Abstract
Although impulsivity is suggested as a possible link to explain the association of Attention-Deficit/Hyperactivity Disorder (ADHD) with an Eating Disorder (ED), there is little research on how clinical and cognitive/neuropsychological functioning might change when this comorbidity occurs. ADHD individuals are at a higher of developing ED and also obesity. Some research has described the impact of ADHD in clinical treatment-seeking samples of ED patients. Consequently, we investigated how ED impacted on clinical and cognitive variables of a community sample of treatment-naive ADHD individuals. Ninety college students arranged in three groups (ADHD+ED, ADHD only and Controls) were analyzed using semi-structured interviews for ADHD (K-SADS), the Iowa Gambling Task, the Conner's Continuous Performance Test, Digit and Visual span, as well as rating scales for anxiety (STAI), depression (BDI) and impulsivity (BIS-11), and binge eating (BES). We found that ADHD+ED individuals significantly differed from both groups, presenting with a higher body mass index; more hyperactivity-impulsivity symptoms; higher binge eating scores; more omission errors on the Continuous Performance Test; disadvantageous choices on the Iowa Gambling Task. Also, we demonstrated through a moderation/mediation analysis that a greater level of binge eating mediated the increases in body mass index on our sample. There were no significant paths to explain binge-eating severity through changes on any of the neuropsychological tests used. The presence of an ED in normal weight in a community sample of ADHD individuals is associated with higher body mass index and a worse cognitive functioning.
Keywords: ADHD, eating disorders, comorbidity, neuropsychology, bulimia, binge eating, obesity, decision making
Introduction
A recent meta-analysis found that the risk of diagnosing an Eating Disorder (ED) in patients with Attention-Deficit/Hyperactivity Disorder (ADHD) is 3.82 times greater when compared to the general population (1). The heightened risk remains significant after controlling for age and gender, and holds true for all eating disorders syndromes [Anorexia Nervosa (AN), Bulimia Nervosa (BN), and Binge eating Disorder (BED)]. Furthermore, even though EDs are 10 times more prevalent in females, the association is significant for both sexes (2). Interestingly, even before full ED syndromes have developed at adolescence, eating disorder symptoms (e.g., loss of control eating) have also been significantly associated with ADHD (3, 4).
The comorbidity of ADHD with ED is of interest as it defines a subgroup of patients who have greater disordered eating habits (5) and might respond differently to current standard treatments for EDs (6). These patients are also at risk of presenting with a more disrupted mental functioning, exemplified by higher rates of other psychiatric comorbidities, especially greater rates of substance abuse (5, 7). ADHD symptoms can also indicate a higher severity of eating disorder symptoms and personality psychopathology in ED patients (8). Although this has been demonstrated in clinical samples (5, 9), there is scarce evidence to generalize the same phenomenon for community samples. Eating disorders respond poorly to treatment after a 1 year follow up if ADHD symptoms are present at baseline, especially if they present with high inattention symptoms (10).
Of note, the recent approval of lisdexamphetamine for the treatment of Binge Eating Disorder in the United States (11) suggests that psychostimulant medication might have a direct effect on eating behavior regardless of ADHD status. However, this is still poorly understood and concern may arise that populations at-risk for developing an eating disorder might misuse psychostimulants seeking weight regulation or as a compensatory behavior to their disordered eating habits (12, 13).
Results from studies evaluating how comorbid mental disorders influence cognitive functioning of ADHD have had varying results for children (14, 15) and adults (16, 17). Also, most studies have focused on anxiety and mood disorders. The diverse cognitive domains investigated and the varying results for different comorbid disorders prevent us from defining a specific profile of how a comorbid disorder impairs cognitive functioning in ADHD.
Two previous studies explored neuropsychological differences of ADHD individuals comorbid with an eating disorder. Seitz et al. (18) compared a sample of adult women with BN and a past history of ADHD (n = 12) to those without a history of ADHD (n = 45). They found that women comorbid for BN with ADHD presented more pronounced inattention and impulsivity when compared to those with BN only. This study also found that inattention was significantly more associated with these deficits than hyperactivity/impulsivity. One other study investigated if neuropsychological measures could explain the association of ADHD symptoms with BED symptoms. Steadman et al. (19) assessed 44 individuals and reported that impulsivity measured through a Continuous Performance Test (CPT) didn't moderate the correlation of ADHD and Binge Eating symptoms. The scarce literature on the causal pathways to explain this comorbidity have pointed toward impulse regulation deficits but further studies are necessary to corroborate this hypothesis (20).
In the present work, we aimed to test whether the presence of an ED was associated with poorer attentional function and decision-making in individuals with ADHD. Also, as a secondary aim, we tried to replicate findings from studies that evaluated the clinical profile across these disorders, in a community and treatment-naive sample.
Materials and methods
Sample
The present study was an analysis of a larger protocol presented in detail elsewhere in an open access manuscript (21), hereon summarized. A sectional study was conducted using a convenience sample from the fifth year of the medical course from the Federal University of Rio de Janeiro (UFRJ), over four consecutive years (2010–2014 with 8 recruitment waves in total). All students were invited to participate protocol when they initiated the fifth year, during the first class of psychiatry. The protocol was approved by the Institute of Psychiatry—UFRJ Ethics Committee.
All participants provided informed consent to take part, and the Institute of Psychiatry of the Federal University of Rio de Janeiro (IPUB-UFRJ) Ethics Committee approved this study. The exclusion criteria for the present analysis were: Presence of any psychiatric diagnosis other than ADHD or ADHD+ED, epilepsy, and current use of any psychotropic medication.
Procedures
Screening
Participants were screened for participating in the research with:
Clinical assessment
All participants were evaluated through semi-structured interviews using DSM-5 criteria. These evaluations were completed by board certified psychiatrists with more than 10 years of clinical experience in adult ADHD and ED (BPN and PM). All diagnosis were discussed using history, self-report, and semi-structured interviews.
The K-SADS interview adapted for adults with ADHD (24) was used to diagnose ADHD. The participant received a diagnosis of ADHD if they met DSM-5 criteria for at least 5 current inattention or hyperactivity/impulsivity symptoms, associated with at least 5 past inattention or hyperactivity/impulsivity symptoms, with onset before the age of 12 and occurring in at least 2 life-time domains with significant impairment. All ADHD assessments were done blind to the participant's self-report status, and all students with more than 5 current symptoms of inattention or hyperactivity/impulsivity were discussed with the other rater for consideration of a diagnosis.
SCID-P module for ED.
MINI-Plus: for all other psychiatric diagnosis.
Participants completed the following questionnaires:
Beck Depression Inventory (BDI) (25, 26) developed for assessing the severity of depressive symptoms. The BDI contains 21 questions, which are rated on a Likert scale ranging from 0 to 3.
State-Trait Anxiety Inventory (STAI) (27) is composed of two 20-item scales that measure trait and situational anxiety.
Barrat-Impusivity Scale (BIS-11) (28): This scale measures impulsivity in life situations. The original BIS-11 uses three subscales but the Brazillian transcultural adaptation studies validated two subescales—which were used for the present analysis—in two domains: attentional/planning (BIS-ATPLAN), cognitive and motor inhibition (BIS-CINI) impulsivity.
All the mentioned rating scales have been translated and adapted to Brazilian Portuguese [ASRS-18 (29), BES (30), BDI (26), STAI (27) and BIS-11(31)].
Neuropsychological assessment
Neuropsychological evaluation comprised:
IQ, calculated with the four-subtest form of the Wechsler Abbreviated Intelligence Scale (WASI) (32), from which the blocks, vocabulary, matrix, and similarities tests were administered.
The Digit Span and the Visual Span: Both these tests are used to assess executive functioning. In the verbal Digit Span task the subject has to recall forward and reverse orders of digit sequences. It uses the phonological loop to measure working memory, attention, and inhibition. The Visual Span is a visuospatial version of the verbal span in which the subject has to recall forwards and reverse sequences of a visual task (tapping on cubes).
The Conner's Continuous Performance Task II (CPT-II) (33): Continuous performance tests are the most commonly used attention tasks in clinical practice (34) and give the opportunity to evaluate the ability of a subject to maintain consistent responses over time and speed of stimuli presentation (35). Variables of interest in the present study were: number of omission errors (OMI), commission errors (COM), hit reaction time (HRT), HRT block change (HRT BL CHANGE), reaction time by inter-stimulus interval (Hit RT ISI CHANGE), and attentiveness (d′). OMI errors occur when subjects fail to respond on trials containing target letters (all non-“X” letters), COM errors occur when they respond on trials with letters “X.” HRT is the mean response time for all non-X responses over all six time blocks and represents the subject's easier discrimination of the target. HRT BL CHANGE (a vigilance measure) is the slope of change in the reaction time over the six time blocks; a positive slope indicates a slowing RT, and a negative slope indicates a quicker RT as the test progresses. Hit RT ISI CHANGE (capacity to adjust to presentation speed) is calculated by computing the slope of change in RT over the three ISIs (1, 2, and 4 s). The ISIs are block-randomized so that all three ISI conditions occur every block but in a different order; by varying the inter-stimulus intervals (1, 2, and 4 s), it is possible to assess the ability to adjust to changing tempo and task demand. The index d′ reflects the subject's perceptual sensitivity to targets as a measure of how well the individual discriminates between targets (signals) and non-targets (noise). Higher d′-values indicate greater sensitivity and better discrimination between targets and non-targets.
The Iowa Gambling Task (IGT) (36): We used a computerized version of the IGT, in which subjects had to choose a card from four decks. They were told that in order to win the largest sum of money some decks were advantageous while others were disadvantageous. Two decks brought large immediate gains with large future losses (decks A and B), while two decks lead to small wins but also small future losses (Decks C and D). After 100 trials, a net score was calculated using the equation [(Decks C+D) – (Decks A+B)], which produces a measure of the total number of advantageous decks minus the total number of disadvantageous decks. This was also calculated for 5 blocks of 20 trials, which enables the assessment of learning over the test. This test measures decision making and non-planning impulsivity.
Sample
A total of 726 students were eligible for the study but only 662 (91.1%) were screened using the ASRS-18 and BES as some students were not present during research presentation and screening procedures. From the 662 screened students, board certified psychiatrists using the semi-structured interviews interviewed a total of 344 students for mental disorders. All students with positive ASRS-18 or BES were interviewed as well as a random equal proportion of negative screenings. The final sample analyzed in the present study final sample consisted of 90 students. There were no statistically significant differences between the age and gender profile of protocol completers and non-completers.
Statistical analysis
Statistical tests were performed using SPSS v.20. Subjects were classified into one of three diagnostic groups (Control, ADHD only, ADHD+ED). We have included subjects in the “Control” group that didn't have any psychiatric diagnosis assessed using the MINI-Plus and didn't fulfill criteria for either ADHD or ED. In the ADHD group we included subjects that fulfilled criteria for ADHD but didn't have ED. For the ADHD+ED group we included patients presenting the comorbidity with both diagnoses. The differences between groups were considered significant if p < 0.05. The demographic characteristics were tested across groups using a one-way analysis of variance (ANOVA). Afterwards, pairwise contrasts were obtained using independent-samples t-tests. Post-hoc with Fisher's LSD correction was used.
For the mediation analysis we used the paramed command from STATA (37). The significance of the indirect and total effects was tested via bias-corrected bootstrapping, which is robust to violations of the assumption of homoscedasticity.
Results
Sample characteristics
The sociodemographic characteristics for the final sample are detailed in Table 1. The three diagnostic groups were proportional and had non-significant differences regarding gender distribution (p = 0.215), socioeconomic status (p = 0.976), and global IQ (p = 0.46). None of the ADHD subjects from the ADHD-only or from the ADHD+ED groups were currently taking any medication.
Table 1.
Subjects sociodemographic and clinical characteristics.
| Total Sample (n = 90) | Controls (n = 39) | ADHD (n = 35) | ADHD+E (n = 16) | p-valuea | Effect-Size (Partial Eta2)a | |
|---|---|---|---|---|---|---|
| Age | 23.71 (±1.9) | 23.3 (1 ± 0.2) | 24 (±2.3) | 24 (±1.6) | 0.215 | 0.035 |
| Gender: % (n) | 0.976 | 0.001 | ||||
| Female | 81% (68) | 81.8% (27) | 80% (28) | 81.3% (13) | ||
| Weight, kgs | 62.6 (±12.2) | 60.2 (±9) | 60.5 (±9.7) | 73.2 (±17.5) | <0.0001b | 0.167 |
| B.M.I. | 22.37 (±3.4) | 21.6 (±2.7) | 21.8 (±2.8) | 25.7 (±4.3) | <0.0001c | 0.205 |
| Overweight | 13% (n = 11) | 10.4% (4) | 8.7% (3) | 37.8% (6) | ||
| Obese | 2.3% (n = 2) | 0% (0) | 2.9% (1) | 6.3% (1) | ||
| SES: % (n) | 0.184 | 0.038 | ||||
| ≥ 32,500 $/y (A1) | 6.7% (n = 6) | 7.7% (3) | 8.6% (3) | 0% (0) | ||
| ≥ 21,900 $/y (A2) | 11.1% (10) | 2.6% (1) | 20% (7) | 12.5% (2) | ||
| ≥ 6,800 $/y (B 1 e 2) | 30% (27) | 28.2% (11) | 28.6% (10) | 37.5% (6) | ||
| ≥ 2,500 $/y (C e D) | 52.2% (n = 47) | 61.5% (24) | 42.9% (15) | 50% (8) | ||
| Global IQ | 113 (9) | 112 (10) | 113 (8) | 116 (8.2) | 0.46 | 0.021 |
Univariate Analysis. Omnibus p-values and effect sizes. LSD post-hoc correction.
Control = ADHD, p = 0.889; Control < ADHD + ED and ADHD < ADHD + ED, p < 0.001.
Control = ADHD, p = 0.786; Control < ADHD + ED and ADHD < ADHD + ED, p < 0.001.
The ADHD+ED group consisted of five participants with bulimia Nervosa; three participants with BEDs; three participants with subclinical Bulimia Nervosa (didn't fulfill the frequency criteria for binge and purging episodes); and five participants with subclinical BED (didn't fulfill the frequency criteria for binge episodes).
The proportion of obese and overweight participants in the ADHD+ED group was significantly higher when compared to both the ADHD only and to the Control groups (p = 0.004). Also, the mean BMI in this group was significantly greater than the other two groups: 4.1 points higher than the control group and 3.9 points higher than ADHD-only group. The ADHD+ED group was 13 kg (28.6 lbs) heavier than the control group and 12.7 kg (28 lbs) heavier than ADHD-only group on average (Table 1).
The analysis of ADHD symptoms revealed that the ADHD+ED group had significantly greater current Hyperactivity/Impulsivity than the ADHD only group. All other comparisons were non-significant between the ADHD+ED and the ADHD only group, with a trend for greater past Inattention symptoms in the ADHD+ED group (Table 2).
Table 2.
ADHD symptoms and self-report psychopathological measures.
| Total Sample (n = 90) | Controls (n = 39) | ADHD (n = 35) | ADHD+ED (n = 16) | Omnibus p-valuea | Contrasts | Omnibus Effect-Size (Partial Eta2)a | |
|---|---|---|---|---|---|---|---|
| Current Inatt | N.A. | 1.5 (±1.9) | 5.9 (±1.5) | 6.8 (±1.3) | <0.001 | 1<2=3b | 0.657 |
| Current H/I | N.A. | 1.2 (±1.2) | 4 (±2.2) | 5.6 (±2.7) | <0.001 | 1<2<3c | 0.433 |
| Past Inatt | N.A. | 1.1 (±1.3) | 5 (±1.9) | 6.1 (±1.9) | <0.001 | 1<2=3d | 0.607 |
| Past H/I | N.A. | 1.1 (±1.4) | 4.2 (±2.4) | 4.4 (±2.8) | <0.001 | 1<2=3e | 0.349 |
| BIS - Total | 66.6 (±12) | 58.9 (±8.8) | 71.8 (±10.3) | 71.4 (±15.8) | <0.001 | 1<2=3f | 0.256 |
| BIS-ATTPL | 18.3 (±4.1) | 17.9 (±4.7) | 18.8 (±3.9) | 18 (±3.6) | 0.73 | 1 = 2 = 3 | 0.010 |
| BIS-CINI | 43.5 (±8.4) | 36 (±4.8) | 48 (±6.6) | 50 (±5.2) | <0.001 | 1<2=3g | 0.547 |
| STAI-T | 41.7 (±9.9) | 38.4 (±9.4) | 44.2 (±9.6) | 44.6 (±9.8) | 0.032 | 1<2h | 0.089 |
| STAI-S | 42.2 (±10.4) | 38 (±8.7) | 45.3 (±11.5) | 44.5 (±8.1) | 0.23 | 1<2i | 0.087 |
| BES | 8.7 (6.3) | 6,22 (5.3) | 8.82 (6) | 15 (5.3) | <0.001 | 1=2<3j | 0.234 |
| BDI | 7 (±6.9) | 4.5 (±4.5) | 8.8 (±7.2) | 8.5 (±9.1) | 0.03 | 1<2=3l | 0.092 |
Mean (SD). N.A, Not Applicable; Current Inatt, KSADS Current Inattention symptoms; Current H/I, KSADS.Current Hyperactivity/Impulsivity symptoms; Past Inatt, KSADS Past Inattention symptoms; Past H/I KSADS Past Hyperactivity/Impulsivity symptoms; BIS, Barrat Impulsivity Scale; BIS-ATTPLAN, Attention and Planning BIS subscale; BIS-CINI, Inhibitory Control BIS subscale; STAI-T and –S, State-Trait Anxiety Inventory – Trait and –State; BES, Binge Eating Scale; BDI, Beck depression inventory.
1 = Controls; 2 = ADHD; 3 = ADHD+ED.
Univariate Analysis. Omnibus p-values. LSD post hoc correction.
1 < 2, p < 0.001; 1 < 3, p < 0.001; 2 = 3, p = 0.11.
1 < 2, p < 0.001; 1 < 3, p < 0.001; 2 < 3, p = 0.007.
1 < 2, p < 0.001; 1 < 3, p < 0.001; 2 = 3, p = 0.53.
1 < 2, p < 0.001; 1 < 3, p < 0.001; 2 = 3, p = 0.75.
1 < 2, p < 0.001; 1 < 3, p = 0.002; 2 = 3, p = 0.92.
1 < 2, p < 0.001; 1 < 3, p < 0.001; 2 = 3, p = 0.34.
1 < 2, p = 0.01; 1 = 3, p = 0.052; 2 = 3, p = 0.89.
1 = 2, p = 0.059; 1 = 3, p = 0.065; 2 = 3, p = 0.69.
1 = 2, p = 0.07; 1 < 3, p < 0.001; 2 < 3, p = 0.002.
1 < 2, p = 0.025; 1 < 3, p = 0.031; 2 = 3, p = 0.757.
Self-report psychopathology scales
Regarding anxiety symptoms, there were no significant differences on state anxiety (p = 0.23) and there was a significant difference on trait anxiety between controls and ADHD only (p = 0.01) or between controls and ADHD+ED (p = 0.05). The ADHD only and ADHD+ED groups didn't present significant differences on state (p = 0.69) or trait (p = 0.89) anxiety. The same occurred with depressive symptoms. Although controls had significantly lower BDI score than ADHD only (p = 0.02) and than ADHD+ED (p = 0.03), the last two groups didn't differed significantly among themselves (p = 0.75). In the analysis of self-report impulsivity, only the BIS-Total (p < 0.001) and BIS-CINI (p < 0.001) scores presented significant differences with ADHD only and ADHD+ED groups having higher scores than the control group.
Neuropsychological assessment
Results are reported by means and standard deviations for each group and total, for each test, in the Supplementary Materials. Hereon are reported mean differences and group comparisons with post-hoc tests. The neuropsychological assessment of verbal and non-verbal IQ (Table 1), Digit and Visual Span (Table 3), and the IGT (Table 4) did not show significant differences across groups. However, there was a trend for the ADHD+ED group having more disadvantageous choices than the other two groups across block 2 and in the net score of the IGT (Figure 1). Of note, only the deck B measure from the IGT was found to be to be significantly different in the ADHD+ED vs. Controls (p = 0.05).
Table 3.
Interactions among groups for digit and visual span.
| Omnibus F (Eta2), p-valuea | ADHD vs. Control | ADHD+ED vs. Control | ADHD+ED vs. ADHD | ||
|---|---|---|---|---|---|
| Mean Difference (Cohen's d) | Mean Difference (Cohen's d) | Mean Difference (Cohen's d) | |||
| Digit span | Raw score | 0.93 (0.02), p = 0.39 | −1.38 (1.87) | 0.19 (−0.20) | 1.19 (−1.24) |
| Straight sequence | 0.83 (0.02), p = 0.44 | −0.72 (1.75) | −0.05 (0.09) | 0.67 (1.24) | |
| Higher straight sequence | 0.57 (0.01), p = 0.56 | −0.19 (.82) | 0.27 (−0.93) | 0.46 (−1.53) | |
| Reverse sequence | 0.63 (0.01), p = 0.53 | −0.65 (1.56) | −0.13 (0.26) | 0.51 (−0.93) | |
| Higher reverse sequence | 0.73 (0.02), p = 0.48 | −0.31 (1.33) | 0.19 (−0.58) | 0.50 (−1.58) | |
| Visual span | Raw score | 0.13 (0.004), p = 0.87 | −0.36 (0.74) | −1.42 (0.22) | 0.22 (−0.34) |
| Straight sequence | 0.004 (0.000), p = 0.99 | 0.93 (0.13) | 0.00 (0) | 0.03 (0.10) | |
| Higher straight sequence | 0.17 (0.005), p = 0.83 | 0.09 (−0.46) | −0.12(.47) | −0.21 (0.82) | |
| Reverse sequence | 0.30 (0.009), p = 0.73 | −0.32 (1.13) | −0.14 (.37) | 0.18 (0.49) | |
| Higher reverse sequence | 0.56 (0.01), p = 0.57 | −0.26 (1.52) | −0.09 (.41) | 0.16 (−0.76) |
Mean difference (Cohen's d).
Univariate Analysis. Omnibus p-values. LSD post-hoc correction.
Table 4.
Interactions among groups for Iowa gambling task.
| Omnibus F (Eta2), p-valuea | ADHD vs. Control | ADHD+ED vs. Control | ADHD+ED vs. ADHD | |
|---|---|---|---|---|
| Mean Difference (Cohen's d) | Mean Difference (Cohen's d) | Mean Difference (Cohen's d) | ||
| IGT deck A | 0.32 (0.009), p = 0.727 | 0.049 (−0.75) | 1.63 (−0.29) | 1.13 (−0.20) |
| IGT deck B | 2.50 (0.067), p = 0.089 | 2.8 (−0.37) | 5 (−0.71) * | 2.25 (−0.33) |
| IGT deck C | 1.65 (0.045), p = 0.19 | −2.46 (0.34) | −3.8 (0.34) | −1.33 (0.19) |
| IGT deck D | 0.48 (0.014), p = 0.61 | −0.61 (0.07) | −2.67 (0.35) | −2.05 (0.26) |
| IGT Block 1 | 0.90 (0.025), p = 0.41 | −0.53 (0.08) | 2.31 (−0.41) | 2.83 (−0.44) |
| IGT Block 2 | 2 (0.054), p = 0.14 | 0.14 (−0.02) | −3.48 (0.69) | −3.62 (0.66)a |
| IGT Block 3 | 0.98 (0.027), p = 0.37 | −1.6 (0.22) | −3.09 (0.41) | −1.49 (0.23) |
| IGT Block 4 | 2.12 (0.057), p = 0.12 | −3.15 (0.35) | −5.19 (0.70) | −2.03 (0.29) |
| IGT Block 5 | 1.07 (0.03), p = 0.34 | −0.76 (0.09) | −3.67 (0.53) | −2.91 (0.42) |
| Net score | 2.12 (0.057), p = 0.12 | −6.98 (0.30) | −13.77 (0.73) | −6.79 (0.37)b |
Mean difference (Cohen's d).
p < 0.05;
p = 0.068;
p = 0.053.
Univariate Analysis. Omnibus p-values. LSD post-hoc correction.
Decks A and B = disadvantageous; Decks C and D = advantegeous; Net score for all 100 trials = [(Decks C+D) – (Decks A+B)]; Block refers to Net score of only 20 trials.
Figure 1.
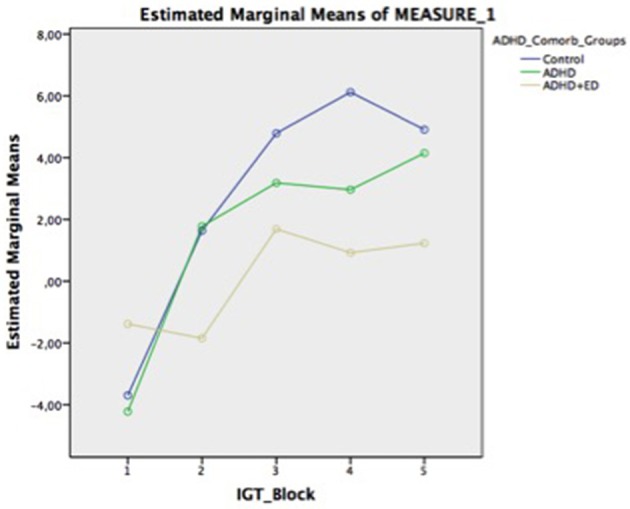
Iowa Gambling Task across groups over time-in-task.
In terms of vigilance testing, measures from the CPT (Table 5) yielded significant differences only for the Omission errors. There were significant differences when analyzing ADHD+ED vs. Controls (p = 0.031) and the ADHD+ED vs. ADHD only (p = 0.042). This difference was of a moderate effect size in both contrasts. All other CPT measures were non-significantly different in all comparisons (Table 5).
Table 5.
Interactions among groups for Conner's continuous performance test.
| Omnibus | ADHD vs. Control | ADHD+ED vs. Control | ADHD+ED vs. ADHD | |
|---|---|---|---|---|
| F (Eta2), p-valuea | Mean Difference (Cohen's d) | Mean Difference (Cohen's d) | Mean Difference (Cohen's d) | |
| Omission | 2.65 (0.072), p = 0.07 | −0.02 (0.009) | 9.05 (−0.42)b | 9.07 (−0.42)c |
| Comission | 1.45 (0.040), p = 0.24 | 2.56 (−0.31) | 4.26 (−0.50) | 1.69 (−0.19) |
| Standard error | 0.85 (0.024), p = 0.43 | 0.24 (−0.002) | 70.36 (−0.33) | 70.11 (−0.32) |
| D Prime | 1.74 (0.048), p = 0.18 | −12.72 (0.29) | −25.41 (0.60) | −12.68 (0.29) |
| Variability | 1.61 (0.045), p = 0.20 | −85 (−0.89) | −304 (−2.54) | −218 (−1.76) |
| HRT | 0.01 (0), p = 0.98 | −285.13 (0.04) | −130.20 (0.02) | 154.92 (−0.02) |
| HRT Block change | 0.56 (0.016), p = 0.74 | 0.26(−0.29) | 0.27(−0.21) | 0.01 (−0.01) |
| HRT ISI change | 0.29 (0.009), p = 0.74 | 0.26 (−0.09) | 0.69 (−0.24) | 0.42 (−0.09) |
Mean difference (Cohen's d). HRT, Hit Reaction time; HRT ISI Change, HRT Inter Stimulus Interval Change.
Univariate Analysis. Omnibus p-values. LSD post-hoc correction.
p = 0.035.
p = 0.041.
Correlations between BMI, BES, BDI, and ADHD symptoms
Pearson correlations between BMI, BES, BDI, and ADHD symptoms (current KSADS inattention + current KSADS hyperactivity/ impulsivity as a single composite score) revealed that binge eating was positively correlated with depression (r = 0.025; p < 0.05) and ADHD (r = 0.43; p < 0.001) with a moderate effect size. Binge eating was positively correlated with BMI with a strong effect size (r = 0.48; p < 0.001). ADHD was positively correlated with depression (r = 0.33; p < 0.01) with a moderate effect size and didn't correlate with BMI (r = 0.23, p = 0.062). None of the correlations were so high as to suggestion redundancy (all correlations < |0.8|).
Mediational analysis
The data were first analyzed for missing cases, outliers, and assumptions of normality. Seventeen cases were missing from the BES variable and eight cases were missing from the BDI variable. Cases with missing data on one or more variable were excluded from relevant analyses. One univariate outlier (Z > |3.00|) was observed in the BMI variable and one outlier was observed in the BDI variable. These cases were also excluded listwise from all further analyses. No univariate outliers were observed in the ADHD or BES variables. All variables were normally distributed (skew < |2.00|, kurtosis < |9.00|). The descriptive statistics associated with BES, BMI, BDI, and ADHD symptoms are reported in Table 6.
Table 6.
Descriptive Statistics associated with binge eating, body mass index, depression, and ADHD symptoms.
| M | SD | Min | Max | Skew | Kurtosis | |
|---|---|---|---|---|---|---|
| BES | 8.86 | 6.42 | 0.00 | 22.00 | 0.38 | −0.89 |
| BMI | 22.23 | 2.57 | 16.49 | 29.59 | 0.77 | 0.66 |
| BDI | 7.06 | 6.82 | 0.00 | 27.00 | 1.29 | 0.85 |
| ADHD | 7.19 | 4.86 | 0.00 | 17.00 | 0.18 | −0.93 |
BES, binge eating specific score; BMI, body mass index; BDI, Beck Depression Inventory; ADHD, current composite score of inattentive and hyperactive/impulsive symptoms. N = 69.
Pearson correlations between BES, BMI, BDI, and ADHD symptoms are presented in Table 7. Binge eating was positively correlated with depression and ADHD (moderate effect size). Binge eating was positively correlated with BMI with a strong effect size. ADHD was positively correlated with depression (moderate effect size). None of the correlations were so high as to suggestion redundancy (all correlations < |0.8|).
Table 7.
Correlations between binge eating, body mass index, depression, and ADHD symptoms.
BES, binge eating specific score; BMI, body mass index; BDI, Beck Depression Inventory; ADHD, current composite score of inattentive and hyperactive/impulsive symptoms.
N = 69.
p < 0.05;
p < 0.01;
p < 0.001.
Mediation model 1: Binge eating mediates the relationship between ED diagnostic status and BMI, after controlling for depression.
In the first mediation model we tested, we tested the hypothesis that binge eating mediates the effects of ADHD/ED comorbidity on BMI. This was investigated with a mediation analysis was conducted using the paramed command (37) in STATA (StataCorp, 2015). Assumptions of the mediation analysis for H1 were tested using two regression analyses in SPSS (IBM Corp., 2013): one bivariate regression of BES on eating disorder status and BDI (path a) and a multiple regression of BMI on BES, BDI, and eating disorder status (paths b′ and c′, respectively). Visual inspection of histograms of the standardized residuals revealed that they were approximately normally distributed for both regressions. Visual inspection of a scatterplot of standardized residuals plotted against standardized predicted values revealed that the assumption of homoscedasticity was violated for both regressions. A dummy regression did not reveal any multivariate outliers (Mahalanobis distance > 13.82, df = 2, p < 0.001) among the ED and BDI variables. A second dummy regression did not reveal any multivariate outliers (Mahalanobis distance > 16.27, df = 3, p < 0.001) among the ED, BDI, and BES variables. Neither regression was associated with excessive multicollinearity (tolerance > 0.10 for all predictor variables and covariates).
The mediation analysis was then conducted in STATA using the paramed command. The significance of the indirect and total effects was tested via bias-corrected bootstrapping, which is robust to violations of the assumption of homoscedasticity.
Both path a (coefficient = 7.86, SE = 1.76, 95% CI [4.34, 11.38] and path b′ (coefficient = 0.13, 0.05, 95% CI [0.04, 0.23]) were associated with significant but small positive effects. The analysis revealed that having an eating disorder was significantly positively associated with higher BMI (total effect = 3.31, bootstrap standard error = 0.63, 95% CI [2.06, 4.52]). Binge eating significantly mediated this effect (indirect effect = 1.04, bootstrap standard error = 0.58, 95% CI [0.09, 2.30]).
Mediation model 2: Impulsivity mediates the relationship between ADHD and binge eating, after controlling for depression.
For this mediation, we first sought to establish the existence of significant relationships between the predictor, mediator, and dependent variables. We therefore carried out a regression in which BES was entered as the dependent variable and the BDI, BIS, current KSADS inattention, and current KSADS hyperactivity/impulsivity were entered using the forced entry method. This model predicting binge eating from ADHD symptoms and impulsivity was significant, R2 = 0.21, Adj. R2 = 0.16, SE = 5.88, ΔF(4, 60) = 9.64, p = 0.006. However, none of the included variables significantly predicted binge eating. The regression was not affected by multicollinearity (tolerance > 0.1 for all variables).
Therefore, given that a relationship between the mediator and dependent variable could not be established after controlling for the independent variable, the current model did not meet Baron and Kenny's necessary assumptions for a mediated model (38). While modern research has indicated that not all of Baron and Kenny's assumptions are necessary to establish a significant indirect effect (39), we concluded that an indirect effect in the absence of a significant effect between the mediator and dependent variable was not of theoretical interest in the current model.
Discussion
In the present research we have demonstrated that individuals comorbid for ADHD and ED presented greater omission errors in the CPT and a tendency for impaired decision-making using the IGT. Also, these patients presented higher number of current Hyperactivity/Impulsivity symptoms than the ADHD-only group. Furthermore, we demonstrated that comorbid individuals had a higher BMI and that a greater level of binge eating mediated this relationship.
Previous research in the field of ADHD and Obesity has suggested that weight gain in individuals with ADHD might be due to a sleep disorder (e.g., sleep apnea) in obese patients mimicking ADHD (40), a genetic variant with impaired reward processing or altered eating habits due to impulsivity (41). We have presented evidence that the presence of a comorbid ED might contribute to weight gain in ADHD subjects. Even in the presence of mild eating binges, as measured by the BES, our ADHD+ED subjects had a significantly higher BMI than ADHD only. This falls in line with findings from clinical samples, where obese subjects presenting for weight loss comorbid with ADHD had higher BMI (5, 42–44).
The higher number of current HI symptoms in the ADHD+ED group cannot be regarded as an indication of higher impulsive traits or higher anxiety levels since these measures didn't differ from the other groups in self-report questionnaires. This contradicts the findings that ADHD-ED comorbid individuals had higher anxiety levels found in clinical samples of obese (5). ADHD individuals usually change their clinical presentation over time (45) with waning of HI behaviors to more socially age appropriate presentations.
Two previous studies have investigated cognitive function in participants with ADHD+ED. Reinblatt et al. (46), has investigated if children comorbid for Loss of Control Eating (a subclinical form of BED) differed from ADHD-only or Control participants using a Continuous Performance Test (The GNG neurobehavioural task) and a motor inhibition task. They didn't find any differences between groups. Furthermore, Seitz et al. (18) compared a sample of women with current bulimia nervosa with a history of childhood ADHD to a separate sample without a childhood history of ADHD on a continuous performance test, a task for divided attention, and a task for executive functioning. Although they didn't find significant results on the neuropsychological tests, there was a trend greater for omission errors in the comorbid group.
In accordance with the results found by Seitz et al. (18), our ADHD+ED group presented significantly higher omission errors on the CPT. Although this would indicate inattention, distractibility the number of current inattention symptoms didn't differ from ADHD only. Also, this index can denote a slower motor response. Apparently, the presence of an ED further impairs attentional systems in ADHD individuals.
The findings in the IGT suggest that ADHD+ED subjects have their decision-making skills impaired, when compared to ADHD only subjects. This might be explained by the presence of an ED. The IGT could be representative of an information processing mediated by the insula (47). A triadic model of impulse control postulates that abnormal functioning in different parts of the brain impairs this function. These cognitive systems control habitual and salient behaviors processed by the amygdala-striatum; self-regulation modulated by the prefrontal cortex; and translation of interoceptive states to feelings (urges, cravings) by the insula. It might be that ED impairs decision-making of ADHD individuals by the well-documented deficits of ED individuals in insular function (48, 49).
Binge eating was not significantly predicted by depression, impulsivity as measured by the BIS, inattention, or hyperactivity/impulsivity measured by the KSADS. Perhaps low sample size prevented the model from uncovering significant results. This effect might also reflect the poor ecological validity of these self-report measures, given the well-cited associated between impulsivity and binge eating (5, 18).
Limitations
Our findings are limited by the small sample size, relatively mild-moderate severity of eating behavior disturbances. The high cognitive functioning of all samples, as they are college students, could interfere with cognitive testing as it could induce a ceiling effect of results. Differences could be more pronounced in a clinical sample with severe symptoms and a poorer cognitive functioning. On the other hand, our study has several strengths, exemplified by the control group without any mental disorder, and the use of a treatment-naive sample, as medication could be a factor that could interfere with cognitive testing.
Author contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. BN has been funded with a scholarship from CAPES-PDSE (Comissão de Aperfeiçoamento de Pessoal do Nível Superior - Programa de Doutorado Sanduiche no Exterior).
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2018.00531/full#supplementary-material
References
- 1.Nazar BP, Bernardes C, Peachey G, Sergeant J, Mattos P, Treasure J. The risk of eating disorders comorbid with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Int J Eat Disord. (2016) 49:1045–57. 10.1002/eat.22643 [DOI] [PubMed] [Google Scholar]
- 2.Brewerton TD, Duncan AE. Associations between attention deficit hyperactivity disorder and eating disorders by gender: results from the national comorbidity survey replication. Eur Eat Disord Rev. (2016) 24:536–40. 10.1002/erv.2468 [DOI] [PubMed] [Google Scholar]
- 3.Egbert AH, Wilfley DE, Eddy KT, Boutelle KN, Zucker N, Peterson CB, et al. Attention-deficit/hyperactivity disorder symptoms are associated with overeating with and without loss of control in youth with overweight/obesity. Child Obes. (2018) 14:50–7. 10.1089/chi.2017.0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinblatt SP, Leoutsakos J-MS, Mahone EM, Forrester S, Wilcox HC, Riddle MA. Association between binge eating and attention-deficit/hyperactivity disorder in two pediatric community mental health clinics. Int J Eat Disord. (2015) 48:505–11. 10.1002/eat.22342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nazar BP, Suwwan R, de Sousa Pinna CM, Duchesne M, Freitas SR, Sergeant J, et al. Influence of attention-deficit/hyperactivity disorder on binge eating behaviors and psychiatric comorbidity profile of obese women. Compr Psychiatry (2014) 55:572–8. 10.1016/j.comppsych.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 6.Levy LD, Fleming JP, Klar D. Treatment of refractory obesity in severely obese adults following management of newly diagnosed attention deficit hyperactivity disorder. Int J Obes. (2009) 33:326–34. 10.1038/ijo.2009.5 [DOI] [PubMed] [Google Scholar]
- 7.Alfonsson S, Parling T, Ghaderi A. Self-reported symptoms of adult attention deficit hyperactivity disorder among obese patients seeking bariatric surgery and its relation to alcohol consumption, disordered eating and gender. Clin Obes. (2013) 3:124–31. 10.1111/cob.12025 [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Aranda F, Agüera Z, Castro R, Jiménez-Murcia S, Ramos-Quiroga JA, Bosch R, et al. ADHD symptomatology in eating disorders: a secondary psychopathological measure of severity? BMC Psychiatry (2013) 13:166. 10.1186/1471-244X-13-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nazar BP, de Sousa Pinna CM, Suwwan R, Duchesne M, Freitas SR, Sergeant J, et al. ADHD rate in obese women with binge eating and bulimic behaviors from a weight-loss clinic. J Atten Disord. (2016) 20:610–6. 10.1177/1087054712455503 [DOI] [PubMed] [Google Scholar]
- 10.Svedlund NE, Norring C, Ginsberg Y, von Hausswolff-Juhlin Y. Are treatment results for eating disorders affected by ADHD symptoms? A one-year follow-up of adult females Eur Eat Disord Rev. (2018) 26:337–45. 10.1002/erv.2598 [DOI] [PubMed] [Google Scholar]
- 11.McElroy SL, Hudson JI, Mitchell JE, Wilfley D, Ferreira-Cornwell MC, Gao J, et al. Efficacy and safety of lisdexamfetamine for treatment of adults with moderate to severe binge-eating disorder: a randomized clinical trial. JAMA Psychiatry (2015) 72:235–46. 10.1001/jamapsychiatry.2014.2162 [DOI] [PubMed] [Google Scholar]
- 12.Gibbs EL, Kass AE, Eichen DM, Fitzsimmons-Craft EE, Trockel M, Wilfley DE. Attention-deficit/hyperactivity disorder-specific stimulant misuse, mood, anxiety, and stress in college-age women at high risk for or with eating disorders. J Am Coll Health (2016) 64:300–8. 10.1080/07448481.2016.1138477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry (2008) 47:21–31. 10.1097/chi.0b013e31815a56f1 [DOI] [PubMed] [Google Scholar]
- 14.Garon N. Decision making in children with ADHD only, ADHD-anxious/depressed, and control children using a child version of the Iowa Gambling Task. J Atten Disord. (2006) 9:607–19. 10.1177/1087054705284501 [DOI] [PubMed] [Google Scholar]
- 15.Narvaez JC, Zeni CP, Coelho RP, Wagner F, Pheula GF, Ketzer CR, et al. Does comorbid bipolar disorder increase neuropsychological impairment in children and adolescents with ADHD? Rev Bras Psiquiatr. (2014) 36:53–9. 10.1590/1516-4446-2013-1085 [DOI] [PubMed] [Google Scholar]
- 16.Crunelle CL, Veltman DJ, van Emmerik-van Oortmerssen K, Booij J, van den Brink W. Impulsivity in adult ADHD patients with and without cocaine dependence. Drug Alcohol Depend. (2013) 129:18–24. 10.1016/j.drugalcdep.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 17.Silva KL, Rovaris DL, Guimarães-da-Silva PO, Victor MM, Salgado CA, Vitola ES, et al. Could comorbid bipolar disorder account for a significant share of executive function deficits in adults with attention-deficit hyperactivity disorder? Bipolar Disord. (2014) 16:270–6. 10.1111/bdi.12158 [DOI] [PubMed] [Google Scholar]
- 18.Seitz J, Kahraman-Lanzerath B, Legenbauer T, Sarrar L, Herpertz S, Salbach-Andrae H, et al. The role of impulsivity, inattention and comorbid ADHD in patients with bulimia nervosa. PLoS ONE (2013) 8:e63891. 10.1371/journal.pone.0063891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steadman KM, Knouse LE. Is the relationship between ADHD symptoms and binge eating mediated by impulsivity? J Atten Disord. (2014) 20:907–12. 10.1177/1087054714530779 [DOI] [PubMed] [Google Scholar]
- 20.Reinblatt SP. are eating disorders related to attention deficit/hyperactivity disorder? Curr Treat Options Psychiatry (2015) 2:402–12. 10.1007/s40501-015-0060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattos P, Nazar BP, Tannock R. By the book: ADHD prevalence in medical students varies with analogous methods of addressing DSM items. Rev Bras Psiquiatr. (2018) 40:382–7. 10.1590/1516-4446-2017-2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. The world health organization adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. (2005) 35:245–56. 10.1017/S0033291704002892 [DOI] [PubMed] [Google Scholar]
- 23.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. (1982) 7:47–5. 10.1016/0306-4603(82)90024-7 [DOI] [PubMed] [Google Scholar]
- 24.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry (1997) 36:980–8. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod. Probl Pharmacopsychiatry (1974) 7:151–69. 10.1159/000395074 [DOI] [PubMed] [Google Scholar]
- 26.Gorenstein C, Andrade L, Vieira Filho AH, Tung TC, Artes R. Psychometric properties of the portuguese version of the beck depression inventory on Brazilian college students. J Clin Psychol. (1999) 55, 553–62. [DOI] [PubMed] [Google Scholar]
- 27.Andrade L, Gorenstein C, Vieira Filho AH, Tung TC, Artes R. Psychometric properties of the Portuguese version of the state-trait anxiety inventory applied to college students: factor analysis and relation to the beck depression inventory. Braz J Med Biol Res. (2001) 34:367–74. 10.1590/S0100-879X2001000300011 [DOI] [PubMed] [Google Scholar]
- 28.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. (1995) 51:768–74. [DOI] [PubMed] [Google Scholar]
- 29.Mattos P, Segenreich D, Saboya E, Louzã M, Dias G, Romano M. Adaptação transcultural para o português da escala Adult Self-Report Scale para avaliação do transtorno de déficit de atenção/hiperatividade (TDAH) em adultos. Arch Clin Psychiatry (2006) 33:188–94. 10.1590/S0101-60832006000400004 [DOI] [Google Scholar]
- 30.Freitas S, Lopes CS, Coutinho W, Appolinario JC. Tradução e adaptação para o português da escala de compulsão alimentar periódica translation and adaptation into Portuguese of the Binge-Eating Scale. Rev Bras Psiquiatr. (2001) 23:215–20. 10.1590/S1516-44462001000400008 [DOI] [Google Scholar]
- 31.Malloy-diniz LF, Mattos P, Leite WB, Abreu N, Paula JJ, De Tavares H, et al. Tradução e adaptação cultural da Barratt Impulsiveness Scale (BIS-11) para aplicação em adultos brasileiros. J Bras Psiquiatr. (2010) 59:99–105. 10.1590/S0047-20852010000200004 [DOI] [Google Scholar]
- 32.Heck VS, Yates DB, Poggere LC, Tosi SMVD, Bandeira DR, Trentini CM. Validação dos subtestes verbais da versão de adaptação da WASI. Aval Psicol. (2009) 8:33–42. [Google Scholar]
- 33.Conners BCK. Conners ' Continuous Performance Test II (CPT II V. 5) (2004) [Google Scholar]
- 34.Rabin LA, Barr WB, Burton LA. Assessment practices of clinical neuropsychologists in the United States and Canada: a survey of INS, NAN, and APA Division 40 members. Arch Clin Neuropsychol. (2005) 20:33–65. 10.1016/j.acn.2004.02.005 [DOI] [PubMed] [Google Scholar]
- 35.Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. “Oops!”: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia (1997) 35:747–58. [DOI] [PubMed] [Google Scholar]
- 36.Bechara A. Iowa Gambling Task Professional Manual. Lutz, FL: Psychological Assessment Resources; (2007). [Google Scholar]
- 37.Liu H, Emsley R, Dunn G, VanderWeele T, Valeri L. Paramed: a command to perform causal mediation analysis using parametric models. Unkn J. (2014). [Google Scholar]
- 38.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. (1986) 51:1173–82. 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 39.Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods (2009) 41:924–36. 10.3758/BRM.41.3.924 [DOI] [PubMed] [Google Scholar]
- 40.Cortese S, Morcillo Peñalver C. Comorbidity between ADHD and obesity: exploring shared mechanisms and clinical implications. Postgrad Med. (2010) 122:88–96. 10.3810/pgm.2010.09.2205 [DOI] [PubMed] [Google Scholar]
- 41.Blum K, Chen ALC, Braverman ER, Comings DE, Chen TJ, Arcuri V, et al. Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatr Dis Treat. (2008) 4:893–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altfas JR. Prevalence of attention deficit/hyperactivity disorder among adults in obesity treatment. BMC Psychiatry (2002) 2:9. 10.1186/1471-244X-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Peñalver C, Rohde LA, Faraone SV. Association between ADHD and obesity: a systematic review and meta-analysis. Am J Psychiatry (2016) 173:34–43. 10.1176/appi.ajp.2015.15020266 [DOI] [PubMed] [Google Scholar]
- 44.Nigg JT, Johnstone JM, Musser ED, Long HG, Willoughby MT, Shannon J. Attention-deficit/hyperactivity disorder (ADHD) and being overweight/obesity: new data and meta-analysis. Clin Psychol Rev. (2016) 43:67–79. 10.1016/j.cpr.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volkow ND, Swanson JM. Clinical practice: adult attention deficit – hyperactivity disorder. N Engl J Med. (2013) 369:1935–44. 10.1056/NEJMcp1212625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinblatt SP, Mahone EM, Tanofsky-Kraff M, Lee-Winn AE, Yenokyan G, Leoutsakos J-MS, et al. Pediatric loss of control eating syndrome: association with attention-deficit/hyperactivity disorder and impulsivity. Int J Eat Disord. (2015) 48:580–8. 10.1002/eat.22404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noël X, Brevers D, Bechara A. A triadic neurocognitive approach to addiction for clinical interventions. Front Psychiatry (2013) 4:179. 10.3389/fpsyt.2013.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esposito R, Cieri F, di Giannantonio M, Tartaro A. The role of body image and self-perception in anorexia nervosa: the neuroimaging perspective. J Neuropsychol. (2018) 12:41–52. 10.1111/jnp.12106 [DOI] [PubMed] [Google Scholar]
- 49.Frank GKW, Kaye WH. Current status of functional imaging in eating disorders. Int J Eat Disord. (2012) 45:723–36. 10.1002/eat.22016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


