralis females
S. litura females
AUTHOR CONTRIBUTIONS
Behavioural assays in the wind tunnel inluding statistical analysis were done by Felipe Borrero‐Echeverry. Marie Bengtsson acquired, prepared and analysed all chemicals. Kiyoshi Nakamuta field‐collected Spodoptera litura. Felipe Borrero‐Echeverry, Marie Bengtsson and Peter Witzgall designed the study, all authors contributed to the writing of the manuscript.
ACKNOWLEDGMENTS
Supported by the Linnaeus initiative "Insect Chemical Ecology, Ethology and Evolution" (Formas, SLU).
DATA ARCHIVING
The doi for our data is https://doi.org/10.5061/dryad.v54v8.
LITERATURE CITED
- Anderson, P. , Sadek M. M., Larsson M., Hansson B. S., and Thöming G.. 2013. Larval host plant experience modulates both mate finding and oviposition choice in a moth. Anim. Behav. 85:1169–1175. [Google Scholar]
- Badeke, E. , Haverkamp A., Hansson B. S., and Sachse S.. 2016. A challenge for a male noctuid moth? Discerning the female sex pheromone against the background of plant volatiles. Front. Physiol. 7:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher, P. G. , Bengtsson M., Hansson B. S., and Witzgall P.. 2010. Flying the fly: long‐range flight behavior of Drosophila melanogaster to attractive odors. J. Chem. Ecol. 36:599–607. [DOI] [PubMed] [Google Scholar]
- Bengtsson, M. , Jaastad G., Knudsen G., Kobro S., Bäckman A.‐C., Pettersson E., and Witzgall P.. 2006. Plant volatiles mediate attraction to host and non‐host plant in apple fruit moth, Argyresthia conjugella . Entomol. Exp. Appl. 118:77–85. [Google Scholar]
- Bengtsson, M. , Boutitie A., Jósvai J., Toth M., Andreadis S., Rauscher S., Unelius C. R., and Witzgall P.. 2014. Pheromone races of Cydia splendana (Lepidoptera, Tortricidae) overlap in host plant association and geographic distribution. Front. Ecol. Evol. 2:33. [Google Scholar]
- Berlocher, S. H. , and Feder J. L.. 2002. Sympatric speciation in phytophagous insects: moving beyond controversy? Ann. Rev. Entomol. 47:773–815. [DOI] [PubMed] [Google Scholar]
- Beyaert, I. , and Hilker M.. 2014. Plant odour plumes as mediators of plant‐insect interactions. Biol. Rev. 89:68–81. [DOI] [PubMed] [Google Scholar]
- Binyameen, M. , Hussain A., Yousefi F., Birgersson G., and Schlyter F.. 2013. Modulation of reproductive behaviors by non‐host volatiles in the polyphagous Egyptian Cotton Leafworm, Spodoptera littoralis . J. Chem. Ecol. 39:1273–1283. [DOI] [PubMed] [Google Scholar]
- Blows, M. W. 2002. Interaction between natural and sexual selection during the evolution of mate recognition. Proc. R Soc. B 269:1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick, D. , Svanbäck R., Fordyce J., Yang L., Davis J., Hulsey C. D., and Forister M. L.. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161:1–28. [DOI] [PubMed] [Google Scholar]
- Borrero‐Echeverry, F. , Becher P. G., Birgersson G. Å. O., Bengtsson M., Witzgall P., and Saveer A. M.. 2015. Flight attraction of Spodoptera littoralis (Lepidoptera, Noctuidae) to cotton headspace and synthetic volatile blends. Front. Ecol. Evol. 3:56. [Google Scholar]
- Boughman, J. W. 2002. How sensory drive can promote speciation. Trends Ecol. Evol. 17:571–577. [Google Scholar]
- Boughman, J. W. , and Svanbäck R.. 2016. Synergistic selection between ecological niche and mate preference primes diversification. Evolution 71:6–22. [DOI] [PubMed] [Google Scholar]
- Butlin, R. , Debelle A., Kerth C., Snook R. R., Beukeboom L. W., Castillo C. R., Diao W., Maan M. E., Paolucci S., and Weissing F. J.. 2012. What do we need to know about speciation? Trends Ecol. Evol. 27:27–39. [DOI] [PubMed] [Google Scholar]
- Cardé, R. T. , Baker T. C.. 1984. Sexual communication with pheromones Pp. 355–383 in Bell W. J. and Cardé R. T., eds. Chemical ecology of insects. Springer, Boston, MA. [Google Scholar]
- Cardé, R. T. , Roelofs W. L., Harrison R. G., Vawter A. T., Brussard P. F., Mutuura A., and Munroe E.. 1978. European corn borer: pheromone polymorphism or sibling species? Science 199:555–556. [DOI] [PubMed] [Google Scholar]
- Chaffiol, A. , Kropf J., Barrozo R., Gadenne C., Rospar J. P., and Anton S.. 2012. Plant odour stimuli reshape pheromonal representation in neurons of the antennal lobe macroglomerular complex of a male moth. J. Exp. Biol. 215:1670–1680. [DOI] [PubMed] [Google Scholar]
- Chaffiol, A. , Dupuy F., Barrozo R. B., Kropf J., Renou M., Rospars J. P., and Anton S.. 2014. Pheromone modulates plant odor responses in the antennal lobe of a moth. Chem. Senses 39:451–463. [DOI] [PubMed] [Google Scholar]
- Dickens, J. , Jang E., Light D., and Alford A. 1990. Enhancement of insect pheromone responses by green leaf volatiles. Naturwissenschaften 77:29–31. [Google Scholar]
- Dickens, J. C. , Smith J. W., and Light D. M.. 1993. Green leaf volatiles enhance sex attractant pheromone of the tobacco budworm, Heliothis virescens (Lep.: Noctuidae). Chemoecology 4:175–177. [Google Scholar]
- Drès, M. , and Mallet J.. 2002. Host races in plant–feeding insects and their importance in sympatric speciation. Philos. Trans. R Soc. Lond. B 357:471–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droney, D. C. , Musto C. J., Mancuso K., Roelofs W. L., and Linn C. E. Jr. 2012. The response to selection for broad male response to female sex pheromone and its implications for divergence in close‐range mating behavior in the European corn borer moth, Ostrinia nubilalis . J. Chem. Ecol. 38:1504–1512. [DOI] [PubMed] [Google Scholar]
- El‐Sayed, A. 2017. The Pherobase: database of pheromones and semiochemicals. http://www.pherobase.com.
- El‐Sayed, A. , Godde J., and Arn H.. 1999. Sprayer for quantitative application of odor stimuli. Environ. Entomol. 28:947–953. [Google Scholar]
- Endler, J. A. 1992. Signals, signal conditions, and the direction of evolution. Am. Nat. 139:S125–S153. [Google Scholar]
- Forbes, A. A. , Devine S. N., Hippee A. C., Tvedte E. S., Ward A. K., Widmayer H. A., and Wilson C. J.. 2017. Revisiting the particular role of host shifts in initiating insect speciation. Evolution 71:1126–1137. [DOI] [PubMed] [Google Scholar]
- Gavrilets, S. 2004. Fitness landscapes and the origin of species. Princeton Univ. Press, Princeton, NJ. [Google Scholar]
- Groot, A. T. , Marr M., Schoefl G., Lorenz S., Svatos A., and Heckel D. G.. 2008. Host strain specific sex pheromone variation in Spodoptera frugiperda . Front. Zool. 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano, E. , Saveer A., Borrero‐Echeverry F., Strauch M., Zakir A., Bengtsson M., Ignell R., Anderson P., Becher P., Witzgall P., and Dekker T.. 2015. A herbivore‐induced plant volatile interferes with host plant and mate location in moths through suppression of olfactory signaling pathways. BMC Biol. 13:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckel, D. G. 2010. Smells like a new species: gene duplication at the periphery. Proc. Natl. Acad. Sci. USA 107:9481–9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks, C. , and Byers J.. 1976. Biosystematics of the genus Euxoa (Lepidoptera: Noctuidae): V. Rearing procedures, and life cycles of 36 species. Canad. Entomol. 108:1345–1357. [Google Scholar]
- Ian, E. , Kirkerud N. H., Galizia C. G., and Berg B. G.. 2017. Coincidence of pheromone and plant odor leads to sensory plasticity in the heliothine olfactory system. PloS One 12:e0175513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, C. R. , Jung J. K., and Kim Y.. 2013. Effects of different sex pheromone compositions and host plants on the mating behavior of two Grapholita species. J. Asia‐Pacific Entomol. 16:507–512. [Google Scholar]
- Kergoat, G. J. , Prowell D. P., Le Ru B. P., Mitchell A., Dumas P., Clamens A. L., Condamine F. L., and Silvain J. F.. 2012. Disentangling dispersal, vicariance and adaptive radiation patterns: a case study using armyworms in the pest genus Spodoptera (Lepidoptera: Noctuidae). Mol. Phylogenet. Evol. 65:855–870. [DOI] [PubMed] [Google Scholar]
- Krieger, J. , Grosse‐Wilde E., Gohl T., Dewer Y. M. E., Raming K., and Breer H.. 2004. Genes encoding candidate pheromone receptors in a moth (Heliothis virescens). Proc. Natl. Acad. Sci. USA 101:11845–11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromann, S. H. , Saveer A. M., Binyameen M., Bengtsson M., Birgersson G., Hansson B. S., Schlyter F., Witzgall P., Ignell R., and Becher P. G.. 2015. Concurrent modulation of neuronal and behavioural olfactory responses to sex and host plant cues in a male moth. Proc. R Soc. B 282:20141884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt, P. J. , and Phillips T. W.. 1997. Host plant influences on sex pheromone behavior of phytophagous insects. Ann. Rev. Entomol. 42:371–391. [DOI] [PubMed] [Google Scholar]
- Lebreton, S. , Borrero‐Echeverry F., Gonzalez F., Solum M., Wallin E., Hedenström E., Hansson B. S., Gustavsson A.‐L., Bengtsson M., Birgersson G., et al. 2017. A Drosophila female pheromone elicits species‐specific long‐range attraction via an olfactory channel with dual specificity for sex and food. BMC Biol. 15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppik, E. , and Frérot B.. 2012. Volatile organic compounds and host‐plant specialization in European corn borer E and Z pheromone races. Chemoecology 22:119–129. [Google Scholar]
- Light, D. M. , Flath R. A., Buttery R. G., Zalom F. G., Rice R. E., Dickens J. C., and Jang E. B.. 1993. Host‐plant green‐leaf volatiles synergize the synthetic sex pheromones of the corn earworm and codling moth (Lepidoptera). Chemoecology 4:145–152. [Google Scholar]
- Linn, C. E. , Campbell M. G., and Roelofs W. L.. 1986. Male moth sensitivity to multicomponent pheromones: critical role of female‐released blend in determining the functional role of components and active space of the pheromone. J. Chem. Ecol. 12:659–668. [DOI] [PubMed] [Google Scholar]
- Lofstedt, C. , and van der Pers J. N. C.. 1985. Sex pheromones and reproductive isolation in four European small ermine moths. J. Chem. Ecol. 11:649–666. [DOI] [PubMed] [Google Scholar]
- Loughrin, J. H. , Manukian A., Heath R. R., and Tumlinson J. H.. 1995. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J. Chem. Ecol. 21:1217–1227. [DOI] [PubMed] [Google Scholar]
- M'Gonigle, L. K. , Mazzucco R., Otto S. P., and Dieckmann U.. 2012. Sexual selection enables long‐term coexistence despite ecological equivalence. Nature 484:506–509. [DOI] [PubMed] [Google Scholar]
- Malausa, T. , Bethenod M.‐T., Bontemps A., Bourguet D., Cornuet J.‐M., and Ponsard S.. 2005. Assortative mating in sympatric host races of the European corn borer. Science 308:258–260. [DOI] [PubMed] [Google Scholar]
- Matsubayashi, K. W. , Ohshima I., and Nosil P.. 2010. Ecological speciation in phytophagous insects. Entomol. Exp. Appl. 134:1–27. [Google Scholar]
- Namiki, S. , Iwabuchi S., and Kanzaki R.. 2008. Representation of a mixture of pheromone and host plant odor by antennal lobe projection neurons of the silkmoth Bombyx mori . J. Comp. Physiol. A 194:501–515. [DOI] [PubMed] [Google Scholar]
- Party, V. , Hanot C., Büsser D. S., Rochat D. & Renou M.. 2013. Changes in odor background affect the locomotory response to pheromone in moths. PLoS One 8:e52897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, H. 1978. More evidence against speciation by reinforcement. S. Afri. J. Sci. 74:369–371. [Google Scholar]
- Paterson, H. E. 1980. A comment on “mate recognition systems.” Evolution 34:330–331. [DOI] [PubMed] [Google Scholar]
- Pfennig, K. S. , and Pfennig D. W.. 2009. Character displacement: ecological and reproductive responses to a common evolutionary problem. Quart. Rev. Biol. 84:253–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan, P. 1992. Evolution of sex pheromones and the role of asymmetric tracking Pp. 265–314 in Roitberg B. D. and Isman M. B., eds. Insect chemical ecology: An evolutionary approach. Chapman and Hall, New York. [Google Scholar]
- Pinheiro, J. , Bates D., DebRoy S., Sarkar D., and R Core Team . 2018. NLME: linear and nonlinear mixed effects models. R package version 3.1‐137. https://CRAN.R-project.org/package=nlme.
- Pogue, M. 2002. A world revision of the genus Spodoptera Guenée (Lepidoptera: Noctuidae). Memoirs Am. Entomol. Soc. 43:1–202. [Google Scholar]
- Pregitzer, P. , Schubert M., Breer H., Hansson B. S., Sachse S., and Krieger J.. 2012. Plant odorants interfere with detection of sex pheromone signals by male Heliothis virescens . Front. Cell. Neurosci. 6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proffit, M. , Khallaf M. A., Carrasco D., Larsson M. C., and Anderson P.. 2015. Do you remember the first time? Host plant preference in a moth is modulated by experiences during larval feeding and adult mating. Ecol. Lett. 18:365–374. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Rebar, D. , and Rodríguez R. L.. 2015. Insect mating signal and mate preference phenotypes covary among host plant genotypes. Evolution 69:602–610. [DOI] [PubMed] [Google Scholar]
- Reddy, G. V. P. , and Guerrero A.. 2004. Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 9:253–261. [DOI] [PubMed] [Google Scholar]
- Ritchie, M. G. 2007. Sexual selection and speciation. Ann. Rev. Ecol. Evol. Syst. 38:79–102. [Google Scholar]
- Rosenthal, G. G. 2017. Mate choice: the evolution of sexual decision making from microbes to humans. Princeton Univ. Press, Princeton. [Google Scholar]
- Rouyar, A. , Deisig N., Dupuy F., Limousin D., Wycke M.‐A., Renou M., and Anton S.. 2015. Unexpected plant odor responses in a moth pheromone system. Front. Physiol. 6:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueffler, C. , Van Dooren T. J., Leimar O., and Abrams P. A.. 2006. Disruptive selection and then what? Trends Ecol. Evol. 21:238–245. [DOI] [PubMed] [Google Scholar]
- Safran, R. J. , Scordato E. S. C., Symes L. B., Rodriguez R. L., and Mendelson T. C.. 2013. Contributions of natural and sexual selection to the evolution of premating reproductive isolation: a research agenda. Trends Ecol. Evol. 28:643–650. [DOI] [PubMed] [Google Scholar]
- Sakurai, T. , Nakagawa T., Mitsuno H., Mori H., Endo Y., Tanoue S., Yasukochi Y., Touhara K., and Nishioka T.. 2004. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori . Proc. Natl. Acad. Sci. USA 101:16653–16658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans, A. , Moran M., Riba M., Guerrero A., Roig J., and Gemeno C.. 2016. Plant volatiles challenge inhibition by structural analogs of the sex pheromone in Lobesia botrana (Lepidoptera: Tortricidae). Eur. J. Entomol. 113:579–586. [Google Scholar]
- Saveer, A. M. , Kromann S. H., Birgersson G., Bengtsson M., Lindblom T., Balkenius A., Hansson B. S., Witzgall P., Becher P. G., and Ignell R.. 2012. Floral to green: mating switches moth olfactory coding and preference. Proc. R Soc. B 279:2314–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saveer, A. M. , Becher P. G., Birgersson G., Hansson B. S., Witzgall P., and Bengtsson M.. 2014. Mate recognition and reproductive isolation in the sibling species Spodoptera littoralis and Spodoptera litura . Front. Ecol. Evol. 218. [Google Scholar]
- Schmidt‐Büsser, D. , Von Arx M., and Guerin P. M.. 2009. Host plant volatiles serve to increase the response of male European grape berry moths, Eupoecilia ambiguella, to their sex pheromone. J. Comp. Physiol. A 195:853–864. [DOI] [PubMed] [Google Scholar]
- Scordato, E. S. , Symes L. B., Mendelson T. C., and Safran R. J.. 2014. The role of ecology in speciation by sexual selection: a systematic empirical review. J. Heredity 105:782–94. [DOI] [PubMed] [Google Scholar]
- Servedio, M. R. , Van Doorn G. S., Kopp M., Frame A. M., and Nosil P.. 2011. Magic traits in speciation: ‘magic’ but not rare? Trends Ecol. Evol. 26:389–397. [DOI] [PubMed] [Google Scholar]
- Smadja, C. , and Butlin R.. 2009. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102:77–97. [DOI] [PubMed] [Google Scholar]
- Tauber, C. A. , and Tauber M. J.. 1989. Sympatric speciation in insects: perception and perspective Pp. 307–344 in Otte D. and Endler D. S., eds. Speciation and its consequences. Sinauer, Sunderland. [Google Scholar]
- Thibert‐Plante, X. , and Gavrilets S.. 2013. Evolution of mate choice and the so‐called magic traits in ecological speciation. Ecol. Lett. 16:1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thöming, G. , Larsson M. C., Hansson B. S., and Anderson P.. 2013. Comparison of plant preference hierarchies of male and female moths and the impact of larval rearing hosts. Ecology 94:1744–1752. [DOI] [PubMed] [Google Scholar]
- Trona, F. , Anfora G., Bengtsson M., Witzgall P., and Ignell R.. 2010. Coding and interaction of sex pheromone and plant volatile signals in the antennal lobe of the codling moth Cydia pomonella . J. Exp. Biol. 213:4291–4303. [DOI] [PubMed] [Google Scholar]
- Trona, F. , Anfora G., Balkenius A., Bengtsson M., Tasin M., Knight A., Janz N., Witzgall P., and Ignell R.. 2013. Neural coding merges sex and habitat chemosensory signals in an insect herbivore. Proc. R Soc. B 280:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unbehend, M. , Hänniger S., Meagher R. L., Heckel D. G., and Groot A. T.. 2013. Pheromonal divergence between two strains of Spodoptera frugiperda . J. Chem. Ecol. 39:364–376. [DOI] [PubMed] [Google Scholar]
- Varela, N. , Avilla J., Anton S., and Gemeno C. 2011. Synergism of pheromone and host‐plant volatile blends in the attraction of Grapholita molesta males. Entomol. Exp. Appl. 141:114–122. [Google Scholar]
- Velasquez‐Velez, M. I. , Saldamando‐Benjumea C. I., and Rios‐Diez J. D.. 2011. Reproductive isolation between two populations of Spodoptera frugiperda (Lepidoptera: Noctuidae) collected in corn and rice fields from central Colombia. Annal. Entomol. Soci. Am. 104:826–833. [Google Scholar]
- Von Arx, M. , Schmidt‐Busser D., and Guerin P. M.. 2012. Plant volatiles enhance behavioral responses of grapevine moth males, Lobesia botrana to sex pheromone. J. Chem. Ecol. 38:222–225. [DOI] [PubMed] [Google Scholar]
- Wang, F. , Deng J., Schal C., Lou Y., Zhou G., Ye B., Yin X., Xu Z., and Shen L.. 2016. Non‐host plant volatiles disrupt sex pheromone communication in a specialist herbivore. Sci. Rep. 6:32666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzgall, P. , Bengtsson M., Buser H. R., Chambon P. J., Priesner E., Wildbolz T., and Arn H.. 1991. Sex pheromones of Spilonota ocellana and Spilonota laricana . Entomol. Exp. Appl. 60:219–223. [Google Scholar]
- Yang, Z. H. , Bengtsson M., and Witzgall P.. 2004. Host plant volatiles synergize response to sex pheromone in codling moth, Cydia pomonella . J. Chem. Ecol. 30:619–629. [DOI] [PubMed] [Google Scholar]
- Yang, C. Q. , Wu X. M., Ruan J. X., Hu W. L., Mao Y. B., Chen X. Y., and Wang L. J.. 2013. Isolation and characterization of terpene synthases in cotton (Gossypium hirsutum). Phytochemistry 96:46–56. [DOI] [PubMed] [Google Scholar]
- Zakir, A. , Bengtsson M., Sadek M. M., Hansson B. S., Witzgall P., and Anderson P.. 2013a. Specific response to herbivore‐induced de novo synthesized plant volatiles provides reliable information for host plant selection in a moth. J. Exp. Biol. 216:3257–3263. [DOI] [PubMed] [Google Scholar]
- Zakir, A. , Sadek M. M., Bengtsson M., Hansson B. S., Witzgall P., and Anderson P.. 2013b. Herbivore‐induced plant volatiles provide associational resistance against an ovipositing herbivore. J. Ecol. 101:410–417. [Google Scholar]
- Zakir, A. , Khallaf M. A., Hansson B. S., Witzgall P., and Anderson P.. 2017. Herbivore‐induced changes in cotton modulates reproductive behavior in the moth Spodoptera littoralis . Front. Ecol. Evol. 5:49. [Google Scholar]
- Zhang, D. D. , and Löfstedt C.. 2015. Moth pheromone receptors: gene sequences, function, and evolution. Front. Ecol. Evol. 3:105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treatment
Single host plant volatiles
Main pheromone component (MPC) + single host plant volatiles
4 component pheromone (4CP) + single host plant volatiles
Main pheromone component (MPC) + plant blends
4 component pheromone (4CP) + plant blends
S. littoralis females
S. litura females
Data Availability Statement
Data available at the Dryad Digital Repository https://doi.org/10.5061/dryad.v54v8.


