Abstract
While bats carry a diverse range of ectoparasites, little research has been conducted on the effects these organisms may have on bat populations. The southern bent-winged bat (Miniopterus orianae bassanii) is a critically endangered subspecies endemic to south-eastern Australia, whose numbers have declined over the past 50 years for unknown reasons. As part of a larger study to investigate the potential role of disease in these declines, southern bent-winged bats from four locations were captured and examined for the presence of bat flies, mites, ticks and the nematode Riouxgolvania beveridgei (previously associated with skin nodules in bent-winged bats). Results were compared with those obtained from the more common eastern bent-winged bat (Miniopterus orianae oceanensis), sampling animals from three different locations. All four types of parasite were found on both subspecies. There was no correlation between the presence of ectoparasites, body weight or any signs of disease. However, prevalence of tick and R. beveridgei infections were greater in Victorian southern bent-winged bats than South Australian southern bent-winged bats and eastern bent-winged bats, possibly indicative of some type of chronic stress impacting the immune system of this subspecies.
Keywords: Bat fly, Miniopterus orianae bassanii, Miniopterus orianae oceanensis, Mite, Tick, Nematode, Riouxgolvania beveridgei
Graphical abstract
Highlights
-
•
Ectoparasites not associated with ill health in bent-winged bats.
-
•
Greater prevalence of ticks and R. beveridgei infections on Victorian southern bent-winged bats.
-
•
Bat flies and mites more common in summer. Ticks more common in spring. R. beveridgei more common in winter and spring.
-
•
Mites more common on juvenile bats.
1. Introduction
Historically, the perception among ecologists was that ‘well-adapted’ parasites do not harm their hosts (McCallum, 2012). However, models have shown that parasites may be capable of making significant contributions to species extinctions (de Castro and Bolker, 2005). These models are supported by clinical evidence of parasites exerting substantial negative effects, not just on individuals, but also on populations. Sarcoptic mange, caused by the mite Sarcoptes scabiei, has caused population declines of 86% in Barbary sheep (Ammotragus lervia) in Spain (Gonzalez-Candela et al., 2004), 85% in red foxes (Vulpes vulpes) in Britain (Soulsbury et al., 2007) and local extinctions of common wombat (Vombatus ursinus) populations (Martin et al., 1998) in Australia. Infection with Varroa destructor, a parasitic mite of the Asian honeybee (Apis cerana), has caused the collapse of European honeybee (Apis mellifera) colonies worldwide due to its consumption of haemolymph and transmission of pathogens, such as the deformed wing virus (Rosenkranz et al., 2010).
Bats, often seen as carriers of many disease agents (Wibbelt et al., 2010), have also experienced the negative consequences of infectious and parasitic diseases. The recent appearance of white nose syndrome (caused by the fungus, Pseudogymnoascus destructans) has caused mass mortalities of insectivorous bats in North America (Blehert, 2012). Angiostrongyliasis (caused by the rat lungworm, Angiostrongylus cantonensis) was diagnosed as a cause of neurological disease and death in flying foxes in Australia (Barrett et al., 2002).
Bent-winged bats are small, cave-roosting, insectivorous bats (Richards and Reardon, 2008). In south-eastern Australia there are two subspecies of the large bent-winged bat (Miniopterus orianae) that form separate maternity colonies (Cardinal and Christidis, 2000). The southern bent-winged bat (M. orianae bassanii) occurs only in south-western Victoria and south-eastern South Australia (SA). There are three maternity caves, one near Warrnambool, one near Cape Bridgewater (both in Victoria) and the other near Naracoorte in SA. In the last 50 years, the size of the Naracoorte population of southern bent-winged bats has declined from an estimated 100,000–200,000 in 1963 (Dwyer and Hamilton-Smith, 1965) to 25,000–35,000 in 2011 (Lear, 2012). The Warrnambool population is thought to have declined from 15,000 to 10,000 over the same time period (DELWP, 2017). The subspecies was listed as critically endangered under the Environment Protection and Biodiversity Conservation Act in 2007. The eastern bent-winged bat (M. orianae oceanensis) is more common and widespread, being distributed along the east coast of Australia (Richards and Reardon, 2008). Although numbers appear to be stable in Victoria, the subspecies is listed as vulnerable due to the use of a single maternity site in this state.
While disease has been identified as a possible cause for the declines in southern bent-winged bat populations (DELWP, 2017), there has been only one published disease investigation of this subspecies: in 2009, southern bent-winged bats with pale nodular cutaneous lesions were detected at Naracoorte (McLelland et al., 2013). These were caused by Riouxgolvania beveridgei, a nematode from the Order Muspiceida. Muspicioid parasites have been found previously in bats, rodents, deer, kangaroos, humans and crows (Anderson, 2000), but Riouxgolvania are only parasitic on bats (Hasegawa et al., 2012). Larvae leave the parent worm by migrating between layers of the cuticle of the head, and ultimately escape by perforating the cuticle. This kills the parent, which remains within the nodule (Bain and Chabaud, 1979; Rausch and Rausch, 1983). The nodule formed by the worm in the skin of the bat contains a tiny opening, which allows the larvae to emerge onto the skin. Infection of hosts presumably occurs by direct contact between bats, leading to larval skin penetration (Anderson, 2000). Pathogenicity for bats is unknown, but related parasites have caused severe polymyositis in humans (McKelvie et al., 2013).
Insectivorous bats are also known to host many other ectoparasites, such as bat flies, mites and ticks. Bat flies are a highly specialised group of dipteran ectoparasites that are related to the sheep ked and hippoboscid flies (Dick and Patterson, 2006). They are only found on bats, consuming the blood of their hosts (referred to as haematophagy). There are no reports of bat fly infections causing anaemia, but they have been associated with weight loss (Linhares and Komeno, 2000), increased grooming behaviour (Obame-Nkoghe et al., 2016) and the transmission of potential pathogens, such as dengue virus (Abundes-Gallegos et al., 2018), rhabdoviruses (Goldberg et al., 2017), Bartonella spp. (Wilkinson et al., 2016) and Polychromophilus spp. (Obame-Nkoghe et al., 2016). Transmission of these agents can potentially occur via the feeding activity of the bat flies or by ingestion of the ectoparasites by their bat hosts (Ramanantsalama et al., 2018). There are two families, the Streblidae and the Nycteribiidae (which are wingless) (Maa, 1971). Bat flies reproduce via viviparous puparity, in which eggs are fertilized internally and all larval stages develop within the female (Dick and Patterson, 2006). Larvae moult twice inside the female, and gravid females deposit a single, third instar larva on the roosting substrate. Once deposited, the larva immediately forms a puparium. Following a pupal stage that lasts three to four weeks, an adult fly emerges, which must locate and colonize a bat host (Dick and Patterson, 2006).
Bent-winged bats are parasitised by a number of species of mites. The most common family is the mesostigmatid Spinturnicidae, which is found only on bats, but a number of other mesostigmatid (Rhinonyssidae and Macronyssidae) and prostigmatid (Myobiidae) families are also known to be parasitic on Australian bent-winged bats (Domrow, 1987, 1991). Spinturnix mites remain on the bats year round, including during hibernation, are only present on the wing membranes and consume blood. Minimal pathology has been attributed to mite infections on bats, but recent studies found that they increase grooming behaviour (Godinho et al., 2013) and could act as mechanical vectors of P. destructans (Lucan et al., 2016).
Ticks also parasitise bats, with Ixodes holocyclus, the paralysis tick, causing high mortality and morbidity in flying foxes in northern Australia (Olsson and Woods, 2008). This species of tick has not been found on bent-winged bats, but other Ixodes species have, without causing any apparent ill effects (Arthur, 1956; Roberts, 1970).
As part of a larger disease investigation (Holz et al., 2018a, 2018b), the aims of this study were to compare the presence of each of these parasites in southern bent-winged bats with the more common eastern bent-winged bats and to determine whether there is an association between the parasites and bat health.
2. Material and methods
2.1. Study population and sites
Sampling was undertaken during summer (January–February), autumn (March–April), late winter (August) and early spring (September), between April 2015 and August 2017. Trapping for southern bent-winged bats occurred at the Naracoorte (36.9602° S, 140.7413° E) breeding cave, but, because of the difficult access to the breeding cave near Warrnambool, no trapping occurred there. Instead, those southern bent-winged bats were trapped at nearby non-breeding caves (Allansford (38.3861° S, 142.5931° E) and Portland 1 and 2 (38.3609° S, 141.6041° E)). Eastern bent-winged bats were trapped at abandoned mines at Christmas Hills (37.6515° S, 145.3173° E) and Eildon (37.2343° S, 145.8976° E) and the only Victorian breeding cave near Lakes Entrance (37.8511° S, 147.9958° E) in eastern Victoria. [Note that due to concerns that members of the public may enter caves and disturb bats, this paper uses a generic description of the cave locations, rather than the specific name of each cave.] For details on sampling periods and sample sizes see Table 1.
Table 1.
Prevalence (%) of ectoparasites in southern and eastern bent-winged bats in south-east Australia by species, location and month. All bats are adults unless indicated. Some individuals were infected with multiple species of the same parasite group. n = bat sample size; ND = species not determined (the presence of bat flies and mites on bats were recorded but no specimens were collected for species determination).
| Southern Bent-winged Bats |
Eastern Bent-winged Bats |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Allansford |
Portland 1 |
Portland 2 |
Naracoorte |
Christmas Hills |
Eildon |
Lakes Entrance |
||||
| Sep 2015 |
Sep 2016 |
Feb 2017 |
Aug 2017 |
Jan 2016 |
Sep 2016 |
Apr 2015 |
Sep 2015 |
Sep 2016 |
Mar 2017 |
|
| n = 32 | n = 45 | n = 44 | n = 67a | n = 63 | n = 75 | n = 35 | n = 26 | n = 39 | n = 51b | |
| Bat Flies | 19 | 36 | 52 | 21 | 87 | 17 | 6 | 15 | 18 | 29 |
| Penicillidia tectisentis | 6 | 18 | 27 | ND | 48 | 16 | 0 | 0 | 0 | 0 |
| P. oceanica | 0 | 0 | 0 | ND | 0 | 0 | 6 | 8 | 15 | 18 |
| Nycteribia parilis vicaria | 13 | 7 | 32 | ND | 37 | 3 | 0 | 0 | 3 | 16 |
| Species not determinedc | 0 | 13 | 0 | 21 | 19 | 0 | 0 | 8 | 0 | 0 |
| Mites | 9 | 4 | 95 | 43 | 95 | 8 | 0 | 12 | 0 | 82 |
| Spinturnix loricata | 0 | 4 | 86 | ND | 89 | 1 | 0 | 0 | 0 | 0 |
| S. psi | 0 | 0 | 0 | ND | 0 | 0 | 0 | 4 | 0 | 82 |
| Ichoronyssus miniopteri | 3 | 0 | 20 | ND | 3 | 7 | 0 | 0 | 0 | 10 |
| Macronyssus aristippe | 3 | 0 | 0 | ND | 2 | 0 | 0 | 0 | 0 | 0 |
| Species not determinedc | 3 | 0 | 0 | 43 | 6 | 0 | 0 | 8 | 0 | 0 |
| Ixodes simplex | 16 | 27 | 0 | 3 | 2 | 5 | 0 | 0 | 26 | 0 |
| Riouxgolvania beveridgei | 44 | 53 | 9 | 54 | 6 | 11 | 0 | 8 | 0 | 0 |
A 68th bat was examined for bat flies but escaped before being checked for other ectoparasites.
20 juvenile and 31 adult bats.
It was not possible to determine the species of some of the bat flies and mites collected.
2.2. Sample collection
Individuals were caught as they flew out of the caves/mines, using harp traps (Austbat, Bairnsdale, Victoria (Tidemann and Woodside, 1978)) set at dusk at the entrances. Traps were monitored continually with the bats either left in the harp trap bag, or transferred in small numbers to cloth bags, prior to sampling. All bats were examined for any external signs of disease, aged as juveniles or adults (based on the presence or absence of a cartilaginous core at the metacarpal-phalangeal joint (Brunet-Rossinni and Wilkinson, 2009)), sexed, forearm length measured from carpus to elbow, and weighed. As adults were the focus of this study, much of the sampling occurred outside of the breeding season (October to March), with only a small number of juveniles sampled in March at Lakes Entrance (Table 1).
The body and wings of each bat were scanned for the presence of bat flies, mites and ticks by parting the fur and extending both wings. Where found, a sample of these were removed and placed in an Eppendorf tube containing 70% ethanol. The species of bat fly was determined under a stereo microscope (Nikon SMZ 745T, Tokyo, Japan) using a morphological key (Maa, 1971). Mites were placed on a glass slide, cleared over at least 48 h in lactophenol, and species determined under a compound microscope (Olympus BH-2, Tokyo, Japan) using morphological keys (Domrow, 1971, 1987). Tick species was determined under a stereo microscope using a morphological key (Roberts, 1970). All identifications were undertaken in conjunction with taxon experts.
Nodules found on wings and legs appeared similar grossly to those reported previously from southern bent-winged bats from Naracoorte (McLelland et al., 2013). To confirm their identity, nodules were microscopically examined from six southern bent-winged bats from Portland 1 cave. Bats were anaesthetised by mask induction using isoflurane (Forane, Baxter, Old Toongabbie, Australia) and oxygen. Once anaesthetised, a single nodule was surgically removed from each bat and placed in 70% ethanol. Skin was closed using tissue glue (Vetbond, 3M, St. Paul, USA).
2.3. Statistical analyses
A range of potential internal and external predictor variables were screened for association with presence of infection with bat flies, mites, ticks, and R. beveridgei using univariable logistic regression (using maximum likelihood estimates) and calculating Odds Ratios (OR) (using Wald estimates). Internal variables examined here included location group (South Australian southern bent-winged bat, Victorian southern bent-winged bat and Victorian eastern bent-winged bat), body weight, sex, age (adult or juvenile) and absence/presence of co-infestation with bat flies, mites, ticks, and R. beveridgei nodules (internal factors). Season (spring, summer, autumn, winter) was the only external factor included. The effect of age could only be examined for mites, as this was the one parasite where sufficient numbers were found on juveniles (no ticks and R. beveridgei, and only two juveniles with bat flies) to enable comparisons. Similarly, there were no cases of infection with R. beveridgei or ticks in autumn, so for those two parasites, the predictor “season” included winter, spring and summer only. Residuals were examined to check that model assumptions were met. All factors significant at p < 0.20 were subsequently included in a multivariable logistic regression model, using backward stepping. The final model only included those variables significant at p < 0.05; again, residuals were examined to check that model assumptions were met. All statistics were performed using Minitab 18 (Minitab, USA).
3. Results
None of the bats examined showed external evidence of clinical disease, such as low body weight, fur loss or dermatitis. The nodules removed from the six bats at the Portland 1 cave all contained parasites that were identified morphologically as R. beveridgei (Bain and Chabaud, 1979), the same species as that described from the Naracoorte bats. As a result of these findings, all nodules were assumed to represent infection with R. beveridgei due to their similar appearance and location on affected bats.
Bat fly, mite, tick and R. beveridgei prevalence varied widely between the various bat groups, with 6–87% (bat flies), 0–95% (mites), 0–27% (ticks) and 0–54% (R. beveridgei) of the sampled bats infected, depending on location group and sampling period (Table 1).
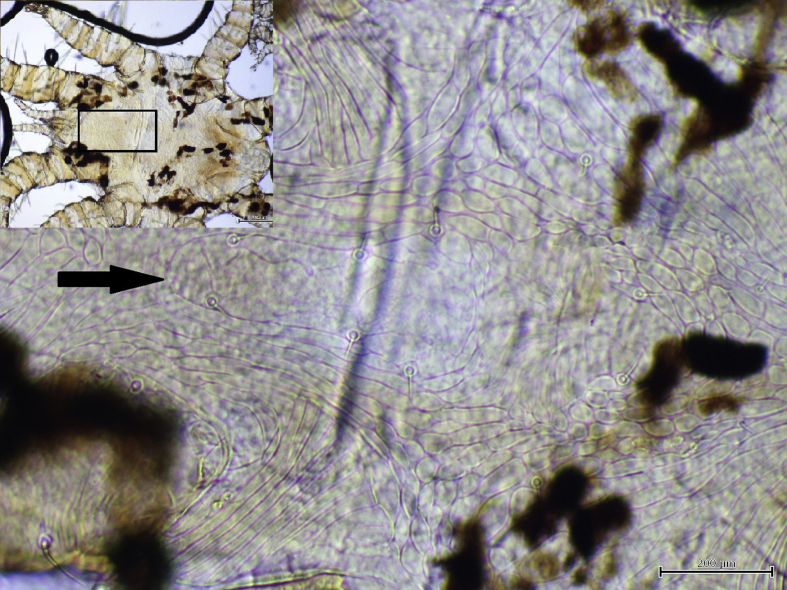
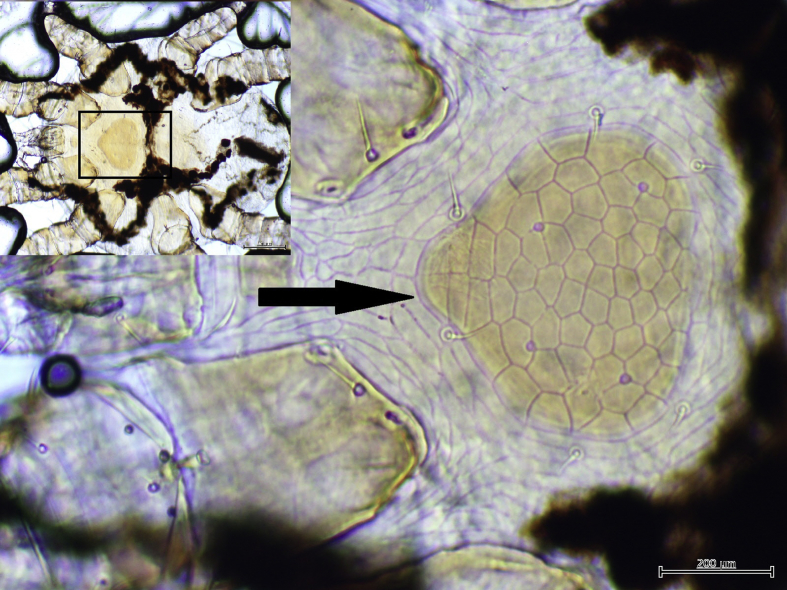
Three species of bat flies were identified: Penicillidia tectisentis (Fig. 1) was only found on southern bent-winged bats (at all locations this subspecies was sampled); Penicillidia oceanica (Fig. 2) was only found on eastern bent-winged bats (at all locations that subspecies was sampled); while Nycteribia parilis vicaria was found on both bat subspecies and at all locations sampled, except Christmas Hills. Mite species identified were Spinturnix loricata (Fig. 3), found on southern bent-winged bats at three of the four locations sampled, and Spinturnix psi (Fig. 4), found on eastern bent-winged bats at two of the three locations sampled. While these represented the majority of mites found, two other species were also detected: Ichoronyssus miniopteri and Macronyssus aristippe (Table 1). All ticks were identified as Ixodes simplex.
Fig. 1.
Dorsal view of Penicillidia tectisentis. Note notopleural setae (Arrow).
Fig. 2.
Dorsal view of Penicillidia oceanica. Note absence of notopleural setae (Arrow).
Fig. 3.
Ventral view of idiosoma of female Spinturnix loricata. Rectangle in inset photograph denotes position of sternal shield. Note elongate sternal shield (Arrow).
Fig. 4.
Ventral view of idiosoma of female Spinturnix psi. Rectangle in inset photograph denotes position of sternal shield. Note subcircular sternal shield (Arrow).
After univariable screening, a range of predictors were significant at the p < 0.20 level. However, in the final, multivariable models, body weight and co-infection with other ectoparasites were not significant (p < 0.05) predictors of infection with any of the parasites examined. The final, multivariable, models showed significant associations between the presence of ectoparasites and age, season and location group (for details on ORs, confidence intervals and p-values of all models, see Table 2). None of the 20 juvenile bats examined were found to carry ticks or R. beveridgei, and only two juveniles were parasitised by bat flies. However, mite infections were more common on juvenile bats than adults (OR = 9.3). Season was a significant predictor of all ectoparasite infections (all p < 0.001). Infection with bat flies and mites was most likely in summer, and while there were no infections in autumn, tick infections were most prevalent in spring, and R. beveridgei infections least prevalent in summer (Table 2). Sex was only significantly associated with infection with R. beveridgei, with males more likely to be parasitised (OR = 2.1). While bat fly and mite infections were not associated with location, Victorian southern bent-winged bats were more likely than South Australian southern bent-winged bats to carry ticks (OR = 6.4) and R. beveridgei (OR = 6.1). Eastern bent-winged bats were less likely to be infected with R. beveridgei than either of the southern bent-winged bat groups, however, they were more likely to carry ticks than the South Australian southern bent-winged bats (Table 2).
Table 2.
Multivariable logistic regression models for bat flies, ticks, mites and Riouxgolvania beveridgei infections in southern (SBWB) and eastern (EBWB) bent-winged bats from Victoria (Vic) and South Australia (SA), for variables significant at the p < 0.05 level.
| Variable | Bat Flies |
Ticks |
Mites |
R. beveridgei |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Location Group | 0.003 | <0.01 | ||||||||||
| Vic EBWB vs SA SBWB |
4.7 | 1.2, 18.1 | 0.20 | 0.04, 0.95 | ||||||||
| Vic SBWB vs SA SBWB |
6.4 | 1.8, 22.8 | 6.1 | 2.9, 12.8 | ||||||||
| Vic SBWB vs Vic EBWB |
1.3 | 0.56, 3.2 | 30.1 | 6.8, 132.6 | ||||||||
| Juvenile vs Adult | 9.3 | 2.5, 34.9 | <0.01 | |||||||||
| Male vs Female | 2.1 | 1.2, 3.7 | 0.009 | |||||||||
| Season | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||
| Spr vs Aut | 0.8 | 0.4, 1.5 | 0.06 | 0.02, 0.15 | ||||||||
| Sum vs Aut | 5.6 | 2.9, 10.8 | 14.3 | 6.4, 31.8 | ||||||||
| Win vs Aut | 1.1 | 0.5, 2.3 | 1.2 | 0.6, 2.4 | ||||||||
| Sum vs Spr | 7.0 | 4.1, 11.8 | 0.08 | 0.01, 0.59 | 229.1 | 89.3, 587.8 | 0.1 | 0.06, 0.35 | ||||
| Win vs Spr | 1.3 | 0.7, 2.6 | 0.12 | 0.03, 0.56 | 19.5 | 8.3, 45.9 | 1.1 | 0.56, 2.1 | ||||
| Win vs Sum | 0.2 | 0.1, 0.4 | 1.6 | 0.14, 18.4 | 0.11 | 0.03, 0.40 | 7.3 | 3.0, 18.1 | ||||
OR = odds ratio; CI – confidence interval; Spr = Spring; Sum = Summer; Aut = Autumn; Win = Winter; significant OR values in bold.
4. Discussion
The aim of this study was to compare parasite types and prevalence between two bent-winged bat subspecies, in order to detect differences and possible associations with indicators of clinical disease that may have contributed to the population decline of the southern bent-winged bat. Both subspecies were found to carry bat flies, mites, ticks and R. beveridgei, with location and season significantly affecting occurrence. None of the bats demonstrated any correlation between infection, low body weight or obvious clinical signs of disease.
While low body weight was not associated with parasitic infections in our study, shortened periods of rest and sleep, as a result of increased time spent grooming, can lead to decreased survival and reproductive success (Giorgi et al., 2001). This has been reported to occur for bat fly and mite infections (Giorgi et al., 2001; Godinho et al., 2013; Linhares and Komeno, 2000; Lucan, 2006; ter Hofstede and Fenton, 2005), but not for ticks or R. beveridgei (Evans, 2009; McLelland et al., 2013). Increased grooming reduces the amount of time bats spend resting and increases their metabolic rate (Giorgi et al., 2001). Southern bent-winged bats at Naracoorte have been found to spend 16% of their roosting time grooming, with 62% at rest, and 22% active (Codd et al., 2003). Grooming activity was not assessed in the current study but it is possible for bat fly and mite infections to have significant consequences for their hosts without necessarily causing weight loss. It is also possible that only very heavy levels of infestation produce clinical disease and, once clinical disease becomes apparent on external observation, mortality is rapid making it difficult to detect affected bats.
A greater proportion of juvenile bats than adults were infected with mites in this study. Mite and bat fly reproduction has been reported to be synchronised with bat reproduction leading to greater parasitic burdens in juveniles and pregnant and lactating females (Lourenço and Palmeirim, 2008; Zahn and Rupp, 2004). This is due to decreased grooming behaviour (an energy expensive activity that declines to compensate for the demands of lactation) and lower immunity in young bats and lactating females, leading to potentially very high burdens (Christe et al., 2000; McLean and Speakman, 1997). Unfortunately only 20 juveniles (none of which were positive for ticks or R. beveridgei and only two were positive for bat flies) were sampled during the breeding season at Lakes Entrance (Mar 2017), so it was not possible to determine any association between parasite infection rate and juvenile body weight.
The seasonality of bat fly and mite infections we observed was also consistent with synchronisation with bat reproduction as mentioned above (Lourenço and Palmeirim, 2008), exposing the greatest number of new hosts to parasitic colonisation. Southern and eastern bent-winged bats give birth in late spring/early summer, depending on location (Dwyer and Hamilton-Smith, 1965), probably resulting here in the significantly increased prevalence of bat fly and mite infections found during the summer season (Table 1, Table 2). However, exceptions to the increased parasite prevalence associated with reproductive cycle hypothesis also occur. Gould's wattled bats (Chalinolobus gouldii) in Melbourne, Victoria, which usually give birth in late spring, were more heavily infected with Trichonyssus womersleyi mites during winter (Evans, 2009). Tick infections in our and the Evans (2009) study, were most common in spring, and R. beveridgei infections were most prevalent in winter and spring. Clearly, parasitic infection prevalence is influenced by multiple factors and determinants likely vary between parasite species.
Environmental conditions, for example, may significantly affect parasite abundance. Ticks require high environmental humidity in order to develop (Randolph and Storey, 1999). Evans (2009) speculated that high humidity during winter may permit nymphal tick development, with a peak in adult numbers occurring during spring. Humidity measured in August 2015 in the Victorian breeding cave and in September 2015 in the nearby Allansford cave was above 80% (unpublished data). However, humidity measured in September 2014 in the South Australian breeding cave was around 60%, and did not climb above 80% until 31 October. Therefore, if humidity is a significant factor in tick development, these climatic differences could explain why significantly more Victorian southern bent-winged bats carried ticks in the spring compared with South Australian southern bent-winged bats. If there were differences in humidity within eastern bent-winged bat roosts this might also explain why the population sampled at Christmas Hills in the spring was negative for ticks, while 26% of the group at Eildon was positive. Unfortunately, humidity data for these study sites was not available.
Little is known about the life history of R. beveridgei, including its prepatent period. The prepatent period of Muspicea borreli, a related parasite found in mice, is 50–60 days (Spratt et al., 2002). It is possible that R. beveridgei transmission may have occurred during summer or autumn but the parasite did not develop to the point of producing visible skin nodules until later in the year, hence the peaks observed in winter and spring. The only previous survey of R. beveridgei infection in bent-winged bats, undertaken during spring 2009, found a higher prevalence of R. beveridgei (45%) in the South Australian bent-winged bats (McLelland et al., 2013) than the in the bats sampled during spring in our study (11%). Due to a dearth of information about the parasite's life cycle, it is not possible to determine why prevalence has declined, or why it was significantly higher for the Victorian southern bent-winged bats in the current study compared with the other two bent-winged bat populations.
In conclusion, significant differences were detected between bent-winged bat populations for tick and R. beveridgei prevalence, and between seasons for all parasite groups. While there was no correlation between these findings and any obvious detrimental effects, factors such as grooming behaviour and resting times were not assessed. These should be examined in future work to determine if ectoparasite infections could be influencing these activities leading to potentially reduced survival and breeding rates.
Previous work has shown that bats living in fragmented habitat can suffer chronic stress leading to reduced immune function (Seltmann et al., 2017) and higher parasite burdens (Christe et al., 2000, 2007; Lourenço and Palmeirim, 2008). The habitat occupied by southern bent-winged bats is highly fragmented. Only 16% of the original native vegetation remains in the South Australian range of the species (South East Natural Resources Management Board, 2010), while the Victorian southern bent-winged bat habitat contains only 17% of its original vegetation (Glenelg Hopkins Catchment Management Authority, 2013). This compares with native vegetation percentages of 45–62% for the eastern bent-winged bat environments (Victorian Environmental Assessment Council, 2010). While the current study examined parasite prevalence, it did not assess parasite burdens on individual bats or their immunocompetence. The difficulties inherent in estimating immune function and ectoparasite load in live animals would make these types of studies problematic. However, the results could inform future management decisions as they may reveal a correlation between habitat quality, parasite infection rates and burdens and bat immune capability.
5. Permits
Samples were collected with approval from the Faculty of Veterinary and Agricultural Science Animal Ethics Committee, University of Melbourne, Victoria (ethics approval 1513456.1), Department of Environment, Land, Water and Planning, Victoria (permit number 0007644), Wildlife Ethics Committee, South Australia (permit number 37/2015) and the Department of Environment, Water and Natural Resources, South Australia (permit number Q26488-1).
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors would like to thank the Holsworth Wildlife Research Endowment, Wildlife Disease Association Australasia, Australian Government’s Threatened Species Discretionary Grants Program, Department of Environment, Land, Water and Planning Victoria, Karst Conservation Fund and David Middleton for providing generous financial support for this project. The lead author is supported by an Australian Postgraduate Award scholarship. The authors acknowledge the valuable assistance provided by Melanie Archer (Victorian Institute of Forensic Medicine) for bat fly species confirmation, Bruce Halliday (CSIRO Canberra) for mite species confirmation and Stephen Barker (University of Queensland) for tick identification. The assistance provided by Ian Beveridge, Amanda Bush, David McLelland and the numerous volunteers involved with the bat trapping and sampling trips is also gratefully acknowledged.
References
- Abundes-Gallegos J., Salas-Rojas M., Galvez-Romero G., Perea-Martinez L., Obregon-Morales C.Y., Morales-Malacara J.B., Chomel B.B., Stuckey M.J., Moreno-Sandoval H., Garcia-Baltazar A., Nogueda-Torres B., Zuniga G., Aguilar-Setien A. Detection of dengue virus in bat flies (Diptera: Streblidae) of common vampire bats, Desmodus rotundus, in Progreso, Hidalgo, Mexico. Vector Borne Zoonotic Dis. 2018;18:70–73. doi: 10.1089/vbz.2017.2163. [DOI] [PubMed] [Google Scholar]
- Anderson R.C. second ed. Centre for Agriculture and Biosciences International; 2000. The Superfamily Muspiceoidea, Nematode Parasites of Vertebrates: Their Development and Transmission; pp. 622–624. [Google Scholar]
- Arthur D.R. The Ixodes ticks of chiroptera (Ixodoidea, Ixodidae) J. Parasitol. 1956;42:180–196. [PubMed] [Google Scholar]
- Bain O., Chabaud A.-G. Sur les Muspiceidae (Nematoda—Dorylaimina) Ann. Parasitol. Hum. Comp. 1979;54:207–225. doi: 10.1051/parasite/1979542207. [DOI] [PubMed] [Google Scholar]
- Barrett J.L., Carlisle M.S., Prociv P. Neuro-angiostrongylosis in wild black and grey-headed flying foxes (Pteropus spp.) Aust. Vet. J. 2002;80:554–558. doi: 10.1111/j.1751-0813.2002.tb11039.x. [DOI] [PubMed] [Google Scholar]
- Blehert D.S. Fungal disease and the developing story of bat white-nose syndrome. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet-Rossinni A.K., Wilkinson G.S. Methods for age estimation and the study of senescence in bats. In: Kunz T.H., Parsons S., editors. Ecological and Behavioral Methods for the Study of Bats. Johns Hopkins University Press; Baltimore: 2009. pp. 315–325. [Google Scholar]
- Cardinal B.R., Christidis L. Mitochondrial DNA and morphology reveal three geographically distinct lineages of the large bentwing bat (Miniopterus schreibersii) in Australia. Aust. J. Zool. 2000;48:1–19. [Google Scholar]
- Christe P., Arlettaz R., Vogel P. Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis) Ecol. Lett. 2000;3:207–212. [Google Scholar]
- Christe P., Glaizot O., Evanno G., Bruyndonckx N., Devevey G., Yannic G., Patthey P., Maeder A., Vogel P., Arlettaz R. Host sex and ectoparasites choice: preference for, and higher survival on female hosts. J. Anim. Ecol. 2007;76:703–710. doi: 10.1111/j.1365-2656.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- Codd J.R., Sanderson K.J., Branford A.J. Roosting activity budget of the southern bent-wing bat (Miniopterus schreibersii bassanii) Aust. J. Zool. 2003;51:307–316. [Google Scholar]
- de Castro F., Bolker B. Mechanisms of disease-induced extinction. Ecol. Lett. 2005;8:117–126. [Google Scholar]
- DELWP . Department of Environment; Land, Water and Planning, Melbourne: 2017. National Recovery Plan for the Southern Bent-wing Bat Miniopterus Schreibersii Bassanii Draft. [Google Scholar]
- Dick C.W., Patterson B.D. Bat flies - obligate ectoparasites of bats. In: Morand S., Krasnov B.R., Poulin R., editors. Micromammals and Macroparasites. Springer; Tokyo: 2006. pp. 179–194. [Google Scholar]
- Domrow R. Acari Spinturnicidae from Australia and new Guinea. Acarologia. 1971;13:552–584. [Google Scholar]
- Domrow R. Acari Mesostigmata parasitic on Australian vertebrates: an annotated checklist. keys and bibliography. Invertebr. Taxon. 1987;1:817–948. [Google Scholar]
- Domrow R. Acari Prostigmata (excluding Trombiculidae) parasitic on Australian vertebrates: an annotated checklist, keys and bibliography. Invertebr. Taxon. 1991;4:1283–1376. [Google Scholar]
- Dwyer P.D., Hamilton-Smith E. Breeding caves and maternity colonies of the bent-winged bat in south-eastern Australia. Helictite. 1965;4:3–21. [Google Scholar]
- Evans L.N. Faculty of Veterinary Science. University of Melbourne; Melbourne: 2009. Roosting Behaviour of Urban Microbats: the Influence of Ectoparasites, Roost Microclimate and Sociality; p. 226. [Google Scholar]
- Giorgi M.S., Arlettaz R., Christe P., Vogel P. The energetic grooming costs imposed by a parasitic mite (Spinturnix myoti) upon its bat host (Myotis myotis) Proc. Biol. Sci. 2001;268:2071–2075. doi: 10.1098/rspb.2001.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenelg Hopkins Catchment Management Authority . 2013. Glenelg Hopkins Regional Catchment Strategy 2013-2019, Hamilton; p. 84. [Google Scholar]
- Godinho L.N., Cripps J.K., Coulson G., Lumsden L.F. The effect of ectoparasites on the grooming behaviour of Gould's wattled bat (Chalinolobus gouldii): an experimental study. Acta Chiropterol. 2013;15:463–472. [Google Scholar]
- Goldberg T.L., Bennett A.J., Kityo R., Kuhn J.H., Chapman C.A. Kanyawara virus: a novel rhabdovirus infecting newly discovered Nycteribiid bat flies infesting previously unknown pteropodid bats in Uganda. Sci. Rep. 2017;7:5287. doi: 10.1038/s41598-017-05236-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Candela M., Leon-Vizcaino L., Cubero-Pablo M.J. Population effects of sarcoptic mange in barbary sheep (Ammotragus lervia) from Sierra Espuna regional park, Spain. J. Wildl. Dis. 2004;40:456–465. doi: 10.7589/0090-3558-40.3.456. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Sato M., Maeda K., Murayama Y. Description of Riouxgolvania kapapkamui sp. N. (Nematoda: Muspiceoidea: Muspiceidae), a peculiar intradermal parasite of bats in Hokkaido, Japan. J. Parasitol. 2012;98:995–1000. doi: 10.1645/GE-2710.1. [DOI] [PubMed] [Google Scholar]
- Holz P.H., Lumsden L.F., Druce J., Legione A.R., Vaz P., Devlin J.M., Hufschmid J. Virus survey in populations of two subspecies of bent-winged bats (Miniopterus orianae bassanii and oceanensis) in south-eastern Australia reveals a high prevalence of diverse herpesviruses. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz P.H., Lumsden L.F., Marenda M.S., Browning G.F., Hufschmid J. Two subspecies of bent-winged bats (Miniopterus orianae bassanii and oceanensis) in southern Australia have diverse fungal skin flora but not Pseudogymnoascus destructans. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear K. An American in Australia: monitoring the maternity colony of southern bent-wing bats (Miniopterus schreibersii bassanii) at Naracoorte Caves National Park, South Australia. J Aust Cave Karst Manage Assoc. 2012;86:15–18. [Google Scholar]
- Linhares A.X., Komeno C.A. Trichobius joblingi, Aspidoptera falcata, and Megistopoda proxima (Diptera : Streblidae) parasitic on Carollia perspicallata and Sturnia lillium (Chiroptera : Phyllostomidae) in southeastern Brazil: sex ratios, seasonality, host site preference, and effect of parasitism on the host. J. Parasitol. 2000;86:167–170. doi: 10.1645/0022-3395(2000)086[0167:TJAFAM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lourenço S., Palmeirim J.M. Which factors regulate the reproduction of ectoparasites of temperate-zone cave-dwelling bats? Parasitol. Res. 2008;104:127–134. doi: 10.1007/s00436-008-1170-6. [DOI] [PubMed] [Google Scholar]
- Lucan R.K. Relationships between the parasitic mite Spinturnix andegavinus (Acari: Spinturnicidae) and its bat host, Myotis daubentonii (Chiroptera: Vespertilionidae): seasonal, sex- and age-related variation in infestation and possible impact of the parasite on the host condition and roosting behaviour. Folia Parasitol (Praha) 2006;53:147–152. doi: 10.14411/fp.2006.019. [DOI] [PubMed] [Google Scholar]
- Lucan R.K., Bandouchova H., Bartonicka T., Pikula J., Zahradnikova A., Jr., Zukal J., Martinkova N. Ectoparasites may serve as vectors for the white-nose syndrome fungus. Parasit Vectors. 2016;9:16. doi: 10.1186/s13071-016-1302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa T.C. Revision of the Australian batflies. Pac. Insects Monogr. 1971;28:1–118. [Google Scholar]
- Martin R.W., Handasyde K.A., Skerratt L.F. Current distribution of sarcoptic mange in wombats. Aust. Vet. J. 1998;76:411–414. doi: 10.1111/j.1751-0813.1998.tb12391.x. [DOI] [PubMed] [Google Scholar]
- McCallum H. Disease and the dynamics of extinction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:2828–2839. doi: 10.1098/rstb.2012.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvie P., Reardon K., Bond K., Spratt D.M., Gangell A., Zochling J., Daffy J. A further patient with parasitic myositis due to Haycocknema perplexum, a rare entity. J. Clin. Neurosci. 2013;20:1019–1022. doi: 10.1016/j.jocn.2012.08.009. [DOI] [PubMed] [Google Scholar]
- McLean J.A., Speakman J.R. Non-nutritional maternal support in the brown long-eared bat. Anim. Behav. 1997;54:1193–1204. doi: 10.1006/anbe.1997.0498. [DOI] [PubMed] [Google Scholar]
- McLelland D.J., Reardon T., Bourne S., Dickason C., Kessell A., Boardman W. Outbreak of skin nodules associated with Riouxgolvania beveridgei (Nematoda: Muspiceida) in the southern bentwing bat (Miniopterus schreibersii bassanii), South Australia. J. Wildl. Dis. 2013;49:1009–1013. doi: 10.7589/2012-11-288. [DOI] [PubMed] [Google Scholar]
- Obame-Nkoghe J., Rahola N., Bourgarel M., Yangari P., Prugnolle F., Maganga G.D., Leroy E.M., Fontenille D., Ayala D., Paupy C. Bat flies (Diptera: Nycteribiidae and Streblidae) infesting cave-dwelling bats in Gabon: diversity, dynamics and potential role in Polychromophilus melanipherus transmission. Parasit Vectors. 2016;9:333. doi: 10.1186/s13071-016-1625-z. 310.1186/s13071-13016-11625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A., Woods R. Bats. In: Vogelnest L., Woods R., editors. Medicine of Australian Mammals. CSIRO Publishing; Collingwood, Australia: 2008. pp. 465–502. [Google Scholar]
- Ramanantsalama R.V., Andrianarimisa A., Raselimanana A.P., Goodman S.M. Rates of hematophagous ectoparasite consumption during grooming by an endemic Madagascar fruit bat. Parasit Vectors. 2018;11:330. doi: 10.1186/s13071-018-2918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph S.E., Storey K. Impact of microclimate on immature tick-rodent host interactions (Acari: Ixodidae): implications for parasite transmission. J. Med. Entomol. 1999;36:741–748. doi: 10.1093/jmedent/36.6.741. [DOI] [PubMed] [Google Scholar]
- Rausch R.L., Rausch V.R. Maseria vespertilionis n.g., n.sp. (Dorylaimina : Muspiceidae), a nematode from nearctic bats (Vespertilionidae) Ann. Parasitol. Hum. Comp. 1983;58:361–376. doi: 10.1051/parasite/1983584361. [DOI] [PubMed] [Google Scholar]
- Richards G.C., Reardon T.B. Family Miniopteridae. In: Van Dyck S., Strahan R., editors. The Mammals of Australia. third ed. Reed New Holland; Sydney: 2008. pp. 503–510. [Google Scholar]
- Roberts F.H.S. CSIRO; Melbourne: 1970. Australian Ticks. [Google Scholar]
- Rosenkranz P., Aumeier P., Ziegelmann B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010;103(Suppl. 1):S96–S119. doi: 10.1016/j.jip.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Seltmann A., Czirjak G.A., Courtiol A., Bernard H., Struebig M.J., Voigt C.C. Habitat disturbance results in chronic stress and impaired health status in forest-dwelling paleotropical bats. Conserv Physiol. 2017;5:cox020. doi: 10.1093/conphys/cox020. doi:010.1093/conphys/cox1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulsbury C.D., Iossa G., Baker P.J., Cole N.C., Funk S.M., Harris S. The impact of sarcoptic mange Sarcoptes scabiei on the British fox Vulpes vulpes population. Mamm Rev. 2007;37:278–296. [Google Scholar]
- South East Natural Resources Management Board . 2010. South East Natural Resources Management Plan, Mt. Gambier; p. 158. [Google Scholar]
- Spratt D.M., Haycock P., Boyden J.M., Nicholas W.L. Aspects of the life history of Muspicea borreli (Nematoda: Muspiceidae), parasite of the house mouse (Mus domesticus) in Australia. Parasite. 2002;9:199–205. doi: 10.1051/parasite/2002093199. [DOI] [PubMed] [Google Scholar]
- ter Hofstede H.M., Fenton M.B. Relationships between roost preferences, ectoparasite density, and grooming behaviour of neotropical bats. J Zool Lond. 2005;266:333–340. [Google Scholar]
- Tidemann C.R., Woodside D.P. A collapsible bat-trap and a comparison of results obtained with the trap and with mist-nets. Aust. Wildl. Res. 1978;5:355–362. [Google Scholar]
- Victorian Environmental Assessment Council . 2010. Remnant Native Vegetation Investigation Discussion Paper, East Melbourne; pp. 57–117. [Google Scholar]
- Wibbelt G., Moore M.S., Schountz T., Voigt C.C. Emerging diseases in Chiroptera: why bats? Biol. Lett. 2010;6:438–440. doi: 10.1098/rsbl.2010.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D.A., Duron O., Cordonin C., Gomard Y., Ramasindrazana B., Mavingui P., Goodman S.M., Tortosa P. The bacteriome of bat flies (Nycteribiidae) from the Malagasy region: a community shaped by host ecology, bacterial transmission mode, and host-vector specificity. Appl. Environ. Microbiol. 2016;82:1778–1788. doi: 10.1128/AEM.03505-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn A., Rupp D. Ectoparasite load in European vespertilionid bats. J Zool Lond. 2004;262:383–391. [Google Scholar]