Abstract
The use of biomaterials has substantially contributed to both our understanding of tumorigenesis and our ability to identify and capture tumour cells in vitro and in vivo. Natural and synthetic biomaterials can be applied as models to recapitulate key features of the tumour microenvironment in vitro, including architectural, mechanical and biological functions. Engineered biomaterials can further mimic the spatial and temporal properties of the surrounding tumour niche to investigate the specific effects of the environment on disease progression, offering an alternative to animal models for the testing of cancer cell behaviour. Biomaterials can also be used to capture and detect cancer cells in vitro and in vivo to monitor tumour progression. In this Review, we discuss the natural and synthetic biomaterials that can be used to recreate specific features of tumour microenvironments. We examine how biomaterials can be applied to capture circulating tumour cells in blood samples for the early detection of metastasis. We highlight biomaterial-based strategies to investigate local regions adjacent to the tumour and survey potential applications of biomaterial-based devices for diagnosis and prognosis, such as the detection of cellular deformability and the non-invasive surveillance of tumour-adjacent stroma.
Tumours are complex and heterogeneous structures. Understanding tumour progression and cancer metastasis requires the investigation of not only the tumour itself but also of the dynamic and reciprocal interactions between cancer cells and the adjacent tumour stroma, that is, the tumour microenvironment (or niche). This microenvironment is very heterogeneous but generally contains certain cell types (for example, cancer-associated fibroblasts (CAFs)), extracellular matrix (ECM) proteins and signalling molecules, which change as tumours grow and metastasize throughout the body (BOX 1). The tumour microenvironment properties are modulated, in part, as a result of alterations to the 3D fibrillar ECM that surrounds tumour tissue and to the 2D basement membrane that underlies epithelia. For example, the ECM can be modified by CAFs1,2 and tumour cells alike, causing the matrix to become stiffer3, more dense4, crosslinked5, aligned3 and less porous5. In the case of larger breast tumours, patients can actually feel the stiffened tumour stroma.
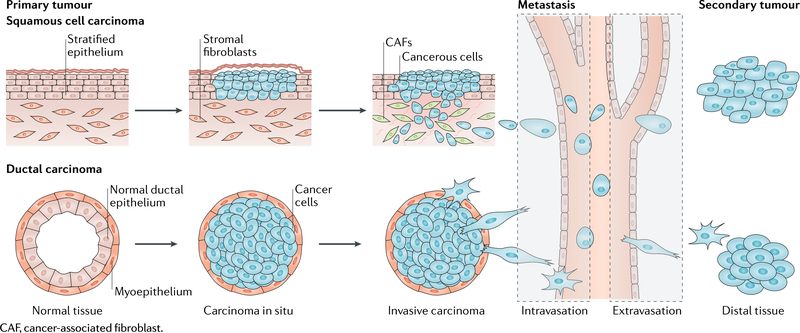
Box 1 |. Cancer and metastasis.
Squamous and ductal carcinoma share basic stages of cancer metastasis. These cancers originate from epithelial cells, which line surfaces and vessels of the body.
Primary tumour
The mutation of a single cell leads to uncontrolled division, resulting in an excess of abnormal cells. As the mass grows, the cells can acquire additional mutations and remodel the surrounding tissue, forming a primary tumour. tumours are heterogeneous and often lack the polarity and cellular organization of the original tissue.
Epithelial-to-mesenchymal transition
Epithelial-to-mesenchymal transition (EMT) is a cellular programme that causes cells within a primary tumour to lose characteristic cell-cell adhesions, to break the basement membrane associated with an epithelial phenotype, to transition to a mesenchymal phenotype that lacks cell polarity and to upregulate and/or activate specific transcription factors, such as Twist family bHLH transcription factor 1 (TWiSTl). The EMT programme enables cells of the primary tumour to locally invade the surrounding stroma and is characterized by a shape change of the cells in the primary tumour.
Intravasation
Intravasation is the migration of cancer cells from tumour-adjacent stroma into a blood or lymphatic vessel. this is a multistep process, during which metastatic tumour cells migrate through the extracellular matrix and between cells in the vessel as well as through the water-tight junctions between endothelial cells to reach the fluid in the lumen of the vessel.
Extravasation
Extravasation is the exit of cancer cells from a blood or lymphatic vessel through the endothelial cell layer lining the vessel and into a secondary site distant from the primary tumour. This is also a multistep process, during which circulating tumour cells slow down and stop along the vessel wall through adhesion to endothelial cells. Cells break through the water-tight junctions between endothelial cells and the matrix within the vessel to invade new tissue.
Secondary tumour
A malignant tumour that grows in a secondary organ from cells originating from a primary tumour.
Animal models are powerful systems to study the dynamic stromal properties of tumours, but it is difficult to dissect the specific contributions of individual microenvironmental cues to tumour development and progression6. However, reducing the in vivo niche to its major biochemical and biophysical components offers a possibility to model the tumour microenvironment in vitro. Identifying and recreating specific aspects of the tumour stroma, for example, stiffness, topography or nutrient exchange, using biomaterials allows for the fabrication of reductionist in vitro systems to study basic mechanisms that regulate cancer cell plasticity, dissemination and repopulation of the niche (Box 2).
Box 2 |. Key aspects of biomaterials for cancer biology.
Biomaterial
A natural or synthetic substance that is compatible with biological systems. it can be engineered for research, diagnostic or therapeutic purposes.
Hydrogel
A polymer gel in which natural or synthetic hydrophilic polymers can be physically or chemically crosslinked to produce a hydrogel that contains different volume fractions of water. The physical and chemical properties of hydrogels can be modulated, for example, by altering the crosslink density or bulk polymer concentration to increase stiffness or by adding peptides or degradation enzymes.
Stiffness
The resistance of a material to deflection or deformation in response to an applied force. stiffness is a term synonymously used in the biological literature for Young’s modulus or elasticity. the stiffness of tumour tissue is higher than that of healthy stromal tissue, leading to alterations of mechanosignalling pathways in cancer cells. Therefore, it is important to model the correct stiffness of tumour tissue in vitro to recreate relevant biomaterial-based cancer models. the stiffness of tissue culture plastic (GPa) is orders of magnitude higher than that of human tissues (kPa), and the stiffness of tumours and of their adjacent stroma is usually an order of magnitude higher than that of healthy tissues; for example, the stiffness of mammary tumours is ~5 kPa, and the stiffness of adjacent stroma is ~0.1 kPa (REF3).
Topography
A parameter that corresponds to the shape and features of the surface of materials. the topography changes with the architecture of the extracellular matrix (ECM). For example, hydrogels and fibrillar matrices have generally smooth and rough topographies, respectively. increasing collagen deposition increases migration and invasion of tumour cells up to the point at which pore size becomes the limiting factor.
Porosity
Porous or empty spaces within a material are formed as a result of polymer crosslinking. in hydrogels and fibrillar matrices, pores are filled with fluid, and tumour cells can migrate through them to invade the material. the minimum size limitation for cells to pass through pores is <5 pm2 (REF23); however, cancer cells can release matrix-cleaving enzymes to degrade the ECM and make room to migrate, which can be recreated in biomaterials using enzyme-degradable peptides as crosslinkers.
Biomaterials have been used to study tumour biology since the early 1980s, when scientists questioned whether signals from the extracellular compartment could regulate cell behaviour in a distinct and/or similar way as to how genetics can dictate cell fate. In particular, seminal work demonstrating that changes to the extracellular milieu could affect gene expression in mammary glands7 has triggered unprecedented interest in how the ECM regulates cell behaviour in development. Pioneering work by the group of Mina Bissell established a ‘dynamic reciprocity’ between the cell and its microenvironment, showing that components of the ECM, such as collagen or fibronectin, associate with the plasma membrane and connect to the intracellular cytoskeleton through specific structures (later identified as focal adhesions). Signals from the ECM are then relayed to the nucleus to affect gene expression and to regulate the expression of ECM molecules or their modification through the expression of ECM-modifying enzymes. However, the detailed mechanisms of cell-ECM interactions are still under intense investigation, and much remains to be understood.
In this Review, we discuss how biomaterials can be applied to model tumours and their microenvironments in vitro. We examine different materials that can be used to capture and measure cancer cells for diagnostics and prognostics and investigate biomaterials for their potential to be used for cancer treatment in vivo.
First attempts to model the tumour ECM
Matrigel
The discovery of dynamic reciprocity was made possible, in part, through the use of tissue-derived biomaterials, which mimic an in vivo microenvironment for in vitro studies of cell-ECM interactions. Matrigel is a solubilized, gelatinous protein mixture composed of reconstituted basement membrane, which was originally isolated from Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells8,9 and is still routinely used to support the formation of epithelial structures. Matrigel mainly consists of assorted ECM proteins such as laminin, type IV collagen, heparin sulfate proteoglycans and entactin. However, Matrigel also contains growth factors that can potentially interfere with cell signalling events and thus affect the interpretation of results10. Therefore, growth factor-reduced versions of Matrigel have been developed to enable 3D cell culture characterization that focuses on the material properties alone. The use of Matrigel partly allows for the in vitro recreation of the architectural and biochemical complexity of an in vivo cell microenvironment. For example, the first 3D culture of primary mammary epithelial cells (MECs) was achieved using Matrigel, demonstrating that the basement membrane plays a crucial role for the 3D organization of MECs and for the generation of stable and functional hollow-lumen acinar structures11. 3D culture of MECs using Matrigel allows the cells to aggregate, remodel the ECM and self-organize into a layer of polarized cells — often with a hollow lumen — through the establishment of epithelial junctions and polarity. This approach enabled the first in vitro differentiated functional alveolar organoid, paving the way for morphogenesis and developmental studies in vitro using biomaterials. These recombinant basement membrane-derived systems have also been used to assess differences in gene expression profiles between cell lines12. The use of Matrigel in combination with collagen further enabled the identification of cellular differences between normal and malignant cells in 3D13.
The seed and soil hypothesis of metastasis
Originally, biomaterials were mainly used to understand how the adjacent tumour ECM regulates tum- origenesis. An equally important aspect — albeit less well studied — is the cellular and ECM composition of the microenvironment at distant sites of metastasis. The distant microenvironment was described by Stephen Paget as the ‘soil’ in his ‘seed and soil hypothesis’14. On the basis of the analysis of the data of a large cohort of patients with breast cancer, he hypothesized that the microenvironment plays a crucial role in regulating the seeding and growing of secondary tumours. Similar to disease progression-associated changes of the tumour ECM, Paget suggested that unique features of the soil can cause cancer cells to metastasize to specific locations. Stromal and immune cells are also part of the soil, migrating to distal sites prior to the arrival of tumour seeds15. Extracted stromal ECM components can further promote or prevent tumour progression16,17, demonstrating that the ECM plays a role in seed implantation and can remodel tumour stroma18. Both in the tumour microenvironment and at distant sites of metastasis, a complex network of ECM proteins contributes to tumour progression and impacts cancer cell behaviour. Natural biomaterials can be applied to recreate these microenvironments, incorporating different stromal and ECM features to improve in vitro disease models and to develop new generations of therapeutics and diagnostics. However, there is a veritable balance between preserving the native ECM structure and composition to precisely resemble the in vivo architecture and the removal of cellular and antigenic material, such as nucleic acids, membrane lipids and cytosolic proteins, to be able to reproducibly use these biomaterials in vitro. These caveats have led to the development of new natural matrices as well as synthetic hydrogels that are more reductionist than these initially used natural biomaterials.
Engineering the tumour microenvironment
Natural biomaterials
Mimicking the microenvironment of tumours requires the use of 3D rather than 2D architectures to enable morphogenesis. Collagen gels were first used as 3D scaffolds to demonstrate how normal murine MECs form lumens in 3D as opposed to monolayers on 2D substrates7, emphasizing the importance of 3D materials to recreate in vivo cell morphologies in vitro. The first ECM-specific behaviour observed using 3D biomaterials was cancer cell dissemination from tumour cell aggregates. In collagen gels, mammary carcinoma cells migrate as single cells with larger protrusions and higher local dissemination than cells embedded in Matrigel, in which cells migrate in a collective pattern19. These data indicate that protein composition of the matrix is an important property of neoplastic cell invasion. Unlike invasive carcinomas, malignant cells establish a vasculogenic network when embedded in collagen matrices with small pores and short fibres; tumours that feature such a tumour-adjacent matrix are correlated with poor prognosis. Such a short fibre-based network is not established if cells are exposed to increasing amounts of recombinant basement membrane20, and thus the vasculogenic network is not formed. This effect can be titrated, and increasing collagen concentration restores vascular network formation21.
Natural matrices containing collagen and/or recombinant basement membrane can be crosslinked or fabricated at different concentrations to modulate their stiffness and thus enable the assessment of the influence of stiffness in concert with specific genetic alterations. For example, MECs respond to increasing collagen matrix stiffness, which is achieved through adding collagen proteins, by breaking the acinar structure and invading into the ECM. If the genome of the MECs contains specific cancer-driving oncogenes, for example, receptor tyrosine-protein kinase ERBB2 (REF5), they display an even more aggressive phenotype when interacting with a stiff matrix. MicroRNAs also play a role in regulating the expression of genes that favour tumour progression and are implicated in the increased stiffness sensitivity of MECs22. In addition to stiffness, ECM porosity further plays a central role in cancer cell migration and tumour growth. Small pore sizes reduce the migration speed of cells in natural ECMs, such as collagen, by acting as barriers for nuclei deformation. A similar behaviour has been observed using synthetic materials23. However, in contrast to synthetic materials, cells can use matrix metalloproteinases (MMPs) to degrade natural ECM and increase the pore size to migrate through dense collagen gels24. Beyond a specific pore size threshold, myosin-mediated traction forces can propel the nucleus forward and allow migration through a dense ECM25,26. These data indicate that ECM fibre assembly, porosity and composition affect ECM architecture and material properties and, consequently, cancer cell migration and dissemination. However, in a natural matrix, the biochemical and biophysical parameters of the ECM cannot be decoupled; that is, individual matrix properties can only be varied relative to each other. This makes it challenging to accurately predict the impact of individual effects of natural ECMs on cell migration27,28. For example, altering ECM stiffness by adding more matrix protein also affects the adhesive properties of the matrix29. Moreover, batch-to-batch variations can influence the reproducibility of experiments; even in commercial products, such as Matrigel, variation in matrix protein composition, for example, fibronectin, can drive differences in cell behaviour30. Therefore, although natural ECM mimics the microenvironment of native tissue very well, coupled variation of ECM parameters and inconsistent composition are valid concerns.
Given these issues, a clear consensus on the relationship of migration and ECM parameters has not yet been achieved. For example, the concentration of specific ECM components has been shown to have either biphasic31 or direct32 effects on cancer cell migration. Cell contractility is also required for migration along a matrix, but how specific ECM properties guide cell contractility is still under debate. In collagen matrices, the forces generated by mammary carcinoma cells are independent of collagen concentration and matrix stiffness33. However, invasive cancer cells, which transition to a more mesenchymal phenotype with a spindle-like morphology, exhibit more processive or directed migration, making them more invasive with increasing collagen concentration34.
These (sometimes controversial) observations have also been made using pristine natural matrices made from recombinant or animal-derived proteins. A better suitable ECM model is matrix exposed to clinically relevant doses of radiation. Irradiated matrices exhibit altered structures that substantially reduce metastatic cancer cell adhesion, spreading and migration35. In addition to the interest in using more relevant and reductionist materials, there is an equal interest in moving from common cell lines to their primary human tumour cell counterparts owing to their different and potentially more relevant behaviours. Together, this has created the push to move to mainly synthetic material systems.
Synthetic biomaterials
Natural materials have been key for initial investigations of ECM and cancer, but owing to their above-mentioned disadvantages, synthetic materials are increasingly used to mimic tumour ECM (FIG. 1). Synthetic materials have the advantage that parameters can be decoupled36; tuning one parameter, such as substrate stiffness, does not affect other parameters, such as fibre architecture or pore size37 (BOX 2). They can also serve as a platform for cell adhesion by providing different ECM proteins or peptides, such as arginine-glycine-aspartic acid (RGD), glycine-phenylalanine-hydroxyproline- glycine-glutamate-arginine (GFOGER) or isoleucine- lysine-valine-alanine-valine (IKVAV), to understand how specific ECM components regulate tumorigenesis (FIG. 1 a). For example, polyethylene glycol chains decorated with peptides of laminin 1 and type I collagen, but not of fibronectin, support invasive behaviours of metastatic prostate cancer cells, which is not observed for non-metastatic cancer cell lines38. Therefore, such systems can be potentially used to separate neoplastic cells from a mixed cell population. Synthetic materials can be easily functionalized with not only adhesive ligands but also a variety of other signalling proteins and peptides; for example, materials can be crosslinked with protease-degradable linkers, thus allowing the cells to control local matrix properties in a similar way as in natural matrices39. However, synthetic materials enable variation and individual control of ECM properties, although the combination of specific properties or proteins does not necessarily result in a linear cell response30,40. For example, cancer cells show different sensitivity to combinations of matrix proteins than to the individual proteins41 and can be more or less responsive to specific matrix properties if they adhere to more or less permissive matrix proteins30.
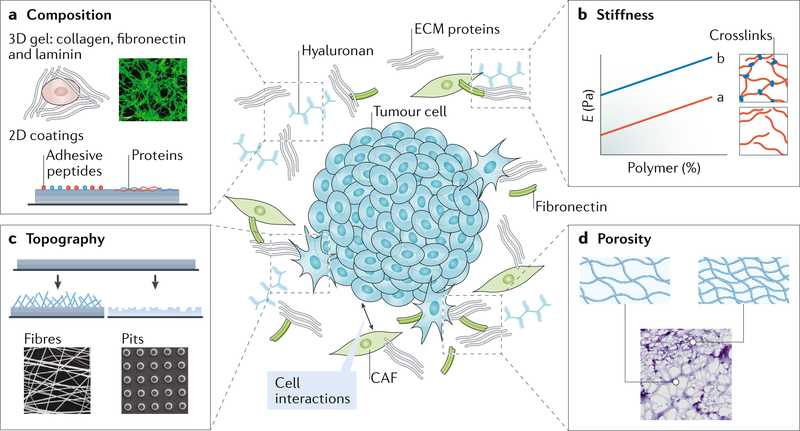
Fig. 1 |. Modelling the tumour microenvironment.
The tumour microenvironment constitutes the niche that surrounds a tumour, including extracellular matrix (ECM), cells and signalling molecules. The niche is characterized by specific dynamic ECM properties. a | The composition of the ECM can vary in terms of both ligand type and ligand presentation. 3D hydrogels made of ECM proteins or 2D materials can be used to recreate a specific ECM composition. The ligand type41 and concentration3 affect cell behaviour and can induce an epithelial-to-mesenchymal transition (EMT). b | Stiffness, that is, the Young’s modulus, can also impact EMT3,45. The Young’s modulus (E) of a material can be modified by changing chain entanglements (line a) or crosslinking (line b). The stiffness is measured as the force per cross-sectional area of the material. c | Topographical features of the niche can be recreated by spinning polymers into fibres and depositing them as a thin layer on a surface, to which a cell can adhere. Alternatively, a material can be etched to create specific nanotopographical or microtopographical features, such as pits. Such topographies can be used to induce cell transformations58–62 or to capture cancer ceils82,85,86. d | The pore size and pore connectivity of the tumour microenvironment can be modelled by modulating bulk polymer density or droplet size in emulsions. Non-malignant cells are highly sensitive to pore size63,64; materials with small pores can inhibit migration and proliferation, and large pores are felt by the cells as 2D surfaces. CAF, cancer-associated fibroblast.
Modulating matrix stiffness.
A breast tumour mass is routinely identified by manual palpation; the patient or doctor identifies a stiff lump relative to the compliant surrounding tissue. In epithelial tumours, a direct correlation between stiffness and metastatic potential has been reported3,5,42–45; however, this correlation has not been observed in all animal models46. To tune stiffness in natural ECMs, matrix concentration is increased, which also affects porosity and ligand density3. By contrast, in synthetic materials, changing crosslink density or bulk polymer concentration allows for the variation of stiffness by several orders of magnitude without modifying adhesion ligand density47 (FIG. 1 b). Most epithelial tumour models use a combination of naturally derived or natural and synthetic matrices in 3D48,49. These approaches using materials with increasing stiffness have been applied to study the mechano sensitivity of mammary epithelia during their transition to a mesenchymal phenotype, that is, the epithelial-to-mesenchymal transition (EMT). A stiff matrix triggers focal adhesion assembly through stress-induced elastic deformation, which in combination with cell contractility activates extracellular signal-regulated kinase (ERK) and the RHO family of GTPases, driving MECs towards EMT3 (FIG. 2). Increasing matrix stiffness also triggers the release of the EMT transcription factor Twist family bHLH transcription factor 1 (TWIST1) from its cytoplasmic binding partner RAS GTPase-activating protein-binding protein 2 (G3BP2), its translocation to the nucleus and initiation of an EMT transcription programme45.
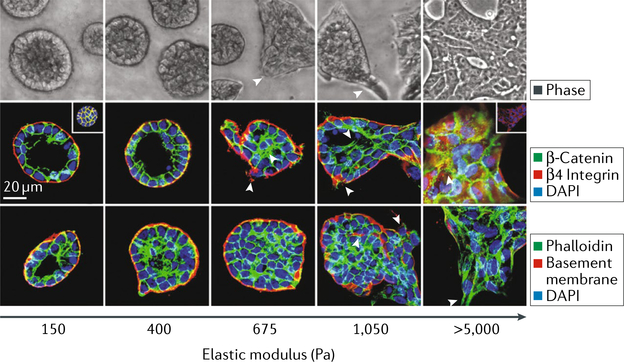
Fig. 2 |. Matrix stiffness regulates the epithelial-to-mesenchymal transition.
Phase contrast and fluorescent images of mammary epithelial ceLL colonies on polyacrylamide hydrogels of indicated stiffness (150–5,000 Pa) with Matrigel overlay are shown. Microscopy images show colony morphology after 20 days. The fluorescent images show β-catenin (green) before and after (inset) triton extraction, β4 integrin (red), epithelial cadherin (E-cadherin) (red; inset) and nuclei (blue). In the bottom images, actin (green), laminin 5 (basement membrane; red) and nuclei (blue) are shown. DAPI, 4’,6-diamidi- no-2-phenylindole. Figure is reproduced with permission from REF.3, Elsevier.
Additional evidence suggests that hydrogel stiffness regulates not only malignant transformation but also dissemination and migration of invading cancer cells50. Metastatic cells have tumour-specific stiffness preferences; at an optimal stiffness, corresponding to the stiffness of a specific tumour type, they express markers consistent with highly migratory cells and migrate faster than at sub-optimal stiffness51.
Synthetic materials can also be designed as dynamic systems, in which crosslinking can be gradually52,53 changed or modified on demand44,54,55, thus better mimicking slow disease progression. Collective cancer cell behaviours can be substantially different in materials that stiffen following polarization than in materials with static stiffness56. Controlled degradation57 can also provide a strategy to examine cell behaviour in response to an environment that becomes increasingly softer and to identify mechanotransduction pathways that can slow tumorigenesis. Therefore, matrix stiffness and the timing of its presentation are important ECM properties that influence neoplastic cell behaviour.
Fibre architecture, topography and porosity.
The architecture and topography of ECM fibres also affect the behaviour of neoplastic cells. Cancer cells can sense whether the surface is atomically flat or has a roughened topography (FIG. 1 c), which can induce invasion and metastasis. For example, fibrillar matrix structures can be synthetically recreated using electrospun fibres, such as silk, to support 3D cell migration of both malignant and non-malignant cell lines58,59. Alternatively, poly- dimethylsiloxane (PDMS) is a commonly used polymer for topographical studies. Using patterned PDMS substrates, it has been shown that neoplastic cells are less sensitive to geometrical cues than non-malignant cells60,61. On micrografted surfaces, MECs enter a dormant state, whereas their neoplastic counterparts continue to proliferate through a RHO-RHO-associated protein kinase (ROCK)-myosin-dependent pathway62. This principle also extends to other roughened surfaces, on which malignant cells appear less sensitive and continue to grow and migrate independent of roughness60,62.
Similarly, ECM porosity, which dictates cell spreading, can differentially affect non-malignant and metastatic cells (FIG. 1 d). For example, metastatic cells can migrate through PDMS channels that are smaller than the diameter of their nuclei by breaking and reforming their nuclear envelope23. 3D material systems containing collagen and agarose can be used to independently modulate stiffness, porosity and ligand density. If the porosity of the material is decreased independent of other properties, glioblastoma cell migration is steri- cally hindered63. Conversely, non-malignant cells sense porosity together with other properties, such as stiffness; for example, in channels of decreasing width, the migration speed of non-malignant cells increases with stiffer channel walls64. These data suggest complex and often coupled interactions and therefore do not yet allow an overarching conclusion or propose the ideal material for modelling the tumour microenvironment. However, individual ECM properties have already been identified that can be modulated using biomaterials to study their effects on cancer cells (TABLE 1).
Table 1 |.
Biomaterials for modelling the tumour microenvironment
| Biomaterial | Model | Advantages | Disadvantages | Refs |
|---|---|---|---|---|
| Synthetic material | ||||
| Polydimethylsiloxane (PDMS) | 2D micropatterns | Flexible substrate with patterns promoting cancer cell alignment | Uncertain viscoelastic mechanics and protein attachment | 37,148 |
| Microchannels and microfluidics | • Directed cell migration in channels • Cell confinement • Confined fluid flow for controlled application of shear stress to cells |
• Curing ratios often create materials that are less flexible • Static substrate |
23,114 | |
| Polyethylene glycol diacrylate (PEGDA) | 3D culture | • Wide stiffness range • Direct conjugation of many types of adhesive ligands • Can be used to identify tumour-specific stiffness |
– | 51 |
| Polyethylene glycol (PEG) | 3D culture | • Wide stiffness range • Direct conjugation of many types of adhesive ligands or degradable linkers • Inert and biocompatible |
Backbone is not degradable | 39,149,150 |
| Poly(lactide-co-glycolide) acid (PLGA) | 3D culture | • Porous scaffold • Biocompatible • Biodegradable |
Methyl side groups increase hydrophobicity | 151,152 |
| Implantable material | Recruitment and capture of metastatic cells | Degradation prior to cell capture | 93 | |
| Poly(ε-caprolactone) (PCL) | Implantable material | Recruitment and capture of metastatic and immune cells | Degradation prior to cell capture | 92 |
| Polyacrylamide | Substrate gradients | • Sequential polymerization to create spatial patterns • Indication of metastatic cell ‘memory’ • Small well polymerization for high-throughput drug screens |
Substrate stiffness does not change with time | 153–155 |
| Used with Matrigel overlay for 3D culture | • Wide stiffness range • Conjugation of individual or multiple ligands resulting in nonlinear cell responses |
• Cytotoxic prior to polymerization, preventing cell encapsulation • Difficult to measure forces in 3D |
3,30,40,45 | |
| Elastic 2D substrate | Measurement of traction forces in cancer cells | • Cytotoxic prior to polymerization, preventing cell encapsulation • Difficult to measure forces in 3D |
42 | |
| Synthetic-natural hybrid materials | ||||
| Polyethylene glycol-heparin | 3D culture | • Direct conjugation of adhesive ligands • Enzymatically degradable |
Limited degradation control | 38 |
| Methacrylated hyaluronic acid (MeHA) | 3D culture | • Direct conjugation of adhesive ligands • Enzymatically degradable • Temporal gradients through sequential crosslinking |
• Radical polymerization limits in vivo application • Can induce DNA damage • Modifications can reduce bioactivity |
44,54,55 |
| Natural materials | ||||
| Matrigel | 3D culture | • Established fibrillar model system • Temperature-based polymerization • Easy encapsulation methods • Growth factor-reduced version • 3D organization of acinar structures |
• Batch-to-batch variation • Difficult to independently modulate parameters • Tumour-derived (inductive composition) • Temperature sensitive |
8,9,11,30 |
| Alginate | 3D culture | • Stiffness can be modulated independently of architecture • Time-dependent stiffening with calcium crosslinking • Enables mammary epithelial cells to polarize before EMT |
Calcium-dependent covalent bonds | 56,156 |
| Type I collagen | 3D culture | • Fibrillar • Adhesion of multiple cell types • Facilitates cell invasion • Shows same radiation damage as tumours |
• Transglutaminases and oxidases can crosslink with limited range Harsh organics are more common crosslinkers with a wider range • Limited stiffness range of ~1–1,000 Pa |
23,35,69,157 |
| Matrigel-impregnated | Migration is biphasic and directly dependent on concentration | • Pore size changes with Matrigel concentration • Limited ligand presentation |
31,32 | |
| Agarose-impregnated | • Stiffness can be modulated independently of ligand density • Restricted invasion of glioma cells |
Pore size changes with agarose concentration | 63 | |
EMT, epithelial-to-mesenchymal transition.
Model requirements beyond materials
Tumours are often described as organs that contain different cell types, including CAFs65, endothelial cells, pericytes and immune inflammatory cells66. The vast majority of biomaterial-based models are incomplete because they do not incorporate these important cell types that modify the microenvironment. Cancer cells secrete soluble factors that activate CAFs, leading to a change in CAF protein expression and an increase in MMP secretion and CAF contractility67–69. CAF- generated forces promote angiogenesis70 and generate holes in the matrix to facilitate cell invasion69. CAFs can also directly bind to cancer cells through heterotypic epithelial cadherin (E-cadherin; also known as CDH1) and neural cadherin (N-cadherin; also known as CDH2) junctions and pull cancer cells away from the tumour71. CAF contractility further promotes the nuclear translocation of Yes-associated protein YAP65 homologue (YAP1), which in turn results in matrix stiffening, angiogenesis and cancer cell invasion. This positive feedback loop drives tumour progression72. However, most current biomaterial approaches to the niche lack these important interactions and signalling events.
Metastasis of cancer cells further depends on the ability of cancer cells to migrate through the stroma, intravasate blood vessels, survive in the circulation and extravasate into new matrix to colonize distant tissues (BOX 1). Although no hydrogel system to date mimics all these stages, materials-based microphysiological systems have been explored to mimic specific steps in this process, such as extravasation, in which cancer cells pass through the endothelium; for example, microphysiological systems can be fabricated using PDMS to engineer a perfusable microvascular network with hydrogel regions and media channels. Such systems are thin and composed of neo-vessels, allowing imaging analysis to study transendothelial migration73. By applying this in vitro approach, it has been shown that tumour necrosis factor (TNF)-a increases endothelial cell permeability, facilitates tumour cell intravasation74 and modulates extrava- sation75,76. Microphysiological systems can also be used to investigate metastasis of certain cancer cells to specific secondary sites. For example, a microenvironment containing osteoblasts can be used to elucidate why breast cancer cells preferentially metastasize to bone. A higher number of breast cancer cells extravasate into the bone cell-conditioned microenvironment than into a collagen matrix, suggesting that bone-secreted chemokines such as CXC-chemokine ligand 5 (CXCL5) play a role in the chemotactic migration of breast cancer cells77. These systems enable the investigation of the contribution of specific families of cell-secreted cytokines to cancer cell metastasis, which is difficult to dissect in animal models. Further development of microfluidic devices and incorporation of various materials will make in vitro models increasingly relevant for cancer biologists as reductionist systems to recreate more steps of the metastatic process within one system.
Capturing cells in blood and stroma
Biomaterials can be applied for diagnostic and prognostic screening of cancer in vivo and ex vivo (FIG. 3). The current standard of care primarily consists of regular screenings, such as mammograms for breast cancer, flexible sigmoidoscopy or faecal occult blood test for colorectal cancer78 and computed tomography and chest radiography scans for lung cancer79. However, by the time the disease is observable, the tumour has often already metastasized. To detect tumours in patients earlier and more accurately, biopsy samples can be taken and genetically tested for prognostic markers, for example, breast cancer markers breast cancer type 1 susceptibility protein (BRCA1) and ERBB2 by using mRNA microarrays80. Such assays have dramatically reduced cancer occurrence; however, they do not directly detect disease-causing cells.
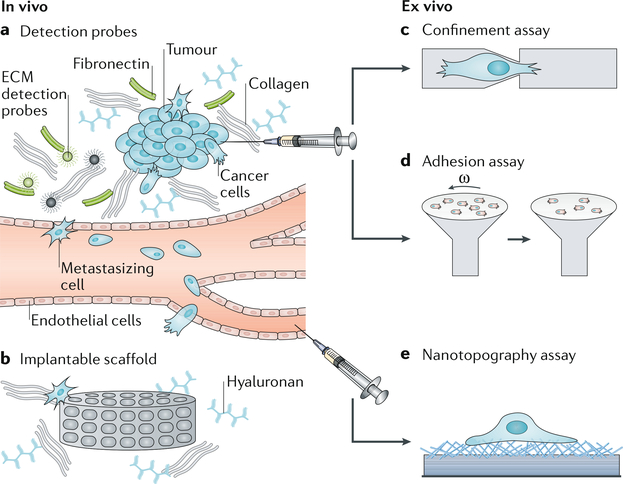
Fig. 3 |. Next-generation material-based cancer technologies.
The specific interactions between cancer cells and the tumour stroma can be exploited for the detection of cancer cells. a | Magnetic resonance imaging (MRI) or positron emission tomography (PET) contrast agents can be conjugated with extracellular matrix (ECM)-affinity peptides to create specific probes to target the dense ECM of the tumour stroma for the detection of mature tumours in vivo. b | Implantable scaffolds can be used to recreate a pre-metastatic niche at the implant site, recruiting cells for capture and therapy and at the same time lowering the tumour burden in typical secondary metastasis sites. c,d | Confinement assays or adhesion assays can be applied to test cells obtained from tumour biopsy samples for their aggressiveness by measuring cellular deformation or adhesion to specific ECM molecules. Omega (ω) is the angular velocity that defines the shear stress applied to cells. e | Circulating tumour cells (CTCs) can be isolated from patient blood samples using nanotopography assays that take advantage of the affinity of CTCs for nano-roughed substrates.
Biomaterial-based technologies have primarily focused on capturing circulating tumour cells (CTCs). CTCs are a small fraction of cells that disseminate from primary tumours and are thought to be responsible for the haematogenic spread of cancer to distant sites81,82. Increased CTC levels in the blood are correlated with negative prognosis. Therefore, CTC isolation and quantification are essential for the early detection of metastasis and subsequent treatment82. However, CTCs are difficult to isolate with high efficiency and purity81 and thus their unique molecular signatures remain elusive82. The most commonly used CTC isolation method relies on increased epithelial cell adhesion molecule (EPCAM) expression on the surface of CTCs81, which is used by the US Food and Drug Administration (FDA)-approved CellSearch System. However, this system requires a very large sample volume, has low sensitivity and is time consuming81.
Ex vivo detection using nanotopographies
CTC capture efficiency can be improved by increasing the local concentration of capture substrate or by coupling the substrate with surface-functionalizing molecules, such as antibodies or aptamers. For example, microfluidic chip assays composed of PDMS microposts with a surface coating of anti-EPCAM antibody can concentrate CTCs in smaller sample vol- umes79 than systems without antibody coating. Silicon nanopillars further improve CTC capture by clustering antibodies through binding to streptavidin or gold83. Aptamer-functionalized gold nanopillar arrays show efficient cell release through cleavage of the sulfur-gold bonds between the aptamers and the gold nanopillars84.
CTC purification and capture can also be achieved using artificial nanoscale topographies, mimicking structural features and dimensions of ECM81. Cancer cells preferentially adhere to nanostructured rough substrates compared with smooth substrates, even in the absence of surface functionalization with antibodies82. For example, fractal nanostructures have an uneven topography and a crystalline structure, which increase cancer cell binding to the surface85,86. Fractal nanostructures can be generated from synthetic materials, such as TiO2, with inverse opal photonic crystals to mimic cellular components or natural materials, such as hydroxyapatite nanostructures of seashells86,87. Alternatively, rough nanoscale substrates can be fabricated with an anti-EPCAM antibody-coated, mesh-like silicon nanowire substrate and overlaid with a PDMS-based chaotic mixer88,89. These systems show a >95% capture efficiency of EPCAM-positive MCF7 breast cancer cells, which is more than 20-fold higher than EPCAM antibody-coated smooth substrates90,91. The addition of electrospun thermoresponsive nanofibres enables an even higher capture efficiency and allows on-demand release and single-CTC analysis, for example, for next-generation sequencing88. Cell release can also be achieved by using degradable zinc-phosphate nanosubstrates92.
Nanostructured surfaces enable high capture efficiency but cannot provide high cell purity owing to nonspecific cell adhesion. Dual-functional lipid coating can be applied to improve the capture specificity of nanopillars owing to the higher concentration of antibody on the surface and inhibition of nonspecific cell adhesion93. Poly(carboxybetaine methacrylate) brushes also decrease nonspecific cell adhesion, and the active carboxyl groups capture CTC-specific biomolecules94. These nanostructure-based methods enable ex vivo detection of CTCs, demonstrating how specific ECM properties, such as topography, can be exploited to increase capture efficiency and provide a strategy for proactive disease monitoring. It has been suggested that CTC detection probability scales with patient mortality79 and, thus, technologies for the continuous detection of CTCs could provide a strategy to detect cancer cell metastasis early enough to substantially increase patient survival.
In vivo cell detection using implantable materials
Biomaterials can also be implanted to monitor tumour progression in vivo95,96. According to Paget’s seed and soil hypothesis, secondary metastases do not occur randomly14. Specific microenvironments are primed for tumour cell colonization through the presence of tumour-supportive fibroblasts, endothelial progenitor cells, immune cell-secreted factors and ECM-remodelling events95–97. Current imaging techniques are limited in their ability to detect micrometastases that form at distal sites95–97, which reduces their prognostic capabilities and offers an area of opportunity for biomaterial-based solutions.
For example, microporous scaffolds such as poly(lactide-co-glycolide) acid (PLGA) can be implanted to recruit and capture metastasized cells. Breast cancer cells that have metastasized to the brain can be injected into the fat pads of mice and entrapped in an implanted PLGA scaffold. Mice with scaffolds implanted to capture circulating cells develop fewer lung tumours96 than animals without any implanted material, indicating that the scaffolds reduce secondary metastases formation. Poly(ε-caprolactone) (PCL) has similar physical properties to PLGA but degrades more slowly95. PCL scaffolds can also be used to recruit tumour and immune cells, which are implicated in establishing a pre-metastatic niche, and to decrease the number of detectable tumour cells in common secondary sites95,98–100. Additional modifications, such as graphene oxide (GO) functionalization, can further increase cancer cell adhesion compared with non-functionalized scaffolds101. GO addition to the scaffold can also enable photothermal ablation of cancer cells within the scaffold owing to the near-infrared absorbance of GO101,102, demonstrating how implantable scaffolds can be used for both cancer cell capture and therapy. Besides chemical modifications, scaffolds can also be coated with ECM proteins, including fibronectin and type IV collagen, to improve scaffold capture efficiency. Each tumour type is characterized by specific ECM combinations and thus scaffolds can be coated with a tumour-specific ECM that supports metastases41 to improve cancer cell recruitment. For example, coating with decellularized lung or liver matrix of metastatic tumours substantially increases capture efficiency97.
Matrix is not the only niche component that can be used to improve cell capture. Cancer cell-secreted exosomes or haptoglobin can also be incorporated into synthetic scaffolds to create a bioengineered niche that captures metastatic cells more effectively than tissues to which cells commonly metastasize and increases survival in animals implanted with these scaffolds103,104. Natural materials such as silk can also be functionalized with proteins, such as bone morphogenetic protein 2 (BMP2), to mimic a bone marrow microenvironment. This material can serve as a surrogate for a pre-metastatic niche and recruit metastasizing cancer cells that would normally home to bone marrow105. In particular, BMP2 increases the adhesion of metastatic prostate and breast cancer cell lines to the scaffold105,106. Such scaffolds can be implanted to capture tumour cells, reduce the tumour burden on standard metastatic organs and prevent the local remodelling of tissue into a pre-metastatic niche, making them potent therapeutic tools to detect, capture and ablate metastasized cancer cells. However, these scaffolds do not have an inherent proclivity to capture specific cell types.
Ex vivo cell detection using physical properties
Cells migrate through the stromal ECM through confined pores, which can be smaller than the nucleus of the cell. To achieve this, cells can either degrade adjacent matrix using MMPs24 or physically deform it107. Increased MMP expression and decreased nuclear size108,109 are associated with aggressive cancers and thus cell deformability is emerging as a marker for the invasive potential of cancer cells110. Assays for the investigation of cellular deformability exploit the variable pore size in the ECM to shed light on the relationship between the degree of deformation and the corresponding invasive and metastatic potential. The most common strategy is to micro-fabricate channels — for example, in PDMS — with defined geometries and track cellular movement. Cells with low expression of nuclear lamina proteins, which contribute to nuclear stiffness, pass more quickly through narrow regions107 than cells with high lamin A and/or lamin C expression and stiff nuclei. Specific deformation tolerances can be assessed using funnel-shaped constrictions in series111 or in parallel to analyse cell transition effects112. Metastatic cells modulate their morphology, as they are forced into confined spaces more than their non-metastatic counterparts, resulting in faster and larger deformation events112. Highly metastatic cells can even rupture and reassemble their nuclear envelops when they encounter transit constrictions23. Intravasation constitutes one of the most restrictive parts of the journey of a metastasizing cell. Microfluidic devices with cell and nutrient chambers separated by microchannels of varying width can be used to determine the minimum gap that cancer cells can migrate through in confined environments. Such a device has been applied to demonstrate that the nucleus is a crucial limiting factor for a cell to be able to traverse confined environments113.
Constrictive devices rely on cell-generated forces; alternatively, external hydrodynamic forces can be applied to deform cells. Opposing flows, that is, hydrodynamic stretching, can uniformly deform cells, and the degree of deformation can be controlled by simply changing the flow rate114 or through pinched-flow stretching in a single inlet115. The latter design forces cells to flow in the centre of the channel, siphons fluid on the sides of the channel away from the cells and then compresses the cells when the fluid is added back to the channel115. These assays can be applied to analyse cell deformability of single cells or populations of cells using pressure-driven microfiltration systems. Using these systems, it has been observed that induction of EMT or drug resistance leads to an increase in cell deformability116. Such microfiltration devices enable high-throughput assessment oftransit time and deformability117 to investigate a population of cells from a tumour. These assays, applying forces either internally or externally, measure internal features of the cytoskeleton that are found in metastatic but not in non-metastatic cells. Therefore, microchannel assays can be useful as diagnostic tools to assess the aggressiveness of cells isolated from tumour biopsy samples and to observe the effect of cancer therapies on cell deformability and thus disease progression.
Adhesion properties and mechanisms provide another physical metric to determine the metastatic potential of cancer cells. Assays that apply negative pressure to detach cells118, to assess binding efficiencies to ECMs119 or to analyse adhesion turnover120 have demonstrated that adhesion is modulated differentially in metastatic cancer cells compared with in non-metastatic cells. For example, metastatic cancer cells can move rapidly through tissue through increased cation sensitivity that leads to more rapid formation and disassembly of focal adhesions than in their non-metastatic counterparts121. Cell-matrix adhesions are directly modulated by magnesium, manganese and calcium cations, which increase integrin affinities for matrix proteins in proportion to their concentration. The concentration of cations is tenfold lower in the stroma than in the tumour122,123. Thus, once metastatic cells reach the stroma, only cells with labile adhesions can migrate. Indeed, cancer cell adhesion strength to fibronectin and type I collagen at low cation conditions correlates with metastatic potential; within a highly metastatic cell population, the subset of cells with high adhesion strength is less migratory and invasive than malignant and non-cancerous epithelial cells or strongly adherent metastatic cells121. Analysing the weakly adherent cell fraction enables the determination of the metastatic potential of a tumour in situ. Each of the above-discussed assays yields valuable information about the metastatic potential of cancer cells, which could make such devices useful diagnostic tools for prognostic assessment and for determining a course of treatment.
Non-invasive surveillance of tumour-adjacent stroma
Interaction with the surrounding matrix is an important regulator of cell dissemination, and various matrix properties can act as markers to detect and/or capture highly invasive cells that are predisposed towards tumour formation. Exploiting the similarities of tumour microenvironments across different cancer types opens up avenues for monitoring the presence and growth of primary tumours. For example, overexpression of inte- grins, common matrix signatures41 and overexpression of specific MMPs can act as prognostic indicators of the metastatic potential of tumours in patients with primary breast tumours124. Unlike most physical parameters of the ECM, the composition of the tumour-adjacent stroma can be non-invasively monitored, making it an attractive property for the assessment of tumour progression in patients.
In addition to biochemical surveillance, imaging methods are also being explored using material-based probes. For example, a combination ofhigh-affinity fibrin peptides and tracer molecules (that is, radioisotopes) that are detectable by magnetic resonance imaging (MRI) or single photon emission computed tomography (SPECT) are being developed to assess increased fibrin deposition in tumours125·126. Antigen-binding fragment (Fab) probes can be combined with a radioisotope to image fibrin clots in the tumour microenvironment127. Such probes also demonstrate low retention times in non-target tissue in vivo126,128. Fibronectin is also overexpressed during EMT, making it a prime target for early cancer detection probes124,129–131. Similar to MRI contrast agents for fibrin, gadolinium-based MRI contrast agents can be used to target fibronectin-fibrin complexes, demonstrating robust detection of the primary tumour and of >0.5 mm3 micrometastases129. Most current strategies target major ECM components; however, probes that target more tumour-specific ECM elements, such as periostin in oesophageal cancer132, could improve detection specificity, decrease background signalling through rapid clearance of non-bound contrast agents124 and increase tissue penetration depth owing to their small size. These approaches, which are still being developed, enable us to image tumours with increasing spatial resolution, but they do not provide information about the aggressiveness of tumours.
Perspectives and conclusion
Strategies to understand and detect tumours have greatly improved our ability to recognize and assess specific tumour pathways and cell behaviours that are indicative of disease progression. As the field matures, cancer diagnosis and treatment will most certainly involve more materials-based approaches to address shortcomings in our ability to model, detect and treat cancer. Despite the development of a variety of dynamic, synthetic biomaterials applicable for the modelling and study of cancer, Matrigel is still most commonly used by cancer biologists for 3D cell culture systems even though it is highly variable, difficult to purify and derived from a mouse tumour. Therefore, the field of material science must continue to evolve and incorporate tuneable synthetic materials to help understand the cell behaviours induced by these increasingly complex materials.
As the biomaterials community, we also aim to clinically translate lessons learned from in vitro models to diagnostic assays. The substantial progress made in our understanding of the tumour as a material and in detecting and capturing cancer cells makes this an exciting time for material-based cancer research. There are great opportunities to improve our basic understanding of cancer and also our detection and treatment capabilities, for example, investigating tumour-stroma interactions in reductionist matrix systems, developing a complete tumour-in-a-dish model (including intrava- sation and extravasation) and understanding how animal models reflect clinical outcomes. Improvement of detection probes using biomaterials, whether invasive or not, is also a growing research area, which is reflected in the expanding body of literature. For example, during tumour growth, collagen, fibrin and hyaluronan concentrations increase in the surrounding ECM, and the matrix stiffens and is aligned by lysyl oxidases5,133,134 to facilitate invasion124,135. Potential therapeutic avenues include the use of proteases to degrade matrix proteins and decrease stiffness to improve drug penetration. Conversely, hyaluronidase, which degrades the extracellular glycosamino- glycan hyaluronan, can be inhibited to limit tumour growth and metastasis136,137. Clinical trials of hyaluronidase delivery have demonstrated its safety138, and a phase III study is currently being conducted (NCT02715804). Finally, future improvements in treatment options using biomaterials will ultimately impact clinical outcomes. For example, altering ECM structure could improve nanoparticle and drug delivery, resulting in more effective, deeper-penetrating therapies and improved patient outcomes133,139–142. In addition to enzymatic strategies, physical disruption of the matrix using high-intensity ultrasound can be used to improve the penetration of nanoparticles into the tumour tissue without damaging surrounding tissues139. Thermal strategies with nanotubes143 or gold nanorods144 can also be applied to denature the collagen matrix and increase tumour diffusivity.
Using biomaterials for the modelling, detection and treatment of cancer is a promising strategy. Another important contribution of material science in the near future will be to help rectify the differences in disease progression and treatment between humans, animal models and patient-derived xenografts145. Biomaterial-based models are reductionist in nature; thus, their application in vivo could improve the reliability of animal models, making them more predictive of patient outcomes146. Animal models are considered the standard assay for tumour biology, and material-based in vivo strategies are required to understand the differences between humans and animal models. For example, recombinant, chemically defined natural147 or synthetic45 biomaterials could be used that can actively modify tissue properties5. Such materials have already enabled the identification of cancer stem cells and mechanotransduction mechanisms and have demonstrated how material properties can drive tumorigenesis, making future applications in vivo promising.
The examples discussed in this Review demonstrate that biomaterials can serve as powerful tools to replicate mechanisms of disease and the response to treatments in vitro. The materials-based strategies that have enabled these discoveries should be broadly applied in the future to further improve our understanding of cancer biology and to begin to impact clinical outcomes.
Acknowledgements
Funding for this work was provided by US National Institutes of Health grants R01CA206880 (A.J.E. and J.Y.) and R21CA217735 (A.J.E.), a US National Science Foundation grant 1463689 (A.J.E.) and the Graduate Research Fellowship programme (P.B.). Additional fellowship support was provided by Brazilian Federal Agency for Support and Evaluation of Graduate Education award 88881.135357/2016-01 (B.F.M.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Affo S, Yu LX & Schwabe RF The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu. Rev. Pathol 12, 153–186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pankova D et al. Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol. Cancer Res 14, 287–295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). This paper uses a hydrogel-Matrigel sandwich culture system to show that initial matrix stiffness can drive loss of mammary epithelial polarity. [DOI] [PubMed] [Google Scholar]
- 4.Pickup MW, Mouw JK & Weaver VM The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243–1253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levental KR et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009). This paper demonstrates that stiffer tissues can drive tumour growth and metastasis in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mak IW, Evaniew N & Ghert M Lost in translation: animal models and clinical trials in cancer treatment. Am. J. Transl Res 6, 114–118 (2014). [PMC free article] [PubMed] [Google Scholar]
- 7.Bissell MJ, Hall HG & Parry G How does the extracellular matrix direct gene expression? J. Theor. Biol 99, 31–68 (1982). [DOI] [PubMed] [Google Scholar]
- 8.Kleinman HK et al. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 21, 6188–6193 (1982). [DOI] [PubMed] [Google Scholar]
- 9.Orkin RW et al. A murine tumor producing a matrix of basement membrane. J. Exp. Med 145, 204–220 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vukicevic S et al. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res 202, 1–8 (1992). [DOI] [PubMed] [Google Scholar]
- 11.Barcellos-Hoff MH, Aggeler J, Ram TG & Bissell MJ Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105, 223–235 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenny PA et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol 1, 84–96 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen OW, Ronnovjessen L, Howlett AR & Bissell MJ Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl Acad. Sci. USA 89, 9064–9068 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paget S The distribution of secondary growths in cancer of the breast. Lancet 133, 571–573 (1889). [PubMed] [Google Scholar]
- 15.Kalluri R & Zeisberg M Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Ozdemir BC et al. Depletion of carcinoma- associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhim AD et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vennin C et al. Reshaping the tumor stroma for treatment of pancreatic cancer. Gastroenterology 54, 820–838 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Nguyen-Ngoc KV et al. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc. Natl Acad. Sci. USA 109, E2595–E2604 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velez DO et al. 3D collagen architecture induces a conserved migratory and transcriptional response linked to vasculogenic mimicry. Nat. Commun 8, 1651 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman A, Ziperstein MJ & Kaufman LJ The effect of fibrillar matrix architecture on tumor cell invasion of physically challenging environments. Biomaterials 35, 6954–6963 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Mouw JK et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med 20, 360–367 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denais CM et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016). This manuscript is the first to describe the mechanisms that enable cancer cells to migrate through pores in matrix that are smaller than the diameter of the nucleus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sodek KL, Brown TJ & Ringuette MJ Collagen I but not Matrigel matrices provide an MMP-dependent barrier to ovarian cancer cell penetration. BMC Cancer 8, 223 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf K et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol 201, 1069–1084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey SP, Martin KE & Reinhart-King CA Three-dimensional collagen matrix induces a mechanosensitive invasive epithelial phenotype. Sci. Rep 7, 42088 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedl P & Wolf K Plasticity of cell migration: a multiscale tuning model. J. Cell Biol 188, 11–19 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaman MH et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl Acad. Sci. USA 103, 10889–10894 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason BN, Starchenko A, Williams RM, Bonassar LJ & Reinhart-King CA Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 9, 4635–4644 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams CM, Engler AJ, Slone RD, Galante LL & Schwarzbauer JE Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res. 68, 3185–3192 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraley SI et al. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat. Cell Biol 12, 598–604 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anguiano M et al. Characterization of three-dimensional cancer cell migration in mixed collagen-Matrigel scaffolds using microfluidics and image analysis. PLOS One 12, e0171417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinwachs J et al. Three-dimensional force microscopy of cells in biopolymer networks. Nat. Methods 13, 171–176 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Koch TM, Munster S, Bonakdar N, Butler JP & Fabry B 3D Traction Forces in Cancer Cell Invasion. PLOS One 7, e33476 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller JP et al. Clinical doses of radiation reduce collagen matrix stiffness. APL Bioeng. 2, 031901 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck JN, Singh A, Rothenberg AR, Elisseeff JH & Ewald AJ The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials 34, 9486–9495 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen JH et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater 13, 979–987 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taubenberger AV et al. 3D extracellular matrix interactions modulate tumour cell growth, invasion and angiogenesis in engineered tumour microenvironments. Acta Biomater. 36, 73–85 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Loessner D et al. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 31, 8494–8506 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Alcaraz J et al. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 27, 2829–2838 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reticker-Flynn NE et al. A combinatorial extracellular matrix platform identifies cell-extracellular matrix interactions that correlate with metastasis. Nat. Commun 3, 1122 (2012). This paper highlights how non-additive matrix properties, specifically the combination of multiple matrix proteins, can drive metastatic behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraning-Rush CM, Califano JP & Reinhart-King CA Cellular traction stresses increase with increasing metastatic potential. PLOS One 7, e32572 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leight JL, Wozniak MA, Chen S, Lynch ML & Chen CS Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol. Biol. Cell 23, 781–791 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stowers RS, Allen SC & Suggs LJ Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl Acad. Sci. USA 112, 1953–1958 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei SC et al. Matrix stiffness drives epithelial-mesenchymal transition and tumor metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol 17, 678–688 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fenner J et al. Macroscopic stiffness of breast tumors predicts metastasis. Sci. Rep 4, 5512 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tse JR & Engler AJ Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol 47, 10.16.1–10.16.16 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Emerman JT & Pitelka DR Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro 13, 316–328 (1977). [DOI] [PubMed] [Google Scholar]
- 49.Bissell MJ The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int. Rev. Cytol 70, 27–100 (1981). [DOI] [PubMed] [Google Scholar]
- 50.Pang MF et al. Tissue stiffness and hypoxia modulate the integrin-linked kinase ILK to control breast cancer stem-like cells. Cancer Res. 76, 5277–5287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jabbari E, Sarvestani SK, Daneshian L & Moeinzadeh S Optimum 3D matrix stiffness for maintenance of cancer stem cells is dependent on tissue origin of cancer cells. PLOS One 10, e0132377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shu XZ, Ahmad S, Liu Y & Prestwich GD Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J. Biomed. Mater. Res. A 79, 902–912 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Young JL & Engler AJ Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 32, 1002–1009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guvendiren M & Burdick JA Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun 3, 792 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Ondeck MG & Engler AJ Mechanical characterization of a dynamic and tunable methacrylated hyaluronic acid hydrogel. J. Biomech. Eng 138, 021003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stowers RS et al. Extracellular matrix stiffening induces a malignant phenotypic transition in breast epithelial cells. Cell. Mol. Bioeng 10, 114–123 (2017). Temporal changes in matrix stiffness can modulate mammary epithelial cell responses in a different way than static materials, which when stiff, always induce phenotype transitions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kloxin AM, Kasko AM, Salinas CN & Anseth KS Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maghdouri-White Y, Elmore LW, Bowlin GL & Dreau D Breast epithelial cell infiltration in enhanced electrospun silk scaffolds. J. Tissue Eng. Regen Med 10, E121–131 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Chen Z et al. Electrospun nanofibers for cancer diagnosis and therapy. Biomater. Sci 4, 922–932 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Kushiro K, Yaginuma T, Ryo A & Takai M Differences in three-dimensional geometric recognition by non-cancerous and cancerous epithelial cells on microgroove-based topography. Sci. Rep 7, 4244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ning D et al. Mechanical and morphological analysis of cancer cells on nanostructured substrates. Langmuir 32, 2718–2723 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Chaudhuri PK, Pan CQ, Low BC & Lim CT Topography induces differential sensitivity on cancer cell proliferation via Rho-ROCK-Myosin contractility. Sci. Rep 6, 19672 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulrich TA, Jaim A, Tanner K, MacKay JL & Kumar S Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials 31, 1875–1884 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Pathak A & Kumar S Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl Acad. Sci. USA 109, 10334–10339 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Attieh Y & Vignjevic DM The hallmarks of CAFs in cancer invasion. Eur. J. Cell Biol 95, 493–502 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Gaggioli C et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol 9, 1392–1400 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Goetz JG et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146, 148–163 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glentis A et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun 8, 924 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang K et al. Stiffening and unfolding of early deposited-fibronectin increase proangiogenic factor secretion by breast cancer-associated stromal cells. Biomaterials 54, 63–71 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Labernadie A et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol 19, 224–237 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calvo F et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol 15, 637–646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen MB et al. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat. Protoc 12, 865–880 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee H, Park W, Ryu H & Jeon NL A microfluidic platform for quantitative analysis of cancer angiogenesis and intravasation. Biomicrofluidics 8, 054102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zervantonakis IK et al. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl Acad. Sci. USA 109, 13515–13520 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen MB, Whisler JA, Jeon JS & Kamm RD Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr. Biol 5, 1262–1271 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bersini S et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials 35, 2454–2461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saquib N, Saquib J & Ioannidis JP Does screening for disease save lives in asymptomatic adults? Systematic review of meta-analyses and randomized trials. Int. J. Epidemiol 44, 264–277 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Zhang Z et al. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget 5, 12383–12397 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mehta S et al. Predictive and prognostic molecular markers for cancer medicine. Ther. Adv. Med. Oncol 2, 125–148 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qian WY, Zhang Y & Chen WQ Capturing cancer: emerging microfluidic technologies for the capture and characterization of circulating tumor cells. Small 11, 3850–3872 (2015). [DOI] [PubMed] [Google Scholar]
- 82.Chen WQ et al. Nanoroughened surfaces for efficient capture of circulating tumor cells without using capture antibodies. ACS Nano 7, 566–575 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park GS et al. Full surface embedding of gold clusters on silicon nanowires for efficient capture and photothermal therapy of circulating tumor cells Nano Lett. 12, 2176–2176 (2012). [DOI] [PubMed] [Google Scholar]
- 84.Zhai TT, Ye DK, Zhang QW, Wu ZQ & Xia XH Highly efficient capture and electrochemical release of circulating tumor cells by using aptamers modified gold nanowire arrays. ACS Appl. Mater. Interfaces 9, 34706–34714 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Zhang W, Zhao K, Banks CE & Zhang Y Antibody-modified hydroxyapatite surfaces for the efficient capture of bladder cancer cells in a patient’s urine without recourse to any sample pre-treatment. J. Mater. Chem. B 5, 8125–8132 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Xu HW et al. Three-dimensional inverse opal photonic crystal substrates toward efficient capture of circulating tumor cells. ACS Appl. Mater. Interfaces 9, 30510–30518 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Zhang J et al. Surface chemistry induces mitochondria-mediated apoptosis of breast cancer cells via PTEN/PI3K/AKT signaling pathway. Biochim. Biophys. Acta Mol. Cell Res 1865, 172–185 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Jan YJ et al. NanoVelcro rare-cell assays for detection and characterization of circulating tumor cells. Adv. DrugDeliv. Rev 125, 78–93 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang LX, Asghar W, Demirci U & Wan Y Nanostructured substrates for isolation of circulating tumor cells. Nano Today 8, 374–387 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang ST et al. Three-dimensional nanostructured substrates toward efficient capture of circulating tumor cells. Angew. Chem. Int. Ed 48, 8970–8973 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang ST et al. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew. Chem. Int. Ed 50, 3084–3088 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo S et al. Degradable zinc-phosphate-based hierarchical nanosubstrates for capture and release of circulating tumor cells. ACS Appl. Mater. Interfaces 8, 15917–15925 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Lou HY et al. Dual-functional lipid coating for the nanopillar-based capture of circulating tumor cells with high purity and efficiency. Langmuir 33, 1097–1104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun N et al. Chitosan nanofibers for specific capture and nondestructive release of CTCs assisted by pCBMA brushes. Small 12, 5090–5097 (2016). [DOI] [PubMed] [Google Scholar]
- 95.Rao SS et al. Enhanced survival with implantable scaffolds that capture metastatic breast cancer cells in vivo. Cancer Res. 76, 5209–5218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Azarin SM et al. In vivo capture and label-free detection of early metastatic cells. Nat. Commun 6, 8094 (2015). This paper describes an implantable hydrogel-based method to capture cells that have intravasated from tumours. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aguado BA et al. Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche. Acta Biomater. 33, 13–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qian BZ et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–U129 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kitamura T, Qian BZ & Pollard JW Immune cell promotion of metastasis. Nat. Rev. Immunol 15, 73–86 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hiratsuka S, Watanabe A, Aburatani H & Maru Y Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol 8, 1369–U1331 (2006). [DOI] [PubMed] [Google Scholar]
- 101.Mauro N, Scialabba C, Pitarresi G & Giammona G Enhanced adhesion and in situ photothermal ablation of cancer cells in surface-functionalized electrospun microfiber scaffold with graphene oxide. Int. J. Pharm 526, 167–177 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Ma HS et al. A bifunctional biomaterial with photothermal effect for tumor therapy and bone regeneration. Adv. Funct. Mater 26, 1197–1208 (2016). [Google Scholar]
- 103.Aguado BA et al. Secretome identification of immune cell factors mediating metastatic cell homing. Sci. Rep 5, 17566 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de la Fuente A et al. M-trap: exosome-based capture of tumor cells as a new technology in peritoneal metastasis. J. Natl Cancer Inst 107, djv184 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seib FP, Berry JE, Shiozawa Y, Taichman RS & Kaplan DL Tissue engineering a surrogate niche for metastatic cancer cells. Biomaterials 51,313–319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aguado BA, Bushnell GG, Rao SS, Jeruss JS & Shea LD Engineering the pre-metastatic niche. Nat. Biomed. Eng 1,0077 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davidson PM, Denais C, Bakshi MC & Lammerding J Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cell. Mol. Bioeng 7, 293–306 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yao N et al. Structure and function analysis in circulating tumor cells: using nanotechnology to study nuclear size in prostate cancer. Am. J. Clin. Exp. Urol 6, 43–54 (2018). [PMC free article] [PubMed] [Google Scholar]
- 109.Chen JF et al. Subclassification of prostate cancer circulating tumor cells by nuclear size reveals very small nuclear circulating tumor cells in patients with visceral metastases. Cancer 121,3240–3251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alibert C, Goud B & Manneville JB Are cancer cells really softer than normal cells? Biol. Cell 109, 167–189 (2017). [DOI] [PubMed] [Google Scholar]
- 111.Guo Q, Park S & Ma HS Microfluidic micropipette aspiration for measuring the deformability of single cells. Lab. Chip 12, 2687–2695 (2012). [DOI] [PubMed] [Google Scholar]
- 112.Mak M & Erickson D A serial micropipette microfluidic device with applications to cancer cell repeated deformation studies. Integr. Biol 5, 1374–1384 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Malboubi M, Jayo A, Parsons M & Charras G An open access microfiuidic device for the study of the physical limits of cancer cell deformation during migration in confined environments. Microelectron. Eng 144, 42–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gossett DR et al. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl Acad. Sci. USA 109, 7630–7635 (2012). This paper demonstrates a high-throughput single cell microfluidic-based deformability cytometry assay showing that mechanical properties of cells can be used to separate populations with different metastatic potentials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dudani JS, Gossett DR, Tse HTK & Di Carlo D Pinched-flow hydrodynamic stretching of single-cells. Lab. Chip 13, 3728–3734 (2013). [DOI] [PubMed] [Google Scholar]
- 116.Qi D et al. Screening cell mechanotype by parallel microfiltration. Sci. Rep 5, 17595 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nyberg KD et al. The physical origins of transit time measurements for rapid, single cell mechanotyping. Lab. Chip 16, 3330–3339 (2016). [DOI] [PubMed] [Google Scholar]
- 118.Palmer CP et al. Single cell adhesion measuring apparatus (SCAMA): application to cancer cell lines of different metastatic potential and voltage-gated Na+ channel expression. Eur. Biophys. J 37, 359–368 (2008). [DOI] [PubMed] [Google Scholar]
- 119.Veiseh M et al. Cellular heterogeneity profiling by hyaluronan probes reveals an invasive but slow-growing breast tumor subset. Proc. Natl Acad. Sci. USA 111, E1731–E1739 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bijian K et al. Targeting focal adhesion turnover in invasive breast cancer cells by the purine derivative reversine. Br. J. Cancer 109, 2810–2818 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fuhrmann A, Banisadr A, Beri P, Tlsty TD & Engler AJ Metastatic state of cancer cells may be indicated by adhesion strength. Biophys. J 112, 736–745 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seltzer MH, Rosato FE & Fletcher MJ Serum and tissue magnesium levels in human breast carcinoma. J. Surg. Res 10, 159–162 (1970). [DOI] [PubMed] [Google Scholar]
- 123.Seltzer MH, Rosato FE & Fletcher MJ Serum and tissue calcium in human breast carcinoma. Cancer Res 30, 615–616 (1970). [PubMed] [Google Scholar]
- 124.Zhou ZX & Lu ZR Molecular imaging of the tumor microenvironment. Adv. Drug Deliv. Rev 113, 24–48 (2017). [DOI] [PubMed] [Google Scholar]
- 125.Chaabane L et al. In vivo MR imaging of fibrin in a neuroblastoma tumor model by means of a targeting Gd-containing peptide. Mol. Imag. Biol 17, 819–828 (2015). [DOI] [PubMed] [Google Scholar]
- 126.Starmans LWE et al. Noninvasive visualization of tumoral fibrin deposition using a peptidic fibrin-binding single photon emission computed tomography tracer. Mol. Pharm 12, 1921–1928 (2015). [DOI] [PubMed] [Google Scholar]
- 127.Obonai T et al. Tumour imaging by the detection of fibrin clots in tumour stroma using an anti-fibrin Fab fragment. Sci. Rep 6, 23613 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Mourik TR, Claesener M, Nicolay K & Grull H Development of a novel, fibrin-specific PET tracer. J. Labelled Comp. Radiopharm 60, 286–293 (2017). [DOI] [PubMed] [Google Scholar]
- 129.Zhou ZX et al. MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nat. Commun 6, 7984 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li CL et al. Fibronectin induces epithelial-mesenchymal transition in human breast cancer MCF-7 cells via activation of calpain. Oncol. Lett 13, 3889–3895 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Han Z & Lu ZR Targeting fibronectin for cancer imaging and therapy. J. Mater. Chem. B 5, 639–654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heidari P et al. Imaging of secreted extracellular periostin, an important marker of invasion in the tumor microenvironment in esophageal cancer. J. Nuclear Med 56, 1246–1251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khawar IA, Kim JH & Kuh HJ Improving drug delivery to solid tumors: priming the tumor microenvironment. J. Control. Release 201, 78–89 (2015). [DOI] [PubMed] [Google Scholar]
- 134.Grossman M et al. Tumor cell invasion can be blocked by modulators of collagen fibril alignment that control assembly of the extracellular matrix. Cancer Res. 76, 4249–4258 (2016). [DOI] [PubMed] [Google Scholar]
- 135.Theocharis AD, Skandalis SS, Gialeli C & Karamanos NK Extracellular matrix structure. Adv. DrugDeliv. Rev 97, 4–27 (2016). [DOI] [PubMed] [Google Scholar]
- 136.Provenzano P P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jacobetz MA et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hingorani SR et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin. Cancer Res 22, 2848–2854 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee S et al. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound. J. Control. Release 263, 68–78 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Baronzio G, Parmar G & Baronzio M Overview of methods for overcoming hindrance to drug delivery to tumors, with special attention to tumor interstitial fluid. Front. Oncol 5, 165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yhee JY et al. Effects of tumor microenvironments on targeted delivery of glycol chitosan nanoparticles. J. Control. Release 267, 223–231 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Nicolas-Boluda A, Silva AKA, Fournel S & Gazeau F Physical oncology: new targets for nanomedicine. Biomaterials 150, 87–99 (2018). [DOI] [PubMed] [Google Scholar]
- 143.Marangon I et al. Tumor stiffening, a key determinant of tumor progression, is reversed by nanomaterial-induced photothermal therapy. Theranostics 7, 329–343 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Raeesi V & Chan WCW Improving nanoparticle diffusion through tumor collagen matrix by photo-thermal gold nanorods. Nanoscale 8, 12524–12530 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Schuh JC Trials, tribulations, and trends in tumor modeling in mice. Toxicol. Pathol 32, 53–66 (2004). [DOI] [PubMed] [Google Scholar]
- 146.Day CP, Merlino G & Van Dyke T Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 163, 39–53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Quintana E et al. Efficient tumour formation by single human melanoma cells. Nature 456, 593–598 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tzvetkova-Chevolleau T et al. The motility of normal and cancer cells in response to the combined influence of the substrate rigidity and anisotropic microstructure. Biomaterials 29, 1541–1551 (2008). [DOI] [PubMed] [Google Scholar]
- 149.Zhu J Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 31, 4639–4656 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lewis KJR et al. Epithelial-mesenchymal crosstalk influences cellular behavior in a 3D alveolus-fibroblast model system. Biomaterials 155, 124–134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fischbach C et al. Engineering tumors with 3D scaffolds. Nat. Methods 4, 855–860 (2007). [DOI] [PubMed] [Google Scholar]
- 152.Makadia HK & Siegel SJ Poly lactic- co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3, 1377–1397 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Choi YS et al. The alignment and fusion assembly of adipose-derived stem cells on mechanically patterned matrices. Biomaterials 33, 6943–6951 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nasrollahi S et al. Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory. Biomaterials 146, 146–155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zustiak S, Nossal R & Sackett DL Multiwell stiffness assay for the study of cell responsiveness to cytotoxic drugs. Biotechnol. Bioeng 111, 396–403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chaudhuri O et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 13, 970–978 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Fang JY, Tan SJ, Yang Z, Tayag C & Han B Tumor bioengineering using a transglutaminase crosslinked hydrogel. PLOS One 9, e105616 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]