Animal, plant, and fungal cells occupy environments that impose changes in oxygen tension. Consequently, many species have evolved mechanisms that permit robust adaptation to these changes. The fungal pathogen Candida albicans can colonize hypoxic (low oxygen) niches in its human host, such as the lower gastrointestinal tract and inflamed tissues, but to colonize its host, the fungus must also evade local immune defenses. We reveal, for the first time, a defined link between hypoxic adaptation and immune evasion in C. albicans. As this pathogen adapts to hypoxia, it undergoes changes in cell wall structure that include masking of β-glucan at its cell surface, and it becomes better able to evade phagocytosis by innate immune cells. We also define the signaling mechanisms that mediate hypoxia-induced β-glucan masking, showing that they are dependent on mitochondrial signaling and the cAMP-protein kinase pathway. Therefore, hypoxia appears to trigger immune evasion in this fungal pathogen.
KEYWORDS: hypoxia, Candida albicans, cell wall, β-glucan masking, mitochondrial signaling, cAMP-protein kinase A signaling
ABSTRACT
Organisms must adapt to changes in oxygen tension if they are to exploit the energetic benefits of reducing oxygen while minimizing the potentially damaging effects of oxidation. Consequently, organisms in all eukaryotic kingdoms display robust adaptation to hypoxia (low oxygen levels). This is particularly important for fungal pathogens that colonize hypoxic niches in the host. We show that adaptation to hypoxia in the major fungal pathogen of humans Candida albicans includes changes in cell wall structure and reduced exposure, at the cell surface, of β-glucan, a key pathogen-associated molecular pattern (PAMP). This leads to reduced phagocytosis by murine bone marrow-derived macrophages and decreased production of IL-10, RANTES, and TNF-α by peripheral blood mononuclear cells, suggesting that hypoxia-induced β-glucan masking has a significant effect upon C. albicans-host interactions. We show that hypoxia-induced β-glucan masking is dependent upon both mitochondrial and cAMP-protein kinase A (PKA) signaling. The decrease in β-glucan exposure is blocked by mutations that affect mitochondrial functionality (goa1Δ and upc2Δ) or that decrease production of hydrogen peroxide in the inner membrane space (sod1Δ). Furthermore, β-glucan masking is enhanced by mutations that elevate mitochondrial reactive oxygen species (aox1Δ). The β-glucan masking defects displayed by goa1Δ and upc2Δ cells are suppressed by exogenous dibutyryl-cAMP. Also, mutations that inactivate cAMP synthesis (cyr1Δ) or PKA (tpk1Δ tpk2Δ) block the masking phenotype. Our data suggest that C. albicans responds to hypoxic niches by inducing β-glucan masking via a mitochondrial cAMP-PKA signaling pathway, thereby modulating local immune responses and promoting fungal colonization.
INTRODUCTION
The relationship between an opportunistic pathogen and its human host is strongly influenced by the immune status of the host and the ability of the pathogen to evade immune detection and clearance. This is particularly evident for major fungal pathogens such as Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans which are contained or cleared by most healthy individuals but which can cause life-threatening disease in immunocompromised individuals, killing more than a million people worldwide each year (1).
In immunocompetent individuals, potent innate immune defenses provide a first line of defense against these pathogenic fungi once they have penetrated external physical barriers. Myeloid cells express an array of pattern recognition receptors (PRRs) that recognize fungal cells by interacting with specific pathogen-associated molecular patterns (PAMPs), some of which lie on the fungal cell surface (2, 3). The formation of an immunological synapse between a PRR and its cognate PAMP triggers signaling events in the myeloid cell that promote the phagocytosis and killing of the fungal cell and the activation of downstream immunological effectors (4, 5).
Meanwhile, the fungal pathogen attempts to evade and resist these immunological defenses. A. fumigatus expresses the RodA hydrophobin on the surfaces of spores to mask the PAMPs melanin and β-glucan, which would otherwise be detected by the phagocytic PRRs Dectin-1, Dectin-2, and MelLec (6). C. neoformans attempts to evade immune detection by enveloping itself in a polysaccharide capsule to mask β-glucan in its cell wall (7). Similarly, C. albicans modulates PAMP exposure on its cell surface in response to host-mediated and environmental signals (8–11). The degree of β-glucan exposure on the surfaces of C. albicans cells changes during the course of systemic infection (8), and C. albicans appears to actively modify β-glucan exposure at its surface. For example, the relatively low ambient pHs associated with vulvovaginal niches have been reported to trigger elevated β-glucan exposure, leading to enhanced innate recognition of C. albicans cells by macrophages and neutrophils (10). In contrast, host-derived lactate activates β-glucan masking via a noncanonical signaling pathway involving the lactate receptor Gpr1 and the transcription factor Crz1, and this leads to reduced phagocytic recognition and attenuated cytokine responses (9).
Further observations in mice and humans reinforce the importance of the PAMP β-glucan for the immune recognition of C. albicans. In humans, a Dectin-1 polymorphism that truncates this β-glucan receptor has been associated with aberrant cytokine responses to C. albicans and susceptibility to recurrent vulvovaginitis (12). In mice, the inactivation of Dectin-1 attenuates inflammatory responses to C. albicans and permits fungal proliferation in models of systemic, gastrointestinal, and mucosal infection (13–16). However, the degree to which Dectin-1 defects affect host immunity depends on the genetic background of the host and the adaptation of C. albicans in vivo (15, 17).
Once a C. albicans cell has been recognized and phagocytosed, the phagocyte attempts to kill the pathogen by launching a chemical assault upon the phagosome contents, which includes a burst of reactive oxygen, nitrogen, and other species (18). Certain combinations of stress appear to promote the killing of C. albicans cells (19). Nevertheless, the fungus attempts to resist killing by mounting robust oxidative, nitrosative stress responses that promote fungal survival (20–24).
Hypoxia (low oxygen) represents an additional stress that fungal pathogens are exposed to in the host (25). C. albicans displays a robust response to hypoxia (26–29), and consequently, this fungus is able to colonize hypoxic niches such as the gastrointestinal tract (30, 31), as well as aerobic niches such as the skin and mucosa. C. albicans cells appear to induce both short- and long-term transcriptional responses to hypoxia. Sellam and coworkers identified Sit4, Ccr4, and Sko1 as potential regulators of the short-term response, which includes the induction of the transcription factors Tye7 and Upc2 (29). C. albicans sit4, ccr4, and sko1 mutants display hypoxic transcriptional signatures even under normoxic conditions, presumably because these mutations compromise mitochondrial functionality and oxygen utilization (29). The long-term response to hypoxia involves the upregulation of several pathways: glycolysis via Tye7 (32, 33), unsaturated fatty acid metabolism via Efg1 (26, 27), and sterol biosynthesis via Upc2 (34, 35).
We reasoned that, as low oxygen levels represent a significant input signal for fungal cells within certain host niches (25), and as hypoxia affects the expression of cell wall genes and proteins in C. albicans (26, 36), hypoxia might affect β-glucan exposure at the cell surface. Here we show that hypoxia induces β-glucan masking in C. albicans, we identify key signaling pathways that mediate hypoxia-induced β-glucan masking, and we demonstrate that hypoxia-induced β-glucan masking attenuates phagocytic recognition, uptake, and cytokine responses. This phenotype is likely to be important in the context of fungal immune detection and clearance during infection.
RESULTS
Hypoxia induces β-glucan masking at the C. albicans cell surface.
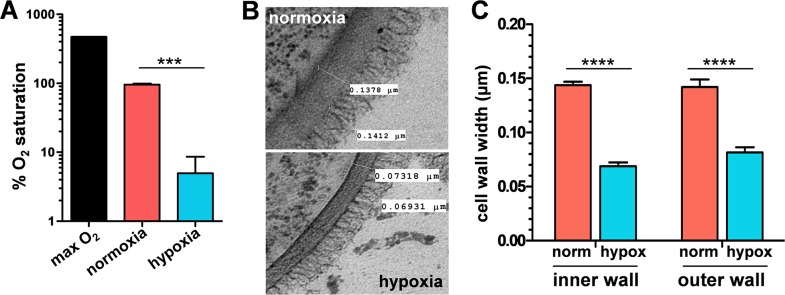
To test the impact of hypoxia on the C. albicans cell wall, we grew cells under analogous conditions to those used to examine lactate-induced β-glucan masking (9). Wild-type cells (SC5314; see Table S1 in the supplemental material) were grown in minimal media under normoxic conditions and transferred to hypoxic conditions for five hours, and then cells were harvested for analysis while still in exponential growth phase (Materials and Methods). Dissolved oxygen levels were approximately 1% of the maximum levels under these conditions (Fig. 1A). Transmission electron microscopy (TEM) revealed that hypoxia affects the architecture of the C. albicans cell wall (Fig. 1B). Quantification of these TEM images revealed significant differences between hypoxic cells and their normoxic controls with respect to the thickness of the inner glucan-chitin and outer mannan layers of their cell walls. Hypoxic cells had thinner cell walls. This is consistent with the observations that hypoxia leads to changes in cell wall gene expression and the cell wall proteome (26, 36).
FIG 1.
Hypoxia affects the architecture of the C. albicans cell wall. (A) Oxygen levels under the normoxic (pink) and hypoxic (blue) growth conditions used in this study. Means and standard deviations from three independent replicate experiments are shown. (B) Transmission electron micrographs of the cell walls of wild-type C. albicans cells (SC5314; see Table S1 in the supplemental material) grown under these normoxic and hypoxic conditions. (C) Quantification of the thickness of the inner and outer layers of the C. albicans cell wall using ImageJ from TEM images of SC5314 cells such as those shown in panel B. Means and standard deviations from images of cells (n = >30) from three independent replicate experiments are shown. The data were analyzed using ANOVA with Tukey’s multiple-comparison test and are indicated by asterisks as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
C. albicans strains used in this study. Download Table S1, PDF file, 0.1 MB (74.5KB, pdf) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
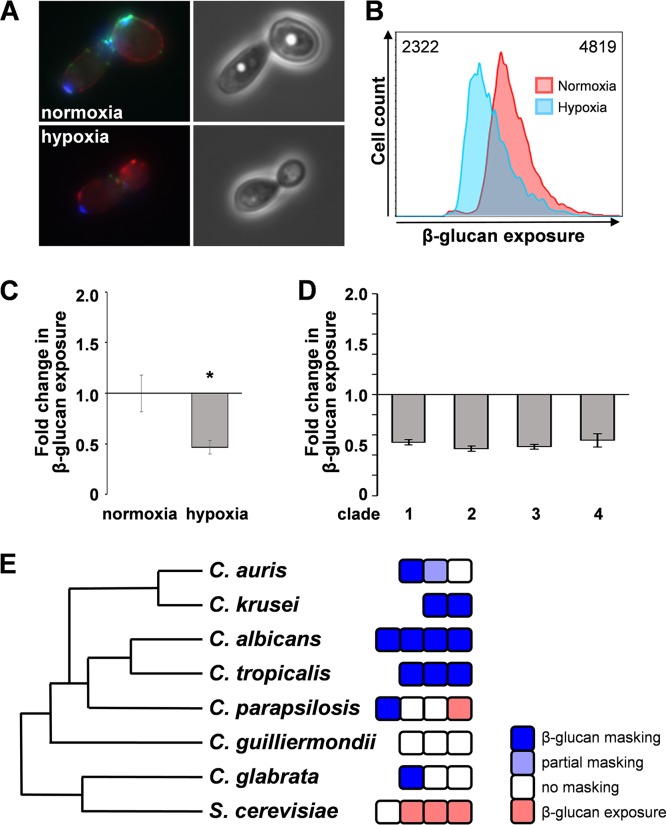
We then tested whether hypoxia affects the degree of exposure of the major PAMP β-glucan at the C. albicans cell surface. First, β-glucan exposure on hypoxic and normoxic cells was examined by microscopy. Cells growing exponentially under normoxic and hypoxic conditions were harvested and fixed, and the exposed β-glucan was stained with Fc-Dectin-1 (Fig. 2A). The cells were also stained with wheat germ agglutinin (for chitin) and concanavalin A (for mannan). Hypoxic cells displayed less Fc-Dectin-1 staining than the control normoxic cells. This was quantified by flow cytometry (Fig. 2B), and the change in β-glucan exposure was then expressed in terms of the fold change in median fluorescence intensity (MFI) for the hypoxic cell population compared to the control normoxic population (Fig. 2C). Hypoxic cells displayed a significant decrease in MFI, indicating that hypoxia induces β-glucan masking in C. albicans.
FIG 2.
Hypoxia induces β-glucan masking in C. albicans. (A) Fluorescence microscopy of β-glucan exposure on C. albicans wild-type cells (SC5314: Table S1) grown under normoxic or hypoxic conditions and stained for exposed β-glucan (Fc-dectin-1; green), mannan (concanavalin A; red), and chitin (wheat germ agglutinin; blue). (B) Analysis of β-glucan exposure on C. albicans SC5314 cells grown under normoxic or hypoxic conditions by Fc-dectin-1 staining and flow cytometry. The median fluorescence intensity (MFI) for each population is indicated. (C) The fold change in β-glucan exposure for C. albicans SC5314 cells grown under hypoxic conditions was calculated relative to the values for control normoxic cells. Means and standard deviations from three independent replicate experiments are shown, and the data were analyzed using ANOVA with Tukey’s multiple-comparison test: *, P ≤ 0.05. (D) Quantification of hypoxia-induced β-glucan masking in C. albicans clinical isolates from four major clades: clade 1, SC5314; clade 2, IHEM16614; clade 3, J990102; clade 4, AM2005/0377 (Table S1). (E) Analysis of hypoxia-induced β-glucan masking in other pathogenic Candida species and in S. cerevisiae. Each box represents a different isolate (Table S1) (the data for C. albicans were taken from panel D). Masking was defined as a change in β-glucan exposure to <0.6 fold change (dark blue); partial masking was defined as a decrease in β-glucan exposure to between 0.6- and 0.8-fold change (light blue); no masking was defined as a change in β-glucan exposure between 0.8- and 1.2-old change decrease (white); β-glucan exposure was defined as an increase in β-glucan exposure to >1.4-fold change (pink).
Hypoxia-induced β-glucan masking was observed reproducibly in representative clinical isolates from four major epidemiological clades of C. albicans (Fig. 2D), indicating that this phenotype is not specific to clade 1 (SC5314). We also observed hypoxia-induced β-glucan masking in some other pathogenic Candida species, notably in Candida tropicalis and Candida krusei. However, most Candida glabrata, Candida guilliermondii, and Candida parapsilosis isolates did not display masking, and one of the Candida parapsilosis isolates we tested even displayed β-glucan exposure in response to hypoxia. The lack of a consistent β-glucan masking phenotype in these C. parapsilosis isolates might relate to this species’ apparent habitation of diverse environmental niches as well as being a skin commensal (37). There was no clear correlation between the hypoxia-induced β-glucan masking phenotype and phylogenetic relatedness (Fig. 2E).
Hypoxia-induced β-glucan masking is not dependent on the pathway that mediates lactate-induced β-glucan masking.
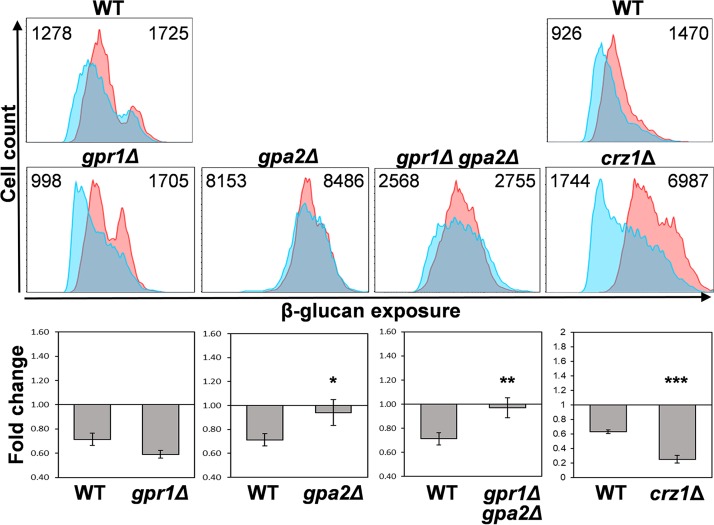
Previously we showed that lactate-induced β-glucan masking is mediated by a noncanonical signaling pathway that involves lactate receptor Gpr1 and transcription factor Crz1 (9). Therefore, we tested whether hypoxia-induced β-glucan masking is mediated by the same pathway. β-Glucan masking was quantified by flow cytometry in C. albicans mutants that lack Gpr1, its Gα protein Gpa2, or Crz1 (Fig. 3) (Table S1). The deletion of CRZ1 affected β-glucan exposure: both hypoxic and normoxic crz1Δ cell populations displayed elevated levels of exposure relative to wild-type control cells. However, the loss of Crz1 did not block hypoxia-induced β-glucan masking. Also, gpr1Δ cells retained the β-glucan masking phenotype (Fig. 3). Therefore, neither Gpr1 nor Crz1 is required for hypoxia-induced β-glucan masking, indicating that the different cellular inputs, lactate and hypoxia, trigger β-glucan masking via different signaling pathways. Interestingly, the Gα protein Gpa2 is required for hypoxia-induced β-glucan masking (Fig. 3).
FIG 3.
Hypoxia-induced β-glucan masking is not dependent on Gpr1 or Crz1. Analysis of β-glucan exposure on C. albicans mutants by flow cytometry of Fc-dectin-1-stained cells grown under normoxic (pink) or hypoxic conditions (cyan). The median fluorescence intensity (MFI) for each population is shown at the top right and left of each panel, respectively: WT, wild type, SC5314; gpr1Δ, LR2; gpa2Δ, NM6; gpr1Δ gpa2Δ, NM23; crz11Δ, DSY2195 (Table S1). The wild-type control for each experiment is shown above the mutants examined in that same experiment. The gpr1Δ and gpa2Δ mutants (middle panels) were compared together in the same experiment with the wild-type control (upper left panel), whereas the crz1Δ mutant (middle panel) was compared with wild-type cells in a different experiment (upper right panel). The fold changes in β-glucan exposure for each strain (lower panels) were calculated by dividing the MFI under hypoxic conditions by the MFI for the corresponding normoxic cells. Means and standard deviations from three independent replicate experiments are shown, and the data were analyzed using ANOVA with Tukey’s multiple-comparison test: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Hypoxia-induced β-glucan masking is not dependent on key morphogenetic or stress regulators.
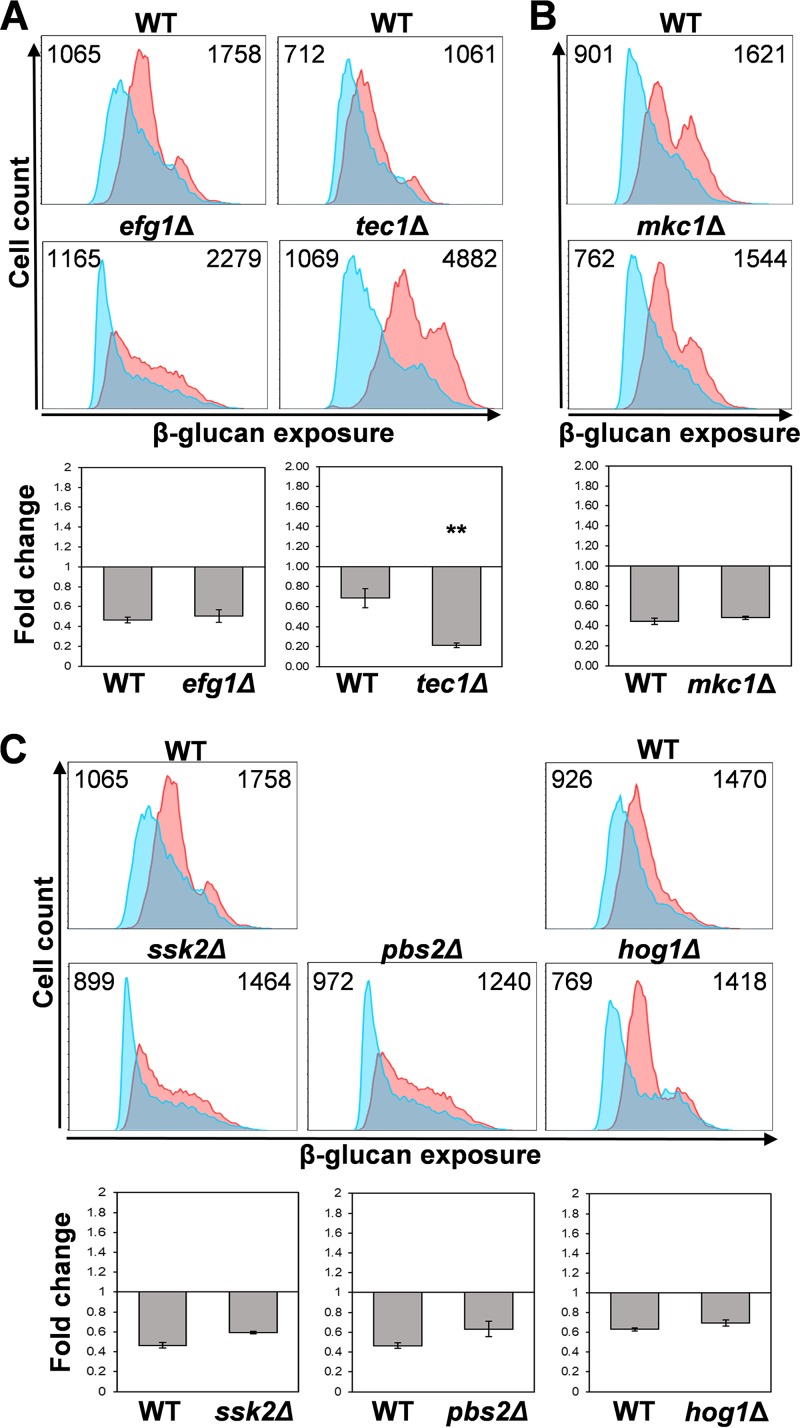
Key morphogenetic regulators such as Efg1, stress regulators such as Hog1, and the cell integrity signaling pathway are known to influence cell wall gene expression and cell wall structure in C. albicans (26, 38–44). Furthermore, Efg1 contributes to the regulation of the hypoxic response in C. albicans (26, 27), and Hog1 orthologues contribute to hypoxic responses in Saccharomyces cerevisiae and human cells (45, 46). Therefore, we tested whether these and other related regulators are required for hypoxia-induced β-glucan masking.
Hypoxia-induced β-glucan masking was retained in efg1Δ cells (Fig. 4A). Furthermore, the phenotype was maintained in tec1Δ, cph1Δ, and bcr1Δ cells (Fig. 4A; see also Fig. S1 in the supplemental material). We note that the basal levels of β-glucan exposure were perturbed in tec1Δ and efg1Δ cells, presumably because these mutations perturb the yeast cell wall (41, 47). Nevertheless, the tec1Δ and efg1Δ mutants still displayed β-glucan masking in response to hypoxia (Fig. 4A and Fig. S1). Therefore, the morphogenetic regulators Efg1, Tec1, Cph1, and Bcr1 are not required for hypoxia-induced β-glucan masking.
FIG 4.
Hypoxia-induced β-glucan masking is not dependent on key regulators of morphogenesis, cell integrity, or stress adaptation. Analysis of β-glucan exposure on C. albicans mutants by flow cytometry of Fc-dectin-1-stained cells (upper panels) grown under normoxic (pink) or hypoxic conditions (cyan). Median fluorescence intensities (MFIs) for hypoxic and normoxic cell populations are shown (top right and left of each panel, respectively). The corresponding wild-type control is shown above each mutant. The fold change in β-glucan exposure (lower panels) for each strain was calculated by dividing the MFI under hypoxic conditions by the MFI for the control normoxic cells. Means and standard deviations from three independent replicate experiments are shown, and the data were analyzed using ANOVA with Tukey’s multiple-comparison test: *, P ≤ 0.05; **, P ≤ 0.01. (A) Select morphogenetic mutants are shown in WT (wild type) (SC5314) and efg1Δ (HLC52) and tec1Δ (CaAS18) mutants (Table S1). Additional mutants are shown in Fig. S1. (B) Cell integrity pathway in the WT (SN95) and mkc1Δ (CaLC700) cells. (C) Stress-activated protein kinase pathway in WT (SC5314) and ssk2Δ (JC482), pbs2Δ (JC74), and hog1Δ (JC50) mutants. The efg1Δ, ssk1Δ, and pbs2Δ strains were analyzed in the same experiment against the same wild-type control.
Hypoxia-induced β-glucan masking in specific C. albicans mutants. Analysis of β-glucan masking by C. albicans mutants by Fc-dectin-1 staining of cells grown under normoxic (pink) or hypoxic conditions (cyan) (upper panels). Median fluorescence intensities (MFIs) for hypoxic and normoxic cell populations are shown. The corresponding wild-type control is shown above each mutant or set of mutants. Fold changes in β-glucan exposure (lower panels) represent the MFI under hypoxic conditions divided by the MFI for the corresponding control normoxic cells. Means and standard deviations from three independent replicate experiments are shown, and the data were analyzd using ANOVA with Tukey’s multiple-comparison test: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. (A) Morphogenetic mutants. WT, wild type, SC5314; cph1Δ, JKC19; efg1Δ, HLC52; bcr1Δ, CNJ702; tec1Δ, CaAS18 (Table S1). (B) Mutants affecting mitochondrial functionality. WT, wild type, DAY152; pop2Δ, YCAT51; ccr4Δ, YCAT39. The data for the efg1 Δ, tec1Δ, and ccr4Δ mutants also appear in Fig. 4 and 6. Download FIG S1, PDF file, 0.2 MB (180.7KB, pdf) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The Mkc1 MAP kinase is critical for signaling via the cell integrity pathway (39, 40). However, hypoxia-induced β-glucan masking was not perturbed in mkc1Δ cells (Fig. 4B), indicating that this masking is not dependent on the cell integrity pathway.
C. albicans cells lacking the Hog1 stress-activated protein kinase, its MAP kinase kinase Pbs2, or its MAP kinase kinase kinase Ssk2 (48, 49) retained hypoxia-induced β-glucan masking (Fig. 4C). Therefore, despite the fact that Hog1 signaling contributes to cell wall remodelling in C. albicans and to hypoxic responses in other eukaryotes (43, 45, 46), this signaling pathway is not essential for hypoxia-induced β-glucan masking in C. albicans.
Hypoxia-induced β-glucan masking is dependent on cAMP-protein kinase A signaling.
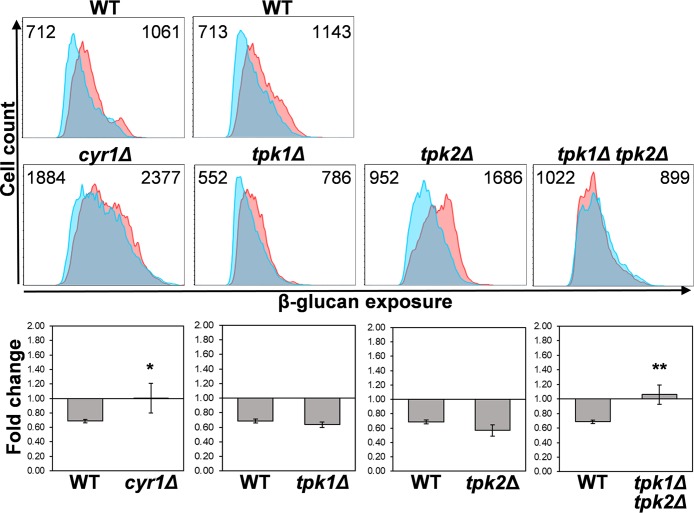
The cAMP-protein kinase A (PKA) pathway plays important roles in yeast-hypha morphogenesis, stress adaptation, and cell wall integrity in C. albicans (50–56). Therefore, we tested whether cAMP-PKA signaling is required for hypoxia-induced β-glucan masking. First, we examined C. albicans cyr1Δ cells which lack adenylyl cyclase (51), and then we tested tpk1Δ and tpk2Δ mutants in which one or both types of PKA catalytic subunit have been inactivated (56). Significantly, hypoxia-induced β-glucan masking was blocked in cyr1Δ cells and also in the tpk1Δ tpk2Δ double mutant, which lacks any PKA activity (Fig. 5). This indicates that cAMP-PKA signaling is critical for this phenotype. Interestingly, the phenotype was retained in the single tpk1Δ and tpk2Δ mutants (Fig. 5), showing that each type of catalytic subunit of PKA is capable of mediating hypoxia-induced β-glucan masking.
FIG 5.
Hypoxia-induced β-glucan masking is dependent on cAMP-PKA signaling. Cytometric analysis of β-glucan exposure on C. albicans cAMP-PKA mutants by Fc-dectin-1 staining of cells grown under normoxic (pink) or hypoxic conditions (cyan) (upper panels). Median fluorescence intensities (MFIs) for hypoxic and normoxic cell populations are shown. The corresponding wild-type control is shown above each mutant: WT, wild type (SN152) and cyr1Δ (CR323), tpk1Δ, tpk2Δ, and tpk1Δ tpk2Δ mutants (Table S1). The fold change in β-glucan exposure (lower panels) for each strain was calculated by dividing the MFI under hypoxic conditions by the MFI for the corresponding control normoxic cells. Means and standard deviations from three independent replicate experiments are shown, and the data were analyzed using ANOVA with Tukey’s multiple-comparison test: *, P ≤ 0.05; **, P ≤ 0.01.
Mutations that attenuate mitochondrial respiration affect hypoxia-induced β-glucan masking.
Next, we investigated possible mechanisms by which hypoxia might activate cAMP-PKA signaling. Structural and functional alterations in mitochondrial respiratory chain complexes are known to increase superoxide levels. Mitochondrial reactive oxygen species (ROS), such as superoxide, become elevated under hypoxic conditions, and these mitochondrial ROS are thought to contribute to hypoxic signaling (57, 58). Therefore, we examined whether mutations that affect mitochondrial functionality in C. albicans perturb hypoxia-induced β-glucan masking.
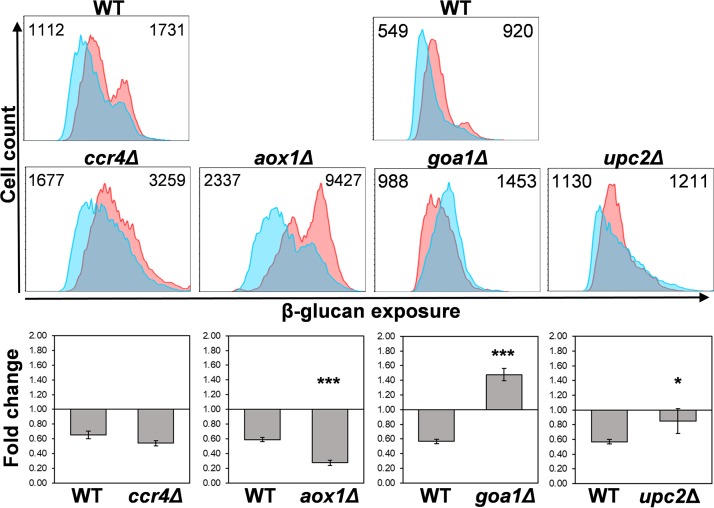
We examined ccr4Δ and pop2Δ mutants because the inactivation of this Ccr4-Pop2 mRNA deadenylase (Table S1), which regulates mRNA decay and translation, has been reported to affect mitochondrial phospholipid homeostasis and to confer sensitivity to cell wall stressors in C. albicans (59). Presumably, these pleiotropic effects account for the relatively high levels of β-glucan exposure observed for the ccr4Δ and pop2Δ cells under basal, normoxic conditions (Fig. 6 and Fig. S1). Nevertheless, hypoxia-induced β-glucan masking was retained in ccr4Δ and pop2Δ strains and was even enhanced in pop2Δ cells (Fig. 6 and Fig. S1).
FIG 6.
Mutations that perturb mitochondrial functionality affect hypoxia-induced β-glucan masking. Quantification of β-glucan exposure on C. albicans mutants by Fc-dectin-1 staining and flow cytometry of cells grown under normoxic (pink) or hypoxic conditions (cyan) (upper panels): WT, wild type (DAY185), ccr4Δ (YCAT39), aox1Δ (WH324), goa1Δ (GOA31), upc2Δ (UPC2M4A) (Table S1). Additional mutants are shown in Fig. S1. The cytometry data for the corresponding wild-type control is shown above each set of mutants analyzed in the same experiment. Median fluorescence intensities (MFIs) for hypoxic and normoxic cell populations are shown. The fold change in β-glucan exposure (lower panels) for each strain represents the MFI under hypoxic conditions divided by the MFI for the corresponding normoxic cells. Means and standard deviations from three independent replicate experiments are shown, and the data were analyzed using ANOVA with Tukey’s multiple-comparison test: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
AOX1A and AOX1B are nonsynonymous alleles which encode alternative oxidases that protect the C. albicans mitochondrion against oxidative damage (60–62), as do their homologues in other fungi and plants (63, 64). We observed that, like pop2Δ cells, a C. albicans aox1AΔ/aox1BΔ (hereafter aox1Δ) mutant that lacks both isoforms of Aox1, display significantly enhanced β-glucan masking in response to hypoxia compared to the wild-type control (Fig. 6). Normoxic aox1Δ cells displayed high basal levels of β-glucan exposure, relative to the wild-type control (Fig. 6), potentially because of abnormally high levels of mitochondrial ROS (60–64).
We then determined whether Goa1 or Upc2 influences the phenotype because both of these proteins have been implicated in mitochondrial functionality. The exact biochemical role of Goa1 remains obscure, but a GOA1 gene deletion confers oxidative stress sensitivity and results in the loss of mitochondrial membrane potential (65). In addition to its roles in the regulation of ergosterol biosynthesis, the transcription factor Upc2 regulates the expression of essential mitochondrial chaperones, mediates lipid homeostasis, and contributes to the hypoxic response in C. albicans (33, 35, 66–68). Hypoxia-induced β-glucan masking was blocked in C. albicans goa1Δ and upc2Δ mutants (Fig. 6 and Table S1). This further implicates mitochondrial signaling in activating this phenotype.
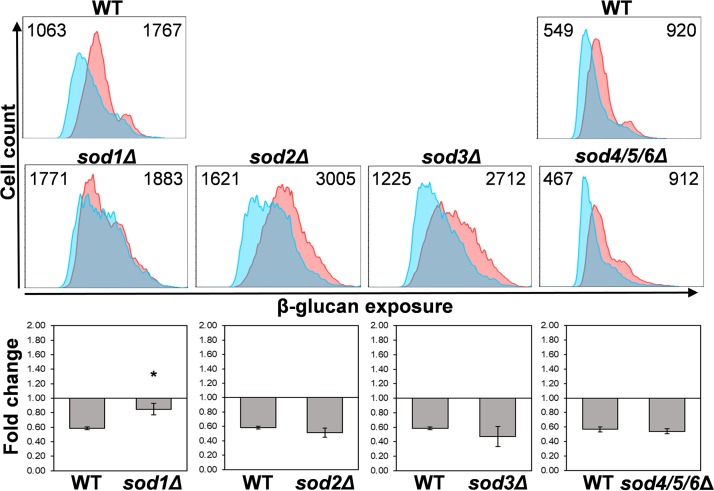
Changes in mitochondrial superoxide levels have been suggested to contribute to hypoxic signaling (57, 58). Therefore, we tested whether specific C. albicans superoxide dismutases affect hypoxia-induced β-glucan masking. C. albicans expresses six superoxide dismutases: Sod1 is localized to the intermembrane space and mitochondrial matrix, Sod2 to the mitochondrial matrix and Sod3 to the cytoplasm, whereas Sod4 to 5 are secreted (21, 62, 69–72). Mutants lacking Sod2, Sod3, Sod4, Sod5, and/or Sod6 displayed no obvious defects in hypoxia-induced β-glucan masking (Fig. 7). Although no masking defect was observed for sod2Δ and sod3Δ cells, they did display relatively high levels of β-glucan exposure under basal normoxic conditions (Fig. 7). The basis for this is not clear, but it might be related to the perturbation of intracellular ROS under normal growth conditions. However, C. albicans sod1Δ cells, which lack the only superoxide dismutase that is localized to the intermembrane space (62), displayed a significant reduction in hypoxia-induced β-glucan masking (Fig. 7). This is consistent with the view that ROS signaling in the mitochondrial intermembrane space is involved in activating β-glucan masking.
FIG 7.
Sod1 is required for hypoxia-induced β-glucan masking. C. albicans superoxide dismutase mutants were grown under normoxic (pink) or hypoxic conditions (cyan), stained with Fc-dectin-1, and analyzed by flow cytometry to examine their β-glucan exposure (upper panels). Median fluorescence intensities (MFIs) for hypoxic and normoxic cell populations are shown. The corresponding wild-type control is shown above each set of mutants, the triple sod4-6Δ mutant having been analyzed in a separate experiment from the other sodΔ mutants: WT, SC5314; sod1Δ, CA-IF003; sod2Δ, CA-IF007; sod3Δ, CA-IF011 single mutants; sod4/5/6Δ triple mutant, CA-IF070 (Table S1). The fold change in β-glucan exposure (lower panels) for each strain represents the MFI under hypoxic conditions relative to the MFI for the corresponding normoxic control. Means and standard deviations from three independent replicate experiments are shown, and the data were analyzed using ANOVA with Tukey’s multiple-comparison test: *, P ≤ 0.05.
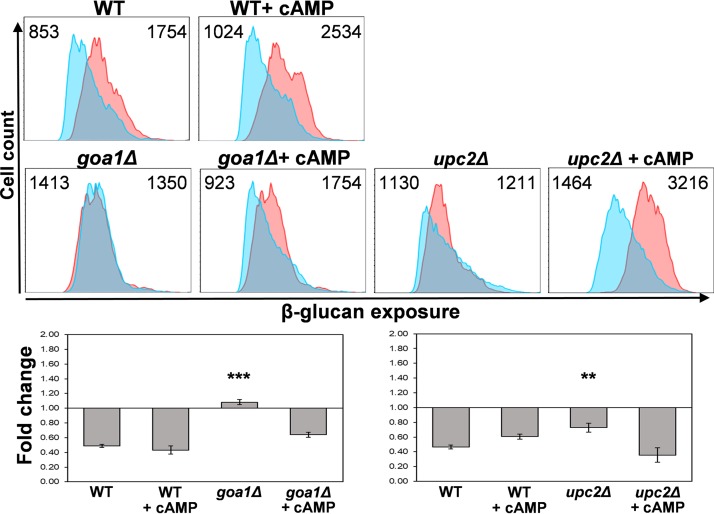
Both the cAMP-PKA pathway (Fig. 5) and mitochondrial signaling (Fig. 6) are required for hypoxia-induced β-glucan masking. To test whether the cAMP signaling might lie upstream or downstream of mitochondrial signaling, we asked whether exogenous cAMP can suppress the defect in masking observed for goa1Δ and upc2Δ cells. We used the membrane-permeable derivative, dibutyryl-cAMP (db-cAMP) for these experiments as described previously (73). Interestingly, exogenous db-cAMP suppressed the masking defect of goa1Δ and upc2Δ cells (Fig. 8), suggesting that mitochondrial signaling might lie upstream of the cAMP-PKA pathway.
FIG 8.
Exogenous dibutyryl-cAMP suppresses the defects in hypoxia-induced β-glucan masking caused by mitochondrial mutants. C. albicans (wild type [SC5314] [Table S1]), goa1Δ (GOA31), and upc2Δ cells (UPC2M4A) were grown under normoxic (pink) or hypoxic conditions (cyan) for 5 h, as described above, with 0 or 5 mM dibutyryl-cAMP (cAMP). The cells were then stained with Fc-dectin-1 and analyzed by flow cytometry to quantify their β-glucan exposure (upper panels). Median fluorescence intensities (MFIs) are shown. The fold changes in β-glucan exposure are shown (lower panels): means and standard deviations from three independent replicate experiments are analyzed using ANOVA with Tukey’s multiple-comparison test: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Hypoxia-induced β-glucan masking attenuates immune recognition and host responses.
The interactions of C. albicans with innate immune cells are affected when β-glucan masking is activated by lactate (9). In particular, lactate-induced β-glucan masking reduces neutrophil recruitment, decreases phagocytosis by macrophages, and attenuates cytokine responses. Therefore, we tested whether hypoxia-induced β-glucan masking exerts similar effects upon immune responses.
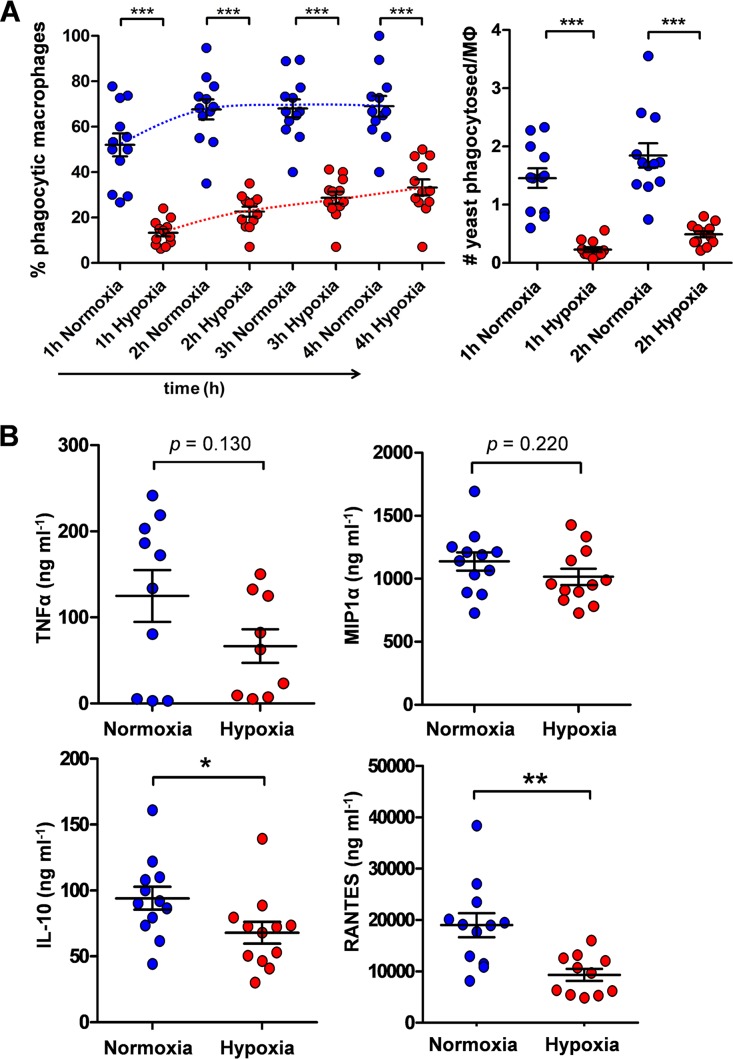
We quantified the phagocytosis of wild-type C. albicans cells by primary murine bone marrow-derived macrophages (BMDMs) from time-lapse spinning disc video microscopy. Representative videos can be viewed in Movies S1 and S2 in the supplemental material. Over the 4-h period examined, significantly fewer macrophages phagocytosed the C. albicans cells that had undergone hypoxia-induced β-glucan masking compared with the unmasked control cells (Fig. 9A). Furthermore, these macrophages ingested fewer of the masked C. albicans cells than the unmasked cells (Fig. 9A). This was consistent with the view that hypoxia-induced β-glucan masking renders C. albicans cells less visible to phagocytes. It was not possible to monitor the fate of these C. albicans cells after phagocytosis because, for technical reasons, these cells were fixed (Materials and Methods).
FIG 9.
Growth under hypoxia attenuates immune responses against C. albicans. Wild-type C. albicans cells (SC5314 [Table S1]) were grown for 5 h under normoxic (blue) or hypoxic conditions (red) and fixed. (A) At t = 0, these C. albicans cells were mixed with murine bone marrow-derived macrophages (BMDMs) at a ratio of 3:1 (yeast cells/macrophages), and the host-fungus interactions monitored by time-lapse video microscopy. The proportion of BMDMs that had phagocytosed at least one C. albicans cell (percent phagocytic macrophages) was quantified at t = 1, 2, 3, and 4 h. Also, the number of C. albicans cells phagocytosed per BMDM were quantified at t = 1 and 2 h. (B) Duplicate samples of human PBMCs from 6 different individuals were mixed with the C. albicans cells (ratio of 5:1, yeast cells/PBMCs), and TNF-α, MIP-1α, IL-10, and RANTES levels were assayed after 24 h. These data were analyzed using ANOVA with Bonferroni’s post hoc test: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Time-lapse video of BMDM interactions with normoxic C. albicans cells. Movies S1 and S2, which are representative of 12 movies in total (4 movies from 3 mice), show the first two hours of interactions between murine BMDMs and normoxic C. albicans interactions. Download Movie S1, AVI file, 18.8 MB (18.8MB, avi) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Time-lapse video of BMDM interactions with normoxic C. albicans cells. Movies S1 and S2, which are representative of 12 movies in total (4 movies from 3 mice), show the first two hours of interactions between murine BMDMs and normoxic C. albicans interactions. Download Movie S2, AVI file, 18.7 MB (18.7MB, avi) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We then quantified cytokine and chemokine responses for peripheral blood mononuclear cells (PBMCs) isolated from blood samples from healthy human volunteers. We observed a slight reduction in the levels of TNF-α and possibly MIP-1α induced by the masked C. albicans cells compared to the control unmasked cells, but these changes were not statistically significant (Fig. 9B). However, the masked cells induced significantly lower levels of the anti-inflammatory cytokine IL-10, and of the chemokine RANTES (Fig. 9B), which plays an important role in the homing and migration of effector and memory T cells. Taken together, the data clearly show that hypoxia-induced β-glucan masking affects immune responses to C. albicans cells.
DISCUSSION
Oxygen levels can vary greatly in healthy tissues, but tissues can become hypoxic during infection, and oxygen levels approach zero in the lumen of the healthy lower gastrointestinal tract, permitting colonization by obligate anaerobes (25, 74–78). C. albicans displays robust adaptation to hypoxic environments, and consequently is able to colonize such niches (26–29, 31, 79, 80). As hypoxia has been shown to affect the expression of cell wall genes and proteins in C. albicans (26, 36), we hypothesized that this host input might affect cell wall architecture, and in particular, PAMP exposure at the C. albicans cell surface. We tested this and showed that hypoxia induces significant changes in the thickness of the inner glucan-chitin and outer mannan layers of the cell wall (Fig. 1) and that hypoxia also induces β-glucan masking (Fig. 2).
Previously we showed that host-derived lactate triggers β-glucan masking in C. albicans (9). We reasoned that, given the different nature of these host inputs, lactate and hypoxia might mediate β-glucan masking via different upstream regulators. As we predicted, hypoxia-induced β-glucan masking is not dependent on the lactate receptor Gpr1 (Fig. 3) (9). However, the Gpr1-associated G-alpha protein, Gpa2, does contribute to both lactate-induced (9) and hypoxia-induced β-glucan masking (Fig. 3). This implies that Gpa2 must also be regulated by Gpr1-independent mechanisms.
Our data suggest that the hypoxic signal is mediated via the mitochondrion (Fig. 10), which would be consistent with data from fungal, plant, and mammalian systems (57, 58, 81–84). First, the inhibition of mitochondrial functionality in C. albicans (goa1, upc2) blocked hypoxia-induced β-glucan masking (Fig. 6).
FIG 10.
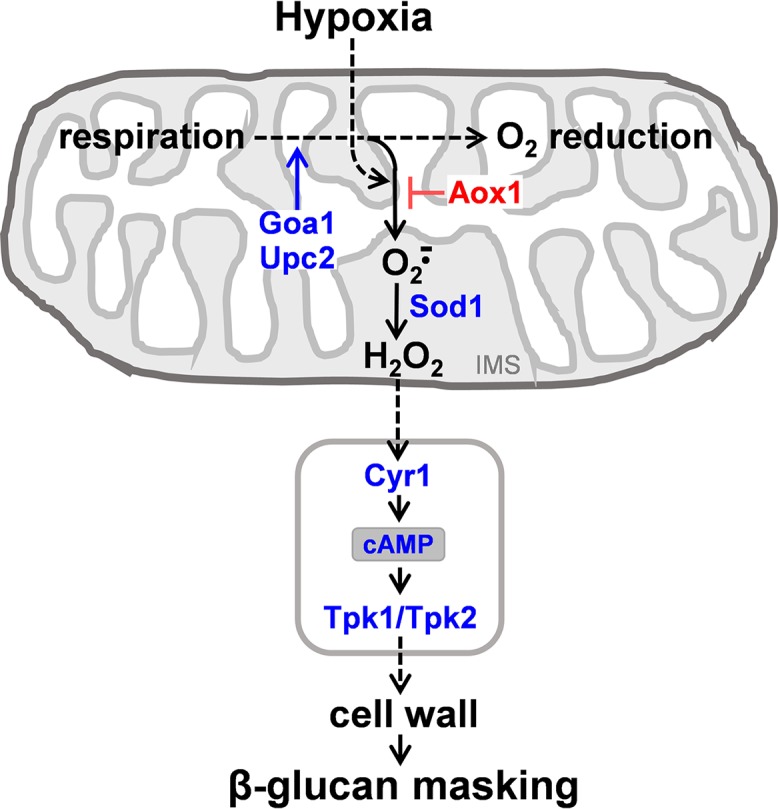
Mechanisms by which hypoxia induces β-glucan masking in C. albicans. Combining our observations with those of others, we propose the following working model. Hypoxia triggers an increase in the formation of mitochondrial superoxide by the respiratory apparatus (57, 58). Inactivating Goa1 or Upc2, which promote mitochondrial functionality, reduces overall respiration rates and hence mitochondrial ROS production. The alternative oxidase (Aox1) acts to limit mitochondrial ROS production (60–62) and therefore inactivating Aox1 enhances the signal. Superoxide dismutase within the mitochondrial inner membrane space (IMS) converts superoxide into diffusible hydrogen peroxide, which leads to the generation of a mitochondrial signal that transduces to the cytoplasm (see text). This possibly leads to the activation of adenylyl cyclase (Cyr1) and cAMP-PKA (Tpk1/2) signaling, which triggers remodelling of the cell wall and masking of cell surface β-glucan by mechanisms that remain to be elaborated.
Second, C. albicans cells that lack the mitochondrial alternative oxidase, Aox1, display enhanced hypoxia-induced β-glucan masking (Fig. 6). In C. albicans and other fungi and in plants, alternative oxidases such as Aox1 limit the superoxide generation in the respiratory chain, thereby protecting the mitochondrion against oxidative damage (60–64). Therefore, hypoxia-induced β-glucan masking is probably enhanced in response to the elevated superoxide levels in the mitochondrion of aox1Δ cells.
Third, the masking phenotype is blocked in sod1Δ cells, but not in C. albicans cells that lack any of the other superoxide dismutases (Fig. 7). Sod1 is the only superoxide dismutase that localizes to the mitochondrial intermembrane space (62). This is particularly significant because respiratory complex III releases superoxide into the mitochondrial intermembrane space (57, 85–87) and complex III is critical for hypoxic signaling (57, 58, 81, 84, 88, 89). Normally, in wild-type cells, Sod1 converts the charged, nondiffusible superoxide anion into the diffusible ROS, hydrogen peroxide. Thus, in sod1Δ cells, hydrogen peroxide production would be lowered in the mitochondrial intermembrane space.
Taken together, these data suggest that the transduction of the hypoxic signal depends on the generation of hydrogen peroxide in the mitochondrial intermembrane space (Fig. 10). Hydrogen peroxide is viewed as a candidate signaling molecule because of its relatively long half-life, its membrane permeability, and its ability to oxidize cysteines in target proteins (90, 91). Certainly, cysteine oxidative modifications have been shown to regulate the activities of key proteins in involved in gene expression, metabolism, cell differentiation, and growth (92, 93). Indeed, perturbations of cytochrome c oxidase or cytochrome c itself could conceivably trigger downstream signaling events, as the inactivation of these proteins has been shown to affect signaling in other systems (94, 95). Therefore, we suggest that hydrogen peroxide might trigger downstream signaling events in hypoxic C. albicans cells.
The downstream transduction of the mitochondrial signal generated is not dependent on Hog1 signaling or Efg1 (Fig. 4), which has been implicated previously in transcriptional responses to hypoxia (26, 29, 45, 46). Instead, hypoxic signal transduction is dependent on the cAMP-PKA pathway (Fig. 5, 8, and 10). It is conceivable that, in some way, the mitochondrial and cAMP-PKA pathways act in parallel. However, hypoxia-induced glucan β-masking is dependent upon both signaling modules.
The involvement of cAMP-PKA signaling in β-glucan masking is entirely consistent with previous work showing that this pathway influences cell wall gene expression and integrity (52, 55, 56). Furthermore, our observations reinforce the view that the mitochondrion and adenylyl cyclase control virulence phenotypes in C. albicans (96). The mechanisms by which β-glucan masking is achieved at the cell surface remain obscure and are under investigation. However, transcript profiling studies of adenylyl cyclase and PKA mutants suggest that this pathway modulates the synthesis and assembly of cell wall mannoproteins and mannan (ALS1, ALS2, ALS4, CCW14, CSP37, ECM4, KTR1, SCW10, WSC1), glucan (KRE6, KRE9, PHR2) as well as chitin (CHS7, CHT3) (52, 55), potentially providing clues as to these mechanisms.
Changes in β-glucan exposure on C. albicans cells have been observed in vivo, during systemic infection (8), and in vitro in response to changes in ambient pH or host-derived lactate (9, 10). It is well-known that β-glucan recognition by Dectin-1 plays a major role in fungal recognition by innate immune cells (4, 13–16, 97). It has been reported that C. albicans cells grown overnight under hypoxia with high carbon dioxide levels (5% CO2) display enhanced immune recognition (17). Here we report that exponentially growing C. albicans cells, which were exposed specifically to hypoxia before fixing, elicit attenuated immune responses (Fig. 9), like cells exposed to lactate (9). We reason that differences in CO2 concentration, cell morphology, and/or growth state might account for the different immunological outputs in these two studies. Certainly, high CO2 concentrations are known to affect C. albicans morphology and physiology (98), and C. albicans morphology affects innate immune responses (99, 100).
We observed different phagocytic responses for BMDMs toward hypoxic C. albicans cells compared to normoxic control cells. Hypoxic cells evaded phagocytic uptake despite numerous contacts between yeast cells and phagocytes during their dynamic interactions over the period examined (see Movies S1 and S2 in the supplemental material). This was presumably because of the reduced availability of fungal target sites for host cell Dectin-1 engagement. The impact of hypoxia upon innate immune responses against C. albicans cells was more subtle than for those previously observed for lactate exposure. Exposing the fungal cells to lactate led to significant reductions in the levels of TNF-α and MIP1α released by human macrophages (9). Hypoxia-grown C. albicans cells also elicited reduced levels of these cytokines compared to control normoxic cells, but these changes were less dramatic and not statistically significant (Fig. 9). However, statistically significant decreases in IL-10 and RANTES production were observed for hypoxic cells (Fig. 9), indicating that hypoxia does affect immune responses against C. albicans. These differences in cytokine responses between lactate- and hypoxia-treated C. albicans cells might relate to the different signaling mechanisms that are activated in response to these host inputs (above). No doubt these different signaling mechanisms drive subtly different patterns of cell wall remodelling, in addition to the common β-glucan masking phenotype we have described.
We argue that the effects of hypoxia on β-glucan masking by C. albicans and upon the innate immune responses against this pathogen will almost certainly have a significant impact upon host-fungus interactions during colonization and infection. This view is supported by the accompanying paper (101), which shows that oxygen deprivation enhances the successful colonization of host niches by C. albicans in vivo.
MATERIALS AND METHODS
Strains and growth conditions.
Strains are listed in Table S1 in the supplemental material. All C. albicans strains were grown overnight at 30°C and 200 rpm in minimal medium (GYNB [2% glucose, 0.65% yeast nitrogen base without amino acids, containing the appropriate supplements]) (102). On the day of an experiment, overnight cultures were diluted into fresh minimal medium to an OD600 of 0.2, and incubated at 30°C at 200 rpm for 5 h for analysis. Normoxic cells were grown with aeration, whereas hypoxic cells were grown in screw cap conical flasks under nitrogen. Dissolved O2 was measured using a Thermo Fisher Scientific Orion RDO probe 3M (087010MD).
Microscopy.
For fluorescence microscopy, cells were fixed in 50 mM thimerosal (Sigma-Aldrich) and stained for β-glucan (1.5 µg/ml Fc-Dectin-1 plus anti-human IgG conjugated to Alexa Fluor 488; green), chitin (50 µg/ml wheat germ agglutinin conjugated to Alexa Fluor 350; blue), and mannan (25 µg/ml concanavalin A conjugated to Texas Red; red). All samples were examined by phase differential interference contrast (DIC) and fluorescence microscopy using a Zeiss Axioplan 2 microscope. Images were recorded digitally using the Openlab system (Openlab v 4.04: Improvision, Coventry, UK) with a Hamamatsu C4742- 95 digital camera (Hamamatsu Photonics, Hamamatsu, Japan).
High-pressure freeze substitution transmission electron microscopy on normoxic and hypoxic C. albicans cells was performed as described previously (103, 104), cutting ultrathin sections of 100 nm in thickness. Samples were imaged with a Philips CM10 transmission microscope (FEI, United Kingdom) equipped with a Gatan Bioscan 792 camera, and the images were recorded using a Digital Micrograph (Gatan, Abingdon Oxon, United Kingdom). The thicknesses of the inner chitin-glucan and outer mannan layers of the cell wall were measured by averaging >30 measurements for each cell (n > 30 cells) using ImageJ.
β-Glucan exposure.
To assess the exposure of β-glucan on the C. albicans cell surface, strains were grown in YNB plus 2% glucose overnight and then grown in fresh medium for 5 h under hypoxic or normoxic conditions. These exponentially growing cells were fixed immediately with 50 mM thimerosal (Sigma-Aldrich, Dorset, UK) to capture the cell surface architecture. They were then stained for β-glucan exposure using Fc-Dectin-1 and anti-human IgG conjugated to Alexa Fluor 488, and their fluorescence was quantified using a BD Fortessa flow cytometer as described previously (9). The plots represent three biological replicate experiments, in each of which 10,000 events were acquired. Normoxic cells of the congenic wild-type control were used as a control for each run. As a secondary control, cells were treated as descrbied above but without the addition of Fc-Dectin-1. Median fluorescence intensities (MFI) were determined using FlowJo v. 10 software.
Cytokine assays.
Cytokine assays on PBMCs were performed as described previously (9). Briefly, PBMCs were isolated from nonheparinized whole-blood samples (20 ml) collected from healthy donors using Ficoll-Paque centrifugation according to the manufacturer’s instructions (Sigma-Aldrich). Purified PBMCs were cultured for 5 days in MACS medium (Dulbecco’s modified Eagle’s medium containing 10% serum, 2 mM glutamine, 5 mg/ml penicillin and streptomycin). Normoxic and hypoxic C. albicans cells were fixed with 50 mM thimerosal (Sigma) and washed 4 times with sterile 1× PBS (Sigma-Aldrich). These yeast cells were incubated with PBMCs (ratio of 5:1, yeast cells/PBMCs) for 24 h, whereupon 100 µl of supernatant was collected and the cytokines and chemokines were quantified using the Luminex screening kit (R&D Systems, Abingdon, UK) in the BioPlex 200 system (Bio-Rad, Watford, UK) according to the manufacturer’s recommendations.
Phagocytosis assays.
Bone marrow-derived macrophages (BMDMs) were prepared following extraction of bone marrow from the femurs and tibias of 12-week-old male C57BL/6 mice aged and differentiated for 7 days as described previously (105). Normoxic and hypoxic C. albicans cells were fixed with thimerosal (described above), mixed with macrophages at a ratio of 3:1 (yeast cells/macrophages), and imaged at 1-min intervals for up to 4 h using established protocols with a Nikon Eclipse Ti UltraVIEW VoX spinning disk microscope (99, 106, 107). The C. albicans cells were fixed to allow subsequent confirmation, by cytometry, that these specific hypoxic cell populations displayed β-glucan masking. The percentages of macrophages phagocytosing yeast cells and the number of yeast cells engulfed per macrophage were quantified at hourly time intervals. The difference between conditions for each time point was determined using ANOVA with Bonferroni’s post hoc test.
Ethics statement.
Blood samples from healthy volunteers were collected with the informed consent of these donors and according to local guidelines and regulations that were approved by the College Ethics Review Board of the University of Aberdeen (CERB/2012/11/676).
Three 7-week-old male C57BL/6 mice were used for the preparation of BMDMs. These mice, which were selected randomly, were bred in-house, housed in stock cages under specific-pathogen-free conditions. They underwent no surgical procedures prior to culling by cervical dislocation. All animal experimentation was approved by the UK Home Office and by the University of Aberdeen Animal Welfare and Ethical Review Body.
Statistical analyses.
Statistical analyses were performed in GraphPad Prism 5. Results from independent replicate experiments are expressed as means ± standard deviations. One-way ANOVA (Tukey’s multiple-comparison test) was used to test the statistical difference between two sets of data with a nonparametric distribution. The following P values were considered statistically significant and indicated as follows: *, P < 0.05; **, P <0.01; ***, P < 0.001; ****, P < 0.0001.
Time-lapse video of BMDM interactions with hypoxic C. albicans cells. Movies S3 and S4 are representative of 12 movies (4 movies from 3 mice), that illustrate the first two hours of interactions between BMDMs and hypoxic C. albicans interactions. Download Movie S3, AVI file, 19.0 MB (19MB, avi) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Time-lapse video of BMDM interactions with hypoxic C. albicans cells. Movies S3 and S4 are representative of 12 movies (4 movies from 3 mice), that illustrate the first two hours of interactions between BMDMs and hypoxic C. albicans interactions. Download Movie S4, AVI file, 19.4 MB (19.4MB, avi) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
We are very grateful to the members of our Iain Fraser Cytometry Centre and Microscopy and Histology Core Facility for their superb help, advice, and support. We also thank our generous colleagues in the Candida community, and in particular Ana Traven, Jan Quinn, Guanghua Huang, Suzanne Noble, Donna MacCallum, Liz Johnson, Karl Kuchler, Patrick van Dijck, Rich Calderone, and Malcolm Whiteway for providing strains used in this study.
This work was funded by grants from the UK Medical Research Council (www.mrc.ac.uk) to A.J.P.B., N.A.R.G., L.P.E., and M.G.N. (MR/M026663/1) and by Ph.D. studentships from the University of Aberdeen to A.P. and D.W.L. The work was also supported by the Wellcome Trust (www.wellcome.ac.uk) (N.A.R.G., G.D.B., A.J.P.B. [097377] and G.D.B. [102705]) and by the Medical Research Council Centre for Medical Mycology at the University of Aberdeen (MR/N006364/1).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/mBio.02120-18.
Citation Pradhan A, Avelar GM, Bain JM, Childers DS, Larcombe DE, Netea MG, Shekhova E, Munro CA, Brown GD, Erwig LP, Gow NAR, Brown AJP. 2018. Hypoxia promotes immune evasion by triggering β-glucan masking on the Candida albicans cell surface via mitochondrial and cAMP-protein kinase A signaling. mBio 9:e01318-18. https://doi.org/10.1128/mBio.01318-18.
REFERENCES
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Hardison SE, Brown GD. 2012. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol 13:817–822. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erwig LP, Gow NA. 2016. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol 14:163–176. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- 4.Dambuza IM, Levitz SM, Netea MG, Brown GD. 2017. Fungal recognition and host defense mechanisms. Microbiol Spectr 5(4). [DOI] [PubMed] [Google Scholar]
- 5.Lionakis MS, Levitz SM. 2018. Host control of fungal infections: lessons from basic studies and human cohorts. Annu Rev Immunol 36:157–191. doi: 10.1146/annurev-immunol-042617-053318. [DOI] [PubMed] [Google Scholar]
- 6.Stappers MHT, Clark AE, Aimanianda V, Bidula S, Reid DM, Asamaphan P, Hardison SE, Dambuza IM, Valsecchi I, Kerscher B, Plato A, Wallace CA, Yuecel R, Hebecker B, da Glória Teixeira Sousa M, Cunha C, Liu Y, Feizi T, Brakhage AA, Kwon-Chung KJ, Gow NAR, Zanda M, Piras M, Zanato C, Jaeger M, Netea MG, van de Veerdonk FL, Lacerda JF, Campos A, Carvalho A, Willment JA, Latgé J-P, Brown GD. 2018. Recognition of DHN-melanin by a C-type lectin receptor is required for immunity to Aspergillus. Nature 555:382–386. doi: 10.1038/nature25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Meara TR, Alspaugh JA. 2012. The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev 25:387–408. doi: 10.1128/CMR.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheeler RT, Kombe D, Agarwala SD, Fink GR. 2008. Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog 4:e1000227. doi: 10.1371/journal.ppat.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballou ER, Avelar GM, Childers DS, Mackie J, Bain JM, Wagener J, Kastora SL, Panea MD, Hardison SE, Walker LA, Erwig LP, Munro CA, Gow NAR, Brown GD, MacCallum DM, Brown AJP. 2016. Lactate signalling regulates fungal beta-glucan masking and immune evasion. Nat Microbiol 2:16238. doi: 10.1038/nmicrobiol.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherrington SL, Sorsby E, Mahtey N, Kumwenda P, Lenardon MD, Brown I, Ballou ER, MacCallum DM, Hall RA. 2017. Adaptation of Candida albicans to environmental pH induces cell wall remodelling and enhances innate immune recognition. PLoS Pathog 13:e1006403. doi: 10.1371/journal.ppat.1006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopke A, Brown AJP, Hall RA, Wheeler RT. 2018. Dynamic fungal cell wall architecture in stress adaptation and immune evasion. Trends Microbiol 26:284–295. doi: 10.1016/j.tim.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morré SA, Vriend G, Williams DL, Perfect JR, Joosten LAB, Wijmenga C, van der Meer JWM, Adema GJ, Kullberg BJ, Brown GD, Netea MG. 2009. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galès A, Conduché A, Bernad J, Lefevre L, Olagnier D, Béraud M, Martin-Blondel G, Linas M-D, Auwerx J, Coste A, Pipy B. 2010. PPARgamma controls Dectin-1 expression required for host antifungal defense against Candida albicans. PLoS Pathog 6:e1000714. doi: 10.1371/journal.ppat.1000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho A, Giovannini G, De Luca A, D'Angelo C, Casagrande A, Iannitti RG, Ricci G, Cunha C, Romani L. 2012. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell Mol Immunol 9:276–286. doi: 10.1038/cmi.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sem X, Le GTT, Tan ASM, Tso G, Yurieva M, Liao WWP, Lum J, Srinivasan KG, Poidinger M, Zolezzi F, Pavelka N. 2016. Beta-glucan exposure on the fungal cell wall tightly correlates with competitive fitness of Candida species in the mouse gastrointestinal tract. Front Cell Infect Microbiol 6:186. doi: 10.3389/fcimb.2016.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, Hopke A, Mora-Montes HM, Kerrigan A, Netea MG, Murray GI, MacCallum DM, Wheeler R, Munro CA, Gow NAR, Cramer RA, Brown AJP, Brown GD. 2013. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog 9:e1003315. doi: 10.1371/annotation/7821bda1-dde3-4e72-b688-447b6bca20ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown GD. 2011. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol 29:1–21. doi: 10.1146/annurev-immunol-030409-101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaloriti D, Jacobsen M, Yin Z, Patterson M, Tillmann A, Smith DA, Cook E, You T, Grimm MJ, Bohovych I, Grebogi C, Segal BH, Gow NAR, Haynes K, Quinn J, Brown AJP. 2014. Mechanisms underlying the exquisite sensitivity of Candida albicans to combinatorial cationic and oxidative stress that enhances the potent fungicidal activity of phagocytes. mBio 5:e01334-14. doi: 10.1128/mBio.01334-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorenz MC, Bender JA, Fink GR. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fradin C, De Groot P, MacCallum D, Schaller M, Klis F, Odds FC, Hube B. 2005. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol 56:397–415. doi: 10.1111/j.1365-2958.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheetham J, MacCallum DM, Doris KS, da Silva Dantas A, Scorfield S, Odds F, Smith DA, Quinn J. 2011. MAPKKK-independent regulation of the Hog1 stress-activated protein kinase in Candida albicans. J Biol Chem 286:42002–42016. doi: 10.1074/jbc.M111.265231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miramon P, Kasper L, Hube B. 2013. Thriving within the host: Candida spp. interactions with phagocytic cells. Med Microbiol Immunol 202:183–195. doi: 10.1007/s00430-013-0288-z. [DOI] [PubMed] [Google Scholar]
- 24.Patterson MJ, McKenzie CG, Smith DA, da Silva Dantas A, Sherston S, Veal EA, Morgan BA, MacCallum DM, Erwig L-P, Quinn J. 2013. Ybp1 and Gpx3 signaling in Candida albicans govern hydrogen peroxide-induced oxidation of the Cap1 transcription factor and macrophage escape. Antioxid Redox Signal 19:2244–2260. doi: 10.1089/ars.2013.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grahl N, Shepardson KM, Chung D, Cramer RA. 2012. Hypoxia and fungal pathogenesis: to air or not to air? Eukaryot Cell 11:560–570. doi: 10.1128/EC.00031-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setiadi ER, Doedt T, Cottier F, Noffz C, Ernst JF. 2006. Transcriptional response of Candida albicans to hypoxia: linkage of oxygen sensing and Efg1p-regulatory networks. J Mol Biol 361:399–411. doi: 10.1016/j.jmb.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 27.Stichternoth C, Ernst JF. 2009. Hypoxic adaptation by Efg1 regulates biofilm formation by Candida albicans. Appl Environ Microbiol 75:3663–3672. doi: 10.1128/AEM.00098-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Synnott JM, Guida A, Mulhern-Haughey S, Higgins DG, Butler G. 2010. Regulation of the hypoxic response in Candida albicans. Eukaryot Cell 9:1734–1746. doi: 10.1128/EC.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sellam A, van Het Hoog M, Tebbji F, Beaurepaire C, Whiteway M, Nantel A. 2014. Modeling the transcriptional regulatory network that controls the early hypoxic response in Candida albicans. Eukaryot Cell 13:675–690. doi: 10.1128/EC.00292-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miranda LN, van der Heijden IM, Costa SF, Sousa API, Sienra RA, Gobara S, Santos CR, Lobo RD, Pessoa VP, Levin AS. 2009. Candida colonisation as a source for candidaemia. J Hosp Infect 72:9–16. doi: 10.1016/j.jhin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Rosenbach A, Dignard D, Pierce JV, Whiteway M, Kumamoto CA. 2010. Adaptations of Candida albicans for growth in the mammalian intestinal tract. Eukaryot Cell 9:1075–1086. doi: 10.1128/EC.00034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Askew C, Sellam A, Epp E, Hogues H, Mullick A, Nantel A, Whiteway M. 2009. Transcriptional regulation of carbohydrate metabolism in the human pathogen Candida albicans. PLoS Pathog 5:e1000612. doi: 10.1371/journal.ppat.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonhomme J, Chauvel M, Goyard S, Roux P, Rossignol T, d'Enfert C. 2011. Contribution of the glycolytic flux and hypoxia adaptation to efficient biofilm formation by Candida albicans. Mol Microbiol 80:995–1013. doi: 10.1111/j.1365-2958.2011.07626.x. [DOI] [PubMed] [Google Scholar]
- 34.MacPherson S, Akache B, Weber S, De Deken X, Raymond M, Turcotte B. 2005. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob Agents Chemother 49:1745–1752. doi: 10.1128/AAC.49.5.1745-1752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Znaidi S, Weber S, Al-Abdin OZ, Bomme P, Saidane S, Drouin S, Lemieux S, De Deken X, Robert F, Raymond M. 2008. Genomewide location analysis of Candida albicans Upc2p, a regulator of sterol metabolism and azole drug resistance. Eukaryot Cell 7:836–847. doi: 10.1128/EC.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sosinska GJ, de Groot PWJ, Teixeira de Mattos MJ, Dekker HL, de Koster CG, Hellingwerf KJ, Klis FM. 2008. Hypoxic conditions and iron restriction affect the cell-wall proteome of Candida albicans grown under vagina-simulative conditions. Microbiology 154:510–520. doi: 10.1099/mic.0.2007/012617-0. [DOI] [PubMed] [Google Scholar]
- 37.Trofa D, Gacser A, Nosanchuk JD. 2008. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev 21:606–625. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paravicini G, Mendoza A, Antonsson B, Cooper M, Losberger C, Payton MA. 1996. The Candida albicans PKC1 gene encodes a protein kinase C homolog necessary for cellular integrity but not dimorphism. Yeast 12:741–756. doi:. [DOI] [PubMed] [Google Scholar]
- 39.Navarro-Garcia F, Alonso-Monge R, Rico H, Pla J, Sentandreu R, Nombela C. 1998. A role for the MAP kinase gene MKC1 in cell wall construction and morphological transitions in Candida albicans. Microbiology 144:411–424. doi: 10.1099/00221287-144-2-411. [DOI] [PubMed] [Google Scholar]
- 40.Navarro-Garcia F, Eisman B, Fiuza SM, Nombela C, Pla J. 2005. The MAP kinase Mkc1p is activated under different stress conditions in Candida albicans. Microbiology 151:2737–2749. doi: 10.1099/mic.0.28038-0. [DOI] [PubMed] [Google Scholar]
- 41.Sohn K, Urban C, Brunner H, Rupp S. 2003. EFG1 is a major regulator of cell wall dynamics in Candida albicans as revealed by DNA microarrays. Mol Microbiol 47:89–102. [DOI] [PubMed] [Google Scholar]
- 42.Enjalbert B, Smith DA, Cornell MJ, Alam I, Nicholls S, Brown AJP, Quinn J. 2006. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol Biol Cell 17:1018–1032. doi: 10.1091/mbc.e05-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munro CA, Selvaggini S, de Bruijn I, Walker L, Lenardon MD, Gerssen B, Milne S, Brown AJP, Gow NAR. 2007. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol Microbiol 63:1399–1413. doi: 10.1111/j.1365-2958.2007.05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Pena JM, Garcia R, Nombela C, Arroyo J. 2010. The high-osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: a yeast dialogue between MAPK routes. Yeast 27:495–502. doi: 10.1002/yea.1792. [DOI] [PubMed] [Google Scholar]
- 45.Jin N, Hatton N, Swartz DR, Xia X, Harrington MA, Larsen SH, Rhoades RA. 2000. Hypoxia activates jun-N-terminal kinase, extracellular signal-regulated protein kinase, and p38 kinase in pulmonary arteries. Am J Respir Cell Mol Biol 23:593–601. doi: 10.1165/ajrcmb.23.5.3921. [DOI] [PubMed] [Google Scholar]
- 46.Hickman MJ, Spatt D, Winston F. 2011. The Hog1 mitogen-activated protein kinase mediates a hypoxic response in Saccharomyces cerevisiae. Genetics 188:325–338. doi: 10.1534/genetics.111.128322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.García R, Bermejo C, Grau C, Pérez R, Rodríguez-Peña JM, Francois J, Nombela C, Arroyo J. 2004. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J Biol Chem 279:15183–15195. doi: 10.1074/jbc.M312954200. [DOI] [PubMed] [Google Scholar]
- 48.Arana DM, Nombela C, Alonso-Monge R, Pla J. 2005. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology 151:1033–1049. doi: 10.1099/mic.0.27723-0. [DOI] [PubMed] [Google Scholar]
- 49.Cheetham J, Smith DA, da Silva Dantas A, Doris KS, Patterson MJ, Bruce CR, Quinn J. 2007. A single MAPKKK regulates the Hog1 MAPK pathway in the pathogenic fungus Candida albicans. Mol Biol Cell 18:4603–4614. doi: 10.1091/mbc.e07-06-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bockmuhl DP, Krishnamurthy S, Gerads M, Sonneborn A, Ernst JF. 2001. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol Microbiol 42:1243–1257. [DOI] [PubMed] [Google Scholar]
- 51.Rocha CR, Schröppel K, Harcus D, Marcil A, Dignard D, Taylor BN, Thomas DY, Whiteway M, Leberer E. 2001. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell 12:3631–3643. doi: 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harcus D, Nantel A, Marcil A, Rigby T, Whiteway M. 2004. Transcription profiling of cyclic AMP signaling in Candida albicans. Mol Biol Cell 15:4490–4499. doi: 10.1091/mbc.e04-02-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson D, Tutulan-Cunita A, Jung W, Hauser NC, Hernandez R, Williamson T, Piekarska K, Rupp S, Young T, Stateva L. 2007. Deletion of the high-affinity cAMP phosphodiesterase encoded by PDE2 affects stress responses and virulence in Candida albicans. Mol Microbiol 65:841–856. doi: 10.1111/j.1365-2958.2007.05788.x. [DOI] [PubMed] [Google Scholar]
- 54.Giacometti R, Kronberg F, Biondi RM, Passeron S. 2009. Catalytic isoforms Tpk1 and Tpk2 of Candida albicans PKA have non-redundant roles in stress response and glycogen storage. Yeast 26:273–285. doi: 10.1002/yea.1665. [DOI] [PubMed] [Google Scholar]
- 55.Fanning S, Xu W, Beaurepaire C, Suhan JP, Nantel A, Mitchell AP. 2012. Functional control of the Candida albicans cell wall by catalytic protein kinase A subunit Tpk1. Mol Microbiol 86:284–302. doi: 10.1111/j.1365-2958.2012.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao C, Wu M, Bing J, Tao L, Ding X, Liu X, Huang G. 2017. Global regulatory roles of the cAMP/PKA pathway revealed by phenotypic, transcriptomic and phosphoproteomic analyses in a null mutant of the PKA catalytic subunit in Candida albicans. Mol Microbiol 105:46–64. doi: 10.1111/mmi.13681. [DOI] [PubMed] [Google Scholar]
- 57.Hamanaka RB, Chandel NS. 2009. Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr Opin Cell Biol 21:894–899. doi: 10.1016/j.ceb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waypa GB, Smith KA, Schumacker PT. 2016. O2 sensing, mitochondria and ROS signaling: the fog is lifting. Mol Aspects Med 47-48:76–89. doi: 10.1016/j.mam.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dagley MJ, Gentle IE, Beilharz TH, Pettolino FA, Djordjevic JT, Lo TL, Uwamahoro N, Rupasinghe T, Tull DL, McConville M, Beaurepaire C, Nantel A, Lithgow T, Mitchell AP, Traven A. 2011. Cell wall integrity is linked to mitochondria and phospholipid homeostasis in Candida albicans through the activity of the post-transcriptional regulator Ccr4-Pop2. Mol Microbiol 79:968–989. doi: 10.1111/j.1365-2958.2010.07503.x. [DOI] [PubMed] [Google Scholar]
- 60.Huh WK, Kang SO. 1999. Molecular cloning and functional expression of alternative oxidase from Candida albicans. J Bacteriol 181:4098–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruy F, Vercesi AE, Kowaltowski AJ. 2006. Inhibition of specific electron transport pathways leads to oxidative stress and decreased Candida albicans proliferation. J Bioenerg Biomembr 38:129–135. doi: 10.1007/s10863-006-9012-7. [DOI] [PubMed] [Google Scholar]
- 62.Broxton CN, Culotta VC. 2016. An adaptation to low copper in Candida albicans involving SOD enzymes and the alternative oxidase. PLoS One 11:e0168400. doi: 10.1371/journal.pone.0168400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maxwell DP, Wang Y, McIntosh L. 1999. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci U S A 96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cárdenas-Monroy CA, Pohlmann T, Piñón-Zárate G, Matus-Ortega G, Guerra G, Feldbrügge M, Pardo JP. 2017. The mitochondrial alternative oxidase Aox1 is needed to cope with respiratory stress but dispensable for pathogenic development in Ustilago maydis. PLoS One 12:e0173389. doi: 10.1371/journal.pone.0173389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bambach A, Fernandes MP, Ghosh A, Kruppa M, Alex D, Li D, Fonzi WA, Chauhan N, Sun N, Agrellos OA, Vercesi AE, Rolfes RJ, Calderone R. 2009. Goa1p of Candida albicans localizes to the mitochondria during stress and is required for mitochondrial function and virulence. Eukaryot Cell 8:1706–1720. doi: 10.1128/EC.00066-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silver PM, Oliver BG, White TC. 2004. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot Cell 3:1391–1397. doi: 10.1128/EC.3.6.1391-1397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoehamer CF, Cummings ED, Hilliard GM, Morschhauser J, Rogers PD. 2009. Upc2p-associated differential protein expression in Candida albicans. Proteomics 9:4726–4730. doi: 10.1002/pmic.200900176. [DOI] [PubMed] [Google Scholar]
- 68.Joshua IM, Hofken T. 2017. From lipid homeostasis to differentiation: old and new functions of the zinc cluster proteins Ecm22, Upc2, Sut1 and Sut2. Int J Mol Sci 18:E772. doi: 10.3390/ijms18040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martchenko M, Alarco AM, Harcus D, Whiteway M. 2004. Superoxide dismutases in Candida albicans: transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol Biol Cell 15:456–467. doi: 10.1091/mbc.e03-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang CS, Rhie GE, Oh JH, Huh WK, Yim HS, Kang SO. 2002. Copper- and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology 148:3705–3713. doi: 10.1099/00221287-148-11-3705. [DOI] [PubMed] [Google Scholar]
- 71.Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. 2009. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol Microbiol 71:240–252. doi: 10.1111/j.1365-2958.2008.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang CS, Rhie G, Kim ST, Kim YR, Huh WK, Baek YU, Kang SO. 1999. Copper- and zinc-containing superoxide dismutase and its gene from Candida albicans. Biochim Biophys Acta 1427:245–255. doi: 10.1016/S0304-4165(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 73.Tripathi G, Wiltshire C, Macaskill S, Tournu H, Budge S, Brown AJ. 2002. Gcn4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. EMBO J 21:5448–5456. doi: 10.1093/emboj/cdf507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Messmer K, Sunder-Plassmann L, Jesch F, Gornandt L, Sinagowitz E, Kessler M. 1973. Oxygen supply to the tissues during limited normovolemic hemodilution. Res Exp Med 159:152–166. doi: 10.1007/BF01851543. [DOI] [PubMed] [Google Scholar]
- 75.Oill PA, Roser SM, Galpin JE, Ziment I, Selecky PA, Schofferman J. 1976. Infectious disease emergencies. Part III: patients presenting with respiratory distress syndromes. West J Med 125:452–478. [PMC free article] [PubMed] [Google Scholar]
- 76.Savage DC. 1977. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 77.Ernst JF, Tielker D. 2009. Responses to hypoxia in fungal pathogens. Cell Microbiol 11:183–190. doi: 10.1111/j.1462-5822.2008.01259.x. [DOI] [PubMed] [Google Scholar]
- 78.Espey MG. 2013. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic Biol Med 55:130–140. doi: 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- 79.Perez JC, Kumamoto CA, Johnson AD. 2013. Candida albicans commensalism and pathogenicity are intertwined traits directed by a tightly knit transcriptional regulatory circuit. PLoS Biol 11:e1001510. doi: 10.1371/journal.pbio.1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pierce JV, Dignard D, Whiteway M, Kumamoto CA. 2013. Normal adaptation of Candida albicans to the murine gastrointestinal tract requires Efg1p-dependent regulation of metabolic and host defense genes. Eukaryot Cell 12:37–49. doi: 10.1128/EC.00236-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. 2005. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 82.Bailey-Serres J, Chang R. 2005. Sensing and signalling in response to oxygen deprivation in plants and other organisms. Ann Bot 96:507–518. doi: 10.1093/aob/mci206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN. 2006. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol 141:357–366. doi: 10.1104/pp.106.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guzy RD, Mack MM, Schumacker PT. 2007. Mitochondrial complex III is required for hypoxia-induced ROS production and gene transcription in yeast. Antioxid Redox Signal 9:1317–1328. doi: 10.1089/ars.2007.1708. [DOI] [PubMed] [Google Scholar]
- 85.Turrens JF, Alexandre A, Lehninger AL. 1985. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Yu L, Yu CA. 1998. Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J Biol Chem 273:33972–33976. doi: 10.1074/jbc.273.51.33972. [DOI] [PubMed] [Google Scholar]
- 87.Muller FL, Liu Y, Van Remmen H. 2004. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 88.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. 1998. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. 2000. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 90.Bienert GP, Schjoerring JK, Jahn TP. 2006. Membrane transport of hydrogen peroxide. Biochim Biophys Acta 1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 91.Riemer J, Schwarzlander M, Conrad M, Herrmann JM. 2015. Thiol switches in mitochondria: operation and physiological relevance. Biol Chem 396:465–482. doi: 10.1515/hsz-2014-0293. [DOI] [PubMed] [Google Scholar]
- 92.Brandes N, Schmitt S, Jakob U. 2009. Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal 11:997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herrero-de-Dios C, Day AM, Tillmann AT, Kastora SL, Stead D, Salgado PS, 2018. Redox regulation, rather than stress-induced phosphorylation, of a Hog1 mitogen-activated protein kinase modulates its nitrosative-stress-specific outputs. mBio 9:e02229-17. doi: 10.1128/mBio.02229-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. 2005. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leadsham JE, Sanders G, Giannaki S, Bastow EL, Hutton R, Naeimi WR, Breitenbach M, Gourlay CW. 2013. Loss of cytochrome c oxidase promotes RAS-dependent ROS production from the ER resident NADPH oxidase, Yno1p, in yeast. Cell Metab 18:279–286. doi: 10.1016/j.cmet.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 96.Grahl N, Demers EG, Lindsay AK, Harty CE, Willger SD, Piispanen AE, Hogan DA. 2015. Mitochondrial activity and Cyr1 are key regulators of ras1 activation of C. albicans virulence pathways. PLoS Pathog 11:e1005133. doi: 10.1371/journal.ppat.1005133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown GD, Gordon S. 2001. Immune recognition. A new receptor for beta-glucans. Nature 413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 98.Klengel T, Liang W-J, Chaloupka J, Ruoff C, Schröppel K, Naglik JR, Eckert SE, Mogensen EG, Haynes K, Tuite MF, Levin LR, Buck J, Mühlschlegel FA. 2005. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol 15:2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lewis LE, Bain JM, Lowes C, Gillespie C, Rudkin FM, Gow NAR, Erwig L-P. 2012. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog 8:e1002578. doi: 10.1371/journal.ppat.1002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mukaremera L, Lee KK, Mora-Montes HM, Gow NAR. 2017. Candida albicans yeast, pseudohyphal, and hyphal morphogenesis differentially affects immune recognition. Front Immunol 8:629. doi: 10.3389/fimmu.2017.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lopes JP, Stylianou M, Backman E, Holmberg S, Jass J, Claesson R, Urban CF. 2018. Evasion of immune surveillance in low oxygen environments enhances Candida albicans virulence. mBio 9:e02120-18. doi: 10.1128/mBio.02120-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sherman F. 1991. Getting started with yeast. Methods Enzymol 194:3–21. doi: 10.1016/0076-6879(91)94004-V. [DOI] [PubMed] [Google Scholar]
- 103.Netea MG, Gow NAR, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, McKinnon A, MacCallum DM, Odds FC, Van der Meer JWM, Brown AJP, Kullberg BJ. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 116:1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ene IV, Adya AK, Wehmeier S, Brand AC, MacCallum DM, Gow NAR, Brown AJP. 2012. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol 14:1319–1335. doi: 10.1111/j.1462-5822.2012.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davies JQ, Gordon S. 2005. Isolation and culture of murine macrophages. Methods Mol Biol 290:91–103. [DOI] [PubMed] [Google Scholar]
- 106.Lewis LE, Bain JM, Okai B, Gow NA, Erwig LP. 2013. Live-cell video microscopy of fungal pathogen phagocytosis. J Vis Exp 9:50196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bain J, Gow NA, Erwig LP. 2015. Novel insights into host-fungal pathogen interactions derived from live-cell imaging. Semin Immunopathol 37:131–139. doi: 10.1007/s00281-014-0463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
C. albicans strains used in this study. Download Table S1, PDF file, 0.1 MB (74.5KB, pdf) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Hypoxia-induced β-glucan masking in specific C. albicans mutants. Analysis of β-glucan masking by C. albicans mutants by Fc-dectin-1 staining of cells grown under normoxic (pink) or hypoxic conditions (cyan) (upper panels). Median fluorescence intensities (MFIs) for hypoxic and normoxic cell populations are shown. The corresponding wild-type control is shown above each mutant or set of mutants. Fold changes in β-glucan exposure (lower panels) represent the MFI under hypoxic conditions divided by the MFI for the corresponding control normoxic cells. Means and standard deviations from three independent replicate experiments are shown, and the data were analyzd using ANOVA with Tukey’s multiple-comparison test: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. (A) Morphogenetic mutants. WT, wild type, SC5314; cph1Δ, JKC19; efg1Δ, HLC52; bcr1Δ, CNJ702; tec1Δ, CaAS18 (Table S1). (B) Mutants affecting mitochondrial functionality. WT, wild type, DAY152; pop2Δ, YCAT51; ccr4Δ, YCAT39. The data for the efg1 Δ, tec1Δ, and ccr4Δ mutants also appear in Fig. 4 and 6. Download FIG S1, PDF file, 0.2 MB (180.7KB, pdf) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Time-lapse video of BMDM interactions with normoxic C. albicans cells. Movies S1 and S2, which are representative of 12 movies in total (4 movies from 3 mice), show the first two hours of interactions between murine BMDMs and normoxic C. albicans interactions. Download Movie S1, AVI file, 18.8 MB (18.8MB, avi) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Time-lapse video of BMDM interactions with normoxic C. albicans cells. Movies S1 and S2, which are representative of 12 movies in total (4 movies from 3 mice), show the first two hours of interactions between murine BMDMs and normoxic C. albicans interactions. Download Movie S2, AVI file, 18.7 MB (18.7MB, avi) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Time-lapse video of BMDM interactions with hypoxic C. albicans cells. Movies S3 and S4 are representative of 12 movies (4 movies from 3 mice), that illustrate the first two hours of interactions between BMDMs and hypoxic C. albicans interactions. Download Movie S3, AVI file, 19.0 MB (19MB, avi) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Time-lapse video of BMDM interactions with hypoxic C. albicans cells. Movies S3 and S4 are representative of 12 movies (4 movies from 3 mice), that illustrate the first two hours of interactions between BMDMs and hypoxic C. albicans interactions. Download Movie S4, AVI file, 19.4 MB (19.4MB, avi) .
Copyright © 2018 Pradhan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.