Abstract
Mouse urinary behavior is quantifiable and is used to pinpoint mechanisms of voiding dysfunction and evaluate potential human therapies. Approaches to evaluate mouse urinary function vary widely among laboratories, however, complicating cross-study comparisons. Here, we describe development and multi-institutional validation of a new tool for objective, consistent, and rapid analysis of mouse void spot assay (VSA) data. Void Whizzard is a freely available software plugin for FIJI (a distribution of ImageJ) that facilitates VSA image batch processing and data extraction. We describe its features, demonstrate them by evaluating how specific VSA method parameters influence voiding behavior, and establish Void Whizzard as an expedited method for VSA analysis. This study includes control and obese diabetic mice as models of urinary dysfunction to increase rigor and ensure relevance across distinct voiding patterns. In particular, we show that Void Whizzard is an effective tool for quantifying nonconcentric overlapping void spots, which commonly confound analyses. We also show that mouse genetics are consistently more influential than assay design parameters when it comes to VSA outcomes. None of the following procedural modifications to reduce overlapping spots masked these genetic-related differences: reduction of VSA testing duration, water access during the assay period, placement of a wire mesh cage bottom on top of or elevated over the filter paper, treatment of mesh with a hydrophobic spray, and size of wire mesh opening. The Void Whizzard software and rigorous validation of VSA methodological parameters described here advance the goal of standardizing mouse urinary phenotyping for comprehensive urinary phenome analyses.
Keywords: diabetic mice, free open-source software, urinary dysfunction, void spot assay, voiding behavior
INTRODUCTION
A majority of older adults experience lower urinary tract symptoms (LUTS), which may include increased voiding frequency (both day and night), incomplete bladder emptying, urgency, weak stream, post-void dribble, and urinary incontinence. LUTS are costly to manage, reduce quality of life, and associate with depression, sexual dysfunction, and sleep disturbance (2, 33–35). New research is needed to identify LUTS underpinnings and develop new and effective therapies.
Laboratory mice are increasingly used as LUTS research models. Mice are highly tractable, and a vast offering of strains enables definitive identification of genes and signaling networks involved in urinary function and dysfunction. However, because patient-reported symptoms underlie human LUTS diagnoses, a formidable challenge of using mice for human LUTS research is to accurately phenotype mouse urinary physiology and understand how it relates to human voiding function.
The void spot assay (VSA; also known as the void spotting assay and voiding spot on paper assay) has been used for decades to phenotype mouse voiding behavior (9, 11, 13, 22–26) but until recently has not been rigorously characterized or validated. The environment in which mice are housed substantially impacts their voiding behaviors (1, 6, 14), but it is unclear which, if any, VSA procedural parameters influence voiding. We and others are seeking to examine the impact of major VSA assay parameters, such single or group housing, shape of the cage in which VSA is performed, age of mice, breeding behaviors, and others (5, 7, 16, 43), with the long-term goal of establishing mouse urinary function as a quantifiable trait for phenotypic analyses.
There are many reasons why the VSA should be adopted as one of the standard methods for mouse urinary phenotyping. It is inexpensive, does not require specialized equipment, can be conducted multiple times on the same mouse, and does not require introduction of instruments into the body (it is noninvasive). To advance VSA testing, it is necessary to overcome several limitations. There is no standardized VSA protocol, making comparisons across studies tenuous. There are also analytical challenges. Urine spots often overlap, and there is no consistent method for quantifying overlapping spot areas. It is also unclear whether the diversity of urinary phenotypes presented by mice can be accurately quantified using a single standard assay, whether results can be compared across laboratories, and whether behavioral responses to the assay environment overshadow baseline voiding function.
All previous VSA procedural optimization studies were performed on genetically normal mice with the assumption that results are generalizable to other mouse strains. This study includes obese diabetic and control mice to address the specific technical and analytical challenge of overlapping urine spots. Obesity and diabetes are human risk factors for LUTS (10, 18–20, 31, 45) and increase urine production (polyuria) and frequency (pollakiuria) in mice and humans. These diabetic urinary sequelae coupled with inactivity make overlapping urine spots especially common in VSA testing. Glucosuria is also a problem in obese diabetic mice as it has been postulated to cause mice to chew and damage VSA papers. Here, we report the outcomes of VSA technical remediation to reduce frequency of overlapping spots and curtail chewing damage to VSA papers by obese diabetic mice. We found little evidence substantiating previous concerns that voiding behavioral changes caused by the VSA testing environment overshadow physiological differences between mice. Voiding behaviors consistently differed between obese diabetic and control male mice, and none of the following procedural modifications to reduce overlapping spots and curtail paper chewing masked these differences: reduction of the VSA testing duration, restriction of water during the assay period, placement of a wire mesh cage bottom on top of or suspended over the filter paper, treatment of mesh with a hydrophobic spray, and size of wire mesh opening.
Although urinary function testing platforms like the VSA render mouse voiding behavior quantifiable, approaches to evaluate mouse urinary function vary widely across laboratories, complicating cross-study comparisons. Here, we also describe development and multi-institutional validation of a new tool for objective, consistent, and rapid analysis of mouse VSA data. Void Whizzard is a freely available software plugin for ImageJ that standardizes and automates VSA image batch processing and data extraction. We describe its features and demonstrate its increased speed compared with traditional analysis methods. We also use this resource to evaluate how specific VSA method parameters influence voiding behavior. Furthermore, we demonstrate that Void Whizzard is an effective tool for quantifying nonconcentric overlapping void spots, which commonly confound analyses. The Void Whizzard software and rigorous validation of VSA methodological parameters described here advance the goal of standardizing mouse urinary phenotyping for comprehensive urinary phenome analyses.
MATERIALS AND METHODS
Mice.
BTBR.Cg-Lepob/WiscJ mice were purchased from Jackson Laboratory (Bar Harbor, ME, strain no. 004824) (12) to establish a breeding colony at the University of Wisconsin-Madison. Mice were housed in static polysulfone cages containing a mix of corn cob and Alpha-Dri bedding and maintained on a 12 h light and dark cycle at 25°C and 20%–50% relative humidity. Mice were group housed, and feed (irradiated Diet 2920X, Harlan Teklad, Madison, WI) and water were available ad libitum except during the testing period, when mice were housed individually and only feed was available unless otherwise indicated. All procedures were approved by the University of Wisconsin Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
All experiments compared 8- to 10-wk-old obese diabetic BTBR Lepob/ob (ob/ob) males to BTBR wild- type control male littermates. We used males because male urinary tract symptoms are a primary research focus of our laboratory and because the two goals of this study were 1) to develop a tool to aid in consistent parsing and quantification of complex void pattern data that may arise during VSA, and 2) to test VSA procedural modifications that may reduce complexity of these void data. Male mice have been reported to exhibit more complex void parameters than females, including increased void frequency and volume (5) and, as such, were ideal candidates to address our study goals. Diabetic mice were determined by genotype (ob/ob) and measured blood glucose levels of at least 300 mg/dl at beginning of study. Average blood glucose levels were 222.4 ± 6.4 mg/dl for wild type and 520.3 ± 25.8 mg/dl for ob/ob mice for which a reading could be obtained (n = 8 of 34 ob/ob mice yielded glucose readings that exceeded the range of the glucose meter or levels >700 mg/dl; these 8 mice were distributed randomly across experiments).
Blood glucose measurements.
Blood glucose levels were measured between 1 and 3 PM one day before VSA. Mice were fasted for 4 h, removed from cage, and placed in a mouse restrainer. The base of the tail was swabbed with a 70% isopropyl alcohol pad, and a single incision was made through the tail vein with a 28G sterile lancet. Blood was tested using an AlphaTRAK 2 blood glucose monitoring system and AlphaTRAK 2 glucose test strips.
VSA and procedural modifications.
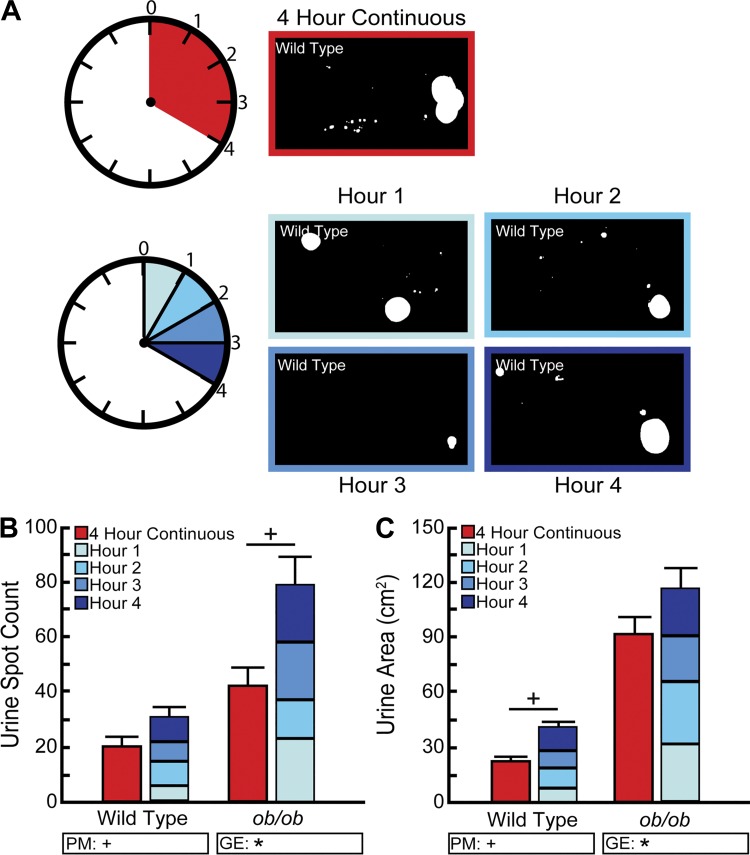
Testing was performed in the vivarium where mice were housed. Whatman grade 540 (Fisher Scientific no. 057163-W) filter papers (27 × 16 cm) were fitted to bottoms of clean and empty mouse cages and secured with masking tape. Mice were introduced to the cage (singly housed), the food hopper (containing standard rodent chow) and cage lid were secured, and testing was performed for a duration of 4 h. Testing time was standardized (10:00 AM to 2:00 PM GMT). Mice did not have access to water during the testing period unless otherwise specified. A single experimenter performed all tests to minimize stress to the mice during the testing period (16).
To test whether voiding behavior changes over the 4-h testing period, mice were tested twice: one time for each experimental condition on two successive days. Either a single filter paper was used for four continuous hours (10:00 AM to 2:00 PM GMT), or the mouse was placed in a cage with a clean filter paper and each hour after hours 1, 2, and 3 transferred to a new cage containing a new filter paper. The starting environment (4 h continuous vs. hourly paper changes) was randomized. Group sizes of nine ob/ob and nine wild-type mice were used for this experiment.
Group sizes of seven ob/ob and seven wild-type mice were used to test the impact of drinking water access during the testing period. Water was either provided ad libitum from a standard water bottle for the duration of the assay, or the bottle was removed for that period to enforce water restriction. Testing was conducted one time for each experimental condition on two successive days, and mice were randomized to starting environment.
To test if a wire mesh cage floor influences voiding patterns, tests were performed by placing mice directly on the filter paper (without a wire mesh), on top of a wire mesh (galvanized steel mesh hardware cloth) fitted directly over the filter paper, or on top of a wire mesh elevated 1.5 cm or 12.5 cm above the filter paper. Wire mesh opening size was 0.635 cm (0.25 in.) unless otherwise indicated. Each mouse was tested one time on each cage floor variation (total of four tests per mouse) over four successive days, and the starting environment was randomized to account for acclimation to the mesh. Seven ob/ob and seven wild-type mice were used.
Testing of the effect of a wire mesh cage floor with a hydrophobic barrier coating on urinary end points was conducted one time for each experimental modification on two successive days for eight ob/ob and seven wild-type mice. Wire mesh was left untreated or was spray-coated with Rust-Oleum Clear NeverWet Superhydrophobic Coating Product and allowed to dry thoroughly. Hydrophobicity was tested by immersing wire mesh in water, removing it immediately, and visually inspecting it for clinging water droplets. Spray coating was reapplied before every use. Wire mesh was elevated 1.5 cm above the filter paper for testing. The starting environment was randomized.
To test whether wire mesh opening size influences voiding patterns, cage floors were fashioned from wire mesh with either 0.635 cm (0.25 in.) or 1.27 cm (0.5 in.) openings. Wire mesh was elevated 1.5 cm above the filter paper for testing, which took place one time for each experimental modification on two successive days with a randomized starting environment. Group sizes of seven ob/ob and seven wild-type mice were used.
VSA paper imaging.
Filter papers were imaged with an Autochemi AC1 Darkroom ultraviolet imaging cabinet (UVP, Upland, CA) equipped with an Auto Chemi Zoom lens 2UV and an epi-illuminator. Image capture settings were adjusted using UVP VisonWorksLS image acquisition software. Images were captured using an Ethidium Bromide filter set (570–640 nm) and 365 nm epi-illumination. Exposure settings were optimized to maximize signal over noise.
Software development and implementation.
We designed Void Whizzard as a plugin for FIJI (a packaged distribution of ImageJ) as a means to rapidly and objectively process VSA filter paper images and extract data. FIJI (and Void Whizzard by association) are public domain software and are compatible with Mac, Windows, and Linux operating systems. The Void Whizzard plugin packages several existing macros for background subtraction, thresholding, dividing overlapping spots, and quantification features within a VSA paper image. Raw image files are noise reduced using the despeckle filter in the standard FIJI download. A Kuwahara filter is used for image smoothing while maintaining urine spot integrity (40). A Gaussian Mixture Modeling plugin analyzes pixel intensity distribution and establishes thresholds to separate urine spots from background (27). The Ellipse Split plugin applies best-fit ellipses to each urine spot and separates nonconcentric overlapping spots (41). Data output is specified by the experimenter. The defaults are total ellipse number, total ellipse area (overlapping area is quantified twice), ellipse location (center vs. corners), imputed urine volume, and categorical distribution of ellipse sizes. This study focuses on two of these parameters: total ellipse (spot) number and total ellipse (spot) area. Experimenters can optionally exclude features from analysis according to their size and circularity to eliminate image artifacts. Void Whizzard installation instructions and user guide are available at http://imagej.net/Void_Whizzard.
Multi-institutional use and validation of Void Whizzard software.
Individuals with previous experience performing VSA from four different institutions were selected to serve as experimenters for preliminary testing of Void Whizzard. Each experimenter was provided with 20 raw VSA image files to analyze using their existing laboratory methods for quantification and then to repeat using Void Whizzard for analysis. The 20 raw images were divided into 2 groups of 10. One group of papers had at least one nonconcentric overlapping spot, and the remaining papers had no overlapping spots. Experimenters were blinded to which papers had overlapping spots. Experimenters were instructed to quantify spot number, total urine area from the papers, and time required to quantify all 20 images. When using laboratory-specific methodology, three of the four institutions performed the analysis using methods described previously (5, 32, 43). The fourth group utilized ImageJ to invert the images and apply a threshold for separation of spots from background, and used the analyze particles feature of ImageJ to quantify the number and area of urine spots. When using Void Whizzard, all experimenters used the default settings.
Statistical analyses.
Data are reported as mean ± standard error unless otherwise indicated. Statistical analyses were performed using RStudio version 1.1.442. A significant difference is considered to be P < 0.05. Levene’s test was used to determine homogeneity of variance with P < 0.05 indicating inequality of variance. Parametric data were tested using two-way ANOVA, followed by Tukey’s honestly significant difference post hoc test to identify significant differences. Type III sum of squares ANOVA was run for nonparametric data, followed by Tukey’s honestly significant difference. Categorical data was analyzed using Fisher’s exact test. The Shapiro-Wilk test was used to assess normality of residuals with P < 0.05 indicating non-normal data. Data that did not meet the criteria for homogeneity of variance or normality were transformed using either a base-10 log transformation (count data, e.g., void number) or a square-root transformation (size data, e.g., void area). Where necessary, 0.5 was added to data before log transformation to yield nonzero values.
RESULTS AND DISCUSSION
The VSA is an accessible, inexpensive, nontechnical platform that we have used for rodent urinary function testing and that others have used for behavioral testing. Although these characteristics make it attractive for widespread application, measuring and quantifying VSA results can be time consuming, especially for rodents with high-frequency or high-volume voids. Subjectivity in VSA analysis further complicates comparisons between assays and makes extrapolation to different mouse strains or alternate testing platforms nearly impossible.
Void Whizzard software design and functionality.
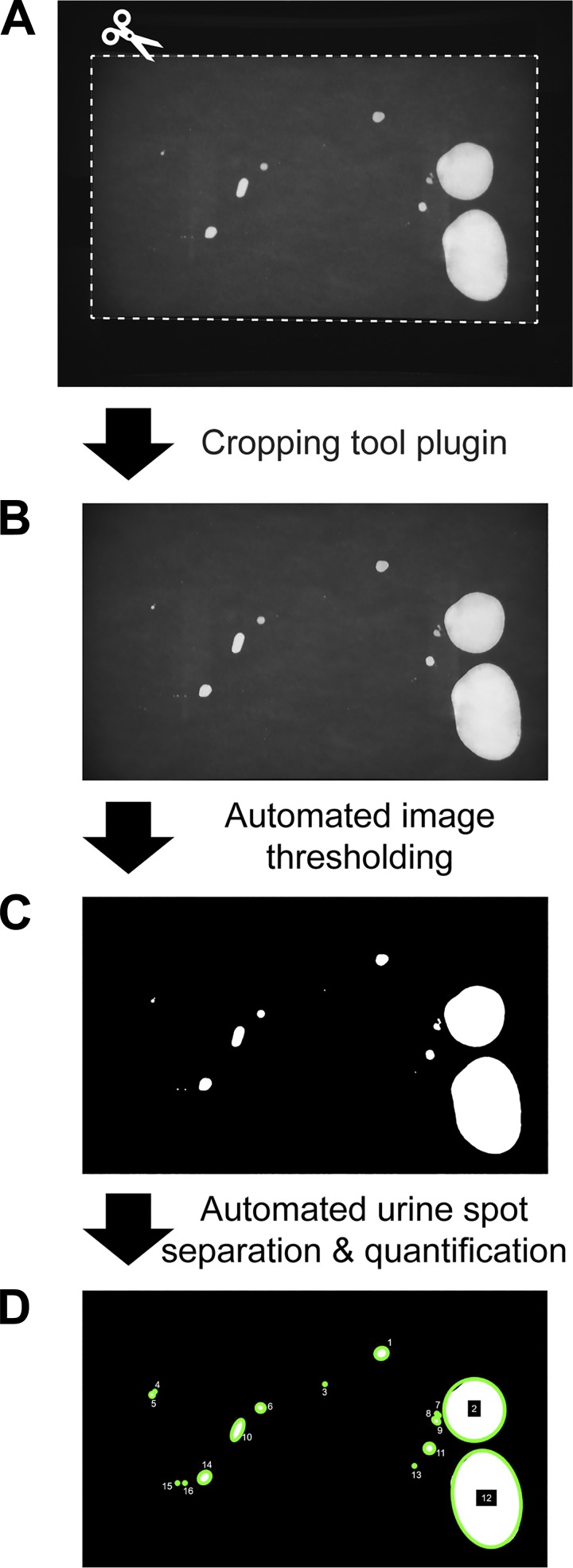
Void Whizzard was created to increase efficiency and objectivity of VSA analysis. Void Whizzard is a software plugin for FIJI, a bundled distribution of the publicly available image-processing application, ImageJ. Following VSA testing and filter paper image acquisition, Void Whizzard simplifies image straightening and cropping, then automates batch image thresholding, urine spot separation, and quantification (Fig. 1; also see materials and methods for image processing details). We incorporated flexibility into our design, allowing for custom user input regarding filter paper size, units of measure, and thresholds for spot size and circularity. Void Whizzard also accommodates images of ultraviolet light-illuminated urine spots (light spots on dark background) or ninhydrin-stained urine spots (dark spots on light background). Void Whizzard is free and open source, meaning it is available for distribution and can be modified by users wishing to extend its functionality.
Fig. 1.
Void Whizzard design and functionality. Void Whizzard is designed to standardize and expedite data extraction from void spot assay images. Experimenters use built-in tools to crop and straighten images. Void Whizzard then automatically converts images to binary, separates nonconcentric overlapping spots, optionally excludes spots based on user-defined circularity and size thresholds, and calculates spot number, area, volume, location, and categorical distribution based on size.
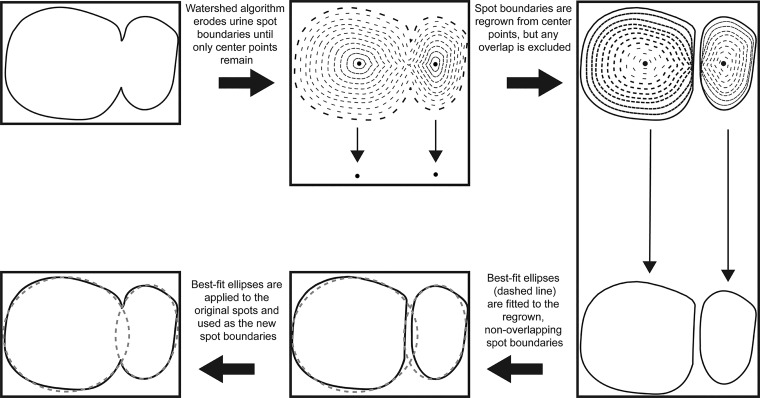
Overlapping urine spots are a confounder for VSA analysis. Spots may be completely overlapping (concentric spots, or one spot deposited within another) or may have partially or substantially overlapping borders (nonconcentric spots). Nonconcentric overlapping spots could be a source of variation among laboratories: where one experimenter may see a complex spot pattern and measure one spot, another experimenter may identify and measure two or more overlapping spots. This concern can be exacerbated in rodent models of urinary dysfunction, such as mouse models of diabetes that exhibit diabetic diuresis resulting in frequent and excessive urination. For these reasons, we designed Void Whizzard to address the overlapping spot limitation specifically, introducing functionality to objectively identify, separate, and measure nonconcentric overlapping spots (Fig. 2).
Fig. 2.
Void Whizzard method for separating nonconcentric overlapping urine spots. Overlapping spots are separated using Void Whizzard. The watershed algorithm erodes spot boundaries until spot center points are identified. Center points are then dilated to reconstruct spot boundaries excluding areas of overlap. The split ellipse algorithm segments and fits ellipses to each spot. Ellipse boundaries match original spot curvatures but maintain integrity, even in overlapping regions. The best fit ellipses are then used for subsequent spot quantification.
Void Whizzard expedites and reduces variability between VSA analyses.
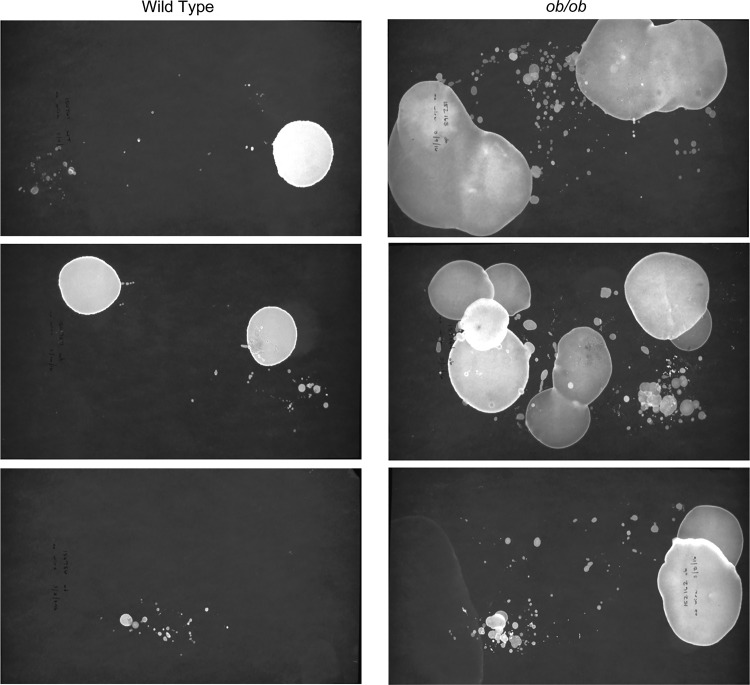
Void Whizzard was designed and tested by one laboratory and validated by testers from four external laboratories. All software testers were experienced in VSA analysis. Results described in this section are exclusively from external testers after each was provided with Void Whizzard, an instruction manual, and 20 VSA images (10 images with and 10 without overlapping spots). All images were captured from filter papers generated from VSA testing of obese diabetic ob/ob or BTBR wild-type mice (Fig. 3), a classification to which testers were blinded. ob/ob mice are a model of urinary dysfunction and exhibit diabetic diuresis, including increased frequency and volume, which often results in overlapping urine spots. Testers were instructed to analyze images twice, once using their own laboratory-specific method and once using Void Whizzard with default settings (i.e., testers were instructed against customizing analysis or outputs). Testers were then instructed to report the following for each method: 1) urine spot number per image, 2) urine spot area per image, and 3) time elapsed to complete analysis of all 20 images.
Fig. 3.
Sample images of representative void spot assays (VSAs) from BTBR wild-type and ob/ob mice. BTBR wild-type and ob/ob male mice were tested for 4 h without a wire mesh. Three representative VSA images are shown from each genotype. ob/ob mice produce more urine and exhibit more overlapping spots than wild-type mice.
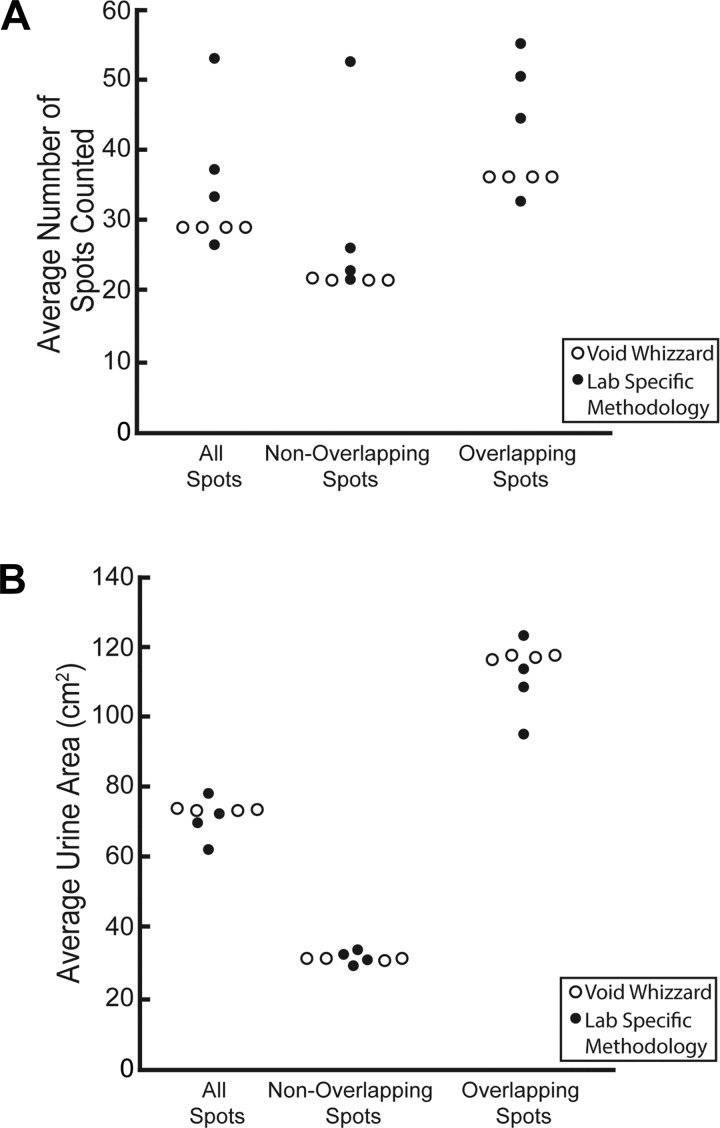
We compared laboratory-specific and Void Whizzard analyses in terms of tester-reported urine spot number and total urine area for all 20 images. We focused on variability within laboratory-specific and Void Whizzard analyses, as such variability affects statistical power and mouse experimental sample size. We observed considerably more variability within laboratory-specific than Void Whizzard analyses (Fig. 4). The range in spot number averages for laboratory-specific analyses is 27 spots, and for Void Whizzard is 0 (Fig. 4A). The range in total spot area averages for laboratory-specific analyses is 15.5 cm2 and for Void Whizzard is 0.3 cm2 (Fig. 4B). Notably, reported spot areas modestly differ among Void Whizzard testers, differences that likely derive from image straightening and cropping, the only parameter requiring user input. User variability in crop area selection affects spots near filter paper edges by reducing their boundaries or removing spots entirely. Our most important finding is that Void Whizzard is more consistent and reproducible than individual laboratory VSA analyses.
Fig. 4.
Laboratory-specific methods for void spot assay (VSA) image analysis give rise to considerable variability in end point measurements; Void Whizzard diminishes between-laboratory variability. Experimenters from 4 laboratories were given 20 preselected VSA images (10 with and 10 without at least one nonconcentric overlapping spot). Experimenters used a laboratory standard method and then Void Whizzard to calculate average spot number (A) and total spot area (B). Results from laboratory standard VSA analyses varied more widely than Void Whizzard analyses.
We hypothesized that difficulties inherent in manual separation of overlapping spots would result in a greater range of reported values among test images containing such spots compared with images lacking them. For test images lacking nonconcentric overlapping spots, the range of spot number averages for laboratory-specific analyses is 30 spots and for Void Whizzard is 1 spot (Fig. 4A). The range of total spot area averages for laboratory-specific analyses is 3.3 cm2 and for Void Whizzard is 0.6 cm2 (Fig. 4B). We observed a similar trend for images containing overlapping spots. The range of total spot number averages for laboratory-specific analyses is 22 spots and for Void Whizzard is 1 spot (Fig. 4A). Meanwhile, the range of total spot area for laboratory-specific analyses is 27.8 cm2 and for Void Whizzard is 0.6 cm2 (Fig. 4B). Thus, laboratory-specific methods give rise to substantial variability in void number determination, regardless of whether analyzed images contain overlapping spots. Laboratory-specific methods also vary in void area determination but may be more precise for images with nonoverlapping spots (compare range of 3.3 cm2 for images with nonoverlapping spots to a range of 27.8 cm2 for images with overlapping spots). As with the collective results for all 20 images discussed above, Void Whizzard increases VSA analysis precision.
We hypothesized that by streamlining and automating VSA image quantification, Void Whizzard would reduce analysis time. Each tester measured the time needed to analyze all 20 test images using their own method and using Void Whizzard (not including installation time). The average time ± standard error for laboratory-specific methods was 64.5 ± 17.4 min compared with only 5 ± 0.4 min for Void Whizzard. These results indicate that Void Whizzard dramatically increases VSA analysis efficiency, thereby saving personnel time and effort.
Decreasing time of exposure to filter paper results in significant differences in urine spot number and spot area.
In addition to standardizing and expediting VSA analysis, Void Whizzard is specifically designed to consistently and objectively identify and separate nonconcentric overlapping spots. However, this tool cannot separate concentric overlapping spots (spots deposited within another). VSA method procedural modifications are one way to minimize the concentric spot confounder. We tested several different VSA procedural modifications by comparing results between ob/ob mice, which we knew would produce overlapping urine spots, and wild-type control animals, which produce no or few overlapping spots (Fig. 3).
We began by testing assay duration. Published studies have used testing periods from 1 to 24 h (4, 8, 16, 17, 37, 38, 43, 46). Our standard testing period is four continuous hours and involves placing mice in direct contact with a single filter paper for the entire testing period. This experimental design may contribute to overlapping spots because the longer a mouse voids on the same paper, the more likely a new void spot will be deposited on top of an existing one. Overlap obscures both frequency (spot number) and volume (spot area) of voids deposited, leading to inaccurate analyses. We examined whether changing the filter paper after each hour during a 4-h test would ameliorate this problem. BTBR wild-type or ob/ob mice were evaluated by VSA utilizing one filter paper for four continuous hours or four filter papers with one paper replaced after each hour during four consecutive hours (Fig. 5A). Papers were imaged, and total urine spot number and urine spot area quantified using Void Whizzard. Spot number and area measurements for the four-consecutive-hour test were totaled to provide cumulative measures to be compared with the 4-h continuous test. The spot number for wild-type mice did not significantly differ for continuous (20 ± 3 spots) or cumulative (29 ± 3 spots, P = 0.8) tests (Fig. 5B). However, the spot number for ob/ob mice did differ, yielding 42 ± 6 spots for the continuous test and 77 ± 10 spots (P < 0.01) for the cumulative test. This trend is reversed for spot area. Wild-type mice yield a smaller total urine area during the continuous test (21.8 ± 1.8 cm2) than during the cumulative test (38.2 ± 2.8 cm2, P < 0.05), whereas ob/ob mice show no difference in spot area (continuous = 90.9 ± 8.6 cm2; cumulative = 113.7 ± 11.1 cm2; P = 0.2) (Fig. 5C). These data reveal that reducing the time a mouse is evaluated on a single filter paper increases sensitivity of the VSA for both spot number and area, presumably because of reduction of concentric overlapping spots. However, we cannot rule out potential behavioral changes incited by introducing new stimuli (filter papers) into the caging environment.
Fig. 5.
Void spot assay (VSA) filter paper testing interval changes urine frequency and volume. A: BTBR wild-type and ob/ob male mice were tested using a single paper for four continuous hours or using papers replaced after each hour of a 4-h cumulative testing period. B: continuous test yielded fewer spots than the cumulative test for ob/ob mice, but there was no difference between tests for wild-type mice. C: continuous test yielded a smaller total urine area than the cumulative for wild-type mice, but there was no difference between tests for ob/ob mice. Results are mean ± SE of nine wild-type and nine ob/ob mice. +Significant difference observed by VSA procedural modification (PM). *Significant differences detected due to genotypic effects (GE). A significant difference between groups is considered to be P < 0.05. Data were tested using two-way ANOVA, followed by Tukey’s honestly significant difference.
Decreasing assay duration preserves differences in urinary outputs for BTBR mice.
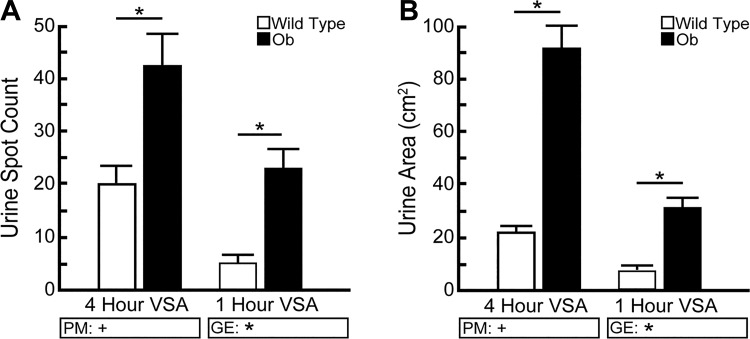
The preceding result demonstrating decreased assay sensitivity with increased evaluation time led us to question whether decreasing VSA duration overall would be sufficient to reveal phenotypic differences between wild-type and ob/ob mice with diuretic urinary dysfunction, while greatly reducing or eliminating concentric overlapping spots. To answer this question, we compared continuous four-hour test results to the first hour of cumulative-test results. Indeed, both results reveal significant differences between genotypes. ob/ob mice produce more urine spots than wild-type mice in 4 h of continuous testing (ob/ob = 42 ± 6 spots; wild type = 20 ± 3 spots; P < 0.5) and in the first hour of cumulative testing (ob/ob = 23 ± 4 spots; wild type = 5 ± 2 spots; P < 0.001) (Fig. 6A). Likewise, ob/ob mice yield a greater total spot area than wild-type mice in 4 h of continuous testing (ob/ob = 90.9 ± 8.6 cm2; wild type = 21.9 ± 1.9 cm2; P < 0.001) and in 1 h of the cumulative testing (ob/ob = 30.9 ± 3.8 cm2; wild type = 6.8 ± 1.7 cm2; P < 0.001) (Fig. 6B). We conclude that shortening the duration of the VSA from 4 h to 1 h is an effective remediation that addresses a limitation of the assay, that of concentric overlapping spots, while maintaining the ability to distinguish phenotypic differences between wild-type and diabetic mice, a model of rodent urinary dysfunction. However, despite the benefits of a shorter testing window (reduced personnel time and concentric overlapping spots), it is worth noting that a shorter testing window might not be optimal for some mouse strains. Specifically, it may reduce statistical power for mice that void infrequently. Some laboratories have utilized a continuously rolling filter paper as another method to decrease exposure time to the same filter paper area (28).
Fig. 6.
Voiding behavioral differences between BTBR wild-type and ob/ob mice are detectable regardless of whether assay duration is 4 h or 1 h. BTBR wild-type and ob/ob male mice were tested using a single paper for a 1-h or 4-h testing period. A: ob/ob mice yield more spots than wild-type mice for both testing periods. B: ob/ob mice produce more urine volume than wild-type mice in the 4-h continuous test and in the 1-h test. Results are mean ± SE of seven wild-type and seven ob/ob mice. +Significant difference observed by void spot assay (VSA) procedural modification (PM). *Significant differences detected due to genotypic effects (GE). A significant difference between groups is considered to be P < 0.05. Data were tested using two-way ANOVA, followed by Tukey’s honestly significant difference.
Water access during VSA does not affect urinary end points.
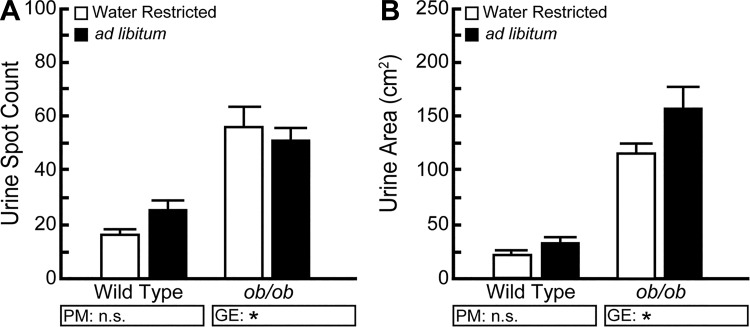
We routinely restrict water access during a 4-h VSA testing period but had not considered the impact. Four hours of water restriction is relatively brief, as other studies have deprived mice of water for up to 48 h, and previous work has shown that water restriction for 4 h did not significantly alter voiding behavior (3, 7). We tested whether restricting or providing water ad libitum for the testing period affected urine spot number or area. Water access did not significantly affect spot number or area for wild-type or ob/ob mice (Fig. 7). We monitored mice for signs of hydration distress upon assay completion and observed no gross differences in behavior or appearance of water-restricted mice compared with mice provided water ad libitum.
Fig. 7.
Drinking water access during the void spot assay (VSA) testing period does not significantly change VSA outcomes. BTBR wild-type and ob/ob male mice were tested for 4 h without water or with water available ad libitum. Water access does not significantly affect spot number (A) or total spot area (B). Results are mean ± SE of seven mice per group. *Significant differences detected due to genotypic effects (GE). A significant difference between groups is considered to be P < 0.05. Data were tested using two-way ANOVA, followed by Tukey’s honestly significant difference. n.s., no significant difference.
Placement of a wire mesh over the VSA filter paper affects urine frequency.
A frequent critique of the VSA is that placing mice in contact with a filter paper onto which they urinate for extended periods of time will allow mice to wander through voids, creating artifactual spots or extending natural spot boundaries to inflate the number of void spots observed. We tested whether placing mice directly on the filter paper or on a wire mesh fitted over the filter paper would change urine frequency or volume.
As we prepared for this experiment, we saw utility in testing an additional aspect of the wire mesh. The VSA is one of several platforms available for testing urinary function; others include metabolic cage assays, uroflowmetry, cystometry, etc. Several of these platform designs involve placing mice on a wire mesh elevated over collection vessels (e.g., metabolic cages) or a balance (e.g., cystometry, hybrid VSA-cystometry caging systems) to allow analysis of urine biomarkers, concentration, frequency, volume, and more (12, 24, 26, 32, 46). We expect urinary physiology to be the same across methods, yet comparisons between methods is confounded by lack of standardized protocols. Testing procedural modifications that align parameters across platforms (e.g., presence of wire mesh cage floor) could elucidate physiological end points common across test methods, enabling comparisons between them. To examine this question, we also elevated a wire mesh at different heights over the VSA filter paper to mimic elevation of mice over collection vessels or a balance and examined effects on urine spot number and area.
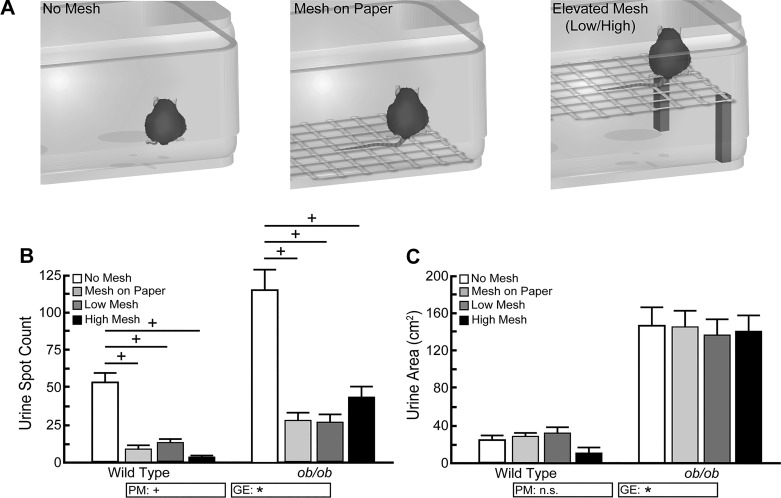
BTBR wild-type and ob/ob mice were placed directly in contact with the filter paper, on top of a wire mesh fitted directly on top of the filter paper, or on a wire mesh elevated over the filter paper at a height of 1.5 cm (low mesh) or 12.5 cm (high mesh) to mimic other urinary function testing platforms (Fig. 8A). Wild-type mice produce more urine spots when in direct contact with the filter paper (53 ± 6 spots) compared with mesh on paper (9 ± 3 spots, P < 0.001), low mesh (12 ± 2 spots, P < 0.001), and high mesh (3 ± 1 spots, P < 0.001). Similarly, ob/ob mice urinate more frequently when directly on top of the filter paper (114 ± 14 spots) than when a mesh cage floor is present (mesh on paper = 27 ± 5 spots, P < 0.001; low mesh = 26 ± 5 spots, P < 0.001; high mesh = 43 ± 7 spots, P < 0.01) (Fig. 8B). Urine area does not change significantly for wild-type or for ob/ob mice (Fig. 8C), thus implying that average voided volumes were larger. These results show that addition of a wire mesh to the VSA design, regardless of height of that mesh over the filter paper, decreases urine frequency but increases volume per void.
Fig. 8.
Presence of a wire mesh over the void spot assay (VSA) filter paper significantly alters urine frequency. A: BTBR wild-type and ob/ob male mice were tested in cages containing a filter paper alone, a wire mesh placed directly on the paper, a wire mesh elevated 1.5 cm above the paper (low mesh), or a wire mesh elevated 12.5 cm above the paper (high mesh). B: wild-type and ob/ob mice void more frequently when in direct contact with a filter paper than when on a wire mesh cage floor. C: total urine area does not significantly differ when mice are in direct contact with paper or placed on a mesh. Results are mean ± SE of seven wild-type and seven ob/ob mice. +Significant difference observed by VSA procedural modification (PM). *Significant differences detected due to genotypic effects (GE). A significant difference between groups is considered to be P < 0.05. Data were tested using two-way ANOVA, followed by Tukey’s honestly significant difference. n.s., no significant difference.
It is important to consider that mouse voiding patterns, like those in the human, are affected by behavioral and physiological factors that we are only beginning to understand. We focused on the influence of a wire mesh, placed directly on the cage bottom or elevated above it, because it has been speculated that a wire mesh deprives mice of enrichment, creating an environment to which they cannot acclimatize (15) and because it had been reported previously that mice are fearful of perceived elevation (42). These wire mesh cage floors are used in a variety of mouse void function testing methods, the results of which can contradict each other, raising questions of assay validity. In this study, placing mice on a wire mesh in contact with or elevated above the filter paper substantially changed voiding behavior of mice, reducing total void number by as much as 96%. It is therefore likely that presence or absence of a wire mesh floor appreciably contributes to behavioral voiding differences between assays and should be considered when comparing results of assays with differing test conditions. These results also indicate that presence of a wire mesh during VSA testing is a confounding behavioral variable that may interfere with accurate assessments of physiological voiding behaviors. Although not examined in this study, it is also possible that mice acclimatize to the presence of a mesh over repeated days of testing. Therefore, users should carefully consider whether or not to incorporate a wire mesh into void assay experimental design.
Small void spots are not caused by mice tracking through deposited voids.
Although presence of a wire mesh reduced urine spot number, we do not know what led to this decrease. One explanation is that our data substantiate the VSA critique that mice track their urine around when in direct contact with the filter paper. To combat this critique, experimenters often take preemptive (and potentially unnecessary) steps to reduce the impact of potential artifacts. Strategies used to reduce artifacts include empirical cutoffs based on spot shape or size (32), arbitrary cutoffs based on spot area (5, 43), and volume cutoffs based on physiological data (21, 29). Yet other experimenters ignore these cutoffs and quantify all spots without exclusions for size or shape (39).
We wanted to determine the validity of the urine tracking critique and subsequent preventative measures by testing whether presence of the mesh in the previous experiment reduced small spots that could be attributed to mouse paw or tail marks. To address this question, we used a built-in feature of Void Whizzard called “binning.” This feature allows users to input custom values to group spot size data into “bins.” We looked to existing literature to inform our bin cut-off values for urine area. Bjorling et al. (5) use a cut-off corresponding to 0.5 μl of urine, “the lower limit to eliminate particles arising from claw or tooth marks, footprints, or that resulted from tail dragging.” Thus, we separated our data into two bins: one including spots less than or equal to 0.066 cm2 (0.5 μl urine as determined by a standard curve) and one including all spots greater than 0.066 cm2 in area.
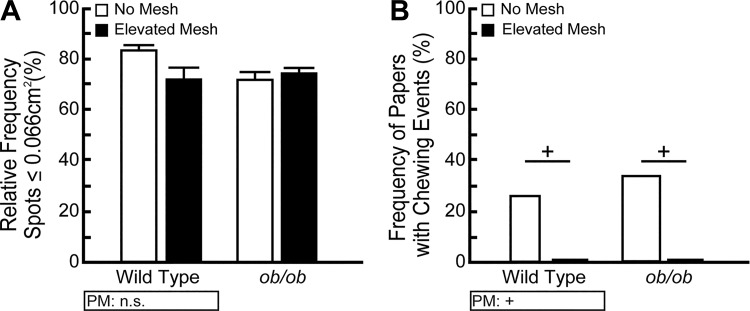
We hypothesized that mice elevated on a wire mesh above the filter paper would not be able to directly contact either the paper or deposited voids, thus eliminating artifactual spots. We compared urine frequency for mice directly in contact with the filter paper to those on a raised mesh (low mesh). Neither wild-type nor ob/ob mice show any difference in relative occurrence of small spots (<0.066 cm2) to total spots (Fig. 9A). These data demonstrate that the greater number of urine spots observed when mice directly contact the filter paper is not caused by urine tracking. To emphasize, addressing an unfounded critique by excluding data based solely on spot size results in loss of considerable amounts of valid urine function data. In our low mesh experiment, no less than 72.3% of total wild-type and 74.2% of total ob/ob urine spots would have been eliminated based on size alone had we instituted the 0.066 cm2 cut-off.
Fig. 9.
Small void spots are not void spot assay (VSA) testing artifacts; a wire mesh eliminates filter paper chewing. BTBR wild-type and ob/ob male mice were tested in cages fitted with a bare filter paper or with a wire mesh elevated 1.5 cm above the filter paper. A: frequency of small urine spots (<0.066 cm2) to total urine spots, previously attributed to footprints or tail dragging, does not differ between groups. B: elevating the mouse above the paper completely eliminates paper chewing. Frequency of chewing events is the number of unchewed or chewed filter papers divided by the total number of papers utilized. +Significant difference observed by VSA procedural modification (PM). Results are mean ± SE of seven mice per group. A significant difference between groups is considered to be P < 0.05. Data were tested using Fisher’s exact test. n.s., no significant difference.
Void Whizzard was created to enable user flexibility, including the ability to exclude features from analysis based on spot size and shape (spot circularity). In some circumstances, removing small spots from further analysis is useful for resolving voiding differences between experimental groups (44). However, arbitrarily removing small spots from downstream analyses reduces data dimensionality and potentially obscures important phenotypes. For example, we previously used VSA and uroflowmetry to characterize voiding dysfunction in male mice treated with slow-release implants of testosterone and estradiol (30). Void size and frequency measurements failed to reveal statistically significant differences between hormone-treated mice and controls, even though physiological differences had been identified with other methods. It was not until data were treated as categorical that a pattern of dysfunction, involving a shift from large to small voids, emerged. This is not the only mouse model in which small volume voiding is indicative of urinary dysfunction. Mice with spinal cord injury are prone to urinary leakage, fur wetting, and urine scald (36). For these and other models, small volume voids are an important component of urinary phenotype, and VSA is one tool that can be used in conjunction with others for comprehensive quantitative phenotyping.
Presence of a raised wire mesh eliminates filter paper chewing.
Another limitation of the VSA is that some mice chew the filter paper during the assay, confounding analysis of deposited void data. Chewing behavior may be of particular concern in diabetic mice, which have sweetened urine (due to glucosuria) that may encourage chewing of void spots. We asked whether elevating mice on a wire mesh (low or high mesh) altered chewing behavior. To determine frequency of chewing events, the number of filter papers with no chewing or evidence of chewing was each divided by the total number of filter papers utilized for all no-mesh experiments and was compared with frequency results from all elevated-mesh experiments. As expected, elevation of the mouse over the paper completely eliminates paper chewing in both wild-type and ob/ob mice. Wild type and ob/ob mice directly on paper chew 25.8% and 33.3% of the time, respectively, but incidence drops to 0% for both when elevated on mesh (P < 0.01) (Fig. 9B).
Coating a wire mesh with a hydrophobic barrier spray does not change urinary outputs.
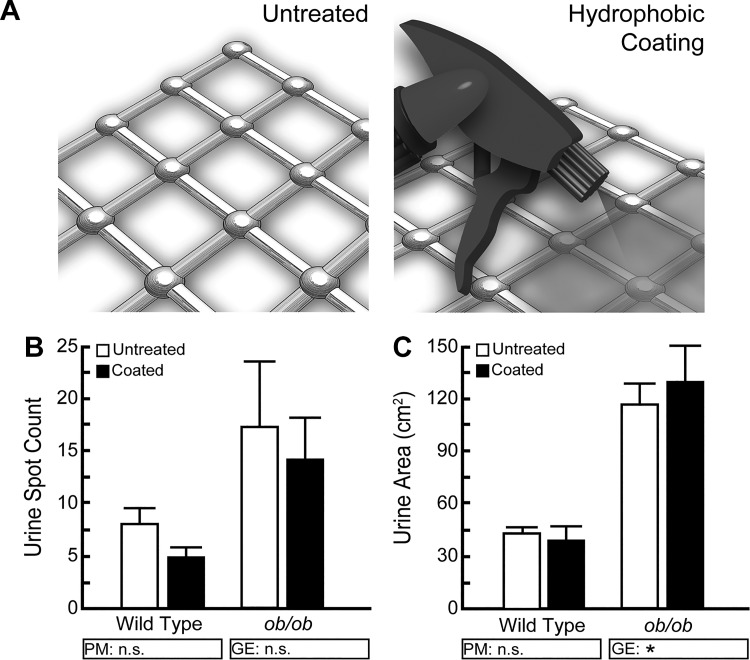
Increased urine frequency when mice are in direct contact with the filter paper (Fig. 8) does not appear to be due to mouse urine tracking (Fig. 9). Another explanation for how wire mesh may reduce urine spot number is that small droplet voids adhere to the mesh and do not fall to the filter paper. We hypothesized that coating the mesh with a hydrophobic barrier spray would eliminate adherence of void droplets. To test this question, wire mesh was left untreated or coated thoroughly with a hydrophobic barrier spray and placed at the low mesh height (1.5 cm) above a filter paper. Addition of the hydrophobic barrier does not change void frequency or void area for either wild-type or ob/ob mice (Fig. 10). Therefore, urine droplets do not appear to cling to the wire mesh in an amount sufficient to alter spot number or area.
Fig. 10.
A hydrophobic spray applied to a wire mesh cage bottom does not significantly change void spot assay (VSA) outcomes. A: BTBR wild-type and ob/ob male mice were tested using an elevated wire mesh cage bottom either untreated or treated with a hydrophobic spray to prevent urine adherence. B and C: application of the hydrophobic barrier to the wire mesh does not change the frequency of voids or total urine area for either wild-type or ob/ob mice. Graphical results are mean ± SE of seven wild-type and eight ob/ob mice. +Significant difference observed by VSA procedural modification (PM). *Significant differences detected due to genotypic effects (GE). A significant difference between groups is considered to be P < 0.05. Data were tested using Type III sum of squares ANOVA for nonparametric data, followed by Tukey’s honestly significant difference (HSD). n.s., no significant difference.
Wire mesh opening size does not affect urine spot number or area.
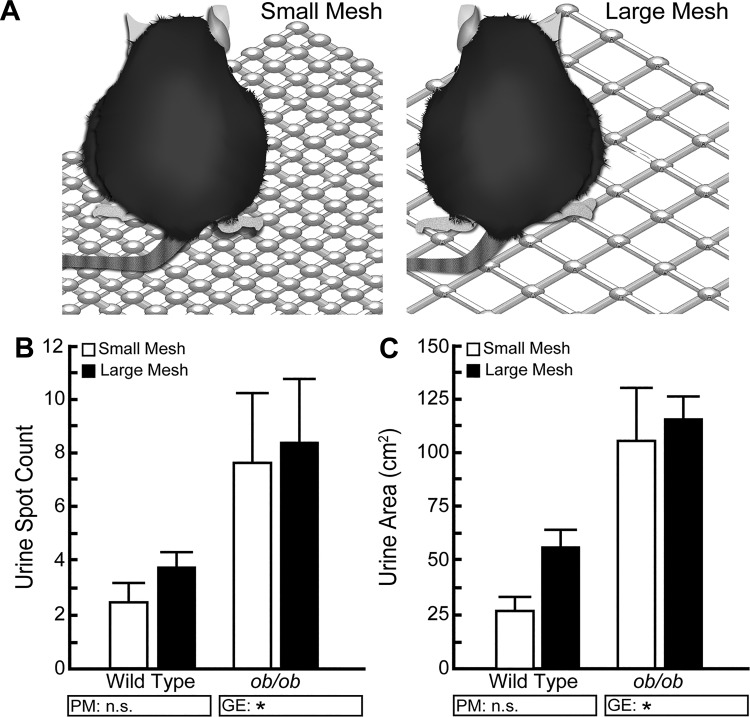
We recognized that wire mesh opening size could be another source of variability between experimental parameters. We compared the effect of a 0.635-cm mesh opening size (0.25 in., small mesh) to a 1.27-cm mesh opening size (0.5 in., large mesh) elevated at the low mesh height (1.5 cm) above a filter paper. We see no difference in spot number or spot area based on wire mesh opening size (Fig. 11).
Fig. 11.
Opening size of a wire mesh cage bottom does not significantly affect void spot assay (VSA) outcomes. A: BTBR wild-type and ob/ob male mice were tested using an elevated wire mesh cage bottom with opening sizes of either 0.635 cm (0.25 in., small mesh) or 1.27 cm (0.5 in., large mesh). B and C: changing the size of the mesh openings has no effect on the frequency of voids or total urine area for either wild-type or ob/ob mice. Graphical results are mean ± SE of seven mice per group. +Significant difference observed by VSA procedural modification (PM). *Significant differences detected due to genotypic effects (GE). A significant difference between groups is considered to be P < 0.05. Data were tested using two-way ANOVA, followed by Tukey’s honestly significant difference. n.s., no significant difference.
VSA results reveal expected phenotypic differences in urinary end points.
Throughout much of this section, we focused on effects of procedural modifications on urinary end points for BTBR wild-type and ob/ob mice to determine whether these modifications address the VSA limitation of concentric overlapping spots. We discovered that a couple of modifications did alter spot number and/or area (e.g., assay duration, presence of a wire mesh), but several modifications had no effect (e.g., water access, coating wire mesh with a hydrophobic barrier spray, wire mesh opening size).
We did not detail statistically significant genetic differences between wild-type and ob/ob mice, aside from consideration of VSA duration (Fig. 6). Significant differences in urinary function have been demonstrated previously in another Lepob/ob model, so we expected urine frequency and volume to be increased consistently in our BTBR ob/ob mice (9). As we compiled our data, however, we observed an interesting trend. Without fail, every procedural modification we tested revealed significant phenotypic differences in urinary end points between wild-type and ob/ob mice (genotype effect). To highlight these results, we created a table summarizing statistically significant differences due to procedural modification and/or genotype effect (Table 1). Furthermore, we summarized experiment-specific procedural modification and genotype effect significance for each experimental parameter tested within the corresponding figure (see Figs. 5–11). Despite criticism and acknowledged limitations of the VSA method, ultimately this platform performed exactly as required for testing urinary function-based hypotheses, reliably revealing physiological differences that can be attributed to biologically driven mechanisms, such as genotype. This is perhaps the most important conclusion from this study. Even though some procedural modifications do have significant impacts on voiding behaviors, they do not interfere with our ability to observe a genetic difference in voiding patterns. These results provide validation for the use of VSA as a rigorous method for examining urinary function in rodents. The rigor of the VSA is further bolstered by Void Whizzard and the power of automated analysis. Together, this study and accompanying software advance the long-term goal of establishing the VSA as a standardized component of mouse urinary phenome analysis.
Table 1.
Significance of results summary
| Wild-Type Procedural Modification | ob/ob Procedural Modification | Wild-Type: ob/ob Genotype Effect | |
|---|---|---|---|
| Assay duration (Figs. 5 and 6) | |||
| 4 h continuous vs. 4 h cumulative | + | + | * |
| 4 h continuous vs. 1 h | + | + | * |
| Water access (Fig. 7) | ns | ns | * |
| Presence/height of wire mesh (Fig. 8) | |||
| No mesh vs. mesh on paper | + | + | * |
| No mesh vs. low mesh | + | + | * |
| No mesh vs. high mesh | + | + | * |
| Paper chewing (Fig. 9) | + | + | not examined |
| Hydrophobic spray (Fig. 10) | ns | ns | * |
| Wire mesh opening size (Fig. 11) | ns | ns | * |
Procedural modification may, and genotype effect consistently does, affect VSA urinary outcomes (urine spot count and/or urine area). Statistical significance due to
VSA procedural modification, and
Wild-type vs. ob/ob genotype effect.
A significant difference between groups is considered to be P < 0.05. ns, no significant difference; VSA, void spot assay.
GRANTS
This work was supported by the National Institutes of Health Grants R01-DK-099328, R01-ES-001332, R01-DK-078158, U54-DK-104310, K01-DK-114334, F31-ES-028594, and T32-ES-007015.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A.W., L.L.A., S.R.O., G.S.M., D.E.B., W.A.R., J.M., P.C.M., K.W.E., and C.M.V. conceived and designed research; K.A.W., L.L.A., S.R.O., G.S.M., K.E.R., W.G.H., B.M.Z., L.E.L., Z.W., and E.M.S.-S. performed experiments; K.A.W., L.L.A., S.R.O., and C.M.V. analyzed data; K.A.W., L.L.A., S.R.O., D.E.B., and C.M.V. interpreted results of experiments; K.A.W., L.L.A., and C.M.V. prepared figures; K.A.W., L.L.A., and C.M.V. drafted manuscript; K.A.W., L.L.A., K.E.R., W.G.H., B.M.Z., L.E.L., Z.W., D.E.B., W.A.R., J.M., P.C.M., E.M.S.-S., K.W.E., and C.M.V. edited and revised manuscript; K.A.W., L.L.A., S.R.O., K.E.R., W.G.H., B.M.Z., L.E.L., Z.W., D.E.B., W.A.R., J.M., P.C.M., E.M.S.-S., K.W.E., and C.M.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the Aikens Center for Neurology Research for their support.
REFERENCES
- 1.Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57BL/6J mice: sexual and developmental determination. Behav Brain Res 182: 73–79, 2007. doi: 10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bal K, Ayik S, Issi Y, Bolukbasi A, Akhan G. Sleep analysis of patients with nocturia and benign prostatic obstruction. Urology 80: 383–388, 2012. doi: 10.1016/j.urology.2011.12.067. [DOI] [PubMed] [Google Scholar]
- 3.Bekkevold CM, Robertson KL, Reinhard MK, Battles AH, Rowland NE. Dehydration parameters and standards for laboratory mice. J Am Assoc Lab Anim Sci 52: 233–239, 2013. [PMC free article] [PubMed] [Google Scholar]
- 4.Biallosterski BT, Prickaerts J, Rahnama’i MS, de Wachter S, van Koeveringe GA, Meriaux C. Changes in voiding behavior in a mouse model of Alzheimer’s disease. Front Aging Neurosci 7: 160, 2015. doi: 10.3389/fnagi.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorling DE, Wang Z, Vezina CM, Ricke WA, Keil KP, Yu W, Guo L, Zeidel ML, Hill WG. Evaluation of voiding assays in mice: impact of genetic strains and sex. Am J Physiol Renal Physiol 308: F1369–F1378, 2015. doi: 10.1152/ajprenal.00072.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang A, Butler S, Sliwoski J, Valentino R, Canning D, Zderic S. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am J Physiol Renal Physiol 297: F1101–F1108, 2009. doi: 10.1152/ajprenal.90749.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Zhang L, Hill WG, Yu W. Evaluating the voiding spot assay in mice: a simple method with complex environmental interactions. Am J Physiol Renal Physiol 313: F1274–F1280, 2017. doi: 10.1152/ajprenal.00318.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelissen LL, Misajet B, Brooks DP, Hicks A. Influence of genetic background and gender on bladder function in the mouse. Auton Neurosci 140: 53–58, 2008. doi: 10.1016/j.autneu.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182: 939–941, 1973. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- 10.Frimodt-Møller C. Diabetic cystopathy: epidemiology and related disorders. Ann Intern Med 92: 318–321, 1980. doi: 10.7326/0003-4819-92-2-318. [DOI] [PubMed] [Google Scholar]
- 11.Gharaee-Kermani M, Rodriguez-Nieves JA, Mehra R, Vezina CA, Sarma AV, Macoska JA. Obesity-induced diabetes and lower urinary tract fibrosis promote urinary voiding dysfunction in a mouse model. Prostate 73: 1123–1133, 2013. doi: 10.1002/pros.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudkins KL, Pichaiwong W, Wietecha T, Kowalewska J, Banas MC, Spencer MW, Mühlfeld A, Koelling M, Pippin JW, Shankland SJ, Askari B, Rabaglia ME, Keller MP, Attie AD, Alpers CE. BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J Am Soc Nephrol 21: 1533–1542, 2010. doi: 10.1681/ASN.2009121290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurst JL. The functions of urine marking in a free-living population of house mice, Mus domesticus Rutty. Anim Behav 35: 1433–1442, 1987. doi: 10.1016/S0003-3472(87)80016-7. [DOI] [Google Scholar]
- 14.Hurst JL. Urine marking in populations of wild house mice Mus domesticus Rutty. III. Communication between the sexes. Anim Behav 40: 233–243, 1990. doi: 10.1016/S0003-3472(05)80918-2. [DOI] [Google Scholar]
- 15.Kalliokoski O, Jacobsen KR, Darusman HS, Henriksen T, Weimann A, Poulsen HE, Hau J, Abelson KS. Mice do not habituate to metabolism cage housing–a three week study of male BALB/c mice. PLoS One 8: e58460, 2013. doi: 10.1371/journal.pone.0058460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keil KP, Abler LL, Altmann HM, Bushman W, Marker PC, Li L, Ricke WA, Bjorling DE, Vezina CM. Influence of animal husbandry practices on void spot assay outcomes in C57BL/6J male mice. Neurourol Urodyn 35: 192–198, 2016. doi: 10.1002/nau.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keil KP, Abler LL, Altmann HM, Wang Z, Wang P, Ricke WA, Bjorling DE, Vezina CM. Impact of a folic acid-enriched diet on urinary tract function in mice treated with testosterone and estradiol. Am J Physiol Renal Physiol 308: F1431–F1443, 2015. doi: 10.1152/ajprenal.00674.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JH, Sun HY, Park SY, Soh MJ, Kim YJ, Song YS. Association between obesity and lower urinary tract symptoms: propensity score matching study between healthy controls and obese patients seeking bariatric surgery. Surg Obes Relat Dis 12: 1585–1593, 2016. doi: 10.1016/j.soard.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Kupelian V, McVary KT, Kaplan SA, Hall SA, Link CL, Aiyer LP, Mollon P, Tamimi N, Rosen RC, McKinlay JB. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston area community health survey. J Urol 189, Suppl: S107–S116, 2013. doi: 10.1016/j.juro.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Laven BA, Orsini N, Andersson SO, Johansson JE, Gerber GS, Wolk A. Birth weight, abdominal obesity and the risk of lower urinary tract symptoms in a population based study of Swedish men. J Urol 179: 1891–1896, 2008. doi: 10.1016/j.juro.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Yang G, Bushman W. Prostatic inflammation induces urinary frequency in adult mice. PLoS One 10: e0116827, 2015. doi: 10.1371/journal.pone.0116827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann ML, Geddes CE, Lee JL, Herkenham M. Urine scent marking (USM): a novel test for depressive-like behavior and a predictor of stress resiliency in mice. PLoS One 8: e69822, 2013. doi: 10.1371/journal.pone.0069822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumley LA, Sipos ML, Charles RC, Charles RF, Meyerhoff JL. Social stress effects on territorial marking and ultrasonic vocalizations in mice. Physiol Behav 67: 769–775, 1999. doi: 10.1016/S0031-9384(99)00131-6. [DOI] [PubMed] [Google Scholar]
- 24.Mann EA, Alam Z, Hufgard JR, Mogle M, Williams MT, Vorhees CV, Reddy P. Chronic social defeat, but not restraint stress, alters bladder function in mice. Physiol Behav 150: 83–92, 2015. doi: 10.1016/j.physbeh.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruniak JA, Owen K, Bronson FH, Desjardins C. Urinary marking in female house mice: effects of ovarian steroids, sex experience, and type of stimulus. Behav Biol 13: 211–217, 1975. doi: 10.1016/S0091-6773(75)91920-3. [DOI] [PubMed] [Google Scholar]
- 26.Maruniak JA, Owen K, Bronson FH, Desjardins C. Urinary marking in male house mice: responses to novel environmental and social stimuli. Physiol Behav 12: 1035–1039, 1974. doi: 10.1016/0031-9384(74)90151-6. [DOI] [PubMed] [Google Scholar]
- 27.Mei C, Dauphin M. Mixture Modeling ImageJ Plugin, 2003. https://imagej.nih.gov/ij/plugins/mixture-modeling.html.
- 28.Negoro H, Kanematsu A, Doi M, Suadicani SO, Matsuo M, Imamura M, Okinami T, Nishikawa N, Oura T, Matsui S, Seo K, Tainaka M, Urabe S, Kiyokage E, Todo T, Okamura H, Tabata Y, Ogawa O. Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Nat Commun 3: 809, 2012. doi: 10.1038/ncomms1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson TM, Moses MA, Uchtmann KS, Keil KP, Bjorling DE, Vezina CM, Wood RW, Ricke WA. Estrogen receptor-α is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J Urol 193: 722–729, 2015. doi: 10.1016/j.juro.2014.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson TM, Ricke EA, Marker PC, Miano JM, Mayer RD, Timms BG, vom Saal FS, Wood RW, Ricke WA. Testosterone and 17β-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology 153: 5556–5565, 2012. doi: 10.1210/en.2012-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penson DF, Munro HM, Signorello LB, Blot WJ, Fowke JH; Urologic Diseases in America Project . Obesity, physical activity and lower urinary tract symptoms: results from the Southern Community Cohort Study. J Urol 186: 2316–2322, 2011. doi: 10.1016/j.juro.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritter KE, Wang Z, Vezina CM, Bjorling DE, Southard-Smith EM. Serotonin receptor 5-HT3A affects development of bladder innervation and urinary bladder function. Front Neurosci 11: 690, 2017. doi: 10.3389/fnins.2017.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rom M, Schatzl G, Swietek N, Rücklinger E, Kratzik C. Lower urinary tract symptoms and depression. BJU Int 110: E918–E921, 2012. doi: 10.1111/j.1464-410X.2012.11552.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosen RC, Fitzpatrick JM; ALF-LIFE Study Group . Ejaculatory dysfunction in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. BJU Int 104: 974–983, 2009. doi: 10.1111/j.1464-410X.2009.08503.x. [DOI] [PubMed] [Google Scholar]
- 35.Saigal CS, Joyce G. Economic costs of benign prostatic hyperplasia in the private sector. J Urol 173: 1309–1313, 2005. doi: 10.1097/01.ju.0000152318.79184.6f. [DOI] [PubMed] [Google Scholar]
- 36.St Claire SL, St Claire MC, Davis JA, Chang L, Miller GF. Barrier film protects skin of incontinent rats. Contemp Top Lab Anim Sci 36: 46–48, 1997. [PubMed] [Google Scholar]
- 37.Stewart FA, Lundbeck F, Oussoren Y, Luts A. Acute and late radiation damage in mouse bladder: a comparison of urination frequency and cystometry. Int J Radiat Oncol Biol Phys 21: 1211–1219, 1991. doi: 10.1016/0360-3016(91)90278-C. [DOI] [PubMed] [Google Scholar]
- 38.Stewart FA, Michael BD, Denekamp J. Late radiation damage in the mouse bladder as measured by increased urination frequency. Radiat Res 75: 649–659, 1978. doi: 10.2307/3574851. [DOI] [PubMed] [Google Scholar]
- 39.Sugino Y, Kanematsu A, Hayashi Y, Haga H, Yoshimura N, Yoshimura K, Ogawa O. Voided stain on paper method for analysis of mouse urination. Neurourol Urodyn 27: 548–552, 2008. doi: 10.1002/nau.20552. [DOI] [PubMed] [Google Scholar]
- 40.Tischer C, Schindelin J. Linear Kuwahara, 2017. https://imagej.net/Linear_Kuwahara.
- 41.Wagner T. Elipse Split ImageJ Plugin, 2015. https://imagej.net/Ellipse_split.
- 42.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2: 322–328, 2007. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, Hill WG, Zeidel ML. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol 306: F1296–F1307, 2014. doi: 10.1152/ajprenal.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu W, Hill WG, Robson SC, Zeidel ML. Role of P2X4 receptor in mouse voiding function. Sci Rep 8: 1838, 2018. doi: 10.1038/s41598-018-20216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zamuner M, Laranja WW, Alonso JC, Simões FA, Rejowski RF, Reis LO. Is metabolic syndrome truly a risk factor for male lower urinary tract symptoms or just an epiphenomenon? Adv Urol 2014: 203854, 2014. doi: 10.1155/2014/203854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zwaans BM, Krueger S, Bartolone SN, Chancellor MB, Marples B, Lamb LE. Modeling of chronic radiation-induced cystitis in mice. Adv Radiat Oncol 1: 333–343, 2016. doi: 10.1016/j.adro.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]