Abstract
Amino acid contents and their derived volatile compositions in Cabernet Sauvignon grapes and wines after regulated deficit irrigation (RDI) were investigated during the 2015 and 2016 growing seasons in Yinchuan (NingXia, China). High-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) were used for amino acid and volatile compound analyses. Three RDI strategies were tested: 60% (RDI-1), 70% (RDI-2), and 80% (RDI-3) of grapevine estimated evapotranspiration (ETc), and 100% ETc was used as the control group (CK). RDI-treated vines had lower yields and berry weights with higher total soluble solids than the control treatment. RDI-1 increased proline levels in berries and wines. RDI-2 enhanced tyrosine and asparagine levels in wines. RDI-3 enhanced arginine, alanine, valine, leucine, and isoleucine levels in berries and wines. RDI-2 and RDI-3 increased the concentrations of 2-methyl-1-butyl acetate, benzaldehyde, 3-methyl-1-pentanol, and 3-methyl-1-butanol in wines. The accumulation of volatile compounds was closely related to the amino acid concentrations—especially isoleucine, valine, and leucine—in grapes. Our results showed that RDI treatments altered amino acid concentrations and their derived volatile compositions in wines.
Keywords: water deficit irrigation, secondary metabolism, red wine, winegrape
1. Introduction
Amino acids present in grapes serve as a nitrogen source for yeasts during alcoholic fermentation [1]. Amino acids in grapes are precursors of volatile compounds, such as leucine, valine, isoleucine, and phenylalanine [2]; and the branched-chain amino acids (valine, leucine, and isoleucine), aromatic amino acids (phenylalanine, tyrosine, and tryptophan), and methionine in grapes can be converted into α-ketoacids and metabolized into higher alcohols and higher acids in yeast cells through the Ehrlich pathway [2,3]. These compounds can be further metabolized into alcohol esters and acetates [3]. These fermentative volatile compounds account for approximately 50% of the total volatile concentration in wines [4]. Thus, amino acid profiles in grapes affect fermentation development and have an important role in the production of volatile compositions [2]. Many previous studies have reported how changes in grape amino acid content can affect wine volatile composition [1,5,6] because phenylalanine, leucine, isoleucine, valine, tyrosine, and methionine seem to be precursors of volatile compounds (2-phenylethanol, 3-methyl-1-butanol, 2-methyl-1-butanol, isobutanol, tyrosol, and methionol, respectively). The addition of branched-chain amino acids (such as valine, leucine, and isoleucine) during fermentation increases higher alcohol production. In comparison, adding phenylalanine increases the concentrations of 2-phenylethanol, 2-phenylethyl acetate, and ethyl esters in wine [7]. Therefore, future work is needed to reveal the relationship between amino acids and aromas in grapes and wines. Amino acids and volatile compositions in grapes and wines are affected by many factors, including viticultural practices, climate conditions, and nitrogen fertilization [7,8,9,10]. Recently, great research effort has been devoted to assessing changes in the amino acids and aroma components in grapes and wines after different treatments. Sánchez-Gómez et al. [8] reported that the application of aqueous extracts of vine-shoot waste could enhance the amino acid content of wine. They also reported that a higher amino acid concentration in must resulted in higher consumption during alcoholic fermentation, and the resultant wines have a lower concentration of amino acids. Bouzas-Cid et al. [9] reported that irrigation alters the concentration of methionine in musts, and the vintage is closely related to the amino acid content in must.
Grape is an important fruit with economic and nutritional value and is cultivated in China. Northwest China is the main grape cultivation area, especially Ningxia, Xinjiang, and Gansu. However, the shortage of fresh water resources restricts the development of the grape industry in China. Regulated deficit irrigation (RDI) can save water and improve crop quality [11,12,13], alter grape and wine flavors and phenolic compounds, and plays an important role in improving vine canopy shapes, vigorous vines, and canopy microclimates [14,15,16,17,18]. However, few reports exist on the effect of RDI on amino acid contents and their derived volatiles in grapes and wines. Exploring whether this influence is positive or negative could provide a theoretical foundation for producing high-quality grapes and wines.
Consequently, the aim of this work was to gain insight on the effect of RDI grapevine treatment on the amino acid composition in grapes and wine and fermentative volatile compounds in wine since previous studies suggest that using RDI as a strategy to promote efficient water use in agriculture alters metabolites in grapes. The relationship between amino acids and the fermentative volatile compositions in wine was also elucidated using three different RDI strategies.
2. Materials and Methods
2.1. Field Conditions and Materials
The study was conducted with own-rooted red wine grape (Vitis vinifera L.) cv. Cabernet Sauvignon from a commercial vineyard (Chateau Lilan, 38°28′ N 105°97′ E, elevation 1169 m, semiarid continental climate) in Yinchuan (Ningxia, China). The experiments were conducted for two continuous vintages from 2015 to 2016. Vines were oriented in the north–south direction with drip irrigation (2.4 L/h, irrigation time determined by the amount of irrigation). The vines were spaced at 3.0 × 0.6 m and had been planted in 2010. A completely randomized block design using three replicates of five lines with 100 vines each (Three central lines were used for experiments and sampling, whereas the remaining lines acted as buffers.) was performed. Three RDI strategies were used: 60% (RDI-1), 70% (RDI-2), and 80% (RDI-3) of the estimated evapotranspiration (ETc), and 100% ETc was used for the control group (CK). The vines were irrigated from fruit set until approximately two weeks prior to harvest. Irrigation was applied when vines reached a midday stem water potential value of −0.6 MPa. The ETc was calculated using the Pennman–Monteith model [19], and meteorological data were obtained from a microweather station located within the experiment vineyard. Vine water status was monitored by measuring the leaf water potential at midday (ψmd) using a pressure chamber after treatments.
2.2. Samples and Vinifications
When the grapes attained 22–24 Brix, samples were manually collected for each replicate on the same day and transported to the laboratory in ice boxes within 30 min. In total, 600 grapes were collected for each replicate. The yield was measured, and one hundred grapes were used to analyze the physicochemical parameters according to International Organisation of Vine and Wine [20] methods. Samples were immediately frozen in liquid nitrogen and stored at −40 °C before further analysis. All treatments were carried out in triplicate, and the results are the average of three analyses (n = 3).
Approximately 100 kg of grapes from each treatment group were used for vinification in stainless steel tanks, and all vinifications were performed in triplicate. Before alcoholic fermentation (AF), the total acidity of must was adjusted to 5.5 g/L, and pectolytic enzymes (30 mg/L) and SO2 (50 mg/L) were added. After 24 h, a commercial yeast Saccharomyces cerevisiae strain BO 213 (200 mg/L, Laffort, Bordeaux, France) was added according to direction. All the fermentation was controlled at the same condition (25 ± 1 °C). The temperature and must density were automatically monitored. At the end of alcoholic fermentation and without malolactic fermentation, filtration, tartaric stabilization, and natural clarification were manually performed, and the resultant wine samples were collected and stored at −40 °C before further analysis.
2.3. HPLC Determination of Amino Acids
Derivatization of Amino Acids
The HPLC analysis was performed using a Shimadzu LA-20AT system (Shimadzu, Kyoto, Japan) equipped with an automatic liquid sampler. Chromatographic separation was performed on a C18 column (AJS-01, 3 μm, 150 × 4.6 mm; Agela Technologies, Tianjin, China). The column was operated at 30 °C. The derivatization of amino acids was carried out according to the methods of Sánchez-Gómez et al. [8] and Liu et al. [21]. Briefly, fifty grape berries were deseeded and turned into powder under liquid nitrogen. Grape musts were centrifuged at 4 °C and 10,000 rpm for 10 min to collect clear juice. For precolumn derivatization, the clear juice or wine was filtered through 0.22 μm nylon 66 organic membranes (Micro Pes, Membrana, Wuppertal, Germany), and 100 μL of norvaline and 100 μL of sarcosine (internal standards) were added to each sample (5 mL). The samples were then submitted to an automatic precolumn derivatization with o-phthaldialdehyde (OPA reagent 1, Agilent) for primary amino acids and with 9-fluorenylmethylchloroformate (FMOC reagent 2, Agilent) for proline. 10 μL from the derivatized sample were injected at 40 °C, and total reaction time was about 90 s.
Two eluents were used as the mobile phases: eluent A: 75 mM sodium acetate, 0.018% triethylamine (pH 6.9), and 0.3% tetrahydrofuran; eluent B: ultra-pure water, methanol, and acetonitrile (10:40:50 v/v/v). Phases A and B were filtered through 0.22 μm nylon-66 organic membranes.
All samples were eluted according to a previous method reported by Garde-Cerdán, et al. [22] with some modifications. The total analysis time was approximately 40 min. Briefly, a 1.6 mL/min flow rate was used with the following elution program: 6% B for 10 min, 6 to 10% B in 6 min, maintained for 2 min, from 10 to 16% B in 2 min, from 16 to 40% B in 13 min, from 40 to 50% B in 7 min, from 50 to 100% B in 1 min, and maintained for 4 min. The elution program was followed by balancing the column with Phases A and B for about 1 min. The injection amount was 10 μL, and for detection, a photodiode array detector monitored for primary and secondary amino acids at 338 and 262 nm, respectively. The identification and quantification of amino acids were performed using pure reference standards. All external chemical standards (tryptophan (trp, 98%), glycine (gly, 98.5%), phenylalanine (phe, 98%), methionine (met, 98%), leucine (leu, 98%), arginine (arg, 98%), proline (pro, 99%), lysine (lys, 98%), glutamic acid (glu, 99%), asparagine (asp, 99%), isoleucine (ile, 99%), valine (val, 98%), histidine (his, 98%), serine (ser, 98%), alanine (ala, 98%), threonine (thr, 98%), and cysteine (cys, 97%)) used for the identification and quantification of amino acids were purchased from Sigma-Aldrich (Shanghai, China). The concentrations of the amino acids of the standard are shown in Table S1.
2.4. Determination of Volatile Compounds by GC-MS
2.4.1. Extraction of Volatile Compounds
The extraction of volatile compounds was carried out according to previous research [23]. Briefly, 100 randomly selected grapes were deseeded and ground under liquid nitrogen. Fifty grams of the obtained powder was mixed with 1 g polyvinylpolypyrrolidone (PVPP), and the mixture was macerated at 4 °C for 3 h under a nitrogen atmosphere. Then, the mixture was centrifuged at 8000 rpm (4 °C) for 20 min. The clear juice or wine was filtered twice through 0.45 μm nylon 66 organic membranes. For volatile compound determination, 10 mL of wine with 40 μL of 2-octanol as an internal standard (0.32 g/L dissolved in ethanol, Sigma-Aldrich (Shanghai, China) was blended with 2 g NaCl in a 20 mL sample vial containing a magnetic stirrer. The vial was tightly capped with a PTFE-silicon septum for further analysis.
2.4.2. GC-MS Analysis
GC-MS analyses were performed using a Thermo-Finnigan Trace 2000 gas chromatograph (Thermo Finnigan, Waltham, MA, USA) with an HP-INNWAX column (60 m, 0.25 mm I.D., 0.25 μm; Agilent, Shanghai, China). The solid-phase micro-extraction fiber (SPME, DVB/CAR/PDMS 2CM, 50/30-μm) was heated at 250 °C for 2 h. The vial containing the sample (wine) was extracted in a 40 °C water bath for 30 min while being stirred and then desorbed at 230 °C for 3 min into the splitless injection port of the GC-MS instrument. Helium was used as the carrier gas (1 mL/min). The chromatographic conditions consisted of an initial oven temperature of 40 °C for 2.5 min, increasing the temperature to 150 °C at a rate of 5 °C/min, increasing to 220 °C at a rate of 3 °C/min and holding for 30 min. The temperatures of the ion source and MS transfer line were 250 °C and 280 °C, respectively. The scan range was 33–450 m/z with electron impact (EI) mode at 70 eV.
The NIST 2002 mass spectroscopy library (National Institute of Standards and Technology, Gaithersburg, MD, USA) and the retention times of the authentic standards were used to identify the volatile compounds. Quantification was performed using the peak areas on the total ion chromatogram. The concentrations of the volatile compounds were calculated based on their calibration curves (the relative response rate was greater than 98%), which were built by plotting the area ratio of the target compounds to the internal standard against the concentration ratio [24]. All determinations were performed in triplicate.
2.5. Statistical Analysis
All analyses were performed in triplicate, and mean values were calculated. Duncan’s multiple range tests were used to determine significant differences (p < 0.05) with SPSS 19.0 software for Windows (SPSS Inc., Chicago, IL, USA). The heatmap was created by Metabo-Analyst 3.0 [25].
3. Results and Discussion
3.1. Weather Conditions and Physicochemical Parameters of Grape Berries
Growing season rainfall in 2015 was lower than that in 2016 (Table S2). The mean growing season daily temperature and growing season ET0 in 2015 were higher than those in 2016. The weather during the years of this study was typical of a semiarid continental climate, which is very suitable for cultivation of Cabernet Sauvignon (V. vinifera L.) [26].
The effect of RDI on the physicochemical parameters of grape berries is shown in Table 1. The full irrigation grapes had the highest yield, total acidity (TA), and berry weight and the lowest total soluble solids (TSS) and pH for both years. The RDI-1 and RDI-2 treatment groups had significantly lower yields, berry fresh weights, and TA for both years, which agreed with a previous report [13]. RDI treatments increased the TSS for the two years but not significantly. RDI-1 and RDI-2 treatments significantly increased the pH both years. The RDI treatments reducing the TA and increasing the pH were in accordance with previous reports and could be caused by a reduction in malic acid content [18,27]. The physicochemical parameters of the RDI treatment groups were different for the two years, which might because the growing season rainfall in 2016 was higher than that in 2015 (Table S2).
Table 1.
Physicochemical parameters of grape berries from the different treatment groups.
| Parameter | RDI-1 | RDI-2 | RDI-3 | Control | ||||
|---|---|---|---|---|---|---|---|---|
| R | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 |
| Yield (ton/ha) | 7.12b | 7.70b | 7.77ab | 7.94b | 8.36a | 8.58ab | 9.06a | 9.57a |
| Weight (g/100 berries) | 110.14b | 118.18b | 105.01c | 110.10c | 118.38a | 124.81a | 122.50a | 122.70a |
| Total soluble solids (Brix) | 23.92a | 23.45a | 23.53ab | 23.95a | 23.56ab | 23.31a | 22.30b | 23.26a |
| Titratable acidity (g/L tartaric acid) | 4.42b | 3.25b | 4.81ab | 3.14b | 5.12ab | 3.51a | 5.46a | 3.71a |
| pH | 3.90a | 4.13b | 3.72b | 4.31a | 3.69c | 3.92c | 3.67c | 3.86c |
| Midday stem water potential from fruit set to harvest (MPa) | −0.56 | −0.58 | −0.49 | −0.52 | −0.38 | −0.43 | −0.30 | −0.34 |
Different letters in the row indicate significant differences (p < 0.05) among treatments for the same vintage.
3.2. Amino Acid Profiles of Grape Berries and Wines
Amino acids are the main precursors for secondary metabolites, including flavonoids, in grapes [11], and are also a nitrogen source for yeast during alcoholic fermentation [28,29]. The amino acid profile of grape berries affects the production of polyphenol compounds, ethanol and glycerol [30]. The amino acid profiles of the grape berries and wines in the current experiment are shown in Table 2 and Table 3, respectively. Chromatograms of the amino acid are shown in Figure S1. According to amino acid biosynthesis pathways, 17 of the amino acids present in grape berries and wines can be classified into five families, including serines (Cys, Ser, and Gly), aromatic amino acids (Phe and Trp), aspartates (Ile, Asp, Lys, Met, and Thr), glutamates (Arg, Glu, His, and Pro), and pyruvates (Leu, Ala, and Val) [31].
Table 2.
Amino acid concentrations (mg/L) in grapes from untreated (CK) and treated vineyards.
| Family | Amino Acid | 2015 | 2016 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RDI-1 | RDI-2 | RDI-3 | CK | RDI-1 | RDI-2 | RDI-3 | CK | ||
| Serine | Cys | 5.96 ± 1.81a | 7.92 ± 1.13a | 10.78 ± 0.05a | 8.55 ± 0.40a | 5.76 ± 0.07a | 6.70 ± 0.69a | 8.43 ± 0.49a | 6.17 ± 0.84a |
| Ser | 35.26 ± 1.21b | 38.56 ± 1.02ab | 41.24 ± 3.01a | 39.25 ± 1.78ab | 33.58 ± 0.24ab | 28.543 ± 2.01b | 42.75 ± 2.06a | 30.52 ± 0.78b | |
| Gly | 3.18 ± 0.45c | 3.40 ± 0.20c | 7.26 ± 0.43a | 7.82 ± 0.10b | 4.92 ± 0.01ab | 2.08 ± 0.29b | 8.96 ± 0.18a | 2.77 ± 0.11b | |
| Aromatic amino acids | Phe | 15.58 ± 0.85c | 16.85 ± 0.45c | 26.41 ± 0.77a | 20.00 ± 0.88b | 17.12 ± 1.39b | 15.26 ± 1.33c | 18.71 ± 0.97a | 16.18 ± 0.90bc |
| Trp | 11.24 ± 0.48ab | 6.95 ± 0.21c | 15.49 ± 0.89a | 9.54 ± 0.32b | 15.88 ± 0.54a | 6.21 ± 0.81c | 14.39 ± 0.31a | 8.82 ± 0.35b | |
| Aspartate | Ile | 8.61 ± 1.04c | 7.62 ± 0.64c | 17.62 ± 1.05a | 12.34 ± 0.62b | 10.65 ± 0.95b | 12.06 ± 0.95b | 19.17 ± 0.92a | 8.14 ± 0.98b |
| Asp | 20.16 ± 0.86b | 25.31 ± 0.73a | 25.08 ± 0.76a | 21.55 ± 0.56b | 18.99 ± 1.61bc | 18.28 ± 0.79c | 23.16 ± 0.44a | 21.34 ± 1.08ab | |
| Lys | 2.51 ± 0.81b | 2.45 ± 0.21b | 5.21 ± 0.21a | 3.45 ± 0.12b | 3.01 ± 0.32b | 1.98 ± 0.08b | 4.30 ± 0.05a | 2.68 ± 0.0.31b | |
| Met | 1.64 ± 0.04c | 1.58 ± 0.15c | 4.95 ± 0.75a | 3.21 ± 0.09b | 2.41 ± 0.21b | 1.02 ± 0.01d | 5.01 ± 0.02a | 1.95 ± 0.04c | |
| Thr | 47.62 ± 2.68b | 51.26 ± 1.53b | 57.24 ± 0.92a | 57.32 ± 1.46a | 47.76 ± 1.23ab | 47.85 ± 0.69ab | 49.56 ± 0.60a | 44.55 ± 3.15b | |
| Glutamate | Arg | 330.19 ± 2.61b | 336.47 ± 3.13b | 354.54 ± 1.52a | 346.57 ± 1.26a | 320.54 ± 1.01b | 308.47 ± 0.30c | 334.85 ± 6.09a | 317.55 ± 4.37bc |
| Glu | 69.74 ± 1.53c | 82.42 ± 2.13b | 89.85 ± 0.84a | 70.45 ± 0.17c | 64.45 ± 2.01a | 40.54 ± 0.99c | 66.37 ± 0.66a | 54.25 ± 1.45b | |
| His | 16.32 ± 1.04c | 15.21 ± 0.98c | 27.39 ± 2.30a | 21.52 ± 1.51b | 22.17 ± 1.64ab | 15.05 ± 0.95c | 25.45 ± 2.01a | 18.95 ± 1.01bc | |
| Pro | 195.77 ± 1.61a | 139.45 ± 2.59b | 122.26 ± 10.36b | 105.12 ± 5.526c | 155.49 ± 0.82a | 145.46 ± 2.04b | 119.89 ± 3.80c | 115.64 ± 1.84c | |
| Pyruvate | Leu | 15.95 ± 1.58c | 24.58 ± 1.08b | 31.05 ± 22.85a | 21.95 ± 2.84b | 17.82 ± 1.84b | 15.24 ± 2.01b | 29.54 ± 2.54a | 14.85 ± 0.54b |
| Ala | 35.78 ± 4.18b | 63.83 ± 3.69a | 74.70 ± 59.24a | 67.94 ± 0.67a | 51.65 ± 2.75b | 31.20 ± 0.87d | 71.03 ± 2.74a | 43.57 ± 0.72c | |
| Val | 13.51 ± 1.59c | 12.95 ± 1.52c | 27.62 ± 2.81a | 20.12 ± 0.43b | 22.57 ± 1.24a | 20.14 ± 0.76a | 24.32 ± 2.31a | 15.89 ± 0.81b | |
Different letters in the row indicate significant differences (p < 0.05) among treatments for the same vintage.
Table 3.
Amino acid concentrations (mg/L) in wines from untreated (CK) and treated vineyards.
| Family | Amino Acid | 2015 | 2016 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RDI-1 | RDI-2 | RDI-3 | CK | RDI-1 | RDI-2 | RDI-3 | CK | ||
| Serine | Cys | 3.18 ± 0.13b | 4.12 ± 0.06b | 7.22 ± 0.13a | 6.41 ± 0.26ab | 2.94 ± 0.05b | 3.21 ± 0.67b | 5.99 ± 0.02a | 5.96 ± 0.19a |
| Ser | 1.40 ± 0.03c | 3.75 ± 0.43b | 6.21 ± 0.61a | 2.11 ± 0.15c | 1.05 ± 0.05c | 5.66 ± 0.43b | 8.82 ± 0.42a | 4.30 ± 0.40b | |
| Gly | 2.32 ± 0.77c | 4.62 ± 0.93b | 6.32 ± 0.42a | 3.05 ± 0.38c | 2.74 ± 0.10c | 4.41 ± 0.69c | 5.83 ± 0.06a | 3.75 ± 0.77b | |
| Aromatic amino acids | Trp | 2.06 ± 0.22c | 3.34 ± 0.37b | 4.07 ± 0.75a | 1.73 ± 0.51c | 2.55 ± 0.50b | 4.04 ± 0.37b | 5.51 ± 0.74a | 2.51 ± 0.54c |
| Phe | 1.88 ± 0.02c | 2.62 ± 0.09b | 4.14 ± 0.52a | 1.03 ± 0.04c | 2.05 ± 0.22c | 1.51 ± 0.33c | 3.67 ± 0.12a | 2.62 ± 0.11b | |
| Aspartate | Ile | 0.24 ± 0.06c | 2.03 ± 0.26b | 3.64 ± 0.17a | 1.83 ± 0.29c | 0.65 ± 0.16d | 1.63 ± 0.05c | 2.21 ± 0.05a | 1.46 ± 0.15b |
| Lys | 1.59 ± 0.01c | 2.08 ± 0.12b | 3.7 ± 0.07a | 1.86 ± 0.18c | 1.04 ± 0.17b | 2.91 ± 0.34ab | 3.29 ± 0.09a | 1.69 ± 0.15b | |
| Met | 0.4 ± 0.03c | 0.49 ± 0.09c | 1.56 ± 0.06a | 0.69 ± 0.04b | 0.55 ± 1.09c | 0.51 ± 0.08c | 1.82 ± 0.04a | 0.66 ± 0.07b | |
| Asp | 2.79 ± 0.21c | 3.63 ± 0.15b | 4.6 ± 0.29a | 2.07 ± 0.10c | 1.06 ± 0.09c | 3.55 ± 0.18a | 3.19 ± 0.41a | 1.89 ± 0.26b | |
| Thr | 1.12 ± 0.07c | 2.78 ± 0.14b | 2.12 ± 0.07a | 1.05 ± 0.02c | 1.48 ± 0.14c | 1.84 ± 0.02c | 2.4 ± 0.15a | 1.85 ± 0.20b | |
| Glutamate | Pro | 16.24 ± 0.89c | 15.57 ± 2.39b | 14.51 ± 3.48a | 9.47 ± 0.55c | 13.49 ± 1.14c | 12.74 ± 1.51b | 13.47 ± 2.60a | 10.06 ± 1.10c |
| His | 1.43 ± 0.14a | 1.59 ± 0.04a | 1.91 ± 0.07a | 0.55 ± 0.02b | 1.56 ± 0.05b | 1.86 ± 0.01b | 2.51 ± 0.06a | 0.56 ± 0.01c | |
| Arg | 12.59 ± 1.98b | 10.52 ± 1.12c | 15.00 ± 0.16a | 10.75 ± 0.91c | 15.06 ± 1.51b | 13.28 ± 1.00b | 17.87 ± 1.73a | 14.11 ± 1.28b | |
| Glu | 4.65 ± 1.73b | 2.44 ± 0.53c | 7.74 ± 0.17a | 5.74 ± 0.92ab | 3.41 ± 2.13c | 2.44 ± 0.13c | 8.66 ± 1.04a | 6.26 ± 1.07b | |
| Pyruvate | Leu | 5.4 ± 0.79c | 8.67 ± 0.59b | 10.66 ± 1.28a | 6.76 ± 0.51c | 6.09 ± 0.14c | 7.06 ± 0.40b | 9.23 ± 0.26a | 5.88 ± 0.54c |
| Ala | 5.72 ± 0.28c | 6.13 ± 0.48b | 9.49 ± 0.94a | 4.31 ± 0.14c | 6.91 ± 1.36c | 7.11 ± 1.55c | 10.55 ± 0.43a | 8.51 ± 0.96b | |
| Val | 1.27 ± 0.06c | 2.45 ± 0.18b | 4.71 ± 0.45a | 1.1 ± 0.12c | 1.09 ± 0.71b | 1.33 ± 0.05b | 3.78 ± 0.49a | 1.08 ± 0.41b | |
Different letters in the row indicate significant differences (p < 0.05) among treatments for the same vintage.
For the serine family, serine was the most abundant amino acid in grape berries, accounting for 50% of the amino acids from this family (Table 2). RDI-3 treatments significantly increased the levels of serine in both years; lower levels of serine were detected in the RDI-2 treatment group. The highest levels of cysteine were found in 2015 wines produced from RDI-3-treated vines; however, RDI-1 and RDI-2 treatments decreased cysteine values in both vintages of wine (Table 3). Phenylalanine and tryptophan are the main aromatic amino acid families in grape berries and wines. RDI-1 and RDI-3 treatments significantly increased tryptophan levels in the two vintages; however, the lowest tryptophan levels were detected with the RDI-2 treatment for both years (Table 2 and Table 3). The highest levels of phenylalanine were detected in the RDI-3 group for both years, which means that the RDI-3 treatment might give wine more rose, floral, and honey flavors [2]. The concentration of phenylalanine amino acid in berries with the RDI-2 in 2016 was higher compared to the control group. Higher values of aspartic acid in grape berries after RDI-3 treatment might provide more nitrogen for yeast [30,32]. The RDI-1 and RDI-2 treatments decreased the levels of methionine and lysine in berries; however, increased levels of methionine and lysine were found in berries after the RDI-3 treatment (Table 2). Methionine is the precursor of glutathione [29], and the RDI-3 treatment increased the level of methionine in the two vintages wines, which was consistent with a high level of glutathione. In contrast, the RDI-1 and RDI-2 treatments decreased the level of methionine in the two vintages wines, which was consistent with a low level of glutathione (Table 3 and Figure S2). Threonine was the most abundant member of the aspartate amino acid family in berries.
For the glutamate family, proline and arginine were the most abundant amino acids in grape berries (Table 2). Proline plays an important role in enhancing plant tolerance to water deficits [32,33]. The RDI treatments enhanced proline biosynthesis in both vintages, which was consistent with previous reports [32]. Arginine levels were enhanced in berries after the RDI-3 treatment; however, the RDI-2 treatment decreased the arginine levels in both vintages. The levels of arginine were lower in the wines than the berries, which might be due to yeast consuming arginine during fermentation [2]. The leucine and valine levels increased in berries and wines from the RDI-3 treatment for both vintages. The RDI-2 treatment increased the levels of leucine and valine in the 2016 grape berries; however, higher leucine and valine levels were only found in the 2015 wines.
3.3. Volatile Compounds Derived from Amino Acids in Grape Berries and Wines
3.3.1. Volatile Compounds Derived from Amino Acids in Grape Berries
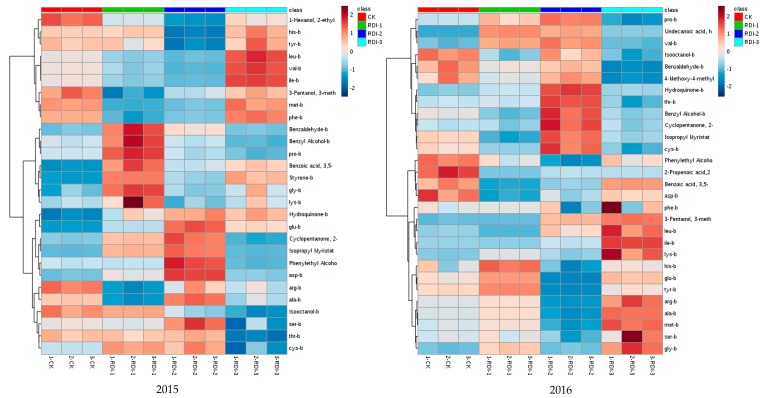
The volatile compounds derived from amino acids in the grapes and wines in this experiment are shown in Figure 1 and Table S3. The total amount of aromatic compounds in berries was higher in the RDI-3 treatment group than the other experimental groups (Figure 1 and Table S3). The polyphenol compounds were mainly produced by phenylalanine through the shikimic acid pathway [2]. The results showed that the level of phenylalanine in the berries from the RDI-3 treatment was significantly higher than that in the other treatment groups (Table 2) and, thus, promotes the production of polyphenol [2]. Increased levels of aromatic compounds—such as 3,5-dimethyl-benzoic acid, styrene—and hydroquinone, were detected in berries after the RDI-1 and RDI-2 treatments in 2015, and the increase was significant (Figure 1 and Table S3). Increased levels of aromatic compounds, such as benzyl alcohol, 3,5-dimethyl-benzoic acid, and hydroquinone, were detected in berries after the RDI-3 treatment for both years, and the increase was significant (Figure 1 and Table S3). RDI treatments increased the branched ketone content in berries for both vintages. Interestingly, RDI treatments increased the branched ester content, which could enhance the fruity aroma in berries.
Figure 1.
Clustered heatmaps of amino acids and volatile compounds derived from grape berries subjected to different treatments. Data were normalized by a pooled sample from the control group.
3.3.2. Volatile Compounds Derived from Amino Acids in Wines
The volatile compounds derived from amino acids in wines in this experiment are shown in Table 4. According to volatile compound biosynthesis pathways, the volatile compounds derived from amino acids in wines can be classified into four families, aromatic compounds, branched alcohols, branched ketones, and branched esters. The total amount of aromatic compounds in wines was higher in the RDI-3 treatment group than the other experimental groups (Table 4). The RDI-1 and RDI-2 treatments significantly increased the levels of aromatic compounds, such as benzaldehyde, benzyl alcohol, and phenylethyl alcohol, in the two vintages wines (Table 4). Increased levels of phenylethyl alcohol, 2-methyl-1-propanol and 3-methyl-1-butanol were detected in wines created from the RDI treatment groups, and these compounds might give wines more floral, rose, honey, and fruity flavors [3]. The quantity of branched ketones in wines was significantly higher than that in berries, and RDI treatments increased the branched ketone content in wines for both vintages. Many future works are needed to elucidate the potential effects of different RDI techniques on the accumulation of volatile precursors and amino acids in grapes and wines and their derived volatile compounds.
Table 4.
Volatile compound concentrations (μg/L) in wines from untreated (CK) and treated vineyards.
| Compounds | 2015 | 2016 | ||||||
|---|---|---|---|---|---|---|---|---|
| RDI-1 | RDI-2 | RDI-3 | CK | RDI-1 | RDI-2 | RDI-3 | CK | |
| Benzeneacetic acid, methyl ester | 3.74 ± 0.23a | 1.62 ± 0.10c | 4.2 ± 0.26a | 2.56 ± 0.16b | 1.43 ± 0.09c | 1.72 ± 0.10c | 4.05 ± 0.24a | 2.54 ± 0.15b |
| 1-propenyl-benzene | 0.52 ± 0.03c | 1.02 ± 0.06a | 0.77 ± 0.05b | 0.8 ± 0.05b | 1.29 ± 0.08b | 1.89 ± 0.11b | 1.11 ± 0.07a | 1.33 ± 0.08b |
| Benzaldehyde | 2.86 ± 0.18b | 2.98 ± 0.18b | 4.96 ± 0.31a | 1.87 ± 0.12c | 3.17 ± 0.19b | 2.11 ± 0.13c | 5.37 ± 0.32a | 2.97 ± 0.18bc |
| 3,4-Dimethyl-benzoic acid | 2.53 ± 0.16c | 4.77 ± 0.3a | 3.37 ± 0.21bc | 3.6 ± 0.22b | 4.99 ± 0.3a | 5.13 ± 0.31a | 4.52 ± 0.27a | 4.68 ± 0.28a |
| Styrene | 2.76 ± 0.17ab | 3 ± 0.19a | 2.65 ± 0.16ab | 2.29 ± 0.14b | 5.34 ± 0.32a | 6.75 ± 0.4a | 6.43 ± 0.38a | 5.6 ± 0.33a |
| Benzyl alcohol | 0.48 ± 0.03b | 0.85 ± 0.05a | 0.89 ± 0.06a | 0.63 ± 0.04b | 0.65 ± 0.04ab | 0.61 ± 0.04b | 0.79 ± 0.05a | 0.57 ± 0.03b |
| Phenylethyl alcohol | 56.43 ± 3.5a | 52.1 ± 3.23a | 55.35 ± 3.43a | 49.57 ± 3.07a | 58.27 ± 3.48a | 57.16 ± 3.41a | 55.83 ± 3.33a | 53.71 ± 3.21a |
| Furfural | 2.46 ± 0.15a | 2.31 ± 0.14a | 2.32 ± 0.14a | 2.41 ± 0.15a | ndb | nd | nd | nd |
| 3-Furaldehyde | 0.27 ± 0.02b | 0.61 ± 0.04a | 0.56 ± 0.03a | 0.16 ± 0.01b | nd | nd | nd | nd |
| 2,3,4,6-Tetramethylphenol | 1.85 ± 0.11a | 0.85 ± 0.05b | 1.55 ± 0.1a | 1.88 ± 0.12a | 2.6 ± 0.16ab | 2.65 ± 0.16ab | 3.21 ± 0.19a | 2.36 ± 0.14b |
| Total amount of aromatic compounds | 73.89 ± 4.58a | 70.11 ± 4.35a | 76.62 ± 4.75a | 65.78 ± 4.08a | 78.19 ± 4.67a | 78.45 ± 4.68a | 81.87 ± 4.89a | 74.26 ± 4.43a |
| Dihydro-3-methyl-2,5-furandione | nd | nd | nd | nd | 0.45 ± 0.03ab | 0.42 ± 0.03b | 0.55 ± 0.03a | 0.49 ± 0.03ab |
| 2-Methyl-1-propanol | 12 ± 0.74a | 11.34 ± 0.7ab | 10.57 ± 0.66ab | 9.25 ± 0.57b | 22.74 ± 1.36a | 16.22 ± 0.97b | 22.2 ± 1.33a | 16.16 ± 0.96b |
| 3-Methyl-1-pentanol | 2.73 ± 0.17c | 5.41 ± 0.34a | 4.41 ± 0.27ab | 3.42 ± 0.21bc | nd | nd | nd | nd |
| 3-Methyl-1-butanol | 192.09 ± 11.91a | 203.06 ± 12.59a | 222.58 ± 13.8a | 202.78 ± 12.57a | 315.86 ± 18.85ab | 305.71 ± 18.25ab | 353.97 ± 21.13a | 275.11 ± 16.42b |
| 3-Methyl-2-pentanol | 0.86 ± 0.05b | 0.59 ± 0.04c | 1.21 ± 0.07a | 0.98 ± 0.06ab | 1.28 ± 0.08bc | 1.08 ± 0.06c | 1.63 ± 0.1a | 1.55 ± 0.09ab |
| 2-Propanol, 1-butoxy- | 0.54 ± 0.03b | 1.31 ± 0.08a | 0.55 ± 0.03b | 0.35 ± 0.02b | nd | nd | nd | nd |
| Total amount of branched alcohols | 208.22 ± 12.91a | 221.72 ± 13.75a | 239.31 ± 14.84a | 216.79 ± 13.44a | 339.87 ± 20.29ab | 323.01 ± 19.28ab | 377.8 ± 22.55a | 292.82 ± 17.48b |
| 2-Butanone, 3-hydroxy- | 0.95 ± 0.06b | 0.42 ± 0.03c | 1.74 ± 0.11a | 0.88 ± 0.05b | 0.88 ± 0.05b | 1.43 ± 0.09a | 1.38 ± 0.08a | 1.25 ± 0.07a |
| Total amount of branched ketones | 0.95 ± 0.06b | 0.42 ± 0.03c | 1.74 ± 0.11a | 0.88 ± 0.05b | 0.88 ± 0.05b | 1.43 ± 0.09a | 1.38 ± 0.08a | 1.25 ± 0.07a |
| 2-Methyl-1-butyl acetate | 10.5 ± 0.03b | 10.8 ± 0.05a | 10.7 ± 0.04a | 10.53 ± 0.03b | 10.78 ± 0.05b | 11.07 ± 0.06a | 10.93 ± 0.06ab | 10.94 ± 0.06ab |
| Phenylmethyl acetate | 10.24 ± 0.01a | 10.14 ± 0.01b | 10.24 ± 0.01a | 10.15 ± 0.01b | 10.33 ± 0.02b | 10.24 ± 0.01c | 10.41 ± 0.02a | 10.27 ± 0.02bc |
| Phenethyl acetate | 11.34 ± 0.01b | 12.98 ± 0.03a | 11.47 ± 0.02b | 11.59 ± 0.01b | 11.29 ± 0.05b | 12.85 ± 0.06a | 11.39 ± 0.01b | 11.41 ± 0.02b |
| Isoamyl octanoate | 15.36 ± 0.03b | 19.21 ± 0.01a | 19.38 ± 0.11a | 20.62 ± 0.06a | 14.98 ± 0.07b | 20.35 ± 0.05a | 21.68 ± 0.09a | 21.92 ± 0.03a |
| Isoamyl hexanoate | 12.35 ± 0.06b | 15.34 ± 0.13a | 15.03 ± 0.1a | 14.95 ± 0.11a | 12.68 ± 0.05b | 16.92 ± 0.19a | 16.28 ± 0.14a | 15.62 ± 0.02a |
| Total amount of branched esters | 59.79 ± 0.05b | 68.47 ± 0.06a | 66.82 ± 0.06a | 67.84 ± 0.04a | 60.06 ± 0.07b | 71.43 ± 0.08a | 70.69 ± 0.08a | 70.16 ± 0.07a |
Different letters in the row indicate significant differences (p < 0.05) among treatments for the same vintage. nd: not detected.
3.4. The Correlation Analysis between Volatile Compounds and Amino Acids in Grape Berries and Wines
The analysis correlation ship between volatile compounds and amino acids in grape berries and wines was shown in Figure S2. The RDI-2 and RDI-3 treatment significantly increased the level of leucine in the two vintages berries and wines (Table 2 and Table 3). The concentration of leucine in berries was positively related to the concentration of leucine in wines (Figure S2). The concentration of leucine was closely related to the concentrations of 2-methyl-1-butyl acetate, benzaldehyde, 3-methyl-1-pentanol and 3-methyl-1-butanol in wines (Figure S2). RDI-3 treatment significantly enhanced the concentrations of arginine, alanine, valine, and isoleucine in both vintages wines and RDI-2 significantly increased the concentrations of valine and isoleucine in both vintages wines (Table 3). The concentrations of arginine, alanine, valine, and isoleucine were positively related to the accumulation of volatile compounds—such as 3,4-dimethyl-benzoic acid, 3-hydroxy-2-butanone, 1-butoxy-2-propanol, 2-methyl-1-butyl acetate, and 3-methyl-1-pentanol—in wines (Figure S2). The accumulation of volatile compounds, after RDI treatments, was closely related to the amino acid concentrations—especially isoleucine, valine, and leucine—in wines.
4. Conclusions
The effects of RDI on amino acids and their derived volatiles in Cabernet Sauvignon grapes and wines were evaluated for two consecutive vintages. The RDI-1-treated vines had the lowest yield and berry weight but higher TSS compared with that of the RDI-2 and RDI-3 groups. The RDI-2 and RDI-3 treatments increased the concentrations of amino acids in berries as well as certain volatile components in wines, including 3-methyl-2-pentanol and 3-methyl-1-butanol. The accumulation of volatile compounds in wines is closely related to the content of amino acids, especially isoleucine, valine, and leucine. Overall, the RDI-2 and RDI-3 treatments improved the qualities of grapes and wines based on their amino acid and derived volatile compositions.
Acknowledgments
This work was supported by the Key Research and Development Program of Ningxia (grant no. 2016BZ0602) and the China Agriculture Research System for Grape (grant no. CARS-29-zp-6).
Supplementary Materials
The following are available online: Figure S1–S2, Table S1–S3.
Author Contributions
Y.-l.J. and Y.-l.F. designed the research; Y.-l.J., G.-q.X., and X.-f.Y. conducted the experiments; Y.-l.J. and X.-f.Z. analyzed the data; Y.-l.J. and X.-f.Y. wrote the manuscript; T.-y.T., J.-x.Z., and Y.-l.F. promoted the manuscript. All authors have read and approved the final manuscript.
Funding
This research was funded by [the Key Research and Development Program of Ningxia] grant number [2016BZ0602] and The APC was funded by [the Key Research and Development Program of Ningxia].
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Bell S.J., Henschke P. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005;11:242–295. doi: 10.1111/j.1755-0238.2005.tb00028.x. [DOI] [Google Scholar]
- 2.Styger G., Prior B., Bauer F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011;38:1145. doi: 10.1007/s10295-011-1018-4. [DOI] [PubMed] [Google Scholar]
- 3.Bisson L.F., Karpel J.E. Genetics of yeast impacting wine quality. Annu. Rev. Food Sci. Technol. 2010;1:139–162. doi: 10.1146/annurev.food.080708.100734. [DOI] [PubMed] [Google Scholar]
- 4.Rapp A. Volatile flavour of wine: Correlation between instrumental analysis and sensory perception. Food. 1998;42:351–363. doi: 10.1002/(SICI)1521-3803(199812)42:06<351::AID-FOOD351>3.3.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 5.Ardo Y. Flavour formation by amino acid catabolism. Biotechnol. Adv. 2006;24:238–242. doi: 10.1016/j.biotechadv.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Procopio S., Krause D., Hofmann T., Becker T. Significant amino acids in aroma compound profiling during yeast fermentation analyzed by pls regression. LWT. Food Sci. Technol. 2013;51:423–432. [Google Scholar]
- 7.Wang Y.Q., Ye D.Q., Liu P.T., Duan L.L., Duan C.Q., Yan G.L. Synergistic effects of branched-chain amino acids and phenylalanine addition on major volatile compounds in wine during alcoholic fermentation. S. Afr. J. Enol. Viticult. 2016;37:169–175. doi: 10.21548/37-2-683. [DOI] [Google Scholar]
- 8.Sánchez-Gómez R., Garde-Cerdán T., Zalacain A., Garcia R., Cabrita M.J., Salinas M.R. Vine-shoot waste aqueous extract applied as foliar fertilizer to grapevines: Effect on amino acids and fermentative volatile content. Food Chem. 2016;197:132–140. doi: 10.1016/j.foodchem.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Bouzas-Cid Y., Díaz-Losada E., Trigo-Córdoba E., Falqué E., Orriols I., Garde-Cerdán T., Mirás-Avalos J.M. Effects of irrigation over three years on the amino acid composition of Albariño (Vitis vinifera L.) musts and wines in two different terroirs. Sci. Hortic. 2018;227:313–325. doi: 10.1016/j.scienta.2017.05.005. [DOI] [Google Scholar]
- 10.Gutiérrez-Gamboa G., Portu J., Santamaría P., López R., Garde-Cerdán T. Effects on grape amino acid concentration through foliar application of three different elicitors. Food Res. Int. 2017;99:688–692. doi: 10.1016/j.foodres.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Torres N., Hilbert G., Luquin J., Goicoechea N., Antolín M.C. Flavonoid and amino acid profiling on Vitis vinifera L. cv Tempranillo subjected to deficit irrigation under elevated temperatures. J. Food Compost. Anal. 2017;62:51–62. doi: 10.1016/j.jfca.2017.05.001. [DOI] [Google Scholar]
- 12.Gutiérrez-Gamboa G., Garde-Cerdán T., Gonzalo-Diago A., Moreno-Simunovic Y., Martínez-Gil A.M. Effect of different foliar nitrogen applications on the must amino acids and glutathione composition in Cabernet Sauvignon vineyard. LWT-Food Sci. Technol. 2017;75:14–154. [Google Scholar]
- 13.Ji X.W., Cheng Z.Y., Zhao X. Effect of regulated deficit drip irrigation on yield and quality of wine grape in desert oasis. J. Arid Land Res. Environ. 2015;4:184–188. [Google Scholar]
- 14.Li Y.S., Zhao X.H., Wang H. Research advance and prospect of regulated deficit irrigation on grapevines. Agric. Res. Arid Areas. 2013;1:23–241. [Google Scholar]
- 15.Wang S.J., Liu Q.B., Yu H.Y., Sun R. The Theory and Technological System of Regulated Deficit Irrigation for Grape. Agric. Mech. Res. 2005;2:8–9. [Google Scholar]
- 16.Fang Y.L., Sun W., Wan L. Effects of Regulated Deficit Irrigation (RDI) on Wine Grape Growth and Fruit Quality. Sci. Agric. Sin. 2013;46:2730–2738. [Google Scholar]
- 17.Ju Y.L., Wang T.M., Zhao X.F., Liu M., Min Z., Zhang J.X., Fang Y.L. Effects of regulated deficit irrigation on fruit development and seed phenolic compounds of Cabernet Sauvignon. Sino-Overseas Grapevine Wine. 2017;4:18–24. [Google Scholar]
- 18.Song J., Shellie K.C., Wang H., Qian M.C. Influence of deficit irrigation and kaolin particle film on grape composition and volatile compounds in Merlot grape (Vitis vinifera L.) Food Chem. 2012;134:841–850. doi: 10.1016/j.foodchem.2012.02.193. [DOI] [PubMed] [Google Scholar]
- 19.Allen R.G., Pereira L.S., Raes D., Smith M. Crop Evapotranspiration Guidelines for Computing Crop Water Requirements (FAO Irrigation and Drainage Paper no. 56) Food and Agriculture Organization of the United Nations; Rome, Italy: 1998. [Google Scholar]
- 20.OIV International Code of Oenological Practices. [(accessed on 1 January 2012)];2012 Available online: http://www.oiv.int/oiv/info/enpratiquesoenologiques.
- 21.Liu R., Tian Y., Wen Y., Gao Q., Pan Q. Effects of rain-shelter cultivation on the contents of amino acids in wine grape berry. Sino-Overseas Grapevine Wine. 2012;4:15–19. [Google Scholar]
- 22.Garde-Cerdán T., López R., Portu J., González-Arenzana L., López-Alfaro I., Santamaría P. Study of the effects of proline, phenylalanine, and urea foliar application to tempranillo vineyards on grape amino acid content. comparison with commercial nitrogen fertilisers. Food Chem. 2014;163:136–141. doi: 10.1016/j.foodchem.2014.04.101. [DOI] [PubMed] [Google Scholar]
- 23.Ju Y.L., Liu M., Zhao X.F., Zeng J., Min Z., Fang Y.L. Effects of Filed Management Practices and Harvest Time on Fatty Acid Composition of Cabernet Sauvignon and Chardonnay (Vitis vinifera L.) Berries Skins. Food Sci. 2017;38:107–113. [Google Scholar]
- 24.Traverso J.A., Pulido A., Rodríguez-García M.I., Alché J.D. Thiol-based redox regulation in sexual plant reproduction: New insights and perspectives. Front. Plant Sci. 2013 doi: 10.3389/fpls.2013.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia J., Wishart D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis Current Protocols in Bioinformatics. Curr. Protoc. Bioinform. 2016;55:14.10.1–14.10.91. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 26.Shellie K.C. Vine and berry response of Merlot (Vitis vinifera L.) to differential water stress. Am. J. Enol. Vitic. 2006;57:514–518. [Google Scholar]
- 27.Koundouras S., Marinos V., Gkoulioti A., Kotseridis Y., van Leeuwen C. Influence of vineyard location and vine water status on fruit maturation of nonirrigated cv. Agiorgitiko (Vitis vinifera L.). Effects on wine phenolic and aroma components. J. Agric. Food Chem. 2006;54:5077–5086. doi: 10.1021/jf0605446. [DOI] [PubMed] [Google Scholar]
- 28.Valero E., Millan C., Ortega J.M., Mauricio J.C. Concentration of amino acids in wine after the end of fermentation by Saccharomyces cerevisiae strains. J. Sci. Food Agric. 2003;83:830–835. doi: 10.1002/jsfa.1417. [DOI] [Google Scholar]
- 29.Martinez-Rodriguez A.J., Carrascosa A.V., Martin-Alvarez P.J., Moreno-Arribas V., Polo M.C. Influence of the yeast strain on the changes of the amino acids, peptides and proteins during sparkling wine production by the traditional method. J. Ind. Microbiol. Biotechnol. 2002;29:314–322. doi: 10.1038/sj.jim.7000323. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Orte P., Ibarz M., Cacho J., Ferreira V. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005;89:163–174. doi: 10.1016/j.foodchem.2004.02.021. [DOI] [Google Scholar]
- 31.Guan L., Wu B., Hilbert G., Li S., Gomès E., Delrot S., Dai Z. Cluster shading modifies amino acids in grape (Vitis vinifera L.) berries in a genotype-and tissue-dependent manner. Food Res. Int. 2017;98:2–9. doi: 10.1016/j.foodres.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Castellarin S.D., Matthews M.A., Di Gaspero G., Gambetta G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta. 2007;227:101–112. doi: 10.1007/s00425-007-0598-8. [DOI] [PubMed] [Google Scholar]
- 33.Garde-Cerdán T., Lorenzo C., Martínez-Gil A.M., Lara J.F., Pardo F., Salinas R. Evolution of nitrogen compounds during grape ripening from organic and nonorganic Monastrell–nitrogen consumption and volatile formation in alcoholic fermentation. Res. Org. Farming. 2009;8:123–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.