Abstract
Primary human keratinocytes isolated from fresh skin tissues and their expansion in vitro have been widely used for laboratory research and for clinical applications. The conventional isolation method of human keratinocytes involves a two-step sequential enzymatic digestion procedure, which has been proven to be inefficient in generating primary cells from adult tissues due to the low cell recovery rate and reduced cell viability. We recently reported an advanced method to isolate human primary epidermal progenitor cells from skin tissues that utilizes the Rho kinase inhibitor Y-27632 in the medium. Compared with the traditional protocol, this new method is simpler, easier, and less time-consuming, and increases epithelial stem cell yield and enhances their stem cell characteristics. Moreover, the new methodology does not require the separation of the epidermis from the dermis, and, therefore, is suitable for isolating cells from different types of adult tissues. This new isolation method overcomes the major shortcomings of conventional methods and is more suitable for producing large numbers of epidermal cells with high potency both for laboratory and for clinical applications. Here, we describe the new method in detail.
Keywords: Developmental Biology, Issue 138, Human keratinocytes, epidermal stem cells, dermal cells, primary skin cell isolation, keratinocyte culture, Y-27632, Rho-associated kinase inhibitor, skin regeneration
Introduction
The goal was to develop a simple and efficient protocol to isolate primary human keratinocytes (HKCs) from adult tissues, especially for clinical applications. Skin epidermal stem cells, localized in the basal layer of the skin, possess a high potential to proliferate and differentiate and provide keratinocytes to maintain the functions of the skin1,2,3,4. HKCs isolated from skin tissues are widely used for skin tissue engineering and regeneration purposes, especially in the repair of damaged skin and in gene therapy for clinical applications5,6. The key issue for HKC-based applications is to efficiently isolate and expand large numbers of HKCs with high potential in vitro7,8. Although various research groups have developed methods to produce cultures of stem-like HKCs, these methods are sometimes time-consuming and complicated to perform and have other limitations, such as low cell yields and being limited by the type of skin specimen used9. For instance, the traditional method to isolate HKCs from skin tissues involves a two-step enzymatic digestion with a separation of the epidermis from the dermis6. That method usually works well for neonatal tissues, but it becomes very difficult when used to isolate cells from adult tissues.
Y-27632, an inhibitor of Rho-associated protein kinase (ROCK), has been reported to significantly enhance the efficiency of epidermal stem cell isolation and colony growth10,11,12. In a previous study, we discovered that Y-27632 facilitates the clonal growth of epidermal cells but reduces the yield of dermal cells by differentially controlling the expression of adhesion molecules13. We also established a new conditioned inoculation medium, called G-medium, which supports the growth and yield of primary epidermal cells. By combining G-medium with Y-27632, this novel method can spontaneously separate epidermal and dermal cells after enzyme digestion, thus removing the step of epidermis-dermis separation13,14. Based on previous reports, we now describe the detailed procedure of this new method to isolate HKCs from adult skin tissue.
Protocol
Human tissues used in this protocol have been handled according to the guidelines of the Institution's human research ethics committee (NO.2015120401, date: May 12, 2015).
1. Preparations
Collect fresh adult abdominal skin tissues discarded from plastic surgery at the hospital in a 50-mL tube with 10 mL of ice-cold Dulbecco's modified Eagle medium (DMEM). The specimen can be kept at 4 °C for up to 72 h without significantly affecting the cell viability.
- Prepare the reagents and culture medium as described below.
- Add 1 mL of penicillin (100 U/mL) and streptomycin (100 mg/L) to 50 mL of phosphate-buffered solution (PBS) to prepare the washing solution.
- Prepare two sets of enzyme digestion solutions: (1) prepare 50 mL of dispase (2.5 mg/mL in DMEM) and 50 mL of type I collagenase (2.5 mg/mL in DMEM) separately for the conventional method; and (2) prepare 50 mL of a mixture of dispase and type I collagenase (dissolve 125 mg of dispase powder and 125 mg of type I collagenase powder in 50 mL of DMEM) for the new method. NOTE: All enzyme solutions should be filtered with a 0.22-µm strainer and kept at4 °C.
- Prepare digestion solutions for both methods: 0.05% trypsin and 0.25% trypsin were commercially purchased. Prepare a 10 mg/mL DNase I solution by dissolving 50 mg of DNase I powder in 5 mL of PBS, filter it with a 0.22-µm strainer, and store it at -20 °C.
- Prepare 500 mL of medium to neutralize the enzymatic digestion: DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
- Prepare 500 mL of inoculation medium (growth-factor-containing medium, called G-medium): DMEM/F12 (3:1) medium containing 1% penicillin/streptomycin, 40 µg/mL fungizone, 40 ng/mL fibroblast growth factor 2 (FGF2), 20 ng/mL epidermal growth factor (EGF), and 2% B27 supplement.
- Pretreat 100-mm cell culture dishes with 2 mL of coating matrix containing type I collagen for 30 min at room temperature.
2. Conventional Method
- Skin tissue pretreatment
- Take 1.5 cm2 of skin tissue and wash it 1x with 10 mL of PBS; then, rinse it with 10 mL of 70% ethanol for 30 s and incubate it 2x with 10 mL of washing solution (PBS containing 2% penicillin and streptomycin), 5 min each. NOTE: All washes are performed in 100-mm cell culture dishes inside a laminar flow hood.
- Trim the tissue with sterile scissors to remove the subcutaneous fat layer in a 100-mm cell culture dish and, then, weigh the skin tissue (the weight is 1 g in this protocol). NOTE: Sufficient trimming is very crucial for the efficient separation of the epidermis from the dermis. The yellowish fat layer can be easily distinguished visually.
- Transfer the tissue to another 100-mm sterile dish with the dermis side down and, then, cut the skin tissue into 3- to 4-mm-wide strips using a scalpel blade. NOTE: The dermis side with the fat layer is easily recognized visually.
- Add 10 mL of 2.5 mg/mL dispase solution to the skin tissues in a 100-mm culture dish and, then, incubate the dish overnight at 4 °C (no more than 20 h). NOTE: The amount of dispase solution can be calculated by using 10 mL of dispase solution for the digestion of each gram of tissue.
- Separation of the epidermis from the skin tissue
- On the second day, peel off the epidermis from the dermis using fine tweezers. NOTE: If it is difficult to peel off the epidermis, the dispase digestion was not sufficient, which is usually due to insufficient trimming of the tissue. In this case, the tissue needs a longer incubation time.
- Mince the peeled epidermis with 500 µL of cell culture medium into a tissue slurry using scalpel blades; then, resuspend it with 10 mL of 0.25% trypsin in a 50-mL tube and incubate it 20 min in a water bath at 37 °C, with shaking.
- Collection and culturing of primary cells
- Neutralize the trypsin activity by adding an equal volume of neutralization solution (10 mL in this protocol).
- Dissociate the epidermal cells by pipetting the solution up and down 20x with a 10-mL serological pipette and pass the cell solution through a 100-µm cell strainer to remove any residual tissue debris.
- Centrifuge the cell solution at 200 x g for 5 min after filtration; resuspend the cell pellet in neutralization medium for washing and, then, centrifuge it again at 200 x g to obtain the cell pellet.
- Resuspend the cell pellet in 10 mL of low-calcium, serum-free keratinocyte medium (SFM). Take 10 µL of this solution to count the total cell number and, then, plate about 2 x 106 cells with 10 mL of SFM into 100-mm cell culture dishes pretreated with coating matrix (containing type I collagen). NOTE: Coating is a necessary step for the conventional method.
- Change the culture medium every 2 d. NOTE: Check the cell adhesion under a microscope before and after changing the culture medium.
- Passage the cells when they reach about 80% confluence. NOTE: All culture dishes are pretreated with the coating matrix.
3. The New Method
- Skin tissue pretreatment
- Collect the tissue as described above (step 2.1) for the conventional method.
- Wash the skin tissue 1x with 10 mL of PBS and, then, weigh it in a sterile plastic container by an electronic scale (the weight is around 1 g in this protocol). NOTE: The minimum weight of tissue needed for this protocol is 0.1 g, since it is difficult to obtain a sufficient number of cells if the tissue specimen used is too small. There is no maximum weight of tissue for this protocol, which ultimately depends on the handling capacity of the operator.
- Use forceps to rinse the skin tissue with 10 mL of 70% ethanol for 30 s.
- Incubate the tissue 2x with 10 mL of washing solution (prepared in step 2.1.1), for 5 min each time. NOTE: All the washing steps noted above are performed in 100-mm cell culture dishes inside a laminar flow hood (a tissue culture hood).
- Transfer the tissue to another 100-mm cell culture sterile dish.
- Using scalpel blades, mince the tissue thoroughly into a tissue slurry.
- Add 200 µL of PBS every 5 min to keep the tissues wet. NOTE: Take around 15 min for steps 3.1.5 - 3.1.7. All the above steps are performed at room temperature.
- Digestion of skin tissue
- Transfer the homogenized tissue solution into a 50-mL tube. Add 10 mL of enzyme mixture for every 1 g of skin tissue. Mix the enzymes thoroughly with the homogenized tissues.
- Incubate the mixture with shaking in a water bath at 37 °C for 1 h.
- Add a 1/5 volume of 0.25% trypsin (2.5 mL in this protocol) to the digestion mixture for another 30 min in a water bath at 37 °C.
- Add DNase I solution to the enzyme mixture at a 1:100 (v/v) ratio (250 µL in this protocol) and incubate it for 5 min at room temperature.
- Collection and culturing of primary cells
- Stop the digestion process by adding 12.5 mL of neutralization medium in a 1:1 ratio. Pipette the solution up and down for about 20x, using a 10-mL serological pipette to dissociate the cells.
- Filter the dissociated cells through a 100-µm cell strainer to remove the tissue debris. Centrifuge the supernatant at 200 x g for 5 min. Observe the pellet at the bottom of the tube.
- Discard the supernatant and wash the cell pellet with 10 mL of neutralization medium, and centrifuge again at 200 x g for 5 min to collect the cells.
- Resuspend the cell pellet in 10 mL of inoculation medium (G-medium from step 1.2.5) with 10 µM of Y-27632.
- Take 10 µL of the solution to count the total cell number and, then, plate about 2 x 106 cells with 10 mL of G-medium into a 100-mm cell culture dish. NOTE: The precoating step is not necessary for the new method, and no removal of the dermal layer is required either.
- After 3 d, replace the inoculation medium with low-calcium SFM medium. Change the culture medium every 2 d. NOTE: Check the cell adhesion before and after changing the medium.
- Passage the cells when they reach about 80% confluence. NOTE: If necessary, pretreat the cells with 0.05% trypsin for 2 min and wash the cells with PBS to remove contaminating dermal cells before trypsinizing the epidermal cells.
4. Cell Passaging
Remove the medium, wash the cells with PBS, and, then, add 2 mL of 0.05% trypsin (per each 100-mm dish).
Incubate the cells in an incubator (37 °C with 5% CO2) for 5 min.
Check the cells using a microscope to make sure that all cells have detached from the culture dish.
Add 8 mL of DMEM with 10% FBS to neutralize the enzymatic reaction and, then, collect the cells in a 15-mL tube.
Centrifuge at 200 x g for 5 min to obtain the cell pellet.
Remove the supernatant slowly and, then, resuspend the cell pellet with 10 mL of SFM medium and count the number of cells.
Plate 1 x 106 cells with 10 mL of SFM into each 100-mm cell dish.
Change the medium every 2 d.
Representative Results
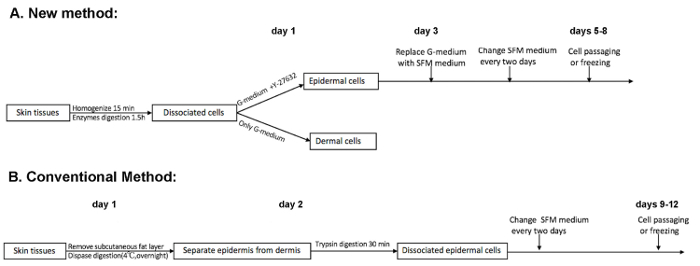
Schematic diagrams of the new method (Figure 1A) and the conventional method (Figure 1B) are presented in Figure 1. The conventional method is a two-step digestion, which requires a 2-day procedure. By contrast, the new method is a one-step digestion, which takes around 3 hours to perform. Importantly, the one-step new method can obtain two populations (epidermal and dermal cells) at the same time, which depends on the presence of Y-27632 in the inoculation medium. Moreover, the initial culture time of epidermal cells to reach around 80% confluence usually takes at least 3 days less than the conventional method if the same number of cells is inoculated after isolation.
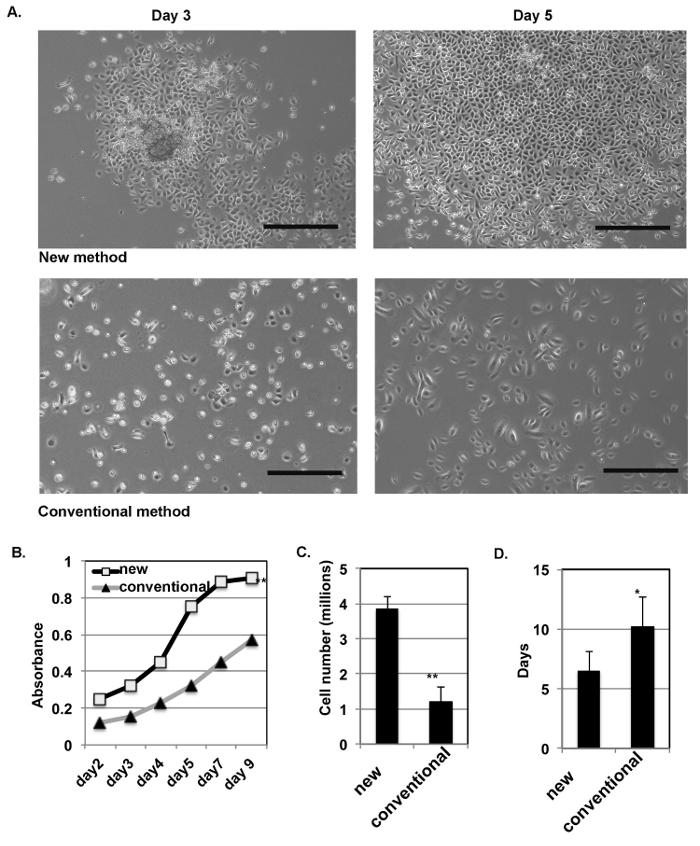
The new method yields more primary epidermal cells, which grow in a colony morphology. The isolated HKCs from human skin tissues were inoculated with G-medium containing Y-27632. The same number of cells were plated following the conventional method as the control. Representative images of the epidermal cells at days 3 and 5 after the initial inoculation are shown in Figure 2A. The cells obtained from the new method tended to grow as a colony morphology. The growth curve after inoculation was assessed using a cell counting kit-8 (CCK-8) at different time points after inoculation (Figure 2B), and the doubling time is around 3 - 4 days for cells prepared by the new method but is around 6 - 8 days for the conventional method. At day 7, the yields of the cells were calculated. The results are shown in Figure 2C, which shows that the new method yielded three times more cells than the conventional method and that the time needed to reach confluence after the initial inoculation was at least 3 days less (Figure 2D). At day 7, the epidermal cells reached confluence with the new method, but not with the conventional method (Figure 2D).
The new method enhanced the expression of α6-integrin by the primary cells. α6-integrin has been shown to be highly expressed by epidermal stem cells15. Cells isolated by the new method maintained the characteristics of skin basal cells after several passages. Colony growth is one of the characteristics of epidermal stem cells derived from the basal layer of the skin, and a high level of α6-integrin expression is another feature of these epidermal stem cells. We found that the addition of Y-27632 significantly increased the expression of α6-integrin in epidermal cells at passage 3 (Figure 3A-B), and at passage 3, epidermal cells isolated using the new method show no contamination by dermal cells13. It is very important to maintain the basal cell features after several passages during in vitro expansion. Immunofluorescence (IF) analysis of the basal cell marker keratin 5 and the terminal differentiation marker loricrin16 was performed with those cells after three passages. We found that 90% of all cells were K5-positive but less than 10% of them expressed loricrin (Figure 3C - 3D). These results indicate that primary HKCs isolated using the new method contain an epidermal stem cell population and can maintain the characteristic features of basal cells. But the longer that HKCs are passaged, the more they begin to differentiate. By the new method, we found that epidermal cells cultured in vitro could maintain their proliferation ability until passage 8. Finally, we tested the purity of epidermal cells isolated using the new method, since a mixture of epidermal and dermal cells was inoculated right after isolation. With a short-time (2 - 3 min) treatment with trypsin to remove a few dermal cells contaminating the initial culture, a relatively pure population of epidermal cells was obtained after one passage (Figure 4).
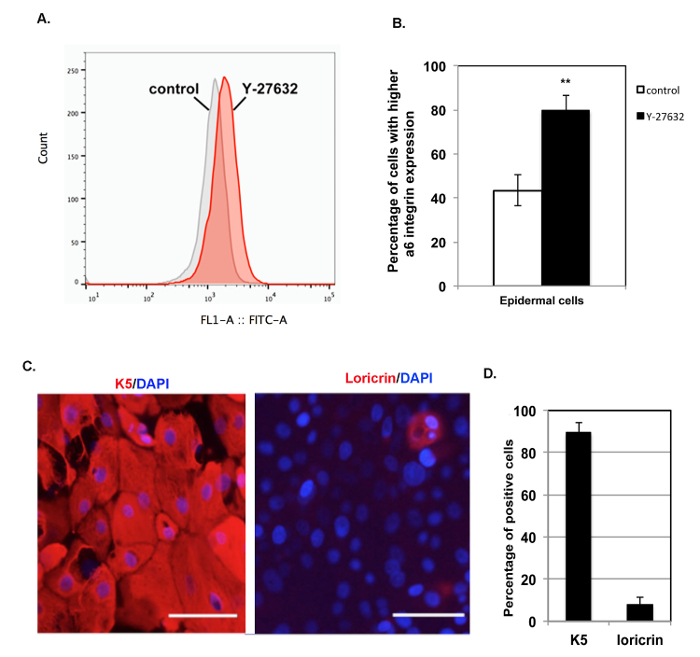
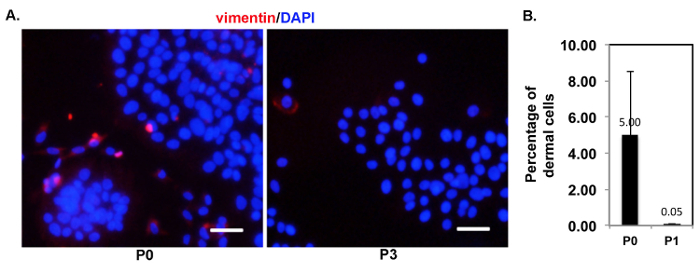
Figure 1: Comparison of the new and conventional isolation procedures. (A) This panel shows a schematic representation of the new isolation method. At day 1, the dissociated skin cells are inoculated in G-medium with or without Y-27632. In the presence of Y-27632 for 2 days, the epidermal cells selectively expand. At around day 6, the cells reach a density sufficient enough for passaging. (B) This panel shows a schematic representation of the conventional isolation method. The epidermis is separated from the dermis and the epidermal cells are isolated on day 2, and usually, the cells reach a density sufficient enough for passaging at around 10 days. Please click here to view a larger version of this figure.
Figure 2: The new isolation method increases the yield of primary epidermal cells. (A) This panel shows representative images of epidermal cells prepared by the new method (top row) and by the conventional method (bottom row) at days 3 and 5 after the initial inoculation. The scale bars = 200 µm. (B) Primary HKCs prepared by the new and the conventional methods were collected at the indicated time points for the analysis of viable cells using a cell counting kit-8 (CCK-8). (C) The total number of cells prepared by the new method and by the conventional method were counted at day 7 after inoculation. (D) The average time to reach confluence of primary epidermal cells (passage 0) is shown for both the new isolation method and the conventional method. For panels B - D: Student's t-test was used; the error bars show the standard variation; n = 4; ** p < 0.01, * p < 0.05 when comparing the new method with the conventional method. Please click here to view a larger version of this figure.
Figure 3: Primary epidermal cells obtained by the new method are able to maintain basal cell features during in vitro expansion. (A) This panel shows an FACS analysis of α6-integrin expression of epidermal cells at passage 3 treated with Y-27632 (Y-27632, red) or without Y-27632 (control, grey) for 48 h. (B) This panel shows a quantification analysis of cells with high expression levels of α6-integrin from panel A; high α6-integrin expression is defined as a signal > 103, ** p < 0.01. (C) This panel shows representative images of immunofluorescence staining of K5 (red) and loricrin (red); DAPI (blue) was used to stain nuclei. The scale bars = 100 µm. (D) This panel shows a quantification analysis of K5- and loricrin-positive cells. A total of 400 cells were counted to quantify each group, and the average numbers of K5- and loricrin-positive cells are shown. The experiment was independently repeated 4 times. Please click here to view a larger version of this figure.
Figure 4: After the initial passage, relatively pure epidermal cells were obtained using the new method. (A) This panel shows representative images of immunofluorescence staining of vimentin (red) for epidermal cells at passages 0 (P0) and 3 (P3); DAPI (blue) was used to stain nuclei. The scale bars = 50 µm. (B) This panel shows a quantification analysis of vimentin-positive cells at passages 0 (P0) and 1 (P1). A total of 400 cells were counted to quantify each group, and the average number of vimentin-positive cells is shown. The experiment was independently repeated 4 times. Please click here to view a larger version of this figure.
Discussion
Cultured primary HKCs have been widely utilized to treat wounds in clinics for more than three decades and, since that time, it has been always important to efficiently obtain sufficient numbers of cells for clinical applications in a timely manner. Therefore, in practice, the conventional isolation method, which requires the separation of the epidermis from the dermis, makes it difficult to meet these demands, due to the low yield of cells and the low ability to passage adult cells. Here, we describe a new simple method we developed to efficiently obtain HKCs from adult tissue based on previous reports13,14, which is more suitable both for laboratory and for clinical applications.
Attention must be paid to the following critical steps to achieve the best results for the new method. First, the homogenization procedure is a crucial step; insufficient homogenization will clearly affect the efficiency of enzymatic digestion and result in a low yield of dissociated cells. Second, the trypsin digestion step should not take more than 30 min; otherwise, the viability of the dissociated cells will decrease. Third, to efficiently collect the dissociated cells, the pore size of the cell strainer used for filtration should be 100 µm, and a single strainer should be used to filter no more than 20 mL of the cell solution. Fourth, the density of the initial plating is an important factor to minimize contamination with dermal cells, because a high cell density will significantly reduce the inhibitory effect of Y-27632 on dermal cells. Therefore, it is crucial to accurately count the total number of dissociated cells at the end of the isolation procedure before plating.
First, the new method simplifies the isolation procedure: the two-step conventional isolation procedure usually takes 2 days to perform, and the trimming procedure is crucial to completely remove the fat tissue from the skin, especially for adult tissues (Figure 1); otherwise, there will be an inefficient separation of the epidermis from the dermis after overnight digestion with dispase. By taking advantage of Y-27632, which inhibits the growth of dermal cells after inoculation13, the new method does not need to separate the epidermis from the dermis and, therefore, the trimming procedure to remove the fat tissue is not necessary for the new method, which only needs half a day to be performed. Second, the new method also simplifies the culture procedure since, with the traditional method, the dissociated cells are usually cultured on a feeder layer of irradiated mouse fibroblasts in medium containing 10% FBS or in a collagen-precoated dish. Animal-derived components are always risk factors that should be avoided for clinical applications. We developed a serum-free conditioned inoculation medium (G-medium), which does not require a precoating step for culture dishes and also enhances the yield of primary keratinocytes13 because G-medium combined with Y-27632 promotes the attachment of epidermal cells to the culture dish13,17,18. Therefore, the new method facilitates the culture procedure and is also a relatively safe procedure for clinical applications without the addition of animal-derived components. Third, the new method improves the yield of primary epidermal cells and shortens the initial culture time needed to reach confluence. Compared with the traditional method, the yield of cells produced by the new method is around 3 times higher, and the initial passage of epidermal cells reached confluence at least 3 days earlier (Figure 2). Finally, the new method can be utilized for the isolation of epidermal cells from all types of skin specimens, such as hairy scalp skin and adult skin tissues with a thick fat layer, which have been tested in a previous report13.
High-potential primary HKCs have been widely used for laboratory research and for clinical applications. This new method enhances the potential of cultured cells with characteristics of epidermal stem cells13. Y-27632 enhances the α6-integrin expression by HKCs, and after three passages, more than 90% of the cultured epidermal cells expressed the basal cell marker keratin 5, and fewer than 10% of the cells expressed the differentiation marker Loricrin, indicating that these cells keep the characteristics of skin basal cells after several passages. The culture-expanded epidermal cells can reconstitute skin in vivo after grafting13, suggesting that they possess regeneration potential after expansion in culture. Notably, the new method is ideal to isolate cells from adult tissues, as mentioned above. Therefore, epidermal cells prepared by this method can be used for in vitro and in vivo models to study skin diseases and can be developed into autologous cell-based products for clinical applications such as the treatment of skin wounds.
In summary, the new protocol provides a simplified and effective procedure to isolate and expand primary epidermal cells from adult skin tissue. This advanced method is more suitable for producing large numbers of high-potential epidermal cells both for laboratory and for clinical applications.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFA0104604), the General Program of National Natural Science Foundation of China (NSFC, 81772093), the Science and Technology Development Program of Suzhou (ZXL2015128), the Natural Science Foundation of Jiangsu Province (BK20161241), and a Shandong Taishan Scholar Award (tshw201502065).
References
- Ojeh N, Pastar I, Tomic-Canic M, Stojadinovic O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. International Journal of Molecular Sciences. 2015;16(10):25476–25501. doi: 10.3390/ijms161025476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulou PA, Blanpain C. Development and homeostasis of the skin epidermis. Cold Spring Harbor Perspectives in BIology. 2012;4(7):a008383. doi: 10.1101/cshperspect.a008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamstrup M, Faurschou A, Gniadecki R, Wulf HC. Epidermal stem cells - role in normal, wounded and pathological psoriatic and cancer skin. Current Stem Cell Research & Therapy. 2008;3(2):146–150. doi: 10.2174/157488808784223087. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annual Review of Cell and Developmental Biology. 2006;22(22):339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, et al. Building a microphysiological skin model from induced pluripotent stem cells. Stem Cell Research & Therapy. 2013;4(S1):S2. doi: 10.1186/scrt363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aasen T, Izpisua Belmonte JC. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nature Protocols. 2010;5(2):371–382. doi: 10.1038/nprot.2009.241. [DOI] [PubMed] [Google Scholar]
- Bayati V, Abbaspour MR, Neisi N, Hashemitabar M. Skin-derived precursors possess the ability of differentiation into the epidermal progeny and accelerate burn wound healing. Cell Biology International. 2017;41(2):187–196. doi: 10.1002/cbin.10717. [DOI] [PubMed] [Google Scholar]
- Hirsch T, et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551(7680):327–332. doi: 10.1038/nature24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer B, Kobayasi T. Separation of human epidermal cells from fibroblasts in primary skin culture. Archiv für dermatologische Forschung. 1975;252(1):39–46. doi: 10.1007/BF00582429. [DOI] [PubMed] [Google Scholar]
- Terunuma A, Limgala RP, Park CJ, Choudhary I, Vogel JC. Efficient procurement of epithelial stem cells from human tissue specimens using a Rho-associated protein kinase inhibitor Y-27632. Tissue Engineering Part A. 2010;16(4):1363–1368. doi: 10.1089/ten.tea.2009.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, et al. ROCK inhibitor Y-27632 increases the cloning efficiency of limbal stem/progenitor cells by improving their adherence and ROS-scavenging capacity. Tissue Engineering Part C: Methods. 2013;19(7):531–537. doi: 10.1089/ten.tec.2012.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa H. Application of Rho-associated protein kinase (ROCK) inhibitor to human pluripotent stem cells. Journal of Bioscience and Bioengineering. 2012;114(6):577–581. doi: 10.1016/j.jbiosc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Wen J, Zu T, Zhou Q, Leng X, Wu X. Y-27632 simplifies the isolation procedure of human primary epidermal cells by selectively blocking focal adhesion of dermal cells. Journal of Tissue Engineering and Regenerative Medicine. 2018;12(2):e1251–e1255. doi: 10.1002/term.2526. [DOI] [PubMed] [Google Scholar]
- Zou D, Pan J, Zhang P, Wu X. A new method to isolate human epidermal keratinocytes. Journal of Clinical Dermatology (in Chinese) 2016;6:424–429. [Google Scholar]
- Cerqueira MT, Frias AM, Reis RL, Marques AP. Interfollicular epidermal stem cells: boosting and rescuing from adult skin) Methods in Molecular Biology. 2013;989:1–9. doi: 10.1007/978-1-62703-330-5_1. [DOI] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nature Reviews Molecular Cell Biology. 2005;6(4):328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Breyer J, et al. Inhibition of Rho kinases increases directional motility of microvascular endothelial cells. Biochemical Pharmacology. 2012;83(5):616–626. doi: 10.1016/j.bcp.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochimica et Biophysica Acta. 2004;1692(2-3):103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]


