Abstract
Isothiocyanates from cruciferous vegetables have been studied extensively in cells and in animals for their disease preventive and therapeutic effects. However, translating their utility to human populations has been both limited and challenging. Herein, clinical trials employing two isothiocyanates, sulforaphane (SFN; 1-Isothiocyanato-4-(methylsulfinyl) butane) and phenethyl isothiocyanate (PEITC; 2-isothiocyanatoethylbenzene) that are isolated principally from broccoli and watercress, respectively, are summarized and discussed. Both of these compounds have been used in small human clinical trials, either within food matrices or as single agents, against a variety of diseases ranging from cancer to autism. Results suggest an opportunity to incorporate them, or more likely preparations derived from their source plants, into larger human disease mitigation efforts. The context for the applications of these compounds and plants in evidence-based food and nutritional policy is also evaluated.
Keywords: Broccoli, watercress, isothiocyanate, sulforaphane, phenethyl isothiocyanate, clinical trials
1. Introduction
Origin and synthesis
Isothiocyanates are stress-response chemicals formed from glucosinolates in plants often belonging to the cruciferae family, and more broadly the Brassica genus. A plethora of diverse plants belong to this family - broccoli, watercress, kale, cabbage, collard greens, Brussels sprouts, bok choy, mustard greens and cauliflower to name a few. The agricultural use of cruciferous vegetables dates back many centuries. Farmers likely started cultivating wild forms of the mustard plant and via artificial selection were able to produce the large variety of genetically similar yet visibly different species that we see today [1] [2].
Nitrogen and sulfur-containing glucosinolates, present in cruciferous vegetables are hydrolyzed, by the action of the plant myrosinase enzyme, into nitriles, indoles, thiocyanates and isothiocyanates upon cutting, cooking, chewing and digestion [3]. Hydrolysis of glucosinolates provides an important defense mechanism against pathogen attacks, changes in the climate and other stresses [4]. Over 120 different glucosinolates have been identified [5] [6] and it has been conjectured that almost all of them originate from four plant species - Barbarea vulgaris, Arabidopsis thaliana, Eruca sativa and Isatis tinctoria [7]. Different glucosinolates produce distinct isothiocyanates. For example, glucoraphanin (GR) is the glucosinolate precursor of sulforaphane (SFN; 1-Isothiocyanato-4-(methylsulfinyl) butane) while gluconasturtiin is the biogenic source of phenylethyl isothiocyanate (PEITC; 2-isothiocyanatoethylbenzene). The types and concentrations of glucosinolates vary significantly between crucifers and are also subject to change based on temperature, age, soil chemistry, solar irradiance, season, genetics, and plant ontogeny. Therein lies a major challenge for their use in science-driven interventional studies.
Chemical structures
The formations of SFN from glucoraphanin and PEITC from gluconasturtiin are illustrated in Figure 1. Early reports of identification of SFN in cruciferous plants date back to the 1950s [8]. SFN isolated from broccoli was shown to induce carcinogen detoxication enzymes by Talalay and colleagues in 1992 [9]. The functional group responsible for this biological action is the strongly electrophilic central carbon of the (N=C=S). Concurrently, a series of structural analogs of SFN were synthesized, but none showed superior cytoprotective enzyme inducer activity to SFN [10]. None of these analogs have transitioned into clinical studies. Reports of cancer preventive properties of PEITC dates back to the 1970s [11]. It is an aromatic isothiocyanate and the biological reactivity of PEITC is also largely governed by (N=C=S).
Figure 1.
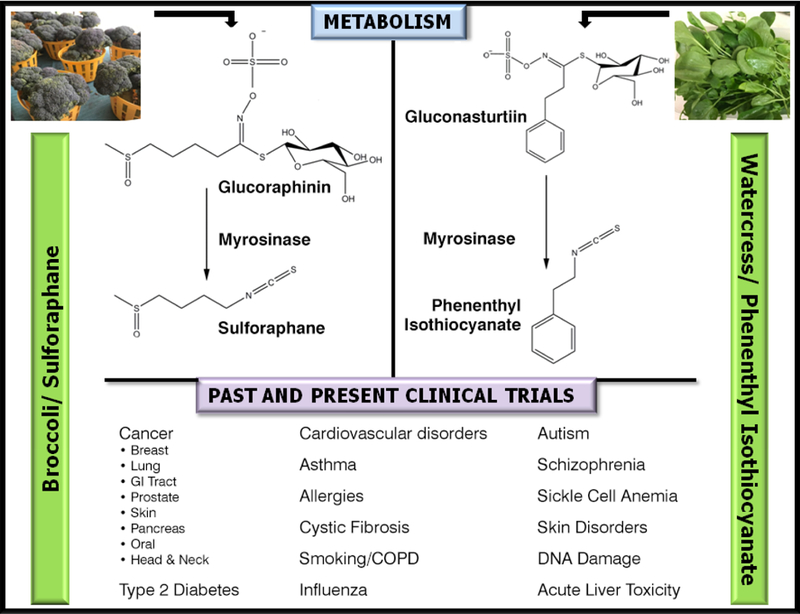
Schematic of glucosinolate metabolism in broccoli and watercress; and a summary of conditions for which clinical trials have been or are currently being carried out using broccoli or watercress preparations, SFN or PEITC. Information on current clinical trials was obtained from clinicaltrials.gov.
2. Pharmacology
Metabolism, bioavailability and elimination
The precursor glucosinolates are metabolized into isothiocyanates by the action of plant myrosinase and undergo further metabolism, initially through conjugation with glutathione (GSH) by glutathione S-transferases (GSTs) [12]. This conjugation is followed by the formation of isothiocyanate-Cys-Gly by gamma glutamyltranspeptidase (GTP). The mercapturic acid, isothiocyanate-N-acetyl cysteine (isothiocyanate-NAC) is ultimately formed by the sequential reactions catalyzed by cysteinylglycinase (CGase) and acetyltransferase (AT). While all of these metabolites of isothiocyanates have been detected in urine and plasma after ingestion of cruciferous vegetable preparations or isothiocyanates themselves in humans [13] [14], the mercapturic acids seem to be the most predominant species.
Critical to the formation of isothiocyanates is the plant enzyme myrosinase and β-thioglucosidases occurring in the human gastrointestinal microbiome [15], which convert precursor glucosinolates into bioactive isothiocyanates. A human homolog of myrosinase has not been described. Directly administered isothiocyanates have much higher bioavailability than glucosinolates [16], reflecting their intrinsic lipophilicity, but more importantly reflecting the need for ingested glucosinolates to first be converted to isothiocyanates prior to absorption and further metabolism. Measurement of urinary excretion of SFN and SFN metabolites has been used routinely to determine bioavailability in various trials. In a randomized, cross-over clinical trial where participants were administered either SFN- or GR-rich (GRR) beverages, it was observed that participants who received SFN-rich beverage had substantially higher rates of urinary excretion of SFN metabolites than those who received GRR beverage [17]. Only 5% of the administered GR was recovered as SFN metabolites as compared to 70% when SFN was administered. Egner and colleagues also showed in this cohort of healthy Chinese adults that there was rapid clearance of urinary metabolites of SFN, a finding that had been reported by others previously [18]. Four healthy volunteers who were fed an extract of 3-day old broccoli sprouts that had been hydrolyzed with myrosinase (mean dose of isothiocyanates ~201 μmol) showed rapid excretion rates of isothiocyanates (mean cumulative 8 hour excretion ~117 μmol; about 58% of the administered dose) [19].
Others have shown that consumption of fresh broccoli sprouts resulted in significantly higher plasma and urinary concentrations of SFN than achieved following consumption of a commercial dietary supplement claiming to be rich in GR, but lacking myrosinase [20]. In another small clinical study, a different commercial supplement rich in GR but without myrosinase, provided identical crude pharmacokinetics as a laboratory-prepared, freeze-dried broccoli sprout extract [21], indicating inter-individual variability observed in GR delivery. Similarly, it was shown that high myrosinase activity corresponded with higher bioavailability of SFN and somewhat shorter excretion half lives (2.2 hours for high activity compared with 3.1 hours for low activity) of urinary SFN conjugates compared to heat-applied broccoli florets with low myrosinase activity [22].
The finding that myrosinase-containing preparations of broccoli have higher bioavailability than those that have no myrosinase has been echoed by other studies [21]. A comparison of fresh broccoli sprouts, glucosinolate-rich broccoli powder lacking myrosinase and a combination of both, yielded the highest urinary SFN recovery from the sprouts followed by the combination and the powder, respectively [23]. In this study, appearance of SFN metabolites in urine and plasma was delayed after consumption of the powders compared to that from consumption of broccoli sprouts, likely due to the lack of active and readily available, ingested myrosinase and a dependence on the gut microbiome to supply it. Not surprisingly, methods of cooking broccoli and other cruciferous vegetables can have a significant impact on the formation and content of isothiocyanates. Fresh broccoli yields approximately 3 times higher levels of isothiocyanates compared to cooked broccoli [24], leading to the clear suggestion that retention of endogenous myrosinase activity by avoiding heat is an important consideration for isothiocyanate delivery from cruciferous vegetables. These concerns are supported by epidemiologic data reviewed by Tang and colleagues [25] [26].
When 3 subjects were given one oral dose of 40 mg of PEITC, the highest plasma concentrations of total isothiocyanates ranging from 0.64 to 1.40 μM was detected between 3 and 5.5 hours [27]. A separate study evaluated four healthy volunteers who were instructed to eat 30 g of watercress (estimated to contain approximately 9.79 mg/g dry weight of gluconasturtiin) for breakfast and PEITC-NAC ranging from 4.6 to 10.2 mg was detected in 24 hour urine collections with peak excretion at 2 to 4 hours post ingestion [28]. In rats, peak plasma concentrations (9 μM) of PEITC were observed ~44 minutes after oral administration of 10 μmol/ kg [29]. When rats were dosed repeatedly with high doses of PEITC (1 or 5 mg/ kg; 6 or 30 μmol/kg), bioavailability of PEITC was enhanced significantly compared to a single dosing regimen [30]. When the same strain of rats was orally supplied with the same dose of SFN, Cmax values for PEITC were much higher than that of SFN, suggesting that the latter reaches higher concentrations in tissue [31]. Whether this holds true for human bioavailability of PEITC and SFN is a matter for further investigation. As might be expected, work two decades ago demonstrated that no myrosinase activity could be detected after cooking watercress in boiling water for 3 minutes compared to its uncooked counterpart [32].
Collectively, isothiocyanate pharmacology is dependent on multiple different factors - type of food matrix (plant characteristics like species and age), type of starting material (food vs. supplement), method of preparation (raw vs. cooked) and secondary processing (subject to chewing and/ or gut microbiome). Variability that results from these factors makes isothiocyanate research challenging but it also presents excellent opportunities for further research and applications in multiple different areas.
3. Clinical trials with disease-related outcomes, using isothiocyanates
Several clinical trials have shown that isothiocyanates, given orally as glucosinolate precursors within cruciferous vegetables or directly in its bioactive form, have provided evidence for protective or therapeutic effects against disease. In particular, broccoli/ SFN (delivered as mature broccoli, broccoli sprout extract, broccoli seed extract, and broccoli powder) have been tested expansively in some studies with promising results (summarized in Table 1). Though the literature for watercress/ PEITC is not as extensive, and is mostly limited to lung cancer and respiratory disease, some studies (summarized in Table 2) have identified its potential as a preventive and therapeutic agent. Observational/epidemiological studies have also been carried out to determine whether consumption of particular food items leads to altered disease outcomes in target populations. While these studies do not translate bench science to clinical evidence, they sometimes provide a basis for conducting robust clinical studies. In this light, select epidemiologic studies have been briefly described, where suitable, alongside clinical trials in the following text. In addition to clinical trials that have been published in peer-reviewed journals, there are multiple clinical trials that are in the pipeline (as indicated on clinicaltrials.gov) with broccoli/ SFN and watercress/PEITC (Figure 1) and are discussed in a separate section.
Table 1.
Summary of clinical trials using broccoli and SFN for disease indications.
| Agent | Disease/ condition | Participants, agent, dose and schedule |
outcome | Reference |
|---|---|---|---|---|
| Broccoli/ SFN | Cancer - breast | 54 breast biopsy candidates; Broccoli seed extract; 514 μmol GRR/ day; 56 days) | Lower Ki67, lower HDAC3 in benign tissue, lower HDAC in PBMC compared to placebo | [45] |
| Cancer - lung | 30 healthy, young smokers; steam-cooked broccoli; 250 g per day; 10 days | Increased DNA repair activity in PBMC compared to control diet | [52] | |
| 291 healthy participants; broccoli sprout beverages; GR (600 μmol) and SFN (40 μmol); 84 days | Rapid and sustained increases in urinary excretion of benzene (61%) and acrolein (23%) | [56] | ||
| 50 healthy participants; GR- or SFN-rich broccoli sprout beverage; 7 days | 20-50% increase in excretion levels of glutathione conjugates of acrolein, crotonaldehyde and benzene in GR, SFN or both compared to baseline | [57] | ||
| Cancer - GI tract | 40 H. pylori-infected subjects; broccoli sprouts; 70 g/ day/ 8 weeks | Reduced urease, inflammation and bacterial colonization in broccoli intervention group compared to alfalfa control group | [61] | |
| Cancer - prostate | 90 men with biochemical recurrence after radial prostatectomy; 60 mg of prostaphane daily; 6 months | Significantly lower log PSA slope compared to placebo | [68] | |
| Diabetes | 103 Scandinavian T2D patients; broccoli sprout extract; 150 μmol SFN per dose; 12 weeks | Improved fasting glucose and HbA1C in obese participants | [69] | |
| 81 T2D patients; broccoli sprout powder (22.5 μmol /g SFN) ; 5 g or 10 g per day; 4 weeks | Reduced fasting glucose, reduced inflammatory markers and serum insulin compared to placebo | [70] [71] | ||
| Skin disorders | 5 subjects with Epidermolysis bullosa simplex; topical application of broccoli sprout extract (500 nmol SFN/ ml); 1 week | Increase in K17 expression, variable but induced expression on K6 and K16 | [72] | |
| 6 volunteers; topical application with broccoli sprout extract (200 or 400 nmol SFN); 3 doses every 24 hours | Reduced erythema (mean = 37.7%) caused by UV radiation | [73] | ||
| Heart and vascular disease | 37 subjects with high CVD risk; standard or high GRR broccoli; 400 g per week; 12 weeks | Reduced plasma LDL-C in high GRR group | [74] | |
| 77 T2D patients with a positive H.pylori stool antigen test; broccoli sprout powder or in combination with standard therapy; 6 g/ day; 28 days | Improvement in systolic and diastolic blood pressure in the combination group | [75] | ||
| 14 adults with sickle cell disease; broccoli sprout homogenate; 50-150 μmol dose escalation for 21 days | Increase in whole blood mRNA levels of heme oxygenase 1 and trend for same with subunit of fetal hemoglobin | [78] | ||
| Developmental/ behavioral disorders | 27 young males with moderate to severe ASD; SFN derived from lyophilized broccoli sprout; 50-150 μmol SFN; 18 weeks | Improvement in social interaction, abnormal behavior and verbal communication | [79] | |
| 10 schizophrenia patients; broccoli seed extract; 69 μmol GRR (3 tablets, daily); 54 days | Improvement in cognitive function tests | [82] | ||
| Respiratory conditions | 29 subjects inoculated with FluMist live attenuated influenza virus; broccoli sprout homogenate; 100 μmol SFN; 21 days | Increase in peripheral blood NK cell expression and reduction in circulating influenza RNA | [83] | |
| 16 young, healthy smokers; broccoli sprout homogenate; 200 g per day; 4 days | Reduction in virus-induced inflammation; reduction in influenza sequences in nasal lavage fluid from smokers | [84] | ||
| 45 moderate asthmatics; SFN; 100 μmol daily; 14 days | Reduction in bronchoconstrictor effects of methacholine; reduction in airway resistance | [85] | ||
| 29 healthy subjects who tested positive for cat allergens; SFN-rich broccoli sprout extract; 100 μmol per day; 4 days | 54% reduction in diesel exhaust particle-induced nasal white blood cell counts | [86] |
Table 2.
Summary of clinical trials using watercress and PEITC for disease indications.
| Agent | Disease/ condition | Participants, agent, dose and schedule |
outcome | Reference |
|---|---|---|---|---|
| Watercress/ PEITC | Cancer - lung | 11 healthy smokers; watercress; 56.8 g per meal for 3 meals/ day; 3 days | Increased urinary NNAL plus NNAL-Gluc (33.5%) during feeding period | [53] |
| 82 healthy smokers; PEITC; 10 mg; 4 times a day for 5 weeks | Reduced NNK metabolic activation ratio with PEITC (7.7%) | [54] | ||
| DNA damage | 60 subjects; raw watercress; 85 g daily; 8 weeks | Reduction in basal DNA damage in lymphocytes and reduction in DNA damage in response to ex-vivo H2O2 | [102] | |
| 10 healthy males; raw watercress; 85 g per day; 8 weeks | Reduction in exercise-induced PMBC DNA damage; reduction in lipid peroxidation | [38] | ||
| Acute liver toxicity | 10 healthy volunteers; watercress homogenate; 50 g per day 10 hours before acetaminophen | Decrease in urinary and plasma metabolites of acetaminophen (Cys- and Mer-) | [103] |
Cancer
Isothiocyanates have in pre-clinical carcinogenesis studies demonstrated remarkable chemopreventive efficacy [33] [34]. Clinical studies are much more limited; those addressing a variety of different cancer sites are discussed below. The mechanisms by which isothiocyanates exert their anti-carcinogenic effects are being investigated by many groups, and point to key mechanisms including induction of carcinogen detoxication and elimination through elevated NRF2 signaling [35] [36], enhanced DNA damage repair [37] [38], elimination of cancer stem cells [39] and others [40]. One of the challenges involved in studying the effect of isothiocyanates on the development of cancer in humans using randomized clinical trials, is the absence of reliable biomarkers to predict cancer risk - given that cancer incidence itself cannot be effectively or affordably evaluated as an endpoint for prevention.
Breast cancer
Observational/ epidemiological studies:
Breast cancer risk is driven by levels and duration of exposure to estrogen over a life course. SFN is known to modulate the metabolism of estradiol in human mammary epithelial cells, including dampening the formation of promutagenic DNA adducts from 4-hydroxyestradiol [41]. A higher urinary 2-hydroxyestrone:16-hydroxyestrone ratio was observed in healthy, postmenopausal women with the consumption of Brassica vegetables for 4 weeks [42], which we and others believe is a favorable trend that mitigates estrogen-mediated mammary carcinogenesis. Epidemiologic studies evaluating the effect of cruciferous vegetable consumption on breast cancer development are not in complete agreement. A pooled analysis of cohort studies conducted in 2001 showed that consuming fruits and vegetables, including cruciferous vegetables had no effect on breast cancer risk [43]. However, in a subsequent Swedish study, women who consumed higher amounts of Brassica vegetables (median = 1.5 servings per day) had a significantly lower risk of developing breast cancer compared to women who consumed virtually no Brassica vegetables [44], a protective outcome that is not seen with overall consumption of fruits and vegetables.
Clinical trials:
A few small clinical trials have been conducted to determine whether consumption of broccoli sprout extracts shows efficacy against breast cancer biomarkers in women without invasive breast cancer who were scheduled for breast biopsies after a mammogram [45] [46] with intriguing results on epigenetic effects that require further investigation with larger and more comprehensive trials. In subjects with a history of breast cancer, intake of ≥14 cups/week of cruciferous vegetables for 3 weeks was seen to significantly reduce urinary 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress [47], likely indicating the utility of cruciferous vegetables in cancer patient nutrition.
Lung cancer
Observational/epidemiological studies:
Some epidemiologic studies have shown that there is an inverse relationship between consumption of Brassica vegetables and lung cancer risk [48] [49] [50]. Yet, at the epidemiologic level, it is important to note that there are some cohorts that don’t show favorable lung cancer outcomes associated with consumption of cruciferous vegetables, for example men in the United States [49] and Europeans [51].
Clinical trials:
Increased DNA repair activity was observed after intake of 250 g of broccoli/day for 10 days in young smokers [52] suggesting that such a diet could counter DNA damage leading to lung cancer caused by cigarette smoke. Multiple studies have suggested that isothiocyanates in cruciferous vegetables modify the metabolism of lung carcinogens [53]. In a recent study in which smokers were administered PEITC (10 mg, 4 times a day), the PEITC arm reduced metabolic activation of NNK, one of the most potent lung carcinogens present in cigarettes by 7.7% [54]. Larger increases in rates of excretion of detoxication metabolites (often mercapturic acids) of combustion pollutants such as benzene and aldehydes were observed following PEITC intervention [55] (Figure 2). Similar trends in modulating metabolism of air pollutants by broccoli sprout beverages had been observed in our group in two previous trials. When a broccoli sprout-derived beverage containing 600 μmol GR and 40 μmol of SFN was given to study participants in Qidong, China for 12 weeks, higher urinary excretion levels for benzene and acrolein were observed [56] compared to the placebo beverage. Similar results in air pollutant metabolism were observed in another study where GR or SFN-rich beverages were administered to participants in Qidong for a week [57].
Figure 2.
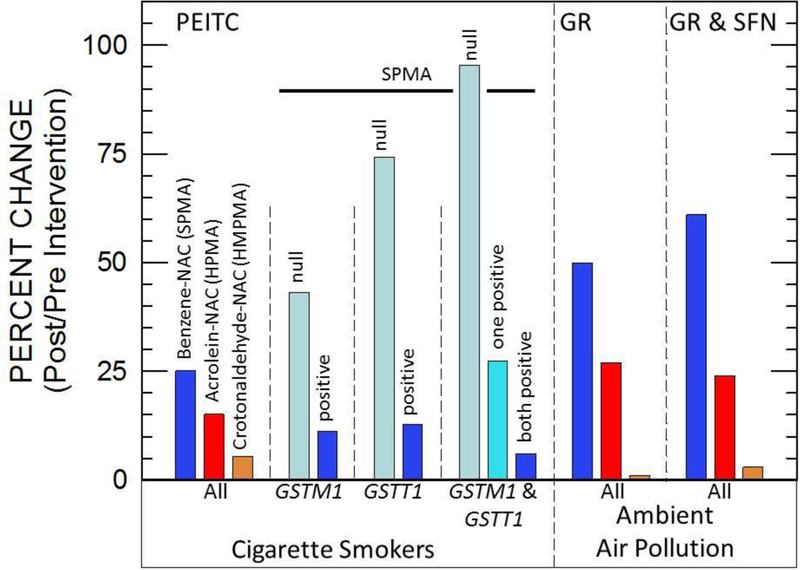
Percent change in excretion of benzene, acrolein and crotonaldehyde mercapturic acid conjugates by PEITC for 21 or 35 days, GR for 7 days, and GR and SFN for 84 days. Adapted from [54]-[57].
Cancers of the GI tract
Observational/ epidemiological studies:
In the Netherlands Cohort Study, it was observed that consumption of Brassica vegetables was inversely associated with development of gastric cardia adenocarcinoma [58]. In an ecologic study where colorectal cancer rates between Maori and non-Maori people in New Zealand were compared, the authors attributed the low prevalence of cancer amongst Maori people to the consumption of vegetables like watercress [59], in spite of their high red meat consumption. Interestingly, another study showed that consumption of cruciferous vegetables increased the urinary excretion of PhIP, a DNA adduct forming carcinogen present in cooked meat [60].
Clinical trials:
Reduced urease and inflammation was seen in Helicobacter pylori-infected humans fed a broccoli sprout-rich diet [61] indicating that isothiocyanates could likely be used to block stomach carcinogenesis in high-risk individuals. Similar encouraging results have been shown in smaller trials with a lower number of participants [62]. However, in another trial using 250 mg standardized broccoli sprout yielding 1000 μg SFN twice daily for four weeks, no significant changes were observed in urea breath test and gastric juice ammonia concentrations but modest changes were seen in lipid peroxidation in the gastric mucosa compared to placebo [63].
Prostate cancer
Observational/ epidemiological studies:
The most comprehensive epidemiologic review (now 15 years old), evaluated evidence for the effect of cruciferous vegetable consumption on prostate cancer incidence and found no conclusive effect [64]. However, a decreased prostate cancer risk was observed with increased intake of vegetables like broccoli in a cohort of men previously exposed to asbestos in Western Australia [66]. There are specific challenges associated in using markers of prostate cancer to predict risk. The use of PSA as a marker of prostate cancer is highly controversial and might add confounders to studies that look at cruciferous vegetables and prostate cancer risk at the population level [65].
Clinical trials:
The efficacy of SFN against prostate cancer has been tested in a few small clinical trials. Treating 20 prostate cancer patients with SFN-rich extracts (200 μmol/ day) for 20 weeks did not result in a significant decline (≥50%) in PSA levels [67]. However, in another study, men with biochemical recurrence after radical prostatectomy showed promising results after daily ingestion of 60 mg (~340 μmol) of stabilized free SFN in a commercial dietary supplement for 6 months [68].
Diabetes
In a randomized, double-blind, placebo-controlled study, 103 Scandinavian type 2 diabetes (T2D) patients were given daily oral broccoli sprout extract (containing 150 μmol SFN per dose) for 12 weeks, and improved fasting glucose and HbA1C was observed with the most robust improvement occurring in dysregulated, obese participants [69]. Most of these patients were concurrently on metformin treatment, therefore the therapeutic effect of SFN alone in T2D is unknown from this study. Previous studies in Iran showed that broccoli sprout powder consumption (112 or 225 μmol/day of SFN equivalents given as a commercial glucoraphanin-rich dietary supplement) for 4 weeks, reduced serum insulin concentration in T2D patients [70]. The same intervention resulted in favorable lipid profiles in T2D patients [71] suggesting that isothiocyanates have utility in reducing diabetes-related complications too. Whether or not SFN and other isothiocyanates could replace or complement current diabetes medications such as metformin requires further investigation.
Skin disorders
Topical application of broccoli sprout extract for keratin-based disorders was tested by Kerns and colleagues in a small study [72]. Here, five subjects with epidermolysis bullosa simplex (EBS; caused by mutations in keratin 14 or 5) applied the extract (500 nmol SFN/ mL) daily. Variable but induced expression of keratins 16 and 6 were observed after application indicating the potential of broccoli sprout extract to be used in similar keratin-associated disorders. SFN-rich broccoli sprout extract application was shown to protect skin of volunteers against erythema caused by ultraviolet radiation [73]. However, more extensive studies with larger sample sizes are needed to understand the broad spectrum of possible uses of isothiocyanates in skin disease.
Heart, blood and vascular disease
In 2015, a study showed that a broccoli diet containing high levels of GR reduced plasma LDL cholesterol levels significantly [74]. Cardiovascular disease risk in Helicobacter pylori-infected T2D patients was shown to be reduced after administration of broccoli sprouts powder [75]. However, in 40 hypertension patients consuming 10 g of dried broccoli sprouts for 4 weeks, no changes in blood pressure or flow mediated dilation were detected [76]. The exact mechanism by which SFN/ broccoli is able to protect against heart and vascular disease is currently unknown but is likely related to redox changes associated with NRF2 signaling [77]. This is an active area of research that warrants more clinical studies. In a phase 1 study that used SFN-containing broccoli sprout homogenate (50-150 μmol dose escalation for 14 days) in adults with sickle cell disease, increases in whole blood mRNA levels of heme oxygenase1 and subunit of fetal hemoglobin were observed [78].
Developmental/ behavioral disorders
SFN treatment (50-150 μmol daily, for 18 weeks followed by 4 weeks without treatment) improved autism (ASD)-related outcomes (largely based on the Social Responsiveness Scale) in young, male patients [79]. This finding was particularly important because SFN potentially addressed the pathophysiological hallmarks of ASD [80] (oxidative stress and antioxidant deficiency) instead of treating symptoms of ASD as done by standard therapy. Such results are in alignment with the findings from a recent report that showed that SFN was able to reduce damage caused to mouse cortical cultures by chemicals that mimicked the action in several brain disorders, including ASD [81]. There are multiple clinical trials in the pipeline evaluating the utility of sulforaphane/ broccoli preparations in ASD and findings from these studies would add valuable insight to incorporating these compounds into ASD treatment methods. Dietary broccoli sprout extract also improved outcomes related to schizophrenia in patients [82], suggesting that isothiocyanates may collectively have a very important role to play in treating neurological and developmental conditions.
Respiratory conditions
A broccoli sprout homogenate was shown to reduce influenza-related outcomes in human volunteers [83]. Influenza virus-induced markers of inflammation were significantly lower in smokers after consumption of broccoli sprout homogenates [84]. In another study, daily 100 μmol SFN for 14 days was shown to improve the bronchoprotective response in asthmatics [85]. Broccoli sprout extract (dose equivalent to consumption of 100 - 200 g broccoli) was also shown to reduce the nasal allergic response to diesel exhaust particles in human subjects [86]. When 25 or 150 μmol SFN was orally administered to smokers with chronic obstructive pulmonary disorder (COPD) for four weeks, no significant changes in inflammatory markers were observed compared to placebo [87]. This outcome may be related to the fact that baseline oxidative stress and inflammation is already very high in such a patient population and cannot be reversed by a dietary agent.
Ongoing clinical trials
As per clinicaltrials.gov, there are a number of clinical trials in the pipeline that are using broccoli/SFN and watercress/PEITC and some of them are highlighted here- SFN against tobacco-related head and neck cancer (NCT03268993, 03182959); SFN against lung cancer in former smokers (NCT03232138); SFN against autism (NCT02677051, 02909959; 02879110, 02654743, 02561481); SFN against schizophrenia (NCT02810964, 02880462); SFN against cystic fibrosis (NCT01315665); Watercress juice against oral cancer (NCT01790204); PEITC jelly in head and neck cancer (safety and efficacy study; NCT03034603); Watercress diet against long-term effects secondary to cancer therapy in adults (NCT02468882). Results from these studies will likely further bolster the already known disease-preventive and therapeutic effects of these isothiocyanates and will reveal important aspects of how to best administer them to target populations.
4. Future directions
Gaps in research
Overall, there has been an expanding number of well-designed albeit small clinical studies that use SFN/PEITC or broccoli/watercress preparations. Major challenges lie in defining and optimizing formulations for the plant preparations, including sourcing, composition, elucidating critical pharmacokinetic parameters and in dose selection. Many trials employ maximum tolerated doses where in fact lower doses may prove equally or even more effective. Recruiting high numbers of participants, using safe, effective and clinically interpretable doses as well as identifying disease endpoints/ patient populations suitable for intervention are essential points to consider. Results from some studies have shown that it is important to identify limitations for the use of compounds like SFN [87] [45]. Reporting findings that suggest that SFN is not effective as a therapeutic against conditions that are associated with high oxidative and inflammatory stress, or disease that is too advanced will allow us to identify conditions that can perhaps be best addressed by interventions with isothiocyanates.
Not all individuals may respond in the same manner to isothiocyanate interventions. In a randomized, 3-phase cross-over study where 16 subjects were given different varieties of broccoli, it was seen that highest excretion of SFN metabolites occurred in GSTM1-positive individuals [88]. Higher and more rapid excretion of this sort likely means lesser biological benefits will be exerted at the tissue level in GSTM1-positive people. In another study, it was shown that induction of GST-alpha and GST-mu by Brassica vegetables was dependent on GSTM1 genotype of participants [89]. However, with broccoli-based beverages in our China trials, no effect of GST genotypes was observed, perhaps reflecting that such effects were masked by the high doses of GR/SFN that were used. In PBMC obtained from subjects who consumed 85 g of watercress for 8 weeks, it was observed that small but significant increases in GPX and SOD enzyme activity were observed in GSTM1-null but not in GSTM1-positive individuals [90]. Larger-scale interventions should therefore take these genetic polymorphisms into account to resolve their impact on individuals so that those who will best benefit from cruciferous vegetables can be identified. Effects of GST genotypes on the pharmacodynamic action of PEITC have also been reported [55] (Figure 2).
Identifying biomarkers of not only SFN/ PEITC but more broadly of the ingestion of the plants themselves will allow for more accurate quantification of intake, especially in epidemiologic studies. Urinary isothiocyanate measurements have been routinely used in several studies but there is large variability in isothiocyanate concentration between various plant products (SFN is much higher in broccoli compared to other crucifers) as well as between different preparations of the same genus of plant. Furthermore, glucosinolate metabolism rates are not the same in a given cohort which also introduces an additional variability to studying cruciferous vegetables at large. Some other biomarkers of cruciferous vegetables have been proposed in a few studies - lutein [91], urinary 3,3’-diindolylmethane (DIM) [92]. However, they are yet to be incorporated into large nutritional studies using crucifers.
New technologies
Advances have been made to circumvent some of the challenges associated with isothiocyanate delivery. To account for the variability - or lack - in myrosinase activity in preparations or individuals that could lead to lower or higher isothiocyanate concentrations, tablets containing both the GR as well as active myrosinase have been manufactured and are sold in the US and internationally. Additionally, cruciferous crops that produce higher content of glucosinolates can been selected-for and emerging technologies in the field are evaluating how bacteria can be engineered to bind specific cells and secrete myrosinase in a targeted chemoprevention approach [93]. A recent study compared the delivery efficiency of an alpha-cyclodextrin inclusion SFN preparation with a commercial SFN-rich nutritional supplement [94]. Collectively, findings from research studies need to be effectively disseminated to the general public so that this knowledge can drive day-to-day nutritional practices of people.
5. Broader implications of isothiocyanate research
Over-the-counter supplements
Isothiocyanate dietary supplements present a potentially beneficial strategy to introduce key nutritional components to people, particularly with regard to addressing issues related to poor bioavailability/absorption of cooked cruciferous vegetables. However, some serious challenges persist and need to be urgently dealt with before moving forward.
In the United States, both broccoli and watercress are available in the form of dietary supplement capsules. Based on growth in sales of general supplements over the past few years [95], it is likely that broccoli, watercress and other cruciferous vegetable extract supplement sales will continue to grow in the coming years. This trend is likely to be amplified by online sales via the internet and social media. Even though some supplements are evolving with scientific research (e.g., introducing “myrosinase-activated” versions in place of standard extracts), many of these products have not been tested for efficacy in randomized clinical trials or monitored for bioactive content, and therefore present areas of research or manufacturing that require more robust work.
While most manufacturers don’t directly make health or disease treatment claims on the labels of these products, some do so overtly. The Food and Drug Administration (FDA) recently issued a series of warnings to companies that produce supplements that claim cancer preventive and therapeutic properties [96]. Amongst the common statements found on broccoli and watercress supplements are claims to support detoxification - a finding that has been essentially extrapolated from the larger body of research on these plants and their bioactives and not necessarily by direct testing the respective products. Hence, it is important not only to conduct rigorous scientific experimentation on these products but also to raise public awareness so that people know what to consume, how and when.
Isothiocyanates in evidence-based food policy
In 2003, the World Health Organization (WHO)’s International Agency for Cancer Research (IARC) published a chapter on cruciferous vegetables as a recommendation for cancer prevention. Two of the public health recommendations specifically advised that cruciferous vegetables not be promoted more than other types of vegetables and that caution should be taken when ingesting high amounts of crucifers (either in the form of supplements or consuming large amounts of vegetables). The second point has since been addressed by several clinical trials showing the little to no side effects, at least when reasonable amounts of cruciferous vegetables are consumed. In any case, it is generally unadvisable and impractical for individuals to consume diets that focus solely on one food type. Instead, the public should be encouraged to consistently consume moderate amounts of a diverse array of cruciferous vegetables as part of the diet. Yet, according to 2015 United States Agricultural Department data, cruciferous vegetables are not within the top seven mostly consumed vegetables in the US [97], suggesting that the message of cruciferous vegetables-isothiocyanates is not being delivered to the American public effectively.
At the agricultural level, growing Brassica plants require a lot of water and might not be feasible in areas of the world where water is scarce. In fact, research has shown that using alternatives like wastewater to grow Brassica plants increases exposure to pathogens which introduces an unnecessary health threat to consumers [98]. Given that Brassica plants have a highly evolved response to external stress stimuli, it is likely that exposure to heavy metals via contaminated soil could affect the quality of the plant [99], and might certainly be unhealthy for people to consume. Furthermore, the consumption of conventionally grown cruciferous vegetables may come with the added exposure to pesticides and herbicides. Therefore, safer, affordable and sustainable agricultural methods will need to be promoted for growing Brassica and cruciferous plants in order to harness their full disease-mitigating potential.
One glaringly obvious challenge when it comes to incorporating isothiocyanates into nutritional and food policies around the world is the fact that the most common plants that are known sources of isothiocyanates don’t readily grow in many parts of the world. Several varieties of such plants including standard broccoli and watercress that have been studied extensively require a cooler climate to grow and would not fare well in tropical countries. Encouragingly though, the family of cruciferous vegetables is extremely large and includes several dozen plant types that grow well under different climates. Research efforts focusing on native cruciferous or Brassica plants that could potentially provide comparable, if not superior health effects to those of the more common plants will be useful. The identification of isothiocyanates and their biological effects from Moringa oleifera, which is a plant that grows well in the tropics, is an important step forward in this direction [100] [101].
Acknowledgements
Our work on isothiocyanates is supported by the National Institutes of Health [R35 CA197222, R01 CA190610, R01 CA213123, P50 CA097190, P30 CA047904], The Lewis B. and Dorothy Cullman Foundation, and the Breast Cancer Research Foundation.
Abbreviations:
- ASD
Autism Spectrum Disorder
- DIM
diindoylmethane
- DNA
deoxyribonucleic acid
- GPx
glutathione peroxidase
- GR
glucoraphanin
- GST
glutathione S-transferase
- HbA1C
hemoglobin A1C
- HMPMA
3-hydroxy-1-methylpropylmercapturic acid
- HPMA
2-hydroxypropylmercapturic acid
- LDL
low-density lipoprotein
- NAC
N-acetyl cysteine
- NNK
nicotine-derived nitrosamine ketone
- NRF2
nuclear factor erythroid 2 [NF-E2]-related factor 2
- PBMC
peripheral blood mononuclear cells
- PEITC
phenethyl isothiocyanate
- PhIP
2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- PSA
prostate-specific antigen
- SPMA
S-phenylmercapturic acid
- SFN
sulforaphane
- SOD
superoxide dismutase
- T2D
Type 2 Diabetes
References
- 1.Fahey J Brassica: characteristics and properties; in Caballero B, Finglas P, Toldrá F, (eds): The Encyclopedia of Food and Health. Oxford: Academic Press, 2016;1:469–477. [Google Scholar]
- 2.Johnson T, Dinkova-Kostova A, Fahey J. Glucosinolates from the Brassica vegetables and their health effects; in Caballero B, Finglas P, Toldrá F, (eds): The Encylopedia of Food and Health. Oxford: Academic Press, 2016;3:248–255. [Google Scholar]
- 3.Hayes JD, Kelleher MO, Eggleston IM. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr 2008;47 Suppl 2:73–88. [DOI] [PubMed] [Google Scholar]
- 4.Del CM-B, Moreno DA, Carvajal M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int J Mol Sci 2013;14:11607–11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001;56:5–51. [DOI] [PubMed] [Google Scholar]
- 6.Dinkova-Kostova AT, Kostov RV. Glucosinolates and isothiocyanates in health and disease. Trends Mol Med 2012;18:337–347. [DOI] [PubMed] [Google Scholar]
- 7.Agerbirk N, Olsen CE. Glucosinolate structures in evolution. Phytochemistry 2012;77:16–45. [DOI] [PubMed] [Google Scholar]
- 8.Kjaer A, Christensen B. Isothiocyanates XXX*. Glucohirsutin, a new naturally occurring glucoside furnishing (−)-8-Methylsulphinyloctyl isothiocyanate on enzymatic hydrolysis. Acta Chem Scand. 1958;12:833–838. [Google Scholar]
- 9.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A 1992;89:2399–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posner GH, Cho CG, Green JV, Zhang Y, Talalay P. Design and synthesis of bifunctional isothiocyanate analogs of sulforaphane: correlation between structure and potency as inducers of anticarcinogenic detoxication enzymes. J Med Chem 1994;37:170–176. [DOI] [PubMed] [Google Scholar]
- 11.Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst 1977;58:395–398. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Kolm RH, Mannervik B, Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem Biophys Res Commun 1995;206:748–755. [DOI] [PubMed] [Google Scholar]
- 13.Egner PA, Kensler TW, Chen JG, Gange SJ, Groopman JD, Friesen MD. Quantification of sulforaphane mercapturic acid pathway conjugates in human urine by high-performance liquid chromatography and isotope-dilution tandem mass spectrometry. Chem Res Toxicol 2008;21:1991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer 2006;55:53–62. [DOI] [PubMed] [Google Scholar]
- 15.Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA, Talalay P. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res (Phila) 2012;5:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev 2001;10:501–508. [PubMed] [Google Scholar]
- 17.Egner PA, Chen JG, Wang JB, Wu Y, Sun Y, Lu JH, Zhu J, Zhang YH, Chen YS, Friesen MD, Jacobson LP, Munoz A, Ng D, Qian GS, Zhu YR, Chen TY, Botting NP, Zhang Q, Fahey JW, Talalay P, Groopman JD, Kensler TW. Bioavailability of sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res (Phila) 2011;4:384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanlon N, Coldham N, Gielbert A, Sauer MJ, Ioannides C. Repeated intake of broccoli does not lead to higher plasma levels of sulforaphane in human volunteers. Cancer Lett 2009;284:15–20. [DOI] [PubMed] [Google Scholar]
- 19.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta 2002;316:43–53. [DOI] [PubMed] [Google Scholar]
- 20.Clarke JD, Hsu A, Riedl K, Bella D, Schwartz SJ, Stevens JF, Ho E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res 2011;64:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahey JW, Holtzclaw WD, Wehage SL, Wade KL, Stephenson KK, Talalay P. Sulforaphane bioavailability from glucoraphanin-rich broccoli: control by active endogenous myrosinase. PLoS One 2015;10:e0140963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliviero T, Verkerk R, Vermeulen M, Dekker M. In vivo formation and bioavailability of isothiocyanates from glucosinolates in broccoli as affected by processing conditions. Mol Nutr Food Res 2014;58:1447–1456. [DOI] [PubMed] [Google Scholar]
- 23.Cramer JM, Jeffery EH. Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr Cancer 2011;63:196–201. [DOI] [PubMed] [Google Scholar]
- 24.Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DK, Botero-Omary M, Chung FL. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer 2000;38:168–178. [DOI] [PubMed] [Google Scholar]
- 25.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, McCann SE. Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol Biomarkers Prev 2008;17:938–944. [DOI] [PubMed] [Google Scholar]
- 26.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, McCann SE. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol Biomarkers Prev 2010;19:1806–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liebes L, Conaway CC, Hochster H, Mendoza S, Hecht SS, Crowell J, Chung FL. High-performance liquid chromatography-based determination of total isothiocyanate levels in human plasma: application to studies with 2-phenethyl isothiocyanate. Anal Biochem 2001;291:279–289. [DOI] [PubMed] [Google Scholar]
- 28.Chung FL, Morse MA, Eklind KI, Lewis J. Quantitation of human uptake of the anticarcinogen phenethyl isothiocyanate after a watercress meal. Cancer Epidemiol Biomarkers Prev 1992;1:383–388. [PubMed] [Google Scholar]
- 29.Ji Y, Kuo Y, Morris ME. Pharmacokinetics of dietary phenethyl isothiocyanate in rats. Pharm Res 2005;22:1658–1666. [DOI] [PubMed] [Google Scholar]
- 30.Konsue N, Kirkpatrick J, Kuhnert N, King LJ, Ioannides C. Repeated oral administration modulates the pharmacokinetic behavior of the chemopreventive agent phenethyl isothiocyanate in rats. Mol Nutr Food Res 2010;54:426–432. [DOI] [PubMed] [Google Scholar]
- 31.Hanlon N, Coldham N, Gielbert A, Kuhnert N, Sauer MJ, King LJ, Ioannides C. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br J Nutr 2008;99:559–564. [DOI] [PubMed] [Google Scholar]
- 32.Getahun SM, Chung FL. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol Biomarkers Prev 1999;8:447–451. [PubMed] [Google Scholar]
- 33.Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P. Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A 2002;99:7610–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morse MA, Wang CX, Stoner GD, Mandal S, Conran PB, Amin SG, Hecht SS, Chung FL. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in the lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res 1989;49:549–553. [PubMed] [Google Scholar]
- 35.Dinkova-Kostova A, Fahey J, Kostov R, Kensler T. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci. Technol. 2017;69(B):257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Palliyaguru DL, Kensler TW. Frugal chemoprevention: targeting Nrf2 with foods rich in sulforaphane. Semin Oncol 2016;43:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charron CS, Clevidence BA, Albaugh GA, Kramer MH, Vinyard BT, Milner JA, Novotny JA. Assessment of DNA damage and repair in adults consuming allyl isothiocyanate or Brassica vegetables. J Nutr Biochem 2013;24:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fogarty MC, Hughes CM, Burke G, Brown JC, Davison GW. Acute and chronic watercress supplementation attenuates exercise-induced peripheral mononuclear cell DNA damage and lipid peroxidation. Br J Nutr 2013;109:293–301. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res 2010;16:2580–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis 2012;33:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang L, Zahid M, Liao Y, Rogan EG, Cavalieri EL, Davidson NE, Yager JD, Visvanathan K, Groopman JD, Kensler TW. Reduced formation of depurinating estrogen-DNA adducts by sulforaphane or KEAP1 disruption in human mammary epithelial MCF-10A cells. Carcinogenesis 2013;34:2587–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fowke JH, Longcope C, Hebert JR: Brassica vegetable consumption shifts estrogen metabolism in healthy postmenopausal women. Cancer Epidemiol Biomarkers Prev 2000;9:773–779. [PubMed] [Google Scholar]
- 43.Smith-Warner SA, Spiegelman D, Yaun SS, Adami HO, Beeson WL, van den Brandt PA, Folsom AR, Fraser GE, Freudenheim JL, Goldbohm RA, Graham S, Miller AB, Potter JD, Rohan TE, Speizer FE, Toniolo P, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hunter DJ. Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. JAMA 2001;285:769–776. [DOI] [PubMed] [Google Scholar]
- 44.Terry P, Wolk A, Persson I, Magnusson C. Brassica vegetables and breast cancer risk. JAMA 2001;285:2975–2977. [DOI] [PubMed] [Google Scholar]
- 45.Atwell LL, Zhang Z, Mori M, Farris P, Vetto JT, Naik AM, Oh KY, Thuillier P, Ho E, Shannon J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev Res (Phila) 2015;8:1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Atwell LL, Farris PE, Ho E, Shannon J. Associations between cruciferous vegetable intake and selected biomarkers among women scheduled for breast biopsies. Public Health Nutr 2016;19:1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirth MD, Murphy EA, Hurley TG, Hebert JR. Effect of cruciferous vegetable intake on oxidative stress biomarkers: differences by breast cancer status. Cancer Invest 2017;35:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kvale G, Bjelke E, Gart J. Dietary habits and lung cancer risk. Int J Cancer 1983;31(4):397–405. [DOI] [PubMed] [Google Scholar]
- 49.Feskanich D, Ziegler RG, Michaud DS, Giovannucci EL, Speizer FE, Willett WC, Colditz GA. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J Natl Cancer Inst 2000;92:1812–1823. [DOI] [PubMed] [Google Scholar]
- 50.Voorrips LE, Goldbohm RA, Verhoeven DT, van Poppel GA, Sturmans F, Hermus RJ, van den Brandt PA. Vegetable and fruit consumption and lung cancer risk in the Netherlands Cohort Study on diet and cancer. Cancer Causes Control 2000;11:101–115. [DOI] [PubMed] [Google Scholar]
- 51.Miller AB, Altenburg HP, Bueno-de-Mesquita B, Boshuizen HC, Agudo A, Berrino F, Gram IT, Janson L, Linseisen J, Overvad K, Rasmuson T, Vineis P, Lukanova A, Allen N, Amiano P, Barricarte A, Berglund G, Boeing H, Clavel-Chapelon F, Day NE, Hallmans G, Lund E, Martinez C, Navarro C, Palli D, Panico S, Peeters PH, Quiros JR, Tjonneland A, Tumino R, Trichopoulou A, Trichopoulos D, Slimani N, Riboli E. Fruits and vegetables and lung cancer: findings from the European prospective investigation into cancer and nutrition. Int J Cancer 2004;108:269–276. [DOI] [PubMed] [Google Scholar]
- 52.Riso P, Martini D, Moller P, Loft S, Bonacina G, Moro M, Porrini M. DNA damage and repair activity after broccoli intake in young healthy smokers. Mutagenesis 2010;25:595–602. [DOI] [PubMed] [Google Scholar]
- 53.Hecht SS, Chung FL, Richie JP Jr, Akerkar SA Borukhova A, Skowronski L, Carmella SG. Effects of watercress consumption on metabolism of a tobacco-specific lung carcinogen in smokers. Cancer Epidemiol Biomarkers Prev 1995;4:877–884. [PubMed] [Google Scholar]
- 54.Yuan JM, Stepanov I, Murphy SE, Wang R, Allen S, Jensen J, Strayer L, Adams-Haduch J, Upadhyaya P, Le C, Kurzer MS, Nelson HH, Yu MC, Hatsukami D, Hecht SS. Clinical trial of 2-phenethyl isothiocyanate as an inhibitor of metabolic activation of a tobacco-specific lung carcinogen in cigarette smokers. Cancer Prev Res (Phila) 2016;9:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan JM, Murphy SE, Stepanov I, Wang R, Carmella SG, Nelson HH, Hatsukami D, Hecht SS. 2-phenethyl isothiocyanate, glutathione S-transferase M1 and T1 polymorphisms, and detoxification of volatile organic carcinogens and toxicants in tobacco smoke. Cancer Prev Res (Phila) 2016;9:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Munoz A, Johnson JL, Groopman JD, Fahey JW, Talalay P, Zhu J, Chen TY, Qian GS, Carmella SG, Hecht SS, Kensler TW. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila) 2014;7:813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Munoz A, Egner PA, Chen JG, Qian GS, Chen TY, Fahey JW, Talalay P, Groopman JD, Yuan JM, Hecht SS. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis 2012;33:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer 2011;129:2681–2693. [DOI] [PubMed] [Google Scholar]
- 59.Thomson B, Shaw I. A Comparison of risk and protective factors for colorectal cancer in the diet of New Zealand Maori and non-Maori. Asian Pac J Cancer Prev 2002;3:319–324. [PubMed] [Google Scholar]
- 60.Walters DG, Young PJ, Agus C, Knize MG, Boobis AR, Gooderham NJ, Lake BG. Cruciferous vegetable consumption alters the metabolism of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in humans. Carcinogenesis 2004;25:1659–1669. [DOI] [PubMed] [Google Scholar]
- 61.Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila) 2009;2:353–360. [DOI] [PubMed] [Google Scholar]
- 62.Galan MV, Kishan AA, Silverman AL. Oral broccoli sprouts for the treatment of Helicobacter pylori infection: a preliminary report. Dig Dis Sci 2004;49:1088–1090. [DOI] [PubMed] [Google Scholar]
- 63.Chang YW, Jang JY, Kim YH, Kim JW, Shim JJ. The Effects of Broccoli Sprout Extract Containing Sulforaphane on Lipid Peroxidation and Helicobacter pylori Infection in the Gastric Mucosa. Gut Liver 2015;9:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer 2002;42:1–9. [DOI] [PubMed] [Google Scholar]
- 65.Kristal AR, Stanford JL. Cruciferous vegetables and prostate cancer risk: confounding by PSA screening. Cancer Epidemiol Biomarkers Prev 2004;13:1265. [PubMed] [Google Scholar]
- 66.Ambrosini GL, de Klerk NH, Fritschi L, Mackerras D, Musk B. Fruit, vegetable, vitamin A intakes, and prostate cancer risk. Prostate Cancer Prostatic Dis 2008;11:61–66. [DOI] [PubMed] [Google Scholar]
- 67.Alumkal JJ, Slottke R, Schwartzman J, Cherala G, Munar M, Graff JN, Beer TM, Ryan CW, Koop DR, Gibbs A, Gao L, Flamiatos JF, Tucker E, Kleinschmidt R, Mori M. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Invest New Drugs 2015;33:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cipolla BG, Mandron E, Lefort JM, Coadou Y, Della NE, Corbel L, Le SR, Azzouzi AR, Mottet N. Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Cancer Prev Res (Phila) 2015;8:712–719. [DOI] [PubMed] [Google Scholar]
- 69.Axelsson AS, Tubbs E, Mecham B, Chacko S, Nenonen HA, Tang Y, Fahey JW, Derry JMJ, Wollheim CB, Wierup N, Haymond MW, Friend SH, Mulder H, Rosengren AH. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med 2017;9 DOI: 10.1126/scitranslmed.aah4477. [DOI] [PubMed] [Google Scholar]
- 70.Bahadoran Z, Tohidi M, Nazeri P, Mehran M, Azizi F, Mirmiran P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: a randomized double-blind clinical trial. Int J Food Sci Nutr 2012;63:767–771. [DOI] [PubMed] [Google Scholar]
- 71.Bahadoran Z, Mirmiran P, Hosseinpanah F, Rajab A, Asghari G, Azizi F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Diabetes Res Clin Pract 2012;96:348–354. [DOI] [PubMed] [Google Scholar]
- 72.Kerns ML, Guss L, Fahey J, Cohen B, Hakim JM, Sung S, Lu RG, Coulombe PA. Randomized, split-body, single-blinded clinical trial of topical broccoli sprout extract: Assessing the feasibility of its use in keratin-based disorders. J Am Acad Dermatol 2017;76:449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, Dinkova-Kostova AT. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci U S A 2007;104:17500–17505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Armah CN, Derdemezis C, Traka MH, Dainty JR, Doleman JF, Saha S, Leung W, Potter JF, Lovegrove JA, Mithen RF. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: evidence from randomised controlled trials. Mol Nutr Food Res 2015;59:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mirmiran P, Bahadoran Z, Golzarand M, Zojaji H, Azizi F. A comparative study of broccoli sprouts powder and standard triple therapy on cardiovascular risk factors following H.pylori eradication: a randomized clinical trial in patients with type 2 diabetes. J Diabetes Metab Disord 2014;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Christiansen B, Bellostas MN, Petersen AM, Kveiborg B, Madsen CR, Thomas H, Ihlemann N, Sorensen JC, Kober L, Sorensen H, Torp-Pedersen C, Dominguez H. Ingestion of broccoli sprouts does not improve endothelial function in humans with hypertension. PLoS One 2010;5:e12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mann GE. Nrf2-mediated redox signalling in vascular health and disease. Free Radic Biol Med 2014;75 Suppl 1:S1. [DOI] [PubMed] [Google Scholar]
- 78.Doss JF, Jonassaint JC, Garrett ME, Ashley-Koch AE, Telen MJ, Chi JT. Phase 1 study of a sulforaphane-containing broccoli sprout homogenate for sickle cell disease. PLoS One 2016;11:e0152895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh K, Connors SL, Macklin EA, Smith KD, Fahey JW, Talalay P, Zimmerman AW. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci U S A 2014;111:15550–15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu H, Talalay P, Fahey JW. Biomarker-Guided Strategy for Treatment of Autism Spectrum Disorder (ASD). CNS Neurol Disord Drug Targets 2016;15:602–613. [DOI] [PubMed] [Google Scholar]
- 81.Pearson BL, Simon JM, McCoy ES, Salazar G, Fragola G, Zylka MJ. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat Commun 2016;7:11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shiina A, Kanahara N, Sasaki T, Oda Y, Hashimoto T, Hasegawa T, Yoshida T, Iyo M, Hashimoto K. An open Study of sulforaphane-rich broccoli sprout extract in patients with schizophrenia. Clin Psychopharmacol Neurosci 2015;13:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muller L, Meyer M, Bauer RN, Zhou H, Zhang H, Jones S, Robinette C, Noah TL, Jaspers I. Effect of broccoli sprouts and live attenuated influenza virus on peripheral blood natural killer cells: a randomized, double-blind study. PLoS One 2016;11:e0147742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noah TL, Zhang H, Zhou H, Glista-Baker E, Muller L, Bauer RN, Meyer M, Murphy PC, Jones S, Letang B, Robinette C, Jaspers I. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: a randomized, double-blind study. PLoS One 2014;9:e98671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown RH, Reynolds C, Brooker A, Talalay P, Fahey JW. Sulforaphane improves the bronchoprotective response in asthmatics through Nrf2-mediated gene pathways. Respir Res 2015;16:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heber D, Li Z, Garcia-Lloret M, Wong AM, Lee TY, Thames G, Krak M, Zhang Y, Nel A. Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food Funct 2014;5:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wise RA, Holbrook JT, Criner G, Sethi S, Rayapudi S, Sudini KR, Sugar EA, Burke A, Thimmulappa R, Singh A, Talalay P, Fahey JW, Berenson CS, Jacobs MR, Biswal S. Lack of effect of oral sulforaphane administration on Nrf2 expression in COPD: a randomized, double-blind, placebo controlled trial. PLoS One 2016;11:e0163716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr 2005;82:1283–1291. [DOI] [PubMed] [Google Scholar]
- 89.Lampe JW, Chen C, Li S, Prunty J, Grate MT, Meehan DE, Barale KV, Dightman DA, Feng Z, Potter JD. Modulation of human glutathione S-transferases by botanically defined vegetable diets. Cancer Epidemiol Biomarkers Prev 2000;9:787–793. [PubMed] [Google Scholar]
- 90.Hofmann T, Kuhnert A, Schubert A, Gill C, Rowland IR, Pool-Zobel BL, Glei M. Modulation of detoxification enzymes by watercress: in vitro and in vivo investigations in human peripheral blood cells. Eur J Nutr 2009;48:483–491. [DOI] [PubMed] [Google Scholar]
- 91.Martini MC, Campbell DR, Gross MD, Grandits GA, Potter JD, Slavin JL. Plasma carotenoids as biomarkers of vegetable intake: the University of Minnesota Cancer Prevention Research Unit feeding studies. Cancer Epidemiol Biomarkers Prev 1995;4:491–496. [PubMed] [Google Scholar]
- 92.Fujioka N, Ransom BW, Carmella SG, Upadhyaya P, Lindgren BR, Roper-Batker A, Hatsukami DK, Fritz VA, Rohwer C, Hecht SS. Harnessing the power of cruciferous vegetables: developing a biomarker for Brassica vegetable consumption using urinary 3,3’-diindolylmethane. Cancer Prev Res (Phila) 2016;9:788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ho C, Tan H, Chua K, Kang A, Lim K, Ling K, Yew W, Lee Y, Thiery J, Chang M. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat Biomed Eng. 2018;2:27–37. [DOI] [PubMed] [Google Scholar]
- 94.Fahey JW, Wade KL, Wehage SL, Holtzclaw WD, Liu H, Talalay P, Fuchs E, Stephenson KK. Stabilized sulforaphane for clinical use: phytochemical delivery efficiency. Mol Nutr Food Res 2017;61. [DOI] [PubMed] [Google Scholar]
- 95.Garcia-Cazarin M, Wambogo E, Regan K, Davies C. Dietary supplement research portofolio at the NIH, 2009–2011. J Nutr 2014;144(4):414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Food The and Administration Drug. FDA takes action against 14 companies for selling illegal cancer treatments. (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm554698.htm ) Last accessed 10–25-2017.
- 97.United States Department of Agriculture: U.S. per capita loss-adjusted vegetable availability, 2015. (https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=58340 ) Last accessed 10–30-2017.
- 98.Mok HF, Hamilton AJ. Exposure factors for wastewater-irrigated Asian vegetables and a probabilistic rotavirus disease burden model for their consumption. Risk Anal 2014;34:602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mourato MP, Moreira IN, Leitao I, Pinto FR, Sales JR, Martins LL. Effect of heavy metals in plants of the genus Brassica. Int J Mol Sci 2015;16:17975–17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waterman C, Rojas-Silva P, Tumer TB, Kuhn P, Richard AJ, Wicks S, Stephens JM, Wang Z, Mynatt R, Cefalu W, Raskin I. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol Nutr Food Res 2015;59:1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doerr B, Wade KL, Stephenson KK, Reed SB, Fahey JW. Cultivar effect on Moringa oleifera glucosinolate content and taste: a pilot study. Ecol Food Nutr 2009;48:199–211. [DOI] [PubMed] [Google Scholar]
- 102.Gill CI, Haldar S, Boyd LA, Bennett R, Whiteford J, Butler M, Pearson JR, Bradbury I, Rowland IR. Watercress supplementation in diet reduces lymphocyte DNA damage and alters blood antioxidant status in healthy adults. Am J Clin Nutr 2007;85:504–510. [DOI] [PubMed] [Google Scholar]
- 103.Chen L, Mohr SN, Yang CS. Decrease of plasma and urinary oxidative metabolites of acetaminophen after consumption of watercress by human volunteers. Clin Pharmacol Ther 1996;60:651–660. [DOI] [PubMed] [Google Scholar]


