Abstract
In humans, depression is often associated with low-grade inflammation, activation of the tryptophan/kynurenine pathway, and mild lymphopenia. Preclinical research confirms that inflammation induces depression-like behavior through activation of the tryptophan/kynurenine pathway. However, the mechanisms governing recovery from depression are unknown. Understanding the pathways leading to resolution of depression will likely lead to identification of novel targets for treatment. We investigated the contribution of T lymphocytes to the resolution of lipopolysaccharide-induced depression-like behavior. Duration of depression-like behavior was markedly prolonged in mice without mature T or B lymphocytes (Rag1−/− mice). This prolonged depression-like behavior was associated with persistent upregulation of the tryptophan-metabolizing enzyme indoleamine-2,3-dioxygenase (Ido)1 in the prefrontal cortex (PFC). Reconstitution of Rag1−/− mice with T lymphocytes normalized resolution of depression-like behavior and expression of Ido1 in the PFC. During resolution of inflammation-induced depression-like behavior, T lymphocytes accumulated in the meninges and were required for induction of interleukin (IL)-10 in the meninges and the PFC. Inhibition of IL-10 signaling by nasal administration of neutralizing anti–IL-10 antibody to WT mice led to persistent upregulation of Ido1 in the PFC and prolonged depression-like behavior. Conversely, nasal administration of recombinant IL-10 in Rag1−/− mice normalized Ido1 expression and resolution of depression-like behavior. In conclusion, the present data show for the first time that resolution of inflammation-induced depression is an active process requiring T lymphocytes acting via an IL-10–dependent pathway to decrease Ido1 expression in the brain. We propose that targeting the T lymphocyte/IL-10 resolution pathway could represent a novel approach to promote recovery from major depressive disorder.
Introduction
According to the World Health Organization, ~350 million people suffer from major depressive disorder (MDD), and 76 million years are lost to disability worldwide owing to depression [1]. The treatment of MDD remains a major challenge, and antidepressant drugs are effective in only half of patients [2]. We propose that understanding the mechanism of spontaneous resolution of inflammation-induced depression can lead to development of more effective drugs to treat depression related to inflammation.
MDD is often associated with alterations in the immune system, including increased circulating levels of biomarkers of inflammation [3, 4], reduced blood lymphocyte counts, and reduced proliferative responses of lymphocytes to mitogens [5–8]. Inflammatory mediators induce depression in patients treated with recombinant cytokines. Administration of interferon (IFN)-α and interleukin (IL)-2, to treat hepatitis C virus infection [9] or cancer, is associated with development of depressive symptoms [10]. In these patients, development of depressive symptoms associated with a reduced level of circulating tryptophan [11]. Tryptophan is metabolized into kynurenine by the cytokine-inducible enzyme indoleamine 2,3-dioxygenase (IDO)1. A positive correlation between circulating IDO1 enzymatic activity and depression scores was found in patients with mastocytosis [12]. Higher IDO1 enzymatic activity was also observed in suicidal adolescents with MDD [13]. Moreover, several studies report positive associations between depressive symptoms and plasma or cerebrospinal fluid concentrations of the neurotoxic kynurenine pathway metabolite quinolinic acid [14].
The mechanisms that mediate development of symptoms of depression in response to inflammation have been studied extensively at the preclinical level [15–18]. In rodents, transient depression-like behavior can be induced by administration of lipopolysaccharide (LPS) [15, 16, 19]. LPS-treated mice first develop sickness behavior characterized by reductions in body weight, food intake, and locomotor activity that resolves after 14–18 h and results from the production of proinflammatory cytokines at the periphery and in the brain [15]. This transient episode of sickness behavior is followed by a phase of depression-like behavior, evidenced as increased immobility in the forced swim test (FST) and tail-suspension test (TST). Proinflammatory cytokines such as IFN-γ, tumor necrosis factor (TNF)-α, and IL-1β [20, 21] increase the expression of Ido1, thereby activating the kynurenine pathway resulting in depression-like behavior. IDO1 activity is necessary for LPS-induced depression-like behavior but not for sickness behavior in response to LPS [16, 22]. Spontaneous resolution of LPS-induced depression-like behavior occurs after 1 day [15, 23], but the mechanisms governing this resolution are unknown.
The contribution of innate immunity to depression has been well investigated. However, much less is known about the role of adaptive immunity. T lymphocytes have been reported to survey the brain from the meninges and contribute to central nervous system homeostasis [24]. T lymphocytes regulate social interactions [25], cognitive functions [26], stress resilience [27–29], and neuronal repair in different nerve-injury models [30–33]. However, it is not known how resolution of inflammation-induced depression is regulated. One possibility is that T lymphocytes induced an anti-inflammatory response [28]. Here we investigated the possible role of T lymphocytes and IL-10 signaling in the resolution of inflammation-induced depression-like behavior in a mouse model.
Materials and methods
Animals
Male (10–14 weeks old) wild-type (WT), Rag1−/−(no mature T and B cells) [34], and Il10−/− mice on a C57BL/6J genetic background (Jackson Laboratory, Bar Harbor, ME) were housed and bred at The University of Texas MD Anderson Cancer Center. All procedures were approved by the Institutional Animal Care and Use Committee.
Peripheral inflammation was induced with LPS (serotype 0127:B8; cat#L3129, Sigma-Aldrich, St Louis, USA) 0.83 mg/kg intraperitoneally [19, 35]. Sickness was quantified by measuring food intake, locomotor activity, and body-weight loss. Depression-like behavior was assessed by measuring duration of immobility in the FST and TST (Supplementary Information).
Adoptive T lymphocyte transfer with CD3+ T lymphocytes (8 × 106 in 100 µL 0.1% bovine serum albumin/phosphate-buffered saline (PBS) (w/v) into the tail vein) isolated using negative selection kit II (#130-095-130; Miltenyi Biotec Inc, San Diego, CA, USA) was performed 1 week before LPS injection, as described [36]. Control mice received vehicle.
Nasal administration of neutralizing anti–IL-10 antibody and recombinant IL-10
Thirty min before antibody or recombinant IL-10 fusion of human IL-10 and human IL-4 connected via a linker sequence to increase its stability [36–38], A concentration of 3 µl of hyaluronidase (total 100 U; Sigma-Aldrich) in PBS was delivered to each nostril [39]. Four doses of 3 µl immunoglobulin (Ig)G or anti–IL-10 (#l5145 produced in goat; Sigma-Aldrich), 1 µg/µl in PBS, total 12 µg/mouse/day, were administered to the nostrils daily from day 0 to day 3. The nasal route of administration has been used before for antibodies [40, 41]. Six µg of recombinant IL-10 was injected intranasally at 2 days and 3 days after LPS injection. PBS was used as control.
Quantitative reverse-transcription polymerase chain reaction
Mice were euthanized by CO2 exposure and transcardially perfused with ice-cold PBS; tissues were snap-frozen in liquid nitrogen. Meninges were collected as described by Louveau et al [42]. (Supplementary information). Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) Taqman was used to measure mRNA levels (supplementary information).
Staining
The whole meninges were mounted and stained with DAPI and rat anti-mouse anti-CD3 antibody (eBioscience #14-0032-85, dilution 1:1000) and then incubated with secondary antibody goat anti-rat (Alexa-488, Invitrogen, dilution 1:500).
Cell culture
BV2 microglia (200,000 cells per well into a 12-well plate) were cultured with 1 ng/ml of murine recombinant IFN-γ (#315-05; Peprotech, Rocky Hill, NJ, USA) [20] ±10 ng/ml of recombinant IL-10 [36, 37] in Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum and antibiotics (Invitrogen, Carlsbad, CA, USA) at 37 °C with 5% CO2.
Statistical analysis
Differences in behavioral activity and mRNA expression levels were assessed by Student’s t test, one-way or repeated-measure two-way ANOVA followed by Bonferroni correction for multiple tests, depending on experimental design. Significance was indicated as***P < 0.001, **P < 0.01, *P < 0.05.
Results
Contribution of T lymphocytes to LPS-induced depression-like behavior
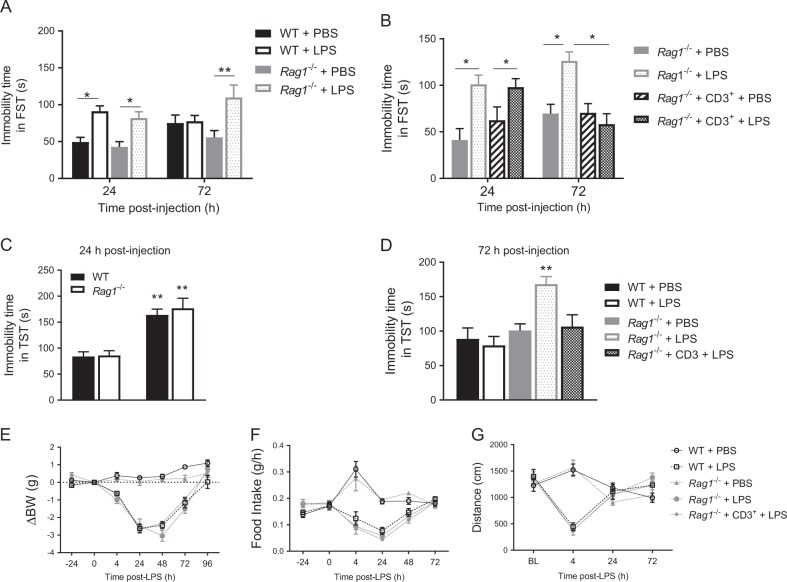
To evaluate the contribution of T lymphocytes to depression-like behavior, we compared the course of the LPS-induced increase in immobility in the FST between WT mice and Rag1−/− mice, which lack mature T and B lymphocytes. At 24 h after LPS injection, the increase in immobility in the FST was similar in WT and Rag1−/− mice (Fig. 1a). At 72 h after LPS, WT mice had fully recovered, but Rag1−/− mice still showed increased immobility in the FST, indicating that resolution of depression-like behavior is impaired in the absence of T and B lymphocytes (Fig. 1a). To determine whether T lymphocytes are required and sufficient for resolution of depression-like behavior, CD3+ T lymphocytes isolated from the spleens of WT mice were adoptively transferred to Rag1−/− mice 1 week before LPS injection. Reconstitution of Rag1−/− mice with T lymphocytes normalized the resolution of depression-like behavior; as shown by a return to baseline levels of the immobility time in the FST 72 h post-LPS (Fig. 1b). Reconstitution of Rag1−/− mice with CD3+ T lymphocytes did not affect behavior in the FST in the PBS-treated mice at 24 or 72 h or in the LPS-treated mice at 24 h (Fig. 1b). In separate cohorts of mice, we obtained similar results when using the TST as a measure of depression-like behavior; at 24 h the increase in immobility in the TST was similar in WT and Rag1−/− mice (Fig. 1c). At 72 h after LPS, Rag1−/− mice showed increased immobility in the TST while WT and reconstituted Rag1−/− mice did not show evidence of depression-like behavior at this time point (Fig. 1d). These data indicate that CD3+ T lymphocytes are crucial for resolution of LPS-induced depression-like behavior but do not contribute to its onset and severity.
Fig. 1.
T lymphocytes are necessary for resolution of lipopolysaccharide (LPS)-induced depression-like behavior. a The forced-swim test (FST) was performed in wild-type (WT) and Rag1−/− mice (n = 6 mice/group) 24 h and 72 h after injection of phosphate-buffered saline (PBS) or LPS. Two-way ANOVA followed by Bonferroni’s multiple correction test (treatment effect F(3,40) = 9.31, P < 0.0001). b FST was performed in Rag1−/− and reconstituted Rag1−/− mice (n = 8 mice/group) 24 h and 72 h after injection of LPS. Two-way ANOVA followed by Bonferroni’s correction (time × treatment interaction F(3,56) = 4.09, P = 0.011). c The tail-suspension test (TST) was performed in WT and Rag1−/− mice at 24 h post-injection (n = 7 mice/group). Two-way ANOVA followed by Bonferroni’s correction (LPS effect F(1,24) = 45.1, P = 0.0001). d TST in WT, Rag1−/− and reconstituted Rag1−/− mice (n = 6 mice/group) at 72 h after injection of LPS. One-way ANOVA followed by Bonferroni’s correction (F(4,25) = 6.49, P = 0.001). e Body weight, (f) food intake, and (g) spontaneous locomotor activity were monitored after injection of PBS or LPS (n = 8 mice/group). The data are presented as mean ± standard error of the mean. **P < 0.01, *P < 0.05
There were no differences in the course of LPS-induced reductions in body weight, food intake, and spontaneous locomotor activity between WT mice, Rag1−/− mice, and Rag1−/− mice reconstituted with T lymphocytes (Fig. 1e–g and Supplementary Figure S1A, B). These data imply that T lymphocytes do not contribute to the onset and resolution of LPS-induced sickness behavior.
The homing and survival of transferred T lymphocytes in the Rag1−/− mice was confirmed by flow cytometric analysis of peripheral blood (Supplementary Figure S2A) and qRT-PCR analysis of Cd3 expression in the spleen and the meninges (Supplementary Figure S2B, C).
Contribution of T lymphocytes to normalization of Ido1 expression in the prefrontal cortex
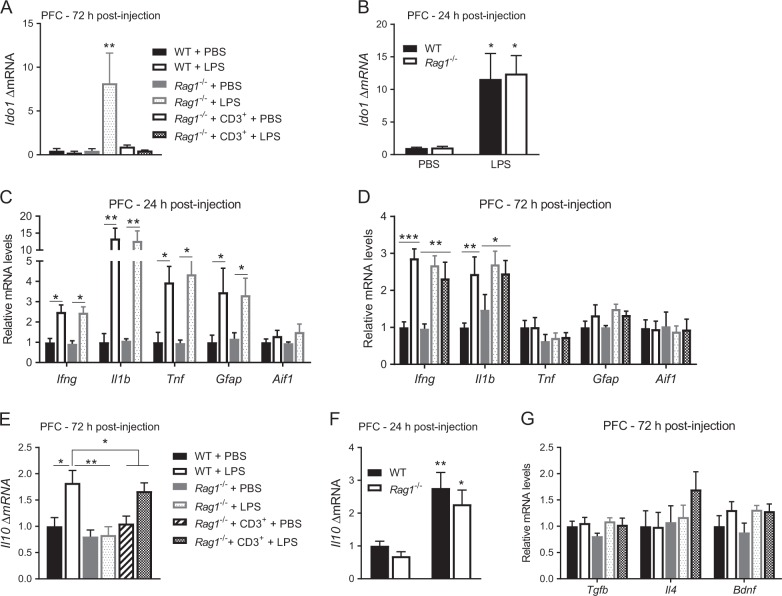
The tryptophan-metabolizing enzyme IDO1 is necessary for LPS-induced depression-like behavior [22]. We determined whether the prolongation of depression-like behavior observed in T lymphocyte-deficient mice was associated with prolonged upregulation of brain Ido1. In the prefrontal cortex (PFC) of Rag1−/− mice, Ido1 levels were still upregulated 72 h post-LPS, whereas Ido1 levels had returned to basal levels in WT mice and in Rag1−/− mice reconstituted with T lymphocytes (Fig. 2a). Importantly, 24 h post-LPS, Ido1 mRNA levels in the PFC were similar in WT and Rag1−/− mice (Fig. 2b). In contrast, at 72 h post-LPS, Ido1 mRNA in the hippocampus of WT and Rag1−/− mice had returned to baseline levels (Figure S3A). These data indicate that T lymphocytes are required for normalization of both Ido1 expression in the PFC and resolution of depression-like behavior.
Fig. 2.
T lymphocytes regulate Il10 and Ido1 expression in the prefrontal cortex (PFC). a Ido1 mRNA level in the PFC 72 h after injection of lipopolysaccharide (LPS) (n = 6 mice per group). One-way ANOVA followed by Bonferroni’s multiple correction test (F(4,25) = 5.02, P = 0.004). b Ido1 mRNA level in the PFC 24 h after injection of LPS (n = 6–7 mice per group). Two-way ANOVA followed by Bonferroni’s multiple correction test (genotype x LPS interaction F(1,22) = 0.02, p = 0.89). mRNA levels of glial cell markers and proinflammatory cytokines in the PFC 24 h (c) and 72 h (d) post-LPS (n = 6–8 mice per group). e Il10 mRNA level in the PFC 72 h after injection in WT, Rag1−/− and reconstituted Rag1−/− mice (n = 8 mice/group). One-way ANOVA followed by Bonferroni’s multiple correction test (F(5,42) = 6.86, P = 0.0001). f Il10 mRNA level in the PFC 24 h after injection in WT and Rag1−/− mice (n = 7 mice/group). Two-way ANOVA followed by Bonferroni’s multiple correction test (genotype × LPS interaction F(1,21) = 0.06, p = 0.81). g Anti-inflammatory cytokine expression in the PFC 72 h post-LPS (n = 6–8 mice/group). Data are normalized to expression levels in the PBS group. The gene Aif1 encodes IBA1. The data are presented as mean ± standard error of the mean. ***P < 0.001, **P < 0.01, *P < 0.05
Contribution of T lymphocytes to cytokine expression
Glial-cell activation and increased production of proinflammatory cytokines in the brain contribute to Ido1 upregulation [43]. We investigated whether the T lymphocyte-dependent normalization of Ido1 in the PFC and resolution of depression-like behavior were associated with normalization of markers of glial activity and cytokine production in the PFC. At 24 h post-LPS, upregulation of glial activation markers Gfap and Aif3 (encoding Iba1) or proinflammatory cytokines such as Ifng, Il1b and Tnf, did not differ between WT mice and Rag1−/− mice (Fig. 2c). At 72 h post-LPS, Ifng, Il1b, Gfap and Aif1 expression in the PFC were upregulated similarly in WT mice and Rag1−/− mice with or without T lymphocyte reconstitution (Fig. 2d). Tnf expression in the PFC was not increased at this time point. These data indicate that the prolonged depression-like behavior and upregulation of Ido1 mRNA in the PFC of LPS-treated Rag1−/− mice did not result from an exaggerated acute and/or a prolonged increase in glial activation or in expression of proinflammatory cytokines.
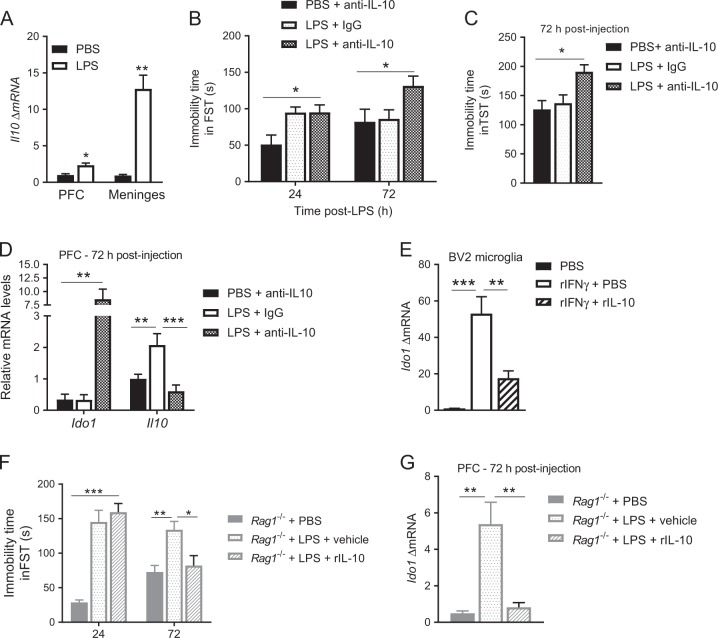
Next, we examined the expression of anti-inflammatory cytokines and brain-derived neurotrophic factor (BDNF) in the PFC. At 72 h after LPS, expression of Il10 was upregulated in WT mice but not in the PFC of Rag1−/− mice. Interestingly, reconstitution of Rag1−/− mice with T lymphocytes restored the upregulation of Il10 expression 72 h after LPS (Fig. 2e). Interestingly, Il10 mRNA is upregulated in both WT and Rag1−/− mice at 24 h (Fig. 2f). At 72 h post-LPS, Tgfb, Il4, and Bdnf mRNA levels were similar to baseline levels in WT, Rag1−/−, and reconstituted Rag1−/− mice (Fig. 2g). The expression patterns of these anti-inflammatory cytokines were similar in the hippocampus (Figure S3B).
In the liver, expressions of proinflammatory and anti-inflammatory cytokines were similar in all LPS-treated groups at 72 h post-LPS, indicating that peripheral inflammation is not dependent on T lymphocytes (Supplementary Figure S3C).
Contribution of T lymphocytes to the upregulation of IL-10 during resolution of depression-like behavior
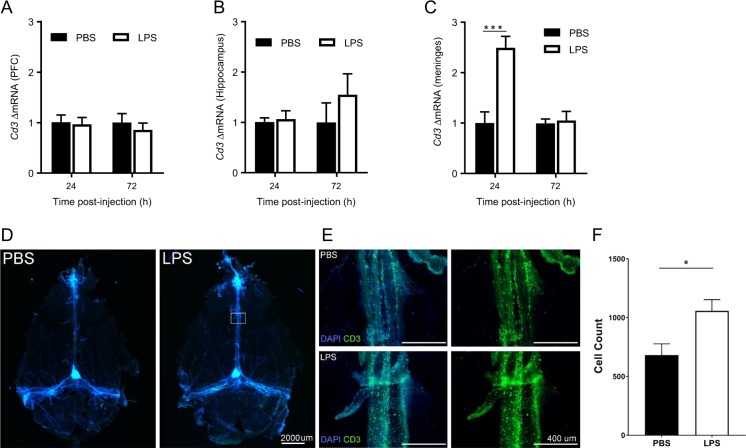
To determine whether T lymphocytes are the source of the increase in Il10 in the PFC 72 h after LPS, we first determined whether Cd3 mRNA increases in the PFC during resolution of LPS-induced depression-like behavior. LPS did not alter Cd3 levels in the PFC and the hippocampus (Fig. 3a, b). However, LPS induced a significant increase in the expression of Cd3 in the meninges 24 h post-LPS in WT mice. Meningeal Cd3 levels had returned to baseline 72 h post-LPS (Fig. 3c). Immunofluorescence analysis of whole mounted meninges confirmed accumulation of CD3+ T lymphocytes in the meninges at 24 h after LPS. (Fig. 3d–f).
Fig. 3.
T lymphocytes transiently accumulate in the meninges prior resolution of depression-like behavior. a Cd3 mRNA level in the prefrontal cortex (PFC) 24 h and 72 h after lipopolysaccharide (LPS) injection in wild-type (WT) mice (n = 8 mice/group). b Cd3 mRNA level in the hippocampus 24 h and 72 h after (c) Cd3 mRNA level in the meninges 24 h and 72 h post-LPS in WT mice (n = 6 mice/group). Two-way ANOVA followed by Bonferroni’s correction (time × treatment interaction F(1,10) = 24.1, P = 0.0006). d Representative images of the whole mounted meninges (×4) stained with DAPI and anti-CD3 (green) 24 h after PBS or LPS. e Representative images of higher magnification (×10) of the central sinus of the meninges indicated by a square in the previous panel. f Quantification of the CD3+ T lymphocytes in the 3 sinus of the meninges 24 h after LPS (n = 4 mice/group). T test (df = 6, p = 0.03). The data are presented as mean ± standard error of the mean. ***P < 0.001, **P < 0.01, *P < 0.05
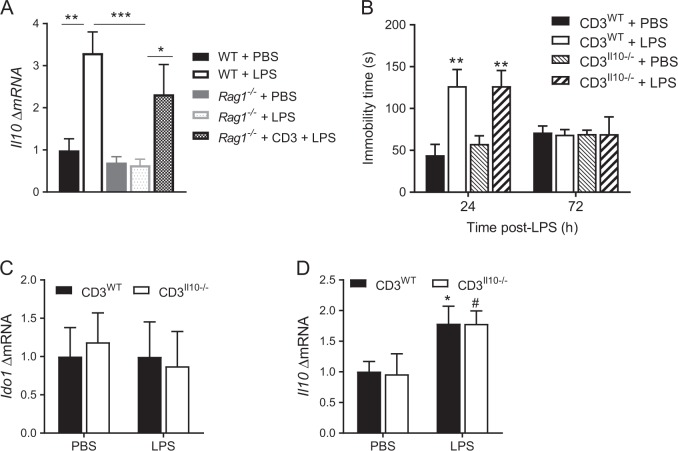
LPS increased meningeal Il10 expression in WT and reconstituted Rag1−/− mice 72 h post-LPS (Fig. 4a). However, T lymphocytes are not the source of the IL-10 because the transfer of Il10−/− T lymphocytes normalized the resolution of depression-like behavior similarly to the way WT T lymphocytes did (Fig. 4b). Moreover, the lack of Il10 in the T lymphocytes did not impair normalization of Ido1 expression nor the upregulation of Il10 mRNA in the PFC 72 h post-LPS (Fig. 4c, d). WT or Il10−/− T lymphocytes did not alter onset of depression-like behavior or onset and resolution of the sickness response (Supplementary Figure S4A–C).
Fig. 4.
T lymphocytes are not the source of IL-10 to induce resolution. a Il10 mRNA level in the meninges 72 h post-LPS (n = 6 mice per group). One-way ANOVA followed by Bonferroni’s correction (F(4,20) = 9.76, P = 0.0002). b The forced-swim test was performed 24 h and 72 h post-injection in Rag1−/− mice reconstituted with WT or Il10−/− T lymphocytes (n = 5 mice/group). Two-way ANOVA followed by Bonferroni’s correction (T lymphocytes genotype × time interaction, F(3,32) = 5.26, P = 0.005). c Ido1 mRNA level in the PFC 72 h post-LPS in Rag1−/− mice reconstituted with WT or Il10−/− T lymphocytes (n = 5 mice/group). d Il10 mRNA level in the PFC 72 h post-LPS in Rag1−/− mice reconstituted with WT or Il10−/− T lymphocytes (n = 5 mice/group). Two-way ANOVA followed by Bonferroni’s correction (LPS effect, F(1,16) = 9.75, P = 0.007). The data are presented as mean ± standard error of the mean. Significant effects of LPS are shown by * for Rag1−/− mice reconstituted with WT and # for Il10−/− T lymphocytes; ***P < 0.001, **P < 0.01, */#P < 0.05
Contribution of IL-10 to normalization of Ido1 expression and depression-like behavior
Upregulation of Il10 during resolution of LPS-induced depression-like behavior was more pronounced in the meninges than in the PFC (Fig. 5a). To investigate whether endogenous IL-10 signaling is necessary for resolution of LPS-induced depression-like behavior, we administered a neutralizing anti–IL-10 antibody intranasally to WT mice treated with LPS or PBS. The anti–IL-10 antibody prolonged the LPS-induced increase in immobility in the FST and the TST in WT mice (Fig. 5b, c). Nasal anti–IL-10 antibody administration also prolonged Ido1 upregulation in the PFC. Interestingly, anti–IL-10 antibody blocked the induction of Il10 in the PFC as well (Fig. 5d). Nasal administration of anti–IL-10 antibody did not affect cytokine and Ido1 expression in the liver (Supplementary Figure S5A, B), did not affect onset and resolution of sickness behavior (Supplementary Figure S5C), and did not interfere with immobility in the FST as measured 24 h post-LPS (Fig. 5b).
Fig. 5.
Interleukin (IL)-10 signaling is necessary and sufficient to downregulate Ido1 expression and for resolution of LPS-induced depression-like behavior. (a) Il10 mRNA level in the prefrontal cortex (PFC) and the meninges 24 h post-LPS (n = 6 mice per group). Two-way ANOVA followed by Bonferroni’s correction (tissue × treatment interaction, F(1,17) = 51.5, P < 0.0001). b The FST was performed 24 h and 72 h post-injection in WT mice after nasal administration of neutralizing anti–IL-10 antibody or immunoglobulin (Ig)G (n = 7 mice/group). Two-way ANOVA followed by Bonferroni’s correction (treatment, F(2,18) = 7.52, P = 0.004). c The TST was performed at 72 h post-LPS after nasal administration of anti-IL-10 (n = 7 mice per group). One-way ANOVA followed by Bonferroni’s correction (F(2, 18) = 6.42, P = 0.008). d Il10 and Ido1 mRNA levels in the PFC 72 h post-LPS in WT mice after nasal administration of anti–IL-10 antibody or IgG (n = 7 mice per group). Two-way ANOVA followed by Bonferroni’s correction (tissue × treatment interaction, F(2,18) = 4.75, P = 0.02). e Ido1 mRNA level in BV2 cells 6 h after treatment with recombinant interferon (rIFN)-γ in the presence or absence of recombinant interleukin (IL)-10 fusion protein (n = 5 per group). One-way ANOVA followed by Bonferroni’s correction (F(2, 12) = 20.8, P = 0.0001). f FST was performed 24 h and 72 h post-injection in Rag1−/− mice (n = 7 mice per group) after nasal injection of recombinant IL-10 or PBS. Two-way ANOVA followed by Bonferroni’s correction (treatment, F(2,18) = 28.5, P < 0.0001). g Ido1 mRNA levels in the PFC of Rag1−/− mice 72 h post-LPS after nasal injection of recombinant IL-10 (n = 7 mice/group). One-way ANOVA followed by Bonferroni’s correction (F(2, 15) = 14.6, P = 0.0003). The data are presented as mean ± standard error of the mean. ***P < 0.001, **P < 0.01, *P < 0.05
Our findings suggested that brain IL-10 may mediate the T lymphocyte-dependent normalization of Ido1 expression in the PFC after LPS. Therefore, we first tested the capacity of recombinant IL-10 to regulate Ido1 expression in vitro. BV2 microglial cells were cultured with IFN-γ to induce Ido1 expression in the presence or absence of IL-10. Administration of IL-10 significantly suppressed the IFN-γ–induced increase in Ido1 expression (Fig. 5e). Next, we investigated whether nasal administration of recombinant IL-10 promotes resolution of depression-like behavior in the absence of T cells. Rag1−/− mice were intranasally injected with recombinant IL-10 or PBS on day 2 and 3 after LPS. IL-10 injection induced resolution of LPS-induced increase in immobility in the FST (Fig. 5f). Nasal administration of IL-10 downregulated Ido1 mRNA in the PFC (Fig. 5g). Nasal administration of IL-10 did not affect sickness behavior in response to LPS (Supplementary Figure S5D). Taken together, these data indicate that resolution of LPS-induced depression and normalization of brain Ido1 depends on brain IL-10 signaling.
Discussion
Our study is the first to demonstrate that resolution of inflammation-induced depression-like behavior requires activation of an endogenous resolution pathway that critically depends on T lymphocytes and IL-10 signaling in the meninges and/or brain. T lymphocyte-dependent IL-10 signaling suppresses PFC Ido1 expression to resolve depression-like behavior. IL-10 is the main effector downstream of T lymphocytes as IL-10 alone is sufficient to normalize the resolution of depression-like behavior and Ido1 expression in the absence of T lymphocytes. However, T lymphocytes are not the source of the IL-10. We propose that T lymphocytes accumulate in the meninges prior resolution to promote IL-10 production in the meninges and induce a long lasting increase in IL-10 in the PFC, leading to suppression of Ido1 expression in the PFC and subsequent resolution of inflammation-induced depression.
T lymphocytes also play a crucial modulatory role in social behavior, cognitive functioning, and stress resilience [25–28, 44]. For example, adoptive transfer of lymphocytes from stressed mice protects against stress-induced depression-like behavior in naïve Rag2−/− mice, indicating a role for lymphocytes in stress resilience [28]. Our current findings add to the notion that T lymphocytes play an essential role in depression-like behavior. Moreover, the involvement of T lymphocytes in resolution mechanisms is not restricted to depression, but also applies to pain. We have shown that resolution of chemotherapy-induced neuropathic pain is delayed in mice deficient in T lymphocytes or IL-10 signaling [36]. These findings suggest that pain and depression share T lymphocyte-dependent endogenous resolution pathways. Deficiencies in T lymphocyte function and IL-10 production may underlie the high comorbidity of pain and depression [35, 45].
Although T lymphocytes are required for resolution of depression-like behavior, they do not contribute to the onset and resolution of sickness behavior (weight loss, reduced food intake, reduced locomotor activity) that is driven by proinflammatory cytokines independently of IDO1 activation. Consistently, LPS-induced changes in proinflammatory cytokine expression in the PFC at 24 and 72 h after LPS are independent of T lymphocytes. In addition, the presence of T lymphocytes does not influence LPS-induced cytokine production in the liver. These data are in line with previous reports showing that LPS injection increased brain and peripheral proinflammatory cytokines in WT, Rag1−/−, and Rag2−/− mice in the same manner [46, 47]. Therefore, we propose that T lymphocytes promote resolution of depression-like behavior via a pathway that is independent of regulation of peripheral or central proinflammatory cytokine production.
IL-10 can be produced by multiple cell types in brain and meninges including endothelial cells, astrocytes, neurons, microglia, T lymphocytes, and macrophages. Although we show here that T lymphocytes are required for induction of IL-10 in meninges and the PFC, they are not the source of the IL-10. Interestingly, in models of neuropathic pain [36], spinal cord injury [48], and facial axotomy [33], the neuroprotective effects of T lymphocytes are also mediated via IL-10, while this IL-10 is not produced by the T lymphocytes. Collectively, our findings indicate that T lymphocytes in the meninges induce Il10 mRNA expression by other cells, such as macrophages in the meninges and glial cells in the PFC [49]. In support of this meninges to brain communication model, others has been shown meningeal cells can release cytokines to induce IL-10 production by glial cells in response to peripheral inflammation [50, 51].
Notably, nasal administration of anti-IL-10 not only prevented resolution of depression-like behavior but also counteracted the upregulation of Il10 mRNA in the PFC. IgG antibodies do not cross the intact blood–brain interface, making it unlikely that the anti–IL-10 antibody acted within the PFC. These findings may imply that the meningeal IL-10 signal propagates to the brain via an unknown mechanism that leads to increased Il10 mRNA expression in the PFC. Alternatively, T lymphocytes may serve to induce IL-10 production by the macrophages in the meninges. These macrophages could subsequently penetrate the brain parenchyma [52]. Interestingly, IL-10 produced by peripheral macrophages is necessary for resolution of inflammatory pain [53]. It is also possible that T lymphocytes induce perivascular macrophages to produce IL-10. Future studies should aim at identifying the exact cellular source of the IL-10 required for resolution of depression-like behavior.
IDO1 activity is critical to the development of inflammation-induced depression-like behavior [16, 20]. Induction of Ido1 during onset of depression depends on proinflammatory cytokines like IFN-γ [20]. Our data show that Ifng is still upregulated at the time point when Ido1 expression has normalized in both WT mice and Rag1−/− mice reconstituted with T lymphocytes. We show here for the first time that IL-10 signaling downregulates Ido1 expression during resolution of depression without affecting Ifng expression. In vitro, IL-10 inhibits Ido1 expression by microglia even in the presence of IFN-γ. Similarly, in vivo, administration of IL-10 to Rag1−/− mice reduces Ido1 expression. Collectively, our findings indicate that IL-10 inhibits IFN-γ receptor signaling-induced upregulation of Ido1. Indeed, IL-10 inhibits IFN-γ receptor signaling through activation of suppressor of cytokine signaling (SOCS3) [54].
The potential clinical relevance of the present findings is supported by a recent case–control whole-blood microarray study showed that MDD is associated with downregulation of genes related to T lymphocyte function and adaptive immunity [55]. Moreover, mild lymphopenia is a common observation in patients with MDD [5–8]. In addition, patients with MDD have lower levels of circulating IL-10 [56], and a genetic predisposition to produce less IL-10 is associated with a higher risk for depressive symptoms [57]. Taken together, these findings indicate that reduced T lymphocyte numbers/function and lower IL-10 production in patients with MDD may contribute to the disorder because of an impaired resolution.
In conclusion, the results of our experiments based on model of inflammation-induced depression-like behavior demonstrate that resolution of depression-like behavior is an active process that requires T lymphocytes and downstream induction of IL-10 signaling in the brain. We propose that a reduced capacity to induce IL-10 by T lymphocytes in the meninges negatively impacts the capacity to resolve depression and may ultimately contribute to MDD. Our findings also indicate that inhibition of proinflammatory cytokine signaling may not be sufficient to treat MDD. In contrast, we propose that promoting IL-10 signaling should be considered as a novel, efficacious therapeutic strategy to promote resolution of depression.
Electronic supplementary material
Acknowledgements
We thank Dr. Eric C. Hack (UMC Utrecht, Netherlands) for providing the recombinant IL-10 fusion protein. We thank Bryan LaVergne for technical assistance. We thank Drs. Jonathan Kipnis and Antoine Louveau (University of Virginia at Charlottesville) for sharing their expertise on harvesting the meninges. The authors acknowledge Jeanie F. Woodruff, BS, ELS, for editorial support. This work was supported by NIH grant R01 NS073939 (AK, RD, and CJH), a Cyrus Scholar Award (GL) and the American Pain Society Future Leader in Pain Research grant (GL).
Competing interests
RD has received honorarium from Danone Nutricia Research France. The other authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0154-1).
References
- 1.The burden of depression. Nature. 2014;515:163. [DOI] [PubMed]
- 2.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosse L, Hoogenboezem T, Ambree O, Bellingrath S, Jorgens S, de Wit HJ, et al. Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain Behav Immun. 2016;54:38–44. doi: 10.1016/j.bbi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Miller AH. Depression and immunity: a role for T cells? Brain Behav Immun. 2010;24:1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snijders G, Schiweck C, Mesman E, Grosse L, De Wit H, Nolen WA, et al. A dynamic course of T cell defects in individuals at risk for mood disorders. Brain Behav Immun. 2016;58:11–7. doi: 10.1016/j.bbi.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Toben C, Baune BT. An act of balance between adaptive and maladaptive immunity in depression: a role for T lymphocytes. J Neuroimmune Pharmacol. 2015;10:595–609. doi: 10.1007/s11481-015-9620-2. [DOI] [PubMed] [Google Scholar]
- 9.Renault PF, Hoofnagle JH, Park Y, Mullen KD, Peters M, Jones DB, et al. Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med. 1987;147:1577–80. doi: 10.1001/archinte.1987.00370090055011. [DOI] [PubMed] [Google Scholar]
- 10.Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient’s initial affective state. N Engl J Med. 1999;340:1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- 11.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–73. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 12.Georgin-Lavialle S, Moura DS, Salvador A, Chauvet-Gelinier JC, Launay JM, Damaj G, et al. Mast cells’ involvement in inflammation pathways linked to depression: evidence in mastocytosis. Mol Psychiatry. 2016;21:1511–6. doi: 10.1038/mp.2015.216. [DOI] [PubMed] [Google Scholar]
- 13.Bradley KA, Case JA, Khan O, Ricart T, Hanna A, Alonso CM, et al. The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res. 2015;227:206–12. doi: 10.1016/j.psychres.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savitz J. Role of Kynurenine metabolism pathway activation in major depressive disorders. Curr Top Behav Neurosci. 2017;31:249–67. doi: 10.1007/7854_2016_12. [DOI] [PubMed] [Google Scholar]
- 15.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Chen L, Lim G, Sung B, Wang S, McCabe MF, et al. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012a;122:2940–54. [DOI] [PMC free article] [PubMed]
- 17.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remus JL, Dantzer R. Inflammation models of depression in rodents: relevance to psychotropic drug discovery. Int J Neuropsychopharmacol. 2016;19:pyw028. [DOI] [PMC free article] [PubMed]
- 19.Maciel IS, Silva RB, Morrone FB, Calixto JB, Campos MM. Synergistic effects of celecoxib and bupropion in a model of chronic inflammation-related depression in mice. PLoS ONE. 2013;8:e77227. doi: 10.1371/journal.pone.0077227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29:4200–9. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou W, Dantzer R, Budac DP, Walker AK, Mao-Ying QL, Lee AW, et al. Peripheral indoleamine 2,3-dioxygenase 1 is required for comorbid depression-like behavior but does not contribute to neuropathic pain in mice. Brain Behav Immun. 2015;46:147–53. doi: 10.1016/j.bbi.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–22. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, et al. Illness, cytokines, and depression. Ann N Y Acad Sci. 2000;917:478–87. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 24.Ellwardt E, Walsh JT, Kipnis J, Zipp F. Understanding the role of T cells in CNS homeostasis. Trends Immunol. 2016;37:154–65. doi: 10.1016/j.it.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, et al. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature. 2016;535:425–9. doi: 10.1038/nature18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–80. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, et al. Vaccination as a novel approach for treating depressive behavior. Biol Psychiatry. 2009;65:283–8. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci. 2015;35:1530–8. doi: 10.1523/JNEUROSCI.2278-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, et al. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4 + CD25 + cells. J Neurobiol. 2006;66:552–63. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- 30.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 31.Raposo C, Graubardt N, Cohen M, Eitan C, London A, Berkutzki T, et al. CNS repair requires both effector and regulatory T cells with distinct temporal and spatial profiles. J Neurosci. 2014;34:10141–55. doi: 10.1523/JNEUROSCI.0076-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh JT, Hendrix S, Boato F, Smirnov I, Zheng J, Lukens JR, et al. MHCII-independent CD4 + T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125:699–714. doi: 10.1172/JCI76210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xin J, Wainwright DA, Mesnard NA, Serpe CJ, Sanders VM, Jones KJ. IL-10 within the CNS is necessary for CD4 + T cells to mediate neuroprotection. Brain Behav Immun. 2011;25:820–9. doi: 10.1016/j.bbi.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77. doi: 10.1016/0092-8674(92)90030-G. [DOI] [PubMed] [Google Scholar]
- 35.Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev. 2014;66:80–101. doi: 10.1124/pr.113.008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, et al. CD8 + T Cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J Neurosci. 2016;36:11074–83. doi: 10.1523/JNEUROSCI.3708-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eijkelkamp N, Steen-Louws C, Hartgring SA, Willemen HL, Prado J, Lafeber FP, et al. IL4-10 fusion protein is a novel drug to treat persistent inflammatory pain. J Neurosci. 2016;36:7353–63. doi: 10.1523/JNEUROSCI.0092-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Vulpen LFD, Popov-Celeketic J, van Meegeren MER, Coeleveld K, van Laar JM, Hack CE, et al. A fusion protein of interleukin-4 and interleukin-10 protects against blood-induced cartilage damage in vitro and in vivo. J Thromb Haemost. 2017;15:1788–98. doi: 10.1111/jth.13778. [DOI] [PubMed] [Google Scholar]
- 39.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr Res. 2010;68:419–22. doi: 10.1203/00006450-201011001-00834. [DOI] [PubMed] [Google Scholar]
- 40.Mayo L, Cunha AP, Madi A, Beynon V, Yang Z, Alvarez JI, et al. IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain. 2016;139(Pt 7):1939–57. doi: 10.1093/brain/aww113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosso P, Moreno S, Fracassi A, Rocco ML, Aloe L. Nerve growth factor and autophagy: effect of nasal anti-NGF-antibodies administration on Ambra1 and Beclin-1 expression in rat brain. Growth Factors. 2015;33:401–9. doi: 10.3109/08977194.2015.1122002. [DOI] [PubMed] [Google Scholar]
- 42.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jo WK, Zhang Y, Emrich HM, Dietrich DE. Glia in the cytokine-mediated onset of depression: fine tuning the immune response. Front Cell Neurosci. 2015;9:268. doi: 10.3389/fncel.2015.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SJ, Lee H, Lee G, Oh SJ, Shin MK, Shim I, et al. CD4+ CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS ONE. 2012b;7:e42054. [DOI] [PMC free article] [PubMed]
- 45.Poole H, White S, Blake C, Murphy P, Bramwell R. Depression in chronic pain patients: prevalence and measurement. Pain Pract. 2009;9:173–80. doi: 10.1111/j.1533-2500.2009.00274.x. [DOI] [PubMed] [Google Scholar]
- 46.Bodeman CE, Dzierlenga AL, Tally CM, Mulligan RM, Lake AD, Cherrington NJ, et al. Differential regulation of hepatic organic cation transporter 1, organic anion-transporting polypeptide 1a4, bile-salt export pump, and multidrug resistance-associated protein 2 transporter expression in lymphocyte-deficient mice associates with interleukin-6 production. J Pharmacol Exp Ther. 2013;347:136–44. doi: 10.1124/jpet.113.205369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark SM, Michael KC, Klaus J, Mert A, Romano-Verthelyi A, Sand J, et al. Dissociation between sickness behavior and emotionality during lipopolysaccharide challenge in lymphocyte deficient Rag2(-/-) mice. Behav Brain Res. 2015;278:74–82. doi: 10.1016/j.bbr.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishii H, Tanabe S, Ueno M, Kubo T, Kayama H, Serada S, et al. ifn-gamma-dependent secretion of IL-10 from Th1 cells and microglia/macrophages contributes to functional recovery after spinal cord injury. Cell Death Dis. 2013;4:e710. doi: 10.1038/cddis.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M. Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflamm. 2016;13:297. doi: 10.1186/s12974-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Z, Tokuda Y, Zhang XW, Nakanishi H. Age-dependent responses of glial cells and leptomeninges during systemic inflammation. Neurobiol Dis. 2008;32:543–51. doi: 10.1016/j.nbd.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Wu Z, Zhang J, Nakanishi H. Leptomeningeal cells activate microglia and astrocytes to induce IL-10 production by releasing pro-inflammatory cytokines during systemic inflammation. J Neuroimmunol. 2005;167:90–8. doi: 10.1016/j.jneuroim.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 52.Weber MD, Godbout JP, Sheridan JF. Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology. 2017;42:46–61. doi: 10.1038/npp.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, et al. Monocytes/Macrophages control resolution of transient inflammatory pain. J Pain. 2014;15:496–506. doi: 10.1016/j.jpain.2014.01.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002;168:6404–11. doi: 10.4049/jimmunol.168.12.6404. [DOI] [PubMed] [Google Scholar]
- 55.Leday GGR, Vertes PE, Richardson S, Greene JR, Regan T, Khan S, et al. Replicable and coupled changes in innate and adaptive immune gene expression in two case-control studies of blood microarrays in major depressive disorder. Biol Psychiatry. 2017;83:70–80. doi: 10.1016/j.biopsych.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, Xiao B, Qiu W, Yang L, Hu B, Tian X, et al. Altered expression of CD4( + )CD25( + ) regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. J Affect Disord. 2010;124:68–75. doi: 10.1016/j.jad.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 57.Holtzman S, Abbey SE, Chan C, Bargman JM, Stewart DE. A genetic predisposition to produce low levels of IL-10 is related to depressive symptoms: a pilot study of patients with end stage renal disease. Psychosomatics. 2012;53:155–61. doi: 10.1016/j.psym.2011.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.