Abstract
Background
New technology has resulted in bronchoscopy being increasingly used for diagnosing pulmonary lesions. Reported yield from these procedures varies widely with few randomized clinical trials. This study compares the diagnostic yield of a thin bronchoscope and radial endobronchial ultrasound (R-EBUS) with standard bronchoscopy and fluoroscopy (SB-F) in lung lesions.
Methods
Patients presenting for diagnostic bronchoscopic evaluation at five centers were randomized to undergo SB-F or R-EBUS with a thin bronchoscope (TB-EBUS). If SB-F was nondiagnostic, crossover to the TB-EBUS arm was allowed. Data on patient demographics, radiographic features, and final pathologic or radiographic follow-up were collected. Statistical comparisons were made by Fisher exact test, χ2 test, and Student t test. Bivariate and multivariate analyses were performed to determine predictors of diagnostic yield.
Results
One hundred and ninety-seven patients were included in the final analyses. There was no difference in demographics, lesion size, or location between study arms. The average lesion size was 31.2 mm (SD, 10.8 mm). Bronchoscopy was diagnostic in 87 patients (44%). Although the diagnostic yield was higher in the TB-EBUS arm compared with the SB-F arm (49% vs 37%), this difference was not statistically significant (P = .11). Among those with nondiagnostic bronchoscopic findings in the standard arm, 87% (n = 46) crossed over to TB-EBUS, resulting in a diagnosis in seven additional patients (15% of 46).
Conclusions
Bronchoscopy with or without a thin scope and R-EBUS had a poor diagnostic yield for pulmonary lesions. Future work should focus on improvements in technique and technology advances that ensure a higher likelihood of obtaining a diagnosis.
Key Words: bronchoscopy, guided bronchoscopy, lung lesion, radial endobronchial ultrasound
Abbreviations: AQuIRE, American College of Chest Physicians Quality Improvement Registry, Evaluation, and Education; EMN, electromagnetic navigation; pCA, probability of malignancy; R-EBUS, radial endobronchial ultrasound; ROSE, rapid on-site evaluation; SB-F, standard bronchoscopy and fluoroscopy; TBBX, transbronchial biopsy; TB-EBUS, thin bronchoscope and radial endobronchial ultrasound
One of the most important indications for undergoing a bronchoscopy is undiagnosed pulmonary lesions. Historically, this was accomplished by utilizing standard bronchoscopy under fluoroscopic guidance (SB-F) with either transbronchial brush, forceps, or needle aspiration. The yield of this technology varies depending on the size of the lesion, with pooled reports ranging from 14% to 34% for lesions < 2 cm and to 64% for larger lesions.1 Over the past 15 years, newer technologies have emerged to improve the yield of bronchoscopy by more reliably sampling pulmonary lesions that were once less attainable by the standard approach. These include electromagnetic navigation (EMN), ultrathin bronchoscopy, radial endobronchial ultrasound (R-EBUS), virtual bronchoscopy, and guide sheaths. A meta-analysis of 39 studies of guided-bronchoscopy technologies in more than 3,000 patients demonstrated a pooled diagnostic yield of 70% with few differences in yield by approach.2 These data and additional studies provided support for the 2013 American College of Chest Physicians nodule treatment guidelines recommending that guided bronchoscopy utilizing R-EBUS or EMN be performed where the technology and expertise is available.1
In the years following the publication of the meta-analysis, however, a number of studies have reported significantly lower yields from bronchoscopy for the diagnosis of pulmonary lesions.3, 4 Analysis of the American College of Chest Physicians Quality Improvement Registry, Evaluation, and Education (AQuIRE) registry reported the yield for guided bronchoscopy to be between 38% and 57% depending on the type of guidance used.3 Similarly, a prospective multicenter trial evaluating the effectiveness of a bronchial airway gene expression classifier on the diagnostic performance of bronchoscopy revealed a diagnostic yield of only 57% despite a cancer prevalence of 61%.4
Despite the discrepancy in the literature, bronchoscopy is being increasingly used to attempt diagnoses for pulmonary lesions, particularly those that are small and peripheral. The development of thinner bronchoscopes has provided access to more peripheral areas of the lung compared with standard sized bronchoscopes, and the addition of R-EBUS allows for enhanced visualization of these lesions. We report here the results of a randomized controlled trial of standard flexible bronchoscopy with fluoroscopy compared with guided bronchoscopy with thin bronchoscope and R-EBUS in the evaluation of lung lesions.
Methods
Participants
Patients 22 years of age and older with a solid lung lesion (1.5 to 5 cm identified on chest CT scan obtained within the previous 3 months) and presenting to an outpatient pulmonologist were eligible for study enrollment. Patients with lesions having an intermediate pretest probability of malignancy (pCA, 0.05 to 0.65) as determined by clinical judgment and in whom bronchoscopic biopsy was determined to be the next best treatment step were deemed eligible. This decision was made by the treating pulmonologist, and study protocol did not require input from interventional radiology as to the accessibility for a CT scan-guided biopsy approach. In lesions determined to be partially solid, the solid component must have made up > 75% of the lesion and measure 1.5 to 5 cm in order to be eligible. Patients with higher risk lesions (pCA > 0.65) in need of a diagnosis for nonsurgical treatment or prior to surgery were also eligible to participate. Patients who were pregnant, lacked fitness to undergo bronchoscopy, or had a target lesion not readily visualized by fluoroscopy on the day of bronchoscopy were excluded from participation. Consecutive patients meeting inclusion/exclusion criteria were invited to participate in this study.
Randomization schedule was determined in advance by computer with the intent of a 1:1 randomization scheme. Participant assignments were placed in sealed envelopes and distributed to sites. This study was approved by the institutional review board at all sites (e-Table 1). The study procedures were performed beginning in July 2014 and continued through April 2017.
Bronchoscopy Procedure
Following confirmation of fluoroscopic view of the pulmonary lesion, moderate or deep sedation was used per site standard of care. Participants were randomized to either the SB-F arm or the thin bronchoscopy plus R-EBUS (TB-EBUS) arm. In the SB-F arm, bronchoscopy was performed with a standard sized bronchoscope (outer channel diameter, 4.9 mm) (BF-180 or BF-190; Olympus). Lesions were localized by fluoroscopy guidance and brushing was performed. Bronchial brushing was standardized to 10 strokes per brushing. A slide was made from the brush and reviewed via rapid on-site evaluation (ROSE). The tip of the brush was cut and placed into CytoLyt solution (Hologic Inc). Regardless of ROSE determination, five transbronchial biopsies were then performed (FB-233D; Olympus). Those participants with ROSE that was nondiagnostic for malignancy or without an alternative benign diagnosis (eg, granulomatous inflammation) were crossed over to the TB-EBUS arm.
Participants randomized to the TB-EBUS arm underwent bronchoscopy, using a thin bronchoscope (BF-P190; Olympus) with a 4.2-mm outer diameter. The bronchoscopist had the option to use a guide sheath (external diameter, 2 mm [K201]; Olympus) to extend the working channel of the bronchoscope where indicated. The 20-MHz mechanical R-EBUS probe (UM-S20-17S; Olympus) with or without the guide sheath covering was introduced into the working channel of the bronchoscope and advanced to the lesion. Fluoroscopy was used to aid lesion location, and an attempt to definitively locate the lesion by R-EBUS was made. If the ultrasound image seen following R-EBUS probe insertion was eccentric (eg, only part of the target lesion was visualized by ultrasound), the bronchoscopist had the option to introduce a double-hinged curette in an attempt to manipulate the position such that the ultrasound image of the lesion was concentric (eg, the ultrasound image demonstrated the target lesion completely surrounding the probe in a 360-degree fashion). A deidentified JPEG image of the ultrasound image was captured. Following lesion location, R-EBUS probe was removed and samples were taken through the bronchoscope working channel or guide sheath. The brush tip was cut directly into CytoLyt solution. Smooth transbronchial forceps (FB-233D; Olympus) were then introduced to obtain five grossly visible transbronchial biopsy specimens.
Data Collection
Study participant demographic information including sex, race, age, and smoking history was collected. Radiographic information collected included lesion location, size (longest axis diameter), and nodule contour when available. Diagnostic yield was determined from the finalized pathology results of the bronchoscopy. A procedure was considered diagnostic when a malignant or specific benign diagnosis was made on the basis of either the brushing or transbronchial biopsy (TBBX), or both.
Those who underwent a nondiagnostic procedure and those with pathology noting inflammation were monitored until a definitive diagnosis was made by an additional procedure (eg, CT scan-guided biopsy or surgical resection) or the lesion demonstrated 1 year of stability or resolution on repeat CT imaging.
Sample Size
The study was initially designed with 85% power to detect an absolute 20% difference between diagnostic procedure arms in diagnostic yield (ie, assuming 50% yield for SB-F vs 70% yield for TB-EBUS), with two-sided hypothesis testing and an α level of 0.05. The final actual sample sizes of n = 85 (SB-F) and n = 112 (TB-EBUS) provided 81% power to detect an absolute difference of 20% in diagnostic yield.
Data Analysis
Descriptive statistics were generated to characterize study participants. Baseline comparisons between the study arms were conducted using χ2 tests, Fisher exact tests, two-sample Student t tests, or Wilcoxon rank sum tests, as appropriate. Each procedure was characterized as diagnostic or nondiagnostic, and diagnostic yield was compared between study arms in the primary analysis, using a χ2 test. Sensitivity analyses were conducted to investigate whether the primary findings were markedly different when (1) analyses included adjustment for site, using site as a random effect within a generalized linear mixed model to account for within-site clustering, and (2) analyses excluded all subjects enrolled at the site where the large majority of protocol deviations occurred. χ2 tests were used to determine whether findings were consistent across patient subgroups. Using all baseline variables (ie, demographics, clinical characteristics, and study site), a backwards selection procedure was used to develop a multivariable logistic regression model to identify variables associated with diagnostic yield. Diagnostic procedure arm was forced into these models, and variables were removed one by one if they were not significantly (P < .05) associated with the outcome. Incidentally, a forwards selection procedure yielded a model identical to the backwards model. All analyses were conducted with SAS version 9.4 (SAS Institute).
Results
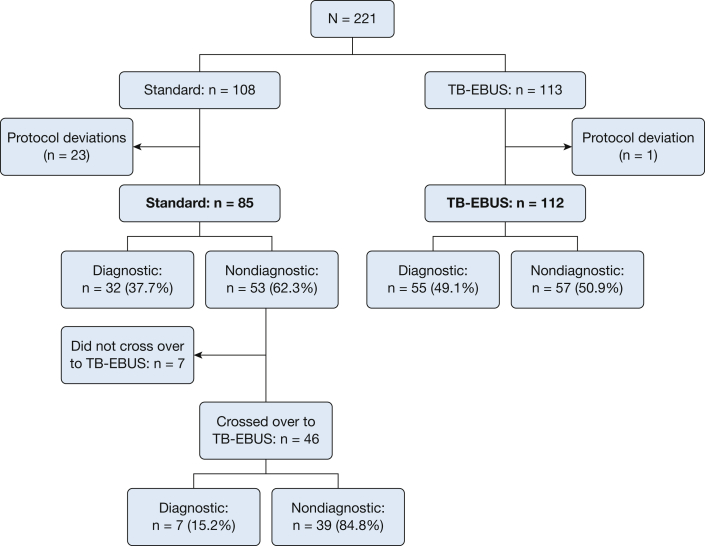
There were 221 patients who met entry criteria and agreed to participate in the trial. Of these, 108 were randomized to the SB-F and 113 to the TB-EBUS. The final analysis included 197 patients: 85 in the SB-F arm and 112 in the TB-EBUS arm. Figure 1 demonstrates patient randomization, exclusions, and diagnostic yield. In one site, n = 13 patients in the SB-F arm were inappropriately crossed over to the TB-EBUS arm after brushings were performed but without subsequent TBBX. To avoid a potential bias in our findings, study investigators decided to exclude all SB-F patients (n = 19) from that site. Four additional patients randomized to the SB-F arm at other sites were also excluded from the primary analysis due to protocol deviations.
Figure 1.
CONSORT diagram and diagnostic yields. CONSORT = Consolidated Standards of Reporting Trials; TB-EBUS = thin bronchoscope and radial endobronchial ultrasound.
Patient demographics and radiographic characteristics of target lesions are presented in Table 1. There was no difference between groups based on age, sex, race, smoking status, or pack-year history. The average pulmonary lesion size was 31.2 mm (SD, 10.8). Most lesions were located in the upper lobes, were solid in appearance, and had a radiographic bronchus sign (n = 136, 69%). There was no difference in size or radiographic characteristics of pulmonary lesions between groups.
Table 1.
Participant Demographics and Lesion Characteristics
| Variable | All Subjects (N = 197) | Standard Bronchoscopy (N = 85) | Thin Bronchoscope + R-EBUS (N = 112) | P Value |
|---|---|---|---|---|
| Patient demographics | ||||
| Age, mean (SD) | 67.0 (10.9) | 65.4 (11.8) | 68.2 (10.1) | .07 |
| Male sex, No. (%) | 101 (51.30) | 39 (45.90) | 62 (55.40) | .19 |
| Race, No. (%) | .34 | |||
| White | 140 (71.10) | 56 (65.90) | 84 (75.00) | |
| Black | 52 (26.40) | 26 (30.60) | 26 (23.20) | |
| Other | 5 (2.50) | 3 (3.50) | 2 (1.80) | |
| Smoking status, No. (%) | .69 | |||
| Current | 56 (28.40) | 25 (29.40) | 31 (27.70) | |
| Former | 90 (45.70) | 36 (42.40) | 54 (48.20) | |
| Never | 51 (25.90) | 24 (28.20) | 27 (24.10) | |
| Pack-years, mean (SD) | 39.7 (29.4) | 39.3 (29.9) | 40.0 (29.2) | .88 |
| Medical history, No. (%) | ||||
| Lung cancer (personal) | 23 (11.70) | 7 (8.20) | 16 (14.30) | .19 |
| Lung cancer (family) | 23 (11.70) | 10 (11.80) | 13 (11.60) | .97 |
| Emphysema | 57 (28.93) | 25 (29.40) | 32 (28.60) | .9 |
| Lesion Characteristics | ||||
| Size, mean (SD), mm | 31.2 (10.8) | 30.4 (11.1) | 31.8 (1.5) | .39 |
| No. of lesions present | ||||
| Mean, SD | 1.84 (2.73) | 1.81 (2.06) | 1.86 (3.16) | .77 |
| Median, IQR | 1 (1-2) | 1 (1-2) | 1 (1-2) | |
| Location of primary lesion, No. (%) | .2 | |||
| LLL | 21 (10.66) | 9 (10.60) | 12 (1.70) | |
| LUL | 59 (29.95) | 24 (28.20) | 35 (31.30) | |
| RLL | 27 (13.71) | 17 (20.00) | 10 (8.90) | |
| RML | 21 (10.66) | 10 (11.80) | 11 (9.80) | |
| RUL | 69 (35.03) | 25 (29.40) | 44 (39.30) | |
| Bronchus sign | 136 (69.00) | 62 (72.90) | 74 (66.10) | .3 |
| Nodule type, No. (%) | ||||
| Ground glass | 5 (2.50) | 3 (3.50) | 2 (1.80) | .65 |
| Semisolid | 29 (14.70) | 13 (15.30) | 16 (14.30) | .84 |
| Solid | 164 (83.30) | 70 (82.40) | 94 (83.90) | .77 |
| Lesion contour, No. (%) | ||||
| Spiculation | 105 (53.30) | 48 (56.50) | 57 (5.90) | .44 |
| Lobulation | 55 (27.90) | 23 (27.10) | 32 (28.60) | .81 |
| Smooth | 45 (26.80) | 20 (28.60) | 25 (25.50) | .66 |
IQR = interquartile range; LLL = left lower lobe; LUL = left upper lobe; R-EBUS = radial endobronchial ultrasound; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe.
Bronchoscopy was diagnostic in 87 participants (44%). Although the diagnostic yield was higher in the TB-EBUS arm when compared with the SB-F arm (49% vs 37%), this difference was not statistically significant (P = .11). In the sensitivity analyses, our conclusions remained unchanged when analyses included adjustment for study site (P = .12 when comparing treatment arms) or when analyses excluded all subjects enrolled at the site where the majority of protocol deviations occurred (P = .25 when comparing treatment arms).
e-Table 2 lists comparisons between the treatment arm diagnostic yields by various patient subgroups (ie, lesion size, location, spiculation and lobulation, and type of sedation); in none of these subgroups was the diagnostic yield significantly different between treatment arms. e-Table 3 lists comparisons of diagnostic test characteristics (ie, sensitivity and specificity) between treatment arms; neither of these was significantly different between treatment arms. By design, positive and negative predictive values were assumed to be 100% for both SB-F and TB-EBUS, since positive (malignant) and negative (benign) test findings were made by ROSE in combination with transbronchial biopsies.
In the SB-F arm, 13 of the 85 patients (15.3%) had brushings that were diagnostic by ROSE at the time of the procedure (six malignant, one suspicious for malignancy, six chronic inflammation). Of the 53 patients with nondiagnostic bronchoscopy results in the SB-F arm, 87% (n = 46) subsequently crossed over to the TB-EBUS arm. This resulted in a diagnosis in seven additional patients (15%). Of the 179 patients who underwent TB-EBUS either because of randomization or subsequent crossover, 174 (97%) had ultrasound confirmation of lesion localization. A concentric image was seen in 113 (65%). Diagnostic yield was significantly higher when the ultrasound image was concentric compared with eccentric (50% vs 31%; P = .014). In the TB-EBUS arm, a guide sheath was used in 67 of 112 patients (59.8%). Diagnostic yield was not significantly different (P = .67) among patients when a guide sheath was used (yield, 50.8%) compared with patients when a guide sheath was not used (yield, 46.7%). Among the n = 46 subjects who crossed over to TB-EBUS, the diagnostic yield was not statistically significantly different (P = .45) between the n = 20 in whom a guide sheath was used (10% yield) and the n = 26 in whom a guide sheath was not used (19.2% yield).
Table 2 demonstrates factors associated with diagnostic yield. As lesion size increased so did the diagnostic yield. A diagnosis was achieved in lesions measuring 36 to 50 mm in 57.1% of patients vs 30.8% in lesions 15 to 25 mm (P = .002). Lesions that were not lobulated were more likely to yield a diagnostic bronchoscopy than those with lobulation (50% vs 29%, respectively; P = .008). In the TB-EBUS arm, lesions with a concentric appearance on ultrasound imaging were also more likely to result in a diagnostic bronchoscopy (50.4% vs 31.2%, respectively; P = .014). In addition, there was no difference in diagnostic yield based on center (P = .38 [SB-F], P = .38 [TB-EBUS]) (data not shown). In the multivariable analysis, only size and absence of lobulation were independently predictive of diagnostic yield. The odds of a diagnosis being able to be made were 3.6 times higher among patients with nodules that lacked lobulation compared with those that were lobulated, and every 1-mm increase in nodule size was associated with a 6% increase in the odds of a diagnosis being able to be made.
Table 2.
Bivariate and Multivariate Analyses for Predictors of Diagnostic Yield
| Bivariate Analyses | ||
|---|---|---|
| Factors | Bronchoscopic Diagnosis |
|
| No. (%) | P Value | |
| Lobulated | ||
| No (n = 142) | 142 (50.0) | .008 |
| Yes (n = 55) | 55 (29.1) | |
| Size of lesion | ||
| 15-25 mm (n = 65) | 20 (30.8) | .002 |
| 26-35 mm (n = 62) | 27 (43.6) | |
| 36-50 mm (n = 70) | 40 (57.1) | |
| R-EBUS imaging | ||
| Ecentric (n = 61) | 19 (31.2) | .014 |
| Concentric (n = 113) | 57 (50.4) | |
| Multivariable Logistic Regression Model | ||
|---|---|---|
| Factors | OR | 95% CI |
| Study arm (TB-EBUS vs SB-F) | 1.74 | 0.87-3.46 |
| Lobulated (no vs yes) | 3.35 | 1.51-7.43 |
| Size of lesion | 1.06 | 1.02-1.09 |
SB-F = standard bronchoscopy and fluoroscopy; TB-EBUS = thin bronchoscope and radial endobronchial ultrasound. See Table 1 legend for expansion of other abbreviation.
Table 3 shows the final pathologic and clinical diagnoses for participants up to 1 year following enrollment. Of the 94 patients who received a diagnosis as a result of bronchoscopic procedure, 86% (n = 81) had a malignancy. While 13 (6%) were lost to follow-up, at 1 year 66% (n = 58) of those with nondiagnostic bronchoscopies were diagnosed with malignancy. In addition, six lesions were treated empirically by stereotactic body radiotherapy for presumed cancer.
Table 3.
Final Diagnoses
| Bronchoscopic Diagnoses | |||
|---|---|---|---|
| All (n = 94) | SB-F (n = 32) | TB-EBUS (n = 62) | |
| Malignant | 81 | 27 | 54 |
| NSCLC | 11 | 6 | 5 |
| Adenocarcinoma | 43 | 13 | 30 |
| Squamous cell | 18 | 4 | 14 |
| Small cell | 1 | 1 | 0 |
| Large cell | 0 | 0 | 0 |
| Neuroendocrine | 4 | 0 | 4 |
| Other | 4 | 3 | 1 |
| Benign | 13 | 5 | 8 |
| Granuloma | 1 | 0 | 1 |
| Inflammationa | 7 | 5 | 2 |
| Pneumonia | 4 | 0 | 4 |
| Other | 1 | 0 | 1 |
| Follow-up Diagnoses (Up to 1 y After Procedure) | |||
|---|---|---|---|
| All (n = 90) | SB-F (n = 40) | TB-EBUS (n = 50) | |
| Malignant | 58 | 28 | 30 |
| NSCLC | 2 | 2 | 0 |
| Adenocarcinoma | 35 | 13 | 22 |
| Squamous cell | 4 | 3 | 1 |
| Small cell | 3 | 2 | 1 |
| Large cell | 1 | 0 | 1 |
| Neuroendocrine | 3 | 2 | 1 |
| Other | 10 | 6 | 4 |
| Benign | 22 | 8 | 14 |
| Granuloma | 1 | 0 | 1 |
| Inflammation | 0 | 0 | 0 |
| Pneumonia | 4 | 3 | 1 |
| Other | 4 | 2 | 2 |
| Lesion resolved | 3 | 1 | 2 |
| Lesion stable in size | 5 | 2 | 3 |
| Lesion smaller in size | 5 | 0 | 5 |
| No formal diagnosis | 10 | 4 | 6 |
| Enlarging | 4 | 3 | 1 |
| Presumed malignant/treated empirically | 6 | 1 | 5 |
| Lost to follow-up | 13 | 6 | 7 |
NSCLC = non-small cell lung cancer. See Table 2 legend for expansion of other abbreviations.
Inflammation was considered benign with a bronchoscopic diagnosis and resolution on 1-y follow-up.
Discussion
The role of the pulmonologist as a diagnostician has increased due to the frequent use of CT imaging and resulting incidental findings.5 This makes newly available novel guided bronchoscopy techniques and platforms as a means for diagnosis enticing. However, the range of diagnostic yield in the literature varies greatly, leaving clinicians with uncertainty as to the usefulness of the procedure. Our study, the first to compare standard bronchoscopy with fluoroscopy to a newer thin bronchoscope with radial EBUS, is important in providing clarity and has several important findings. First, standard bronchoscopy with fluoroscopy is poor for diagnosing pulmonary lesions. Second, the use of a thinner bronchoscope with the addition of radial EBUS, while better, provides a diagnosis slightly less than one-half the time, a finding more in line with recent reports than a previous meta-analysis.2 These findings highlight the need for improvements in technology to increase diagnostic yield and the importance of continued prospective randomized studies of new bronchoscopy platforms and techniques.
Standard bronchoscopy with fluoroscopy has been a mainstay for the pulmonologist. While this technology has its utility in evaluating for infection and diagnosing endobronchial lesions, there has been much variability in yield for diagnosing central and peripheral pulmonary lesions.1, 2, 4, 6 In central lesions, diagnostic yield is higher with an overall sensitivity of 88% as seen by analysis of 35 studies that included 4,507 patients.1 In peripheral lesions, studies have demonstrated a yield for flexible bronchoscopy with fluoroscopy and transbronchial biopsies ranging from 27% to 75%.1 The presence of a CT scan bronchus sign is a predictor of higher diagnostic yield, with a recent meta-analysis demonstrating threefold higher odds of making a diagnosis, although this was lower in prospective studies.7 Another approach, particularly for smaller, more peripheral lesions, is CT scan-guided biopsy, which has a diagnostic yield ranging from 70% to 90% depending on size,8 but pneumothorax and major hemorrhage rates of 14% and 1%, respectively.9 While the lower complication profile for bronchoscopy is appealing it must be weighed against a trade-off in yield.
While the meta-analysis had a pooled diagnostic yield of 70% for guided bronchoscopy,2 a much lower yield was seen in the AQuIRE registry where 518 patients undergoing bronchoscopy for peripheral lesions at 15 centers had an overall diagnostic yield of just greater than 50%.3 In another large (639 patients), prospective multicenter (28 sites) trial evaluating a bronchial genomic classifier the yield of bronchoscopy was only 57%.4 Our findings are much more in line with the two trials than the meta-analysis.
It is worth considering why these discrepancies exist. Our study did not include the use of navigation (EMN or virtual) or use of a needle for biopsy. While the addition of a navigational platform has been suggested to provide increased yield, in our study when TB-EBUS was utilized the lesion could be seen in 97% of the patients, suggesting that the addition of such platforms might not provide additional benefit in reaching the target lesion. Size of the bronchoscope is another factor to consider; while our study utilized a thin bronchoscope with a 4-mm outer diameter, there is an even smaller ultrathin bronchoscope with a 3-mm outer diameter that is not yet available for use in the United States. A four-center prospective, randomized study in Japan compared the diagnostic yield of the thin 4-mm scope with TBBX under R-EBUS, fluoroscopy, and virtual navigational bronchoscopy with that of the ultrathin scope in pulmonary lesions measuring 3 cm or less.6 While the thin scope demonstrated an overall diagnostic yield of 59%, the ultrathin scope had a significantly better yield at 74% (P = .04).6 In addition, it may be that yield was lower because of the sampling tools used in this trial. We did not perform needle biopsy in this study because at the time there were no needles approved for use through the guide sheath, preventing consistent use of needles for all patients enrolled; the use of needle aspiration has been associated with a higher diagnostic yield in both the AQuIRE registry and other studies.3, 10 In addition, we did not use bronchial washings or lavage in this study. Previous studies have shown a yield of 43% and 48% for lavage/washings in peripheral and central malignant pulmonary lesions, respectively.1 It is a limitation in that including lavage may have improved diagnostic yield in benign disease. Last, we may have failed to identify a difference because of the exclusion of 25 patients, which reduced the final power to detect a difference in diagnostic yield from the designed 85% to 81%. Another possible explanation is that a meta-analysis pools single-site studies of investigators with interest, expertise, and high bronchoscopy volume, which could all lead to improved diagnostic yield, as opposed to large multicenter randomized trials with variable expertise and volumes, which may lead to lower yield. This may only partially explain our results as the sites included in this study are all high-volume centers with interest and expertise in bronchoscopy. Nonetheless, a comparison of meta-analyses with large prospective randomized controlled trials points out that this discrepancy exists.
There are some findings in our study that mirror previous trials. Diagnostic yield is improved when the size of the lesion is large and when the R-EBUS image reveals that the lesion is concentric.
How should we place the findings of this and other recent studies into clinical context? First, it appears that standard bronchoscopy with fluoroscopy alone has extremely poor performance characteristics for pulmonary lesions and should be reserved for evaluation of presumed infection or larger masses. Second, for patients with smaller lesions consideration should be given to using transthoracic needle aspiration as opposed to bronchoscopy, especially when the adverse event rate would be expected to be lower (eg, peripheral lesions in patients without emphysema). This is likely to provide a higher diagnostic yield and decrease the chances of the patient having to undergo a second procedure and a delay in diagnosis. As new technology evolves, the pulmonary community should commit to rigorously evaluating its usefulness in a multicenter randomized fashion.
In conclusion, in a randomized prospective multicenter setting, bronchoscopy with or without a thin scope and R-EBUS had a poor diagnostic yield for pulmonary lesions. Future work should focus on improvements in technique and advances in technology that ensure a higher likelihood of obtaining a tissue diagnosis.
Acknowledgments
Author contributions: N. T. T. and G. A. S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. N. T. T., L. Y., A. C., J. W. M., H. M., P. J. N., and G. A. S. contributed substantially to the study design, data analysis, and interpretation, and the writing of the manuscript. N. J. P. and H. L. contributed substantially to data interpretation and the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: N. T. T., L. Y., A. C., and J. W. M. have received consulting fees from Olympus America. G. A. S. has received grant funding from Olympus America. None declared (H. J. M., N. J. P., H. L., M. A. J., P. J. N.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Layne Walker, BS, Katherine Taylor, MS, Jenna Los, MLA, Lexi Barriere, BS, and Timothy Rodriguez, BS, for their contributions to study recruitment and data entry.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Funded by Olympus America. P. J. N.’s time on this manuscript was also funded, in part, by grants from the National Institutes of Health (National Center for Advancing Translational Sciences [Grant UL1 TR001450] and the National Institute of General Medical Sciences [Grant U54-GM104941]).
Supplementary Data
References
- 1.Rivera M.P., Mehta A.C., Wahidi M.M. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e142S–e165S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 2.Wang Memoli J.S., Nietert P.J., Silvestri G.A. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142(2):385–393. doi: 10.1378/chest.11-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ost D.E., Ernst A., Lei X. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. results of the AQuIRE Registry. Am J Respir Crit Care Med. 2016;193(1):68–77. doi: 10.1164/rccm.201507-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silvestri G.A., Vachani A., Whitney D. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med. 2015;373(3):243–251. doi: 10.1056/NEJMoa1504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould M.K., Tang T., Liu I.L. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208–1214. doi: 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 6.Oki M., Saka H., Ando M. Ultrathin bronchoscopy with multimodal devices for peripheral pulmonary lesions: a randomized trial. Am J Respir Crit Care Med. 2015;192(4):468–476. doi: 10.1164/rccm.201502-0205OC. [DOI] [PubMed] [Google Scholar]
- 7.Ali M.S., Sethi J., Taneja A., Musani A., Maldonado F. Computed tomography bronchus sign and the diagnostic yield of guided bronchoscopy for peripheral pulmonary lesions: a systematic review and meta-analysis. Ann Am Thorac Soc. 2018;15(8):978–987. doi: 10.1513/AnnalsATS.201711-856OC. [DOI] [PubMed] [Google Scholar]
- 8.Bach P.B., Gould M.K., Silvestri G.A. Computed tomography screening for lung cancer. Ann Intern Med. 2013;159(2):155–156. doi: 10.7326/0003-4819-159-2-201307160-00017. [DOI] [PubMed] [Google Scholar]
- 9.Wiener R.S., Schwartz L.M., Woloshin S., Welch H.G. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Internal Med. 2011;155(3):137–144. doi: 10.1059/0003-4819-155-3-201108020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen A., Chenna P., Loiselle A., Massoni J., Mayse M., Misselhorn D. Radial probe endobronchial ultrasound for peripheral pulmonary lesions: a 5-year institutional experience. Ann Am Thorac Soc. 2014;11(4):578–582. doi: 10.1513/AnnalsATS.201311-384OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.