Abstract
Objective:
To characterize the severity and natural history of hearing loss, and the prevalence of having a cochlear implant in a maturing cohort of individuals with enlarged vestibular aqueduct (EVA) and 0 or 1 mutant allele of SLC26A4.
Study Design:
Prospective cohort study of subjects ascertained between 1998 and 2015 at the NIH Clinical Center.
Methods:
Study subjects were 127 individuals (median age, 8 years; range, 0-59 years) with EVA in at least one ear.
Results:
Ears with EVA and 0 or 1 mutant allele of SLC26A4 had mean 0.5/1/2/4-kHz pure-tone averages of 62.6 and 52.9 dB HL, respectively, in contrast to EVA ears with 2 mutant alleles of SLC26A4 (88.1 dB HL; p < 0.01). This association was independent of age, sex, or side of EVA (P < 0.001). Natural history of hearing loss was not associated with number of mutant alleles (p = 0.94). The prevalence of having a cochlear implant was 9 (12%) of 76, 2 (13%) of 15, and 12 (38%) of 32 subjects with 0, 1, and 2 mutant alleles, respectively (P = 0.00833). This association was not independent (P = 0.534) but reflected underlying correlations with age at time of first audiogram (P = 0.003) or severity of hearing loss (P = 0.000).
Conclusions:
Ears with EVA and 0 or 1 mutant allele of SLC26A4 have less severe hearing loss, no difference in prevalence of fluctuation, and a lower prevalence of cochlear implantation in comparison to ears with 2 mutant alleles of SLC26A4.
Keywords: cochlear implant, congenital anomalies, fluctuation, hearing loss, natural history, non-syndromic, otology, pediatric otology, Pendred syndrome, progression, SLC26A4
INTRODUCTION
Enlargement of the vestibular aqueduct (EVA) is a commonly detected malformation in inner ears with early-onset sensorineural or mixed hearing loss1. Both syndromic EVA (related to Pendred syndrome) and non-syndromic EVA can be associated with hearing loss fluctuation, progression, or both (reviewed in ref. 2). Pendred syndrome is an autosomal recessive disorder comprised of hearing loss, EVA, and goiter2. Pendred syndrome is caused by SLC26A4 mutations, whereas only some cases of non-syndromic EVA are associated with SLC26A4 mutations. Conversely, approximately one-half of all individuals with unilateral or bilateral hearing loss with EVA will have either one (“M1”) or two (“M2”) mutant alleles of SLC26A43–5. The detection of only one mutant allele of SLC26A4 is an incompletely diagnostic result. Cosegregation studies of SLC26A4-linked genetic markers, and an observed familial recurrence risk of approximately 1 in 4 in M1 families, indicate that undetected mutations are likely to exist in noncoding regions of SLC26A4 in M1 patients6. In contrast, a much lower recurrence risk and discordant segregation of SLC26A4-linked markers with EVA suggests the existence of other etiologies in M0 patients6.
There are surprisingly few studies that quantitatively analyze the effects and correlations of age, SLC26A4 genotype status, and audiometric thresholds in patients with EVA. Albert et al.7 investigated SLC26A4-related hearing loss in a cohort of 109 children ascertained in France with EVA and bilateral pre-lingual or early post-lingual hearing loss. In comparison to M2 patients, patients with no detectable SLC26A4 mutations (“M0”) were older at the time of “discovery” of hearing loss, had a lower prevalence of hearing loss that was severe or profound in degree (severity), and had a lower prevalence of hearing fluctuations. However, the ages of the subjects in the different SLC26A4 genotype groups (M0, M1 or M2) were not presented so the observed differences could reflect underlying correlations of age with genotype status. Azaiez et al.8 analyzed SLC26A4 genotype-phenotype correlations in a North American majority-Caucasian cohort of 349 patients with either unilateral or bilateral EVA. They did not find a significant difference in the degree of hearing loss in the M0 and M1 groups when compared to the M2 group. Subject age was not included in the analysis or presented data. Rah et al.9 examined the correlation of audiometric phenotype with SCL26A4 genotype in 56 Korean patients with EVA (47 M2, 9 M1, 0 M0) followed by pure-tone audiometry for an average of 30.3 months. M1 and M2 subjects were similar in their average age at initial visit and demonstrated severe-to-profound hearing loss with a slightly downward sloping pattern. There was no significant difference in mean threshold, degree of fluctuation, or progression of hearing between the M1 and M2 groups. The absence of M0 subjects in the Rah et al.9 study cohort is remarkably different from North American and European majority-Caucasian EVA cohorts in which M0 is the predominant SLC26A4 genotype status. The overall difference in proportions of SLC26A4 genotype groups raises the possibility of differences in the prevalence or identities of other factors contributing to EVA in the Korean population10. Therefore ethnic background is an important consideration in genotypic and phenotypic studies of EVA.
In 2010, we reported the influence of SLC26A4 genotype on the severity and natural history of hearing loss for 139 EVA ears in 84 subjects11. Nearly all of the subjects were self-reported Caucasians of North American or European ancestry. The M2 ears had more severe hearing loss than M0 and M1 ears. Hearing loss severity was not independently associated with goiter, cochlear malformations or any other radiologic parameters11. Thirty-seven percent of all EVA ears showed some type of fluctuation in hearing, but SLC26A4 genotype status did not have a detectable correlation with natural history of hearing loss. The mean age of M0 and M1 subjects in our 2010 study was 9.0 years and 8.7 years, respectively.
The rationale for our current study was to collect and analyze data on natural history of hearing loss and prevalence of cochlear implantation for our previously enrolled M0 and M1 patients, as well as data for M0 and M1 patients ascertained since King et al.11. We included M2 subjects for comparison since their natural history has been characterized11. The mean ages of M0 and M1 subjects in our current study are approximately 13 years old, four years older than those in King et al.11. This maturation and expansion into adolescence of our cohort provides a more comprehensive and novel source of data on the natural history and outcomes of hearing loss for M0 and M1 patients with EVA.
MATERIALS AND METHODS
This study was approved by the Combined Neuroscience Institutional Review Board, National Institutes of Health (NIH), Bethesda, Maryland. Written informed consent was obtained for all subjects. The eligibility criterion for an affected subject was enlargement of the vestibular aqueduct (EVA) on a CT or MRI scan in at least one ear without consideration of hearing status. We originally defined a vestibular aqueduct (EVA) as enlarged if its diameter exceeded 1.5 mm at the midpoint between the posterior cranial fossa and the vestibule of the inner ear. We subsequently revised our midpoint diameter criterion to >1.0 mm1. Subjects were initially evaluated at the NIH Clinical Center as described. We relied upon records from outside health care providers for subjects who did not come to the NIH Clinical Center. For subjects evaluated prior to April 2014, we attempted to contact 132 subjects between November 2014 and March 2015 to request additional audiology records since the time of last contact. Additional longitudinal data was thus collected from 25 subjects. Five of the 132 subjects who were re-contacted were initially ascertained as siblings of EVA probands but had an indeterminate EVA phenotype at the time of initial ascertainment. The EVA phenotype of those 5 subjects was re-classified as unaffected or unknown upon evaluation of additional data. Our total study cohort thus comprised 127 subjects: 29 with unilateral EVA and 98 with bilateral EVA (Fig. 1). Seventy-one (56%) were female and 56 (44%) were male. Self-reported race and ethnicity were classified according to our institutional review board guidelines. Three (2.4%) of the 127 subjects were African American/Black, 114 (89.8%) were Caucasian/White, three (2.4%) were more than one race, and seven (5.5%) did not report their race. Two subjects (1.6%) were Hispanic or Latino, 119 (93.7%) were not Hispanic or Latino, and six (4.7%) did not report their ethnicity. We classified SLC26A4 genotype status (M0, M1 or M2) as described in Muskett et al.12.
Figure 1. Study paradigm.
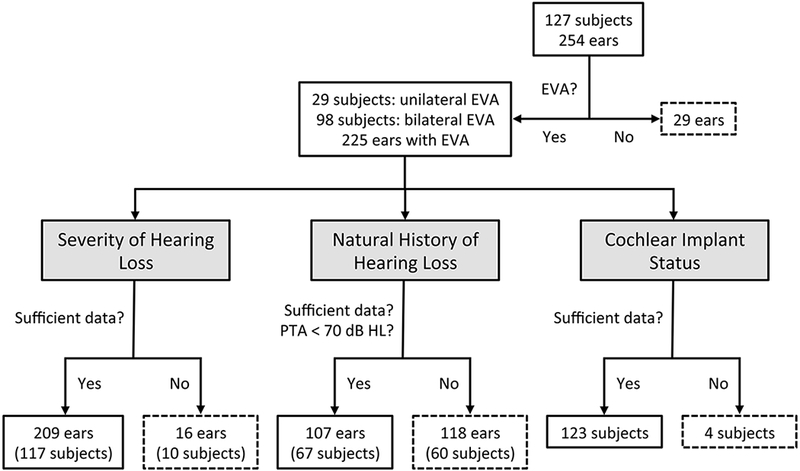
Workflow for selection and numbers of subjects and ears for analyses of severity of hearing loss, natural history of hearing loss, and cochlear implant status.
Severity of Hearing Loss
Severity of hearing loss was determined by the four-frequency (500, 1000, 2000, and 4000 Hz) pure-tone average (PTA) calculated from the most recent complete audiogram for each ear (Fig. 1). Ears were excluded from this analysis if our database did not include: (1) at least one pure-tone air-conduction audiogram for the ear without or prior to a cochlear implant; and (2) an audiogram with recorded thresholds for 500-, 1000-, 2000-, and 4000-Hz test stimuli. Audiometric data are shown in Supplemental Figures S1 to S4 according to the standard reporting format of the American Academy of Otolaryngology-Head and Neck Surgery13.
Natural History of Hearing Loss
Pure-tone air-conduction thresholds for 250-, 500-, 1000-, 2000-, 4000- and 8000-Hz test stimuli were analyzed for improvement, stability, progression, or fluctuation (Fig. 1). Ears were excluded from this analysis if our database had: (1) less than three pure-tone air-conduction audiograms, and (2) no audiogram with a four-frequency PTA of 70 dB HL or better (to prevent a ceiling effect). Test reports were reviewed for tympanometric evidence of middle ear pathology. If a tympanogram suggested negative middle ear pressure exceeding −100 daPa or a peak compensated admittance of less than 0.3 ml, then the data from that audiogram was not included in natural history analyses. For one analysis, we sub-categorized fluctuating hearing loss as fluctuating, fluctuating/progressive, or fluctuating/variable as previously described11.
Prevalence of Cochlear Implantation
We defined cochlear implant status as zero, one, or two implanted ears. We calculated the four-frequency PTA at the time of the first audiogram in the better-hearing ear as an indicator of initial functional hearing status.
Statistical Analysis
We performed one-way independent weighted ANOVA with post hoc Tukey testing to detect two-point correlations between the hearing loss severity (four-frequency PTA) and other variables. Significant correlations were further analyzed by post hoc Tukey testing. We used Fisher’s exact test to detect two-point correlations between the natural history of hearing loss or cochlear implantation status and other variables. We performed linear regression to analyze associations of hearing loss severity with other aspects of EVA. We used logistic regression to analyze associations of natural history or cochlear implant status with other aspects of EVA.
RESULTS
Severity of Hearing Loss
The degree of hearing loss was analyzed in 209 ears with EVA in 117 subjects (Fig. 1, Table 1). The mean four-frequency (0.5/1/2/4-kHz) pure-tone average (PTA) was 62.6, 59.2, and 88.1 dB HL for M0, M1, and M2 ears, respectively (p < 0.0001; one-way ANOVA). The mean four-frequency PTA for M2 ears is greater than that for either the M0 or M1 ears (p < 0.01; Tukey test). However, the mean ages were 12.6. 10.5 and 17.4 years for M0, M1 and M2 ears, respectively (Table 1). This difference (p < 0.022, one-way ANOVA) in mean age raises the possibility that the differences in mean PTA reflect an underlying correlation of age with genotype status. In order to identify independent correlations, we performed a linear regression analysis of the four-frequency PTA with age, sex, side of EVA (right versus left) and SLC26A4 genotype status. Hearing loss severity was not associated with sex (P = 0.296) or side (P = 0.805), but it was associated with age (P < 0.001) and genotype status (P < 0.001).
Table 1:
Age and severity of hearing loss in EVA ears at time of most recent audiogram
| Number of mutant alleles of SLC26A4 | |||||
|---|---|---|---|---|---|
| M0 | M1 | M2 | Total (M0 + M1 + M2) | ||
| Number of ears | 125 | 25 | 59 | 209 | |
| Mean (± s.d.) age (y) | 12.6 (± 10.0) | 10.5 (± 8.7) | 17.4 (± 17.2) | 13.8 (± 12.4) | |
| Median age (y) | 8.4 | 7.2 | 7.3 | 8.2 | |
| Mean (± s.d.) pure-tone average* (dBHL) | 62.6 (± 29.7) | 59.2 (± 30.3) | 88.1 (± 23.4) | 69.4 (± 30.4) | |
calculated for responses to 0.5-, 1-, 2- and 4-kHz stimuli
Natural History of Hearing Loss
The natural history of hearing loss was analyzed in 107 EVA ears in 67 subjects (Fig. 1, Table 2). We detected fluctuation in 77%, stable hearing in 9%, and progressive hearing loss in 14% of all EVA ears. The natural history was independent of genotype status (p = 0.94, Fisher’s exact test). Among the 82 EVA ears broadly categorized as having fluctuating hearing, we observed a prevalence of fluctuating hearing in 38%, fluctuating/progressive hearing loss in 41%, and fluctuating/variable hearing loss in 21% of all EVA ears (Table 3). The prevalence of fluctuating hearing loss sub-types was not different among genotype groups (P = 0.6765, Fisher’s exact test).
Table 2:
Natural history of hearing loss for all EVA ears*
| Number of mutant alleles of SLC26A4 | ||||
|---|---|---|---|---|
| M0 | M1 | M2 | Total (M0 + M1 + M2) | |
| Number of ears | 72 | 20 | 15 | 107 |
| Median duration of audiometric follow-up (y) | 4.0 | 3.5 | 4.0 | 4.0 |
| Mean (± s.d.) number of audiograms | 8.1 (± 5.8) | 6.8 (± 3.0) | 8.9 (± 3.7) | 8.0 (± 5.1) |
| Number (%) of ears with fluctuations in hearing | 53 (74) | 17 (85) | 12 (80) | 82 (77) |
| Number (%) of ears with stable hearing | 8 (11) | 1 (5) | 1 (7) | 10 (9) |
| Number (%) of ears with progressive hearing loss | 11 (15) | 2 (10) | 2 (13) | 15 (14) |
excluding ears with any pure-tone average greater than 70 dBHL
Table 3:
Natural history of hearing loss fluctuation in EVA ears*
| Number (%) of ears | M0 | M1 | M2 | Total (M0 + M1 + M2) |
|---|---|---|---|---|
| Total Number of ears | 53 | 17 | 12 | 82 |
| Number (%) of ears with fluctuation | 21 (40) | 7 (41) | 3 (25) | 31 (38) |
| Number (%) of ears with fluctuation-progression | 21 (40) | 8 (47) | 5 (42) | 34 (41) |
| Number (%) of ears with fluctuation-variable | 11 (20) | 2 (12) | 4 (33) | 17 (21) |
excluding ears with any pure-tone average greater than 70 dBHL
In order to identify independent correlates with natural history, we performed a logistic regression analysis of the natural history (stable, progressive, fluctuating, fluctuating/variable or fluctuating/progressive) with sex, side of EVA, genotype status and length of temporal interval of audiometric data. The only significant association was with the length of the temporal interval of audiometric data (P < 0.050): progressive hearing loss was associated with an increased temporal interval of audiometric data. There was no association of natural history with sex (P = 0.991), side of EVA (P = 0.532) or genotype status (P = 0.849).
Prevalence of Cochlear Implantation
The prevalence of a cochlear implant in one or both ears was calculated for 123 subjects with EVA: 76 M0, 15 M1, and 32 M2 subjects (Fig. 1, Table 4). The number of subjects with either one or two implanted ears was nine (12%), two (13%), and 12 (38%) for M0, M1, and M2 subjects, respectively. Among these subjects with cochlear implants, seven of nine M0 subjects had unilateral implants and two had bilateral implants, both of the M1 subjects had unilateral implants, and six of 12 M2 subjects had unilateral implants and six had bilateral implants. There was a significant difference in prevalence of cochlear implants between groups (P = 0.00833, Fisher’s exact test) that could reflect the difference (P = 0.011, Fisher’s exact test) in prevalence of bilateral EVA among the genotype groups: 52 (68%), 12 (80%), and 30 (94%) for M0, M1, and M2 subjects, respectively. The difference could also reflect differences in age or severity of hearing loss among the genotype groups. In order to identify independent correlations, we performed a logistic regression analysis of cochlear implant status with sex, laterality of EVA (unilateral versus bilateral), genotype status, age at first audiogram, and four-frequency PTA on the first audiogram. Younger age at the time of first audiogram and a higher four-frequency PTA on the first audiogram were each associated with having a cochlear implant (P = 0.003 and 0.000, respectively). There was no association of cochlear implant status with sex (P = 0.660), laterality of EVA (P = 0.889) or genotype status (P = 0.534).
Table 4:
EVA subjects and cochlear implant status
| M0 | M1 | M2 | Total (M0 + M1 + M2) | |
|---|---|---|---|---|
| Number of subjects | 76 | 15 | 32 | 123 |
| Mean (± s.d.) age (y) at 1st audiogram | 8.4 (± 8.7) | 7.4 (± 9.5) | 10.6 (± 13.2) | 8.9 (± 10.1) |
| Median age (y) at 1st audiogram | 5.1 | 3.8 | 4.6 | 4.9 |
| Number (%) of subjects with 1 or 2 ears implanted | 9 (12) | 2 (13) | 12 (38) | 23 (18) |
| Number (%) of subjects with bilateral EVA | 52 (68) | 12 (80) | 30 (94) | 94 (76) |
| Number (%) of subjects with hearing loss* at 1st audiogram | 45 (59) | 10 (67) | 29 (91) | 84 (68) |
25 dBHL or greater
DISCUSSION
The rationale for this study was to characterize long-term outcomes of hearing loss and cochlear implantation in patients with enlargement of the vestibular aqueduct (EVA) and identify prognostic indicators (genotype status, degree of hearing loss at initial detection) for these outcomes. It continues to appear that SLC26A4 mutation status is predictive of auditory outcome. Severity of M0 and M1 hearing losses are not statistically different from one another; however, the M0 and M1 groups differ significantly from the M2 group (Table 1). In general, M2 hearing loss already presents as more severe than M0 or M1 hearing loss in childhood. These results are consistent with those of our earlier study11.
Our data indicate that the SLC26A4 genotype is not correlated with the natural history of hearing loss in EVA patients. About 80% of patients in our study show fluctuations in hearing (Table 2). This is higher than we previously reported11, which may reflect the longer duration of the study, ascertainment bias, or a combination of these factors. However, our estimates of the prevalence of fluctuation are almost certainly below the actual prevalence due to incomplete detection of fluctuation by our methodology. It is likely that some episodes of fluctuation were missed due to delays or lack of audiometric testing.
M0 and M1 groups do not differ from one another among the prevalence of cochlear implantation; however, the M0 and M1 groups differ significantly from the M2 group (Table 4). The difference is over three-fold: the prevalence of implantation is 12% in M0 or M1 subjects versus 38% in M2 subjects. The hearing loss in the better hearing ear tends to be less severe at the time of the first audiogram in M0 or M1 individuals when compared to the severity of hearing loss in M2 subjects at the time of the first audiogram. The higher prevalence of cochlear implantation in the M2 patients likely reflects the greater severity of hearing loss they have in comparison to patients in the M0 and M1 groups, as indicated by the logistic regression analysis.
Caveats to this study include an incomplete prospective data collection. The 19% response rate to our requests for updated audiograms likely, in part, reflected the long time interval since the time of last contact. Our study was initiated over 15 years ago and many subjects were lost to follow-up since we are not their primary hearing health care providers. Furthermore, many of the subjects who were minors at the time of initial participation in our study, when the point of contact was their parent or legal guardian, are currently adults no longer living with their parent or legal guardian. Nevertheless, the response rate may have led to bias in the results. There was an ascertainment bias toward older M2 patients and younger M1 patients, as well as a recent ascertainment bias toward familial M0 cases.
A second caveat is that the study families were ascertained on the basis of having at least one member with EVA and known hearing loss, and this could introduce a bias toward ears with more severe hearing loss. Although some of our study subjects underwent temporal bone imaging because they had a sibling with EVA and not because they were known to have hearing loss themselves, our ascertainment may have skewed the data toward greater amounts of hearing loss in ears with EVA.
Another caveat is that we did not attempt to determine the total numbers of subjects that progressed to cochlear implant candidacy independent of actually having the procedure. Criteria for cochlear implant candidacy are multifactorial and have evolved during the course of our study. Current audiometric criteria require data on aided sentence recognition, benefit from hearing aids, and degree of hearing loss. For the purposes of our study, data collection was limited to degree of hearing loss and unaided word recognition. Moreover, designation of cochlear implant candidacy based on this limited data for the patients our cohort would require presumptive use of our own criteria.
A final caveat to our study is the relatively young age (mean age = 13.8 years for the entire cohort) at most recent audiogram for our subjects. Future goals of our study and those at other centers should include a continuing longitudinal analysis of outcomes of M0 and M1 EVA ears to increase the numbers of ears at older ages. This information would provide for more effective counseling as well as baseline data for clinical trials.
CONCLUSION
In ears with EVA, decreased severity of hearing loss is independently associated with the presence of zero or one mutant allele of SLC26A4 in comparison to more severe hearing loss associated with two mutant alleles. Hearing fluctuation affects a majority of ears and is independent of the number of mutant alleles of SLC26A4. The prevalence of having a cochlear implant is lower in subjects with zero or one mutant allele of SLC26A4. This association is not independent and reflects underlying correlations of less severe hearing loss with zero or one mutant alleles of SLC26A4.
Supplementary Material
Acknowledgments
We thank the study subjects for their participation, staff members of the NIDCD clinic and NIH Clinical Center for their support of the study and subjects, Jessica Ratay for research assistance, and Michael Hoa, Thomas Friedman and Jessica Ratay for critical review of the manuscript. This study was supported by the NIDCD intramural research program.
Funding: NIH intramural research funds Z01-DC-000060-13, Z01-DC-000064-13 and Z01-DC-000082-02.
Footnotes
Conflicts of Interest: the authors have no relevant financial interests to disclose.
Bibliography
- 1.Boston M, Halsted M, Meinzen-Derr J et al. The large vestibular aqueduct: a new definition based on audiologic and computed tomography correlation. Otolaryngol Head Neck Surg 2007; 136:972–977. [DOI] [PubMed] [Google Scholar]
- 2.Pendred V Deaf-Mutism and Goitre. Lancet 1896; ii:532. [Google Scholar]
- 3.Pryor SP, Madeo AC, Reynolds JC et al. SLC26A4/PDS genotype-phenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. J Med Genet 2005; 42:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi BY, Stewart AK, Madeo AC et al. Hypo-functional SLC26A4 variants associated with nonsyndromic hearing loss and enlargement of the vestibular aqueduct: genotype-phenotype correlation or coincidental polymorphisms? Hum Mutat 2009; 30:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chattaraj P, Reimold FR, Muskett JA et al. Use of SLC26A4 mutation testing for unilateral enlargement of the vestibular aqueduct. JAMA Otolaryngol Head Neck Surg 2013; 139:907–913. [DOI] [PubMed] [Google Scholar]
- 6.Choi BY, Madeo AC, King KA et al. Segregation of enlarged vestibular aqueducts in families with non-diagnostic SLC26A4 genotypes. J Med Genet 2009; 46:856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert S, Blons H, Jonard L et al. SLC26A4 gene is frequently involved in nonsyndromic hearing impairment with enlarged vestibular aqueduct in Caucasian populations. Eur J Hum Genet 2006; 14:773–779. [DOI] [PubMed] [Google Scholar]
- 8.Azaiez H, Yang T, Prasad S et al. Genotype-phenotype correlations for SLC26A4-related deafness. Hum Genet 2007; 122:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rah YC, Kim AR, Koo JW, Lee JH, Oh SH, Choi BY Audiologic presentation of enlargement of the vestibular aqueduct according to the SLC26A4 genotypes. Laryngoscope 2015; 125:E216–222. [DOI] [PubMed] [Google Scholar]
- 10.Park HJ, Lee SJ, Jin HS et al. Genetic basis of hearing loss associated with enlarged vestibular aqueducts in Koreans. Clin Genet 2005; 67:160–165. [DOI] [PubMed] [Google Scholar]
- 11.King KA, Choi BY, Zalewski C et al. SLC26A4 genotype, but not cochlear radiologic structure, is correlated with hearing loss in ears with an enlarged vestibular aqueduct. Laryngoscope 2010; 120:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muskett JA, Chattaraj P, Heneghan JF et al. Atypical patterns of segregation of familial enlargement of the vestibular aqueduct. Laryngoscope 2016; 126:E240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurgel RK, Jackler RK, Dobie RA, Popelka GR. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg 2012; 147:803–807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


