1. Introduction
Human-induced global change is increasingly affecting life on our planet, including living conditions for humans themselves as well as the resources we depend on [1,2]. As a result, species diversity is strongly declining [3–5]. The Living Planet Index shows a 58% global decline in populations of amphibians, fish, reptiles, mammals and birds between 1970 and 2012, varying from 36 to 38% in terrestrial and marine ecosystems to 81% in freshwater habitat [6]. Habitat loss or degradation and overexploitation are the main causes of these steep declines. Since the worldwide expansion of modern humans (Homo sapiens) began, humans have overexploited vertebrates, with a bias to the largest animals being extirpated first, from the Late Pleistocene extinctions of terrestrial megafauna to the ongoing declines of terrestrial, marine and freshwater large-bodied animals [7–11]. There is increasing evidence that this global wildlife loss, or defaunation, does not only imply the loss of charismatic animals but also the functions they have in ecosystems [12–16]. To restore these missing functions, a novel ecological restoration technique has emerged, referred to as rewilding [17]. Rewilding aims to restore natural processes in ecosystems in general, and often focuses on re-introduction of missing large wildlife species or, in case these went extinct, their proxies [18]. Rewilding is increasingly implemented in practice globally, with a strong emphasis on Europe and the re-introduction of large herbivores [19,20].
2. Impacts of rewilding
Rewilding has recently been described as ‘a captivating, controversial, twenty-first century concept to address ecological degradation’ [20]. Most rewilding initiatives fit the concept of trophic rewilding, defined as an ecological restoration strategy that uses species introductions to restore top-down trophic interactions and associated trophic cascades to promote self-regulating biodiverse ecosystems [18]. If it works, this would both turn around defaunation, by re-introducing missing wildlife, and halt biodiversity declines. Furthermore, rewilding may have positive effects on multiple ecosystem services [21,22]. As such, rewilding is a narrative of hope, which is the captivating part (e.g. [23–25]). The controversial part is that opponents fear that rewilding is the new Pandora's box in conservation and may harm biodiversity, with particular concern about exotic species proliferation as a consequence of rewilding ([26,27]; but see [28] for a response). However, at present, data on the effects of explicit rewilding efforts are scarce and the scientific literature on rewilding is strongly dominated by essays, perspectives and opinion papers [18,20]. At the same time rewilding is increasingly implemented in practice by initiatives such as Rewilding Europe (http://www.rewildingeurope.com). Rewilding is also increasingly discussed and implemented in other parts of the world, e.g. Australia [29], oceanic islands in the Pacific [30] and South America [31]. Therefore, there is an urgent need for science to move on from ideological debates to actual data on the impacts of rewilding.
3. Obtaining data on rewilding impacts
Whereas data on explicit rewilding projects may be scarce, our understanding of rewilding impacts can be expanded by studying non-intentional rewilding events. Indeed, Tanentzap and Smith [32] demonstrate that the top-down regulation of lower trophic levels is equally strong in trophic rewilding through intentional and non-intentional species introductions. Furthermore, much relevant information exists from studies not explicitly about rewilding. There is, for instance, lots of experience with ecological effects of megafauna reintroductions in South Africa, such as white rhinoceros in Kruger National Park [33], even if these have not been called rewilding projects. Similarly, there is ample experience with impacts of free-ranging horses and bovids in Europe, which could be seen as replacement of the extinct native wild grazer populations, i.e. the aurochs (extinct in 1627) and the tarpan (extinct 1887).
In this issue, we bring together the state-of-the-art of trophic rewilding research, using the multitude of opportunities to obtain data on trophic rewilding impacts as described above. Furthermore, we address the policy and societal sides of trophic rewilding, such as which herbivores can be introduced where and how to measure rewilding success? Importantly, we here synthesize the main outcomes of the impact of trophic rewilding on ecosystems in the light of global change and provide an outlook of the opportunities of trophic rewilding as a new restoration technique as well as research avenues.
4. Effects on biodiversity and invasive species
Trophic rewilding potentially has positive effects on biodiversity, and could thus mitigate the biodiversity crisis, but data to test this are very limited. Van Klink and WallisDeVries [34] review the evidence of the impacts of rewilding with large herbivores on arthropods, the most species-rich group of eukaryotic organisms, but rarely considered in rewilding. They found that systematic monitoring is rare and that a comparison with a relevant control treatment is usually lacking. Still, they can conclude that at moderate densities large herbivores increase grassland arthropod diversity, whereas they decrease diversity at high densities [34]. These results are largely in line with large herbivore impact on arthropod diversity in non-rewilding areas [35]. In wetlands, Eurasian beavers (Castor fiber) strongly affect wetland plants and wetland ecosystem functioning [13]. Beavers are increasingly being introduced in the context of rewilding projects [36], but empirical evidence of their effects is often lacking. Willby et al. [37] show that by creating within-habitat heterogeneity beavers increase plants diversity, whereas they increase the abundance, but not the diversity of aquatic beetles. By creating heterogeneity within ponds, beavers likely contribute to increased biodiversity across scales [38].
A concern of trophic rewilding is the impact on invasive species. Derham et al. [39] show three cases in which rewilding native carnivores helps suppressing invasive smaller carnivores and releasing native prey species; in contrast rewilding a mesoherbivore (Tule elk Cervus canadensis nannodes) sometimes slows down grassland plant invasions, sometimes not. Trophic rewilding can work to prevent biological invasions, mitigate their impacts and promote the coexistence of newcomer species with long-time residents. However, the conditions for success will vary from case to case and will be hard to predict. In this respect trophic rewilding shows parallels with invasion biology itself [39].
5. Can trophic rewilding serve as a climate change mitigation strategy?
Large herbivores can interact with climate change in a number of direct and indirect ways [40,41]. In the Arctic, caribou or reindeer (Rangifer tarandus) and muskoxen (Ovibos moschatus) are the only large herbivores. With rising temperature, thermophilic plant species are invading the tundra and these are preferred by the herbivores. Therefore, reindeer and muskoxen are able to maintain the present vegetation composition even in a warmer climate [42]. Furthermore, they prevent woody plant encroachment with increasing temperatures, which in turn leads to a higher albedo [43], with a cooling effect. Large herbivores may increase soil carbon sequestration, when they consolidate the persistence of the permafrost [44]; however, this is at present uncertain [41]. Andriuzzi & Wall [45] also indicate uncertainty and major knowledge gaps about the impact of large herbivores on soil responses and feedbacks. Despite this uncertainty, rewilding the Arctic with a larger density and diversity of large herbivores may mitigate the impact of temperature rise in this region [40,41]. In tropical rainforest, defaunation leads to lower dispersal of tree species with megafaunal fruits, which have a higher wood density and, therefore, contribute strongly to carbon storage in tropical forests [16,46]. Rewilding with large herbivores could thus increase the carbon storage potential of the forest [40]. In rangelands, greenhouse gas emissions could be strongly reduced when ruminant livestock are replaced by non-ruminant wildlife [40,47]. Cromsigt et al. [40] argue that trophic rewilding as a climate change mitigation strategy should focus on restoring populations of large (greater than 100 kg) non-ruminant herbivores. Furthermore, trophic rewilding should be integrated into intergovernmental climate change mitigation funding schemes to allow wide enough implementation of rewilding to make a difference.
Trophic rewilding can mitigate the incidence of wildfires which may be increasing in areas which are more prone to droughts as a result of climate change [48]. Especially large grazers are suitable for this purpose, as they consume large amounts of potential fuel, whereas browsers increase the fuel load by consuming woody plants, not herbaceous plants. The frequency and intensity of fires, in turn, has a myriad of effects on climate, but the net effect remains uncertain [40].
6. Which animals to introduce where in trophic rewilding
To introduce species into systems to restore extinct top-down interactions requires knowledge of their former distribution as well as their diet. As historic and pre-historic human-driven extinctions have reshaped global patterns of mammal diversity [49], it is unsure where these animals may be able to live under current climate conditions, and thus which sites are suitable for trophic rewilding. Furthermore, these anthropogenic range contractions bias species climate change forecasts [50]. Using species distribution modelling Jarvie & Svenning [51] demonstrate that for 17 large-bodied mammalian taxa near-future climate change should not generally prohibit the implementation of megafauna-based trophic rewilding, reflecting the broad environmental tolerances of many megafauna species. However, this may be different for ectotherms, which in contrast to mammalian megafauna, need to be able to thermoregulate efficiently. Extant giant tortoises are used as substitutes for their extinct relatives in trophic rewilding projects on oceanic islands to restore their role of ecosystem engineers [52]. In these seasonally dry environments, mitigation measures may be needed to allow the tortoises to successfully maintain their water balance and execute their role in seed dispersal, nutrient cycling and herbivory at the landscape scale [53]. In the boreal forest, moose (Alces americanus) fulfil an important role in fertilizing the forest with nitrogen, the limiting plant nutrient, by transporting nitrogen across the aquatic-terrestrial ecotone [54]. If moose were to follow the boreal-deciduous forest ecotone, which moves north with increasing temperatures [55], deer would replace moose, but these are much less effective as nutrient transporters [54].
Then, when the climate is suitable, how to identify areas that would profit most from restoration of trophic interactions? Marjakangas et al. [56] use species distribution modelling in combination with occurrence data and species interaction records to quantify the potential to restore seed-dispersal interactions through rewilding in the Brazilian Atlantic forest, a biodiversity hotspot strongly impoverished in large animals [16]. By ranking bird and mammal species based on their seed-dispersal potential they identify which species can best be used. More species-rich and larger forest fragments have many more interactions to cash in on than the deteriorated ones, hence could be prioritized for re-introductions of missing seed dispersers [56].
The traits of the animals and plants involved in the interaction that should be restored are central to these types of analyses, as well as the pre-human distribution of the plants and animals. However, when it comes to grassland conservation Fuhlendorf et al. [57] argue that an essential component is missing, namely the role of humans. As evidenced by the degradation that included the loss of large native herbivores and replacement with domestic livestock [58], a focus on restoring an appropriate herbivore for a restoration context is important for both the conservation of the herbivore species, as well as their recognized role in conserving biodiversity broadly [59]. Future grassland conservation efforts including the decision which herbivores to use, must depend on the development of a model that better integrates societal, economic and policy objectives and recognizes climate change, fragmentation and humans as an integral part of grassland ecosystems [57].
7. How to study rewilding impacts?
From the studies presented in this issue, a number of different ways emerge to evaluate the impact of trophic rewilding. It is essential to compare the state of the rewilding area with a non-rewilding situation at a given time and as they develop [34]. This is a challenge in many cases, as rewilding projects are commonly not designed as scientific experiments, but in practice rather a certain site is selected where subsequently animals are released, and/or other restoration actions are implemented. Sometimes some additional land use is also continued or implemented. A classical before/after comparison can be used in some cases to evaluate the change in state. This has for instance been done to evaluate the impact of the release of wolves in Yellowstone National Park and the subsequent impact on elk and tree density in the landscape [60,61]. However, a site may be subject to autonomous changes over time, which may interfere with assessing the effects of rewilding. Furthermore, certain measures may be necessary to implement trophic rewilding, such as altering the fencing around the site, changing the hydrology of the site to give more room to natural water level dynamics, or taking it out of former agricultural use, which may alter the site irrespective of the (re-) introduction of animals [62]. Lastly, and most often, before-rewilding data may simply not be available. In such cases, one can attempt to find neighbouring similar sites, which are not subject to rewilding, to allow a paired comparison. This can be challenging as well, as often sites differ in more aspects then the rewilding management and the site subject to rewilding may be chosen because it was different from other sites to begin with. Still, variations on this theme are possible, as similar sites with different densities of introduced animals may be suitable for comparison of the impact of the animals. The more sites, the more robust the analysis (see for instance [63]). Another way to evaluate the impact of trophic rewilding is to use exclosures, e.g. fenced areas inside the rewilding site where the animals have no access (figure 1a,b). In this way, the impact of the animals can be deduced from the differences in site characteristics in the presence of the animals and inside the exclosures [13,14]. When no such comparisons are possible, then animal movement within a site, using GPS collars, can be used to identify low- and high-impact areas (figure 1c,d).
Figure 1.
Examples of how to collect data in trophic rewilding research. (a,b) Using exclosures to study the impact of the presence of large herbivores in rewilding projects. (a) Exclosure in the Lauwersmeer, The Netherlands, where Konik horses and bovids are used as proxies for extinct native horses and bovids and maintain short lawn grasses, whereas upon exclusion of the large herbivores inside the exclosure a tall wetland reed (Phragmites australis) vegetation emerges. (b) Exclosure on the island of Tiengemeten, The Netherlands, where Scottish highland cattle prevent the encroachment of woody plants; inside the exclosure willow species have established. (c,d) Using collars to track animal movement and quantify areas used with high and low intensity. (c) Eurasian elk (Alces alces) in a rewilding project in the largest raised bog area in Denmark, Lille Vildmose (Little Wild Bog). (d) Tapir (Tapirus terrestris) with young from the Iberá Rewilding Program, Argentina [64]. Photo credits: (a,b) E.S.B and (c,d) J.-C.S. (Online version in colour.)
8. How to evaluate rewilding progress?
Trophic rewilding can be seen as a form of open-ended management [65], as it focuses on promoting natural processes rather than pre-determined targets [62]. However, this may falsely suggest that rewilding is without ambition and measurable output as a classical evaluation of targets is not possible in open-ended management. To overcome this challenge Torres et al. [66] developed a rewilding scale, through which rewilding progress can be measured. The rewilding scale evaluates the recovery of processes and their natural dynamics based on decreasing human influence on ecological processes and increasing ecological integrity of the ecosystem [66]. Instead of achieving the highest score per se, a gradual increase in naturalness of the ecosystem over time could be a suitable goal of local management.
9. Opportunities for trophic rewilding
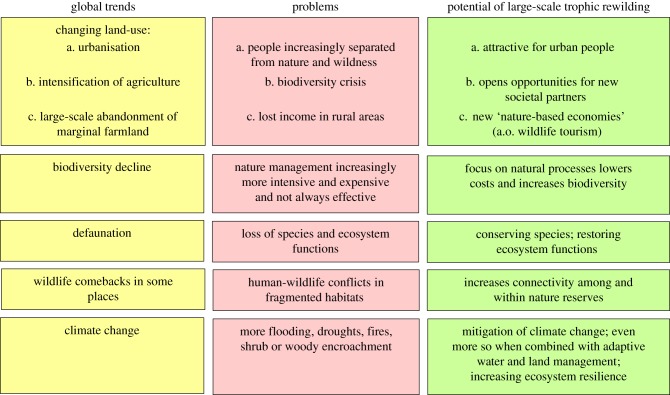
Ecosystems worldwide are increasingly affected by human-induced global change, including over-exploitation of living systems, temperature rise, eutrophication and exotic species proliferation, resulting in a biodiversity crisis [6]. Furthermore, human societies increasingly experience disasters as an increase in flooding events and wildfires, with further increases likely in the near future [67,68]. Therefore, new thinking is needed to address these pressing societal and biodiversity conservation challenges. Rewilding could potentially offer such a new way of thinking, as it is a future-oriented process-oriented and non-static restoration strategy [62] (figure 2). Through its focus on natural processes, rewilding advocates new ways of land management, focused on restoring diversity-maintaining autonomous mechanisms in nature. By restoring more complete ecosystems, trophic rewilding—if successful—could (i) confer greater robustness (resistance or resilience) to the pressures from global change and (ii) by restoring (more) self-managing ecosystems help reduce needs for people and domestic animals for ongoing management, which overall should release economic and human resources for other uses. By combining functions such as water storage capacity and giving room to natural processes, rewilding could contribute to finding solutions for flooding and drought problems as well as increase biodiversity. Such examples are now becoming available (e.g. [62]). Furthermore, rewilding aims to pursue both fundamental aims of restoration – biodiversity conservation and wildness, which has a lot of value to many people [25,69].
Figure 2.
Scheme with outlook of potential of trophic rewilding under global change for nature and society. The potential of large-scale trophic rewilding in relation to global trends and problems offers new research avenues for ecology, sociology and economy. (Online version in colour.)
Trophic rewilding can potentially provide solutions to problems which are the result of current global trends (figure 2). This special issue is an overview of where we stand at the moment. There are promising examples of climate change mitigation, of mitigation of invasive species and mitigation of biodiversity decline. But all authors agree that we are in strong need of more data, for which we hope this theme issue will serve as an inspiration and motivation. Furthermore, it becomes increasingly clear how trophic rewilding is a highly interdisciplinary field, where scientists in ecology, sociology and economy are all involved (see figure 2 and [40,57,62]).
Acknowledgements
E.S.B. thanks Wouter Helmer for explaining the philosophy and practice of trophic rewilding as implemented by Rewilding Europe.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
J.-C.S. considers this work a contribution to his Carlsberg Foundation Semper Ardens project MegaPast2Future (grant no. CF16-0005) and to his VILLUM Investigator project ‘Biodiversity Dynamics in a Changing World’ funded by VILLUM FONDEN (grant no. 16549).
References
- 1.Röckstrom J, et al. 2009. A safe operating space for humanity. Nature 461, 472–475. ( 10.1038/461472a) [DOI] [PubMed] [Google Scholar]
- 2.Ripple WJ, Wolf C, Newsome TM, Galetti M, Alamgir M, Crist E, Mahmoud MI, Laurance WF. 2017. World scientists' warning to humanity: a second notice. Bioscience 67, 1026–1028. ( 10.1093/biosci/bix125) [DOI] [Google Scholar]
- 3.Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM. 2015. Accelerated modern human–induced species losses: entering the sixth mass extinction. Sci. Adv. 1, e1400253 ( 10.1126/sciadv.1400253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCallum ML. 2015. Vertebrate biodiversity losses point to a sixth mass extinction. Biodiver. Conserv. 24, 2497–2519. ( 10.1007/s10531-015-0940-6) [DOI] [Google Scholar]
- 5.Régnier C, Achaz G, Lambert A, Cowie RH, Bouchet P, Fontaine B. 2015. Mass extinction in poorly known taxa. Proc. Natl Acad. Sci. USA 112, 7761–7766. ( 10.1073/pnas.1502350112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WWF. 2016. Living Planet Report 2016. Risk and resilience in a new era. Gland, Switzerland: WWF.
- 7.Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F. 1998. Fishing down marine food webs. Science 279, 860–863. ( 10.1126/science.279.5352.860) [DOI] [PubMed] [Google Scholar]
- 8.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 9.Sandom C, Faurby S, Sandel B, Svenning JC. 2014. Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc. R. Soc. B 281, 20133254 ( 10.1098/rspb.2013.3254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR. 2015. Marine defaunation: animal loss in the global ocean. Science 347, 1255641 ( 10.1126/science.1255641) [DOI] [PubMed] [Google Scholar]
- 11.Ripple WJ, et al. 2015. Collapse of the world's largest herbivores. Sci. Adv. 1, e1400103 ( 10.1126/sciadv.1400103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill JL. 2014. Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 201, 1163–1169. ( 10.1111/nph.12576) [DOI] [PubMed] [Google Scholar]
- 13.Bakker ES, Pagès JF, Arthur R, Alcoverro T. 2016. Assessing the role of large herbivores in the structuring and functioning of freshwater and marine angiosperm ecosystems. Ecography 39, 162–179. ( 10.1111/ecog.01651) [DOI] [Google Scholar]
- 14.Bakker ES, Gill JL, Johnson CN, Vera FWM, Sandom CJ, Asner GP, Svenning JC. 2016. Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl Acad. Sci. USA 113, 847–855. ( 10.1073/pnas.1502545112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnosky AD, Lindsey EL, Villavicencio NA, Bostelmann E, Hadly EA, Wanket J, Marshall CR. 2016. Variable impact of late-Quaternary megafaunal extinction in causing ecological state shifts in North and South America. Proc. Natl Acad. Sci. USA 113, 856–861. ( 10.1073/pnas.1505295112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galetti M, et al. 2018. Ecological and evolutionary legacy of megafauna extinctions. Biol. Rev. 93, 845–862. ( 10.1111/brv.12374) [DOI] [PubMed] [Google Scholar]
- 17.Corlett RT. 2016. Restoration, reintroduction, and rewilding in a changing World. Trends Ecol. Evol. 31, 453–462. ( 10.1016/j.tree.2016.02.017) [DOI] [PubMed] [Google Scholar]
- 18.Svenning JC, et al. 2016. Science for a wilder Anthropocene: synthesis and future directions for trophic rewilding research. Proc. Natl Acad. Sci. USA 113, 898–906. ( 10.1073/pnas.1502556112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stokstad E. 2015. Bringing back the aurochs. Science 350, 1144–1147. ( 10.1126/science.350.6265.1144) [DOI] [PubMed] [Google Scholar]
- 20.Pettorelli N, Barlow J, Stephens PA, Durant SM, Connor B, Schulte to Bühne H, Sandom CJ, Wentworth J, du Toit JT. 2018. Making rewilding fit for policy. J. Appl. Ecol. 55, 1114–1125. ( 10.1111/1365-2664.13082) [DOI] [Google Scholar]
- 21.Navarro LM, Pereira HM. 2012. Rewilding abandoned landscapes in Europe. Ecosystems 15, 900–912. ( 10.1007/s10021-012-9558-7) [DOI] [Google Scholar]
- 22.Cerqueira Y, Navarro LM, Maes J, Marta-Pedroso C, Honrado JP, Pereira HM. 2015. Ecosystem services: the opportunities of rewilding in Europe. In Rewilding European landscapes (eds Pereira HM, Navarro LM). New York, NY: Springer Open. [Google Scholar]
- 23.Donlan CJ, et al. 2006. Pleistocene rewilding: an optimistic agenda for twenty-first century conservation. Am. Nat. 168, 660–681. ( 10.2307/3873461) [DOI] [PubMed] [Google Scholar]
- 24.Monbiot G. 2013. Feral: searching for enchantment on the frontiers of rewilding. London, UK: Penguin Random House. [Google Scholar]
- 25.Jepson P. In press. Recoverable Earth: a twenty-first century environmental narrative. Ambio. ( ) [DOI] [PMC free article] [PubMed]
- 26.Nogues-Bravo D, Simberloff D, Rahbek C, Sanders NJ. 2016. Rewilding is the new Pandora's box in conservation. Curr. Biol. 26, R87–R91. ( 10.1016/j.cub.2015.12.044) [DOI] [PubMed] [Google Scholar]
- 27.Rubenstein DR, Rubenstein DI. 2016. From Pleistocene to trophic rewilding: a wolf in sheep's clothing. Proc. Natl Acad. Sci. USA 113, E1 ( 10.1073/pnas.1521757113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svenning JC, et al. 2016. Reply to Rubenstein and Rubenstein: time to move on from ideological debates on rewilding. Proc. Natl Acad. Sci. USA 113, E2–E3. ( 10.1073/pnas.1521891113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter DO, Britz T, Jones M, Letnic M. 2015. Reintroduction of Tasmanian devils to mainland Australia can restore top-down control in ecosystems where dingoes have been extirpated. Biol. Conserv. 191, 428–435. ( 10.1016/j.biocon.2015.07.030) [DOI] [Google Scholar]
- 30.Heinen JH, van Loon EE, Hansen DM, Kissling WD. 2018. Extinction-driven changes in frugivore communities on oceanic islands. Ecography 41, 1245–1255. ( 10.1111/ecog.03462) [DOI] [Google Scholar]
- 31.Galetti M, Root-Bernstein M, Svenning JC. 2017. Challenges and opportunities for rewilding South American landscapes. Perspect. Ecol. Conserv. 15, 245–247. ( 10.1016/j.pecon.2017.10.002) [DOI] [Google Scholar]
- 32.Tanentzap AJ, Smith BR. 2018. Unintentional rewilding: lessons for trophic rewilding from other forms of species introductions. Phil. Trans. R. Soc. B 373, 20170445 ( 10.1098/rstb.2017.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cromsigt J, te Beest M. 2014. Restoration of a megaherbivore: landscape-level impacts of white rhinoceros in Kruger National Park, South Africa. J. Ecol. 102, 566–575. ( 10.1111/1365-2745.12218) [DOI] [Google Scholar]
- 34.van Klink R, WallisDeVries MF. 2018. Risks and opportunities of trophic rewilding for arthropod communities. Phil. Trans. R. Soc. B 373, 20170441 ( 10.1098/rstb.2017.0441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Klink R, Van der Plas F, Van Noordwijk CGET, WallisDeVries MF, Olff H. 2015. Effects of large herbivores on grassland arthropod diversity. Biol. Rev. 90, 347–366. ( 10.1111/brv.12113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Law A, Gaywood MJ, Jones KC, Ramsay P, Willby NJ. 2017. Using ecosystem engineers as tools in habitat restoration and rewilding: beaver and wetlands. Sci. Total Environ. 605, 1021–1030. ( 10.1016/j.scitotenv.2017.06.173) [DOI] [PubMed] [Google Scholar]
- 37.Willby NJ, Law A, Levanoni O, Foster G, Ecke F. 2018. Rewilding wetlands: beaver as agents of within-habitat heterogeneity and the responses of contrasting biota. Phil. Trans. R. Soc. B 373, 20170444 ( 10.1098/rstb.2017.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Law A, McLean F, Willby NJ. 2016. Habitat engineering by beaver benefits aquatic biodiversity and ecosystem processes in agricultural streams. Freshwater Biol. 61, 486–499. ( 10.1111/fwb.12721) [DOI] [Google Scholar]
- 39.Derham TT, Duncan RP, Johnson CN, Jones ME. 2018. Hope and caution: rewilding to mitigate the impacts of biological invasions. Phil. Trans. R. Soc. B 373, 20180127 ( 10.1098/rstb.2018.0127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cromsigt JPGM, te Beest M, Kerley GIH, Landman M, le Roux E, Smith FA. 2018. Trophic rewilding as a climate change mitigation strategy? Phil. Trans. R. Soc. B 373, 20170440 ( 10.1098/rstb.2017.0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olofsson J, Post E. 2018. Effects of large herbivores on tundra vegetation in a changing climate, and implications for rewilding. Phil. Trans. R. Soc. B 373, 20170437 ( 10.1098/rstb.2017.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaarlejärvi E, Olofsson J. 2014. Concurrent biotic interactions influence plant performance at their altitudinal distribution margins. Oikos 123, 943–952. ( 10.1111/oik.01261) [DOI] [Google Scholar]
- 43.Te Beest T, Sitters J, Menard CB, Olofsson J. 2018. Reindeer grazing increases summer albedo by reducing shrub abundance in Arctic tundra. Environ. Res. Lett. 11, 125013 ( 10.1088/1748-9326/aa5128) [DOI] [Google Scholar]
- 44.Zimov NS, Zimov SA, Zimova AE, Zimova GM, Chuprynin VI, Chapin FS. 2009. Carbon storage in permafrost and soils of the mammoth tundra-steppe biome: role in the global carbon budget. Geophys. Res. Lett. 36, L02502 ( 10.1029/2008GL036332) [DOI] [Google Scholar]
- 45.Andriuzzi WS, Wall DH. 2018. Soil biological responses to, and feedbacks on, trophic rewilding. Phil. Trans. R. Soc. B 373, 20170448 ( 10.1098/rstb.2017.0448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bello C, Galetti M, Pizo MA, Magnago LFS, Rocha MF, Lima RA, Peres CA, Ovaskainen O, Jordano P. 2015. Defaunation affects carbon storage in tropical forests. Sci. Adv. 1, e1501105 ( 10.1126/sciadv.1501105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hempson GP, Archibald S, Bond WJ. 2017. The consequences of replacing wildlife with livestock in Africa. Sci. Rep. 7, 17196 ( 10.1038/s41598-017-17348-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson CN, Prior LD, Archibald S, Poulos HM, Barton AM, Williamson GJ, Bowman DMJS. 2018. Can trophic rewilding reduce the impact of fire in a more flammable world? Phil. Trans. R. Soc. B 373, 20170443 ( 10.1098/rstb.2017.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faurby S, Svenning J-C. 2015. Historic and prehistoric human-driven extinctions have reshaped global mammal diversity patterns. Diversity Distrib. 21, 1155–1166. ( 10.1111/ddi.12369) [DOI] [Google Scholar]
- 50.Faurby S, Araújo MB. 2018. Anthropogenic range contractions bias species climate change forecasts. Nat. Clim. Change 8, 252–256. ( 10.1038/s41558-018-0089-x) [DOI] [Google Scholar]
- 51.Jarvie S, Svenning J-C. 2018. Using species distribution modelling to determine opportunities for trophic rewilding under future scenarios of climate change. Phil. Trans. R. Soc. B 373, 20170446 ( 10.1098/rstb.2017.0446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen DM, Donlan CJ, Griffiths CJ, Campbell KJ. 2010. Ecological history and latent conservation potential: large and giant tortoises as a model for taxon substitutions. Ecography 33, 272–284. ( 10.1111/j.1600-0587.2010.06305.x) [DOI] [Google Scholar]
- 53.Falcón W, Hansen DM. 2018. Island rewilding with giant tortoises in an era of climate change. Phil. Trans. R. Soc. B 373, 20170442 ( 10.1098/rstb.2017.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bump JK. 2018. Fertilizing riparian forests: nutrient repletion across ecotones with trophic rewilding. Phil. Trans. R. Soc. B 373, 20170439 ( 10.1098/rstb.2017.0439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans P, Brown CD. 2017. The boreal–temperate forest ecotone response to climate change. Environ. Rev. 25, 423–431. ( 10.1139/er-2017-0009) [DOI] [Google Scholar]
- 56.Marjakangas E-L, et al. 2018. Estimating interaction credit for trophic rewilding in tropical forests. Phil. Trans. R. Soc. B 373, 20170435 ( 10.1098/rstb.2017.0435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuhlendorf SD, Davis CA, Elmore RD, Goodman LE, Hamilton RG. 2018. Perspectives on grassland conservation efforts: should we rewild to the past or conserve for the future? Phil. Trans. R. Soc. B 373, 20170438 ( 10.1098/rstb.2017.0438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allred BW, Fuhlendorf SD, Hamilton RG. 2011. The role of herbivores in Great Plains conservation: comparative ecology of bison and cattle behavior. Ecosphere 2, 1–17. ( 10.1890/ES10-00152.1) [DOI] [Google Scholar]
- 59.Fuhlendorf SD, Engle DM, Elmore RD, Limb RF, Bidwell TG. 2012. Conservation of pattern and process: developing an alternative paradigm for rangeland management. Rangeland Ecol. Manage. 65, 579–589. ( 10.2111/REM-D-11-00109.1) [DOI] [Google Scholar]
- 60.Ripple WJ, Larsen EJ, Renkin RA, Smith DW. 2001. Trophic cascades among wolves, elk and aspen on Yellowstone National Park's northern range. Biol. Conserv. 102, 227–234. ( 10.1016/S0006-3207(01)00107-0) [DOI] [Google Scholar]
- 61.Beschta RL, Ripple WJ. 2015. Divergent patterns of riparian cottonwood recovery after the return of wolves in Yellowstone, USA. Ecohydrology 8, 58–66. ( 10.1002/eco.1487) [DOI] [Google Scholar]
- 62.Jepson P, Schepers F, Helmer W. 2018. Governing with nature: a European perspective on putting rewilding principles into practice. Phil. Trans. R. Soc. B 373, 20170434 ( 10.1098/rstb.2017.0434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crête M. 1999. The distribution of deer biomass in North America supports the hypothesis of exploitation ecosystems. Ecol. Lett. 2, 223–227. ( 10.1046/j.1461-0248.1999.00076.x) [DOI] [Google Scholar]
- 64.Zamboni T, Di Martino S, Jiménez-Pérez I. 2017. A review of a multispecies reintroduction to restore a large ecosystem: the Iberá Rewilding Program (Argentina). Perspect. Ecol. Conserv. 15, 248–256. ( 10.1016/j.pecon.2017.10.001) [DOI] [Google Scholar]
- 65.Higgs E, Falk DA, Guerrini A, Hall M, Harris J, Hobbs RJ, Jackson ST, Rhemtulla JM, Throop W. 2014. The changing role of history in restoration ecology. Front. Ecol. Environ. 12, 499–506. ( 10.1890/110267) [DOI] [Google Scholar]
- 66.Torres A, et al. 2018. Measuring rewilding progress. Phil. Trans. R. Soc. B 373, 20170433 ( 10.1098/rstb.2017.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.IPCC. 2014. Climate Change 2014: Synthesis report Contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change (eds Core Writing Team, Pachauri RK, Meyer LA). Geneva, Switzerland: IPCC. [Google Scholar]
- 68.Willner SN, Levermann A, Zhao F, Frieler K. 2018. Adaptation required to preserve future high-end river flood risk at present levels. Sci. Adv. 4, eaao1914 ( 10.1126/sciadv.aao1914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ridder B. 2007. The naturalness versus wildness debate: ambiguity, inconsistency, and unattainable objectivity. Restor. Ecol. 15, 8–12. ( 10.1111/j.1526-100X.2006.00184.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.