Significance
Global demand for species used in traditional medicine is increasing among wealthy urban consumers. This growing trade provides livelihood opportunities for harvesters, but also risks causing resource overexploitation. A dearth of reliable data hinders assessments of whether these species are declining, and why. We investigate these issues for Himalayan caterpillar fungus—one of the world’s most expensive medicinal species—by integrating local harvesters’ knowledge of production trends with ecological modeling. We find that harvesters increasingly attribute declining production to overexploitation, while models indicate that climate warming is also contributing to this decline. Our results underscore the “double whammy” threatening highly valuable species, and demonstrate the complementarity of different knowledge systems for assessing the sustainability of the medicinal resource trade.
Keywords: local ecological knowledge, niche commodities, species distribution modeling, Ophiocordyceps sinensis, Tibetan Plateau
Abstract
Demand for traditional medicine ingredients is causing species declines globally. Due to this trade, Himalayan caterpillar fungus (Ophiocordyceps sinensis) has become one of the world’s most valuable biological commodities, providing a crucial source of income for hundreds of thousands of collectors. However, the resulting harvesting boom has generated widespread concern over the sustainability of its collection. We investigate whether caterpillar fungus production is decreasing—and if so, why—across its entire range. To overcome the limitations of sparse quantitative data, we use a multiple evidence base approach that makes use of complementarities between local knowledge and ecological modeling. We find that, according to collectors across four countries, caterpillar fungus production has decreased due to habitat degradation, climate change, and especially overexploitation. Our statistical models corroborate that climate change is contributing to this decline. They indicate that caterpillar fungus is more productive under colder conditions, growing in close proximity to areas likely to have permafrost. With significant warming already underway throughout much of its range, we conclude that caterpillar fungus populations have been negatively affected by a combination of overexploitation and climate change. Our results underscore that harvesting is not the sole threat to economically valuable species, and that a collapse of the caterpillar fungus system under ongoing warming and high collection pressure would have serious implications throughout the Himalayan region.
Ecosystem changes and associated biodiversity losses have disproportionately negative consequences for marginalized peoples who rely on ecosystems for their livelihoods and well-being (1, 2). Species that have become niche commodities (3), such as those viewed as luxury goods or used in traditional medicine, are especially threatened (4–7). In particular, the rapidly expanding exploitation of resources to meet Asia’s demand for biological niche commodities has been likened to a “disease epidemic” responsible for causing species declines globally (8), in part through rampant poaching of charismatic and endangered species, such as black rhino, pangolin, and jaguar (4, 9, 10). Although not all wild-harvested, commercially traded species are overexploited (11), other anthropogenic factors may synergistically contribute to species’ population declines (12). For example, climate warming often exacerbates the effects of habitat loss and harvesting (13–15). Predicting the effects of these interacting factors remains challenging, with the principal threats of harvesting pressure, habitat degradation, and climate change each playing out at different scales (12, 13).
A more complete understanding of how these factors are affecting species and ecosystems can be achieved by bringing together insights from different knowledge systems in a “multiple evidence base” approach (16). While Western science is well-suited to quantifying phenomena across large spatial extents, particularly through the use of remotely sensed data, local ecological knowledge (LEK) qualitatively integrates observations across many variables and longer timescales than are typically possible for scientific field studies (17, 18). Because people who depend on an ecosystem for their livelihoods over many years tend to be well-attuned to its dynamics, their LEK can give insight into complex issues, such as climate change and species declines (19–21). This bridging of knowledge systems is particularly useful in situations of high uncertainty, as when resources are affected by multiple factors with potentially diverse outcomes across locations and scales (16, 22, 23).
The Case of Caterpillar Fungus
Ecosystems of the Himalayan region are especially vulnerable to climate change (24). Their high biodiversity supports a large commercial trade in wild-harvested medicinal species (25, 26). Among these, caterpillar fungus (Ophiocordyceps sinensis, formerly called Cordyceps sinensis) (Fig. 1), an entomopathogenic fungus that parasitizes over 50 species of Thitarodes (Hepialidae) moth larvae in the Himalayan region (27), has become one of the most valuable biological niche commodities in the world (3). Although it has been used in Tibetan and Chinese medicine for centuries, this fungus has experienced a surge in demand in recent decades, primarily among urban Chinese consumers (28, 29). Used to treat a growing list of ailments, including cancer (30), its price increased by 20% per year on average from 1997–2012 (31). By 2017, high-quality pieces sold for more than 140,000 USD per kilogram in Beijing, more than three times the price of gold (caterpillar fungus prices from Tong Ren Tang Pharmaceutical Co. in Beijing on May 20, 2017; gold prices for May 2017 from https://goldprice.org/). For Bhutan, despite producing far less caterpillar fungus than its larger neighbors, the hundreds of kilograms that it sells each year make this niche commodity one of its most valuable exports (32). Consequently, caterpillar fungus sale generates a substantial proportion of regional gross domestic product (29), and harvesting has become a primary source of income for hundreds of thousands of collectors (33–35).
Fig. 1.
Caterpillar fungus emerges after snowmelt (A) and is harvested by collectors (B). They dig up the caterpillar with the fungus still attached (C); cleaned and reproductively immature individuals (Left) are more valuable than uncleaned and sporulating individuals (Right).
The intensity of the harvesting boom in environmentally and economically marginal areas has led to widespread concern over the ecological and social sustainability of caterpillar fungus collection (36). Each May to June, collectors dig infected caterpillars out of the ground with the fungus still attached, typically before it begins producing spores, because reproductively mature specimens are considered undesirable by consumers (Fig. 1) (29, 37). This harvesting strategy is expected to have a negative effect on long-term population dynamics. Assessing trends in caterpillar fungus remains difficult due to spatially and temporally patchy records of collection amounts, with even less data on collection effort (31). Although case studies assert that production is decreasing because of high collection pressure and ecosystem degradation (37, 38), such claims are location-specific and often rely on inconclusive evidence or an incomplete understanding of the fungus’ life cycle (39). Climate change has also been invoked as causing changes in caterpillar fungus abundance (40). While correlative modeling studies found associations between climatic conditions and caterpillar fungus habitat, they predicted both net range expansions and contractions under future climate scenarios (41, 42). Little attention has been paid to how recent climate change may have already affected caterpillar fungus (40), despite a growing understanding of how it has altered Himalayan ecosystems, causing vegetation changes and permafrost thaw in the regions inhabited by the fungus (24, 43–45).
Here, we examine how multiple anthropogenic factors are affecting a niche commodity throughout its range. We investigate whether caterpillar fungus production is decreasing regionally, and if so, why. We follow a multiple evidence base approach that makes use of data from field interviews in Tibet, a systematic literature review, official collection records, and regional climate data. We weigh the evidence for whether overexploitation and climate change are affecting its production by synthesizing findings from the LEK of those involved in its collection and trade, assessing the environmental conditions that determine both the spatial extent of its range and its production within a location, and analyzing how the climatic factors important to its growth have changed in recent decades. We demonstrate how complementary insights from LEK and Western science can improve understanding of valuable biological resources, illuminate multiple threats to their sustainability, and identify hotspots of vulnerability.
Results
LEK of Production Trends.
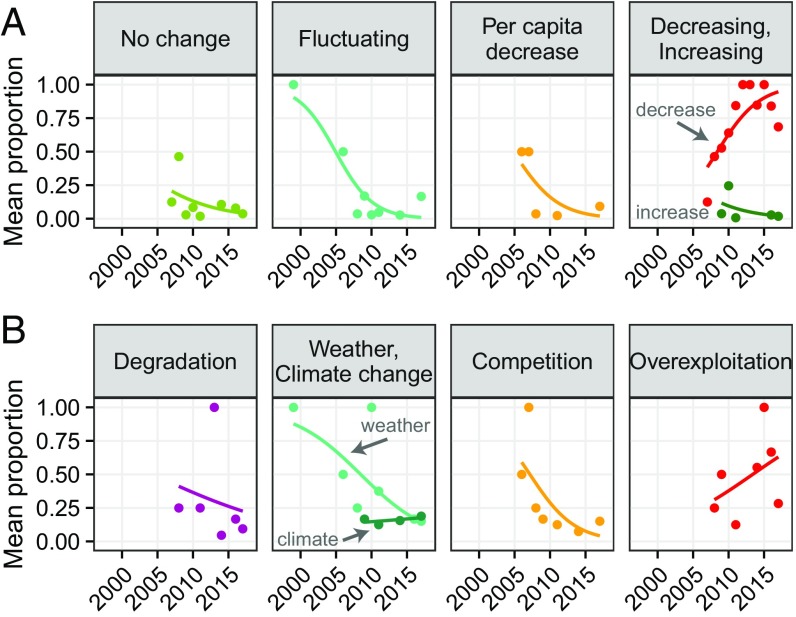
Interviews with caterpillar fungus collectors and traders throughout the Himalayan region about their LEK reveal that in the past decade, the majority of respondents observed that caterpillar fungus production has decreased (n > 816) (Fig. 2A and SI Appendix, Supplementary Methods). The proportion of “decreasing” trends reported has risen over time, while the proportion of responses that production is “not changing,” “fluctuating” interannually, or that “per capita” collection has decreased (due to higher competition among collectors; i.e., a detectable change in harvest but not production amounts) have each declined (Fig. 3). Within a single region of Dolpa, Nepal, studies bookending nearly two decades also evince this shift from little cause for concern, with traditional doctors reporting its “common” occurrence in the 1990s, to nearly unanimous reports of “decreasing” production in recent years (38, 46).
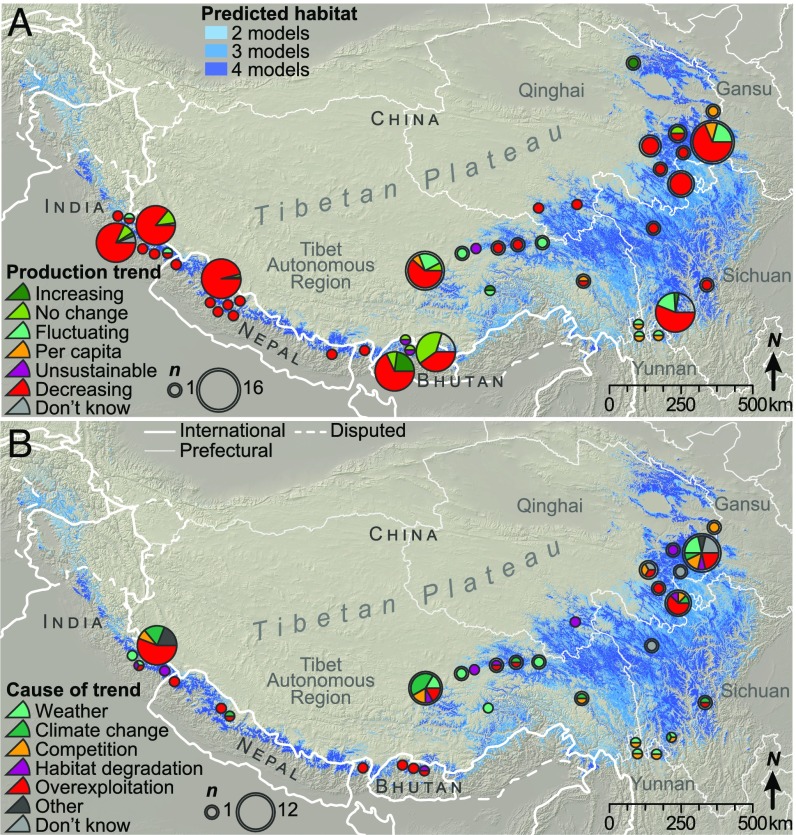
Fig. 2.
LEK of changes in caterpillar fungus (A) production and their (B) causes are shown throughout its geographic range. Symbols for data from our interviews have a ring around them and are scaled by the number of respondents (n). Symbols for data from the literature are displayed as large for quantitative data and small for qualitative. Areas shaded blue indicate where two, three, or four of the species distribution models predicted suitable habitat. Only LEK data collected from 2008–2017 are shown. Change data (A) are from 24 studies (including this one) and >816 interviewees; cause data (B) are from 17 studies and >413 interviewees. (See SI Appendix, Supplementary Methods for additional details on sample size.)
Fig. 3.
LEK of caterpillar fungus production through time. Points represent the mean proportion of responses for each type of change observation (A) and their perceived causes (B), calculated across all studies conducted in a given year, and weighted by the level of confidence in each study. Regression lines show temporal trends for each response but should not be used for inference. Annually aggregated responses about changes are based on data from 29 studies (including this one) and >817 interviewees (A); responses about causes are from 22 studies and >413 interviewees (B). (See SI Appendix, Supplementary Methods for additional details on sample size.)
LEK of the causal factors affecting production is more heterogeneous than LEK of the production trends themselves, but nearly all responses (n > 413) (SI Appendix, Supplementary Methods) can be categorized into several factors related to land use and climate (Fig. 2B). Of the one-third of our interviewees who reported multiple factors as contributing to the changes they observed, the majority cited a combination of climatic and land use causes. Results from the literature review and our interviews reveal that the proportion of responses attributing changes in the amount of locally available caterpillar fungus to competition among collectors or to transient, interannual weather conditions has declined over time, while attribution of decreasing production to overexploitation has risen (Fig. 3).
In the past decade, overexploitation was the dominant cause reported in Bhutan, Nepal, and India. Within China, overexploitation was also the dominant cause reported in Qinghai, while climate change was dominant in Tibet and Sichuan, followed closely by interannual weather fluctuations in Tibet and overexploitation in Sichuan. Habitat degradation was noted, although less frequently, in all countries except Nepal, mostly in the form soil degradation. This was related to impacts of caterpillar fungus harvesting in about half of the cases. The highest proportion of climate change responses were from our interviewees in central Tibet, who mentioned warming and drying trends as problematic for caterpillar fungus production. Collectors in Dolpa, Nepal who cited climate change reported decreasing winter snow (66%), earlier spring snowmelt (52%), and warming (38%) as causes of decreasing production (40). LEK of how weather affects interannual variations in production focused nearly unanimously on high snowfall and rainfall in the preceding winter and spring as important factors promoting higher yields (e.g., refs. 47 and 48).
Environmental Predictors of Suitable Habitat.
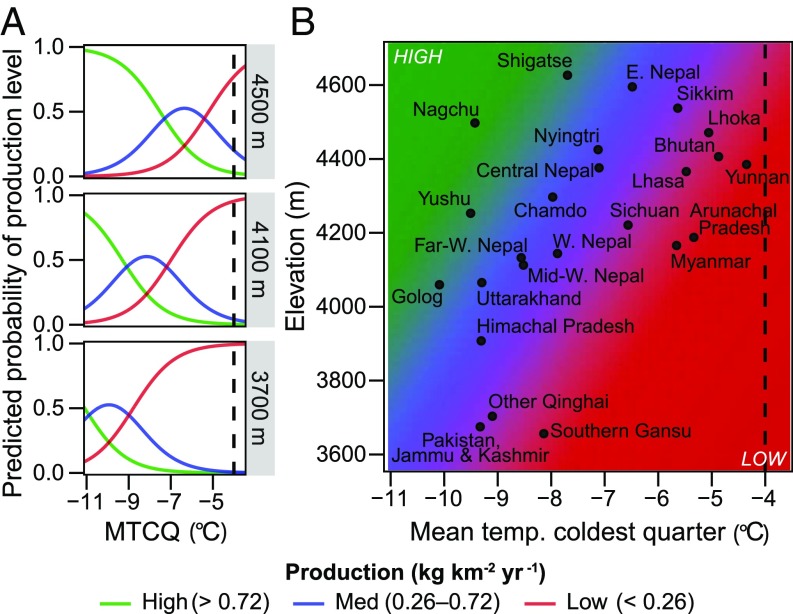
To increase our understanding of the environmental conditions associated with suitable caterpillar fungus habitat throughout the Himalayan region, we created four species-distribution models. Each of the models showed strong predictive performance according to a suite of evaluation statistics (SI Appendix, Table S1). They captured nuances related to terrain characteristics and microclimates, showing high suitability along alpine valley walls and ridgelines while avoiding nearby glaciers and floodplains (SI Appendix, Fig. S1). The models consistently ranked precipitation of the wettest or coldest quarter (i.e., monsoon rainfall or winter precipitation), nonvegetated cover, and elevation as the most important predictors of habitat, followed by mean temperature of the coldest quarter (i.e., winter temperature) (SI Appendix, Fig. S2). Precipitation seasonality and isothermality were least influential overall and were removed by two of the four models. Several of the variables’ response curves exhibit well-defined ranges, indicating that, with all other variables held at their means, habitat suitability is highest at elevations between 3,200 and 4,900 m, with ∼15–55% nonvegetated cover, and with mean winter temperatures between −15 °C and −5 °C, declining steeply at temperatures warmer than −4 °C (SI Appendix, Fig. S2). Suitability also increases with higher winter precipitation.
Despite the prevalence of permafrost in these cold and mountainous environments (43), 94.5% of our 400 caterpillar fungus occurrences and 93.9% of the habitat predicted by the species distribution models fell outside of areas likely to have permafrost. In regions where permafrost is present (excluding three provinces on the eastern Tibetan Plateau with little permafrost), caterpillar fungus occurs at its margin, with a median distance of 3.2 km between caterpillar fungus occurrence locations and their nearest likely permafrost areas (mean = 5.6 km, SD = 7.4 km, n = 331).
Environmental Predictors of Production Level.
We then examined how the key environmental predictors of caterpillar fungus habitat relate to its production level at a subregional scale. We grouped administrative units into low (<0.26), medium (0.26–0.72), and high (>0.72) production levels (kg km−2 y−1) based on mean collection amounts across multiple years, where available, divided by the predicted habitat area in each administrative unit. Ordered logistic regressions to determine the environmental conditions associated with these production levels generated three models with similar levels of support (SI Appendix, Table S2). Mean temperature of the coldest quarter (hereafter “winter temperature”) was the most significant effect in each model (P < 0.001). Other significant predictors included elevation and precipitation of the coldest quarter (hereafter “winter precipitation”; P < 0.05). The models indicated that within suitable habitat areas, the production level of caterpillar fungus increases at higher elevations and where there are drier and colder winters. Inclusion of winter precipitation led to higher production estimates in the drier, central Tibetan Plateau. The most parsimonious model showed that the odds of caterpillar fungus production moving to a higher production level increase by 57%, on average, with every 100-m increase in elevation and decrease by 64% with every 1° increase in mean winter temperature, with the other variables held constant (Fig. 4A).
Fig. 4.
Caterpillar fungus production increases at higher elevations and colder winter temperatures. The probability of high, medium, and low production levels along a winter temperature gradient is shown at three representative elevations (A). Points depict where regions fall along the production gradient, based on their habitat areas’ mean environmental conditions (B). Production probabilities are the result of ordered logistic regressions using collection data from 33 administrative units. Dashed vertical lines mark −4 °C, the approximate temperature threshold beyond which the species distribution models predicted little suitable habitat. “Other Qinghai” and “Southern Gansu” include habitat south of the Yellow River. Northern areas of Qinghai and Gansu have mean temperatures below −12 °C during the coldest quarter. MTCQ, mean temperature of the coldest quarter. (See SI Appendix, Fig. S1A for region locations.)
On average, core production areas on the Tibetan Plateau (Nagchu, Yushul, and Golog) were predicted to be among the highest-producing due to their cold winter temperatures and relatively high elevations (Fig. 4B). Low-producing areas closest to the −4 °C winter temperature threshold, above which the species distribution models predicted little suitable habitat, include Arunachal Pradesh, Yunnan, and Bhutan. However, there is more environmental heterogeneity within these regions than can be captured by their means: for example, along steep elevation gradients in the southern Himalaya (SI Appendix, Fig. S3A).
Recent Climate Trends.
Climate variables found to be important for caterpillar fungus habitat and production have changed significantly within collectors’ lifetimes (SI Appendix, Fig. S3 C, E, and G). Linear regressions of climatic conditions each year from 1979–2013 revealed that winter temperatures have warmed significantly during this period across nearly all caterpillar fungus habitats in India, Nepal, and Bhutan, as well as around Lhasa and Qinghai Lake on the Tibetan Plateau. Winter warming has been strongest in Bhutan, with mean winter temperatures increasing by 3.5–4 °C across most of its predicted habitat (+1.1 °C per decade, on average), pushing all but the highest-elevation areas close to the −4 °C winter temperature threshold identified by the species distribution models. Changes in precipitation have been spatially heterogeneous, with large areas of caterpillar fungus habitat experiencing significant reductions in summer precipitation, while others have experienced significant increases. Winter precipitation has also increased significantly over much of the Tibetan Plateau. However, parts of Nepal and Bhutan have undergone significant winter drying trends, with the largest proportional decreases in winter precipitation concentrated in Bhutan’s most productive caterpillar fungus administrative unit, Wangduephodrang.
Discussion
Our multiple evidence base approach using data spanning nearly two decades and four countries revealed that caterpillar fungus production is declining throughout much of its range. While collectors increasingly attribute the decline in caterpillar fungus to overharvesting, habitat and production modeling suggest that climate change is also likely playing a role. Fewer collectors attributed population declines to climate change or habitat degradation. However, climate change may be amplifying the negative effects of harvesting and playing a role in the ecosystem degradation observed by some collectors, thus interacting with collection pressure to affect the status of this resource (12, 13). Collectors’ reports of fluctuating production among years, which they linked to interannual variations in weather, are also consistent with the oscillating production predicted by a theoretical host–pathogen model of caterpillar fungus population dynamics (49). That data-free, mathematical model indicates that sustainable harvesting of caterpillar fungus should be possible, but that constant, high collection pressure could reduce the system’s ability to recover from perturbations, reduce yields, and eventually drive the fungus to extinction (49). Our finding of a temporal shift from a larger proportion of collectors reporting production fluctuations toward a larger proportion reporting a persistent decline in production lends empirical support for these theory-based model predictions (49) playing out under unsustainable harvesting pressures.
LEK of production trends may be affected by various sources of perception bias. Two of the trends most pertinent to the caterpillar fungus system are shifting baselines (through which knowledge of past conditions is lost, thereby masking evidence of longer-term trends) and interference among harvesters (through which increased competition exaggerates perceptions of population declines) (50). We mitigated the influence of the former by integrating LEK collected at different points in time, and the latter by distinguishing per capita decreases in collection from declining production wherever possible. Our interviewees primarily referred to their harvesting rate, or “catch per unit effort,” as a reliable indicator of overall caterpillar fungus population trends (17). They were attuned to the growing number of collectors vying to find it and took this into account when ascribing caterpillar fungus’ decreasing availability either to true declines in production or to fewer pieces per capita due to higher competition. The shift toward attributing decreasing harvest amounts to overexploitation rather than merely to competition reveals the growing perception among collectors that their harvest rates are now negatively affecting production of the fungus.
LEK of environmental factors referred most frequently to the effects of winter precipitation on caterpillar fungus yields. The apparent discrepancy between the regression result that caterpillar fungus production is higher in areas that receive less winter precipitation, and the LEK result that production is higher in years with more winter precipitation (47, 48) may reflect their different scales of analysis (16, 23). While the regressions elucidate a cross-regional pattern of production level based on average environmental conditions—indicating that drier, colder, and higher-elevation habitats are generally more productive—LEK primarily reflects local-scale, temporal patterns of how interannual variations in winter weather affect relative production amounts within a specific location. Significant winter drying trends in Nepal and Bhutan are therefore unlikely to have created a wholesale shift toward more productive habitats, but they may be shifting the timing of snowmelt and reducing moisture availability needed to promote fungal fruiting (51), thus potentially contributing to the decreasing production observed locally. However, the factors controlling fungal development are complex, and likely also include the timing of winter precipitation and its interaction with temperature (51).
Winter temperature emerged as a key control on caterpillar fungus, limiting not only its distribution, as reported previously in Nepal (41), but also its production level. The species distribution models detected upper and lower winter temperature thresholds beyond which they predicted little suitable habitat. Within this temperature range, the logistic regressions found that production levels decrease as winter temperatures increase. Significant winter warming to date throughout habitat areas in India, Nepal, and especially Bhutan is therefore likely to have caused production declines in those countries, possibly pushing some areas beyond the viable temperature range. This interpretation is further supported by genetic evidence that natural selection on the fungus toward higher elevations and concomitant adaptations for cold tolerance have resulted in a loss of ability to cope with more heterogeneous environments (52), thus potentially lowering its fitness under warmer temperatures. In contrast, significant winter warming in the exceptionally cold northeastern Tibetan Plateau may have shifted new areas above the low-temperature threshold, thereby promoting the increased production observed there. In several instances, LEK explicitly linked climate warming to decreasing caterpillar fungus production (40). However, causally linking gradual temperature changes to the complex caterpillar fungus life cycle may be beyond the scope of most collectors’ LEK, thus underscoring the complementary role of Western science in identifying additional causes of the population trends that collectors observe (16, 17).
Our results also identify a universal upper constraint on caterpillar fungus habitat that aligns closely with a single landscape characteristic—presence of permafrost—which appears to influence the distribution of the species. A previous suggestion of its distribution around a climatically defined potential treeline is also temperature-based (53). However, our finding of its balancing act between preferring higher elevations and winter temperatures well below 0 °C, and its need to avoid areas likely to have permafrost, suggest the likely importance of key microclimatic controls, such as seasonal timing of soil thaw, temperature, and moisture availability.
Although the spatial resolution of our models cannot fully capture the microclimates inhabited by the fungi and their caterpillar hosts, these organisms are nonetheless likely to be affected by the substantial climatic and cryospheric changes occurring in this region. Since the 1980s, the number of days with frozen soil on the Tibetan Plateau decreased by 15–22 d per decade within caterpillar fungus’ elevation range (54), the permafrost limit has moved upward in elevation by 25–100 m (55), and the number of frost days is projected to continue decreasing throughout the Himalayan region as it continues to warm (56). Climate warming can also cause more oscillations above and below freezing, which is damaging even for freeze-tolerant species (57). An increase in the frequency of freeze–thaw cycling or a shift in the timing of soil thaw with climate warming could thus reduce caterpillar fungus fitness and potentially begin to introduce phenological mismatches between the timing of caterpillar, fungus, and vegetation activity (58, 59). In theory, caterpillar fungus should be able to shift its range upward in response to changing climate (41, 42), but it is unknown whether all of the necessary elements of the ecosystem—including edaphic conditions, vegetation, caterpillar species, and the fungus itself—will respond in the same ways and at the same pace to climate change, nor how these climate responses may interact with habitat degradation or other factors (60, 61). Evidence that vegetation on the Tibetan Plateau did not shift upward in response to climate warming from 2000 to 2014 (62) suggests that a lag in creation of a new suitable habitat may prohibit caterpillar fungus from simply “moving up the mountain” as climatic conditions change.
A collapse in caterpillar fungus populations would have both ecological and social ramifications. Although the potential cascade of ecological effects are not yet well understood, due to the diversity of plants eaten by the caterpillar hosts and the dozens of species of caterpillars parasitized by this fungus (27), the economic impacts on many harvesting-dependent communities would undoubtedly be profound (33–35).
Conclusions
The sustainability of the caterpillar fungus ecology and economy is threatened by the combined pressures of climate change and overexploitation for traditional medicine. Based on the geographic distribution of the caterpillar fungus, we infer that it requires winter temperatures well below 0 °C, while avoiding permanently frozen soil. Such conditions are typically present at the margin of permafrost areas. Our study revealed that, outside of the eastern Tibetan Plateau, nearly all recorded caterpillar fungus locations are within a narrow distance of areas likely underlain by permafrost. Given that winter temperatures have warmed significantly from 1979 to 2013 across much of its range, and especially in Bhutan, its populations are likely to have been negatively affected. However, an upward shift in the elevation of permafrost and thus of potential caterpillar fungus habitat in response to changing climate will not necessarily be accompanied by simultaneous shifts in the caterpillar species, the fungus, and the vegetation and edaphic conditions on which they depend.
Combining Western scientific methods with local environmental knowledge proved crucial for understanding recent trends in caterpillar fungus in the Himalayas. While environmental modeling highlighted the influence of climate change on the decline in caterpillar fungus populations, local environmental knowledge emphasized the role of local harvesting pressure. Harvesters gave insight into population trends where quantitative data were largely lacking or underestimated due to hidden flows, such as those associated with poaching. Our integration of LEK from multiple locations provided a synoptic view and more complete understanding across the species’ range and through time. Meanwhile, Western scientific methods allowed relationships to be quantitatively analyzed at a regional scale to understand the role of global change factors, which are not always well-captured by site-specific knowledge.
While ongoing climate change is likely to reduce caterpillar fungus production throughout its range, implementation of more sustainable management policies intended to counteract overexploitation will also reduce the amount available for legal collection. Both of these pathways toward reduced caterpillar fungus availability will limit people’s access to this highly valuable resource, thus underscoring the need for alternative livelihood options in the communities that depend on this niche commodity.
Methods
Our study spans the full habitat range of caterpillar fungus, including the Tibetan Plateau in China and Himalayan regions of Bhutan, India, and Nepal.
In June 2017, we conducted semistructured interviews with caterpillar fungus collectors, local traders, and community leaders in Sichuan, Qinghai, and the Tibet Autonomous Region (Tibet), China (with approval from Stanford University’s Internal Review Board, protocol 41289). After obtaining oral informed consent, we asked interviewees open-ended questions about their perceptions of whether caterpillar fungus production amounts were changing, and why. If they reported decreasing amounts, we asked them to specify whether these were true decreases in production or only decreases in the amount collected per capita. Forty-nine people responded to our questions about changes, and 39 also responded to questions about causes.
Our systematic literature review covered 396 publications on the caterpillar fungus social and ecological systems, published between 1723 and 2017, in five languages. From these, we identified 73 publications that reported trends in its production, which we narrowed to 29 for further analysis, based on criteria to ensure that the studies met our quality standards and captured LEK rather than author assertions. Ten of these studies reported quantitative LEK responses from a total of 768 interviewees, 6 studies reported qualitative LEK responses from up to an additional 3,919 interviewees, and 13 studies gave qualitative data from an undefined number of interviewees. For these studies and our interviews, we coded LEK into seven categories of production trends and seven categories of causal factors (SI Appendix, Tables S3 and S4). We assigned confidence weightings to all LEK data.
We used the Software for Assisted Habitat Modeling to map the current suitable habitat for caterpillar fungus (63). For presence points, we used a unique dataset assembled from locations reported in the literature and supplemented by GPS locations collected during field work in June 2017 (n = 400) (SI Appendix, Fig. S1A) (data available at https://purl.stanford.edu/ww909xk7776). Environmental predictor variables included elevation, MODIS Vegetation Continuous Fields, and CHELSA (Climatologies at High Resolution for the Earth’s Land Surface Areas) bioclimatic variables (SI Appendix, Figs. S4 and S5 and Table S5). We used the point data and environmental variables to develop predictions from four species distribution models. We used areas where all four models’ habitat predictions overlapped as a conservative input for further analysis.
To triangulate how the habitat area relates to permafrost—another aspect of Himalayan ecosystems affected by climate and topography—we overlaid the caterpillar fungus presence points and predicted habitat area on a global permafrost map (64). We then calculated the proportion of points and habitat area in regions likely to have permafrost, as well as the average distance from presence points to likely permafrost areas. Because the permafrost map itself is a modeled output derived from similar inputs to those used in our species distribution models, we did not include it in our habitat models to avoid confounding the caterpillar fungus–climate relationships.
We estimated caterpillar fungus production (kg km−2 y−1) by administrative unit for each state (India), district (Nepal), dzongkhag (Bhutan), and prefecture or county (China) for which collection data were available from the literature or official reports (SI Appendix, Table S6). Although collection amounts are subject to underreporting and cannot perfectly approximate the amount of caterpillar fungus growing each year, we assume that collection quantities are a reasonable proxy for true production quantities, given that harvesters in many areas search exhaustively for it. To determine how environmental factors relate to production amounts, we performed ordered logistic regressions with production bin as the outcome, and with the variables selected by a majority of the species distribution models as the predictors.
We assessed changes in climatic conditions that control caterpillar fungus distribution and production. For this, we calculated annual bioclimatic variables equivalent to those used in the species distribution models and logistic regression analyses, using monthly CHELSA precipitation and temperature data from 1979–2013 (65). We then conducted pixel-wise linear regressions for each bioclimatic variable through time and detected significant trends at P < 0.05. Additional details are provided in the SI Appendix, Supplementary Methods.
Supplementary Material
Acknowledgments
We thank P. Tsering, T. Dolma, T. Dorje, W. Xu, L. Tso, and Kabzung for their assistance with interviews; X. Yang, Olo, and Y. Nyima for translations; E. Lyon for data processing; A. Tredennick for statistical guidance; and S. Schachat for feedback on the manuscript.
Footnotes
The authors declare no conflict of interest.
Data deposition: All caterpillar fungus location coordinates and their sources have been deposited in the Stanford Digital Repository, https://purl.stanford.edu/ww909xk7776.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811591115/-/DCSupplemental.
References
- 1.Hooper DU, et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486:105–108. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 2.Díaz S, Fargione J, Chapin FS, 3rd, Tilman D. Biodiversity loss threatens human well-being. PLoS Biol. 2006;4:e277. doi: 10.1371/journal.pbio.0040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.le Polain de Waroux Y, Lambin EF. Niche commodities and rural poverty alleviation: Contextualizing the contribution of Argan oil to rural livelihoods in Morocco. Ann Assoc Am Geogr. 2013;103:589–607. [Google Scholar]
- 4.Graham-Rowe D. Biodiversity: Endangered and in demand. Nature. 2011;480:S101–S103. doi: 10.1038/480S101a. [DOI] [PubMed] [Google Scholar]
- 5.Burgess MG, et al. Range contraction enables harvesting to extinction. Proc Natl Acad Sci USA. 2017;114:3945–3950. doi: 10.1073/pnas.1607551114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courchamp F, et al. Rarity value and species extinction: The anthropogenic Allee effect. PLoS Biol. 2006;4:e415. doi: 10.1371/journal.pbio.0040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Law W, Salick J. Human-induced dwarfing of Himalayan snow lotus, Saussurea laniceps (Asteraceae) Proc Natl Acad Sci USA. 2005;102:10218–10220. doi: 10.1073/pnas.0502931102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksson H, et al. Contagious exploitation of marine resources. Front Ecol Environ. 2015;13:435–440. [Google Scholar]
- 9.Fraser B. China’s lust for jaguar fangs imperils big cats. Nature. 2018;555:13–14. doi: 10.1038/d41586-018-02314-5. [DOI] [PubMed] [Google Scholar]
- 10.Cheng W, Xing S, Bonebrake TC. Recent pangolin seizures in China reveal priority areas for intervention. Conserv Lett. 2017;10:757–764. [Google Scholar]
- 11.Larsen HO, Olsen CS. Unsustainable collection and unfair trade? Uncovering and assessing assumptions regarding central Himalayan medicinal plant conservation. Biodivers Conserv. 2007;16:1679–1697. [Google Scholar]
- 12.Brook BW, Sodhi NS, Bradshaw CJ. Synergies among extinction drivers under global change. Trends Ecol Evol. 2008;23:453–460. doi: 10.1016/j.tree.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Mora C, Metzger R, Rollo A, Myers RA. Experimental simulations about the effects of overexploitation and habitat fragmentation on populations facing environmental warming. Proc Biol Sci. 2007;274:1023–1028. doi: 10.1098/rspb.2006.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segan DB, Murray KA, Watson JE. A global assessment of current and future biodiversity vulnerability to habitat loss–climate change interactions. Glob Ecol Conserv. 2016;5:12–21. [Google Scholar]
- 15.Carroll C. Interacting effects of climate change, landscape conversion, and harvest on carnivore populations at the range margin: Marten and lynx in the northern Appalachians. Conserv Biol. 2007;21:1092–1104. doi: 10.1111/j.1523-1739.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 16.Tengö M, Brondizio ES, Elmqvist T, Malmer P, Spierenburg M. Connecting diverse knowledge systems for enhanced ecosystem governance: The multiple evidence base approach. Ambio. 2014;43:579–591. doi: 10.1007/s13280-014-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moller H, Berkes F, Lyver POB, Kislalioglu M. Combining science and traditional ecological knowledge: Monitoring populations for co-management. Ecol Soc. 2004;9:2. [Google Scholar]
- 18.Berkes F. Sacred Ecology. 4th Ed Routledge; New York: 2018. [Google Scholar]
- 19.Klein JA, et al. Unexpected climate impacts on the Tibetan Plateau: Local and scientific knowledge in findings of delayed summer. Glob Environ Change. 2014;28:141–152. [Google Scholar]
- 20.Turvey ST, et al. Can local ecological knowledge be used to assess status and extinction drivers in a threatened freshwater cetacean? Biol Conserv. 2013;157:352–360. [Google Scholar]
- 21.Reyes-García V, et al. Local indicators of climate change: The potential contribution of local knowledge to climate research. Wiley Interdiscip Rev Clim Change. 2016;7:109–124. doi: 10.1002/wcc.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JT, et al. Weaving indigenous and sustainability sciences to diversify our methods. Sustain Sci. 2016;11:1–11. [Google Scholar]
- 23.Gagnon C, Berteaux D. Integrating traditional ecological knowledge and ecological science: A question of scale. Ecol Soc. 2009;14:19. [Google Scholar]
- 24.Xu J, et al. The melting Himalayas: Cascading effects of climate change on water, biodiversity, and livelihoods. Conserv Biol. 2009;23:520–530. doi: 10.1111/j.1523-1739.2009.01237.x. [DOI] [PubMed] [Google Scholar]
- 25.Olsen CS, Larsen HO. Alpine medicinal plant trade and Himalayan mountain livelihood strategies. Geogr J. 2003;169:243–254. [Google Scholar]
- 26.Salick J, Fang Z, Byg A. Eastern Himalayan alpine plant ecology, Tibetan ethnobotany, and climate change. Glob Environ Change. 2009;19:147–155. [Google Scholar]
- 27.Wang XL, Yao YJ. Host insect species of Ophiocordyceps sinensis: A review. ZooKeys. 2011:43–59. doi: 10.3897/zookeys.127.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh ET, Lama KT. Following the caterpillar fungus: Nature, commodity chains, and the place of Tibet in China’s uneven geographies. Soc Cult Geogr. 2013;14:318–340. [Google Scholar]
- 29.Winkler D. Yartsa gunbu (Cordyceps sinensis) and the fungal commodification of Tibet’s rural economy. Econ Bot. 2008;62:291–305. [Google Scholar]
- 30.Xu J, et al. The mechanisms of pharmacological activities of Ophiocordyceps sinensis fungi. Phytother Res. 2016;30:1572–1583. doi: 10.1002/ptr.5673. [DOI] [PubMed] [Google Scholar]
- 31.Winkler D. Caterpillar fungus production and sustainability on the Tibetan Plateau and in the Himalayas. In: Gruschke A, Breuer I, editors. Tibetan Pastoralists and Development: Negotiating the Future of Grassland Livelihoods. Reichert; Wiesbaden, Germany: 2017. pp. 45–62. [Google Scholar]
- 32.Royal Monetary Authority 2017. Royal Monetary Authority of Bhutan, Annual Report 2016/17 (Royal Monetary Authority, Thimphu, Bhutan.
- 33.Childs G, Choedup N. Indigenous management strategies and socioeconomic impacts of Yartsa gunbu (Ophiocordyceps sinensis) harvesting in Nubri and Tsum, Nepal. Himalaya J Assoc Nepal Himalayan Stud. 2014;34:7. [Google Scholar]
- 34.Shrestha UB, Dhital KR, Gautam AP. Economic dependence of mountain communities on Chinese caterpillar fungus Ophiocordyceps sinensis (yarsagumba): A case from western Nepal. Oryx. 2017:1–9. [Google Scholar]
- 35.Pouliot M, Pyakurel D, Smith-Hall C. High altitude organic gold: The production network for Ophiocordyceps sinensis from far-western Nepal. J Ethnopharmacol. 2018;218:59–68. doi: 10.1016/j.jep.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 36.Cannon PF, et al. Steps towards sustainable harvest of Ophiocordyceps sinensis in Bhutan. Biodivers Conserv. 2009;18:2263–2281. [Google Scholar]
- 37.Negi CS, Joshi P, Bohra S. Rapid vulnerability assessment of Yartsa gunbu (Ophiocordyceps sinensis [Berk.] G.H. Sung et al) in Pithoragarh district, Uttarakhand state, India. Mt Res Dev. 2015;35:382–391. [Google Scholar]
- 38.Shrestha UB, Bawa KS. Trade, harvest, and conservation of caterpillar fungus (Ophiocordyceps sinensis) in the Himalayas. Biol Conserv. 2013;159:514–520. [Google Scholar]
- 39.Stewart MO, Bushley KE, Yongping Y. Regarding the social-ecological dimensions of caterpillar fungus (Ophiocordyceps sinensis) in the Himalayas—Reply to Shrestha and Bawa. Biol Conserv. 2013;167:446–447. [Google Scholar]
- 40.Shrestha UB, Bawa KS. Harvesters’ perceptions of population status and conservation of Chinese caterpillar fungus in the Dolpa region of Nepal. Reg Environ Change. 2015;15:1731–1741. [Google Scholar]
- 41.Shrestha UB, Bawa KS. Impact of climate change on potential distribution of Chinese caterpillar fungus (Ophiocordyceps sinensis) in Nepal Himalaya. PLoS One. 2014;9:e106405. doi: 10.1371/journal.pone.0106405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan Y, et al. Range shifts in response to climate change of Ophiocordyceps sinensis, a fungus endemic to the Tibetan Plateau. Biol Conserv. 2017;206:143–150. [Google Scholar]
- 43.Gruber S, et al. Inferring permafrost and permafrost thaw in the mountains of the Hindu Kush Himalaya region. Cryosphere. 2017;11:81–99. [Google Scholar]
- 44.Yang M, Nelson FE, Shiklomanov NI, Guo D, Wan G. Permafrost degradation and its environmental effects on the Tibetan Plateau: A review of recent research. Earth Sci Rev. 2010;103:31–44. [Google Scholar]
- 45.Shrestha UB, Gautam S, Bawa KS. Widespread climate change in the Himalayas and associated changes in local ecosystems. PLoS One. 2012;7:e36741. doi: 10.1371/journal.pone.0036741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lama YC, Ghimire SK, Aumeeruddy-Thomas Y. Medicinal Plants of Dolpo: Amchis’ Knowledge and Conservation. WWF-Nepal Program; Kathmandu, Nepal: 2001. [Google Scholar]
- 47.Weckerle CS, Yang Y, Huber FK, Li Q. People, money, and protected areas: The collection of the caterpillar mushroom Ophiocordyceps sinensis in the Baima Xueshan Nature Reserve, Southwest China. Biodivers Conserv. 2010;19:2685–2698. [Google Scholar]
- 48.Kuniyal CP, Sundriyal RC. Conservation salvage of Cordyceps sinensis collection in the Himalayan mountains is neglected. Ecosyst Serv. 2013;3:e40–e43. [Google Scholar]
- 49.Woodall H, Bullock JM, White SM. Modelling the harvest of an insect pathogen. Ecol Modell. 2014;287:16–26. [Google Scholar]
- 50.Daw TM. Shifting baselines and memory illusions: What should we worry about when inferring trends from resource user interviews? Anim Conserv. 2010;13:534–535. [Google Scholar]
- 51.Boddy L, et al. Climate variation effects on fungal fruiting. Fungal Ecol. 2014;10:20–33. [Google Scholar]
- 52.Xia E-H, et al. The caterpillar fungus, Ophiocordyceps sinensis, genome provides insights into highland adaptation of fungal pathogenicity. Sci Rep. 2017;7:1806. doi: 10.1038/s41598-017-01869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winkler D. Yartsa gunbu–Cordyceps sinensis: Economy, ecology & ethno-mycology of a fungus endemic to the Tibetan Plateau. Memorie della Societá Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano. 2005;33:69–85. [Google Scholar]
- 54.Li X, Jin R, Pan X, Zhang T, Guo J. Changes in the near-surface soil freeze–thaw cycle on the Qinghai-Tibetan Plateau. Int J Appl Earth Obs Geoinf. 2012;17:33–42. [Google Scholar]
- 55.Cheng G, Wu T. Responses of permafrost to climate change and their environmental significance, Qinghai‐Tibet Plateau. J Geophys Res Earth Surf. 2007;112:F02S03. [Google Scholar]
- 56.Panday PK, Thibeault J, Frey KE. Changing temperature and precipitation extremes in the Hindu Kush‐Himalayan region: An analysis of CMIP3 and CMIP5 simulations and projections. Int J Climatol. 2015;35:3058–3077. [Google Scholar]
- 57.Dillon ME, et al. Life in the frequency domain: The biological impacts of changes in climate variability at multiple time scales. Integr Comp Biol. 2016;56:14–30. doi: 10.1093/icb/icw024. [DOI] [PubMed] [Google Scholar]
- 58.Burgess MD, et al. Tritrophic phenological match-mismatch in space and time. Nat Ecol Evol. 2018;2:970–975. doi: 10.1038/s41559-018-0543-1. [DOI] [PubMed] [Google Scholar]
- 59.Ernakovich JG, et al. Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob Change Biol. 2014;20:3256–3269. doi: 10.1111/gcb.12568. [DOI] [PubMed] [Google Scholar]
- 60.Pearson RG. Climate change and the migration capacity of species. Trends Ecol Evol. 2006;21:111–113. doi: 10.1016/j.tree.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 61.Guo F, Lenoir J, Bonebrake TC. Land-use change interacts with climate to determine elevational species redistribution. Nat Commun. 2018;9:1315. doi: 10.1038/s41467-018-03786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang N, He J-S, Chen L, Wang L. No upward shift of alpine grassland distribution on the Qinghai-Tibetan Plateau despite rapid climate warming from 2000 to 2014. Sci Total Environ. 2018;625:1361–1368. doi: 10.1016/j.scitotenv.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 63.Morisette JT, et al. VisTrails SAHM: Visualization and workflow management for species habitat modeling. Ecography. 2013;36:129–135. [Google Scholar]
- 64.Gruber S. Derivation and analysis of a high-resolution estimate of global permafrost zonation. Cryosphere. 2012;6:221–233. [Google Scholar]
- 65.Karger DN, et al. Climatologies at high resolution for the earth’s land surface areas. Sci Data. 2017;4:170122. doi: 10.1038/sdata.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.