Abstract
Numerous epidemiologic studies have identified an association between occupational exposures to organophosphorus pesticides (OPs) and asthma or asthmatic symptoms in adults. Emerging epidemiologic data suggest that environmentally relevant levels of OPs may also be linked to respiratory dysfunction in the general population and that in utero and/or early life exposures to environmental OPs may increase risk for childhood asthma. In support of a causal link between OPs and asthma, experimental evidence demonstrates that occupationally and environmentally relevant OP exposures induce bronchospasm and airway hyperreactivity in preclinical models. Mechanistic studies have identified blockade of autoinhibitory M2 muscarinic receptors on parasympathetic nerves that innervate airway smooth muscle as one mechanism by which OPs induce airway hyperreactivity, but significant questions remain regarding the mechanism(s) by which OPs cause neuronal M2 receptor dysfunction and, more generally, how OPs cause persistent asthma, especially after developmental exposures. The goals of this review are to 1) summarize current understanding of OPs in asthma; 2) discuss mechanisms of OP neurotoxicity and immunotoxicity that warrant consideration in the context of OP-induced airway hyperreactivity and asthma, specifically, inflammatory responses, oxidative stress, neural plasticity, and neurogenic inflammation; and 3) identify critical data gaps that need to be addressed in order to better protect adults and children against the harmful respiratory effects of low-level OP exposures.
Keywords: airway hyperreactivity, asthma, eosinophils, macrophages, nerve-immune interactions, neurotoxicity, organophosphorus pesticides
INTRODUCTION
Asthma is a chronic inflammatory lung disease characterized by episodic and reversible bronchoconstriction, mucus hypersecretion, airway inflammation, and airway hyperreactivity (AHR), all of which interfere with breathing. According to the US Centers for Disease Control and Prevention, in 2015 approximately 18.4 million adults and 6.2 million children in the United States had asthma, with 10 people dying from asthma each day on average (https://www.cdc.gov/asthma/most_recent_data.htm, accessed April 2018). Asthma prevalence and severity have increased markedly over the past two decades, especially in urban settings (25, 57, 100, 218). Many hypotheses have been proposed to explain the increased susceptibility of urban residents to asthma, including exposure to allergens, air pollution, differences in health care, and stress (57, 64, 89, 102, 125, 128). An environmental factor associated with occupational asthma in agriculture (105, 109, 111–113, 115, 155, 248) that is beginning to receive increased attention in the context of urban asthma (107, 156, 249) is exposure to organophosphorus pesticides (OPs).
OPs are among the most widely used pesticides worldwide, and they have been applied extensively in not only agricultural but also suburban and urban settings to control insects (43). Although residential uses of OPs have been largely phased out in the United States and many other countries over the past decade, OPs are still used heavily in agricultural, industrial, commercial, and military settings. As a result, OPs are ubiquitous in the human chemosphere, as confirmed by the widespread detection of OP metabolites in urine samples from the general US population (14, 34, 66). Occupational exposures, which are mainly associated with the production, distribution, and application of OPs, occur primarily via dermal absorption, with more limited exposure via inhalation (72, 106). The general population is exposed to OPs via ingestion of food and water contaminated with OPs and by dermal and inhalational exposure to pesticide drift and “overspray” (247). The latter is not an insignificant source of exposure—studies conducted in communities living near agricultural fields sprayed with OPs have found extensive OP contamination in the air (98), in homes (99), and in urine from pregnant women (33) and children (185) in these communities.
OPs are potent inhibitors of the enzyme acetylcholinesterase (AChE), which hydrolyzes the neurotransmitter acetylcholine to terminate cholinergic signaling. The phosphate form of the insecticide, which may be the parent compound or its active metabolite depending on the specific pesticide, inhibits AChE by phosphorylating the serine residue within the catalytic triad of the enzyme’s active site (179). Inhibition of AChE increases the amount and residence time of acetylcholine at nicotinic and muscarinic receptors in target tissues, resulting in cholinergic overstimulation in the brain and peripheral tissues. Acute OP poisoning in humans has been extensively documented and includes both central and peripheral cholinergic effects that collectively contribute to a clinical toxidrome known as “cholinergic crisis” (118). Respiratory failure is the primary cause of death in cholinergic crisis and is thought to be mediated by both peripheral and central mechanisms (118). In the periphery, OPs induce bronchoconstriction via cholinergic overstimulation of muscarinic receptors on airway smooth muscle (31). Although it is unclear whether central mechanisms of respiratory depression are predominantly muscarinic or nicotinic, OPs disrupt respiratory control in the brain stem, thereby triggering central apnea. Although AChE inhibition initiates the pathogenic mechanisms that ultimately cause respiratory dysfunction, observations in preclinical models indicate that central control of breathing often stabilizes before recovery of AChE activity and recurrent AChE inhibition after the initial intoxication does not retrigger central apnea (131). These observations suggest that mechanisms in addition to AChE are involved in OP-triggered respiratory collapse during cholinergic crisis.
In OP-poisoned humans, respiratory failure can occur during early or late stages of cholinergic crisis. It is thought that early respiratory failure, which manifests within the first 24 h after exposure, is caused by a combination of central nervous system (CNS) and peripheral (outside the CNS) mechanisms, whereas late respiratory failure, which occurs 24–96 h after exposure, is triggered predominantly by peripheral mechanisms (62, 222). While the mechanisms of delayed respiratory failure are poorly understood, they likely include delayed neuromuscular dysfunction secondary to desensitization of nicotinic receptors and increased vascular permeability that leads to severe bronchorrhea and inflammation in the lungs (118). In addition to respiratory failure, other pulmonary complications can arise and/or persist in patients who survive acute OP intoxication. For example, persistent asthma has been reported in individuals accidentally poisoned by OPs (54).
Although the respiratory effects of acute intoxication with OPs at levels that cause rapid and profound depression of AChE activity resulting in cholinergic crisis are well documented, emerging studies suggest that subchronic or chronic exposures to OPs at levels that cause minimal depression of AChE activity may also be associated with adverse respiratory outcomes, specifically, asthma and asthmatic symptoms. The evidence for and the mechanisms mediating adverse effects of low-level OP pesticide exposures, defined as exposures that do not cause significant AChE inhibition, on lung function are the focus of this review.
EPIDEMIOLOGIC EVIDENCE OF OP-INDUCED ASTHMA
Early case reports provided the first indication that exposures to OPs at levels that do not cause cholinergic crisis may trigger asthma or asthmatic symptoms in adults (26, 234). Subsequent cross-sectional studies of farmers and their families, farmworkers, and commercial pesticide applicators in multiple countries around the world provided further evidence that occupational exposures to OP pesticides are associated with decreased lung function, wheezing, and adult-onset asthma (73, 105, 109–111, 113, 114, 166, 172, 175). Matched case-control studies of pesticide applicators in India, who were assessed for blood AChE activity and lung function before and after spraying season, similarly found significant inverse associations between subacute exposures to OPs and key lung function parameters that persisted for weeks after occupational exposures ceased (36, 174). Recently published case-control studies of adolescent pesticide applicators in Egypt concluded that results were consistent with an association between exposure to the OP chlorpyrifos (CPF; see Table 1 for a list of common abbreviations for OPs) and reduced lung function (27). An interesting relationship between OP exposure, allergy, and asthma has emerged from these studies of occupational OP exposures: stronger associations are observed between OP exposures and allergic (atopic) asthma compared with nonallergic (nonatopic) asthma (110, 111).
Table 1.
Pesticide abbreviations
| BRP | Bromofos |
| CPF/CPO | Chlorpyrifos/chlorpyrifos-oxon |
| DZN | Diazinon |
| FEN | Fenthion |
| MAL/MO | Malathion/malaoxon |
| PTH/PO | Parathion/paraoxon |
Although not as extensively studied as occupational OP exposures, environmental OP exposures have also been associated with increased risk of asthma and asthmatic symptoms in adults and adolescents in the general public (156, 249). More recently, concerns have grown that children may be particularly vulnerable to effects of environmental OPs on respiratory health. Growing appreciation of the unique susceptibility of the developing lung to environmental chemicals (160), coupled with the observation that children and adolescents disproportionately contribute to the increased prevalence of asthma worldwide (25, 236), has prompted investigations of whether prenatal and early life exposures to environmental contaminants, including pesticides, increase individual risk for asthma. Initial studies demonstrated an association between early life exposures to pesticides as a general class of environmental factors and increased risk of asthma and wheeze in children (201, 202). Subsequent prospective epidemiologic studies have considered the respiratory impacts of early life exposures to OPs specifically. Much of the data relevant to this question has come from The Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), the longest-running longitudinal birth cohort study of pesticide effects on children’s health, which has been studying children in a farmworker community in the Salinas Valley of northern California (66). Data from the CHAMACOS study supports the hypothesis that OP exposures during pregnancy and the first year of life, as determined by analysis of urinary OP metabolites in pregnant women and their infants, are associated with respiratory symptoms in children at 5 and 7 yr of age (185, 186).
The epidemiologic studies published to date vary considerably with respect to the populations assessed and the methods used to quantify OP exposure (typically via questionnaire, less often by quantification of urinary OP metabolites or blood AChE activity) and to assess respiratory health (self-reported respiratory symptoms vs. clinically diagnosed respiratory disease/symptoms vs. spirometry and other functional tests). These studies face significant challenges in assessing exposure—existing biomarkers of OP exposure (e.g., urinary OP metabolites and blood AChE activity) reflect only very recent exposures (197)—and controlling for potentially confounding coexposures (other classes of pesticides, allergens, livestock, etc.). Nonetheless, systematic reviews of the published epidemiologic literature generally support an association between OP pesticide exposure and respiratory disease, including asthma. Four of five meta-analyses (3, 58, 107, 248) concluded that the weight of evidence supports a strong association between OP exposure and increased risk of asthma and/or asthma exacerbations. The fifth study (155) concluded that there was a weak association but noted that because of significant methodological differences in quantifying pesticide exposure and lung function across studies, further research with more accurate assessments of OP pesticide exposure and robust measures of respiratory disease are needed before ruling out an association between OP exposure and asthma. Better understanding of the mechanisms underlying OP effects on respiratory function, and the factors (both genetic and environmental) that modulate respiratory responses to OPs, would further enable more robust study designs for epidemiologic studies.
EXPERIMENTAL EVIDENCE SUPPORTS CAUSAL LINK BETWEEN LOW-LEVEL OPS AND ASTHMA
While there are comparatively few preclinical studies of the respiratory effects of occupationally and environmentally relevant levels of OPs, the available experimental data support the epidemiologic evidence linking low-level OP exposures to asthma. Early publications by a research team in Mexico demonstrated that OPs induce bronchospasm in a variety of animals when administered at doses that significantly inhibit AChE activity but do not cause cholinergic crisis (96, 206). Specifically, intravenous injection of OPs increased total respiratory impedance in calves (96), while intraperitoneal injection of the OP parathion (PTH) produced a dose-dependent increase in lung resistance in guinea pigs as measured by plethysmography (206). Importantly, the latter study also found that subclinical doses of PTH caused airway hyperresponsiveness to acetylcholine. This effect was replicated in isolated perfused rabbit lung and prevented by the muscarinic receptor antagonist atropine (206).
Subsequent research led by US investigators Fryer and Lein provided more direct evidence of a causal link between low-level OP exposure and AHR. The preclinical model used by these investigators was the guinea pig exposed to OPs via subcutaneous injection to simulate dermal exposure, which is the predominant route of occupational exposure (72). Administration via subcutaneous injection also eliminated the potential confounding effect of reflex bronchoconstriction to irritation upon inhalation or tracheal instillation of OPs (107). With this guinea pig model, three structurally distinct diethyl phosphorothioate OPs—CPF, diazinon (DZN), and PTH—were observed to significantly potentiate vagally induced bronchoconstriction measured 24 h after a single injection of the OP (83, 141). Consistent with the epidemiologic data suggesting that atopy increases susceptibility to OP-induced asthma (110, 111), antigen sensitization without challenge significantly decreased the threshold dose for OP-induced AHR and exacerbated OP effects on vagally induced bronchoconstriction in animals exposed to either CPF (180) or PTH (182). In contrast, sensitization did not alter DZN-induced AHR (180). Subcutaneous exposure to the pyrethroid insecticide permethrin did not potentiate vagally induced bronchoconstriction in either sensitized or nonsensitized animals (141, 180), indicating that induction of AHR may be common to diethyl phosphorothioate OP pesticides but is not a generalized toxic effect of all pesticides. Important questions yet to be addressed are 1) whether OPs that are not phosphorothioates, which include a number of widely used OPs, also induce AHR and 2) the mechanism(s) underlying the differential influence of sensitization on AHR induced by CPF or PTH versus DZN.
As discussed above, the canonical mechanism of acute OP toxicity involves AChE inhibition (44); however, several lines of evidence from the studies conducted by Fryer and Lein established that OPs induce AHR independent of AChE inhibition. First, direct measurement of AChE activity in the lung, blood, and brain confirmed that CPF, PTH, and DZN caused AHR at doses that did not significantly inhibit AChE in these tissues (83, 141). Second, acute administration of eserine, a nonorganophosphate cholinesterase inhibitor, at a dose that significantly inhibited AChE did not potentiate vagally induced bronchoconstriction (83). Third, at doses that caused AHR in response to vagal stimulation, the OPs CPF, PTH, and DZN did not potentiate bronchoconstriction induced by intravenously administered acetylcholine in vagotomized guinea pigs (83, 141). Importantly, across all experimental conditions (vehicle, OP exposed, and eserine exposed), vagally mediated bronchoconstriction was frequency dependent and blocked by atropine, indicating that bronchoconstriction was mediated by release of acetylcholine from prejunctional nerves onto postjunctional M3 receptors on airway smooth muscle. Collectively, these studies provide proof-of-concept evidence that at levels such as might be encountered in occupational or residential settings OPs could feasibly contribute to asthma pathogenesis or exacerbation of asthmatic symptoms. The observation that these OPs induced AHR independent of AChE inhibition is of significant importance for risk assessments, because many regulatory agencies, including the US Environmental Protection Agency and the US Occupational Safety and Health Administration, use AChE inhibition as a point of departure for determining safe levels of OPs.
NEURAL MECHANISMS OF OP-INDUCED AHR
The vagus nerves provide the dominant autonomic and sensory control of airway smooth muscle tone and reactivity (28). Preganglionic parasympathetic nerve fibers within the vagus nerves synapse onto postganglionic parasympathetic nerve cell bodies clustered within the airways (Fig. 1A). Release of acetylcholine from postganglionic parasympathetic nerves causes bronchoconstriction by activating M3 muscarinic receptors on airway smooth muscle. However, acetylcholine also binds to autoinhibitory M2 muscarinic receptors on the postganglionic, prejunctional parasympathetic nerves (71, 84), which decreases further release of acetylcholine, thereby limiting vagally induced bronchoconstriction (Fig. 1B). The physiological importance of autoinhibitory M2 receptors was clearly established by demonstrating that M2-selective antagonists, such as gallamine and methoctramine, increased, by as much as 10-fold, bronchoconstriction in response to electrical stimulation of the vagus nerves (84) and reflex bronchoconstriction induced by histamine (45). The autoinhibitory function of prejunctional M2 receptors, which was first described in guinea pig lung (84), has since been demonstrated in autonomic target organs of all species studied thus far (205), including humans (161, 162).
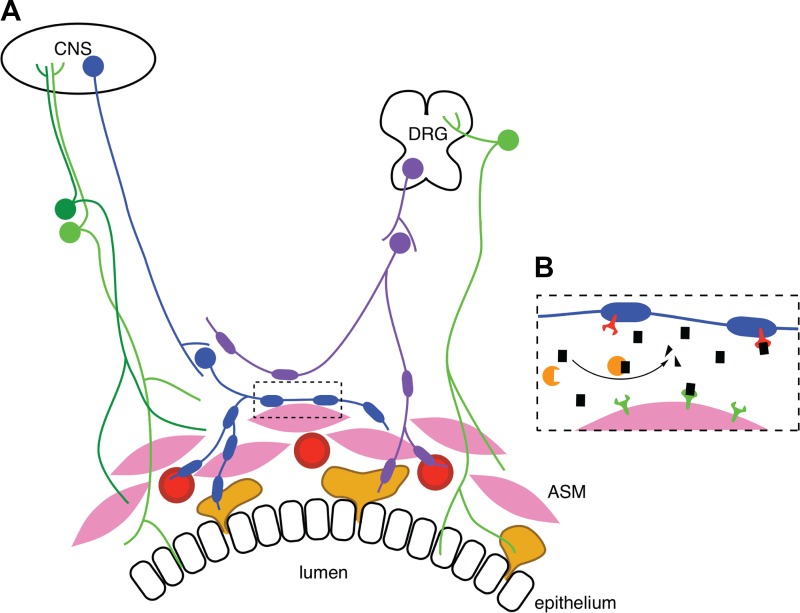
Fig. 1.
Innervation of the conducting airways. A: airway smooth muscle (ASM) contraction is directly controlled by vagal parasympathetic nerves (blue) arising from the brain stem in the central nervous system (CNS). These nerves synapse onto postganglionic parasympathetic neurons that supply ASM (pink). Blood vessels (red) and submucosal glands (yellow) are also innervated by postganglionic parasympathetic nerves. Sympathetic nerves (purple) innervate blood vessels and submucosal glands and synapse onto postganglionic parasympathetic fibers. In some nonhuman species, sympathetic nerves also directly innervate ASM. Sensory C fibers arising from dorsal root ganglia (DRG) (green) innervate the ASM, epithelium, and submucosal glands. B: at parasympathetic-ASM junctions, muscle contraction is mediated by ACh (black rectangles) released from the postganglionic nerves. ACh binding to postjunctional M3 muscarinic receptors (green) causes ASM contraction. Cholinergic signaling is terminated by enzymatic cleavage of ACh by acetylcholinesterase (orange) and by ACh binding to prejunctional autoregulatory M2 muscarinic receptors (red) expressed by postganglionic parasympathetic nerves that inhibit further release of ACh.
A significant body of literature provides evidence that OPs can interfere with the function of muscarinic receptors independent of AChE inhibition via direct interactions with receptors or modulation of downstream signaling pathways (reviewed in Refs. 124, 179; see Table 2). The outcome of these interactions (potentiation or blockade of receptor function) varies according to the OP, the muscarinic receptor subtype, and the target cell. This extensive literature suggests that OPs may induce AHR either by potentiating M3 receptor activity in airway smooth muscle or by blocking autoinhibitory M2 receptors on airway parasympathetic nerves. These hypotheses have been tested in the guinea pig model of OP-induced AHR using methacholine, a nonspecific muscarinic receptor agonist that activates postsynaptic M3 receptors on airway smooth muscle to cause bronchoconstriction, and pilocarpine, a muscarinic agonist with 100-fold selectivity for prejunctional M2 vs. postjunctional M3 receptors (84, 137) that binds neuronal M2 receptors to attenuate vagally induced bronchoconstriction in control animals (83, 141). At doses that caused AHR in the absence of significant AChE inhibition, CPF, DZN, and PTH had no effect on methacholine-induced bronchoconstriction 24 h after OP exposure. These data suggest that OP-induced AHR at this time point is not due to changes in either postsynaptic M3 receptors or airway smooth muscle contractility. In contrast, pilocarpine dose-response curves were shifted significantly to the right in OP-exposed animals relative to vehicle control animals, demonstrating decreased responsiveness of neuronal M2 receptors at 24 h after exposure. Importantly, at doses that did not decrease M2 receptor function OPs also did not cause AHR.
Table 2.
Experimental evidence of OP-induced muscarinic receptor dysfunction
| OP | Model | Exposure Paradigm | Key Findings | Reference |
|---|---|---|---|---|
| Sarin | Rat | Inhalation, 0.2 or 0.4 mg/m3 for 1 h/day; 1, 5, or 10 days | ↓M1 and M3 receptor densities in multiple brain regions when exposed during heat stress | (103) |
| CPF/CPO | Neonatal rat | Oral 1.5 or 3 mg·kg−1·day−1 CPF, 0.25 or 0.35 mg·kg−1·day−1 CPO; PND 1–6 | ↓Forebrain mAChR density by CPF but not CPO | (18, 19) |
| CPF | Guinea pig | sc, 70 or 390 mg/kg | ↑AHR via neuronal M2 receptor dysfunction | (83) |
| PTH, DZN | Guinea pig | sc, 1–10 mg/kg PTH, 0.75–75 mg/kg DZN | ↑AHR via neuronal M2 receptor dysfunction | (141) |
| CPF/CPO, PTH/PO | In vitro protein preparation | CPO IC50 70 µM | ↓GRK2 phosphorylation of M2 by CPF/CPO but not PTH/PO | (256) |
| CPO | Primary neuronal cell culture | 100 µM | ↑Agonist-stimulated M2 internalization | (225) |
AHR, airway hyperreactivity; CPF, chlorpyrifos; CPO, chlorpyrifos-oxon; DZN, diazinon; GRK2, G protein-coupled receptor kinase-2; mAChR, muscarinic acetylcholine receptor; OP, organophosphorus pesticide; PO, paraoxon; PND, postnatal day; PTH, parathion.
Interestingly, OP blockade of M2 receptors appears to be relatively specific to neuronal autoinhibitory receptors since the function of cardiac postjunctional M2 receptors was not altered in OP-exposed guinea pigs (83, 141). Furthermore, there was no evidence of effects on other organ systems predominantly controlled by muscarinic neurotransmission (e.g., pupils, salivary glands, gut). The biological reason(s) as to why the lung appears to be uniquely susceptible to OPs after systemic exposure to doses that do not cause cholinergic crisis is not known. However, these observations are consistent with literature suggesting that regulation of M2 receptors (17, 81, 136) and the effects of OPs on muscarinic receptors (50, 123, 215, 225, 243) vary with tissue and cell type.
These data support a model in which low-level OPs cause AHR by interfering with negative-feedback control of cholinergic signaling in parasympathetic nerves, thereby potentiating vagally induced bronchoconstriction. Resting cholinergic tone (163), reflex bronchoconstriction triggered by lung irritation (32, 227), and mucin secretion in the airways (187, 196) are also regulated by postganglionic parasympathetic nerves. Thus it seems likely that OPs also potentiate these physiological processes, which would be consistent with the hypothesis that OPs cause and/or exacerbate asthma. Although it has yet to be determined whether OPs cause neuronal M2 receptor dysfunction in the airways of species other than the guinea pig, the potential relevance of this model to human asthma is strongly supported by clinical studies demonstrating that neuronal M2 receptors are dysfunctional in the airways of asthmatic patients (10, 162). Additional questions that have yet to be systematically addressed are the persistence of OP effects on AHR—currently it has been shown that CPF-induced AHR persists for at least 7 days after a single injection in the guinea pig model (141)—and whether the mechanism of OP-induced AHR changes at varying times after exposure. The latter is relevant given experimental data demonstrating that the mechanism of ozone-induced AHR shifts from increased acetylcholine release as a result of M2 dysfunction at 1 day after exposure to increased substance P (SP) release coupled with airway smooth muscle hyperreactivity at 3 days after exposure (251).
The mechanism(s) by which OPs block neuronal M2 muscarinic receptor function in airways currently is not well understood. Initial efforts to address this question tested the hypothesis that OPs interact directly with airway nerves to pharmacologically antagonize M2 receptor function or downregulate M2 receptor expression (183), analogous to OP effects on muscarinic receptors in the brain (reviewed in Refs. 124, 179). However, several lines of experimental evidence argue against a significant role for these mechanisms in OP-induced AHR: 1) Neither PTH nor its oxon metabolite, paraoxon (PO), altered vagally induced bronchoconstriction in guinea pigs or isolated trachea when administered acutely at levels that did not inhibit AChE. 2) Neither PTH nor PO downregulated transcript levels of M2 receptors in human neuroblastoma cells or primary guinea pig parasympathetic nerve cultures. 3) Neither PTH nor PO decreased protein levels or inhibited carbachol-induced internalization of M2 receptors in primary autonomic neurons or COS cells transfected with cDNA encoding full-length human M2 receptor (183). Whereas the effect of low-level OP exposures on M2 receptor expression or ligand binding to neuronal M2 receptors in the intact lung has yet to be determined, collectively the published data support an alternative model in which OPs directly target intermediary cell type(s), which in turn release factors that act on parasympathetic nerves to cause neuronal M2 muscarinic receptor dysfunction and AHR. This proposed model of an indirect effect of OPs on neuronal M2 receptors in airways would be consistent with the growing evidence of noncholinergic mechanisms of OP neurotoxicity (43, 124, 179).
INFLAMMATORY MECHANISMS OF OP-INDUCED AHR
A key characteristic of asthma is lung inflammation. Type 2 (TH2) immune responses, which are classically associated with allergy, play a predominant role in human asthma (reviewed in Refs. 70, 91). Type 2 immunity is largely regulated by IL-1, IL-5, and IL-13 secreted by TH2 cells and is characterized by high IgE titers and eosinophilia (6). There is a strong relationship between airway eosinophilia and atopic asthma (11, 23, 52, 59, 78). The numbers of eosinophils in lung tissue, and levels of eosinophil cationic protein in bronchoalveolar lavage, are significantly increased in patients with atopic asthma (4, 129). Severe asthma is also associated with the presence of an increased and persistent population of eosinophils in the lungs, even in the absence of acute exacerbation (237). In a guinea pig model of antigen-induced AHR, antigen sensitization, without challenge, has been shown to recruit eosinophils to the lungs and cause them to cluster around airway nerves (47, 65), a phenomenon also observed in human asthma (47). Blocking eosinophil influx with antibodies to either IL-5 (65) or VLA-4 (80) or inhibiting eosinophil migration to the nerves with low doses of dexamethasone (69) prevents AHR and neuronal M2 receptor dysfunction in antigen-challenged guinea pigs. The mechanism by which eosinophils block neuronal M2 receptor activity involves release of major basic protein (MBP) from eosinophils activated by antigen challenge (46, 47) or viral infection (1, 2). MBP acts as an allosteric inhibitor of the neuronal M2 receptors (121), thereby potentiating vagally stimulated bronchoconstriction. Antibody blockade of MBP protects M2 function and inhibits AHR (68), and removal of MBP from M2 receptors by heparin acutely restores M2 receptor function and reverses AHR (82) in a guinea pig model of antigen-induced AHR.
Low-level OP exposures have been observed to influence allergic disease in experimental models. Specifically, OPs were shown to enhance immune responses to other chemical allergens in the local lymph node assay (86, 87) and to exacerbate eosinophilia in a mouse model of ovalbumin-induced allergic airway inflammation (169). However, preclinical evaluations in guinea pigs have not yielded a clear answer regarding the role of eosinophils in OP-induced AHR. In support of a role for eosinophils in OP-induced AHR, pretreatment with function-blocking antibody to IL-5 prevented OP-induced AHR in sensitized guinea pigs exposed to PTH (182) or CPF (180). However, neither OP increased the number of eosinophils in airways or associated with airway nerves or stimulated release of MBP from eosinophils in airways. Moreover, although IL-5 antibody decreased eosinophils and MBP in the airways of sensitized animals exposed to vehicle, it had no effect on these end points in sensitized animals exposed to either CPF or PTH. Interestingly, IL-5 antibody decreased MBP in the trachea of sensitized animals exposed to CPF, but heparin did not reverse CPF-induced AHR (180). There are at least two possible interpretations of these data: 1) eosinophils mediate CPF- and PTH-induced AHR in sensitized animals, but these OPs trigger eosinophils to release mediators other than MBP to cause AHR, or 2) IL-5 antibody prevents OP-induced AHR in sensitized animals via eosinophil-independent mechanism(s). Distinguishing between these possibilities has yet to be addressed experimentally; however, in support of the latter, IL-5 receptors are expressed by airway smooth muscle (189) and other inflammatory cells found in the lungs (55, 177).
In contrast to its significant block of OP-induced AHR in sensitized animals exposed to CPF or PTH, IL-5 antibody had no effect on AHR in sensitized animals exposed to DZN (180), consistent with the observation that DZN-induced AHR was not altered by sensitization. Pretreatment with IL-5 antibody also had no effect on CPF- or PTH-induced AHR in nonsensitized guinea pigs (180, 182). These data indicate that IL-5-independent mechanisms, and thus presumably eosinophil-independent mechanisms, mediate DZN-induced AHR and the effects of CPF and PTH on AHR and M2 receptor dysfunction in nonsensitized animals. A similar switch in mechanism depending upon sensitization status has been observed in preclinical models of virus-induced AHR (2). These findings have significant translational implications for predicting physiological responses of individuals exposed to low-level OP exposures and for designing individualized therapeutic interventions. They also raise the question of whether inflammatory mechanisms mediate OP-induced AHR in nonsensitized individuals. Inflammatory cell types other than eosinophils that are resident in the lungs, including macrophages, mast cells, and neutrophils, have been implicated in AHR triggered by other stimuli (74), including viral infection (138) and ozone exposure (250). It is also known that proinflammatory cytokines such as IFN-γ, TNF-α, and IL-1β can decrease in vivo neuronal M2 receptor expression and function in airways (122, 168, 230).
Evidence from diverse experimental models demonstrates that low-level OP exposure can activate many of the inflammatory mediators implicated in AHR (reviewed in Ref. 13; see Table 3). For example, inhalation of the OP nerve agent sarin triggers a robust inflammatory response in the lungs of guinea pigs (142) and rats (176), measured as significantly increased levels of inflammatory mediators, including histamine and prostaglandins, increased numbers of inflammatory cells, specifically eosinophils and macrophages, and increased mRNA expression for proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α. In the rat brain, low levels of sarin were observed to increase gene expression of TNF-α, IL-1β, and IL-6, coincident with decreased expression of M1 muscarinic receptors (103). Similarly, low-level CPF exposure increased expression of TNF-α and IL-1β in mouse brain (108). Ex vivo exposure to the OP malathion (MAL) activated mouse macrophages (193–195) and potentiated macrophage phagocytosis (75). In vivo exposure to low-level MAL stimulated mast cell degranulation in the intestine and skin (192), whereas ex vivo MAL exposure triggered histamine release from mast cells (190) and basophils (242). Studies of neutrophil activity in OP-exposed workers suggested that OPs may decrease neutrophil chemotaxis (104), although ex vivo CPF exposure of human whole blood cultures containing neutrophils and other inflammatory cells potentiated LPS-induced release of IFN-γ (60). The molecular mechanisms by which OPs activate immune cells remain largely unknown. There is evidence that OPs trigger nonneuronal cholinergic signaling of inflammatory cells (90, 144, 220), and mechanisms involving activation of signaling pathways by OP metabolites as well as OP-induced cytotoxicity/cell death of immune cells have also been implicated (56).
Table 3.
Experimental evidence of OP-induced inflammation and immune modulation
| OP | Model | Exposure Paradigm | Key Findings | Reference |
|---|---|---|---|---|
| Multiple | Occupationally exposed human serum | na | ↑Incidence of upper respiratory infections in applicators, ↓serum neutrophil chemotaxis | (104) |
| MAL | Mouse | Oral; chronic 0.1–100 mg·kg−1·day−1, 14 days acute 450–600 mg/kg | ↑Macrophage activity and mast cell degranulation | (191, 193) |
| CPF | Rat | Oral; 10 or 25 mg/kg | Systemic ↑ in TNF-α and core body temperature | (198) |
| Sarin | Rat | Inhalation; 0.2 or 0.4 mg/m3 for 1 h/day; 1, 5 or 10 days | ↑Expression of IL-1β, TNF-α, IL-6 mRNA in brain | (103) |
| CPF | Primary human fetal astrocytes | 25 µM; 7 days | ↑Transcripts of IL-6, GFAP, MAPK | (158) |
| BRP | Mouse, dermal challenge and LLNA | Dermal sensitization (0.3%); dermal or intrathecal challenge (0.03 or 0.003%); LLNA 0.1–3% | ↑Inflammatory cells and IFN-γ | (87) |
| PTH | Mouse, LLNA | Oral; 0.4–1.2 mg/kg | ↑Allergic potential of environmental allergens; ↑TH1 cytokines | (86) |
| PTH | Mouse OVA allergic inflammation model | Oral; 0.15 or 15 mg·kg−1·day−1, 5 days | Exacerbated allergic inflammation; ↑IgE, cytokines, chemokines, and eosinophilia | (169) |
| CPF, methyl-PTH | HepG2 cells | 2–8 µM, 24–72 h | ↓PON1 mRNA and protein, increased inflammatory cytokines | (157) |
| CPF/CPO | Human blood in vitro | 1–1,000 µg/ml | CPO ↑IFN-γ response to LPS | (60) |
BRP, bromofos; CPF, chlorpyrifos; CPO, chlorpyrifos-oxon; GFAP, glial fibrillary acidic protein; LLNA, local lymph node assay; MAL, malathion; na, not applicable; OP, organophosphorus pesticide; OVA, ovalbumin; PON1, paraoxonase 1; PTH, parathion.
Collectively, these observations provide biological plausibility for the hypothesis that OPs induce AHR by increasing inflammatory cytokines in the airways via stimulation of resident inflammatory cells other than eosinophils. In support of this hypothesis, recent studies have demonstrated that macrophages are required for OP-induced AHR in nonsensitized animals. Pretreatment with liposome-encapsulated clodronate induced alveolar macrophage apoptosis and prevented AHR in nonsensitized guinea pigs exposed to PTH (181). Transcripts for TNF-α and IL-1β were upregulated in alveolar macrophages isolated from PTH-treated guinea pig lungs, and although ex vivo exposure to PTH did not significantly increase IL-1β and TNF-α mRNA, it did increase TNF-α protein release from alveolar macrophages isolated from the lungs of naive guinea pigs. Consistent with these observations, pretreatment with the TNF-α inhibitor etanercept, but not the IL-1β receptor inhibitor anakinra, prevented PTH-induced AHR and protected neuronal M2 receptor function in the airways of PTH-exposed guinea pigs (181). These data are consistent with a model in which low-level OPs activate macrophages to release TNF-α, which causes M2 receptor dysfunction and AHR. However, the results from these studies also raise significant questions regarding the mechanistic relationship between OPs, macrophages, TNF-α, and parasympathetic nerves that lead to AHR, e.g., what is the cellular origin of TNF-α, and what factor(s) mediates increased TNF-α expression in alveolar macrophages in vivo? OP activation of macrophages in vivo may occur indirectly via activation of mast cells or upregulation of prostaglandins, since depletion of the former or blockade of the latter prevented MAL-induced macrophage activation in mice (191). The published data also do not rule out potential roles for other inflammatory mediators known to be influenced by OPs, such as IFN-γ.
OXIDATIVE STRESS AS A POTENTIAL MEDIATOR OF OP-INDUCED AHR
Oxidative stress plays an important role in the pathogenesis of asthma (reviewed in Refs. 39, 184, 200). Oxidative stress occurs when the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) exceeds the antioxidant capacity of the system. This imbalance can lead to oxidative and nitrative damage to macromolecules, which in turn can cause cell damage and elicit robust inflammatory responses (22). Tight control of ROS and RNS is essential for maintaining lung homeostasis, as indicated by observations that the redox system in the asthmatic lung is clearly unbalanced toward an oxidative state (41). This is likely due in part to the observation that resident and recruited immune cells in the asthmatic lung produce excess ROS and RNS (61). Preclinical studies have demonstrated that ROS directly triggers airway smooth muscle contraction (224) and causes a shift from a TH1 to a TH2 immune response (167). Higher levels of reactive molecules, such as nitric oxide, are associated with increased risk and severity of asthma, and fractional exhaled nitric oxide is used as a marker of TH2 inflammation to confirm atopic asthma and to diagnose cough-variant asthma and eosinophilic bronchitis (214, 232). Asthma is also associated with decreased levels of endogenous antioxidant molecules, both enzymatic, such as superoxide dismutase and catalase, and nonenzymatic, such as glutathione and vitamin E (41, 239). Evidence for the latter is the association between decreased glutathione levels in exhaled breath condensate and asthma exacerbation in children (42).
Relatively low levels of OPs have been shown to induce oxidative stress in a variety of experimental models (Table 4). Of particular relevance to OP-induced AHR: 1) CPF was observed to trigger rapid and reversible, concentration-dependent production of ROS in differentiated PC12 cells, a neuronal cell line used to model autonomic neurons, and CPF sensitized PC12 cells to other prooxidant stressors (48); 2) ex vivo exposure to MAL stimulated ROS generation in macrophages (194, 195); and 3) epidemiologic studies of pesticide workers identified an association between occupational OP exposure and biomarkers of oxidative stress (132, 135, 139, 207, 231). A major question in the field of OP neurotoxicology is whether oxidative stress mediates the neurotoxic effects of OPs. A 2009 review of the human and animal literature concluded that oxidative stress does contribute to chronic OP neurotoxicity (213). This conclusion was based on evidence of increased levels of protein nitration and lipid peroxidation, decreased total antioxidant capacity, and protective effects of antioxidants against OP-mediated histopathological and biochemical alterations. Thus there is strong experimental evidence linking OPs to oxidative stress and data to support the hypothesis that oxidative stress mediates OP neurotoxicity.
Table 4.
Experimental evidence of OP-induced oxidative stress
| OP | Model | Exposure Paradigm | Key Findings | Reference |
|---|---|---|---|---|
| Multiple | Occupational exposure | na | ↑Malondialdehyde (antioxidant enzyme) levels and bronchial obstruction | (132) |
| CPF | Human monocyte cell line U937 | 4.45–570 µM, 0.5–24 h | ↑Time- and concentration-dependent apoptosis mediated by caspase activation (caspase 3) | (164) |
| Multiple | Occupational exposure | na | ↑Urinary and leukocyte 8-OHdG levels during spraying, correlated with urinary metabolite levels | (139) |
| CPF, dichlorvos | Human natural killer cell lines | 0–100 ppm, 1–72 h | ↑Time- and concentration-dependent apoptosis mediated by caspase activation (caspase 3) | (145) |
| CPF | Oligodendrocyte progenitor cells | 3.9–250 µM, 24–72 h | ↑Oxidative stress and caspase activation | (203) |
| Multiple | Occupational exposure | na | ↑Oxidative stress and DNA damage, higher in applicators, same in cultured lymphocytes | (135) |
| CPO | Human and mouse neuroprogenitor cell lines | 0.001–100 µM | ↓Proliferation in human cells, increased caspase 3 activation in mouse cells | (49) |
| CPF | Mouse | Oral; 3–12 mg/kg | ↑Oxidative damage in hepatic and renal tissue (ROS, DNA-protein cross-linking, 8-OHdG, malondialdehyde, ↓glutathione) | (153) |
| PTH/PO, MAL/MO | Primary cultured human airway epithelium cells | 0.25–10 mM, 24 h | ↑Oxon metabolites produced dose-dependent cytotoxicity | (5) |
| PO | EL4 cells | 10 nM, 0–16 h | ↑Apoptosis via ER and mitochondrial mechanisms, mediated by calcium | (143) |
| CPF, methyl-PTH | HepG2 cells | 2–8 µM, 24–72 h | ↓PON1 mRNA, increased inflammatory cytokines | (157) |
| DZN | Tilapia | 0.97–3.95 ppm, 12 or 24 h | ↑Oxidative damage to proteins in liver and gills | (221) |
| CPF | Occupational exposure | Average exposure estimated at 3.7 µg·kg−1·day−1 | ↑In urinary 8-OHdG 1 day after spraying, returning to baseline after 2 days; marker for ox stress to DNA | (231) |
CPF, chlorpyrifos; CPO, chlorpyrifos-oxon; DZN, diazinon; ER, endoplasmic reticulum; MAL, malathion; MO, maloxon; na, not applicable; 8-OHdG, 8-hydroxydeoxyguanosine; OP, organophosphorus pesticide; PTH, parathion; PO, paraoxon; PON1, paraoxonase 1; ROS, reactive oxygen species.
It remains controversial, however, as to whether OPs can induce oxidative stress in the absence of significant AChE inhibition. Experimental evidence clearly demonstrates that acute AChE inhibition is associated with oxidative stress in the brain (146, 159) and that increased oxidative stress may contribute to (159) or exacerbate (148) cholinergic toxicity elicited by acute OP inhibition of AChE. However, in vitro studies demonstrate that OP-induced oxidative stress is antagonized by coexposure to antioxidants but not cholinergic antagonists (92), suggesting that AChE inhibition is not required for OP-induced oxidative stress. In at least one study of pesticide workers (207), increased levels of antioxidant enzymes and lipid peroxidation in blood leukocytes and erythrocytes were observed in the absence of blood cholinesterase inhibition, providing further evidence that OPs may induce oxidative stress independent of AChE inhibition. Such observations raise the question, however, of how OPs increase ROS/RNS production in the absence of significant AChE inhibition. It has been posited that ROS formed during cytochrome P-450-mediated metabolism of OPs predisposes toward an oxidative environment that evolves into a positive feedforward cycle of inflammation and oxidative stress (152). Since cytochrome P-450 enzymes involved in OP metabolism are expressed in the lung (94), this suggests the novel hypothesis that pulmonary metabolism of OPs creates a local prooxidant environment that promotes asthma or exacerbation of asthmatic symptoms. The more immediate data gap, however, is that although there is experimental evidence to support the plausibility of oxidative stress as a mechanism underlying OP-induced AHR, to date there are no studies that have directly tested this hypothesis.
MECHANISMS OF NEURONAL PLASTICITY AS MEDIATORS OF OP-INDUCED AHR
Neuronal plasticity is a key characteristic of a functional nervous system that enables fully differentiated, postmitotic neurons to adapt to changing environmental stimuli. Neuronal plasticity can be broadly categorized as changes in neuronal structure or morphology and changes in neurochemical properties. Both types of neuronal plasticity have been implicated in the pathogenesis of AHR in preclinical models. For instance, hyperinnervation in the lung has been linked to AHR in a murine model of early life allergen exposure (9) and in a nonhuman primate model of early life allergen and ozone exposure (127). In guinea pigs, increased dendritic arborization of neurons in postganglionic parasympathetic neurons correlated with increased excitability of lower airway parasympathetic nerves (101). Collectively, these studies suggest that structural changes in pulmonary nerves may contribute to AHR. Neurochemical changes, and in particular increased expression of tachykinins such as SP, have also been linked to AHR (67, 229, 241).
Neurotrophins play a key role in modulating neuroplasticity in the peripheral nervous system. Neurotrophins, which include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3, and neurotrophin-4/5, are critically involved in nervous system development and maintenance, influencing neuronal cell survival, differentiation, and target innervation (8, 21, 37, 40, 117, 199). Many studies have shown that neurotrophins, particularly NGF, contribute to AHR by altering cholinergic and/or sensory innervation in the airways (12, 24, 38, 53, 101, 119, 171, 173, 246). For example, NGF can increase expression of neuropeptides, such as SP, calcitonin gene-related peptide (CGRP), and neurokinin A, in sensory nerve fibers in the lung; increased levels of these neuropeptides can increase smooth muscle contraction via direct action on airway smooth muscle or indirectly via action on cholinergic nerve fibers (15, 29, 30, 51, 126, 154). NGF has also been shown to cause a phenotypic switch in sensory neurons innervating the trachea in the guinea pig, resulting in more cells expressing SP (120), and in the mouse NGF directly affects synaptic transmission in airway parasympathetic nerves (233).
Neurotrophins and their receptors are also expressed by many immune cell types, including macrophages and mast cells (165, 228), as well as by airway smooth muscle and airway epithelium during inflammatory events (38, 77). Additionally, eosinophils clustered around airway nerves in preclinical asthma models can increase expression of NGF and neuropeptides, contributing to airway hyperresponsiveness (59). The connection between inflammation and neuroplasticity is evident in a number of in vivo and in vitro systems. For example, ovalbumin sensitization in the guinea pig, a widely used model of allergic inflammation, increases excitability of vagal sensory nerves (147, 149, 253). The proinflammatory cytokines TNF-α and IL-1β have been shown to increase expression and release of neurotrophins in many in vitro preparations, including human monocytes (204), human and mouse pulmonary epithelial cells (76, 97), human bronchial smooth muscle cells (130), and rat astrocytes (88). Furthermore, IL-1β has been shown to induce NGF expression leading to AHR in both in vivo (229) and ex vivo (79) models. In addition to effects on neurochemistry, proinflammatory cytokines have been shown to modulate neuronal cytoarchitecture in autonomic neurons (95, 134).
Thus neuroimmune cross talk has proven to be important in airway physiology, especially in disease states (77, 93, 150, 165, 217). When this neuroimmune cross talk results in a positive feedback loop to further increase release of proinflammatory neuropeptides, it is classified as neurogenic inflammation. Neurotrophins have been shown to play a role in neurogenic inflammation and AHR, and bronchoalveolar lavage fluid levels of neurotrophins were found to positively correlate with asthma severity in children (216). Most notably, early life allergen exposure has been shown to result in persistent AHR and altered neuropeptide expression in several animal models (9, 24, 38, 77, 93, 127), with some of these studies directly implicating neurotrophin-dependent mechanisms (9, 24, 38).
OPs have several well-documented direct effects on both morphological and neurochemical plasticity of peripheral neurons (Table 5). At levels that do not inhibit the enzymatic activity of AChE, CPF promotes dendritic growth but inhibits axonal growth in sympathetic neurons cultured from rat superior cervical ganglia (116). Low levels of CPF also inhibit axonal growth in sensory neurons cultured from rat dorsal root ganglia (244) and neurite outgrowth in neuronal PC12 cells (209), while the oxon metabolite of CPF inhibits axonal growth of sensory neurons in developing zebrafish (245). Determining whether and how OPs affect the morphology of nerves that innervate airways and how such changes might influence AHR is an important area of future research. A number of neurochemical changes have also been described after OP exposure. OPs upregulate SP expression in the brain (170), and CPF and DZN alter transcriptional profiles of genes related to neurotransmitter phenotype differentiation in PC12 cells (208, 210, 212). OPs also affect a number of factors known to modulate neuronal plasticity. Preclinical models of low-dose chronic OP exposure have reported that OPs alter the expression of the neurotrophins NGF and BDNF in the brain (18–20, 140, 211, 219). DZN has been shown to alter transcriptional profiles of neurotrophic factors (208, 212) and to change the neurotransmitter phenotype of PC12 cells (210). However, whether low-level OP exposures alters expression or function of neurotrophins in the periphery remains an understudied question. Also not known is whether OPs upregulate SP or CGRP in the airways or alter vagal or dorsal root ganglion nociceptors.
Table 5.
Experimental evidence of OP effects on neuroplasticity
| OP | Model | Exposure Paradigm | Key Findings | Reference |
|---|---|---|---|---|
| PTH, FEN | Chick DRG explant | 1 µM, 72 h | ↓Cell membrane integrity; retraction of pseudopodia, induction of lipid vacuoles, lipid accumulation, disruption of tubular structures in growth cone | (223) |
| CPF/CPO | Neonatal rat | Oral; 1.5 or 3 mg·kg−1·day−1 CPF, 0.25 or 0.35 mg·kg−1·day−1 CPO, PND 1–6 | CPF but not CPO ↓forebrain NGF expression | (18, 19) |
| CPF/CPO | Rat SCG primary neuronal cell culture | 0.001–1 µM CPF, 0.001–1 nM CPO | ↓Axonal outgrowth but enhanced BMP-induced dendritic growth | (116) |
| CPF, Methyl-PTH | Neonatal rat | Oral, 4 or 6 mg/kg CPF, 0.6 or 0.9 mg/kg methyl-PTH, PND 10–20 | ↑NGF and BDNF expression in multiple brain regions; ↑marker of neuronal activity in hippocampus and cortex | (20) |
| CPF, DZN | Neonatal rat | sc, 1 mg/kg CPF, 1 or 2 mg/kg DZN, PND 1–4 | ↓Transcripts of neurotrophic growth factors in the FGF family in multiple brain regions | (211) |
| CPF | Rat | sc, 2.5–18 mg/kg, every other day for 30 days | ↓NGF expression in multiple brain regions, ↓in axonal transport in sciatic nerves ex vivo | (219) |
| CPF/CPO | Rat DRG primary neuronal cell culture | 0.001–10 µM CPF, 0.001–10 nM CPO, 24 h | ↓Axonal outgrowth | (244) |
| CPF/CPO | Zebrafish | 0.003–1 µM, 24–72 h | CPO but not CPF ↓axonal growth of sensory neurons and motor neurons and affected swimming behavior | (245) |
BDNF, brain-derived neurotrophic factor; BMP, bone morphogenetic protein; CPF, chlorpyrifos; CPO, chlorpyrifos-oxon; DRG, dorsal root ganglion; DZN, diazinon; FEN, fenthion; FGF, fibroblast growth factor; NGF, nerve growth factor; OP, organophosphorus pesticide; PND, postnatal day; PTH, parathion; SCG, superior cervical ganglia.
Although OPs have not yet been directly implicated in neurogenic inflammation in the airways, it seems like a logical line of inquiry given their known effects on inflammation and neuronal signaling (Table 3, Table 5). A testable hypothesis derived from these observations is that OP-induced inflammation alters NGF levels in the lung leading to changes in innervation and, subsequently, AHR (Fig. 2). Although it is unlikely that OP-induced changes in neurotrophin expression or action mediate effects of OPs on AHR 24 h after exposure, this mechanism could feasibly contribute to persistent effects of OPs on AHR. Convergence of OP effects on neurogenic inflammation and neuroplasticity could also be particularly significant in the context of developmental OP exposure. The developing lung is especially susceptible to environmental insult (178), and developmental exposure to other environmental contaminants has been shown to permanently affect pulmonary innervation in preclinical models (9, 240, 241, 252).
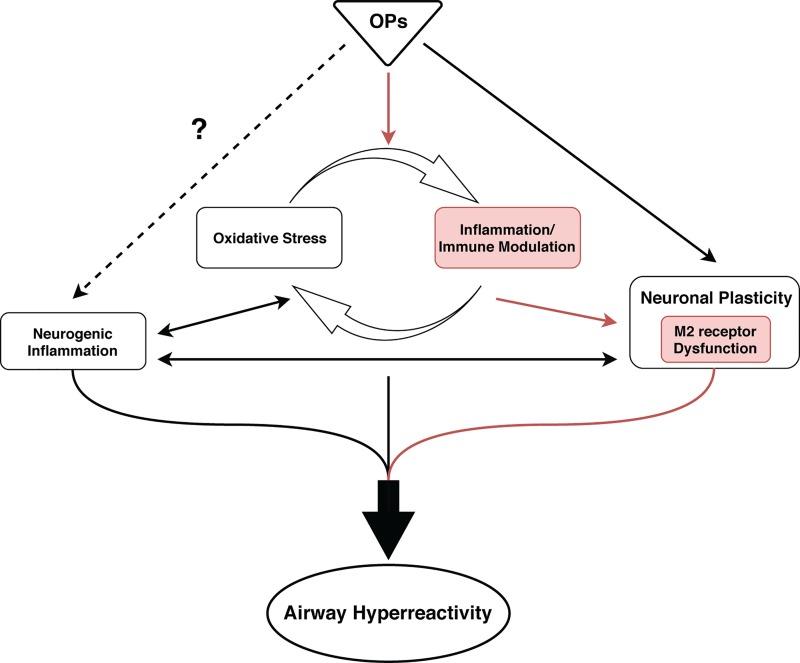
Fig. 2.
Multiple mechanisms of organophosphorus pesticide (OP) toxicity likely contribute to OP-induced airway hyperreactivity (AHR). Experimental evidence demonstrates that OPs cause dysfunction of prejunctional neuronal M2 muscarinic receptors via TNF-α-dependent mechanisms (red pathway). Additional proposed mechanisms of OP-induced AHR include neurogenic inflammation, oxidative stress, and altered neuronal plasticity. Although OPs have been shown to influence these processes in the central nervous system, OP effects on these processes in the periphery have yet to be causally linked to OP-induced AHR.
CONCLUSIONS
Both epidemiologic and clinical studies have linked exposures to occupational (73, 105, 109–111, 113, 114, 166, 172, 175) and environmental (156, 185, 186, 249) levels of OPs to increased incidence of asthma. Preclinical studies demonstrate that diethyl phosphorothioate OPs trigger AHR at doses that do not significantly inhibit AChE (83, 141), corroborating a causal relationship between low-level OPs and asthma. The working model of OP-induced AHR derived from the existing mechanistic data is that OPs cause dysfunction of autoinhibitory M2 muscarinic receptors expressed on postganglionic parasympathetic nerves in the airways (83, 141). Dysfunction of these neuronal M2 receptors results in increased release of acetylcholine in response to nerve stimulation, thereby increasing cholinergic drive on M3 muscarinic receptors expressed by airway smooth muscle. OP effects on neuronal M2 receptors appear to be mediated indirectly (183) via OP modulation of immune cells resident in the lung (180–182). There remain outstanding data gaps in this model, including 1) the identity and cause-effect relationship(s) between inflammatory cells, soluble mediators, and neuronal M2 muscarinic receptors in the airways; 2) whether the mechanism(s) of OP-induced AHR change with time after exposure; and 3) how they change with sensitization status. Given the significant overlap between mechanisms of OP neurotoxicity elucidated for target organs other than the lung and mechanisms of AHR in asthma, it seems likely that multiple mechanisms of OP neurotoxicity, including inflammation, oxidative stress, and neuronal plasticity, are involved in OP-induced AHR. Thus focusing on the interplay between these processes in future preclinical studies may provide important mechanistic insights, which in turn will inform epidemiologic studies as well as preventive or therapeutic measures.
Significant questions also remain regarding clinical aspects of OP-induced AHR. How long after a single exposure does OP-induced AHR persist? Do OPs other than the diethyl phosphorothioate OPs cause AHR? What factors in addition to atopy (180–182) influence respiratory responses to OPs—do sex, age, specific gene mutations, and polymorphisms influence outcome? But perhaps the most significant data gaps involve our understanding of whether, and how, developmental or early life OP exposures contribute to individual risk for adolescent or adult asthma. Although the epidemiologic evidence suggests that early life exposure to OPs increases the risk of developing asthma later in life (185, 186), this relationship has yet to be demonstrated in a preclinical model. Moreover, the mechanisms by which OPs influence the developing lung to increase susceptibility to asthma are not obvious from the current mechanistic understanding of OP-induced AHR. However, “borrowing” from the literature of OP neurotoxicity, plausible hypotheses can be derived. First, as discussed above, OPs can modulate in vitro and in vivo axonal and dendritic morphogenesis of autonomic and sensory neurons (116, 244, 245). Experimental evidence from preclinical models of asthma indicates that increased dendritic arborization of postganglionic parasympathetic neurons (101) or increased sensory innervation of the lung is correlated with AHR (59). Collectively, these data suggest the testable hypothesis that early life exposures to OPs elicit lasting changes in the morphology of airway nerves, thereby altering functional patterns of neuronal connectivity to increase the susceptibility of the lung to AHR. A second possibility for how developmental OP exposures might cause persistent asthma derives from literature demonstrating that OPs can cause epigenetic changes (133, 188, 254, 255).
The reproducible observation that OPs induce AHR independent of AChE inhibition in both humans and preclinical models is of translational significance because many regulatory agencies, in both the United States and Europe, use peripheral cholinesterase inhibition as a regulatory point of departure for OP risk assessments. It is important in this context that current data indicate that OP-induced AHR may occur at OP levels below current regulatory thresholds for human health and safety. A more comprehensive understanding of the mechanisms of OP-induced AHR may provide a scientifically rational basis for reassessment of safe exposure limits for OPs. Another important area of future research is the identification of susceptible subpopulations and understanding how mechanism(s) of OP-induced AHR vary in different populations. This information will be critical for predicting individual physiological responses to OP exposures and for designing more effective therapeutic interventions. The evolving literature on OP-induced AHR also raises significant questions regarding the current use of OP insecticides in the inner cities to control cockroach antigen (16, 35, 151, 235, 238), which itself is considered a predominant trigger of asthma (7, 63). Specifically, these data suggest that exposure to OP insecticides may be contributing to, rather than ameliorating, asthma.
GRANTS
This work was supported by National Institutes of Health Grants (NIH) R01-ES-017592, R01-HL-131525, R01-HL-113023, P30-ES-023513, and U54-HD-079125 and NIH Predoctoral Fellowship Grant T32-HL-07013 (to F. C. Shaffo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.J.L. conceived and designed research; F.C.S. and P.J.L. prepared figures; F.C.S. and P.J.L. drafted manuscript; A.C.G. and P.J.L. edited and revised manuscript; F.C.S., A.C.G., A.D.F., and P.J.L. approved final version of manuscript.
REFERENCES
- 1.Adamko DJ, Fryer AD, Bochner BS, Jacoby DB. CD8+ T lymphocytes in viral hyperreactivity and M2 muscarinic receptor dysfunction. Am J Respir Crit Care Med 167: 550–556, 2003. doi: 10.1164/rccm.200206-506OC. [DOI] [PubMed] [Google Scholar]
- 2.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m2 muscarinic receptor dysfunction, and antiviral effects. J Exp Med 190: 1465–1478, 1999. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral AF. Pesticides and asthma: challenges for epidemiology. Front Public Health 2: 6, 2014. doi: 10.3389/fpubh.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin K, Lúdvíksdóttir D, Janson C, Nettelbladt O, Björnsson E, Roomans GM, Boman G, Sevéus L, Venge P; BHR Group . Inflammation and structural changes in the airways of patients with atopic and nonatopic asthma. Am J Respir Crit Care Med 162: 2295–2301, 2000. doi: 10.1164/ajrccm.162.6.9912001. [DOI] [PubMed] [Google Scholar]
- 5.Angelini DJ, Moyer RA, Cole S, Willis KL, Oyler J, Dorsey RM, Salem H. The pesticide metabolites paraoxon and malaoxon induce cellular death by different mechanisms in cultured human pulmonary cells. Int J Toxicol 34: 433–441, 2015. doi: 10.1177/1091581815593933. [DOI] [PubMed] [Google Scholar]
- 6.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol 135: 626–635, 2015. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomés A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol 107: 419–428, 2001. doi: 10.1067/mai.2001.112854. [DOI] [PubMed] [Google Scholar]
- 8.Aven L, Ai X. Mechanisms of respiratory innervation during embryonic development. Organogenesis 9: 194–198, 2013. doi: 10.4161/org.24842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aven L, Paez-Cortez J, Achey R, Krishnan R, Ram-Mohan S, Cruikshank WW, Fine A, Ai X. An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyperreactivity. FASEB J 28: 897–907, 2014. doi: 10.1096/fj.13-238212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayala LE, Ahmed T. Is there loss of protective muscarinic receptor mechanism in asthma? Chest 96: 1285–1291, 1989. doi: 10.1378/chest.96.6.1285. [DOI] [PubMed] [Google Scholar]
- 11.Azzawi M, Johnston PW, Majumdar S, Kay AB, Jeffery PK. T lymphocytes and activated eosinophils in airway mucosa in fatal asthma and cystic fibrosis. Am Rev Respir Dis 145: 1477–1482, 1992. doi: 10.1164/ajrccm/145.6.1477. [DOI] [PubMed] [Google Scholar]
- 12.Bachar O, Adner M, Uddman R, Cardell LO. Nerve growth factor enhances cholinergic innervation and contractile response to electric field stimulation in a murine in vitro model of chronic asthma. Clin Exp Allergy 34: 1137–1145, 2004. doi: 10.1111/j.1365-2222.2004.1868.x. [DOI] [PubMed] [Google Scholar]
- 13.Banks CN, Lein PJ. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology 33: 575–584, 2012. doi: 10.1016/j.neuro.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr DB, Allen R, Olsson AO, Bravo R, Caltabiano LM, Montesano A, Nguyen J, Udunka S, Walden D, Walker RD, Weerasekera G, Whitehead RD Jr, Schober SE, Needham LL. Concentrations of selective metabolites of organophosphorus pesticides in the United States population. Environ Res 99: 314–326, 2005. doi: 10.1016/j.envres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Belvisi MG. Airway sensory innervation as a target for novel therapies: an outdated concept? Curr Opin Pharmacol 3: 239–243, 2003. doi: 10.1016/S1471-4892(03)00048-1. [DOI] [PubMed] [Google Scholar]
- 16.Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, Landrigan PJ, Wolff MS. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect 111: 79–84, 2003. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard V, Décossas M, Liste I, Bloch B. Intraneuronal trafficking of G-protein-coupled receptors in vivo. Trends Neurosci 29: 140–147, 2006. doi: 10.1016/j.tins.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Betancourt AM, Burgess SC, Carr RL. Effect of developmental exposure to chlorpyrifos on the expression of neurotrophin growth factors and cell-specific markers in neonatal rat brain. Toxicol Sci 92: 500–506, 2006. doi: 10.1093/toxsci/kfl004. [DOI] [PubMed] [Google Scholar]
- 19.Betancourt AM, Carr RL. The effect of chlorpyrifos and chlorpyrifos-oxon on brain cholinesterase, muscarinic receptor binding, and neurotrophin levels in rats following early postnatal exposure. Toxicol Sci 77: 63–71, 2004. doi: 10.1093/toxsci/kfh003. [DOI] [PubMed] [Google Scholar]
- 20.Betancourt AM, Filipov NM, Carr RL. Alteration of neurotrophins in the hippocampus and cerebral cortex of young rats exposed to chlorpyrifos and methyl parathion. Toxicol Sci 100: 445–455, 2007. doi: 10.1093/toxsci/kfm248. [DOI] [PubMed] [Google Scholar]
- 21.Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 14: 2919–2937, 2000. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 22.Biswas SK. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev 2016: 5698931, 2016. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel FB. Eosinophilic inflammation in asthma. N Engl J Med 323: 1033–1039, 1990. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 24.Braun A, Lommatzsch M, Neuhaus-Steinmetz U, Quarcoo D, Glaab T, McGregor GP, Fischer A, Renz H. Brain-derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br J Pharmacol 141: 431–440, 2004. doi: 10.1038/sj.bjp.0705638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brozek G, Lawson J, Szumilas D, Zejda J. Increasing prevalence of asthma, respiratory symptoms, and allergic diseases: four repeated surveys from 1993-2014. Respir Med 109: 982–990, 2015. doi: 10.1016/j.rmed.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Bryant DH. Asthma due to insecticide sensitivity. Aust NZ J Med 15: 66–68, 1985. doi: 10.1111/j.1445-5994.1985.tb02740.x. [DOI] [PubMed] [Google Scholar]
- 27.Callahan CL, Al-Batanony M, Ismail AA, Abdel-Rasoul G, Hendy O, Olson JR, Rohlman DS, Bonner MR. Chlorpyrifos exposure and respiratory health among adolescent agricultural workers. Int J Environ Res Public Health 11: 13117–13129, 2014. doi: 10.3390/ijerph111213117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canning BJ, Fischer A. Neural regulation of airway smooth muscle tone. Respir Physiol 125: 113–127, 2001. doi: 10.1016/S0034-5687(00)00208-5. [DOI] [PubMed] [Google Scholar]
- 29.Canning BJ, Reynolds SM, Anukwu LU, Kajekar R, Myers AC. Endogenous neurokinins facilitate synaptic transmission in guinea pig airway parasympathetic ganglia. Am J Physiol Regul Integr Comp Physiol 283: R320–R330, 2002. doi: 10.1152/ajpregu.00001.2002. [DOI] [PubMed] [Google Scholar]
- 30.Canning BJ, Spina D (Editors). Sensory Nerves. Berlin: Springer Science & Business Media, 2009, p. 623. doi: 10.1007/978-3-540-79090-7. [DOI] [Google Scholar]
- 31.Carey JL, Dunn C, Gaspari RJ. Central respiratory failure during acute organophosphate poisoning. Respir Physiol Neurobiol 189: 403–410, 2013. doi: 10.1016/j.resp.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Carr MJ, Undem BJ. Pharmacology of vagal afferent nerve activity in guinea pig airways. Pulm Pharmacol Ther 16: 45–52, 2003. doi: 10.1016/S1094-5539(02)00179-7. [DOI] [PubMed] [Google Scholar]
- 33.Castorina R, Bradman A, Fenster L, Barr DB, Bravo R, Vedar MG, Harnly ME, McKone TE, Eisen EA, Eskenazi B. Comparison of current-use pesticide and other toxicant urinary metabolite levels among pregnant women in the CHAMACOS cohort and NHANES. Environ Health Perspect 118: 856–863, 2010. doi: 10.1289/ehp.0901568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CDC Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables (July 2014). Atlanta, GA: Centers for Disease Control and Prevention, 2014. [Google Scholar]
- 35.CDC Second National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention National Center for Environmental Health, 2003. [Google Scholar]
- 36.Chakraborty S, Mukherjee S, Roychoudhury S, Siddique S, Lahiri T, Ray MR. Chronic exposures to cholinesterase-inhibiting pesticides adversely affect respiratory health of agricultural workers in India. J Occup Health 51: 488–497, 2009. doi: 10.1539/joh.L9070. [DOI] [PubMed] [Google Scholar]
- 37.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4: 299–309, 2003. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 38.Chen YL, Huang HY, Lee CC, Chiang BL. Small interfering RNA targeting nerve growth factor alleviates allergic airway hyperresponsiveness. Mol Ther Nucleic Acids 3: e158, 2014. doi: 10.1038/mtna.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho YS, Moon HB. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol Res 2: 183–187, 2010. doi: 10.4168/aair.2010.2.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorentz CU, Alston EN, Belcik T, Lindner JR, Giraud GD, Habecker BA. Heterogeneous ventricular sympathetic innervation, altered beta-adrenergic receptor expression, and rhythm instability in mice lacking the p75 neurotrophin receptor. Am J Physiol Heart Circ Physiol 298: H1652–H1660, 2010. doi: 10.1152/ajpheart.01128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 12: 93–124, 2010. [Erratum in Antioxid Redox Signal 12: 321, 2010.] doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corradi M, Folesani G, Andreoli R, Manini P, Bodini A, Piacentini G, Carraro S, Zanconato S, Baraldi E. Aldehydes and glutathione in exhaled breath condensate of children with asthma exacerbation. Am J Respir Crit Care Med 167: 395–399, 2003. doi: 10.1164/rccm.200206-507OC. [DOI] [PubMed] [Google Scholar]
- 43.Costa LG. Organophosphorus compounds at 80: some old and new issues. Toxicol Sci 162: 24–35, 2018. doi: 10.1093/toxsci/kfx266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa LG, Giordano G, Guizzetti M, Vitalone A. Neurotoxicity of pesticides: a brief review. Front Biosci 13: 1240–1249, 2008. doi: 10.2741/2758. [DOI] [PubMed] [Google Scholar]
- 45.Costello RW, Evans CM, Yost BL, Belmonte KE, Gleich GJ, Jacoby DB, Fryer AD. Antigen-induced hyperreactivity to histamine: role of the vagus nerves and eosinophils. Am J Physiol Lung Cell Mol Physiol 276: L709–L714, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Costello RW, Jacoby DB, Gleich GJ, Fryer AD. Eosinophils and airway nerves in asthma. Histol Histopathol 15: 861–868, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol Lung Cell Mol Physiol 273: L93–L103, 1997. doi: 10.1152/ajplung.1997.273.1.L93. [DOI] [PubMed] [Google Scholar]
- 48.Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Brain Res Dev Brain Res 121: 189–195, 2000. doi: 10.1016/S0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- 49.Culbreth ME, Harrill JA, Freudenrich TM, Mundy WR, Shafer TJ. Comparison of chemical-induced changes in proliferation and apoptosis in human and mouse neuroprogenitor cells. Neurotoxicology 33: 1499–1510, 2012. doi: 10.1016/j.neuro.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Dabisch PA, To F, Kerut EK, Horsmon MS, Mioduszewski RJ. Multiple exposures to sarin vapor result in parasympathetic dysfunction in the eye but not the heart. Toxicol Sci 99: 354–361, 2007. doi: 10.1093/toxsci/kfm167. [DOI] [PubMed] [Google Scholar]
- 51.Dakhama A, Park JW, Taube C, El Gazzar M, Kodama T, Miyahara N, Takeda K, Kanehiro A, Balhorn A, Joetham A, Loader JE, Larsen GL, Gelfand EW. Alteration of airway neuropeptide expression and development of airway hyperresponsiveness following respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 288: L761–L770, 2005. doi: 10.1152/ajplung.00143.2004. [DOI] [PubMed] [Google Scholar]
- 52.De Monchy JG, Kauffman HF, Venge P, Koëter GH, Jansen HM, Sluiter HJ, De Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis 131: 373–376, 1985. [DOI] [PubMed] [Google Scholar]
- 53.de Vries A, Engels F, Henricks PA, Leusink-Muis T, McGregor GP, Braun A, Groneberg DA, Dessing MC, Nijkamp FP, Fischer A. Airway hyper-responsiveness in allergic asthma in guinea-pigs is mediated by nerve growth factor via the induction of substance P: a potential role for trkA. Clin Exp Allergy 36: 1192–1200, 2006. doi: 10.1111/j.1365-2222.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 54.Deschamps D, Questel F, Baud FJ, Gervais P, Dally S. Persistent asthma after acute inhalation of organophosphate insecticide. Lancet 344: 1712, 1994. doi: 10.1016/S0140-6736(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 55.Dewachi O, Joubert P, Hamid Q, Lavoie JP. Expression of interleukin (IL)-5 and IL-9 receptors on neutrophils of horses with heaves. Vet Immunol Immunopathol 109: 31–36, 2006. doi: 10.1016/j.vetimm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Díaz-Resendiz KJ, Toledo-Ibarra GA, Girón-Pérez MI. Modulation of immune response by organophosphorus pesticides: fishes as a potential model in immunotoxicology. J Immunol Res 2015: 213836, 2015. doi: 10.1155/2015/213836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding G, Ji R, Bao Y. Risk and protective factors for the development of childhood asthma. Paediatr Respir Rev 16: 133–139, 2015. [DOI] [PubMed] [Google Scholar]
- 58.Doust E, Ayres JG, Devereux G, Dick F, Crawford JO, Cowie H, Dixon K. Is pesticide exposure a cause of obstructive airways disease? Eur Respir Rev 23: 180–192, 2014. doi: 10.1183/09059180.00005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drake MG, Lebold KM, Roth-Carter QR, Pincus AB, Blum ED, Proskocil BJ, Jacoby DB, Fryer AD, Nie Z. Eosinophil and airway nerve interactions in asthma. J Leukoc Biol 104: 61–67, 2018. doi: 10.1002/JLB.3MR1117-426R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duramad P, Tager IB, Leikauf J, Eskenazi B, Holland NT. Expression of Th1/Th2 cytokines in human blood after in vitro treatment with chlorpyrifos, and its metabolites, in combination with endotoxin LPS and allergen Der p1. J Appl Toxicol 26: 458–465, 2006. doi: 10.1002/jat.1162. [DOI] [PubMed] [Google Scholar]
- 61.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA 98: 2622–2627, 2001. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eddleston M, Mohamed F, Davies JO, Eyer P, Worek F, Sheriff MH, Buckley NA. Respiratory failure in acute organophosphorus pesticide self-poisoning. QJM 99: 513–522, 2006. doi: 10.1093/qjmed/hcl065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eggleston PA, Arruda LK. Ecology and elimination of cockroaches and allergens in the home. J Allergy Clin Immunol 107, Suppl: S422–S429, 2001. doi: 10.1067/mai.2001.113671. [DOI] [PubMed] [Google Scholar]
- 64.Eggleston PA, Buckley TJ, Breysse PN, Wills-Karp M, Kleeberger SR, Jaakkola JJ. The environment and asthma in U.S. inner cities. Environ Health Perspect 107, Suppl 3: 439–450, 1999. doi: 10.1289/ehp.99107s3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elbon CL, Jacoby DB, Fryer AD. Pretreatment with an antibody to interleukin-5 prevents loss of pulmonary M2 muscarinic receptor function in antigen-challenged guinea pigs. Am J Respir Cell Mol Biol 12: 320–328, 1995. doi: 10.1165/ajrcmb.12.3.7873198. [DOI] [PubMed] [Google Scholar]
- 66.Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect 107, Suppl 3: 409–419, 1999. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans CM, Belmonte KE, Costello RW, Jacoby DB, Gleich GJ, Fryer AD. Substance P-induced airway hyperreactivity is mediated by neuronal M2 receptor dysfunction. Am J Physiol Lung Cell Mol Physiol 279: L477–L486, 2000. doi: 10.1152/ajplung.2000.279.3.L477. [DOI] [PubMed] [Google Scholar]
- 68.Evans CM, Fryer AD, Jacoby DB, Gleich GJ, Costello RW. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest 100: 2254–2262, 1997. doi: 10.1172/JCI119763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evans CM, Jacoby DB, Fryer AD. Effects of dexamethasone on antigen-induced airway eosinophilia and M2 receptor dysfunction. Am J Respir Crit Care Med 163: 1484–1492, 2001. doi: 10.1164/ajrccm.163.6.2007047. [DOI] [PubMed] [Google Scholar]
- 70.Fahy JV. Type 2 inflammation in asthma𠀓present in most, absent in many. Nat Rev Immunol 15: 57–65, 2015. [Erratum in Nat Rev Immunol 15: 129, 2015.] doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Faulkner D, Fryer AD, Maclagan J. Postganglionic muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 88: 181–187, 1986. doi: 10.1111/j.1476-5381.1986.tb09485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fenske RA, Farahat FM, Galvin K, Fenske EK, Olson JR. Contributions of inhalation and dermal exposure to chlorpyrifos dose in Egyptian cotton field workers. Int J Occup Environ Health 18: 198–209, 2012. doi: 10.1179/1077352512Z.00000000030. [DOI] [PubMed] [Google Scholar]
- 73.Fieten KB, Kromhout H, Heederik D, van Wendel de Joode B. Pesticide exposure and respiratory health of indigenous women in Costa Rica. Am J Epidemiol 169: 1500–1506, 2009. doi: 10.1093/aje/kwp060. [DOI] [PubMed] [Google Scholar]
- 74.Finkelman FD, Boyce JA, Vercelli D, Rothenberg ME. Key advances in mechanisms of asthma, allergy, and immunology in 2009. J Allergy Clin Immunol 125: 312–318, 2010. doi: 10.1016/j.jaci.2009.12.936. [DOI] [PubMed] [Google Scholar]
- 75.Flipo D, Bernier J, Girard D, Krzystyniak K, Fournier M. Combined effects of selected insecticides on humoral immune response in mice. Int J Immunopharmacol 14: 747–752, 1992. doi: 10.1016/0192-0561(92)90071-R. [DOI] [PubMed] [Google Scholar]
- 76.Fox AJ, Patel HJ, Barnes PJ, Belvisi MG. Release of nerve growth factor by human pulmonary epithelial cells: role in airway inflammatory diseases. Eur J Pharmacol 424: 159–162, 2001. doi: 10.1016/S0014-2999(01)01138-4. [DOI] [PubMed] [Google Scholar]
- 77.Freund-Michel V, Frossard N. The nerve growth factor and its receptors in airway inflammatory diseases. Pharmacol Ther 117: 52–76, 2008. doi: 10.1016/j.pharmthera.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Frigas E, Loegering DA, Solley GO, Farrow GM, Gleich GJ. Elevated levels of the eosinophil granule major basic protein in the sputum of patients with bronchial asthma. Mayo Clin Proc 56: 345–353, 1981. [PubMed] [Google Scholar]
- 79.Frossard N, Naline E, Olgart Höglund C, Georges O, Advenier C. Nerve growth factor is released by IL-1beta and induces hyperresponsiveness of the human isolated bronchus. Eur Respir J 26: 15–20, 2005. doi: 10.1183/09031936.05.00047804. [DOI] [PubMed] [Google Scholar]
- 80.Fryer AD, Costello RW, Yost BL, Lobb RR, Tedder TF, Steeber DA, Bochner BS. Antibody to VLA-4, but not to L-selectin, protects neuronal M2 muscarinic receptors in antigen-challenged guinea pig airways. J Clin Invest 99: 2036–2044, 1997. doi: 10.1172/JCI119372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fryer AD, el-Fakahany EE. An endogenous factor induces heterogeneity of binding sites of selective muscarinic receptor antagonists in rat heart. Membr Biochem 8: 127–132, 1989. doi: 10.3109/09687688909025826. [DOI] [PubMed] [Google Scholar]
- 82.Fryer AD, Jacoby DB. Function of pulmonary M2 muscarinic receptors in antigen-challenged guinea pigs is restored by heparin and poly-L-glutamate. J Clin Invest 90: 2292–2298, 1992. doi: 10.1172/JCI116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fryer AD, Lein PJ, Howard AS, Yost BL, Beckles RA, Jett DA. Mechanisms of organophosphate insecticide-induced airway hyperreactivity. Am J Physiol Lung Cell Mol Physiol 286: L963–L969, 2004. doi: 10.1152/ajplung.00343.2003. [DOI] [PubMed] [Google Scholar]
- 84.Fryer AD, Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 83: 973–978, 1984. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukuyama T, Kosaka T, Tajima Y, Ueda H, Hayashi K, Shutoh Y, Harada T. Prior exposure to organophosphorus and organochlorine pesticides increases the allergic potential of environmental chemical allergens in a local lymph node assay. Toxicol Lett 199: 347–356, 2010. doi: 10.1016/j.toxlet.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 87.Fukuyama T, Tajima Y, Ueda H, Hayashi K, Shutoh Y, Harada T, Kosaka T. Allergic reaction induced by dermal and/or respiratory exposure to low-dose phenoxyacetic acid, organophosphorus, and carbamate pesticides. Toxicology 261: 152–161, 2009. doi: 10.1016/j.tox.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 88.Gadient RA, Cron KC, Otten U. Interleukin-1 beta and tumor necrosis factor-alpha synergistically stimulate nerve growth factor (NGF) release from cultured rat astrocytes. Neurosci Lett 117: 335–340, 1990. doi: 10.1016/0304-3940(90)90687-5. [DOI] [PubMed] [Google Scholar]
- 89.Gaffin JM, Kanchongkittiphon W, Phipatanakul W. Perinatal and early childhood environmental factors influencing allergic asthma immunopathogenesis. Int Immunopharmacol 22: 21–30, 2014. doi: 10.1016/j.intimp.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galloway T, Handy R. Immunotoxicity of organophosphorous pesticides. Ecotoxicology 12: 345–363, 2003. doi: 10.1023/A:1022579416322. [DOI] [PubMed] [Google Scholar]
- 91.Gillissen A, Paparoupa M. Inflammation and infections in asthma. Clin Respir J 9: 257–269, 2015. doi: 10.1111/crj.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giordano G, Afsharinejad Z, Guizzetti M, Vitalone A, Kavanagh TJ, Costa LG. Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol Appl Pharmacol 219: 181–189, 2007. doi: 10.1016/j.taap.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 93.Groneberg DA, Quarcoo D, Frossard N, Fischer A. Neurogenic mechanisms in bronchial inflammatory diseases. Allergy 59: 1139–1152, 2004. doi: 10.1111/j.1398-9995.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 94.Gundert-Remy U, Bernauer U, Blömeke B, Döring B, Fabian E, Goebel C, Hessel S, Jäckh C, Lampen A, Oesch F, Petzinger E, Völkel W, Roos PH. Extrahepatic metabolism at the body’s internal-external interfaces. Drug Metab Rev 46: 291–324, 2014. doi: 10.3109/03602532.2014.900565. [DOI] [PubMed] [Google Scholar]
- 95.Guo X, Chandrasekaran V, Lein P, Kaplan PL, Higgins D. Leukemia inhibitory factor and ciliary neurotrophic factor cause dendritic retraction in cultured rat sympathetic neurons. J Neurosci 19: 2113–2121, 1999. doi: 10.1523/JNEUROSCI.19-06-02113.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gustin P, Dhem AR, Lomba F, Lekeux P, Van de Woestijne KP, Làndsér FJ. Measurement of total respiratory impedance in calves by the forced oscillation technique. J Appl Physiol (1985) 64: 1786–1791, 1988. doi: 10.1152/jappl.1988.64.5.1786. [DOI] [PubMed] [Google Scholar]
- 97.Hahn C, Islamian AP, Renz H, Nockher WA. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J Allergy Clin Immunol 117: 787–794, 2006. doi: 10.1016/j.jaci.2005.12.1339. [DOI] [PubMed] [Google Scholar]
- 98.Harnly M, McLaughlin R, Bradman A, Anderson M, Gunier R. Correlating agricultural use of organophosphates with outdoor air concentrations: a particular concern for children. Environ Health Perspect 113: 1184–1189, 2005. doi: 10.1289/ehp.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harnly ME, Bradman A, Nishioka M, McKone TE, Smith D, McLaughlin R, Kavanagh-Baird G, Castorina R, Eskenazi B. Pesticides in dust from homes in an agricultural area. Environ Sci Technol 43: 8767–8774, 2009. doi: 10.1021/es9020958. [DOI] [PubMed] [Google Scholar]
- 100.Hartert TV, Peebles RS Jr. Epidemiology of asthma: the year in review. Curr Opin Pulm Med 6: 4–9, 2000. doi: 10.1097/00063198-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 101.Hazari MS, Pan JH, Myers AC. Nerve growth factor acutely potentiates synaptic transmission in vitro and induces dendritic growth in vivo on adult neurons in airway parasympathetic ganglia. Am J Physiol Lung Cell Mol Physiol 292: L992–L1001, 2007. doi: 10.1152/ajplung.00216.2006. [DOI] [PubMed] [Google Scholar]
- 102.Heinrich J. Can prenatal maternal stress increase the risk of asthma? Expert Rev Respir Med 9: 379–381, 2015. doi: 10.1586/17476348.2015.1066249. [DOI] [PubMed] [Google Scholar]
- 103.Henderson RF, Barr EB, Blackwell WB, Clark CR, Conn CA, Kalra R, March TH, Sopori ML, Tesfaigzi Y, Ménache MG, Mash DC. Response of rats to low levels of sarin. Toxicol Appl Pharmacol 184: 67–76, 2002. doi: 10.1006/taap.2002.9495. [DOI] [PubMed] [Google Scholar]
- 104.Hermanowicz A, Kossman S. Neutrophil function and infectious disease in workers occupationally exposed to phosphoorganic pesticides: role of mononuclear-derived chemotactic factor for neutrophils. Clin Immunol Immunopathol 33: 13–22, 1984. doi: 10.1016/0090-1229(84)90288-5. [DOI] [PubMed] [Google Scholar]
- 105.Hernández AF, Casado I, Pena G, Gil F, Villanueva E, Pla A. Low level of exposure to pesticides leads to lung dysfunction in occupationally exposed subjects. Inhal Toxicol 20: 839–849, 2008. doi: 10.1080/08958370801905524. [DOI] [PubMed] [Google Scholar]
- 106.Hernandez AF, Paron T, Serrano JL, Marin P. Epidemiological studies: Spain. In: Anticholinesterrase Pesticides: Metabolism, Neurotoxicity and Epidemiology, edited by Satoh T, Gupta RC. Hoboken, NJ: Wiley, 2010, p. 495–508. [Google Scholar]
- 107.Hernández AF, Parrón T, Alarcón R. Pesticides and asthma. Curr Opin Allergy Clin Immunol 11: 90–96, 2011. doi: 10.1097/ACI.0b013e3283445939. [DOI] [PubMed] [Google Scholar]
- 108.Hirani A, Lee WH, Kang S, Ehrich M, Lee YW. Chlorpyrifos induces pro-inflammatory environment in discrete regions of mouse brain (Abstract). FASEB J 21: A988, 2007. [Google Scholar]
- 109.Hoppin JA, Umbach DM, London SJ, Alavanja MC, Sandler DP. Chemical predictors of wheeze among farmer pesticide applicators in the Agricultural Health Study. Am J Respir Crit Care Med 165: 683–689, 2002. doi: 10.1164/ajrccm.165.5.2106074. [DOI] [PubMed] [Google Scholar]
- 110.Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Alavanja MC, Sandler DP. Pesticides and atopic and nonatopic asthma among farm women in the Agricultural Health Study. Am J Respir Crit Care Med 177: 11–18, 2008. doi: 10.1164/rccm.200706-821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Coble J, Alavanja MC, Beane Freeman LE, Sandler DP. Pesticide use and adult-onset asthma among male farmers in the Agricultural Health Study. Eur Respir J 34: 1296–1303, 2009. doi: 10.1183/09031936.00005509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hoppin JA, Umbach DM, London SJ, Lynch CF, Alavanja MC, Sandler DP. Pesticides and adult respiratory outcomes in the agricultural health study. Ann NY Acad Sci 1076: 343–354, 2006. doi: 10.1196/annals.1371.044. [DOI] [PubMed] [Google Scholar]
- 113.Hoppin JA, Umbach DM, London SJ, Lynch CF, Alavanja MC, Sandler DP. Pesticides associated with wheeze among commercial pesticide applicators in the Agricultural Health Study. Am J Epidemiol 163: 1129–1137, 2006. doi: 10.1093/aje/kwj138. [DOI] [PubMed] [Google Scholar]
- 114.Hoppin JA, Umbach DM, Long S, London SJ, Henneberger PK, Blair A, Alavanja M, Freeman LE, Sandler DP. Pesticides are associated with allergic and non-allergic wheeze among male farmers. Environ Health Perspect 125: 535–543, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoppin JA, Umbach DM, Long S, Rinsky JL, Henneberger PK, Salo PM, Zeldin DC, London SJ, Alavanja MC, Blair A, Beane Freeman LE, Sandler DP. Respiratory disease in United States farmers. Occup Environ Med 71: 484–491, 2014. doi: 10.1136/oemed-2013-101983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Howard AS, Bucelli R, Jett DA, Bruun D, Yang D, Lein PJ. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol Appl Pharmacol 207: 112–124, 2005. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 117.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736, 2001. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hulse EJ, Davies JO, Simpson AJ, Sciuto AM, Eddleston M. Respiratory complications of organophosphorus nerve agent and insecticide poisoning. Implications for respiratory and critical care. Am J Respir Crit Care Med 190: 1342–1354, 2014. doi: 10.1164/rccm.201406-1150CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hunter DD, Carrell-Jacks LA, Batchelor TP, Dey RD. Role of nerve growth factor in ozone-induced neural responses in early postnatal airway development. Am J Respir Cell Mol Biol 45: 359–365, 2011. doi: 10.1165/rcmb.2010-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med 161: 1985–1990, 2000. doi: 10.1164/ajrccm.161.6.9908051. [DOI] [PubMed] [Google Scholar]
- 121.Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest 91: 1314–1318, 1993. doi: 10.1172/JCI116331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jacoby DB, Xiao HQ, Lee NH, Chan-Li Y, Fryer AD. Virus- and interferon-induced loss of inhibitory M2 muscarinic receptor function and gene expression in cultured airway parasympathetic neurons. J Clin Invest 102: 242–248, 1998. doi: 10.1172/JCI1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jett DA, Fernando JC, Eldefrawi ME, Eldefrawi AT. Differential regulation of muscarinic receptor subtypes in rat brain regions by repeated injections of parathion. Toxicol Lett 73: 33–41, 1994. doi: 10.1016/0378-4274(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 124.Jett DA, Lein PJ. Non-cholinesterase mechanisms of central and peripheral neurotoxicity: muscarinic receptors and other targets. In: Toxicology of Organophosphate and Carbamate Compounds, edited by Gupta RC. Amsterdam: Elsevier, 2006, p. 233–245. doi: 10.1016/B978-012088523-7/50018-1. [DOI] [Google Scholar]
- 125.Jie Y, Isa ZM, Jie X, Ju ZL, Ismail NH. Urban vs. rural factors that affect adult asthma. Rev Environ Contam Toxicol 226: 33–63, 2013. [DOI] [PubMed] [Google Scholar]
- 126.Kajekar R, Myers AC. Calcitonin gene-related peptide affects synaptic and membrane properties of bronchial parasympathetic neurons. Respir Physiol Neurobiol 160: 28–36, 2008. doi: 10.1016/j.resp.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kajekar R, Pieczarka EM, Smiley-Jewell SM, Schelegle ES, Fanucchi MV, Plopper CG. Early postnatal exposure to allergen and ozone leads to hyperinnervation of the pulmonary epithelium. Respir Physiol Neurobiol 155: 55–63, 2007. doi: 10.1016/j.resp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 128.Karimi P, Peters KO, Bidad K, Strickland PT. Polycyclic aromatic hydrocarbons and childhood asthma. Eur J Epidemiol 30: 91–101, 2015. doi: 10.1007/s10654-015-9988-6. [DOI] [PubMed] [Google Scholar]
- 129.Karjalainen EM, Lindqvist A, Laitinen LA, Kava T, Altraja A, Halme M, Laitinen A. Airway inflammation and basement membrane tenascin in newly diagnosed atopic and nonatopic asthma. Respir Med 97: 1045–1051, 2003. doi: 10.1016/S0954-6111(03)00136-7. [DOI] [PubMed] [Google Scholar]
- 130.Kemi C, Grunewald J, Eklund A, Höglund CO. Differential regulation of neurotrophin expression in human bronchial smooth muscle cells. Respir Res 7: 18, 2006. doi: 10.1186/1465-9921-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Adams GK 3rd, Yamamura HI, O’Leary JF. Recovery of central respiratory function following anticholinesterase intoxication. Eur J Pharmacol 38: 101–112, 1976. doi: 10.1016/0014-2999(76)90206-5. [DOI] [PubMed] [Google Scholar]
- 132.Kesavachandran C, Singh VK, Mathur N, Rastogi SK, Siddiqui MK, Reddy MM, Bharti RS, Khan AM. Possible mechanism of pesticide toxicity-related oxidative stress leading to airway narrowing. Redox Rep 11: 159–162, 2006. doi: 10.1179/135100006X116673. [DOI] [PubMed] [Google Scholar]
- 133.Kim HY, Wegner SH, Van Ness KP, Park JJ, Pacheco SE, Workman T, Hong S, Griffith W, Faustman EM. Differential epigenetic effects of chlorpyrifos and arsenic in proliferating and differentiating human neural progenitor cells. Reprod Toxicol 65: 212–223, 2016. doi: 10.1016/j.reprotox.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim IJ, Beck HN, Lein PJ, Higgins D. Interferon gamma induces retrograde dendritic retraction and inhibits synapse formation. J Neurosci 22: 4530–4539, 2002. doi: 10.1523/JNEUROSCI.22-11-04530.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kisby GE, Muniz JF, Scherer J, Lasarev MR, Koshy M, Kow YW, McCauley L. Oxidative stress and DNA damage in agricultural workers. J Agromed 14: 206–214, 2009. doi: 10.1080/10599240902824042. [DOI] [PubMed] [Google Scholar]
- 136.Koenig JA, Edwardson JM. Intracellular trafficking of the muscarinic acetylcholine receptor: importance of subtype and cell type. Mol Pharmacol 49: 351–359, 1996. [PubMed] [Google Scholar]
- 137.Langley JN. On the physiology of the salivary secretion: Part II. On the mutual antagonism of atropin and pilocarpin, having especial reference to their relations in the sub-maxillary gland of the cat. J Physiol 1: 339–369, 1878. doi: 10.1113/jphysiol.1878.sp000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee AM, Fryer AD, van Rooijen N, Jacoby DB. Role of macrophages in virus-induced airway hyperresponsiveness and neuronal M2 muscarinic receptor dysfunction. Am J Physiol Lung Cell Mol Physiol 286: L1255–L1259, 2004. doi: 10.1152/ajplung.00451.2003. [DOI] [PubMed] [Google Scholar]
- 139.Lee CH, Kamijima M, Kim H, Shibata E, Ueyama J, Suzuki T, Takagi K, Saito I, Gotoh M, Hibi H, Naito H, Nakajima T. 8-Hydroxydeoxyguanosine levels in human leukocyte and urine according to exposure to organophosphorus pesticides and paraoxonase 1 genotype. Int Arch Occup Environ Health 80: 217–227, 2007. doi: 10.1007/s00420-006-0128-1. [DOI] [PubMed] [Google Scholar]
- 140.Lee YS, Lewis JA, Ippolito DL, Hussainzada N, Lein PJ, Jackson DA, Stallings JD. Repeated exposure to neurotoxic levels of chlorpyrifos alters hippocampal expression of neurotrophins and neuropeptides. Toxicology 340: 53–62, 2016. doi: 10.1016/j.tox.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lein PJ, Fryer AD. Organophosphorus insecticides induce airway hyperreactivity by decreasing neuronal M2 muscarinic receptor function independent of acetylcholinesterase inhibition. Toxicol Sci 83: 166–176, 2005. doi: 10.1093/toxsci/kfi001. [DOI] [PubMed] [Google Scholar]
- 142.Levy A, Chapman S, Cohen G, Raveh L, Rabinovitz I, Manistersky E, Kapon Y, Allon N, Gilat E. Protection and inflammatory markers following exposure of guinea pigs to sarin vapour: comparative efficacy of three oximes. J Appl Toxicol 24: 501–504, 2004. doi: 10.1002/jat.1008. [DOI] [PubMed] [Google Scholar]
- 143.Li L, Du Y, Ju F, Ma S, Zhang S. Calcium plays a key role in paraoxon-induced apoptosis in EL4 cells by regulating both endoplasmic reticulum- and mitochondria-associated pathways. Toxicol Mech Methods 26: 211–220, 2016. doi: 10.3109/15376516.2016.1156796. [DOI] [PubMed] [Google Scholar]
- 144.Li Q. New mechanism of organophosphorus pesticide-induced immunotoxicity. J Nippon Med Sch 74: 92–105, 2007. doi: 10.1272/jnms.74.92. [DOI] [PubMed] [Google Scholar]
- 145.Li Q, Kobayashi M, Kawada T. Organophosphorus pesticides induce apoptosis in human NK cells. Toxicology 239: 89–95, 2007. doi: 10.1016/j.tox.2007.06.100. [DOI] [PubMed] [Google Scholar]
- 146.Liang LP, Pearson-Smith JN, Huang J, McElroy P, Day BJ, Patel M. Neuroprotective effects of AEOL10150 in a rat organophosphate model. Toxicol Sci 162: 611–621, 2018. doi: 10.1093/toxsci/kfx283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lieu TM, Myers AC, Meeker S, Undem BJ. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol 302: L941–L948, 2012. doi: 10.1152/ajplung.00366.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu J, Gupta RC, Goad JT, Karanth S, Pope C. Modulation of parathion toxicity by glucose feeding: Is nitric oxide involved? Toxicol Appl Pharmacol 219: 106–113, 2007. doi: 10.1016/j.taap.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 149.Liu Z, Hu Y, Yu X, Xi J, Fan X, Tse CM, Myers AC, Pasricha PJ, Li X, Yu S. Allergen challenge sensitizes TRPA1 in vagal sensory neurons and afferent C-fiber subtypes in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 308: G482–G488, 2015. doi: 10.1152/ajpgi.00374.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lommatzsch M, Braun A, Renz H. Neurotrophins in allergic airway dysfunction: what the mouse model is teaching us. Ann NY Acad Sci 992: 241–249, 2003. doi: 10.1111/j.1749-6632.2003.tb03154.x. [DOI] [PubMed] [Google Scholar]
- 151.Lu C, Knutson DE, Fisker-Andersen J, Fenske RA. Biological monitoring survey of organophosphorus pesticide exposure among pre-school children in the Seattle metropolitan area. Environ Health Perspect 109: 299–303, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lukaszewicz-Hussain A. Role of oxidative stress in organophosphate insecticide toxicity—short review. Pestic Biochem Physiol 98: 145–150, 2010. doi: 10.1016/j.pestbp.2010.07.006. [DOI] [Google Scholar]
- 153.Ma P, Wu Y, Zeng Q, Gan Y, Chen J, Ye X, Yang X. Oxidative damage induced by chlorpyrifos in the hepatic and renal tissue of Kunming mice and the antioxidant role of vitamin E. Food Chem Toxicol 58: 177–183, 2013. doi: 10.1016/j.fct.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 154.Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol 45: 1–98, 1995. doi: 10.1016/0301-0082(94)E0017-B. [DOI] [PubMed] [Google Scholar]
- 155.Mamane A, Baldi I, Tessier JF, Raherison C, Bouvier G. Occupational exposure to pesticides and respiratory health. Eur Respir Rev 24: 306–319, 2015. doi: 10.1183/16000617.00006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mamane A, Raherison C, Tessier JF, Baldi I, Bouvier G. Environmental exposure to pesticides and respiratory health. Eur Respir Rev 24: 462–473, 2015. doi: 10.1183/16000617.00006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Medina-Díaz IM, Ponce-Ruiz N, Ramírez-Chávez B, Rojas-García AE, Barrón-Vivanco BS, Elizondo G, Bernal-Hernández YY. Downregulation of human paraoxonase 1 (PON1) by organophosphate pesticides in HepG2 cells. Environ Toxicol 32: 490–500, 2017. doi: 10.1002/tox.22253. [DOI] [PubMed] [Google Scholar]
- 158.Mense SM, Sengupta A, Lan C, Zhou M, Bentsman G, Volsky DJ, Whyatt RM, Perera FP, Zhang L. The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol Sci 93: 125–135, 2006. doi: 10.1093/toxsci/kfl046. [DOI] [PubMed] [Google Scholar]
- 159.Milatovic D, Gupta RC, Aschner M. Anticholinesterase toxicity and oxidative stress. Sci World J 6: 295–310, 2006. doi: 10.1100/tsw.2006.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miller MD, Marty MA. Impact of environmental chemicals on lung development. Environ Health Perspect 118: 1155–1164, 2010. doi: 10.1289/ehp.0901856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Minette PA, Barnes PJ. Prejunctional inhibitory muscarinic receptors on cholinergic nerves in human and guinea pig airways. J Appl Physiol (1985) 64: 2532–2537, 1988. doi: 10.1152/jappl.1988.64.6.2532. [DOI] [PubMed] [Google Scholar]
- 162.Minette PA, Lammers JW, Dixon CM, McCusker MT, Barnes PJ. A muscarinic agonist inhibits reflex bronchoconstriction in normal but not in asthmatic subjects. J Appl Physiol (1985) 67: 2461–2465, 1989. doi: 10.1152/jappl.1989.67.6.2461. [DOI] [PubMed] [Google Scholar]
- 163.Myers AC, Undem BJ. Muscarinic receptor regulation of synaptic transmission in airway parasympathetic ganglia. Am J Physiol Lung Cell Mol Physiol 270: L630–L636, 1996. doi: 10.1152/ajplung.1996.270.4.L630. [DOI] [PubMed] [Google Scholar]
- 164.Nakadai A, Li Q, Kawada T. Chlorpyrifos induces apoptosis in human monocyte cell line U937. Toxicology 224: 202–209, 2006. doi: 10.1016/j.tox.2006.04.055. [DOI] [PubMed] [Google Scholar]
- 165.Nassenstein C, Kutschker J, Tumes D, Braun A. Neuro-immune interaction in allergic asthma: role of neurotrophins. Biochem Soc Trans 34: 591–593, 2006. doi: 10.1042/BST0340591. [DOI] [PubMed] [Google Scholar]
- 166.Ndlovu V, Dalvie MA, Jeebhay MF. Asthma associated with pesticide exposure among women in rural Western Cape of South Africa. Am J Ind Med 57: 1331–1343, 2014. doi: 10.1002/ajim.22384. [DOI] [PubMed] [Google Scholar]
- 167.Nesi RT, Barroso MV, Souza Muniz V, de Arantes AC, Martins MA, Brito Gitirana L, Neves JS, Benjamim CF, Lanzetti M, Valenca SS. Pharmacological modulation of reactive oxygen species (ROS) improves the airway hyperresponsiveness by shifting the Th1 response in allergic inflammation induced by ovalbumin. Free Radic Res 51: 708–722, 2017. [DOI] [PubMed] [Google Scholar]
- 168.Nie Z, Jacoby DB, Fryer AD. Etanercept prevents airway hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. Br J Pharmacol 156: 201–210, 2009. doi: 10.1111/j.1476-5381.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nishino R, Fukuyama T, Tajima Y, Miyashita L, Watanabe Y, Ueda H, Kosaka T. Prior oral exposure to environmental immunosuppressive chemicals methoxychlor, parathion, or piperonyl butoxide aggravates allergic airway inflammation in NC/Nga mice. Toxicology 309: 1–8, 2013. doi: 10.1016/j.tox.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 170.O’Neill JJ. Non-cholinesterase effects of anticholinesterases. Fundam Appl Toxicol 1: 154–160, 1981. doi: 10.1016/S0272-0590(81)80052-8. [DOI] [PubMed] [Google Scholar]
- 171.Ogawa H, Azuma M, Uehara H, Takahashi T, Nishioka Y, Sone S, Izumi K. Nerve growth factor derived from bronchial epithelium after chronic mite antigen exposure contributes to airway hyperresponsiveness by inducing hyperinnervation, and is inhibited by in vivo siRNA. Clin Exp Allergy 42: 460–470, 2012. doi: 10.1111/j.1365-2222.2011.03918.x. [DOI] [PubMed] [Google Scholar]
- 172.Ohayo-Mitoko GJ, Kromhout H, Simwa JM, Boleij JS, Heederik D. Self reported symptoms and inhibition of acetylcholinesterase activity among Kenyan agricultural workers. Occup Environ Med 57: 195–200, 2000. doi: 10.1136/oem.57.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pan J, Rhode HK, Undem BJ, Myers AC. Neurotransmitters in airway parasympathetic neurons altered by neurotrophin-3 and repeated allergen challenge. Am J Respir Cell Mol Biol 43: 452–457, 2010. doi: 10.1165/rcmb.2009-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pathak MK, Fareed M, Srivastava AK, Pangtey BS, Bihari V, Kuddus M, Kesavachandran C. Seasonal variations in cholinesterase activity, nerve conduction velocity and lung function among sprayers exposed to mixture of pesticides. Environ Sci Pollut Res Int 20: 7296–7300, 2013. doi: 10.1007/s11356-013-1743-5. [DOI] [PubMed] [Google Scholar]
- 175.Peiris-John RJ, Ruberu DK, Wickremasinghe AR, van-der-Hoek W. Low-level exposure to organophosphate pesticides leads to restrictive lung dysfunction. Respir Med 99: 1319–1324, 2005. doi: 10.1016/j.rmed.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 176.Peña-Philippides JC, Razani-Boroujerdi S, Singh SP, Langley RJ, Mishra NC, Henderson RF, Sopori ML. Long- and short-term changes in the neuroimmune-endocrine parameters following inhalation exposures of F344 rats to low-dose sarin. Toxicol Sci 97: 181–188, 2007. doi: 10.1093/toxsci/kfm017. [DOI] [PubMed] [Google Scholar]
- 177.Pierrot C, Bègue A, Szpirer C, Capron A, Capron M, Khalife J. Cloning of the rat IL-5Ralpha gene: analysis of 5′-upstream region and expression by B cells. Biochem Biophys Res Commun 288: 328–339, 2001. doi: 10.1006/bbrc.2001.5782. [DOI] [PubMed] [Google Scholar]
- 178.Pinkerton KE, Joad JP. Influence of air pollution on respiratory health during perinatal development. Clin Exp Pharmacol Physiol 33: 269–272, 2006. doi: 10.1111/j.1440-1681.2006.04357.x. [DOI] [PubMed] [Google Scholar]
- 179.Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health B Crit Rev 2: 161–181, 1999. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- 180.Proskocil BJ, Bruun DA, Garg JA, Villagomez CC, Jacoby DB, Lein PJ, Fryer AD. The influence of sensitization on mechanisms of organophosphorus pesticide-induced airway hyperreactivity. Am J Respir Cell Mol Biol 53: 738–747, 2015. doi: 10.1165/rcmb.2014-0444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Proskocil BJ, Bruun DA, Jacoby DB, van Rooijen N, Lein PJ, Fryer AD. Macrophage TNF-α mediates parathion-induced airway hyperreactivity in guinea pigs. Am J Physiol Lung Cell Mol Physiol 304: L519–L529, 2013. doi: 10.1152/ajplung.00381.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Proskocil BJ, Bruun DA, Lorton JK, Blensly KC, Jacoby DB, Lein PJ, Fryer AD. Antigen sensitization influences organophosphorus pesticide-induced airway hyperreactivity. Environ Health Perspect 116: 381–388, 2008. doi: 10.1289/ehp.10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Proskocil BJ, Bruun DA, Thompson CM, Fryer AD, Lein PJ. Organophosphorus pesticides decrease M2 muscarinic receptor function in guinea pig airway nerves via indirect mechanisms. PLoS One 5: e10562, 2010. doi: 10.1371/journal.pone.0010562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qu J, Li Y, Zhong W, Gao P, Hu C. Recent developments in the role of reactive oxygen species in allergic asthma. J Thorac Dis 9: E32–E43, 2017. doi: 10.21037/jtd.2017.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Raanan R, Balmes JR, Harley KG, Gunier RB, Magzamen S, Bradman A, Eskenazi B. Decreased lung function in 7-year-old children with early-life organophosphate exposure. Thorax 71: 148–153, 2016. doi: 10.1136/thoraxjnl-2014-206622. [DOI] [PubMed] [Google Scholar]
- 186.Raanan R, Harley KG, Balmes JR, Bradman A, Lipsett M, Eskenazi B. Early-life exposure to organophosphate pesticides and pediatric respiratory symptoms in the CHAMACOS cohort. Environ Health Perspect 123: 179–185, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ramnarine SI, Haddad EB, Khawaja AM, Mak JC, Rogers DF. On muscarinic control of neurogenic mucus secretion in ferret trachea. J Physiol 494: 577–586, 1996. doi: 10.1113/jphysiol.1996.sp021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ray DE, Richards PG. The potential for toxic effects of chronic, low-dose exposure to organophosphates. Toxicol Lett 120: 343–351, 2001. doi: 10.1016/S0378-4274(01)00266-1. [DOI] [PubMed] [Google Scholar]
- 189.Rizzo CA, Yang R, Greenfeder S, Egan RW, Pauwels RA, Hey JA. The IL-5 receptor on human bronchus selectively primes for hyperresponsiveness. J Allergy Clin Immunol 109: 404–409, 2002. doi: 10.1067/mai.2002.122459. [DOI] [PubMed] [Google Scholar]
- 190.Rodgers K, Ellefson D. Mechanism of the modulation of murine peritoneal cell function and mast cell degranulation by low doses of malathion. Agents Actions 35: 57–63, 1992. doi: 10.1007/BF01990952. [DOI] [PubMed] [Google Scholar]
- 191.Rodgers K, Xiong S. Contributions of inflammatory mast cell mediators to alterations in macrophage function after malathion administration. Int J Immunopharmacol 19: 149–156, 1997. doi: 10.1016/S0192-0561(96)00073-2. [DOI] [PubMed] [Google Scholar]
- 192.Rodgers K, Xiong S. Effect of acute administration of malathion by oral and dermal routes on serum histamine levels. Int J Immunopharmacol 19: 437–441, 1997. doi: 10.1016/S0192-0561(97)00098-2. [DOI] [PubMed] [Google Scholar]
- 193.Rodgers K, Xiong S. Effect of administration of malathion for 14 days on macrophage function and mast cell degranulation. Fundam Appl Toxicol 37: 95–99, 1997. doi: 10.1006/faat.1997.2302. [DOI] [PubMed] [Google Scholar]
- 194.Rodgers KE, Ellefson DD. Modulation of macrophage protease activity by acute administration of O,O,S trimethyl phosphorothioate. Agents Actions 29: 277–285, 1990. doi: 10.1007/BF01966458. [DOI] [PubMed] [Google Scholar]
- 195.Rodgers KE, Ellefson DD. Modulation of respiratory burst activity and mitogenic response of human peripheral blood mononuclear cells and murine splenocytes and peritoneal cells by malathion. Fundam Appl Toxicol 14: 309–317, 1990. doi: 10.1016/0272-0590(90)90210-B. [DOI] [PubMed] [Google Scholar]
- 196.Rogers DF. Motor control of airway goblet cells and glands. Respir Physiol 125: 129–144, 2001. doi: 10.1016/S0034-5687(00)00209-7. [DOI] [PubMed] [Google Scholar]
- 197.Rohlman DS, Anger WK, Lein PJ. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology 32: 268–276, 2011. doi: 10.1016/j.neuro.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rowsey PJ, Gordon CJ. Tumor necrosis factor is involved in chlorpyrifos-induced changes in core temperature in the female rat. Toxicol Lett 109: 51–59, 1999. doi: 10.1016/S0378-4274(99)00122-8. [DOI] [PubMed] [Google Scholar]
- 199.Ruit KG, Osborne PA, Schmidt RE, Johnson EM Jr, Snider WD. Nerve growth factor regulates sympathetic ganglion cell morphology and survival in the adult mouse. J Neurosci 10: 2412–2419, 1990. doi: 10.1523/JNEUROSCI.10-07-02412.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O. Oxidative stress in asthma. World Allergy Organ J 4: 151–158, 2011. doi: 10.1097/WOX.0b013e318232389e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Salam MT, Li YF, Langholz B, Gilliland FD; Children’s Health Study . Early-life environmental risk factors for asthma: findings from the Children’s Health Study. Environ Health Perspect 112: 760–765, 2004. doi: 10.1289/ehp.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Salameh PR, Baldi I, Brochard P, Raherison C, Abi Saleh B, Salamon R. Respiratory symptoms in children and exposure to pesticides. Eur Respir J 22: 507–512, 2003. doi: 10.1183/09031936.03.00107403a. [DOI] [PubMed] [Google Scholar]
- 203.Saulsbury MD, Heyliger SO, Wang K, Johnson DJ. Chlorpyrifos induces oxidative stress in oligodendrocyte progenitor cells. Toxicology 259: 1–9, 2009. doi: 10.1016/j.tox.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 204.Schulte-Herbrüggen O, Nassenstein C, Lommatzsch M, Quarcoo D, Renz H, Braun A. Tumor necrosis factor-α and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J Neuroimmunol 160: 204–209, 2005. doi: 10.1016/j.jneuroim.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 205.Scott GD, Fryer AD. Role of parasympathetic nerves and muscarinic receptors in allergy and asthma. Chem Immunol Allergy 98: 48–69, 2012. doi: 10.1159/000336498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Segura P, Chávez J, Montaño LM, Vargas MH, Delaunois A, Carbajal V, Gustin P. Identification of mechanisms involved in the acute airway toxicity induced by parathion. Naunyn Schmiedebergs Arch Pharmacol 360: 699–710, 1999. doi: 10.1007/s002109900101. [DOI] [PubMed] [Google Scholar]
- 207.Shadnia S, Azizi E, Hosseini R, Khoei S, Fouladdel S, Pajoumand A, Jalali N, Abdollahi M. Evaluation of oxidative stress and genotoxicity in organophosphorus insecticide formulators. Hum Exp Toxicol 24: 439–445, 2005. doi: 10.1191/0960327105ht549oa. [DOI] [PubMed] [Google Scholar]
- 208.Slotkin T, Seidler F. Transcriptional profiles reveal similarities and differences in the effects of developmental neurotoxicants on differentiation into neurotransmitter phenotypes in PC12 cells. Brain Res Bull 78: 211–225, 2009. doi: 10.1016/j.brainresbull.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect 107, Suppl 1: 71–80, 1999. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Slotkin TA, Seidler FJ. Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol Appl Pharmacol 233: 211–219, 2008. doi: 10.1016/j.taap.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Slotkin TA, Seidler FJ, Fumagalli F. Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily. Environ Health Perspect 115: 909–916, 2007. doi: 10.1289/ehp.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Slotkin TA, Seidler FJ, Fumagalli F. Unrelated developmental neurotoxicants elicit similar transcriptional profiles for effects on neurotrophic factors and their receptors in an in vitro model. Neurotoxicol Teratol 32: 42–51, 2010. doi: 10.1016/j.ntt.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Soltaninejad K, Abdollahi M. Current opinion on the science of organophosphate pesticides and toxic stress: a systematic review. Med Sci Monit 15: RA75–RA90, 2009. [PubMed] [Google Scholar]
- 214.Song WJ, Kim HJ, Shim JS, Won HK, Kang SY, Sohn KH, Kim BK, Jo EJ, Kim MH, Kim SH, Park HW, Kim SS, Chang YS, Morice AH, Lee BJ, Cho SH. Diagnostic accuracy of fractional exhaled nitric oxide measurement in predicting cough-variant asthma and eosinophilic bronchitis in adults with chronic cough: a systematic review and meta-analysis. J Allergy Clin Immunol 140: 701–709, 2017. doi: 10.1016/j.jaci.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 215.Sun T, Ma T, Ho IK. Differential modulation of muscarinic receptors in the rat brain by repeated exposure to methyl parathion. J Toxicol Sci 28: 427–438, 2003. doi: 10.2131/jts.28.427. [DOI] [PubMed] [Google Scholar]
- 216.Szczepankiewicz A, Rachel M, Sobkowiak P, Kycler Z, Wojsyk-Banaszak I, Schöneich N, Skibinska M, Bręborowicz A. Serum neurotrophin-3 and neurotrophin-4 levels are associated with asthma severity in children. Eur Respir J 39: 1035–1037, 2012. doi: 10.1183/09031936.00136611. [DOI] [PubMed] [Google Scholar]
- 217.Takei Y, Laskey R. Interpreting crosstalk between TNF-alpha and NGF: potential implications for disease. Trends Mol Med 14: 381–388, 2008. doi: 10.1016/j.molmed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 218.Tarlo SM. Trends in incidence of occupational asthma. Occup Environ Med 72: 688–689, 2015. doi: 10.1136/oemed-2015-102852. [DOI] [PubMed] [Google Scholar]
- 219.Terry AV Jr, Gearhart DA, Beck WD Jr, Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J Pharmacol Exp Ther 322: 1117–1128, 2007. doi: 10.1124/jpet.107.125625. [DOI] [PubMed] [Google Scholar]
- 220.Toledo-Ibarra GA, Díaz-Resendiz KJ, Pavón-Romero L, Rojas-García AE, Medina-Díaz IM, Girón-Pérez MI. Effects of diazinon on the lymphocytic cholinergic system of Nile tilapia fish (Oreochromis niloticus). Vet Immunol Immunopathol 176: 58–63, 2016. doi: 10.1016/j.vetimm.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 221.Toledo-Ibarra GA, Díaz Resendiz KJ, Ventura-Ramón GH, González-Jaime F, Vega-López A, Becerril-Villanueva E, Pavón L, Girón-Pérez MI. Oxidative damage in gills and liver in Nile tilapia (Oreochromis niloticus) exposed to diazinon. Comp Biochem Physiol A Mol Integr Physiol 200: 3–8, 2016. doi: 10.1016/j.cbpa.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 222.Tsao TC, Juang YC, Lan RS, Shieh WB, Lee CH. Respiratory failure of acute organophosphate and carbamate poisoning. Chest 98: 631–636, 1990. doi: 10.1378/chest.98.3.631. [DOI] [PubMed] [Google Scholar]
- 223.Tuler SM, Bowen JM. Toxic effects of organophosphates on nerve cell growth and ultrastructure in culture. J Toxicol Environ Health 27: 209–223, 1989. doi: 10.1080/15287398909531292. [DOI] [PubMed] [Google Scholar]
- 224.Tuo QR, Ma YF, Chen W, Luo XJ, Shen J, Guo D, Zheng YM, Wang YX, Ji G, Liu QH. Reactive oxygen species induce a Ca2+-spark increase in sensitized murine airway smooth muscle cells. Biochem Biophys Res Commun 434: 498–502, 2013. doi: 10.1016/j.bbrc.2013.03.102. [DOI] [PubMed] [Google Scholar]
- 225.Udarbe Zamora EM, Liu J, Pope CN. Effects of chlorpyrifos oxon on M2 muscarinic receptor internalization in different cell types. J Toxicol Environ Health A 71: 1440–1447, 2008. doi: 10.1080/15287390802328887. [DOI] [PubMed] [Google Scholar]
- 227.Undem BJ, Carr MJ. The role of nerves in asthma. Curr Allergy Asthma Rep 2: 159–165, 2002. doi: 10.1007/s11882-002-0011-4. [DOI] [PubMed] [Google Scholar]
- 228.Vega JA, García-Suárez O, Hannestad J, Pérez-Pérez M, Germanà A. Neurotrophins and the immune system. J Anat 203: 1–19, 2003. doi: 10.1046/j.1469-7580.2003.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Verhein KC, Hazari MS, Moulton BC, Jacoby IW, Jacoby DB, Fryer AD. Three days after a single exposure to ozone, the mechanism of airway hyperreactivity is dependent on substance P and nerve growth factor. Am J Physiol Lung Cell Mol Physiol 300: L176–L184, 2011. doi: 10.1152/ajplung.00060.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Verhein KC, Jacoby DB, Fryer AD. IL-1 receptors mediate persistent, but not acute, airway hyperreactivity to ozone in guinea pigs. Am J Respir Cell Mol Biol 39: 730–738, 2008. doi: 10.1165/rcmb.2008-0045OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang L, Liu Z, Zhang J, Wu Y, Sun H. Chlorpyrifos exposure in farmers and urban adults: metabolic characteristic, exposure estimation, and potential effect of oxidative damage. Environ Res 149: 164–170, 2016. doi: 10.1016/j.envres.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 232.Wedes SH, Khatri SB, Zhang R, Wu W, Comhair SA, Wenzel S, Teague WG, Israel E, Erzurum SC, Hazen SL. Noninvasive markers of airway inflammation in asthma. Clin Transl Sci 2: 112–117, 2009. doi: 10.1111/j.1752-8062.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Weigand LA, Kwong K, Myers AC. The effects of nerve growth factor on nicotinic synaptic transmission in mouse airway parasympathetic neurons. Am J Respir Cell Mol Biol 53: 443–449, 2015. doi: 10.1165/rcmb.2014-0280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Weiner A. Bronchial asthma due to the organic phosphate insecticides; a case report. Ann Allergy 19: 397–401, 1961. [PubMed] [Google Scholar]
- 235.Weisenburger DD. Human health effects of agrichemical use. Hum Pathol 24: 571–576, 1993. doi: 10.1016/0046-8177(93)90234-8. [DOI] [PubMed] [Google Scholar]
- 236.Weitzman M, Gortmaker SL, Sobol AM, Perrin JM. Recent trends in the prevalence and severity of childhood asthma. JAMA 268: 2673–2677, 1992. doi: 10.1001/jama.1992.03490190073034. [DOI] [PubMed] [Google Scholar]
- 237.Wenzel S. Severe asthma: epidemiology, pathophysiology and treatment. Mt Sinai J Med 70: 185–190, 2003. [PubMed] [Google Scholar]
- 238.Whyatt RM, Camann DE, Kinney PL, Reyes A, Ramirez J, Dietrich J, Diaz D, Holmes D, Perera FP. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ Health Perspect 110: 507–514, 2002. doi: 10.1289/ehp.02110507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wood LG, Garg ML, Blake RJ, Simpson JL, Gibson PG. Oxidized vitamin E and glutathione as markers of clinical status in asthma. Clin Nutr 27: 579–586, 2008. doi: 10.1016/j.clnu.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 240.Wu ZX, Benders KB, Hunter DD, Dey RD. Early postnatal exposure of mice to side-steam tobacco smoke increases neuropeptide Y in lung. Am J Physiol Lung Cell Mol Physiol 302: L152–L159, 2012. doi: 10.1152/ajplung.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wu ZX, Hunter DD, Kish VL, Benders KM, Batchelor TP, Dey RD. Prenatal and early, but not late, postnatal exposure of mice to sidestream tobacco smoke increases airway hyperresponsiveness later in life. Environ Health Perspect 117: 1434–1440, 2009. doi: 10.1289/ehp.0800511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xiong S, Rodgers K. Effects of malathion metabolites on degranulation of and mediator release by human and rat basophilic cells. J Toxicol Environ Health 51: 159–175, 1997. doi: 10.1080/00984109708984019. [DOI] [PubMed] [Google Scholar]
- 243.Yagle K, Costa LG. Effects of organophosphate exposure on muscarinic acetylcholine receptor subtype mRNA levels in the adult rat. Neurotoxicology 17: 523–530, 1996. [PubMed] [Google Scholar]
- 244.Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol Appl Pharmacol 228: 32–41, 2008. doi: 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yang D, Lauridsen H, Buels K, Chi LH, La Du J, Bruun DA, Olson JR, Tanguay RL, Lein PJ. Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol Sci 121: 146–159, 2011. doi: 10.1093/toxsci/kfr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yang YG, Tian WM, Zhang H, Li M, Shang YX. Nerve growth factor exacerbates allergic lung inflammation and airway remodeling in a rat model of chronic asthma. Exp Ther Med 6: 1251–1258, 2013. doi: 10.3892/etm.2013.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ye M, Beach J, Martin JW, Senthilselvan A. Associations between dietary factors and urinary concentrations of organophosphate and pyrethroid metabolites in a Canadian general population. Int J Hyg Environ Health 218: 616–626, 2015. doi: 10.1016/j.ijheh.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 248.Ye M, Beach J, Martin JW, Senthilselvan A. Occupational pesticide exposures and respiratory health. Int J Environ Res Public Health 10: 6442–6471, 2013. doi: 10.3390/ijerph10126442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ye M, Beach J, Martin JW, Senthilselvan A. Urinary dialkyl phosphate concentrations and lung function parameters in adolescents and adults: results from the Canadian Health Measures Survey. Environ Health Perspect 124: 491–497, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yoon HK, Cho HY, Kleeberger SR. Protective role of matrix metalloproteinase-9 in ozone-induced airway inflammation. Environ Health Perspect 115: 1557–1563, 2007. doi: 10.1289/ehp.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Yost BL, Gleich GJ, Jacoby DB, Fryer AD. The changing role of eosinophils in long-term hyperreactivity following a single ozone exposure. Am J Physiol Lung Cell Mol Physiol 289: L627–L635, 2005. doi: 10.1152/ajplung.00377.2004. [DOI] [PubMed] [Google Scholar]
- 252.Yu M, Zheng X, Peake J, Joad JP, Pinkerton KE. Perinatal environmental tobacco smoke exposure alters the immune response and airway innervation in infant primates. J Allergy Clin Immunol 122: 640–7.e1, 2008. doi: 10.1016/j.jaci.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 253.Yu S, Kollarik M, Ouyang A, Myers AC, Undem BJ. Mast cell-mediated long-lasting increases in excitability of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 293: G850–G856, 2007. doi: 10.1152/ajpgi.00277.2007. [DOI] [PubMed] [Google Scholar]
- 254.Zhang X, Wallace AD, Du P, Kibbe WA, Jafari N, Xie H, Lin S, Baccarelli A, Soares MB, Hou L. DNA methylation alterations in response to pesticide exposure in vitro. Environ Mol Mutagen 53: 542–549, 2012. doi: 10.1002/em.21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang X, Wallace AD, Du P, Lin S, Baccarelli AA, Jiang H, Jafari N, Zheng Y, Xie H, Soares MB, Kibbe WA, Hou L. Genome-wide study of DNA methylation alterations in response to diazinon exposure in vitro. Environ Toxicol Pharmacol 34: 959–968, 2012. doi: 10.1016/j.etap.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zou LM, Li SY, Zhang J. [Effects of organophosphorus insecticides on G protein-coupled receptor kinase-2 mediated phosphorylation of M2 muscarinic receptors]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 24: 352–355, 2006. [PubMed] [Google Scholar]