Abstract
In addition to stimulating the visual system, light incident on the retina stimulates other biological functions, also referred to as non-visual responses. Among the most notable biological functions are human circadian rhythms, which are bodily rhythms that, in constant darkness, oscillate with a period close to, but typically slightly longer than 24 hours. Twenty-four-hour light–dark patterns incident on the retina are the major synchronizer of circadian rhythms to the local time on Earth. Entrainment of circadian rhythms has been implicated in health and well-being. Light can also elicit an acute alerting effect on people, similar to a “cup of coffee.” This review summarizes the literature on how light affects entrainment and alertness and how it can be used to achieve these aims.
1. Introduction
Light incident on the retina has a profound effect on our health and well-being because it sets the timing of our biological clock and promotes entrainment to the local time on Earth. This review begins with a discussion of the circadian system, the lighting characteristics that affect its outputs, and the analytical metrics that have been proposed to characterize those outputs in terms of their spectral and absolute sensitivities. Measurement devices and techniques for accurately quantifying the circadian system’s outputs in the field are then described, followed by a discussion of how they have been applied in practical, real-life settings. Recent applied and field research among various populations such as older adults, adolescents, and daytime office workers is then described. The review concludes with a discussion of possible research directions for the next 50 years, and how lighting research might be employed to maintain circadian entrainment, avoid circadian disruption, and continue to improve human health and well-being.
2. Non-visual responses to light
2.1. Circadian rhythms
Nearly all creatures are exposed to 24-hour light–dark cycles to which they have adapted by developing biological rhythms, called circadian rhythms. In mammals, these rhythms are generated and regulated by the body’s biological clock, which is located in the suprachiasmatic nuclei (SCN) of the brain’s hypothalamus region. In humans, the biological clock oscillates with an average periodicity of about 24.2 hours in the absence of any external time cues. Daily light–dark patterns incident on the retina reset the biological clock and entrain its timing to match the local 24-hour light–dark pattern. When local light–dark patterns become asynchronous with a person’s activity–rest patterns, as can occur with travel across time zones or night-shift work, circadian disruption or misalignment may occur. Such disruption can lead to maladies like poor sleep and performance as well as increased risk for more serious diseases such as diabetes, obesity, and even cancer. In addition to entraining or phase shifting the timing of the biological clock, light can also acutely affect the biological clock’s outputs, such as hormone production (e.g., melatonin and cortisol) and measures of performance and alertness. Much less is known about light’s acute effects on humans, but recent studies suggest the lighting characteristics that elicit these effects may differ from those that promote entrainment and phase shifting. 1–3
In 2002, Rea et al. proposed a new framework for lighting practice and discussed lighting characteristics affecting the circadian system, as measured by light’s ability to suppress the hormone melatonin. 4 Prior to that time, few publications in Lighting Research and Technology had focused on light’s non-visual effects. In 1992, Stone reviewed the health effects of fluorescent lighting, specifically discussing hazards such as skin cancer and sick building syndrome along with other factors such as glare and flicker. 5 Soon thereafter in 1993, Küller and Wetterberg investigated the impact of two light levels from daylight and warm-light fluorescent light sources on endocrine, neurophysiological, and subjective responses. 6 Even though the spectral sensitivity of the circadian system was then unknown, this study nonetheless showed that light had an arousing effect on people and that a daylight source was more effective at eliciting that response.
Recent discoveries in this area of research have led to a series of studies investigating the lighting characteristics that affect the circadian system, mainly in respect to its spectral and absolute sensitivities. While the research consensus does not include the spectral weighting functions for any specific response to light, researchers and metrologists agree that the photopic luminous efficiency function, V(λ), and commercially available light meters are inappropriate for specifying and measuring circadian-effective light. Some developments in metrology have occurred in the past few years with the introduction of new measurement devices as research tools. Finally, researchers have also started exploring applications that could benefit from implementing circadian-effective light, mostly in laboratory studies, although a growing number of field studies are now being published, as discussed below.
2.2. Lighting characteristics affecting the outputs of the circadian system
Current lighting technologies, standards, measurement devices, and applications generally have been based on the fovea’s response to light stimulus. Some key differences between light’s effects on vision and the circadian system must be considered, however, especially when specifying light’s effects on the latter. 7, 8 In 1980, Lewy et al. demonstrated that a two-hour exposure to high levels (2500 lux at the cornea from an incandescent light source) of light at night (LAN) significantly suppressed melatonin production in healthy subjects.9 Following this seminal publication, a series of studies were performed to better understand the absolute and spectral sensitivities of acute melatonin suppression by LAN exposure. 10
2.2.1 Spectral characteristics
In terms of the circadian system’s spectral sensitivity, a major discovery was made in 2002 by Berson et al., who identified a new class of photoreceptor known as intrinsically photosensitive retinal ganglion cells (ipRGCs). 11 While not the retina’s sole photoreceptor, the ipRGCs are the primary ones involved in circadian phototransduction, which is the process used by the retina to convert light into electrical signals for the SCN. This discovery confirmed earlier studies by Foster and Hankins, 12 among others, 13–15 who showed that genetically manipulated rodents lacking functional rods and cones nonetheless acutely suppressed melatonin and phase shifted the onset of wheel running activity after a pulse of light. Subsequent research, however, has shown that the classical photoreceptors (rods and cones) and the ipRGCs are all involved in circadian phototransduction. 16, 17 These studies have led to an agreement that the human circadian system, as measured by acute melatonin suppression and phase shifting of dim light melatonin onset (DLMO), is a “blue sky” detector with a peak spectral sensitivity close to 460 nm, while V(λ), which is based on the responses of long- and middle-wavelength cones, peaks at the “yellow-green” (555 nm) region.
A crucial consideration when discussing the spectral sensitivity of the circadian system is that studies have shown that the circadian system’s response to polychromatic light sources slightly differs from its response to narrowband light sources due to spectral opponent behavior resulting from color vision. 18–21 Neurons in the retina’s outer layer provide vertical and lateral connections between the photoreceptors and the ganglion cells, and thereby form the first step in spectral opponent colour vision in humans. In 2004, Figueiro et al. hypothesized that the human circadian system exhibited spectral opponent behavior. 18 Subadditivity is a characteristic of the spectral opponent colour channels whereby the net response of these channels to polychromatic light cannot be predicted from summing responses to the fractional amounts of the component spectra. Therefore, an action spectrum developed using monochromatic light sources was not expected to predict the response of the circadian system to polychromatic light sources. As described in Rea et al., three independent studies demonstrated that acute melatonin suppression exhibits a subadditive response to polychromatic light. 18–20, 22
Since the discovery of the ipRGCs, scientists, biologists, and metrologists have explored the development of a spectral efficiency function for the circadian system. While various spectral response functions for the circadian system have been proposed, none has been formally recognized. In 2009, soon after the seminal publications in this area by Brainard et al.23 and Thapan et al., 24 the German Institute for Standardization released a prestandard DIN V 5031-100:2009-03 (revised and finalized in 2015) 25, 26 defining an action spectrum for nocturnal melatonin suppression (sms(λ)) using the empirical Gall and Bieske function (c(λ)). 27 This function, however, did not take into account the neurophysiology and neuroanatomy of the retina nor the operating characteristics of the circadian system.
In 2014, Lucas et al. proposed a toolbox that would permit researchers to report the effective irradiance experienced by each of the photoreceptors (i.e., rods, cones, and ipRGCs) involved in non-visual responses. 7 While this toolbox can be used for equating the stimulus–response relationships employed in different studies, as well as for relating research findings to lighting conditions in the field, it unfortunately provides no indication of the circadian system’s response to light stimulus. In other words, although it can produce valuable data, reporting how light stimulus excites the five photoreceptors does not provide any insight into how that stimulus will affect clock outputs, such as the suppression of the hormone melatonin or circadian clock phase change.
Rea et al. have forwarded a model of human circadian phototransduction that is based on current knowledge of the neuroanatomy and neurophysiology of the human retina, while also taking into account that the relative contribution of each photoreceptor class to that process differs among species. 22, 28 Using empirical, light-induced nocturnal suppression data from Brainard et al.23 and Thapan et al., 24 this model characterizes the absolute and spectral sensitivities of the human circadian system to light, as measured by acute melatonin suppression.
As noted above, the ipRGCs are central to circadian phototransduction but are not the sole photoreceptors involved. In the Rea et al. model, rods indirectly control the absolute threshold of the synthesized circadian phototransduction pathway through the AII amacrine neuron, which is the central neuron that controls rod or cone signals to the ganglion cells. 22 When rods begin to saturate in response to higher light levels, cones become the dominant photoreceptor for photic input to the ganglion cells and, subsequently, to different parts of the brain. According to the model, cones participate in circadian phototransduction through the “blue versus yellow” (b-y) bipolar neurons. These spectrally opponent neurons can provide photic input to the circadian system through synapses with the ipRGCs, the axons of which form the retinohypothalamic tract that directly innervates the SCN. Functionally, only S-cones output can add to the ipRGC’s direct response to light. A second type of amacrine neuron restricts input to the ipRGCs only to the “blue” signal from the b-y spectrally opponent bipolar.
Thus, as the spectral power distributions of narrowband sources shift from short to long wavelengths, the bipolar switches its response from a “blue” signal that adds to the ipRGC response to a “yellow” signal that provides no contribution to circadian phototransduction. The model predicts the human circadian system’s response to broadband, polychromatic sources. Depending upon their spectral power distribution, the model also predicts a non-linear, sub-additive response to polychromatic light. 18–20
Two simpler, additive functions have been proposed to characterize the spectral sensitivity of the human circadian system. A spectral efficiency envelope peaking at 460 nm was proposed by Gall and Bieske in 2004, 27 and one based only upon the ipRGC’s functional photopigment, melanopsin (an opsin identified by Provencio et al.29), was proposed by Enezi et al. in 2010.30–32 As recently discussed by Rea and Figueiro in 2016, neither of these spectral efficiency functions cohesively represents the neurophysiology underlying circadian phototransduction. 33 The authors’ quantitative comparison of these simplified functions, specifically concerning their ability to characterize the spectral sensitivity of nocturnal melatonin suppression using narrowband and polychromatic spectra, concludes that accuracy is lost when predicting light-induced nocturnal melatonin suppression by narrowband and broadband spectra. 34
Amundadottir et al. recently proposed a unified framework to evaluate the spectral effectiveness of light sources for stimulating the five known photoreceptors, along with the resulting physiological impacts. 35 Following Lucas et al., 7 the model assumes an equal-area normalization for the photoreceptors’ spectral sensitivity functions, where the sensitivity curves are scaled to have equal areas under the curve. Studies have shown, however, that the model cannot accurately represent ocular light induced non-visual responses based on the participation of photoreceptors (whether just one alone or all in equal participation), and its practical application is therefore limited. 17, 36 Furthermore, although the Amundadottir et al. model can evaluate photometric and radiometric responses (both relative and absolute) with respect to a light source’s spectral effectiveness, it does not predict, a priori, the absolute biological responses. In other words, the model fails to address how a stimulated photoreceptor’s response can functionally integrate and inherently affect any biological rhythm. Nor does the model factor in the dynamic participation of all photoreceptors with respect to changes in light stimulus, despite studies which have shown that the contribution of photoreceptors involved in phototransduction varies with light level and spectral power distribution. 22, 28, 31, 37
Attempting to characterize the human circadian response while at the same time simplify non-linear approaches such as the Rea et al. model for practical application, Bellia and Seraceni proposed an “intermediate” model that is based on the Gall and Bieske efficiency function and adheres to classical photometric principles. 38 This model evaluates circadian-effective light (CLA) as a linear function of corneal illuminance by computing two unique constants (the circadian action factor [acv] and spectral opponency [opp]) based on the spectral power distribution of a given light source. Other constant parameter values were obtained by minimizing the mean square deviations between results obtained using the revised Rea et al.28 model and the proposed simplified model. Similar to the Rea et al. model, the intermediate model encourages estimation of acute melatonin suppression as a surrogate for the impact of light on the circadian system. Thus, upon estimation of the CLA, in order to predict absolute biological response, the authors recommended using the Rea et al.28 model to evaluate the corresponding percent melatonin suppression expressed in terms of circadian stimulus (CS). Here, however, one must realise that even though this simplified model embodies a photometric approach and seems to partly match the response to the Rea et al. model’s predictions, past studies have shown that the human circadian response to light stimuli is inherently subadditive. 18–20 Although this model would be practical for use in commercial applications, it is important to note that it is primarily mathematical and not based on biophysical response data. Hence, it is critical to assess whether this model can predict how circadian-effective light correlates to actual melatonin suppression. Moreover, this simplified model has not been used to predict response from monochromatic light sources.
2.2.2 Absolute characteristics
In addition to its spectral sensitivity, researchers also have investigated the human circadian system’s absolute sensitivity by measuring acute melatonin suppression and phase shifting of the timing of DLMO. Laboratory studies have now demonstrated that light levels lower than those used by Lewy et al. can suppress melatonin and phase shift the onset of evening melatonin secretion. 10 The absolute sensitivity of the human circadian system as measured by acute melatonin suppression, however, is still lower than the daytime absolute sensitivity of the human visual system as measured by flicker photometry. In other words, more light is required to affect night-time melatonin levels than is needed for one to read black text on white paper. 8
In 1989, McIntyre et al. demonstrated the human circadian system’s dose–response relationship to light, showing that as light levels increased, so did the circadian response, as measured by acute melatonin suppression. 39 Almost 10 years later, a laboratory study by Aoki et al. evaluated the minimum amount of light that is required to suppress nocturnal melatonin by exposing five subjects to light levels ranging from < 10 to 5000 lux of cool-white fluorescent light. 40 The results showed that for exposure durations of 30, 60, 90 and 120 minutes, the minimum amount of light needed to significantly suppress melatonin was 393, 366, 339 and 285 lux respectively.
Shortly thereafter in 2000, Zeitzer et al. assessed the effects of light exposures on acute melatonin suppression and phase shifting of core body temperature (CBT).10 Twenty-three participants were administered a 6.5-hour cool white light exposure between 3 and 9100 lux over the course of nine nights. The authors used a four-parameter logistic model to estimate the maximal response of the circadian system to a light pulse and the half-maximal phase shifting response was predicted to be between 80 and 160 lux. A four-parameter logistic model was also used to fit the acute melatonin suppression data and this model predicted a half-maximal response at approximately 50 to 130 lux.
More recently, West et al. evaluated narrowband short-wavelength light to determine whether it could induce dose-dependent melatonin suppression by exposing eight subjects to eight irradiance levels (0.1 to 600 μW/cm2 [0.09 to 562 lux]) of a 469 nm LED light and a control 4000 K white fluorescent light.41 They found that an irradiance of 20 μW/cm2 (18.7 lux) of the same light resulted in significantly stronger melatonin suppression than the lower irradiances (0.1, 0.5, and 2 μW/cm2 [0.09, 0.47, and 1.87 lux, respectively]), and 10 μW/cm2 (9.4 lux) resulted in significantly stronger melatonin suppression than the lowest irradiance of 0.1 μW/cm2 (0.09 lux). For the 4000 K white fluorescent source, an irradiance of 40 μW/cm2 (85.4 lux) elicited melatonin suppression that was: (1) significantly lower than that recorded for the 300 and 600 μW/cm2 (281 and 562 lux, respectively) of 469 nm LED light, (2) significantly higher than that for the lowest 469 nm LED irradiance of 0.1 μW/cm2 (0.09 lux), and (3) not significantly different from the LED irradiances of 0.5 through 75 μW/cm2 (0.47 through 70.2 lux, respectively).
Figueiro et al. measured the dose effectiveness of narrowband short-wavelength light (460–470 nm) for acute melatonin suppression and found significant main effects of corneal irradiance and exposure duration, as well as a significant interaction between the two variables. 42Post hoc t-tests revealed reliable suppression of melatonin by mean corneal irradiances as low as 2 μW/cm2 (1.5 lux), but only after a 90-min exposure, whereas a mean corneal irradiance of 20 μW/cm2 (14 lux) resulted in a significant suppression after a 15-min exposure.
To date, the Rea et al.22, 28 model is the only one that accounts for both the spectral and absolute sensitivities of the circadian system in quantifying CLA and its relationship with CS. (The Lighting Research Center (LRC) at Rensselaer Polytechnic Institute has released a free online Circadian Stimulus Calculator to determine CS for any combination of source type and light level in photopic lux, available at http://www.lrc.rpi.edu/programs/lightHealth/index.asp
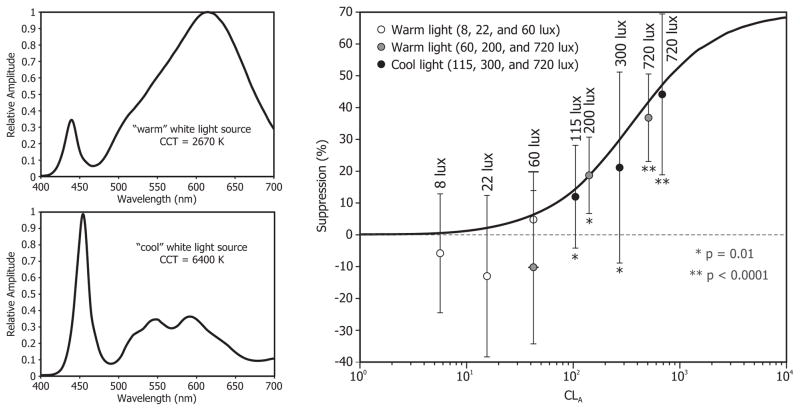
In 2013, Rea and Figueiro investigated a working threshold for acute melatonin suppression from white light sources commonly used in indoor and outdoor architectural applications.43 This study was also a test of the a priori prediction obtained using the Rea et al. model. In two laboratory studies, 28 subjects were administered (via LED light goggles) warm white light (2760 K) for one hour. The first study exposed subjects to targeted corneal illuminances of 8, 22, and 60 lux, and the second study exposed subjects to targeted corneal illuminances of 60, 200, and 720 lux. The authors sampled plasma melatonin before and immediately after the one-hour light exposure. While the first study’s results showed no significant melatonin suppression from any of the lighting conditions, the second study’s results showed significant post-exposure melatonin suppression from the 200 and 720 lux conditions compared to the dim light, control night.
Using a similar protocol, additional studies have been performed using a cool white light source (6400 K). In one of those studies, which is presently unpublished, subjects were exposed to one hour of targeted corneal illuminances of 115, 300, and 720 lux. Figure 1 shows the mean ± standard error of the mean of the melatonin suppression values observed in this study and in the two above-cited warm white light source studies. 43 The solid line shows the model’s predictions (note that it is not a curve fitting to the data); the actual melatonin suppression values obtained from subjects were very close to the model’s predictions, especially at higher light levels. In respect to a working threshold, given the large individual differences in responses to light, the authors advised that a stringent working threshold was appropriate until more is known about all factors influencing light’s effects on melatonin production. They proposed that 30 lux of a “warm” color polychromatic light source for 30 minutes should be used as a working threshold for acute melatonin suppression. It should be noted that if one is using short-wavelength (460–470 nm) light, the threshold for activation of the circadian system is much lower, as shown by Figueiro et al.42
Figure 1.
Mean ± standard error of the mean (SEM) nocturnal melatonin suppression from one-hour exposures to two warm white and cool white light polychromatic sources (spectral power distributions shown on left) as a function of CLA. Photopic lux values are also shown for reference. The solid line shows the Rea et al. model’s predictions (note that it is not a curve fitting to the data); the actual melatonin suppression values obtained from subjects were very close to the model’s predictions, especially at higher light levels.
Although some of the model’s predictions have been tested in laboratory conditions and in the field, as detailed below, Rea et al. are careful to note that future work will be required to extend the model to incorporate the various important attributes of circadian phototransduction mentioned earlier, including the impacts of duration of exposure, prior photic history, and potential changes in spectral sensitivity throughout the night. 28 It is not known, however, whether any of these proposed models can predict other outcomes of the circadian system, such as phase shifting of DLMO and acute alertness.10, 28, 38 Undoubtedly, this topic will continue to be explored.
2.2.3 Temporal characteristics
In addition to quantity and spectrum, the temporal aspects of light exposure (i.e., timing and duration) are just as important for understanding light’s impact on the circadian system. Although much less research has been conducted on how light’s temporal characteristics may affect outputs of the circadian system, it is nonetheless known that if applied at different times, the same quantity and spectrum of light will have different effects on the circadian system. Moreover, the duration of exposure will also influence the efficacy of light treatment, as lower light levels at longer duration exposures can have the same effect on the circadian system as higher light levels of shorter durations. 44 In respect to timing, light exposure can advance or delay (i.e., phase shift) the timing of the biological clock. Phase response curves have been constructed to estimate the direction and degree to which a given individual will phase shift in response to a light pulse. Earlier studies demonstrated that polychromatic white light received after waking will cause one to go to bed earlier and wake up earlier, while light applied before bedtime will cause one to go to bed later and wake up later.45, 46 While these studies also explored the notion that a “dead zone” or a photic insensitivity exists in the temporal response of the human circadian system, the results indicated that there are no times when light has no effect on the circadian system. Instead, it appears that light affects the human circadian system over the entire course of the 24-hour day, even though its maximum effect is close to the minimum CBT, which typically occurs two to three hours prior to natural waking. 47 Furthermore, one’s prior history of light exposure over days and weeks is also crucial when considering light’s temporal aspects, as that history appears to reduce the sensitivity of the circadian system in terms of acute and phase shifting responses.48–50
2.3. Collecting and analysing light exposure data using field measurement devices
To better understand and quantify the human circadian system’s response to light, there is a need for standards and practices that are replicable, translatable between studies, and readily employable in the field. Devices and techniques employed in the field, therefore, should consistently and accurately record and characterize the light stimulus as it may affect the circadian system (i.e., amount, spectrum, timing, and duration) as well as the circadian system’s outputs (e.g., activity–rest patterns). Most commercial photometers, however, use V(λ) to determine luminance (cd/m2) or illuminance (lm/m2, or lux). The majority of circadian-related research conducted from 1980 to 2000 quantified light stimuli using commercially available light meters, which were a popular and inexpensive tool of choice for lighting and photographic professionals. The discovery that the circadian system, as measured by acute melatonin suppression and phase shifting of the timing of DLMO, is maximally sensitive to short wavelengths, however, has shown that: (1) quantifying light stimuli in terms of V(λ) is inadequate and (2) commercially available light meters have only limited accuracy in measuring light’s effect on the circadian system.7, 8
As discussed below, to address these limitations, the LRC has developed various versions of the Daysimeter, which have been used successfully in numerous studies to collect calibrated personal light exposures and activity–rest patterns.51–58 Also discussed below are techniques used to quantify circadian entrainment and disruption in the field using these devices.
2.3.1. Actigraphs
Actigraphs are small wearable autonomous devices that measure and record the activity–rest patterns of individuals over extended periods (i.e., activity time series [ATS]). For circadian and sleep research, the actigraph is usually worn on the wrist and measures activity–rest patterns that can be used to estimate sleep parameters such as total sleep time, sleep efficiency, sleep onset latency, and wake after sleep onset. They typically use accelerometers and data loggers, but can also be designed to measure personal light exposure profiles (i.e., irradiance time series [ITS]) with or without ATS. Some actigraphs also measure other time series, such as temperature and heart rate, and they all measure time (some better than others).
Studies have shown a strong correlation between actigraphy data and both melatonin and CBT rhythms.59 Although polysomnography remains the “gold standard” method for evaluating sleep parameters, it often needs to be conducted in a laboratory or hospital setting. Actigraphy, on the other hand, is a useful tool for providing data about an individual’s sleep in their own natural sleep setting, at home. As there is no standard of measurement in actigraphy, however, actigraphy reports cannot easily be compared between laboratories and the resulting data must be considered as qualitative.60 In addition, the actigraph may not provide an accurate estimation of sleep onset latency and daytime sleeping.61 In view of this difficulty, it is common for researchers to ask study subjects to keep logs or diaries to verify sleep and wake times to supplement actigraphy data, but these self-reports are not always accurate and are therefore of limited use.
In a 2003 literature review, Ancoli-Israel et al. explored the validity of actigraphy and actigraphy-based technology.59 The authors found actigraphy to be a reliable method for evaluating variations in sleep patterns and treatment effects on sleep, diagnosing circadian rhythm disorders such as delayed sleep phase syndrome, and evaluating sleep when polysomnography cannot be used. However, the actigraph may not provide accurate data when sleep becomes fragmented, and thus overestimates sleep times. Additionally, an accurate bedtime marker may be required in order to calculate sleep onset latency. Ancoli-Israel et al. suggested that some studies might need more than one week of actigraphy data collection for accurate analysis. 59
In respect to light measurements, actigraphs and other photometric devices that are typically used by researchers are not calibrated to measure light for the circadian system. In 2012, Price et al. assessed the performance of 16 Actiwatch Spectrum (Philips Respironics, Murraysville, Pennsylvania) devices for spectral response, directional response, and dynamic range, and proposed techniques for calibration, deployment, and data analysis for the use of these devices in studies assessing circadian rhythms.62 To determine their spectral sensitivity and linearity, the devices were exposed to a variety of narrowband and broadband polychromatic illumination conditions, across a spectral range of 360 to 720 nm from 100 W (2900 K) halogen lamps. The devices were oriented both horizontally and vertically. In terms of spectral sensitivity, each of the red-green-blue (RGB) sensors showed a broadband response to light. The blue sensor had a peak response at 460 nm (full width at half maximum [FWHM] = 395 to 500 nm); the green sensor had a peak response at 500 nm (FWHM = 475 to 550 nm); and the red sensor had a peak response at 655 nm (FWHM 600 to 695 nm). Spectral response variability was generally high among all sensors. The cosine response errors ranged from 30 to 50% in the horizontal plane and 60 to 65% in the vertical plane. With regard to linearity, there was a non-linear, variable RGB response at low light levels; the actual response was much higher than expected at lower transmittances. The authors noted that the directional and non-linear responses of the blue sensor were higher than desired for short wavelengths. Price et al. cautioned that for individuals with aged, yellowed lenses, the shift toward longer wavelengths would be more difficult to analyse using these devices. In a 2013 evaluation of field measurement devices, Figueiro et al.63 also found that the Actiwatch does not measure blue light well, which supported the findings of Price et al.
The principal difficulty associated with using a wrist-worn actigraph to measure light–dark exposures is the high likelihood that the device will be covered by clothing. Thorne et al. investigated light exposure across seasons and noted that some Actiwatch readings were zero.64 The authors felt that the accuracy of the Actiwatch was limited due to the possibility that the wrist-worn device had been covered by garment sleeves, and thus the measured light levels were lower than they should have been.
In 2017, Price et al. quantified the spectral, angular, and dynamic performance of 11 actigraph models from eight manufacturers or research groups, including the Daysimeter and new Actiwatch Spectrum models. 65 Price and Lyachev also described a subsequent modification to the ActTrust device (Condor Instruments, Sao Paulo, Brazil), which is designed to match the spectral response of melanopsin. 66 As noted earlier, the spectral sensitivity of the human circadian system is not limited to the melanopsin response only, however, so the usability of such devices for circadian research may still be limited.
2.3.2. Daysimeter
The first version of the Daysimeter was developed by the LRC to fill a known gap in the ability to accurately obtain human circadian light exposure data.51 The Daysimeter-D was designed to be worn at a wide variety of locations on the body that experimenters and subjects might deem appropriate or comfortable. The Daysimeter-D’s three light sensors are calibrated in terms of the retinal photic input to the human visual (i.e., photopic) and circadian systems. Its three, orthogonally oriented, solid-state accelerometers are calibrated in terms of gravitational forces on the device. Not only do the continuous measurements provide quantitative light exposure and activity level data, but the recorded light–dark and activity–rest patterns make it possible, using phasor analyses (discussed below), to quantitatively assess the degree of circadian entrainment and disruption exhibited by the person (or animal) wearing the device.67
In 2013, Figueiro et al. discussed the effectiveness of three light measurement devices used in the field: the Actiwatch Spectrum, Daysimeter-S, and Daysimeter-D. 63 Each device was designed to measure light exposure and activity levels over extended periods of time, and report photopic illuminance values in lux. To compare recorded light and activity measurements for all three devices, the research team recruited 12 test subjects to wear four Daysimeters (at the eye, on the wrist, as a pendant, and on the torso as a pin) and one Actiwatch Spectrum (on the wrist) simultaneously for seven consecutive days. Differences between the Actiwatch Spectrum and the Daysimeters were assessed by comparing photopic illuminance and activity levels when the devices were worn on the wrist. To determine the impact of the light sensor placement, data from the Daysimeter worn on the wrist, as a pendant, and as a pin were compared to those from the Daysimeter-D, which was worn at eye level. Figueiro et al. found that compared to a commercial-grade illuminance meter, significant photometric errors were recorded for common discharge light sources using the Actiwatch Spectrum. The Actiwatch Spectrum also produced significant errors in photopic sensitivity at both the short- and long-wavelength regions of the spectrum.
Figueiro et al. concluded that the Actiwatch Spectrum’s added sensitivity outdoors V(λ) could lead to large photometric measurement errors for some sources with significant radiant power in tails of V(λ) (e.g., daylight). 63 For example, the device’s outdoor light measurements showed values > 150,000 lux, a value 50% higher than the light actually available on the surface of Earth at noon under a clear sky. The device also produced significant photometric errors for common light sources, showing nearly complete insensitivity to fluorescent and high-intensity light discharge (570 to 600 nm) compared to a commercial illuminance meter. While the differences in CS measured with the Daysimeter at the eye and other locations were typically less than 10%, the magnitude of difference became quite large when comparing measurements of photopic illuminance (lux), illustrating the potential problem in measuring light exposures at the wrist as surrogates for corneal light exposure. The Daysimeter also showed better spatial sensitivities and a photopic response more similar to V(λ) than the Actiwatch Spectrum. Figueiro et al. concluded that the absolute spectral and spatial calibrations of a light measurement device were important for drawing valid inferences in sleep and circadian rhythms research, noting that measurement devices’ operating characteristics should be both clearly defined and explicitly related to visual and non-visual biological systems. 63
2.3.3. Estimating circadian disruption in the field using phasor analysis
In 2008, Rea et al. proposed a quantitative method for estimating circadian entrainment and disruption in the field.68 Called phasor analysis, this method permits examination of the relationship between the 24-hour (circadian) light–dark exposure pattern, the stimulus, and the activity–rest pattern. The response is quantified in terms of the phase and magnitude of their joint circular correlation function. The joint circular correlation function is determined by calculating correlations (r, not r2) between the entire, continuously repeating time series of light–dark exposure data and the entire, continuously repeating time series of activity–rest data as one time series is rotated with respect to the other. The circular correlation function is decomposed into its Fourier components from which the 24-hour frequency component can be represented by a vector, called a phasor. The vector length, or phasor magnitude, represents the amount of circadian entrainment exhibited in the light–dark pattern and the associated activity–rest pattern; the greater the phasor magnitude, the stronger the correlation between the light stimulus and the activity response. The vector angle, or phasor angle, reflects the phase relationship between the 24-hour light–dark exposure pattern and the 24-hour activity–rest pattern.
The LRC has conducted several studies to evaluate phasor analysis as a measure of circadian entrainment or disruption in humans and nocturnal animals. The first study used phasor analyses to estimate circadian disruption in nurses and rats in the field and laboratory. 68 In the field, 43 day-shift and rotating-shift nurses wore a Daysimeter at work for two to three consecutive days. In the laboratory, individually housed rats experienced either a 12:12-hour light–dark pattern or a “jet-lagged” condition where that pattern was reversed every 48 hours. The field results showed a consistent relationship between activity–rest rhythms and light exposure in day-shift nurses, while rotating-shift nurses were less synchronized to their light–dark cycle. The cross-species laboratory comparison showed that the 12:12 light–dark pattern rats’ phasor magnitudes were similar to those of day-shift nurses, while the jet-lagged rats’ phasor magnitudes were similar to those of the rotating-shift nurses.
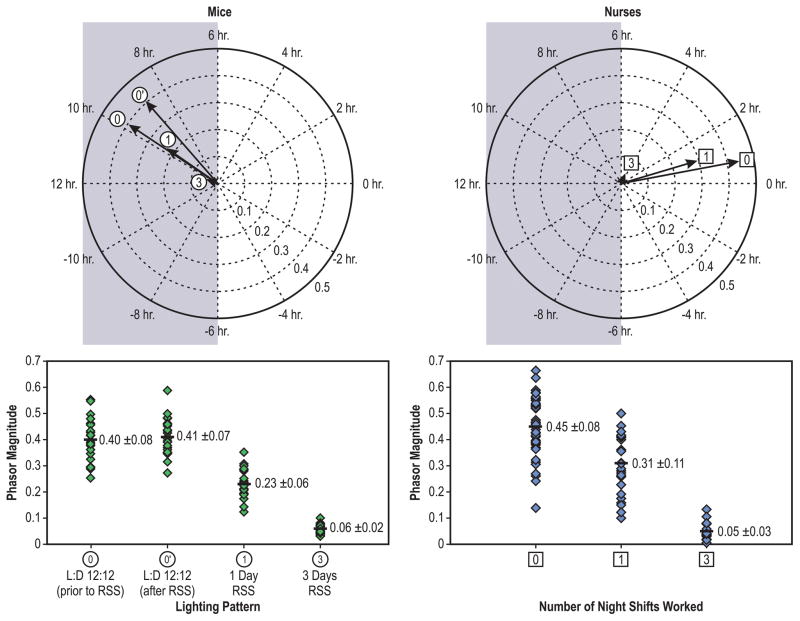
In a follow up study, Radetsky et al. compared ecological data from day-shift and rotating-shift nurses with data from mice that experienced light–dark patterns emulating those experienced by the nurses.69 Special lighting systems were installed to ensure that the spectrum and amount of light inside the animals’ cages were tuned to the spectral and absolute sensitivities of the nocturnal murine circadian system. The results showed that individual phasor magnitudes were very similar for both species when exposed to light–dark patterns simulating either day-shift or rotating-shift schedules (Figure 2). The authors proposed that the high level of congruence between the mouse and human phasor magnitudes offered support for a bridge between ecological studies of light exposure in humans and controlled light exposures in animal models.
Figure 2.
Circadian disruption for mice and for human nurses exposed to different light–dark patterns associated with shift work. Top Left: Phasor averages for mouse data for different light–dark (L:D) patterns; the first (0) and the second (0’) 12:12-hour (12:12) light–dark pattern and the one-day (1) and the three-day (3) rotating shift schedule (RSS) patterns. Bottom Left: Individual mouse phasor magnitudes for the different light–dark patterns. Phasor magnitudes become statistically shorter with an increasing number of days of the rotating shift schedule. Top Right: Phasor averages for day-shift (0) and rotating-shift nurses who worked one (1) and who worked three (3) consecutive nightshifts during a week. Bottom Right: Individual nurse phasor magnitudes for three different light–dark patterns. As with the mouse data, phasor magnitudes for the nurses become statistically shorter (p <0.05) as the number of nights worked during the week increased.
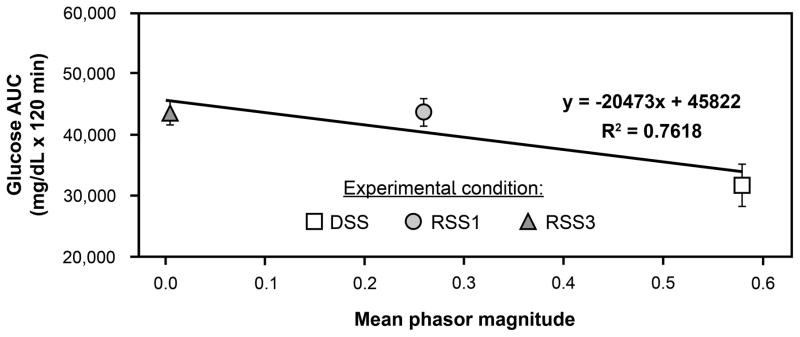
Most recently, Figueiro et al. exposed mice to lighting conditions mimicking light–dark exposures experienced by day-shift nurses, rotating-shift nurses working one night per week, and rotating-shift nurses working three nights per week. 70 After three weeks under these conditions, oral glucose tolerance tests (measured over 120 minutes) showed that glucose area under the curve (AUC) was significantly higher after animals experienced the simulated shift-work schedules compared to the day-shift schedule. More importantly, the results showed a significant negative correlation between phasor magnitude (a measure of circadian entrainment) and glucose AUC (Figure 3). A higher glucose AUC suggests a lower glucose tolerance, typically associated with higher risk for type 2 diabetes.
Figure 3.
Mean glucose area under the curve (AUC) and phasor magnitudes for mice that experienced lighting schedules simulating day shift (DSS) and rotating shifts involving one night (RSS1) and three nights (RSS3) per week. Higher glucose AUC is associated with lower glucose tolerance. Glucose tolerance significantly (p < 0.0001) decreased after exposure to the lighting conditions that simulated rotating shift work schedules and was inversely correlated with phasor magnitude, which is a measure of circadian entrainment.
These studies are of utmost importance as they demonstrate how the discussion about light’s effect on human health needs to shift to the relationship between light–dark and activity–rest patterns, and not just focus on LAN exposure and its impact on circadian disruption. Phasor analysis provides a method for quantifying circadian disruption in field and laboratory settings, as well as a bridge between ecological measurements of circadian entrainment in humans and parametric studies of circadian disruption in animal models, including nocturnal rodents.
2.4 Acute alertness and light
The effects of light on alertness at night are well documented 71–74 and have been associated with light’s ability to suppress melatonin. 75 Earlier studies showed that high levels (> 2500 lux at the cornea) of white light were needed to affect objective and subjective measures of alertness. 73, 74 As it has now been established that the spectral sensitivity of acute melatonin suppression 23, 24 (a marker of the circadian clock) peaks close to 460 nm, researchers have also been investigating the impact of short-wavelength (blue) light on measures of alertness. 76 Research has shown that the light levels required to affect measures of alertness can be greatly reduced when low levels of blue light are used, rather than polychromatic white light. 76, 77 These studies suggest that the ipRGCs mediate, at least in part, the alerting effects of light at night and that the suppression of melatonin may indeed play a role in eliciting alertness in humans by “fooling the body” into thinking that it is daytime.
Studies examining the effects of light on measures of alertness and performance have also been conducted during the daytime, when melatonin levels are low. 78–80 Functional magnetic resonance imaging studies show that bright (> 7000 lux) white light as well as lower levels (7.5 lux) of short-wavelength (peak close to 473 nm) light were more effective at activating brain regions associated with alertness than either remaining in dim light or being exposed to higher levels (24.5 lux) of 527 nm light. 81, 82 These results suggest that melatonin suppression is not needed to affect measures of alertness while nonetheless suggesting that the effects of light on measures of alertness during the daytime could still be mediated, at least in part, by the responses of the ipRGCs.
A new line of study, however, has demonstrated that long-wavelength (red, peak close to 630 nm) light can increase objective and subjective measures of alertness at night 3, 83 and during the day. 1, 2 Given that the ipRGCs are not sensitive to long-wavelength light, the results of these studies suggest that long-wavelength cones mediate red light’s alerting effects. 11 While the spectral and absolute sensitivity functions for acute alertness have not been established, these results clearly suggest that the pathways in the brain associated with acute alertness differ from those associated with acute melatonin suppression. Several other studies using light that was filtered to remove short-wavelength content also showed that acute melatonin suppression is not needed to maintain subjective alertness. 84, 85
In addition to the well-known effects of spectral irradiance incident on the retina on physiological and endocrine processes, studies have claimed that colour itself can affect a range of physiological, psychological, and behavioral responses in humans. 86 The precise effect elicited by colour on humans, however, remains under debate. For example, while a few studies have claimed that the colour red is more arousing and alerting than the colour blue, other studies have not shown any significant difference between the physiological arousal elicited by the two colours. 87 Future studies investigating the effects of saturated coloured lights on humans, however, should take into account these possible psychological effects.
3. Using light to promote circadian entrainment and elicit alertness
Numerous and diverse populations are in a position to benefit from these advances in the study of light and its effects on the human circadian system. The majority of laboratory research and field studies published over the years include lighting for offices, older adults, and adolescents. While there is continuing debate in the field as to whether we are ready for applications, a number of studies have attempted to demonstrate the benefits of using higher circadian stimulation in buildings during the daytime. In some cases, this lighting solution has been combined with a reduction in circadian stimulation during evening hours. A few other studies also examined how light can be used to increase alertness, regardless of its impact on circadian phase. Based on the scientific research, this lighting scheme should promote entrainment, which in turn should result in better sleep, mood, and perhaps performance.
3.1. Older adults with and without dementia
Seniors residing in assisted living facilities are perhaps the best example of a population at risk for circadian disorders; 88 due to age-dependent reduced retinal light exposures and to fixed lighting conditions in their living environments, seniors are less likely to experience the necessary, robust 24-hour light–dark pattern needed for circadian entrainment. Exposure to bright white light (at least 3000 lux and as high as 8000 lux at the cornea) for at least one hour in the morning for a period of at least two weeks was found to improve or consolidate night-time sleep of individuals with Alzheimer’s disease and related dementias (ADRD).89, 90 Research demonstrated that, in ADRD patients, continuous, bright, indirect white light (at least 1000 lux at the cornea) during daytime hours consolidated activity–rest rhythms,91 attenuated cognitive deterioration, ameliorated depressive symptoms, and attenuated the increase in functional limitations over time.92 Recently, Figueiro et al. showed that much lower levels (400 lux at the cornea) of a high correlated colour temperature (CCT) light source (which emits more short-wavelength content) improved objective and subjective sleep and reduced agitation and depression in ADRD patients living in long-term care facilities.93
Two landmark studies from the same research group in the Netherlands clearly showed the benefits of delivering a robust light–dark pattern to ADRD patients. In a 1997 article, Van Someren et al. explored the effects of increased levels of bright light during the day on 22 institutionalized older adults with severe ADRD.91 A ceiling-mounted luminaire containing high-intensity white fluorescent tubes was installed in the common areas where the patients spent most of their waking time. Patients received a mean ± standard error of the mean of 1136 ± 89 lux from the new lighting, although the amount of light received ranged from 790 to 2190 lux depending on the patient’s position relative to a window. The results showed that bright light exposure over the course of a day could improve disturbed circadian activity–rest rhythms in older adults with severe dementia. Bright light treatment was most effective in patients with relatively unimpaired vision, as opposed to those with severe visual deficits. Interdaily stability, which is a measure of the consistency in activity–rest patterns over the course of many days, increased (i.e., coupling of the rhythm to environmental zeitgebers, such as the time of meals), indicating a more steadfast organization of the circadian activity–rest rhythm. This was the first field study demonstrating that light could improve activity–rest rhythms in ADRD patients.
In a follow up study, Riemersma-van der Lek et al. were the first to investigate the effect of long-term (maximum of 3.5 years) light exposure and melatonin pills in a study of 189 ADRD patients.92 Ceiling-mounted, fluorescent luminaires were installed in a common living room. The patients received either light or melatonin, light and melatonin in combination, or neither light nor melatonin. Melatonin pills were given before bed, and a light exposure of > 1000 lux at eye level was presented throughout the day. Results showed that the light exposure attenuated cognitive declines as measured by Mini–Mental State Examination scores, ameliorated depressive symptoms on the Cornell Scale for Depression in Dementia, and attenuated the increase in functional limitations, as measured by the activity of daily living scale, by 53%. Melatonin increased negative mood and withdrawn behavior, but shortened sleep latency and increased sleep duration. The authors concluded that as light improves cognitive and non-cognitive symptoms of dementia, and melatonin has an adverse effect on mood, melatonin should only be used in combination with light therapy.
In 2015, Sloane et al. used a tailored lighting intervention that delivered more short-wavelength content and reduced the light levels to 17 pairs of ADRD patients and caregivers living at home. 94 In a crossover, within-subjects design, subjects received a tailored and a placebo light in living spaces where they were most likely to spend their time during the day. Subjects experienced each condition for six weeks, separated by a four-week washout period. The tailored lighting intervention did not significantly affect actigraphic and self-reports of sleep measures in ADRD patients, but it did improve sleep and reduced strain in caregivers.
Figueiro et al. tested the effectiveness of a lighting intervention delivering a CS of > 0.3 during the day and < 0.1 in the evening in both residences and in more-controlled environments, showing that this lighting intervention implemented in the homes of ADRD patients and their caregivers reduced depression symptoms in patients. 95 They also showed that the tailored lighting intervention installed in nursing homes, where total light exposures are better controlled, increased sleep time and reduced depression and agitation scores in ADRD patients. 93 One of the biggest challenges is finding a practical method for effectively delivering the lighting intervention to the residents’ eyes. Observing that ADRD patients spend a considerable portion of the day sitting around tables in common areas, Figueiro et al. built and tested the effectiveness of a light table delivering very high circadian stimulus (CS > 0.6) on sleep quality and mood in ADRD patients living in nursing homes. 96 Residents who sat at the light table for four weeks showed significantly increased sleep duration and reduced agitation and depression scores. The study was performed with a small subject sample and is currently being replicated in a larger sample.
A 24-hour lighting scheme has been proposed that delivers high circadian stimulation during the daytime hours, low circadian stimulation in the evening hours (Figure 4), and nightlights that provide perceptual cues to decrease falls risks at night without disrupting sleep.97 Arguably, light therapy in assisted living facilities should be quite effective because it is possible to fully control the 24-hour light–dark pattern, as residents spend the majority of their time indoors in a defined space.
Figure 4.
The Lighting Research Center’s 24-hour lighting scheme demonstration room provides cycled electric lighting delivering high circadian stimulus during the day (left) and low circadian stimulus in the evening (right). Construction of the room was made possible by the Light and Health Alliance: Acuity Brands; Cree; Current, powered by GE; Ketra; OSRAM; Philips Lighting; and USAI Lighting.
3.2. Adolescents
Adolescents can be chronically sleep deprived because of their inability to fall asleep early in combination with fixed wake-up times on school days. According to the Centers for Disease Control and Prevention, more than 70% of school children get insufficient sleep—less than eight hours on school nights. 98 This type of restricted sleep schedule has been linked with depression, behavioral problems, poor performance at school, and automobile accidents.99 There are a number of reasons why children and adolescents often do not retire early enough to sleep a minimum of eight hours before rising for school, ranging from academic pressures to engagement in electronic media (e.g., tablets and video games) and endogenous circadian sleep disorders. Many schools begin classes quite early in the morning, particularly middle and high schools. When days are short, as is experienced during the winter months in the northern U.S., classes may start before sunrise. Furthermore, if young people spend the majority of their days indoors in exclusively dim lighting, the circadian system may not be able to synchronize with the solar day and thus their sleep, performance in school, and general health may decline.
Figueiro et al. conducted three studies investigating the impact of morning and evening light on DLMO, a well-known circadian marker in adolescents. 57, 100, 101 In two of these studies, the use of orange-tinted glasses that removed short-wavelength light from waking until 3:00 p.m. for five consecutive days delayed DLMO by about 30 minutes. 100, 101 There was a slight delay in bedtimes, but there were no observed effects of the intervention on mood and short-term performance. In the third study, Figueiro and Rea demonstrated that evening light exposure during spring months significantly delayed DLMO compared to winter months, when students were in circadian darkness during evening hours. 57 The results from these studies underscore the importance of controlling the entirety of 24-hour light–dark patterns to effectively promote circadian entrainment and reduce sleep restriction in adolescents. Consistently, Figueiro and Overington showed that adolescents who were exposed to self-luminous displays in the evening significantly suppressed melatonin after one- and two-hour exposures. 101 More importantly, they demonstrated that adolescents will suppress significantly more melatonin than college students when exposed to the same circadian-effective light.
As it is unlikely that adolescents will stop using self-luminous displays prior to bedtime, the use of techniques such as software or filters for removing or reducing short-wavelength emission from these devices is becoming more common. Escofet and Bará reviewed the existing software applications and hardware filters and concluded that software applications seem to offer the best trade-off for controlling light spectra from self-luminous displays. 102 While software applications that reduce emissions in the short-wavelengths can help to reduce circadian-effective light emitted by these devices, however, they also compromise colour. The authors showed that one very effective approach is to simply dim the devices without changing the display’s spectral composition. Regardless, when developing lighting schemes for adolescents, it is important that both daytime and evening light be specified. When designing lighting for schools, moreover, educational materials offering guidelines for evening light exposures should be made available to students and parents.
3.3. Daytime workers
Light can promote entrainment in daytime workers and elicit an acute alerting effect that may not affect circadian phase. Studies to date have mainly focused on the acute alerting effects of light, but more recently, studies have investigated the impact of short-wavelength light on entrainment and its impact on sleep and mood, as detailed below.
In 2015, Borisuit et al. compared self-reports of visual acceptance and alertness in two realistic office environments illuminated with daylight (without a view) and electric lighting (ceiling fluorescent lights). 103 Subjective glare was found to be lower under the daylight condition than under the electric lighting condition. Subjective alertness decreased over the course of the afternoon under both conditions, but less so under the daylight conditions.
In 2016, Figueiro and Rea collected circadian light exposures in office workers in Grand Junction, Colorado, during winter and summer months. 104 The office building used as a test site was designed to provide daylight availability in the space, and therefore was expected to provide good circadian stimulation to workers. Their overall hypothesis was that office workers would receive a significantly greater amount of circadian stimulation during summer than during winter months, leading to better sleep and mood during the summer. One interesting finding from this study was that the circadian stimulus received by the office workers was below 0.2 during both winter and summer months, suggesting that they were not receiving high amounts of circadian-effective light (CS ≥ 0.3) during the day. The study also showed that circadian-effective light exposures were significantly higher in summer than in winter, resulting in greater sleep duration and sleep efficiency and reduced sleep onset latency, which suggested that receiving greater circadian-effective light exposures during the day was associated with better sleep at night. These results are consistent with a second, more recent study from the same research group showing that morning exposure to a circadian stimulus ≥ 0.3, compared to an exposure ≤ 0.15, was associated with better sleep quality and less depression in office workers. 105
Canazei et al. investigated the impact of a dynamic lighting system, which delivered approximately 1000 to 2000 lux from a light source varying from 4000 to 6500 K, over the course of the morning shift on alertness, mood, heart rate variability, sleep quality, and productivity among a group of Austrian workers. 106 Data were collected during winter and summer months. The authors showed that a dynamic lighting system during morning shift work had a calming effect (i.e., increased high-frequency power heart rate variability and decreased subjective ratings of arousal) and improved productivity-related measures during winter months only. These seasonal effects could be expected, given that people working morning shifts arrive at work before sunrise in winter months, which deprives them of morning entraining stimulus. The results also showed decreases in anxiety, depression, and sleep onset latency after four weeks of exposure to the dynamic lighting intervention. The authors argued that the dynamic lighting intervention’s greater short-wavelength emissions conferred greater benefits on morning shift workers than did a 4100 K source. The mechanisms through which this effect was achieved are not clear, however, given that the control condition delivered 1000 lux at the cornea, which is close to saturation levels for activation of the circadian system. 22
Another field study reported positive outcomes from exposure to very high CCT light sources (17,000 K) compared to low CCT sources (2900 K) on office workers’ concentration, level of fatigue, alertness, daytime sleepiness, and performance. 107 Yet another study, however, did not find any beneficial effect of dynamic lighting (500 to 700 lux; 3000 to 4700 K) on workers’ need for recovery, vitality, sleep quality, mental health, headache and eyestrain, or subjective performance, even though the subjects expressed preference for the dynamic lighting system. 108
4. Where do we go next?
The past 50 years have witnessed a large body of research into how light affects human health and well-being, which has grown even more over the 15 years that have passed since the discovery of melanopsin and the ipRGCs. By and large, researchers have focused on the spectral sensitivity of the human circadian system, but this aspect is not the whole story. Additional work is required to better understand the temporal aspects of lighting. Indeed, the variables of timing and duration have not always been considered in lighting, and yet, they are key elements in specifying lighting for the circadian system.
Future studies should also determine the brain mechanisms associated with light’s acute alerting effects. Recent research indicates that long-wavelength light, which is not as effective at suppressing melatonin, can elicit alertness, suggesting brain mechanisms that are different from those involved in acute melatonin suppression.1–3
To date, most of the work investigating the impact of light on health and well-being has been performed in laboratory settings, although more recently, as discussed here, a growing number of field studies have been conducted and published. It is important that we continue to investigate the robustness of light’s impacts on health and well-being outside laboratory conditions. It might also be important to start publishing negative results, as they may be just as revealing. It will be challenging to make these studies reproducible, but better specification of the stimulus and the use of calibrated field measurement devices will be key to successful studies.
5. Conclusions
It is well established that a regular 24-hour light–dark pattern minimizes circadian disruption, which in turn minimizes negative health and performance outcomes. Circadian disruption can be observed and become an issue when people travel across multiple time zones, use self-luminous displays in the evenings, spend most of their days in dim daytime interiors, stay up late to watch hockey, and move from building to building and space to space throughout the day. The 24-hour light–dark pattern is no longer regular and predictable.
The challenge for lighting researchers and professionals is that they have been so closely tied to thinking about a particular building—that is, a single place where one needs to see for tasks and perceive ambience instantaneously. Circadian hygiene is not instantaneous, but cumulative. Today, because people have luminous displays and active lives that change their 24-hour pattern of light and dark, they do not have a single lighting entity that is responsible for total 24-hour light exposure patterns, and therefore cannot address 24-hour light exposure issues. As shown by Rea et al., 68 however, it is the temporal relationship between the total circadian light–dark and activity–rest patterns that needs to be measured and controlled to reduce circadian disruption.
A new lighting profession needs to emerge, such as personal light and health coaching, or new software applications need to be developed to keep track of light–dark exposures and provide recipes for maintaining entrainment or correcting circadian disruption. This can already be accomplished in spaces where users do not change their living space across the 24-hour day (e.g., senior living facilities and submarines). A new standard and practice can now be implemented in senior living facilities. Another area of research for real impact could be lighting for schoolchildren, who have a regular routine at school. The next steps would be to educate teachers and parents about the significance of a robust 24-hour light–dark pattern. Office spaces pose a greater challenge, but one could start envisioning the use of a “light oasis” (Figure 5), where workers could receive their circadian light exposures during the daytime with the aid of a circadian software application that informs them about what they need and when they need it. It is likely that people like airline pilots, flight attendants, and shift workers will never have an ideal lighted environment, but at least researchers can use phasor analyses and animal models to investigate ways to minimize disruption, and therefore, improve health and well-being.
Figure 5.
Light oases of varying configuration are practical solutions for providing circadian stimulus for office workers. Such oases could be used in conjunction with a personalized circadian application that informs workers about what light they might need and when they need it.
Time will tell if lighting will indeed go through this transition. For now, the lighting community should celebrate all the great achievements that have been made in this area over the past 50 years.
Acknowledgments
Funding
The National Institute on Aging (R01AG034157), National Institute of Occupational, Safety and Health (NIOSH – R01OH01668), US General Services Administration, and Office of Naval Research funded this research.
The authors would like to acknowledge Mark S. Rea, David Pedler and Rebekah Mullaney for their technical and editorial support. The authors would also like to thank Dr. Peter Boyce, LR&T editor, for his insightful and helpful comments on the manuscript.
References
- 1.Sahin L, Figueiro MG. Alerting effects of short-wavelength (blue) and long-wavelength (red) lights in the afternoon. Physiol Behav. 2013;116–117:1–7. doi: 10.1016/j.physbeh.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Sahin L, Wood B, Plitnick B, Figueiro MG. Daytime light exposure: Effects on biomarkers, measures of alertness, and performance. Behav Brain Res. 2014;274:176–85. doi: 10.1016/j.bbr.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Figueiro MG, Sahin L, Wood B, Plitnick B. Light at night and measures of alertness and performance: Implications for shift workers. Biol Res Nurs. 2016;18:90–100. doi: 10.1177/1099800415572873. [DOI] [PubMed] [Google Scholar]
- 4.Rea MS, Bullough JD, Figueiro MG. Phototransduction for human melatonin suppression. J Pineal Res. 2002;32:209–13. doi: 10.1034/j.1600-079x.2002.01881.x. [DOI] [PubMed] [Google Scholar]
- 5.Stone PT. Fluorescent lighting and health. Light Res Technol. 1992;24:55–61. [Google Scholar]
- 6.Küller R, Wetterberg L. Melatonin, cortisol, EEG, ECG and subjective comfort in healthy humans: Impact of two fluorescent lamp types at two light intensities. Light Res Technol. 1993;25:71–81. [Google Scholar]
- 7.Lucas RJ, Peirson SN, Berson DM, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rea MS, Figueiro MG, Bullough JD. Circadian photobiology: An emerging framework for lighting practice and research. Light Res Technol. 2002;34:177–87. [Google Scholar]
- 9.Lewy A, Wehr T, Goodwin T, Newsome D, Markey S. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–9. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 10.Zeitzer JM, Dijk D-J, Kronauer R, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 12.Foster RG, Hankins MW. Non-rod, non-cone photoreception in the vertebrates. Prog Retin Eye Res. 2002;21:507–27. doi: 10.1016/s1350-9462(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 13.Freedman MS, Lucas RJ, Soni B, et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–4. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 14.Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–7. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 15.Lucas RJ, Foster RG. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology. 1999;140:1520–4. doi: 10.1210/endo.140.4.6672. [DOI] [PubMed] [Google Scholar]
- 16.Panda S, Sato TK, Castrucci AM, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–6. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 17.Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figueiro MG, Bullough JD, Parsons RH, Rea MS. Preliminary evidence for spectral opponency in the suppression of melatonin by light in humans. Neuroreport. 2004;15:313–6. doi: 10.1097/00001756-200402090-00020. [DOI] [PubMed] [Google Scholar]
- 19.Figueiro MG, Bullough JD, Bierman A, Rea MS. Demonstration of additivity failure in human circadian phototransduction. Neuro Endocrinol Lett. 2005;26:493–8. [PubMed] [Google Scholar]
- 20.Figueiro MG, Bierman A, Rea MS. Retinal mechanisms determine the subadditive response to polychromatic light by the human circadian system. Neurosci Lett. 2008;438:242–5. doi: 10.1016/j.neulet.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 21.Walmsley L, Hanna L, Mouland J, et al. Colour as a signal for entraining the mammalian circadian clock. PLoS Biology. 2015;13:e1002127. doi: 10.1371/journal.pbio.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Rev. 2005;50:213–28. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(DIN) DIfN. DIN V 5031-100: Strahlungsphysik im optischen Bereich und Lichttechnik -Teil 100: Über das Auge vermittelte, nichtvisuelle Wirkung des Lichts auf den Menschen - Größen, Formelzeichen und Wirkungsspektren. Berlin: Deutsches Institut für Normung; 2009. [Google Scholar]
- 26.(DIN) DIfN. DIN V SPEC 5031-100: Strahlungsphysik im optischen Bereich und Lichttechnik - Teil 100: Über das Auge vermittelte, nichtvisuelle Wirkung des Lichts auf den Menschen - Größen, Formelzeichen und Wirkungsspektren. Berlin: Deutsches Institut für Normung; 2015. [Google Scholar]
- 27.Gall D, Bieske K. Definition and measurement of circadian radiometric quantities. Proceedings of the CIE Symposium ‘04 on Light and Health; Vienna, Austria: Commission Internationale de l’Éclairage; 2004. pp. 129–32. [Google Scholar]
- 28.Rea MS, Figueiro MG, Bierman A, Hamner R. Modelling the spectral sensitivity of the human circadian system. Light Res Technol. 2012;44:386–96. [Google Scholar]
- 29.Provencio I, Jiang G, De Grip W, Hayes W, Rollag M. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95:340–5. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enezi Ja, Revell V, Brown T, Wynne J, Schlangen L, Lucas R. A “melanopic” spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights. J Biol Rhythms. 2011;26:314–23. doi: 10.1177/0748730411409719. [DOI] [PubMed] [Google Scholar]
- 31.Lall GS, Revell VL, Momiji H, et al. Distinct Contributions of Rod, Cone, and Melanopsin Photoreceptors to Encoding Irradiance. Neuron. 2010;66:417–28. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailes HJ, Lucas RJ. Melanopsin and inner retinal photoreception. Cell Mol Life Sci. 2010;67:99–111. doi: 10.1007/s00018-009-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rea MS, Figueiro MG. Light as a circadian stimulus for architectural lighting. Light Res Technol. 2016 In press. [Google Scholar]
- 34.Rea MS, Figueiro MG. Quantifying light-dependent circadian disruption in humans and animal models. Chronobiol Int. 2014;31:1239–46. doi: 10.3109/07420528.2014.957302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amundadottir ML, Lockley SW, Andersen M. Unified framework to evaluate non-visual spectral effectiveness of light for human health. Light Res Technol. 2016 In press. [Google Scholar]
- 36.Panda S, Provencio I, Tu DC, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–7. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 37.Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. J Circadian Rhythms. 2010;8:2. doi: 10.1186/1740-3391-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellia L, Seraceni M. A proposal for a simplified model to evaluate the circadian effects of light sources. Light Res Technol. 2014;46:493–505. [Google Scholar]
- 39.McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Human melatonin suppression by light is intensity dependent. J Pineal Res. 1989;6:149–56. doi: 10.1111/j.1600-079x.1989.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 40.Aoki H, Yamada N, Ozeki Y, Yamane H, Kato N. Minimum light intensity required to suppress nocturnal melatonin concentration in human saliva. Neurosci Lett. 1998;252:91–4. doi: 10.1016/s0304-3940(98)00548-5. [DOI] [PubMed] [Google Scholar]
- 41.West KE, Jablonski MR, Warfield B, et al. Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J Appl Physiol. 2011;110:619–26. doi: 10.1152/japplphysiol.01413.2009. [DOI] [PubMed] [Google Scholar]
- 42.Figueiro MG, Lesniak NZ, Rea MS. Implications of controlled short-wavelength light exposure for sleep in older adults. BMC Res Notes. 2011;4:334. doi: 10.1186/1756-0500-4-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rea MS, Figueiro MG. A Working Threshold for Acute Nocturnal Melatonin Suppression from “White” Light Sources used in Architectural Applications. J Carcinog Mutagen. 2013;4:1000150. [Google Scholar]
- 44.Chang AM, Santhi N, St Hilaire M, et al. Human responses to bright light of different durations. J Physiol (Lond) 2012;590:3103–12. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jewett ME, Rimmer DW, Duffy JF, Klerman EB, Kronauer RE, Czeisler CA. Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am J Physiol. 1997;273:R1800–9. doi: 10.1152/ajpregu.1997.273.5.r1800. [DOI] [PubMed] [Google Scholar]
- 46.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Figueiro MG. Disruption of circadian rhythms by light during day and night. Curr Sleep Med Rep. 2017;3:76–84. doi: 10.1007/s40675-017-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–4. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- 49.Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. J Physiol. 2011;589:1095–102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bierman A, Klein TR, Rea MS. The Daysimeter: A device for measuring optical radiation as a stimulus for the human circadian system. Meas Sci Technol. 2005;16:2292–9. [Google Scholar]
- 52.Figueiro MG. Lessons from the Daysimeter: can circadian disruption in individuals with Alzheimer’s disease be measured? Neurodegener Dis Manag. 2012;2:553–6. [Google Scholar]
- 53.Rea MS, Figueiro MG, Jones GE, Glander KE. Daily activity and light exposure levels for five species of lemurs at the duke lemur center. Am J Phys Anthropol. 2014;153:68–77. doi: 10.1002/ajpa.22409. [DOI] [PubMed] [Google Scholar]
- 54.Higgins PA, Hornick TR, Figueiro MG. Rest-activity and light exposure patterns in the home setting: a methodological case study. Am J Alzheimers Dis Other Dementias. 2010;25:353–61. doi: 10.1177/1533317510363467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med. 2011;12:685–92. doi: 10.1016/j.sleep.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood B, Rea MS, Plitnick B, Figueiro MG. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl Ergon. 2013;44:237–40. doi: 10.1016/j.apergo.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 57.Figueiro MG, Rea MS. Short-wavelength light enhances cortisol awakening response in sleep-restricted adolescents. Int J Endocrinol. 2012;2012:301935. doi: 10.1155/2012/301935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Figueiro MG, Hamner R, Higgins P, Hornick T, Rea MS. Field Measurements of Light Exposures and Circadian Disruption in Two Populations of Older Adults. J Alzheimers Dis. 2012;31:711–5. doi: 10.3233/JAD-2012-120484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 60.Figueiro MG, Rea MS. New Tools to Measure Light Exposure, Activity, and Circadian Disruption in Older Adults. SLEEP 2011 25th Annual Meeting of the Associated Professional Sleep Societies, LLC; Minneapolis, MN. 2011. [Google Scholar]
- 61.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514–27. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price L, Khazova M, O’Hagan J. Performance assessment of commercial circadian personal exposure devices. Light Res Technol. 2012;44:17–26. [Google Scholar]
- 63.Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res Technol. 2013;45:421–34. doi: 10.1177/1477153512450453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thorne HC, Jones KH, Peters SP, Archer SN, Dijk DJ. Daily and seasonal variation in the spectral composition of light exposure in humans. Chronobiol Int. 2009;26:854–66. doi: 10.1080/07420520903044315. [DOI] [PubMed] [Google Scholar]
- 65.Price LLA, Lyachev A, Khazova M. Optical performance characterization of light-logging actigraphy dosimeters. J Opt Soc Am. 2017;34:545–57. doi: 10.1364/JOSAA.34.000545. [DOI] [PubMed] [Google Scholar]
- 66.Price LLA, Lyachev A. Research Note: Modification of a personal dosimetry device for logging melanopic irradiance. Light Res Technol. 2017 In press. [Google Scholar]
- 67.Miller D, Figueiro MG, Bierman A, Schernhammer E, Rea MS. Ecological measurements of light exposure, activity and circadian disruption. Light Res Technol. 2010;42:271–84. doi: 10.1177/1477153510367977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rea MS, Bierman A, Figueiro MG, Bullough JD. A new approach to understanding the impact of circadian disruption on human health. J Circadian Rhythms. 2008;6:7. doi: 10.1186/1740-3391-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radetsky LC, Rea MS, Bierman A, Figueiro MG. Circadian Disruption: Comparing humans with mice. Chronobiol Int. 2013;30:1066–71. doi: 10.3109/07420528.2013.797428. [DOI] [PubMed] [Google Scholar]
- 70.Figueiro MG, Radetsky L, Plitnick B, Rea MS. Glucose tolerance in mice exposed to light–dark stimulus patterns mirroring dayshift and rotating shift schedules. Sci Rep. 2017;7:40661. doi: 10.1038/srep40661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dawson D, Campbell S. Bright light treatment: are we keeping our subjects in the dark? Sleep. 1990;13:267–71. [PubMed] [Google Scholar]
- 72.Daurat A, Foret J, Benoit O, Mauco G. Bright light during nighttime: effects on the circadian regulation of alertness and performance. Biol Signals Recept. 2000;9:309–18. doi: 10.1159/000014654. [DOI] [PubMed] [Google Scholar]
- 73.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 74.Badia P, Myers B, Boecker M, Culpepper J, Harsh JR. Bright light effects on body temperature, alertness, EEG and behavior. Physiol Behav. 1991;50:583–8. doi: 10.1016/0031-9384(91)90549-4. [DOI] [PubMed] [Google Scholar]
- 75.Chellappa SL, Steiner R, Blattner P, Oelhafen P, Götz T, Cajochen C. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PLoS One. 2011;6:e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cajochen C, Munch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 77.Figueiro MG, Bullough JD, Bierman A, Fay CR, Rea MS. On light as an alerting stimulus at night. Acta Neurobiol Exp (Warsz) 2007;67:171–8. doi: 10.55782/ane-2007-1645. [DOI] [PubMed] [Google Scholar]
- 78.Smolders KC, de Kort YA, Cluitmans PJ. A higher illuminance induces alertness even during office hours: findings on subjective measures, task performance and heart rate measures. Physiol Behav. 2012;107:7–16. doi: 10.1016/j.physbeh.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 79.Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol. 2006;290:R1413–20. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- 80.Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SM. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 2003;26:695–700. doi: 10.1093/sleep/26.6.695. [DOI] [PubMed] [Google Scholar]
- 81.Vandewalle G, Balteau E, Phillips C, et al. Daytime light exposure dynamically enhances brain responses. Curr Biol. 2006;16:1616–21. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 82.Vandewalle G, Schmidt C, Albouy G, et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS One. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Figueiro MG, Bierman A, Plitnick B, Rea MS. Preliminary evidence that both blue and red light can induce alertness at night. BMC Neurosci. 2009;10:105. doi: 10.1186/1471-2202-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van de Werken M, Gimenez MC, de Vries B, Beersma DG, Gordijn MC. Short-wavelength attenuated polychromatic white light during work at night: limited melatonin suppression without substantial decline of alertness. Chronobiol Int. 2013;30:843–54. doi: 10.3109/07420528.2013.773440. [DOI] [PubMed] [Google Scholar]
- 85.Papamichael C, Skene DJ, Revell VL. Human nonvisual responses to simultaneous presentation of blue and red monochromatic light. J Biol Rhythms. 2012;27:70–8. doi: 10.1177/0748730411431447. [DOI] [PubMed] [Google Scholar]
- 86.Elliot AJ, Maier MA, Moller AC, Friedman R, Meinhardt J. Color and psychological functioning: the effect of red on performance attainment. J Exp Psychol Gen. 2007;136:154–68. doi: 10.1037/0096-3445.136.1.154. [DOI] [PubMed] [Google Scholar]
- 87.O’Connor Z. Colour psychology and colour therapy: Caveat emptor. Color Res Appl. 2011;36:229–34. [Google Scholar]
- 88.Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Tiemeier H. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int. 2013;30:1223–30. doi: 10.3109/07420528.2013.813528. [DOI] [PubMed] [Google Scholar]
- 89.Mishima K, Hishikawa Y, Okawa M. Randomized, dim light controlled, crossover test of morning bright light therapy for rest-activity rhythm disorders in patients with vascular dementia and dementia of Alzheimer’s type. Chronobiol Int. 1998;15:647–54. doi: 10.3109/07420529808993200. [DOI] [PubMed] [Google Scholar]
- 90.Yamadera H, Ito T, Suzuki H, Asayama K, Ito R, Endo S. Effects of bright light on cognitive and sleep–wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2000;54:352–3. doi: 10.1046/j.1440-1819.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- 91.Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955–63. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 92.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–55. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 93.Figueiro MG, Plitnick B, Lok A, et al. Tailored lighting intervention improves measures of sleep, depression and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527–37. doi: 10.2147/CIA.S68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sloane P, Figueiro M, Garg S, et al. Effect of home-based light treatment on persons with dementia and their caregivers. Light Res Technol. 2015;47:161–76. doi: 10.1177/1477153513517255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Figueiro MG, Hunter CM, Higgins PA, et al. Tailored lighting intervention for persons with dementia and caregivers living at home. Sleep Health. 2015;1:322–30. doi: 10.1016/j.sleh.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Figueiro M, Plitnick B, Rea M. Research Note: A self-luminous light table for persons with Alzheimer’s disease. Light Res Technol. 2016;48:253–9. doi: 10.1177/1477153515603881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Figueiro MG. A proposed 24 h lighting scheme for older adults. Light Res Technol. 2008;40:153–60. [Google Scholar]
- 98.Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance — United States, 2015. Morb Mortal Weekly Rep. 2016;65:43–4. [Google Scholar]
- 99.McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, Perry GS. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med. 2011;53:271–3. doi: 10.1016/j.ypmed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 100.Figueiro MG, Brons JA, Plitnick B, Donlan B, Leslie RP. Measuring circadian light and its impact on adolescents. Light Res Technol. 2011;43:201–15. doi: 10.1177/1477153510382853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Figueiro MG, Overington D. Self-luminous devices and melatonin suppression in adolescents. Light Res Technol. 2016;48:966–75. [Google Scholar]
- 102.Escofet J, Bará S. Reducing the circadian input from self-luminous devices using hardware filters and software applications. Light Res Technol. 2015 In press. [Google Scholar]
- 103.Borisuit A, Linhart F, Scartezzini J-L, Münch M. Effects of realistic office daylighting and electric lighting conditions on visual comfort, alertness and mood. Light Res Technol. 2015;47:192–209. [Google Scholar]
- 104.Figueiro MG, Rea MS. Office lighting and personal light exposures in two seasons: Impact on sleep and mood. Light Res Technol. 2016;48:352–64. [Google Scholar]
- 105.Figueiro MG, Steverson B, Heerwagen J, et al. The impact of daytime light exposures on sleep and mood in office workers. Sleep Health. 2017;3:204–15. doi: 10.1016/j.sleh.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 106.Canazei M, Dehoff P, Staggla S, Pohl W. Effects of dynamic ambient lighting on female permanent morning shift workers. Light Res Technol. 2014;46:140–56. [Google Scholar]
- 107.Mills PR, Tomkins SC, Schlangen LJM. The effect of high correlated colour temperature office lighting on employee wellbeing and work performance. J Circadian Rhythms. 2007;5:2. doi: 10.1186/1740-3391-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Kort YA, Smolders KC. Effects of dynamic lighting on office workers: First results of a field study with monthly alternating settings. Light Res Technol. 2010;42:345–60. [Google Scholar]