Abstract
Study Objective
Telavancin and vancomycin are both approved for treatment of hospital‐acquired and ventilator‐associated bacterial pneumonias caused by Staphylococcus aureus, and both agents can cause renal dysfunction. The objective of this study was to assess renal function changes by performing renal shift table analyses of telavancin‐ and vancomycin‐treated patients in phase III trials.
Design
Retrospective, descriptive analysis of data from the safety population from the Assessment of Telavancin for Treatment of Hospital‐Acquired Pneumonia (ATTAIN) trials.
Patients
A total of 1503 adults with hospital‐acquired or ventilator‐associated bacterial pneumonia primarily caused by gram‐positive pathogens and who received telavancin (n = 751) or vancomycin (n = 752).
Measurements and Main Results
Decline or improvement in creatinine clearance (CrCl) across seven defined categories (≤30, >30–40, >40–50, >50–60, >60–70, >70–80, and >80 ml/min) was classified as negative or positive shifts, respectively. The number of categories crossed (either positive or negative) determined the grade of shift (of a potential grades 1–6, with crossing from one category to the next adjacent category defined as a grade 1 shift) at specific time points compared with baseline: day 4, day 7, and end of therapy (EOT). Approximately 77%–91.6% of patients had either no change or improvement of CrCl across all time points for both treatments. Negative shifts were consistent for telavancin (day 4, 19.3%; day 7, 19.0%; EOT, 23.0%) but increased over time for vancomycin (day 4, 8.4%; day 7, 12.3%; EOT, 19.3%). A significantly lower proportion of patients receiving vancomycin showed renal function decline on day 4 and day 7. At EOT, negative shift rates were similar between treatments (treatment difference 3.6% [95% CI −0.7 to 7.9]). At day 7 and EOT, a higher percentage of vancomycin‐treated patients experienced high‐grade negative shifts relative to telavancin (day 7, vancomycin 2.8% vs telavancin 1.9%; EOT, vancomycin 4.7% vs telavancin 4.1%), though differences were not statistically significant.
Conclusion
Use of shift tables revealed differences in timing of renal function changes in patients receiving telavancin and vancomycin. Telavancin‐related declines in renal function were similar at day 4 and day 7, with a slight increase by EOT. This differed from vancomycin, which caused a steady increase in the percentage of patients with renal function decline over time. A significant difference in negative renal shifts between treatments occurred at day 4 and day 7 and favored vancomycin; however, the difference was minimal and not significant at EOT.
Keywords: telavancin, vancomycin, renal function, pneumonia, Staphylococcus aureus
Hospital‐acquired and ventilator‐associated bacterial pneumonia (HABP/VABP) are serious infections with substantial related morbidity and mortality, especially when caused by organisms such as methicillin‐resistant Staphylococcus aureus (MRSA).1, 2, 3 Vancomycin and linezolid are the Infectious Diseases Society of America–recommended antibacterial agents used for the treatment of HABP and VABP caused by MRSA4; however, vancomycin has slow bactericidal activity and variable lung penetration.5, 6, 7 Linezolid is associated with potentially significant adverse drug interactions.8, 9 Daptomycin, an alternative antibiotic used to treat MRSA, is not an effective treatment for pneumonia, as it is inhibited by pulmonary surfactant, a major component of epithelial lining fluid (ELF).10 The newer, long‐acting lipoglycopeptides, oritavancin and dalbavancin, are currently untested for the treatment of serious infections.
Telavancin, a synthetic lipoglycopeptide antibacterial, is 32‐fold more potent than vancomycin against MRSA strains when tested under in vitro conditions.11 In addition, telavancin displays good penetration into ELF and alveolar macrophages (AM), with concentrations exceeding the current minimum inhibitory concentration for 90% of MRSA strains (0.06 μg/ml)12 by several‐fold (ELF, 15‐fold; AM, 700‐fold) at 24 hours.13 In other studies, median telavancin ELF penetration was approximately 75% of the plasma concentration,14 whereas vancomycin ELF levels were around 50%.15 Data for vancomycin AM penetration in humans are limited. Telavancin is approved in the United States for the treatment of HABP and VABP caused by MRSA and other susceptible isolates of S. aureus when alternative treatments are not suitable.16 Furthermore, in a post hoc analysis of phase III trials, comparable efficacy to vancomycin was observed in a limited number of patients with HABP/VABP and concurrent S. aureus bacteremia.16, 17
Adverse event data from clinical trials have demonstrated that both telavancin and vancomycin are associated with a decline in renal function in some patients.18, 19 Other factors may also contribute to the observed nephrotoxicity, including the disease process itself or the use of concomitant medications.20 Often, changes in renal function require careful dosage adjustment of the antibacterial agent, particularly for those cleared or eliminated through the kidney. An understanding of the time course and magnitude of changes in renal function during antibacterial use may prevent unnecessary treatment termination or changes in treatment.
The time course of renal function changes was not characterized in pivotal telavancin and vancomycin clinical trials.19, 21 Shift table analyses are used to describe various drug‐related laboratory abnormalities, such as the hematologic effects of linezolid during clinical trials.22 Renal shift tables are a tool used to observe renal function changes over time based on estimated creatinine clearance (CrCl) values. Thus, the objective of this study was to compare the timing and extent of changes in renal function during treatment with telavancin or vancomycin in the HABP/VABP safety population from the Assessment of Telavancin for Treatment of Hospital‐Acquired Pneumonia (ATTAIN) trials by performing a retrospective renal shift analysis.
Methods
Study Design and Population
This was a retrospective, descriptive analysis of the safety population from the ATTAIN trials (http://ClinicalTrials.gov identifiers NCT00107952 and NCT00124020). The study methods and principal results have been published previously.19 Briefly, the ATTAIN trials were two identical double‐blind, randomized, global studies involving adult patients with HABP or VABP primarily caused by gram‐positive pathogens. The trials were conducted in accordance with the principles of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guideline for Good Clinical Practice and the principles of the Declaration of Helsinki, or with country‐specific laws and regulations if these afforded greater protection to the patient. All patients or their authorized representatives gave written informed consent.
The safety population comprised 1503 patients who received at least one dose of telavancin (n = 751) or vancomycin (n = 752). Telavancin was administered as a 60‐minute infusion of 10 mg/kg (based on actual body weight) every 24 hours, with dosage adjustments made if CrCl was ≤50 ml/minute (7.5 mg/kg every 24 hrs if CrCl 30–50 ml/min; 10 mg/kg every 48 hrs if CrCl <30 ml/min; 10 mg/kg every 48 hrs if receiving hemodialysis). Vancomycin was administered as a 60‐minute infusion at a dosage of 1 g every 12 hours or according to the study site‐specific protocol for vancomycin dosing based on body weight and/or renal function or trough level monitoring. Infusion time may have been longer based on the standard of care of the study site. Of the vancomycin‐treated patients, 242 (32%) began treatment at a total of <1.8 g per 24 hours, 479 (64%) at 1.8–2.2 g per 24 hours, and 31 (4%) at >2.2 g per 24 hours. Patients were to receive study medication for a minimum of 7 days and a maximum of 21 days. Most patients (1247/1503 [83%]) received between 3 and 14 days of study medication, and median duration of therapy for both treatments was 9 days.
Depending on the duration of treatment, renal function was to be assessed by measuring serum creatinine concentration before receiving study treatment (baseline); on days 4, 7, 10, 14, 17, and 21; at end of therapy; and at follow‐up. The end of therapy visit was performed within 3 days after receiving the last dose of study medication. Serum creatinine values were not available for all study visits since patients may have withdrawn or discontinued study treatment, or laboratory data were otherwise not available for a particular day. Creatinine clearance was estimated using the Cockcroft‐Gault equation.23 If actual body weight was less than ideal body weight, then actual body weight was used in the equation; otherwise, ideal body weight was used.
Development of Renal Function Shift Tables
Estimates of renal function as determined by CrCl at baseline, on days 4 and 7, and at end of therapy, and the lowest CrCl observed after baseline were used in this analysis to assess the timing and degree of change in renal function. Due to the small numbers of patients in the ATTAIN studies at days 10 and 14, it was decided to focus on the baseline, day 4, day 7, and end of therapy in the present analysis. Creatinine clearance levels for each time point were partitioned into seven categories (≤30, >30–40, >40–50, >50–60, >60–70, >70–80, and >80 ml/min). These categories were selected to capture renal function changes based on baseline CrCl and patients who may have required a telavancin dosage change if the CrCl dropped below 50 ml/minute.
Tables were created to assess changes in renal function between baseline and the defined time points for telavancin‐ or vancomycin‐treated patients. Changes in renal function were classified by the direction of the shift and the number of categories shifted. If no change in renal function was observed between baseline and the specified time point, it was defined as “no shift”; if CrCl improved (e.g., >30–40 ml/min at baseline to >40–50 ml/min), it was defined as a “positive shift”; and if CrCl declined (e.g., >50–60 ml/min at baseline to >40–50 ml/min), it was defined as a “negative shift.” Negative or positive shifts in CrCl were grouped into six categories termed grades 1–6. Decline or improvement in renal function from one CrCl category to the next adjacent category was defined as a grade 1 shift. Using these categories, there was the potential for the maximum of a grade 6 shift. For example, a shift of >30–40 ml/minute at baseline to >60–70 ml/minute was defined as a grade 3 positive shift.
Statistical Analysis
The number and percentage of telavancin‐ or vancomycin‐treated patients in each category were compared. Patients with missing CrCl values at baseline or at a specified time point were not included in the analysis for the specific time point. Two‐sided 95% confidence intervals on the difference (percent telavancin minus percent vancomycin) were derived for each category using the Newcombe‐Wilson method.24 This was a retrospective analysis, and all summaries are considered descriptive and exploratory.
Results
Complete baseline characteristics of the all‐treated population have been reported previously.19 In summary, >50% of patients were ≥65 years old, male, white, and in intensive care units at baseline.19 Patients in the telavancin and vancomycin groups weighed an average of 74.5 kg and 71.1 kg, respectively, at baseline.19 Comorbidities included diabetes mellitus (telavancin 27%, vancomycin 25%), congestive heart failure (telavancin 17%, vancomycin 19%), chronic obstructive pulmonary disease (telavancin 23%, vancomycin 24%), and acute renal failure (telavancin 10%, vancomycin 8%) and/or chronic renal failure (telavancin 6%, vancomycin 7%).19 Although the number of patients with CrCl measurements changed at each time point in this post hoc analysis, the demographics of the telavancin and vancomycin populations remained relatively consistent (data not shown).
The majority of patients in the ATTAIN trials had either no change or improvement of renal function at days 4 and 7, and end of therapy, when treated with either telavancin or vancomycin. The numbers and percentages of patients experiencing CrCl shifts at each time point are reported in Tables 1, 2, and 3. Irrespective of baseline renal function, the percentage of patients who had no change or improved renal function while receiving telavancin was 80.7% (478/592) at day 4, 81.0% (340/420) at day 7, and 77.0% (527/684) at end of therapy. For patients receiving vancomycin, the percentage of patients that had no change or improved renal function was 91.6% (559/610) at day 4, 87.7% (377/430) at day 7, and 80.7% (563/698) at end of therapy. For all time points, significantly more vancomycin‐treated patients experienced a positive shift in renal function, and the negative shifts were significantly higher in the telavancin‐treated patients on days 4 and 7 (Table 3). At the end of therapy, the difference between the percentages of telavancin‐ and vancomycin‐treated patients who experienced a negative shift diminished (Table 3). When comparing the lowest CrCl level measured after baseline, telavancin‐treated patients experienced significantly more negative shifts than vancomycin‐treated patients (Table 3). The proportion of patients who had a CrCl >50 ml/minute at baseline that then fell to CrCl ≤50 ml/minute at any time point during the study was similar between treatment groups, except for day 4, when the percentage was significantly higher in telavancin‐treated patients (8.5%) relative to vancomycin‐treated patients (2.9%) (Table 4).
Table 1.
Shifts in Renal Function Categories from Baseline to End of Therapy in Patients Treated with Telavancin
| End of Therapy CrCl Category (ml/min) | Baseline CrCl Category (ml/min) | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤30 | >30–40 | >40–50 | >50–60 | >60–70 | >70–80 | >80 | ||
| ≤30 | 60 (8.8) | 15 (2.2) | 8 (1.2) | 11 (1.6) | 5 (0.7) | 6 (0.9) | 4 (0.6) | 109 (15.9) |
| >30–40 | 8 (1.2) | 21 (3.1) | 10 (1.5) | 6 (0.9) | 3 (0.4) | 1 (0.1) | 9 (1.3) | 58 (8.5) |
| >40–50 | 6 (0.9) | 12 (1.8) | 19 (2.8) | 11 (1.6) | 5 (0.7) | 4 (0.6) | 3 (0.4) | 60 (8.8) |
| >50–60 | 1 (0.1) | 5 (0.7) | 16 (2.3) | 15 (2.2) | 7 (1.0) | 5 (0.7) | 12 (1.8) | 61 (8.9) |
| >60–70 | 2 (0.3) | 4 (0.6) | 8 (1.2) | 10 (1.5) | 13 (1.9) | 8 (1.2) | 12 (1.8) | 57 (8.3) |
| >70–80 | 3 (0.4) | 1 (0.1) | 3 (0.4) | 3 (0.4) | 12 (1.8) | 10 (1.5) | 12 (1.8) | 44 (6.4) |
| >80 | 0 | 1 (0.1) | 7 (1.0) | 3 (0.4) | 16 (2.3) | 12 (1.8) | 256 (37.4) | 295 (43.1) |
| Total | 80 (11.7) | 59 (8.6) | 71 (10.4) | 59 (8.6) | 61 (8.9) | 46 (6.7) | 308 (45.0) | 684 (100) |
CrCl = creatinine clearance.
The no. (%) of patients who began therapy within a baseline renal function category (displayed horizontally) and ended therapy within a renal function category (displayed vertically) is shown. White shading represents no change in renal function, dark gray shading represents improvement in renal function, and light gray shading represents worsening renal function. Patients with missing values at baseline or end of therapy were not included in the analysis.
Table 2.
Shifts in Renal Function Categories from Baseline to End of Therapy in Patients Treated with Vancomycin
| End of Therapy CrCl Category (ml/min) | Baseline CrCl Category (ml/min) | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤30 | >30–40 | >40–50 | >50–60 | >60–70 | >70–80 | >80 | Total | |
| ≤30 | 50 (7.2) | 10 (1.4) | 2 (0.3) | 1 (0.1) | 4 (0.6) | 2 (0.3) | 7 (1.0) | 76 (10.9) |
| >30–40 | 15 (2.1) | 16 (2.3) | 11 (1.6) | 2 (0.3) | 2 (0.3) | 1 (0.1) | 9 (1.3) | 56 (8.0) |
| >40–50 | 9 (1.3) | 24 (3.4) | 11 (1.6) | 10 (1.4) | 4 (0.6) | 1 (0.1) | 10 (1.4) | 69 (9.9) |
| >50–60 | 4 (0.6) | 11 (1.6) | 19 (2.7) | 14 (2.0) | 9 (1.3) | 3 (0.4) | 11 (1.6) | 71 (10.2) |
| >60–70 | 3 (0.4) | 5 (0.7) | 8 (1.1) | 6 (0.9) | 12 (1.7) | 6 (0.9) | 11 (1.6) | 51 (7.3) |
| >70–80 | 2 (0.3) | 3 (0.4) | 1 (0.1) | 9 (1.3) | 14 (2.0) | 6 (0.9) | 19 (2.7) | 54 (7.7) |
| >80 | 0 | 3 (0.4) | 9 (1.3) | 13 (1.9) | 15 (2.1) | 32 (4.6) | 249 (35.7) | 321 (46.0) |
| Total | 83 (11.9) | 72 (10.3) | 61 (8.7) | 55 (7.9) | 60 (8.6) | 51 (7.3) | 316 (45.3) | 698 (100) |
CrCl = creatinine clearance.
The no. (%) of patients who began therapy within a baseline renal function category (displayed horizontally) and ended therapy within a renal function category (displayed vertically) is shown. White shading represents no change in renal function, dark gray shading represents improvement in renal function, and light gray shading represents worsening renal function. Patients with missing values at baseline or end of therapy were not included in the analysis.
Table 3.
Summary of Changes in Renal Function of the Safety Population
| Change in Renal Function | Day 4 | Day 7 | End of Therapy | Lowest CrCla | ||||
|---|---|---|---|---|---|---|---|---|
| TLV Group (n = 592) | VAN Group (n = 610) | TLV Group (n = 420) | VAN Group (n = 430) | TLV Group (n = 684) | VAN Group (n = 698) | TLV Group (n = 696) | VAN Group (n = 703) | |
| Positive shift, no. (%) | 135 (22.8) | 179 (29.3) | 93 (22.1) | 146 (34.0) | 133 (19.4) | 205 (29.4) | 92 (13.2) | 122 (17.4) |
| % difference (95% CI) | −6.5 (−11.5, −1.6)b | −11.8 (−17.7, −5.8)b | −9.9 (−14.4, −5.4)b | −4.1 (−7.9, −0.4)b | ||||
| No shift, no. (%) | 343 (57.9) | 380 (62.3) | 247 (58.8) | 231 (53.7) | 394 (57.6) | 358 (51.3) | 380 (54.6) | 392 (55.8) |
| % difference (95% CI) | −4.4 (−9.9, 1.2) | 5.1 (−1.6, 11.7) | 6.3 (1.1, 11.5)b | −1.2 (−6.4, 4.0) | ||||
| Negative shift, no. (%) | 114 (19.3) | 51 (8.4) | 80 (19.0) | 53 (12.3) | 157 (23.0) | 135 (19.3) | 224 (32.2) | 189 (26.9) |
| % difference (95% CI) | 10.9 (7.0, 14.8)b | 6.7 (1.8, 11.6)b | 3.6 (−0.7, 7.9) | 5.3 (0.5, 10.0)b | ||||
% difference = %TLV–%VAN * 100; TLV = telavancin; VAN = vancomycin; CrCl = creatinine clearance; CI = confidence interval.
Lowest CrCl was estimated from serum creatinine concentration measured at any time after baseline (including times not reported in this analysis).
Statistically significant difference (95% CI does not include zero).
Table 4.
Summary of Changes in Patients with Baseline CrCl >50 ml/min that Shifted to ≤50 ml/min
| Day 4 | Day 7 | End of Therapy | Lowest CrCla | |||||
|---|---|---|---|---|---|---|---|---|
| TLV Group (n = 412) | VAN Group (n = 416) | TLV Group (n = 296) | VAN Group (n = 291) | TLV Group (n = 474) | VAN Group (n = 482) | TLV Group (n = 481) | VAN Group (n = 486) | |
| No. (%) | 35 (8.5) | 12 (2.9) | 23 (7.8) | 21 (7.2) | 68 (14.3) | 53 (11.0) | 92 (19.1) | 77 (15.8) |
| % difference (95% CI) | 5.6 (2.5, 8.9)b | 0.6 (−3.8, 4.9) | 3.4 (−0.9, 7.6) | 3.3 (−1.5, 8.1) | ||||
% difference = %TLV–%VAN * 100. TLV = telavancin; VAN = vancomycin; CrCl = creatinine clearance; CI = confidence interval.
Lowest CrCl was estimated from serum creatinine concentration measured at any time after baseline (including times not reported in this analysis).
Statistically significant difference (95% CI does not include zero).
Most negative changes in renal function for both treatments were low grade (shift of one or two categories) (Figure 1A and Table S1). In the telavancin treatment group, 15.7% of patients experienced a grade 1 or 2 negative shift on day 4, 15.5% on day 7, and 14.5% at end of therapy, whereas in the vancomycin treatment group, 7.2% of patients experienced a grade 1 or 2 negative shift on day 4, 7.9% on day 7, and 12.5% at end of therapy. Larger shifts in renal function decline (grades 4–6) were observed more often at day 4 with telavancin (1.9%) compared with vancomycin (0.7%) (Figures 1A and B). However, at day 7 and end of therapy, smaller proportions of telavancin‐treated patients than vancomycin‐treated patients experienced these large shifts (1.9% vs 2.8% on day 7; 4.1% vs 4.7% at end of therapy) (Figures 1A and B;Table S1). The percent difference in negative shifts between the telavancin and vancomycin groups was 3.6% at end of therapy.
Figure 1.
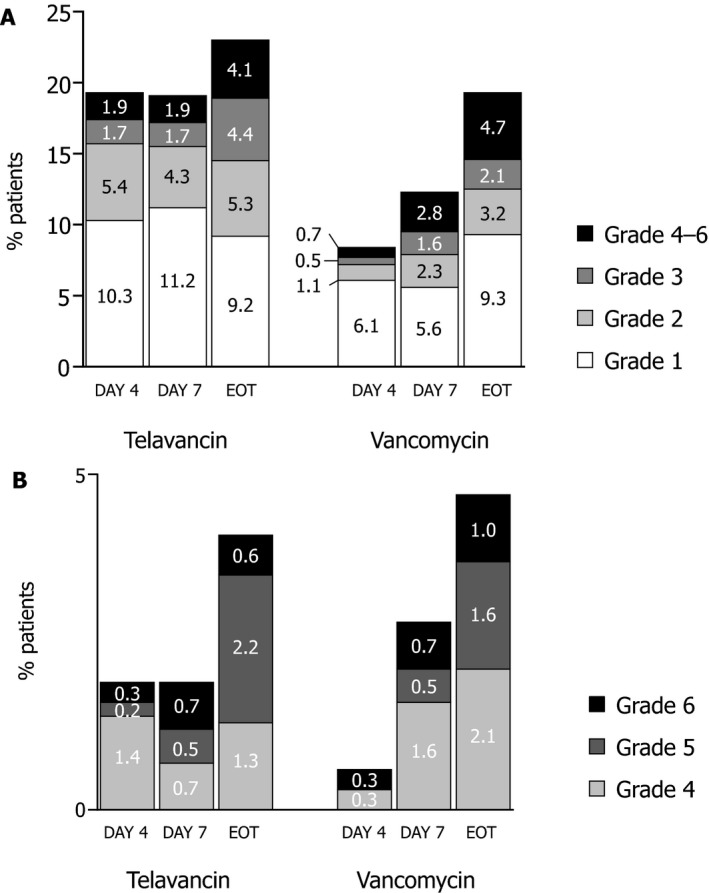
Distribution of the negative shifts in renal function by grade in the telavancin and vancomycin groups. The proportions of patients who experienced a negative shift of grades 1–6 on days 4 and 7 after the initial dose of telavancin or vancomycin and at the end of therapy (EOT) are shown in panel A. The breakdowns of the larger negative shifts in renal function (grades 4–6) are shown in panel B.
Discussion
The methodology of capturing renal adverse events in clinical trials is limited by using terms from the Medical Dictionary for Regulatory Activities (MedDRA), which do not reliably characterize the extent, duration, or severity of kidney injury. The current study describes changes in renal function, which cannot be captured by traditional adverse event reporting of clinical trials. Renal shift tables revealed statistically significant differences in renal impairment during treatment of HABP and VABP with telavancin compared with vancomycin; however, at the end of therapy, the overall differences between the two treatments were minimal and not statistically significantly different. Of note, there was a slightly higher percentage of grade 4–6 negative shifts in the vancomycin‐treated group at day 7 and end of therapy.
In the ATTAIN trials, per‐protocol changes in serum creatinine concentration were captured and characterized as potentially clinically significant when levels increased by >50% from baseline and were >1.5 mg/dl.19 A greater percentage of patients in the telavancin treatment arm experienced a clinically significant increase in serum creatinine concentration, which is reflected in the higher percentage of negative shifts observed in this analysis. The mechanism underlying the early increases in serum creatinine concentration with telavancin treatment compared with vancomycin treatment is unknown. There are distinct pharmacokinetic differences in the volume of distribution (Vd) of telavancin and vancomycin. The telavancin Vd is narrow (0.13–0.15 L/kg),16 whereas the vancomycin Vd is broad (0.4–1 L/kg).25 Furthermore, reduced vancomycin clearance has been reported with longer durations of vancomycin therapy.26 This reduction in clearance during prolonged use may contribute to the potential delayed effects of vancomycin on kidney function by allowing vancomycin accumulation.26, 27, 28, 29 Vancomycin dosage adjustment is required to maintain optimal concentrations based on trough levels throughout therapy. Alternatively, telavancin demonstrates predictable and consistent pharmacokinetics; therefore, drug level monitoring is not required.16
Overall, most patients in both treatment groups demonstrated no decline in renal function across all time points. Except for day 4, there was no significant difference between treatment groups in shifts of CrCl levels that would have resulted in a telavancin dose modification (CrCl ≤50 ml/min). A similar effect with regard to the time course has been shown in a rat model of nephrotoxicity, in which telavancin was administered at clinically relevant exposures.30 The study demonstrated that elevations in KIM‐1, a marker of early renal injury, were most pronounced within the first 5 days of therapy, with a peak at about day 3, and then gradually returned to baseline levels.
There are several limitations to this retrospective analysis. First, renal shift table analyses could produce false‐positive findings. For example, a 1‐ml/minute decrease in CrCl can be reported as a grade 1 shift if the change resulted in a value that moved from one category to the next lowest category. These small alterations have the potential to lead to differential misclassification, particularly at the higher end of CrCl categories. In addition, our analysis does not take into account the clinical significance of the actual change in renal function. For example, a grade 1 change from 60 to 49 ml/minute may be more clinically significant than a grade 1 change from >80 to 75 ml/minute. Second, changes in renal function that occurred in those patients with CrCl values that remained >80 or <30 ml/minute throughout the study were not analyzed. Therefore, changes suggesting hyperfiltration or improvement in those with more advanced renal failure may have been missed. Furthermore, use of the Cockcroft‐Gault formula to estimate CrCl is a potential limitation, as this method is known to be influenced by patient age, weight, and body mass index23; however, it is consistent with the telavancin and vancomycin labeled dosing.16, 31 Third, the increased rates of incomplete renal function data at certain time points (e.g., day 4) present an added issue; however, these data were included because they were important in evaluating and understanding the time course of renal function changes. Another limitation stems from the possibility of information censoring, in which case there is a lack of renal data reported for excluded patients at subsequent time points following departure from the study. Due to the low number of patients who were treated with study drug for greater than 7 days, we were unable to determine the trend of grade 4–6 negative renal shifts for telavancin and vancomycin for longer courses of treatment. In addition, the ATTAIN trials were not designed to prospectively capture reversibility of renal function changes; however, the data from follow‐up visits showed that the majority of telavancin‐treated patients with significant increases in serum creatinine concentration demonstrated improvement or resolution of renal failure.19
Another issue, as discussed in a recent meta‐analysis,32 is that the vancomycin dose was lower in the trials conducted from 2005 to 2007 than the dose currently used in practice. Current recommendations for treating MRSA pneumonia advise dosing vancomycin to maintain trough concentrations of 15–20 μg/ml33; however, mean vancomycin trough concentrations were ≥10 μg/ml in only 66% of patients in the ATTAIN trials.19 Therefore, it is possible that rates of decline in renal function ascribed to vancomycin were understated when considering current approaches to dosing. In fact, recent studies indicate that vancomycin use is associated with a high risk of nephrotoxicity. High vancomycin trough levels (≥15 μg/ml) likely result in an increased risk of nephrotoxicity without improved clinical response.34, 35 On the other hand, recent data from the Telavancin Observational Use Registry (TOUR) suggest that in patients with lower respiratory tract infection, including HABP and VABP, telavancin is often administered at doses lower than prescribed in the label (median 9.1 [range 2.9–14.2] mg/kg in TOUR vs 10 mg/kg in ATTAIN).36 It is likely that real‐world dosing of telavancin and vancomycin would alter the rates of renal dysfunction described in this analysis.
It should also be noted that nearly 37% of patients in the ATTAIN trials had either a mixed infection with both gram‐positive and gram‐negative pathogens or only infection with a gram‐negative pathogen.37 Therefore, patients with suspected polymicrobial infections could have received aztreonam or piperacillin‐tazobactam. The common empirical combination therapy of vancomycin and piperacillin‐tazobactam has been shown to increase the incidence of acute kidney injury.38, 39, 40 Concurrent use of piperacillin‐tazobactam in the ATTAIN trials has been described previously.37 Briefly, for telavancin, piperacillin‐tazobactam was started early (within first 3 days) in the course of treatment for 13% of patients (93/751), and on day 4 or later in 10% of patients (73/751). For vancomycin, the percentages were similar, with early piperacillin‐tazobactam initiation for 12% of patients (88/752) and 7% of patients (54/752) at day 4 or later. Whereas the concurrent use of piperacillin‐tazobactam was similar for both telavancin and vancomycin in the ATTAIN trials, the actual rates of nephrotoxicity for each combination has not been analyzed to date. In a single‐dose, pharmacokinetic study of telavancin combined with aztreonam or piperacillin‐tazobactam in healthy volunteers, no renal adverse events were reported.41 Further study is needed to understand whether renal toxicity is affected by the combination of telavancin with piperacillin‐tazobactam.
It is recommended that future antibacterial trials are designed to prospectively capture data required to assess the time course of renal function changes. This would provide clinicians with an improved understanding of how the changes progress with different treatments and offer guidance for appropriate dosage adjustments.
Conclusion
A retrospective renal shift analysis on the HABP/VABP safety population from the ATTAIN trials compared the timing and extent of renal function changes during treatment with either telavancin or vancomycin. Although significantly more telavancin‐treated patients had overall negative shifts in renal function at days 4 and 7 of treatment, those differences were diminished by the end of therapy. This granular analysis revealed that the majority of negative shifts that occurred with telavancin or vancomycin therapy were of grade 1 or 2. Although not statistically significant, at day 7 and end of therapy, vancomycin‐treated patients experienced greater negative shifts (grades 4–6) in renal function. This analysis may provide clinicians with a better understanding of the expected time course and extent of changes of renal function with these two antibacterial agents.
Supporting information
Table S1. Summary of Shifts in Creatinine Clearance from Baseline to Specified Times After Treatment
Acknowledgments
The authors would like to acknowledge Thomas Hardin, Pharm.D., for his interpretation and clinical correlations of the renal shift table data, Dr. Anna Osmukhina for her direction in the biostatistical analyses and intellectual contributions to the interpretation of data, and Chris Barnes, Ph.D., for his assistance with statistical analysis. Editorial and medical writing support was provided by Nicole Seneca, Ph.D., of AlphaBioCom, LLC, and funded by Theravance Biopharma R&D, Inc.
Funding: This study was funded by Theravance Biopharma R&D, Inc.
Conflict of interest: Dr. Dwyer participated on scientific advisory boards for and received personal fees from Theravance during the conduct of the study; outside of the submitted work, he has received personal fees from Achaogen, Cerexa Inc., and ContraFect. Dr. Jacobs received fees for data collection and submission of patients to a Theravance registry and fees for participation in Theravance advisory boards and speakers’ bureau during the conduct of this study; outside of the submitted work, he has received fees for participation on advisory boards or speakers’ bureaus for Allergan Pharmaceutical and Merck and Company (including Cubist Pharmaceuticals). Dr. Lacy is an employee of Theravance Biopharma US, Inc., and holds stock in the company. Drs. Bruss and Nogid were employees of Theravance Biopharma US, Inc., at the time of the study, and they currently hold stock in the company.
Data were presented in part as a poster at the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Annual Meeting, IDWeek, New Orleans, Louisiana, October 26–30, 2016.
References
- 1. Magill SS, Edwards JR, Bamberg W, et al. Multistate point‐prevalence survey of health care‐associated infections. N Engl J Med 2014;370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sopena N, Sabria M, Neunos 2000 Study Group . Multicenter study of hospital‐acquired pneumonia in non‐ICU patients. Chest 2005;127:213–9. [DOI] [PubMed] [Google Scholar]
- 3. Vidaur L, Planas K, Sierra R, et al. Ventilator‐associated pneumonia: impact of organisms on clinical resolution and medical resources utilization. Chest 2008;133:625–32. [DOI] [PubMed] [Google Scholar]
- 4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital‐acquired and ventilator‐associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis 2016;63:e61–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lamp KC, Rybak MJ, Bailey EM, Kaatz GW. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob Agents Chemother 1992;36:2709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sweeney D, Shinabarger DL, Smart JI, Bruss J, Pillar CM. Evaluation of the bactericidal activity of telavancin against Staphylococcus aureus using revised testing guidelines. Diagn Microbiol Infect Dis 2017;89:83–5. [DOI] [PubMed] [Google Scholar]
- 7. Kiem S, Schentag JJ. Interpretation of epithelial lining fluid concentrations of antibiotics against methicillin resistant Staphylococcus aureus . Infect Chemother 2014;46:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramsey TD, Lau TT, Ensom MH. Serotonergic and adrenergic drug interactions associated with linezolid: a critical review and practical management approach. Ann Pharmacother 2013;47:543–60. [DOI] [PubMed] [Google Scholar]
- 9. Viswanathan P, Iarikov D, Wassel R, Davidson A, Nambiar S. Hypoglycemia in patients treated with linezolid. Clin Infect Dis 2014;59:e93–5. [DOI] [PubMed] [Google Scholar]
- 10. Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149–52. [DOI] [PubMed] [Google Scholar]
- 11. Mendes RE, Sader HS, Smart JI, Castanheira M, Flamm RK. Update of the activity of telavancin against a global collection of Staphylococcus aureus causing bacteremia, including endocarditis (2011‐2014). Eur J Clin Microbiol Infect Dis 2017;36:1013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duncan LR, Sader HS, Smart JI, Flamm RK, Mendes RE. Telavancin activity in vitro tested against a worldwide collection of Gram‐positive clinical isolates (2014). J Glob Antimicrob Resist 2017;10:271–6. [DOI] [PubMed] [Google Scholar]
- 13. Gotfried MH, Shaw JP, Benton BM, et al. Intrapulmonary distribution of intravenous telavancin in healthy subjects and effect of pulmonary surfactant on in vitro activities of telavancin and other antibiotics. Antimicrob Agents Chemother 2008;52:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lodise TP Jr, Gotfried M, Barriere S, Drusano GL. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother 2008;52:2300–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lodise TP, Drusano GL, Butterfield JM, Scoville J, Gotfried M, Rodvold KA. Penetration of vancomycin into epithelial lining fluid in healthy volunteers. Antimicrob Agents Chemother 2011;55:5507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Theravance Biopharma Antibiotics Inc . VIBATIV (telavancin) package insert. South San Francisco, CA; 2016. [Google Scholar]
- 17. Wilson SE, Graham DR, Wang W, Bruss JB, Castaneda‐Ruiz B. Telavancin in the treatment of concurrent Staphylococcus aureus bacteremia: a retrospective analysis of ATLAS and ATTAIN studies. Infect Dis Ther 2017;6(3):413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta‐analysis of vancomycin‐induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 2013;57:734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rubinstein E, Lalani T, Corey GR, et al. Telavancin versus vancomycin for hospital‐acquired pneumonia due to gram‐positive pathogens. Clin Infect Dis 2011;52:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mehta RL, Pascual MT, Soroko S, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004;66:1613–21. [DOI] [PubMed] [Google Scholar]
- 21. Stryjewski ME, Graham DR, Wilson SE, et al. Telavancin versus vancomycin for the treatment of complicated skin and skin‐structure infections caused by gram‐positive organisms. Clin Infect Dis 2008;46:1683–93. [DOI] [PubMed] [Google Scholar]
- 22. Gerson SL, Kaplan SL, Bruss JB, et al. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother 2002;46:2723–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft‐Gault, MDRD, and new CKD‐EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 2010;5:1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newcombe RG. Two‐sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998;17:857–72. [DOI] [PubMed] [Google Scholar]
- 25. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health‐System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009;66:82–98. [DOI] [PubMed] [Google Scholar]
- 26. Nakayama H, Echizen H, Tanaka M, Sato M, Orii T. Reduced vancomycin clearance despite unchanged creatinine clearance in patients treated with vancomycin for longer than 4 weeks. Ther Drug Monit 2008;30:103–7. [DOI] [PubMed] [Google Scholar]
- 27. Carreno JJ, Kenney RM, Lomaestro B. Vancomycin‐associated renal dysfunction: where are we now? Pharmacotherapy 2014;34:1259–68. [DOI] [PubMed] [Google Scholar]
- 28. Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care‐associated methicillin‐resistant Staphylococcus aureus pneumonia. Clin Ther 2007;29:1107–15. [DOI] [PubMed] [Google Scholar]
- 29. Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration‐time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 2009;49:507–14. [DOI] [PubMed] [Google Scholar]
- 30. Tam VH, Ledesma KR, Bowers DR, Zhou J, Truong LD. Kidney injury associated with telavancin dosing regimen in an animal model. Antimicrob Agents Chemother 2015;59:2930–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hospira Inc . Vancomycin hydrochloride for injection USP [package insert]. Lake Forest, IL; 2015. [Google Scholar]
- 32. Chuan J, Zhang Y, He X, et al. Systematic review and meta‐analysis of the efficacy and safety of telavancin for treatment of infectious disease: are we clearer? Front Pharmacol 2016;7:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011;52:e18–55. [DOI] [PubMed] [Google Scholar]
- 34. Barriere SL, Stryjewski ME, Corey GR, Genter FC, Rubinstein E. Effect of vancomycin serum trough levels on outcomes in patients with nosocomial pneumonia due to Staphylococcus aureus: a retrospective, post hoc, subgroup analysis of the Phase 3 ATTAIN studies. BMC Infect Dis 2014;14:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin‐resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012;54:621–9. [DOI] [PubMed] [Google Scholar]
- 36. Jacobs M, Shields R, Ansari‐Orlando Z, Osmukhina A, Nogid B, Castaneda‐Ruiz B. Real‐world treatment of lower respiratory tract infections in the Telavancin Observational Use Registry (TOUR). Chest 2017;152:A201–2. [Google Scholar]
- 37. Lacy MK, Stryjewski ME, Wang W, et al. Telavancin hospital‐acquired pneumonia trials: impact of Gram‐negative infections and inadequate Gram‐negative coverage on clinical efficacy and all‐cause mortality. Clin Infect Dis 2015;61(Suppl 2):S87–93. [DOI] [PubMed] [Google Scholar]
- 38. Burgess LD, Drew RH. Comparison of the incidence of vancomycin‐induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin‐tazobactam. Pharmacotherapy 2014;34:670–6. [DOI] [PubMed] [Google Scholar]
- 39. Gomes DM, Smotherman C, Birch A, et al. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin‐tazobactam or cefepime. Pharmacotherapy 2014;34:662–9. [DOI] [PubMed] [Google Scholar]
- 40. Meaney CJ, Hynicka LM, Tsoukleris MG. Vancomycin‐associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy 2014;34:653–61. [DOI] [PubMed] [Google Scholar]
- 41. Wong SL, Sorgel F, Kinzig M, Goldberg MR, Kitt MM, Barriere SL. Lack of pharmacokinetic drug interactions following concomitant administration of telavancin with aztreonam or piperacillin/tazobactam in healthy participants. J Clin Pharmacol 2009;49:816–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of Shifts in Creatinine Clearance from Baseline to Specified Times After Treatment


