Enteric virus, such as norovirus, infections cause significant morbidity and mortality worldwide. However, direct antiviral infection prevention strategies are limited. Blocking host entry and initiation of infection provides an established avenue for intervention. Here, we investigated the role of the polymeric immunoglobulin receptor (pIgR)-secretory immunoglobulin (sIg) cycle during enteric virus infections. The innate immune functions of sIg (agglutination, immune exclusion, neutralization, and expulsion) were not required during control of acute murine norovirus (MNV) infection. Instead, lack of pIgR resulted in increased IFN-γ levels, which contributed to reduced MNV titers. Another enteric virus, reovirus, also showed decreased infection in pIgR KO mice. Collectively, our data point to a model in which sIg-mediated microbial sensing promotes norovirus and reovirus infection. These data provide the first evidence of the proviral role of natural sIg during enteric virus infections and provide another example of how intestinal bacterial communities indirectly influence MNV pathogenesis.
KEYWORDS: RNA virus, enteric viruses, gastrointestinal infection, pathogenesis
ABSTRACT
Noroviruses are enteric pathogens causing significant morbidity, mortality, and economic losses worldwide. Secretory immunoglobulins (sIg) are a first line of mucosal defense against enteric pathogens. They are secreted into the intestinal lumen via the polymeric immunoglobulin receptor (pIgR), where they bind to antigens. However, whether natural sIg protect against norovirus infection remains unknown. To determine if natural sIg alter murine norovirus (MNV) pathogenesis, we infected pIgR knockout (KO) mice, which lack sIg in mucosal secretions. Acute MNV infection was significantly reduced in pIgR KO mice compared to controls, despite increased MNV target cells in the Peyer's patch. Natural sIg did not alter MNV binding to the follicle-associated epithelium (FAE) or crossing of the FAE into the lymphoid follicle. Instead, naive pIgR KO mice had enhanced levels of the antiviral inflammatory molecules interferon gamma (IFN-γ) and inducible nitric oxide synthase (iNOS) in the ileum compared to controls. Strikingly, depletion of the intestinal microbiota in pIgR KO and control mice resulted in comparable IFN-γ and iNOS levels, as well as MNV infectious titers. IFN-γ treatment of wild-type (WT) mice and neutralization of IFN-γ in pIgR KO mice modulated MNV titers, implicating the antiviral cytokine in the phenotype. Reduced gastrointestinal infection in pIgR KO mice was also observed with another enteric virus, reovirus. Collectively, our findings suggest that natural sIg are not protective during enteric virus infection, but rather, that sIg promote enteric viral infection through alterations in microbial immune responses.
IMPORTANCE Enteric virus, such as norovirus, infections cause significant morbidity and mortality worldwide. However, direct antiviral infection prevention strategies are limited. Blocking host entry and initiation of infection provides an established avenue for intervention. Here, we investigated the role of the polymeric immunoglobulin receptor (pIgR)-secretory immunoglobulin (sIg) cycle during enteric virus infections. The innate immune functions of sIg (agglutination, immune exclusion, neutralization, and expulsion) were not required during control of acute murine norovirus (MNV) infection. Instead, lack of pIgR resulted in increased IFN-γ levels, which contributed to reduced MNV titers. Another enteric virus, reovirus, also showed decreased infection in pIgR KO mice. Collectively, our data point to a model in which sIg-mediated microbial sensing promotes norovirus and reovirus infection. These data provide the first evidence of the proviral role of natural sIg during enteric virus infections and provide another example of how intestinal bacterial communities indirectly influence MNV pathogenesis.
INTRODUCTION
The mucosal surface of the gastrointestinal (GI) tract is a potential entry point for many pathogens. To protect itself from pathogen attack, the host has evolved multiple mechanisms, including the secretion of immunoglobulins, i.e., secretory immunoglobulins (sIg). sIg neutralize microorganisms in the intestinal lumen and reduce the immunogenicity of the remaining bacteria (1). Intestinal epithelial cells transcytose polymeric IgA (pIgA) and pIgM from the lamina propria via the basolaterally expressed polymeric immunoglobulin receptor (pIgR). Once the pIgR-pIgA/M complex reaches the intestinal lumen, the receptor is cleaved, and sIgA and sIgM are released (1). Pathogens that have crossed the epithelial barrier and those present in intestinal epithelial cells can also be expelled by this transcytotic process (2). Highlighting the defense function of the process are studies demonstrating that deletion of pIgR results in increased pathogen loads for Helicobacter pylori (3), Giardia muris (4), Salmonella spp. (5), and Clostridium difficile (6).
sIgA are the predominant species of immunoglobulins in the intestine (7). In addition to their host defense function, they also play an immunomodulatory role (7). The follicle-associated epithelium (FAE) of Peyer’s patches (PP) and other mucosa-associated lymphoid follicles contain transcytotic microfold (M) cells. sIgA aid in luminal sampling and the initiation of mucosal immune responses. Selective adherence of luminal sIgA to the M cell surface triggers uptake of sIgA immune complexes into the PP (8), resulting in “retrograde” sIgA sampling by dendritic cells (DC) (9), noninflammatory activation of DC, and induction of regulatory T cells (7). The sIgA-induced antipathogenic immune responses also nonspecifically reduce the inflammatory potential of macrophages via upregulation of inhibitory receptors (10).
Noroviruses (NoV) are the leading cause of acute gastroenteritis worldwide (11, 12). Targeting host entry and infection initiation may provide an avenue for intervention, as they are instrumental in determining host range, initiation of immune responses, and pathogenesis. However, limited information is available about factors that promote or inhibit norovirus infection. To gain a better understanding of the early events during norovirus infection in a natural host, we took advantage of murine norovirus (MNV), a well-established and highly tractable animal model for studying norovirus biology (13–15). The first MNV strain to be discovered, MNV-1 (16), initiates infection in the ileum (17) but is cleared within days (18). Infection is primarily detected in antigen-presenting cells (APCs) (i.e., DC and macrophages) and lymphocytes (T and B cells) (19–21). To reach these target cells, MNV-1 hijacks M cells for targeted delivery (17, 22, 23). Reovirus is another enteric virus that uses M cells during infection of the intestine (17, 24), and both viruses require enteric bacteria for optimal pathogenesis (25–27).
No information is available about the role of natural sIg during enteric virus infection. Therefore, we infected mice deficient in pIgR (pIgR knockout [KO] mice) with MNV-1 and reovirus. Surprisingly, and contrary to previous studies (4, 5, 28), MNV-1 and reovirus T1L loads were reduced in the gastrointestinal tracts of pIgR KO mice compared to C57BL/6 controls (wild type [WT]). This was despite enhanced numbers of DC, macrophages, and B cells—MNV target cells—in the PP of pIgR KO mice. However, compared to WT mice, naive pIgR KO mice had enhanced baseline levels of inducible nitric oxide synthase (iNOS) and interferon gamma (IFN-γ), a cytokine with known anti-MNV and anti-reovirus activities (29–33). Depleting the microbiota from pIgR KO and WT mice resulted in comparable intestinal IFN-γ and iNOS levels and equalized MNV-1 loads. Neutralization of IFN-γ in pIgR KO mice or addition of IFN-γ to WT mice resulted in enhanced or reduced MNV-1 loads, respectively. Taken together, our findings support a model whereby the presence of the microbiota and immune cell sensing of sIg downmodulates antiviral cytokine levels, thereby promoting enteric viral infection.
(This article was submitted to an online preprint archive [34].)
RESULTS
Acute norovirus infection is reduced in pIgR KO mice.
To investigate the role of sIg during norovirus infection, we analyzed MNV-1 infection in mice lacking the pIgR (35). Since assessment of viral replication in the intestine is confounded by the lingering presence of the inoculum, neutral red, a light-sensitive dye, was incorporated into MNV-1 virions (MNV-1-NR). Exposure to light inactivates MNV-1-NR virions, but not newly replicated MNV-1 particles devoid of NR, providing a means to differentiate between input (i.e., light-sensitive) and replicated (i.e., light-tolerant) virus (36).
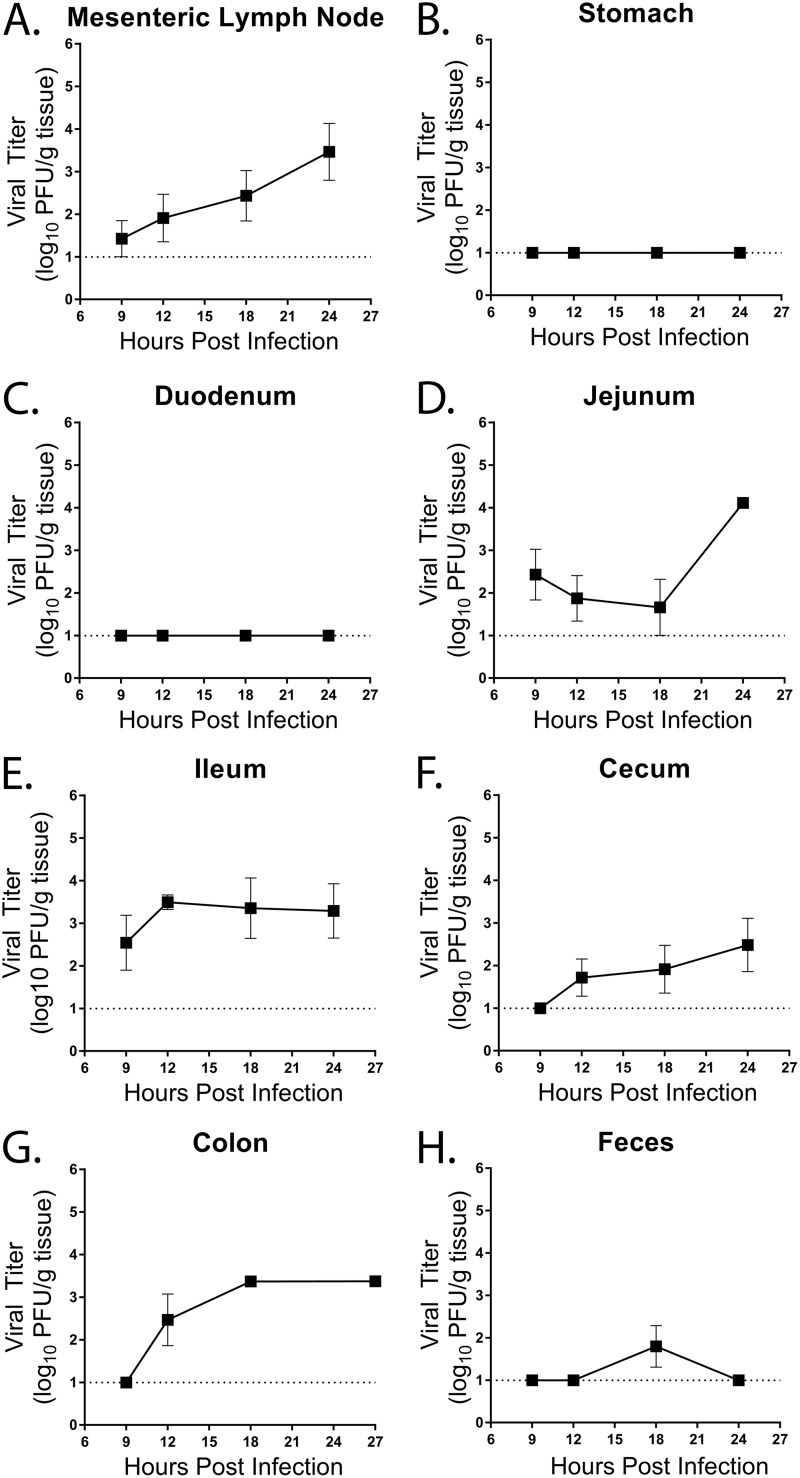
To determine the kinetics of acute MNV-1 infection in WT mice, we first performed a time course of MNV-1-NR infection. Mice were infected by oral gavage (o.g.) with 3.8 × 105 PFU, and viral loads were measured at 9, 12, 18, and 24 hours postinfection (hpi). A small amount of replicated virus was detected at 9 hpi in the mesenteric lymph node (MLN) and increased steadily throughout the time course (Fig. 1A). No replication was detected in the stomach and duodenum throughout the 24-h time course (Fig. 1B and C). Replicating MNV-1 was first detected at 9 hpi in the jejunum and ileum in WT mice (Fig. 1D and E). Titers of replicating virus in the ileum peaked at 12 hpi and then plateaued, whereas jejunal titers did not increase until 24 hpi. Replicating viral titers in the cecum and ascending colon were first detected 12 hpi and increased until 24 hpi (Fig. 1F and G). Shedding of replicating virus in the feces was observed in only one animal at 18 hpi (Fig. 1H). Taken together, these results suggest that the first round of MNV-1 replication occurs within 9 hpi in the distal small intestine and MLNs after oral infection of WT mice.
FIG 1.
Kinetics of MNV-1-NR infection in WT mice. WT mice were infected by oral gavage with 3.8 × 105 PFU/animal of neutral-red-labeled MNV-1. Mesenteric lymph nodes (A), stomach (B), duodenum (C), jejunum (D), ileum (E), cecum (F), colon (G), and feces (H) of five mice per time point were harvested at the indicated times in a darkened room using a red photolight. The tissue homogenate was serially diluted and exposed to white light for 30 min. Replicated viral titers in the indicated tissues were assessed via plaque assay. The detection thresholds are indicated by dotted lines. The data are from two independent experiments. The error bars represent standard errors of the mean (SEM).
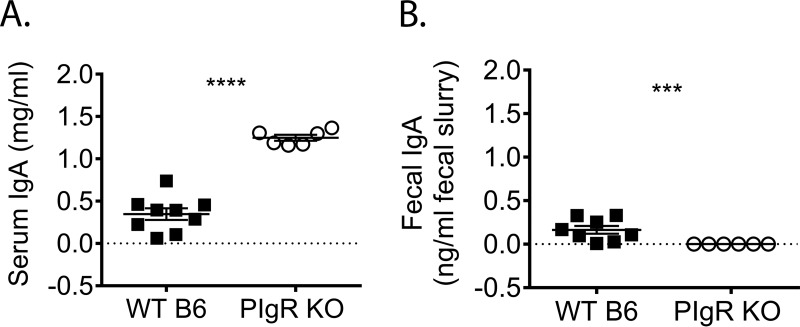
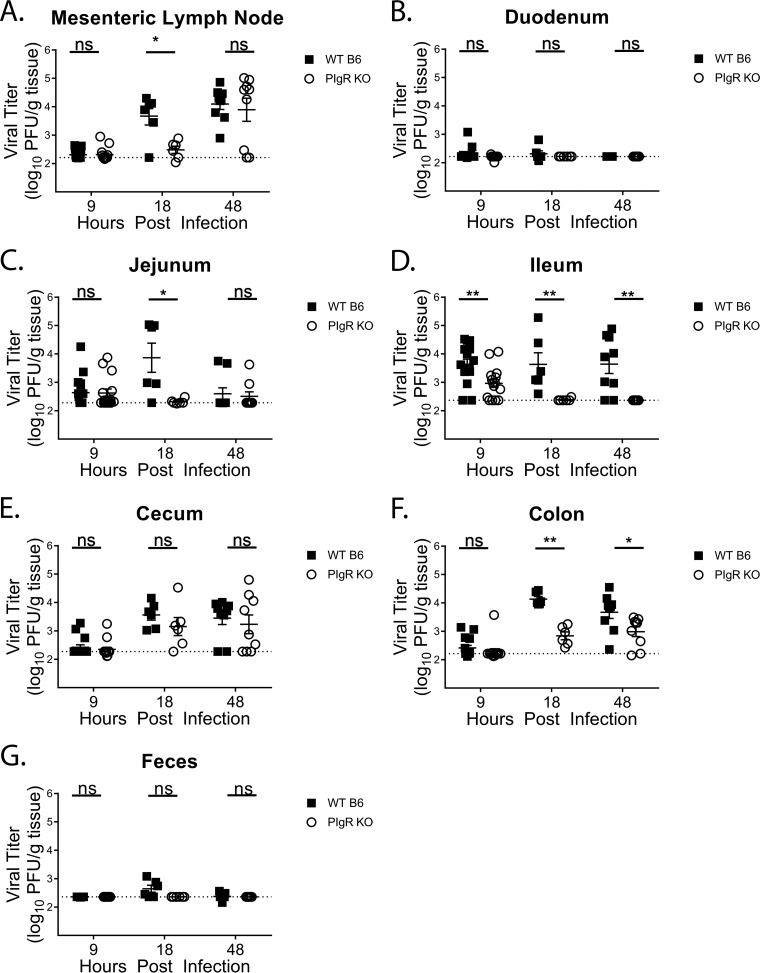
Next, we compared MNV-1 infection in naive pIgR KO and WT mice. We first confirmed that fecal levels of IgA were undetectable in pIgR KO mice, while serum IgA levels were significantly increased compared to WT mice (Fig. 2A and B), in accordance with the literature (37, 38). Then, we measured MNV-1-NR loads in the intestinal tract and draining lymph nodes of pIgR KO and WT mice at 9, 18, and 48 hpi. Consistent with the results shown in Fig. 1, infection was detected in the MLNs at 18 hpi, and levels were maintained at 48 hpi in WT mice. However, infection in pIgR KO mice was delayed and did not reach WT levels until 48 hpi (Fig. 3A). Consistent virus replication was not detected in the duodenum at any time point tested for either mouse strain (Fig. 3B). Jejunal MNV-1 replication peaked at 18 hpi in WT mice, while no consistent replication was observed in pIgR KO mice after 9 hpi (Fig. 3C). Surprisingly, MNV-1 replication was significantly reduced in the ileum in pIgR KO mice compared to WT mice at all time points tested (Fig. 3D). Cecal MNV-1 replication increased throughout the time course in pIgR KO mice but was not significantly different from that in WT mice (Fig. 3E). In contrast, colon titers were significantly reduced in pIgR KO mice at 18 and 48 hpi compared to WT mice (Fig. 3F). Fecal shedding was detectable in WT mice only at 18 hpi and was below the limit of detection in pIgR KO mice (Fig. 3G). Taken together, these data demonstrate that pIgR KO mice have reduced viral loads, suggesting that natural, non-MNV-specific sIg are not protective but instead enhance acute norovirus infection in vivo.
FIG 2.
Characterization of pIgR KO mice. Sera and feces were collected from naive pIgR KO and WT mice, and IgA concentrations were measured via ELISA. (A) Serum diluted 1:500. (B) Ten percent fecal slurries diluted 1:200. The limit of detection (LOD) was set at 0. Each symbol is a data point from an individual animal. The error bars represent SEM. The data were analyzed using the Mann-Whitney U test. ***, P < 0.001; ****, P < 0.0001.
FIG 3.
Acute norovirus infection is reduced in pIgR KO mice. Mice were infected by oral gavage with 3.8 × 105 PFU/animal neutral-red-labeled MNV-1, and tissues were harvested at 9, 18, and 48 hpi in a darkened room using a red photolight. The tissue homogenates were serially diluted and exposed to white light for 30 min. Replicated viral titers in the indicated tissues of WT and pIgR KO mice were assessed via plaque assay. The sensitivity threshold for each graph, indicated by the dotted line, is as follows (log PFU per gram of tissue): 2.17 (A), 2.21 (B), 2.3 (C), 2.37 (D), 2.27 (E), 2.22 (F), and 2.37 (G). The data are pooled from at least two independent experiments, and each symbol is a data point from an individual animal. The error bars represent SEM. The data were analyzed using the Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ns, not statistically significant.
Natural sIg do not facilitate MNV-1 access to Peyer's patches.
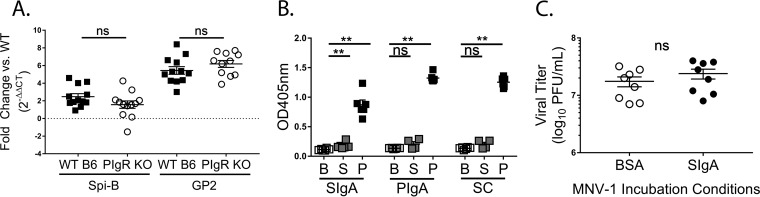
To determine the mechanism of reduced MNV-1 infection in pIgR KO mice, we investigated M cell-associated gene expression, since M cells are necessary for efficient MNV infection (17, 22). Host mRNA was isolated from PP in the ilea of naive pIgR KO and WT mice and analyzed for transcript levels of Spi-B (a transcription factor required during M cell development) and GP2 (a marker of mature M cells) (39). No differences in expression levels were observed for either gene between the two groups (Fig. 4A). These data suggest that the virus encounters similar levels of functional M cells during infection of both mouse strains and that the lack of pIgR and the associated lack of immune cell sensing of immune complexes do not significantly affect M cell gene transcription.
FIG 4.
Natural sIgA binding to MNV-1 fails to neutralize infection in vitro. (A) Transcript levels of M cell-related Spi-B and GP2 genes from WT and pIgR KO Peyer's patches over GAPDH transcript levels are shown as 2−ΔΔCT in relation to the WT. ΔCT values were analyzed for statistical significance. (B) ELISA was performed by coating microtiter plates with 0.1 mg/ml of recombinant murine secretory sIgA, murine pIgA, or murine secretory component (SC) and incubated with bacterially expressed P or S domain of the MNV-1 capsid or ELISA coating buffer. Protein domains were detected using an MNV-1 capsid antibody, followed by a peroxidase-conjugated secondary antibody. Absorbance (optical density [OD]) was read at 405 nm. Six replicates are shown. (C) MNV-1 (2.8 × 107 PFU) was preincubated with 0.25 mg/ml recombinant sIgA or bovine serum albumin for 1 h in a 37°C water bath, and neutralization was assessed via plaque assay. The error bars represent SEM. The data were analyzed using the Mann-Whitney U test. **, P < 0.01; ns, not statistically significant.
sIg are taken up by M cells and sampled by an underlying network of MNV-susceptible APCs (7). To determine whether natural sIg aid in viral targeting to and transcytosis across the FAE, we first determined whether MNV-1 was capable of interacting with non-NoV-specific recombinant sIgA. Recombinant anti-rotavirus sIgA, the secretory component alone, or anti-rotavirus dimeric IgA (40) was used to coat enzyme-linked immunosorbent assay (ELISA) plates and incubated with bacterium-expressed MNV-1 P and S domains (41), followed by detection with a polyclonal anti-MNV antibody (Fig. 4B). The P domain of the MNV-1 capsid protein bound to non-MNV-specific sIgA as a whole and to both components, the non-MNV-specific IgA dimer and the secretory component. Binding of the S domain to sIgA or its components was similar to that of the negative control, bovine serum albumin (BSA). These data demonstrate that the interaction with natural sIgA is restricted to the P domain, which also interacts with host cell receptors and contains neutralizing antibody epitopes (42). Interestingly, sIgA did not affect viral infection in vitro, since no difference in MNV-1 titers was observed by plaque assay when complexed with recombinant, non-antigen-specific sIgA compared to a protein control (Fig. 4C).
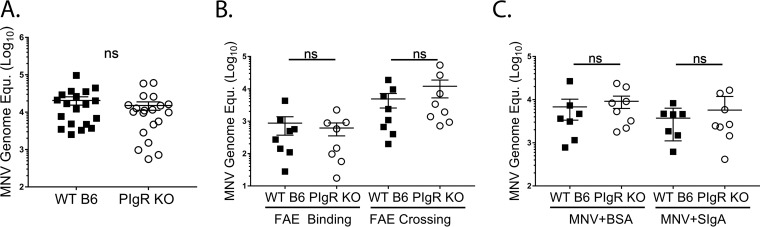
Since MNV-1 was able to bind to recombinant, non-MNV-specific sIgA, we next performed ligated-ileal-loop assays to determine whether MNV-1 uptake was altered by the presence or absence of sIg. Ileal PP of naive pIgR KO and WT mice were ligated to create an ∼2-cm closed loop, and virus was injected as the loop was sealed. After 25 min, whole PP were excised, and mucus-bound virions were removed prior to measuring viral genome titers by quantitative real-time (qRT)-PCR. No difference was observed in viral genome copies between the two groups (Fig. 5A), suggesting similar numbers of virions bound to and/or entered the PP in both mouse strains. Since MNV-1 primarily infects immune cells (19), we further assessed the numbers of virions that reached PP immune cells. The FAE was digested from the PP lamina propria, and viral genome levels were determined by qRT-PCR. There were no significant differences in the amounts of virus that associated with the PP FAE, or that crossed the FAE into the lamina propria, in pIgR KO compared to WT mice (Fig. 5B). However, one limitation of the previous experiment is the short interaction time between MNV-1 and sIg compared to a natural oral infection, during which the virus travels through the intestinal tract. Therefore, we hypothesized that preincubation of MNV-1 with natural sIgA may boost virus internalization if performed prior to the ileal loop ligation. Nevertheless, similar MNV levels were detected in the PP lamina propria in WT and pIgR KO mice when MNV-1 was precomplexed with non-MNV-specific sIgA or BSA as a negative control for MNV-1 binding (Fig. 5C). Taken together, these data demonstrate that, although MNV-1 can interact with recombinant nonspecific sIg, natural sIg does not facilitate MNV-1 binding to or crossing of the intestinal epithelial barrier. These findings indicate that natural sIg does not modulate norovirus interaction with the epithelial barrier.
FIG 5.
Natural secretory immunoglobulins do not aid in MNV access to the Peyer’s patch. Intestinal ileal loop assays were performed in WT and pIgR KO mice. MNV-1 (100 μl; 3.8 × 105 PFU) was injected into the closed loop and incubated for 25 min. Viral genome copy equivalents (Equ.) were measured by qRT-PCR in Peyer’s patches. (A) MNV-1 bound to and was internalized in PP. The data were pooled from four independent experiments. (B) MNV-1 genomes bound to the follicle-associated epithelium (FAE Binding) and were internalized in the PP lamina propria (FAE Crossing). The data were pooled from four independent experiments. (C) MNV-1 was preincubated with rotavirus-specific sIgA or BSA for 1 h at 37°C before injecting the complex into the loop. The data are pooled from two independent experiments. Each symbol is a data point from an individual PP. The data mean is indicated for each group. The error bars represent SEM. The data were analyzed using the Mann-Whitney U test. ns, not statistically significant.
pIgR KO mice have increased numbers of small-intestinal APC subsets.
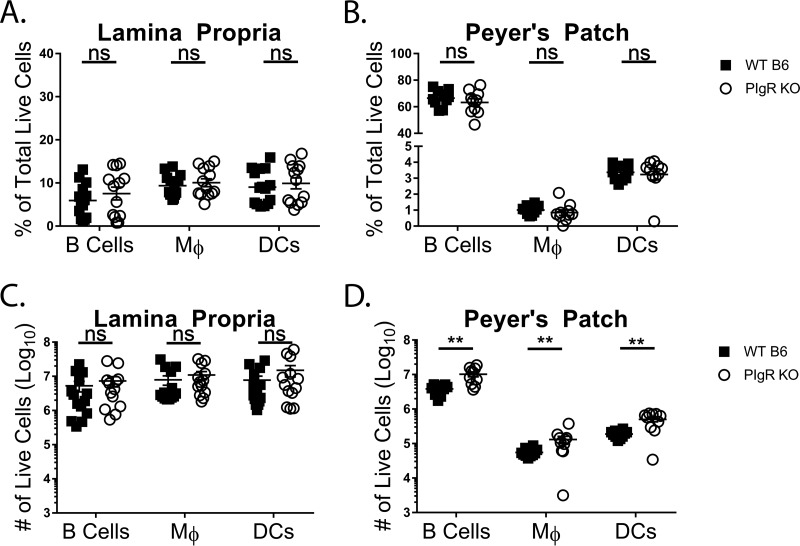
Since access to the site of primary viral infection, the PP, could not account for the reduced infection seen in pIgR KO mice, we next investigated whether an absolute or relative reduction in the numbers of B cells, macrophages, and DC, established MNV target cells (20, 21) in the small intestine, might provide an explanation. Small intestines and their PP were collected from naive mice, tissues were digested to create single-cell suspensions, and cellularity was analyzed via flow cytometry. No differences were detected in the percentages of B cells (CD19+ CD11b−), macrophages (CD19− CD11b+ CD64+), and DC (CD19− CD11b+ CD64− CD11c+) in the villous or PP lamina propria (Fig. 6A and B). No differences were observed in the numbers of the three APC subsets in the villous lamina propria (Fig. 6C). Interestingly, all three APC subsets were increased roughly 2.5-fold in the PP lamina propria of pIgR KO mice compared to controls (Fig. 6D). These data demonstrate that pIgR KO mice have increased numbers of MNV-susceptible cell types at the site of infection. Taken together with data from Fig. 5, these findings suggest that access to and availability of norovirus target cells cannot account for the reduced MNV-1-NR infection seen in pIgR KO mice.
FIG 6.
pIgR KO mice have increased small-intestinal APC subsets. Peyer’s patches and small-intestinal lamina propria cells were isolated from naive pIgR KO and WT mice. Live cells were analyzed via flow cytometry. The gating strategy was as follows. APCs were defined as singlets, live, CD45+ I-A/I-E (MHC-II)+. APCs were further gated into B cells (CD19+ CD11b−), macrophages (CD19− CD11b+ CD64+) (Mφ), and DC (CD19− CD11b+ CD64− CD11c+). (A and B) Percentages of live lamina propria (A) and Peyer’s patch (B) cells. (C and D) Absolute numbers of live lamina propria (C) and Peyer’s patch (D) cells. The data shown are pooled from two or three independent experiments, and each symbol is a data point from an individual animal. The data mean is indicated for each group. The error bars represent SEM. The data were analyzed using the Mann-Whitney U test. **, P < 0.01; ns, not statistically significant.
IFN-γ and iNOS levels are enhanced in naive pIgR KO mice.
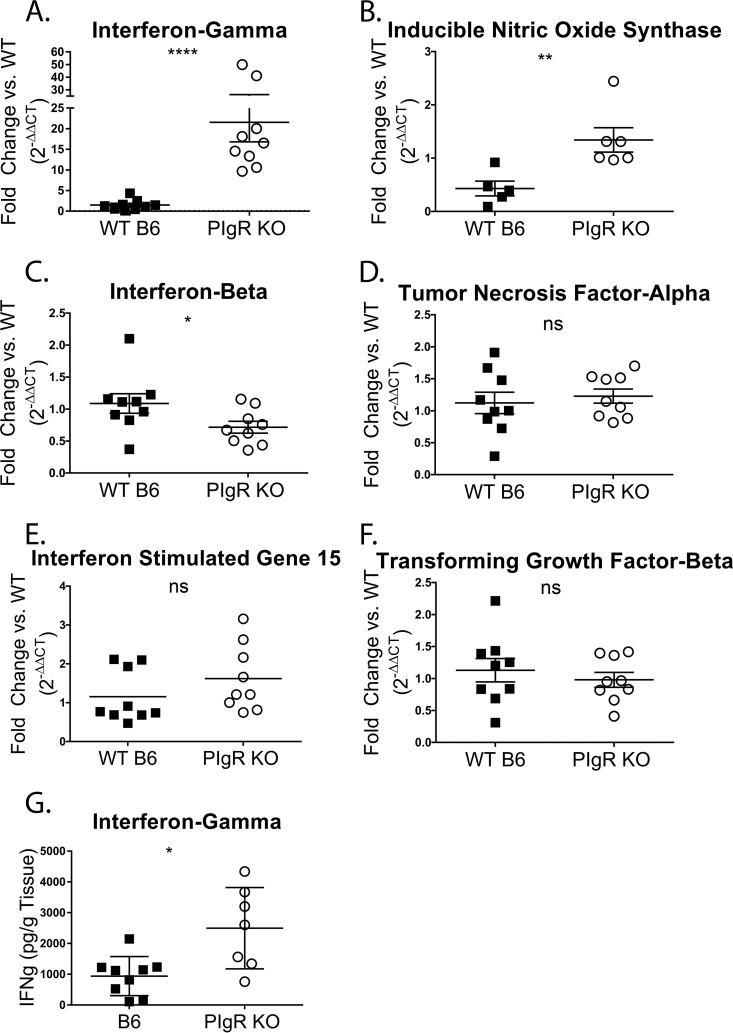
sIg complexes have immunomodulatory functions, and when enteric bacteria are recognized in complex with sIg, an anti-inflammatory response is initiated (7). Therefore, we investigated whether pIgR KO mice exhibit an altered intestinal immune landscape. Toward that end, transcript levels of a panel of cytokines and chemokines were analyzed in naive pIgR KO mice and compared to those from WT mice. The ileum was chosen for assessment, as it is the initial site of MNV-1 replication and the organ in which MNV-1 titers were significantly reduced at all time points in pIgR KO mice (Fig. 3D). Host mRNA was isolated from naive ilea of both groups of mice and analyzed by qRT-PCR for transcript levels of the anti-inflammatory transforming growth factor beta (TGF-β) and interleukin 10 (IL-10) genes and the proinflammatory and antiviral tumor necrosis factor alpha (TNF-α), iNOS, IFN-γ, IFN-β, IFN-λ, and interferon-stimulated gene 15 (ISG15) genes. Both IFN-γ and iNOS transcript levels were significantly enhanced in pIgR KO mice compared to WT controls (Fig. 7A and B). In contrast, IFN-β transcript levels were significantly reduced in pIgR KO mice compared to WT controls (Fig. 7C), while transcript levels of the inflammatory molecules TNF-α and ISG15 were comparable (Fig. 7D and E). Transcript levels of TGF-β, a cytokine that promotes IgA class switching (43), were also equivalent between strains (Fig. 7F). IFN-λ and IL-10 were undetectable in either group (data not shown). Consistent with increased IFN-γ transcript levels, IFN-γ protein levels were also significantly higher in pIgR KO mice versus WT controls (Fig. 7G).
FIG 7.
IFN-γ and iNOS levels are enhanced in pIgR KO mice. (A to F) Total ileal RNA was isolated from naive conventionally housed pIgR KO and WT mice to determine host gene levels relative to gapdh. The data are shown relative to WT mice and are displayed as 2−ΔΔCT. ΔCT values were analyzed for significance. (G) Ilea from naive pIgR KO and WT mice were harvested to determine host IFN-γ protein levels via ELISA. Each symbol is the data point from an individual animal. The data mean is indicated for each group. The error bars represent SEM. *, P < 0.05; **, P < 0.01; ****, P < 0.0001; ns, not statistically significant.
These data demonstrate that pIgR KO mice have enhanced levels of iNOS and IFN-γ, consistent with previous findings that sIgA dampens inflammatory signals (7). Both molecules have established antiviral activities (44–47). No significant mortality is seen in iNOS-deficient mice infected with MNV-1 (16), and MNV-1 replicates similarly in bone marrow-derived macrophages from WT and iNOS-deficient mice (20). In contrast, IFN-γ is a cytokine that effectively blocks MNV infection (29–32). Thus, these findings suggest that elevated levels of some antiviral cytokines, in particular IFN-γ, may limit MNV-1 replication in pIgR KO mice.
Intestinal microbial communities are altered in pIgR KO mice.
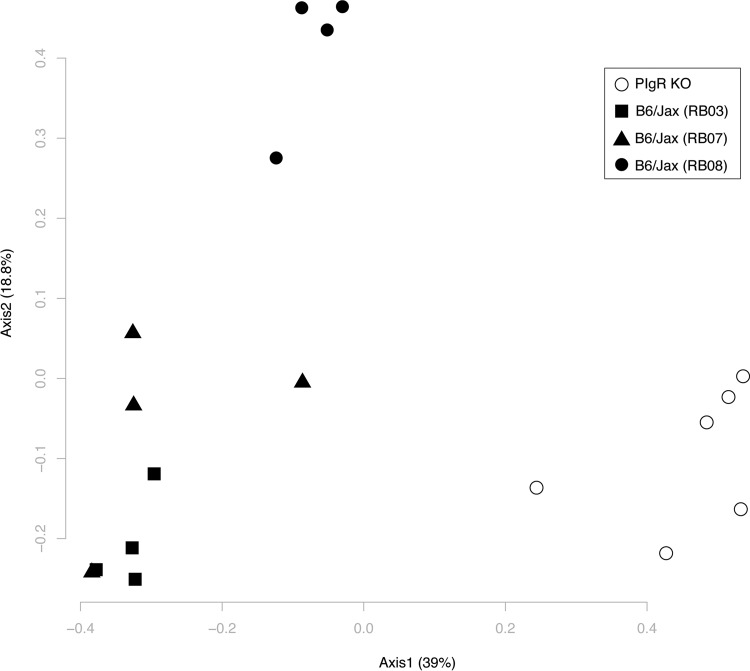
Alterations in the intestinal microbiota in the absence of sIg, coupled with the lack of sIg sensing by intestinal immune cells, may skew immune activation and account for the upregulation of IFN-γ and iNOS seen in naive pIgR KO mice compared to controls. Previous reports regarding microbial alterations in the gastrointestinal tract in pIgR KO mice are conflicting (48, 49). Therefore, we determined the microbial composition via 16S rRNA gene sequencing in naive pIgR KO and WT mice originating from the same breeding locations as the mice used in the infection experiments. The intestinal microbial communities in the ileal content were significantly altered in pIgR KO mice compared to WT controls using analysis of molecular variance (AMOVA) (P = 0.001) (Fig. 8). Interestingly, WT mice also had significant differences in microbial communities based on breeding room (RB03 versus RB07, P = 0.023; RB03 versus RB08, P = 0.018; RB07 versus RB08, P = 0.026; AMOVA). However, differences between WT and pIgR KO mice were much greater than differences between WT mice from different breeding locations. Similar results were also seen in the ileal and cecal mucosa, cecal contents, and feces (data not shown). Our findings are consistent with the study by Reikvam et al. (49) and demonstrate that intestinal microbial communities are significantly altered in pIgR KO mice. These alterations may contribute to a skewed intestinal cytokine milieu.
FIG 8.
Intestinal microbial communities are altered in pIgR KO mice. Ileal contents were isolated from naive pIgR KO and WT mice, and intestinal microbial communities were analyzed via 16S rRNA gene sequencing. A PCoA plot of θYC distances between bacterial communities is shown. Each symbol represents the bacterial community from the ileal content of an individual animal. The solid symbols represent WT mice, and the shapes distinguish between WT breeding rooms. The open circles represent pIgR KO mice.
IFN-γ and iNOS levels are similar in germfree pIgR KO and WT mice.
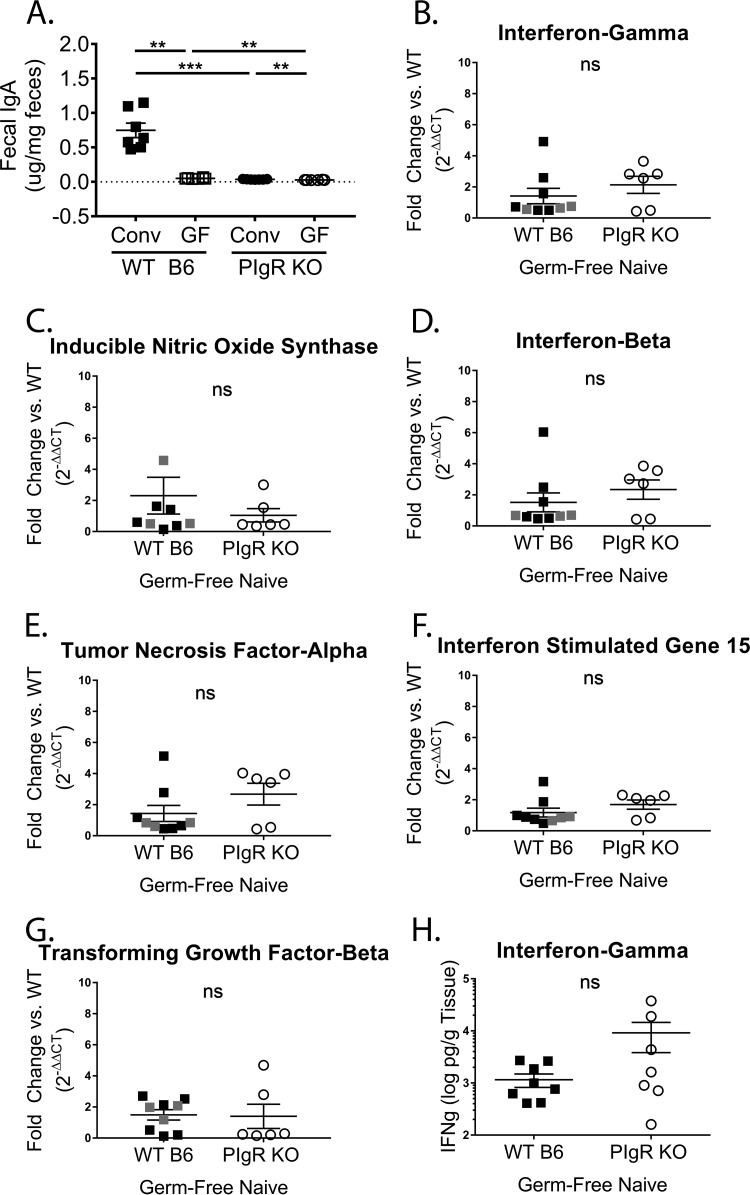
To test whether enhanced levels of cytokines limit MNV-1 infection in pIgR KO mice, we first sought to identify conditions under which the levels of IFN-γ and iNOS were similar between pIgR KO and WT mice. We hypothesized that germfree mice of both backgrounds might represent one such condition. Thus, both mouse strains were rederived germfree, and IgA levels in the feces of germfree and conventional WT and pIgR KO mice were analyzed. We found that fecal IgA levels were significantly reduced in germfree mice compared to conventionally housed mice for both strains (Fig. 9A). Next, transcript levels from the ilea of naive germfree mice were analyzed for the above-mentioned panel of cytokines. Ileal IFN-γ, iNOS, and IFN-β transcript levels were comparable between pIgR KO mice and WT controls devoid of bacterial stimulation (Fig. 9B to D). Similar to conventionally housed mice, TNF-α, ISG15, and TGF-β transcript levels were equivalent between the two groups of germfree mice (Fig. 9E to G). In addition, and consistent with transcript levels, IFN-γ protein levels were also equivalent (Fig. 9H). These data demonstrate that removal of the microbiota results in similar small-intestinal IFN-γ and iNOS levels in pIgR KO and WT mice, highlighting the immunomodulatory functions of sIg.
FIG 9.
IFN-γ and iNOS levels are reduced in germfree pIgR KO mice. (A) Ten percent fecal slurries diluted 1:200 from naive conventional (Conv) and germfree (GF) pIgR KO and WT mice were analyzed for IgA concentrations via ELISA. The conventional samples were distinct from those in Fig. 2B. The LOD was set at 0. (B to G) Total ileal RNA was isolated from naive germfree pIgR KO and germfree WT mice. Data points from WT mice housed at University of Michigan are indicated in black, and at Emory University in gray. Host gene levels were normalized to gapdh. The data are shown relative to WT mice and displayed as 2−ΔΔCT. ΔCT values were analyzed for significance. (H) Ilea from naive germfree pIgR KO and WT mice were harvested to determine host IFN-γ protein levels via ELISA. Each symbol is a data point from an individual animal. The error bars represent SEM. The data were analyzed using the Mann-Whitney U test. **, P < 0.01; ***, P < 0.001; ns, not statistically significant.
MNV-1 infections are similar in germfree pIgR KO and WT mice.
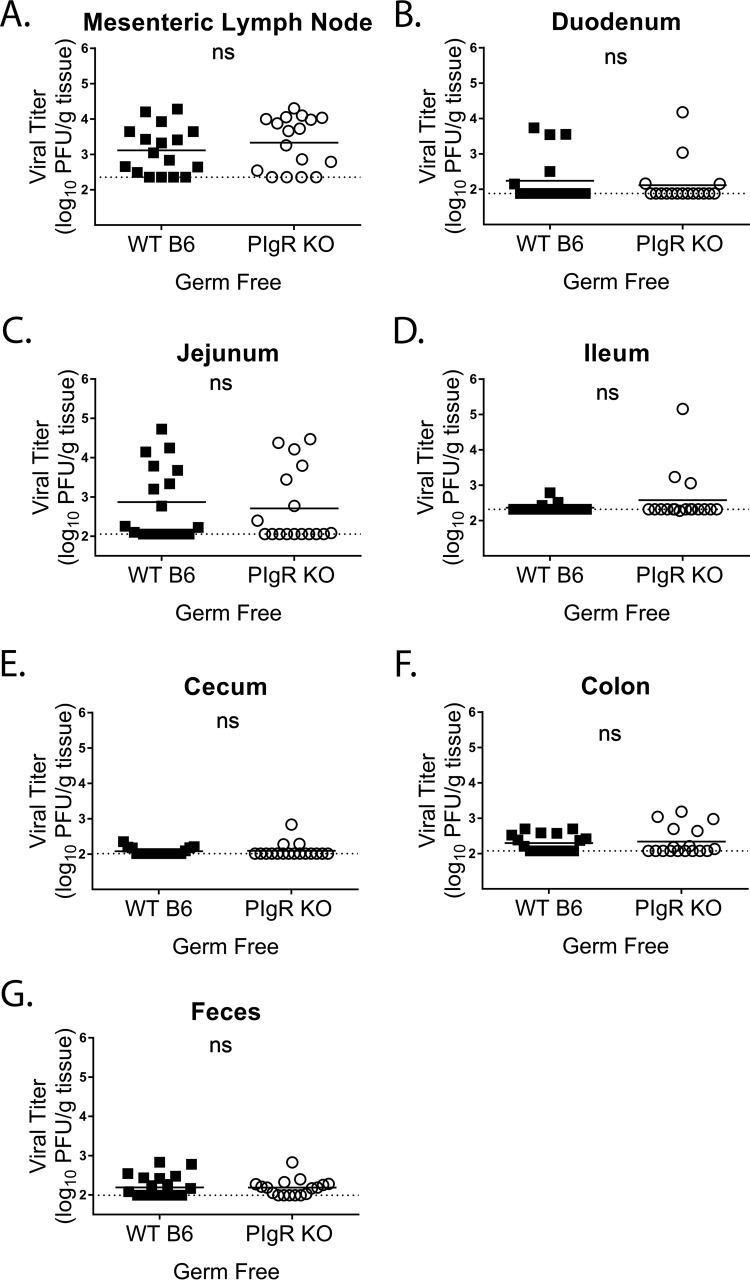
The above-mentioned findings suggested that in the absence of pIgR and sIg (i.e., pIgR KO mice), inflammatory responses to enteric bacteria may create an inhospitable environment for MNV-1 infection. To test this hypothesis, we infected germfree pIgR KO and WT mice with MNV-1-NR and assessed the titers of replicated virus via plaque assay at 18 hpi. Consistent with our hypothesis, germfree pIgR KO and WT mice had similar viral loads throughout the gastrointestinal tract (i.e., duodenum, jejunum, ileum, cecum, and colon) and MLNs, as well as similar viral shedding in the feces (Fig. 10A to G). In addition, it is worth noting that, similar to previous studies using antibiotic-depleted WT mice (21), overall virus loads were reduced in germfree compared to conventional WT mice. These data demonstrate that MNV-1 replicates similarly in pIgR KO and WT mice under conditions where inflammatory markers such as IFN-γ and iNOS are equivalent.
FIG 10.
MNV-1 infections are similar in germfree pIgR KO and WT mice. Germfree mice were infected by oral gavage with 3.8 × 105 PFU/animal of neutral-red-labeled MNV-1, and tissues were harvested at 18 hpi in a darkened room using a red photolight. The tissue homogenate was serially diluted and exposed to white light for 30 min. Replicated viral titers in the indicated tissues of germfree WT and pIgR KO mice were assessed via plaque assay. The sensitivity threshold for each graph, indicated by a dotted line, is as follows (log PFU per gram of tissue): 2.356 (A), 2.881 (B), 2.057 (C), 2.317 (D), 2.014 (E), 2.077 (F), and 1.993 (G). The data were pooled from four independent experiments, and each symbol is a data point from an individual animal. The error bars represent SEM. The values were analyzed using the Mann-Whitney U test. ns, not statistically significant.
IFN-γ reduces MNV-1 infection in vivo.
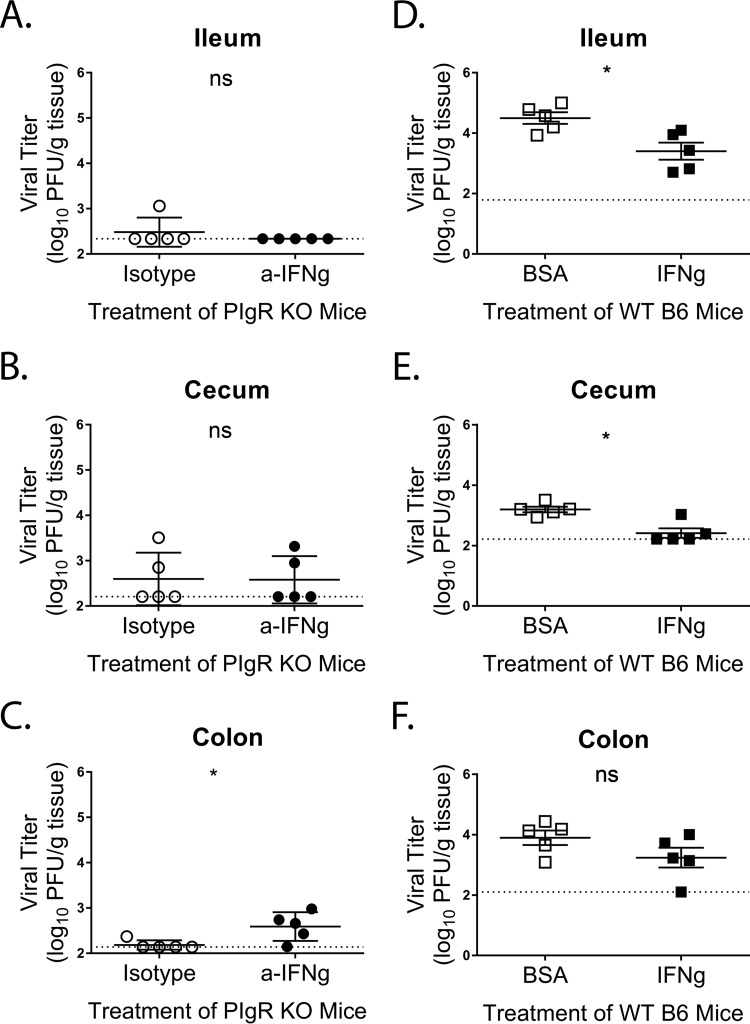
To test whether enhanced levels of IFN-γ limit MNV-1 infection in pIgR KO mice, we neutralized intestinal IFN-γ in pIgR KO mice via intraperitoneal (i.p.) treatment with 500 µg anti-IFN-γ or isotype control antibody 18 h prior to MNV-1-NR infection. Viral replication was measured 18 hpi via plaque assay in intestinal segments as before. While no differences were observed in viral titers in either the ilea or the ceca of anti-IFN-γ- or isotype control-treated pIgR KO mice (Fig. 11A and B), there was significant enhancement of MNV-1 replication in the colons of pIgR KO mice following IFN-γ neutralization (Fig. 11C). No differences were detected in viral loads in the mesentery, duodenum, jejunum, and feces between the two groups of mice (data not shown). These data suggested that the enhanced levels of IFN-γ in pIgR KO mice might directly inhibit MNV-1 replication. To confirm these findings, we next treated WT mice i.p. with 104 units of IFN-γ 18 h prior to MNV-1-NR infection. Viral loads were again determined 18 hpi by plaque assay. Significantly reduced titers were observed in both the ilea and the ceca of IFN-γ-treated WT mice compared to controls (Fig. 11D to E). No difference in viral replication was seen in the colon (Fig. 11F). Similar to our findings in pIgR KO mice, no differences were detected in viral loads in the mesentery, duodenum, jejunum, and feces of IFN-γ- or control-treated WT mice (data not shown). These data confirm the anti-MNV properties of IFN-γ in vivo. Taken together, these results point to a model in which altered intestinal microbial sensing in the absence of sIg results in enhanced IFN-γ sufficient to limit MNV-1 replication in the GI tract.
FIG 11.
IFN-γ modulates MNV-1 infection in vivo. (A to C) PIgR KO mice were treated with 500 mg of anti-IFN-γ antibody (XMG1.2) or isotype control antibody via intraperitoneal injection 18 h prior to infection. Mice were infected by oral gavage with 3.8 × 105 PFU/animal of neutral-red-labeled MNV-1. Tissues were harvested at 18 hpi in a darkened room using a red photolight. The tissue homogenates were serially diluted and exposed to white light for 30 min. Replicated viral titers in the indicated tissues were assessed via plaque assay. (D to F) WT mice were treated with 104 units/animal of IFN-γ or equivalent protein (BSA) via intraperitoneal injection 18 h prior to infection. Infection was performed as before. The sensitivity threshold for each graph, indicated by a dotted line, is as follows (log PFU per gram of tissue): 2.34 (A), 2.21 (B), 2.14 (C), 1.79 (D), 2.22 (E), and 2.1 (F). The data were pooled from two independent experiments, and each symbol is a data point from an individual animal. The error bars represent SEM. The values were analyzed using the Mann-Whitney U test. *, P < 0.05; ns, not statistically significant.
Acute reovirus infection is reduced in pIgR KO mice.
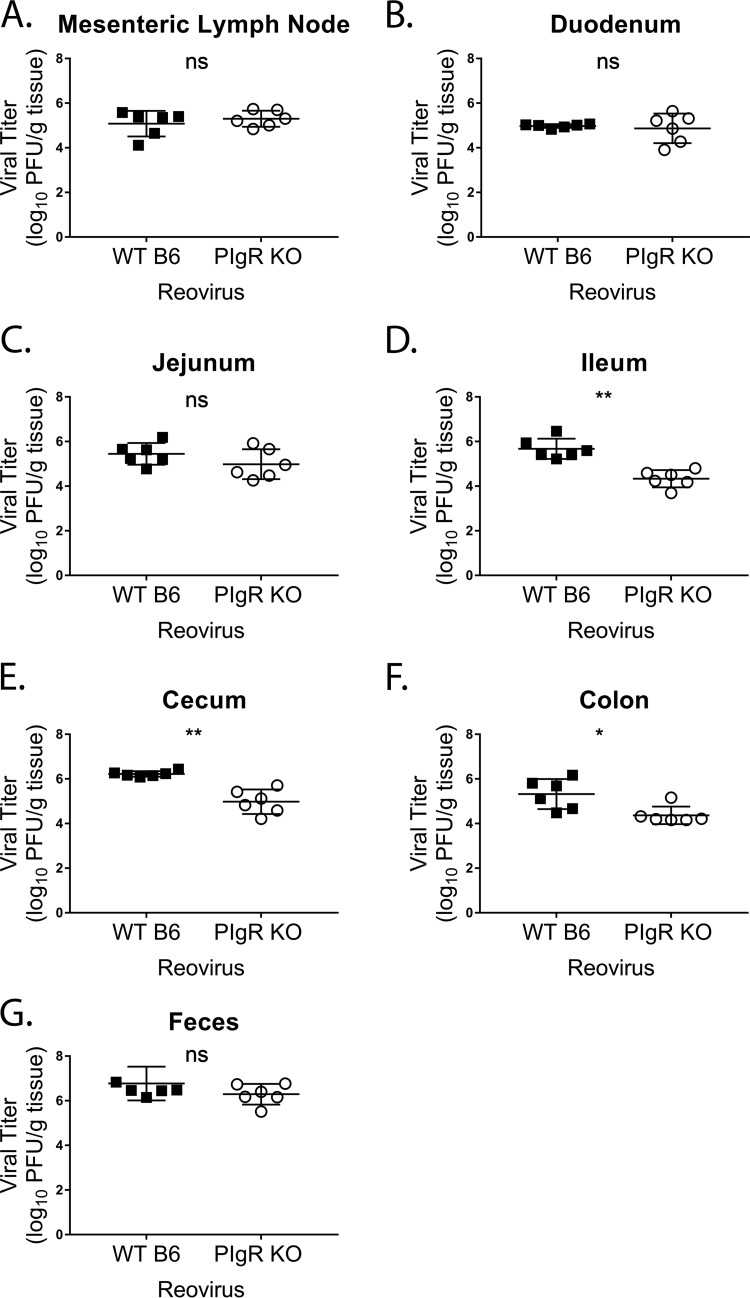
IFN-γ has broad-spectrum antiviral activities. To determine if the observed pIgR KO phenotype extended to another virus, we measured reoviral loads in the intestinal tracts and mesenteric lymph nodes of pIgR KO and WT mice at 24 hpi. Reovirus T1L infections were similar in the mesenteric lymph nodes, duodenum, jejunum, and feces of both mouse strains (Fig. 12A to C and G). However, pIgR KO mice had significantly reduced infection in the ileum, cecum, and ascending colon compared to controls (Fig. 12D to F). Taken together, these data demonstrate that, similar to MNV, natural sIg are not protective during reovirus infection. In summary, our findings show that pIgR and sIg do not directly inhibit enteric viral infection; instead, sIg promote enteric viral infection through alterations in intestinal immune responses, which are modulated by the microbiota.
FIG 12.
Acute reovirus infection is reduced in pIgR KO mice. Mice were infected by oral gavage with 2 × 106 PFU/animal of reovirus T1L, and indicated tissues, i.e., mesenteric lymph node (A), duodenum (B), jejunum (C), ileum (D), cecum (E), colon (F), and feces (G), were harvested at 24 hpi. Viral titers in the indicated tissues of WT and pIgR KO mice were assessed via plaque assay. The data were pooled from two independent experiments, and each symbol is a data point from an individual animal. The error bars represent SEM. The data were analyzed using the Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ns, not statistically significant.
DISCUSSION
sIg provides a first line of defense against several enteric pathogens by reducing the immunogenicity of lumenal bacterial communities and mediating immune exclusion and expulsion from the host (reviewed in references 7, 50, and 51). Despite the ability of recombinant, non-MNV-specific pIgA, secretory component, and sIgA to bind MNV, MNV-1 infection was not altered in vitro, and removing sIg-mediated innate intestinal defenses did not alter MNV-1 binding to the FAE or internalization into PP. Instead, our findings demonstrated that pIgR KO mice, which lack sIg, showed reduced MNV-1 infection. This suggests natural sIg do not exclude MNV from the intestine or mediate intraepithelial expulsion of the virus. More importantly, these data indicate that natural sIg do not protect the host from norovirus infection, a finding that also extended to reovirus. This result is surprising given that other enteric pathogens are controlled in this manner (3–6). To our knowledge, this represents the first example in which pIgR and natural sIg indirectly promote infection by enteric viruses.
Our current analysis of pIgR KO mice also revealed previously unrecognized features of the mouse strain. First, the mice had ∼2.5-fold greater numbers of macrophages, DC, and B cells in their PP than did WT controls, suggesting larger PP. Second, key M cell transcripts (i.e., Spi-B and GP2) were similar to those of controls, suggesting that M cell development is independent of sIg. Third, analysis of host mRNA levels in the small intestine revealed that IFN-γ and iNOS were selectively elevated in naive conventional, but not germfree, pIgR KO mice compared to WT controls. Previous studies have also reported enhanced inflammatory activation in conventional pIgR KO mice, resulting in lethal systemic hyperactivity (52), spontaneous chronic obstructive pulmonary disease (COPD) (53), and enhanced susceptibility to chemically induced colitis (54).
A recent study determined that innate immune cell detection of serum IgA via the IgA receptor (FcαR1), in conjunction with pattern recognition receptor activation, resulted in a synergistic enhancement of proinflammatory cytokine production and release (55). That study provides a possible explanation for the enhanced levels of inflammatory molecules we found in pIgR KO animals, as we (Fig. 2A) and others (37, 38) have detected elevated serum IgA levels compared to WT controls. We posit that enhanced serum IgA levels, coupled with altered bacterial communities and a lack of sIg sensing, may result in an improperly controlled immune response to enteric commensals in pIgR KO mice.
Interestingly, the observed increase in inflammatory immune responses was not uniform, i.e., elevated iNOS and IFN-γ levels in conventional pIgR KO mice, reduced IFN-β, and no change in transcript levels of other pro- and anti-inflammatory mediators (Fig. 7). The reason for this remains unclear. One potential explanation may come from the observed increases in the number of APC subsets (DC, macrophages, and B cells) in the PP of pIgR KO mice compared to WT controls. These cell types have both FcαR1 and pathogen recognition receptors and are capable of proinflammatory cytokine secretion (55–59). Another potential source for the enhanced proinflammatory cytokine levels in pIgR KO mice may be intraepithelial lymphocytes (IELs), which are enhanced in the small intestines of these mice (60, 61) and which upon activation exhibited more abundant IFN-γ secretion than WT IELs (60). Of note, activation of IELs via the T cell receptor in vivo resulted in significant reduction of MNV titers in the small intestines and MLNs of WT mice (62). That study may point to a potential cell type that could mediate the antiviral activity in pIgR KO mice, but further studies are required to investigate this hypothesis.
Furthermore, our study represents another example of enteric bacteria indirectly influencing MNV pathogenesis. Norovirus infections are modulated directly and indirectly by the presence of commensal bacteria (63). In the case of MNV, infection of antibiotic-treated mice results in decreased MNV loads in the ileum (21, 64), and commensal bacteria promote MNV persistence in the intestine via modulation of type III interferon responses (65). Our microbiome analysis revealed that different bacterial communities could support optimal MNV infection in WT mice (Fig. 8). However, MNV infection was inhibited in pIgR KO mice when the intestines were inflamed and a different microbial community was present. Further studies will be needed to determine whether and how specific bacterial species promote or inhibit enteric viral infections.
Our study further highlights the importance of the intestinal cytokine milieu for optimal MNV pathogenesis. MNV infection was reduced only in conventional pIgR KO mice, which exhibited elevated levels of intestinal iNOS and IFN-γ, but not in their germfree counterparts, where cytokine levels were similar to those in WT mice. IFN-γ has well-recognized antinorovirus activity (29–32). Our study provides direct evidence for IFN-γ-mediated inhibition of MNV replication in vivo. Under both conditions of IFN-γ modulation, neutralization and addition, MNV-1 titers were altered in some regions of the intestine (Fig. 11). Interestingly, viral loads in intestinal segments were differentially affected by each treatment, which may be due to regionalization of the intestinal immune system (66). These data suggest that the immune response generated toward the enteric microbiota in the absence of sIg was capable of inhibiting norovirus replication. Reovirus titers in the lower gastrointestinal tract were also reduced in pIgR KO mice compared to WT controls, further emphasizing the role of pIgR in facilitation of enteric viral infection.
Collectively, our findings point to a model in which the immunomodulatory functions of sIg aid in norovirus and reovirus replication. Further work is required to test whether interaction of enteric viruses with sIg complexes could skew the inflammatory viral immune response, promoting tolerogenic responses and intestinal homeostasis. Such a response may account for the weak inflammatory response and poor lasting immunity observed in norovirus infections (67). Norovirus binding to multivalent sIg complexes may also promote coinfections of APCs, which in turn can increase viral fitness via complementation or recombination (68–70). However, additional work is needed to test these hypotheses.
Taken together, our study demonstrates that, unlike other enteric pathogens, sIg do not protect from MNV or reovirus infection, but instead, the presence of pIgR and sIg promotes infection. Thus, both molecules are host factors that can have either anti- or promicrobial functions, depending on the pathogen. In addition, these findings represent another example in which the microbiota modulates enteric virus pathogenesis. Lastly, our data further highlight the fact that MNV and reovirus, as enteric pathogens, have optimized infection under conditions of intestinal homeostasis. When these conditions are perturbed, such as by the absence of pIgR, sIg, or enteric bacteria, virus infection is compromised. In the future, it will be interesting to test whether this holds true for other enteric viral infections.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed under specific-pathogen-free (SPF) and MNV-free conditions. A breeder pair of pIgR knockout mice on a C57BL/6 background (B6.129P2-Pigrtm1Fejo/Mmmh; stock number 030988-MU) was obtained from T. Stappenbeck and H. W. Virgin (Washington University, St. Louis, MO). All the mice were bred and housed under SPF and MNV- and reovirus-free conditions. Age- and sex-matched experimental mice between 6 and 16 weeks of age were used. Germfree C57/BL6 and pIgR KO mice were derived and housed in University of Michigan (UM) and Emory University germfree and gnotobiotic facilities. Their germfree status was verified regularly through fecal analysis and necropsy. The mice used in the study were seronegative for anti-MNV antibodies by ELISA, as described previously (20). Animal studies were performed in accordance with local and federal guidelines. The protocol was approved by the University of Michigan Committee on Use and Care of Animals (UCUCA number PRO00006658).
Viral stocks.
The plaque-purified MNV-1.CW3 (GV/MNV1/2002/USA) virus stock (here referred to as MNV-1) was generated as previously described (71). A neutral-red-labeled MNV-1 stock (MNV-1-NR) (3.8 × 106 PFU/ml) was generated from MNV-1, passage 6, as previously described (36). MNV-1-NR was handled in a darkened room using a red photolight (Premier Omni) and stored in a light-safe box at −80°C. Reovirus recombinant T1L stocks were generated with reovirus cDNAs in baby hamster kidney T7 cells (a kind gift from Terence Dermody, University of Pittsburgh) (72, 73), followed by plaque purification and passage in L929 cells (74).
Animal infections.
Mice were infected via o.g. with 3.8 × 105 PFU MNV-1-NR at 100 μl/mouse in a red light only room. For neutralizing antibody treatment of pIgR KO mice, 500 µg anti-IFN-γ antibody (rat IgG, clone XMG1.2; eBioscience) or isotype control (rat IgG, clone HRPN; InVivoMab) was administered intraperitoneally 18 h before MNV-1-NR infection. For IFN-γ treatment of B6 mice, 104 units IFN-γ (PeproTech) or an equivalent weight of BSA fraction V (Roche) was diluted in phosphate-buffered saline (PBS), filtered with a 0.22-μm filter (Fisher Scientific), and administered intraperitoneally 18 h before MNV-1-NR infection. Mice were infected via o.g. with 3.8 × 105 PFU MNV-1-NR at 100 μl/mouse in a red light only room. Tissues were harvested at 9, 18, or 48 hpi, as previously described (17), with the following modifications: 2 cm of tissue was collected in preweighed tubes containing 1.0-mm-diameter zirconia/silica beads (BioSpec), flash-frozen in an ethanol-dry ice bath, weighed, and stored at −80°C. The mice were infected via o.g. with 2 × 106 PFU of reovirus T1L. Tissues were harvested at 24 hpi and processed as described for MNV, with the modification that 1 cm of tissue was collected.
Plaque assay.
Homogenized samples were exposed to white light for 30 min to inactivate the input virus. Light exposure reduced MNV-1-NR titers by 3 log units. The plaque assay was performed as previously described (75). The reovirus titers of homogenized samples were determined by plaque assay as described previously, using L929 cells (74). Samples without detectable replicated virus were assigned 10 PFU/ml as the lowest detectable unit. Data were normalized to the tissue weight and expressed as PFU per gram of tissue. The sensitivity threshold was determined for each organ by averaging the numbers of PFU per gram of tissue of all samples without detectable viral titers.
RNA isolation.
Total RNA was extracted from tissues using TRIzol reagent (ThermoFisher Scientific) following the manufacturer’s guidelines. Contaminating genomic DNA was removed by treating samples with a Turbo DNA-free DNase kit (ThermoFisher Scientific). Total RNA was quantified using a spectrophotometer (NanoDrop) and stored at −80°C.
Quantitative real-time PCR.
To measure host cell transcripts, cDNA was generated with 100 μg of total RNA using iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad; number 1708841) in a thermocycler (Eppendorf Mastercycler Epgradient PCR machine) and stored at −20°C. cDNA was analyzed for levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (76), GP2 (75), Spi-B (76), IFN-γ (77), IFN-β (77), TNF-α (77), iNOS (78), ISG15 (79), TGF-β (80), IL-10 (81), and IFN-λ (79) in a Bio-Rad CFX96 real-time system qPCR machine using Sso Advanced Universal SYBR Green Supermix (Bio-Rad). Gene expression was normalized to the transcript levels of the endogenous host gene gapdh, and the fold change was calculated relative to WT controls using the ΔΔCT method (82). Quantification of MNV-1 genome equivalents in host tissue was performed as previously described (71).
MNV-1 neutralization assay.
MNV-1 (2.8 × 107 PFU) was preincubated with 0.25 mg/ml recombinant sIgA (83) or 0.25 mg/ml bovine serum albumin fraction V (Roche) diluted in 1× PBS for 1 h in a 37°C water bath, and neutralization was assessed via plaque assay.
Ligated intestinal loops.
Mice were anesthetized and placed on a 37°C heating pad. An ∼2-cm section of the ileum was tied gently on either side of a PP using silk surgical-suture thread. Approximately 0.1 ml MNV-1 (6.7 × 107 PFU/ml) was injected as the loop was closed. The PP was excised 25 min later. Mucus was removed by a 10-min incubation with 5 mM dithiothreitol (Sigma-Aldrich) in 1× PBS (Gibco) in a 37°C water bath. The FAE was removed by shaking the PP at 250 rpm (MaxQ 600; Thermo Scientific) for 20 min in 1× PBS containing 5 mM EDTA (Fluka Analytical) and washed twice with 1× PBS. Disassociation of the FAE from the lamina propria was confirmed via flow cytometry using Ep-Cam (diluted 1:50; clone G8.8; phycoerythrin [PE]; BioLegend). Ep-Cam-negative cells (i.e., PP lamina propria) were washed twice with 1× PBS by gently inverting the tubes. The cells were placed in 500 μl RNALater (Sigma) and stored at −80°C.
For sIgA-MNV complex formation, MNV-1 (2.8 × 107 PFU) was preincubated with 0.25 mg/ml recombinant sIgA (83) or 0.25 mg/ml bovine serum albumin fraction V (Roche) diluted in 1× PBS for 1 h at 37°C before being added to the loop and treated as described above.
Tissue digestion/flow cytometry.
PP and small-intestine lamina propria were dissected from naive pIgR KO and WT mice and digested as previously described (84). Cells (2 × 106) were then used for flow cytometric analysis. Viable cells were identified (Invitrogen LIVE/DEAD fixable Aqua dead dell stain kit) and then stained for the following surface markers: CD45 (diluted 1:200; clone 30-F11; AF700; BioLegend), I-A/I-E (diluted 1:50; clone M5/114.15.2; PE/Dazzle 594; BioLegend), CD19 (diluted 1:50; clone 6D5; peridinin chlorophyll protein [PerCP]/Cy5.5; BioLegend), CD11b (diluted 1:50; clone M1/70; allophycocyanin [APC]/Cy7; BioLegend), CD64 (diluted 1:50; clone X54-5/7.1; fluorescein isothiocyanate [FITC]; BioLegend), and CD11c (diluted 1:50; clone N418; PE/Cy7; BioLegend). All the antibodies were diluted in fluorescence-activated cell sorter (FACS) buffer, 5% fetal bovine serum (HyClone), and 0.1% sodium azide (Sigma) in 1× PBS for 30 min on ice. After surface staining, the cells were fixed for 10 min on ice using Cytofix/Cytoperm (BD Biosciences) and washed, and the fluorescence was analyzed with a BD LSR Fortessa. Data were analyzed using FlowJo software (BD Biosciences) with the following gating strategy: live cells, CD45- and I-A/I-E (major histocompatibility complex class II [MHC-II])-positive APCs were characterized into B cells (CD19+) and mononuclear phagocytes (CD19− CD11b+), which were further delineated into macrophages (CD64+ CD11c−) and DC (CD64− CD11c+).
Expression and purification of proteins.
MNV-1 capsid protein protruding (P) and shell (S) domains were bacterially expressed, and recombinant proteins were purified as previously described (41). Recombinant rotavirus-specific mouse sIgA, IgA dimer, or secretory component was expressed and purified as described previously (40).
ELISAs.
The sIgA ELISA was performed as described previously (16) with the following modifications. Microtiter plates (Immulon II HB flat-bottom ELISA plate; Thermo Labsystems) were coated with 0.1 mg/ml recombinant mouse sIgA, IgA dimer, or secretory component in coating buffer overnight at 4°C.The plates were washed and blocked overnight at 4°C. Recombinant P and S domains were then added (0.1 mg/ml) for 1 h at 37°C. Bound recombinant MNV-1 capsid protein domains were detected with a rabbit polyclonal anti-MNV-1 virus-like particle (VLP) antibody (diluted 1:5,000) (20), followed by a peroxidase-conjugated goat anti-rabbit secondary antibody (IgG; diluted 1:1,000; Jackson Laboratories), and absorbance was recorded at 405 nm. Mouse IgA was analyzed in feces and serum using a mouse-specific IgA kit (Bethyl Laboratories, Montgomery, TX) following the manufacturer’s protocol. For interferon-gamma ELISA, ileal tissues were weighed, homogenized, and diluted 1:2 in PBS, and ELISA was performed by the UM Cancer Center ELISA Core following the manufacturer's protocol.
16S rRNA gene sequencing.
The Microbial Systems Molecular Biology Laboratory at the University of Michigan provided 16S rRNA gene sequencing and analysis of samples. DNA was isolated with a MagAttract PowerMicrobiome DNA/RNA kit (Qiagen) using an epMotion 5075 liquid-handling system. The V4 region of the 16S rRNA gene was amplified and sequenced as described previously, except using a MiSeq Reagent Nano kit v2 (500 cycles) (85). The 16S rRNA gene sequence data were processed and analyzed using the software package mothur (v.1.40.2) and the most recent MiSeq standard operating procedure (SOP) (86, 87). After sequence processing and alignment to the SILVA reference alignment (release 128) (88), sequences were binned into operational taxonomic units (OTUs) based on 97% sequence similarity using the OptiClust method (89). A total of 2,212 sequences per sample were subsampled, and samples with fewer than 2,212 sequences were not included in the analysis. By calculating θYC distances (a metric that takes the relative abundances of both shared and nonshared OTUs into account) (90) between communities and using AMOVA (91), it was possible to determine if there were statistically significant differences between the microbiota of different groups. Principal-coordinate analysis (PCoA) was used to visualize the θYC distances between samples by plotting in R (version 3.5.1). We also investigated the taxonomic compositions of the bacterial communities by classifying sequences within mothur using a modified version of the Ribosomal Database Project (RDP) training set (version 16) (92, 93).
Statistical analysis.
Unless otherwise stated, data were analyzed using Mann-Whitney U tests with GraphPad Prism software version 7 (GraphPad Software, La Jolla, CA).
Accession number(s).
16S sequence primary data were deposited to BioProject and are available under accession number PRJNA489290.
ACKNOWLEDGMENTS
We are indebted to Thaddeus Stappenbeck (Washington University, St. Louis, MO) for the generous gift of pIgR KO breeders, to Kathryn Eaton and the germfree mouse core at the University of Michigan, and to Gary Huffnagel (University of Michigan, Ann Arbor, MI) for the anti-IFN-γ serum.
This work was funded in part by NIH R21 AI103961 to C.E.W. and the University of Michigan Host-Microbiome Initiative. H.T. was supported in part by a University of Michigan Rackham Merit fellowship and NIH training grants T32AI007413 and T32DK094775. B.A.M. was supported by funds from Children’s Healthcare of Atlanta and Emory Children’s Pediatric Center.
We declare no conflict of interest.
REFERENCES
- 1.Kaetzel CS. 2014. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol Lett 162:10–21. doi: 10.1016/j.imlet.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corthesy B. 2009. Secretory immunoglobulin A: well beyond immune exclusion at mucosal surfaces. Immunopharmacol Immunotoxicol 31:174–179. doi: 10.1080/08923970802438441. [DOI] [PubMed] [Google Scholar]
- 3.Gorrell RJ, Wijburg OL, Pedersen JS, Walduck AK, Kwok T, Strugnell RA, Robins-Browne RM. 2013. Contribution of secretory antibodies to intestinal mucosal immunity against Helicobacter pylori. Infect Immun 81:3880–3893. doi: 10.1128/IAI.01424-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davids BJ, Palm JE, Housley MP, Smith JR, Andersen YS, Martin MG, Hendrickson BA, Johansen FE, Svard SG, Gillin FD, Eckmann L. 2006. Polymeric immunoglobulin receptor in intestinal immune defense against the lumen-dwelling protozoan parasite Giardia. J Immunol 177:6281–6290. doi: 10.4049/jimmunol.177.9.6281. [DOI] [PubMed] [Google Scholar]
- 5.Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA. 2006. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med 203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston PF, Gerding DN, Knight KL. 2014. Protection from Clostridium difficile infection in CD4 T cell- and polymeric immunoglobulin receptor-deficient mice. Infect Immun 82:522–531. doi: 10.1128/IAI.01273-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corthesy B. 2007. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol 178:27–32. doi: 10.4049/jimmunol.178.1.27. [DOI] [PubMed] [Google Scholar]
- 8.Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. 2002. Selective adherence of IgA to murine Peyer's patch M cells: evidence for a novel IgA receptor. J Immunol 169:1844–1851. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 9.Rey J, Garin N, Spertini F, Corthesy B. 2004. Targeting of secretory IgA to Peyer's patch dendritic and T cells after transport by intestinal M cells. J Immunol 172:3026–3033. doi: 10.4049/jimmunol.172.5.3026. [DOI] [PubMed] [Google Scholar]
- 10.Mantis NJ, Rol N, Corthesy B. 2011. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartsch SM, Lopman BA, Ozawa S, Hall AJ, Lee BY. 2016. Global economic burden of norovirus gastroenteritis. PLoS One 11:e0151219. doi: 10.1371/journal.pone.0151219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall AJ, Lopman BA, Payne DC, Patel MM, Gastanaduy PA, Vinje J, Parashar UD. 2013. Norovirus disease in the United States. Emerg Infect Dis 19:1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karst SM, Wobus CE, Goodfellow IG, Green KY, Virgin HW. 2014. Advances in norovirus biology. Cell Host Microbe 15:668–680. doi: 10.1016/j.chom.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wobus CE, Thackray LB, Virgin HWT. 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol 80:5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karst SM, Wobus CE. 2015. Viruses in rodent colonies: lessons learned from murine noroviruses. Annu Rev Virol 2:525–548. doi: 10.1146/annurev-virology-100114-055204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karst SM, Wobus CE, Lay M, Davidson J, Virgin IVHW. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Hernandez MB, Liu T, Payne HC, Stencel-Baerenwald JE, Ikizler M, Yagita H, Dermody TS, Williams IR, Wobus CE. 2014. Efficient norovirus and reovirus replication in the mouse intestine requires microfold (M) cells. J Virol 88:6934–6943. doi: 10.1128/JVI.00204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mumphrey SM, Changotra H, Moore TN, Heimann-Nichols ER, Wobus CE, Reilly MJ, Moghadamfalahi M, Shukla D, Karst SM. 2007. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J Virol 81:3251–3263. doi: 10.1128/JVI.02096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grau KR, Roth AN, Zhu S, Hernandez A, Colliou N, DiVita BB, Philip DT, Riffe C, Giasson B, Wallet SM, Mohamadzadeh M, Karst SM. 2017. The major targets of acute norovirus infection are immune cells in the gut-associated lymphoid tissue. Nat Microbiol 2:1586–1591. doi: 10.1038/s41564-017-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. 2004. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol 2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinje J, Tibbetts SA, Wallet SM, Karst SM. 2014. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Hernandez MB, Liu T, Blanco LP, Auble H, Payne HC, Wobus CE. 2013. Murine norovirus transcytosis across an in vitro polarized murine intestinal epithelial monolayer is mediated by M-like cells. J Virol 87:12685–12693. doi: 10.1128/JVI.02378-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karst SM, Wobus CE. 2015. A working model of how noroviruses infect the intestine. PLoS Pathog 11:e1004626. doi: 10.1371/journal.ppat.1004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf JL, Rubin DH, Finberg R, Kauffman RS, Sharpe AH, Trier JS, Fields BN. 1981. Intestinal M cells: a pathway for entry of reovirus into the host. Science 212:471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]
- 25.Jones MK, Grau KR, Costantini V, Kolawole AO, de Graaf M, Freiden P, Graves CL, Koopmans M, Wallet SM, Tibbetts SA, Schultz-Cherry S, Wobus CE, Vinje J, Karst SM. 2015. Human norovirus culture in B cells. Nat Protoc 10:1939–1947. doi: 10.1038/nprot.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldridge MT, Turula H, Wobus CE. 2016. Norovirus regulation by host and microbe. Trends Mol Med 22:1047–1059. doi: 10.1016/j.molmed.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. 2011. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz-Cornil I, Benureau Y, Greenberg H, Hendrickson BA, Cohen J. 2002. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins. J Virol 76:8110–8117. doi: 10.1128/JVI.76.16.8110-8117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Changotra H, Jia Y, Moore TN, Liu G, Kahan SM, Sosnovtsev SV, Karst SM. 2009. Type I and type II interferons inhibit the translation of murine norovirus proteins. J Virol 83:5683–5692. doi: 10.1128/JVI.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maloney NS, Thackray LB, Goel G, Hwang S, Duan E, Vachharajani P, Xavier R, Virgin HW. 2012. Essential cell-autonomous role for interferon (IFN) regulatory factor 1 in IFN-gamma-mediated inhibition of norovirus replication in macrophages. J Virol 86:12655–12664. doi: 10.1128/JVI.01564-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang S, Maloney NS, Bruinsma MW, Goel G, Duan E, Zhang L, Shrestha B, Diamond MS, Dani A, Sosnovtsev SV, Green KY, Lopez-Otin C, Xavier RJ, Thackray LB, Virgin HW. 2012. Nondegradative role of Atg5-Atg12/Atg16L1 autophagy protein complex in antiviral activity of interferon gamma. Cell Host Microbe 11:397–409. doi: 10.1016/j.chom.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biering SB, Choi J, Halstrom RA, Brown HM, Beatty WL, Lee S, McCune BT, Dominici E, Williams LE, Orchard RC, Wilen CB, Yamamoto M, Coers J, Taylor GA, Hwang S. 2017. Viral replication complexes are targeted by LC3-guided interferon-inducible GTPases. Cell Host Microbe 22:74–85 e7. doi: 10.1016/j.chom.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubin BY, Bartal AH, Anderson SL, Millet SK, Hirshaut Y, Feit C. 1983. The anticellular and protein-inducing activities of human gamma interferon preparations are mediated by the interferon. J Immunol 130:1019–1020. [PubMed] [Google Scholar]
- 34.Turula H, Bragazzi Cunha J, Mainou BA, Ramakrishnan SK, Wilke CA, Gonzalez-Hernandez MB, Pry A, Fava J, Bassis CM, Edelman J, Shah YM, Corthesy B, Moore BB, Wobus CE. 2018. Natural secretory immunoglobulins promote enteric viral infections. bioRxiv doi: 10.1101/253286. [DOI] [PMC free article] [PubMed]
- 35.Uren TK, Johansen FE, Wijburg OL, Koentgen F, Brandtzaeg P, Strugnell RA. 2003. Role of the polymeric Ig receptor in mucosal B cell homeostasis. J Immunol 170:2531–2539. doi: 10.4049/jimmunol.170.5.2531. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Hernandez MB, Perry JW, Wobus CE. 2013. Neutral red assay for murine norovirus replication and detection in a mouse. Bio Protoc 3:e415. doi: 10.21769/BioProtoc.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, Betsholtz C, Brandtzaeg P. 1999. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med 190:915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimada S, Kawaguchi-Miyashita M, Kushiro A, Sato T, Nanno M, Sako T, Matsuoka Y, Sudo K, Tagawa Y, Iwakura Y, Ohwaki M. 1999. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J Immunol 163:5367–5373. [PubMed] [Google Scholar]
- 39.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. 2013. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol 6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crottet P, Cottet S, Corthesy B. 1999. Expression, purification and biochemical characterization of recombinant murine secretory component: a novel tool in mucosal immunology. Biochem J 341:299–306. doi: 10.1042/bj3410299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taube S, Rubin JR, Katpally U, Smith TJ, Kendall A, Stuckey JA, Wobus CE. 2010. High-resolution x-ray structure and functional analysis of the murine norovirus 1 capsid protein protruding domain. J Virol 84:5695–5705. doi: 10.1128/JVI.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolawole AO, Smith HQ, Svoboda SA, Lewis MS, Sherman MB, Lynch GC, Pettitt BM, Smith TJ, Wobus CE. 2017. Norovirus escape from broadly neutralizing antibodies is limited to allostery-like mechanisms. mSphere 2:e00334-17. doi: 10.1128/mSphere.00334-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerutti A. 2008. The regulation of IgA class switching. Nat Rev Immunol 8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karupiah G, Xie QW, Buller RM, Nathan C, Duarte C, MacMicking JD. 1993. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science 261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 45.Saura M, Zaragoza C, McMillan A, Quick RA, Hohenadl C, Lowenstein JM, Lowenstein CJ. 1999. An antiviral mechanism of nitric oxide: inhibition of a viral protease. Immunity 10:21–28. doi: 10.1016/S1074-7613(00)80003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persichini T, Colasanti M, Fraziano M, Colizzi V, Medana C, Polticelli F, Venturini G, Ascenzi P. 1999. Nitric oxide inhibits the HIV-1 reverse transcriptase activity. Biochem Biophys Res Commun 258:624–627. doi: 10.1006/bbrc.1999.0581. [DOI] [PubMed] [Google Scholar]
- 47.Samuel CE. 2001. Antiviral actions of interferons. Clin Microbiol Rev 14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sait L, Galic M, Strugnell RA, Janssen PH. 2003. Secretory antibodies do not affect the composition of the bacterial microbiota in the terminal ileum of 10-week-old mice. Appl Environ Microbiol 69:2100–2109. doi: 10.1128/AEM.69.4.2100-2109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reikvam DH, Derrien M, Islam R, Erofeev A, Grcic V, Sandvik A, Gaustad P, Meza-Zepeda LA, Jahnsen FL, Smidt H, Johansen FE. 2012. Epithelial-microbial crosstalk in polymeric Ig receptor deficient mice. Eur J Immunol 42:2959–2970. doi: 10.1002/eji.201242543. [DOI] [PubMed] [Google Scholar]
- 50.Kaetzel CS. 2005. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev 206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 51.Corthesy B, Benureau Y, Perrier C, Fourgeux C, Parez N, Greenberg H, Schwartz-Cornil I. 2006. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J Virol 80:10692–10699. doi: 10.1128/JVI.00927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karlsson MR, Johansen FE, Kahu H, Macpherson A, Brandtzaeg P. 2010. Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy 65:561–570. doi: 10.1111/j.1398-9995.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 53.Richmond BW, Brucker RM, Han W, Du RH, Zhang Y, Cheng DS, Gleaves L, Abdolrasulnia R, Polosukhina D, Clark PE, Bordenstein SR, Blackwell TS, Polosukhin VV. 2016. Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat Commun 7:e11240. doi: 10.1038/ncomms11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murthy AK, Dubose CN, Banas JA, Coalson JJ, Arulanandam BP. 2006. Contribution of polymeric immunoglobulin receptor to regulation of intestinal inflammation in dextran sulfate sodium-induced colitis. J Gastroenterol Hepatol 21:1372–1380. doi: 10.1111/j.1440-1746.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- 55.Hansen IS, Hoepel W, Zaat SAJ, Baeten DLP, den Dunnen J. 2017. Serum IgA immune complexes promote proinflammatory cytokine production by human macrophages, monocytes, and Kupffer cells through FcalphaRI-TLR cross-talk. J Immunol 199:4124–4131. doi: 10.4049/jimmunol.1700883. [DOI] [PubMed] [Google Scholar]
- 56.Sakamoto N, Shibuya K, Shimizu Y, Yotsumoto K, Miyabayashi T, Sakano S, Tsuji T, Nakayama E, Nakauchi H, Shibuya A. 2001. A novel Fc receptor for IgA and IgM is expressed on both hematopoietic and non-hematopoietic tissues. Eur J Immunol 31:1310–1316. doi:. [DOI] [PubMed] [Google Scholar]
- 57.Otten MA, Groenveld I, van de Winkel JG, van Egmond M. 2006. Inefficient antigen presentation via the IgA Fc receptor (FcalphaRI) on dendritic cells. Immunobiology 211:503–510. doi: 10.1016/j.imbio.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 58.Munder M, Mallo M, Eichmann K, Modolell M. 1998. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med 187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cherdantseva LA, Potapova OV, Sharkova TV, Belyaeva YY, Shkurupiy VA. 2014. Association of Helicobacter pylori and iNOS production by macrophages and lymphocytes in the gastric mucosa in chronic gastritis. J Immunol Res 2014:762514. doi: 10.1155/2014/762514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kato-Nagaoka N, Shimada S, Yamakawa Y, Tsujibe S, Naito T, Setoyama H, Watanabe Y, Shida K, Matsumoto S, Nanno M. 2015. Enhanced differentiation of intraepithelial lymphocytes in the intestine of polymeric immunoglobulin receptor-deficient mice. Immunology 146:59–69. doi: 10.1111/imm.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamazaki K, Shimada S, Kato-Nagaoka N, Soga H, Itoh T, Nanno M. 2005. Accumulation of intestinal intraepithelial lymphocytes in association with lack of polymeric immunoglobulin receptor. Eur J Immunol 35:1211–1219. doi: 10.1002/eji.200425627. [DOI] [PubMed] [Google Scholar]
- 62.Swamy M, Abeler-Dorner L, Chettle J, Mahlakoiv T, Goubau D, Chakravarty P, Ramsay G, Reis e Sousa C, Staeheli P, Blacklaws BA, Heeney JL, Hayday AC. 2015. Intestinal intraepithelial lymphocyte activation promotes innate antiviral resistance. Nat Commun 6:7090. doi: 10.1038/ncomms8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karst SM. 2016. The influence of commensal bacteria on infection with enteric viruses. Nat Rev Microbiol 14:197–204. doi: 10.1038/nrmicro.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kernbauer E, Ding Y, Cadwell K. 2014. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516:94–98. doi: 10.1038/nature13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW. 2015. Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science 347:266–269. doi: 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mowat AM, Agace WW. 2014. Regional specialization within the intestinal immune system. Nat Rev Immunol 14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 67.Newman KL, Leon JS. 2015. Norovirus immunology: of mice and mechanisms. Eur J Immunol 45:2742–2757. doi: 10.1002/eji.201545512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erickson AK, Jesudhasan PR, Mayer MJ, Narbad A, Winter SE, Pfeiffer JK. 2017. Bacteria facilitate enteric virus co-infection of mammalian cells and promote genetic recombination. Cell Host Microbe doi: 10.1016/j.chom.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allison R, Thompson C, Ahlquist P. 1990. Regeneration of a functional RNA virus genome by recombination between deletion mutants and requirement for cowpea chlorotic mottle virus 3a and coat genes for systemic infection. Proc Natl Acad Sci U S A 87:1820–1824. doi: 10.1073/pnas.87.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen YH, Du W, Hagemeijer MC, Takvorian PM, Pau C, Cali A, Brantner CA, Stempinski ES, Connelly PS, Ma HC, Jiang P, Wimmer E, Altan-Bonnet G, Altan-Bonnet N. 2015. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell 160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taube S, Perry JW, McGreevy E, Yetming K, Perkins C, Henderson K, Wobus CE. 2012. Murine noroviruses bind glycolipid and glycoprotein attachment receptors in a strain-dependent manner. J Virol 86:5584–5593. doi: 10.1128/JVI.06854-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kobayashi T, Antar AA, Boehme KW, Danthi P, Eby EA, Guglielmi KM, Holm GH, Johnson EM, Maginnis MS, Naik S, Skelton WB, Wetzel JD, Wilson GJ, Chappell JD, Dermody TS. 2007. A plasmid-based reverse genetics system for animal double-stranded RNA viruses. Cell Host Microbe 1:147–157. doi: 10.1016/j.chom.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boehme KW, Ikizler M, Kobayashi T, Dermody TS. 2011. Reverse genetics for mammalian reovirus. Methods 55:109–113. doi: 10.1016/j.ymeth.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Virgin HWT, Bassel-Duby R, Fields BN, Tyler KL. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol 62:4594–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalez-Hernandez MB, Bragazzi Cunha J, Wobus CE. 2012. Plaque assay for murine norovirus. J Vis Exp doi: 10.3791/4297:e4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wood MB, Rios D, Williams IR. 2016. TNF-alpha augments RANKL-dependent intestinal M cell differentiation in enteroid cultures. Am J Physiol Cell Physiol 311:C498–C507. doi: 10.1152/ajpcell.00108.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohanty SK, Shivakumar P, Sabla G, Bezerra JA. 2006. Loss of interleukin-12 modifies the pro-inflammatory response but does not prevent duct obstruction in experimental biliary atresia. BMC Gastroenterol 6:14. doi: 10.1186/1471-230X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stoolman JS, Vannella KM, Coomes SM, Wilke CA, Sisson TH, Toews GB, Moore BB. 2011. Latent infection by gammaherpesvirus stimulates profibrotic mediator release from multiple cell types. Am J Physiol Lung Cell Mol Physiol 300:L274–L285. doi: 10.1152/ajplung.00028.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopusna K, Benkoczka T, Luptak J, Matuskova R, Lukacikova L, Oveckova I, Rezuchova I. 2016. Murine gammaherpesvirus targets type I IFN receptor but not type III IFN receptor early in infection. Cytokine 83:158–170. doi: 10.1016/j.cyto.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 80.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. 2006. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A 103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Terashima A, Watarai H, Inoue S, Sekine E, Nakagawa R, Hase K, Iwamura C, Nakajima H, Nakayama T, Taniguchi M. 2008. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med 205:2727–2733. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Higgins PD, Johnson LA, Sauder K, Moons D, Blanco L, Taube S, Wobus CE. 2011. Transient or persistent norovirus infection does not alter the pathology of Salmonella typhimurium induced intestinal inflammation and fibrosis in mice. Comp Immunol Microbiol Infect Dis 34:247–257. doi: 10.1016/j.cimid.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crottet P, Peitsch MC, Servis C, Corthesy B. 1999. Covalent homodimers of murine secretory component induced by epitope substitution unravel the capacity of the polymeric Ig receptor to dimerize noncovalently in the absence of IgA ligand. J Biol Chem 274:31445–31455. doi: 10.1074/jbc.274.44.31445. [DOI] [PubMed] [Google Scholar]
- 84.Geem D, Medina-Contreras O, Kim W, Huang CS, Denning TL. 2012. Isolation and characterization of dendritic cells and macrophages from the mouse intestine. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seekatz AM, Theriot CM, Molloy CT, Wozniak KL, Bergin IL, Young VB. 2015. Fecal microbiota transplantation eliminates Clostridium difficile in a murine model of relapsing disease. Infect Immun 83:3838–3846. doi: 10.1128/IAI.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schloss PD. 2009. A high-throughput DNA sequence aligner for microbial ecology studies. PLoS One 4:e8230. doi: 10.1371/journal.pone.0008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Westcott SL, Schloss PD. 2017. OptiClust, an improved method for assigning amplicon-based sequence data to operational taxonomic units. mSphere 2:e00073-17. doi: 10.1128/mSphereDirect.00073-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yue JC, Clayton MK. 2005. A similarity measure based on species proportions. Commun Stat Theory Methods 34:2123–2131. doi: 10.1080/STA-200066418. [DOI] [Google Scholar]
- 91.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x. [DOI] [Google Scholar]
- 92.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM. 2014. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]