Abstract
Objective(s)
MTN-020/ASPIRE and IPM-027/Ring Study recently proved the dapivirine vaginal ring was safe and effective with consistent use. To optimize the ring’s impact, the barriers and facilitators to ring adherence must be understood and addressed.
Methods
Former ASPIRE participants were stratified by age group (18–21; 22–45) and randomly selected at seven sites in Malawi, South Africa, Uganda and Zimbabwe, 12–17 months after trial exit. Using in-depth interviews or focus group discussions, ring use barriers were explored using structured guides and visual tools including individual-level depictions of dapivirine levels detected in plasma and returned rings.
Results
187 were enrolled; 37% were 18–21 when they began ASPIRE. Most (75%) had drug-level results suggesting inconsistent ring use throughout ASPIRE. Participants viewed themselves as adherent, while simultaneously describing regular instances and reasons for ring removal (e.g. for sex or menses). Less adherent women reported fears that partners would oppose the ring or feel it during sex. High adherers expressed altruistic motivations for ring use. Women of all ages attributed young women’s non-adherence to their tendency to be less “serious” about the future, HIV prevention and the study; motivated predominantly by benefits; more fearful of fertility-related consequences; and to having less relationship control.
Conclusions
When presented with objective adherence data, participants provided reasons for intermittent ring use, while simultaneously portraying themselves as consistent ring users. Further research is needed to understand how women could use the ring in a way that fits into the context of their relationships and their lives while still conferring adequate HIV prophylaxis.
INTRODUCTION
The dapivirine vaginal ring, a candidate for HIV prevention, was proven safe and effective in two recent clinical trials sponsored by the International Partnership for Microbicides (IPM) and the Microbicide Trials Network (MTN).[1, 2] Open-label studies to establish ongoing safety and adherence are underway. Existing contraceptive research and emerging findings from end-user research in HIV prevention have illustrated women’s need for method choice.[3–5] Although oral pre-exposure prophylaxis (PrEP) offers a safe, effective, and critical means for women to protect themselves from HIV, the daily use regimen can pose adherence and other challenges.[6–11] The ring provides sustained release of dapivirine over a 30 day period thereby presenting women with an alternative to daily oral PrEP.
Adolescent girls and young women are key populations at risk of HIV acquisition, and a priority group for research and programmatic efforts.[12] In the MTN-020 (ASPIRE) trial, the ring did not prevent HIV acquisition amongst less adherent women aged 18–21.[1, 13] This was also found true of any population with low adherence.[1, 13] Understanding and addressing reasons for non-use and motivators for use is critical to the overall success of the ring research agenda. We aimed to understand former ASPIRE trial participants’ adherence behaviors by presenting them with their individual residual drug levels and discussion of actual use.
METHODS
The MTN-020/ASPIRE trial, a phase III, randomized, double-blind, placebo-controlled trial with a nested qualitative component,[14] was conducted between August 2012 and June 2015; 2629 women participated at 15 sites in Malawi, South Africa, Uganda and Zimbabwe.[1, 15]
MTN-032/AHA study is an exploratory sub-study of the ASPIRE trial in which 187 former ASPIRE participants were randomly selected at seven of the 15 sites in Malawi, South Africa, Uganda and Zimbabwe to discuss their objective adherence data derived from dapivirine levels measured in plasma and returned rings. This paper includes data collected from June 2016 through October 2016: 12–17 months after ASPIRE trial exit.
Randomly pre-selected AHA participants were recruited from those who had re-contact permission, been on active product in ASPIRE, and had at least three quarterly visits, or at least one visit if seroconversion occurred. Young women were intentionally over-sampled in both in-depth interviews (IDI) and focus group discussions (FGD) because they were less adherent and not as well protected by the ring in ASPIRE.[1] FGD were homogenous by age group at time of ASPIRE enrollment (18–21, 22–45).
Measures and Procedures
Challenges and facilitators to adherence were explored using structured guides and two visual tools, designed and pre-tested in collaboration with study management and site teams.
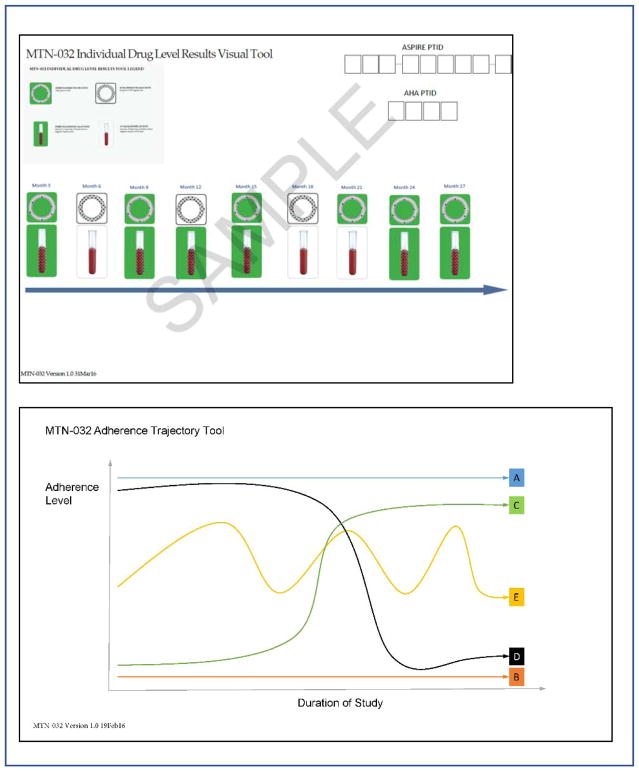
The “individual-level” tool depicted plasma levels and returned ring adherence data for quarterly visits with available data (Figure 2a). Images were color-coded as green and described as “ring appears used” when plasma dapivirine >95pg/mL or returned ring dapivirine was <22mg; or color-coded as white and described as “ring does NOT appear used” when plasma was <95pg/mL and returned ring was >22mg. The “trajectory tool” depicted five sample trajectories of ring adherence over time (Figure 2b). Prior to receiving individual adherence results, IDI participants were asked to indicate their projected ring use during ASPIRE. During FGD, participants discussed the trajectory tool in relation to perceptions of ring use in ASPIRE.
Figure 2.
Data collection Tools used to discuss adherence
Following informed consent, interviewers administered demographic and sexual behavior questionnaires and presented drug adherence results individually prior to the FGD or during the IDI. Afterwards, interviewers documented participants’ emotional reaction(s) from a list of options, with an option for other responses.[16] Participants were subsequently probed on whether the results made sense, how they felt about the results, and whether the results agreed with how they remember using the ring. To frame the discussion around adherence, interviewers read the following introduction to IDI participants: “While you were in the study, you were counseled/advised by study staff to use the ring for the full month and then to replace with a new ring every month. As you may remember, we took samples of your blood during some of the clinic visits. Looking at your blood tests, here are the results showing how often there was drug detected in your system” and the following for FGD participants: “The blood samples and returned rings showed that some participants did not use their rings or have drug in their blood all or most of the time. We would like to find out from you, since you participated in the trial, why this may have been.” Additional questions in the guides explored how well participants thought they used the ring (defined as “for the full month, every month”), and about circumstances where the ring was intentionally removed or fell out involuntarily.
IDI and FGD were led by trained social scientists in English or the local African language. All interview guides were translated into African languages and back-translated by a second, blinded translator into English to verify accuracy. Interviews were audio-recorded and later translated and transcribed into English.
Analysis
Coding of transcribed interviews was conducted in Dedoose software[17] by a team of analysts. Inter-coder consistency was confirmed for 10% of transcripts with a mean kappa score of 0.77[17, 18]. The analysis team met weekly to discuss coding questions, emerging themes, and to reach consensus on coding issues and interpretation of findings. Demographic and behavioral data were captured on case report forms (CRFs) and summarized in SAS®[19].
The proportion of plasma and/or adherence measurements classified as “adherent” was computed for each participant, and based on the distribution for this sample, four adherence groups were defined: low (0–60% adherent); middle-low (61–80% adherent); middle-high (81–99% adherent); and high (100% adherent). Analysis was stratified by adherence group, interview mode and age group. Code reports were summarized into memos and analyzed for content related to reasons for removal of or adherence to the ring, patterns of use and explanation of differences in adherence between age groups. The study was approved by the Institutional Review Boards at RTI International and each study site, and was overseen by the regulatory infrastructure of the U.S. National Institutes of Health and the MTN.
RESULTS
Study Sample
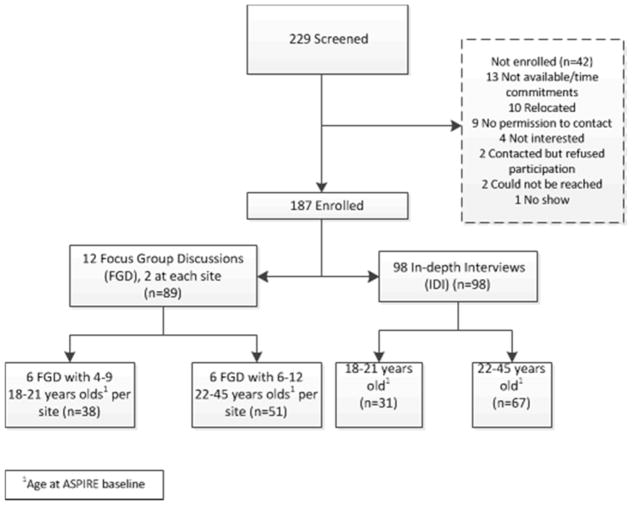
Across the seven research sites, 229 women were screened and 187 enrolled (Figure 1). Ninety-eight women had single IDIs; 89 participated in one of 12 FGDs. Characteristics of the study sample are presented in aggregate, and by adherence and age groups in Table 1. Participants averaged 27 years of age, 62% were unmarried and just under half (48%) had completed secondary school. The majority (88%) of the older age group were with the same partner as when they exited ASPIRE, compared to 61% of the younger age group. Forty-six women (25%) were classified as high-adherers, suggesting consistent use at every visit measured, and 46 (25%) were classified as low-adherers. The rest of the participants were classified as either middle-low (n=39, 21%) or middle-high (n=56, 29%) adherers (Table 1). More participants in the highest two adherence groups were in the lowest socio-economic status bracket compared to lower adherers. More than half (57%) reported their primary partners were HIV-negative, although many (37%) didn’t know their partner’s status, or other possible sex partners (58%).
Figure 1.
Participant disposition
Table 1.
Participant characteristics
| All sites (n=187) | By Cumulative Adherence | By MTN020 Age | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Low (n=46) | Middle-Low (n=39) | Middle-High (n=56) | High (n=46) | 18–21 (n=69) | 22–45 (n=118) | ||
| Country of enrollment | |||||||
| South Africa | 93 (50%) | 25 (54%) | 27 (69%) | 22 (39%) | 19 (41%) | 44 (47%) | 49 (53%) |
| Zimbabwe | 31 (17%) | 8 (17%) | 4 (10%) | 14 (25%) | 5 (11%) | 7 (23%) | 24 (77%) |
| Uganda | 29 (16%) | 8 (17%) | 4 (10%) | 11 (20%) | 6 (13%) | 5 (17%) | 24 (83%) |
| Malawi | 34 (18%) | 5 (11%) | 4 (10%) | 9 (16%) | 16 (35%) | 13 (38%) | 21 (62%) |
| Age at time of 032 interview, years1 | 27 (28, 19–48) | 26 (28, 20–48) | 27 (28, 20–43) | 27 (28, 19–42) | 27 (28, 21–44) | 23 (23, 19–26) | 30 (31, 24–48) |
| Age at time of 020 baseline1 | 24 (25, 18–44) | 23 (25, 18–44) | 24 (25, 18–40) | 24 (25, 18–40) | 24 (25, 18–42) | 20 (20, 18–21) | 27 (28, 22–44) |
| 18–21 | 69 (37%) | 17 (37%) | 15 (38%) | 20 (36%) | 17 (37%) | 69 (100%) | - |
| 22–45 | 118 (63%) | 29 (63%) | 24 (62%) | 36 (64%) | 29 (63%) | - | 118 (100%) |
| Completed secondary school or more | 48% | 25 (54%) | 24 (62%) | 30 (54%) | 11 (24%) | 37 (54%) | 53 (45%) |
| Earns own income | 109 (58%) | 26 (57%) | 23 (59%) | 35 (63%) | 25 (54%) | 37 (54%) | 72 (61%) |
| SES Score2 | |||||||
| low (lowest 40%) | 75 (40%) | 13 (28%) | 9 (23%) | 32 (57%) | 21 (46%) | 23 (33%) | 52 (44%) |
| middle | 66 (35%) | 19 (41%) | 19 (49%) | 11 (20%) | 17 (37%) | 25 (36%) | 41 (35%) |
| high (highest 20%) | 46 (25%) | 14 (30%) | 11 (28%) | 13 (23%) | 8 (17%) | 21 (30%) | 25 (21%) |
| Marital Status | |||||||
| Legally | 9 (5%) | 3 (7%) | 1 (3%) | 1 (2%) | 4 (10%) | 1 (2%) | 8 (7%) |
| Traditionally | 56 (33%) | 15 (36%) | 9 (24%) | 20 (38%) | 12 (30%) | 15 (25%) | 41 (37%) |
| Unmarried | 107 (62%) | 24 (57%) | 27 (73%) | 32 (60%) | 24 (60%) | 45 (74%) | 62 (56%) |
| Same partner as when exited ASPIRE | 135 (78%) | 34 (81%) | 28 (76%) | 39 (74%) | 34 (85%) | 37 (61%) | 98 (88%) |
| Currently living with partner | 91 (53%) | 25 (60%) | 14 (38%) | 28 (53%) | 24 (60%) | 20 (33%) | 71 (64%) |
| Primary sex partner has other sex partners | 32 (19%) | 10 (24%) | 4 (11%) | 15 (28%) | 3 (8%) | 8 (13%) | 24 (22%) |
| Don’t know | 99 (58%) | 27 (64%) | 25 (68%) | 26 (49%) | 21 (53%) | 39 (64%) | 60 (54%) |
| Primary partner’s HIV status | |||||||
| HIV positive | 10 (6%) | 3 (7%) | 3 (8%) | 1 (2%) | 3 (8%) | 3 (5%) | 7 (6%) |
| HIV negative | 98 (57%) | 29 (69%) | 18 (49%) | 29 (55%) | 22 (55%) | 39 (64%) | 59 (53%) |
| Participant does not know | 64 (37%) | 10 (24%) | 16 (43%) | 23 (43%) | 15 (38%) | 19 (31%) | 45 (41%) |
median (mean, min-max)
An SES indicator variable was created using principal component analysis (PCA) of 10 demographic assets from the demographic case report form including: home ownership; number of rooms in household; household assets of electricity, radio, television, mobile telephone, non-mobile telephone, refrigerator; toilet facilities; and drinking water sources[32]. A tri-level categorical variable (lowest 40%, middle 40%, and highest 20%) was created based on the first eigenvalue and the SAS-generated PRIN1 score[33].
Perceptions of adherence
When presented with the trajectory tool in IDIs, most (80%) identified high levels of adherence (line A) for the trial duration, or increasingly high levels after the first few months (line C). Only two participants chose low use throughout the study (line B), and a small number of middle-low and low-adherers (n=12) chose inconsistent use (line E). In FGD women discussed the trajectory tool, and among those who spoke about their own use, most said they were best represented by lines A or C. In several FGD participants expressed the belief that most others had inconsistent use (line E).
Individual-level tools (figure 2a) of objective drug levels depicted adherence that varied widely across visits. The two most commonly documented reactions to results were happiness and acceptance for the high, middle-high and middle-low adherence groups and embarrassment/discomfort and acceptance for the low adherence group.
Reasons for non-adherence
Non-adherence reported by participants included removing the ring briefly for sex or bathing, multi-day removals for menses, or multi-week removals with reinsertion one to three days before the clinic visit. Reasons for removing the ring, and frequency thereof, are in Table 2, stratified by age and adherence group. Older women often complained of side effects from ring use, such a vaginal discharge, womb pain, vaginal itching, and headaches. They articulated fears of potential long term effects of ring use, such as cancer and vaginal stretching. Participant concerns were informed or exacerbated by research- or ring-related rumors spread by community members, peers and family members. With the notable exception of the high adherers, all participants, irrespective of age, cited partner opposition to the ring or fear of partner opposition, particularly during sex, as a dominant reason for non-adherence.
Table 2.
Most Frequently Reasons for Ring Removals and Non-adherence1, by Age Group and Drug Level Adherence Grouping
| Younger Women (18–21; n=69) | Older Women(22–45; n=118) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| IDI | IDI | |||||||||
|
|
|
|||||||||
| Adherence group | Low | Middle –low | Middle-high | High | FGD2 | Low | Middle-Low | Middle-High | High | FGD2 |
|
|
||||||||||
| n | 7 | 8 | 6 | 10 | 38 | 22 | 15 | 18 | 13 | 51 |
| Health symptom-related | ||||||||||
|
| ||||||||||
| Health problem attributed to ring | x | x* | x | x | x | x | x* | |||
|
| ||||||||||
| Concern ring will cause side effects/other diseases (e.g. cancer) | x | x | x | x | x* | x | x* | x | ||
|
| ||||||||||
| Partner & Sex-Related | ||||||||||
|
| ||||||||||
| Partner Opposition | x | x | x | x | x | x | ||||
|
| ||||||||||
| Fear of partner opposition/feeling ring during sex | x | x* | x | x | x* | x* | x* | x | ||
|
| ||||||||||
| Partner removed ring | x | x | x | x | x | |||||
|
| ||||||||||
| Pain/discomfort during sex (her and/or her partner) | x | x* | x | |||||||
| Rumor/beliefs-related | ||||||||||
|
| ||||||||||
| Concerns about rumors spread by community/peers/family | x | x | x* | x* | ||||||
| Believes ring was placebo/not effective | x | x* | x | |||||||
| Menses-related | ||||||||||
|
| ||||||||||
| Thinks ring blocks menstrual flow | x | x | x | x | x | |||||
| Removed to clean during menses | x | x | x | x | ||||||
| Removed during menses | x | x | x | x* | x | x | ||||
| Hygiene-related | ||||||||||
|
| ||||||||||
| To clean ring while bathing/concern about ring being dirty/smelly | x | x | x | x | x | |||||
| Removes ring to clean vagina/bathe | x | x | x | x | x | x | ||||
| External influence-related | ||||||||||
|
| ||||||||||
| Opposition from peers/family | x | x | x | x* | ||||||
| Motivation-related | ||||||||||
|
| ||||||||||
| Motivated by money/benefits, not ring | x | x | x | x | x | |||||
X = reported by 1–3; X*=reported by more than 3 participants
FGD had women from mixed adherence group
Reasons for ring removal during menses were to clean the ring and themselves; concerns that the ring would block the flow of menstrual blood, and menstrual pain that was attributed to the ring. Doubt about the efficacy of the ring also contributed to non-use. Women of all ages suggested that the ring’s unknown efficacy may have contributed to non-adherence with an intention to use the “proven” and active ring more consistently in the subsequent open-label study (MTN 025/HOPE). Additional reasons for nonadherence (Table 2) related to hygiene concerns, external-influence from peers and family members and sole interest in study benefits.
Others’ non-adherence
Women commented on other ASPIRE participants’s adherence patterns and portrayed them as lacking knowledge about the trial and being disinterested in its outcome and motivated solely by reimbursement.
Participants also suggested others may have removed the ring because they wanted to get pregnant or use other vaginal products to prepare for sex. Additionally, women described a tendency amongst other women of removing the ring after their clinic visit and re-inserting it prior to their subsequent clinic visit – a pattern of behavior participant’s rarely acknowledged in reference to their own use.
Finally, participants suggested younger women were less “serious” about their futures, more concerned about pregnancy than HIV prevention, and interested in the monetary rewards of participation rather than the public health impact of their participation. They were characterized as being only interested in ‘partying’ and unconcerned about the consequences of their actions. However, participants acknowledged that young women had less confidence and control in their relationships with men.
A young Malawian woman associated other young women’s naivety and impressionability with their low adherence:
Because their reasoning is still poor and so they will not use the ring as they are supposed to because of their immaturity … their peers can easily discourage them… It is harder for an older person to get discouraged. (FGD, Lilongwe, Malawi, ASPIRE age group 18–21)
Motivators of adherence
In interviews, participants across all adherence groups discussed what motivated them to use the ring. Women in high adherence groups more frequently reported motivation by their contribution to science, including ring success and helping other women to be protected from HIV. In the following statement, a participant expresses the social importance of her adherence:
I think knowing that like in the future I am going to empower other young ladies like me not to be exposed to the HIV virus. That was my goal and it was at the back of my mind. (IDI, Johannesburg, South Africa, High adherence group, ASPIRE age group 18–21)
Another participant from Uganda expresses a similar sentiment:
I care for our young sisters and also my fellow women and that’s why I cared while in this research and hope to get good news from this study that will help us (women). (IDI, Kampala, Uganda, High adherence group, ASPIRE age group 22–45)
Additionally, women described personal and internalized ways in which their own sense of self-worth and belief in the protective effect of the ring motivated them:
While I was using the ring, I was very proud of myself I use to pride myself in my ring use. I told myself that this ring that I am using, is protecting me. (IDI, Durban, South Africa, Middle-high adherence group, ASPIRE age group 22–45.)
Encouragement and positive feedback from others helped participants adhere. The clinical trial waiting rooms and study-orchestrated participant engagement activities, such as social events and tea parties, provided a forum for exchanging ring use experiences and having access to staff in a less structured setting. Hearing about their peers’ positive experiences with the ring diluted women’s initial reluctance and led to more consistent use. Describing the support she had from her peers, one woman commented:
I never had any fear because I would get confidence from my friends who I often asked (about) their experience and they would confirm that they haven’t had any problem; in a way it would make you strong. (IDI, Kampala, Uganda, Middle-high adherence group, ASPIRE age group 22–45)
Many participants described adherence that was motivated by addressing or experiencing the converse of the commonly listed reasons for non-adherence. For example, several participants described adherence that was motivated by having disclosed ring use to partners and by not feeling or being disturbed by the ring during sex. Similarly, descriptions of non-experience of side effects, feeling protected, and regular HIV testing were expressed as motivators to consistent use.
DISCUSSION
We aimed to elicit candid accounts of participants’ non-adherence behavior by discussing ring use trajectories during their time in the ASPIRE trial and sharing their individual drug level results from plasma and returned rings. Through the use of these tools and in-depth discussion, three important insights about ring use emerged:
First, there are fundamental differences between how “consistent use” and “adherence” were conceptualized by the researchers and the participants. It is clear from the drug level adherence data and interviews that many participants were using the ring intermittently throughout study participation. Nevertheless, participants generally described themselves as consistent ring users. Similar to previous microbicide and ring studies, the most common reasons for non-use related to non-disclosure to male partners, hygiene and menstrual concerns, negative community rumors, and attribution of side effects to the ring.[14, 20–22] Thus, while generally perceiving themselves as adherent, and consistently so after a learning curve period, participants simultaneously described a set of salient challenges that were regular, episodic (e.g. use of the ring during sex and menses), or pervasive (e.g. peer influence and community rumors) reasons for non-use that corroborated the use patterns observed in the residual drug level data. The apparent discrepancy between residual drug data and participant narratives in defining “adherent” can be understood as neither a contradiction nor a deliberate misrepresentation, but rather as a complementary interpretation: participants generally felt they used the ring consistently once they got used to it, and they may have removed it regularly. Innovative approaches to adherence measurement – both novel biomarker techniques[23] and less rigid or binary interview questions - may help researchers elucidate a richer understanding of how ring use was actually – and often intermittently – managed.
It has been noted in previous trials that women used other HIV prevention products in modified ways. In the VOICE trial, women sometimes took oral tablets several times a week rather than daily, or inserted only a little vaginal gel instead of full doses, because they felt that fully adhering to the proposed regimen might cause bodily harm.[22, 24] Additional research is needed to better understand how women use the ring, and further pharmacokinetic research is needed to better characterize the protection offered by different patterns of actual usage. Oral PrEP, which has been shown to be effective with intermittent use for men who have sex with men,[25] but less effective with intermittent use for women,[26] offers a valuable case study in reacting to and researching desired use patterns.
The most commonly cited reasons for removal offer important insight into what the ring meant to women in this setting. As a novel foreign object that gets inserted into their vaginas – the epicenter of reproduction and fertility – the ring was both literally and figuratively located in an important and delicate position.[27] The most common reasons for removal suggest that maintenance of sexual relationships and healthy well-being was more important than following study-prescribed ring-use guidelines, resulting in adaptations to consistent ring use. Participants preserved their sense of sexuality and health by removing the ring during sex, thereby minimizing any actual or feared physical or psychological disturbance of the ring to: a) their relationship or b) the pleasure of themselves or their partner.[28] They removed the ring during menses to allow for a natural and “unblocked” flow, while also cleaning themselves and the ring.[29] Participants and other community members were sometimes wary of how the ring might threaten their health or ability to have children. Future messaging and promotion of ring use must be mindful that the ring, while designed to deliver antiretroviral drug and prevent disease, can be perceived as a threat to women’s socio-cultural needs to embody reproductive healthiness.
Finally, our stratified analysis of adherence groups revealed nuanced differences in how women with consistently high drug levels spoke about ring use. Irrespective of age group, none of the “high adhering” women reported fears of partner opposition, nor fears that their male partner would feel the ring during sex. These women’s inherent character (e.g. higher self-efficacy), their circumstances, or their relationships may have differed from other women in these communities in ways that could be informative to future ring promotion. While the focus of inquiry on this study was reasons for non-adherence, it is equally important to understand motivators for using the ring. Women with consistently high residual drug levels expressed a greater sense of altruism and pride in their personal contribution to science than less adherent women. Stadler et. al. have noted that women have used clinical trials to express moral subjectivities, and show themselves to be good trial participants and societal members.[30]
We did not unpack altruistic statements to explore whether women gained additional benefits, e.g. individual, societal, from their participation. Of note, our data suggest that young women, who were not protected in ASPIRE and were less adherent, were perceived by others to lack an appreciation for the importance of the research – the larger good – and to hold only selfish considerations of study benefits. Young women were depicted by both older and younger women, as more self-interested, with less confidence in their relationships. By contrast, consistently adherent women in this analysis (some of whom were young) were generally less concerned with the direct effects of their behavior on their partners, and more concerned with the ethical implications of their actions in HIV prevention; a concept previously called ‘ethical intercorporeality’ when describing men’s motivations for participating in a HPV study in Mexico.[31] Participating in a trial is thus a tangible demonstration of ones’ commitment to the broader society.
There are limitations the reader should consider when interpreting these study results. Interviews were conducted post-trial, and data were subject to recall bias. We provided visual tools of participants’ adherence record throughout the study; nevertheless, participants may not have accurately remembered their behavior. Secondly, participants may have felt compelled to provide socially desirable responses about their ring use. While the residual drug feedback is subject to error, misreporting of adherence is a well-documented challenge in HIV prevention trials; thus, we do not expect laboratory error to explain every discrepancy between biomarker and self-reported measures of adherence. Further, due to the timing and design of this study, we cannot assess causality between the reasons provided and adherence biomarkers. Finally, our interviews focused disproportionately on barriers to ring use; participants were not systematically asked about facilitators. Much of our data pertaining to motivators of adherence were extracted from indirect narratives of barriers to use, rather than direct enquiry and exploration of motivators.
In conclusion, when presented with objective individual-level adherence data, participants provided a variety of explanations for ring non-adherence during ASPIRE. Non-disclosure and disapproval of ring use by male partners, hygiene-related worries (especially related to menses) and adverse health concerns were salient reasons for ring removals and non-adherence among almost all adherence and age subgroups, suggesting the need for pre-emptive counseling and messaging in future ring studies/activities, and attention towards involving male partners or designing messages geared towards men. High adherers spoke of a broader sense of altruistic commitment to science and a desire to identify a solution to mitigate HIV in their communities – a message that could be used to motivate future ring users. Despite plasma and ring data demonstrating variable use, most women believed themselves to be adherent or mostly adherent to the ring. While some of this discrepancy may be indicative of a social desirability bias, further research is needed to better understand how women envision the ring working in their bodies, and their personal judgments about how and when to use the ring. Ultimately, we aim to identify a “sweet spot” whereby women could use the ring such that it fits into the context of their relationships and their lives, while also being aligned with the optimal pharmacokinetic profile of the ring to prevent HIV acquisition.
Acknowledgments
Funding: The MTN-020/ASPIRE study was designed and implemented by the Microbicide Trials Network (MTN). The MTN is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The vaginal rings used in this study were supplied by the International Partnership for Microbicides (IPM).
Footnotes
Role of authors:
ETM, JS, SN were involved with study design, study implementation, data interpretation, and manuscript writing. MG, AWK and LST were involved with study implementation and review of the manuscript. NL and AWK oversaw data coding and analysis and were involved with writing of the manuscript and data interpretation. KR, LEM, JE, CZ and ME were involved with data collection, data interpretation, and manuscript review
References
- 1.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. The New England journal of medicine. 2016 doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nel A, van Niekerk N, Kapiga S, Bekker L-G, Gama C, Gill K, et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. New England Journal of Medicine. 2016;375(22):2133–2143. doi: 10.1056/NEJMoa1602046. [DOI] [PubMed] [Google Scholar]
- 3.Gollub EL. Choice Is Empowering: Getting Strategic About Preventing HIV Infection in Women. International Family Planning Perspectives. 2006;32(4):209–212. doi: 10.1363/3220906. [DOI] [PubMed] [Google Scholar]
- 4.van der Straten A, Cheng H, Agot K, Ahmed K, Weinrib R, Manenzhe K, et al. Preference and choice of multipurpose prevention technologies (MPT) in young African women. Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2017. [Google Scholar]
- 5.Nel AM, Mitchnick LB, Risha P, Muungo LT, Norick PM. Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among African women. J Womens Health. 2011;20(8):1207–1214. doi: 10.1089/jwh.2010.2476. [DOI] [PubMed] [Google Scholar]
- 6.Corneli A, Perry B, McKenna K, Agot K, Ahmed K, Taylor J, et al. Participants’ Explanations for Nonadherence in the FEM-PrEP Clinical Trial. J Acquir Immune Defic Syndr. 2016;71(4):452–461. doi: 10.1097/QAI.0000000000000880. [DOI] [PubMed] [Google Scholar]
- 7.Corneli A, Perry B, Agot K, Ahmed K, Malamatsho F, Van Damme L. Facilitators of adherence to the study pill in the FEM-PrEP clinical trial. PLoS One. 2015;10(4):e0125458. doi: 10.1371/journal.pone.0125458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. New England Journal of Medicine. 2012:1–12. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeten JM, Grant R. Use of Antiretrovirals for HIV Prevention: What Do We Know and What Don’t We Know? Current HIV/AIDS Reports. 2013:1–10. doi: 10.1007/s11904-013-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. The Lancet Infectious Diseases. 2014;14(9):820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UNAIDS. Fast-Track: ending the AIDS epidemic by 2030. Geneva: UNAIDS; 2014. [Google Scholar]
- 13.Brown E, Palanee-Philips T, Marzinke M, Hendrix C, Dezutti C, Soto-Torres L, et al. Residual dapivirine ring levels indicate higher adherence to vaginal ring is associated with HIV-1 protection. IAS; Durban: 2016. [Google Scholar]
- 14.Montgomery ET, van der Straten A, Chitukuta M, Reddy K, Woeber K, Atujuna M, et al. Acceptability and use of a dapivirine vaginal ring in a phase III trial. AIDS. 2017;31(8):1159–1167. doi: 10.1097/QAD.0000000000001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palanee-Phillips T, Schwartz K, Brown ER, Govender V, Mgodi N, Kiweewa FM, et al. Characteristics of Women Enrolled into a Randomized Clinical Trial of Dapivirine Vaginal Ring for HIV-1 Prevention. PloS one. 2015;10(6):e0128857. doi: 10.1371/journal.pone.0128857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckman P. Basic Emotions. In: Dalgleish T, Power M, editors. Handbook of Cognition and Emotion. Sussex, UK: John Wiley & Sons Ltd; 1999. [Google Scholar]
- 17.Dedoose Version 7.6.15. web application for managing, analyzing, and presenting qualitative and mixed method research data. Los Angeles, CA: SocioCultural Research Consultants, LLC; 2017. ( www.dedoose.com) [Google Scholar]
- 18.Flowers SC. Perceptions of Fidelity and Adaptation in Evidence-Informed Interventions by Women of Color Sexuality Health Educators. CUNY Academic Works. 2016 http://academicworkscunyedu/gc_etds/1586.
- 19.SAS® Institute Inc. Version 9.0 for Windows. Cary, NC, USA: 2017. [Google Scholar]
- 20.Montgomery ET, van der Straten A, Cheng H, Wegner L, Masenga G, von Mollendorf C, et al. Vaginal ring adherence in sub-Saharan Africa: expulsion, removal, and perfect use. AIDS and behavior. 2012;7:1787–1798. doi: 10.1007/s10461-012-0248-4. [DOI] [PubMed] [Google Scholar]
- 21.van der Straten A, Montgomery E, Cheng H, Wegner L, Masenga G, von Mollendorf C, et al. High Acceptability of a Vaginal Ring Intended as a Microbicide Delivery Method for HIV Prevention in African Women. AIDS and behavior. 2012:1–12. doi: 10.1007/s10461-012-0215-0. [DOI] [PubMed] [Google Scholar]
- 22.van der Straten A, Montgomery ET, Musara P, Etima J, Naidoo S, Laborde N, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS. 2015;29(16):2161–2171. doi: 10.1097/QAD.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stalter RM, Moench TR, MacQueen KM, Tolley EE, Owen DH. Biomarkers and biometric measures of adherence to use of ARV-based vaginal rings. J Int AIDS Soc. 2016;19(1):20746. doi: 10.7448/IAS.19.1.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery ET, Mensch B, Musara P, Hartmann M, Woeber K, Etima J, et al. Misreporting of Product Adherence in the MTN-003/VOICE Trial for HIV Prevention in Africa: Participants’ Explanations for Dishonesty. AIDS and behavior. 2017;21(2):481–491. doi: 10.1007/s10461-016-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. The New England journal of medicine. 2015;373(23):2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 26.Bekker LG, Roux S, Sebastien E, Yola N, Amico KR, Hughes JP, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open-label, phase 2 trial. The lancet HIV. 2017 doi: 10.1016/S2352-3018(17)30156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilber; AM, Kenter E, Redmond S, Merten S, Bagnol B, Low N, et al. Vaginal practices as women’s agency in Sub-Saharan Africa: A synthesis of meaning and motivation through meta-ethnography. Social Science & Medicine. 2012;74(9):1311–1323. doi: 10.1016/j.socscimed.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Laborde ND, Pleasants E, Reddy K, Atujuna M, Nakyanzi T, Chitukuta M, et al. Impact of the Dapivirine Vaginal Ring on Sexual Experiences and Intimate Partnerships of Women in an HIV Prevention Clinical Trial: Managing Ring Detection and Hot Sex. AIDS and behavior. 2017 doi: 10.1007/s10461-017-1977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saethre E, Stadler J. Negotiating Pharmaceutical Uncertainty: Women’s Agency in a South African HIV Prevention Trial. Nashville: Vanderbilty University Press; 2017. [Google Scholar]
- 30.Stadler J, Scorgie F, van der Straten A, Saethre E. Adherence and the Lie in a HIV Prevention Clinical Trial. Med Anthropol. 2015:1–14. doi: 10.1080/01459740.2015.1116528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wentzell E. Medical Research Participation as “Ethical Intercorporeality”: Caring for Bio-Social Bodies in a Mexican Human Papillomavirus (HPV) Study. Medical anthropology quarterly. 2017;31(1):115–132. doi: 10.1111/maq.12326. [DOI] [PubMed] [Google Scholar]
- 32.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health policy and planning. 2006;21(6):459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 33.Luecke EH, Cheng H, Woeber K, Nakyanzi T, Mudekunye-Mahaka IC, van der Straten A, et al. Stated product formulation preferences for HIV pre-exposure prophylaxis among women in the VOICE-D (MTN-003D) study. J Int AIDS Soc. 2016;19(1):20875. doi: 10.7448/IAS.19.1.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]