Abstract
The cerebellum has a well-established role in controlling motor functions such coordination, balance, posture, and skilled learning. There is mounting evidence that it might also play a critical role in non-motor functions such as cognition and emotion. It is therefore not surprising that cerebellar deficits are associated with a wide array of diseases including ataxia, dystonia tremor, schizophrenia, dyslexia, and autism spectrum disorder. What is intriguing is that a seemingly uniform circuit that is often described as being “simple” should carry out all of these behaviors. Analyses of how cerebellar circuits develop have revealed that such descriptions massively underestimate the complexity of the cerebellum. The cerebellum is in fact highly patterned and organized around a series of parasagittal stripes and transverse zones. This topographic architecture partitions all cerebellar circuits into functional modules that are thought to enhance processing power during cerebellar dependent behaviors. What are arguably the most remarkable features of cerebellar topography are the developmental processes that produce them. This review is concerned with the genetic and cellular mechanisms that orchestrate cerebellar patterning. We place a major focus on how Purkinje cells control all aspects of cerebellar circuit assembly. Using this model, we discuss evidence for how “zebra-like” patterns in Purkinje cells sculpt the cerebellum, how specific genetic cues mediate the process, and how activity refines the patterns into an adult map that is capable of executing various functions. We will also discuss how defective Purkinje cell patterning might impact the pathogenesis of neurological conditions.
Keywords: Purkinje cell, development, stripes, patterning, connectivity, activity, motor function
The functional architecture of the cerebellum
The histological simplicity of the cerebellum and the long-standing assumption that it is primarily a motor structure massively underestimates the breadth of its capabilities, most of which we have yet to fully understand. We are now beginning to appreciate its role in behaviors previously thought to be strictly dedicated to higher order function, including emotion, language, and cognition (Koziol et al., 2014; Marien et al., 2014; Baumann et al., 2015; Adamaszek et al., 2017). In parallel with advances in understanding cerebellar functional diversity, the development of the cerebellum is becoming an important focal point in solving how this conserved structure works. But before we consider the embryonic manipulations, fate mapping tools, and sophisticated genetic techniques that have greatly enhanced our ability to investigate the dynamics of cerebellar development, we must first consider its most basic anatomical plan.
Gross anatomy distinguishes three main divisions of the cerebellum. The middle portion, called the vermis, is named for its worm-like appearance (Figure 1D). On either side of the vermis is a region called the paravermis, and in most species it is not a structurally separate area. However, the paravermis does execute distinct behaviors, although it performs its functions using the canonical circuit components of the cerebellum (Figure 1A). The most lateral portions of the cerebellum adjacent to each paravermis are simply known as the hemispheres. Surface examination of the cerebellum reveals what is perhaps the most recognizable feature of the cerebellum in all species, its highly folded architecture. Note however, that the embryonic cerebellum is smooth compared to the adult structure (Figure 1B-E).
Figure 1.
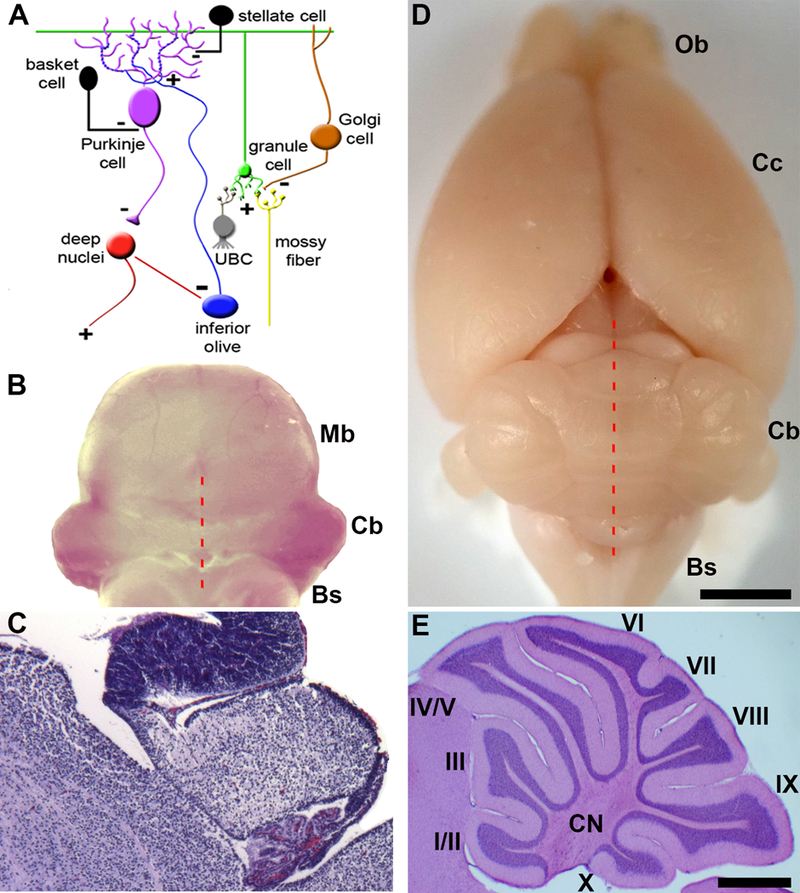
Connectivity and gross morphology of the cerebellum. A) Cellular composition and circuitry of the cerebellum. (+) indicates excitatory synapses and (−) indicates inhibitory synapses. B) Poster view of an embryonic day 16 mouse hindbrain. C) Sagittal tissues section of an embryonic day 16 mouse cerebellum stained with H&E. Provided are general anatomical landmarks for orientation with respect to the cerebellum, with the choroid plexus located posteriorly, the midbrain anteriorly, and the brainstem ventrally. D) Dorsal view of an adult mouse brain. The red broken lines in panels B and C indicate the midline of the cerebellum. E) Sagittal section of the mouse cerebellum showing the structure of the lobules. The lobules are labeled with Roman numerals. Abbreviations: UBC = unipolar brush cell, Bs = brainstem, Cb = cerebellum, Cc = cerebral cortex, CN = cerebellar nuclei, Cp = choroid plexus, fl/pfl = flocculus/paraflocculus, gl = granular layer, Mb = midbrain, ml = molecular layer, Ob = olfactory bulb, V= vermis, H = hemisphere. Scale bar in D = 2mm (applies to B where it = 1mm) and the scale bar in E = 500μm (applies to C where it = 200μm). Panel A was reused with permission from Reeber et al. (2013; Frontiers in Syst Neurosci, 7:83).
The adult cerebellum is anatomically divided into distinct folds called lobules (Larsell, 1952). Mammals and birds have ten lobules that are separated from one another by a series of fissures. Because each fissure extends to a specific depth in the cerebellum, each lobule develops with a unique shape (Figure 1E). The invariance of lobule structure within a species and their conservation across species are in accordance with the idea that complex morphogenetic programs spatially and temporally control lobule/fissure formation (Sudarov and Joyner, 2007).
The connectivity within the cerebellum is understood at a considerable level of detail, with each cell type forming stereotypical connections with its neighbors (Rámon y Cajal, 1909). The cerebellum has three distinct layers, with each layer comprised of distinct cell types (Figure 1A). The most superficial layer contains inhibitory stellate and basket cell interneurons and excitatory climbing fibers. All three project onto Purkinje cells, which make up a middle monolayer called the Purkinje cell layer. The Purkinje cell layer also contains interneurons called candelabrum cells as well as specialized astrocytes called Bergmann glia. The Purkinje cells perform the main computations in the cerebellum. The deepest layer is called the granular layer and it contains millions of small excitatory neurons called granule cells, inhibitory Golgi cells, inhibitory Lugaro cells, mossy fibers that deliver sensory signals to the cerebellum, and a peculiar excitatory cell type called unipolar brush cells. Unlike all other cell types that are found in all regions of the cerebellum, the unipolar brush cells are localized mainly to lobules IX and X (Mugnaini et al., 2011). In contrast, modulatory “beaded” afferent fibers terminate in all layers and in all lobules. Below the three layers is the white matter that contains a dense network of fiber tracts. Embedded in this network are three pairs of cerebellar nuclei on each side of the cerebellar midline (Figure 1E). These nuclei contain specialized neurons that transmit the final output of the cerebellum. From medial to lateral, they are the fastigial, interposed, and dentate nuclei, all of which link the cerebellum to the rest of the brain and spinal cord (Sillitoe and Joyner, 2007).
The presence of different circuitries, multiple cell lineages, and intricate folding raises a key question – how does the cerebellum integrate these different facets of its anatomy into a crystalline three-dimensional structure? We argue that Purkinje cell development holds the answer. We frame Purkinje cell development within a discussion of their patterning mechanisms.
Development and specification of the cerebellar primordium
Following the neural tube’s morphological division into distinct neuromeres, including the forebrain, midbrain, and hindbrain, the coordination of various transcription factors and mobilization of secreted morphogens demarcate the neuroepithelium that will give rise to cerebellar tissue and specify its neurons. In mice, the cerebellar primordium arises between E8.5 and E9.5 entirely from within the metencephalon (Wassef and Joyner, 1997; Zervas et al., 2004). In the earliest phase of cerebellar development, abutting domains of the mutually repressive homeobox genes Orthodenticle homolog 2 (Otx2) and Gastrulation brain homeobox 2 (Gbx2) regionalize the mid-hindbrain boundary (MHB) and form the isthmic organizer (IsO). At the IsO, the secreted morphogen Fibroblast growth factor 8 (Fgf8) is necessary and sufficient for differentiation of cerebellar cell types and the initiation of its general morphology, even when ectopically transplanted (reviewed in Zervas et al., 2005). This is likely due to the inductive power of Fgf8 in activating and cooperating with various other genetic players, including engrailed 1 (En1), engrailed 2 (En2), and the paired box genes Pax2 and Pax5 (reviewed by Sillitoe and Joyner, 2007). Upon demarcating the cerebellar territory, genetic cues initiate the commitment of cells within the germinal zones. The mechanism by which the pools of neuronal progenitors give rise to the distinct cell types of the cerebellum and their ordered position in space, however, has proven to be complex. For this reason, we will focus on Purkinje cells and mainly discuss the mouse cerebellum given the wealth of genetic data in this model. The entire Purkinje cell population in the adult is thought to arise from ~100 to 150 precursors and they are likely specified at around E7–E8 (Baader et al., 1996; Mathis et al., 1997; Hawkes et al., 1998; Watson et al., 2005). The mechanisms of Purkinje cells specification are poorly understood, especially from the perspective of how Purkinje cells with different molecular signatures are produced. That is, there is no evidence to suggest that Purkinje cell precursors are restricted to different Purkinje cell sub-lineages. However, it is clear that differentiated Purkinje cells are quickly restricted to distinct subsets that fall into the pattern of stripes and zones (Figure 2A, B; Hawkes and Gravel, 1991; Hawkes and Eisenman, 1997; Herrup and Kuemerle, 1997; Oberdick et al., 1998; Armstrong and Hawkes 2000; Larouche and Hawkes 2006; Sillitoe and Joyner, 2007; White and Sillitoe, 2013). These patterns guide cerebellar development.
Figure 2.
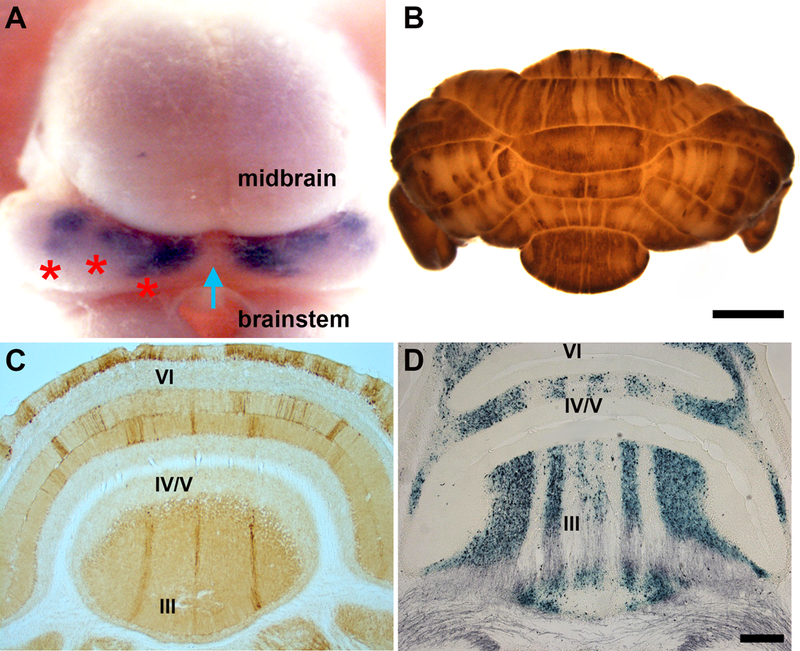
Patterned architecture of the developing and adult mouse cerebellum. A) Dorsal view of a Pcp2-CreER-IRES-hAP embryonic day 16 transgenic mouse showing clusters of Purkinje cells after alkaline phosphatase histochemistry (purple). The blue arrow points to the cerebellar midline and the red asterisks mark the Purkinje cell clusters on one side of the cerebellum. B) Dorsal view of an adult mouse cerebellum wholemount stained for zebrin II. C) Coronal tissue section through the adult mouse cerebellum showing stripes of zebrin II expression in the anterior lobules (indicated by Roman numerals. D) Coronal tissue section through the adult mouse cerebellum showing stripes of spinocerebellar mossy fiber terminal fields after anterograde tracing using WGA-HRP and histochemical processing (see Sillitoe et al., 2010). Abbreviations: ml = molecular layer, gl = granular layer, pcl = Purkinje cell layer. The lobules are labeled with Roman numerals. Scale bar in B = 2mm (applies to A where it = 500μm) and scale bar in D = 500μm. Panel A was reused with permission from Sillitoe et al. (2009; Neuroscience, 162:574–588), panel B from Reeber et al. (2013; Frontiers in Syst Neurosci, 7:83), and panel C from Levy et al. (2015; Neuromethods 92, Wheat Germ Agglutinin (WGA) Tracing: A Classic Approach to Unraveling Neural Circuitry in Neural Tracing Methods Tracing Neurons and Their Connections, Springer Protocols, Ed Benjamin R. Arenkiel, Humana Press).
The cerebellum is patterned into an array of stripes and zones
The cytoarchitecture of the cerebellum is not uniform. Instead, its circuits are organized into a remarkable array of patterns that are best revealed using molecular expression patterns, although afferent terminal field organization, genetic insults, and cell lineage all conform to the same plan. The most well understood pattern is revealed by the expression of zebrin II (Figure 2B, C), an antigen located on the aldolaseC (AldoC) protein (Brochu et al., 1990; Ahn et al., 1994). In the cerebellum, zebrin II is expressed only in Purkinje cells. There are two subsets of Purkinje cells that form the zebrin II map: zebrin II-immunopositive (zebrin II+) and zebrin II-immunonegative (zebrin II-). Purkinje cells in each subset are organized into an alternating pattern of parasagittal stripes (Figures 2B-2D; Brochu et al., 1990; Sillitoe and Hawkes, 2002). Stripes are symmetrically distributed about the midline, highly reproducible between individuals of the same species, and conserved through evolution (Hawkes et al., 1985; Hawkes and Leclerc, 1987; Brochu et al., 1990; Sillitoe et al., 2005; Marzban et al., 2010). The regional differences in stripe patterns further compartmentalize the cerebellum into four transverse domains (Ozol et al., 1999). These divisions are clearly seen in the vermis (Sillitoe and Hawkes 2002): the striped anterior zone (AZ: ~lobules I–V), the uniformly zebrin II+ central zone (CZ: ~lobules VI–VII), the striped posterior zone (PZ: ~lobules VIII–dorsal IX), and the uniformly zebrin II+ nodular zone (NZ: ~lobules IX ventral and X). The unique complement of stripes in the vermis reveals a similar alternation of transverse zones in the paravermis and hemispheres (Figure 2B; Sarna et al., 2006).
There are a growing number of molecular markers that are co-expressed with either the zebrin II+ or zebrin II- subsets of Purkinje cells. For example, the GABA-B receptor is expressed by the zebrin II+ subset (Chung et al., 2008a) whereas phospholipase C(PLC)ß4 is expressed by the zebrin II- subset (Sarna et al., 2006). However, detailed comparisons between zebrin II expression and other antigenic, genetic, and transgenic markers indicate that the parasagittal stripes are much more elaborate than the expression of any one marker might reveal. For example, although zebrin II and the glycoprotein epitope HNK1 are largely co-expressed in the same Purkinje cells (Eisenman and Hawkes 1993), in some areas each marker is expressed in distinct Purkinje cells (Marzban et al., 2004). More globally, the 25 kDa small heat shock protein (HSP)25 is expressed in parasagittal Purkinje cell stripes in the CZ and NZ – transverse zones in which zebrin II is uniformly expressed in all Purkinje cells (Armstrong et al., 2000). Taking all known stripes and sub-stripes together, the prediction is that the adult cerebellar cortex in mouse is subdivided into several hundred distinct units, each typically comprising several hundred Purkinje cells (Hawkes, 1997; Sarna and Hawkes, 2003; Apps and Hawkes, 2009). Afferent topography respects the Purkinje cell striped patterns. Afferent terminal fields are therefore restricted by zone and stripe boundaries. Climbing fibers and mossy fibers both terminate in stripes, and depending on the subset, align with either zebrin II+ or zebrin II- Purkinje cell stripes (Gravel et al., 1987; Gravel and Hawkes, 1990; Reeber et al., 2011; Reeber and Sillitoe, 2011). The anatomical distribution of the afferent termination patterns reflects the functional properties of the cerebellar map (Chockkan and Hawkes, 1994; Hallem et al., 1999; Ebner et al., 2012).
Mutations that affect cerebellar development often result in phenotypes that are restricted by zone and stripe boundaries. Notable examples are found in spontaneous mutants such as swaying (Thomas et al., 1991), rostral cerebellar malformation/Unc5h3 (Napieralski and Eisenman, 1996), cerebellar deficient folia (Cook et al., 1997; Beierbach et al., 2001; Park et al., 2002), and meander tail (Ross et al., 1990), which all cause alterations that are restricted primarily to the AZ. In lurcher (Lc/+), gain of function of the δ2 glutamate receptor causes the developmental restriction of a zebrin II expression domain at the CZ/PZ boundary (Tano et al., 1992), and the weaver mutation induces a Purkinje cell ectopia that is primarily restricted to the CZ (Eisenman et al., 1998; Armstrong and Hawkes, 2001). Strikingly, there are a growing number of disease-related genetic mutations and insults that manifest as stripes, ranging from disease mutations of the Niemann Pick type C gene to viral infection and the spreading of prions (Sarna and Hawkes, 2003; Armstrong et al., 2010; Stromme et al., 2011; Williams et al., 2007; Ragagnin et al., 2017). In all cases, the Purkinje cells that are degenerating in stripes typically conform, as expected, to either the zebrin II+ or the zebrin II- subset. It is therefore not only genetic history that obeys the basic stripe plans, but also cellular sensitivity to toxic stimuli.
The rhombic lip and ventricular zone produce distinct cerebellar cell types
Much effort in recent years has been tailored towards defining the molecular properties within the germinal zones of the cerebellum (Morales and Hatten, 2006; Yamada et al., 2014). There are two cerebellar germinal zones that produce all of its cell types: the rhombic lip and the ventricular zone. The dorsal and extreme posterior aspect of the cerebellum contains the rhombic lip. Genetic fate mapping studies using the Atoh1 locus showed that the rhombic lip gives rise to all glutamatergic neurons including the large projection neurons of the cerebellar nuclei, cerebellar granule cells, and unipolar brush cells (Wingate 2001; Machold and Fishell 2005; Wang et al. 2005; Englund et al. 2006). The other germinal zone, the ventricular zone, is located at the base of the fourth ventricle. The ventricular zone gives rise to all the GABAergic neurons of the cerebellum including the different classes of interneurons, the small inhibitory cerebellar nuclei neurons, and all Purkinje cells. In contrast to the glutamatergic neurons that come from the Atoh1 lineage, the GABAergic neurons derive from progenitors expressing the transcription factor Ptf1a. PTF1A is required for their specification (Hoshino et al., 2005; Pascual et al., 2007). Gene expression is highly patterned in both germinal zones, as evidenced by the multiple molecular domains in the rhombic lip (Machold et al., 2007; Chizhikov et al., 2010; Green et al., 2014; Yeung et al., 2014) and the ventricular zone (Chizhikov et al. 2006; Zordan et al. 2008; Lundell et al., 2009; Seto et al., 2014).
Migratory routes and assembly of a circuit platform
Proper functioning of the cerebellar circuit largely depends upon the correct placement of neurons and glia in different regions, as well as the correct layering of its constituent cell types. Though the histological three-layered topology is seemingly simple, the developmental steps to obtain this organization are complex. The temporal and spatial properties of cells from each germinal zone–Purkinje cells, granule cells, and all their connected interneurons–determine how they navigate from the ventricular zone or rhombic lip to eventually settle into their prospective regions. The availability of new genetic tools has allowed us to uncover how different molecular players instruct the early and late phases of cerebellar cellular placement and circuit formation.
Purkinje cells are one of the first, and from the perspective of circuit formation arguably most important, neuronal class to begin migrating following cellular differentiation. In the adult, the Purkinje cell bodies form a monolayer sandwiched between the granule cell layer and the molecular layer. Correct localization of Purkinje cells is crucial to proper cerebellar function, as Purkinje cells serve as the sole output of the cerebellar cortex: disruption of their location could result in movement diseases (Reeber et al., 2013; Louis et al., 2017). Immediately after their birth between ~E10-E13, Purkinje cells begin to migrate up projections of radial glial processes that extend from the ventricular zone to the pial surface (Morales and Hatten, 2006). Genetic and in vitro studies have recently demonstrated that waves of transiently expressed proteins such as GFRa1 and the interacting extracellular matrix proteins, particularly NCAM (neural cell adhesion molecule), are essential for this movement (Sergaki and Ibanez, 2017). Serving as a resistor to cell movement, NCAM appears to control Purkinje cell migration by slowing the process, as its removal results in enhanced migratory speed (Sergaki and Ibanez, 2017).
Molecular signals dictate the ultimate location of the Purkinje cell and its striking monolayer architecture. In the cerebral cortex, the large 388kDa extracellular secreted glycoprotein, Reelin, is thought to function as a migratory control signal that instructs cortical layering (D’Arcangelo and Curran, 1998; Hack et al., 2002). However, its roles during brain development have expanded greatly to include synaptic function (Wasser and Herz, 2017). In the cerebellum, granule cell precursors express Reelin whereas Purkinje cells express its binding partners, including ApoER2, VLDLR, and Dab1 (Leto et al., 2015). Recent studies of Reelin c-terminal knockout mice indeed confirmed that interruption of Reelin signaling causes ectopic localization of Purkinje cell pools in deep layers of the cerebellar anlage. However, not all adult Purkinje cells were absent from their nascent Purkinje cell plate, indicating that different pools of Purkinje cells may be variably influenced by Reelin signaling (Nakamura et al., 2016). These data are consistent with previous findings that showed a role for VLDLR and ApoER2 receptors during the dispersal of distinct, but overlapping subsets of Purkinje cells (Larouche et al., 2008). In that study, the authors demonstrated convincingly that the subset dispersal defects impact Purkinje cells with specific stripe phenotypes. The idea of a patterned scaffold raises the possibility that, as other cell types are produced, they too might be under a similar compartmental influence.
Most differentiated cerebellar interneurons, including granule cells, unipolar brush cells, basket cells, and stellate cells, undergo most of their migratory journey after P0 (Leto et al., 2015). Although Golgi cells differentiate embryonically, they too must leave the white matter and organize in parallel with the massive influx of granule cells that invade the core of the postnatal cerebellum (Zhang and Goldman, 1996a; Zhang and Goldman, 1996b). For GABAergic interneurons, each sub-class is produced from a common pool of precursors (Leto et al., 2006). Heterochronic transplant and genetic fate mapping approaches have shown that the different classes then differentiate in an inside-out fashion, with the earliest born Golgi cells eventually residing in the internal granular layer and the latest born stellate cells taking their position in the outer two-thirds of the molecular layer (Leto et al., 2009; Sudarov et al., 2011). A recent elegant paper has further shown that the migration of inhibitory neurons is finely controlled by synaptic activity in the developing cerebellum. Using time-lapse video microscopy and electrophysiology, the authors demonstrate that classic synaptic neurotransmission influences the directionality of interneuron migration (Wefers et al., 2017). Of the eight distinct types of neurons that emerge from embryonic through postnatal development, granule cells are among the last to differentiate and settle into their correct cerebellar location, but their precursors are among the first to leave their original primary germinal zone. Unlike UBCs, which migrate directly from the rhombic lip to the inner granule layer through the white matter, granule cell precursors first migrate tangentially towards the roof of the developing cerebellar anlage and form a transient germinal layer known as the external granular layer (EGL) (Leto et al., 2015). There have been many excellent studies on how granule cells (and unipolar brush cells) are produced, divide, and differentiate (Butts et al., 2014), so we will not cover the details here, but suffice it to say that they require critical factors such as Atoh1 (Ben-Arie et al., 1997) and Lmx1a (Chizhikov et al., 2010). More pertinent to this discussion is the finding of a beautiful genetic clonal analysis study that uncovered a modular mode of development for granule cell development (Legue et al., 2015). Interestingly, mosaic analysis with double markers (MADM) showed that during postnatal development, granule cell parallel fibers stack in an inside to outside temporal manner in the molecular layer. This is in contrast to the cell bodies of the clonally related granule cells that are randomly positioned in the internal granular layer (Espinosa and Luo, 2008; Zong et al., 2005). A major player in the proper migration of granule cells to the internal granular layer is Bergmann glia, a class of unipolar astrocytes derived from radial glia (Anthony et al., 2004; Morales and Hatten, 2006). The Bergmann glia themselves migrate dorsally from the ventricular zone and intercalate between Purkinje cell bodies before extending long processes to the pial surface. They serve as a scaffold for radial migration of differentiated granule cells from the external to internal granular layer (Xu et al., 2013). There are several molecules that coordinate the process between both cell types, such as PTEN in the Bergmann glia (Yue et al., 2005) and fibroblast growth factor 9 (FGF9), which is secreted from the granule cells (Lin et al., 2009).
Generating the patterned architecture of the developing cerebellum
Purkinje cells have several levels of heterogeneity (Cerminara et al., 2015). Their molecular heterogeneity is perhaps the best understood and zebrinII expression is indeed a primary example. However, in any discussion of stripes, the general question arises: what do stripes do? It is appealing to postulate that the stripe and zone architecture of the cerebellum controls or determines all aspects of its development and function. Purkinje cell patterns are highly reproducible between individuals and conserved through evolution (Sillitoe et al., 2005; Pakan et al., 2007; Marzban et al., 2010; Marzban and Hawkes, 2011; Wylie et al., 2016). Cerebellar afferents also terminate into a striped topography that respects the Purkinje cell map (Voogd and Ruigrok, 2004; Sillitoe and Joyner, 2007; Apps and Hawkes, 2009). In the adult brain, it is the combination of the Purkinje cell stripes and afferent topography and their connectivity that has inspired the search for functional correlates of the cerebellar maps (Chockkan and Hawkes, 1994; Hallem et al., 1999; Ebner et al., 2012). To fully appreciate cerebellar function, we must also ask key questions about its formation. Therefore, a more specific question can be asked about stripes: how do stripe patterns develop in the embryonic and postnatal cerebellum?
Similar to other developmental processes, it was assumed that manipulating and altering the patterns would provide insight as to how they are constructed. True as this may be, many strategies failed to alter the stripe phenotype. This quickly raised the question, when is the Purkinje cell stripe phenotype specified in the first place? The data showed that surgical interventions in the neonate have no effect on the expression of different stripe markers and transgenes (zebrin I – Leclerc et al., 1988; L7/pcp2-LacZ – Oberdick et al., 1993; HSP25 – Armstrong et al., 2001). Interestingly, Purkinje cell subtypes develop normally in cultured explants of cerebellar tissue taken from E13 brain (Oberdick et al., 1993; Seil et al., 1995; Rivkin and Herrup, 2003; Furutama et al., 2010) or when embryonic tissue is transplanted (Wassef et al., 1990). Moreover, even though Purkinje cells reside in ectopic locations in various mouse mutants, at the molecular level they still express their correct adult phenotypes (e.g., reeler – Edwards et al., 1994; disabled – Gallagher et al., 1998; weaver – Armstrong and Hawkes, 2001). These data suggest that specification of Purkinje cell phenotypes towards distinct zebrin subtypes is controlled by cell autonomous mechanisms that are initiated early in cerebellar development. This hypothesis is supported by viral and genetic lineage tracing studies that link Purkinje cell birth date to mediolateral patterning (Hashimoto and Mikoshiba, 2003; Sudarov et al., 2011).
There is one experimental manipulation that does alter Purkinje cell subtypes. Genetic deletion of the transcription factor coding gene Early B-cell Factor 2 revealed the long-awaited defect (Ebf2: Croci et al., 2006; Chung et al., 2008b). Ebf2 expression is restricted to zebrin II negative Purkinje cells. When Ebf2 is lost, several cerebellar developmental processes are affected, although the most intriguing is that a subset of Purkinje cells that do not express zebrin II are erroneously triggered to express zebrin II lineage markers plus those that define the zebrin II negative population (Croci et al., 2006; Chung et al., 2008b). Ebf2 is therefore an important gene to further understand in future studies since it is suggested that its encoded protein, EBF2, suppresses the zebrin II phenotype.
The structure of the Purkinje cell stripes, with sharp boundaries and clear distinctions from their neighbors, forms from a developmental plan of “clusters” that are shaped over time (Figure 2A; White and Sillitoe, 2013). However, gene expression and lineage tracing suggest a direct relationship between embryonic clusters and adult stripes. This has not been straightforward to test because the pattern of developmental markers is dynamic with patterns either changing or disappearing around birth, perhaps because they are downregulated (neurogranin – Larouche et al., 2006) or because expression expands to include all Purkinje cells (calbindin – Wassef et al., 1985). This is in contrast to most adult stripe markers that are not expressed in the mature pattern of stripes until ~P15 (zebrin II – Lannoo et al., 1991; HSP25 – Armstrong et al., 2001). While the evidence suggests that the basic cerebellar stripe plan is laid down in the embryo, the maturation of stripe phenotypes does not seem to be completed until about P15. Zebrin II is a prime example: though it is first expressed around P6, it is not evident in all Purkinje cells until P10-P12. In the days following, the stripes actually emerge. Between P12 and P15, zebrin II is downregulated in specific Purkinje cells, resulting in the mature stripe pattern (Brochu et al., 1990; Lannoo et al., 1991; Rivkin and Herrup, 2003). The genetic and molecular mechanisms that mediate zebrin II regulation to form stripes remain unknown. However, based on marker analysis, we do know that clusters are intimately related to stripes (Larouche et al., 2006; Marzban et al., 2007; Sillitoe et al., 2009; White and Sillitoe, 2013, Vibulyasek et al., 2017). The current working hypothesis is that adult stripes are derived from structural and molecular correlates that are the embryonic clusters. Although it is tempting to imagine a direct relationship, experimental evidence indicates that the process is more complex. It has been calculated that the embryonic markers together form a schema with ~10 clusters on each side of the cerebellar midline. These clusters are then likely subjected to additional patterning, taking the crude embryonic clusters and reshaping/sculpting them into sharper adult stripes. The 20 clusters may in fact be partitioned into 50 during postnatal development, a step that perhaps transitions the very rudimentary embryonic map into the more precise adult stripe topography (Fujita et al., 2012). Furthermore, recent evidence suggests that specific pools of embryonic Purkinje cells migrate differentially to form stripes in distinct areas of the cerebellum, which ultimately leads to their specific positioning in different lobules (Vibulyasek et al., 2017). Although it may not be a simple one to one relationship between clusters and stripes, there is anatomical and expression data to support the idea that multiple clusters coalescence to form stripes (Ji and Hawkes, 1994; Marzban et al., 2007). These studies are supported by genetic fate mapping that used an L7/pcp2-CreER-IRES-hAP inducible transgenic allele to show that at least some of the embryonic clusters contribute Purkinje cells to multiple stripes in the adult cerebellum (see Figure 2A; Sillitoe et al., 2009). The decisions that determine how the Purkinje cells clusters are segmented and how their cellular dynamics are orchestrated to contribute to each stripe are not known; however, we assume that the process is under tight genetic control.
Afferent topography
The Purkinje cell patterns must somehow integrate with the afferent fiber system in order to have a functional influence. It is therefore postulated that the Purkinje cell map serves as a scaffold around which climbing fiber and mossy fiber afferent projections (reviewed in Sotelo, 2004; White and Sillitoe, 2013) as well as interneurons including granule cells, Golgi cells, and unipolar brush cells (e.g., Chung et al., 2009; reviewed in Apps and Hawkes, 2009) organize.
Climbing fibers
Climbing fibers originate from a brainstem nucleus called the inferior olive. The inferior olive projects climbing fibers to the contralateral cerebellum where it terminates on the dendrites of Purkinje cells. Each inferior olive subnucleus projects to Purkinje cells located in a limited number of stripes (Voogd and Ruigrok, 2004; Sugihara and Quy, 2007; Apps and Hawkes, 2009). The inferior olive neurons that communicate with the cerebellum are generated in the caudal rhombic lip. After they leave this germinal zone, they migrate ventrally in the submarginal stream (Sotelo and Chédotal, 2005) and enter the embryonic cerebellum at ~E15 in mice. It is interesting that the inferior olive neurons, like their target Purkinje cells, require Ptf1a for determining their fate, survival, differentiation, and migration (Yamada et al., 2007). There is a long-standing hypothesis that climbing fibers immediately recognize the patterned architecture of Purkinje cell clusters upon their arrival in the cerebellar cortex (Chédotal and Sotelo, 1992; Paradies et al., 1996). Since Purkinje cell clusters are thought to disperse into stripes, it would be reasonable to hypothesize that climbing fibers reorganize to form parasagittal terminal fields that precisely innervate Purkinje cell stripes (Gravel et al., 1987; Apps and Hawkes, 2009). In the neonatal and early postnatal cerebellum, each Purkinje cell is innervated by several climbing fibers. A pruning process eliminates all but one to achieve the adult mono-innervation pattern (Cesa and Strata, 2009; Carrillo et al., 2013). However, the mechanism of climbing fiber poly- to mono-innervation may not shape mature stripes (Crépel, 1982; Sotelo et al., 1984).
The prevailing hypothesis put forward by Sotelo and colleagues argues that the inferior olive to Purkinje cell connectivity is established by matching gene expression domains that contain cues for guiding the afferents to specific stripes (Wassef et al., 1992; Chédotal et al., 1997; Sotelo and Chédotal, 2005). There is some compelling experimental evidence to support such a model. Nishida et al. (2002) used retroviral vectors to alter olivocerebellar topography by overexpressing Ephrin-A2. Importantly, they showed that high Eph receptor activity in inferior olivary axons mediated their exclusion from entering into domains that expressed ectopic Ephrin-A2 ligand (Nishida et al., 2002). These data strongly support a role for the Eph/Ephrin signaling pathway during afferent to target matching and indicate that the process is dependent on positional information. However, it is still unclear if these or related molecular signals influence connectivity that directly shapes the parasagittal zone topography per se.
Mossy fibers
Mossy fibers provide a major source of afferents to the cerebellum. They originate from a diverse number of areas in the brain and spinal cord and terminate in a unique structure called a glomerulus, which contains their target granule cell dendrites. Mossy fibers also terminate into a series of parasagittal domains (Figure 2D; Gravel and Hawkes, 1990; Ji and Hawkes, 1994; Armstrong et al., 2009; Sillitoe et al., 2010; Ruigrok, 2011; Gebre et al., 2012). However, unlike climbing fibers that have very sharp stripe boundaries, the mossy fiber patterns are somewhat less defined, which is perhaps related to the architecture and function of the granular layer (Shambes et al., 1978). Regardless, mossy fiber terminal fields align with Purkinje cell stripes (Armstrong et al., 2009), although in some cases they split Purkinje cell stripes into smaller domains (cuneocerebellar/spinocerebellar terminal fields in the anterior lobules: Ji and Hawkes, 1994; Reeber et al., 2011).
Mossy fibers follow a similar developmental trajectory to climbing fibers. The earliest mossy fibers enter the cerebellar anlage by around E12 in rat (Ashwell and Zhang, 1992; Ashwell and Zhang, 1998). What is interesting and different compared to climbing fibers is that the mossy fibers start to form parasagittal patterns before most of their granule cell targets cells are born (Nunes and Sotelo, 1985). Even more peculiar is that as the mossy fiber projection map is forming, they make direct but transient contacts with clusters of Purkinje cells in the embryonic and early postnatal cerebellum (Mason and Gregory, 1984; Takeda and Maekawa, 1989; Grishkat and Eisenman, 1995; Kalinovsky et al., 2011; Sillitoe, 2016). Similar to climbing fibers, mossy fibers are presumed to disperse along with the Purkinje cells as the developmental clusters transform into adult stripes. However, a switch in targets must occur such that as granule cells are born and migrate past the Purkinje cells en route to the internal granular layer, the mossy fiber terminals would be displaced from the Purkinje cells to make synapses with differentiated granule cells below. In the end, the domains of mossy fiber terminal fields remain aligned with the Purkinje cell stripes in the layer above. These data are consistent with observations from spontaneous mutant mice in which the cerebellum is described as being “agranular” and lacking a mossy fiber-granule cell-Purkinje cell pathway (Nunes and Sotelo, 1985; Nunes et al., 1988; Eisenman and Arlinghaus, 1991). The functional diversity of mossy fibers and their sequential entry into the cerebellum raised the question of whether the different mossy fibers might compete for territory as they form a map. Richard Hawkes and colleagues performed lesion studies in neonatal rats and noted a lack of terminal sprouting into regions that had lost their mossy fiber input (Ji and Hawkes, 1995). They interpreted these data as indicating that the molecular properties within the map, and not competition, sculpt mossy fiber to Purkinje cell topography. The molecular basis of such connectivity has not been fully resolved, although loss of retinoic acid receptor-related orphan receptor alpha (RORalpha: Nunes et al., 1988) and En1/2 (Sillitoe et al., 2010), and overexpression of En2 in Purkinje cells (Baader et al., 1999) all result in mossy fiber patterning defects. Although these studies provide powerful evidence that the process of establishing afferent patterns is genetically controlled (Sillitoe et al., 2010), there is also evidence that the refinement of the map may be in part dependent on neuronal activity (Tolbert et al., 1994; White et al., 2014).
Interneurons
Although the different classes of cerebellar interneurons show evidence of parasagittal stripe organization, it has been a challenge to fully decipher their patterning mechanisms. This is partly due to the lack of genetic tools with which to selectively manipulate each class independently. However, in the case of granule cells, their distinct mode of development from the rhombic lip and external granular layer has provided key clues. The external granular layer in the developing cerebellum and the adult internal granular layer are subdivided by transverse boundaries. These boundaries were revealed using lineage tracing, gene expression, and mice with genetic mutations (Hawkes et al. 1999; Ozol and Hawkes 1997; Yaguchi et al. 2009). Although these tools revealed only a portion of the complexity, we learned that that the cerebellar granular layer is derived from at least two precursor pools that generate distinct lineages on either side of a boundary that partitions the cerebellum (Hawkes et al., 1999). Interestingly, previous chimera studies in mouse found similar divisions in the granule cell lineage (Goldowitz, 1989). These studies were supported by mutant phenotypes such scrambler (Goldowitz et al., 1997) and disabled (Gallagher et al. 1998), which have an incomplete fusion of the anterior and posterior granular layers in lobule VI. This defect leaves distinct but overlapping anterior and posterior extensions. More recently, Atoh1CreER genetic inducible fate mapping showed that granule cell progenitors are destined to populate specific anterior–posterior zones (Machold and Fishell, 2005). One marking scheme that was used labeled lineages at E12.5 and followed them into the adult AZ whereas those marked at E15.5 settled in the NZ. Together, these data suggest that the allocation of cells to specific granule cell compartments may be dependent on spatial and temporal regulation of gene expression and perhaps also specific cellular movements.
But, the process of generating stripes may not be dependent on a simple process of differential migration of external granular layer lineages. As for Purkinje cells, genetics may play a primary role. For example, neuronal nitric oxide synthase (nNOS – or its surrogate marker nicotinamide adenine dinucleotide phosphate (NADPH)-diaphorase) is expressed in patches in the adult granular layer. The patches are generated during postnatal development (Yan et al., 1993; Schilling et al., 1994) and align with Purkinje cell stripes (Hawkes and Turner, 1994). It was postulated that differential nNOS expression during granule cell patch formation was under the control of either Purkinje cells or their mossy fiber inputs (Schilling et al., 1994). The mode of migration of granule cells from outer to inner layers raised queries as to whether at least some aspects of granule cell architecture were in fact determined by a more physical mechanism. Indeed the migration of granule cells within parasagittal “raphes” could provide a key framework to support the segregation of granule cells into topographic domains as they settle into a mature circuit (Lin and Cepko, 1998; Karam et al., 2001).
Unipolar brush cells, the other rhombic lip derived excitatory cell type, also show a restriction to parasagittal stripes. Differential molecular expression distinguishes them into at least three subtypes, calretinin+, mGluR1α+ and PLCβ4+, and mGluR1α– and PLCβ4+, which all respect the parasagittal Purkinje cell stripes (Apps and Hawkes, 2009). Again, unipolar brush cell patterning is not simply correlated to Purkinje cells, but perhaps mechanistically linked. Unipolar brush cells lose their restriction to lobules IX and X when Purkinje cell patterning is disrupted in Ebf2 and Reelin mutant mice (Croci et al., 2006; Chung et al., 2008b; Lee et al., 2015).
The compartmentalization of inhibitory interneurons has remained largely unexplored. However, we know that Golgi cell dendrites are restricted by Purkinje cell stripe boundaries (Sillitoe et al. 2008a). Importantly, both the Golgi cells and unipolar brush cells are thought to physically intermingle with Purkinje cells during the cluster stage. Like Golgi cells, basket and stellate cell soma exhibit a morphological restriction to stripes (Sillitoe et al., 2008a), but for basket cells and less so for stellate cells, their axons also seem to extend in the parasagittal plane (Eccles et al., 1967). To achieve this restriction, it is possible that the parasagittal projection of basket and stellate cell axons could also follow the spreading of Purkinje cell clusters into stripes.
From stripes and spikes to complex motor behaviors
Most theories of cerebellar function account only for the relatively well-known connectivity based on its few cell types. This is clearly not how the cerebellum works. We argue that these theories should take into account the remarkable stripe patterns and the circuitries located within. Certainly, there has been a growing list of excellent functional studies that paid close attention to stripes. These studies have considered cerebellar functional compartmentalization, information processing, and the control of behaviors (Horn et al., 2010; Graham and Wylie, 2012; Attwell et al., 1999; Mostofi et al., 2010). This is interesting since motor deficits arise in disease models where stripe formation is delayed or stripe boundaries are not fully refined (White et al., 2016; White et al., 2014; Welsh and Oristaglio, 2016; Sarna and Hawkes, 2003; Strømme et al., 2011).
The mechanistic basis of any functional and behavioral differences as they relate to stripes could be that Purkinje cells in distinct stripes have intrinsically different molecular signatures and synaptic properties (Wadiche and Jahr, 2005; Kano et al., 2008; Mateos et al., 2001; Wang et al., 2011; Nagao et al., 1997; Dehnes et al., 1998; Furuta et al., 1997; Paukert et al., 2010). For example, zebrin II positive Purkinje cell parallel fiber synapses are enriched for mGluR1 and their cell bodies, dendrites, and spines express neuronal glutamate transporter (EAAT4). Importantly, Purkinje cell stripes may be associated with different levels of vesicular glutamate transporter (VGLUT2) at climbing fibers terminals. These differences in protein localization seem to mediate domains of increased Ca2+ release, which affect parallel fiber–Purkinje cell and climbing fiber–Purkinje cell synaptic function in a stripe-dependent manner. These molecular differences then control synaptic plasticity according to the stripe identity (Wadiche and Jahr, 2005; Wang et al., 2011; Paukert et al., 2010). It is intriguing that a number of stripe marker proteins are required for the expression of long-term depression (LTD) at parallel fiber – Purkinje cells synapses (Hawkes, 2014).
The potential for stripe-related plasticity is supported by recent work that demonstrated fundamental differences in the baseline firing of Purkinje cells. Single-unit extracellular recordings were used to show that zebrin II positive Purkinje cells fire at a lower rate and with a more regular firing pattern compared to zebrin II negative Purkinje cells (mouse - Zhou et al., 2014; rat - Xiao et al., 2014). Although the mechanism by which Purkinje cells acquire distinct firing properties is unknown, it may involve the function of the transient receptor cation channels (TRPC3; Zhou et al., 2014). It will be interesting to test when the Purkinje cells become segregated based on activity and whether the clusters have a developmental correlate. Moreover, it will important to test whether disease-related developmental defects alter stripe activity.
The anatomical, molecular, and genetic mechanisms that control stripe formation are becoming well established as we build on core findings discussed above. However, we are only now beginning to appreciate how activity within and between subsets of Purkinje cells might shape clusters and stripes. There are several possible mechanisms for how activity might impact stripes. Before considering these mechanisms, it is useful to first understand the basic properties of Purkinje cell activity. Purkinje cells fire two types of action potentials: simple spikes, which are generated intrinsically and modulated by mossy fiber-granule cells inputs, and complex spikes, which are triggered by synaptic input from climbing fibers (Cerminara and Rawson, 2004). Both types of action potentials are evident in neonatal and early postnatal rodents (Mariani and Changeux, 1981; Arancillo et al., 2015; Sokoloff et al., 2015). The loss of, or the disruption of, proper Purkinje cell firing may affect the development of proper stripe patterning by eliminating traveling waves in the developing cerebellum (Watt et al., 2009). Because of the Purkinje cell collateral system, direct Purkinje cell-to-Purkinje cell communication could influence stripe formation through inhibitory GABA function, or potentially even through an early excitatory GABA connection (although its is still not clear if Purkinje cells exhibit an excitatory to inhibitory GABA switch; Sotelo, 2008). This is not the only temporal change that occurs in Purkinje cell activity. Recent work in anesthetized and awake mice used in vivo electrophysiology to record Purkinje cells in postnatal mice (Arancillo et al., 2015). The study found that complex spike firing rate increased sharply at three weeks of age, whereas simple spike firing rate increased gradually until four weeks of age. They also found that compared to adult Purkinje cells, the pattern of simple spike firing during development was more irregular as periods of steady firing are interrupted by long pauses. The regularity in simple spike firing eventually reached maturity at four weeks of age. These data show that Purkinje cell activity is dynamically sculpted throughout postnatal development, traversing several critical events that are required for cerebellar morphogenesis and circuit formation. Importantly, the establishment of Purkinje cell firing properties seems to overlap with the final stages of stripe maturation (Armstrong and Hawkes, 2000). In accordance with this, conditional genetic silencing of GABAergic neurotransmission only in Purkinje cells alters the sharpness of Purkinje cell stripe boundaries and the segregation of mossy fibers into topographic domains (White et al., 2014).
One of the major challenges now facing us is to link the formation of stripe patterns to the execution of behaviors that recruit circuits within the highly topographic map. Along the same lines though, we also need to fully establish whether stripes controls motor behavior (Horn et al., 2010; Cerminara and Apps, 2011). In this regard, it is not clear whether each stripe controls a specific behavior, or whether stripes communicate and cooperate to execute a particular task. These models must take into account that synchronous activity might be important for cerebellar function. Synchrony within stripes is dependent on olivocerebellar connectivity (Welsh, 2002; Schultz et al., 2009). However, even a stripe synchrony model must deal with the complication that parallel fibers extend long distances and can cross several stripes to interact with Purkinje cells (Valera et al., 2016; Levy et al., 2017). One can take the alternate view that the parallel fibers actually allow for communication across synchrony stripes and perhaps the Purkinje cell collaterals could even play a similar role in a more cell autonomous manner (Tsutsumi et al 2015; Witter et al. 2016). The impact of Purkinje cell collaterals could extend to the rest of the circuit with contacts to granule cells and inhibitory interneurons. Such local microcircuit connectivity could provide the means to finely regulate neural activity and enhance signal processing (Watt et al., 2009; Witter et al., 2016; Guo et al., 2016). Ultimately, synchronizing the cellular behavior of Purkinje cells within and between specific stripes might provide flexibility for the cerebellar nuclei to drive motor and non-motor behaviors (Welsh et al., 1995; Yamamoto et al., 2001; De Zeeuw et al., 2011; Gauck and Jaeger, 2000; Person and Raman, 2012). Resolving these issues will likely require further analysis of how stripes and circuits form.
Acknowledgements
This work was supported by funds from Baylor College of Medicine (BCM) and Texas Children’s Hospital, BCM IDDRC Grant U54HD083092 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (The IDDRC Neuropathology Sub-Core contributed to the tissue staining experiments), and by National Institutes of Neurological Disorders and Stroke (NINDS) R01NS089664 and R01NS100874 to RVS.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, … Verhoeven J (2017). Consensus Paper: Cerebellum and Emotion. Cerebellum. https://doi.org/10.1007/s12311-016-0815-8 [DOI] [PubMed] [Google Scholar]
- 2.Ahn AH, Dziennis S, Hawkes R, & Herrup K (1994). The cloning of zebrin II reveals its identity with aldolase C. Development (Cambridge, England), 120(8), 2081–90. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7925012 [DOI] [PubMed] [Google Scholar]
- 3.Anthony TE, Klein C, Fishell G, & Heintz N (2004). Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron, 41(6), 881–90. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15046721 [DOI] [PubMed] [Google Scholar]
- 4.Apps R, & Hawkes R (2009). Cerebellar cortical organization: a one-map hypothesis. Nature Reviews Neuroscience, 10(9), 670–681. https://doi.org/10.1038/nrn2698 [DOI] [PubMed] [Google Scholar]
- 5.Arancillo M, White JJ, Lin T, Stay TL, & Sillitoe RV (2015). In vivo analysis of Purkinje cell firing properties during postnatal mouse development. Journal of Neurophysiology, 113(2), 578–591. https://doi.org/10.1152/jn.00586.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong C, & Hawkes R (2001). Selective Purkinje cell ectopia in the cerebellum of the weaver mouse. The Journal of Comparative Neurology, 439(2), 151–61. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11596045 [DOI] [PubMed] [Google Scholar]
- 7.Armstrong CL, Chung S-H, Armstrong JN, Hochgeschwender U, Jeong Y-G, & Hawkes R (2009). A novel somatostatin-immunoreactive mossy fiber pathway asssociated with HSP25-immunoreactive purkinje cell stripes in the mouse cerebellum. The Journal of Comparative Neurology, 517(4), 524–538. https://doi.org/10.1002/cne.22167 [DOI] [PubMed] [Google Scholar]
- 8.Armstrong CL, Duffin CA, McFarland R, & Vogel MW (2011). Mechanisms of Compartmental Purkinje Cell Death and Survival in the Lurcher Mutant Mouse. The Cerebellum, 10(3), 504–514. https://doi.org/10.1007/s12311-010-0231-4 [DOI] [PubMed] [Google Scholar]
- 9.Armstrong RA, Ellis W, Hamilton RL, MacKenzie IRA, Hedreen J, Gearing M, … Cairns NJ (2010). Neuropathological heterogeneity in frontotemporal lobar degeneration with TDP-43 proteinopathy: A quantitative study of 94 cases using principal components analysis. Journal of Neural Transmission, 117(2), 227–239. https://doi.org/10.1007/s00702-009-0350-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong CL, & Hawkes R (2000). Pattern formation in the cerebellar cortex. Biochem Cell Biol, 78(5), 551–562. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11103945 [PubMed] [Google Scholar]
- 11.Armstrong CL, Krueger-Naug AM, Currie RW, & Hawkes R (2000). Constitutive expression of the 25-kDa heat shock protein Hsp25 reveals novel parasagittal bands of purkinje cells in the adult mouse cerebellar cortex. The Journal of Comparative Neurology, 416(3), 383–97. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10602096 [DOI] [PubMed] [Google Scholar]
- 12.Armstrong CL, Krueger-Naug AM, Currie RW, & Hawkes R (2001). Expression of heat-shock protein Hsp25 in mouse Purkinje cells during development reveals novel features of cerebellar compartmentation. The Journal of Comparative Neurology, 429(1), 7–21. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11086286 [DOI] [PubMed] [Google Scholar]
- 13.Ashwell KW, & Zhang LI (1998). Prenatal development of the vestibular ganglion and vestibulocerebellar fibres in the rat. Anatomy and Embryology, 198(2), 149–61. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9725774 [DOI] [PubMed] [Google Scholar]
- 14.Ashwell KW, & Zhang LL (1992). Ontogeny of afferents to the fetal rat cerebellum. Acta Anatomica, 145(1), 17–23. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1414208 [DOI] [PubMed] [Google Scholar]
- 15.Attwell PJ, Rahman S, Ivarsson M, & Yeo CH (1999). Cerebellar cortical AMPA-kainate receptor blockade prevents performance of classically conditioned nictitating membrane responses. J Neurosci, 19(24), RC45 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10594089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baader SL, Schilling ML, Rosengarten B, Pretsch W, Teutsch HF, Oberdick J, & Schilling K (1996). Purkinje Cell Lineage and the Topographic Organization of the Cerebellar Cortex: A View from X Inactivation Mosaics. Developmental Biology, 174(2), 393–406. https://doi.org/10.1006/dbio.1996.0083 [DOI] [PubMed] [Google Scholar]
- 17.Baader SL, Vogel MW, Sanlioglu S, Zhang X, & Oberdick J (1999). Selective disruption of “late onset” sagittal banding patterns by ectopic expression of engrailed-2 in cerebellar Purkinje cells. J.Neurosci. Retrieved from http://www.jneurosci.org.ezproxyhost.library.tmc.edu/content /jneuro/19/13/5370.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, … Sokolov AA (2015). Consensus Paper: The Role of the Cerebellum in Perceptual Processes. Cerebellum, 14(2), 197–220. https://doi.org/10.1007/s12311-014-0627-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beierbach E, Park C, Ackerman SL, Goldowitz D, & Hawkes R (2001). Abnormal dispersion of a purkinje cell subset in the mouse mutant cerebellar deficient folia (cdf). The Journal of Comparative Neurology, 436(1), 42–51. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11413545 [PubMed] [Google Scholar]
- 20.Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, … Zoghbi HY. (1997). Math1 is essential for genesis of cerebellar granule neurons. Nature, 390(6656), 169–172. https://doi.org/10.1038/36579 [DOI] [PubMed] [Google Scholar]
- 21.Brochu G, Maler L, & Hawkes R (1990). Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. The Journal of Comparative Neurology, 291(4), 538–52. https://doi.org/10.1002/cne.902910405 [DOI] [PubMed] [Google Scholar]
- 22.Butts T, Green MJ, & Wingate RJT (2014). Development of the cerebellum: simple steps to make a “little brain.” Development, 141(21), 4031–4041. https://doi.org/10.1242/dev.106559 [DOI] [PubMed] [Google Scholar]
- 23.Carrillo J, Cheng S-Y, Ko KW, Jones TA, & Nishiyama H (2013). The Long-term Structural Plasticity of Cerebellar Parallel Fiber Axons and Its Modulation by Motor Learning. Journal of Neuroscience, 33(19), 8301–8307. https://doi.org/10.1523/JNEUROSCI.3792-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerminara NL, & Apps R (2011). Behavioural Significance of Cerebellar Modules. The Cerebellum, 10(3), 484–494. https://doi.org/10.1007/s12311-010-0209-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerminara NL, Lang EJ, Sillitoe RV, & Apps R (2015). Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nature Reviews. Neuroscience, 16(2), 79–93. https://doi.org/10.1038/nrn3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerminara NL, & Rawson JA (2004). Evidence that climbing fibers control an intrinsic spike generator in cerebellar Purkinje cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(19), 4510–7. https://doi.org/10.1523/JNEUROSCI.4530-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cesa R, & Strata P (2009). Axonal competition in the synaptic wiring of the cerebellar cortex during development and in the mature cerebellum. Neuroscience, 162(3), 624–632. https://doi.org/10.1016/j.neuroscience.2009.02.061 [DOI] [PubMed] [Google Scholar]
- 28.Chédotal A, Bloch-Gallego E, & Sotelo C (1997). The embryonic cerebellum contains topographic cues that guide developing inferior olivary axons. Development (Cambridge, England), 124(4), 861–70. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9043067 [DOI] [PubMed] [Google Scholar]
- 29.Chedotal A, & Sotelo C (1992). Early Development of Olivocerebellar Projections in the Fetal Rat Using CGRP Immunocytochemistry. The European Journal of Neuroscience, 4(11), 1159–1179. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12106421 [DOI] [PubMed] [Google Scholar]
- 30.Chizhikov VV, Lindgren AG, Currle DS, Rose MF, Monuki ES, & Millen KJ (2006). The roof plate regulates cerebellar cell-type specification and proliferation. Development, 133(15), 2793–2804. https://doi.org/10.1242/dev.02441 [DOI] [PubMed] [Google Scholar]
- 31.Chizhikov VV, Lindgren AG, Mishima Y, Roberts RW, Aldinger KA, Miesegaes GR, … Millen KJ (2010). Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. Proc Natl Acad Sci U S A, 107(23). https://doi.org/10.1073/pnas.0910786107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chockkan V, & Hawkes R (1994). Functional and antigenic maps in the rat cerebellum: zebrin compartmentation and vibrissal receptive fields in lobule IXa. The Journal of Comparative Neurology, 345(1), 33–45. https://doi.org/10.1002/cne.903450103 [DOI] [PubMed] [Google Scholar]
- 33.Chung S-H, Kim C-T, & Hawkes R (2008a). Compartmentation of GABA-B receptor2 expression in the mouse cerebellar cortex. The Cerebellum, 7(3), 295–303. https://doi.org/10.1007/s12311-008-0030-3 [DOI] [PubMed] [Google Scholar]
- 34.Chung S-H, Marzban H, Watanabe M, & Hawkes R (2009). Phospholipase Cβ4 Expression Identifies a Novel Subset of Unipolar Brush Cells in the Adult Mouse Cerebellum. The Cerebellum, 8(3), 267–276. https://doi.org/10.1007/s12311-009-0092-x [DOI] [PubMed] [Google Scholar]
- 35.Chung S-HS-H, Marzban H, Croci L, Consalez GGG, & Hawkes R (2008b). Purkinje cell subtype specification in the cerebellar cortex: early B-cell factor 2 acts to repress the zebrin II-positive Purkinje cell phenotype. Neuroscience, 153(3). https://doi.org/10.1016/j.neuroscience.2008.01.090 [DOI] [PubMed] [Google Scholar]
- 36.Cook SA, Bronson RT, Donahue LR, Ben-Arie N, & Davisson MT (1997). Cerebellar deficient folia (cdf): a new mutation on mouse chromosome 6. Mammalian Genome: Official Journal of the International Mammalian Genome Society, 8(2), 108–12. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9060409 [DOI] [PubMed] [Google Scholar]
- 37.Crepel F (1982). Regression of functional synapses in the immature mammalian cerebellum. Trends in Neurosciences, 5, 266–269. https://doi.org/10.1016/0166-2236(82)90168-0 [Google Scholar]
- 38.Croci L, Chung S-H, Masserdotti G, Gianola S, Bizzoca A, Gennarini G, … Consalez GG (2006). A key role for the HLH transcription factor EBF2COE2,O/E-3 in Purkinje neuron migration and cerebellar cortical topography. Development (Cambridge, England), 133(14), 2719–29. https://doi.org/10.1242/dev.02437 [DOI] [PubMed] [Google Scholar]
- 39.D’Arcangelo G, & Curran T (1998). Reeler: new tales on an old mutant mouse. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, 20(3), 235–44. https://doi.org/10.1002/(SICI)1521-1878(199803)20:3<235::AID-BIES7>3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- 40.De Zeeuw CI, Hoebeek FE, Bosman LWJ, Schonewille M, Witter L, & Koekkoek SK (2011). Spatiotemporal firing patterns in the cerebellum. Nature Reviews Neuroscience, 12(6), 327–344. https://doi.org/10.1038/nrn3011 [DOI] [PubMed] [Google Scholar]
- 41.Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, & Danbolt NC (1998). The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 18(10), 3606–19. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9570792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duffin CA, McFarland R, Sarna JR, Vogel MW, & Armstrong CL (2010). Heat shock protein 25 expression and preferential Purkinje cell survival in the lurcher mutant mouse cerebellum. The Journal of Comparative Neurology, 518(11), 1892–907. https://doi.org/10.1002/cne.22309 [DOI] [PubMed] [Google Scholar]
- 43.Ebner TJ, Wang X, Gao W, Cramer SW, & Chen G (2012). Parasagittal Zones in the Cerebellar Cortex Differ in Excitability, Information Processing, and Synaptic Plasticity. The Cerebellum, 11(2), 418–419. https://doi.org/10.1007/s12311-011-0347-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eccles JC, Sasaki K, & Strata P (1967). A comparison of the inhibitory actions of Golgi cells and of basket cells. Experimental Brain Research, 3(1), 81–94. https://doi.org/10.1007/BF00234471 [DOI] [PubMed] [Google Scholar]
- 45.Edwards MA, Leclerc N, Crandall JE, & Yamamoto M (1994). Purkinje cell compartments in the reeler mutant mouse as revealed by Zebrin II and 90-acetylated glycolipid antigen expression. Anatomy and Embryology, 190(5), 417–28. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7887492 [DOI] [PubMed] [Google Scholar]
- 46.Eisenman LM, & Arlinghaus LE (1991). Spinocerebellar projection in the meander tail mutant mouse: organization in the granular posterior lobe and the agranular anterior lobe. Brain Research, 558(1), 149–52. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1718567 [DOI] [PubMed] [Google Scholar]
- 47.Eisenman LM, Gallagher E, & Hawkes R (1998). Regionalization defects in the weaver mouse cerebellum. The Journal of Comparative Neurology, 394(4), 431–44. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9590553 [PubMed] [Google Scholar]
- 48.Eisenman LM, & Hawkes R (1993). Antigenic compartmentation in the mouse cerebellar cortex: zebrin and HNK-1 reveal a complex, overlapping molecular topography. The Journal of Comparative Neurology, 335(4), 586–605. https://doi.org/10.1002/cne.903350410 [DOI] [PubMed] [Google Scholar]
- 49.Englund C, Kowalczyk T, Daza RAM, Dagan A, Lau C, Rose MF, & Hevner RF (2006). Unipolar brush cells of the cerebellum are produced in the rhombic lip and migrate through developing white matter. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(36), 9184–95. https://doi.org/10.1523/JNEUROSCI.1610-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Espinosa JS, & Luo L (2008). Timing Neurogenesis and Differentiation: Insights from Quantitative Clonal Analyses of Cerebellar Granule Cells. Journal of Neuroscience, 28(10), 2301–2312. https://doi.org/10.1523/JNEUROSCI.5157-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujita H, Morita N, Furuichi T, & Sugihara I (2012). Clustered Fine Compartmentalization of the Mouse Embryonic Cerebellar Cortex and Its Rearrangement into the Postnatal Striped Configuration. Journal of Neuroscience, 32(45), 15688–15703. https://doi.org/10.1523/JNEUROSCI.1710-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Furuta A, Rothstein JD, & Martin LJ (1997). Glutamate transporter protein subtypes are expressed differentially during rat CNS development. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 17(21), 8363–75. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9334410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furutama D, Morita N, Takano R, Sekine Y, Sadakata T, Shinoda Y, … Furuichi T (2010). Expression of the IP3R1 promoter-driven nls-lacZ transgene in Purkinje cell parasagittal arrays of developing mouse cerebellum. Journal of Neuroscience Research, 88(13), 2810–25. https://doi.org/10.1002/jnr.22451 [DOI] [PubMed] [Google Scholar]
- 54.Gallagher E, Howell BW, Soriano P, Cooper JA, & Hawkes R (1998). Cerebellar abnormalities in the disabled (mdab1-1) mouse. The Journal of Comparative Neurology, 402(2), 238–251. https://doi.org/10.1002/(SICI)1096-9861(19981214)402:2<238::AID-CNE8>3.0.CO;2-H [PubMed] [Google Scholar]
- 55.Gauck V, & Jaeger D (2000). The control of rate and timing of spikes in the deep cerebellar nuclei by inhibition. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(8), 3006–3016. https://doi.org/20024069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gebre SA, Reeber SL, & Sillitoe RV (2012). Parasagittal compartmentation of cerebellar mossy fibers as revealed by the patterned expression of vesicular glutamate transporters VGLUT1 and VGLUT2. Brain Structure & Function, 217(2), 165–80. https://doi.org/10.1007/s00429-011-0339-4 [DOI] [PubMed] [Google Scholar]
- 57.Goldowitz D (1989). Cell allocation in mammalian CNS formation: Evidence from murine interspecies aggregation chimeras. Neuron, 3(6), 705–713. https://doi.org/10.1016/0896-6273(89)90239-0 [DOI] [PubMed] [Google Scholar]
- 58.Goldowitz D, Cushing RC, Laywell E, D ‘arcangelo G, Sheldon M, Sweet HO, … Curran T (1997). Cerebellar Disorganization Characteristic of Reeler in Scrambler Mutant Mice Despite Presence of Reelin. J Neurosci, 15;17(22), 8767–77. Retrieved from http://www.jneurosci.org.ezproxyhost.library.tmc.edu/content/jneuro/17/22/8767.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graham DJ, & Wylie DR (2012). Zebrin-immunopositive and -immunonegative stripe pairs represent functional units in the pigeon vestibulocerebellum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32(37), 12769–79. https://doi.org/10.1523/JNEUROSCI.0197-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gravel C, & Hawkes R (1990). Parasagittal organization of the rat cerebellar cortex: Direct comparison of purkinje cell compartments and the organization of the spinocerebellar projection. The Journal of Comparative Neurology, 291(1), 79–102. https://doi.org/10.1002/cne.902910107 [DOI] [PubMed] [Google Scholar]
- 61.Gravel C, Leclerc N, Rafrafi J, Sasseville R, Thivierge L, & Hawkes R (1987). Monoclonal antibodies reveal the global organization of the cerebellar cortex. Journal of Neuroscience Methods, 21(2-4), 145–57. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3316852 [DOI] [PubMed] [Google Scholar]
- 62.Green MJ, Myat AM, Emmenegger BA, Wechsler-Reya RJ, Wilson LJ, & Wingate RJT (2014). Independently specified Atoh1 domains define novel developmental compartments in rhombomere 1. Development, 141(2), 389–398. https://doi.org/10.1242/dev.099119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grishkat HL, & Eisenman LM (1995). Development of the spinocerebellar projection in the prenatal mouse. The Journal of Comparative Neurology, 363(1), 93–108. https://doi.org/10.1002/cne.903630109 [DOI] [PubMed] [Google Scholar]
- 64.Guo C, Witter L, Rudolph S, Elliott HLL, Ennis KAA, & Regehr WGG (2016). Purkinje Cells Directly Inhibit Granule Cells in Specialized Regions of the Cerebellar Cortex. Neuron, 91(6), 1330–1341. https://doi.org/10.1016/j.neuron.2016.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hack I, Bancila M, Loulier K, Carroll P, & Cremer H (2002). Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nature Neuroscience, 5(10), 939–45. https://doi.org/10.1038/nn923 [DOI] [PubMed] [Google Scholar]
- 66.Hallem JS, Thompson JH, Gundappa-Sulur G, Hawkes R, Bjaalie JG, & Bower JM (1999). Spatial correspondence between tactile projection patterns and the distribution of the antigenic Purkinje cell markers anti-zebrin I and anti-zebrin II in the cerebellar folium crus IIA of the rat. Neuroscience, 93(3), 1083–94. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10473273 [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto M, & Mikoshiba K (2003). Mediolateral compartmentalization of the cerebellum is determined on the “birth date” of Purkinje cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(36), 11342–11351. https://doi.org/23/36/11342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hawkes R (1997). An anatomical model of cerebellar modules. Progress in Brain Research, 114, 39–52. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9193137 [DOI] [PubMed] [Google Scholar]
- 69.Hawkes R (2014). Purkinje cell stripes and long-term depression at the parallel fiber-Purkinje cell synapse. Frontiers in Systems Neuroscience, 8 https://doi.org/10.3389/fnsys.2014.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hawkes R, Beierbach E, & Tan S-S (1999). Granule cell dispersion is restricted across transverse boundaries in mouse chimeras. European Journal of Neuroscience, 11(11), 3800–3808. https://doi.org/10.1046/j.1460-9568.1999.00812.x [DOI] [PubMed] [Google Scholar]
- 71.Hawkes R, Colonnier M, & Leclerc N (1985). Monoclonal antibodies reveal sagittal banding in the rodent cerebellar cortex. Brain Research, 333(2), 359–65. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3888348 [DOI] [PubMed] [Google Scholar]
- 72.Hawkes R, & Eisenman LM (1997). Stripes and zones: the origins of regionalization of the adult cerebellum. Perspectives on Developmental Neurobiology, 5(1), 95–105. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9509521 [PubMed] [Google Scholar]
- 73.Hawkes R, Faulkner-Jones B, Tam P, & Tan SS (1998). Pattern formation in the cerebellum of murine embryonic stem cell chimeras. The European Journal of Neuroscience, 10(2), 790–3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9749745 [DOI] [PubMed] [Google Scholar]
- 74.Hawkes R, & Gravel C (1991). The modular cerebellum. Progress in Neurobiology, 36(4), 309–27. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1871318 [DOI] [PubMed] [Google Scholar]
- 75.Hawkes R, & Leclerc N (1987). Antigenic map of the rat cerebellar cortex: The distribution of parasagittal bands as revealed by monoclonal anti-purkinje cell antibody mabQ113. The Journal of Comparative Neurology, 256(1), 29–41. https://doi.org/10.1002/cne.902560104 [DOI] [PubMed] [Google Scholar]
- 76.Hawkes R, & Turner RW (1994). Compartmentation of NADPH-diaphorase activity in the mouse cerebellar cortex. The Journal of Comparative Neurology, 346(4), 499–516. https://doi.org/10.1002/cne.903460404 [DOI] [PubMed] [Google Scholar]
- 77.Herrup K, & Kuemerle B (1997). The compartmentalization of the cerebellum. Annual Review of Neuroscience, 20(1), 61–90. https://doi.org/10.1146/annurev.neuro.20.1.61 [DOI] [PubMed] [Google Scholar]
- 78.Horn KM, Pong M, & Gibson AR (2010). Functional relations of cerebellar modules of the cat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(28), 9411–23. https://doi.org/10.1523/JNEUROSCI.0440-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura YV, … Nabeshima Y (2005). Ptf1a, a bHLH Transcriptional Gene, Defines GABAergic Neuronal Fates in Cerebellum. Neuron, 47(2), 201–213. https://doi.org/10.1016/j.neuron.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 80.Ji Z, & Hawkes R (1994). Topography of Purkinje cell compartments and mossy fiber terminal fields in lobules II and III of the rat cerebellar cortex: spinocerebellar and cuneocerebellar projections. Neuroscience, 61(4), 935–54. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7530818 [DOI] [PubMed] [Google Scholar]
- 81.Ji Z, & Hawkes R (1995). Developing mossy fiber terminal fields in the rat cerebellar cortex may segregate because of Purkinje cell compartmentation and not competition. The Journal of Comparative Neurology, 359(2), 197–212. https://doi.org/10.1002/cne.903590202 [DOI] [PubMed] [Google Scholar]
- 82.Kalinovsky A, Boukhtouche F, Blazeski R, Bornmann C, Suzuki N, Mason CA, & Scheiffele P (2011). Development of Axon-Target Specificity of Ponto-Cerebellar Afferents. PLoS Biology, 9(2), e1001013 https://doi.org/10.1371/journal.pbio.1001013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kano M, Hashimoto K, & Tabata T (2008). Type-1 Metabotropic Glutamate Receptor in Cerebellar Purkinje Cells: A Key Molecule Responsible for Long-Term Depression, Endocannabinoid Signalling and Synapse. Source: Philosophical Transactions: Biological Sciences, 363(1500), 2173–2186. Retrieved from http://www.jstor.org/stable/20208626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karam SD, Kim YS, & Bothwell M (2001). Granule cells migrate within raphes in the developing cerebellum: An evolutionarily conserved morphogenic event. The Journal of Comparative Neurology, 440(2), 127–135. https://doi.org/10.1002/cne.1374 [DOI] [PubMed] [Google Scholar]
- 85.Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, … Yamazaki T (2014). Consensus paper: The cerebellum’s role in movement and cognition. Cerebellum. https://doi.org/10.1007/s12311-013-0511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lannoo MJ, Brochu G, Maler L, & Hawkes R (1991). Zebrin II immunoreactivity in the rat and in the weakly electric teleostEigenmannia (gymnotiformes) reveals three modes of purkinje cell development. The Journal of Comparative Neurology, 310(2), 215–233. https://doi.org/10.1002/cne.903100207 [DOI] [PubMed] [Google Scholar]
- 87.Larouche M, Beffert U, Herz J, & Hawkes R (2008). The Reelin receptors Apoer2 and Vldlr coordinate the patterning of Purkinje cell topography in the developing mouse cerebellum. PloS One, 3(2), e1653 https://doi.org/10.1371/journal.pone.0001653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larouche M, Che PM, & Hawkes R (2006). Neurogranin expression identifies a novel array of Purkinje cell parasagittal stripes during mouse cerebellar development. The Journal of Comparative Neurology, 494(2), 215–227. https://doi.org/10.1002/cne.20791 [DOI] [PubMed] [Google Scholar]
- 89.Larouche M, & Hawkes R (2006). From clusters to stripes: The developmental origins of adult cerebellar compartmentation. The Cerebellum, 5(2), 77–88. https://doi.org/10.1080/14734220600804668 [DOI] [PubMed] [Google Scholar]
- 90.Larsell O (1952). The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. Journal of Comparative Neurology, 97(2), 281–356. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12999992 [DOI] [PubMed] [Google Scholar]
- 91.Leclerc N, Gravel C, & Hawkes R (1988). Development of parasagittal zonation in the rat cerebellar cortex: MabQ113 antigenic bands are created postnatally by the suppression of antigen expression in a subset of Purkinje cells. The Journal of Comparative Neurology, 273(3), 399–420. https://doi.org/10.1002/cne.902730310 [DOI] [PubMed] [Google Scholar]
- 92.Lee SK, Sillitoe RV, Silva C, Martina M, & Sekerkova G (2015). α-Synuclein Expression in the Mouse Cerebellum Is Restricted to VGluT1 Excitatory Terminals and Is Enriched in Unipolar Brush Cells. Cerebellum, 14(5), 516–527. https://doi.org/10.1007/s12311-015-0673-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Legué E, Riedel E, & Joyner AL (2015). Clonal analysis reveals granule cell behaviors and compartmentalization that determine the folded morphology of the cerebellum. Development (Cambridge, England), 142(9), 1661–71. https://doi.org/10.1242/dev.120287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leto K, Arancillo M, Becker EBE, Buffo A, Chiang C, Ding B, … Hawkes R (2015). Consensus Paper: Cerebellar Development. Cerebellum, 789–828. https://doi.org/10.1007/s12311-015-0724-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leto K, Bartolini A, Yanagawa Y, Obata K, Magrassi L, Schilling K, & Rossi F (2009). Laminar fate and phenotype specification of cerebellar GABAergic interneurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(21), 7079–91. https://doi.org/10.1523/JNEUROSCI.0957-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leto K, Carletti B, Williams IM, Magrassi L, & Rossi F (2006). Different types of cerebellar GABAergic interneurons originate from a common pool of multipotent progenitor cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(45), 11682–94. https://doi.org/10.1523/JNEUROSCI.3656-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Levy SL, White JJ, Lackey EP, Schwartz L, & Sillitoe RV (2017). WGA-alexa conjugates for axonal tracing. Current Protocols in Neuroscience, 2017, 1.28.1–1.28.24. https://doi.org/10.1002/cpns.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin JC, & Cepko CL (1998). Granule cell raphes and parasagittal domains of Purkinje cells: complementary patterns in the developing chick cerebellum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 18(22), 9342–53. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9801373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin Y, Chen L, Lin C, Luo Y, Tsai RYL, & Wang F (2009). Neuron-derived FGF9 is essential for scaffold formation of Bergmann radial fibers and migration of granule neurons in the cerebellum. Developmental Biology, 329(1), 44–54. https://doi.org/10.1016/j.ydbio.2009.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Louis ED, Kuo S-H, Tate WJ, Kelly GC, Gutierrez J, Cortes EP, … Faust PL (2017). Heterotopic Purkinje Cells: a Comparative Postmortem Study of Essential Tremor and Spinocerebellar Ataxias 1, 2, 3, and 6. The Cerebellum. https://doi.org/10.1007/s12311-017-0876-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lundell TG, Zhou Q, & Doughty ML (2009). Neurogenin1 expression in cell lineages of the cerebellar cortex in embryonic and postnatal mice. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 238(12), 3310–25. https://doi.org/10.1002/dvdy.22165 [DOI] [PubMed] [Google Scholar]
- 102.Machold R, & Fishell G (2005). Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron, 48(1), 17–24. https://doi.org/10.1016/j.neuron.2005.08.028 [DOI] [PubMed] [Google Scholar]
- 103.Machold RP, Kittell DJ, & Fishell GJ (2007). Antagonism between Notch and bone morphogenetic protein receptor signaling regulates neurogenesis in the cerebellar rhombic lip. Neural Development, 2(1), 5 https://doi.org/10.1186/1749-8104-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mariani J, & Changeux JP (1981). Ontogenesis of olivocerebellar relationships. I. Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 1(7), 696–702. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7346578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mariën P, Ackermann H, Adamaszek M, Barwood CHS, Beaton A, Desmond J, … Ziegler W (2014). Consensus paper: Language and the cerebellum: An ongoing enigma. Cerebellum, 13(3), 386–410. https://doi.org/10.1007/s12311-013-0540-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marzban H, Chung S-H, Pezhouh MK, Feirabend H, Watanabe M, Voogd J, & Hawkes R (2010). Antigenic compartmentation of the cerebellar cortex in the chicken (Gallus domesticus). The Journal of Comparative Neurology, 518(12), 2221–39. https://doi.org/10.1002/cne.22328 [DOI] [PubMed] [Google Scholar]
- 107.Marzban H, Chung S, Watanabe M, & Hawkes R (2007). Phospholipase cβ4 expression reveals the continuity of cerebellar topography through development. The Journal of Comparative Neurology, 502(5), 857–871. https://doi.org/10.1002/cne.21352 [DOI] [PubMed] [Google Scholar]
- 108.Marzban H, & Hawkes R (2011). On the Architecture of the Posterior Zone of the Cerebellum. The Cerebellum, 10(3), 422–434. https://doi.org/10.1007/s12311-010-0208-3 [DOI] [PubMed] [Google Scholar]
- 109.Marzban H, Sillitoe RV, Hoy M, Chung S-H, Rafuse VF, & Hawkes R (2004). Abnormal HNK-1 expression in the cerebellum of an N-CAM null mouse. Journal of Neurocytology, 33(1), 117–30. https://doi.org/10.1023/B:NEUR.0000029652.96456.0d [DOI] [PubMed] [Google Scholar]
- 110.Mason CA, & Gregory E (1984). Postnatal maturation of cerebellar mossy and climbing fibers: transient expression of dual features on single axons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 4(7), 1715–35. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6737039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mateos JM, Osorio A, Azkue JJ, Benítez R, Elezgarai I, Bilbao A, … Grandes P (2001). Parasagittal compartmentalization of the metabotropic glutamate receptor mGluR1b in the cerebellar cortex. European Journal of Anatomy, 5(1), 15–21. [Google Scholar]
- 112.Mathis L, Bonnerot C, Puelles L, & Nicolas JF (1997). Retrospective clonal analysis of the cerebellum using genetic laacZ/lacZ mouse mosaics. Development (Cambridge, England), 124(20), 4089–104. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9374405 [DOI] [PubMed] [Google Scholar]
- 113.Morales D, & Hatten ME (2006). Molecular markers of neuronal progenitors in the embryonic cerebellar anlage. J Neurosci, 26(47). https://doi.org/10.1523/JNEUROSCI.3493-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mostofi A, Holtzman T, Grout AS, Yeo CH, & Edgley SA (2010). Electrophysiological localization of eyeblink-related microzones in rabbit cerebellar cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(26), 8920–34. https://doi.org/10.1523/JNEUROSCI.6117-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mugnaini E, Sekerková G, & Martina M (2011). The unipolar brush cell: a remarkable neuron finally receiving deserved attention. Brain Res Rev., 66(1-2). https://doi.org/10.1016/j.brainresrev.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nagao S, Kwak S, & Kanazawa I (1997). EAAT4, a glutamate transporter with properties of a chloride channel, is predominantly localized in Purkinje cell dendrites, and forms parasagittal compartments in rat cerebellum. Neuroscience, 78(4), 929–933. https://doi.org/10.1016/S0306-4522(97)00021-3 [DOI] [PubMed] [Google Scholar]
- 117.Nakamura K, Beppu M, Sakai K, Yagyu H, Matsumaru S, Kohno T, & Hattori M (2016). The C-terminal region of Reelin is necessary for proper positioning of a subset of Purkinje cells in the postnatal cerebellum. Neuroscience, 336, 20–29. https://doi.org/10.1016/j.neuroscience.2016.08.039 [DOI] [PubMed] [Google Scholar]
- 118.Napieralski JA, & Eisenman LM (1996). Further evidence for a unique developmental compartment in the cerebellum of the meander tail mutant mouse as revealed by the quantitative analysis of Purkinje cells. The Journal of Comparative Neurology, 364(4), 718–28. https://doi.org/10.1002/(SICI)1096-9861(19960122)364:4<718::AID-CNE9>3.0.CO;2-6 [DOI] [PubMed] [Google Scholar]
- 119.Nishida K, Flanagan JG, & Nakamoto M (2002). Domain-specific olivocerebellar projection regulated by the EphA-ephrin-A interaction. Development (Cambridge, England), 129(24), 5647–58. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12421705 [DOI] [PubMed] [Google Scholar]
- 120.Nunes MLA, & Sotelo C (1985). Development of the spinocerebellar system in the postnatal rat. The Journal of Comparative Neurology, 237(3), 291–306. https://doi.org/10.1002/cne.902370302 [DOI] [PubMed] [Google Scholar]
- 121.Nunes MLA, Sotelo C, & Wehrlé R (1988). Organization of spinocerebellar projection map in three types of agranular cerebellum: Purkinje cells vs. granule cells as organizer element. The Journal of Comparative Neurology, 273(1), 120–136. https://doi.org/10.1002/cne.902730110 [DOI] [PubMed] [Google Scholar]
- 122.Oberdick J, Baader SL, & Schilling K (1998). From zebra stripes to postal zones: deciphering patterns of gene expression in the cerebellum. Trends in Neurosciences, 21(9), 383–90. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9735946 [DOI] [PubMed] [Google Scholar]
- 123.Oberdick J, Schilling K, Smeyne RJ, Corbin JG, Bocchiaro C, & Morgan JI (1993). Control of segment-like patterns of gene expression in the mouse cerebellum. Neuron, 10(6), 1007–18. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8318226 [DOI] [PubMed] [Google Scholar]
- 124.O’Halloran CJ, Kinsella GJ, & Storey E (2012). The cerebellum and neuropsychological functioning: A critical review. Journal of Clinical and Experimental Neuropsychology, 34(1), 35–56. https://doi.org/10.1080/13803395.2011.614599 [DOI] [PubMed] [Google Scholar]
- 125.Ozol K, Hayden JM, Oberdick J, & Hawkes R (1999). Transverse zones in the vermis of the mouse cerebellum. The Journal of Comparative Neurology, 412(1), 95–111. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10440712 [PubMed] [Google Scholar]
- 126.Ozol K, & Hawkes R (1997). Compartmentation of the granular layer of the cerebellum. Histology and Histopathology, 12(1), 171–84. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9046053 [PubMed] [Google Scholar]
- 127.Pakan JMP, Iwaniuk AN, Wylie DRW, Hawkes R, & Marzban H (2007). Purkinje cell compartmentation as revealed by Zebrin II expression in the cerebellar cortex of pigeons (Columba livia). The Journal of Comparative Neurology, 501(4), 619–630. https://doi.org/10.1002/cne.21266 [DOI] [PubMed] [Google Scholar]
- 128.Paradies MA, Grishkat H, Smeyne RJ, Oberdick J, Morgan JI, & Eisenman LM (1996). Correspondence between L7-lacZ-expressing Purkinje cells and labeled olivocerebellar fibers during late embryogenesis in the mouse. The Journal of Comparative Neurology, 374(3), 451–66. https://doi.org/10.1002/(SICI)1096-9861(19961021)374:3<451::AID-CNE9>3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- 129.Park C, Finger JH, Cooper JA, & Ackerman SL (2002). The cerebellar deficient folia (cdf) gene acts intrinsically in Purkinje cell migrations. Genesis (New York, N.Y.: 2000), 32(1), 32–41. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11835672 [DOI] [PubMed] [Google Scholar]
- 130.Pascual M, Abasolo I, Mingorance-Le Meur A, Martinez A, Del Rio JA, Wright CVE, … Soriano E (2007). Cerebellar GABAergic progenitors adopt an external granule cell-like phenotype in the absence of Ptf1a transcription factor expression. Proceedings of the National Academy of Sciences, 104(12), 5193–5198. https://doi.org/10.1073/pnas.0605699104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paukert M, Huang YH, Tanaka K, Rothstein JD, & Bergles DE (2010). Zones of enhanced glutamate release from climbing fibers in the mammalian cerebellum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(21), 7290–9. https://doi.org/10.1523/JNEUROSCI.5118-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Person AL, & Raman IM (2012). Synchrony and neural coding in cerebellar circuits. Frontiers in Neural Circuits, 6 https://doi.org/10.3389/fncir.2012.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ragagnin A, Ezpeleta J, Guillemain A, Boudet-Devaud F, Haeberlé A-M, Demais V, … Bailly Y (2017). Cerebellar compartmentation of prion pathogenesis. Brain Pathology (Zurich, Switzerland). https://doi.org/10.1111/bpa.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramón y Cajal S (1909). Histologie du Systeme Nerveux de l’Homme et des Vertebres. Maloine, Paris: 1911. chap. II. Demography (Vol. v.1). https://doi.org/10.5962/bhl.title.48637 [Google Scholar]
- 135.Reeber SL, Gebre SA, Filatova N, & Sillitoe RV (2011). Revealing neural circuit topography in multi-color. Journal of Visualized Experiments: JoVE, (57), 4–11. https://doi.org/10.3791/3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reeber SL, Otis TS, & Sillitoe RV (2013). New roles for the cerebellum in health and disease. Frontiers in Systems Neuroscience, 7, 83 https://doi.org/10.3389/fnsys.2013.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reeber SL, & Sillitoe RV (2011). Patterned expression of a cocaine- and amphetamine-regulated transcript peptide reveals complex circuit topography in the rodent cerebellar cortex. The Journal of Comparative Neurology, 519(9), 1781–1796. https://doi.org/10.1002/cne.22601 [DOI] [PubMed] [Google Scholar]
- 138.Rivkin A, & Herrup K (2003). Development of cerebellar modules: extrinsic control of late-phase zebrin II pattern and the exploration of rat/mouse species differences. Molecular and Cellular Neurosciences, 24(4), 887–901. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14697656 [DOI] [PubMed] [Google Scholar]
- 139.Ross ME, Fletcher C, Mason CA, Hatten ME, & Heintz N (1990). Meander tail reveals a discrete developmental unit in the mouse cerebellum. Proceedings of the National Academy of Sciences of the United States of America, 87(11), 4189–92. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2349228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ruigrok TJH (2011). Ins and Outs of Cerebellar Modules. The Cerebellum, 10(3), 464–474. https://doi.org/10.1007/s12311-010-0164-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sarna JR, & Hawkes R (2003). Patterned Purkinje cell death in the cerebellum. Progress in Neurobiology, 70(6), 473–507. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14568361 [DOI] [PubMed] [Google Scholar]
- 142.Sarna JR, Marzban H, Watanabe M, & Hawkes R (2006). Complementary stripes of phospholipase Cbeta3 and Cbeta4 expression by Purkinje cell subsets in the mouse cerebellum. The Journal of Comparative Neurology, 496(3), 303–13. https://doi.org/10.1002/cne.20912 [DOI] [PubMed] [Google Scholar]
- 143.Schilling K, Schmidt HH, & Baader SL (1994). Nitric oxide synthase expression reveals compartments of cerebellar granule cells and suggests a role for mossy fibers in their development. Neuroscience, 59(4), 893–903. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7520135 [DOI] [PubMed] [Google Scholar]
- 144.Schultz SR, Kitamura K, Post-Uiterweer A, Krupic J, & Häusser M (2009). Spatial pattern coding of sensory information by climbing fiber-evoked calcium signals in networks of neighboring cerebellar Purkinje cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(25), 8005–15. https://doi.org/10.1523/JNEUROSCI.4919-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Seil FJ, Johnson ML, & Hawkes R (1995). Molecular compartmentation expressed in cerebellar cultures in the absence of neuronal activity and neuron-glia interactions. The Journal of Comparative Neurology, 356(3), 398–407. https://doi.org/10.1002/cne.903560307 [DOI] [PubMed] [Google Scholar]
- 146.Sergaki MC, & Ibáñez CF (2017). GFRα1 Regulates Purkinje Cell Migration by Counteracting NCAM Function. Cell Reports, 18(2), 367–379. https://doi.org/10.1016/j.celrep.2016.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seto Y, Ishiwata S, & Hoshino M (2014). Characterization of Olig2 expression during cerebellar development. Gene Expression Patterns, 15(1), 1–7. https://doi.org/10.1016/j.gep.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 148.Shambes GM, Gibson JM, & Welker W (1978). Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain, Behavior and Evolution, 15(2), 94–140. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/638731 [DOI] [PubMed] [Google Scholar]
- 149.Sillitoe RV, Gopal N, & Joyner AL (2009). Embryonic origins of ZebrinII parasagittal stripes and establishment of topographic Purkinje cell projections. Neuroscience, 162(3), 574–588. https://doi.org/10.1016/j.neuroscience.2008.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sillitoe RV, & Hawkes R (2002). Whole-mount Immunohistochemistry: A High-throughput Screen for Patterning Defects in the Mouse Cerebellum. Journal of Histochemistry & Cytochemistry, 50(2), 235–244. https://doi.org/10.1177/002215540205000211 [DOI] [PubMed] [Google Scholar]
- 151.Sillitoe RV (2016). Mossy Fibers Terminate Directly Within Purkinje Cell Zones During Mouse Development. Cerebellum (London, England), 15(1), 14–7. https://doi.org/10.1007/s12311-015-0712-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sillitoe RV, Chung S-H, Fritschy J-M, Hoy M, & Hawkes R (2008a). Golgi cell dendrites are restricted by Purkinje cell stripe boundaries in the adult mouse cerebellar cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(11), 2820–6. https://doi.org/10.1523/JNEUROSCI.4145-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sillitoe RV, & Joyner AL (2007). Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu. Rev. Cell Dev. Biol, 23(1), 549–77. https://doi.org/10.1146/annurev.cellbio.23.090506.123237 [DOI] [PubMed] [Google Scholar]
- 154.Sillitoe RV, Marzban H, Larouche M, Zahedi S, Affanni J, & Hawkes R (2005). Conservation of the architecture of the anterior lobe vermis of the cerebellum across mammalian species. Progress in Brain Research, 148, 283–97. https://doi.org/10.1016/S0079-6123(04)48022-4 [DOI] [PubMed] [Google Scholar]
- 155.Sillitoe RV, Stephen D, Lao Z, & Joyner AL (2008b). Engrailed homeobox genes determine the organization of Purkinje cell sagittal stripe gene expression in the adult cerebellum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(47), 12150–12162. https://doi.org/10.1523/JNEUROSCI.2059-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sillitoe RV, Vogel MW, & Joyner AL (2010). Engrailed homeobox genes regulate establishment of the cerebellar afferent circuit map. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(30), 10015–24. https://doi.org/10.1523/JNEUROSCI.0653-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sokoloff G, Plumeau AM, Mukherjee D, & Blumberg MS (2015). Twitch-related and rhythmic activation of the developing cerebellar cortex. Journal of Neurophysiology, 114(3), 1746–1756. https://doi.org/10.1152/jn.00284.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sotelo C (2004). Cellular and genetic regulation of the development of the cerebellar system. Progress in Neurobiology, 72(5), 295–339. https://doi.org/10.1016/j.pneurobio.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 159.Sotelo C (2008). Development of “pinceaux” formations and dendritic translocation of climbing fibers during the acquisition of the balance between glutamatergic and γ-aminobutyric acidergic inputs in developing purkinje cells. Journal of Comparative Neurology, 506(2), 240–262. https://doi.org/10.1002/cne.21501 [DOI] [PubMed] [Google Scholar]
- 160.Sotelo C, Bourrat F, & Triller A (1984). Postnatal development of the inferior olivary complex in the rat. II. Topographic organization of the immature olivocerebellar projection. The Journal of Comparative Neurology, 222(2), 177–199. https://doi.org/10.1002/cne.902220204 [DOI] [PubMed] [Google Scholar]
- 161.Sotelo C, & Chédotal A (2005). Development of the olivocerebellar system: migration and formation of cerebellar maps. In Progress in brain research (Vol. 148, pp. 1–20). https://doi.org/10.1016/S0079-6123(04)48001-7 [DOI] [PubMed] [Google Scholar]
- 162.Strømme P, Dobrenis K, Sillitoe RV, Gulinello M, Ali NF, Davidson C, … Walkley SU (2011). X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal-lysosomal dysfunction. Brain, 134(11), 3369–3383. https://doi.org/10.1093/brain/awr250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sudarov A, & Joyner AL (2007). Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Development, 2(1), 26 https://doi.org/10.1186/1749-8104-2-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sudarov A, Turnbull RK, Kim EJ, Lebel-Potter M, Guillemot F, & Joyner AL (2011). Ascl1 Genetics Reveals Insights into Cerebellum Local Circuit Assembly. Journal of Neuroscience, 31(30), 11055–11069. https://doi.org/10.1523/JNEUROSCI.0479-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sugihara I, & Quy PN (2007). Identification of aldolase C compartments in the mouse cerebellar cortex by olivocerebellar labeling. The Journal of Comparative Neurology, 500(6), 1076–92. https://doi.org/10.1002/cne.21219 [DOI] [PubMed] [Google Scholar]
- 166.Takeda T, & Maekawa K (1989). Olivary branching projections to the flocculus, nodulus and uvula in the rabbit. I. An electrophysiological study. Experimental Brain Research, 74(1), 47–62. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2924841 [DOI] [PubMed] [Google Scholar]
- 167.Tano D, Napieralski JA, Eisenman LM, Messer A, Plummer J, & Hawkes R (1992). Novel developmental boundary in the cerebellum revealed by zebrin expression in the lurcher (Lc/+) mutant mouse. The Journal of Comparative Neurology, 323(1), 128–36. https://doi.org/10.1002/cne.903230111 [DOI] [PubMed] [Google Scholar]
- 168.Thomas KR, Musci TS, Neumann PE, & Capecchi MR (1991). Swaying is a mutant allele of the proto-oncogene Wnt-1. Cell, 67(5), 969–76. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1835670 [DOI] [PubMed] [Google Scholar]
- 169.Tolbert DL, Pittman T, Alisky JM, & Clark BR (1994). Chronic NMDA receptor blockade or muscimol inhibition of cerebellar cortical neuronal activity alters the development of spinocerebellar afferent topography. Developmental Brain Research, 80, 268–274. Retrieved from https://ac-els-cdn-com.ezproxyhost.library.tmc.edu/0165380694901120/1-s2.0-0165380694901120-main.pdf?_tid=479f1e06-e288-11e7-b829-00000aab0f6b&acdnat=1513446240_31d6d3c87d140213c5779754a005173b [DOI] [PubMed] [Google Scholar]
- 170.Tsutsumi S, Yamazaki M, Miyazaki T, Watanabe M, Sakimura K, Kano M, & Kitamura K (2015). Structure-function relationships between aldolase C/zebrin II expression and complex spike synchrony in the cerebellum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(2), 843–52. https://doi.org/10.1523/JNEUROSCI.2170-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Valera AM, Binda F, Pawlowski SA, Dupont J-L, Casella J-F, Rothstein JD, … Isope P (2016). Stereotyped spatial patterns of functional synaptic connectivity in the cerebellar cortex. eLife, 5, e09862 https://doi.org/10.7554/eLife.09862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vibulyaseck S, Fujita H, Luo Y, Tran AK, Oh-Nishi A, Ono Y, … Sugihara I (2017). Spatial rearrangement of Purkinje cell subsets forms the transverse and longitudinal compartmentalization in the mouse embryonic cerebellum. Journal of Comparative Neurology, 525(14), spc1 https://doi.org/10.1002/cne.24284 [DOI] [PubMed] [Google Scholar]
- 173.Voogd J, & Ruigrok TJH (2004). The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: the congruence of projection zones and the zebrin pattern. Journal of Neurocytology, 33(1), 5–21. https://doi.org/10.1023/B:NEUR.0000029645.72074.2b [DOI] [PubMed] [Google Scholar]
- 174.Wadiche JI, & Jahr CE (2005). Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nature Neuroscience, 8(10), 1329–1334. https://doi.org/10.1038/nn1539 [DOI] [PubMed] [Google Scholar]
- 175.Wang B, Harrison W, Overbeek PA, & Zheng H (2011). Transposon mutagenesis with coat color genotyping identifies an essential role for Skor2 in sonic hedgehog signaling and cerebellum development. Development, 138(20), 4487–4497. https://doi.org/10.1242/dev.067264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang VY, Rose MF, & Zoghbi HY (2005). Math1 Expression Redefines the Rhombic Lip Derivatives and Reveals Novel Lineages within the Brainstem and Cerebellum. Neuron, 48(1), 31–43. https://doi.org/10.1016/j.neuron.2005.08.024 [DOI] [PubMed] [Google Scholar]
- 177.Wassef M, Cholley B, Heizmann CW, & Sotelo C (1992). Development of the olivocerebellar projection in the rat: II. Matching of the developmental compartmentations of the cerebellum and inferior olive through the projection map. The Journal of Comparative Neurology, 323(4), 537–550. https://doi.org/10.1002/cne.903230406 [DOI] [PubMed] [Google Scholar]
- 178.Wassef M, & Joyner AL (1997). Early mesencephalon/metencephalon patterning and development of the cerebellum. Perspectives on Developmental Neurobiology, 5(1), 3–16. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9509514 [PubMed] [Google Scholar]
- 179.Wassef M, Sotelo C, Thomasset M, Granholm A-C, Leclerc N, Rafrafi J, & Hawkes R (1990). Expression of compartmentation antigen zebrin I in cerebellar transplants. The Journal of Comparative Neurology, 294(2), 223–234. https://doi.org/10.1002/cne.902940207 [DOI] [PubMed] [Google Scholar]
- 180.Wassef M, Zanetta JP, Brehier A, & Sotelo C (1985). Transient biochemical compartmentalization of Purkinje cells during early cerebellar development. Developmental Biology, 111(1), 129–37. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2993082 [DOI] [PubMed] [Google Scholar]
- 181.Wasser CR, & Herz J (2017). Reelin: Neurodevelopmental Architect and Homeostatic Regulator of Excitatory Synapses. Journal of Biological Chemistry, 292(4), 1330–1338. https://doi.org/10.1074/jbc.R116.766782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Watson CM, Pelka GJ, Radziewic T, Shahbazian MD, Christodoulou J, Williamson SL, & Tam PPL (2005). Reduced proportion of Purkinje cells expressing paternally derived mutant Mecp2308 allele in female mouse cerebellum is not due to a skewed primary pattern of X-chromosome inactivation. Human Molecular Genetics, 14(13), 1851–1861. https://doi.org/10.1093/hmg/ddi191 [DOI] [PubMed] [Google Scholar]
- 183.Watt AJ, Cuntz H, Mori M, Nusser Z, Sjöström PJ, & Häusser M (2009). Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nature Neuroscience, 12(4), 463–473. https://doi.org/10.1038/nn.2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wefers AK, Haberlandt C, Surchev L, Steinhäuser C, Jabs R, & Schilling K (2017). Migration of Interneuron Precursors in the Nascent Cerebellar Cortex. The Cerebellum. https://doi.org/10.1007/s12311-017-0900-7 [DOI] [PubMed] [Google Scholar]
- 185.Welsh JP Functional significance of climbing-fiber synchrony: a population coding and behavioral analysis, 978 Annals of the New York Academy of Sciences (2002). Blackwell Publishing Ltd; https://doi.org/10.1111/j.1749-6632.2002.tb07567.x [DOI] [PubMed] [Google Scholar]
- 186.Welsh JP, Lang EJ, Suglhara I, & Llinás R (1995). Dynamic organization of motor control within the olivocerebellar system. Nature, 374(6521), 453–7. https://doi.org/10.1038/374453a0 [DOI] [PubMed] [Google Scholar]
- 187.Welsh JP, & Oristaglio JT (2016). Autism and Classical Eyeblink Conditioning: Performance Changes of the Conditioned Response Related to Autism Spectrum Disorder Diagnosis. Frontiers in Psychiatry, 7, 137 https://doi.org/10.3389/fpsyt.2016.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.White JJ, Arancillo M, King A, Lin T, Miterko LN, Gebre SA, & Sillitoe RV (2016). Pathogenesis of severe ataxia and tremor without the typical signs of neurodegeneration. Neurobiology of Disease, 86, 86–98. https://doi.org/10.1016/j.nbd.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.White JJ, Arancillo M, Stay TL, George-Jones NA, Levy SL, Heck DH, & Sillitoe RV (2014). Cerebellar zonal patterning relies on Purkinje cell neurotransmission. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(24), 8231–45. https://doi.org/10.1523/JNEUROSCI.0122-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.White JJ, & Sillitoe RV (2013). Development of the cerebellum: from gene expression patterns to circuit maps. Wiley Interdisciplinary Reviews: Developmental Biology, 2(1), 149–164. https://doi.org/10.1002/wdev.65 [DOI] [PubMed] [Google Scholar]
- 191.Williams BL, Yaddanapudi K, Hornig M, & Lipkin WI (2007). Spatiotemporal Analysis of Purkinje Cell Degeneration Relative to Parasagittal Expression Domains in a Model of Neonatal Viral Infection. Journal of Virology, 81(6), 2675–2687. https://doi.org/10.1128/JVI.02245-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wingate RJ (2001). The rhombic lip and early cerebellar development. Current Opinion in Neurobiology, 11(1), 82–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11179876 [DOI] [PubMed] [Google Scholar]
- 193.Witter L, Rudolph S, Pressler RT, Lahlaf SI, & Regehr WG (2016). Purkinje Cell Collaterals Enable Output Signals from the Cerebellar Cortex to Feed Back to Purkinje Cells and Interneurons. Neuron, 91(2). https://doi.org/10.1016/j.neuron.2016.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wylie DR, Hoops D, Aspden JW, & Iwaniuk AN (2016). Zebrin II Is Expressed in Sagittal Stripes in the Cerebellum of Dragon Lizards (Ctenophorus sp.). Brain, Behavior and Evolution, 88(3-4), 177–186. https://doi.org/10.1159/000452857 [DOI] [PubMed] [Google Scholar]
- 195.Xiao G, Pan B, Tian X, Li Y, Li B, & Li Z (2014). Synovial sarcoma in cerebellum: a case report and literature review. Brain Tumor Pathology, 31(1), 68–75. https://doi.org/10.1007/s10014-012-0126-9 [DOI] [PubMed] [Google Scholar]
- 196.Xu H, Yang Y, Tang X, Zhao M, Liang F, Xu P, … Fan X (2013). Bergmann Glia Function in Granule Cell Migration During Cerebellum Development. Molecular Neurobiology, 47(2), 833–844. https://doi.org/10.1007/s12035-013-8405-y [DOI] [PubMed] [Google Scholar]
- 197.Yaguchi Y, Yu T, Ahmed MU, Berry M, Mason I, & Basson MA (2009). Fibroblast growth factor (FGF) gene expression in the developing cerebellum suggests multiple roles for FGF signaling during cerebellar morphogenesis and development. Developmental Dynamics, 238(8), 2058–2072. https://doi.org/10.1002/dvdy.22013 [DOI] [PubMed] [Google Scholar]
- 198.Yamada M, Seto Y, Taya S, Owa T, Inoue YU, Inoue T, … Hoshino M (2014). Specification of Spatial Identities of Cerebellar Neuron Progenitors by Ptf1a and Atoh1 for Proper Production of GABAergic and Glutamatergic Neurons. Journal of Neuroscience, 34(14), 4786–4800. https://doi.org/10.1523/JNEUROSCI.2722-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yamada M, Terao M, Terashima T, Fujiyama T, Kawaguchi Y, Nabeshima Y, & Hoshino M (2007). Origin of climbing fiber neurons and their developmental dependence on Ptf1a. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(41), 10924–34. https://doi.org/10.1523/JNEUROSCI.1423-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yamamoto T, Fukuda M, & Llinás R (2001). Bilaterally synchronous complex spike Purkinje cell activity in the mammalian cerebellum. European Journal of Neuroscience, 13(2), 327–339. https://doi.org/10.1046/j.0953-816X.2000.01395.x [DOI] [PubMed] [Google Scholar]
- 201.Yan XX, Jen LS, & Garey LJ (1993). Parasagittal patches in the granular layer of the developing and adult rat cerebellum as demonstrated by NADPH-diaphorase histochemistry. Neuroreport, 4(11), 1227–30. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7693013 [DOI] [PubMed] [Google Scholar]
- 202.Yeung J, Ha TJ, Swanson DJ, Choi K, Tong Y, & Goldowitz D (2014). Wls Provides a New Compartmental View of the Rhombic Lip in Mouse Cerebellar Development. Journal of Neuroscience, 34(37), 12527–12537. https://doi.org/10.1523/JNEUROSCI.1330-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yue Q, Groszer M, Gil JS, Berk AJ, Messing A, Wu H, & Liu X (2005). PTEN deletion in Bergmann glia leads to premature differentiation and affects laminar organization. Development (Cambridge, England), 132(14), 3281–91. https://doi.org/10.1242/dev.01891 [DOI] [PubMed] [Google Scholar]
- 204.Zervas M, Blaess S, & Joyner AL (2005). Classical Embryological Studies and Modern Genetic Analysis of Midbrain and Cerebellum Development. In Current topics in developmental biology (Vol. 69, pp. 101–138). https://doi.org/10.1016/S0070-2153(05)69005-9 [DOI] [PubMed] [Google Scholar]
- 205.Zervas M, Millet S, Ahn S, & Joyner AL (2004). Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron, 43(3), 345–57. https://doi.org/10.1016/j.neuron.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 206.Zhang L, & Goldman JE (1996a). Developmental fates and migratory pathways of dividing progenitors in the postnatal rat cerebellum. The Journal of Comparative Neurology, 370(4), 536–550. https://doi.org/10.1002/(SICI)1096-9861(19960708)370:4<536::AID-CNE9>3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- 207.Zhang L, & Goldman JE (1996b). Generation of cerebellar interneurons from dividing progenitors in white matter. Neuron, 16(1), 47–54. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8562089 [DOI] [PubMed] [Google Scholar]
- 208.Zhou H, Lin Z, Voges K, Ju C, Gao Z, Bosman LW, … Schonewille M (2014). Cerebellar modules operate at different frequencies. eLife, 3, e02536 https://doi.org/10.7554/eLife.02536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zong H, Espinosa JS, Su HH, Muzumdar MD, & Luo L (2005). Mosaic analysis with double markers in mice. Cell, 121(3), 479–92. https://doi.org/10.1016/j.cell.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 210.Zordan P, Croci L, Hawkes R, & Consalez GG (2008). Comparative analysis of proneural gene expression in the embryonic cerebellum. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 237(6), 1726–35. https://doi.org/10.1002/dvdy.21571 [DOI] [PubMed] [Google Scholar]


