Abstract
Silica is one of the most abundant minerals in the Earth’s crust, and over time it has been introduced first into human life and later into engineering. Silica is present in the food chain and in the human body. As a biomaterial, silica is widely used in dentistry, orthopedics, and dermatology. Recently amorphous sol-gel SiO2 nanoparticles (NPs) have appeared as nanocarriers in a wide range of medical applications, namely in drug/gene target delivery and imaging diagnosis, where they stand out for their high biocompatibility, hydrophilicity, enormous flexibility for surface modification with a high payload capacity, and prolonged blood circulation time. The sol-gel process is an extremely versatile bottom-up methodology used in the synthesis of silica NPs, offering a great variety of chemical possibilities, such as high homogeneity and purity, along with full scale pH processing. By introducing organic functional groups or surfactants during the sol-gel process, ORMOSIL NPs or mesoporous NPs are produced. Colloidal route, biomimetic synthesis, solution route and template synthesis (the main sol-gel methods to produce monosized silica nanoparticles) are compared and discussed. This short review goes over some of the emerging approaches in the field of non-porous sol-gel silica NPs aiming at medical applications, centered on the syntheses processes used.
Keywords: sol-gel, silica, nanoparticles, Stöber, LaMer, reverse emulsion, biogeochemical cycle
1. Introduction
Silica (SiO2) has been used throughout history in the manufacturing of glass, ceramics, concrete, mortar, sandstone and silicones [1]. Today, silica is present in an impressive variety of everyday life products, such as glass and tableware, vitroceramics, domestic and industrial water membranes, plastics, paper, paints, silicones, semiconductors, fiber and optical glasses, electronic, optoelectronic, aerospace, and defense products [2,3,4], to mention some of them. As regards healthcare and medicine, SiO2 is a current material in dentistry (tooth implants, ceramic pastes) [5,6], orthopedics (bone implants, scaffolds) [7,8], dermatology [9] and specialized medical devices (ophthalmological and bio-glasses, scaffolds) [10,11,12,13]. The coming of nanomedicine opened the door to new engineered materials/devices opportunities, colloidal silica having pride of place among micro and nanoparticles (NPs) [14,15,16,17,18,19,20,21,22,23,24].
Nanomedicine initiated a new attitude towards conventional medicine, where challenges are addressed using a bottom-up rather than top-down approach, medical actions are performed at a single cell level, tailor made therapeutic prescription are performed, theranosis (where diagnosis and therapeutic meet) is designed [25]. Nanomedicine is based on nanocarriers (nanosystems, nanoplatforms, NPs) capable of simultaneous targeting, sensing, signaling, and drug releasing distinct drugs/markers, with different pharmacokinetics/pharmacodynamics, to elaborate optimized tailor-made treatments/diagnosis. Cell tracking (in regenerative medicine or in the early detection of cancer) is carried out across all major imaging modalities—like magnetic resonance imaging (MRI), magnetic particle imaging (MPI), computed tomography (CT), positron emission tomography (PET), single-photon emission computed tomography (SPECT), to ultrasound (US), and optical (fluorescent) imaging [26] and journals like Cancer Imaging and Cancer Imaging and Diagnosis. Spatial and temporal controlled drug delivery (throughout injectable or oral nanocarriers) redesigned disease treatment ([27,28,29,30,31,32] and Advanced Drug Delivery Reviews). While introducing thousands of times less drug into the body nanomedicine scaled down the possibility of side effects, like tissue or organ inflammation or destruction, and at the same time increased the localization of pharmaceutical drugs in the diseased tissue. Moreover, engineered NPs maybe fine-tuned relatively to thermal, electrochemical, catalytic, electronic and magnetic properties or designed to respond to micro-environmental (pH sensitivity, enzyme sensitivity) or external (magnetic field, light) triggers [33,34,35,36,37].
As a nanocarrier, silica competes with biological materials (like phospholipids, lipids, lactic acid, sugars, proteins such as dextran or chitosan, virus), synthetic polymers (carbon or silicon-based), and other inorganic materials (ceramics, metals) (Table 1, Figure 1). Silica’s high thermal stability, chemical inertia (at PTN conditions), microbial attack immunity, along with strong surface energy, high hydrophilicity and biocompatibility, impermeability (or weak permeability), great chemical versatility (due to the possibility of in situ/ex situ physical functionalization, through second order chemical bonding, or chemical functionalization, in highly specific conditions), high loading capacity for drugs/chemicals/therapeutic molecules associated with ease of processing [38,39] are the properties that made it so special, a cost-effective, hard to beat material. Furthermore, amorphous silica and silicates are generally recognized as safe by the U.S. Food and Drug Administration [40], to be used as oral delivery ingredients in amounts up to 1500 mg per day, and have been widely applied as an additive in cosmetics, food, and oral drugs [41,42].
Table 1.
Multifunctional NPs: materials and functions (adapted and reproduced with permission from [14]).
| Component | Material | Function |
|---|---|---|
| Biomedical payload | Imaging agents for optical, MRI, MPI, CT, PET, SPECT, US imaging (organic dye, QDs, UCNPs, magnetic materials, metal NPs) | Image enhancement |
| Therapeutic agents (anticancer drugs, DNA, siRNA, hyperthermal/photodynamic materials) | Cancer cell death induction, gene up/down regulation | |
| Carrier | Organic (lipid, natural/synthetic polymers) | Multifunctional (protection of payloads, controlled release of drug/gene, biocompatibility, stimuli responsiveness) |
| Inorganic (hollow metal NPs, hollow metal oxide NPs, C nanostructures, porous, non-porous, core-shell or nanostructured SiO2 NPs) | Multifunctional (imaging ability added to above functions) | |
| Surface modifier | Antibody | Molecular imaging |
| Aptamer | Target specific delivery | |
| Peptid/protein | Uptake enhancement | |
| Small molecules | Penetration of barrier | |
| Charge balancing molecules | Signaling transduction Stimuli responsiveness |
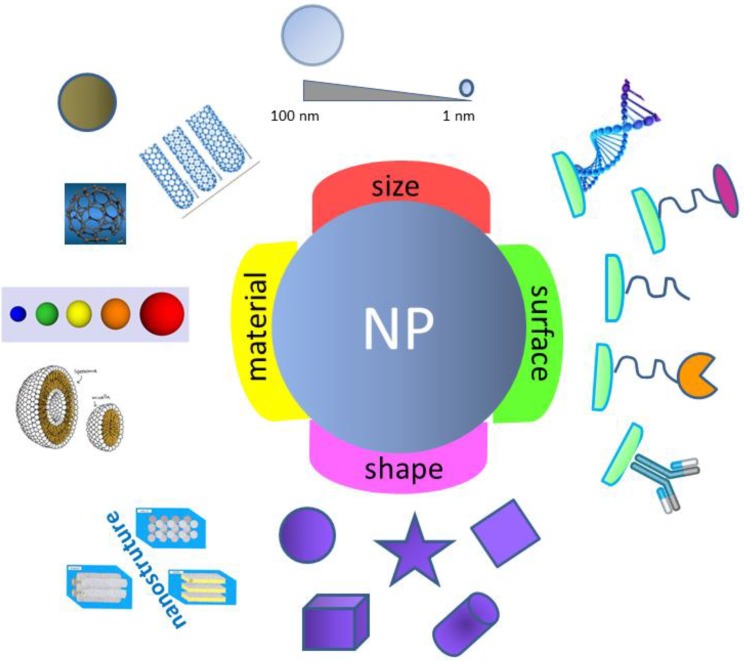
Figure 1.
Engineered multifunctional NP.
Sol Gel Gateway [43] lists the on business sol-gel companies, according to which CeraMem Corporation (Waltham, MA, USA), Quantum Dot Corporation (Hayward, CA, USA), General Engineering and Research (San Diego, CA, USA) and CD Creative Diagnostics (Shirley, NY, USA) stand out for their global market shares in sol-gel silica NPs. As to pharmaceutical and medical applications, the Canadian company PreveCeutical (Vancouver, BC, Canada) has developed (and patented) a sol-gel delivery platform for Nose-to-Brain Delivery of Therapeutic Compounds®, the first FDA approved CBD-based nose-to-brain delivery systems. Sol-gels are taken via nasal (systemic) administration and rapidly gel upon contact with mucosal tissue allowing a direct nose-to-brain delivery by slow releasing of drug/therapeutic molecules. By bypassing the stomach and intestines bioavailability may be improved (even when compared to nasal sprays and other newer delivery systems). Fluorescent ultra-small sol-gel SiO2 NPs (Cornell dots®) have emerged as a particularly fascinating fluorescent probe, which demonstrated excellent outcomes in the first human clinical trials (for cancer imaging) in patients with metastatic melanoma, being commercialized by Hybrid Silica Technologies (HST), a Cornell business startup (Ithaca, NY, USA) [44,45,46,47]. Magnetic (core-shell) sol-gel silica NPs (confering multifunctionality to the carrier) are particularly useful for externally magnetic guided systems in diagnosis, and hyperthermia treatments [48]. USA Cd Creative Diagnostics (Shirley, NY, USA) has commercialized a wide range of plain and core-shell (iron oxide magnetic core) sol-gel silica NPs while the French company NH TherAguix (Crolles, France) provides ultra-small sol-gel silica-based bismuth/gadolinium contrast agents. AuroLase® Therapy (from Nanospectra Biosciences, Houston, TX, USA) utilizes optically tunable sol-gel nanoshells, able to convert light into heat and thermally destroy solid tumors without damaging adjacent healthy tissue. MRI/US Fusion Imaging and Biopsy in Combination with Nanoparticle Directed Focal Therapy for Ablation of Prostate Tissue® and MR/US Fusion Guided Ultra-Focal Gold Nanoparticle Directed Photothermal Ablation of Prostate Gland Tumors® are products in clinical trials. The Israeli (clinical-stage) company Sol Gel Technologies (Ness Ziona, Israel) focuses on developing and commercializing topical dermatological drug products, based on its proprietary sol-gel microencapsulation delivery system. Presently Vered®, Twin® and Sirs-T® are in phase II trials, while a generic candidate is in phase III (Table 2). In contrast to the limited number of sol-gel silica NPs currently under clinical tests a considerable number of sol-gel SiO2 NPs products/processes aiming medical applications are under investigation and some have been recently patented [49] (as proof of concept) and await clinical tests for national agency approval (Table 3).
Table 2.
Sol-gel silica nanocarriers in clinical use and under clinical investigation.
| Trade Mark | Formulation | Company | Application | Phase of Development |
|---|---|---|---|---|
| C-dots® | PEG-coated SiO2 NPs | C-dots Development (USA) | Melanoma (Intravenous) | FDA approved 2011 |
| PreveCeutical® | SiO2 sol-gel delivery platform | PreveCeutical (Canada) | platform for Nose-to-Brain Delivery of Therapeutic Compounds | INC FDA approval |
| Vered® | Patented microencapsulation SiO2 NPs | Sol Gel Technologies (Israel) | Papulopostular Rosacea (Dermatology) | Phase II |
| Twin® | Patented microencapsulation SiO2 NPs | Sol Gel Technologies (Israel) | Acne Vulgaris (Dermatology) | Phase II |
| Sirs-T® | Patented microencapsulation SiO2 NPs | Sol Gel Technologies (Israel) | Acne Vulgaris (Dermatology) | Phase II |
| Generic | Patented microencapsulation SiO2 NPs | Sol Gel Technologies (Israel) | Acne Vulgaris (Dermatology) | Phase III |
| Ultra-small silica-based bismuth gadolinium NPs | NH TherAguix (France) | Dual MR-CT guided radiation therapy | Phase I | |
| AbsolutMag™ | Silica NPs, TiO2-SiO2 coated NPs (10, 20, 30 nm, 20 µm) |
Cd Creative Diagnostics (USA) | Theranostic | Phase I |
| DiagNano™ | Silica magnetic NPs (produced by hydrolysis of orthosilicates in the presence of magnetite) (250 nm–6 µm; 6.0–43 emu/g) |
Cd Creative Diagnostics (USA) | DNA/RNA isolationa and purification | Phase I |
| AuroLase® | PEG-coated silica-gold nanoshells | NanoSpectra Biosciences (USA) | Near-IR light facilitated thermal ablation. Thermal ablation of solid primary and/or metastatic lung tumors | NCT01679470 (Not Provided) |
| AuroLase® | PEG-coated silica-gold nanoshells | NanoSpectra Biosciences (USA) | MR/US Near-IR light facilitated Prostate Gland Tumors thermal ablation. | Phase II |
Table 3.
Sol-gel silica-based NPs therapeutics recently patented (based on [49]).
| Patent | Title | Inventors |
|---|---|---|
| PT20131000062306 PCT/PT2014/000054 |
Multifunctional Superparamagnetic Nanosystem as Contrast Agent for Magnetic Resonance Imaging and Its Production Method | Gonçalve M.C.; Fortes L.M.; Martins B.M.; Carvalho A.D.; Feio G. |
| WO2011003109, 2011 | Fluorescent silica-based NPs | Bradbury M.; Wiesner U.; Penate M.O.; Ow H.; Burns A.; Lewis J. |
| US20100055167 Al, 2010 | Stem cell delivery of antineoplasic medicine | Zhang A.; Guan Y.; Chen L. |
| US20107799303, 2010 | Method of preparing silica NPs from siliceous mudstone | Jang H.-D.; Chang H.-K.; Yoon H.-S. |
| US20100303716 Al., 2010 | Switchable nano-vehicle delivery systems, and methods for making them. | Jin S.; Oh S.; Brammer K.; Kong S. |
| WO2009064964, 2009 | Switchable nano-vehicle delivery systems, and methods for making and using them. | Jin S.; Oh S.; Brammer K.; Kong S. |
| US20110092390, 2010 | Methods for making particles having long spin-lattice relaxation times. | Marcus C.M. |
| US20100040693, 2010 WO2008018716, 2008 |
Silica capsules having nano-holes or nano-pores on their surfaces and method for preparing the same | Chung B.H.; Lim Y.T.; Kim J.K. |
| US20100255103, 2010 | Mesoporous silica NPs for biomedical applications | Liong M.; Lu J.; Tamanoi F.; Zink J.I.; Nel A. |
| US20100104650, 2010 | Charged mesoporous silica naoparticles-based drug delivery system for controlled release and enhanced bioavailability | Lee C.-H.; Lo L.-W.; Yang C.-S., Mou C.-Y. |
| US201001361124, 2010 WO2008128292, 2008 |
Nanoparticle-coated capsule formulation for dermal drug delivery | Prestidge C.A.; Simovic S.; Eskandar N.G. |
| US20090263486, 2009 | Nanoparticle-stabilized capsule formulation for treatment of inflamation | Prestidge C.A.; Simovic S. |
| US20090181076, 2009 | Drug release from nanoparticle-coated capsules | Prestidge C.A.; Simovic S.; Eskandar N.G. |
| WO2009021286, 2009 | Organosilica encapsulated NPs | Qiao S.; Lu G.Q. |
| WO2009091992, 2009 | Repairing damaged nervous system tissue with NPs | Cho Y.; Shi R.; Ivanisevic A.; Borgens R. |
| US20090169482, 2009 | Silica-cored carrier nanoparticle | Zhen S.; Dai L.; Wang R.; Qiao T.A.; Che W.; Harrison W.J. |
| US20090232899, 2009 WO2005117844, 2005 |
Mucoadhesive nanocomposite delivery systems | David A.E.; Zhang R.; Park Y.J.; Yang A.J.-M.; Yang V.C. |
| US20090252811,2009 WO2005009602,2005 |
Capped mesoporous silicates | Lin V.S.-Y.; Lai C.-Y.; Jeftinija S.; Jeftinija D.M. |
| EP 20070829819, 2007 | Mesoporous silica particles. | Yano T.; Sawada T. |
| WO2005044224, 2005 | Drug Delivery system based on polymer nanosshells. | Gao J.; Al H. |
| GB2409160(A), 2005 WO2004GB05203,2005 |
A method of engineering particles for use in the delivery of drugs via inhalation | Okpala J. |
Harkin Holdings (Tauranga, New Zeland) and B. Arkin Bio Ventures (Herzliya Pituach, Israel) are finance agencies that identify, invest in, and follow up innovative early and mid-stage companies directed to nanomedicine (with game-changing breakthroughs) in areas such as immunotherapy, cancer metabolism, microbiome, CNS, autoimmune diseases, orphan diseases and drug delivery nanoplatforms empowering this emerging I&D segment.
2. Silica Biogeochemical Cycle
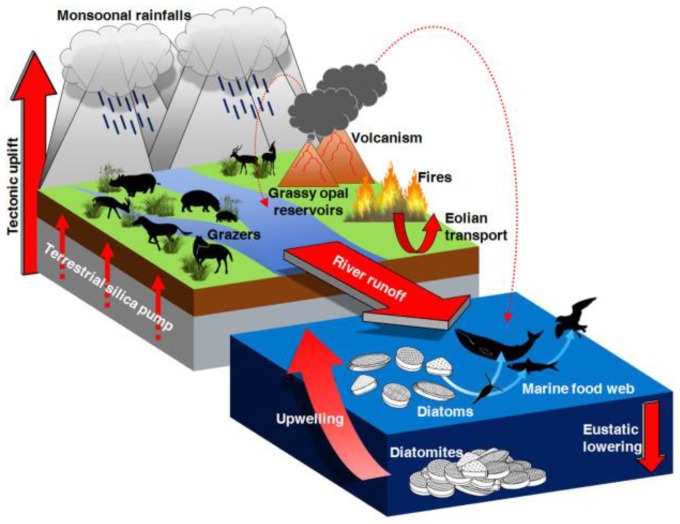
Silica is the most abundant mineral in the Earth’s crust (~75 wt%). In the continental plate, silica’s prevailing geologic crystalline polymorphs are quartz, tridymite and cristobalite, (present in minerals such as feldspars, micas, zeolites or talcs), while the most common amorphous phases are obsidian (present in volcanic rocks like pozzolans, and ashes, and in rocks that suffered meteoric impact) and opals accounts for Gtons per year, vastly outweighing that produced industrially [50].
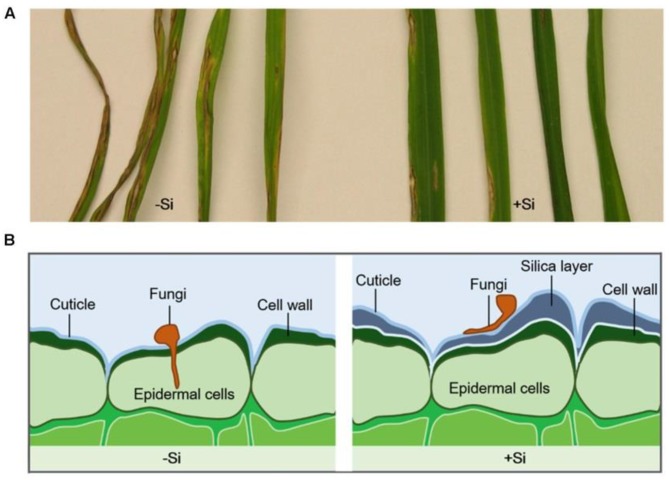
In the ocean plates, diatomaceous earth constitutes a significant amorphous silica (a-SiO2) source. This highly reactive biogenic silica deposit layer resulted from the silica-based skeletal remains of tiny aquatic organisms (diatomaceous and silicified phytoplankton) deposited over millions of years, and may include minor amounts of quartz, oxides of Ca, Mg, Fe and Al, and even some organic matter, depending on their location (Figure 2). More importantly, silica performs an essential role in many, if not all, forms of life [38]. The weathering and deterioration of rocks allows for high silica contend in soils (from <1 to 45% dry weight [52]) in the form of soluble [Si(OH)4] or Si(OH)3O− species (with concentrations ranging from 0.1 to 0.6 mM [53]), allowing its uptake by plants, thus entering the food chain [54,55]. Although not traditionally thought of as an essential element in plants’ life cycles, Si is found in plants at concentrations varying from 0·1 to 10% (~103–105 mg kg−1, dry weight basis), an amount equivalent to, or even exceeding, several macronutrients [53]. Biogenic silica occurs in many single-cell organisms, as natural amorphous structures with the composition SiOn(OH)4−2n. The skeleta of diatoms (Diatomeae, 5 µm up to 60 µm) are formed with silica. Upper plants like Equisetaceae may accumulate 1 up to 10% silica (in dry weight), poaceae, Equisetaceae and Cyperaceae families have silica values >4% wt, the Cucurbitales, Urticales and Commelinaceae families accumulate an intermediate Si content (2–4% wt Si), while most other species contain trace amounts of silica. Nevertheless, silica is known to favor the healthy growth, development and reproduction of plants, increase their resistance to fungi, enhance their mechanical resistance, apart from not being detrimental when excessively collected [56] (Figure 3).
Figure 2.
The diatomite deposition in the Mediterranean region. Reprint with permission from [51].
Figure 3.
Leaf blast symptoms in rice after inoculated with Magnaporthe grisea for 10 days. Rice plants were continuously treated with (+Si) or without silicon (−Si) (A). Silica layer was formed in the cell wall of Si-treated plants and enhanced plant resistance to fungi infection by physical barriers (B). Reprint with permission from [57].
In the human body, silica’s effects in health, aging and disease are still being explored, notwithstanding its average content of ca. 260 ppm, very close to the magnesium level (accounting for 18 g in a person with 70 kg) [58,59]. Silica is pivotal in calcium phosphate nucleation, determinant in bone mineralization, growth and self-healing during dislocations and fractures [59]. Aluminum absorption and (eventual) intoxication in human body (particularly important in Alzheimer’s disease) may be prevented by silica ingestion, through the bonding to Al in the gastrointestinal tract [60,61]. Maintenance of health in the immune system and reduction of the risk of atherosclerosis are other of silica attributes [62]. Silica also accounts for the maintenance of tissue integrity by stabilizing the glycoproteins associated with collagen, thus playing a central role in the structural integrity of nails, hair, and skin [63]. Amorphous silica could enter in the human body via the gastro-intestinal tract (in colloidal form) and has been part of human dietary either as medical clay, or as food additive (labelled E551) [64,65]. Contrary to crystalline silica, associated with lungs cancer and silicosis [66,67], a-SiO2 NPs seem essentially nontoxic [68]. Any (eventual) inflammation process associated with a-SiO2 NPs is dependent on the NP size, shape and synthesis methodology, being no unambiguous linking of physical-chemical properties of a-SiO2 and human toxicity, bioavailability, yet possible [68,69,70,71,72,73,74,75,76].
3. Silica NP Design
Pharmaceutical design is currently based on the Quality by Design (QbD) concept, a new, systematic, risk-based methodology. QbD begins with predefined objectives and dwells on product and process understanding, along with process control [77,78,79,80]. QbD requires: (i) knowledge of the physiologic barriers NPs face within the human body, (ii) complete characterization of NPs materials, and (iii) fully understanding of the NP synthesis process.
3.1. NPs Physiologic Barriers
The biological performance (pharmacokinetics profiles, biodistribution, target recognition, therapeutic efficacy, inflammatory reactions and toxicity) of intravenously injected NPs is controlled by a complex array of interrelated physicochemical and biological factors, starting with opsonization, followed by phagocyte ingestion and ending with NPs clearance. [81,82,83,84]. Rapid blood clearance limits drugs/gene/therapeutic molecules/markers accumulation at target delivery sites, while NPs accumulation in macrophages (within clearance organs) initiates inflammatory responses, inducing toxicity [85].
Opsonization is the process by which a foreign organism or particle becomes covered with biologic proteins (opsonins), forming a coating (named corona by materials scientists or opsonins in pharmaceutics) thereby making it more visible to phagocytic cells. The exact mechanism through which opsonization is activated is complex and is not yet fully understood. When the opsonin proteins (blood serum components like laminin, fibronectin, C-reactive protein, type I collagen, components of the complement proteins such as C3, C4, and C5 and immunoglobulins [81,82]) come into close contact with engineered NPs, typically by random Brownian motion, they may adsorb on NPs surface through van der Walls, electrostatic, ionic or hydrophobic/hydrophilic attractive forces. Protein opsonization usually takes place in the blood circulation system and may hold from few seconds to many days to complete.
After opsonization, phagocytosis occurs. Macrophages (typically Kupffer cells of the liver or spleen) cannot directly identify the NPs themselves, but rather recognize (through specific, non-specific receptors or complement activation) opsonin proteins adsorbed to the NPs surface [81]. Macrophages may uptake foreign materials within a matter of minutes (after opsonization), increasing the phagocytosis rate for positively charged and bacteria-specific proteins and render them ineffective as nanocarriers [83].
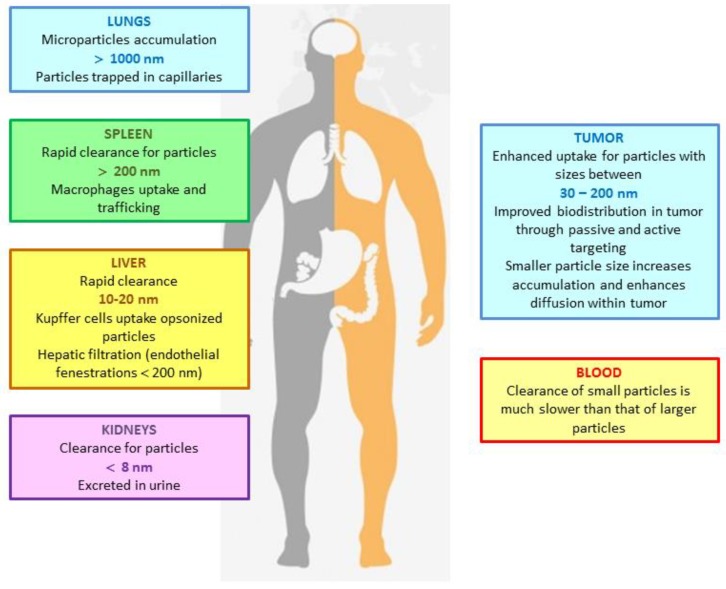
Finally clearance occurs. The phagocytes begin to secret enzymes and other oxidative molecules (like superoxides, oxyhalide molecules, nitric oxide, and hydrogen peroxide) to ingest (chemically break down) the phagocytosed material [81]. Unfortunately, most non-biodegradable NPs cannot be degraded significantly by this process and, depending on their relative size and molecular weight will either be removed by the renal system or sequestered and stored in one of the mononuclear phagocyte system (MPS) organs.
Renal clearance (based on physical filtration, dialysis) is a second optimal method for expelling NPs from the body. As a first approximation, removal by the renal system occurs only for polymeric molecules with a molecular weight of around 5000 or less, or inorganic NPs with hydrodynamic diameters smaller than 8 nm. Dendrimers (with molecular weights as high as 100,000) and NPs larger than 8 nm (if somehow broken down into fragments smaller than 6 nm after drug release) may also be cleared by the renal system [86]. Non-biodegradable NPs and degradation molecules with a molecular weight higher than the renal threshold typically become sequestered in the MPS organs (Figure 4).
Figure 4.
Major organ sites of NPs localization.
Several methods have been developed to mask or camouflage NPs from the MPS or renal clearance. The most preferred of these methods is the adsorption or grafting of poly (ethylene glycol) (PEG) to the surface of NPs. Addition of PEG and PEG-containing copolymers to the surface of NPs resulted in an increase in the blood circulation half-life of the NPs by several orders of magnitude. This method created a hydrophilic protective layer around the NPs that is able to repel the absorption of opsonin proteins via steric hindrance, thereby blocking and delaying the first step in the opsonization process [81]. Moreover, PEGylation prevents NPs aggregation in solution, which helps keep them from forming a cluster once in blood vessels, where they could otherwise embolize and occlude blood flow resulting in microinfarctions at distant sites and organs.
As to toxicity, amorphous silica solubility (~120–170 ppm, at the temperature and pH of the body fluids, 36–37 °C and 7.35–7.45, respectively), allowed an easier silica elimination (as silicic or poly(silicic) acid) which are non-toxic and diffuse through the blood stream or the lymphatic system to be eventually cleared in the urine, preventing its accumulation in kidneys, liver or spleen (contrary to the crystalline polymorphs counterparts) [87,88]. Amorphous silica phases lacked the regular long-range order purposed by the classical crystal growth and dissolution models which difficult the understanding of its dissolution mechanism. Yet, a-SiO2 phases shared with the silica crystalline polymorphs the fundamental unit, the (SiO4)4− tetrahedron, and short range structural order (at length scales up to 20 Å), despite variations in Si-O-Si bond lengths and angles. The a-SiO2 atomic scale disorder enabled the loss of surface (SiO4)4− Q3 units into solution (creating vacancy islands) and keeping unchanged the a-SiO2 surface Gibbs energy. As a consequence, dissolution rates of amorphous silica phases, which involves an equilibrium between the solid phase and dissolve monomer Si(OH)4, scaled linearly with increasing driving force (undersaturation). At pH values above 8, the presence of [H3SiO4] ion in addition to Si(OH)4 is responsible for the high silica solubility at this pH values (as the concentration of Si(OH)4 in equilibrium with the solid silica phase is not pH dependent).
The US National Cancer Institute has pointed out that most engineered NPs are far less toxic than household cleaning products, insecticides used on family pets, or over-the-counter dandruff remedies, which are present at order-of-magnitude higher levels than the engineered NPs [89]. Moreover, in their use as carriers of chemotherapeutics in cancer treatment, engineered NPs are much less toxic than the drugs they carry.
3.2. NPs Characterization
NPs size and morphology, chemical and surface composition, along with crystallinity (or lack of) were used to explain limitations in clinical translation, NPs clearance and inflammatory processes [81,82,83,84,88].
As far as size is concerned, NPs may exhibit different size profiles and different shell thicknesses (in core-shell structures), showing in all cases an outstanding surface-to-volume ratio, responsible for an extremely active/reactive surface performance. Size determines in vivo distribution, intracellular uptake, toxicity, and targeting ability, influencing drug loading, drug release, and in vivo and in vitro stability. Smaller particles have a great risk of aggregation during storage and incubation in vitro, but have higher mobility and longer circulation half-life in vivo. To run an effective and reproducible biomedical NPs system, monosized distribution is required, usually between 10 and less than 200 nm.
As to shape, NPs may exhibit an extensive range of geometries—from spherical to tubular, through centric, eccentric and star like. While spherical NPs are good candidates for drug delivery, anisotropic structures can sometimes provide higher efficiencies in drug deliver (due to a more favorable configuration with the cell), although the sharp edges and corners may induce injuries to blood vessels. NPs may be hollow, dense, nanostructured, or in core-shell (eventually with multiple cores) structures, enhancing the NP load capacity and specific targeting ability.
NPs may differ in surface chemical composition, a critical parameter in determining their drug-loading efficiency, releasing profile, circulation half-life, tumor targeting and clearance from the body. A hydrophilic surface makes the NPs more resistant to the plasma proteins adsorption (preventing the formation of corona) and thus avoiding their recognition and uptake by the MPS. Coating the NP surface with a hydrophilic polymer (like PEG) or directly synthesizing NPs with hydrophilic surface compounds (in situ synthesis) are two strategies to overcome the challenge. Rapid opsonization and clearance is observed for NPs with excess positive surface charges [90].
As regards the renal system, neutral surface charge gives the highest chance to pass through renal filtration (and being excreted in urine), while both positively and negatively charged NPs adsorbed more serum proteins, increasing their hydrodynamic diameter and thus reducing their ability to be eliminated [91]. Unlike the long time clearance process taken by bile (or other MPS organs), the renal system removes the NPs from the body through the urine, with minimal side effects [88].
3.3. NPs Synthesis Methodologies
During the last decades, remarkable efforts have been made to develop novel NPs synthesis methodologies. Today, it is generally accepted that nanosize cannot be efficiently achieved by the traditional top-down methodologies (such as ball milling and lithography) but rather by bottom-up techniques. A bottom-up strategy looks faster, precise, and cost-effective.
A bottom-up strategy holds a large number of techniques (flame spray pyrolysis, chemical vapor deposition, and wet-chemistry methodologies like co-precipitation, hydrothermal, solvothermal and sol-gel) but research has been focused on sol-gel, as the synthesis is straightforward, scalable, easily controllable, time and energy saving. The sol-gel chemistry comprises chemical reactions involving colloidal particles in a sol, or between alkoxide-precursors and water, in a solution, leading to a highly porous amorphous gel product, in which a liquid phase (solvent, catalyst and eventually excess reactants) may be retained in bulks (3D), films (2D), fibers (1D), powders, and NPs (0D) products [39,92]. The sol-gel SiO2 NPs synthesis comprises four common methods: (i) colloidal routes, (ii) biomimetic syntheses, (iii) solution routes (base- and acid catalyzed)) and (iv) templated syntheses (the last one dedicated to mesoporous silica NPs, a topic outside the scope of this short review).
3.3.1. Colloidal Route
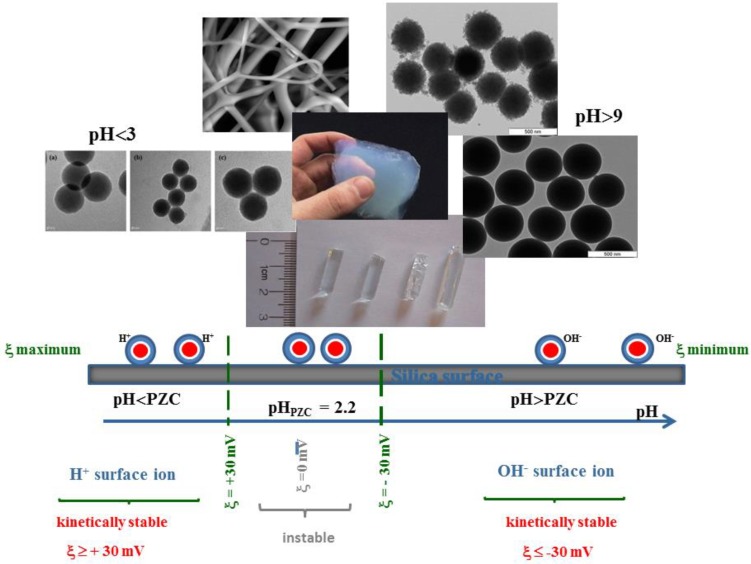
In colloidal routes, sol-gel SiO2 NPs are formed in an aqueous medium through the supersaturation, polymerization and (eventual) precipitation of silica polymorphs. In the geological world silica NPs of ~1 nm (basically orthosilicic acid, a weakly acidic molecule with pKa ~9.8) undergo rapid growth to 2–4 nm, at pH 2–3 (as pHPZC ~2.2; ζ ~0 mV at pH ~2–3, facilitating NPs growth). As silica solubility increases well above pH 7 particles grow up to 4–6 µm by coalescence and Ostwald ripening (pH >> pHPZC, and ζ < −30 mV). At pH > 9 (the ionized form of monomeric silicic acid Si(OH)3O− predominates) silica NPs cement to form a bulk gel, originating opal-like structures [38,39]. Ostwald ripening of silica particles originates from surface instability of silicon dioxide and is driven by differences in chemical potential between particles of different size and shape. The local radius of curvature and ratio of surface area to volume accounts for the particle’s surface energy, which is greater in the case of small particles or those with rough surfaces. Under kinetically favorable conditions these high surface energy particles dissolve preferentially, with the material being deposited onto particles with the largest radius. Silica dissolution proceeds via cleavage of siloxane bonds on the NPs’ surface (which is faster in amorphous structures), resulting in the release of soluble silicic acid (Figure 5).
Figure 5.
Sol-gel silica NPs growth through Ostwald ripening: (a) initial and (b) final stages.
Commercial precipitated silica, formed from sodium silicate solution and sulfuric acid, has the largest share of global market of silica particles (in classical industries), a position that is expected to grow in the next decade [2,3,4].
In the biological world plants, diatoms and sponges are capable of accumulating, storing and processing Si to create biogenic silica (at mild ambient conditions and under-saturated aqueous solutions of silicic acid). Several factors affected the process of natural silica condensation, namely concentration of silicic acid, temperature, pH, and the concentration of co-precipitating/nucleating agents (external small molecules and polymers) [93]. Plants started by taking up Si in the form of Si(OH)4] or Si(OH)3O− (present in soils at concentrations as low as few mg kg−1). When the silicic acid concentration is in excess of 100–200 mg kg−1, polycondensation reactions occur at final location, forming silica polymers equal or higher in size than the critical nuclei size. The viable nuclei grow to form spherical NPs, as the absence of crystallographic patterns promotes isotropic spherical growth. The final SiO2 NPs are amorphous at the 1-nm length scale [94], built up from SiO4 tetrahedron with variable Si-O-Si angles and Si-O bond distances. However, a great variety of medium/long range order patterns may be found in nature (branched chains, structural motifs or even hierarchical patterned structures) resulting in different density, hardness, solubility, viscosity and composition values [54,55,93]. As the silica NPs reach 1–3 nm in size, they interact with plant cell walls (due to the negatively charged silica NPs surfaces, at neutral pH).
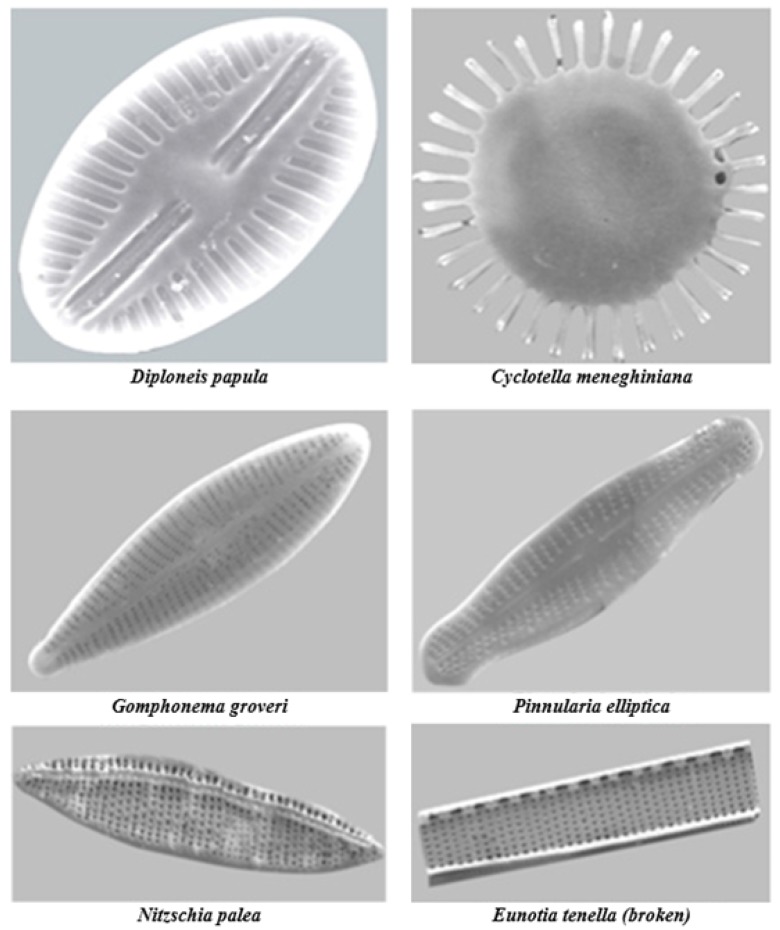
As to diatoms, there are more than 105 species with unique frustule architectures (Figure 6). The micro- and nano-sized diatoms can be also produced by cultivation, and here purification and chemical modification protocols are well established to generate pure active biohybrid materials [95]. Furthermore, the production of diatoms is environmentally friendly (compared to synthetic silica-based NPs), due to absence of toxic waste products and low energy consumption. Diatoms are considered to be harmless thanks to the amorphous silica structure [96], and food grade diatomaceous earth has been approved in the USA to feed animals and there are already several human grade diatomite silica microparticles products on the market in Europe and Australia [97]. The potential of silica diatoms for oral drug delivery applications, in intestinal (pH 7.2) and simulated GIT (pH 1.2–7.4) fluids [98,99,100] was recently demonstrated (Table 4).
Figure 6.
Photomicrographs of diatoms through Scanning Electron Microscope. Reproduce with permission from [101].
Table 4.
Sol-gel silica-based oral delivery NPs (adapted and reproduced with permission from [102]).
| Oral Delivery System | Silica Source | Payload | Coating | Encapsulation Method | Release Mechanism | In Vitro/In Vivo/Ex Vivo | Ref. |
|---|---|---|---|---|---|---|---|
| Non-porous SiO2 NPs | |||||||
| Stober NPs | TEOS | Insulin | PEG 6000PEG 20,000 | Physisorption of insulin to as-synthesized SiO2 NPs–subsequent PEG coating | Passive diffusion | Ex vivo permeation studies with everted rat intestine | [103] |
| Stober NPs | TEOS | Insulin | Chitosan | Physisorption of insulin in chitosan suspension to as-synthesized SiO2 NPs | Passive diffusion | In vitro studies of NPs interactions with porcine mucin | [104] |
| Mesoporous SiO2 NPs | |||||||
| MCM-48 | Luox AS40 | Ibuprofen | Physisorption by immersion | Passive diffusion | In vitro drug release in a simulated body fluid (pH 7.4–7.7) | [105] | |
| Ia3d MSM | TEOS/MPTS | Erythromycin | |||||
| SBA-15 SiO2 | nf | Itraconazole | Physisorption by immersion | Passive diffusion | In vitro drug release in a simulated gastric fluid (pH 1.2) | [106] | |
| SBA-15 and MCM-41 functionalized with –NH2 groups | nf | Bisphosphonates | Electrostatic interaction between drug’s phosphate group and silica’s amnine group at pH 4.8 | Passive diffusion t pH 7.4 | In vitro drug release in phosphate buffer (pH 7.4) | [107] | |
| MCM41 microparticles | TEOS/tri-ethanolamine | Folic acid | Impregnation | pH triggered | Yoghurt in vitro drug release in a simulated GIT fluid (pH 2, 4, 7.5) | [108] | |
| MCM41 NPs | nf | Rhodamine B | a-CD, adamantly ester | Physisorption | Porcine liver esterase triggered | In vitro hydrolysis in HEPES buffer pH 7.5 | [109] |
| MCM48 | TEOS/APTES | Silfalazine | Succinylated soy protein isolate | Physisorption and coating | pH/enzyme triggered | In vitro drug release in simulated GIT fluid at pH 1.2, 5, 7.4 | [110] |
| Hybrid silica microparticles | |||||||
| Core-shell (mesostructured SiO2) | TMOS | Curcumin | 1. Encapsulation of curcumin in SLN by emulsification/sonication 2. sol-gel |
Passive diffusion | In vitro drug release in a simulated GIT fluid (pH 1.2–7.4) | [111] | |
| Core-shell alginate SiO2 | TMOS/APTMS | LGG | 1. Preparation of LGG/alginate microgels by electrospraying 2. mineralization |
Erosion of silica shell | In vitro drug release in a simulated GIT fluid (pH 1.2–7.4) | [111] | |
| Diatoms silica microparticles | |||||||
| Diatom silica | fossile | Indomethacin/gentamicin | Physisorption | Passive diffusion | In vitro drug release in a simulated intestinal fluid (pH 7.2) | [112] | |
| Diatom silica | fossile | Mesalamine/prednisone | Physisorption | Passive diffusion | In vitro drug release in a simulated GIT fluid (pH 1.2–7.4) | [97] | |
3.3.2. Biomimetic SiO2
Mimetic natural SiO2 production is gaining ground, and represents a source of inspiration for green eco-production processes. In biomimetic silica synthesis particle formation can occur by the use of certain co-precipitating/nucleating (biologic or biomimetic) agents, under neutral or acidic conditions. As research on the biogenic silica production has progressed, key molecules (such as silicateins, silaffin R5, proteins, peptides, carbohydrates, lipids, metal ions and phenolic compounds) that participate in the silicification of microorganisms have been found. Several studies have identified alternate amine-molecules as candidates for inducing silica precipitation from precursor compounds in vitro. These amine groups thus impart the silica with a strong positive surface charge (populated with -NH3+ groups, ζ ≥ 30 mV) in acid and neutral pH, thus stabilizing the silica sol and allowing the NPs growth through Ostwald ripening [113,114]. Spherical porphyrin-functionalized SiO2 NPs were biomimetically synthesized with diameters between 50 nm and 800 nm. However, high quality silica NPs with a diameter less than 50 nm still remains a long term challenge [115,116,117,118]. Nearly monodisperse SiO2 NPs, with tunable size between 10 nm and 200 nm, were synthesized in aqueous media by using lysine [119,120] and arginine [121,122] as base catalysis. Cationic block copolymer micelles [123] and cationic poly(acrylamine-co-2-(dimethylamine) ethyl methacrylate, methyl chloride quaternized) (poly (AM-co-DMC)) [124] and polyalylamine hydrochloride (PAH) [125] were used as colloidal template for the biomimetic deposition of 35 nm silica NPs. Protein immobilization (biomolecules encapsulation) within biomimetic silica NPs has been investigated for a wide variety of enzymes [126,127,128,129,130], bovine serum albumin (BSA) protein [131] and has even proved successful for the entrapment of different enzymatic proteins [132].
3.3.3. Solution Route
The solution route is the most common sol-gel synthesis process. Here metallic salts, metal alkoxides, or other organometallic precursors undergo hydrolysis and condensation, to form a wide range of sol-gel products. The right choice of catalyst, pH, water to silica precursor’s ratio (to control hydrolysis rate), type of solvent and solvent to water ratio (to enhance reactants mixing), type of silicon precursor (as R may have inductive and steric effects on hydrolysis rate), the presence of chelating agent (to control the relative hydrolysis to condensation rate) and finally the temperature, allow the control of SiO2 structure, size and/or morphology (Figure 7). Due to the hydrophobic nature of the alkyl groups organometallic precursors and water are not miscible, and the addition of a common solvent (usually an alcohol) becomes mandatory to promote miscibility between reactants. In the case of silica synthesis, the low polarity of the Si-O bond in silicon alkoxide (the Si atom bear δ+ = 0.32 low positive charge in TEOS) is responsible for the slow sol-gel progress, rendering catalysis essential.
Figure 7.
Sol-gel products diversity: the right choice of catalyst, pH value, water to silica precursor’s ratio, type of solvent and solvent to water ratio, type of silicon precursor, presence of chelating agent, and temperature, allow the control of SiO2 topology.
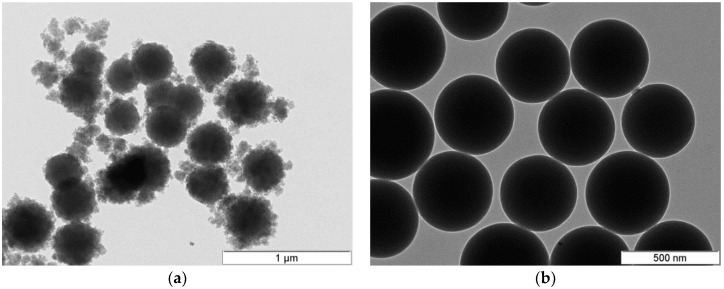
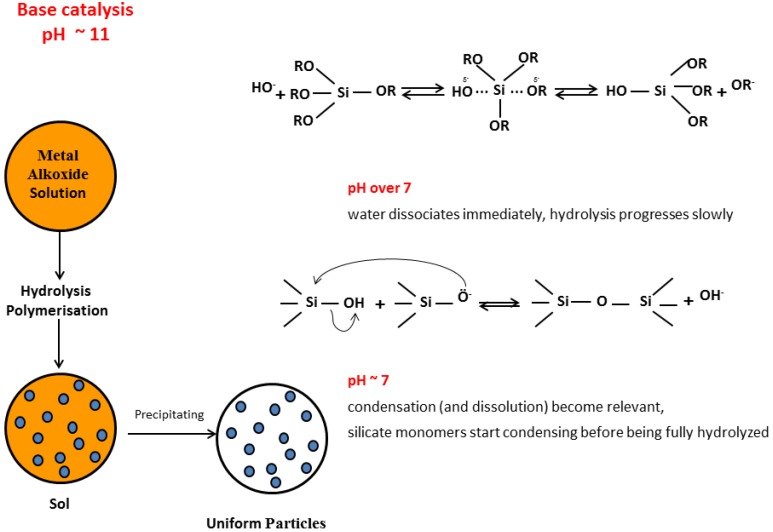
Sol-gel basic conditions confer negative surface charges to the silica monomers (pH >> pHPZC, and ζ ≤ −30 mV), which (kinetically) stabilize the silica suspension, allowing the formation of NPs. Above pH 7, maximum NPs growth is achieved, as a consequence of the increase in silica solubility, which promotes depolymerization of siloxane bonds, and produces monomeric silica necessary for the aging process. As to NPs, Stöber developed a mild synthetic protocol (room temperature, pH ~9–11) for growing (quasi)monodispersed spherical NPs (with diameters between 50 nm and 2 mm) based on sol-gel silicon alkoxides and sodium silicate solution (SSS) as seeds (Figure 8). An alkoxide precursor (such as TEOS) is hydrolyzed (in an ethanol solution) to produce silicic acid, which then undergoes a condensation reaction to form amorphous silica NPs. Arkhireeva and Hay [133] obtained sub-200 nm NPs by slightly modifying the Stöber method. On the other hand, synthesized SiO2 NPs (in sub-100 nm size range) present high polydispersity and irregular shape. Zou et al. [30] proposed a procedure to produce monodisperse spherical SiO2 NPs with sizes ranging between 30–100 nm, based in the classical two-dimensional LaMer [134] model. The strategy is built upon an effective selection of reaction conditions for the Stöber method, and relies on a modification of the conceptual classical LaMer model of nucleation and particle growth. The LaMer methodology, supported on the protocols by Arkhireeva et al. [133] allow the synthesis of NPs at room temperature in less than 1 h.
Figure 8.
NPs sol-gel Stöber synthesis method.
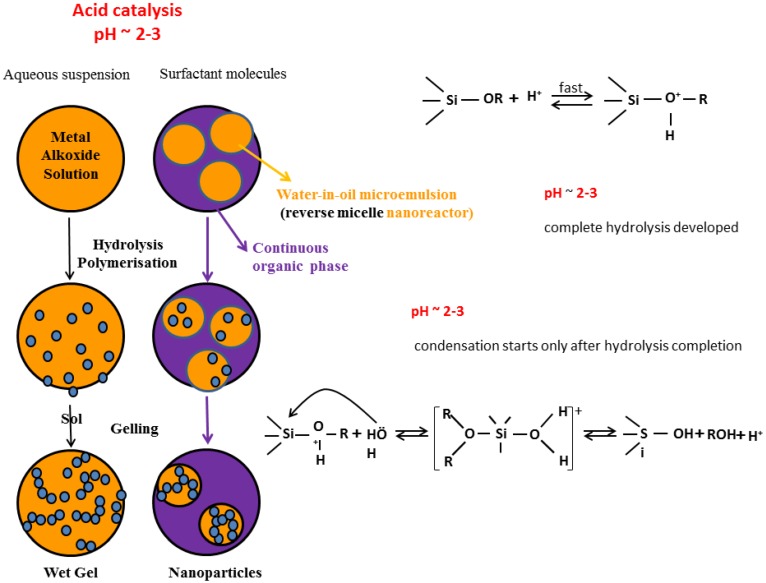
Generally acid conditions favor the production of gels, as the silica formed in acid solutions possesses little or no surface charge (zeta potential will be in the tricky range of ζ < |30 eV|, PZC silica ~ pH = 2.2) facilitating flocculation/connectivity between silica species. Here the hydrolysis step is typically the fastest, but condensation begins before hydrolysis is complete. Condensation often occurs in terminal silanols, resulting in chain like structures in the sol and network-like gels. Linear or highly branched polymeric species are formed, given rise to 3D structures.
To synthetize SiO2 NPs under acid-catalyzed process a reverse-micelle (or water-in-oil microemulsion) system is formed by adding water, oil and surfactant. The hydrolysis and condensation reactions will develop in the confined reaction vessels (formed by the dispersed aqueous phase in the continuous oil matrix (Figure 9)). The confined nanoreactor environment is shown to yield highly monodisperse NPs and allow the incorporation of non-bonded non-polar molecules, which are often difficult to incorporate into the hydrophilic silica matrix. In the last few years, several dye-doped SiO2 NPs have been synthesized by the reverse microemulsion technique in which polar dye molecules are used to ensure successfully encapsulation into SiO2 NPs [135].
Figure 9.
NPs sol-gel reverse emulsion synthesis method.
The reverse microemulsion process is widely used in silica NPs synthesis. However, besides having low yields, the reverse microemulsion process uses a large amount of potentially toxic surfactants and organic solvents, and demands previous washing to biological application, in order to avoid disruption or lyses of biomembranes. The Stöber’s method arises as more eco-friendly alternative, in which the hydrolysis and condensation of a mixture of alkoxysilanes takes place in mild basic aqueous medium, to create monodisperse, spherical, electrostatically-stabilized particles. Recently (ammonia free) Stöber silica NPs were synthesized under basic catalyzed ensured by hydrothermal water (SPA Cabeço de Vide, Portugal, pH ~11) [136].
The Stöber method is a promising method for producing surfactant free silica NPs or coatings; yet the final particles size remain in the hundreds of nanometers to micron regime, which are too large to some of the biological studies. LaMer alternative allows the control of particles size and dispersion, but a regular shape (silica NPs < 100 nm) is still difficult to obtain. NPs prepared through the microemulsion method, exhibited smooth surfaces and low polydispersity. However, for use in biomedicine, the microemulsion method is not as safe as the Stöber one; the use of surfactants in the NPs synthesis carries a higher risk of cytotoxicity.
Stöber silica NPs are largely used in oral applications on account of their chemical stability and intrinsic hydrophilicity, being thus appropriate for biological environments. AEROPERL® 300 Pharma (particle size of 30 mm) is used in formulations of hesperidin oral delivery carrier [137], hydrophylic Aerosil 380 (7 nm in size) is used to stabilize Pickering emulsions in lipid-based oral delivery systems [138,139,140]. Oral insulin bioavailability was tested in a SiO2 nanoplatform (silica NPs associated with insulin and then coated with mucoadhesive polymer, like chitosan or PEG) [100,102] (Table 4).
Sol-gel allows in situ incorporation of a variety of functional (non-hydrolysable) organic groups within the silica matrix, in order to increase their biocompatibility, improve its resistance to enzymatic action, internalization efficiency and gene targeting (either in Stöber or reverse emulsion methods). The ORganically MOdified SILica matrix (known as ORMOSIL [141,142]) is an alternative material with even better and more versatile properties than silica: the presence of non-hydrolysable organic groups in the alkoxisilane precursors makes these behave like glass modifiers, reducing the degree of silica network cross-linking as well as increasing the network flexibility as the unhydrolyzed—Si-R bond apparently dangles, causing higher mobility during gelation and undergoing weaker contraction during drying. A tunable wettability, by a judicious choice of the ratio of hydrophilic to hydrophobic sol-gel precursor monomers, a tailor made porosity (size and shape) and a shell hardness/complacency making ORMOSIL a very competitive material. Furthermore, ORMOSIL NPs surfaces will be populated with both silanol and non-hydrolysable organic groups, allowing an easier chemical conjugation/decoration of biomolecules at the NPs surface and/or be loaded with either hydrophilic or hydrophobic drugs or dyes. Mammalian cells take up and internalize easily silica/ORMOSIL NPs (without any cytotoxic effect) opening the door to its use in health science [143].
Among the commonly used functionalizing groups, amine (–NH2) is the first choice when gene transfection is designed for gene therapy or vaccination. The –NH2 groups electrostatically interact with proteins, enhancing their absorption, biding and protecting pDNA from enzymatic digestion allowing cell transfection in vitro. ORMOSIL NPs have great potential in DNA delivery; ORMOSIL transfection efficiency was equal to or even better than Herpes Simplex Virus-1 (HSV-1)) and does not cause any damage to the tissue nor has immunological side effects that have commonly been observed with viral-mediated gene delivery [144]. ORMOSIL NPs crossed the blood brain barrier (BBB) in fruit fly insects [145] where no toxic effects on the whole insect organism or their neuronal cells were observed. Biodistribution and clearance in vivo studies (mice) using ORMOSIL NPs showed a greater accumulation in liver, spleen and stomach than in kidney, heart and lungs. Although, clearance studies carried out over 15 days period indicated hepatobiliary excretion of the NPs in the same mice [146].
Core-shell structures have great potential in future biomedical applications, since they constitute a scaffold to create multifunctional NPs, applied to several medical fields, from theranosis to gene delivery performance. Sol-gel Stöber method, by simply replacing the nucleating agent SSS (commonly used in the synthesis of plain SiO2 NPs) by another nanosized system, enables its coating. Superparamagnetic iron oxide NPs (SPIONs) [48], and liposomes [147] are selected nanosystems, due to their academic and industrial relevance (Figure 10).
Figure 10.
Examples of sol-gel NPs core-shells possibilities (from left-to-right, up-to-bottom): hollow-spheres (core, core-shell and hollow sphere); nanostructure mesoporous spheres; LIPOSIL structure; SPION-core-silica-shell and ORMOSIL NPs.
SPIONs, the only clinically approved metal oxide NPs [48], have an excellent response to external magnetic fields. However, administration route and SPIONs surface properties dictate their ultimate effect in terms of the efficiency of cellular uptake, biodistribution, and potential toxicity. SPIONs with hydrophobic surfaces are rapidly and efficiently opsonized and cleared from mammal’s circulation system, while SPIONs with hydrophilic surfaces resist these processes being slowly cleared. Silica/ORMOSIL coating emerge as an interesting coating material, granting hydrophilic surface properties, decreasing SPIONs high aggregation tendency, protecting SPIONs from oxidation and thus increasing their blood circulation time [148].
Liposomes are excellent carriers due to their capacity to load hydrophilic and/or hydrophobic molecules, to penetrate in altered vasculatures (due to pathological situations like in cancer or inflammation), to drug release at target sites (over prolonged periods which may vary from hours to weeks) [149]. In clinic, for intravenous administration, there are already several pharmaceutical systems where drugs are encapsulated in liposomal structure. However, when oral administration is envisaged and gastrointestinal tract mucus and epithelium barrier need to be overcome, the protection of liposomes from anticipated disruption becomes a promising strategy [150]. The emerging of silica-based drug delivery carriers for oral route administration was the leitmotiv for silica-coating of liposomes, LIPOSIL for short [151].
Simply silica hollow-sphere NPs (another core-shell possibility) are capable of carrying large amounts of payload or fill their cores with other desirable materials such as polymers, gold or silver along with the gene delivery performance. They can be created through the condensation of alkoxysilanes onto polymer based templates, metal organic frameworks or other nanomaterials, lately removed by chemical etching or thermal degradation [150].
3.3.4. Template Syntheses
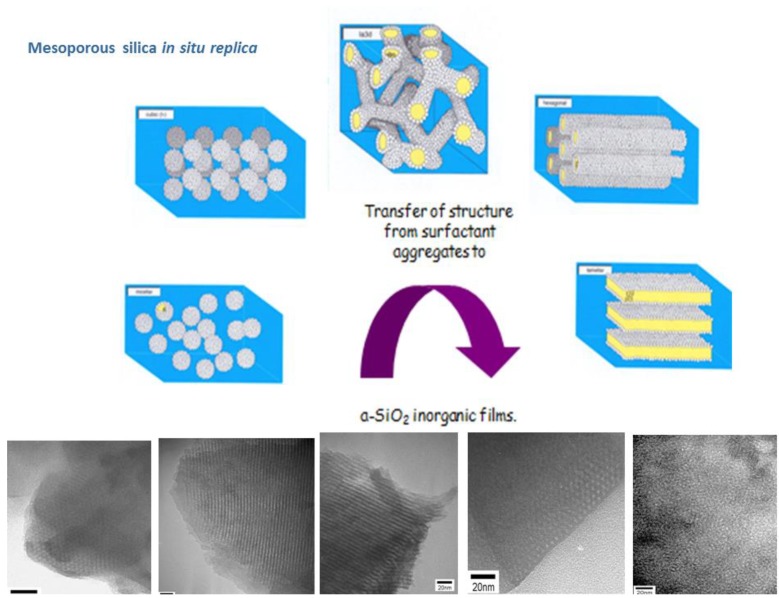
Template synthesis is dedicated to the production of mesoporous materials, topic out of the scope of this short review. A very short summary of synthesis process is presented. The seminal work conducted by researchers at the Mobil Oil Corporation in the early 1990s on the synthesis of mesoporous silicates has led to a number of syntheses in which surfactants are used as templates [152]. Ordered mesoporous materials are unique materials that are defined by an ordered and repetitive mesostructured of pores and disordered arrangement at the atomic level. Their synthesis is based on the use of surfactants that act as templates to direct the morphology of the final amorphous material. Simply, the synthesis process starts with the dissolution of surfactant molecules into polar solvents to yield liquid crystal suspensions. The pair surfactant/solvent defines the working phase diagram. When the surfactant concentration is above the critical micellar concentration (CMC) then the surfactant molecules self-assembly into micelles. Higher surfactant concentrations allow the formation of micellar cubic, hexagonal or lamellar self-assembly structures. Once the (liquid crystal) aggregates are formed, the silica precursors are added to the suspension, the sol-gel reactions occurs and a mesoporous silica material is produced. Finally, the surfactant is removed by chemical or thermal degradation [29] (Figure 11).
Figure 11.
Sol-gel mesoporous silica NPs from in situ replica of self-assembled molecular aggregates.
A definite breakthrough in drug delivery was the use of mesoporous silica NPs to host drugs/therapeutic-molecules/markers. A correct selection of the mesoporous design depends on the molecule to be hosted, and is the first criterion used. The most used mesoporous silica NPs in drug or bioencapsulation are MCM-41 and SBA-15. The synthesis of MCM-41 (from the Mobil Composition of Matter series) involves liquid crystal templating commonly cetyl trimethylammonium bromide (CTAB) that lead to a 2D hexagonal pore channel array with 3.6 nm in size. The diameters of MCM-41 NPs can be controlled in a size range from 25 nm to 100–150 nm. The SBA-15 (Santa Barbara type) is also largely used as biocarrier. This type of mesoporous silica material is prepared by cooperative self-assembly with a pluronic P123 (a non-ionic block co-polymer). The channels adopt also a 2D hexagonal packing with a diameter varying from 6 to 10 nm depending on the synthesis conditions.
The release of the drug from the host mesoporous NP is definitely the big challenge. This may occur through diffusion all through the pore channels (in passive drug delivery) or released under specific stimuli as pH, temperature, ultra-sons or light (in stimuli-responsive systems). Although an impressive variety of mesoporous NPs have design, synthesized and (in vitro and in vivo) tested no products have reached the market so far.
4. Conclusions
Silica, the major component of the Earth’s crust, has entered the food chain and become an essential material in many, if not all, forms of life. Although in humans its role in disease, aging and health is not yet fully understood, its simply presence in the body renders it a biocompatible material. In nanomedicine silica has become an excellent competitor as a nanocarrier, able to target, sense, signal, and drug release drugs/markers with different pharmacokinetics/pharmacodynamics, to elaborate optimized tailor made treatments/diagnosis.
Currently pharmaceutical design is based on Quality by Design concept, which demands accurate knowledge of the physiological barriers nanocarriers face within the human body, of the physical/chemical characteristic of nanocarriers besides fully understand of their synthesis process. A comprehensive revision about these topics is present. In particular, the sol-gel silica nanoparticles synthesis, namely colloidal route, biomimetic production, solution route and template synthesis are discussed, having the ecological inference in consideration. The Stöber and LaMer solution route protocols stand out as time-saving and surfactant free processes. Mimetic natural silica production (either geologic or biologic processes) is gaining ground, and is an inspiration of green eco-production processes. A considerable number of commercial, approved, patented or in clinical stage sol-gel silica NPs are presented. Taken together, the examples shown in this short review emphasize the great potential of silica nanoparticles as nanocarriers in medicine. Improve the synthesis process and design more competitive NPs to comply the rising human health demands are the challenges chemists and materials scientist will face in the near future.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- 1.Gonçalves M.C., Margarido F. Materials for Construction and Civil Engineering: Science, Processing, and Design. Springer; Berlin, Germany: 2015. [Google Scholar]
- 2.IMA Europe. [(accessed on 1 May 2018)]; Available online: https://www.ima-europe.eu/about-industrial-minerals/industrial-minerals-ima-europe/silica.
- 3.ASAP Association of Synthetic Amorphous Silica. [(accessed on 1 May 2018)]; Available online: http://www.asasp.eu/index.php/about-asasp/activities.
- 4.nepSI—The European Network on Silica. [(accessed on 1 May 2018)]; Available online: https://ec.europa.eu/growth/tools-databases/eip-raw-materials/en/content/nepsi-european-network-silica.
- 5.Ha S.-W., Weitzmann M.N., Beck G.R., Jr. Nanobiomaterials in Clinical Dentistry. Elsevier; Kidlington, UK: 2013. Dental and Skeletal Applications of Silica-Based; pp. 69–91. [DOI] [Google Scholar]
- 6.Luhrs A.K., Geurtsen W. Biosilica in Evolution, Morphogenesis, and Nanobiotechnology. Volume 47. Springer; Berlin, Germany: 2009. The application of silicon and silicates in dentistry: A review; pp. 359–380. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X., Zhang N., Mankoci S., Sahai N. Silicates in orthopedics and bone tissue engineering materials. J. Biomed. Mater. Res. A. 2017;105:2090–2102. doi: 10.1002/jbm.a.36061. [DOI] [PubMed] [Google Scholar]
- 8.Zhu M., Zhu Y., Ni B., Xie N., Lu X., Shi J., Zeng Y., Guo X. Mesoporous Silica Nanoparticles/Hydroxyapatite Composite Coated Implants to Locally Inhibit Osteoclastic Activity. ACS Appl. Mater. Interfaces. 2014;6:5456–5466. doi: 10.1021/am405013t. [DOI] [PubMed] [Google Scholar]
- 9.AAA American Academy of Dermatology. [(accessed on 1 May 2018)]; Available online: https://www.aad.org/media/news-releases/--small-changes-in-skin-care-routine-can-significantly-improve-skin-affected-by-acne-and rosacea.
- 10.Navarro M., Serra T. Biomimetic mineralization of ceramics and glasses. Biominer. Biomater. Fundam. Appl. 2016:315–338. doi: 10.1016/B978-1-78242-338-6.00011-9. [DOI] [Google Scholar]
- 11.Brunner T.J., Stark W.J., Boccaccini A.R. Nanoscale Bioactive Silicate Glasses in Biomedical Applications. Nanoscale. 2010 doi: 10.1002/9783527610419.ntls0142. [DOI] [Google Scholar]
- 12.Chen J., Fang L., Zhang Y., Zhu H., Cao S. The Design, Synthetic Strategies and Biocompatibility of Polymer Scaffolds for Biomedical Application. Bentham Science; Emirate of Sharjah, United Arab Emirates: 2014. Silica-Based Scaffolds: Fabrication, Synthesis and Properties in: Frontiers in Biomaterials; pp. 305–324. [Google Scholar]
- 13.Mendoza-Novelo B., Gonzalez-Garcia G., Mata-Mata J.L., Castellano L.E., Cuéllar-Mata P., Ávila E.E. A biological scaffold filled with silica and simultaneously crosslinked with polyurethane. Mater. Lett. 2013;106:369–372. doi: 10.1016/j.matlet.2013.05.088. [DOI] [Google Scholar]
- 14.Lim E.-K., Kim T., Paik S., Haam S., Huh Y.-M., Lee K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015;115:327–394. doi: 10.1021/cr300213b. [DOI] [PubMed] [Google Scholar]
- 15.Matos J.C., Gonçalves M.C., Monteiro G.A. Nano Based Drug Delivery. IAPC Open Book Platform; Zagreb, Croatia: 2015. Silica and ORMOSIL nanoparticles for gene delivery. [Google Scholar]
- 16.Gonçalves M.C., Martins B. Multifunctional core-shell nanostructures. In: Ali N., Seifalian A., editors. Nanomedicine. One Central Press Office; Manchester, UK: 2014. pp. 83–110. [Google Scholar]
- 17.Bharti C., Nagaich U., Pal A.K., Gulati N. Mesoporous silica nanoparticles in target drug delivery: A Review. Int. J. Pharm. Invest. 2015;5 doi: 10.4103/2230-973X.160844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y., Yu C. Advances in Silica based nanoparticles for target cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2016;12:317–332. doi: 10.1016/j.nano.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Argyo C., Weiss V., Brǟuchle C., Bein T. Multifunctional Mesoporous Silica nanoparticles as a Universal Platform for Drug Delivery. Chem. Mater. 2014;26:435–451. doi: 10.1021/cm402592t. [DOI] [Google Scholar]
- 20.Sun T., Shrike Y., Pang B., Hyun D.C., Yang M., Xia Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Rev. Int. Ed. 2014;53:12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 21.Tang L., Cheng J. Nonporous silica nanoparticles for nanomedicine application. Nano Today. 2013;8:290–312. doi: 10.1016/j.nantod.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korzeniowska B., Nooney R., Wencel D., McDonagh C. Silica Nanoparticles for cell imaging and intracellular sensing. Nanotechnology. 2013;24 doi: 10.1088/0957-4484/24/44/442002. [DOI] [PubMed] [Google Scholar]
- 23.Vallet-Regí M. Mesoporous Silica nanoparticles: Their projection in Nanomedicine. ISRN Mater. Sci. 2012 doi: 10.5402/2012/608548. [DOI] [Google Scholar]
- 24.ALOthman Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials. 2012;5:2874–2902. doi: 10.3390/ma5122874. [DOI] [Google Scholar]
- 25.Satalkar P., Elger B.S., Shaw D.M. Defining Nano, Nanotechnology and Nanomedicine: Why Should It Matter? Sci. Eng. Ethics. 2016;5:1255–1276. doi: 10.1007/s11948-015-9705-6. [DOI] [PubMed] [Google Scholar]
- 26.Fass L. Imaging and cancer: A review. Mol. Oncol. 2008;2:115–152. doi: 10.1016/j.molonc.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilczewska A.Z., Niemirowicz K., Markiewicz K.H., Car H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012;64:1020–1037. doi: 10.1016/S1734-1140(12)70901-5. [DOI] [PubMed] [Google Scholar]
- 28.Watermann A., Brieger J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials. 2017;7:189. doi: 10.3390/nano7070189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallet-Regi M., Colilla M., Izquierdo-Barba I., Manzano M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules. 2018;23:47. doi: 10.3390/molecules23010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y., Quan G., Wu Q., Zhang X., Niu B., Wu B., Huang Y., Pan X., Wu C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B. 2018;8:165–177. doi: 10.1016/j.apsb.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mamaeva V., Rosenholm M., TabeBate-Eya L., Bergman L., Peuhu E., Duchanoy A., Fortelius L.E., Sebastian L., Toivola D.M., Lindén M., et al. Mesoporous Silica Nanoparticles as Drug Delivery Systems for Targeted Inhibition of Notch Signaling in Cancer. Mol. Ther. 2011;19:1538–1546. doi: 10.1038/mt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen J., Yang K., Liu F., Li H., Xu Y., Sun S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem. Soc. Rev. 2017;19 doi: 10.1039/C7CS00219J. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand N., Wu J., Xu X., Kamaly N., Farokhzad O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D., Yang F., Xiong F., Gu N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics. 2016;6:1306–1323. doi: 10.7150/thno.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muhammad F., Guo M., Qi W., Sun F., Wang A., Guo Y., Zhu G. pH-Triggered Controlled Drug Release from Mesoporous Silica Nanoparticles via Intracelluar Dissolution of ZnO Nanolids. J. Am. Chem. Soc. 2011;133:8778–8781. doi: 10.1021/ja200328s. [DOI] [PubMed] [Google Scholar]
- 36.Guo C., Yang M., Jing L., Wang J., Yu Y., Li Y., Duan J., Zhou X., Li Y., Sun Z. Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int. J. Nanomed. 2016;11:5257–5276. doi: 10.2147/IJN.S112030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han W., Chilkoti A., López G. Self-assembled hybrid elastin-like polypeptide/silica nanoparticles enable triggered drug release. Nanoscale. 2017;18 doi: 10.1039/C7NR00172J. [DOI] [PubMed] [Google Scholar]
- 38.Iler R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry. John Wiley; Hoboken, NJ, USA: 1978. [Google Scholar]
- 39.Brinker C.J., Scherer G.W. Sol-Gel Science. The Physics and Chemistry of Sol-Gel Processing. Academic Press; Cambridge, MA, USA: 1990. [Google Scholar]
- 40.FDA US Food & Drug Administration CFR—Code of Federal Regulations Title 21. [(accessed on 1 May 2018)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.1711&SearchTerm=silica.
- 41.Kasaai M.R. Nanosized Particles of Silica and Its Derivatives for Applications in Various Branches of Food and Nutrition Sectors. J. Nanotechnol. 2015 doi: 10.1155/2015/852394. [DOI] [Google Scholar]
- 42.Go M.-R., Bae S.-H., Kim H.-J., Yu J., Choi S.-J. Interactions between Food Additive Silica Nanoparticles and Food Matrices. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The Sol Gel Gateway. [(accessed on 1 May 2018)]; Available online: https://www.solgel.com.
- 44.Ow H., Larson D.R., Srivastava M., Baird B.A., Webb W.W., Wiesner U. Bright and Stable Core−Shell Fluorescent Silica Nanoparticles. Nano Lett. 2005;5:113–117. doi: 10.1021/nl0482478. [DOI] [PubMed] [Google Scholar]
- 45.Burns A., Vider J., Ow H., Herz E., Penate-Medina O., Baumgart M., Larson S.M., Wiesner U., Bradbury M. Fluorescent Silica Nanoparticles with Efficient Urinary Excretion for Nanomedicine. Nano Lett. 2009;9:442–448. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim S.E., Zhang L., Ma K., Riegman M., Chen F., Ingold I., Conrad M., Turker M.Z., Gao M., Jiang X., et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat. Nanotechnol. 2016;11:977–985. doi: 10.1038/nnano.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burns A., Ow H., Wiesner U. Fluorescent core–shell silica nanoparticles: towards “Lab on a Particle” architectures for nanobiotechnology. Chem. Soc. Rev. 2016:1028–1042. doi: 10.1039/b600562b. [DOI] [PubMed] [Google Scholar]
- 48.Revia R.A., Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater. Today. 2016;19:157–168. doi: 10.1016/j.mattod.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foglia M.L., Alvarez G.S., Catalano P.N., Mebert A.M., Diaz L.E., Coradin T., Desimone M.F. Recent Patents on the Synthesis and Application of Silica Nanoparticles for Drug Delivery. Recent Pat. Biotechnol. 2011;5 doi: 10.2174/187220811795655887. [DOI] [PubMed] [Google Scholar]
- 50.Müller W.E.G., Grachev M.A. Biosilica in Evolution, Morphogenesis, and Nano-biotechnology. Springer; Berlin, Germany: 2009. [Google Scholar]
- 51.Pellegrino L., Pierre F., Natalicchio M., Carnevale G. The Messinian diatomite deposition in the Mediterranean region and its relationships to the global silica cycle. Earth Sci. Rev. 2018;178:154–176. doi: 10.1016/j.earscirev.2018.01.018. [DOI] [Google Scholar]
- 52.Sommer M., Kaczorek D., Kuzyakov Y., Breuer J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006;169:310–329. doi: 10.1002/jpln.200521981. [DOI] [Google Scholar]
- 53.Epstein E. The anomaly of silicon in plant biology. Proc. Nat. Acad. Sci. USA. 1994;91:11–17. doi: 10.1073/pnas.91.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones L.H.P., Handreck K.A. Silica in Soils, Plants, and Animals. Adv. Agron. 1967;19:107–149. doi: 10.1016/S0065-2113(08)60734-8. [DOI] [Google Scholar]
- 55.Currie A.A.H.A., Perry C.C. Silica in Plants: Biological, Biochemical and Chemical Studies. Ann. Bot. 2007;100:1383–1389. doi: 10.1093/aob/mcm247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epstein E. Silicon. Annual Review of Plant. Physiology and Plant. Mol. Biol. 1999;50:641–664. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- 57.Sun W., Zhang J., Fan Q., Xue G., Li Z., Liang Y. Silicon-enhanced resistance to rice blast is attributed to silicon-mediated defense resistance and its role as physical barrier. Eur. J. Plant. Pathol. 2010;128:39–49. doi: 10.1007/s10658-010-9625-x. [DOI] [Google Scholar]
- 58.Martin K.R. The chemistry of silica and its potential health benefits. J. Nutr. Health Aging. 2007;11:94–98. doi: 10.12691/ajwr-5-2-2. [DOI] [PubMed] [Google Scholar]
- 59.Jugdaohsingh R. Silicon and bone health. J. Nutr. Health Aging. 2007;11:99–110. [PMC free article] [PubMed] [Google Scholar]
- 60.Davenward S., Bentham P., Wright J., Crome P., Job D., Polwart A., Exley C. Silicon-rich mineral water as a non-invasive test of the ‘aluminum hypothesis’ in Alzheimer’s disease. J. Alzheimers Dis. 2013;33:423–430. doi: 10.3233/JAD-2012-121231. [DOI] [PubMed] [Google Scholar]
- 61.Rondeau V., Jacqmin-Gadda H., Commenges D., Helmer C., Dartigues J.-F. Aluminum and silica in drinking water and the risk of Alzheimer’s disease or cognitive decline: findings from 15-year follow-up of the PAQUID cohort. Am. J. Epidemiol. 2009;169:489–496. doi: 10.1093/aje/kwn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwarz K. Silicon, fibre, and atherosclerosis. Lancet. 1977;1 doi: 10.1016/S0140-6736(77)91945-6. [DOI] [PubMed] [Google Scholar]
- 63.Heinemann S., Coradin T., Desimone M.F. Bio-inspired silica–collagen materials: applications and perspectives in the medical field. Biomater. Sci. 2013;7 doi: 10.1039/c3bm00014a. [DOI] [PubMed] [Google Scholar]
- 64.Sripanyakorn S., Jugdaohsingh R., Dissayabutr W., Anderson S.H.C., Thompson R.P.H., Powell J.J. The comparative absorption of silicon from different foods and food supplements. Br. J. Nutr. 2009;102:825–834. doi: 10.1017/S0007114509311757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jurkić L.M., Cepanec I., Pavelić S.K., Pavelić K. Biological and therapeutic effects of ortho-silicic acid and some ortho-silicic acid-releasing compounds: New perspectives for therapy. Nutr. Metab. 2013;10 doi: 10.1186/1743-7075-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.National Cancer Institute; [(accessed on 1 May 2018)]. Cancer-Causing Substances in the Environment: Crystalline Silica. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/crystalline-silica. [Google Scholar]
- 67.Pollard K.M. Silica, Silicosis, and Autoimmunity. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H., Dunphy D.R. Processing Pathway Dependence of Amorphous Silica Nanoparticle Toxicity: Colloidal vs Pyrolytic. J. Am. Chem. Soc. 2012;134:15790–15804. doi: 10.1021/ja304907c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zilaout H., Vlanderen J., Houba R., Kromhout H. 15 years of monitoring occupational exposure to respirable dust and quartz within the European industrial minerals sector. Int. J. Hyg. Environ. Health. 2017;220:810–819. doi: 10.1016/j.ijheh.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Petushkov A., Ndiege N., Salem A.K., Larsen S.C. Advances in Molecular Toxicology. Elsevier; Amsterdam, The Netherlands: 2010. Toxicity of Silica Nanomaterials: Zeolites, Mesoporous Silica, and Amorphous Silica Nanoparticles. [Google Scholar]
- 71.Murugadoss S., Lison D., Godderis L., Van Den Brule S., Mast J., Brassinne F., Sebaihi N., Hoet P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017;91:2967–3010. doi: 10.1007/s00204-017-1993-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu Y., Li Y., Wang W., Jin M., Du Z., Li Y., Duan J., Yu Y., Sun Z. Acute Toxicity of Amorphous Silica Nanoparticles in Intravenously Exposed ICR Mice. PLoS ONE. 2013 doi: 10.1371/journal.pone.0061346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Z., Wang C., Liu S., He W., Wang L., Gan J.J., Huang Z., Wang Z., Wei H., Zhang J., et al. Specifically Formed Corona on Silica Nanoparticles Enhances Transforming Growth Factor β1 Activity in Triggering Lung Fibrosis. ACS Nano. 2017;11:1659–1672. doi: 10.1021/acsnano.6b07461. [DOI] [PubMed] [Google Scholar]
- 74.Merget R., Bauer T., Kupper H.U., Pkilippou S., Bauer H.D., Breitstadt R., Bruening T. Health Hazards due to the inhalation of amorphous silica. Arch. Toxicol. 2001;75:625–634. doi: 10.1007/s002040100266. [DOI] [PubMed] [Google Scholar]
- 75.Yu K.O., Grabinski C.M., Schrand A.M., Murdock R.C., Wang W., Gu B., Schlagen J.J., Hussain S.M. Toxicity of amorphous silica nanoparticles in mouse keratinocytes. J. Nanopart. Res. 2009;11:15–24. doi: 10.1007/s11051-008-9417-9. [DOI] [Google Scholar]
- 76.Rancant F., Gao Q., Graf C., Troppens S., Hadam S., Hackbarth S., Kembuan C., Bluma-Peytavi U., Ruhl E., Lademann J., et al. Skin Penetration and Cellular Uptake of Amorphous Silica Nanoparticles with Variable Size, Surface Functionalization, and Colloidal Stability. ACS Nano. 2012;6:6829–6842. doi: 10.1021/nn301622h. [DOI] [PubMed] [Google Scholar]
- 77.Zhang L., Mao S. Application of quality by design in the current drug development. Asian J. Pharm. Sci. 2017;12:1–8. doi: 10.1016/j.ajps.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amasya G., Badilli U., Aksu B., Tarimci N. Quality by Design case study 1: Design of 5-fluorouracil loaded lipid nanoparticles by the W/O/W double emulsion-solvent evaporation method. Eur. J. Pharm. Sci. 2016;84:92–102. doi: 10.1016/j.ejps.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 79.Yu L.X., Amidon G., Khan M.A., Hoag S.W., Polli J., Raju G.K., Woodcock J. Understanding Pharmaceutical Quality by Design. AAPS J. 2014;16 doi: 10.1208/s12248-014-9598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aksu B., De Beer T., Folestad S., Ketolainen J., Lindén H., Lopes J.A., Matas M., Oostra W., Rantanen J., Weimer M. Strategic funding priorities in the pharmaceutical sciences allied to Quality by Design (QbD) and Process Analytical Technology (PAT) Eur. J. Pharm. Sci. 2012;47:402–405. doi: 10.1016/j.ejps.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 81.Frank M., Fries L. The role of complement in inflammation and phagocytosis. Immunol. Today. 1991;12:322–326. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- 82.Johnson R.J. The complement system. In: Ratner B.D., Hoffman A.S., Schoen F.J., Lemons J.E., editors. Biomaterials Science: An Introduction to Materials in Medicine. Elsevier Academic Press; Amsterdam, NY, USA: 2004. pp. 318–328. [Google Scholar]
- 83.Gref R., Minamitake Y., Peracchia M.T., Trubetskoy V., Torchilin V., Langer R. Biodegradable long circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 84.Mitchell R.N. Innate and adaptive immunity: The immune response to foreign materials. In: Ratner B.D., Hoffman A.S., Schoen F.J., Lemons J.E., editors. Biomaterials Science: An. Introduction to Materials in Medicine. Elsevier Academic Press; Amsterdam, NY, USA: 2004. pp. 304–318. [Google Scholar]
- 85.Owens D.E., III, Peppas N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 86.Hofmann-Amtenbrink M., Grainger D.W., Hofmann H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomed. Nanotechnol. Biol. Med. 2015;11:1689–1694. doi: 10.1016/j.nano.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 87.Alexander G.B., Heston W.M., Iler R.K. The Solubility of Amorphous Silica in Water. J. Phys. Chem. 1954;58:453–455. doi: 10.1021/j150516a002. [DOI] [Google Scholar]
- 88.Croissant J.G., Fatieiev Y., Khashab N.M. Degardability and Clearance of silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017;29 doi: 10.1002/adma.201604634. [DOI] [PubMed] [Google Scholar]
- 89.Prevention/Risk/Substances/Crystalline-Silica. [(accessed on 1 May 2018)]; Available online: https://www.cancer.gov/about-cancer/causes.
- 90.Elsabahy M., Wooley K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi H.S., Liu W., Misra P., Tanaka E., Zimmer J.P., Ipe B.I., Bawendi M.G., Frangioni J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Almeida R.M., Gonçalves M.C. Sol-gel processes and products. In: Pascal R., editor. Encyclopedia of Glass Science, Technology, History, and Culture. John Wiley and Sons; Hoboken, NJ, USA: in press. [Google Scholar]
- 93.Perry C.C., Belton D., Shafran K. Studies of biosilicas; structural aspects, chemical principles, model studies and the future. Prog. Mol. Subcell. Biol. 2003;33:269–299. doi: 10.1007/978-3-642-55486-5_11. [DOI] [PubMed] [Google Scholar]
- 94.Mann S., Perry C.C. Structural Aspects of biogenic silica. Ciba Found. Symp. 1986;121:40–58. doi: 10.1002/9780470513323. [DOI] [PubMed] [Google Scholar]
- 95.Ragni R., Cicco S., Vona D., Leone G., Farinola G. Biosilica from diatoms microalgae: smart materials from bio-medicine to photonics. J. Mater. Res. 2016;32:279–291. doi: 10.1557/jmr.2016.459. [DOI] [Google Scholar]
- 96.Bye E., Davies R., Griffiths D., Gylseth B., Moncrieff C. In vitro cytotoxicity and quantitative silica analysis of diatomaceous earth products. Br. J. Ind. Med. 1984;41:228–234. doi: 10.1136/oem.41.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang H., Shahbazi M., Mǟkilǟ E., da Silva T., Reis R., Salonen J. Diatom silica microparticles for sustained release and permeation enhancement following oral delivery of prednisone and mesalamine. Biomaterials. 2013;3 doi: 10.1016/j.biomaterials.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 98.Losic D., Mitchell J., Voelcker N. Diatomaceous lessons in nanotechnology and advanced materials. Adv. Mater. 2009;21:2947–2958. doi: 10.1002/adma.200803778. [DOI] [Google Scholar]
- 99.Gordon R., Losic D., Tiffany M., Nagy S., Sterrenburg F. The glass menagerie: diatoms for novel applications in nanotechnology. Trends Biotechnol. 2008;27:116–127. doi: 10.1016/j.tibtech.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 100.Noll F., Sumper M., Hampp N. Nanostructure of diatom silica surfaces and of biomimetic analogues. Nano Lett. 2002;2:91–95. doi: 10.1021/nl015581k. [DOI] [Google Scholar]
- 101.Saini E., Khanagwal V.P., Singh R. A systematic databasing of diatoms from different geographical localities and sites of Haryana for advancing validation of forensic diatomology. Data Brief. 2017;10:63–68. doi: 10.1016/j.dib.2016.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diab R., Canilho N., Pavel I.A., Haffner F.B., Girardon M., Pasc A. Silica-based systems for oral delivery of drugs, macomolecules and cells. Adv. Colloid Interface Sci. 2017;249:346–362. doi: 10.1016/j.cis.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 103.Andreani T., Kiill C.P., de Souza A.L.R., Fangueiro J.F., Fernandes L., Doktorovová S. Surface Engineering of silica nanoparticles for oral insulin delivery: Characterization and cell toxicity studies. Colloid. Surf. B. 2014;123:916–923. doi: 10.1016/j.colsurfb.2014.10.047. [DOI] [PubMed] [Google Scholar]
- 104.Andreani T., Miziara I., Lorenzón E.N., De Souza A.R.L., Kiill C.P., Fangueiro J.F. Effect of mucoadhesive polymers on the in vitro performance of insulin-loaded silica nanoparticles: interactions with mucin and biomembrane models. Eur. J. Pharm. Biopharm. 2015;93:118–126. doi: 10.1016/j.ejpb.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 105.Izquierdo-Barba I., Martinez A., Doadrio A.L., Pérez-Pariente J., Vallet-Regí M. Release evaluation of drugs from ordered three-dimensional silica structures. Eur. J. Pharm. Sci. 2005;26:365–373. doi: 10.1016/j.ejps.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 106.Mellaerts R., Aerts C.A., Humbeeck J.V., Augustijns P., Mooter G.V., Martens J.A. Enhanced release of itraconazole from ordered mesoporous SBA-15 silica materials. Chem. Commun. 2007;13:1375–1377. doi: 10.1039/b616746b. [DOI] [PubMed] [Google Scholar]
- 107.Balas F., Manzano M., Horcajada P., Vallet-Regí M. Confinement and Controlled Release of Bisphosphonates on Ordered Mesoporous Silica-Based Materials. J. Am. Chem. 2006;128:8116–8117. doi: 10.1021/ja062286z. [DOI] [PubMed] [Google Scholar]
- 108.Pérez-Esteve E., Ruiz-Rico M., Fuentes A., Marcos M., Sancenon F., Martinez-Manez R. Enrichment of stirred yogurts with folic acid encapsulated in pH-responsive mesoporous silica particles: Bioaccessibility modulation and physico-chemical characterization. LWT Food Sci. Technol. 2016;72 doi: 10.1016/j.lwt.2016.04.061. [DOI] [Google Scholar]
- 109.Patel K., Angelos S., Dichtel W.R., Coskun A., Yang Y.W., Zink J.I., Stoddart J.F. Enzyme-Responsive Snap-Top Covered Silica Nanocontainers. J. Am. Chem. Soc. 2008;130:2382–2383. doi: 10.1021/ja0772086. [DOI] [PubMed] [Google Scholar]
- 110.Popat A., Jambhrunkar S., Zhang J., Yang J., Zhang H., Meka A., Yu C. Programmable drug release using bioresponsive mesoporous silica nanoparticles for site-specific oral drug delivery. Chem. Commun. 2014;50 doi: 10.1039/C4CC00620H. [DOI] [PubMed] [Google Scholar]
- 111.Kim S., Diab R., Joubert O., Canilho N., Pasc A. Core-shell microcapsules of solid lipid nanoparticles and mesoporous silica for enhanced oral delivery of curcumin. Colloids Surf. B. 2016;140:161–168. doi: 10.1016/j.colsurfb.2015.12.040. [DOI] [PubMed] [Google Scholar]
- 112.Aw M.S., Simovic S., Addai-Mensah J., Losic D. Silica microcapsules from diatoms as new carrier for delivery of therapeutics. Nanomedicine. 2011;6:1159–1173. doi: 10.2217/nnm.11.29. [DOI] [PubMed] [Google Scholar]
- 113.Knecht M.R., Wright D.W. Amine-Terminated Dendrimers as Biomimetic Templates for Silica Nanosphere Formation. Langmuir. 2004;20:4728–4732. doi: 10.1021/la0494019. [DOI] [PubMed] [Google Scholar]
- 114.Coradin T., Durupthy O., Livage J. Interactions of Amino-Containing Peptides with Sodium Silicate and Colloidal Silica: A Biomimetic Approach of Silicification. Langmuir. 2002;18:2331–2336. doi: 10.1021/la011106q. [DOI] [Google Scholar]
- 115.Knecht M.R., Wright D.W. Dendrimer-Mediated Formation of Multicomponent Nanospheres. Chem. Mater. 2004;16:4890–4895. doi: 10.1021/cm049058t. [DOI] [Google Scholar]
- 116.Bauer C.A., Robinson D.B., Simmons B.A. Silica particle formation in confined environments via bioinspired polyamine catalysis at near-neutral pH. Small. 2007;3 doi: 10.1002/smll.200600352. [DOI] [PubMed] [Google Scholar]
- 117.Jan J.S., Lee S.L., Carr C.S., Schantz D.F. Biomimetic Synthesis of Inorganic Nanospheres. Chem. Mater. 2005;17 doi: 10.1021/cm0504440. [DOI] [Google Scholar]
- 118.Jin R.H., Yuan J.J. Learning from Biosilica: Nanostructured Silicas and Their Coatings on Substrates by Programmable Approaches. Adv. Biomim. 2009;39 doi: 10.5772/14905. [DOI] [Google Scholar]
- 119.Hartlen K.D., Athanasopoulo A.P.T., Kitaev V. Facile preparation of highly monodisperse small silica spheres (15 to > 200 nm) suitable for colloidal templating and formation of ordered arrays. Langmuir. 2008;24 doi: 10.1021/la7025285. [DOI] [PubMed] [Google Scholar]
- 120.Yokoi T., Sakomoto Y., Terasaki O., Kubota Y., Okubo T., Tatsumi T. Periodic Arrangement of Silica Nanospheres Assisted by Amino Acids. J. Am. Chem Soc. 2006;128 doi: 10.1021/ja065071y. [DOI] [PubMed] [Google Scholar]
- 121.Davis T.M., Snyder M.A., Krohn J.E., Tsapatsis M. Nanoparticles in Lysine−Silica Sols. Chem. Mater. 2006;18 doi: 10.1021/cm061982v. [DOI] [Google Scholar]
- 122.Snyder M.A., Lee J.A., Davis T.M., Scriven L.E., Tsapatsis M. Silica nanoparticle crystals and ordered coatings using lys-sil and a novel coating device. Langmuir. 2007;23 doi: 10.1021/la701063v. [DOI] [PubMed] [Google Scholar]
- 123.Yuan J.J., Mykhaylyk O.O., Ryan A.J., Armes S.P. Cross-Linking of Cationic Block Copolymer Micelles by Silica Deposition. J. Am. Chem. Soc. 2007;129 doi: 10.1021/ja0674946. [DOI] [PubMed] [Google Scholar]
- 124.Li Z., Yang T., Gao Q., Yuan J., Cheng S. Biomimetic synthesis of copolymer-silica nanoparticles with tunable compositions and surface property. J. Colloid Interface Sci. 2009;338:99–104. doi: 10.1016/j.jcis.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 125.Kang K.-K., Oh H.-S., Kim D.-Y., Shim G., Lee C.-S. Synthesis of silica nanoparticles using biomimetic mineralization with polyallylamine hydrochloride. J. Colloid Interface Sci. 2017;507:145–153. doi: 10.1016/j.jcis.2017.07.115. [DOI] [PubMed] [Google Scholar]
- 126.Betancor L., Luckarift H.R. Bioinspired enzyme encapsulation for biocatalysis. Trends Biotechnol. 2008;26:566–572. doi: 10.1016/j.tibtech.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 127.Song X., Jiang Z., Li L., Wu H. Immobilizayion ofb-glucuronidase in lysozyme-induced biosilica particles to imporve its stability. Front. Chem. Sci. Eng. 2014;8:353–361. doi: 10.1007/s11705-014-1421-2. [DOI] [Google Scholar]
- 128.Sheppard V.C., Scheffel A., Poulsen N., Kroger N. Live diatom silica immobilization of multimeric and redox-active enzymes. Appl. Environ. Microbiol. 2012;78:211–218. doi: 10.1128/AEM.06698-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuan I.C., Chuang C.A., Lee S.L., Yu C.Y. Alkyl-substituted methoxylanes enhance the activity and stability of d-amino acid oxidase encapsulated in biomimetic silica. Biotechnol. Lett. 2012;34:1493–1498. doi: 10.1007/s10529-012-0924-5. [DOI] [PubMed] [Google Scholar]
- 130.Berne C., Betancor L., Luckarift H.R., Spain J.C. Application of microfluid reactor for screening cancer produg activation using silica-immmobilized nitrobenzene nitroreductase. Biomacromolecules. 2006;7:2631–2636. doi: 10.1021/bm060166d. [DOI] [PubMed] [Google Scholar]
- 131.Betancor L., Luckarift H.R. Co-immobilization coupled enzyme systems in biotechnology. Biotechnol. Genet. Eng. Rev. 2010;27:95–114. doi: 10.1080/02648725.2010.10648146. [DOI] [PubMed] [Google Scholar]
- 132.Jackson E., Ferrari M., Cuestas-Ayllon C., Fernandez-Pacheco R., Perez-Carvajal J., Fuente J.M., Grazu V., Betancor L. Protein-Templated Biomimetic Silica Nanoparticles. Langmuir. 2015;31:3687–3695. doi: 10.1021/la504978r. [DOI] [PubMed] [Google Scholar]
- 133.Arkhreeva A., Hay J.N. Synthesis of sub-200 nm silsesquioxane particles using a modified Stöber sol–gel route. J. Mater. Chem. 2003;13:3122–3127. doi: 10.1039/B306994J. [DOI] [Google Scholar]
- 134.LaMer V.K., Dinegar R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950;72:4847–4854. doi: 10.1021/ja01167a001. [DOI] [Google Scholar]
- 135.Wei Q., Keck C.M., Muller R.H. CapsMorph® technology for oral delivery-theory, prepatration and characterization. Int. J. Pharm. 2015;482:11–20. doi: 10.1016/j.ijpharm.2014.10.068. [DOI] [PubMed] [Google Scholar]
- 136.Matos J.C., Avelar I., Martins B., Gonçalves M.C. Silica Vectors for Smart Textiles. Carbohydr. Polym. 2017;156:268–275. doi: 10.1016/j.carbpol.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 137.Evonik Power to Create. [(accessed on 1 May 2018)]; Available online: http://www.aerosil.com/product/aerosil/en/industries/pharmaceuticals/aeroperl-300-pharma/
- 138.Tan A., Simovic S., Davey A., Rades T., Prestidge C. Silica-lipid hybrid (SLH) microcapsules: a novel oral delivery system for poorly soluble drugs. J. Control. Release. 2009;134:62–70. doi: 10.1016/j.jconrel.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 139.Tan A., Simonov S., Dvey A.K., Rades T., Boyd B.D., Prestidge C.A. Silica nanoparticles to control the lipase-mediated digestion of lipid-based oral delivery systems. Mol. Pharm. 2010;7:522–532. doi: 10.1021/mp9002442. [DOI] [PubMed] [Google Scholar]
- 140.Joyce P., Tan A., Whitby C., Prestige C.A. The role of porous nanostructure in controlling the lipase-mediated digestion of lipid loaded into silica particles. Langmuir. 2014;30:2779–2788. doi: 10.1021/la500094b. [DOI] [PubMed] [Google Scholar]
- 141.Schmidt H. Organic modification of glass structure, new glass or new polymer? J. Non-Cryst. Solids. 1989;112:419–423. doi: 10.1016/0022-3093(89)90565-6. [DOI] [Google Scholar]
- 142.Chevalier P.M., Corriu R.J.P., Moreau J.J.E., Chi Man M.W. Chemistry of hybrid organic-inorganic acess to silica materials through chemical selectivity. J. Sol-Gel Sci. Technol. 1997;8:603–607. doi: 10.1007/BF02436908. [DOI] [Google Scholar]
- 143.Kumar R., Roy I., Ohulchanskky T.Y., Vathy L.A., Bergey E.J., Sajjad M., Prasad P.N. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano. 2010;4:699–708. doi: 10.1021/nn901146y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mogensen S.C., Andersen H.K. Effect of silica on the pathogenic distinction between herpes simplex virus type 1 and 2 hepatitis in mice. Infect. Immun. 1977;17:274–277. doi: 10.1128/iai.17.2.274-277.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bharali D.J., Klejbor I., Stachowiak E.K., Dutta P., Roy I., Kaur N., Berjey E.J., Prasad P.N. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl. Acad. Sci. USA. 2005;102:11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barandeh F., Nguyen P.L., Kumar R., Iacobucci G.J., Kuznicki M.L., Kosterman A., Bergey E.J., Prasad P.N., Gunawardena S. Organically modified silica nanoparticles are biocompatible and can be targeted to neurons in vivo. PLoS ONE. 2012;7:e29424. doi: 10.1371/journal.pone.0029424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang G. Liposomes as Drug Delivery vehicles. In: Wang B., Hu L., Siahaan T.J., editors. Drug Delivery: Principles and Applications, Drug Discovery and Development. Wiley Series; Hoboken, NJ, USA: 2016. [Google Scholar]
- 148.Gonçalves M.C., Fortes L.M., Pimenta A.R., Pereira J.C.G., Almeida R.M., Carvalho M.D., Ferreira L.P., Cruz M.M., Godinho M. Silica/Ormosil SPIONs for biomedical applications. Curr. Nanosci. 2013;9:599–608. doi: 10.2174/15734137113099990004. [DOI] [Google Scholar]
- 149.Bégu S., Pouessel A.A., Lerner D.A., Tourné-Péteilh C., Devoisselle J.-M. Liposil, a promising composite material for drug storage and realease. J. Control. Realese. 2007;118 doi: 10.1016/j.jconrel.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 150.Fortes L.F., Li Y., Réfega R., Gonçalves M.C. Up-conversion in rare earth-doped silica hollow spheres. Opt. Mater. 2012;34:1440–1446. doi: 10.1016/j.optmat.2012.02.049. [DOI] [Google Scholar]
- 151.Rodrigues A.M., Gonçalves M.C., Corvo M.L., Martins M.B. Liposil as Nanocarriers for Pharmaceutical Applications: Process innovations. J. Nanomed. Nanotechnol. 2017;8 doi: 10.4172/2157-7439.1000450. [DOI] [Google Scholar]
- 152.Kresge C.T., Leonowicz M.E., Roth W.J., Vartuli J.C., Beck J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710–712. doi: 10.1038/359710a0. [DOI] [Google Scholar]