Abstract
Introduction
End-stage renal disease (ESRD) patients with a paradoxical increase in blood pressure (BP) during hemodialysis (HD), termed intradialytic hypertension (ID-HTN), are at significantly increased risk for mortality and adverse cardiovascular events. ID-HTN affects up to 15% of all HD patients, and the pathophysiologic mechanisms remain unknown. We hypothesized that ESRD patients prone to ID-HTN have heightened volume-sensitive cardiopulmonary baroreflex sensitivity (BRS) that leads to exaggerated increases in sympathetic nervous system (SNS) activation during HD.
Methods
We studied ESRD patients on maintenance HD with ID-HTN (n = 10) and without ID-HTN (controls, n = 12) on an interdialytic day, 24 to 30 hours after their last HD session. We measured continuous muscle sympathetic nerve activity (MSNA), beat-to-beat arterial BP, and electrocardiography (ECG) at baseline, and during graded lower body negative pressure (LBNP). Low-dose LBNP isolates cardiopulmonary BRS, whereas higher doses allow assessment of physiologic responses to orthostatic stress.
Results
The ID-HTN patients had significantly higher pre- and post-HD BP, and greater interdialytic fluid weight gain compared to controls. There was a significantly greater increase in MSNA burst incidence (P = 0.044) during graded LBNP in the ID-HTN group, suggesting heightened cardiopulmonary BRS. The ID-HTN group also had a trend toward increased diastolic BP response during LBNP, and had significantly greater increases in BP during the cold pressor test.
Conclusion
Patients with ID-HTN have augmented cardiopulmonary BRS that may contribute to increased SNS activation and BP response during HD.
Keywords: baroreflex, end-stage renal disease, hemodialysis, sympathetic activity
ID-HTN, defined as paradoxical increases in BP during HD, is a major clinical problem that significantly increases morbidity and mortality and affects up to 15% of HD patients.1 ESRD patients with ID-HTN have significantly poorer short-term and long-term outcomes compared to ESRD patients with appropriate decreases in BP during the dialysis procedure.2, 3, 4
The pathogenic mechanisms underlying ID-HTN remain unclear, and thus the best therapeutic approach remains unknown. Although SNS overactivity is presumed to play a role, prior evidence is scarce and conflicting. Some studies report greater increases in total peripheral resistance and reductions in heart rate variability (HRV) suggestive of sympathetic overactivation,5, 6 whereas others report no changes in HRV or plasma catecholamine levels, suggesting that SNS overactivation does not contribute to the pathogenesis of ID-HTN.7 However, HRV and plasma catecholamines, which are indirect markers for sympathetic activity, may not accurately reflect sympathetic nerve activity, particularly in ESRD.
The goal of the current study was to evaluate the mechanistic role of SNS overactivation in the pathogenesis of ID-HTN using intraneural measures of sympathetic nerve activity during orthostatic stress, which simulates the volume shifts during HD.8, 9 Reflex activation of SNS during orthostasis is governed by volume-sensitive cardiopulmonary barorereceptors (“low-pressure” baroreceptors located in the atria, ventricles, and lungs) in response to changes in volume or cardiac preload. Cardiopulmonary baroreceptors are afferent stretch receptors that tonically inhibit central SNS outflow. When cardiac preload is reduced, as during volume loss from ultrafiltration, upright posture, or experimentally via LBNP, the cardiopulmonary baroreceptors become unloaded; this results in a reflex increase in SNS output, in a “feed-forward” mechanism designed to prevent an impending fall in BP. If the cardiopulmonary baroreceptors become sensitized, then the cardiopulmonary baroreflex-mediated control of SNS activity becomes exaggerated, resulting in augmented increases in SNS activation and BP during orthostatic stress. Augmented cardiopulmonary BRS has been described in other patient populations characterized by elevated BP and cardiovascular risk,10, 11 but has never previously been evaluated in ESRD. We hypothesized that ESRD patients with ID-HTN would have exaggerated increases in SNS activity during orthostatic stress due to increased sensitization of the cardiopulmonary baroreflex, resulting in paradoxical increases in BP during volume removal.
Methods
Study Population
A total of 22 patients with ESRD on maintenance HD for ≥6 months were recruited and enrolled from 3 Emory University Dialysis Units. We defined ID-HTN as a failure to reduce BP by at least 10 mm Hg by the end of HD or post-HD, as previously described.1, 2, 3 The ID-HTN group met criteria for ID-HTN in ≥4 of 6 consecutive HD sessions. The control group had a ≥10−mm Hg reduction in systolic BP in ≥4 of 6 consecutive HD sessions. Although no standard definition of intradialytic hypertension exists, prior studies have shown that compared to HD patients whose BP fell by >10 mm Hg, those whose BP did not change (−10 to 10 mm Hg) or rose with HD (>10 mm Hg) both had ∼ 2-fold greater adjusted odds for death or hospitalization at 6 months.2 Exclusion criteria for both groups included drug or alcohol abuse, neuropathy, acute illness, severe anemia with hemoglobin level <8 g/dl, clinical evidence of overt heart failure or volume overload, vascular event within the past 6 months, symptomatic heart disease determined by ECG stress test and/or history, treatment with central α-agonists, autonomic dysfunction or other neurological complaints, treatment with gabapentin, pregnancy, surgery or hospitalization within the past 3 months, and nonadherence to HD or medication regimen. This study was approved by the Emory University Institutional Review Board, and written informed consent was obtained from all participants.
Measurements and Procedures
Blood Pressure
Seated BP was measured in triplicate using standard technique. Beat-to-beat arterial BP was measured continuously and noninvasively using digital pulse photoplethysmography12, 13 (NexFin, Edwards LifeSciences, Irvine, CA).
Muscle Sympathetic Nerve Activity
Multifiber postganglionic MSNA was recorded via a tungsten microelectrode (tip diameter 5−15 μm; Bioengineering, University of Iowa, Iowa City, IA) inserted into the peroneal nerve using microneurography.14, 15 A ground microelectrode was inserted subcutaneously, and nerve activity signals were amplified (gain 50,000–100,000×), filtered (700–2000 Hz), rectified, and integrated (time constant 0.1 second) to obtain a mean voltage display of MSNA (Nerve Traffic Analyzer, Model 662C-4, University of Iowa Bioengineering, Iowa City, IA) that was recorded in real time with beat-to-beat BP and ECG into LabChart 7 (PowerLab 16sp, ADInstruments, Colorado Springs, CO). All MSNA recordings were analyzed by a single investigator (JP) who was blinded to the participant’s group status and who met previously established quality criteria.16, 17, 18 The MSNA was expressed as burst frequency (bursts per minute), and incidence (bursts/100 heart beats).
Lower Body Negative Pressure
Graded LBNP was used to test cardiopulmonary baroreflex sensitivity during low doses, and physiologic responses to increasing orthostatic stress at high doses. The legs and lower abdomen of the participant were sealed in a lower body negative pressure chamber (a chamber that creates a vacuum and has an attached rheostat to measure negative pressure). The LBNP was sequentially applied at −5, −10, −15, −20, −30, and −40 mm Hg for 3 minutes at each level. At low doses (≤−20 mm Hg), only the cardiopulmonary baroreflexes are engaged, and there are no changes in blood pressure. At higher doses (≥−20 mm Hg), both cardiopulmonary and arterial baroreflexes are engaged, and there may be a small change (<10 mm Hg) in blood pressure. Cardiopulmonary BRS is defined as the slope of the linear regression between MSNA burst incidence or burst frequency and dose of LBNP.
Cold Pressor Test
The participant's hand was submerged in cold water (∼0–1 °C) up to the wrist for 1 minute.19 Pain experienced during the cold pressor test (CPT) was rated on a scale of 0 to 4.
Experimental Protocol
Participants were studied in the morning on a nondialysis day, approximately 26 to 30 hours after their last dialysis session. All participants had abstained from food, caffeine, and exercise for at least 12 hours prior to study procedures, but had taken their routinely scheduled medications with sips of water as prescribed. A brief history and physical examination was performed to ensure no history of orthopnea, paroxysmal nocturnal dyspnea, increased dyspnea on exertion, or findings of pulmonary crackles, S3 heart sounds, or greater than trace edema. The study room was quiet, semidark, and temperate (∼21 ° C). Participants were placed in a supine position and were fitted with finger cuffs for continuous BP monitoring and patch electrodes for continuous ECG recordings. The LBNP chamber was fitted and sealed over the lower body, and the leg was accessed for microneurography using an opening in the chamber. The tungsten microelectrode was inserted into the peroneal nerve and manipulated to obtain a satisfactory nerve recording as described above. After 10 minutes of rest, continuous ECG, MSNA, and BP data were measured and recorded in real-time at baseline for 10 minutes and throughout the experimental protocol. After baseline measurements, graded LBNP was applied at the following doses: −5, −10, −15, −20, −30, and −40 mm Hg,10 for 3 minutes at each dose, to simulate orthostatic stress during HD. The LBNP at low doses (≤−20 mm Hg) selectively unloads cardiopulmonary baroreceptors, and allows for assessment of cardiopulmonary BRS. Heart rate, BP, and MSNA were continuously measured throughout the 18 minutes of LBNP, and for an additional 10 minutes of recovery time. After return to baseline conditions, a subset of participants underwent 1 minute of CPT.
Data Analysis
Muscle Sympathetic Nerve Activity
The MSNA, BP, and ECG data were analyzed using WinCPRS (Absolute Aliens, Turku, Finland). R-waves were automatically marked from the continuous ECG and then reviewed and edited manually as needed. The MSNA bursts were detected using the following criteria: 3:1 burst-to-noise ratio within a 0.5-second search window, with an average latency of 1.2 to 1.4 seconds from the previous R-wave, and inspected for accurate detection by a single investigator (JP). The MSNA was expressed as burst frequency (bursts/min) and burst incidence (bursts/100 heart beats).
Statistical Analysis
Statistical analysis was performed with the lme420 and lmerTest21 packages in the R statistical programming environment.22 Baseline measurements were compared using 2-sided t tests and Fisher exact tests. Linear mixed models were used to adjust for repeated measurements on the same individuals during LBNP. These models included not only random intercepts but also random slopes, because the variance increased with the mean during the intervention. Outcome variables in this study were changes in MSNA, SBP, DBP, MAP, and HR. For each outcome variable, 2 separate linear mixed models were run for within-group analysis and between-group analysis respectively. In the within-group analysis, we tested the association between the change of outcome from baseline and the increase in LBNP dose. In the between-group analysis, in addition to comparing baseline between-group differences, we tested for a discrepancy in the rate of change in outcome variables between ID-HTN and control groups. Statistical significance was reported by 2-sided P values with the lmerTest21 package.
Results
Baseline Characteristics
Baseline characteristics are summarized in Table 1. There were no significant differences in age, race, weight, and body mass index (BMI) between the ID-HTN and control groups. Resting muscle sympathetic nerve activity (MSNA) burst frequency tended to be lower in ID-HTN versus controls (27.1 ± 2.9 vs. 36.8 ± 3.9 bursts/min, P = 0.066). There were no significant differences in baseline hemodynamics or in the proportion of subjects with comorbid diabetes. The ID-HTN group had a significantly greater proportion of participants with hypertension as the etiology of ESRD (P = 0.045), whereas the control group tended to have a greater proportion with glomerulonephritis as the etiology of ESRD (P = 0.089). The ID-HTN group had a significantly greater proportion treated with dihydropyridine calcium channel blockers (P = 0.035), and a trend toward a greater proportion treated with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) (P = 0.087). There were no significant differences in β-blocker use between the groups. None of the participants in either group were treated with nondihyropyridine calcium channel blockers. Study participants took their antihypertensive medications after HD on dialysis days, and in the mornings on nondialysis days, and this dosing schedule did not differ between the groups. There were no significant differences in the use of dialyzable antihypertensive medications between the groups (data not shown). Within the ID-HTN group, 4 of 10 were on lisinopril, and 4 of 10 were on metoprolol. Within the control group, 3 of 12 were on lisinopril, and 3 of 12 were on metoprolol.
Table 1.
Baseline characteristics of the study population
| Characteristics | Control n = 12 |
ID-HTN n = 10 |
P value |
|---|---|---|---|
| Age, yr | 47.0 ± 2.8 | 47.1 ± 4.3 | 0.980 |
| Sex, male/female | 5/7 | 6/4 | 0.392 |
| Race, black/white | 12/0 | 9/1 | 0.262 |
| Weight, kg | 90.5 ± 6.3 | 89.6 ± 8.4 | 0.930 |
| Body mass index, kg/m2 | 30.4 ± 1.7 | 29.2 ± 2.0 | 0.651 |
| Baseline muscle sympathetic nerve activity | |||
| Burst frequency, bursts/min | 36.8 ± 3.9 | 27.1 ± 2.9 | 0.066 |
| Burst incidence, bursts/100 heart beats | 52.2 ± 5.2 | 38.4 ± 5.8 | 0.108 |
| Hemodynamics | |||
| Systolic BP, mm Hg | 130 ± 8 | 141 ± 10 | 0.356 |
| Diastolic BP, mm Hg | 76 ± 3 | 78 ± 5 | 0.813 |
| Heart rate, bpm | 79 ± 3 | 75 ± 4 | 0.813 |
| Diabetes, n (%) | 4 (33) | 1 (10) | 0.193 |
| Hypertension, n (%) | 9 (75) | 10 (100) | 0.089 |
| Etiology of end-stage renal disease, n (%) | |||
| Hypertension | 6 (50) | 9 (90) | 0.045 |
| Diabetes | 2 (17) | 1 (10) | 0.650 |
| Glomerulonephritis | 3 (25) | 0 (0) | 0.089 |
| Unspecified | 1 (8) | 0 (0) | 0.350 |
| Antihypertensive medications, n (%) | |||
| Calcium channel blockers | 3 (25) | 7 (70) | 0.035 |
| ACEIs/ARBs | 4 (33) | 7 (70) | 0.087 |
| β-Blockers | 7 (58) | 7 (70) | 0.571 |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BP, blood pressure.
Values with plus-or-minus sign are mean ± SE. Bold indicates statistically significant P values < 0.05.
Hemodialysis Characteristics
Hemodialysis characteristics are summarized in Table 2. The ID-HTN group tended to have shorter dialysis vintage compared to controls (3.1 ± 0.5 vs. 6.0 ± 1.3 years, P = 0.072). There were no significant differences in HD access type between groups. The ID-HTN group had significantly higher pre- and post-HD systolic BP (SBP) and diastolic BP (DBP) compared to the control group, whereas there were no significant differences in pre- and post-HD heart rate (HR) between groups. Although post-HD SBPs were significantly lower in controls compared to participants in the ID-HTN group, 3 of 10 ID-HTN participants had 2 HD sessions in which SBP decreased by >10 mm Hg, resulting in an overall lower mean post-HD systolic BP compared to pre-HD systolic BP within the ID-HTN group. The nadir intradialytic SBP (145 ± 3 vs. 111 ± 3 mm Hg, P < 0.001) and DBP (79 ± 2 vs. 61 ± 2 mm Hg, P < 0.001) were also significantly higher in the ID-HTN group compared to the control group, respectively. The ID-HTN group had significantly higher interdialytic weight gain compared to the control group (2.8 ± 0.2 vs. 2.2 ± 0.2 kg, P = 0.039), and a trend toward higher HD ultrafiltration volume (P = 0.096). There were no significant differences in laboratory measures including serum sodium, potassium, phosphorus, and hemoglobin levels between the groups. There was a trend toward lower hemodialysis adequacy in ID-HTN participants compared to controls assessed as Kt/V (1.41 ± 0.11 vs. 1.63 ± 0.08, P = 0.110) and urea reduction ratio (69.2% ± 3.4% vs. 75.1% ± 1.7%, P = 0.133).
Table 2.
Hemodialysis characteristics of the study population
| Control n = 12 |
ID-HTN n = 10 |
P value | |
|---|---|---|---|
| Dialysis vintage, yr | 6.0 ± 1.3 | 3.1 ± 0.5 | 0.072 |
| Access, n (%) | |||
| AV fistula | 7 (58) | 6 (60) | 0.937 |
| AV graft | 4 (33) | 4 (40) | 0.746 |
| Catheter | 1 (8) | 0 (0) | 0.350 |
| HD hemodynamicsa | |||
| Pre-HD SBP, mm Hg | 148 ± 3 | 178 ± 3 | <0.001 |
| Post-HD SBP, mm Hg | 130 ± 2 | 154 ± 3 | <0.001 |
| Nadir HD SBP, mm Hg | 111 ± 3 | 145 ± 3 | <0.001 |
| Pre-HD DBP, mm Hg | 81 ± 2 | 97 ± 3 | <0.001 |
| Post-HD DBP, mm Hg | 72 ± 2 | 98 ± 14 | <0.001 |
| Nadir HD DBP, mm Hg | 61 ± 2 | 79 ± 2 | <0.001 |
| Pre-HD HR, bpm | 83 ± 2 | 79 ± 3 | 0.334 |
| Post-HD HR, bpm | 82 ± 2 | 84 ± 2 | 0.411 |
| HD characteristicsa | |||
| Interdialytic weight gain, kg | 2.2 ± 0.2 | 2.8 ± 0.2 | 0.039 |
| Dialysis time, min | 223 ± 8 | 223 ± 8 | 0.958 |
| Ultrafiltration volume, L | 2.6 ± 0.1 | 2.9 ± 0.2 | 0.096 |
| Ultrafiltration rate, ml/kg per hr | 8.1 ± 0.4 | 8.5 ± 0.5 | 0.594 |
| Laboratory measures | |||
| Sodium, mEq/l | 138.1 ± 0.9 | 138.3 ± 0.6 | 0.848 |
| Potassium, mEq/l | 4.5 ± 0.1 | 4.8 ± 0.3 | 0.346 |
| Hemoglobin, g/dl | 11.0 ± 0.2 | 11.3 ± 0.5 | 0.600 |
| Phosphorus, mEq/l | 6.2 ± 0.4 | 6.3 ± 0.8 | 0.905 |
| Parathyroid hormone, pg/ml | 810 ± 186 | 598 ± 126 | 0.359 |
| Kt/V | 1.63 ± 0.08 | 1.41 ± 0.11 | 0.110 |
| Urea reduction ratio, % | 75.1 ± 1.7 | 69.2 ± 3.4 | 0.133 |
AV, arteriovenous; DBP, diastolic blood pressure; HD, hemodialysis; HR, heart rate; Kt/V, measure of dialysis adequacy (K = dialyzer clearance of urea, t = dialysis time, V = volume of distribution of urea, which is roughly total body water), SBP, systolic blood pressure.
Values with plus/minus sign are the mean ± SE. Bold indicates statistically significant P values < 0.05.
Mean values averaged across 6 consecutive HD treatments.
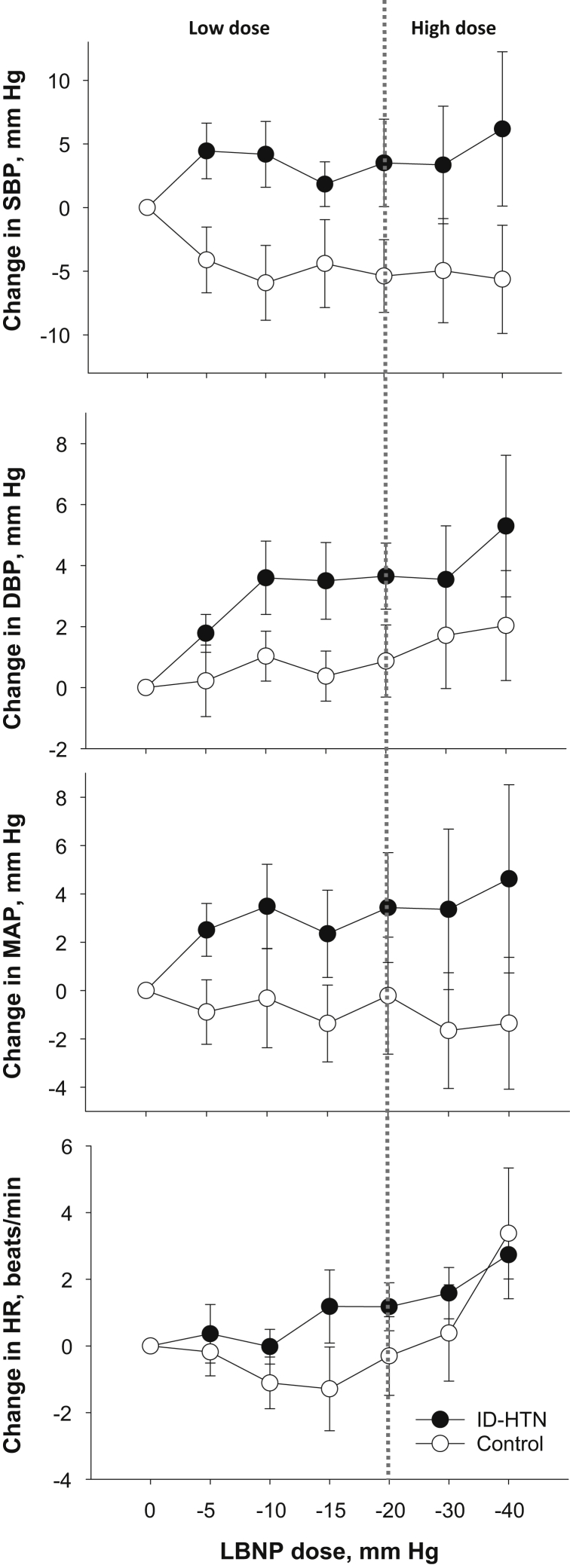
Hemodynamic and Neurocardiovascular Response During Low-Dose LBNP
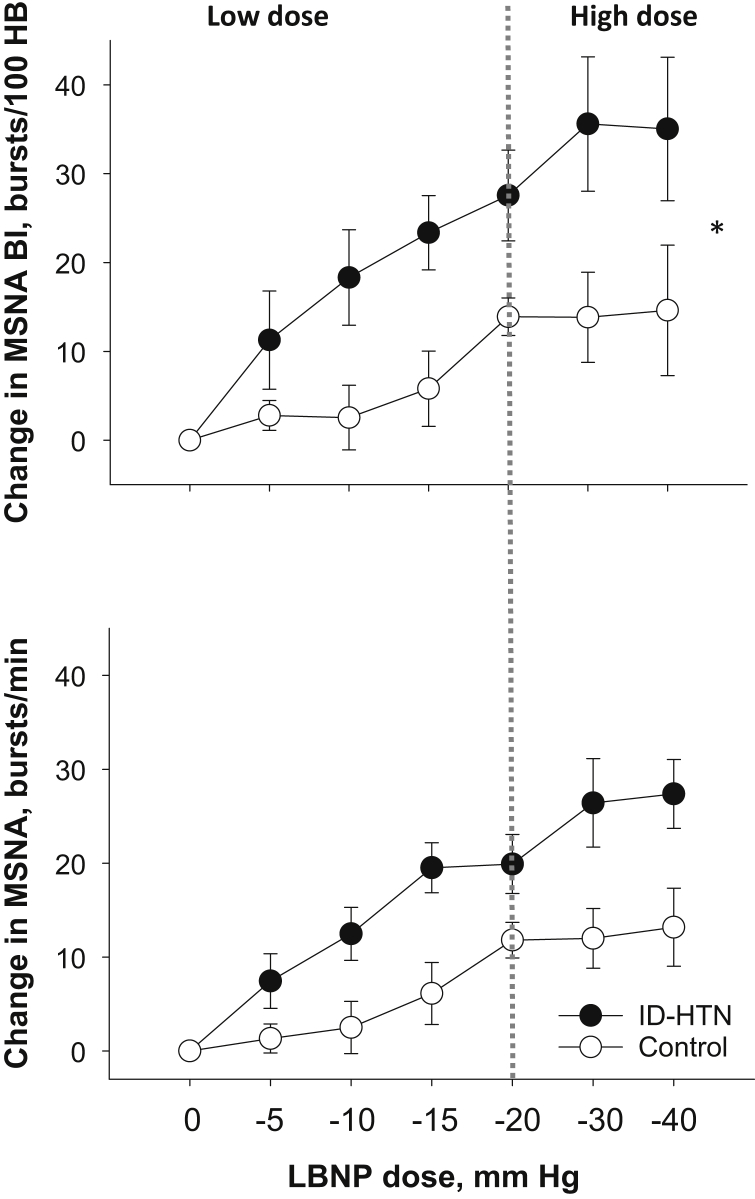
Low-dose LBNP (≤−20 mm Hg) decreases venous return to the heart without a change in BP, and thereby isolates the volume-sensitive low-pressure cardiopulmonary baroreflex.10, 11 There was no significant difference in the SBP, DBP, mean arterial pressure (MAP), or HR response during low-dose LBNP between the ID-HTN and control groups (Figure 1). However, there was a significant increase in DBP within the ID-HTN group during low-dose LBNP (P = 0.011). There was a significantly greater increase in MSNA burst incidence (P = 0.008) and burst frequency (P = 0.006) in ID-HTN participants compared to controls during low-dose LBNP, suggesting heightened cardiopulmonary BRS (Figure 2). These results remained significant when the 3 ID-HTN participants with a >10−mm Hg reduction in SBP during 2 of 6 HD sessions during the screening period were excluded from the analyses.
Figure 1.
Change in systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) during low-dose and high-dose graded lower body negative pressure (LBNP) in end-stage renal disease patients prone to intradialytic hypertension (ID-HTN, n = 10) and controls (n = 12).
Figure 2.
Change in muscle sympathetic nerve activity (MSNA) burst incidence (BI) and burst frequency in the intradialytic hypertension (ID-HTN) group (n = 7) versus controls (n = 7). *P < 0.05 denotes a statistically significant difference in change between groups. HB, heart beat; LBNP, lower body negative pressure.
Hemodynamic and Neurocardiovascular Response During High-Dose LBNP
High-dose LBNP (≤−20 mm Hg) engages both volume-sensitive cardiopulmonary baroreflexes and BP-responsive “high-pressure” arterial baroreflexes.10, 11 High-dose LBNP simulates acute volume loss that occurs with volume removal during HD.8 There was no significant difference in the overall change in SBP, DBP, MAP, or HR from baseline to during high-dose LBNP (≥−20 mm Hg) between the ID-HTN and control groups (Figure 1). ID-HTN group tended to have a greater increase in DBP during high-dose LBNP compared to controls (P = 0.101). There was a significantly greater increase in MSNA burst incidence (P = 0.044), and a trend toward a significantly greater increase in MSNA burst frequency (P = 0.064) in ID-HTN participants compared to controls during high-dose LBNP, suggesting heightened sympathetic response induced by orthostatic stress (Figure 2). These results remained significant when the 3 ID-HTN participants with a reduction of >10 mm Hg in SBP during 2 of 6 HD sessions during the screening period were excluded from the analyses.
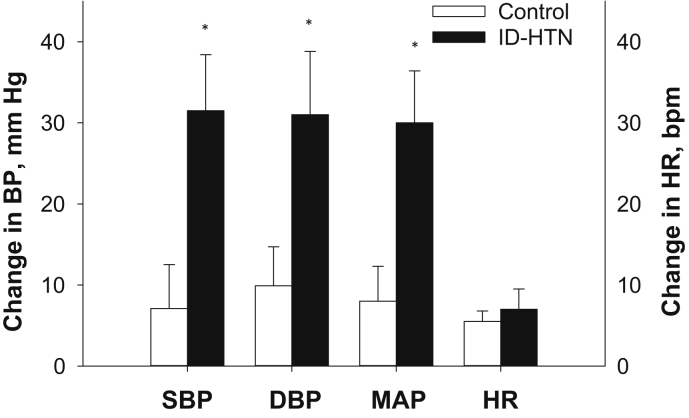
BP Reactivity to Cold Pressor Test
CPT, a non−baroreflex-mediated stimulus, was performed to determine whether ID-HTN was characterized by a generalized increase in BP reactivity, versus a heightened responsiveness due to alterations in BRS alone. There was a significantly greater increase in SBP (+31 ± 7 vs. +7 ± 5 mm Hg, P = 0.020), DBP (+31 ± 8 vs. +10 ± 5 mm Hg, P = 0.040), and MAP (+30 ± 6 vs. +8 ± 4 mm Hg, P = 0.020) during the CPT in ID-HTN participants compared to controls (Figure 3), suggesting that ID-HTN patients may have generalized augmentation in neurohemodynamic reactivity. There was no significant difference in HR responses between the groups (P = 0.576). The perception of pain was similar between the groups (data not shown).
Figure 3.
Change in systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) during the cold pressor test in a subset of participants from the intradialytic hypertension (ID-HTN) group (n = 5) and controls (n = 6). BP, blood pressure. *P < 0.05 denotes a statistically significant difference between groups.
Discussion
ID-HTN is an under-recognized, but relatively common phenomenon, affecting up to 15% of all dialysis procedures.1 Moreover, patients with ID-HTN are at significantly increased risk for short-term and long-term morbidity and mortality. In a large analysis of the United States Renal Data System (USRDS) database, every 10−mm Hg increase in systolic BP during HD was associated with a 6% increased hazard for death at 2 years3 compared to HD patients with a reduction in BP during HD. Similarly, a secondary analysis of the Crit-Line Intradialytic Monitoring Benefit (CLIMB) study revealed that patients whose SBP remained unchanged or rose by ≥10 mm Hg during HD had an 85% greater risk of hospitalization or death at 6 months of follow-up.2 In addition, more frequent episodes of ID-HTN were associated with incremental increases in 30-day mortality and hospitalizations.4 Although no standard definition of ID-HTN exists, criteria that have been used in the literature and correlate with morbidity and interdialytic ambulatory BP include the following: (i) an increase in systolic BP of ≥10 mm Hg from pre- to post-HD, or an increase in MAP ≥15 mm Hg during or immediately after HD; (ii) BP that increases during or immediately after HD and results in a postdialysis BP ≥130/80 mm Hg; (iii) failure to reduce BP by the end of dialysis or postdialysis; and (iv) hypertension during the latter part of HD after significant ultrafiltration has taken place.1, 23, 24
The pathophysiologic mechanisms underlying ID-HTN remain unclear. Some purported hypotheses include volume overload,6, 25, 26, 27 vascular stiffness,28 endothelial dysfunction,29, 30 increased endothelin-1 levels,7, 31 removal of antihypertensive medications,1, 23, 24 hypernatric dialysate1, 23, 32 activation of the renin−angiotensin system,1, 23, 24 and SNS overactivity.1, 5, 6, 23, 24 Of these, SNS overactivation is presumed to play a major pathogenic role in ID-HTN; however, previous evidence was scarce and conflicting. Chou et al. showed that the exaggerated increases in total peripheral vascular resistance during HD in patients with ID-HTN were not accompanied by changes in plasma levels of catecholamines, or the low frequency−to−high frequency ratios of HRV, suggesting that SNS overactivation did not contribute to the pathogenesis of ID-HTN.7 Conversely, in another study, the majority of intradialytic hypertensive episodes were associated with increased BP variability, increased heart rate, and low-frequency interbeat interval variability, suggestive of SNS activation.5 However, HRV and plasma catecholamines are indirect markers for SNS activity and not ideal measures of sympathetic function, especially in patients with impaired renal function.
To our knowledge, the current study is the first to use direct, intraneural recordings of MSNA, the gold-standard method for assessing sympathetic nerve traffic in humans, to demonstrate a significantly augmented SNS response during orthostatic stress in ESRD patients prone to ID-HTN. MSNA correlates with renal, cardiac, and total central sympathetic outflow,33 and is the superior method for quantifying baseline, as well as beat-to-beat changes in SNS activity in humans. Concomitant with exaggerated MSNA reactivity, we observed a trend toward differences in the hemodynamic response to both low-dose and high-dose graded LBNP. Whereas SBP tended to decrease in controls, the ID-HTN group tended to have a lack of fall in SBP, and an increase in DBP and MAP with increasing doses of LBNP. This hemodynamic pattern mirrors the clinical characteristics of ID-HTN in which a lack of fall in BP or a paradoxical increase in BP occurs with volume removal during HD. The finding that MSNA is significantly higher in individuals with ID-HTN during high-dose LBNP suggests that exaggerated increases in SNS activity in response to orthostatic stress may drive this phenomenon.
Our finding that BP and MSNA responses are increased specifically with low-dose LBNP (−20 mm Hg or less) during which cardiopulmonary baroreceptors are selectively unloaded without engagement of arterial baroreceptors, suggests that ID-HTN patients have heightened cardiopulmonary BRS. Augmented cardiopulmonary BRS has been described in other patient populations. including prehypertension,11 aging,10 and salt-sensitive hypertension.34 The mechanisms underlying heightened cardiopulmonary BRS in ID-HTN are unknown, but could be related to volume overload, electrolyte shifts, and the uremic milieu. In the current study, we observed that ID-HTN patients had significantly higher interdialytic fluid weight gain compared to controls. One prior study showed no differences in interdialytic weight gain between patients with ID-HTN versus those with normal reductions in BP during HD,35 whereas another study showed that increased interdialytic weight gain was associated with a significant reduction in the change in systolic BP with HD.36 Greater fluid weight gain, as observed in the current study, with subsequent volume-mediated hypertension and volume overload, may explain the tendency toward lower resting MSNA observed in the ID-HTN group, via suppression by arterial and cardiopulmonary baroreflexes. ID-HTN is known to be linked to volume overload6, 25, 26, 27 and could cause chronic changes to the cardiopulmonary baroreceptors (afferent nerve endings located in the atria), or to altered activation of the receptive field of these stretch receptors in response to volume removal during HD. In salt-sensitive hypertension, high-salt intake changes the ionic environment and sensitizes the cardiopulmonary baroreceptors.34 Similarly, the dialysate sodium concentration is often hypernatric compared to predialysis serum sodium levels,32 resulting in an initial influx of sodium and rapid changes in osmolality that could lead to sensitization of cardiopulmonary BRS. Finally, the Na+-K+ ATPase inhibitor digoxin is known to sensitize cardiopulmonary baroreceptors,37, 38 and digoxin-like immunoreactive substances are uremic toxins that are known to accumulate in ESRD patients.39, 40 Our finding that the ID-HTN group had a trend toward lower Kt/V, although it is unclear whether this association is a consequence or causative factor in intradialytic hypertension, may implicate that differences in the accumulation of uremic toxins may play a role in sensitization of the cardiopulmonary baroreflex. These and other mechanisms underlying heightened cardiopulmonary BRS in ID-HTN should be further investigated to inform the treatment approach to ID-HTN.
Interestingly, we observed that the ID-HTN group had a significantly greater BP response during CPT, a sympathoexcitatory stimulus that is not regulated by baroreflexes.41, 42 This finding suggests that HD patients prone to ID-HTN may have generalized increased neurocardiovascular reactivity, independent of baroreflex control. Although the current study is limited to investigation of cardiopulmonary BRS and SNS activation, the pathogenesis of ID-HTN is likely multifactorial, including baroreflex-mediated, non−baroreflex-mediated, and nonneural factors. In addition, the potential role of abnormal parasympathetic adjustments during orthostasis, as well as impaired “high-pressure” arterial BRS, were not evaluated in this study. Prior studies have shown that when both cardiopulmonary baroreceptors and arterial baroreceptors are concomitantly engaged as during HD ultrafiltration, the 2 afferent inputs are integrated in the same sympathetic control centers in the brainstem, resulting in an augmentation of arterial baroreflex control of SNS output.43 Whereas low-dose LBNP isolates the cardiopulmonary baroreflex, higher-dose LBNP (greater than −20 mm Hg) engages both the cardiopulmonary and arterial baroreflexes and has been used in prior studies to simulate orthostatic stress during hemodialysis.8, 9 Our finding that MSNA and BP reactivity were increased during high-dose LBNP (≥20 mm Hg) when both cardiopulmonary and arterial baroreflexes were engaged suggests that ID-HTN may have a relative blunting of arterial BRS during volume removal in HD, resulting in a failure to diminish the exaggerated pressor and SNS responses during orthostatic stress.
We recognize several limitations. First, ID-HTN was defined in this study as a lack of fall in BP with HD.2 The reference group had a fall in systolic BP by at least 10 mm Hg from pre- to post-HD, whereas the ID-HTN group did not have a fall in systolic BP by at least 10 mm Hg in 4 of 6 consecutive HD sessions. Given the small sample size, a limitation of the current study is a lack of sensitivity analyses to determine whether subgroups with BP increases of >10 mm Hg versus those with increases of >0 mm Hg had greater MSNA reactivity or cardiopulmonary BRS. In addition, using different definitions of ID-HTN, such as a definition requiring an increase in BP by the end of HD ≥10 mm Hg, may have demonstrated greater physiologic differences between the groups. Second, experiments were conducted on an interdialytic day and may not reflect the same physiologic adjustments that occur just before HD when intravascular volume is at its highest. Third, −40 mm Hg was the maximum LBNP dose used in this study. Higher doses of LBNP may have been required to observe greater differences between the groups, particularly in BP responses, given that both ID-HTN and control groups likely had some degree of volume overload. Fourth, there was a trend toward greater use of ACEIs and ARBs within the ID-HTN group that have been shown to decrease MSNA in CKD patients. However, despite the sympathoinhibitory effects of ACEIs and ARBs, there was a significantly greater sympathetic response during cardiopulmonary baroreflex engagement in ID-HTN patients compared to controls. Fifth, although there were no significant differences in the ultrafiltration rate or volume between the groups, we cannot exclude the possibility that there were differences in intradialytic fluid boluses that may have driven increases in the final post-HD BP within the ID-HTN group. Finally, the study population comprised primarily African Americans and may not be generalizable to other racial groups.
In conclusion, ESRD patients prone to ID-HTN had a significant increase in MSNA reactivity during low-dose LBNP, suggesting heightened cardiopulmonary BRS. The ID-HTN patients also had a significantly greater MSNA response and trend toward higher BP response during high-dose LBNP. Together, these findings suggest that augmented reflex SNS activation may drive abnormal BP responses during orthostatic stress induced during HD in patients with ID-HTN. Further studies are needed to elucidate the mechanisms underlying heightened cardiopulmonary BRS to inform the therapeutic approach to this high-risk patient population.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by the National Institutes of Health grant R01 HL135183; Satellite Healthcare, a not-for-profit renal care provider; Merit Review Award number I01CX001065 from the United States Department of Veterans Affairs Clinical Sciences Research and Development Program; American Heart Association National Affiliate, Collaborative Sciences Award 15CSA24340001; resources and the use of facilities at the Clinical Studies Center of the Atlanta VA Medical Center; the Atlanta Research and Education Foundation; and National Institutes of Health training grant T32 DK-00756.
Author Contributions
JP conceived and designed the study. JP, SP, IF, and DD acquired the data. JP, SP, and IF interpreted the results. YL analyzed the data. JP and SP drafted the manuscript. JP, SP, and HM revised the manuscript. JP, SP, IF, YL, DD, and HM approved the final version.
References
- 1.Inrig J.K. Intradialytic hypertension: a less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis. 2010;55:580–589. doi: 10.1053/j.ajkd.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inrig J.K., Oddone E.Z., Hasselblad V. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int. 2007;71:454–461. doi: 10.1038/sj.ki.5002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inrig J.K., Patel U.D., Toto R.D. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis. 2009;54:881–890. doi: 10.1053/j.ajkd.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assimon M.M., Wang L., Flythe J.E. Intradialytic hypertension frequency and short-term clinical outcomes among individuals receiving maintenance hemodialysis. Am J Hypertens. 2018;31:329–339. doi: 10.1093/ajh/hpx186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubinger D., Backenroth R., Sapoznikov D. Sympathetic activation and baroreflex function during intradialytic hypertensive episodes. PLoS One. 2012;7:e36943. doi: 10.1371/journal.pone.0036943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Buren P.N., Zhou Y., Neyra J.A. Extracellular volume overload and increased vasoconstriction in patients with recurrent intradialytic hypertension. Kidney Blood Press Res. 2016;41:802–814. doi: 10.1159/000450565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou K.J., Lee P.T., Chen C.L. Physiological changes during hemodialysis in patients with intradialysis hypertension. Kidney Int. 2006;69:1833–1838. doi: 10.1038/sj.ki.5000266. [DOI] [PubMed] [Google Scholar]
- 8.Ligtenberg G., Blankestijn P.J., Koomans H.A. Hemodynamic response during lower body negative pressure: role of volume status. J Am Soc Nephrol. 1998;9:105–113. doi: 10.1681/ASN.V91105. [DOI] [PubMed] [Google Scholar]
- 9.Nette R.W., Krepel H.P., van den Dorpel M.A. Hemodynamic response to lower body negative pressure in hemodialysis patients. Am J Kidney Dis. 2003;41:807–813. doi: 10.1016/s0272-6386(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 10.Davy K.P., Seals D.R., Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension. 1998;32:298–304. doi: 10.1161/01.hyp.32.2.298. [DOI] [PubMed] [Google Scholar]
- 11.Rea R.F., Hamdan M. Baroreflex control of muscle sympathetic nerve activity in borderline hypertension. Circulation. 1990;82:856–862. doi: 10.1161/01.cir.82.3.856. [DOI] [PubMed] [Google Scholar]
- 12.Ameloot K., Van De Vijver K., Van Regenmortel N. Validation study of Nexfin(R) continuous non-invasive blood pressure monitoring in critically ill adult patients. Minerva Anestesiol. 2014;80:1294–1301. [PubMed] [Google Scholar]
- 13.Martina J.R., Westerhof B.E., van Goudoever J. Noninvasive continuous arterial blood pressure monitoring with Nexfin(R) Anesthesiology. 2012;116:1092–1103. doi: 10.1097/ALN.0b013e31824f94ed. [DOI] [PubMed] [Google Scholar]
- 14.Wallin B.G., Fagius J. Peripheral sympathetic neural activity in conscious humans. Annu Rev Physiol. 1988;50:565–576. doi: 10.1146/annurev.ph.50.030188.003025. [DOI] [PubMed] [Google Scholar]
- 15.Vallbo A.B., Hagbarth K.E., Torebjork H.E. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 16.Delius W., Hagbarth K.E., Hongell A. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand. 1972;84:65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- 17.Delius W., Hongell A., Hongell A. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand. 1972;84:82–94. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- 18.Mano T., Iwase S., Toma S. Microneurography as a tool in clinical neurophysiology to investigate peripheral neural traffic in humans. Clin Neurophysiol. 2006;117:2357–2384. doi: 10.1016/j.clinph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Park J., Campese V.M., Middlekauff H.R. Exercise pressor reflex in humans with end-stage renal disease. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1188–R1194. doi: 10.1152/ajpregu.90473.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates D., Machler M., Bolker B.M. Fitting linear mixed-effects models using lme4. J Stat Software. 2015;67:1–48. [Google Scholar]
- 21.Kuznetsova A., Brockhoff P.B., Christensen R.H.B. lmerTest Package: tests in linear mixed effects models. J Stat Software. 2017;82:1–26. [Google Scholar]
- 22.R Core Team . R Foundation for Statistical Computing; Vienna: 2016. R: a Language and Environment for Statistical Computing. [Google Scholar]
- 23.Van Buren P.N. Pathophysiology and implications of intradialytic hypertension. Curr Opin Nephrol Hypertens. 2017;26:303–310. doi: 10.1097/MNH.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Buren P.N., Inrig J.K. Special situations: intradialytic hypertension/chronic hypertension and intradialytic hypotension. Semin Dial. 2017;30:545–552. doi: 10.1111/sdi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nongnuch A., Campbell N., Stern E. Increased postdialysis systolic blood pressure is associated with extracellular overhydration in hemodialysis outpatients. Kidney Int. 2015;87:452–457. doi: 10.1038/ki.2014.276. [DOI] [PubMed] [Google Scholar]
- 26.Ren H., Gong D., He X. Evaluation of intradialytic hypertension using bioelectrical impedance combined with echocardiography in maintenance hemodialysis patients. Ther Apher Dial. 2018;22:22–30. doi: 10.1111/1744-9987.12605. [DOI] [PubMed] [Google Scholar]
- 27.Sebastian S., Filmalter C., Harvey J. Intradialytic hypertension during chronic haemodialysis and subclinical fluid overload assessed by bioimpedance spectroscopy. Clin Kidney J. 2016;9:636–643. doi: 10.1093/ckj/sfw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgianos P.I., Mpoutsiouki F., Sabani E. Hemodialysis patients with intradialytic rise in blood pressure display higher baseline aortic stiffness and negligible drop in augmentation index with dialysis. Int Urol Nephrol. 2016;48:601–608. doi: 10.1007/s11255-015-1205-8. [DOI] [PubMed] [Google Scholar]
- 29.Inrig J.K., Van Buren P., Kim C. Probing the mechanisms of intradialytic hypertension: a pilot study targeting endothelial cell dysfunction. Clin J Am Soc Nephrol. 2012;7:1300–1309. doi: 10.2215/CJN.10010911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inrig J.K., Van Buren P., Kim C. Intradialytic hypertension and its association with endothelial cell dysfunction. Clin J Am Soc Nephrol. 2011;6:2016–2024. doi: 10.2215/CJN.11351210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng J., Tian J., Lv W.L. Inappropriately elevated endothelin-1 plays a role in the pathogenesis of intradialytic hypertension. Hemodial Int. 2015;19:279–286. doi: 10.1111/hdi.12238. [DOI] [PubMed] [Google Scholar]
- 32.Movilli E., Camerini C., Gaggia P. Role of dialysis sodium gradient on intradialytic hypertension: an observational study. Am J Nephrol. 2013;38:413–419. doi: 10.1159/000355974. [DOI] [PubMed] [Google Scholar]
- 33.Wallin B.G., Esler M., Dorward P. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Victor R.G., Morgan D.A., Thoren P. High salt diet sensitizes cardiopulmonary baroreflexes in Dahl salt-resistant rats. Hypertension. 1986;8:II21–II27. doi: 10.1161/01.hyp.8.6_pt_2.ii21. [DOI] [PubMed] [Google Scholar]
- 35.Van Buren P.N., Kim C., Toto R. Intradialytic hypertension and the association with interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2011;6:1684–1691. doi: 10.2215/CJN.11041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inrig J.K., Patel U.D., Gillespie B.S. Relationship between interdialytic weight gain and blood pressure among prevalent hemodialysis patients. Am J Kidney Dis. 2007;50:108–118. doi: 10.1053/j.ajkd.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imamura T., Takeshita A., Ashihara T. Digitalis-induced augmentation of cardiopulmonary baroreflex control of forearm vascular resistance. Circulation. 1985;71:11–16. doi: 10.1161/01.cir.71.1.11. [DOI] [PubMed] [Google Scholar]
- 38.Schobel H.P., Oren R.M., Roach P.J. Contrasting effects of digitalis and dobutamine on baroreflex sympathetic control in normal humans. Circulation. 1991;84:1118–1129. doi: 10.1161/01.cir.84.3.1118. [DOI] [PubMed] [Google Scholar]
- 39.Kramer H.J., Pennig J., Klingmuller D. Digoxin-like immunoreacting substance(s) in the serum of patients with chronic uremia. Nephron. 1985;40:297–302. doi: 10.1159/000183482. [DOI] [PubMed] [Google Scholar]
- 40.Kelly R.A., O'Hara D.S., Mitch W.E. Endogenous digitalis-like factors in hypertension and chronic renal insufficiency. Kidney Int. 1986;30:723–729. doi: 10.1038/ki.1986.247. [DOI] [PubMed] [Google Scholar]
- 41.Cui J., Wilson T.E., Crandall C.G. Baroreflex modulation of muscle sympathetic nerve activity during cold pressor test in humans. Am J Physiol Heart Circ Physiol. 2002;282:H1717–H1723. doi: 10.1152/ajpheart.00899.2001. [DOI] [PubMed] [Google Scholar]
- 42.Fagius J., Karhuvaara S., Sundlof G. The cold pressor test: effects on sympathetic nerve activity in human muscle and skin nerve fascicles. Acta Physiol Scand. 1989;137:325–334. doi: 10.1111/j.1748-1716.1989.tb08760.x. [DOI] [PubMed] [Google Scholar]
- 43.Ichinose M., Nishiyasu T. Arterial baroreflex control of muscle sympathetic nerve activity under orthostatic stress in humans. Front Physiol. 2012;3:314. doi: 10.3389/fphys.2012.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]