Abstract
The Himalaya is one of the youngest and the loftiest mountain chains of the world; it is also referred to as the water tower of Asia. The Himalayan region harbors nearly 10,000 plant species constituting approximately 2.5% of the global angiosperm diversity of which over 4,000 are endemics. The present-day Himalayan flora consists of an admixture of immigrant taxa and diversified species over the last 40 million years. The interesting questions about the Himalayan flora discussed here are: how did the Himalaya achieve high endemic plant diversity starting with immigrant taxa and what were the main drivers of this diversity? This contribution aims to answer these questions and raise some more. We review and analyze existing information from diverse areas of earth and climate sciences, palaeobiology and phytogeography to evolve a bio-chronological record of plant species divergence and evolution in the Himalaya. From the analysis we infer the effects of major environmental upheavals on plant diversity in the region. The understanding developed in the following discussion is based on the idea that Himalaya experienced at least five phases of major geophysical upheavals, namely: (i) mega-collision between India and Eurasian plates, (ii) tectonic uplift in phases and progressive landform elevation, (iii) onset of southwest (SW) Indian monsoon, (iv) spurring of arid conditions in Central Asia, and (v) cyclic phases of cooling and warming in the Quaternary. The geophysical upheavals that were potentially disrupting for the ecosystem stability had a key role in providing impetus for biological diversification. The upheavals produced new geophysical environments, new ecological niches, imposed physical and physiological isolation barriers, acted as natural selection sieves and led to the formation of new species. This contribution aims to develop a comprehensive understanding of the plant biodiversity profile of the Himalaya in the context of complex, interconnected and dynamic relationship between earth system processes, climate and plant diversity.
Keywords: Biodiversity, Environmental upheavals, Endemics, Himalaya, Species diversification
Introduction
Mountain regions comprise the large majority of the global biodiversity hotspots and it is argued that species diversification is associated with mountain building through changes in landscape and climate followed by formation of varied and heterogeneous habitats along the elevational gradients (Hoorn et al., 2013). It is equally well established that nearly all the mountains on the Earth have experienced a variety of geophysical upheavals in the geological past (Owen, 2004). For relatively younger mountain systems such as the Himalaya and Mount Kinabalu, their progress as biodiverse landscapes has also been shown to be the result of various geophysical upheavals (Pandit, Manish & Koh, 2014; Merckx et al., 2015). Formation of the Himalaya started in early Cenozoic Era around 55–50 million years ago (Mya) with the collision of the Indian and the Eurasian plates (Van Hinsbergen et al., 2012; Favre et al., 2015), an event considered as one of the greatest geophysical episodes in the Earth’s history (Harrison et al., 1992; Che et al., 2010; Wang et al., 2012). The periodic orogenic events led to physiographic and environmental changes (e.g., formation of land bridges, development of monsoon, formation of glaciers and establishment of an elaborate perennial river drainage system) and served as key drivers of the newly evolving ecosystems resulting in geographical isolation of taxa, vicariance, and evolutionary divergence of life forms (Pandit, 2017). Thus, the interconnectedness between geophysical and biological components of the Himalayan ecosystems needs to be unraveled to develop insights into understanding of the build-up of its biodiversity.
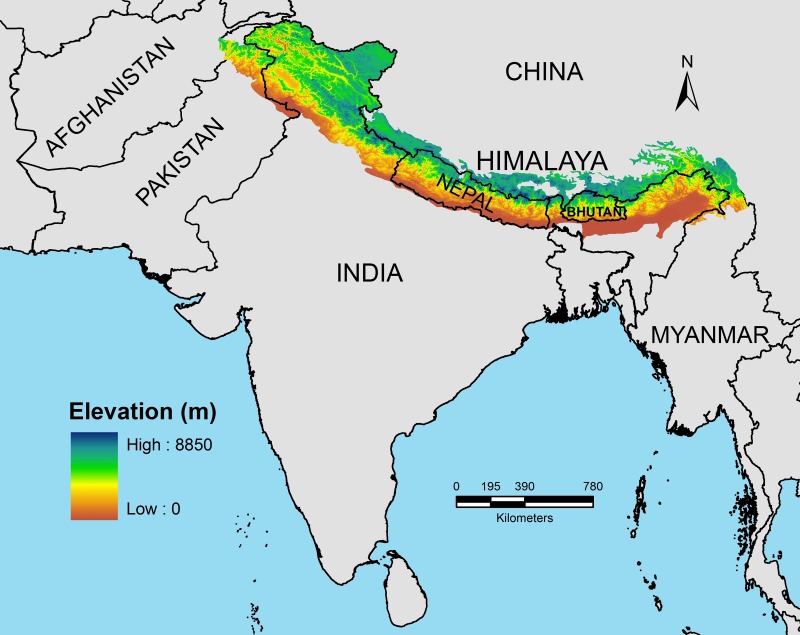
The Himalaya encompasses a geographical area of nearly 3.4 million km2 and is spread across nations of Afghanistan, Pakistan, India, Nepal, China (Tibetan Autonomous Region), Bhutan, and Myanmar (Pandit, Manish & Koh, 2014; Fig. 1). Geographically, the Himalaya extends from Namcha Barwa mountain range in India’s east to Nanga Parbat massif in the west forming an arc of about 2,400 km (Fig. 1). Geologically, Himalaya is divided into four distinct litho-tectonic and physiographic units from north to south, namely Outer Himalaya or Siwaliks, Lesser Himalaya, Greater Himalaya, and Trans-Himalaya (Valdiya, 2002). The average elevational range of Siwaliks is 900–1,500 m, followed by the Lesser Himalaya with an average elevational range of 500–2,500 m. The Greater Himalayan elevations range from 6,000–7,000 m and the northernmost Trans-Himalaya (Hedin, 1909) mostly comprises plateau areas to the north of the Indus and Brahmaputra rivers with average elevation of 5,000–6,000 m (Valdiya, 2002; Pandit, 2017). Eco-climatically, the Himalaya is broadly classified into Eastern and Western Himalaya. The Eastern Himalaya (EH) stretches from 21°–25°N latitudes across the east of Kali Gandaki valley encompassing eastern Nepal, north-eastern Indian states of Sikkim, Arunachal Pradesh and the hill areas of North Bengal, Bhutan, and northern Myanmar. The Western Himalaya (WH) extends from 30°–40°N latitudes across the west of Kali Gandaki valley encompassing western Nepal, Indian states of Uttarakhand, Himachal Pradesh, Jammu and Kashmir and areas of northern Pakistan and Afghanistan (Fig. 1). The EH region experiences heavy annual average rainfall of 3,800–4,000 mm while the WH is comparatively drier with an annual average rainfall of 75–150 mm (see Pandit, 2017). The rainfall plays a major role in determining the east–west bioclimatic gradient of the Himalaya. Some authors have designated a part of the mountain range as the Central Himalaya (Singh & Singh, 1987; Singh, Adhikari & Zobel, 1994; Vetaas, 2000). CH extends from river Kali in the east to river Tons (largest tributary of Yamuna river) in the west encompassing central Nepal and central Uttarakhand (India).
Figure 1. Spatial spread of the Himalayan mountain system across seven nations.
The elevational gradient of the Himalaya represents the longest bioclimatic gradient of the Earth (0–8,500 m) and encompasses a myriad of ecosystems ranging from tropical, temperate and alpine. The base map was prepared using Digital Elevation Models (DEM) in Arc GIS 9.3 sofware (Environmental Systems Research Institute (ESRI), Redlands, CA, USA).
The total number of higher plant species in the Himalaya varies from 8,000–10,000 with about 40% of these taxa as endemics (Pandit, Manish & Koh, 2014). It is well known that majority of the Himalayan flora consists of immigrated plant taxa that have evolved and diversified over millions of years following the Himalayan formation (Singh & Singh, 1987; Pandit & Kumar, 2013; Pandit, Manish & Koh, 2014; Manish, 2017). It is, therefore, of significant interest to evolutionary biologists as to how starting with an immigrant flora, the Himalaya now harbors such a high number of plant endemics. This question, though fascinating, has not been much investigated or discussed in ecological literature. To address this knowledge gap, we need to develop an understanding of the intricate relationship between geodynamic processes of the Himalayan mountain building and its varied biodiversity gradients. To the best of our knowledge, there are only limited studies that have attempted to understand this relationship in an integrated manner (Pandit & Kumar, 2013; Pandit, Manish & Koh, 2014; Favre et al., 2015; Pandit, 2017). The large majority of studies on the Himalaya have mostly focused on the evolutionary consequences of a specific geological period (Miao et al., 2012) or concentrated on a specific geographic region (Wen et al., 2014; Favre et al., 2015). Hence, a broader and a more comprehensive understanding of the evolutionary diversification of Himalayan flora is warranted. In this contribution, we seek to address this knowledge gap by evolving a sequence of plant species divergence episodes during major geological periods in the Himalaya and identifying their relationship with the environmental changes in the region. An overarching goal of this study was to analyze the existing published information on Himalayan plant diversity and understand the intricate relationship between the build-up of plant diversity and physical-climatological variations produced by the geophysical changes during various phases of the Himalaya’s formation.
Survey Methodology
We used four standard databases, namely Web of Science (http://www.webofknowledge.com), Google Scholar (https://scholar.google.co.in/schhp?hl=en), Science Direct (http://www.sciencedirect.com), and PubMed (http://www.ncbi.nlm.nih.gov/pubmed) to systematically identify peer-reviewed journal and book articles using a combination of controlled vocabulary and free text terms based on the following keywords and terms: “Himalaya” AND “Arid”, “Himalaya” AND “Biogeography”, “Himalaya” AND “Ecology”, “Himalaya” AND “Evolution”, “Himalaya” AND “Formation”, “Himalaya” AND “Fossil”, “Himalaya” AND “Glacier”, “Himalaya” AND “Gondwana”, “Himalaya” AND “Ice Age”, “Himalaya” AND “Monsoon”, “Himalaya” AND “Paleo”, “Himalaya” AND “Plants”, “Himalaya” AND “Refugia”, “Himalaya” AND “Tectonic” and “Himalaya” AND “Uplift”. All search fields were considered in the database while searching. Articles were searched for all periods up to, and including, December 2017 in English language irrespective of the number of citations. We ensured that we covered all the peer-reviewed articles that included the term “Himalaya” anywhere in the text, instead of just in the title, abstract or keywords. The resulting list of articles was then screened for whether the study included plant or animal species and the studies dealing with the latter (animal species) were largely excluded from further consideration (unless critical to the discussion). Additionally, we also excluded studies that were not published in peer-reviewed scientific conferences and conference proceedings. To increase the scope and coverage of the present review, we also applied a snowball search technique (Greenhalgh & Peacock, 2005) where we made a manual search for published peer-reviewed studies in the respective references of the selected publications and then included all studies in the present review that matched the above keywords and terms. For each selected publication, we retrieved the following information: author name (s), title, year of publication, journal title, sampling area and studied species.
Geological Backdrop of the Himalaya
Indian continent was once a part of Gondwanaland—a supercontinent formed nearly 600 Mya (Murphy et al., 2008). Gondwanaland covered much of the Southern Hemisphere comprising the present day South America, Africa, Madagascar, Seychelles, India, Australia, and Antarctica. Gondwanaland split around 180 Mya as a result of sea-floor spreading and development of a series of oceanic deep-seated mantle plumes resulting in Western Gondwana (comprising Africa and South America) and Eastern Gondwana (comprising Madagascar, India, Seychelles, Australia and Antarctica). Around 120 Mya, the split of Western Gondwana led to separation of South America from Africa, and another fragment containing India-Madagascar-Seychelles (IMS) separated from Antarctica and Australia (Chatterjee & Scotese, 1999). The newly separated IMS fragment migrated northward across the Tethys ocean towards the Eurasian continent at varying speeds ranging between 5–40 cm/year (Jagoutz et al., 2015). The drifting IMS fragment carried along a host of primitive flora of Gondwanan origin such as seed ferns (Glossopteris, Dicroidium, Sphenobaiera, Linguifolium), conifers (Heidiphyllum, Voltziopsis, araucarians and podocarps) and lycopods (Cyclomeia) (McLoughlin, 2001). Widespread seafloor spreading around 80–90 Mya further widened the central Indian Ocean and resulted in detachment of the Madagascar block from the IMS fragment (Plummer & Belle, 1995). The Indian-Seychelles plate that drifted at ∼5 cm/year suddenly tripled its speed to ∼15 cm/year, the fastest recorded migration speed for any tectonic drift in the geological history (Jagoutz et al., 2015). During its northward traverse around 65 Mya, the Deccan flood basalts erupted and repositioning of the western Indian Ocean spreading ridge occurred that led to the separation of Seychelles from the Indian plate (Duncan & Pyle, 1988; McLoughlin, 2001). Subsequently, the Seychelles block stationed close to Africa while India continued migrating northward. Around 55–50 Mya (Early Eocene), the drifting Indian plate collided with Eurasia along the northeastern corner of Greater India with the collision progressing westwards until 40 Mya (Van Hinsbergen et al., 2012; Bouilhol et al., 2013; Favre et al., 2015). The India-Eurasia collision led to extensive deformation of the northern margin of Indian plate and a major portion of the Indian plate subducted underneath the Asian plate. The collision also led to the draining of the Tethys Sea and upliftment of the long settled Tethyan geosyncline coastal sediments as meta-sedimentary formations. The continental collision also laid the foundation of the youngest and loftiest mountain system of the world—the Himalaya.
The Drifting Indian Plate: Raft or an Island?
From the above account, it is reasonable to guess that post separation from Gondwanaland, the Indian plate may have been an isolated island continent for nearly 45 million years, which could have created conditions for the evolution of a high endemic biodiversity. The fossil record of India, however, provides equivocal evidence on the extent of pre-Himalayan biotic endemism (see Pandit, 2017). Fossil records belonging to the Upper Cretaceous to Lower Tertiary in the Deccan Intertrappean beds of Southern India reveal a mixed flora with wide geographical affinities ranging from disparate regions, namely Africa (Palmocaulon hyphaeneoides, Palmoxylon hyphaenoides), Australia (Eucalyptus dharmendrae, Tristania confertoides), Madagascar (Palmoxylon ghughuense), and South America (Rodietes, Cyclanthodendron) (see Srivastava, 2011; Pandit, 2017). Presence of fossil taxa with such varied geographical affinities indicates the likelihood of biotic exchanges between these disjunct landmasses and also that India may not have been isolated in strict sense to induce high endemism (Pandit, 2017). Isolated or connected, some researchers have reported presence of high endemic biodiversity on the Indian plate that likely developed during 45 million years of its isolation (see Srivastava, 2011). As such, much of the diversity of Indian plate was decimated due to eruption of Deccan volcanoes around the Cretaceous-Tertiary boundary with bulk of these eruptions occurring in the early Paleocene between 67–65 Mya (Officer et al., 1987; Khosla & Sahni, 2003). Notwithstanding these catastrophic events, many ancient Gondwanan lineages did manage to survive and disperse into Asia when India collided with Eurasia (Bossuyt & Milinkovitch, 2001). The proponents of “out-of-India” hypothesis have referred to the Indian plate as a ‘raft’ for ferrying a number of taxa from Gondwanaland to mainland Asia (Bossuyt & Milinkovitch, 2001; Karanth, 2006). The “out-of-India” hypothesis has received support from investigations of the plant family Crypteroniaceae suggesting that the family originated in west Gondwanaland and subsequently reached Asia by rafting on the Indian plate (Conti et al., 2002). Recent fossil leaf impression data from genus Alphonsea (Annonaceae) from the Tertiary sediment deposits of Assam suggests that the genus originated in India during Late Oligocene and migrated to South East Asia via Myanmar during Early Miocene (Srivastava & Mehrotra, 2013).
The First Migration Wave
An immediate consequence of the collision of the Indian and Eurasian plates was the establishment of a contiguous landmass connecting Indian Peninsula with the Sino-Japanese regions in the north and the Malayan Archipelago in the southeast (Pandit, Manish & Koh, 2014). This landmass connectivity was the result of cessation of marine deposition and the beginning of terrestrial sedimentation in the suture zone of India-Eurasia collision (Mehrotra et al., 2005). A biological vacuum was created in the erstwhile nascent Himalayan ecosystems as a result of extinctions caused by Cretaceous-Tertiary volcanism event. This vacuum was gradually filled by large-scale floral migrations from the adjacent connected regions in the east, north and south (Singh & Singh, 1987). Thus, the newly evolved Himalayan landscape started to serve as a ‘intercontinental biological highway’ for migrating flora from all directions (Pandit & Kumar, 2013; Pandit, Manish & Koh, 2014).
The first taxa to colonize the Himalayan landforms were the ones with tropical affinities such as Alangiaceae, Dipterocarpaceae, Ebenaceae, Ericaceae, Gleichneaceae, Rhamnaceae, Malvaceae and Sapotaceae since climatic conditions in the region were essentially tropical in nature at the time of collision (Mehrotra et al., 2005). Faced with no dispersal barriers, either oceanic or climatic, these taxa found novel opportunities to colonize and intermingle in the newly formed Himalayan landmasses with numerous unoccupied niches. Majority of the early migrants which first crossed through the northeastern route (via present day Arunachal Pradesh) were the ones with largely Sino-Japanese and Malayan affinities such as Dalbergia, Dipterocarpus, Lagerstroemia, Myristica, Pittosporum, Shorea, and Terminalia (Singh & Singh, 1987). Fossil records of genera such as Anisoptera, Dipterocarpus, Hopea and Shorea (Dipterocarpaceae), Zizyphus (Rhamnaceae), and Diospyros (Ebenaceae) are abundant in the deposits of northeast India belonging to the Middle Miocene epoch (see Srivastava & Mehrotra, 2010 and references therein). More importantly, no fossil records of these taxa appear anywhere in India during the entire Paleogene Period, but are reported to dominate the fossil deposits found from Middle Miocene onwards. This first phase of plant migration lasted for almost 30 million years and it gradually stopped when connections were lost due to the uplift of Himalaya with a dissected topography as a result of climatic and morpho-tectonic changes in the subsequent epochs (Pandit, 2017).
Uplift of the Himalaya
Formation of the Himalaya and other mountain ranges in Tibet and farther north, such as Qinling, Taihang, Hengduan, and Tianshan started around 45 Mya and continues till now (see Wadia, 1957; Pandit, 2017). There are two contrasting views in literature regarding the timing and sequence of the uplift of the Himalaya. The first one holds that the Himalaya started to rise against a pre-existing proto-Tibetan highland that was already as high as 4,500 m since at least 45 Mya (Ding et al., 2017; Spicer, 2017). The Himalaya have continued to rise in a phased manner against this proto-Tibetan highland, attaining elevations of 1,000 m around 56 Mya, 2,500 m around 23 Mya, 4,000 m around 19 Mya and 5,000 m around 15 Mya (Ding et al., 2017; Spicer, 2017). It was around 15 Mya that Mount Everest came into existence and the average height of the Himalaya became greater than the height of the Tibetan Plateau (Spicer, 2017). Subsequently, the Himalaya continued to rise another 3,000 m due to renewed and considerable tectonic activity in Pleistocene around 3–2.5 and 0.98 Mya (Spicer et al., 2003; Spicer, 2017). The second view of the Himalayan mountain building proposes that the Himalaya along with Tibet rose as one block in a phased manner (Harrison et al., 1992; Molnar, England & Martinod, 1993; Favre et al., 2015). According to this view, four major episodes or windows of uplift have been reported for the Himalaya, namely 45–35 Mya, 35–20 Mya, 20–10 Mya, and 8–6 Mya (Wen et al., 2014; Favre et al., 2015). Notably, the phased elevation episodes were characterized by distinct sets of geophysical developments: 45–35 Mya was characterized by the subduction of Indian plate under the Eurasian plate; 35–20 Mya represented the period during which the Himalaya attained average elevation of 4,000 m and the modern-day southwest (SW) Indian monsoon began to take shape; 20–10 Mya period was accompanied by arrival of wet summer period south of the Himalaya and gradual aridification of the central Asian region to the north; 8–6 Mya represented the period of the major Himalayan uplift at its eastern edge (mostly Tibetan plateau) followed by intensification of monsoon (Zhisheng et al., 2001; Spicer et al., 2003; Wen et al., 2014; Favre et al., 2015).
Despite the equivocal evidence on the phased mode of the Himalaya’s elevation, it is certain that the Himalayan uplift caused three major environmental changes in the region: (i) land connections formed in the preceding epochs between the adjacent landmasses of India, Sino-Japanese and Southeast Asia were lost, (ii) the range of elevational climatic gradient in the Himalaya extended from tropical to temperate and alpine, and (iii) an orographic barrier was formed resulting in the formation of the SW monsoon system (Pandit, 2017). With the development of a cooler temperate environment towards higher elevations, new opportunities arose for the immigration of a number of temperate elements from the Sino-Japanese, European, and Mediterranean regions. Thus, started a second wave of immigration into the Himalaya in which many temperate taxa such as Acer, Alnus, Betula, Desmodium, Meliosma, and Quercus (Sino-Japanese affinities) found their way into the Himalaya from the northwestern end (via present day Jammu & Kashmir) (Mehrotra et al., 2005). Other examples of the immigrant taxa into the Himalaya from different regions include: Anemone, Caltha, Clematis, Ranunculus and Viola (European, Russian, and north Asian), Fagonia (Egyptian), Melilotus (European and Siberian), Seseli (Russian and Siberian), and Trifolium (European, Siberian, and North African) (Mani, 1974). Some researchers have suggested the formation of a “Himalayan corridor” to the south of Great Himalaya and its essentially temperate nature (Kitamura, 1955). It was through this corridor that numerous plant taxa of Sino-Japanese origin migrated westwards and southwards into the Himalaya (see Pandit, 2017). The formation of the “Himalayan corridor” has been confirmed in a number of later studies (Wang, 1992; Sun, 2002; Tabata, 2004).
Onset of Monsoon and Miocene Biodiversity
The onset of SW monsoon system triggered when the average elevation of the Himalaya reached about 4,000 m by the end of Oligocene (28–23 Mya) (Rowley, Pierrehumbert & Currie, 2001; Favre et al., 2015). However, the exact timing of the initiation of monsoon is a matter of much debate. Some researchers argue that the monsoon system originated as early as in Eocene (Srivastava et al., 2012; Shukla et al., 2014; Renner, 2016) or Paleocene epoch when Indian plate reached the Tropic of Capricorn (Patnaik et al., 2012). Irrespective of the timing of its initiation, it is certain that the monsoon system assumed its present-day form (with respect to seasonality and intensity) only by the end of Oligocene and beginning of Miocene when the elevated Himalaya began to act as an orographic barrier to the flow of regional winds in the west-east direction (Favre et al., 2015; Pandit, 2017). Around 8–6 Mya, an extensive uplift of the Tibetan plateau occurred which apparently intensified the monsoonal system (Burbank, Derry & France-Lanord, 1993). Sedimentological records available from the Siwalik foredeep corroborate the intensification of Asian monsoon at 6 Mya, with a prominent peak at 5.4 Mya (Sanyal et al., 2004).
The monsoon played a decisive role in the landscape evolution of the Himalaya. The ensuing heavy rainfall along the frontal ranges of the Himalaya gave rise to numerous streams and rivers which transported vast quantities of sediments and regularly denuded the exposed rock surfaces leading to the formation of deeply incised valleys (Pandit, 2017). The landscape incision was more prominent in the EH than the WH because of its tropical latitudes ensuring prolonged and more intense period of rainfall (Bookhagen, Thiede & Strecker, 2005). As a result, the EH region was transformed into a “region of extreme relief” due to closely clustered high, steep mountains that were dissected by numerous river divides and deep valleys (Irving & Hebda, 1993). The monsoon, therefore, was key to transforming the Himalaya into a highly dissected landscape aided by the tectonic processes that accelerated weathering, denudation and sediment transport driven mainly by precipitation (Pandit, 2017). Differentiated into numerous valleys dissected by rivers, the fragmented Himalayan landscape after the uplift provided novel physical and physiological barriers for gene flow between once continuous plant populations (Favre et al., 2015; Zhao et al., 2016). The combined result of these geographic events and formation of geographic barriers to gene flow culminated in large-scale allopatric speciation and evolutionary diversifications of the Himalayan flora (Pandit, Manish & Koh, 2014; Favre et al., 2015). Various molecular phylogenetic studies provide evidence that many plant species complexes (Caragana, Cyananthus, Koenigia, Meconopsis, Rheum and Rhodiola) originated and diversified in the period after the onset and intensification of modern-day monsoon system in the Himalaya (see Table 1). However, Renner (2016) argued that most of the time-calibrated molecular phylogenetic studies that link clade age of species between 15–0.5 Mya with specific uplift phases of the Tibetan plateau and its associated environmental effects assume that the Tibetan plateau underwent most major uplift during the Miocene, whereas the Tibetan Plateau had already reached the height of 4,000–5,000 m in Paleogene (∼40 Mya). Extensive weathering, erosion and detritus transport along the Himalayan slopes due to monsoon also aided in the formation of a foreland basin and transformation of the vegetation profile of the Himalayan from tropical wet evergreen forests of C3 plants to tall grassland ecosystems comprising predominantly of C4 plants around 8–6 Mya (Hoorn, Ohja & Quade, 2000). This shift from C3 to C4 dominated ecosystem was due to widespread global environmental changes in the Late Cenozoic Era such as global cooling and significant drop in global carbon dioxide (CO2) levels due to increased rates of chemical weathering and trapping of CO2 in the ocean sediments (Raymo & Ruddiman, 1992; Quade & Cerling, 1995). It has, however, been reported that the predominance of C4 plants in the Himalayan ecosystems has declined since the Last Glacial Maximum due to increased CO2 and humidity levels in the atmosphere (Galy et al., 2008).
Table 1. Molecular phylogenetic studies that have related plant species diversifications and evolution to specific phases of the Himalayan mountain formation and its uplift.
The studies have been listed in an alphabetical order according to the author names and year of publication.
| Clade(Family) | Crown age of clade (Mya) | Methodology | Principal Findings | Source |
|---|---|---|---|---|
| Koenigia (Polygonaceae) | 13.72–4.91 | MD and DT | Uplift of the Himalaya promoted species diversification in Koenigia; Himalaya acts as a primary evolutionary centre of Koenigia | Fan et al. (2013) |
| Hippophae tibetana (Elaeagnaceae) | 4–1 | MD and DT | Strong allopatric divergence was promoted in Hippophae tibetana during the Last Interglacial period (0.13–0.115 Mya) by orogenic processes and climate oscillations during the Quaternary | Jia et al. (2011) |
| Spiraea alpina (Rosaceae) | 1.2–0.6 | MD and DT | Uplift of the Tibetan Plateau and severe climatic oscillations during Quaternary promoted intraspecific divergence of Spiraea alpina | Khan et al. (2014) |
| Nannoglottis (Asteraceae) | 1.94–1.02 | MD and DT | Uplift of the Tibetan Plateau and severe climatic oscillations during Quaternary led to origin of several species of Nannoglottis | Liu et al. (2002) |
| Ligularia-Cremanthodium-Parasenecio (Asteraceae) | 13–8 | MD and DT | Uplift of the Tibetan Plateau between Early Miocene to Pleistocene promoted rapid and continuous allopatric speciation in the Ligularia-Cremanthodium-Parasenecio complex | Liu et al. (2006) |
| Taxus wallichiana (Taxaceae) | 6.5–2.0 | MP and SDM | Diversification and evolution of Taxus wallichiana in the Himalaya was promoted by Miocene/Pliocene geological and climatic events, uplift of the Tibetan Plateau and Late Quaternary climatic oscillations | Liu et al. (2013) |
| Ostryopsis intermedia (Betulaceae) | 1.2–0.5 | DT and ENM | Climatic oscillations during Quaternary and uplift of the Tibetan Plateau caused hybrid speciation of Ostryopsis intermedia | Liu et al. (2014) |
| Dasiphora (Rosaceae) | 3.25–0.32 | DT and DET | Uplift of the Tibetan Plateau and severe climatic oscillations during Quaternary caused deep divergences in Dasiphora | Ma et al. (2014) |
| Rheum (Polygonaceae) | 4.2–3.6 | MD and DT | Extensive uplifts of the Tibetan Plateau promoted diversification of species in Rheum | Sun et al. (2012) |
| Fagopyrum tibeticum (Polygonaceae) | 14.8–6.4 | MD and DT | Uplift of the Tibetan Plateau led to species radiation and development of woodiness in Fagopyrum tibeticum | Tian et al. (2011) |
| Rheum (Polygonaceae) | 7 | MD and DT | Uplift of the Tibetan Plateau coupled with climatic oscillations in the Quaternary led to adaptive radiation in Rheum | Wang, Yang & Liu (2005) |
| Dolomiaea (Asteraceae) | 13.6–12.2 | MD and DT | Uplift of the Tibetan Plateau since Miocene led to the evolution of endemic Himalayan flora | Wang, Liu & Miehe (2007) |
| Hippophae tibetana (Elaeagnaceae) | 3.15–1.04 | MD and DT | Rapid uplift of the Tibetan Plateau affected the dispersal potential and species differentiation of Hippophae tibetana | Wang et al. (2010) |
| Pomatosace filicula (Primulaceae) | 2.66–0.73 | MD and DT | Divergence in Pomatosace filicula overlaps with the Quaternary glaciation history in the Tibetan Plateau in the Early and Middle Pleistocene | Wang et al. (2014) |
| Meconopsis (Papaveraceae) | 15–11 | MD and DT | Divergence of Meconopsis was driven by the uplift of the Tibetan Plateau | Xie et al. (2014) |
| Meconopsis integrifolia (Papaveraceae) | 7.86–3.45 | MD and DT | Uplift of the Tibetan Plateau and associated climatic changes triggered the initial divergence of Meconopsis integrifolia | Yang et al. (2012) |
| Isodon (Lamiaceae) | 26.44–14.66 | MD and DT | Uplift of the Tibetan Plateau and associated climatic changes led to rapid radiation of Isodon | Yu et al. (2014) |
| Caragana (Fabaceae) | 16–14 | MD and DT | Uplift of the Tibetan Plateau and onset of the Himalayan motion led to high evolution and diversification of Caragana | Zhang & Fritsch (2010) |
| Stellera chamaejasme (Thymelaeaceae) | 6.5892 | MD and DT | Uplift of the Tibetan Plateau and associated climatic changes led to the origin of Stellera chamaejasme | Zhang, Volis & Sun (2010) |
| Soroseris-Stebbinsia-Syncalathium (Asteraceae) | 8.44–1.56 | MD and DT | Uplift of the Tibetan Plateau and associated changes in climate and habitat fragmentation led to rapid diversification and radiation of Soroseris-Stebbinsia-Syncalathium | Zhang et al. (2011) |
| Phyllolobium (Fabaceae) | 3.96–3.48 | MD and DT | Uplift of the Tibetan Plateau in the Late Pliocene and Early-to-Mid Pleistocene along with Late Pleistocene Glaciation led to rapid diversification of Phyllolobium | Zhang et al. (2012) |
| Rhodiola (Crassulaceae) | 21 | MD and DT | Uplift of the Himalaya and onset of Himalayan motion led to origin of Rhodiola | Zhang et al. (2014) |
| Cyananthus (Campanulaceae) | 23–12 | MD and DT | Onset of the Himalayan motion led to the origin of Cyananthus | Zhou et al. (2013) |
Notes.
- Mya
- million years ago
- MD
- Molecular dating
- DT
- Divergence time analysis
- MP
- Molecular phylogeography
- SDM
- Species distribution modelling
- ENM
- Ecological niche modelling
- DET
- Demographic test analysis
Megafossil evidences including leaf impressions, wood elements, flowers and fruits indicate that until the end of the Oligocene and beginning of Miocene epochs, the uplifting Himalayan landforms were dominated by tropical Indian peninsular flora such as Ficus sp., Mesua ferrea, Mallotus philippensis, Kayea floribunda, Chukrasia tabularis, etc. (see Srivastava et al., 2014). Presence of tropical and humid conditions during Early Miocene epoch has also been confirmed by the discovery of Ficus palaeoracemosa from the Kasauli geological formation (age: 23–10 Mya) in WH (Srivastava, Srivastava & Mehrotra, 2011). During Mid-Miocene when the Himalaya attained an average elevation of 2,200–2,400 m, many sub-tropical and temperate floral elements migrated into the Himalaya. This was revealed by the discovery of Trachycarpus and Prunus from the Miocene sediments of the Ladakh-Karakoram region (Guleria et al., 1983; Lakhanpal et al., 1984; Srivastava et al., 2014). Forests around this time (Mid-Miocene) at higher elevations had numerous temperate taxa such as Alnus, Picea, Pinus and Betula (Singh & Singh, 1987; Phadtare, 2000). The palynological records from the Surai Khola region of Central Nepal reveal that grasslands with predominantly C4 plants dominated the Himalayan foothills from Late Miocene to Pliocene and Early Pleistocene (Hoorn, Ohja & Quade, 2000).
Climate-driven landscape changes resulted in alterations in plant physiology, and geographic isolation and speciation through vicariance. Once these species complexes originated and diversified in the Himalaya, these subsequently dispersed into the neighboring regions of Asia Minor, Central Asia, Mongolian plateau and Europe producing derivative species therein (Jia et al., 2012; Nie et al., 2013). Ohba (1988) proposed the term “Central Asiatic highland corridor” through which the flora originating in the Himalaya migrated northward to the Central Asian highlands. Examples of such emigrant taxa from the Himalaya are: Anaphalis (migrated to eastern Asia, South East Asia and North America) (Nie et al., 2013), Hippophae rhamnoides (migrated to Central Asia and Asia Minor) (Jia et al., 2012), Lagotis (migrated to Central Asia and Arctic highlands) (Li et al., 2014), Leontopodium (migrated to European mountain ranges) (Blöch et al., 2010), Rhodiola (migrated to Northern Hemisphere regions such as Europe and Central Asia) (Zhang et al., 2014) and Solms-laubachia (migrated to Hengduan Mts) (Yue et al., 2009). The advent of the monsoon system is believed to have led to the formation of a mesic corridor in the Indian sub-continental region through which plant species such as Begonia migrated from Africa to Southeast Asia via the Himalaya (Rajbhandary et al., 2011).
Aridification of Central Asia
Following the development of SW monsoon system, aridity in the Central Asian region increased due to blockage of moisture laden winds by the uplift of the Himalaya and the Tibetan Plateau (Miao et al., 2012). The aridification triggered the diversification of many plant species with xerophytic adaptation and colonization of the Tibetan Plateau. Specific examples of species divergence include split of arid palmate Frutescentes section from its sister clades in the Caragana species complex around 8–7 Mya (Zhang & Fritsch, 2010). Similarly, Phyllolobium, a genus diversified as a result of intense uplift, cold climate, and ensuing aridity in the Tibetan Plateau (Table 1; Zhang et al., 2012). Central Asian aridification is also reported to have triggered the divergences of three lineages of Ephedra (eastern Tibetan Plateau, southern Tibetan Plateau, and northern China) (Qin et al., 2013). Likewise, the dominance of Artemisia in the present day vegetation of southwest and southeast regions of Tibetan Plateau is the result of rapid uplift of Tibetan Plateau and consequent prevalence of dry climate during and after the Late Miocene (Yunfa et al., 2011).
Quaternary Climate and the Himalayan Biodiversity
The Quaternary Period starting 2.6 Mya was characterized by expansive climatic fluctuations including repeated advance and retreat of glaciers with intermittent warming stages (Owen, Finkel & Caffee, 2002; Owen, 2009). Almost the entire Northern Hemisphere including Europe, North America and North-west Asia witnessed widespread glaciations with ice sheets descending as far south as to 40°N latitude (Carlson & Winsor, 2012). However, in the Himalaya, glaciations were confined to mountain peaks and high elevations (Owen, 2009). More importantly, unlike other regions of the world, there is no evidence of a uniform ice sheet covering the entire Himalayan range during the Quaternary glacial stages (Owen, 2009). In fact, most of the glaciers are reported to have advanced up to 10 km from their present-day ice margins during various stages of glaciation (Owen, Finkel & Caffee, 2002; Owen, 2009). Thus, the extent of glaciations in the Himalaya has been rather restricted as compared to the other mountain systems such as Alps and Andes, most likely due to the tropical location of the mountain range (Pandit, 2017).
Overall, 3–4 significant glacial advances and retreats have been documented in different regions of the Himalaya during the Quaternary period: Tibetan Plateau (4), Zanskar ranges (3), Swat valley (3), Kanchenjunga ranges (4) and Khumbu mountains (4) (see Owen, Finkel & Caffee, 2002 and references therein). Despite the limited extent of glaciation, the advance and retreat of glaciers had a significant effect on the evolutionary diversification of plant species in the Himalaya (Pandit, 2017). Some species got extinct, some dispersed to new warmer habitats in the south while some survived in glacial refugia in non-glaciated habitats. Habitats with patchy landscape distribution and diverse environmental conditions offered spaces as refugial habitats for numerous plant species. Examples of refugial habitats include low elevation areas of Tibetan Plateau (Aconitum gymnandrum, Juniperus tibetica, Primula secundiflora), south-eastern edge of Tibetan Plateau (Potentilla fruticosa, Metagentiana striata), and neighbouring Hengduan Mts (Pedicularis longiflora, Tsuga dumosa, Juniperus przewalskii, Sinopodophyllum hexandrum) (Zhang et al., 2005; Chen et al., 2008; Wang, 2008; Yang et al., 2008; Li et al., 2009; Wang et al., 2009; Cun & Wang, 2010; Opgenoorth et al., 2010; Li et al., 2011; Hoorn et al., 2013). Moreover, multiple refugia for a single plant species have also been reported such as for Pomatosace filicula (Wang et al., 2014), Hippophae neurocarpa (Kou et al., 2014), Hippophae tibetana (Jia et al., 2011), Rhododendron simsii (Li, Yan & Ge, 2012) and Aconitum gymnandrum (Wang et al., 2009). Development of multiple refugia was facilitated by the steep elevational gradient of the Himalaya that allowed various taxa to rapidly disperse to lower elevation habitats after traversing short geographic distances (Wen et al., 2014). Multiple recolonization events from numerous refugia have been reported after the glacial retreat (Cun & Wang, 2010; Wen et al., 2014; Meng et al., 2015). As a result of episodic glacial advances and retreats, the geographic ranges of the refugial plant taxa underwent repeated contractions and expansions. Consequently, the multiple recolonization events resulted in frequent mixing of floral taxa from different refugia followed by periodic hybridizations, adaptive radiations and speciation resulting in greater species diversity (Pandit, 2017). Hybridizations post recolonization events from refugia have been cited as an important mechanism for the colonizing success of Rhododendron (Zha, Milne & Sun, 2008; Milne et al., 2010), Meconopsis (Yang et al., 2012), and Pinus densata (Gao et al., 2012). Earlier studies have reported that when previously isolated populations come in contact with each other due to large scale glacial dynamics, hybridization followed by polyploidy is a common phenomenon resulting in species divergence (Stebbins, 1984). There is evidence of colonization success and dominance of polyploids in post-Pleistocene plant taxa in the Himalaya (Pandit, Manish & Koh, 2014; Pandit, 2017).
Pollen data from the Pleistocene epoch of Uttarakhand region in the WH indicates that evergreen oaks (Quercus semecarpifolia) and alder (Alnus) dominated the Himalayan landscapes around 0.0078 Mya when climate conditions were cold and wet with moderate monsoon and were subsequently replaced by conifers (Pinus and Abies) around 0.0066 Mya when climate became warmer (Phadtare, 2000; Pandit, 2017). This indicates that repeated climatic oscillations in the Quaternary influenced the vegetation composition of the Himalaya. It has also been postulated that during each glaciation phase, the temperate and alpine flora of the Himalaya moved southwards towards the lower elevations where warmer temperatures prevailed (Mani, 1974; Pandit, 2017). During the interglacial phases with the retreat of glaciers, these taxa subsequently became isolated and diversified. This is evident by the common occurrence of large number of plant species with Sino-Himalayan plants affinities on isolated hilltops of peninsular India, Western and Eastern Ghats and as far south as Sri Lanka. Some examples of such temperate taxa representing Pleistocene relicts are: Anemone rivularis (Himalaya, Nilgiri and Palni Hills), Clematis wightiana (Himalaya, Nilgiri, Shevaroy and Palni Hills), Cnicus wallichii (Himalaya, Nilgiri and Palni Hills), Geranium nepalense (Himalaya, Khasi Hills, Nilgiri and Sri Lanka), Gymnopetalum (Deccan, Chotta Nagpur plateau), Litsea (Himalaya, Western Ghats), Polygala sibirica (Himalaya, Khasi Hills, Western Ghats and Sri Lanka), Rhamnus virgatus (Himalaya, Palni and Tinnevelly Hills), Stellaria media (Himalaya, Nilgiri, Shevaroy, Palni and Sri Lanka), Thalictrum (Himalaya and Anaimalai Hills), Viburnum acuminatum (Mahendragiri, Shevaroy, Palni and Nilgiri Hills), and Viola patrinii (Himalaya, Mahendragiri, Shevaroy, Nilgiri and Palni Hills) (see Mani, 1974).
Present-day Himalayan Biodiversity
The present-day Himalayan ecosystems exhibit a pronounced post-Pleistocene biodiversity, but retain the mixed biotic character of having immigrant elements from different biogeographic regions. Biogeographically, the Himalaya is a transitional zone located at the cusp of three biogeographic realms, namely Palearctic, Afrotropical and Indo-Malayan and Oriental realms in the north, southwest and southeast, respectively (see Mani, 1974). As a result of this unique geographic location, the Himalaya is home to numerous Austro-Polynesian, Sino-Tibetan, Euro-Mediterranean and Malayo-Burman biotic elements. A simplified plant biodiversity profile occupying different Himalayan zones, viz. Western, Central and Eastern Himalaya is presented in Table 2. The elevational limits of different vegetation zones are slightly higher by 300–400 m in the Eastern Himalaya than the Western Himalaya (Pandit, 2017). The principal reason for such an elevational zone shift is the latitudinal difference of nearly 10° between the Eastern and Western Himalaya (see Pandit, 2017).
Table 2. Generalized vegetation profile of the Western, Central and Eastern Himalaya.
Overall, Western Himalaya is characterized by coniferous forests of deodar, pines and silver fir, while Eastern Himalaya shows conspicuous presence of broad-leaved forests of oaks, Rhododendrons and maples (Source: Adapted from Pandit & Kumar, 2013; Pandit, Manish & Koh, 2014; Manish et al., 2017; Pandit, 2017).
| Himalayan zone | Climate zones | ||
|---|---|---|---|
| Tropical and sub-tropical | Temperate | Sub-alpine and alpine | |
| Western | Semi-deciduous forests of Shorea robusta, Acacia catechu, Dalbergia sissoo, Albizia lebbeck, Garuga pinnata, Terminalia bellirica and T. tomentosa are found up to 1,500 m; at higher elevations, Pinus roxburghii occurs | This zone (1,500–3,500 m) is dominated by oaks (Quercus spp.) and Rhododendron spp. Cedrus deodara, Abies pindrow and Picea smithiana dominate elevations between 2,800–3,500 m | This zone (3,500 m and above) show preponderance of herbaceous genera of Anemone, Geranium, Iris, Lloydia, Potentilla, Primula etc. interspersed with dry dwarf alpine scrubs of Berberis, Cotoneaster, Juniperus and Rhododendron |
| Central | Shorea robusta, Acacia catechu and Dalbergia sissoo comprise principal tree species of this zone up to 1,500 m along with Haldina cordifolia, Kydia calycina and Semecarpus anacardium; Pinus roxburghii forests appear at higher elevations | Forest vegetation is similar to Western Himalaya, albeit with lesser number of Rhododendron species and with additional presence of Berberis spp. and Prinsepia utilis | |
| Eastern | Mesua assamica, Mesua ferrea, Albizia procera, Bombax ceiba, Careya arborea, Gmelina arborea, Oroxylum indicum, Duabanga grandiflora dominate up to 1,800 m; at higher elevations, Quercus lamellosa and Mesua forests are found | Evergreen oak forests of Quercus lamellosa and Q. cerris dominate along with Magnolia spp., Lithocarpus pachyphyllus and Acer spp.; between 2,800–3,600 m, Abies delavayi, Abies densa, Tsuga dumosa, Larix griffithii and Rhododendron dominate | |
The development of present-day plant biodiversity in the Himalaya has largely been shaped by the climate of the region. In the tropical and sub-tropical zones (up to 1,500 m), temperature varies from 6 °C to 35 °C in various areas while rainfall variation is from 1,500 to 3,500 mm. These climate conditions are well suited for growth of deciduous and semi-deciduous vegetation. The temperate zone (1,500–3,500 m) experiences an average annual rainfall of 2,400 mm while temperature varies between 5–26 °C. Thus, mainly coniferous forests dominate the temperate zone in the Himalaya. Low temperatures characterize the sub-alpine and alpine zones (3,500 m and above) and the precipitation takes place mainly in the form of snowfall, except in the summer, which lasts for only three months. Thus, only plant communities with specialized adaptations to these harsh conditions can survive in the sub-alpine and alpine zones, such as cushion forming communities (Arenaria polytrichoides, Anaphalis cavei), tussocks or tufts (Kobresia schoenoides, Carex parva), solifluction acrobats (Gentiana urnula, Eriophytum wallichii), and dense woolly forms (Saussurea graminifolia, Glechoma nivalis).
Conclusions
The geophysical upheavals associated with the formation of the Himalaya led to significant climate changes, new geophysical environments, novel ecological niches and formation of physical and physiological isolation barriers that acted as natural selection sieves. These geophysical changes including onset of monsoon, glaciation and glacial advance, and retreat brought about adaptive radiations in the plant taxa. The Himalayan landforms were initially colonized by migrant plant taxa from the neighboring biogeographic regions and in due course, these early taxa established gene exchanges among them and formed new variants that were highly adaptive to the changing physiography and climate of the area. Once the final phase of the Himalayan uplift concluded, the environmental conditions became suitable for the migrant species and the variants to evolve and diversify into new taxa. Disjunct taxa distributions were brought about by the fragmented landscape, while new physical and physiological barriers limited the expansion of species ranges. Newly formed steep gorges and valleys imposed isolation barriers on the recently migrated species enhancing the processes and rates of evolutionary divergence. As a net result, many endemic taxa evolved and the Himalaya became a repository of unique assemblages of plant species. Thus, having started as a ‘sink’ and a ‘biological highway’, the Himalaya turned into a geographical barrier, promoting vicariance, species diversification and endemism. However, only a few studies have empirically tested the above hypotheses and those that have are based on only specific taxa. It is well known that different patterns of diversification may exist for different taxa. Therefore, many of the stated hypotheses here may appear as conjectures. Many unanswered questions still remain, that include: (i) what was the nature of early migrants in the Himalaya; (ii) what were the ancestral distributional areas of these migrants; (iii) what were the precise time periods during which maximum migrations and endemic diversification occurred in the Himalaya; and (iv) were the migrations and diversifications widespread across phylogenies? A complementary and integrative collaboration between researchers from varied backgrounds in earth sciences, atmospheric sciences, palaeobiology, biogeography and ecology are needed to solve these evolutionary riddles in an important global biodiversity hotspot—the Himalaya.
Acknowledgments
We are indebted to late Dr. Virendra Kumar for illuminating ideas on the topic of this review. We also thank the three anonymous reviewers and the Editor for their insightful and constructive suggestions.
Funding Statement
This work was supported by DST INSPIRE Research Fellowship (granted to Kumar Manish) and DU-DST-Purse grant (sanctioned to Maharaj K. Pandit). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Kumar Manish conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Maharaj K. Pandit conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Since this is a Literature Review, the research in this article did not generate any data or code. All the references have been cited in the text itself.
References
- Blöch et al. (2010).Blöch C, Dickoré W, Samuel R, Stuessy T. Molecular phylogeny of the edelweiss (Leontopodium, Asteraceae–Gnaphalieae) Edinburgh Journal of Botany. 2010;67:235–264. doi: 10.1017/S0960428610000065. [DOI] [Google Scholar]
- Bookhagen, Thiede & Strecker (2005).Bookhagen B, Thiede RC, Strecker MR. Abnormal monsoon years and their control on erosion and sediment flux in the high, arid northwest Himalaya. Earth and Planetary Science Letters. 2005;231:131–146. doi: 10.1016/j.epsl.2004.11.014. [DOI] [Google Scholar]
- Bossuyt & Milinkovitch (2001).Bossuyt F, Milinkovitch MC. Amphibians as indicators of early tertiary “out-of-India” dispersal of vertebrates. Science. 2001;292:93–95. doi: 10.1126/science.1058875. [DOI] [PubMed] [Google Scholar]
- Bouilhol et al. (2013).Bouilhol P, Jagoutz O, Hanchar JM, Dudas FO. Dating the India–Eurasia collision through arc magmatic records. Earth and Planetary Science Letters. 2013;366:163–175. doi: 10.1016/j.epsl.2013.01.023. [DOI] [Google Scholar]
- Burbank, Derry & France-Lanord (1993).Burbank DW, Derry LA, France-Lanord C. Reduced Himalayan sediment production 8 Myr ago despite an intensified monsoon. Nature. 1993;364:48–50. doi: 10.1038/364048a0. [DOI] [Google Scholar]
- Carlson & Winsor (2012).Carlson AE, Winsor K. Northern Hemisphere ice-sheet responses to past climate warming. Nature Geoscience. 2012;5:607–613. doi: 10.1038/ngeo1528. [DOI] [Google Scholar]
- Chatterjee & Scotese (1999).Chatterjee S, Scotese CR. The breakup of Gondwana and the evolution and biogeography of the Indian plate. Proceedings of the Indian National Science Academy Part A. 1999;65:397–426. [Google Scholar]
- Che et al. (2010).Che J, Zhou WW, Hu JS, Yan F, Papenfuss TJ, Wake DB, Zhang YP. Spiny frogs (Paini) illuminate the history of the Himalayan region and Southeast Asia. Proceedings of the National Academy of Sciences United States of America. 2010;107:13765–13770. doi: 10.1073/pnas.1008415107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2008).Chen S, Wu G, Zhang D, Gao Q, Duan Y, Zhang F, Chen S. Potential refugium on the Qinghai–Tibet Plateau revealed by the chloroplast DNA phylogeography of the alpine species Metagentiana striata (Gentianaceae) Botanical Journal of the Linnean Society. 2008;157:125–140. doi: 10.1111/j.1095-8339.2008.00785.x. [DOI] [Google Scholar]
- Conti et al. (2002).Conti E, Eriksson T, Schönenberger J, Sytsma KJ, Baum DA. Early Tertiary out-of-India dispersal of Crypteroniaceae: evidence from phylogeny and molecular dating. Evolution. 2002;56:1931–1942. doi: 10.1111/j.0014-3820.2002.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Cun & Wang (2010).Cun YZ, Wang XQ. Plant recolonization in the Himalaya from the southeastern Qinghai-Tibetan Plateau: geographical isolation contributed to high population differentiation. Molecular Phylogenetics and Evolution. 2010;56:972–982. doi: 10.1016/j.ympev.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Ding et al. (2017).Ding L, Spicer RA, Yang J, Xu Q, Cai F, Li S, Lai Q, Wang H, Spicer TEV, Yue Y, Shukla A, Srivastava G, Khan MA, Bera S, Mehrotra RC. Quantifying the rise of the Himalaya orogen and implications for the South Asian monsoon. Geology. 2017;45:215–218. doi: 10.1130/G38583.1. [DOI] [Google Scholar]
- Duncan & Pyle (1988).Duncan R, Pyle D. Rapid eruption of the Deccan flood basalts at the Cretaceous/Tertiary boundary. Nature. 1988;333:841–843. doi: 10.1038/333841a0. [DOI] [Google Scholar]
- Fan et al. (2013).Fan DM, Chen JH, Meng Y, Wen J, Huang JL, Yang YP. Molecular phylogeny of Koenigia L. (Polygonaceae: Persicarieae): implications for classification, character evolution and biogeography. Molecular Phylogenetics and Evolution. 2013;69:1093–1100. doi: 10.1016/j.ympev.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Favre et al. (2015).Favre A, Päckert M, Pauls SU, Jähnig SC, Uhl D, Michalak I, Muellner-Riehl AN. The role of the uplift of the Qinghai-Tibetan Plateau for the evolution of Tibetan biotas. Biological Reviews. 2015;90:236–253. doi: 10.1111/brv.12107. [DOI] [PubMed] [Google Scholar]
- Galy et al. (2008).Galy V, François L, France-Lanord C, Faure P, Kudrass H, Palhol F, Singh SK. C4 plants decline in the Himalayan basin since the Last Glacial Maximum. Quaternary Science Reviews. 2008;27:1396–1409. doi: 10.1016/j.quascirev.2008.04.005. [DOI] [Google Scholar]
- Gao et al. (2012).Gao J, Wang B, Mao JF, Ingvarsson P, Zeng QY, Wang XR. Demography and speciation history of the homoploid hybrid pine Pinus densata on the Tibetan Plateau. Molecular Ecology. 2012;21:4811–4827. doi: 10.1111/j.1365-294X.2012.05712.x. [DOI] [PubMed] [Google Scholar]
- Greenhalgh & Peacock (2005).Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005;331:1064–1065. doi: 10.1136/bmj.38636.593461.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria et al. (1983).Guleria JS, Thakur VC, Virdi NS, Lakhanpal RN. A fossil wood of Prunus from the Kargil (=Liyan) formation of Ladakh. In: Thakur VC, Sharma KK, editors. Geology of indus suture zone of Ladakh. Wadia Institute of Himalayan Geology; Dehradun: 1983. pp. 187–193. [Google Scholar]
- Harrison et al. (1992).Harrison TM, Copeland P, Kidd W, Yin A. Raising Tibet. Science. 1992;255:1663–1670. doi: 10.1126/science.255.5052.1663. [DOI] [PubMed] [Google Scholar]
- Hedin (1909).Hedin S. Trans-Himalaya, discoveries and adventures in Tibet. Macmillan & Co. Ltd; London: 1909. [Google Scholar]
- Hoorn et al. (2013).Hoorn C, Mosbrugger V, Mulch A, Antonelli A. Biodiversity from mountain building. Nature Geoscience. 2013;6:154. doi: 10.1038/ngeo1742. [DOI] [Google Scholar]
- Hoorn, Ohja & Quade (2000).Hoorn C, Ohja T, Quade J. Palynological evidence for vegetation development and climatic change in the Sub-Himalayan Zone (Neogene, Central Nepal) Palaeogeography, Palaeoclimatology, Palaeoecology. 2000;163:133–161. doi: 10.1016/S0031-0182(00)00149-8. [DOI] [Google Scholar]
- Irving & Hebda (1993).Irving E, Hebda R. Concerning the origin and distribution of Rhododendrons. Journal of the American Rhododendron Society. 1993;47:139–146. [Google Scholar]
- Jagoutz et al. (2015).Jagoutz O, Royden L, Holt AF, Becker TW. Anomalously fast convergence of India and Eurasia caused by double subduction. Nature Geoscience. 2015;8:475–478. doi: 10.1038/ngeo2418. [DOI] [Google Scholar]
- Jia et al. (2012).Jia DR, Abbott RJ, Liu TL, Mao KS, Bartish IV, Liu JQ. Out of the Qinghai–Tibet Plateau: evidence for the origin and dispersal of Eurasian temperate plants from a phylogeographic study of Hippophaë rhamnoides (Elaeagnaceae) New Phytologist. 2012;194:1123–1133. doi: 10.1111/j.1469-8137.2012.04115.x. [DOI] [PubMed] [Google Scholar]
- Jia et al. (2011).Jia DR, Liu TL, Wang LY, Zhou DW, Liu JQ. Evolutionary history of an alpine shrub Hippophae tibetana (Elaeagnaceae): allopatric divergence and regional expansion. Biological Journal of the Linnean Society. 2011;102:37–50. doi: 10.1111/j.1095-8312.2010.01553.x. [DOI] [Google Scholar]
- Karanth (2006).Karanth KP. Out-of-India Gondwanan origin of some tropical Asian biota. Current Science. 2006;90:789–792. [Google Scholar]
- Khan et al. (2014).Khan G, Zhang F, Gao Q, Fu PC, Xing R, Wang J, Liu H, Chen S. Molecular phylogeography and intraspecific divergence of Spiraea alpina (Rosaceae) distributed in the Qinghai-Tibetan Plateau and adjacent regions inferred from nrDNA. Biochemical Systematics and Ecology. 2014;57:278–286. doi: 10.1016/j.bse.2014.08.013. [DOI] [Google Scholar]
- Khosla & Sahni (2003).Khosla A, Sahni A. Biodiversity during the Deccan volcanic eruptive episode. Journal of Asian Earth Sciences. 2003;21:895–908. doi: 10.1016/S1367-9120(02)00092-5. [DOI] [Google Scholar]
- Kitamura (1955).Kitamura S. Flowering plants and ferns. In: Kihara H, editor. Fauna and flora of Nepal Himalaya. Fauna and Flora Research Society; Kyoto: 1955. pp. 73–290. [Google Scholar]
- Kou et al. (2014).Kou YX, Wu YX, Jia DR, Li ZH, Wang YJ. Range expansion, genetic differentiation, and phenotypic adaption of Hippophaë neurocarpa (Elaeagnaceae) on the Qinghai–Tibet Plateau. Journal of Systematics and Evolution. 2014;52:303–312. doi: 10.1111/jse.12063. [DOI] [Google Scholar]
- Lakhanpal et al. (1984).Lakhanpal RN, Prakash G, Thussu JL, Guleria JS. A fossil fan palm from the Liyan formation of Ladakh (Jammu and Kaskmir) Palaeobotanist. 1984;31:201–207. [Google Scholar]
- Li et al. (2014).Li GD, Kim C, Zha HG, Zhou Z, Nie ZL, Sun H. Molecular phylogeny and biogeography of the arctic-alpine genus Lagotis (Plantaginaceae) Taxon. 2014;63:103–115. doi: 10.12705/631.47. [DOI] [Google Scholar]
- Li et al. (2009).Li C, Shimono A, Shen H, Tang Y. Phylogeography of Potentilla fruticosa, an alpine shrub on the Qinghai-Tibetan Plateau. Journal of Plant Ecology. 2009;3:9–15. [Google Scholar]
- Li, Yan & Ge (2012).Li Y, Yan HF, Ge XJ. Phylogeographic analysis and environmental niche modeling of widespread shrub Rhododendron simsii in China reveals multiple glacial refugia during the last glacial maximum. Journal of Systematics and Evolution. 2012;50:362–373. doi: 10.1111/j.1759-6831.2012.00209.x. [DOI] [Google Scholar]
- Li et al. (2011).Li Y, Zhai SN, Qiu YX, Guo YP, Ge XJ, Comes HP. Glacial survival east and west of the ‘Mekong–Salween Divide’in the Himalaya–Hengduan Mountains region as revealed by AFLPs and cpDNA sequence variation in Sinopodophyllum hexandrum (Berberidaceae) Molecular Phylogenetics and Evolution. 2011;59:412–424. doi: 10.1016/j.ympev.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2014).Liu B, Abbott RJ, Lu Z, Tian B, Liu J. Diploid hybrid origin of Ostryopsis intermedia (Betulaceae) in the Qinghai-Tibet Plateau triggered by Quaternary climate change. Molecular Ecology. 2014;23:3013–3027. doi: 10.1111/mec.12783. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2002).Liu JQ, Gao TG, Chen ZD, Lu AM. Molecular phylogeny and biogeography of the Qinghai-Tibet Plateau endemic Nannoglottis (Asteraceae) Molecular Phylogenetics and Evolution. 2002;23:307–325. doi: 10.1016/S1055-7903(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2013).Liu J, Möller M, Provan J, Gao LM, Poudel RC, Li DZ. Geological and ecological factors drive cryptic speciation of yews in a biodiversity hotspot. New Phytologist. 2013;199:1093–1108. doi: 10.1111/nph.12336. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2006).Liu JQ, Wang YJ, Wang AL, Hideaki O, Abbott RJ. Radiation and diversification within the Ligularia–Cremanthodium–Parasenecio complex (Asteraceae) triggered by uplift of the Qinghai-Tibetan Plateau. Molecular Phylogenetics and Evolution. 2006;38:31–49. doi: 10.1016/j.ympev.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2014).Ma YZ, Li ZH, Wang X, Shang BL, Wu GL, Wang YJ. Phylogeography of the genus Dasiphora (Rosaceae) in the Qinghai-Tibetan Plateau: divergence blurred by expansion. Biological Journal of the Linnean Society. 2014;111:777–788. doi: 10.1111/bij.12246. [DOI] [Google Scholar]
- Mani (1974).Mani MS. Ecology and biogeography in India. Dr. W Junk Publishers; The Hague: 1974. [Google Scholar]
- Manish (2017).Manish K. Book review: life in the Himalaya: an ecosystem at risk. Frontiers in Ecology and Evolution. 2017;5 doi: 10.3389/fevo.2017.00112. Article 112. [DOI] [Google Scholar]
- Manish et al. (2017).Manish K, Pandit MK, Telwala Y, Nautiyal DC, Koh LP, Tiwari S. Elevational plant species richness patterns and their drivers across non-endemics, endemics and growth forms in the Eastern Himalaya. Journal of Plant Research. 2017;130:829–844. doi: 10.1007/s10265-017-0946-0. [DOI] [PubMed] [Google Scholar]
- McLoughlin (2001).McLoughlin S. The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Australian Journal of Botany. 2001;49:271–300. doi: 10.1071/BT00023. [DOI] [Google Scholar]
- Mehrotra et al. (2005).Mehrotra R, Liu XQ, Li CS, Wang YF, Chauhan M. Comparison of the Tertiary flora of southwest China and northeast India and its significance in the antiquity of the modern Himalayan flora. Review of Palaeobotany and Palynology. 2005;135:145–163. doi: 10.1016/j.revpalbo.2005.03.004. [DOI] [Google Scholar]
- Meng et al. (2015).Meng L, Chen G, Li Z, Yang Y, Wang Z, Wang L. Refugial isolation and range expansions drive the genetic structure of Oxyria sinensis (Polygonaceae) in the Himalaya-Hengduan Mountains. Scientific Reports. 2015;5:10396. doi: 10.1038/srep10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merckx et al. (2015).Merckx VS, Hendriks KP, Beentjes KK, Mennes CB, Becking LE, Peijnenburg KT, Afendy A, Arumugam N, De Boer H, Biun A. Evolution of endemism on a young tropical mountain. Nature. 2015;524:347–350. doi: 10.1038/nature14949. [DOI] [PubMed] [Google Scholar]
- Miao et al. (2012).Miao Y, Herrmann M, Wu F, Yan X, Yang S. What controlled Mid–Late Miocene long-term aridification in Central Asia?—global cooling or Tibetan Plateau uplift: a review. Earth-Science Reviews. 2012;112:155–172. doi: 10.1016/j.earscirev.2012.02.003. [DOI] [Google Scholar]
- Milne et al. (2010).Milne RI, Davies C, Prickett R, Inns LH, Chamberlain DF. Phylogeny of Rhododendron subgenus Hymenanthes based on chloroplast DNA markers: between-lineage hybridisation during adaptive radiation? Plant Systematics and Evolution. 2010;285:233–244. doi: 10.1007/s00606-010-0269-2. [DOI] [Google Scholar]
- Molnar, England & Martinod (1993).Molnar P, England P, Martinod J. Mantle dynamics, uplift of the Tibetan Plateau, and the Indian monsoon. Reviews of Geophysics. 1993;31:357–396. doi: 10.1029/93RG02030. [DOI] [Google Scholar]
- Murphy et al. (2008).Murphy JB, Gutiérrez-Alonso G, Nance RD, Fernández-Suárez J, Keppie JD, Quesada C, Strachan RA, Dostal J. Tectonic plates come apart at the seams. American Scientist. 2008;96:129–137. doi: 10.1511/2008.70.3639. [DOI] [Google Scholar]
- Nie et al. (2013).Nie ZL, Funk V, Sun H, Deng T, Meng Y, Wen J. Molecular phylogeny of Anaphalis (Asteraceae, Gnaphalieae) with biogeographic implications in the Northern Hemisphere. Journal of Plant Research. 2013;126:17–32. doi: 10.1007/s10265-012-0506-6. [DOI] [PubMed] [Google Scholar]
- Officer et al. (1987).Officer CB, Hallam A, Drake CL, Devine JD. Late Cretaceous and paroxysmal Cretaceous/Tertiary extinctions. Nature. 1987;326:143–149. doi: 10.1038/326143a0. [DOI] [Google Scholar]
- Ohba (1988).Ohba H. The alpine flora of the Nepal Himalayas: an introductory note. The Himalayan Plants. 1988;1:19–46. [Google Scholar]
- Opgenoorth et al. (2010).Opgenoorth L, Vendramin GG, Mao K, Miehe G, Miehe S, Liepelt S, Liu J, Ziegenhagen B. Tree endurance on the Tibetan Plateau marks the world’s highest known tree line of the last glacial maximum. New Phytologist. 2010;185:332–342. doi: 10.1111/j.1469-8137.2009.03007.x. [DOI] [PubMed] [Google Scholar]
- Owen (2004).Owen LA. Cenozoic evolution of global mountain systems. In: Owens PN, Slaymaker O, editors. Mountain Geomorphology. Edward Arnold Publication; London: 2004. pp. 33–58. [Google Scholar]
- Owen (2009).Owen LA. Latest Pleistocene and Holocene glacier fluctuations in the Himalaya and Tibet. Quaternary Science Reviews. 2009;28:2150–2164. doi: 10.1016/j.quascirev.2008.10.020. [DOI] [Google Scholar]
- Owen, Finkel & Caffee (2002).Owen LA, Finkel RC, Caffee MW. A note on the extent of glaciation throughout the Himalaya during the global Last Glacial Maximum. Quaternary Science Reviews. 2002;21:147–157. doi: 10.1016/S0277-3791(01)00104-4. [DOI] [Google Scholar]
- Pandit (2017).Pandit MK. Life in the Himalaya: an ecosystem at risk. Harvard University Press; Cambridge: 2017. [Google Scholar]
- Pandit & Kumar (2013).Pandit MK, Kumar V. Land-use change and conservation challenges in the Indian Himalaya. In: Sodhi NS, Gibson L, Raven PH, editors. Conservation biology: voices from the tropics. Wiley Publications; Chichester: 2013. pp. 123–133. [Google Scholar]
- Pandit, Manish & Koh (2014).Pandit MK, Manish K, Koh LP. Dancing on the roof of the world: ecological transformation of the Himalayan landscape. BioScience. 2014;64:980–992. doi: 10.1093/biosci/biu152. [DOI] [Google Scholar]
- Patnaik et al. (2012).Patnaik R, Gupta AK, Naidu PD, Yadav RR, Bhattacharyya A, Kumar M. Indian monsoon variability at different time scales: marine and terrestrial proxy records. Proceedings of the Indian National Science Academy. 2012;78:535–547. [Google Scholar]
- Phadtare (2000).Phadtare NR. Sharp decrease in summer monsoon strength 4,000–3,500 cal yr BP in the Central Higher Himalaya of India based on pollen evidence from alpine peat. Quaternary Research. 2000;53:122–129. doi: 10.1006/qres.1999.2108. [DOI] [Google Scholar]
- Plummer & Belle (1995).Plummer PS, Belle E. Mesozoic tectono-stratigraphic evolution of the Seychelles microcontinent. Sedimentary Geology. 1995;96:73–91. doi: 10.1016/0037-0738(94)00127-G. [DOI] [Google Scholar]
- Qin et al. (2013).Qin AL, Wang MM, Cun YZ, Yang FS, Wang SS, Ran JH, Wang XQ. Phylogeographic evidence for a link of species divergence of Ephedra in the Qinghai-Tibetan Plateau and adjacent regions to the Miocene Asian aridification. PLOS ONE. 2013;8:e56243. doi: 10.1371/journal.pone.0056243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quade & Cerling (1995).Quade J, Cerling TE. Expansion of C4 grasses in the late Miocene of northern Pakistan: evidence from stable isotopes in paleosols. Palaeogeography, Palaeoclimatology, Palaeoecology. 1995;115:91–116. doi: 10.1016/0031-0182(94)00108-K. [DOI] [Google Scholar]
- Rajbhandary et al. (2011).Rajbhandary S, Hughes M, Phutthai T, Thomas D, Shrestha KK. Asian Begonia: out of Africa via the Himalayas. Garden Bulletion of Singapore. 2011;63:277–286. [Google Scholar]
- Raymo & Ruddiman (1992).Raymo ME, Ruddiman WF. Tectonic forcing of late Cenozoic climate. Nature. 1992;359:117–122. doi: 10.1038/359117a0. [DOI] [Google Scholar]
- Renner (2016).Renner SS. Available data point to a 4-km-high Tibetan Plateau by 40 M.A. but 100 molecular-clock papers have linked supposed recent uplift to young node ages. Journal of Biogeography. 2016;43:1479–1487. doi: 10.1111/jbi.12755. [DOI] [Google Scholar]
- Rowley, Pierrehumbert & Currie (2001).Rowley DB, Pierrehumbert RT, Currie BS. A new approach to stable isotope-based paleoaltimetry: implications for paleoaltimetry and paleohypsometry of the High Himalaya since the Late Miocene. Earth and Planetary Science Letters. 2001;188:253–268. doi: 10.1016/S0012-821X(01)00324-7. [DOI] [Google Scholar]
- Sanyal et al. (2004).Sanyal P, Bhattacharya S, Kumar R, Ghosh S, Sangode S. Mio–Pliocene monsoonal record from Himalayan foreland basin (Indian Siwalik) and its relation to vegetational change. Palaeogeography, Palaeoclimatology, Palaeoecology. 2004;205:23–41. doi: 10.1016/j.palaeo.2003.11.013. [DOI] [Google Scholar]
- Shukla et al. (2014).Shukla A, Mehrotra RC, Spicer RA, Spicer TEV, Kumar M. Cool equatorial terrestrial temperatures and the South Asian monsoon in the Early Eocene: evidence from the Gurha Mine, Rajasthan, India. Palaeogeography, Palaeoclimatology, Palaeoecology. 2014;412:187–198. doi: 10.1016/j.palaeo.2014.08.004. [DOI] [Google Scholar]
- Singh, Adhikari & Zobel (1994).Singh SP, Adhikari BS, Zobel DB. Biomass, productivity, leaf longevity, and forest structure in the central Himalaya. Ecological Monographs. 1994;64:401–421. doi: 10.2307/2937143. [DOI] [Google Scholar]
- Singh & Singh (1987).Singh J, Singh S. Forest vegetation of the Himalaya. The Botanical Review. 1987;53:80–192. doi: 10.1007/BF02858183. [DOI] [Google Scholar]
- Spicer (2017).Spicer RA. Tibet, the Himalaya, Asian monsoons and biodiversity-In what ways are they related? Plant Diversity. 2017;39:233–244. doi: 10.1016/j.pld.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer et al. (2003).Spicer RA, Harris NB, Widdowson M, Herman AB, Guo S, Valdes PJ, Wolfe JA, Kelley SP. Constant elevation of southern Tibet over the past 15 million years. Nature. 2003;421:622–624. doi: 10.1038/nature01356. [DOI] [PubMed] [Google Scholar]
- Srivastava & Mehrotra (2010).Srivastava GA, Mehrotra RC. Tertiary flora of northeast India vis-a-vis movement of the Indian plate. Memoir Geological Society of India. 2010;75:123–130. [Google Scholar]
- Srivastava & Mehrotra (2013).Srivastava G, Mehrotra RC. First fossil record of Alphonsea Hk. f. & T. (Annonaceae) from the late Oligocene sediments of Assam, India and comments on its phytogeography. PLOS ONE. 2013;8:e53177. doi: 10.1371/journal.pone.0053177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava et al. (2014).Srivastava G, Mehrotra RC, Shukla AN, Tiwari RP. Miocene vegetation and climate in extra peninsular India: megafossil evidences. Special Publication of the Palaeontological Society of India. 2014;5:283–290. [Google Scholar]
- Srivastava et al. (2012).Srivastava G, Spicer RA, Spicer TEV, Yang J, Kumar M, Mehrotra R, Mehrotra N. Megaflora and palaeoclimate of a Late Oligocene tropical delta, Makum Coalfield, Assam: evidence for the early development of the South Asia Monsoon. Palaeogeography, Palaeoclimatology, Palaeoecology. 2012;342:130–142. [Google Scholar]
- Srivastava, Srivastava & Mehrotra (2011).Srivastava G, Srivastava R, Mehrotra RC. Ficus palaeoracemosa sp. nov.—a new fossil leaf from the Kasauli formation of Himachal Pradesh and its palaeoclimatic significance. Journal of Earth System Science. 2011;120:253–262. doi: 10.1007/s12040-011-0054-9. [DOI] [Google Scholar]
- Srivastava (2011).Srivastava R. Indian Upper Cretaceous-Tertiary flora before collision of Indian Plate: a reappraisal of central and western Indian flora. Memoirs of the Geological Society of India. 2011;77:281–292. [Google Scholar]
- Stebbins (1984).Stebbins G. Polyploidy and the distribution of the arctic-alpine flora: new evidence and a new approach. Botanica Helvetica. 1984;94:1–13. [Google Scholar]
- Sun (2002).Sun H. Tethys retreat and Himalayas-Hengduanshan mountains uplift and their significance on the origin and development of the Sino-Himalayan elements and alpine flora. Acta Botanica Yunnanica. 2002;24:273–288. [Google Scholar]
- Sun et al. (2012).Sun Y, Wang A, Wan D, Wang Q, Liu J. Rapid radiation of Rheum (Polygonaceae) and parallel evolution of morphological traits. Molecular Phylogenetics and Evolution. 2012;63:150–158. doi: 10.1016/j.ympev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Tabata (2004).Tabata H. On the Himalayan uplift and Himalayan corridors. Himalayan Journal of Sciences. 2004;2:256–257. [Google Scholar]
- Tian et al. (2011).Tian X, Luo J, Wang A, Mao K, Liu J. On the origin of the woody buckwheat Fagopyrum tibeticum (= Parapteropyrum tibeticum) in the Qinghai–Tibetan Plateau. Molecular Phylogenetics and Evolution. 2011;61:515–520. doi: 10.1016/j.ympev.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Valdiya (2002).Valdiya KS. Emergence and evolution of Himalaya: reconstructing history in the light of recent studies. Progress in Physical Geography. 2002;26:360–399. doi: 10.1191/0309133302pp342ra. [DOI] [Google Scholar]
- Van Hinsbergen et al. (2012).Van Hinsbergen DJ, Lippert PC, Dupont-Nivet G, McQuarrie N, Doubrovine PV, Spakman W, Torsvik TH. Greater India Basin hypothesis and a two-stage Cenozoic collision between India and Asia. Proceedings of the National Academy of Sciences United States of America. 2012;109:7659–7664. doi: 10.1073/pnas.1117262109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetaas (2000).Vetaas OR. The effect of environmental factors on the regeneration of Quercus semecarpifolia Sm. in Central Himalaya, Nepal. Plant Ecology. 2000;146:137–144. doi: 10.1023/A:1009860227886. [DOI] [Google Scholar]
- Wadia (1957).Wadia DN. Geology of India. Macmillan & Co; London: 1957. [Google Scholar]
- Wang (1992).Wang W. On some distribution patterns and some migration routes found in the eastern Asiatic region (Cont.) Acta Phytotaxonomica Sinica. 1992;30:97–117. [Google Scholar]
- Wang (2008).Wang FY. Phylogeography of an alpine species Primula secundiflora inferred from the chloroplast DNA sequence variation. Journal of Systematics and Evolution. 2008;46:13–22. [Google Scholar]
- Wang et al. (2009).Wang L, Abbott RJ, Zheng W, Chen P, Wang Y, Liu J. History and evolution of alpine plants endemic to the Qinghai-Tibetan Plateau: Aconitum gymnandrum (Ranunculaceae) Molecular Ecology. 2009;18:709–721. doi: 10.1111/j.1365-294X.2008.04055.x. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang GN, He XY, Miehe G, Mao KS. Phylogeography of the Qinghai–Tibet Plateau endemic alpine herb Pomatosace filicula (Primulaceae) Journal of Systematics and Evolution. 2014;52:289–302. doi: 10.1111/jse.12089. [DOI] [Google Scholar]
- Wang, Liu & Miehe (2007).Wang YJ, Liu JQ, Miehe G. Phylogenetic origins of the Himalayan endemic Dolomiaea, Diplazoptilon and Xanthopappus (Asteraceae: Cardueae) based on three DNA regions. Annals of Botany. 2007;99:311–322. doi: 10.1093/aob/mcl259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2010).Wang HA, Qiong LA, Sun KU, Lu FA, Wang Y, Song Z, Wu Q, Chen J, W Phylogeographic structure of Hippophae tibetana (Elaeagnaceae) highlights the highest microrefugia and the rapid uplift of the Qinghai-Tibetan Plateau. Molecular Ecology. 2010;19:2964–2979. doi: 10.1111/j.1365-294X.2010.04729.x. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2012).Wang L, Schneider H, Zhang XC, Xiang QP. The rise of the Himalaya enforced the diversification of SE Asian ferns by altering the monsoon regimes. BMC Plant Biology. 2012;12:210. doi: 10.1186/1471-2229-12-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Yang & Liu (2005).Wang A, Yang M, Liu J. Molecular phylogeny, recent radiation and evolution of gross morphology of the rhubarb genus Rheum (Polygonaceae) inferred from chloroplast DNA trn LF sequences. Annals of Botany. 2005;96:489–498. doi: 10.1093/aob/mci201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen et al. (2014).Wen J, Zhang J, Nie ZL, Zhong Y, Sun H. Evolutionary diversifications of plants on the Qinghai-Tibetan Plateau. Frontiers in Genetics. 2014;5:4. doi: 10.3389/fgene.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie et al. (2014).Xie H, Ash JE, Linde CC, Cunningham S, Nicotra A. Himalayan-Tibetan plateau uplift drives divergence of polyploid poppies: Meconopsis Viguier (Papaveraceae) PLOS ONE. 2014;9:e99177. doi: 10.1371/journal.pone.0099177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2008).Yang FS, Li YF, Ding X, Wang XQ. Extensive population expansion of Pedicularis longiflora (Orobanchaceae) on the Qinghai-Tibetan Plateau and its correlation with the Quaternary climate change. Molecular Ecology. 2008;17:5135–5145. doi: 10.1111/j.1365-294X.2008.03976.x. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2012).Yang FS, Qin AL, Li YF, Wang XQ. Great genetic differentiation among populations of Meconopsis integrifolia and its implication for plant speciation in the Qinghai-Tibetan Plateau. PLOS ONE. 2012;7:e37196. doi: 10.1371/journal.pone.0037196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2014).Yu XQ, Maki M, Drew BT, Paton AJ, Li HW, Zhao JL, Conran JG, Li J. Phylogeny and historical biogeography of Isodon (Lamiaceae): rapid radiation in south-west China and Miocene overland dispersal into Africa. Molecular Phylogenetics and Evolution. 2014;77:183–194. doi: 10.1016/j.ympev.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Yue et al. (2009).Yue JP, Sun H, Baum DA, Li JH, Al-Shehbaz IA, Ree R. Molecular phylogeny of Solms-laubachia (Brassicaceae) s.l. based on multiple nuclear and plastid DNA sequences, and its biogeographic implications. Journal of Systematics and Evolution. 2009;47:402–415. doi: 10.1111/j.1759-6831.2009.00041.x. [DOI] [Google Scholar]
- Yunfa et al. (2011).Yunfa M, Qingquan M, Xiaomin F, Xiaoli Y, Fuli W, Chunhui S. Origin and development of Artemisia (Asteraceae) in Asia and its implications for the uplift history of the Tibetan Plateau: a review. Quaternary International. 2011;236:3–12. doi: 10.1016/j.quaint.2010.08.014. [DOI] [Google Scholar]
- Zha, Milne & Sun (2008).Zha HG, Milne RI, Sun H. Morphological and molecular evidence of natural hybridization between two distantly related Rhododendron species from the Sino-Himalaya. Botanical Journal of the Linnean Society. 2008;156:119–129. doi: 10.1111/j.1095-8339.2007.00752.x. [DOI] [Google Scholar]
- Zhang et al. (2005).Zhang Q, Chiang TY, George M, Liu J, Abbott R. Phylogeography of the Qinghai-Tibetan Plateau endemic Juniperus przewalskii (Cupressaceae) inferred from chloroplast DNA sequence variation. Molecular Ecology. 2005;14:3513–3524. doi: 10.1111/j.1365-294X.2005.02677.x. [DOI] [PubMed] [Google Scholar]
- Zhang & Fritsch (2010).Zhang ML, Fritsch PW. Evolutionary response of Caragana (Fabaceae) to Qinghai–Tibetan Plateau uplift and Asian interior aridification. Plant Systematics and Evolution. 2010;288:191–199. doi: 10.1007/s00606-010-0324-z. [DOI] [Google Scholar]
- Zhang et al. (2012).Zhang ML, Kang Y, Zhong Y, Sanderson SC. Intense uplift of the Qinghai-Tibetan Plateau triggered rapid diversification of Phyllolobium (Leguminosae) in the Late Cenozoic. Plant Ecology & Diversity. 2012;5:491–499. doi: 10.1080/17550874.2012.727875. [DOI] [Google Scholar]
- Zhang et al. (2014).Zhang JQ, Meng SY, Allen GA, Wen J, Rao GY. Rapid radiation and dispersal out of the Qinghai-Tibetan Plateau of an alpine plant lineage Rhodiola (Crassulaceae) Molecular Phylogenetics and Evolution. 2014;77:147–158. doi: 10.1016/j.ympev.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2011).Zhang JW, Nie ZL, Wen J, Sun H. Molecular phylogeny and biogeography of three closely related genera, Soroseris, Stebbinsia, and Syncalathium (Asteraceae, Cichorieae), endemic to the Tibetan Plateau, SW China. Taxon. 2011;60:15–26. [Google Scholar]
- Zhang, Volis & Sun (2010).Zhang YH, Volis S, Sun H. Chloroplast phylogeny and phylogeography of Stellera chamaejasme on the Qinghai-Tibet Plateau and in adjacent regions. Molecular Phylogenetics and Evolution. 2010;57:1162–1172. doi: 10.1016/j.ympev.2010.08.033. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2016).Zhao JL, Xia YM, Cannon CH, Kress WJ, Li QJ. Evolutionary diversification of alpine ginger reflects the early uplift of the Himalayan–Tibetan Plateau and rapid extrusion of Indochina. Gondwana Research. 2016;32:232–241. doi: 10.1016/j.gr.2015.02.004. [DOI] [Google Scholar]
- Zhisheng et al. (2001).Zhisheng A, Kutzbach JE, Prell WL, Porter SC. Evolution of Asian monsoons and phased uplift of the Himalaya–Tibetan plateau since Late Miocene times. Nature. 2001;411:62–66. doi: 10.1038/35075035. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2013).Zhou Z, Hong D, Niu Y, Li G, Nie Z, Wen J, Sun H. Phylogenetic and biogeographic analyses of the Sino-Himalayan endemic genus Cyananthus (Campanulaceae) and implications for the evolution of its sexual system. Molecular Phylogenetics and Evolution. 2013;68:482–497. doi: 10.1016/j.ympev.2013.04.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
Since this is a Literature Review, the research in this article did not generate any data or code. All the references have been cited in the text itself.