Abstract
In many eukaryotes, translation initiation is regulated by proteins that bind to the mRNA cap–binding protein eukaryotic translation initiation factor 4E (eIF4E). These proteins commonly prevent association of eIF4E with eIF4G or form repressive messenger ribonucleoproteins that exclude the translation machinery. Such gene-regulatory mechanisms in plants, and even the presence of eIF4E-interacting proteins other than eIF4G (and the plant-specific isoform eIFiso4G, which binds eIFiso4E), are unknown. Here, we report the discovery of a plant-specific protein, conserved binding of eIF4E 1 (CBE1). We found that CBE1 has an evolutionarily conserved eIF4E-binding motif in its N-terminal domain and binds eIF4E or eIFiso4E in vitro. CBE1 thereby forms cap-binding complexes and is an eIF4E-dependent constituent of these complexes in vivo. Of note, plant mutants lacking CBE1 exhibited dysregulation of cell cycle–related transcripts and accumulated higher levels of mRNAs encoding proteins involved in mitosis than did WT plants. Our findings indicate that CBE1 is a plant protein that can form mRNA cap–binding complexes having the potential for regulating gene expression. Because mammalian translation factors are known regulators of cell cycle progression, we propose that CBE1 may represent such first translation factor–associated plant-specific cell cycle regulator.
Keywords: eukaryotic translation initiation factor 4E (eIF4E), translation initiation factor, translation control, protein synthesis, Arabidopsis thaliana, 4E-binding protein, eIF4E-binding, RNA cap–binding complex, translation, cell cycle, mitosis
Introduction
In eukaryotes, mRNAs are co-transcriptionally capped with a 5′ to 5′ reverse-linked 7-methylguanosine residue; the presence of the cap structure is an important determinant of downstream processing and regulatory fate (1). The cap moiety is recognized by a class of proteins called cap-binding proteins which notably includes eukaryotic initiation factor 4E (eIF4E).2 eIF4E is in turn bound by eIF4G, a large scaffolding protein, to form the eIF4F complex which has important roles in translation initiation. eIF4G contacts eIF4B and the RNA helicase eIF4A which promote unwinding of secondary structure in the mRNA during translation initiation (2, 3). eIF4G recruits the 43S pre-initiation complex to mRNA through contacts with eIF3 and eIF5. The importance of the eIF4F complex is such that mRNA cap recognition by eIF4E is considered a central control point in translation initiation and its regulation (4).
eIF4G contains a well-conserved motif, (Y(x)4Lφ), where x is any amino acid and φ is a hydrophobic residue, which is required for eIF4E binding (5). This motif is shared by a number of other eIF4E-binding proteins (4E-BPs) (6). In mammals, 4E-BPs are regulated by phosphorylation through the mTOR pathway. In the absence of phosphorylation, 4E-BPs compete with eIF4G for binding of eIF4E, sequestering the protein away to reduce levels of translation. Activation of mTOR leads to phosphorylation of 4E-BPs, reducing their affinity for eIF4E and making the protein available for binding by eIF4G, increasing levels of translation initiation (7). To date, there is no evidence of a similar 4E-BP-like mechanism in regulation of plant translation (8).
Other eIF4E-binding proteins are known to contribute to a range of regulatory processes. 4E-transporter in mammals has an important role in mRNA localization by directing nucleocytoplasmic shuttling of eIF4E and also localizes eIF4E to P-bodies (9). Other types of eIF4E-binding proteins target specific mRNAs for translation repression by binding specific sequence motifs internal to the mRNA and binding the eIF4E present at the mRNA's cap, effectively rendering it closed to translational activation. Examples of such activity are found in mammalian Maskin and Neuroguidin, eIF4E-binding proteins which in turn bind the RNA-binding protein cytoplasmic polyadenylation element-binding protein (10, 11). Similarly, in Drosophila, Cup binds eIF4E and RNA-binding proteins such as Smaug and Bruno which recognize specific 3′ UTR elements in mRNAs targeted for translational repression (12, 13). Another Drosophila protein, Mextli, is expressed in ovarian germ line stem cells and binds eIF4E to promote translation in a manner apparently independent of eIF4G (14).
Flowering plants, in addition to the conserved eIF4E and eIF4G, have the plant-specific isoforms eIFiso4E and eIFiso4G which make up a complex called eIFiso4F (2). The exact mechanisms by which translation initiation is regulated by eIF4F and/or eIFiso4F in plants remain unclear by comparison to mammals and fungi. As no proteins similar to 4E-BP have been identified, and binding affinity between eIF4E/eIF4G and eIFiso4E/eIFiso4G is significantly higher than other eukaryotic systems (15), a mechanism of global control of translation initiation by cap-binding protein sequestration has not yet been discovered in plants (8). Arabidopsis thaliana lipoxygenase 2 (LOX2) has been shown to interact with eIFiso4E in vitro (16) and A. thaliana basic transcription factor 3 (BTF3) shows interaction with eIFiso4E in yeast two-hybrid assays (17). However, the biological significance of the observed interaction of these proteins with eIFiso4E is not known. BTF3 lacks a canonical eIF4E-binding motif and the proposed eIF4E-binding site of LOX2 shows poor evolutionary conservation (Fig. S1) (18).
In this study, we describe the discovery of a conserved, plant-specific protein of previously unknown function, conserved binding of eIF4E 1 (CBE1). CBE1 binds eIF4E in vitro and forms cap-binding complexes in an eIF4E-dependent manner in vivo. Plants lacking CBE1 show dysregulation of genes involved in cell cycle processes. Based on these observations, we propose CBE1 and eIF4E may form regulatory messenger ribonucleoproteins in plants.
Results
CBE1 is a conserved plant cap-binding protein
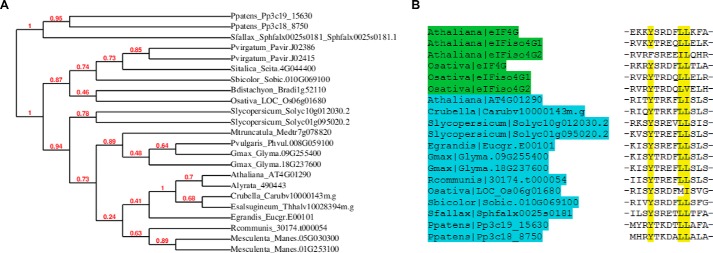
A previous study by Bush et al. (19) employed MS to identify proteins from A. thaliana cell culture that are retained on 7-methylguanosine Sepharose and are presumably associated with cap-binding proteins or complexes. Among the unknown proteins reported, we searched for motifs for interaction with cap-binding proteins. One protein, AT4G01290, appears to have an N-terminal eIF4E-binding motif. To determine whether the protein is an evolutionarily conserved eIF4E-binding protein, homologous plant sequences were gathered and a phylogeny was generated (Fig. 1A). The AT4G01290 protein is encoded in genomes across land plants and appears well conserved; however, it is not found outside of plants or in green algae. Although AT4G01290 is 991 amino acids in length, no identified domains are present in the protein, and it does not have sequence homology to any protein of known function. The N terminus of AT4G01290 features a very well-conserved eIF4E-binding motif (Fig. 1B) and based on its features as a unique plant-specific protein with apparent eIF4E-binding capacity, we named the protein conserved binding of eIF4E 1 (CBE1). The N-terminal domain containing the eIF4E-binding site (amino acids 1–230) was selected to determine whether this protein is an eIF4E-binding protein.
Figure 1.
Conserved binding of eIF4E 1 (CBE1) is a conserved plant-specific protein with a predicted eIF4E-binding motif. A, phylogenetic tree of predicted CBE1 proteins from representative plant species. The phylogenetic tree was generated using the http://www.phylogeny.fr/ pipeline (39) with alignment by MUSCLE and tree construction by PhyML using 500 bootstrap replicates. (Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.) B, alignment of the N-terminal eIF4E-binding motif (Y(x)4Lφ) of selected CBE1 proteins in blue (eIF4G and eIFiso4G motifs shown for comparison in green).
CBE1 forms cap-binding complexes in vitro
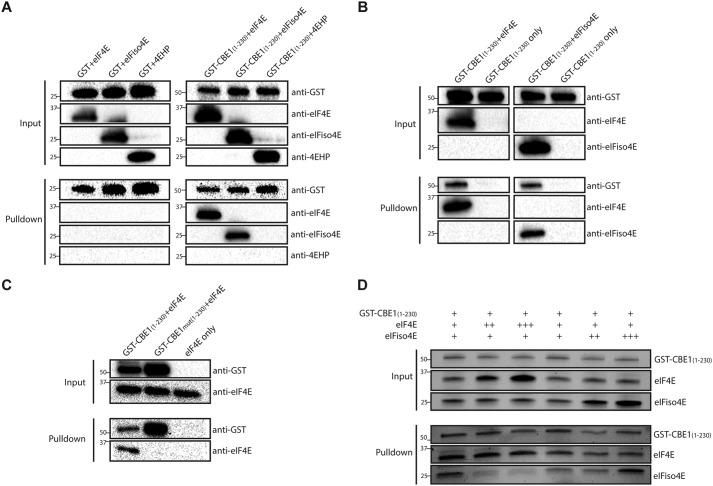
The N-terminal portion of the protein containing the eIF4E-binding motif was expressed as a fusion to GST and purified. GST-CBE11–230 was tested for its ability to bind to the eIF4E-family proteins of A. thaliana, eIF4E, eIFiso4E, and 4EHP (also known as nCBP), by GSH Sepharose pulldown. eIF4E and eIFiso4E were found to bind to GST-CBE11–230 but not to the GST protein control, whereas 4EHP did not bind to GST-CBE11–230 (Fig. 2A). To further test whether the CBE1 protein may form an active cap-binding complex, the ability of eIF4E or eIFiso4E to co-purify GST-CBE11–230 was examined by 7-methylguanosine Sepharose pulldown. GST-CBE11–230 was able to co-purify with the 7-methylguanosine cap analogue beads in a manner dependent on the presence of eIF4E or eIFiso4E (Fig. 2B), supporting CBE1 as a protein that may form a cap-binding complex with these proteins. To confirm the direct interaction between CBE1 and cap-binding proteins, the eIF4E-binding site was mutated from (YTRKFLI) to (ATRKFAA) (GST-CBE1mut1–230) to eliminate the residues essential to forming a complex with eIF4E, as has previously been shown for other eIF4E-binding proteins (20, 21). (Underlined letters indicate residues that were mutated.) The ability to bind eIF4E was lost in the GST-CBE1mut1–230 mutant protein (Fig. 2C) further validating CBE1 as a bona fide 4E-binding protein.
Figure 2.
CBE1 forms cap-binding complexes in vitro. A, GST-tag pulldown of recombinant A. thaliana eIF4E, eIFiso4E, or 4EHP in an equimolar mixture with GST control (left) or GST-CBE11–230 (right). Protein mixtures were incubated with GSH Sepharose 4B beads and washed, and bound complexes were eluted with GSH and detected by Western blotting. The eIFiso4E signal extends into eIF4E panel because of sequential probing with anti-eIFiso4E and then anti-eIF4E antibodies. B, 7-methylguanosine Sepharose bead pulldown of cap-binding complexes. Equimolar mixtures of GST-CBE11–230 with either recombinant A. thaliana eIF4E or eIFiso4E (as well as GST-CBE11–230 only) were incubated with 7-methylguanosine Sepharose beads and washed, and bound complexes were eluted with 2× Laemmli sample buffer. Eluted proteins were detected by Western blotting. C, GST-tag pulldown of recombinant A. thaliana eIF4E, in an equimolar mixture with GST-CBE11–230 or GST-CBE1mut1–230 with eIF4E alone as a control. Protein mixtures were incubated with GSH Sepharose 4B beads and washed; bound complexes were eluted with GSH and detected by Western blotting. D, competition pulldown of recombinant A. thaliana eIF4E and eIFiso4E with GST-CBE11–230. Equimolar amounts of GST-CBE11–230 and the two cap-binding proteins were mixed and incubated with GSH Sepharose 4B beads, followed by washing and elution with GSH. GST-CBE11–230–dependent co-purification of cap-binding proteins was directly measured by Bio-Rad stain free gel imaging. The recovery of the respective cap-binding proteins in the pulldown when challenged with 2-fold (++) or 4-fold (+++) excess levels of either eIF4E or eIFiso4E was observed. All experiments were performed three times with similar results.
The in vitro promiscuity of the eIF4E-binding motifs of eIF4G and eIFiso4G is known (15, 22). It was our hypothesis that despite the ability of CBE1 to bind either eIF4E or eIFiso4E in vitro, it would more likely to form a complex with only eIF4E in vivo. CBE1 evolved in land plants before eIFiso4E was available, as eIFiso4E is not found in the plant lineage until the appearance of flowering plants (23). Additionally, the eIF4E-binding motif of CBE1 bears slightly more similarity to that of eIF4G than eIFiso4G. To test whether eIF4E is the preferred binding partner of CBE1, a mixture of GST-CBE11–230 with eIF4E and eIFiso4E was challenged with excess levels of either eIF4E or eIFiso4E and subjected to GSH Sepharose pulldown (Fig. 2D). Excess levels of eIF4E led to lower levels of eIFiso4E co-purification, but excess eIFiso4E did not diminish eIF4E binding. These results support eIF4E as the most likely preferred binding partner of CBE1.
CBE1 forms cap-binding complexes in vivo
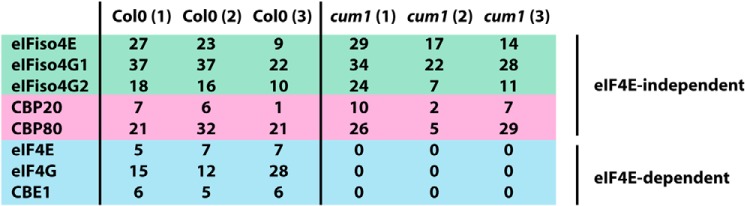
Although in vitro evidence supports CBE1 as an eIF4E-binding protein, we sought evidence that they form a complex in vivo. Cap-binding complexes were co-purified by 7-methylguanosine Sepharose beads from seedling lysates of either WT Col0 or cum1 mutant plants, which contain a nonsense mutation in the gene encoding eIF4E protein and lack detectable eIF4E (24). The purified proteins were subjected to MS (Fig. 3) in order to identify eIF4E-dependent cap-binding complexes. eIFiso4F and nuclear cap-binding complex components CBP20/CBP80 were identified in cap-binding complex eluates from both Col0 and the eIF4E-lacking cum1 plant lysates. However, eIF4E, eIF4G and CBE1 were found only in the Col0 lysate and were not identified in cap-binding complexes purified from the cum1 mutant plants. Based on these observations, we believe that eIF4E and CBE1 likely form a cap-binding complex in vivo.
Figure 3.
CBE1 forms complexes with eIF4E in vivo. Mass spectrometry of native cap-binding complexes isolated from Col0 or cum1 seedlings was performed. 7-Methylguanosine Sepharose bead pulldown of seedling lysates was run briefly by SDS-PAGE, and a gel slice containing the protein mixture was digested and presented for MS. Numbers shown represent total peptide spectra matching the constituents of cap-binding complexes, with the experiment performed in triplicate.
CBE1 contributes to plant development and gene regulation
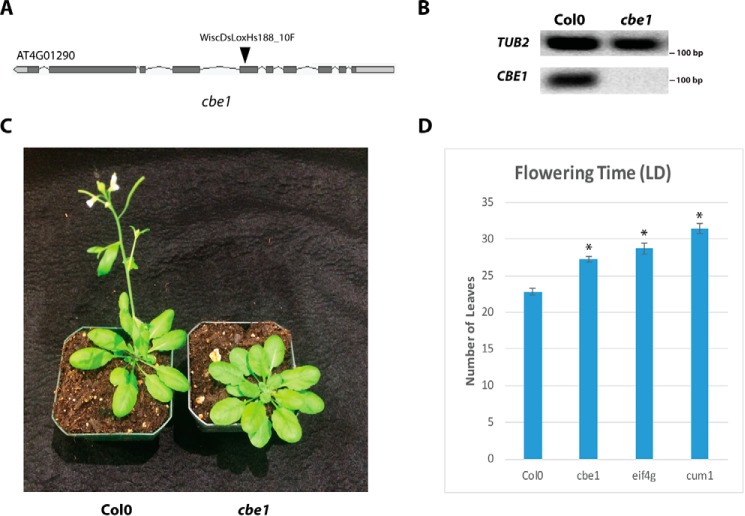
To further investigate the role of CBE1 in plant gene regulation, we obtained the T-DNA line cbe1 with an insertion in the sixth exon of the gene, which was confirmed to eliminate expression of the full-length CBE1 transcript (Fig. 4, A and B). cbe1 plants are viable and robust but show delayed development relative to WT Col0 plants (Fig. 4C). As CBE1 is encoded by a single gene in A. thaliana and has no clear homologues, it appears nonessential, as observed for single gene knockouts of most cap-binding complex components (24–28). As the cbe1 T-DNA insertion occurs downstream of the eIF4E-binding site, it is possible a partial N-terminal product with eIF4E-binding capacity might be present in the mutant; however, the RNA-Seq data (see below) indicate that the transcript formed is not processed correctly and it is unlikely any protein product is made. The effects of the cbe1 mutation on plant development were examined, along with the cum1 mutant lacking eIF4E and a mutant of eif4g, a T-DNA line lacking full-length EIF4G transcript (Fig. S2) (28). A delayed flowering time under long day growth was observed for cbe1, and this delay in flowering is also shared by both the cum1 and eif4g mutants (Fig. 4D). Interestingly, the rate of root growth was unaffected in cbe1, whereas cum1 and eif4g show slower rates of root elongation than WT (Fig. S3). The presence of eIF4E, as well as its large subunit binding partner eIF4G, therefore seems necessary for proper execution of the developmental program in A. thaliana.
Figure 4.
cbe1 mutant of A. thaliana. A, site of T-DNA insertion in the sixth exon of the CBE1 coding region. The eIF4E-binding motif is present in the second exon. B, RT-PCR of 7-day-old WT and cbe1 seedlings grown on MS plates. CBE1 expression is lost in the cbe1 mutant. TUB2 expression serves as a control. C, cbe1 mutants show developmental delay relative to WT Col0 plants. Six-week-old plants grown under long day conditions (16 h light, 8 h dark) shown. D, cap-binding complex mutants show delayed flowering time under long day conditions. Error bars represent S.E. (n = 24–28). Asterisks indicate significantly delayed flowering time in mutant lines compared with Col0 (p value < 0.05, Student's t test).
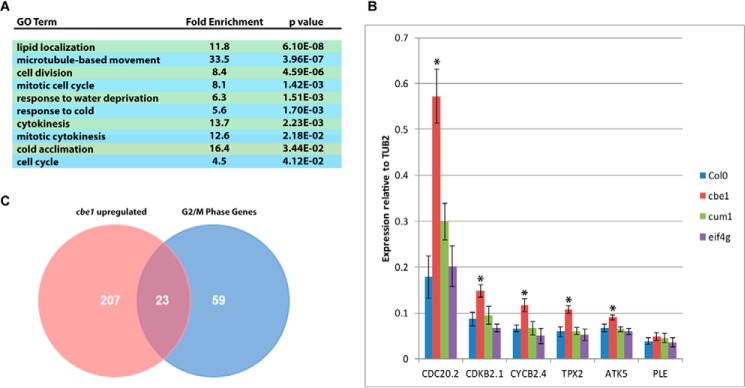
RNA-Seq of mRNA from Col0 and cbe1 seedlings was performed, revealing up-regulation of 230 nuclear-encoded genes and down-regulation of 122 genes in the mutant line (Table S1). Genes overexpressed in the cbe1 mutant have Gene Ontology (GO) term enrichment in cell cycle processes, particularly those related to mitotic plate division (Fig. 5A), whereas down-regulated genes show no significantly enriched gene ontology processes. The G2/M boundary in the cell cycle is an important checkpoint in assuring genome integrity and preparing the cell for division by forming mitotic spindles (29). Previously identified genes with an expression peak at the G2/M boundary (30) show significant enrichment in cbe1 mutant seedlings; 23 out of 82 of these genes are up-regulated in the mutant (Fig. 5C).
Figure 5.
cbe1 mutants show dysregulation of cell cycle gene expression. A, GO term enrichment of cbe1 mutant up-regulated genes by AmiGO 2 (44). No enriched GO terms were observed for down-regulated genes in the mutant. B, qRT-PCR of G2/M phase–specific genes identified by sequencing as overexpressed in cbe1 plants. Gene expression relative to TUB2 was measured in three biological replicates with at least three technical replicates. Error bars represent S.E. (n = 3–6). Asterisks indicate genes with expression significantly varying in the cbe1 mutant compared with Col0 (p value < 0.05, Student's t test). C, Venn diagram showing overlap of up-regulated genes in cbe1 mutant and G2/M phase–specific genes (30).
Overexpression of G2/M-specific genes in cbe1 was confirmed by testing six representative genes by qRT-PCR in WT and mutant plants as well as eIF4E (cum1) and eIF4G (eif4g) knockout backgrounds (Fig. 5B). Five of the six genes showed significant up-regulation in the cbe1 mutant, whereas none were affected in the cum1 or eif4g plants. These genes include the CYCB2.4 and CDKB2.1 genes involved in cyclin-dependent regulation at the G2/M checkpoint (30) and CDC20.2, which is an important component of the mitotic checkpoint complex (31). TPX2 and ATK5, important regulators of mitotic spindle assembly (29, 32), also show increased expression in the cbe1 mutant. The results of the qRT-PCR confirm that disruption of CBE1 activity in A. thaliana leads to overexpression of genes involved in mitotic cytokinesis at the G2/M boundary of the cell cycle, suggesting a role in regulation. Leaf epidermal cells were co-stained with propidium iodide and 4′,6-diamidino-2-phenylindole (DAPI) and imaged with a confocal microscope (Fig. S4); however, there were no significant differences in cell morphology or density noted between cbe1 and Col0.
Discussion
Whereas mechanisms that regulate translation initiation in other eukaryotic systems such as mammals and yeast are well understood, similar mechanisms have largely not been identified in plants. RNA-binding proteins have been described which regulate the translational state of specific transcripts, but global mechanisms for controlling translation remain unclear. In other eukaryotic systems, 4E-BPs control the availability of eIF4E for translation initiation, or by forming complexes with eIF4E that bind to transcripts to repress translation by blocking recognition of the mRNA cap by eIF4F. To date, the presence of similar eIF4E-binding proteins in plants with primary roles in gene regulation has not been substantiated.
This report describes a plant-specific protein, CBE1, with an evolutionarily conserved N-terminal motif for binding eIF4E. A. thaliana CBE1 has the capability to bind eIF4E and eIFiso4E to form cap-binding complexes in vitro and forms binding complexes in vivo in an eIF4E-dependent manner. Plants lacking CBE1 protein show dysregulation of cell cycle transcripts, accumulating higher levels of mRNA-encoding proteins involved in mitotic processes relative to WT plants.
Although the lipoxygenase LOX2 has previously been demonstrated to have the ability to bind eIFiso4E, its potential eIF4E-binding capacity was only shown in a LacZ activity assay (16). CBE1 represents the first bona fide plant protein described outside of eIF4G with strong evidence of direct interaction with eIF4E to form a cap-binding complex that may contribute to gene regulation. Outside of the eIF4E-binding motif, CBE1 contains no known functional domains and the processes in which it may be involved are unclear. It has, however, recently been identified as a member of the mRNA-bound proteome (33), strengthening the case that it serves a role in gene regulation.
CBE1 appears to be required for proper regulation of mitotic cell cycle transcripts, and mutants lacking eIF4E or eIF4G do not appear to have similar effects on these transcripts. Single deletions of eIF4E or eIFiso4E have minor effects in A. thaliana, apparently because of compensation by promiscuous binding of the alternative available protein, whereas double mutants are lethal (23, 34). It is unclear, therefore, whether gene regulatory activity of CBE1 is cap-dependent, based on the observations here. It is also not clear whether CBE1 is involved in an upstream process of regulating G2/M specific genes or whether it acts directly on these transcripts in some way.
Further investigation of the eIF4E-CBE1 complex will be required to determine the mechanism by which the complex contributes to specific gene regulation. As CBE1 is a large protein, it could potentially act as a scaffold similarly to eIF4G and eIFiso4G to recruit other effector proteins to bound transcripts. However, as it lacks the HEAT domains of eIF4G family proteins that provide interaction with eIF4A and other translation initiation components, it may have more in common with proteins involved in translational repression of targeted transcripts. The downstream effects of loss of CBE1 implicate it as a potential regulator of important transcript(s) involved in the G2/M cell cycle checkpoint. Mammalian translation factors are known to be important regulators of cell cycle progression (35–37). CBE1 may constitute the first plant-specific factor acting in a similar manner.
Experimental procedures
Plant lines
A. thaliana T-DNA lines cbe1–1 (WiscDsLoxHs188_10F) and eif4g (SALK_008031) were obtained from the Arabidopsis Biological Resource Center (Ohio State University). The cum1 mutant of eIF4E has been described previously (24).
CBE1 phylogeny
Predicted protein sequences from representative plant species with homology to A. thaliana CBE1 were collected using Phytozome (38). A phylogenetic tree was generated using the http://www.phylogeny.fr/3 pipeline (39) with alignment by MUSCLE and tree construction by PhyML using 500 bootstrap replicates.
CBE1 cloning and expression
A. thaliana CBE1 coding sequence was codon optimized using DNAWorks (40) and cloned by overlap PCR. As the full-length protein was prone to high levels of degradation when expressed in Escherichia coli, the N-terminal coding portion (amino acid residues 1–230) was subcloned into pGEX-4T-1 (GE Healthcare) for expression of GST-tagged construct. GST-CBE11–230 was expressed in BL21(DE3) E. coli and purified using GSH Sepharose 4B (GE Healthcare) and dialyzed in PBS, pH 7.3. Purification of eIF4E, eIFiso4E, and 4EHP were as described previously (22, 41). Inverse PCR with the Q5® Site-Directed Mutagenesis Kit (New England Biolabs) was used to create the CBE1 substitution mutation (GST-CBE1mut1–230). The mutation was confirmed by DNA sequencing. The mutant protein was expressed and purified as described for GST-CBE11–230.
Pulldown assays
For GST pulldown assays, A. thaliana eIF4E, eIFiso4E, or 4EHP was mixed with either GST-CBE11–230, GST-CBE1mut1–230, or GST in equimolar ratio in PBS, pH 7.3. The mixtures were added to pre-equilibrated GSH Sepharose 4B beads and incubated on ice for 10 min with mixing. The beads were then washed three times with PBS, pH 7.3, and the protein was eluted with 10 mm GSH, 50 mm Tris-HCl, pH 7.6. For 7-methylguanosine pulldown assays, GST-CBE11–230 was mixed with eIF4E or eIFiso4E (or buffer control) in equimolar ratio in binding buffer (100 mm HEPES, pH 7.6, 5% glycerol, 50 mm KCl, 5 mm EDTA, 0.1% Triton X-100, 5 mm DTT). The mixture (or control) was added to pre-equilibrated 7-methylguanosine Sepharose beads (Jena Bioscience) and incubated on ice for 20 min with mixing. The beads were then washed four times with buffer, and protein was eluted with 2× Laemmli Sample Buffer (Bio-Rad). All pulldown assays were repeated at least three times with similar results.
Competition assay
Equimolar mixtures of eIF4E, eIFiso4E, and GST-CBE11–230 in PBS, pH 7.3, were subjected to competition with 2-fold or 4-fold molar excess of eIF4E or eIFiso4E. The mixtures were used in a GST pulldown as described above. The input and eluted fractions (10 μl) were separated on a Mini-PROTEAN® TGX Stain-FreeTM gel (Bio-Rad) to directly image the proteins as per the manufacturer's instructions using the ChemiDoc MP Imager (Bio-Rad) and ImageLab software (Bio-Rad).
Western blotting
Samples were separated by SDS-PAGE on 4–20% Mini-PROTEAN TGX (Bio-Rad) gel, turbo blotted onto PVDF, blocked with PBST (8 mm Na2HPO4, 0.15 m NaCl, 2 mm KH2PO4, 3 mm KCl, 0.05% Tween® 20, pH 7.4) containing 5% nonfat dry milk and probed with rabbit antibodies (1:1000 in PBST/milk) to A. thaliana eIF4E (22), A. thaliana eIFiso4E (26), A. thaliana 4EHP (41), or GST (Thermo Fisher Scientific). Horseradish peroxidase–linked secondary antibody (Kirkegarrd-Perry, 1/20,000 in PBST/milk) was detected with SuperSignal West Pico or Femto chemiluminescent substrates (Thermo Fisher Scientific). Blots were imaged with a ChemiDoc MP Imager (Bio-Rad) and ImageLab software (Bio-Rad).
Cap-binding complex MS
Cap-binding complexes were purified from cell lysate of 300 mg tissue from Col0 or cum1 12-day-old seedlings grown on 1% sucrose MS plates. The tissue was frozen in liquid nitrogen, ground, and homogenized in binding buffer described above for 7-methylguanosine pulldown with addition of Pierce Protease Inhibitor (Thermo Fisher Scientific). The homogenized lysate was clarified by centrifugation and added to pre-equilibrated 7-methylguanosine Sepharose beads (Jena Bioscience) for overnight incubation at 4 °C with mixing. The beads were washed four times with buffer and then eluted with 2× Laemmli Sample Buffer (Bio-Rad). The eluate was loaded onto a 4–20% Mini-PROTEAN TGX (Bio-Rad) gel and briefly subjected to electrophoresis (about 10 min) and Coomassie Blue stained, and the protein mixture gel band excised for MS. The gel slice was submitted to The University of Texas at Austin Proteomics Facility for trypsin digest and LC-MS/MS analysis by OrbiTrap Fusion (Thermo Scientific) with spectra visualization by Scaffold 4 (Proteome Software). Cutoff for inclusion of spectra was set at 5% FDR for protein and 0.1% FDR for peptide.
RNA-Seq
Tissue from 7-day-old seedlings grown on 1% sucrose MS plates was frozen in liquid nitrogen and powdered by mortar and pestle, and the RNA was extracted using RNeasy Plant Mini Kit (Qiagen). The RNA was treated with DNase I recombinant RNase-free (Roche) and purified using the RNeasy Mini Kit (Qiagen). Poly(A) RNA libraries were constructed and sequenced by the Genome Sequencing and Analysis Facility at The University of Texas at Austin. Sequencing data were mapped with TopHat to the Arabidopsis thaliana genome release 10 (42), and genes with significantly altered expression were identified with CuffDiff (43). Differentially regulated genes were defined as having a 2-fold change in gene expression and an FDR-adjusted p value (q-value) <0.05. Gene ontology term enrichment analysis was performed with AmiGO 2 (44). RNA sequence data are available in the Gene Expression Omnibus (GEO) GSE114363.
qRT-PCR
Quantitative real-time PCR was performed on a ViiA 7 Real-Time PCR System (Thermo Fisher Scientific) using PowerUp SYBR Green (Applied Biosystems) under standard conditions. RNA was extracted from 7-day-old seedlings as described for RNA-Seq. cDNA was generated with oligo(dT) and Superscript IV Reverse Polymerase (Thermo Fisher Scientific). qRT-PCR primers were designed using Primer3 (45) and are listed in Table S2. TUB2 primers were described previously (46).
Propidium iodide staining
Leaves from 21-day-old A. thaliana Col0 or cbe1 seedlings were floated in 10 μg/ml propidium iodide and 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) in water for 10 min in the dark, then mounted on slides. Confocal microscopy (20×, 514 nm laser) was performed at the Center for Biomedical Research Support at the University of Texas at Austin with a Zeiss LSM 710 confocal microscope (ZEISS).
Author contributions
R. M. P. conceptualization; R. M. P. and J. C. H. L. data curation; R. M. P., J. C. H. L., J. R. J. T., S.-H. Y., G. S. C., and K. S. B. formal analysis; R. M. P., S.-H. Y., G. S. C., and K. S. B. supervision; R. M. P., J. C. H. L., and K. S. B. validation; R. M. P., J. C. H. L., J. R. J. T., S.-H. Y., G. S. C., and K. S. B. investigation; R. M. P. visualization; R. M. P., J. C. H. L., S.-H. Y., G. S. C., and K. S. B. methodology; R. M. P. and K. S. B. writing-original draft; R. M. P., S.-H. Y., G. S. C., and K. S. B. writing-review and editing; K. S. B. resources; K. S. B. funding acquisition; K. S. B. project administration.
Supplementary Material
Acknowledgments
We thank Dr. Andrew Lellis and Anna Webb (Center for Biomedical Research Support) for assistance with the microscopy.
This work was supported by National Science Foundation Grant MCB1052530 (to K. S. B.). The authors declare that they have no conflicts of interest with the contents of this article.
RNA sequences generated in this study have been deposited in the NCBI Gene Expression Omnibus, accession number GSE114363.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- eIF4E
- eukaryotic initiation factor 4E
- BTF3
- basic transcription factor 3
- qRT-PCR
- quantitative RT-PCR
- PBST
- PBS with Tween 20
- FDR
- false discovery rate.
References
- 1. Topisirovic I., Svitkin Y. V., Sonenberg N., and Shatkin A. J. (2011) Cap and cap-binding proteins in the control of gene expression. Wiley. Interdiscip. Rev. RNA 2, 277–298 10.1002/wrna.52 [DOI] [PubMed] [Google Scholar]
- 2. Browning K. S., and Bailey-Serres J. (2015) Mechanism of cytoplasmic mRNA translation. Arabidopsis Book 13, e0176 10.1199/tab.0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson R. J., Hellen C. U., and Pestova T. V. (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 10.1038/nrm2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richter J. D., and Sonenberg N. (2005) Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433, 477–480 10.1038/nature03205 [DOI] [PubMed] [Google Scholar]
- 5. Gross J. D., Moerke N. J., von der Haar T., Lugovskoy A. A., Sachs A. B., McCarthy J. E. G., and Wagner G. (2003) Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell 115, 739–750 10.1016/S0092-8674(03)00975-9 [DOI] [PubMed] [Google Scholar]
- 6. Rhoads R. E. (2009) eIF4E: new family members, new binding partners, new roles. J. Biol. Chem. 284, 16711–16715 10.1074/jbc.R900002200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mamane Y., Petroulakis E., LeBacquer O., and Sonenberg N. (2006) mTOR, translation initiation and cancer. Oncogene 25, 6416–6422 10.1038/sj.onc.1209888 [DOI] [PubMed] [Google Scholar]
- 8. Browning K. S. (2014) Plant translational machinery. in Molecular Biology (Howell S. H. ed), 2nd Ed., pp. 129–151, Springer, New York [Google Scholar]
- 9. Ferraiuolo M. A., Basak S., Dostie J., Murray E. L., Schoenberg D. R., and Sonenberg N. (2005) A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J. Cell Biol. 170, 913–924 10.1083/jcb.200504039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stebbins-Boaz B., Cao Q. P., de Moor C. H., Mendez R., and Richter J. D. (1999) Maskin is a CPEB-associated factor that transiently interacts with eIF-4E. Mol. Cell 4, 1017–1027 10.1016/S1097-2765(00)80230-0 [DOI] [PubMed] [Google Scholar]
- 11. Jung M. Y., Lorenz L., and Richter J. D. (2006) Translational control by neuroguidin, a eukaryotic initiation factor 4E and CPEB binding protein. Mol. Cell Biol. 26, 4277–4287 10.1128/MCB.02470-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson M. R., Leidal A. M., and Smibert C. A. (2004) Drosophila Cup is an eIF4E-binding protein that functions in Smaug-mediated translational repression. EMBO J. 23, 150–159 10.1038/sj.emboj.7600026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakamura A., Sato K., and Hanyu-Nakamura K. (2004) Drosophila cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev. Cell 6, 69–78 10.1016/S1534-5807(03)00400-3 [DOI] [PubMed] [Google Scholar]
- 14. Hernández G., Miron M., Han H., Liu N., Magescas J., Tettweiler G., Frank F., Siddiqui N., Sonenberg N., and Lasko P. (2013) Mextli is a novel eIF4E-binding protein that promotes translation in Drosophila. Mol. Cell Biol. 33, 2854–2864 10.1128/MCB.01354-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayberry L. K., Allen M. L., Nitka K. R., Campbell L., Murphy P. A., and Browning K. S. (2011) Plant Cap-binding complexes eukaryotic initiation factors eIF4F and eIFISO4F: Molecular specificity of subunit binding. J. Biol. Chem. 286, 42566–42574 10.1074/jbc.M111.280099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Freire M. A., Tourneur C., Granier F., Camonis J., El Amrani A., Browning K. S., and Robaglia C. (2000) Plant lipoxygenase 2 is a translation initiation factor-4E-binding protein. Plant Mol. Biol. 44, 129–140 10.1023/A:1006494628892 [DOI] [PubMed] [Google Scholar]
- 17. Freire M. A. (2005) Translation initiation factor (iso) 4E interacts with BTF3, the β subunit of the nascent polypeptide-associated complex. Gene 345, 271–277 10.1016/j.gene.2004.11.030 [DOI] [PubMed] [Google Scholar]
- 18. Miras M., Truniger V., Silva C., Verdaguer N., Aranda M. A., and Querol-Audí J. (2017) Structure of eIF4E in complex with an eIF4G peptide supports a universal bipartite binding mode for protein translation. Plant Physiol. 174, 1476–1491 10.1104/pp.17.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bush M. S., Hutchins A. P., Jones A. M., Naldrett M. J., Jarmolowski A., Lloyd C. W., and Doonan J. H. (2009) Selective recruitment of proteins to 5′ cap complexes during the growth cycle in Arabidopsis. Plant J. 59, 400–412 10.1111/j.1365-313X.2009.03882.x [DOI] [PubMed] [Google Scholar]
- 20. Mader S., Lee H., Pause A., and Sonenberg N. (1995) The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4γ and the translational repressors 4E-binding proteins. Mol. Cell Biol. 15, 4990–4997 10.1128/MCB.15.9.4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dostie J., Ferraiuolo M., Pause A., Adam S. A., and Sonenberg N. (2000) A novel shuttling protein, 4E-T, mediates the nuclear import of the mRNA 5′ cap-binding protein, eIF4E. EMBO J. 19, 3142–3156 10.1093/emboj/19.12.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patrick R. M., Mayberry L. K., Choy G., Woodard L. E., Liu J. S., White A., Mullen R. A., Tanavin T. M., Latz C. A., and Browning K. S. (2014) Two Arabidopsis loci encode novel eukaryotic initiation factor 4E isoforms that are functionally distinct from the conserved plant eukaryotic initiation factor 4E. Plant Physiol. 164, 1820–1830 10.1104/pp.113.227785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patrick R. M., and Browning K. S. (2012) The eIF4F and eIFiso4F complexes of plants: An evolutionary perspective. Comp. Funct. Genomics 2012, 287814 10.1155/2012/287814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshii M., Nishikiori M., Tomita K., Yoshioka N., Kozuka R., Naito S., and Ishikawa M. (2004) The Arabidopsis cucumovirus multiplication 1 and 2 loci encode translation initiation factors 4E and 4G. J. Virol. 78, 6102–6111 10.1128/JVI.78.12.6102-6111.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lellis A. D., Kasschau K. D., Whitham S. A., and Carrington J. C. (2002) Loss-of-susceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF(iso)4E during potyvirus infection. Curr. Biol. 12, 1046–1051 10.1016/S0960-9822(02)00898-9 [DOI] [PubMed] [Google Scholar]
- 26. Duprat A., Caranta C., Revers F., Menand B., Browning K. S., and Robaglia C. (2002) The Arabidopsis eukaryotic initiation factor (iso)4E is dispensable for plant growth but required for susceptibility to potyviruses. Plant J. 32, 927–934 10.1046/j.1365-313X.2002.01481.x [DOI] [PubMed] [Google Scholar]
- 27. Lellis A. D., Allen M. L., Aertker A. W., Tran J. K., Hillis D. M., Harbin C. R., Caldwell C., Gallie D. R., and Browning K. S. (2010) Deletion of the eIFiso4G subunit of the Arabidopsis eIFiso4F translation initiation complex impairs health and viability. Plant Mol. Biol. 74, 249–263 10.1007/s11103-010-9670-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gallie D. R. (2018) Plant growth and fertility requires functional interactions between specific PABP and eIF4G gene family members. PLoS One 13, e0191474 10.1371/journal.pone.0191474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vos J. W., Pieuchot L., Evrard J. L., Janski N., Bergdoll M., de Ronde D., Perez L. H., Sardon T., Vernos I., and Schmit A. C. (2008) The plant TPX2 protein regulates prospindle assembly before nuclear envelope breakdown. Plant Cell 20, 2783–2797 10.1105/tpc.107.056796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Menges M., de Jager S. M., Gruissem W., and Murray J. A. (2005) Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J 41, 546–566 10.1111/j.1365-313X.2004.02319.x [DOI] [PubMed] [Google Scholar]
- 31. Kevei Z., Baloban M., Da Ines O., Tiricz H., Kroll A., Regulski K., Mergaert P., and Kondorosi E. (2011) Conserved CDC20 cell cycle functions are carried out by two of the five isoforms in Arabidopsis thaliana. PLoS One 6, e20618 10.1371/journal.pone.0020618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ambrose J. C., and Cyr R. (2007) The kinesin ATK5 functions in early spindle assembly in Arabidopsis. Plant Cell 19, 226–236 10.1105/tpc.106.047613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reichel M., Liao Y., Rettel M., Ragan C., Evers M., Alleaume A. M., Horos R., Hentze M. W., Preiss T., and Millar A. A. (2016) In planta determination of the mRNA-binding proteome of Arabidopsis etiolated seedlings. Plant Cell 28, 2435–2452 10.1105/tpc.16.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Callot C., and Gallois J. L. (2014) Pyramiding resistances based on translation initiation factors in Arabidopsis is impaired by male gametophyte lethality. Plant Signal. Behav. 9, e27940 10.4161/psb.27940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao Q. P., and Richter J. D. (2002) Dissolution of the maskin-eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation. EMBO J. 21, 3852–3862 10.1093/emboj/cdf353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fingar D. C., Richardson C. J., Tee A. R., Cheatham L., Tsou C., and Blenis J. (2004) mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol. Cell Biol. 24, 200–216 10.1128/MCB.24.1.200-216.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dobrikov M. I., Shveygert M., Brown M. C., and Gromeier M. (2014) Mitotic phosphorylation of eukaryotic initiation factor 4G1 (eIF4G1) at Ser1232 by Cdk1:cyclin B inhibits eIF4A helicase complex binding with RNA. Mol. Cell Biol. 34, 439–451 10.1128/MCB.01046-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodstein D. M., Shu S., Howson R., Neupane R., Hayes R. D., Fazo J., Mitros T., Dirks W., Hellsten U., Putnam N., and Rokhsar D. S. (2012) Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 40, D1178–D1186 10.1093/nar/gkr944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J. F., Guindon S., Lefort V., Lescot M., Claverie J. M., and Gascuel O. (2008) Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–W469 10.1093/nar/gkn180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoover D. M., and Lubkowski J. (2002) DNAWorks: An automated method for designing oligonucleotides for PCR-based gene synthesis. Nucleic Acids Res. 30, e43 10.1093/nar/30.10.e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ruud K. A., Kuhlow C., Goss D. J., and Browning K. S. (1998) Identification and characterization of a novel cap-binding protein from Arabidopsis thaliana. J. Biol. Chem. 273, 10325–10330 10.1074/jbc.273.17.10325 [DOI] [PubMed] [Google Scholar]
- 42. Lamesch P., Berardini T. Z., Li D., Swarbreck D., Wilks C., Sasidharan R., Muller R., Dreher K., Alexander D. L., Garcia-Hernandez M., Karthikeyan A. S., Lee C. H., Nelson W. D., Ploetz L., Singh S., Wensel A., and Huala E. (2012) The Arabidopsis Information Resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 40, D1202–D1210 10.1093/nar/gkr1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D. R., Pimentel H., Salzberg S. L., Rinn J. L., and Pachter L. (2012) Differential gene and transcript expression analysis of RNA-Seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gene Ontology Consortium (2015) Gene ontology consortium: Going forward. Nucleic Acids Res. 43, D1049–D1056 10.1093/nar/gku1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B. C., Remm M., and Rozen S. G. (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115 10.1093/nar/gks596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jiang D., Yang W., He Y., and Amasino R. M. (2007) Arabidopsis relatives of the human lysine-specific Demethylase1 repress the expression of FWA and FLOWERING LOCUS C and thus promote the floral transition. Plant Cell 19, 2975–2987 10.1105/tpc.107.052373 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.