Abstract
Background:
Inorganic arsenic exposure and inter-individual differences in its metabolism have been associated with cardiometabolic risk. A more efficient arsenic metabolism profile (lower MMA%, higher DMA%) has been associated with reduced risk for arsenic-related health outcomes; however, this profile has also been associated with increased risk for diabetes-related outcomes. The mechanism behind these contrasting associations is equivocal; we hypothesized one carbon metabolism (OCM) may play a role.
Methods:
We evaluated the association between OCM-related variables (nutrient intake and genetic variants) and both arsenic metabolism biomarkers (iAs%, MMA% and DMA%) and diabetes-related outcomes (metabolic syndrome, diabetes, HOMA2-IR and waist circumference) in 935 participants free of prevalent diabetes and metabolic syndrome from the Strong Heart Family Study, a family-based prospective cohort comprised of American Indian tribal members aged 14+ years.
Results:
Of the 935 participants free of both diabetes and metabolic syndrome at baseline, 279 (29.8%) developed metabolic syndrome over a median of 5.3 years of follow-up and of the 1,458 participants free of diabetes at baseline, 167 (11.3%) developed diabetes over follow-up. OCM nutrients were not associated with arsenic metabolism, however, higher vitamin B6 was associated with diabetes-related outcomes (higher HOMA2-IR and increased risk for diabetes and metabolic syndrome). A polymorphism in an OCM-related gene, methionine synthase (MTR), was associated with both higher MMA% (β=2.57, 95% CI: 0.22, 4.92) and lower HOMA2-IR (GMR=0.79, 95% CI=0.66, 0.93 per 5 years of follow-up). Adjustment for OCM variables did not affect previously reported associations between arsenic metabolism and diabetes-related outcomes; however, the association between the MTR variant and all diabetes-related outcomes were attenuated or reversed direction after adjustment for arsenic metabolism
Conclusions:
Our findings suggest MMA% may be a partial mediator in the association between OCM and diabetes-related outcomes. Formal mediation analyses with longer follow-up period are needed to confirm this finding. Additional research is needed to determine whether excess B vitamin intake is associated with increased risk for diabetes-related outcomes.
Keywords: Arsenic, Arsenic metabolism, Metabolic Syndrome, diabetes, One carbon metabolism, American Indians
1. INTRODUCTION
One carbon metabolism (OCM) is a network of interrelated biochemical reactions critical to the biosynthesis of purines and thymidylate as well as the generation of methyl groups.1 The transfer of a methyl group to substrates is essential to multiple biological processes including arsenic metabolism.1 OCM functioning, and adequate methyl group availability, is dependent on essential nutrients that support this pathway including folate, vitamin B12, vitamin B6, vitamin B2, and methionine. Therefore, OCM status in epidemiologic and in vivo studies has been evaluated through the measurement of both circulating levels and dietary intake of these nutrients. Low OCM nutrient status in observational studies has been associated with numerous adverse health effects2-8 including diabetes-related outcomes.9-12 However, findings on the efficacy of OCM nutrient supplementation in clinical trials to reduce these outcomes have been mixed. 13-15 Just two trials have evaluated the effect of OCM supplementation (one evaluating folic acid alone,16 one evaluating a B vitamin combination (folic acid, B6 and B12) pill17) on diabetes: results were null except in one high risk group (hypertensive obese participants) sub-analysis.16, 17 Smaller supplementation trials focused on folic acid, on the other hand, have suggested supplementation may improve diabetes indicators and complications among diabetes cases.18-22 Still, some observational studies have suggested high circulating folate levels may actually have deleterious health effects on diabetes-related outcomes, even in the offspring of mothers with high folate during pregnancy.23-27 Studies evaluating the effect of intake of folate from foods or other OCM nutrients on diabetes-related outcomes are limited, mostly cross-sectional in design and findings are even more conflicting,9, 12, 28-35 highlighting the need for more research on this relationship. Single nucleotide polymorphisms (SNPs) in genes encoding enzymes involved in OCM have also been associated with diabetes-related outcomes36-44 and provide a useful, hypothetically unbiased alternative assessment of OCM status.
Evidence suggests arsenic metabolism may also be affected by OCM status, as has been recently reviewed.45 Methylation reactions involved in the metabolism of inorganic arsenic (arsenate and arsenite (iAs)) into mono- and di-methylated arsenicals (MMA and DMA) require the transfer of a methyl group generated by the conversion of S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH) in the OCM pathway.1 Typically, arsenic metabolism in epidemiologic studies is measured as each relative percentage of iAs (iAs%), MMA (MMA%) and DMA (DMA%) out of the three urinary concentrations summed together. DMA has a shorter circulating half-life and is more rapidly excreted than iAs or MMA,46- 51 thus, a urine profile reflecting higher percentages of DMA is generally considered a more efficient arsenic metabolism profile. Randomized clinical trials conducted in highly arsenic-exposed Bangladeshi populations have successfully showed folic acid supplementation can enhance arsenic metabolism, reflected in increases in DMA% and decreases in MMA%, likely through supporting efficiency of the OCM pathway.52, 53 Observational cross-sectional studies also suggest OCM nutrients may enhance arsenic metabolism, including in the Strong Heart Study (SHS), the parent study (comprised of older participants exposed to higher arsenic and recruited 10 years earlier, pre-folic acid fortification) to the cohort this analysis was conducted in, the Strong Heart Family Study (SHFS).54-56 Further, OCM SNPs have been found to influence arsenic metabolism as well.57-60 This is highly important as arsenic metabolism, specifically higher MMA%, has been identified as a risk factor for arsenic-related health outcomes, including cardiovascular disease, skin lesions and cancer.61-68 However, higher MMA% has also been associated with a reduced risk for diabetes-related outcomes. Indeed, in the SHS and SHFS, higher MMA% has been associated with lower risk for diabetes,69 metabolic syndrome70 and elevated waist circumference, as well as lower body mass index (BMI)71 and homeostasis model assessment for insulin resistance (HOMA2-IR)72. The mechanism underlying the contrasting association between arsenic metabolism and diabetes-related outcomes versus other arsenic-related health outcomes is unclear.
The goal of this study was to characterize the role OCM plays in the association between arsenic metabolism and diabetes-related outcomes in an attempt to better understand the contrasting relationships between arsenic metabolism and these outcomes versus other arsenic-related health outcomes. Previous research suggests OCM is strongly tied to both arsenic metabolism and diabetes-related outcomes, making it an ideal candidate to potentially provide a mechanistic pathway between the two variables. In this study, we first evaluated the associations between OCM variables (SNPs in: PEMT, MTR, MTRR, MTHFR, MTHFD1, SHMT1 and CBS; and nutrient intake: vitamin B6, vitamin B2 and folate) and both arsenic metabolism (iAs%, MMA% and DMA%) as well as four diabetes-related outcomes (metabolic syndrome, waist circumference, diabetes, and HOMA2-IR). Diabetes-related outcomes were selected based on previously reported associations between arsenic metabolism and these outcomes in the Strong Heart Study and SHFS.69, 72, 73 We then evaluated whether OCM influences previously reported associations between arsenic metabolism and diabetes-related outcomes in this study population. We used data from participants in the Strong Heart Family Study, a prospective family-based cohort study of cardiometabolic disease in American Indian tribal members from Arizona, Oklahoma, and North/South Dakota.
2. METHODS
2.1. Study population
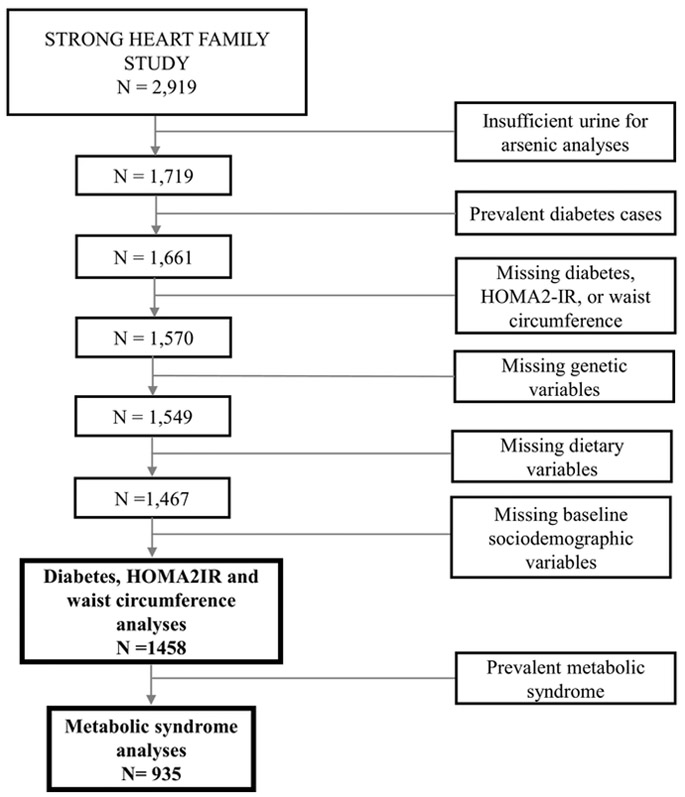
The SHFS recruited 2,919 participants in 1998-1999 (n=428) and 2001-2003 (n=2,491) with follow-up visits conducted in 2006-2009. For this study, we did not include baseline visits conducted in 1998-1999 as OCM nutritional information was only conducted during visits in 2001-2003. We included participants with sufficient urine for arsenic analyses (n=1,719) and who were free of diabetes at baseline (n=1,661). We used urinary arsenic and OCM nutrient data measured from visits conducted in 2001-2003, and diabetes-related outcome (metabolic syndrome, waist circumference, HOMA2-IR, diabetes) data from follow-up visits in 2006-2009. We excluded participants missing information on outcome data (n=91), OCM genetic variant data (n=21) or OCM nutrient status (n=82). We also excluded participants with missing data on education, smoking, alcohol intake, BMI, and estimated glomerular filtration rate (eGFR) (n=9), resulting in 1,458 participants available for analyses evaluating HOMA2-IR, waist circumference or incident diabetes. For incident metabolic syndrome analyses, we further excluded participants with prevalent metabolic syndrome (n=523), resulting in 935 participants available for metabolic syndrome analyses (Figure 1). All participants provided informed consent and study protocols were approved by multiple institutional review boards, participating communities and The Indian Health Service.
Figure 1.
Study Flow Diagram
2.2. Data Collection
Baseline and follow-up visits included bio-specimen collection, physical exam, food frequency questionnaire and an interview-administered questionnaire (age, sex, education, smoking history, alcohol use, medical history).74 Exam measures (waist circumference, blood pressure, height, weight) and collection of urine and fasting blood samples were performed by centrally trained nurses following a standardized protocol.75
2.3. Urine arsenic determinations
Morning spot urine samples were collected in polypropylene tubes, frozen within 1 to 2 hours of collection, shipped buried in dry ice and stored at −70°C in the Penn Medical Laboratory, MedStar Research Institute, Washington, DC for up to 18 years. For arsenic analyses, urine samples were thawed and up to 1.0 mL from each urine sample was transferred to a small vial, transported on dry ice to the Trace Element Laboratory at Graz University, Austria and stored at −80°C until analyses.
Total urine arsenic concentrations were measured using inductively coupled plasma-mass spectrometry (ICPMS), as has been described in detail previously.76 The urine concentrations of arsenite, arsenate, MMA, DMA and arsenobetaine were measured using high performance liquid chromatography/- ICPMS (HPLC/ICPMS). Limits of detection were 0.1 μg/L for all five species. Arsenic species concentrations below the limit of detection (<5% for all species) were imputed as the limit of detection divided by √2. Arsenobetaine levels were low (median=0.52, IQR=0.33-1.13 μg/L), confirming infrequent seafood consumption.
2.4. OCM Nutrient Collection
Dietary intake of OCM-related micronutrients was measured during the baseline visit through estimated daily averages of dietary intake of vitamins B6 and B2 and folate in the past-year. These variables, as well as total caloric intake, were measured through an interviewer-administered Block 119-item food frequency questionnaire (FFQ). The Block questionnaire is one of the most widely used questionnaires with demonstrated reliability and validity.77 To enhance accuracy of the questionnaire in this cohort, additional questions relating to foods commonly consumed by American Indians were added.77
2.5. OCM SNP Selection and Genotyping
DNA was extracted from blood specimens obtained at the baseline visit using organic solvents and was genotyped according to Illumina protocol78 using the Illumina Cardio-Metabo DNA Analysis BeadChip (MetaboChip), which contains 196,725 markers. These markers were selected based on a large-scale meta-analysis for cardiometabolic traits such as coronary artery disease and type 2 diabetes. Markers included in this analysis have been genotyped previously using strict QC methods79 and were selected based on their role in OCM. Single nucleotide polymorphisms (SNPs) were only included if minor allele frequencies were >2%. For genes with multiple SNPs available, SNPs that had been previously associated with arsenic metabolism or diabetes-related outcomes (or SNPs in perfect LD with these SNPs) were selected. SNPs in the following genes were included: rs4646371 in phosphatidylethanolamine N-methyltransferase (PEMT), rs3818239 and rs17751556 in methylenetetrahydrofolate dehydrogenase (MTHFD1), rs12952556 and rs2273027 in serine hydroxymethyltransferase 1 (SHMT1), rs1801131, rs1801133 and rs2274976 in methylenetetrahydrofolate reductase (MTHFR), rs10495387 in methionine synthase (MTR), rs3776464 and rs16879334 in methionine synthase reductase (MTRR) and rs12482221 in cystathionine β-synthase (CBS).
2.6. Diabetes-Related Variable Collection and Definitions
Incident type 2 diabetes was defined as fasting plasma glucose ≥ 126 mg/dL, self-reported physician diagnosis or self-reported use of insulin or oral diabetes treatment. Baseline and follow-up HOMA2-IR values were calculated with the computed solved model for HOMA2-IR80 using fasting glucose and insulin values. Metabolic syndrome was defined according to the National Cholesterol Education Program ATP III guidelines, the most accepted guidelines for the US.81, 82 To be considered as a case of metabolic syndrome, participants had to have at least three of the following criteria: elevated waist circumference (≥40 inches in men and ≥35 inches in women); triglycerides ≥150 mg/dL (or on medication); fasting glucose ≥100 mg/dL (or on medication); HDL cholesterol <40 mg/dL for men and <50 mg/dL for women (or on medication); systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg (or on medication). Metabolic syndrome status was measured at baseline and follow-up visits. Waist circumference was measured at the umbilicus while the participant was in a supine position.75 Other measurements for metabolic syndrome status were measured as follows. For systolic and diastolic blood pressure, three measurements were taken on the right arm in seated position after 5 minutes of rest with an appropriately sized cuff using a Baum mercury sphygmomanometer; the average of the last two measurements was used for analyses.75 Blood samples collected after 12-hour fasting were used to measure triglycerides, glucose, and cholesterol using enzymatic methods.75 High-density lipoprotein cholesterol levels were measured by precipitation with heparin and manganese chloride.75
2.7. Sociodemographic and Diabetes-Related Risk Factor Data
The standardized questionnaire included sociodemographic data (age, sex, education, income), smoking history, alcohol use, physical activity, and medical history.74 Physical exam measures were performed by centrally trained nurses following a standardized protocol and included measurements of height, weight and body fat.75 Estimated glomerular filtration rate (eGFR) was measured from age, sex and recalibrated plasma creatinine using the Chronic Kidney Disease – Epidemiology Collaboration formula.83
2.8. Statistical Analyses
Statistical analyses were conducted in R software (version 3.5.1; R Project for Statistical Computing). Arsenic metabolism was computed by dividing iAs, MMA and DMA over the sum of the three × 100 (iAs%, MMA% and DMA%). Arsenic exposure, calculated as the sum of inorganic and methylated arsenic species (ΣAs) in urine and creatinine-corrected to account for urine dilution, was right-skewed and therefore log-transformed in analyses. OCM nutrients (folate, vitamin B6 and vitamin B2) were adjusted for total caloric intake using a residual analysis approach. This approach has been suggested over including caloric intake as a variable in regression models, as the nutrient and total caloric intake variables are measured through the same questionnaire, and therefore, their errors are correlated.84 For the residual analysis approach, we regressed each log-transformed vitamin intake on log-transformed total caloric intake. We then added the mean log-transformed value of each nutrient to the nutrient residuals to create calorie-corrected nutrient variables. OCM SNPs were included in analyses using co-dominant inheritance models. For the SNPs MTHFD1 (rs3818239), MTHFR (rs2274976), MTHFD1 (rs17751556) and MTR (rs10495387), the homozygous variant genotype was <10 and therefore grouped with the heterozygous genotype in analyses, resulting in just one comparison group to the wild-type genotype.
Sociodemographic, arsenic, vitamin intake and physiological variables were compared in participants with and without metabolic syndrome and diabetes at follow-up, using chi-square and Kruskal Wallis tests for categorical and continuous variables, respectively. Spearman correlations, as well as percentages of participants above the recommended daily intake (RDA) and upper limit (UL), for nutrient variables were evaluated. The associations between OCM status (genetic- and nutrient-related) and arsenic metabolism were evaluated in participants free of prevalent metabolic syndrome and diabetes (n=935) to remove the potential for the effect metabolic state may have on arsenic metabolism. In OCM nutrient analyses, the mean difference and 95% confidence interval (95% CI) of each arsenic species percentage (iAs%, MMA%, DMA%) per interquartile range (IQR) increase in each calorie-corrected nutrient variable (vitamin B6, vitamin B2 and folate) was estimated using mixed linear regression models to account for family-relatedness. In OCM SNP analyses, mixed linear regression models were used to estimate the mean difference (95% CI) of each arsenic species percentage comparing the heterozygous and homozygous variant genotypes to the wild type genotype (reference) for each OCM-related SNP. Models for OCM nutrients and genetic variants association with arsenic metabolism were adjusted for log-transformed ΣAs, age (continuous), sex, region (Arizona, Oklahoma, North/South Dakota), education (<12 years, 12+ years (or above/below appropriate level of schooling if aged <18 years)), BMI (<25, 25-<30, ≥30 kg/m2), smoking status (never, former, current), alcohol use (never, former, current), and eGFR (continuous). In models evaluating waist circumference, BMI was not included as an adjustment for consistency with previous literature.85
The association of OCM status (genetic- and nutrient-related) was also evaluated with each diabetes-related outcome. For diabetes, HOMA2-IR and waist circumference outcomes, analyses were conducted in participants free of baseline diabetes (n=1458). In metabolic syndrome analyses, analyses were conducted in participants free of baseline diabetes and baseline metabolic syndrome (n=935). For diabetes and metabolic syndrome, our dichotomous outcomes, we used modified Poisson regression with robust variance86 using generalized estimating equations with an independence working correlation structure to account for family clustering. Results were reported as relative risk (RR) and 95% confidence intervals (95% CI) of incident diabetes and metabolic syndrome per IQR and quartile increase in calorie-corrected OCM nutrients as well as comparing the heterozygous and homozygous variant genotypes to the wild-type genotype (reference) for each OCM SNP. For our continuous outcomes, HOMA2-IR and waist circumference, we conducted multi-level models (MLM) in which both HOMA2-IR (log-transformed) and waist circumference values at baseline and at follow-up were treated as the outcome and the linear predictor included the interaction of our OCM variable and time since baseline (in years). Specifically, the time variable included two values for each participant: time=0 and time=follow-up duration (in years). In this way, we were able to estimate the GMR of HOMA2-IR and mean difference of waist circumference and 95% CIs by OCM status variables at follow-up (considering 5 years of follow-up, i.e. time=5). Again, we used mixed effects linear regression models to account for family clustering.
Models evaluating the association between OCM SNPs and diabetes-related outcomes were adjusted for age, sex and four of ten population stratification principal components (which have been used previously87, 88 as they describe a significant amount of variation in the population). Models evaluating the association between OCM nutrients and diabetes-related outcomes were adjusted for age, sex, center, education, BMI, kidney function, smoking status and drinking status.
In previous analyses in this cohort, arsenic metabolism has been associated with risk for diabetes, metabolic syndrome and elevated waist circumference, as well as higher HOMA2-IR.73, 89 We re-ran these analyses in this study before and after adjustment for OCM variables to evaluate whether OCM status might be confounding these associations. We also re-ran final models evaluating the association between OCM nutrients and SNPs with diabetes-related outcomes after adjusting for arsenic metabolism (i.e., MMA%) and ΣAs, to evaluate whether arsenic metabolism may act as a partial mediator in these associations. Finally, based on our findings, we conducted an exploratory formal mediation analysis, evaluating the direct, and indirect through MMA%, effects of the MTR SNP on HOMA2-IR at follow-up.
Multiple sensitivity analyses were conducted to better understand the association between vitamin B6 and diabetes-related outcomes. First, we ran Spearman correlations between our calorie-corrected B6 variable and dietary food groups (processed meat, red meat, fried chicken, cereals, vegetables and fruits) to determine if there were any high correlations that might potentially be confounding the associations between vitamin B6 and diabetes-related outcomes; we then used additional adjustments for these food groups separately in the final model. We also tested for potential confounding by niacin by adjusting for it in vitamin B6 and diabetes-related outcome models. Further, we ran models using raw vitamin values with separate adjustment for total caloric intake instead of calorie-corrected vitamins. Finally, we stratified analyses by sex.
3. RESULTS
3.1. Participant Characteristics
Of the 1,458 participants free of diabetes at baseline, 167 (11.3%) developed diabetes over a median of 5.3 years of follow-up, and of the 935 participants free of both diabetes and metabolic syndrome at baseline, 279 (29.8%) developed metabolic syndrome over follow-up. Participants who developed diabetes or metabolic syndrome at follow-up were older, had higher DMA%, BMI, HOMA2-IR and waist circumference, and lower MMA% (p≤0.05) compared with participants that did not develop diabetes or metabolic syndrome (Table 1). Participants who developed diabetes were also exposed to higher levels of ΣAs than those that did not develop diabetes. Participants who developed diabetes or metabolic syndrome did not differ by vitamin intake or other sociodemographic variables. All calorie-corrected nutrients were significantly correlated with each other (p = <0.001) (Supplemental Table 1). The majority of participants were below the RDA for vitamin B2 and folate intake, but above the recommended dietary allowance (RDA) for vitamin B6 intake.
Table 1.
Baseline Participant Characteristics of American Indians in the Strong Heart Family Study by Incident Metabolic Syndrome and Diabetes Status at Follow-up, 1998-2009
| No MetS at Follow-up |
MetS at Follow-up |
P- Value |
No Diabetes at Follow-up |
Diabetes at at Follow-up |
P- Value |
|
|---|---|---|---|---|---|---|
| Total, n (%) | 656(70.2) | 279(29.8) | 1295 (88.8) | 163 (11.2) | ||
| Age (years), median (IQR) | 28.6 (19.4, 41) | 33.7 (24.9, 44.1) | <0.001 | 34.3 (22.4-45.8) | 39.2 (29.6-50.7) | <0.001 |
| Sex, n (%) | ||||||
| Female | 285(43.4) | 114(40.9) | 0.51 | 535(41.3) | 64 (39.3) | 0.68 |
| Male | 371(56.6) | 165(59.1) | 760(58.7) | 99 (60.7) | ||
| Education, n (%) | ||||||
| <12 years | 171(26.1) | 70(25.1) | 0.82 | 313(24.2) | 45 (27.6) | 0.39 |
| 12+ years | 485(73.9) | 209(74.9) | 982(75.8) | 118 (72.4) | ||
| Smoking Status, n (%) | ||||||
| Never | 282(43) | 120(43) | 0.94 | 525(40.5) | 64 (39.3) | 0.87 |
| Ever | 119(18.1) | 53(19) | 264(20.4) | 36 (22.1) | ||
| Current | 255(38.9) | 106(38) | 506(39.1) | 63 (38.7) | ||
| Alcohol Intake, n (%) | ||||||
| Never | 85 (13) | 27(9.7) | 0.21 | 140(10.8) | 19 (11.7) | 0.11 |
| Ever | 137(20.9) | 69(24.7) | 320(24.7) | 52 (31.9) | ||
| Current | 434(66.2) | 183(65.6) | 835(64.5) | 92 (56.4) | ||
| eGFR mL/min/1.73 m2, n(%) | ||||||
| < 60 | 2 (0.3) | 2 (0.7) | 0.737 | 9 (0.7) | 1 (0.6) | 1.00 |
| ≥ 60 | 654(99.7) | 277(99.3) | 1286 (99.3) | 162 (99.4) | ||
|
Caloric Intake (kcal),
median (IQR) |
2228 (1537, 3470) | 2271 (1426, 3252) | 0.32 | 2222 (1516-3345) | 2294 (1561-3309) | 0.92 |
| Vitamin B6 (mg) | ||||||
| Intake, median (IQR) | 1.7 (1.1, 2.8) | 1.7 (1.1, 2.5) | 0.40 | 1.6 (1.1-2.6) | 1.9 (1.1-2.7) | 0.26 |
| Supplement Use, n (%) | 149(22.7) | 77(27.6) | 0.13 | 314(24.2) | 39 (23.9) | 1.00 |
| Vitamin B2 (mg) | ||||||
| Intake, median (IQR) | 1.7 (1.1, 2.6) | 1.6 (1.1, 2.5) | 0.85 | 1.7 (1.1-2.7) | 1.9 (1.2-2.7) | 0.45 |
| Supplement Use, n (%) | 149(22.7) | 77(27.6) | 0.13 | 314(24.2) | 39 (23.9) | 1.00 |
| Folate (μg) | ||||||
| Intake, median (IQR) | 356(226, 550) | 355(222, 514) | 0.49 | 355(232-548) | 409 (248-602) | 0.25 |
| Supplement Use, n (%) | 160(24.4) | 85(30.5) | 0.06 | 340(26.3) | 42.0 (25.8) | 0.97 |
| ΣAs (μg/L), median (IQR) | 4.4 (2.9, 7.1) | 4.7 (3.1, 7.5) | 0.21 | 4.5 (3.0-7.3) | 5.3 (3.2-9.4) | 0.006 |
| iAs%, median (IQR) | 10.9 (7.5, 15.5) | 9.9 (7.3, 13.5) | 0.04 | 10.3 (7.0-14.2) | 9.0 (6.7-13.0) | 0.04 |
| MMA%, median (IQR) | 15.9 (12.6, 19.9) | 14(10.9, 17.8) | <0.001 | 14.8 (11.3-18.5) | 13.1 (10.3-16.8) | <0.001 |
| DMA%, median (IQR) | 72 (65.5, 78.5) | 74.6 (68.9, 80.8) | <0.001 | 74.2 (67.3-80.4) | 78.0 (70.7-82.1) | 0.001 |
| BMI (kg/m2), n (%) | ||||||
| Normal | 276(42.1) | 46(16.5) | <0.001 | 333(25.7) | 9 (5.5) | <0.001 |
| Overweight | 205(31.2) | 92(33) | 385(29.7) | 28 (17.2) | ||
| Obese | 175(26.7) | 141(50.5) | 577(44.6) | 126 (77.3) | ||
| HOMA2-IR, median (IQR) | 1.1 (0.8, 1.6) | 1.5 (1-2.2) | <0.001 | 1.3 (0.9-2.1) | 2.3 (1.5-3.7) | <0.001 |
|
Waist Circumference (cm),
median (IQR) |
90.0 (80.0, 99.0) | 99.0 (91.0, 111) | <0.001 | 97 (87-109) | 111 (100-123) | <0.001 |
Abbreviations: ΣAs (iAs+MMA+DMA); BMI (body mass index); dimethylarsinic acid (DMA); eGFR (estimated glomerular filtration rate); inorganic arsenic (iAs); IQR (interquartile range); metabolic syndrome (MetS); monomethylarsonic acid (MMA)
3.2. Association between OCM Variables and Arsenic Metabolism
In mixed linear regression models, calorie-corrected OCM vitamins (B6, B2 and folate), were not associated with any arsenic metabolism variables (iAs%, MMA%, DMA%) in adjusted models (Table 2). In mixed linear regression models evaluating the association between OCM SNPs and arsenic metabolism, carriers of one or two A alleles in the MTR rs10495387 polymorphism was associated with 2.57% (95% CI: 0.22, 4.92) higher MMA% and non-significantly associated with 4.08% (95% CI -8.32, 0.17) lower DMA% (Table 3). No other OCM SNPs were associated with arsenic metabolism.
Table 2.
Mean Difference in Arsenic Metabolism by IQR Increase (95% CI) in One Carbon Metabolism-Related Nutrient Intake (N=935)
| Variable | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| iAs% | |||
| Vitamin B6 | 0.06(−0.50, 0.61) | 0.05 (−0.48, 0.59) | 0.16 (−0.36, 0.68) |
| Vitamin B2 | 0.23(−0.32, 0.78) | 0.07 (−0.48, 0.61) | 0.15 (−0.41, 0.70) |
| Folate | −0.52 (−0.98, −0.06) | −0.21 (−0.65, 0.24) | −0.12 (−0.56, 0.33) |
| MMA% | |||
| Vitamin B6 | −0.03 (−0.52, 0.46) | −0.09 (−0.56, 0.38) | −0.08 (−0.53, 0.37) |
| Vitamin B2 | 0.47(−0.01, 0.94) | 0.26 (−0.20, 0.72) | 0.26 (−0.19, 0.70) |
| Folate | −0.43 (−0.85, 0.00) | −0.15 (−0.56, 0.25) | −0.14 (−0.52, 0.24) |
| DMA% | |||
| Vitamin B6 | −0.03 (−0.92, 0.87) | 0.04 (−0.80, 0.88) | −0.08 (−0.89, 0.73) |
| Vitamin B2 | −0.70 (−1.55, 0.16) | −0.33 (−1.16, 0.51) | −0.40 (−1.23, 0.42) |
| Folate | 0.95(0.19, 1.70) | 0.36 (−0.35, 1.07) | 0.26 (−0.43, 0.94) |
Model 1: adjusted for log creatine-adjusted urinary total arsenic
Model 2: Model 1 adjustments plus age, sex, center
Model 3: Model 2 adjustments plus education, kidney function, smoking status, drinking status, BMI
Abbreviations: DMA (dimethylarsinic acid); iAs (inorganic arsenic); monomethylarsonic acid (MMA)
Table 3.
Mean Difference (95% CI) in Arsenic Metabolism by One Carbon Metabolism-Related Genetic Polymorphisms (N=935)
| Variable | n | iAs% | MMA% | DMA% |
|---|---|---|---|---|
| CBS (rs12482221) | ||||
| AA | 365 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| AG | 409 | 0.15(−0.7, 0.99) | −0.18 (−0.89, 0.52) | 0.04(−1.28, 1.36) |
| GG | 161 | −0.42 (−1.62, 0.79) | −0.29 (−1.23, 0.66) | 0.70(−1.12, 2.52) |
| SHMT1 (rs12952556) | ||||
| AA | 637 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| AG | 269 | 0.42(−0.51, 1.36) | 0.03(−0.67, 0.72) | −0.45 (−1.75, 0.84) |
| GG | 29 | 0.80(−1.63, 3.23) | 1.54(−0.64, 3.73) | −2.35 (−6.56, 1.87) |
| SHMT1 (rs2273027) | ||||
| AA | 286 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| AG | 463 | 0.51(−0.42, 1.45) | −0.21 (−1.04, 0.62) | −0.31 (−1.82, 1.21) |
| GG | 186 | 0.00(−1.18, 1.19) | −0.33 (−1.31, 0.64) | 0.33(−1.50, 2.15) |
| PEMT (rs4646371) | ||||
| GG | 360 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| GA | 452 | −0.44 (−1.35, 0.47) | 0.18(−0.56, 0.92) | 0.26(−1.13, 1.65) |
| AA | 123 | −0.47 (−1.89, 0.94) | 0.08(−0.99, 1.15) | 0.39(−1.74, 2.52) |
| MTRR (rs3776464) | ||||
| AA | 513 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| AT | 358 | 0.13(−0.79, 1.06) | 0.25(−0.43, 0.93) | −0.38 (−1.75, 0.98) |
| TT | 64 | 0.01(−1.72, 1.75) | 0.25(−1.72, 1.75) | −0.26 (−3.09, 2.57) |
| MTRR (rs16879334) | ||||
| CC | 586 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| CG | 306 | 0.13(−0.79, 1.06) | 0.28(−0.41, 0.96) | −0.41 (−1.78, 0.95) |
| GG | 43 | 0.01(−1.72, 1.75) | 0.26(−1.28, 1.80) | −0.27 (−3.10, 2.55) |
| MTHFR (1801131) | ||||
| CC | 586 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| CT | 306 | 0.07(−0.85, 0.98) | −0.28 (−1.02, 0.46) | 0.21(−1.17, 1.60) |
| TT | 43 | −1 (−2.84, 0.85) | −0.97 (−2.46, 0.52) | 1.97(−0.46, 4.39) |
| MTHFR (1801133) | ||||
| AA | 473 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| AC | 394 | 0.07(−0.73, 0.87) | 0.42(−0.29, 1.13) | −0.49 (−1.79, 0.81) |
| CC | 68 | 1.18(−0.62, 2.98) | 1.02(−0.42, 2.46) | −2.20 (−4.82, 0.43) |
| MTHFR (2274976) | ||||
| GG | 790 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| GA/AA | 145 | −0.89 (−2.03, 0.25) | −0.75 (−1.68, 0.18) | 1.64(−0.10, 3.39) |
| MTHFD1 (rs3818239) | ||||
| AA | 838 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| AG/GG | 77 | −1.08 (−2.32, 0.15) | −0.40 (−1.42, 0.62) | 1.48(−0.38, 3.34) |
| MTHFD1 (rs17751556) | ||||
| AA | 870 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| AG | 97 | −0.32 (−2.00, 1.35) | 0.65(−0.76, 2.06) | −0.32 (−3.05, 2.40) |
| MTR (rs10495387) | ||||
| CC | 892 | 0.00(Reference) | 0.00(Reference) | 0.00(Reference) |
| CA/AA | 43 | 1.51(−0.64, 3.66) | 2.57(0.22, 4.92) | −4.08 (−8.32, 0.17) |
Models adjusted for age, sex, ΣAs, 4 genetic PCs
Abbreviations: DMA (dimethylarsinic acid); iAs (inorganic arsenic); monomethylarsonic acid (MMA)
3.3. Association between OCM Variables and Diabetes-Related Outcomes
In mixed modified Poisson regression models, IQR increases in calorie-corrected vitamin B6 were associated with greater risk for incident metabolic syndrome (RR=1.15; 95% CI=1.02, 1.29) and incident diabetes (RR=1.28; 95% CI=1.10, 1.48) (Table 4). Vitamin B6 was also borderline associated with higher HOMA2-IR in mixed multi-level models (GMR=1.04; 95% CI=1.00, 1.08 per 5-years of follow-up). Results were similar categorizing vitamin B6 into quartiles. Results were consistent in sensitivity analyses stratifying by sex; using non-calorie-corrected vitamin B6 values and adjusting separately for caloric intake in models; adjusting for niacin intake; and further adjusting final models separately for intake of fried chicken, processed meat, red meat, vegetables and fruit (data not shown). Calorie corrected vitamin B2 was also associated with increased risk for incident diabetes (RR per IQR increase in vitamin B2=1.21; 95% CI=1.03, 1.41). Folate was not significantly associated with any diabetes-related outcome.
Table 4.
Association between One Carbon Metabolism-Related Nutrient Intake and Diabetes-related Outcomes
| Variable | Relative Risk for Metabolic Syndrome (n=935) |
Mean Difference in Waist Circumference (N=1458) |
Relative Risk for Diabetes (n=1458) |
GMR for HOMA2-IR (n=1458) |
|---|---|---|---|---|
| Vitamin B6 (mg)a | ||||
| <1.2 | 1 (Reference) | (Reference) | 1 (Reference) | 1 (Reference) |
| 1.2 – <1.8 | 1.17 (0.89 1.53) | 1.18 (−1.21, 3.57) | 1.28 (0.82 2.00) | 1.07 (0.98, 1.17) |
| 1.8 – <2.7 | 1.08 (0.81 1.43) | 1.33 (−1.08, 3.74) | 1.00 (0.62 1.63) | 0.98 (0.89, 1.07) |
| ≥2.7 | 1.40 (1.06 1.85) | 0.47 (−1.94, 2.88) | 1.72 (1.13 2.61) | 1.07 (0.98, 1.18) |
| IQR | 1.15 (1.02 1.29) | 0.03 (−1.01, 1.07) | 1.28 (1.10 1.48) | 1.04 (1.00, 1.08) |
| Vitamin B2 (mg)a | ||||
| <1.2 | 1 (Reference) | (Reference) | 1 (Reference) | 1 (Reference) |
| 1.2 – <1.8 | 1.08 (0.80 1.44) | 0.13 (−2.26, 2.51) | 1.23 (0.80 1.90) | 0.97 (0.88, 1.06) |
| 1.8 – <2.8 | 1.26 (0.96 1.65) | 0.16 (−2.25, 2.57) | 1.17 (0.75 1.82) | 1.00 (0.91, 1.09) |
| ≥2.8 | 0.93 (0.69 1.27) | −1.83 (−4.24, 0.58) | 1.53 (1.00 2.34) | 1.02 (0.93, 1.12) |
| IQR | 1.01 (0.89 1.14) | −0.95 (−2.01, 0.12) | 1.21 (1.03 1.41) | 1.01 (0.97, 1.05) |
| Folate (μg)a | ||||
| <234 | 1 (Reference) | (Reference) | 1 (Reference) | 1 (Reference) |
| 234 - <355 | 0.83 (0.63 1.10) | 1.48 (−0.89, 3.86) | 1.08 (0.71 1.63) | 0.95 (0.87, 1.04) |
| 355 - <560 | 1.03 (0.78 1.35) | 2.11 (−0.27, 4.50) | 1.35 (0.88 2.05) | 1.00 (0.91, 1.09) |
| ≥560 | 0.97 (0.74 1.26) | −0.70 (−3.13, 1.73) | 1.27 (0.83 1.94) | 1.05 (0.96, 1.15) |
| IQR | 1.02 (0.90 1.15) | −0.93 (−1.96, 0.11) | 1.15 (0.97 1.36) | 1.01 (0.97, 1.05) |
Vitamins are calorie-corrected
Models adjusted for age, gender, center, education, BMI, kidney function, smoking status, drinking status.
Abbreviations: GMR (geometric mean ratio);
HOMA2-IR (homeostasis model assessment for insulin resistance)
Three OCM SNPs in CBS, PEMT and MTR genes had significant associations with diabetes-related outcomes. Each addition of the variant G allele in the CBS SNP was associated with an allele-dependent decrease in waist circumference per 5 years of follow-up (βAG= −0.51, 95% CI=−2.36, 1.33; βGG= −0.3.28; 95% CI=−5.82, −0.74) (Table 5). CBS was not associated with any other diabetes-related outcome. For PEMT, each addition of the variant A allele was associated with an allele-dependent increase in both metabolic syndrome (RRGA=1.12, 95% CI= 0.88, 1.43; RRAA=1.37; 95% CI=1.02, 1.85) and waist circumference (βGA= 1.92, 95% CI=−0.13, 3.71; βAA= 2.51, 95% CI=−0.14, 5.17 per 5 years of follow-up). A similar trend was observed for diabetes; however, the association was not significant. For MTR, one or two copies of the variant A allele was associated with lower HOMA2-IR (GMR=0.79, 95% CI=0.66, 0.93 per 5 years of follow-up). The trend was consistent for metabolic syndrome and diabetes, but not significant.
Table 5.
Association between One Carbon Metabolism-Related Genetic Polymorphisms and Diabetes-Related Outcomes
| Variable | n | Relative Risk for Metabolic Syndrome (N=935) |
n | Mean Difference in Waist Circumference (N=1458) |
Relative Risk for Diabetes (N=1458) |
GMR for HOMA2-IR (N=1458) |
|---|---|---|---|---|---|---|
| Total | 935 | 1458 | ||||
| CBS (rs12482221) | ||||||
| AA | 365 | 1.00(Reference) | 558 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| AG | 409 | 1.02(0.81, 1.29) | 658 | −0.51 (−2.36, 1.33) | 1.10 (0.81, 1.51) | 0.99 (0.91, 1.07) |
| GG | 161 | 1.24(0.93, 1.66) | 242 | −3.28 (−5.82, −0.74) | 1.03 (0.67, 1.57) | 0.99 (0.89, 1.11) |
| SHMT1 (rs12952556) | ||||||
| AA | 637 | 1.00(Reference) | 1002 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| AG | 269 | 1.02(0.81, 1.28) | 410 | 0.97(−0.91, 2.86) | 1.03 (0.75, 1.43) | 1.03 (0.95, 1.12) |
| GG | 29 | 1.07(0.59, 1.93) | 46 | 2.08(−2.72, 6.87) | 0.64 (0.22, 1.82) | 1.01 (0.82, 1.24) |
| SHMT1 (rs2273027) | ||||||
| AA | 286 | 1.00(Reference) | 454 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| AG | 463 | 0.89(0.71, 1.11) | 732 | 0.24(−1.68, 2.16) | 1.24 (0.87, 1.78) | 1.05 (0.97, 1.14) |
| GG | 186 | 1.06(0.79, 1.42) | 272 | 1.19(−1.33, 3.71) | 0.93 (0.57, 1.51) | 1.08 (0.96, 1.20) |
| PEMT (rs4646371) | ||||||
| GG | 360 | 1.00(Reference) | 584 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| GA | 452 | 1.12(0.88, 1.43) | 681 | 1.92(0.13, 3.71) | 1.07 (0.80, 1.44) | 1.00 (0.92, 1.08) |
| AA | 123 | 1.37(1.02, 1.85) | 193 | 2.51(−0.14, 5.17) | 1.21 (0.80, 1.84) | 1.01 (0.90, 1.14) |
| MTRR (rs3776464) | ||||||
| AA | 513 | 1.00(Reference) | 775 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| AT | 358 | 0.96(0.78, 1.19) | 579 | −0.09 (−1.91, 1.74) | 1.11 (0.79, 1.55) | 1.01 (0.93, 1.09) |
| TT | 64 | 0.86(0.54, 1.36) | 104 | −0.43 (−3.89, 3.04) | 1.13 (0.61, 2.09) | 0.90 (0.77, 1.04) |
| MTRR (rs16879334) | ||||||
| CC | 514 | 1.00(Reference) | 776 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| CG | 357 | 0.97(0.79, 1.19) | 578 | −0.22 (−2.05, 1.60) | 1.11 (0.79, 1.56) | 1.01 (0.93, 1.09) |
| GG | 64 | 0.86(0.54, 1.36) | 104 | −0.50 (−3.96, 2.96) | 1.13 (0.61, 2.09) | 0.90 (0.77, 1.04) |
| MTHFR (rs1801131) | ||||||
| CC | 586 | 1.00(Reference) | 943 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| CT | 306 | 0.99(0.80, 1.24) | 449 | 0.73(−1.14, 2.59) | 0.80 (0.57, 1.13) | 1.01 (0.93, 1.09) |
| TT | 43 | 0.71(0.37, 1.37) | 66 | 2.03(−2.16, 6.22) | 0.72 (0.28, 1.80) | 1.12 (0.93, 1.34) |
| MTHFR (rs1801133) | ||||||
| AA | 473 | 1.00(Reference) | 748 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| AC | 394 | 1.08(0.89, 1.33) | 599 | −1.03 (−2.79, 0.73) | 0.94 (0.69, 1.28) | 0.95 (0.88, 1.03) |
| CC | 68 | 0.87(0.56, 1.34) | 111 | −2.34 (−5.61, 0.93) | 0.83 (0.47, 1.46) | 0.98 (0.85, 1.13) |
| MTHFR (rs2274976) | ||||||
| GG | 790 | 1.00(Reference) | 1235 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| GA/AA | 145 | 1.07(0.82, 1.40) | 223 | 1.21(−1.11, 3.52) | 0.91 (0.60, 1.37) | 1.08 (0.98, 1.20) |
| MTHFD1 (rs3818239) | ||||||
| AA | 838 | 1.00(Reference) | 1311 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| AG/GG | 77 | 1.00(0.73,1.37) | 147 | 0.95(−1.85, 3.75) | 0.94 (0.50, 1.76) | 1.03 (0.91, 1.16) |
| MTHFD1 (rs17751556) | ||||||
| AA | 870 | 1.00(Reference) | 1362 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| AG/GG | 97 | 0.68(0.40, 1.17) | 96 | −1.90 (−5.33, 1.53) | 0.91 (0.48, 1.70) | 0.94 (0.81, 1.09) |
| MTR (rs10495387) | ||||||
| CC | 892 | 1.00(Reference) | 1390 | 0.00(Reference) | 1.00 (Reference) | 1.00 (Reference) |
| CA/AA | 43 | 0.84(0.54, 1.32) | 68 | −0.03 (−4.01, 3.94) | 0.67 (0.30, 1.50) | 0.79 (0.66, 0.93) |
Models adjusted for age, sex and 4 population stratification PCs
Abbreviations: GMR (geometric mean ratio); HOMA2-IR (homeostasis model assessment for insulin resistance
Of the three OCM nutrients and twelve OCM SNPs, MTR was the only variable associated with both a diabetes-related outcome (HOMA2-IR) and an arsenic metabolism variable (MMA%). Therefore, we ran additional models evaluating the effect the MTR SNP had on diabetes-related outcomes after adjustment for MMA% and ΣAs. Adjustment for arsenic variables attenuated associations, with the association for HOMA2-IR almost losing significance (GMR=0.83, 95% CI=0.71, 0.99). Further, the very slight negative association between MTR and waist circumference before MMA% adjustment reversed to a slightly positive association after adjustment (β=1.35, 95% CI=−2.51, 5.21) (Table 6). Based on these findings, we also used a mediation model to evaluate the direct, and indirect through MMA%, effects of MTR on HOMA2-IR at follow-up. Of the total −0.184 (95% CI: −0.35, 0.02; P-value: 0.092) reduction in log HOMA2-IR at follow-up, the direct effect of MTR accounted for −0.138 (95% CI −0.341, 0.06; P-value: 0.180) and the indirect effect of MTR through MMA% accounted for −0.046 (95% CI −0.095, 0.00; P-value: 0.036). Finally, we attempted to understand the potential role of OCM as a confounder in the relationship between the previously reported associations with arsenic metabolism (lower MMA% and higher DMA%) and increased risk for diabetes-related outcomes by adjusting separately for OCM nutrients and SNPs. Associations remained consistent before and after adjustments (data not shown).
Table 6.
Association Between MTR and Diabetes-Related Outcomes Before and After Adjustment for Arsenic Metabolism
| Final Model | Final Model + MMA% & ΣAs adjustment | |
|---|---|---|
| MTR rs10495387 | ||
| RR of Incident Diabetes (N=1458) | ||
| CC | 1 | 1 |
| CA/AA | 0.67 (0.30, 1.50) | 0.70(0.31, 1.60) |
| GMR of HOMA2-IR (N=1458) | ||
| CC | 1 | 1 |
| CA/AA | 0.79 (0.66, 0.93) | 0.83(0.71, 0.99) |
| Mean Difference in Waist Circumference (N=1458) | ||
| CC | Reference | Reference |
| CA/AA | −0.03 (−4.01, 3.94) | 1.35(−2.51, 5.21) |
| RR of Incident Metabolic Syndrome (N=935) | ||
| CC | 1 | 1 |
| CA/AA | 0.84 (0.54, 1.32) | 0.93(0.58, 1.48) |
All models adjusted for age, sex and 4 population stratification PCs
Abbreviations: ΣAs (sum of iAs, MMA and DMA); GMR (geometric mean ratio); HOMA2-IR (homeostasis model assessment for insulin resistance); MMA (monomethylarsonic acid); RR (relative risk)
4. DISCUSSION
In this study of American Indian men and women aged ≥14 years, OCM nutrients were not associated with arsenic metabolism, however, higher vitamin B6 was consistently associated with three of the four diabetes-related outcomes studied (higher HOMA2-IR and increased risk for diabetes and metabolic syndrome). Adjustment for arsenic metabolism in these models did not affect the associations between vitamin B6 and diabetes related outcomes. Further, previously reported associations between arsenic metabolism and diabetes-related outcomes were not affected by adjustment for any of our OCM (nutrient or genetic) variables. However, the MTR rs10495387 polymorphism was associated with both lower MMA% and higher HOMA2-IR per 5 years of follow-up. After adjustment for MMA% the association between the MTR SNP and all diabetes-related outcomes were attenuated or reversed direction, suggesting MMA% may be a partial mediator in the association between this OCM SNP and diabetes-related outcomes.
Arsenic metabolism has been identified in epidemiological studies as a risk factor for health outcomes associated with arsenic exposure. Indeed, higher MMA% has consistently been associated with greater risk for skin lesions,90-93 cancer68, 94-98 and cardiovascular disease.99-101 As a result of these findings, clinical trials have been conducted in highly arsenic-exposed regions of the world in order to enhance arsenic metabolism (i.e., increase DMA% and decrease MMA%). In contrast, lower MMA% has been identified as protective of diabetes-related outcomes, including diabetes, increased BMI, elevated waist circumference, higher HOMA2-IR and metabolic syndrome.69, 71-73, 102 The mechanism behind these contrasting associations is unclear, but essential to uncover in order to determine if the link between arsenic metabolism and diabetes-related outcomes is an epidemiological artifact or a true association that should be considered in future arsenic health effects prevention trials. Both arsenic metabolism and diabetes-related outcomes are tightly connected to OCM, providing an intriguing potential pathway to explain this equivocal relationship.
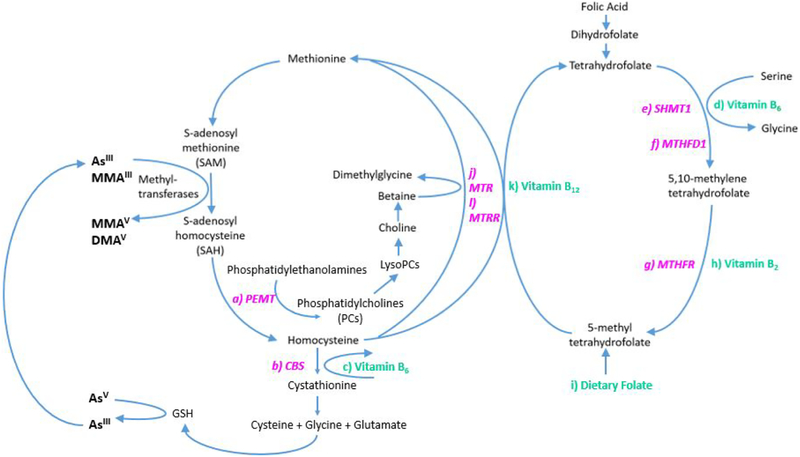
We characterized OCM in our study through two different metrics: OCM SNPs and intake of OCM nutrients. Despite inherent limitations to these variables as proxies to true OCM status that can be obtained through direct measurement of circulating OCM metabolites, they do have a distinct advantage: they are not influenced by a participant’s metabolic state allowing for a better understanding of relationship directions. OCM nutrients including vitamin B6, vitamin B2 and folate were selected for this study based on their role in OCM as outlined in Figure 2. OCM facilitates the generation of methyl groups, through the activation of methionine to S-adenosylmethionine (SAM), which provides the methyl group for most methylation reactions in the body.1 S-adenosylhomocysteine (SAH) is formed as a product of these reactions and inhibits OCM by tightly binding to most methylation transferase enzymes.1 SAH is only removed from these enzymes and hydrolyzed to homocysteine if existing homocysteine is either remethylated to form methionine or rerouted to the transsulfuration pathway.103 Vitamins B6 and B2 are co-enzymes in the conversion of tetrahydrofolate (THF) to 5,10-methylene-THF and the subsequent conversion of 5,10-methylene-THF to 5- methyl tetrahydrofolate (THF), respectively. Dietary folate can enter the OCM pathway as 5-methyl tetrahydrofolate (THF), which can then transfer a methyl donor to homocysteine to generate either THF or methionine. Vitamin B6 also plays a role in the transsulfuration pathway acting as a co-factor in the conversion of homocysteine to cystathionine, which is used in the generation of glutathione.103
Figure 2. Role of One Carbon Metabolism Related Nutrients and Genes in One Carbon Metabolism Pathway.
Genes are identified in pink and nutrients are identified in green. a) the PEMT gene encodes the PEMT enzyme which converts phosphatidylethanolamine to phosphatidylcholine which supports the remethylation of homocysteine through the choline dependent pathway; b) the CBS gene encodes the CBS enzyme which uses c) vitamin B6 as a co-factor to convert homocysteine into cystathionine; d) Vitamin B6 is also used as a co-factor in the e) SHMT1-catalyzed conversion of tetrahydrofolate to 5,10 methylene tetrahydrofolate; f) the MTHFD1 gene encodes a protein with three enzymatic activities, each involved in the conversion of tetrahydrofolate to 5,10 methylene tetrahydrofolate; g) the MTHFR gene encodes the rate limiting enzyme MTHFR which catalyzes the conversion of 5,10 methylene tetrahydrofolate to 5-methyl tetrahydrofolate using h) vitamin B2, in the form of flavin adenine dinucleotide, as a co-factor; 5-methyl tetrahydrofolate can also enter the one carbon metabolism pathway through intake of i) dietary folate; j) MTR catalyzes the conversion of homocysteine to methionine using k) B12 as a co-factor and 5-methyl tetrahydrofolate as the methyl donor; MTR is reactivated through reductive remethylation by l) MTRR. Abbreviations: CBS (Cystathionine beta synthase); MTHFD1 (Methylenetetrahydrofolate Dehydrogenase); MTHFR (Methylenetetrahydrofolate reductase); MTR (Methionine synthase); MTRR (Methionine synthase reductase); PEMT (Phosphatidylethanolamine N-Methyltransferase), SHMT1 (Serine hydroxymethyltransferase)
Our null results on the association between OCM nutrients and arsenic metabolism contrasts with findings from randomized supplementation trials conducted in highly exposed regions in Bangladesh which have shown folic acid supplementation can enhance arsenic metabolism (increase DMA% and reduce MMA% in urine).52, 53 Further, our group has previously reported an association between higher intake of vitamins B6 and B2 and enhanced arsenic metabolism in the Strong Heart Study, the parent study to the SHFS comprised of older American Indian tribal members recruited in 1989-1991.56 It is possible the lack of association in this study is in part due to the relatively high intake levels of these OCM nutrients compared to the parent study, which occurred prior to mandatory folic acid fortification. Some evidence suggests increasing OCM nutrient intake only has an effect on arsenic metabolism when OCM status is low.54, 104 Indeed, a small US-based study evaluating this relationship also reported null findings.105 However, two more recent larger US studies, with B vitamin levels similar to our population, found significant associations between B vitamin intake and arsenic metabolism. Howe et al reported reductions in MMA% with higher weighted sum of B vitamins.106 It is possible our lack of findings is a result of missing key vitamins. Howe’s weighted sum was strongly influenced by both B12 and thiamin, vitamins we did not have data on. A sensitivity analysis evaluating a variable representing higher intakes of all three B vitamins using principal components was also not significantly associated with arsenic metabolism (data not shown). Spencer et al found an association between higher B6 and lower iAs%, but an association between higher B12 and higher iAs% and lower DMA%.107 Together, these contrasting findings highlight the need for additional prospective analyses to better understand the association between B vitamin intake and arsenic metabolism.
We did, however, see strong consistent positive associations between vitamin B6 and our diabetes-related outcomes, including increased risk for metabolic syndrome and diabetes, as well as higher HOMA2- IR. These findings conflict with numerous observational studies which have found low OCM nutrient status to be associated with adverse health effects5-7 including cardiometabolic outcomes.2-4, 8-11 Low levels of OCM nutrients resulting in impaired OCM functioning, in turn causing elevations in homocysteine, has been identified as a potential mechanism behind these findings.108 Still, causality has been questioned as folic acid and B-vitamin combination pill supplementation trials have had inconsistent results for reducing cardiovascular-related outcomes,13, 14 with mostly no significant benefits reported for diabetes incidence.16, 17 Further, some evidence suggests high serum folate may actually increase mortality in diabetes patients.24 Finally, observational associations may also be a result of reverse causality. Animal models have shown diabetes can alter OCM functioning and metabolite levels, including reductions in vitamin B6.109, 110 This could explain the difference in findings, as metabolic state would not necessarily affect intake. Still, it’s unclear why higher B6 intake would be associated with greater risk for diabetes-related outcomes. We conducted several sensitivity analyses to evaluate whether confounding might explain the association. Our calorie-corrected B6 vitamin was not associated with caloric intake or other food patterns that have been associated with diabetes related outcomes (e.g, processed meat77, cereals/carbohydrates) (Supplemental Figure 1). Models evaluating vitamin B6 and diabetes-related outcomes remained consistent after adjustment for these food patterns as well as stratifying by sex. Although residual confounding cannot be disregarded, it is also possible the association is not spurious. Some studies have suggested mandatory B vitamin fortification, resulting in excess intake, has contributed to the obesity epidemic.111-113 In support of this hypothesis, The National Academies Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline has reported that there is some evidence that the B vitamin niacin, when used to treat patients with hypercholesterolemia, can cause impaired glucose tolerance.114 Finally, the association may be tied to cystathionine: vitamin B6 is a co-factor in cystathionine production (Figure 2), and cystathionine has been associated with cardiovascular outcomes.115 Additional research in large prospective cohorts is needed to better understand this relationship.
As with OCM nutrients, twelve OCM SNPs in seven genes were selected based on their role in OCM as outlined in Figure 2, as well as their availability in the MetaboChip illumina, a custom genotyping array of over 200,000 SNPs related to metabolic, cardiovascular and anthropometric traits.78 In our study, MTR was the only gene with a SNP significantly associated with arsenic metabolism. One or two of the variant alleles was associated with higher MMA% and non-significantly associated with higher iAs% and lower DMA% compared to the wild-type genotype, among participants free of baseline metabolic syndrome and diabetes. This is consistent with previous studies which have found the variant genotype of a different MTR SNP to be significantly associated with arsenic metabolism in the same direction (higher iAs% and MMA% and lower DMA%).57, 116 MTR plays a key role in OCM by catalyzing the remethylation of homocysteine to methionine and variants in the gene have been associated with differences in homocysteine levels.117 The MTR SNP in our study was associated with lower HOMA2-IR over 5 years of follow-up and with non-significant decreases in all three other diabetes-related outcomes. Consistently, a common MTR SNP, A2756G, has also been associated with diabetes-related outcomes, including obesity39 and hypertriglyceridemia.40 Because the MTR rs10495387 SNP was associated with both higher MMA% and reduced risk for diabetes-related outcomes, it served as an ideal OCM variable to explore whether OCM influences the association between arsenic metabolism and diabetes-related outcomes. However, adjusting for MTR rs10495387 did not affect any of these previously reported associations. Adjustment for other OCM SNPs as well as OCM nutrients also did not affect any of the previously reported associations, suggesting that in this study population, our measures of OCM were not confounding the relationship between arsenic metabolism and diabetes-related outcomes. In contrast, when we adjusted models evaluating the association between MTR rs10495387 and diabetes-related outcomes for MMA% and ΣAs, associations with diabetes-related outcomes (diabetes, metabolic syndrome and HOMA2IR) were attenuated, providing some evidence MMA% may serve as a partial mediator in the association between OCM and diabetes-related outcomes. This was supported by our exploratory mediation analyses, which showed a significant indirect effect of MTR through MMA% on HOMA2-IR at follow-up. Since a single OCM variable cannot fully represent the pathway functioning, more complex mediation modeling, such as structural equation models that create latent variables for OCM, are needed to confirm, and better understand, these relationships. Further, utilizing Mendelian randomization would provide enhanced understanding of the role OCM SNPs play in these complex intersecting relationships. Finally, we cannot discount the possibility of reverse causality in the observed associations between arsenic metabolism and diabetes-related outcomes. Studies with multiple urine arsenic measurements are needed to understand the effect metabolic state may have on arsenic metabolism.
This study was limited by a reduced sample size for metabolic syndrome analyses due to high prevalence of baseline metabolic syndrome. Our evaluation of dietary OCM intake was limited by lack of measurement of choline, methionine and vitamin B12, all important nutrients in OCM functioning that were not available in the SHFS. Further, despite the strength of FFQ data in its ability to provide OCM nutrient estimates not affected by metabolic state, FFQs are a self-reported dietary assessment tool that is associated with underestimates of intake, particularly energy and proteins.118, 119 Assessment of nutritional biomarkers and OCM metabolites as a third measure of OCM function would have enhanced analyses and study interpretations. Finally, multiple urine arsenic measurements would have allowed for better evaluation of direction of associations.
5. CONCLUSIONS
Our study identified vitamin B6 intake as a strong predictor of development of diabetes-related outcomes, a finding that remained consistent in multiple sensitivity analyses. Additional research is needed to better understand these results and determine whether chronic high intake of vitamin B6 has the potential to disturb metabolic activity. We also identified a SNP in the MTR gene that was associated with both arsenic metabolism and HOMA2-IR. The association between this SNP and diabetes-related outcomes were attenuated after adjustment for arsenic metabolism. Additional research is needed to confirm directions of ssociation; however, these findings add to the evidence that a complex and interconnected relationship exists between arsenic metabolism, OCM and diabetes-related outcomes.
Supplementary Material
Highlights.
One carbon metabolism was assessed as B vitamin intake and genetic polymorphisms
Higher vitamin B6 intake was associated with increased risk for diabetes outcomes
A methionine synthase polymorphism was associated with arsenic metabolism
The same polymorphism was also associated with insulin resistance
Arsenic metabolism, one carbon metabolism and diabetes appear to be interconnected
Acknowledgments
Funding: This work was supported by grants 1F31ES027796-01, 5T32ES007141-33, R01ES025216, 5P30ES009089 and P42ES010349 from NIEHS and grants R01-HL090863, R01-HL109315, R01HL109301, R01HL109284, R01HL109282, R01HL109319, U01-HL41642, U01-HL41652, U01- HL41654, U01-HL65520, U01-HL65521 from NHLBI.
Abbreviations:
- (ΣAs)
Arsenic exposure measured as urine arsenic levels
- (CBS)
Cystathionine β-synthase
- (DMA)
Dimethylarsinic acid
- (eGFR)
Estimated glomerular filtration rate
- (FFQ)
Food frequency questionnaire
- (HOMA2-IR)
Homeostasis model assessment for insulin resistance
- (iAs)
Inorganic arsenic
- (MTR)
Methionine synthase
- (MTRR)
Methionine synthase reductase
- (MTHFD1)
Methylenetetrahydrofolate dehydrogenase
- (MTHFR)
Methylenetetrahydrofolate reductase
- (MMA)
Monomethylarsonic acid
- (OCM)
One carbon metabolism
- (PEMT)
Phosphatidylethanolamine N-methyltransferase
- (SAM)
S- adenosylmethionine
- (SAH)
S-adenosylhomocysteine
- (SHMT1)
Serine hydroxymethyltransferase 1
- (SNP)
Single nucleotide polymorphism
- (SHS)
Strong Heart Study
- (SHFS)
Strong Heart Family Study
Footnotes
Disclaimer: The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ralph Carmel DWJ, ed. Homocysteine in Health and Disease. 2001, Cambridge University Press. [Google Scholar]
- 2.Voutilainen S, et al. , Low dietary folate intake is associated with an excess incidence of acute coronary events: The Kuopio Ischemic Heart Disease Risk Factor Study. Circulation, 2001. 103(22): p. 2674–80. [DOI] [PubMed] [Google Scholar]
- 3.Verhaar MC, Stroes E, and Rabelink TJ, Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol, 2002. 22(1): p. 6–13. [DOI] [PubMed] [Google Scholar]
- 4.Robinson K, et al. , Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation, 1998. 97(5): p. 437–43. [DOI] [PubMed] [Google Scholar]
- 5.Peng Y, Dong B, and Wang Z, Serum folate concentrations and all-cause, cardiovascular disease and cancer mortality: A cohort study based on 1999-2010 National Health and Nutrition Examination Survey (NHANES). Int J Cardiol, 2016. 219: p. 136–42. [DOI] [PubMed] [Google Scholar]
- 6.Araujo JR, et al. , Folates and aging: Role in mild cognitive impairment, dementia and depression. Ageing Res Rev, 2015. 22: p. 9–19. [DOI] [PubMed] [Google Scholar]
- 7.McLean RR, et al. , Plasma B vitamins, homocysteine, and their relation with bone loss and hip fracture in elderly men and women. J Clin Endocrinol Metab, 2008. 93(6): p. 2206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folsom AR, et al. , Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymorphisms, and B vitamins: the Atherosclerosis Risk in Communities (ARIC) study. Circulation, 1998. 98(3): p. 204–10. [DOI] [PubMed] [Google Scholar]
- 9.Bird JK, et al. , Obesity is associated with increased red blood cell folate despite lower dietary intakes and serum concentrations. J Nutr, 2015. 145(1): p. 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun KV, et al. , Dietary Intakes of Folic Acid and Methionine in Early Childhood Are Associated with Body Composition at School Age. J Nutr, 2015. 145(9): p. 2123–9. [DOI] [PubMed] [Google Scholar]
- 11.Mahabir S, et al. , Measures of adiposity and body fat distribution in relation to serum folate levels in postmenopausal women in a feeding study. Eur J Clin Nutr, 2008. 62(5): p. 644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong SM, et al. , A prospective association between dietary folate intake and type 2 diabetes risk among Korean adults aged 40 years or older: the Korean Multi-Rural Communities Cohort (MRCohort) Study. Br J Nutr, 2017. 118(12): p. 1078–1088. [DOI] [PubMed] [Google Scholar]
- 13.Schwingshackl L, et al. , Dietary Supplements and Risk of Cause-Specific Death, Cardiovascular Disease, and Cancer: A Systematic Review and Meta-Analysis of Primary Prevention Trials. Adv Nutr, 2017. 8(1): p. 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, et al. , Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc, 2016. 5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao M, et al. , Meta-analysis of folic acid efficacy trials in stroke prevention: Insight into effect modifiers. Neurology, 2017. 88(19): p. 1830–1838. [DOI] [PubMed] [Google Scholar]
- 16.Qin X, et al. , Effect of folic acid supplementation on risk of new-onset diabetes in adults with hypertension in China: Findings from the China Stroke Primary Prevention Trial (CSPPT). J Diabetes, 2016. 8(2): p. 286–94. [DOI] [PubMed] [Google Scholar]
- 17.Song Y, et al. , Effect of homocysteine-lowering treatment with folic Acid and B vitamins on risk of type 2 diabetes in women: a randomized, controlled trial. Diabetes, 2009. 58(8): p. 1921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao G, et al. , Efficacy of folic acid and enalapril combined therapy on reduction of blood pressure and plasma glucose: a multicenter, randomized, double-blind, parallel-controlled, clinical trial. Nutrition, 2008. 24(11-12): p. 1088–96. [DOI] [PubMed] [Google Scholar]
- 19.Gargari BP, Aghamohammadi V, and Aliasgharzadeh A, Effect of folic acid supplementation on biochemical indices in overweight and obese men with type 2 diabetes. Diabetes Res Clin Pract, 2011. 94(1): p. 33–8. [DOI] [PubMed] [Google Scholar]
- 20.Hunter-Lavin C, et al. , Folate supplementation reduces serum hsp70 levels in patients with type 2 diabetes. Cell Stress Chaperones, 2004. 9(4): p. 344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangoni AA, et al. , Short-term oral folic acid supplementation enhances endothelial function in patients with type 2 diabetes. Am J Hypertens, 2005. 18(2 Pt 1): p. 220–6. [DOI] [PubMed] [Google Scholar]
- 22.Title LM, et al. , Folic acid improves endothelial dysfunction in type 2 diabetes--an effect independent of homocysteine-lowering. Vasc Med, 2006. 11(2): p. 101–9. [DOI] [PubMed] [Google Scholar]
- 23.Kyte B, et al. , High red blood cell folate is associated with an increased risk of death among adults with diabetes, a 15-year follow-up of a national cohort. Nutr Metab Cardiovasc Dis, 2015. 25(11): p. 997–1006. [DOI] [PubMed] [Google Scholar]
- 24.Afriyie-Gyawu E, et al. , Serum folate levels and fatality among diabetic adults: A 15-y follow-up study of a national cohort. Nutrition, 2016. 32(4): p. 468–73. [DOI] [PubMed] [Google Scholar]
- 25.Zhu B, et al. , Folic Acid Supplements Intake in Early Pregnancy Increases Risk of Gestational Diabetes Mellitus: Evidence From a Prospective Cohort Study. Diabetes Care, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Krishnaveni GV, et al. , Association between maternal folate concentrations during pregnancy and insulin resistance in Indian children. Diabetologia, 2014. 57(1): p. 110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yajnik CS, et al. , Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia, 2008. 51(1): p. 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mojtabai R, Body mass index and serum folate in childbearing age women. Eur J Epidemiol, 2004. 19(11): p. 1029–36. [DOI] [PubMed] [Google Scholar]
- 29.Motamed S, et al. , Micronutrient intake and the presence of the metabolic syndrome. N Am J Med Sci, 2013. 5(6): p. 377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazidi M, Pennathur S, and Afshinnia F, Link of dietary patterns with metabolic syndrome: analysis of the National Health and Nutrition Examination Survey. Nutr Diabetes, 2017. 7(3): p. e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bian S, et al. , Dietary nutrient intake and metabolic syndrome risk in Chinese adults: a case-control study. Nutr J, 2013. 12: p. 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otsuka R, et al. , Relationship between number of metabolic syndrome components and dietary factors in middle-aged and elderly Japanese subjects. Hypertens Res, 2010. 33(6): p. 548–54. [DOI] [PubMed] [Google Scholar]
- 33.Roe AJ, et al. , Choline and its metabolites are differently associated with cardiometabolic risk factors, history of cardiovascular disease, and MRI-documented cerebrovascular disease in older adults. Am J Clin Nutr, 2017. 105(6): p. 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunanti IR, et al. , Low serum vitamin B-12 and folate concentrations and low thiamin and riboflavin intakes are inversely associated with greater adiposity in Mexican American children. J Nutr, 2014. 144(12): p. 2027–33. [DOI] [PubMed] [Google Scholar]
- 35.Nakazato M, et al. , Relation of body mass index to blood folate and total homocysteine concentrations in Japanese adults. Eur J Nutr, 2011. 50(7): p. 581–5. [DOI] [PubMed] [Google Scholar]
- 36.Yigit S, Karakus N, and Inanir A, Association of MTHFR gene C677T mutation with diabetic peripheral neuropathy and diabetic retinopathy. Mol Vis, 2013. 19: p. 1626–30. [PMC free article] [PubMed] [Google Scholar]
- 37.Settin A, et al. , Association of ACE and MTHFR genetic polymorphisms with type 2 diabetes mellitus: Susceptibility and complications. J Renin Angiotensin Aldosterone Syst, 2015. 16(4): p. 838–43. [DOI] [PubMed] [Google Scholar]
- 38.Huang T, et al. , Associations of common variants in methionine metabolism pathway genes with plasma homocysteine and the risk of type 2 diabetes in Han Chinese. J Nutrigenet Nutrigenomics, 2014. 7(2): p. 63–74. [DOI] [PubMed] [Google Scholar]
- 39.Terruzzi I, et al. , Are genetic variants of the methyl group metabolism enzymes risk factors predisposing to obesity? J Endocrinol Invest, 2007. 30(9): p. 747–53. [DOI] [PubMed] [Google Scholar]
- 40.Li WX, et al. , Joint associations of folate, homocysteine and MTHFR, MTR and MTRR gene polymorphisms with dyslipidemia in a Chinese hypertensive population: a cross-sectional study. Lipids Health Dis, 2015. 14: p. 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bokor S, et al. , Common polymorphisms in six genes of the methyl group metabolism pathway and obesity in European adolescents. Int J Pediatr Obes, 2011. 6(2-2): p. e336–44. [DOI] [PubMed] [Google Scholar]
- 42.Zhi X, et al. , Additive Interaction of MTHFR C677T and MTRR A66G Polymorphisms with Being Overweight/Obesity on the Risk of Type 2 Diabetes. Int J Environ Res Public Health, 2016. 13(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang B, et al. , Associations of MTHFR C677T and MTRR A66G gene polymorphisms with metabolic syndrome: a case-control study in Northern China. Int J Mol Sci, 2014. 15(12): p. 21687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christensen KE, et al. , The MTHFD1 1958G>A variant is associated with elevated C-reactive protein and body mass index in Canadian women from a premature birth cohort. Mol Genet Metab, 2014. 111(3): p. 390–392. [DOI] [PubMed] [Google Scholar]
- 45.Saxena R, Bozack AK, and Gamble MV, Nutritional Influences on One-Carbon Metabolism: Effects on Arsenic Methylation and Toxicity. Annu Rev Nutr, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayakawa T, et al. , A new metabolic pathway of arsenite: arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch Toxicol, 2005. 79(4): p. 183–91. [DOI] [PubMed] [Google Scholar]
- 47.Naranmandura H, Suzuki N, and Suzuki KT, Trivalent arsenicals are bound to proteins during reductive methylation. Chem Res Toxicol, 2006. 19(8): p. 1010–8. [DOI] [PubMed] [Google Scholar]
- 48.Aposhian HV and Aposhian MM, Arsenic toxicology: five questions. Chem Res Toxicol, 2006. 19(1): p. 1–15. [DOI] [PubMed] [Google Scholar]
- 49.Cullen WR RK, Arsenic Speciation in the Environment. Chemical Reviews, 1989. 89: p. 713–764. [Google Scholar]
- 50.Challenger F, Biological methylation. Adv Enzymol Relat Subj Biochem, 1951. 12: p. 429–91. [DOI] [PubMed] [Google Scholar]
- 51.Vahter M, Mechanisms of arsenic biotransformation. Toxicology, 2002. 181-182: p. 211–7. [DOI] [PubMed] [Google Scholar]
- 52.Gamble MV, et al. , Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am J Clin Nutr, 2006. 84(5): p. 1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters BA, et al. , Folic Acid and Creatine as Therapeutic Approaches to Lower Blood Arsenic: A Randomized Controlled Trial. Environ Health Perspect, 2015. 123(12): p. 1294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heck JE, et al. , Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am J Clin Nutr, 2007. 85(5): p. 1367–74. [DOI] [PubMed] [Google Scholar]
- 55.Hall M, et al. , Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Perspect, 2007. 115(10): p. 1503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spratlen MJ, et al. , Arsenic metabolism and one-carbon metabolism at low-moderate arsenic exposure: Evidence from the Strong Heart Study. Food Chem Toxicol, 2017. 105: p. 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlawicke Engstrom K, et al. , Genetic polymorphisms influencing arsenic metabolism: evidence from Argentina. Environ Health Perspect, 2007. 115(4): p. 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlawicke Engstrom K, et al. , Arsenic metabolism is influenced by polymorphisms in genes involved in one-carbon metabolism and reduction reactions. Mutat Res, 2009. 667(1-2): p. 4–14. [DOI] [PubMed] [Google Scholar]
- 59.Chung CJ, et al. , Polymorphisms in one-carbon metabolism pathway genes, urinary arsenic profile, and urothelial carcinoma. Cancer Causes Control, 2010. 21(10): p. 1605–13. [DOI] [PubMed] [Google Scholar]
- 60.Porter KE, et al. , Association of genetic variation in cystathionine-beta-synthase and arsenic metabolism. Environ Res, 2010. 110(6): p. 580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Del Razo LM, et al. , Altered profile of urinary arsenic metabolites in adults with chronic arsenicism. A pilot study. Arch Toxicol, 1997. 71(4): p. 211–7. [DOI] [PubMed] [Google Scholar]
- 62.Chen YC, et al. , Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control, 2003. 14(4): p. 303–10. [DOI] [PubMed] [Google Scholar]
- 63.Chen YC, et al. , Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med, 2003. 45(3): p. 241–8. [DOI] [PubMed] [Google Scholar]
- 64.Chen YC, et al. , Interaction between environmental tobacco smoke and arsenic methylation ability on the risk of bladder cancer. Cancer Causes Control, 2005. 16(2): p. 75–81. [DOI] [PubMed] [Google Scholar]
- 65.Hsueh YM, et al. , Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol Biomarkers Prev, 1997. 6(8): p. 589–96. [PubMed] [Google Scholar]
- 66.Steinmaus C, et al. , Arsenic methylation and bladder cancer risk in case-control studies in Argentina and the United States. J Occup Environ Med, 2006. 48(5): p. 478–88. [DOI] [PubMed] [Google Scholar]
- 67.Wu MM, et al. , Effect of plasma homocysteine level and urinary monomethylarsonic acid on the risk of arsenic-associated carotid atherosclerosis. Toxicol Appl Pharmacol, 2006. 216(1): p. 168–75. [DOI] [PubMed] [Google Scholar]
- 68.Yu RC, et al. , Arsenic methylation capacity and skin cancer. Cancer Epidemiol Biomarkers Prev, 2000. 9(11): p. 1259–62. [PubMed] [Google Scholar]
- 69.Kuo CC, et al. , Arsenic exposure, arsenic metabolism, and incident diabetes in the strong heart study. Diabetes Care, 2015. 38(4): p. 620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen JW, et al. , Arsenic methylation, GSTO1 polymorphisms, and metabolic syndrome in an arseniasis endemic area of southwestern Taiwan. Chemosphere, 2012. 88(4): p. 432–8. [DOI] [PubMed] [Google Scholar]
- 71.Gribble MO, et al. , Body composition and arsenic metabolism: a cross-sectional analysis in the Strong Heart Study. Environ Health, 2013. 12: p. 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grau-Perez M KC, Gribble MO, Balakrishnan P, Spratlen MS, Vaidya D, Francesconi KA, Goessler W, Guallar E, Silbergeld EK, Umans JG, Best LG, Lee ET, Howard BV, Cole SA, Navas-Acien A, Association of low-moderate arsenic exposure and arsenic metabolism with incident diabetes and insulin resistance in the Strong Heart Family Study. Environmental Health Perspectives, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spratlen MJ, G.-P. M, Best LG, Yracheta J, Lazo M, Vaidya D, Balakrishnan P, Gamble MV, Francesconi KA, Goessler W, Cole SA, Umans JG, Howard BV, Navas-Acien A, The Association of Arsenic Exposure and Arsenic Metabolism with the Metabolic Syndrome and its Individual Components: Prospective Evidence from the Strong Heart Family Study. American Journal of Epidemiology, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yurgalevitch SM, et al. , Physical activity and lipids and lipoproteins in American Indians ages 45-74. Med Sci Sports Exerc, 1998. 30(4): p. 543–9. [DOI] [PubMed] [Google Scholar]
- 75.North KE, et al. , Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol, 2003. 157(4): p. 303–14. [DOI] [PubMed] [Google Scholar]
- 76.Scheer J, et al. , Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal Methods, 2012. 4(2): p. 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fretts AM, et al. , Associations of processed meat and unprocessed red meat intake with incident diabetes: the Strong Heart Family Study. Am J Clin Nutr, 2012. 95(3): p. 752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Voight BF, et al. , The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet, 2012. 8(8): p. e1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balakrishnan P, et al. , Association of Cardiometabolic Genes with Arsenic Metabolism Biomarkers in American Indian Communities: The Strong Heart Family Study (SHFS). Environ Health Perspect, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levy JC, Matthews DR, and Hermans MP, Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care, 1998. 21(12): p. 2191–2. [DOI] [PubMed] [Google Scholar]
- 81.Grundy SM CJ, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F, Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation, 2005. 112(17): p. 2735–2752. [DOI] [PubMed] [Google Scholar]
- 82.de Simone G, et al. , Prognostic impact of metabolic syndrome by different definitions in a population with high prevalence of obesity and diabetes: the Strong Heart Study. Diabetes Care, 2007. 30(7): p. 1851–6. [DOI] [PubMed] [Google Scholar]
- 83.Levey AS, et al. , A new equation to estimate glomerular filtration rate. Ann Intern Med, 2009. 150(9): p. 604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rhee JJ, Cho E, and Willett WC, Energy adjustment of nutrient intakes is preferable to adjustment using body weight and physical activity in epidemiological analyses. Public Health Nutr, 2014. 17(5): p. 1054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holvoet P, et al. , Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA, 2008. 299(19): p. 2287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zou G, A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol, 2004. 159(7): p. 702–6. [DOI] [PubMed] [Google Scholar]
- 87.Balakrishnan P, et al. , Ethnic, Geographic, and Genetic Differences in Arsenic Metabolism at Low Arsenic Exposure: A Preliminary Analysis in the Multi-Ethnic Study of Atherosclerosis (MESA). Int J Environ Res Public Health, 2018. 15(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Balakrishnan P, et al. , Association of Cardiometabolic Genes with Arsenic Metabolism Biomarkers in American Indian Communities: The Strong Heart Family Study (SHFS). Environ Health Perspect, 2017. 125(1): p. 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grau-Perez M, et al. , Association of Low-Moderate Arsenic Exposure and Arsenic Metabolism with Incident Diabetes and Insulin Resistance in the Strong Heart Family Study. Environ Health Perspect, 2017. 125(12): p. 127004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahsan H, et al. , Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol Biomarkers Prev, 2007. 16(6): p. 1270–8. [DOI] [PubMed] [Google Scholar]
- 91.Chen Y, et al. , Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: review of recent findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol Appl Pharmacol, 2009. 239(2): p. 184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei B, et al. , Arsenic methylation and skin lesions in migrant and native adult women with chronic exposure to arsenic from drinking groundwater. Environ Geochem Health, 2017. 39(1): p. 89–98. [DOI] [PubMed] [Google Scholar]
- 93.Yang L, et al. , Associations of arsenic metabolites, methylation capacity, and skin lesions caused by chronic exposure to high arsenic in tube well water. Environ Toxicol, 2017. 32(1): p. 28–36. [DOI] [PubMed] [Google Scholar]
- 94.Steinmaus C, et al. , Arsenic methylation and bladder cancer risk in case-control studies in Argentina and the United States. J Occup Environ Med, 2006. 48(5): p. 478–488. [DOI] [PubMed] [Google Scholar]
- 95.Agusa T, et al. , Exposure, metabolism, and health effects of arsenic in residents from arsenic-contaminated groundwater areas of Vietnam and Cambodia: a review. Rev Environ Health, 2010. 25(3): p. 193–220. [DOI] [PubMed] [Google Scholar]
- 96.Chen YC, et al. , Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med, 2003. 45(3): p. 241–248. [DOI] [PubMed] [Google Scholar]
- 97.Huang YK, et al. , Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study. Cancer Causes Control, 2008. 19(8): p. 829–39. [DOI] [PubMed] [Google Scholar]
- 98.Steinmaus C, et al. , Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol Appl Pharmacol, 2010. 247(2): p. 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang YK, et al. , Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicol Appl Pharmacol, 2007. 218(2): p.135–42. [DOI] [PubMed] [Google Scholar]
- 100.Huang YL, et al. , Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci Total Environ, 2009. 407(8): p. 2608–2614. [DOI] [PubMed] [Google Scholar]
- 101.Chen Y, et al. , A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ Health Perspect, 2013. 121(7): p. 832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuo CC, et al. , The Association of Arsenic Metabolism with Cancer, Cardiovascular Disease, and Diabetes: A Systematic Review of the Epidemiological Evidence. Environ Health Perspect, 2017. 125(8): p. 087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hall MN and Gamble MV, Nutritional manipulation of one-carbon metabolism: effects on arsenic methylation and toxicity. J Toxicol, 2012. 2012: p. 595307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gamble MV, et al. , Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect, 2005. 113(12): p. 1683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steinmaus C, et al. , Dietary intake and arsenic methylation in a U.S. population. Environ Health Perspect, 2005. 113(9): p. 1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Howe CG, et al. , Dietary B Vitamin Intake Is Associated with Lower Urinary Monomethyl Arsenic and Oxidative Stress Marker 15-F2t-Isoprostane among New Hampshire Adults. J Nutr, 2017. 147(12): p. 2289–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kurzius-Spencer M, et al. , Nutrients in one-carbon metabolism and urinary arsenic methylation in the National Health and Nutrition Examination Survey (NHANES) 2003-2004. Sci Total Environ, 2017. 607-608: p. 381–390. [DOI] [PubMed] [Google Scholar]
- 108.Lonn E, et al. , Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med, 2006. 354(15): p. 1567–77. [DOI] [PubMed] [Google Scholar]
- 109.Okada M, et al. , Effect of diabetes on vitamin B6 requirement in experimental animals. Diabetes Obes Metab, 1999. 1(4): p. 221–5. [DOI] [PubMed] [Google Scholar]
- 110.Wijekoon EP, et al. , Homocysteine metabolism in ZDF (type 2) diabetic rats. Diabetes, 2005. 54(11): p. 3245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou SS, et al. , Vitamin paradox in obesity: Deficiency or excess? World J Diabetes, 2015. 6(10): p. 1158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou SS, et al. , B-vitamin consumption and the prevalence of diabetes and obesity among the US adults: population based ecological study. BMC Public Health, 2010. 10: p. 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou SS and Zhou Y, Excess vitamin intake: An unrecognized risk factor for obesity. World J Diabetes, 2014. 5(1): p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.in Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. 1998: Washington (DC). [PubMed] [Google Scholar]
- 115.Dhar I, et al. , Plasma cystathionine and risk of acute myocardial infarction among patients with coronary heart disease: Results from two independent cohorts. Int J Cardiol, 2018. 266: p. 24–30. [DOI] [PubMed] [Google Scholar]
- 116.Niedzwiecki MM, et al. , Serum homocysteine, arsenic methylation, and arsenic-induced skin lesion incidence in Bangladesh: A one-carbon metabolism candidate gene study. Environ Int, 2018. 113: p. 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fredriksen A, et al. , Large-scale population-based metabolic phenotyping of thirteen genetic polymorphisms related to one-carbon metabolism. Hum Mutat, 2007. 28(9): p. 856–65. [DOI] [PubMed] [Google Scholar]
- 118.Mossavar-Rahmani Y, et al. , Applying Recovery Biomarkers to Calibrate Self-Report Measures of Energy and Protein in the Hispanic Community Health Study/Study of Latinos. Am J Epidemiol, 2015. 181(12): p. 996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prentice RL, et al. , Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am J Epidemiol, 2011. 174(5): p. 591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.