Summary
T helper type 17 (Th17) cells and regulatory T (Treg) cells are two distinct T‐cell subsets with opposite effects on immune functions. While Th17 cells are a key effector in the immune response and play critical roles in the development of autoimmunity and inflammation, Treg cells orchestrate the overall immune response and maintain peripheral immune tolerance by regulating the activity of the effector T cells. However, the developmental pathways for Th17 and Treg cells are reciprocally interconnected and there is a significant amount of plasticity between them. Disturbed Th17/Treg balance contributes to the development of autoimmune diseases, like experimental autoimmune encephalomyelitis and multiple sclerosis. MicroRNAs (miRNAs) are small non‐coding RNA molecules that post‐transcriptionally regulate gene expression. Recently, emerging evidence demonstrates that miRNAs play an important role in regulating the pathogenesis of autoimmune diseases through the modulation of Th17/Treg balance. This review will provide an overview of the dysregulated miRNAs and their functions in modulating the Th17/Treg balance in autoimmune diseases.
Keywords: autoimmune disease, immune regulation, microRNAs, T helper type 17/regulatory T balance
Introduction
CD4+ T cells play critical roles in mediating adaptive immunity and are crucial in achieving an effective immune response to pathogens. After interaction with an antigen–MHC complex, naive CD4+ T cells differentiate into different effector cell subsets including T helper type 1 (Th1), Th2, follicular helper T, Th17 and regulatory T (Treg) cells. These T‐cell subsets have distinct cytokine profiles and different effects on immune functions.1 Th17 and Treg cells are two distinct T‐cell subsets with opposing actions. Whereas Th17 cells represent a pro‐inflammatory subset, Treg cells have an antagonist effect. The balance between these two cell populations is essential for immune homeostasis, and the disturbed equilibrium of Th17 and Treg cells has been implicated in a variety of autoimmune and inflammatory diseases.2 Since the discovery of Th17 and Treg cells, many factors have been identified that regulate the differentiation and function of Th17 and Treg cells. MicroRNAs (miRNAs) are a well‐studied class of non‐coding RNA molecules, which post‐transcriptionally regulate gene expression by targeting mRNAs for degradation or by repressing the translation of mRNAs. During the past few years, many miRNAs have been found to play important roles in regulating the function of the immune system, including immune tolerance and autoimmunity. Particularly, miRNAs have been considered as an important new factor in the regulation of Th17/Treg balance that contributes to the development and progression of autoimmune diseases.3 This review will focus on the dysregulated miRNAs leading to disturbed Th17/Treg balance during the development of autoimmune diseases.
Th17, Treg cells and Th17/Treg balance
An overview of Th17 and Treg cells
The Th17 cells require specific cytokines, such as transforming growth factor‐β (TGF‐β), combined with interleukin‐6 (IL‐6) or IL‐21 for their differentiation.4 At the molecular level, the differentiation of Th17 cells requires a unique lineage‐specific transcription factor, retinoid‐related orphan receptor γt (RORγt).5 Th17 cells secrete a characteristic profile of cytokines including IL‐17A, IL‐17F, IL‐21 and IL‐22, which recruit and activate neutrophils and macrophages to fight against extracellular microbial organisms or mediate the development of autoimmune disease.6 Although roles for Th17 cells in promoting inflammation and autoimmune disorders have been extensively demonstrated, it is still controversial whether and how Th17 cells influence tumour immunity.
Regulatory T cells are another lineage of CD4+ T cells with immunosuppressive properties. They require the specific cytokine TGF‐β and the transcription factor Foxp3 for their differentiation.7 In addition, IL‐2 is important for the generation and expansion of Treg cells.8 The Treg cells come in at least two forms according to the site of their maturation: naturally occurring CD4+ CD25+ Treg (nTreg) cells and inducible Treg (iTreg) cells. Although nTreg cells suppress inflammation and immune responses mainly in a cell‐contact‐dependent manner, iTreg cells secrete inhibitory cytokines IL‐10 or TGF‐β to exert their suppressive effects.9 The Treg cells play an important role in the prevention of autoimmunity and in the regulation of immune responses against infections and cancer.
Plasticity of Th17 and Treg cells
Accumulating evidence indicates that CD4+ T cells are more plastic than previously described, particularly Th17 and Treg cells. Although Th17 and Treg cells play opposite roles in the regulation of autoimmunity and inflammation, the TGF‐β pathway is shared by both Th17 and Treg cells for their differentiation. TGF‐β promotes Th17 and iTreg cell development by inducing the expression of both Foxp310 and RORγt4 in T‐cell receptor (TCR)‐stimulated naive CD4+ T cells. These cells can shuttle towards a pro‐inflammatory Th17 phenotype or a regulatory iTreg cell phenotype depending on the surrounding cytokine environment. In the absence of IL‐6 or IL‐21, TGF‐β alone is unable to initiate Th17 differentiation. Foxp3 is able to physically bind RORγt to suppress its transcriptional activity, resulting in the inhibition of Th17 differentiation and favouring the development of the Treg cell lineage. In the presence of IL‐6 or IL‐21, Foxp3 is released from RORγt and then Th17 differentiation can be initiated.11, 12 Therefore, IL‐6 and IL‐21 play a critical role in driving the Th17 differentiation by controlling the Foxp3/RORγt balance. In addition, the cytokine IL‐2 is a potent inducer of Foxp3 but inhibits Th17 cell differentiation via a signal transducer and activator of transcription 5 (STAT5) ‐dependent mechanism.13
Because the developmental pathways for Th17 and Treg cells are reciprocally interconnected, these two cell subsets can interconvert under specific conditions. Foxp3‐expressing Treg cells can convert into IL‐17‐secreting cells and lose their suppressive function under inflammatory conditions.14 Inflammatory Th17 cells can convert into IL‐10‐producing cells, which possess immunosuppressive properties but lack Foxp3 expression.15 In addition, subpopulations of CD4+ Foxp3+ RORγt+ Treg cells that have the capacity to produce IL‐17 have been reported in both humans16 and mice.17 These cells represent a transient population that displays the functional features of both Th17 and Treg cells.
Th17/Treg balance and autoimmune diseases
An imbalance between Th17 cells and Treg cells is often associated with certain autoimmune diseases,2 infectious and allergic diseases,18 as well as cancer.19 Th17 cells with specificity for self‐antigens are highly pathogenic and lead to the development of inflammation and severe autoimmunity. In animal models, the implication of Th17 cells was described in different autoimmune diseases, including experimental autoimmune encephalomyelitis (EAE) and collagen‐induced arthritis. In humans, Th17 cells and their cytokines are also associated with several autoimmune and inflammatory diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), psoriasis and inflammatory bowel disease.20 On the other hand, a reduction in Treg cell numbers and/or a loss of Treg cell function has been observed in many human autoimmune diseases and animal models of autoimmunity.21 So maintaining Th17/Treg balance appears to be critical for the prevention of the development of autoimmune diseases.
MicroRNAs regulates Th17/Treg imbalance in autoimmune diseases
miRNAs that are dysregulated in autoimmune diseases and regulate Th17/Treg balance
Multiple miRNAs have been reported to be dysregulated in autoimmune diseases. Among them, a number of miRNAs are involved in the regulation of Th17/Treg cell balance (Table 1). The miRNAs could regulate Th17/Treg in a T‐cell intrinsic way. Alternatively, miRNAs expressed by non‐T cells could be responsible for the disturbed Th17/Treg balance.
Table 1.
Dysregulated miRNAs in autoimmune diseases that associated with Th17/Treg balance
| Autoimmune disease | miRNA | Cell types | Dysregulation | Function | References |
|---|---|---|---|---|---|
| EAE and MS | miR‐20b | Th17 | ↓ | Th17↓ | 22 |
| miR‐30a | Th17 | ↓ | Th17↓ | 23 | |
| miR‐146a | Th17 | ↓ | Th17↓ | 24 | |
| miR‐214 | CD4+ T cell | ↓ | Th17↓ | 25 | |
| miR‐26a | PBLs | ↓ | Th17↓ | 26 | |
| miR‐17‐92 | T cell | ↑ | Th17↑ | 27 | |
| miR‐326 | Th17 | ↑ | Th17↑ | 28 | |
| miR‐384 | CD4+ T cell | ↑ | Th17↑ | 29 | |
| miR‐181c | CD4+ T cell | ↑ | Th17↑ | 30 | |
| miR‐21 | Th17 | ↑ | Th17↑ | 31 | |
| miR‐132/212 | Th17 | ↑ | Th17↑ | 32 | |
| miR‐155 | Sera | ↑ | Th17↑ | 33 | |
| miR‐27a | CD4+ T cell | ↑ | Th17↑ | 25 | |
| miR‐590 | Th17 | ↑ | Th17↑ | 34 | |
| miR‐448 | Th17 | ↑ | Th17↑ | 35 | |
| miR‐141 | CD4+ T cell | ↑ | Th17↑ | 36 | |
| miR‐200a | CD4+ T cell | ↑ | Th17↑ | 36 | |
| miR‐223 | CD4+ T cell | ↑ | Th17↑ | 37 | |
| miR‐223 | Dendritic cell | ↑ | Th17↑ | 49 | |
| miR‐182 | CD4+ T cell | ↑ | Treg↓ | 38 | |
| miR‐let‐7i | Circulating exosomes | ↑ | Treg↓ | 53 | |
| RA | miR‐301a‐3p | PBMC | ↑ | Th17↑ | 39 |
| miR‐16 | PBMC | ↑ | Th17↑ and Treg↓ | 40 | |
| miR‐21 | PBMC, CD4+ T cell | ↓ | Th17↑ and Treg↓ | 41 | |
| miR‐146a | Treg | ↓ | Treg↑ | 42 | |
| miR‐155 | Treg | ↓ | Treg↑ | 42 | |
| miR‐155 | CD14+ cell, macrophage | ↑ | Th17↑ | 50 | |
| miR‐363 | Dendritic cell | ↓ | Th17↓ | 51 | |
| SLE | miR‐873 | Th17 | ↑ | Th17↑ | 43 |
| miR‐326 | Treg | ↑ | Treg↓ | 44 | |
| miR‐142‐3p | Dendritic cell | ↑ | Th17↑ and Treg↓ | 53 | |
| miR‐663 | BMSC | ↑ | Treg↓ | 52 | |
| EAM | miR‐155 | PBMC, CD4+ T cell | ↑ | Th17↑ | 45 |
| PV | miR‐200a | CD4+ T cell | ↑ | Th17↑ and Treg↓ | 46 |
| miR‐210 | CD4+ T cell | ↑ | Treg↓ | 47 | |
| ITP | miR‐125a‐5p | CD4+ T cell | ↓ | Th17↑ and Treg↓ | 48 |
Th17; T helper type 17; Treg, regulatory T; miRNA, microRNA; EAE, experimental autoimmune encephalomyelitis; MS, multiple sclerosis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; EAM, experimental autoimmune myocarditis; PV, psoriasis vulgaris; ITP, immune thrombocytopenic purpura; PBLs, peripheral blood lymphocytes; PBMC, peripheral blood mononuclear cell; BMSC, bone‐marrow‐derived stroma cell; ↓, Decrease , ↑ Increase; Th17↑, promotes Th17 cell differentiation; Th17↓, inhibits Th17 cell differentiation; Treg↑, promotes Treg cell differentiation; Treg↓, inhibits Treg cell differentiation.
T‐cell intrinsic miRNAs
During the pathogenesis of autoimmune diseases, many miRNAs are dysregulated in T cells and contribute to the disturbed Th17/Treg balance. MS is a chronic inflammatory autoimmune disease that damages the central nervous system and has been characterized by demyelinated regions in the white and grey matter of the brain and spinal cord. As of now, most T‐cell intrinsic miRNAs that regulate Th17/Treg balance were found during the development and progression of MS and EAE. Although miR‐20b,22 miR‐30a,23 miR‐146a,24 miR‐21425 and miR‐26a26 were down‐regulated in CD4+ T cells or Th17 cells during the process of demyelination disease in both patients with MS and EAE mice, the expression of miR‐17‐92,27 miR‐326,28 miR‐384,29 miR‐181c,30 miR‐21,31 miR‐132/212,32 miR‐155,33 miR‐27a,25 miR‐590,34 miR‐448,35 miR‐141,36 miR‐200a36 and miR‐22337 was markedly increased. As a consequence, the dysregulation of these miRNAs contributes to the increased Th17 response. On the other hand, up‐regulation of miR‐182 in CD4+ T cells promotes the development of EAE through the inhibition of Treg cell development.38
Rheumatoid arthritis is an autoimmune disease characterized by a chronic inflammation of the joint synovium membrane leading to bone and cartilage destruction. The chronic inflammatory process in RA indicates that immune regulation in the joint is disturbed and that may be caused by an excessive inflammatory response associated with a deficiency in the control of the immune response. The expression of miR‐301a‐3p,39 miR‐1640 and miR‐2141 was found to be increased in peripheral blood mononuclear cells from RA patients. The up‐regulation of these miRNAs leads to increased Th17 cell frequency and impaired Treg cell development. It has been reported that miR‐146a42 and miR‐15542 promote Treg cell development. In RA patients, decreased expression of miR‐146a and miR‐155 has been associated with reduced frequency of Treg cells.
Several T‐cell intrinsic miRNAs were also found to be dysregulated during the pathogenesis of other autoimmune diseases. The expression of miR‐87343 and miR‐32644 expression in patients with SLE, miR‐155 in patients with experimental autoimmune myocarditis,45 miR‐200a46 and miR‐21047 in patients with psoriasis vulgaris, and miR‐125a‐5p in patients with immune thrombocytopenic purpura48 were all dramatically increased. The dysregulation of these miRNAs contributes to the disturbed Th17/Treg balance through the promotion of Th17 cell development and/or suppression of Treg cell development.
T‐cell extrinsic miRNAs
Non‐T cells, such as dendritic cells (DCs) and macrophages, also express miRNAs that are able to modulate Th17/Treg balance. miR‐223, a myeloid cell‐specific miRNA and one of the most up‐regulated miRNAs in patients with MS, can promote DC‐induced activation of the pathogenic Th17 response.49 miR‐155 is up‐regulated significantly in the RA synovial compartment, particularly in CD68+ macrophages and CD14+ cells. This up‐regulation promotes the development of autoreactive Th17 cells.50 The decreased expression of miR‐363 in DCs from patients with RA leads to increased levels of IL‐6, IL‐17 and TGF‐β in the serum.51 In addition, it has been reported that miR‐663 in bone marrow‐derived mesenchymal stem cells and miR‐142‐3p in monocyte‐derived DCs suppress Treg cell development during the pathogenesis of SLE.52 Recently, studies found that the expression of miRNA let‐7i in circulating exosomes is markedly increased in patients with MS and this leads to the suppression of Treg cell development.53
Mechanisms through which dysregulated miRNAs modulate Th17/Treg balance
During the development of autoimmune diseases, dysregulated miRNAs regulate Th17/Treg balance through targeting the positive or negative regulators of Th17 and/or Treg cell differentiation. In this section, we will summarize different mechanisms through which dysregulated miRNAs modulate Th17/Treg balance during the development of autoimmune diseases (Fig. 1).
Figure 1.
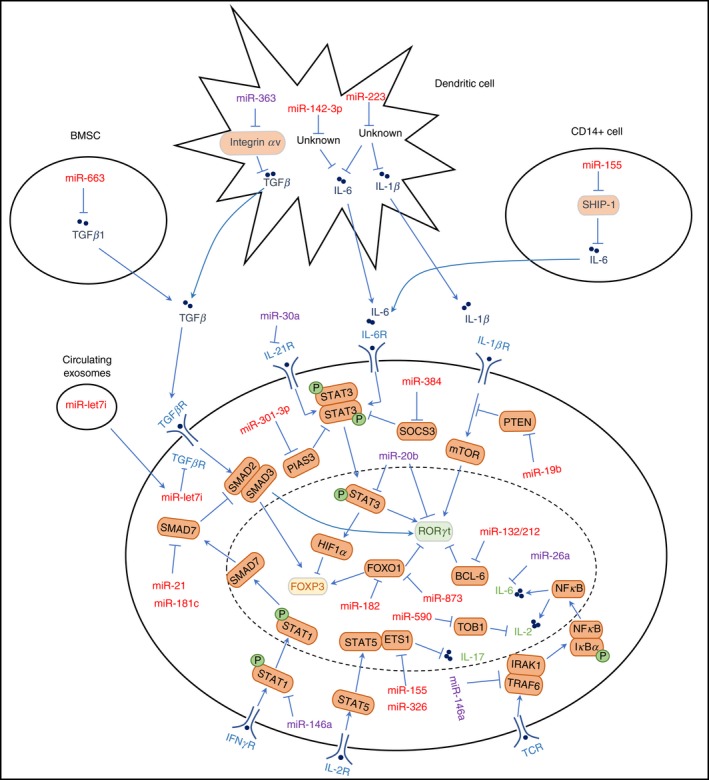
Targets of dysregulated microRNAs (miRNAs) that are involved in the regulation of T helper typ 17 (Th17)/regulatory T (Treg) cell balance. Red colour represents miRNAs that increase the ratio of Th17/Treg; Purple colour represents miRNAs that decrease the ratio of Th17/Treg cells
Targets of dysregulated miRNAs in T cells
RORγt
The master regulator for Th17 cell differentiation, RORγt was identified as one of the direct targets of miR‐20b. miR‐20b suppresses the development of EAE through direct targeting of RORγt, which leads to a decreased Th17 response.22
Foxo1
Forkhead box O1 (Foxo1) was identified as a Th17 suppressor that can interact with RORγt to negatively regulate IL‐17A production54 and suppress the pathogenicity of Th17 cells through the down‐regulation of IL‐1R155 and IL‐23R.54 miR‐873 was found to directly target Foxo1 to facilitate Th17 cell differentiation in patients with SLE.43 On the other hand, Foxo1 is also essential for the induction of Foxp3 expression in Treg cells.56 An elevated level of miR‐182 and decreased Foxo1 expression were observed during the acute phase of EAE. Further studies showed that miR‐182 inhibits Treg cell development through a Foxo1‐dependent pathway.38
Bcl‐6
The miR‐132/212 cluster is induced by aryl hydrocarbon receptor activation, which plays critical roles in autoimmune diseases such as EAE. B‐cell lymphoma 6 (Bcl‐6) is a negative regulator of Th17 differentiation. Further study showed that the miR‐132/212 cluster could target Bcl‐6 to promote Th17 cell differentiation.32
Regulators of IL‐21 and IL‐6 signalling pathways
Interleukin‐21 and IL‐6 are two cytokines that can positively regulate Th17 differentiation. miR‐30a and miR‐26a could inhibit Th17 differentiation through direct targeting of IL‐21R and IL‐6, respectively.23, 26 Because IL‐6 is also a strong suppressor of the TGF‐β‐driven induction of Foxp3,57 miR‐26a could also promote the development and function of Treg cells. STAT3 activation is important for IL‐21 and IL‐6 signal transduction. miR‐20b could directly target STAT3 to suppress the Th17 response and pathogenesis of EAE.22 The protein inhibitor of activated STAT3 (PIAS3) is the main cellular inhibitor of STAT3. miR‐301a‐3p, an inhibitor of PIAS3 expression, promotes Th17 cell differentiation in patients with RA.39 SOCS3 is an important member of the SOCS family and can negatively regulate the IL‐6–STAT3 signalling pathway. miR‐384 could promote the differentiation of Th17 cells through the targeting of SOCS3.29
Regulators of IL1‐β signalling pathway
IL‐1β signalling was required for the early stage of Th17 differentiation by converting Foxp3+ T cells into Th17 cells. After polarization, IL‐1β also favoured Th17 cells to maintain their function.58 IL‐1β promotes Th17 polarization through the activation of MAPK and the Akt/mTOR pathway, which in turn phosphorylates STAT3 and enhances the transcription of RORγt. Phosphatase and Tensin Homology (PTEN) is an antagonist of the PI3K–AKT–mTOR axis and acts as an important anti‐inflammatory mediator by reducing Th17 cell‐mediated pathogenesis. miR‐19b down‐regulates the expression of PTEN, which in turn augments the PI3K–AKT–mTOR axis and promotes Th17 differentiation.27
Regulators of TCR signalling pathway
Upon recognition of autoantigens, autoreactive CD4 T cells initiate TCR signalling that leads to nuclear factor‐ κB (NF‐κB) activation. NF‐κB induces the production of T‐cell autocrine IL‐6 and IL‐21 cytokines that activate STAT3. TRAF6 and IRAK1 are the NF‐κB signalling transducers. miR‐146a targets TRAF6 and IRAK1 and then inhibits the development of pathogenic Th17 cells by blocking the production of IL‐6 and IL‐21 during the development of EAE.24
Regulators of TGF‐β signal pathway
The TGF‐β signalling plays an essential role in the generation of both Th17 and Treg cells. SMAD (an acronym from the fusion of Caenorhabditis elegans Sma genes and the Drosophila Mad, Mothers against decapentaplegic)‐7 inhibits TGF‐β‐induced transcriptional responses by blocking the activation of SMAD‐2/3 and their complex formation with SMAD‐4. miR‐21 and miR‐181c could promote Th17 cell differentiation through direct targeting of SMAD‐7.31, 59 Overexpression of miR‐21 reduced SMAD‐7 expression while up‐regulating SMAD‐2/3 levels, resulting in enhanced IL‐17 production. However, Treg cell differentiation was normal in miR‐21‐deficient mice. TGF‐β signalling regulates Treg cell differentiation and function through both SMAD‐dependent and SMAD‐independent pathways. Although the SMAD‐2/3‐pathway dependent TGF‐β signalling has been demonstrated to partially regulate iTreg cell differentiation, it has also been shown that a combination of SMAD‐2 and SMAD‐3 deficiency does not alter Foxp3 expression or the suppressive activity of iTreg cells in vivo. It has been reported that the level of IL‐2 was enhanced in miR‐21‐deficient mice. Because IL‐2 has been shown to stabilize TGF‐β‐induced Foxp3 expression, it is possible that enhanced IL‐2 expression in miR‐21‐deficient mice could compensate for the loss of defective SMAD‐2/3‐dependent TGF‐β signalling during Treg cell differentiation.
Regulators of IFN‐γ signalling pathway
Interferon‐γ mediates the phosphorylation of STAT1, which leads to the expression of T‐bet, Smad7 and FasL. T‐bet and Smad7 are two negative regulators of Th17 cell polarization. In addition, Smad7 inhibits the regulatory function of Treg cells. STAT1 is a direct target of miR‐146a and decreased expression of miR‐146a facilitates a pro‐inflammatory phenotype of Treg cells because of increased STAT1 activation.24
Regulators of IL‐2 signalling pathway
STAT5 is an essential downstream transcription factor of IL‐2 signalling, which is critical for the survival of T cells but suppresses Th17 cell development.The transcriptional regulator Tob1 (transducer of ERBB2‐1) can impair IL‐2 production in Th17 cells and plays an important role in the activation of encephalitogenic T cells in central nervous system autoimmunity.60 It has been reported that miR‐590 promotes pathogenic Th17 cell differentiation through targeting Tob1.34 Other studies showed that Ets‐1, a target of miR‐155 and miR‐326, may form a protein complex with STAT5, and then inhibit IL‐17 expression. Thereby, miR‐155 and miR‐326 could promote Th17 differentiation through direct targeting of Ets‐1.28, 45
Targets of dysregulated miRNAs in non‐T cells
The miRNAs could modulate the balance of Th17/Treg in a cell non‐autonomous way, that is, through targeting factors important for Th17 and Treg cell development in non‐T cells. miR‐363,51 miR‐142‐3p61 and miR‐22349 were dysregulated in DCs and could promote Th17 cell differentiation during the pathogenesis of autoimmune diseases. miR‐363 suppresses the expression of integrin αv, which is an important positive regulator of TGF‐β activation. However, the targets of miR‐142‐3p and miR‐223, which account for the disturbed Th17/Treg balance, have not been characterized yet. In CD68+ macrophages and CD14+ cells, up‐regulation of miR‐155 leads to decreased expression of SHIP‐1, which in turn promotes the production of Th17 inducing cytokine IL‐6.50 Through targeting TGF‐β 1, miR‐663 in bone‐marrow‐derived mesenchymal stem cells down‐regulates the frequency of Treg cells not only by inhibiting nTreg cell proliferation and iTreg cell differentiation but also by converting Treg cells toward a follicular helper T‐cell phenotype.52 The circulating exosomal miRNA, miR‐let7i, which is markedly increased in patients with MS, suppresses the induction of Treg cells by targeting insulin‐like growth factor 1 receptor and transforming growth factor β receptor.53
Concluding remarks
Although Th17 and Treg cells are both implicated in inflammatory and autoimmune diseases, they are two distinct T‐cell subsets with opposing actions. Th17 and Treg cells share common factors for their development and function, and the plastic nature of these two cell subsets emphasizes the importance of Th17/Treg balance during the development of autoimmunity, inflammation and cancer. The miRNAs play crucial roles in the development and function of both Th17 and Treg cells, and shaping the balance between Th17 and Treg cells has significant biological implications for the development of novel miRNA‐based therapeutic approaches for the treatment of autoimmune and inflammatory diseases.
Funding
This study was supported by the National Natural Science Foundation of China (81471554, 81470611, 81530027); Shenzhen Municipal Science and Technology Innovation Committee (JCYJ20160531185449995, KQJSCX20160301140901, JCYJ20170413165432016); Shenzhen Peacock Plan (1110140040347265); Taishan Scholar Programme (20150215); The Innovation Project of Shandong Academy of Medical Sciences (201608); Shandong Province Key Research and Development Programme (2015GGH318010).
Disclosures
The authors declare no conflict of interest.
Contributor Information
Ting Wang, Email: wt-ting@163.com.
Qingguo Ruan, Email: qg.ruan@siat.ac.cn.
References
- 1. Caza T, Landas S. Functional and phenotypic plasticity of CD4+ T cell subsets. Biomed Res Int 2015; 2015:521957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 2014; 13:668–77. [DOI] [PubMed] [Google Scholar]
- 3. Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell 2009; 136:26–36. [DOI] [PubMed] [Google Scholar]
- 4. McGeachy MJ, Bak‐Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T et al TGF‐β and IL‐6 drive the production of IL‐17 and IL‐10 by T cells and restrain TH‐17 cell‐mediated pathology. Nat Immunol 2007; 8:1390–7. [DOI] [PubMed] [Google Scholar]
- 5. Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F et al A validated regulatory network for Th17 cell specification. Cell 2012; 151:289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Defining the human T helper 17 cell phenotype. Trends Immunol 2012; 33:505–12. [DOI] [PubMed] [Google Scholar]
- 7. Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity 2013; 38:414–23. [DOI] [PubMed] [Google Scholar]
- 8. Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL‐2 controls the stability of Foxp3 expression in TGF‐β‐induced Foxp3+ T cells in vivo . J Immunol 2011; 186:6329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol 2003; 3:253–7. [DOI] [PubMed] [Google Scholar]
- 10. Williams LM, Rudensky AY. Maintenance of the Foxp3‐dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol 2007; 8:277–84. [DOI] [PubMed] [Google Scholar]
- 11. Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T et al IL‐6 programs TH‐17 cell differentiation by promoting sequential engagement of the IL‐21 and IL‐23 pathways. Nat Immunol 2007; 8:967–74. [DOI] [PubMed] [Google Scholar]
- 12. Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB et al IL‐21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 2007; 448:484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL‐2 receptor β‐dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 2007; 178:280–90. [DOI] [PubMed] [Google Scholar]
- 14. Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL‐1β and IL‐2 convert human Treg into TH17 cells. Clin Immunol 2009; 131:298–307. [DOI] [PubMed] [Google Scholar]
- 15. Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW et al Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 2015; 523:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K et al Identification of IL‐17‐producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A 2009; 106:4793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD et al TGF‐β‐induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 2008; 453:236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang X, Chen Y, Zhang F, Yang Q, Zhang G. Peripheral Th17/Treg cell‐mediated immunity imbalance in allergic rhinitis patients. Braz J Otorhinolaryngol 2014; 80:152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duan MC, Zhong XN, Liu GN, Wei JR. The Treg/Th17 paradigm in lung cancer. J Immunol Res 2014; 2014:730380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol 2012; 181:8–18. [DOI] [PubMed] [Google Scholar]
- 21. Tauro S, Nguyen P, Li B, Geiger TL. Diversification and senescence of Foxp3+ regulatory T cells during experimental autoimmune encephalomyelitis. Eur J Immunol 2013; 43:1195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu E, Wang X, Zheng B, Wang Q, Hao J, Chen S et al miR‐20b suppresses Th17 differentiation and the pathogenesis of experimental autoimmune encephalomyelitis by targeting RORγt and STAT3. J Immunol 2014; 192:5599–609. [DOI] [PubMed] [Google Scholar]
- 23. Qu X, Zhou J, Wang T, Han J, Ma L, Yu H et al MiR‐30a inhibits Th17 differentiation and demyelination of EAE mice by targeting the IL‐21R. Brain Behav Immun 2016; 57:193–9. [DOI] [PubMed] [Google Scholar]
- 24. Li B, Wang X, Choi IY, Wang YC, Liu S, Pham AT et al miR‐146a modulates autoreactive Th17 cell differentiation and regulates organ‐specific autoimmunity. J Clin Invest 2017; 127:3702–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahmadian‐Elmi M, Bidmeshki Pour A, Naghavian R, Ghaedi K, Tanhaei S, Izadi T et al miR‐27a and miR‐214 exert opposite regulatory roles in Th17 differentiation via mediating different signaling pathways in peripheral blood CD4+ T lymphocytes of patients with relapsing–remitting multiple sclerosis. Immunogenetics 2016; 68:43–54. [DOI] [PubMed] [Google Scholar]
- 26. Zhang R, Tian A, Wang J, Shen X, Qi G, Tang Y. miR26a modulates Th17/T reg balance in the EAE model of multiple sclerosis by targeting IL6. Neuromolecular Med 2015; 17:24–34. [DOI] [PubMed] [Google Scholar]
- 27. Liu SQ, Jiang S, Li C, Zhang B, Li QJ. miR‐17‐92 cluster targets phosphatase and tensin homology and Ikaros Family Zinc Finger 4 to promote TH17‐mediated inflammation. J Biol Chem 2014; 289:12446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S et al MicroRNA miR‐326 regulates TH‐17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol 2009; 10:1252–9. [DOI] [PubMed] [Google Scholar]
- 29. Qu X, Han J, Zhang Y, Wang Y, Zhou J, Fan H et al MiR‐384 regulates the Th17/Treg ratio during experimental autoimmune encephalomyelitis pathogenesis. Front Cell Neurosci 2017; 11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Guo M, Xin N, Shao Z, Zhang X, Zhang Y et al Decreased microRNA miR‐181c expression in peripheral blood mononuclear cells correlates with elevated serum levels of IL‐7 and IL‐17 in patients with myasthenia gravis. Clin Exp Med 2016; 16:413–21. [DOI] [PubMed] [Google Scholar]
- 31. Murugaiyan G, da Cunha AP, Ajay AK, Joller N, Garo LP, Kumaradevan S et al MicroRNA‐21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J Clin Invest 2015; 125:1069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakahama T, Hanieh H, Nguyen NT, Chinen I, Ripley B, Millrine D et al Aryl hydrocarbon receptor‐mediated induction of the microRNA‐132/212 cluster promotes interleukin‐17‐producing T‐helper cell differentiation. Proc Natl Acad Sci U S A 2013; 110:11964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang J, Cheng Y, Cui W, Li M, Li B, Guo L. MicroRNA‐155 modulates Th1 and Th17 cell differentiation and is associated with multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol 2014; 266:56–63. [DOI] [PubMed] [Google Scholar]
- 34. Liu Q, Gao Q, Zhang Y, Li Z, Mei X. MicroRNA‐590 promotes pathogenic Th17 cell differentiation through targeting Tob1 and is associated with multiple sclerosis. Biochem Biophys Res Commun 2017; 493:901–8. [DOI] [PubMed] [Google Scholar]
- 35. Wu R, He Q, Chen H, Xu M, Zhao N, Xiao Y et al MicroRNA‐448 promotes multiple sclerosis development through induction of Th17 response through targeting protein tyrosine phosphatase non‐receptor type 2 (PTPN2). Biochem Biophys Res Commun 2017; 486:759–66. [DOI] [PubMed] [Google Scholar]
- 36. Naghavian R, Ghaedi K, Kiani‐Esfahani A, Ganjalikhani‐Hakemi M, Etemadifar M, Nasr‐Esfahani MH. miR‐141 and miR‐200a, revelation of new possible players in modulation of Th17/Treg differentiation and pathogenesis of multiple sclerosis. PLoS One 2015; 10:e0124555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hosseini A, Ghaedi K, Tanhaei S, Ganjalikhani‐Hakemi M, Teimuri S, Etemadifar M, et al Upregulation of CD4+ T‐cell derived MiR‐223 in the relapsing phase of multiple sclerosis patients. Cell J 2016; 18:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wan C, Ping CY, Shang XY, Tian JT, Zhao SH, Li L et al MicroRNA 182 inhibits CD4+CD25+Foxp3+ Treg differentiation in experimental autoimmune encephalomyelitis. Clin Immunol 2016; 173:109–16. [DOI] [PubMed] [Google Scholar]
- 39. Tang X, Yin K, Zhu H, Tian J, Shen D, Yi L et al Correlation between the expression of microRNA‐301a‐3p and the proportion of Th17 cells in patients with rheumatoid arthritis. Inflammation 2016; 39:759–67. [DOI] [PubMed] [Google Scholar]
- 40. Wu YH, Liu W, Xue B, Zhang L, Liu XY, Liu B et al Upregulated expression of microRNA‐16 correlates with Th17/Treg cell imbalance in patients with rheumatoid arthritis. DNA Cell Biol 2016; 35:853–60. [DOI] [PubMed] [Google Scholar]
- 41. Dong L, Wang X, Tan J, Li H, Qian W, Chen J et al Decreased expression of microRNA‐21 correlates with the imbalance of Th17 and Treg cells in patients with rheumatoid arthritis. J Cell Mol Med 2014; 18:2213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou Q, Haupt S, Kreuzer JT, Hammitzsch A, Proft F, Neumann C et al Decreased expression of miR‐146a and miR‐155 contributes to an abnormal Treg phenotype in patients with rheumatoid arthritis. Ann Rheum Dis 2015; 74:1265–74. [DOI] [PubMed] [Google Scholar]
- 43. Liu L, Liu Y, Yuan M, Xu L, Sun H. Elevated expression of microRNA‐873 facilitates Th17 differentiation by targeting forkhead box O1 (Foxo1) in the pathogenesis of systemic lupus erythematosus. Biochem Biophys Res Commun 2017; 492:453–60. [DOI] [PubMed] [Google Scholar]
- 44. Sun XG, Tao JH, Xiang N, Li XM, Wang GS, Fang X et al Negative correlation between miR‐326 and Ets‐1 in regulatory T cells from new‐onset SLE patients. Inflammation 2016; 39:822–9. [DOI] [PubMed] [Google Scholar]
- 45. Yan L, Hu F, Yan X, Wei Y, Ma W, Wang Y et al Inhibition of microRNA‐155 ameliorates experimental autoimmune myocarditis by modulating Th17/Treg immune response. J Mol Med (Berl) 2016; 94:1063–79. [DOI] [PubMed] [Google Scholar]
- 46. Wang XY, Chen XY, Li J, Zhang HY, Liu J, Sun LD. MiR‐200a expression in CD4+ T cells correlates with the expression of Th17/Treg cells and relevant cytokines in psoriasis vulgaris: A case control study. Biomed Pharmacother 2017; 93:1158–64. [DOI] [PubMed] [Google Scholar]
- 47. Zhao M, Wang LT, Liang GP, Zhang P, Deng XJ, Tang Q et al Up‐regulation of microRNA‐210 induces immune dysfunction via targeting FOXP3 in CD4+ T cells of psoriasis vulgaris. Clin Immunol 2014; 150:22–30. [DOI] [PubMed] [Google Scholar]
- 48. Li JQ, Hu SY, Wang ZY, Lin J, Jian S, Dong YC et al Long non‐coding RNA MEG3 inhibits microRNA‐125a‐5p expression and induces immune imbalance of Treg/Th17 in immune thrombocytopenic purpura. Biomed Pharmacother 2016; 83:905–11. [DOI] [PubMed] [Google Scholar]
- 49. Satoorian T, Li B, Tang X, Xiao J, Xing W, Shi W et al MicroRNA223 promotes pathogenic T‐cell development and autoimmune inflammation in central nervous system in mice. Immunology 2016; 148:326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kurowska‐Stolarska M, Alivernini S, Ballantine LE, Asquith DL, Millar NL, Gilchrist DS et al MicroRNA‐155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci U S A 2011; 108:11193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pan F, Xiang H, Yan J, Hong L, Zhang L, Liu Y et al Dendritic cells from rheumatoid arthritis patient peripheral blood induce Th17 cell differentiation via miR‐363/integrin αv/TGF‐β axis. Scand J Immunol 2017; 85:441–9. [DOI] [PubMed] [Google Scholar]
- 52. Geng L, Tang X, Zhou K, Wang D, Wang S, Yao G et al MicroRNA‐663 induces immune dysregulation by inhibiting TGF‐β1 production in bone marrow‐derived mesenchymal stem cells in patients with systemic lupus erythematosus. Cell Mol Immunol 2018; 15:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kimura K, Hohjoh H, Fukuoka M, Sato W, Oki S, Tomi C et al Circulating exosomes suppress the induction of regulatory T cells via let‐7i in multiple sclerosis. Nat Commun 2018; 9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y et al Induction of pathogenic TH17 cells by inducible salt‐sensing kinase SGK1. Nature 2013; 496:513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ichiyama K, Gonzalez‐Martin A, Kim B‐S, Jin Hyun Y, Jin W, Xu W et al The microRNA‐183‐96‐182 cluster promotes T helper 17 cell pathogenicity by negatively regulating transcription factor Foxo1 expression. Immunity 2016; 44:1284–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ouyang W, Li MO. Foxo: in command of T lymphocyte homeostasis and tolerance. Trends Immunol 2011; 32:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M et al Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441:235–8. [DOI] [PubMed] [Google Scholar]
- 58. Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS et al Critical regulation of early Th17 cell differentiation by interleukin‐1 signaling. Immunity 2009; 30:576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Z, Xue Z, Liu Y, Liu H, Guo X, Li Y et al MicroRNA‐181c promotes Th17 cell differentiation and mediates experimental autoimmune encephalomyelitis. Brain Behav Immun 2018; 70:305–14. [DOI] [PubMed] [Google Scholar]
- 60. Schulze‐Topphoff U, Casazza S, Varrin‐Doyer M, Pekarek K, Sobel RA, Hauser SL et al Tob1 plays a critical role in the activation of encephalitogenic T cells in CNS autoimmunity. J Exp Med 2013; 210:1301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y, Liang J, Qin H, Ge Y, Du J, Lin J et al Elevated expression of miR‐142‐3p is related to the pro‐inflammatory function of monocyte‐derived dendritic cells in SLE. Arthritis Res Ther 2016; 18:263. [DOI] [PMC free article] [PubMed] [Google Scholar]


