Abstract
Enterotoxigenic Escherichia coli (ETEC), Campylobacter jejuni (CJ), and Shigella sp. are major causes of bacterial diarrhea worldwide, but there are no licensed vaccines against any of these pathogens. Most current approaches to ETEC vaccines are based on recombinant proteins that are involved in virulence, particularly adhesins. In contrast, approaches to Shigella and CJ vaccines have included conjugate vaccines in which Shigella lipopolysaccharides (LPS) or CJ capsule polysaccharides are chemically conjugated to proteins. We have explored the feasibility of developing a multi-pathogen vaccine by using ETEC proteins as conjugating partners for CJ and Shigella polysaccharides. We synthesized three vaccines in which two CJ polysaccharides were conjugated to two recombinant ETEC adhesins based on CFA/I (CfaEB) and CS6 (CssBA), and LPS from Shigella flexneri was also conjugated to CfaEB. The vaccines were immunogenic in mice as monovalent, bivalent and trivalent formulations. Importantly, functional antibodies capable of inducing hemaglutination inhibition (HAI) of a CFA/I expressing ETEC strain were induced in all vaccines containing CfaEB. These data suggest that conjugate vaccines could be a platform for a multi-pathogen, multi-serotype vaccine against the three major causes of diarrheal disease worldwide.
Keywords: ETEC, Shigella, Campylobacter, conjugate vaccines, functional antibodies
Introduction
Enterotoxigenic Escherichia coli (ETEC) Campylobacter jejuni (CJ), and Shigella sp. are major causes of bacterial diarrhea worldwide. Global deaths due to diarrhea have decreased in the last decade, partially due to the introduction of a rotavirus vaccine [1], and the most recent estimates indicate that there 54,900 deaths due to Shigella, 23,600 due to ETEC and 30,900 due to Campylobacter in children under five years of age in low to middle income countries [1]. Nonetheless, there is also an increased awareness of chronic consequences of these infections [2–5] indicating that these three enteric pathogens remain serious health threats to western travelers and young children in resource-limited countries making them apt target populations for vaccines.
ETEC causes diarrhea that can range in severity from mild illness to cholera-like purging. Several well-recognized virulence factors, including colonization factors, EtpA, and the heat-labile and heat-stable enterotoxins have been investigated extensively as vaccine candidates [6, 7]. One of the major colonization factors is colonization factor antigen I (CFA/I) which is composed of a major pilin subunit, CfaB, and a tip adhesin, CfaE, that mediates binding to intestinal cells. Hyperimmune bovine colostral antibodies raised against CFA/I and recombinantly expressed CfaE protected human subjects from diarrhea after challenge with CFA/I-expressing ETEC strain H10407 [8, 9]. We designed a fusion protein stabilized by donor strand complementation (dsc), termed dscCfaEB, but hereafter called CfaEB, containing both CfaE and CfaB, as a vaccine candidate designed to increase efficacy and broaden coverage against ETEC expressing CFA/I and related fimbrial structures [10–12]. Additionally, using a similar rationale, we developed a stable and immunogenic CS6-derived donor strand complemented fusion protein, dscCssBA, or hereafter called CssBA, which combines the two subunits that form the CS6 surface antigen, CssA and CssB [13].
The symptoms of campylobacter enteritis generally include diarrhea, often with leukocytes and blood. The disease is zoonotic, with most sporadic cases associated with contaminated poultry, but major outbreaks have also been associated with water [14] or raw milk contamination [15]. Guillain Barré Syndrome (GBS), a post-infectious polyneuropathy that can result in paralysis, is a complication of CJ infection [16]. The association is due to molecular mimicry between the sialic acid containing-outer core of the lipooligosaccharide (LOS) and human gangliosides [17]. The pathogenesis of CJ remains poorly understood in comparison with other pathogens, but the organism expresses a polysaccharide capsule (CPS) that contributes to virulence [18–22]. The successes of polysaccharide conjugate vaccines against other mucosal pathogens led us to evaluate this approach for CJ. A prototype conjugate vaccine composed of the CPS of CJ strain 81–176 conjugated to CRM197, a carrier protein used in licensed conjugate vaccines, provided 100% efficacy against diarrheal disease in non-human primates [23]. The CPS is the major serodeterminant of the heat stable (HS) serotyping scheme of C. jejuni, a scheme that has 47 serogroups [24]. Thus, a final conjugate vaccine would be multivalent, composed of CPSs from the predominant serotypes.
There are four major species of Shigella and 59 different serotypes based on lipopolysaccharide (LPS), but most disease worldwide is caused by Shigella sonnei and three serotypes of Shigella flexneri [25, 26]. Seroepidemiology studies indicate that antibodies generated against the O-polysaccharide (O-PS) of LPS correlate with protection against the same serotype [27]. There are numerous approaches toward development of Shigella vaccines, including several variations on live attenuated vaccines [28–31]. There are also multiple approaches to vaccines based on the repeating units of the O-PS, including classic conjugate vaccines using detoxified LPS [32], conjugates utilizing synthetic O-PS [33, 34], biological glycoconjugates [35] and mixtures of LPS and Shigella invasion proteins [36].
Since subunit vaccine approaches to both CJ and Shigella involve multiple polysaccharide antigens and all subunit approaches to ETEC involve multiple protein antigens, we have explored the possibility conjugating CJ and Shigella polysaccharides to ETEC proteins. Although a limited number of carriers are found in licensed conjugate vaccines, there are many reports using alternate proteins in an effort to expand the effectiveness of conjugate vaccines [37–40]. The standard CRM197 carrier presents a monomeric species with 7% of its amino acids (39/535) comprising surface-exposed lysines that are available for amino-linked conjugation to activated polysaccharides. Examination of the solved atomic structure of CfaEB shows that this protein is also rich in surface-exposed lysines, constituting 6% of all residues (31/516) [41]. CfaEB and CssBA have bioadhesive proterties, are non-toxic and behave as homogeneous monomeric species in solution, making them favorable candidates for use as carrier proteins [11, 42, 43]. Here, we report on studies evaluating immunogenicity in mice of prototype conjugate vaccines composed of: (i) two CPSs from CJ conjugated to two recombinant ETEC proteins: CfaEB, representing a CFA/I ETEC vaccine candidate, and CssBA, representing a CS6 ETEC vaccine candidate; and (ii) a conjugate of detoxified Shigella O-PS to CfaEB.
Materials and Methods
Bacterial strains and growth for polysaccharide purification.
The following bacterial strains were used for polysaccharide purification: 1) CJ strain PG3208 expressing HS23/36 CPS type [44]; 2) CJ strain BH-01–0142 expressing HS3 CPS type [45]; and 3) S. flexneri 2a strain 2457O [46]. Both CJ strains used lack the ganglioside mimicry in LOS cores that is associated with GBS. Strain PG3208 is a mutant of strain 81–176 (CPS type HS23/36) in which the galT gene was insertionally inactivated with a chloramphenicol cassette resulting in a truncated core [44], while the LOS core of strain BH-01–0142 (CPS type HS3) naturally lacks ganglioside mimicry [45]. CJ strains were grown in porcine Brain Heart Broth for CPS extraction at 37°C under microaerobic conditions. S. flexneri was grown in L-broth at 37°C.
Purification of recombinant ETEC proteins.
The recombinant proteins CfaEB and CfaE (used for ELISA analyses, see below) were produced as previously described [10]. The CssBA clone was constructed from the CS6 producing ETEC strain B7A [13] and the full details of the construction and characterization will be presented elsewhere. All purified proteins were >95% pure based on densitometry analysis on SDS-PAGE, and the endotoxin level of each protein was <20 endotoxin units (EUs)/mg protein by Limulus Amebocyte Lysate assay (LAL: Lonza). Each protein was monomeric in solution based on size exclusion HPLC and reacted positively to anti-sera raised against its components and 6xHis tag on the western blots.
Cloning of CS6.
The CS6 operon was amplified from ETEC strain B7A by PCR with primers pENTR-CS6 F 5’-CACCATGAAGAAAACAATTGGTTTAATTC-3’ and pENTR-CS6 R 5’-CAGGAAGTTTAGTCTCCAGAATTTC-3’ [47]. The resulting fragment was cloned into pENTR™/SD/D-TOPO ® vector using Invitrogen’s pENTR™/SD/D-TOPO® Cloning Kit. Next, the CS6 operon was moved from the pENTR™/SD/D-TOPO® vector into the Gateway® destination expression vector pDEST™14 (Invitrogen) using Gateway® LR Clonase Mix (Invitrogen). The resulting plasmid was expressed in the arabinose-inducible E. coli host strain BL21-AI™ (Invitrogen).
Purification of CS6 fimbriae.
CS6 fimbriae were purified from the recombinant clone described above by a modification of the method of Ghosal et al. [48]. Briefly, a 10 L culture of recombinant BL21AI/pDEST14-CS6-B7A expression strain was grown in a bioreactor (BioFlow 3000, Eppendorf) containing Select APS™ Super Broth (BD Difco), 4 mL/L glucose, and ampicillin (0.1 mg/mL). The culture was grown at 37°C with 850 rpm agitation to an optical density (600nm) of 5 and induced for 3 hours with 0.2% arabinose. Cells were harvested and resuspended in phosphate buffered saline at pH 7.4 (PBS). CS6 was extracted and partially purified from the cells using heat-saline extraction and ammonium sulfate precipitation. The resulting ammonium sulfate pellet was resuspended in 20 mM Tris pH 8.0 and dialyzed overnight in the same buffer. The suspension was then applied to a 5 mL HiTrap Q HP column (GE Healthcare) equilibrated with 20 mM Tris at pH 8.0. The recombinant CS6 protein was then eluted with a 50–500 mM sodium chloride gradient over 30 column volumes using an AKTA Pure chromatography system (GE Healthcare). Fractions containing the protein were pooled and concentrated. Protein concentration was determined by a bicinchoninic acid (BCA) protein assay kit (Pierce).
Polysaccharide extraction and conjugation.
Polysaccharide extraction and purification were carried out with hot phenol as previously described [23]. The Shigella LPS was de-lipidated by treatment with 1% acetic acid at 100 °C for 1.5 hr. Mild centrifugation of the hydrolysate afforded the LPS free of lipid A in the supernatant and the insoluble lipid A as a pellet. The O-PS devoid of lipid A was dialyzed and further purified through a Bio-Gel P-2 column (1cm x 1m) with water as eluent. All polysaccharides had endotoxin levels <100 EUs/μg polysaccharide by LAL. The conjugation of HS23/36 CPS, with the structure α-D-Galp-(1-[→2)-6-deoxy-3-O-Me-α-D-altro-Hepp-(1→3)-β-D-GlcpNAc-(1→3)-α-D-Galp-(1→]n and non-stoichiometric O-methyl phosphoramidate residues at positions 2,4 and 6 of Gal, to CfaEB was achieved as done before with CRM197 [23], in which the non-reducing galactose unit of the CPS was selectively activated by periodate oxidation for conjugation to CfaEB by reductive amination. That of HS3 CPS, with a backbone structure composed of 4-substituted Gal and 3-substituted L-glycero-α-D-ido-Hepp or its derivative 6-deoxy-α-D-ido-Hepp: [→3)-L-glycero/6-deoxy-α-D-ido-Hepp-(1→4)-α-D-Galp-(1→]n and with non-stoichiometric amounts of O-methyl-phosphoramidate at position 2 of the heptoses and 3-hydroxypropanoyl at position 3 of galactose, to CssBA was performed by first selectively activating the primary hydroxyl at C-7 of the CPS 6-deoxy-ido-heptose to a carboxylic acid by 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO)-mediated oxidation, followed by conjugation of the activated CPS to CssBA by carbodiimide chemistry, as described previously [49]. The scheme for the conjugation of S. flexneri 2a de-lipidated LPS, with the O-PS structure [→2)-α-L-Rha-(1→2)-α-L-Rha-(1→3)-{α-D-Glc-(1→4)}α-L-Rha-(1→3)-β-D-GlcNAc-(1→]n, to CfaEB was carried out by stoichiometrically transforming approximately 10% of the branch glucoses to glucuronic acids by TEMPO-mediated oxidation, and then conjugation of the activated S. flexneri de-lipidated O-PS to CfaEB via carbodiimide chemistry [49]. Conjugations were performed using a ratio of 2(CPS):1(protein) by weight. For the CPS-specific ELISA experiments, BSA conjugates of HS3 and S. flexneri de-lipidated O-PS were used, and these were produced by the same methods as just described above for CfaEB and CssBA conjugates.
Determination of polysaccharide and protein concentrations in conjugate vaccines.
Polysaccharide concentration was determined by anthrone assay [50] and protein concentrations were determined using the BCA assay.
Mouse immunizations.
All BALB/cByJ mice were purchased from Jackson Laboratories (Bar Harbor, ME), and experiments were approved under the Naval Medical Research Center Institutional Animal Care and Use Committee. In the pilot experiment mice were immunized with either with a HS23/36-CfaEB conjugate vaccine co-administered with 200 μg of Alhydrogel (alum; Brenntag Biosector) by subcutaneous (S.C.) injection into the scruff of the neck. Mice were given two immunizations at 4-week intervals. There were 4 animals in the low dose group and 5 animals in the high dose group. In subsequent experiments, BALB/cByJ mice were immunized with HS23/36-CfaEB, HS3-CssBA, or Sf-O-PS-CfaEB either as monovalent, bivalent or trivalent formulations with doses that were down selected from individual immunogenicity experiments (data not shown). The amount of polysaccharide and protein contained in each formulation is detailed in Table 1. Animals were immunized S.C. three times, at 4-week intervals, without the use of an adjuvant. Sera were collected from mice prior to immunization and every 2-weeks post-immunization.
Table 1.
Total mass of PS and protein delivered in each combined vaccine.
| Valency | Vaccine Group | Dose µg HS23/36 CPS |
Dose µg HS3 CPS |
Dose µg Sf-O- PS |
Dose µg CfaEB |
Dose µg CssBA |
|---|---|---|---|---|---|---|
| Monovalent | HS23/36-CfaEB | 3.5 | - | - | 1.3 | - |
| HS3-CssBA | - | 1.5 | - | - | 0.8 | |
| Sf-O-PS-CfaEB | - | - | 5.0 | 9.5 | - | |
| Bivalent | HS23/36-CfaEB + HS3-CssBA | 3.5 | 1.5 | - | 1.3 | 0.8 |
| HS23/36-CfaEB + Sf-O-PS-CfaEB | 3.5 | - | 5.0 | 10.8 | - | |
| HS3-CssBA + Sf-O-PS-CfaEB | - | 1.5 | 5.0 | 9.5 | 0.8 | |
| Trivalent | HS23/36-CfaEB + HS3-CssBA + Sf-O-PS-CfaEB | 3.5 | 1.5 | 5.0 | 10.8 | 0.8 |
Polysaccharide-specific ELISAs.
IgG responses to the HS23/36 capsule-were determined by ELISA as previously described using oxidized CPS bound to Carbo-BIND plates (Corning, Corning NY) [20]. Anti-HS3 or anti-Sf-O-PS specific antibody responses were measured by coating HS3-BSA or Sf-O-PS-BSA conjugates, respectively, onto 96-well plates (Nunc Maxisorp, ThermoScientific). Sera were serially diluted, and antigen-specific antibodies were detected using a goat anti-mouse IgG peroxidase conjugated antibody (SeraCare, Gaithersburg, MD) and visualized using ABTS substrate. The optical density (OD) of individual wells at 405 nm was determined using a SpectraMax Plus 384 plate reader (Molecular Devices). The mean OD405 of negative control wells + 3 standard deviations was used to determine the endpoint titer. Individual animal titers were measured. Serum pools were created and analyzed for vaccine groups that did not contain the polysaccharide being measured and were not analyzed for statistical significance compared to groups that contained the antigen being analyzed. Endpoint titers were log10 transformed and analyzed using Prism software (GraphPad). Differences between experimental groups were considered significant at p ≤ 0.05 (t test or one-way ANOVA with multiplicity adjusted P values).
Measurement of ETEC-specific antibodies by ELISA.
Sera were screened for anti-CfaE and anti-CS6 IgG antibodies by ELISA. Briefly, 96-well plates (NUNC Microwell) were coated with recombinant CfaE or purified CS6 fimbriae and blocked. Sera were serially diluted, and antigen-specific antibodies were detected using a goat anti-mouse IgG peroxidase conjugated antibody and visualized using ABTS substrate. The OD405 of individual wells was measured and endpoint titers and statistics were calculated as described above for anti-polysaccharide responses.
Measurement of anti-adhesive antibodies.
A hemagglutination inhibition assay (HAI) was used to measure levels of functional anti-adhesive antibodies in mouse serum samples. Serum samples were tested individually for each animal immunized with a CfaEB containing conjugate, but in pools in vaccine groups where no CfaEB was delivered. The samples were initially diluted 1:8, then diluted two-fold. Each serum dilution was incubated with an equal volume of CFA/I+ ETEC bacteria (strain H10407), further diluting the sera 1:2. The most concentrated dilution tested was 1:16 (lower limit of quantitation). The pre-incubated antibody-bacteria mixture was subsequently mixed with bovine erythrocytes in the presence of 0.5% mannose in U-bottom 96-wells plates (BD Falcon). In the absence of anti-adhesive antibodies, the erythrocytes formed visible agglutinated buttons of cells. In the presence of anti-adhesive antibodies, agglutination was inhibited. The HAI titer was defined as the highest serial dilution that completely inhibited agglutination. If there was no detectable inhibition at the lowest serum dilution of 1:16, the samples were assigned a value of one-half of the quantitation limit (i.e. 8) for computational purposes. Endpoint titers were log2 transformed and analyzed using Prism software. Differences between experimental groups were considered significant at p ≤ 0.05 (t test or one-way ANOVA with multiplicity adjusted P values).
Results
Evaluation of a pilot HS23/36 CPS-CfaEB conjugate vaccine in mice.
Two concerns about the approach of combined conjugate vaccines were if sufficient ETEC protein could be delivered in a conjugate and if chemical treatment of ETEC proteins, particularly adhesins, would affect their structure such that they could no longer elicit functional antibody responses. We administered two different doses of a pilot HS23/36-CfaEB vaccine, given by total weight at 10 µg and 60 µg per dose. Since the mass ratio of polysaccharide to protein in this vaccine was 1.8:1, these doses corresponded to 6.4 µg PS + 3.6 μg protein (“low”) and 38.4 µg PS and 21.6 µg protein (“high”), respectively. The vaccine was given twice at 4-week intervals with alum adjuvant. High levels of anti-HS23/36 CPS IgG and anti-CfaE IgG were detected after two doses (Fig. 1A). Anti-HS23/36 IgG titers were slightly lower in the high dose group, although the difference was not significant. There was also no significant difference in anti-CfaE IgG titers between the low and high dose.
Figure 1.
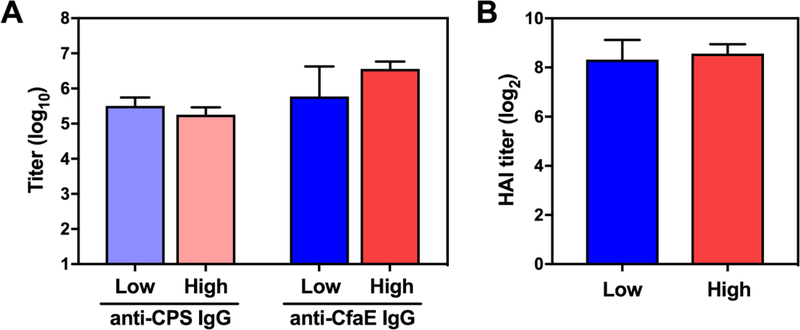
Immunogenicity of an HS23/36-CfaEB conjugate vaccine. CJ-and ETEC-specific IgG antibody responses were measured after two immunizations with a low (10 µg) or high (60 µg) dose of HS23/36-CfaEB. A) Anti-HS23/36 CPS IgG and anti-CfaE IgG endpoint titers were measured by ELISA. B) Functional hemagglutination (HAI) titers were measured against the CFA/I-expressing ETEC strain H10407. Bars represent the mean plus standard deviation of the log10 or log2 transformed titers.
We also measured functional anti-adhesive antibodies in a red blood cell hemagglutination assay (HAI) as a proxy for neutralization of ETEC intestinal adhesion via CFA/I fimbriae using the CFA/I expressing H10407 ETEC strain. High levels of HAI titers were observed after two immunizations with the HS23/36-CfaEB vaccine, and there was no difference in HAI titers between the low and high dose groups (Fig. 1B). These experiments demonstrated that ETEC proteins could be utilized as carrier proteins for CJ capsules, while still eliciting anti-ETEC functional antibodies.
Immunogenicity of other combined conjugate vaccines.
We generated a new batch of HS23/36-CfaEB vaccine and two additional conjugates: an HS3 CJ CPS conjugated to the recombinant ETEC CS6 protein (HS3-CssBA) and S. flexneri 2a O-PS conjugated to CfaEB (Sf-O-PS-CfaEB). The PS:protein mass ratios of these vaccines were: HS23/36-CfaEB (2.6:1); HS3-CssBA (1.8:1); and Sf-O-PS-CfaEB (1:2). Preliminary dose-escalation studies revealed that the monovalent formulations of all three vaccines were immunogenic without the addition of alum (data not shown) and subsequent studies were performed without an adjuvant.
The optimal dose of each conjugate determined by these initial studies was then used to immunize mice as three monovalent vaccines or admixed into three bivalent formulations or a single trivalent formulation, as shown in Table 1. Animals were given three doses subcutaneously at 4-week intervals and bled prior to immunization and two weeks after each immunization. The final IgG titers to the three polysaccharides are shown in Fig. 2 and final IgG titers to CfaE and CS6 are shown in Fig. 3A and 3C, respectively. HAI titers to CFA/I+ H10407 ETEC are shown in Fig. 3B. As expected, the monovalent immunized animals generated IgG antibody responses only to the components of the vaccine with which they were immunized. Mice immunized with HS23/36-CfaEB vaccine generated anti-HS23/36 and anti-CfaE IgG antibody responses (red bars in Figs. 2A and 3A), although the titers were lower than those observed in the pilot experiment which utilized alum adjuvant (Fig. 1A). HS3-CssBA immunized animals generated anti-HS3 CPS IgG and anti-CS6 IgG antibodies (blue bars in Figs. 2B and 3C), and Sf-O-PS-CfaEB immunized animals generated anti-Sf-O-PS IgG and anti-CfaE IgG responses (grey bars in Figs. 2C and 3A). A 7-fold higher dose of CfaEB was delivered in the Sf-O-PS-CfaEB vaccine compared to the HS23/36-CfaEB immunized animals (9.5 vs 1.3 µg, respectively; Table 1), generating significantly higher anti-CfaE IgG titers between these two groups (Fig. 3A, red and grey bars). However, no significant difference in HAI titers were observed (Fig. 3B).
Figure 2.
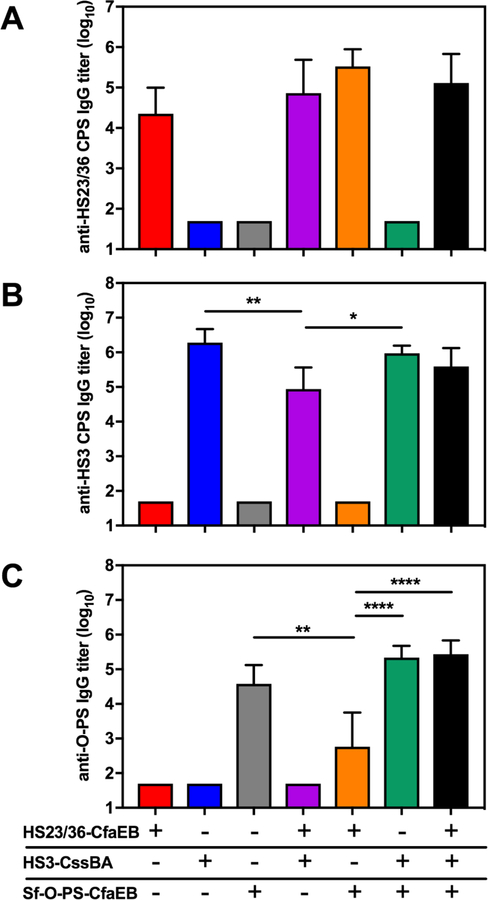
Polysaccharide antibody responses following immunization with multivalent conjugate vaccines. CJ-and Sf-specific polysaccharide IgG responses were measured by ELISA after three immunizations of monovalent, bivalent or a trivalent formulation of three polysaccharide conjugate vaccines. IgG responses were measured against A) HS23/36 CPS, B) HS3 CPS and C) Sf O-polysaccharide (Sf-O-PS). Bars represent the mean plus standard deviation of the log10 transformed titers. Statistical significance was determined using a one-way ANOVA including groups for which individual mouse titers were measured. Asterisks denote a multiplicity adjusted P values between groups (*p ≤ 0.05, ** p ≤ 0.001, ****p < 0.0001).
Figure 3.
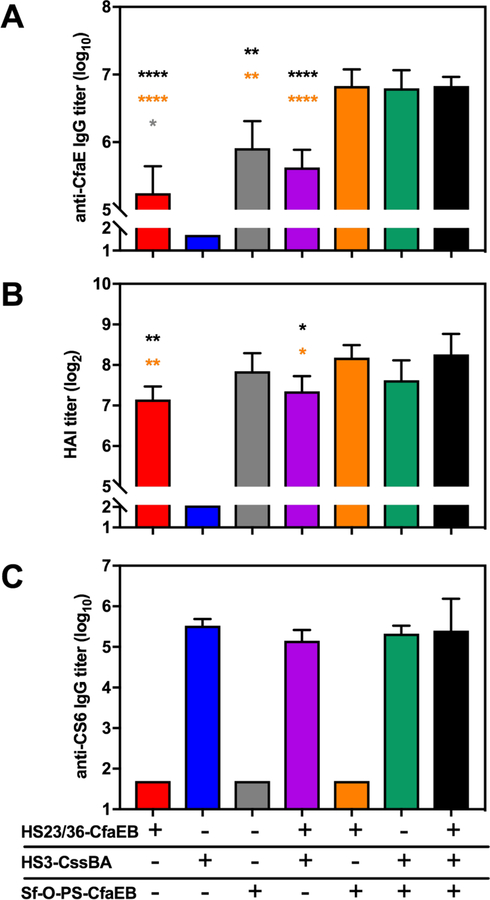
ETEC antibody responses following immunization with multivalent conjugate vaccines. Protein-specific IgG and HAI responses were measured after three immunizations of monovalent, bivalent or a trivalent formulation of three polysaccharide conjugate vaccines. A) Anti-CfaE IgG responses were measured by ELISA. B) Functional HAI titers were measured against H10407. C) anti-CS6 IgG responses were measured by ELISA. Bars represent the mean plus standard deviation of the log10 or log2 transformed titers. Statistical significance was determined using a one-way ANOVA including groups for which individual mouse titers were measured. Symbols denote multiplicity adjusted P values between groups where the color of symbol above the respective bar represents the group that is significantly different. Grey: significantly different from Sf-O-PS-CfaEB. Orange: significantly different from HS23/36-CfaEB + Sf-O-PS-CfaEB. Black: significantly different from the trivalent formulation. * p ≤ 0.05, ** p ≤ 0.001, ****p < 0.0001.
Formulation into bivalent or trivalent vaccines did not dramatically affect polysaccharide-specific antibody generation. High levels of anti-HS23/36 IgG titers were observed in the two bivalent formulations containing HS23/36-CfaEB (Fig 2A, purple and orange bars) and the trivalent formulation (Fig 2A, black bar) compared to the monovalent vaccine (Fig. 2A, red bar). Similarly, high levels of anti-HS3 IgG were detected in the bivalent formulation HS3-CssBA + Sf-O-PS-CfaEB (Fig. 2B, green bar). We observed a significant difference in anti-HS3 IgG in the bivalent HS23/36-CfaEB + HS3-CssBA group with an average titer of 104.9 (Fig. 2B, purple bar) as compared to 106.3 in the monovalent HS3-CssBA group and 106.0 in the HS3-CssBA + Sf-O-PS-CfaEB bivalent group (Fig. 2B, blue and green bars, respectively), although it is not clear if this difference is biologically relevant. Furthermore, combination into a trivalent formulation induced HS3-specific antibody levels equivalent to the monovalent HS3-CssBA vaccine (Fig. 2B, black bar). A similar observation was made after analyzing the Sf-O-PS response where anti-Sf-O-PS IgG titers in the bivalent formulation HS23/36-CfaEB + Sf-O-PS-CfaEB (102.8; Fig. 2C, orange bar) were significantly lower than the Sf-O-PS-CfaEB monovalent vaccine (104.6 ; Fig. 2C, grey bar) and HS3-CssBA + Sf-O-PS-CfaEB bivalent group (105.3, Fig. 2C, green bar). However, a trivalent formulation induced high levels of Sf-O-PS-specific responses (105.4; Fig. 2C, black bar).
We next analyzed the anti-ETEC protein responses in the multivalent vaccine formulations. Two of the conjugate vaccines utilized CfaEB as a carrier and the mass ratio of polysaccharide:protein differed for each conjugate (HS23/36-CfaEB: 2.6:1 and Sf-O-PS-CfaEB: 1:2). These experiments gave us an opportunity to measure the additive effect of CfaEB on anti-CfaE IgG and HAI responses. Combination of HS23/36-CfaEB + HS3-CssBA induced anti-CfaE IgG at levels similar to HS23/36-CfaEB group (Fig. 3A; purple and red bars, respectively), consistent with the same 1.3 µg dose of CfaEB being delivered in these two formulations (Table 1). Similar functional HAI titers were also observed between these two groups (Fig. 3B). Combination of HS23/36-CfaEB with Sf-O-PS-CfaEB (Fig. 3, orange bars) increased the total CfaEB dose delivered to 10.8 µg, and the same dose of CfaEB was delivered in the trivalent group as well (Fig. 3, black bars). Anti-CfaE IgG titers were significantly higher in both the bivalent HS23/36-dscCfaEB + Sf-O-PS-CfaEB and the trivalent groups than any of the groups containing lower amounts of CfaEB including the Sf-O-PS-CfaEB group (Fig. 3A and Table 1) indicating an additive effect of the two CfaEB-containing vaccines. This was also partially true when we measured functional HAI antibody responses (Fig. 3B). The bivalent HS23/36-CfaEB + Sf-O-PS-CfaEB (orange bars) and the trivalent groups (black bars) had significantly higher HAI titers than either the HS23/36-CfaEB (red bar) and the HS23/36-CfaEB + HS3-CssBA (purple bar) groups where only 1.3 µg of CfaEB were delivered per dose. No significant difference in HAI titers was observed between groups dosed with 9.5 (Fig. 3B, grey or green bars) or 10.8 µg (orange and black bars) of CfaEB. As expected, similar levels of anti-CS6 IgG were observed in all bi-and trivalent vaccine formulations containing the HS3-CssBA vaccine as the dose of CssBA was constant in all the formulations (Fig. 3C and Table 1).
Discussion
Vaccine development is a complex and costly process, from basic research through pre-clinical and clinical testing. Although various candidate vaccines against each of the three individual pathogens discussed here have gone into clinical trials, there are no licensed vaccines against ETEC, CJ or Shigella. In this study, we explored whether conjugate vaccines incorporating antigens from these pathogens can induce a multi-target immune response. Although conjugate vaccines are complex, they have been remarkably effective against a variety of other mucosal pathogens. All licensed conjugate vaccines use one of a few carrier proteins such as CRM197 or tetanus toxoid that are unrelated to the targeted pathogen. Since approaches to Shigella and to CJ involve polysaccharides, and all subunit approaches to ETEC involve proteins, we sought to combine the approaches by utilizing ETEC proteins as carriers for CJ and Shigella polysaccharides.
We synthesized three combinations of antigens: CJ HS23/36 CPS-CfaEB, S. flexneri 2a O-PS-CfaEB and CJ HS3 CPS-CssBA and demonstrated immunogenicity against the relevant antigens in monovalent, bivalent, and trivalent formulations. Importantly, we have demonstrated successful conjugation of CfaEB by two distinct chemical mechanisms [23, 49]. The doses of polysaccharide used in these studies ranged from 1.5 µg to 5 µg, and the protein doses ranged from 0.8 µg to 10.8 µg (Table 1). We did see a trend toward an additive effect in terms of protein response when in bivalent and trivalent formulations of the two conjugates containing higher doses of CfaEB. Importantly, even low doses of CfaEB in monovalent formulations resulted in substantial functional antibodies as measured by HAI, indicating that chemical conjugation did not substantially alter the binding domain of the adhesin. Unfortunately, no functional assays exist for the CS6 adhesin. We have recently observed serum bactericidal activity against CJ in rabbits immunized with CJ conjugates + Freund’s adjuvant, non-human primates immunized with CJ conjugates + alum, and mice immunized with conjugates + Toll-like receptor ligand adjuvants. Because the mice in this study were immunized with a CPS conjugate vaccine alone or the conjugate mixed with alum, we were unable to detect CJ bactericidal titers in pooled serum from the mice in this study (data not shown). This is consistent with our observations that CPS-CRM197 vaccines delivered alone or with alum in mice primarily induce a T helper cell type 2 (Th2) immune response characterized by primary induction of anti-CPS IgG1 antibodies which do not activate the classical complement pathway. We also did not detect S. flexneri 2a bactericidal responses in pooled mouse serum (data not shown) utilizing the recently described Shigella SBA assay by Nahm et al. [51]. In contrast, in a clinical study in which a S. flexneri 2a O-PS bioconjugate was delivered alone or with alum [52] SBA activity was detected in the presence or absence of alum. This indicates Th1 activity and generation of complement-fixing antibody isotypes in human volunteers, although O-PS IgG subclasses were not specifically measured in the study. Further studies are required to determine how antibody responses differ between mice and humans vaccinated with the same O-PS and whether it is a reflection of differences in the host immune response and/or carrier proteins and conjugation methods utilized in these two studies. Future studies with the conjugate vaccines described here will test adjuvants known to induce serum bactericidal responses and should allow measurement of anti-CPS bactericidal titers not just against CJ, but also anti-O-PS bacterial responses against Shigella.
Although these preliminary studies show this conjugate approach is feasible, additional studies remain to be done. The ratio of polysaccharide to protein in each combination needs additional experimentation to find an optimum dose of each antigen. Also, as mentioned above, we are currently evaluating different adjuvants to improve antibody responses and to potentially reduce the numbers of doses required to achieve high antibody titers against all vaccine components. An additional factor to consider in our multivalent conjugate approach is the route of administration and generation of mucosal immune responses. Current approaches to generate B and T cell mediated immunity at mucosal sites have primarily centered around direct delivery of live-attenuated vaccines to mucosal sites (oral or intranasal). However, new evidence suggests that recombinant subunit vaccines delivered parenterally with the appropriate adjuvant may stimulate mucosal immunity [53]. ETEC heat-labile toxin (LT) and cholera toxin are some of the few adjuvants identified with these capabilities and future studies are planned to incorporate mutant LT potentially with other immunopotentiating adjuvants to enhance mucosal responses to our conjugate vaccines.
Current estimations of the valencies that would be required for an effective CJ CPS vaccine would be 8 (Poly et al., unpublished) and that for a Shigella O-PS vaccine would be 4 [25]. Since there are 4–5 main classes of ETEC adhesins [54], the final formulation of such a multivalent, multi-pathogen conjugate vaccine would be at maximum 12-valent. Thus, each of the 4 ETEC proteins would be conjugated to three of the 12 relevant polysaccharides, a complexity that is similar to licensed pneumococcal conjugate vaccines. Valency may be reduced by inclusion of a more common ETEC protein (e.g. EtpA) and/or a CJ protein that is shared among strains as carriers.
Acknowledgements
This work was funded by NIAID grant RO1AI089449 (to PG), by Navy Work Unit 6000.RAD1.DA3.A0308, and by the NSERC Canada (to MAM). We thank Danielle Jateng for technical assistance. We thank Drs. Robert Kaminski and Hailey Weerts and Ms. Nina Schumack for their expertise in Shigella-and CJ-specific SBA responses, and Dr. Chad Porter for comments on the manuscript. The opinions and assertions contained herein are the private ones of the authors and do not reflect the official policy of the Department of Navy, Department of Defense, nor the U.S. government. FP, MP, SJS and PG are or were employees of the U. S. government and this work was done as part of their official duties. Title 17 USC 105 provides that ‘Copyright protection under this title is not available for any work the United States government. Title 17 USC 101 defines a U. S. government work as a work prepared by a military service member or employee of the U. S. government as part of that person’s official duties. The animal study protocol was reviewed and approved by the Walter Reed Army Institute of Research/Naval Medical Research Center Institutional Care and Use Committee in compliance with all applicable Federal regulations governing the protection of animals in research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting interests to report.
References
- [1].Collaborators GBDDD. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 2017;17:909–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].The MAL-ED Network Investigators. The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. 2014. Clin. Infect. Dis 59(S4): S193–206. [DOI] [PubMed] [Google Scholar]
- [3].Platts-Mills JA and Kosek M. Update on the burden of Campylobacter in developing countries. 2014. Curr. Opin. Infect. Dis 27:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].George CM, Burrowes V, Perin J, Oldja L, Biswas S, Sack D, Ahmed S, Haque R, Bhuiyan NA, Parvin T, Bhuyian SI, Akter M, Li S, Natarajan G, Shahnaij M, Faruque AG and Stine OC. Enteric infections in young children are associated with environmental enteropathy and impaired growth. 2018. Trop. Med. Internat. Health 23:26–33. [DOI] [PubMed] [Google Scholar]
- [5].Nair P, Okhuysen PC, Jian ZD, Carlin LG, Belkind-Gerson J, Flores J, Paredes M, and DuPont HL. Persistent abdominal symptoms in US adults after short-term stay in Mexico. 2014. J. Travel Med 21:153–158. [DOI] [PubMed] [Google Scholar]
- [6].Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Faraq TH, Panchalingam S, Wu Y, Sow SO, Sur D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O’Reilly CE, Mintz ED, Berkely LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrheoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case control study. Lancet 2013;382:209–22. [DOI] [PubMed] [Google Scholar]
- [7].Roy K H G, Hamilton DJ, Luo J, Ostmann MM and Fleckenstein JM. Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells. Nature 2009;457:594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Freedman DJ T C, Delehanty A, Maneval DR, Nataro J, Crabb JH. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J Infect Dis 1998;177:662–7. [DOI] [PubMed] [Google Scholar]
- [9].Savarino SJ, Riddle M, Tribble DR, Porter CK, O’Dowd A, Cantrell JA, Sincock SA, Poole ST, DeNearing B, Woods CM, Kim H, Grahek SL, Brinkley C, Crabb JH, Bourgeois AL. Prophylactic efficacy of hyperimmune bovine colostral antiadhesin antibodies against enterotoxigenic Escherichia coli diarrhea: a randomized, double-blind, placebo-controlled phase 1 trial. J Infect Dis 2017;216:7–13. [DOI] [PubMed] [Google Scholar]
- [10].Li YF P S, Rasulova F, McVeigh AL, Savarino SJ, Xia D. Crystallization and preliminary X-ray diffraction analyses of several forms of the CfaB major subunit of enterotoxigenic Escherichia coli CFA/I fimbriae. Acta Crystallogr Sect F Struct Biol Cryst Commun 2009;65:242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li YF, Poole S, Nishio K, Jang K, Rasulova F, McVeigh A, et al. Structure of CFA/I fimbriae from enterotoxigenic Escherichia coli. Proceedings of the National Academy of Sciences, USA 2009;106:10793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sakellaris H, Munson G, Scott JR. A conserved residue in the tip proteins of CS1 and CFA/I pili of enterotoxigenic Escherichia coli that is essential for adherence. Proc Natl Acad Sci USA 1999;96:12828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Prouty M, Poole S, Maciel M, Liu Y, Ramakrishnan A, McVeigh A, Fordyce A, Reynolds N, Simons M, Poncet D, Dinadayala P, Joseph S, Riddle M, Heinrichs J, Renauld-Mongenie G, Savarino S. Development of a recombinant CS6 based ETEC vaccine. Vaccines for Enteric Diseases, Albufeira, Portugal, October 2017. [Google Scholar]
- [14].Clark CG, Price L, Ahmed R, Woodward DL, Melito PL, Rodgers FG, Jamieson F, Ciebin B, Li A, Ellis A. Characterization of waterborne-outbreak associated Campylobacter jejuni, Walkerton, Ontario. Emerg Infect Dis 2003;9:1232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Korlath JA, Osterholm MT, Judy JA, Forfang JC, Robinson RA. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J Infect Dis 1985;152:592–6. [DOI] [PubMed] [Google Scholar]
- [16].Allos BM. Association between Campylobacter infection and Guillain Barre Syndrome. J Infect Dis 1997;176 (Suppl 2):S125–8. [DOI] [PubMed] [Google Scholar]
- [17].Ang CW, Lamn JD, Willison HJ, Wagner ER, Endtz HP, DeKlerk MA, Tio-Gillen AP, Van den Braak N, Jacobs PC, Van Doorn PA. Structure of Campylobacter jejuni lipopolysaccharide determines antiganglioside specificity and clinical features of Guillain Barre and Miller Fisher patients. Infect Immun 2002;70:1202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. A phase variable capsule is involved in virulence of Campylobacter jejuni 81–176. Mol Microbiol 2001;40:769–77. [DOI] [PubMed] [Google Scholar]
- [19].Maue AC, Mohawk KL, Giles DK, Poly F, Ewing CP, Jiao Y, Lee G, Ma Z, Monteiro MA, Hill CL, Ferderber JS, Porter CK, Trent MS, Guerry P. The polysaccharide capsule of Campylobacter jejuni modulates the host immune response. Infect Immun 2013;81:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pequegnat B, Laird RM, Ewing CP, Hill CL, Omari E, Poly F, Monteiro MA, Guerry P Phase variable changes in the position of O-methyl phosphoramidate modifications on the polysaccharide capsule of Campylobacter jejuni modulate serum resistance. J Bacteriol 2016;199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van Alphen LB, Wenzel CQ, Richards MR, Fodor C, Ashmus RA, Stahl M, Karlyshev AV, Wren BW, Stinzi A, Miller WG, Lowary TL, Szymanski CM. Biological roles of the O-methyl phosphoramidate capsule modification in Campylobacter jejuni. Plos One 2014;9:e87051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rose A, Kay E., Wren BW, Dallman MJ The Campylobacter jejuni NCTC 11168 capsule prevents excessive cytokine production by dendritic cells. Med Microbiol Immunol 2011;201:137–44. [DOI] [PubMed] [Google Scholar]
- [23].Monteiro MA, Baqar S, Hall ER, Chen YH, Porter CK, Bentzel DE, Applebee L, Guerry P. A capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. Infect Immun 2009;77:1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Penner JL and Hennesy JN. Passive hemaglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat stable antigens. J Clin Microbiol 1980;12:732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine 2016:2887–94. [DOI] [PubMed]
- [26].Livio SSN, Panchalingam S, Tennant SM, Barry EM, Marohn ME, et al. Shigella isolates from the Global Enteric Multicenter Study inform vaccine development. Clin Infect Dis 2014;59:933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cohen D, Green M, Block C, Rouach T, Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis 1988;157:1068–71. [DOI] [PubMed] [Google Scholar]
- [28].Levine M, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol 2007;5:540–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kotloff KLSJ, Pasette M, Sztein MB, Wooden Sl, Livio S et al. Safety and immunogenicity of DVD 1208S, a live, oral guaBA sen set Shigella flexneri 2a vaccine grown on animal-free media. Hum Vaccines 2007;3:268–75. [DOI] [PubMed] [Google Scholar]
- [30].Venkatesan MM R R Live-attenuated Shigella vaccines. Expert Rev Vac 2006;5:669–86. [DOI] [PubMed] [Google Scholar]
- [31].Orr N K N, Atsmon J, Radu P, Yavzori M, Halperin T et al. Community based safety, immunogenicity and transmissibility study of the Shigella sonnei WRSS1 vaccine in Israeli volunteers. Infect Immun 2005;73:8027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pozsgay V, Chu C, Pannell L, Wolfe J, Robbins JB, Schneerson R. Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proc Natl Acad Sci U S A 1999;96:5194–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Belot F, Guerreiro C, Baleuz F, Mulard LA. Synthesis of two linear PADRE conjugates bearing a deca-or pentadecasacchride B epitope as potential synthetic vaccines against Shigella flexerni 2a. Chemistry-Eur J 2005;11:1625–35. [DOI] [PubMed] [Google Scholar]
- [34].Phalipon A, Tanguy M, Grandjean C, Guerreiro C, Belot F, Cohen D, Sansonetti PJ, Mulard LA. A synthetic carbohydrate-protein conjugate vaccine candidate against Shigella flexneri 2a infection. J Immunol 2009;182:2241–7. [DOI] [PubMed] [Google Scholar]
- [35].Kampf MM, Braun M, Sirena D, Ihssen J, Thony-Meyer L, Ren Q. In vivo production of a novel glycoconjugate vaccine against Shigella flexneri 2a in recombinant Escherichia coli: identification of stimulating factors for in vivo glycosylation. Microb Cell Fact 2015;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Turbyfill K, Kaminski R, Oaks EV. Immunogenicity and efficacy of highly purified invasin complex vaccine from Shigella flexneri 2a. Vaccine 2008;26:1353–64. [DOI] [PubMed] [Google Scholar]
- [37].Prymula R, Peeters P, Chrobok V, Friz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels JP, Schuerman L. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a double blind efficacy study. Lancet 2006;367:740–8. [DOI] [PubMed] [Google Scholar]
- [38].Yang HH, Mascuch SJ, Madoff LC, Paoletti LC. Recombinant group B Streptococcus alpha-like protein 3 is an effective immunogen and carrier protein. Clin Vaccine Immunol 2008;15:1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Baraldo K, Mori E, Bartoloni A, Petracca R, Giannozzi A, Norelli F, Rappuoli R, Grandi G, Del Giudice G. N19 polyepitope as a carrier for enhanced immunogenciity and protective efficacy of meningococcal conjugate vaccines. Infect Immun 2004;72:4884–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Szu SC, Li XR, Schneerson R, Vickers JH, Bryla D and Robbins JB. Comparative immunogenicities of Vi polysaccharide protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high or lower molecular weight Vi. Infect Immun 1989;57:3823–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 2004;25:1605–12. [DOI] [PubMed] [Google Scholar]
- [42].Li YF, Poole S, Rasulova F, Esser L, Savarino SJ, Xia D. Crystallization and preliminary X-ray diffraction analysis of CfaE, the adhesive subunit of the CFA/I fimbriae from human enterotoxigenic Escherichia coli. Acta Crystallogr Sect F Struct Biol Cryst Commun 2006;62:121–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hardy SJ, Holmgren J, Johansson S, Sanchez J, Hirst TR. Coordinated assembly of multisubunit proteins: oligomerization of bacterial enterotoxins in vivo and in vitro. Proc Natl Acad Sci USA 1988;85:7109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kanipes MI, Tan X, Akelaitis A, Li J, Rockabrand D, Guerry P, Monteiro MA. Genetic analysis of lipooligosaccharide core biosynthesis in Campylobacter jejuni 81–176. J Bacteriol 2008;190:1568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Poly F, Read TD, Chen YH, Monteiro MA, Serichantalergs O, Pootong P, Bodhidatta L, Mason CJ, Rockabrand D, Baqar S, Porter CK, Tribble D, Darsley M, Guerry P. Characterization of two Campylobacter jejuni strains for use in experimental infection studies. Infect Immun 2008;76:5655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mills J, Venkatesan MM, Baron LS, Buysse JM. Spontaneous insertion of an IS1-like element inot the virF gene is responsible for avirulence in opaque colonial variants of Shigella flexneri 2a. Infect Immun 1992;60:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].McConnell MM T L, Willshaw GA, Smith HR, Rowe B. Gentic control and propterties of coli surface antigens of colonization factor antigen IV (PCF8775) of enterotoxigenic Escherichia coli. Infect Immun 1988;56:1974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ghosal A B R, Banerjee R, Ganguly S, Yamasaki S, Ramamurthy T, Hamabata T, Chatterjee NS. Characterization and studies of the cellular interaciton of native colonization factor CS6 purified from a clinical isolate of enterotoxigenic Escherichia coli. Infect Immun 2009;77:2125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ma Z, Bertolo L, Arar S, Monteiro MA. TEMPO-mediated glycoconjugation: a scheme for the controlled synthesis of polysaccharide conjugates. Carbohy Res 2011;346:343–7. [DOI] [PubMed] [Google Scholar]
- [50].Leyva A, Quintana A, Sanchez M, Rodriguez EN, Cremata J, Sanchez JC. Rapid and sensitive anthrone-sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: method development and validation. Biologicals 2008;36:134–41. [DOI] [PubMed] [Google Scholar]
- [51].Nahm MH, Yu J, Weerts HP, Wenzel H, Tamilselvi CS, Chandrasekaran L, et al. Development, interlaboratory evaluations, and application of a simple, high-throughput Shigella serum bactericidal assay. mSphere 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Riddle MS K R, Di Paolo C, Porter CK, Gutierrez RL, Clarkson KA, Weerts HE, Duplessis C, Castellano A, Alaimo C, Paolino K, Gormley R, Gambillara, Fonck V Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: a single-blind, randomized phase I study. Clin Vaccine Immunol 2016;23:908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Frederick DR, Goggins JA, Sabbagh LM, Freytag LC, Clements JD, McLachlan JB. Adjuvant selection regulates gut migration and phenotypic diversity of antigen-specific CD4(+) T cells following parenteral immunization. Mucosal Immunol 2018;11:549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Isidean SD, Riddle MS, Savarino SJ, Porter CK. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 2011;29:6167–78. [DOI] [PubMed] [Google Scholar]


