Significance
Voltage-gated ion channels, such as CaV2.2, consist of pore-forming and auxiliary subunits that interact through protein–protein interactions. We develop a small-molecule antagonist of the protein–protein interaction between the calcium channel alpha pore-forming domain (CaVα) and beta subunits (CaVβ). The compound suppresses trafficking of CaV2.2 channels to the cell membrane and inhibits CaV2.2 activity by acting intracellularly. This allows peripheral access and eliminates the need of intrathecal administration. Indeed, in vivo systemic administration of the small molecule reduces neuropathic pain behavior in animal models. Our compounds serve as chemical tools to explore the CaVα⋅CaVβ interaction in vivo and as a starting point for the development of therapeutics for the treatment of a range of disorders associated with calcium channels.
Keywords: calcium channel, protein–protein interactions, small-molecule inhibitors, pain, β subunit
Abstract
Extracellular calcium flow through neuronal voltage-gated CaV2.2 calcium channels converts action potential-encoded information to the release of pronociceptive neurotransmitters in the dorsal horn of the spinal cord, culminating in excitation of the postsynaptic central nociceptive neurons. The CaV2.2 channel is composed of a pore-forming α1 subunit (CaVα1) that is engaged in protein–protein interactions with auxiliary α2/δ and β subunits. The high-affinity CaV2.2α1⋅CaVβ3 protein–protein interaction is essential for proper trafficking of CaV2.2 channels to the plasma membrane. Here, structure-based computational screening led to small molecules that disrupt the CaV2.2α1⋅CaVβ3 protein–protein interaction. The binding mode of these compounds reveals that three substituents closely mimic the side chains of hot-spot residues located on the α-helix of CaV2.2α1. Site-directed mutagenesis confirmed the critical nature of a salt-bridge interaction between the compounds and CaVβ3 Arg-307. In cells, compounds decreased trafficking of CaV2.2 channels to the plasma membrane and modulated the functions of the channel. In a rodent neuropathic pain model, the compounds suppressed pain responses. Small-molecule α-helical mimetics targeting ion channel protein–protein interactions may represent a strategy for developing nonopioid analgesia and for treatment of other neurological disorders associated with calcium-channel trafficking.
In the central nervous system, voltage-gated calcium channels (CaVs) play important and diverse roles in the synaptic transmission of electrical signals (e.g., neurotransmitter release), in the integration and modulation of these signals, and in the transduction of membrane depolarization into intracellular signals (1). To accomplish these diverse functions, neurons express a variety of calcium channels that are composed of large heteromeric assemblies of pore-forming α1 (CaVα1), auxiliary α2/δ (CaVα2/δ), β (CaVβ), and γ (CaVγ) subunits (2–8). The auxiliary subunits are believed to modulate channel properties and assist in trafficking of the channels to the plasma membrane (9, 10). A recent cryo-electron microscopy study of the 3D structure of the CaV1.1 voltage-gated calcium channel [Protein Data Bank (PDB) ID code: 5GJV] reveals that the interaction between pore and auxiliary subunits is driven by protein–protein interactions (11). Small molecules that disrupt these interactions could provide tools to study them in vivo and could potentially serve as lead compounds for the development of therapeutic agents to treat a range of neurological disorders associated with CaVs. Specifically, CaV2.2 is a clinically validated target for the treatment of human chronic pain (12–14), neuropathic pain (15), and epilepsy (16) and has been implicated in mechanisms of neuronal excitotoxicity (17).
Among the CaV auxiliary subunits, CaVβ subunits increase the surface expression of CaV channels and regulate their biophysical properties (18, 19). CaVβ subunits are encoded by four different genes, CaVβ1–4, including multiple splice variants. Their 3D structure reveals the presence of Src homology 3 (SH3) and guanylate kinase (GK) domains connected by a HOOK region. One of these structures (PDB ID code: 1VYT) corresponds to the cocrystal structure of the β3-subunit and the α-interacting domain (CaVα1-AID) of CaV channels (20). It shows that CaVα1-AID is a 25-residue α-helical structure that tightly binds to a well-defined groove on the GK domain of the β subunit (21–25). The structure reveals the presence of three subcavities that accommodate the side chains of Tyr-437, Trp-440, and Ile-441, respectively (22). These residues are considered hot spots, amino acids that are critical to the protein–protein interaction (26–30). Hot spots are amino acids that contribute at least 1.4 kcal⋅mol−1 to the Gibbs free energy of binding (28–32). Small molecules that mimic the position of hot spots on CaVα or that bind tightly to hot spots on CaVβ should disrupt the tight CaVα1⋅CaVβ protein–protein interaction. To date, no small-molecule antagonists of the CaVα1⋅CaVβ interaction have been identified.
Disruption of protein–protein interactions with small molecules is generally considered challenging (33) because of the typically large binding interfaces (>1,000 Å2) that result in tightly bound stable complexes. However, there has been a gradual increase in the number of small-molecule antagonists of protein–protein interactions over the past decade (34–38). It has been suggested that these small molecules work by engaging or mimicking hot spots located at the protein–protein interface. Protein–protein interactions such as that of the CaVα1⋅CaVβ protein–protein interaction are classified as secondary interactions, given that the interface is composed primarily of an α-helix binding to a well-defined cavity. In the past, these interactions have been amenable to disruption with peptides or peptidomimetics (34, 39, 40). Several small molecules have been developed to successfully disrupt secondary interactions such as Bcl-xL⋅Bak (41), IL-2⋅IL-2Rα (42), and MDM2⋅p53 interactions (43).
To identify small molecules that inhibit the CaVα1⋅CaVβ protein–protein interaction, we resorted to structure-based computational screening of commercial libraries. Top candidates were purchased and tested for inhibition with fluorescence polarization (FP) using fluorescently labeled CaVα1-AID. Starting with hit compound 1 (BTT-3), we prepared several derivatives, which eventually led to 6 (BTT-266), a small molecule that disrupted the binding of CaVα1-AID to the CaVβ3 subunit with single-digit micromolar inhibition constants. The binding mode of 6 revealed the presence of a salt-bridge interaction between the carboxylate moiety of the compound and the guanidium ion of Arg-307 on CaVβ3. To validate this binding mode, we synthesized compounds lacking a carboxylate and mutated Arg-307 to evaluate its role on the activity of compound 6. We further validated the predicted binding mode of 6 through the synthesis of a series of derivatives. This led to 14 (BTT-369), a derivative with a cell-permeable tetrazole moiety instead of the carboxylic group of 6. Patch-clamp experiments explored the effect of 6, 14, and an inactive analog 2 (BTT-245) on the function and trafficking of the CaV2.2 calcium channel. Finally, 6, 14, and the inactive analog 2 were evaluated in animal models of neuropathic pain.
Results
Structure-Based Virtual Screening.
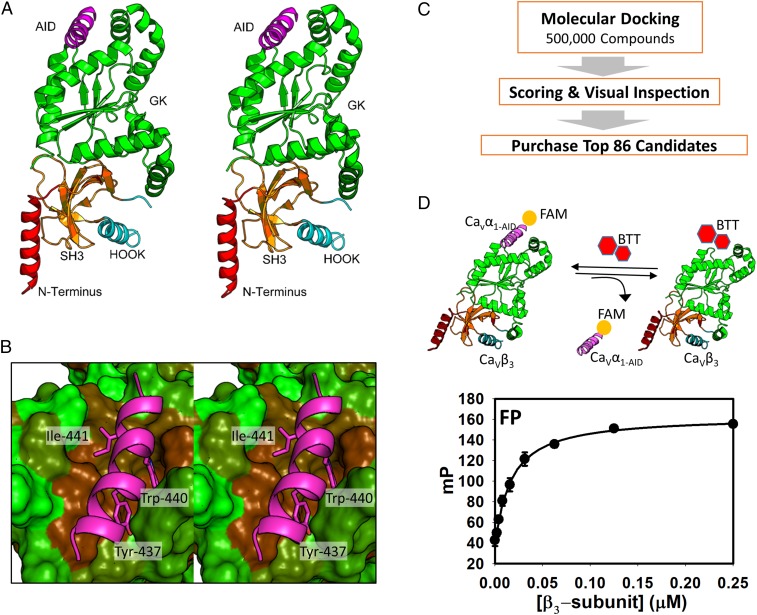
The structural basis of the CaVα1⋅CaVβ interaction was revealed in a cocrystal structure between CaVβ3 and CaVα1-AID (Fig. 1A) (20). CaVα1-AID is a 25-residue α-helix that binds to a well-defined groove on the GK domain of CaVβ3 (Fig. 1B). Previous biophysical studies combined with site-directed mutagenesis on CaVα1-AID show the presence of three hot-spot residues (Tyr-437, Trp-440, and Ile-441) (22). The side chain of these residues is ensconced into three subsites. Small molecules that bind to the subsites and mimic CaVα1-AID hot spots are expected to disrupt this interaction. To identify small molecules that bind to CaVβ at the protein–protein interaction interface, we resorted to virtual screening, focusing on the large binding cavity on CaVβ3. SiteMap (44) scoring of the cavity leads to a SiteScore of 1.01, suggesting that the pocket is highly druggable (45). Therefore, small molecules that bind to this pocket have the potential to exhibit in vivo efficacy similar to those of Food and Drug Administration-approved drugs. We stipulated that a small molecule that binds to this pocket will disrupt the CaVα1⋅CaVβ3 protein–protein interaction. To that end, a total of 500,000 compounds from the ChemDiv commercial library were docked to the druggable pocket on CaVβ3 (Fig. 1C). The resulting protein-compound structures were scored and rank-ordered to select top candidates. The top 500 compounds from the GoldScore and ChemScore scoring functions were visualized, and 86 compounds were selected for purchase by visual inspection.
Fig. 1.
Computational screening followed by biochemical testing identifies a compound that binds to CaVβ3. (A) 3D structure of CaVα1⋅CaVβ (PDB ID code: 1VYT). The AID, the GK domain, the SH3 domain, the HOOK domain that links GK and SH3, and the N terminus are shown in violet, green, orange, cyan, and red ribbon representation, respectively. (B) Stereo close-up view of the CaVα1-AID in complex CaVβ3 (PDB ID code: 1VYT). CaVα1-AID is depicted as a magenta ribbon, and hot spot residues are shown as capped sticks. CaVβ3 is shown in a solvent-accessible surface area color-coded by hydrophobicity ranging from less hydrophobic (green) to more hydrophobic (brown). (C) Schematic depicting the workflow that was used for the structure-based computational screening. (D, Upper) A depiction of the FP assay. (Lower) The plot corresponds to the change in FP as a result of titration of CaVβ3 with increasing concentration of fluorescently labeled CaVα1-AID peptide.
Screening Top Candidates.
To test for binding of the top-ranking compounds, we developed an FP assay. In general, the assay consists of a fluorescently labeled probe, typically a small peptide, and the target protein of interest. Upon binding to the larger protein, the labeled peptide experiences decreased motion, which leads to an increase in light polarization. Small molecules that displace the peptide lead to a decrease in light polarization. The change in polarization can be used to quantify the extent of inhibition. CaVα1⋅CaVβ is ideally suited for an FP assay since the α-helix of CaVα1-AID is the minimum required epitope for binding to CaVβ and the peptide is substantially smaller than CaVβ. The CaVα1-AID peptide was synthesized and fluorescently labeled at its N terminus. The fluorescently labeled CaVα1-AID binds to CaVβ3 with a Kd of 21 ± 2 nM (Fig. 1D). This binding curve was used to select the protein concentration for titration with the compound. The FP assay was used to screen the 86 compounds that emerged from the computational screen at 50 µM. Four compounds inhibited binding of labeled peptide to CaVβ3 (SI Appendix, Fig. S1A). A follow-up concentration-dependent study showed that one compound, 1 (BTT-3), inhibited fluorescently labeled CaVα1-AID binding to CaVβ3 with a Ki of 6.7 ± 0.3 µM (SI Appendix, Fig. S1B) with a predicted binding mode shown in SI Appendix, Fig. S1C.
Design, Synthesis, and Binding Studies of Derivatives.
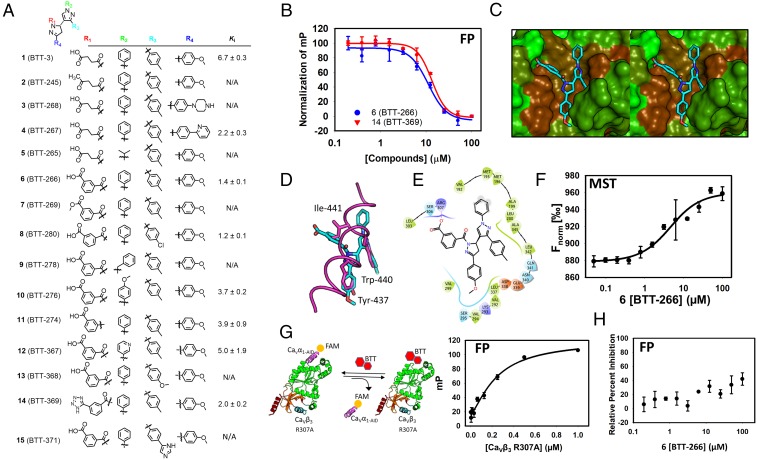
To further probe the predicted binding mode of 1 (SI Appendix, Fig. S1C) and to identify compounds with higher affinity, we designed and synthesized 14 derivatives (Fig. 2A and SI Appendix, Figs. S2–S8). The binding mode of 1 revealed that the R4 group of the compound binds to a pocket on CaVβ3 occupied by Tyr-437 on CaVα1. The R4 pocket is a long, narrow binding site that can accommodate an additional substituent at the para position of the benzene ring. We introduced a piperazine ring at the para position of the benzene ring at R4 in compound 3 (BTT-268) and a pyridine in compound 4 (BTT-267) at the same position. Although 3 did not bind, 4 inhibited the protein–protein interaction.
Fig. 2.
Design and synthesis of a focused library identifies 6 (BTT-266) that inhibits the CaVα⋅CaVβ3 protein–protein interaction. (A) Chemical structure of derivatives that were designed and synthesized based on the structure of 1. (B) The FP assay is used to determine an inhibition constant for 6. (C) Binding mode of 6 to CaVβ3. This model was generated by molecular docking of the compound to the crystal structure of CaVβ3. The compound is shown as capped sticks and is color-coded by atom type (cyan, red, and blue correspond to carbon, oxygen, and nitrogen, respectively). CaVβ3 is shown in a solvent-accessible surface color-coded based on hydrophobicity (green, less hydrophobic; brown, more hydrophobic). (D) The binding mode of 6 superimposed on the binding mode of CaVα1-AID (PDB ID code: 1VYT) shown in magenta tube rendering. Compound 6 is shown in capped-sticks representation color-coded by atom type. Hot spots on CaVα1-AID are shown as magenta capped sticks to illustrate the overlap with substituents on 6. (E) A 2D representation of the binding mode of 6 shown in C and D. The schematic shows the compound chemical structure along with the residues that are involved in intermolecular interactions with the compound. Hydrophobic, positively charged, negatively charged, and polar residues are colored green, blue, red, and cyan, respectively. Solvent-exposed atoms are shown by gray circles in the background. The salt bridge between Arg-307 and the compound is shown by a pink line. (F) The binding curve that emerged from the use of MST to establish direct binding of compound 6. Curve-fitting analysis led to a Kd of 3.6 ± 1.1 µM. (G) An FP assay was developed using the CaVβ3 Arg-307-Ala (R307A) mutant titrated to the CaVα1-AID fluorescently labeled peptide. The binding constant obtained from this analysis is 3.6 ± 1.1 µM. (H) Compound 6 does not inhibit the protein–protein interaction between CaVβ3 Arg-307-Ala as evidenced by a lack of displacement of fluorescently labeled CaVα1-AID bound to the mutated CaVβ3.
In addition to R4, we made modifications at the R2 moiety. The phenyl group of the R2 substituent of 1 is solvent-exposed, and the group is engaged in favorable interactions with several hydrophobic residues that include Val-192, Met-195, Met-196, and Ala-199 (SI Appendix, Fig. S9). We modified this group to an isopropyl moiety in compound 5 (BTT-265), which led to complete loss of binding. This suggests that the R2 aromatic group of 1 is essential for binding.
We stipulated that the rotatable bonds of the R1 moiety may lead to unfavorable entropy of binding, especially considering that this group is likely fixed into the pocket occupied by Ile-441. We converted the butyric acid to a benzoic acid moiety in 6 (BTT-266), which resulted in a substantial increase in affinity to a Ki of 1.4 ± 0.1 µM. Unlike several of the compounds that did not completely inhibit the CaVα1⋅CaVβ3 protein–protein interaction at high concentrations, likely due to poor solubility, 6 showed complete inhibition (Fig. 2B). Compound 6 possesses a chiral center at its core pyrazoline ring. Molecular docking of (R)-6 and (S)-6 resulted in two different binding modes for the enantiomers as expected (Fig. 2C and SI Appendix, Fig. S10). The (S)-6 binding mode appears to be the most plausible, considering the excellent overlap between three of the compound substituents with three of the most critical hot spots on CaVα1-AID, namely Tyr-437, Trp-440, and Ile-441 (Fig. 2D and SI Appendix, Fig. S11). The R3 and R4 substituents of (S)-6 occupy the same position as Tyr-440 and Trp-437 of CaVα1-AID, respectively. The R1 substituent of (S)-6 that bears the benzoic acid moiety occupies the same position as the side chain of Ile-441. Interestingly, just as in compound 1, the carboxylate of 6 is involved in a salt-bridge interaction with Arg-307 (Fig. 2 C and E). Salt-bridge interactions are commonly found in small-molecule protein–protein interaction inhibitors. We have previously shown that disruption of the uPAR⋅uPA protein–protein interaction by IPR-803 was completely dependent on a salt bridge (46) that was later independently confirmed by crystallography (47). To confirm the importance of the salt bridge, we used the FP assay to test whether a methyl ester precursor of 6, namely 7 (BTT-269) or 2 (BTT-245), a ketone derivative of 1, binds to CaVβ3. Both 2 and 7 closely resemble 6, but they do not possess the negative charge of the carboxylate moiety of 6. Both 2 and 7 nearly completely lost their ability to disrupt the CaVα1⋅CaVβ3 interaction, confirming the importance of the salt bridge. These results lend further credence to the predicted binding mode of (S)-6. Carboxylic acids are known to reduce cell permeability. A common medicinal chemistry strategy to maintain the charge of the carboxylate while improving cell permeability is to replace the carboxylate with a tetrazole moiety. To that end, we prepared 14 (BTT-369). The compound possessed a Ki identical to that of 6. Other changes to R1 were made in compound 11 (BTT-274). The twofold reduction in binding affinity suggests that the amide bond is optimal at this position.
We modified additional substituents on 6 to further confirm its binding mode. The importance of a phenyl group at R2 was established by the introduction of a benzyl moiety at R2 in 9 (BTT-278), which led to compete loss of inhibition. Introduction of methoxy group at the para position of the benzene group of 6 in 10 (BTT-276) led to a twofold reduction in binding.
Finally, we modified the R3 group of 6 by replacing the methyl group by a chlorine at the para position in 8 (BTT-280). This resulted in little change to the binding affinity of the compound. A methoxy group in compound 12 (BTT-368), on the other hand, resulted in complex loss of binding. An imidazole phenyl moiety at R3 in compound 15 (BTT-371) also resulted in complete loss of binding, consistent with the predicted binding mode of these compounds showing a constricted binding pocket at R3.
It is worth noting that the inhibition curves for 6 and other compounds are steeper than usual, suggesting potential aggregation. To rule out the possibility that the inhibition of the CaVα1⋅CaVβ3 interaction is due to aggregation, we repeated the concentration-dependent FP study of 6 using 0.05 and 0.25% Triton X-100 (SI Appendix, Fig. S12A). We find that even a 25-fold increase in detergent from 0.01 to 0.25% had no effect on the inhibition curves of 6; the increase of Triton levels to 0.25% appears to have slightly improved the potency of the compound. Next, we used centrifugation to spin down the sample to eliminate all potential aggregates. As shown in SI Appendix, Fig. S12B, there was no difference in inhibition of the CaVα1⋅CaVβ3 interaction by 6 in the presence or absence of spin down. Finally, we tested whether compound 6 inhibits unrelated tight protein–protein interactions, which would be expected if the compounds are inhibiting through aggregation. We used an FP assay that we developed for the tight single-digit nanomolar-affinity protein–protein interaction between the TEAD4 transcription factor and its coactivator Yap1. The compounds showed no inhibition of this interaction (SI Appendix, Fig. S12C). Collectively, these results suggest that inhibition of the CaVα1⋅CaVβ3 interaction by our compounds is not due to aggregation. Steep curves may be more common among compounds that target single-digit nanomolar-affinity interactions that occur over a large protein–protein interaction interface, as reported for ABT-737, a BCl-2 antagonist (48).
Direct Binding Confirmed by Label-Free Microscale Thermophoresis.
To confirm direct binding of 6 to CaVβ3, we resorted to label-free microscale thermophoresis (MST) (Fig. 2F) (49). The method takes advantage of a laser-induced temperature gradient. The molecules migrate along the gradient, and their movement over time is monitored by a fluorescent microscope, resulting in characteristic traces as shown in SI Appendix, Fig. S13. To obtain a binding affinity, a concentration-dependent study is performed with a fixed concentration of protein. If binding occurs, the thermophoretic signal will change with respect to concentration and is used to derive a dissociation constant, Kd. Unlabeled proteins have been successfully used to explore small-molecule binding with MST (50). The raw data shown in SI Appendix, Fig. S13 were used to generate a binding curve shown in Fig. 2F. The curves led to a Kd of 3.6 ± 1.1 µM. The MST binding constant is consistent with the Ki of 1.4 ± 0.1 µM that was obtained from the FP assay.
Site-Directed Mutagenesis Confirms a Critical Salt Bridge.
The binding mode of 6 reveals that the compound forms a salt-bridge interaction with Arg-307 through a benzoic acid moiety. The removal of the charge from the compound, as in methyl ester 7, led to complete loss of inhibition of the CaVα1⋅CaVβ3 interaction. To further confirm the importance of this salt bridge and to establish the predicted binding mode, we mutated Arg-307 to alanine. The resulting CaVβ3 Arg-307-Ala mutant binds to CaVα1-AID with a Kd of 240 ± 59 nM (Fig. 2G). The reduction of Kd by nearly 10-fold suggests that Arg-307 is important for the CaVα1-AID⋅CaVβ3 protein–protein interaction and could potentially be considered a hot spot. We used the CaVβ3 Arg-307-Ala mutant to develop an FP assay to test whether binding of the mutant protein to CaVα1-AID is inhibited by compound 6 (Fig. 2G). Interestingly, compound 6 lost nearly all its affinity to the CaVβ3 Arg-307-Ala mutant, as shown in Fig. 2H. This loss of affinity was similarly observed in the other compounds (SI Appendix, Fig. S14). This further confirms the importance of the salt-bridge interaction and validates the binding mode of the compound to CaVβ3.
Compound 6 Reduces Trafficking of CaV2.2 to the Plasma Membrane.
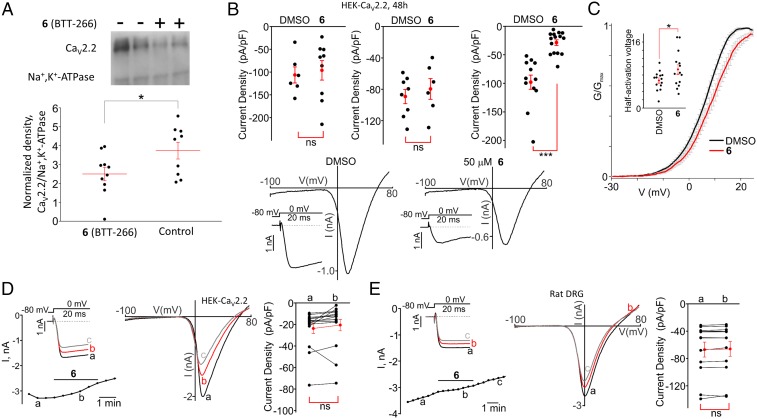
Previous studies have shown that CaVα1⋅CaVβ interaction is responsible for channel trafficking, and other studies have suggested that disruption of this interaction may affect the function of the calcium channel. Here, we use 6 to explore these questions. First, we determined whether 6 affects the targeting of the CaV2.2α1 subunit to the plasma membrane. We employed a surface biotinylation approach (Fig. 3A). We found that at 50 µM, 6 significantly decreased surface presentation of the CaV2.2α1 subunit. To confirm these results, we resorted to the patch-clamp technique. We recorded CaV2.2 currents in HEK-CaV2.2 cells pretreated with 10, 25, and 50 µM of 6 for 48 h (Fig. 3B). We found that the current densities obtained in the cells treated with 50 µM 6 were significantly decreased compared with group treated with vehicle (DMSO) (IDMSO = −98.0 ± 12.5 pA/pF, n = 12 vs. I6 = −28.7 ± 5.0 pA/pF, n = 15; P < 0.001) (Fig. 3B). This is consistent with the surface biotinylation results.
Fig. 3.
Compound 6 decreases the cell-surface presentation of CaV2.2 channels. (A) Western blot analysis of cell-surface biotinylated proteins isolated from HEK-CaV2.2 cells treated with 6 (BTT-266) (+; n = 10) or with the vehicle control DMSO (−; n = 9) for 48 h. (Upper) A representative blot probed with the CaV2.2 antibody and the Na+,K+-ATPase antibody. (Lower) A comparison of the amounts of cell-surface biotinylated CaV2.2 proteins normalized to that of Na+,K+-ATPase. *P < 0.05. (B) (Upper) Summary data of the current densities recorded in HEK-CaV2.2 cells pretreated for 48 h with the vehicle (DMSO) or 10 μM 6 (n = 9) (Left), 25 μM 6 (n = 6) (Center), or 50 μM 6 (n = 15) (Right). *P < 0.001; ns, not significant. (Lower) Sample traces of the current–voltage relationships acquired during the voltage ramps from −100 mV to +80 mV in the DMSO (Left) and 6 at 50 μM (Right) groups. Insets show the CaV2.2 currents activated by depolarizing pulses to 0 mV. (C) Effect of 6 on the voltage dependence of activation of CaV2.2 channels in HEK-CaV2.2 cells pretreated with the vehicle or the indicated compounds for 48 h. The G–V curves are shown. The Inset shows a comparison of half-activation potentials in DMSO- and 50 μM 6-pretreated HEK-CaV2.2 cells (48 h). (D and E, Left) Time courses of CaV2.2 currents recorded in an HEK-CaV2.2 cell (D; n = 15) or in a DRG neuron (E, [BaCl2]out = 2 mM; n = 12) during acute applications of 6 (50 μM). Insets show the superimposed traces of CaV2.2 currents activated by depolarizing pulses to 0 mV in the absence and presence of 6. (Center) The corresponding current–voltage relationships obtained in the absence and presence of 6. (Right) Summaries of the data. Compound 6 was applied as indicated by the horizontal bars. a, b, and c show the times when the current traces were recorded. ns, not significant.
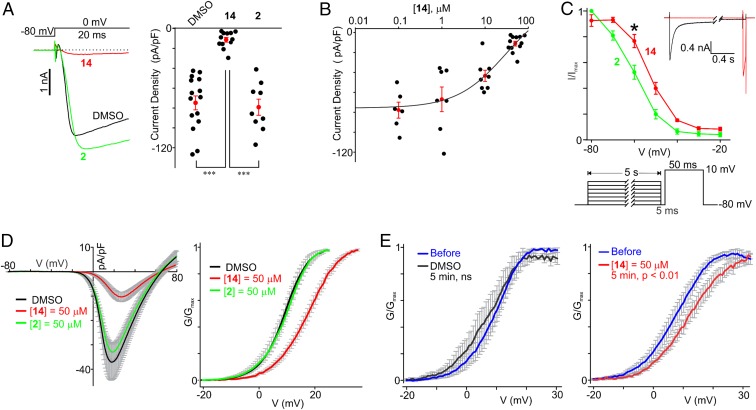
We used 6 to explore whether compounds affected channel function. Notably, the conductance–voltage (G–V) curves for CaV2.2 were shifted toward more positive potentials in HEK-CaV2.2 cells pretreated with 6 for 48 h (Fig. 3C). The mean half-activation potential was greater in the cells pretreated with 6 than in the control cells pretreated with DMSO for 48 h (DMSO: 6.7 ± 0.6 mV, n = 14; 6: 9.4 ± 1.2 mV, n = 14, P < 0.05) (Fig. 3C, Inset). Conversely, acute application of 50 µM 6 did not affect CaV2.2 currents in either in HEK-CaV2.2 cells or rat dorsal root ganglion (DRG) neurons (Fig. 3 D and E), indicating that the compound does not inhibit the channel pore directly. The inactive analog 2, which did not inhibit the CaVα1⋅CaVβ3 protein–protein interaction, did not affect the CaV2.2 current amplitudes in HEK-CaV2.2 cells when it was used acutely or as a long-term treatment (Fig. 4A).
Fig. 4.
Compound 14 (BTT-369) reduces the current density and modulates the voltage-dependence of activation and steady-state inactivation of CaV2.2. (A) The CaV2.2 current densities in HEK-CaV2.2 cells pretreated for 48 h with DMSO (n = 15), 14 (n = 12), or 2 (n = 9). (Left) the superimposed raw sample traces of CaV2.2 currents acquired during the 20-ms depolarizing pulses in HEK-CaV2.2 cells pretreated with the indicated compounds for 48 h. (Right) Summary of the data. ***P < 0.001. (B) The concentration–effect curve for 14. HEK-CaV2.2 cells were pretreated with various concentrations of compound 14 for 48 h. CaV2.2 currents were acquired during 20-ms depolarizing pulses, and the current densities were calculated as a ratio of the peak current amplitude and each cell capacitance (0.1 μM, n = 6; 1 μM, n = 7; 10 μM, n = 8; 50 μM, n = 12). The line is the fit of the data to the four-parameter logistic function. The apparent IC50 value is 31 μM. The same datasets for 50 μM 14 (BTT369) were used in A and B. (C) Effect of 2 (BTT-245) and 14 on the voltage dependence of the steady-state inactivation of CaV2.2 channels. The Inset shows two superimposed traces acquired during the steady-state inactivation protocol shown below the plot. HEK-CaV2.2 cells were pretreated with the indicated compounds for 48 h. *P < 0.05. (D) Effects of 2 and 14 on the voltage dependence of the activation of CaV2.2 channels in HEK-CaV2.2 cells pretreated with the vehicle or the indicated compounds for 48 h. (Left) The average current–voltage relationships acquired during voltage ramps for each test group. (Right) The average G–V curves for each treatment group. The gray vertical lines are error bars indicating SEM. (E) Acute effects of DMSO and 14 on the voltage dependence of the activation of CaV2.2 channels in HEK-CaV2.2 cells. (Left) Average G–V curves before and after 5-min incubation with DMSO (P > 0.05). (Right) Average G–V curves before and after 5-min incubation with 14 (P < 0.01). The gray vertical lines are error bars indicating SEM. ns, not significant.
Compound 14 Modulates the Voltage Dependence of Activation and Steady-State Inactivation of CaV2.2.
When activated by depolarizing pulses to 0 mV from a holding potential of −80 mV, the densities of CaV2.2 currents were significantly smaller in HEK-CaV2.2 cells pretreated for 48 h with 50 μM 14 than in cells treated with 50 μM 2 or DMSO (48 h; 14: −11.1 ± 2.4 pA/pF, n = 12; 2: −79.2 ± 8.2 pA/pF, n = 9; DMSO: −74.8 ± 7.0 pA/pF, n = 15; P < 0.001) (Fig. 4A). The apparent IC50 for inhibition of CaV2.2 currents by 14 is 31 μM (Fig. 4B).
Since the CaVα1⋅CaVβ3 protein–protein interaction may modulate the voltage dependence of activation and steady-state inactivation of CaV2.2α1, and considering that the values of CaVα1 half-activation and half-inactivation potentials were more negative in CaVα1⋅CaVβ3-coexpressing cells (51, 52), we explored whether 14 can modulate the voltage dependence of steady-state inactivation and/or activation of CaV2.2 in HEK-CaV2.2 cells. Fig. 4C shows that the CaV2.2 steady-state inactivation curves were significantly shifted toward more positive potentials in HEK-CaV2.2 cells pretreated for 48 h with 14 (50 μM, V0.5, inact = −52.9 ± 1.3 mV, P < 0.05, n = 10) than in the control group (2; 50 μM, V0.5, inact = −61.6 ± 2.0 mV, n = 10). While analyzing current–voltage relationships obtained during voltage ramps (Fig. 4D), we also found that the mean half-activation potential for Cav2.2 channels exhibited a more positive value in HEK-Cav2.2 cells pretreated for 48 h with 14 (50 μM, V0.5, act = 16.5 ± 1.1 mV, P < 0.001, n = 12) than in the controls (DMSO: V0.5, act = 8.7 ± 1.1 mV, n = 12; 2: 50 μM, V0.5, act = 8.5 ± 1.4 mV, n = 9). These two findings are consistent with the hypothesis that 14 disrupts CaVα1⋅CaVβ3 interactions.
Thus far, we used only long-term pretreatments (48 h) of HEK-CaV2.2 cells with 6 and 14. Since 14 lacks the negatively charged carboxylate group in the R1 substituent of 6, which is replaced with an isosteric tetrazole moiety, 14 is more membrane permeable than 6. The data presented above suggested that 14 may disrupt the intracellular CaVα1⋅CaVβ3 protein–protein interaction in live cells. Therefore, we then asked whether acute short-term treatments with 14 in patch-clamped HEK-Cav2.2 cells could modulate the half-activation potential for Cav2.2 channels. No significant change in the mean half-activation voltage value was observed in patch-clamped HEK-Cav2.2 cells acutely treated with DMSO (before treatment: V0.5, act = 9.1 ± 1.6 mV; 5 min under DMSO: V0.5, act = 6.8 ± 3.3 mV, n = 3, P > 0.05) (Fig. 4E, Left). Conversely, a 5-min incubation with 50 μM 14 was sufficient to significantly shift the G–V curves of Cav2.2 toward more positive potentials (before treatment: V0.5, act = 6.4 ± 1.9 mV; 5 min under 14: V0.5, act = 11.4 ± 1.7 mV, n = 7, P < 0.01) (Fig. 4E, Right), indicating that the Cav2.2 channel requires stronger depolarization to be activated in the presence of 14.
Pharmacokinetic Properties of Compound 14.
Voltage-dependence of activation and steady-state inactivation experiments in HEK-Cav2.2 revealed that compound 14 appears to be more potent than 6 or 2. These observations suggest that 14 may be more efficacious in vivo than 6 and 2. Before testing 14 in nociceptive and neuropathic pain using in vivo assays, we sought to understand the pharmacokinetic properties of the compound in the mouse. For i.v. administration, 14 was formulated as a solution at a final dose of 1 mg/kg. Plasma concentrations at various time points were determined using the LC-MS/MS technique (SI Appendix, Fig. S15). The elimination half-life of 14 was 0.29 h. The amount of time that the maximum concentration of the drug was present in plasma (Tmax) was 0.083 h, and the peak plasma concentration (Cmax) that the drug achieved was 181.36 ng/mL. The area under the curve (AUC0-t) concentration was 57.99 ng⋅mL−1⋅h−1.
For oral gavage administration, 14 was formulated as a solution at a final dose of 10 mg/kg. Plasma concentrations at various time points were again determined using the LC-MS/MS technique (SI Appendix, Fig. S15). The elimination half-life of 14 was 1.99 h. The Tmax in plasma was 1 h, and the Cmax was 164.7 ng/mL. The AUC 0-t concentration was 635.555 ng⋅mL−1⋅h−1. Taken together, these observations indicate that 14 has a significant lifetime in vivo and may exhibit notable therapeutic potential. Thus, there is a structure–activity relationship, 14 > 6 > 2, in the chemical series, which points to potential druggability of the series and the potential to identify an optimal lead compound as well as the pharmacological validation and therapeutic potential.
Effects of Compound 6 on Acute Thermal Nociception in the Rat.
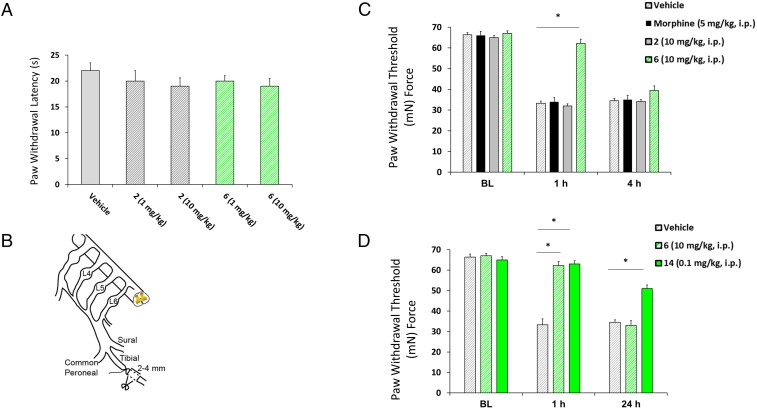
A model of behavioral sensitivity using thermal stimulus was used to determine the acute analgesic properties of the candidate small molecules. Thermal stimulation serves to activate high-threshold primary afferent sensory fibers in the hind paw glabrous skin. These axons carry action potential discharges to the dorsal horn of the spinal cord to activate secondary nociceptive neurons which contribute to a behavioral response of paw withdrawal from the aversive stimulus. Systemic administration of 6 failed to elicit changes in the response latency at 1 or 10 mg/kg (Fig. 5A).
Fig. 5.
Compounds 6 and 14, but not 2, reverse mechanical hypersensitivity in the TNI model of neuropathic pain. (A) Bar graph of paw withdrawal latency (in seconds) of rats (n = 6 per group) demonstrating a lack of analgesic effect for 2 or 6. (B) Diagram of the sural, tibial, and common peroneal terminal nerve branches of the sciatic nerve and their dorsal root origins. Neuropathic pain was induced by ligation of the tibial nerve, and 2–4 mm of the nerve distal to the ligation was removed. (C) PWT (in milli-Newtons, mN) in response to von Frey stimulation to the paw ipsilateral to injury following a single i.p. administration of vehicle, morphine (5 mg/kg), 2, or 6 (10 mg/kg, n = 8) at 4 wk after TNI. The ability of 6 (green striped bar) to reverse TNI-induced mechanical hypersensitivity was significantly different from that of 2 (solid gray bar) and morphine (black striped bar). *P < 0.05 versus 2 and morphine treatment in TNI rodents (mean ± SE; n = 8, repeated measures ANOVA with Tukey’s post hoc test). BL, baseline. (D) At 4 wk, a single bolus injection of 14 (0.1 mg/kg) significantly reversed mechanical hypersensitivity at both 1 and 24 h post dosing. For comparison, reversal of mechanical hypersensitivity by 6 (10 mg/kg) was limited to 1 h. Data are shown as mean ± SE; n = 8; *P < 0.05 versus 6 and vehicle-treated TNI rodents; repeated-measures ANOVA with Tukey’s post hoc test.
Neuropathic Pain Is Attenuated by Treatment with Compounds 6 and 14 but Not Compound 2.
We next examined the effects of morphine and compounds 2 and 6 on established chronic nociceptive behavior in a model of neuropathic pain, tibial nerve injury (TNI), 28 d after surgery (Fig. 5B) (53). TNI induces prolonged chronic behavioral hypersensitivity to mechanical stimuli which lasts for several months and is generally insensitive to opioids such as morphine (53, 54). Before exposure to the compound, all injured animals exhibited pronounced mechanical allodynia (33.1 ± 5.9 mN; n = 8–10) in response to von Frey hair stimulation of the injured hind paw, compared with presurgery levels, which averaged 68.7 ± 3.7 mN (n = 8–10) (Fig. 5C). Pronounced attenuation of tactile hypersensitivity was observed at 1 h but not at 4 h after systemic administration of 6 (10 mg/kg, i.p.; 62.2 ± 3.1 mN; n = 16). The 6 dosing levels of 1 mg/kg and 0.1 mg/kg failed to alter behavior at 1 or 4 h. In contrast, systemic administration of morphine (5 mg/kg) and 2 (10 mg/kg, i.p.) was ineffective in reducing hypersensitivity, with levels averaging 32 ± 2.6 mN (n = 8) (Fig. 5C). That the paw-withdrawal threshold (PWT) returned to baseline by 4 h in 6-injected rats is consistent with the turnover of drug over this period and is a limitation of this drug candidate. PWTs were increased significantly at both 1 and 24 h postinjection in rats exposed to 14 (0.1 mg/kg) 28 d after TNI compared with rats injected with 6 or vehicle control (Fig. 5D).
Discussion
The neuronal CaV2.2 voltage-gated Ca2+ channel is implicated in mediating neurotransmitter release in nociceptive neurons. CaV2.2 channels are large (>400 kDa) heteromeric assemblies composed of pore-forming α subunits and auxiliary α2/δ and β subunits (2–8). The auxiliary subunits are believed to modulate the properties and assist in trafficking of the channels to the plasma membrane (9, 10). The transmembrane α2/δ subunit has recently received renewed interest for being the target of the anticonvulsant drugs gabapentin (Neurontin) and pregabalin (Lyrica), two moderately effective treatments for clinical neuropathic pain (15, 55). There is clear evidence showing dysregulation of Ca2+ homeostasis in many neurological diseases, and studies have demonstrated that Ca2+ channels are a clinically validated target for the treatment of human chronic pain (12–14) and neuropathic pain (15) and have been implicated in mechanisms of neuronal excitotoxicity (17). Most existing drug-discovery efforts targeting calcium channels have concentrated on the α pore-forming subunits. Protein–protein interactions between the pore-forming and auxiliary subunits, particularly the CaVα⋅CaVβ interaction, have been largely ignored.
Here, we report the discovery of a small molecule that modulates the CaVα1⋅CaVβ3 protein–protein interaction of CaVs. We followed a simple approach that consisted first of docking more than 500,000 commercially available compounds to the large pocket at the CaVα·CaVβ3 interface. We visually inspected the top 500 candidates. Testing of 86 compounds led to 6, which inhibited the CaVα1⋅CaVβ3 interaction with a Ki of 1.4 ± 0.1 µM. Using label-free MST, we confirmed that the compound binds directly to CaVβ3 and obtained a Kd that was nearly identical to the Ki from the FP assay. Interestingly, the binding mode of 6 reveals the presence of a salt-bridge interaction between its benzoic acid moiety and Arg-307. To confirm the presence of a salt bridge, we tested a methyl ester derivative of 6, namely 7, as well as a ketone derivative of 1, namely 2, both of which lacked a charged carboxylate. These compounds showed a complete loss of inhibition. To further establish the critical nature of the salt bridge, we mutated Arg-307 to alanine. We found that 6 completely lost its ability to inhibit the CaVα1⋅CaVβ3 interaction in the absence of Arg-307, further confirming the existence of the critical salt-bridge interaction. The importance of this salt bridge was further confirmed by the synthesis of 14, a derivative of 6 that possesses a tetrazole moiety instead of carboxylic acid. The compound exhibited inhibition potency similar to that of 6.
We explored whether the compounds affected calcium-channel trafficking and function. Previous studies have shown that the interaction with CaVβ is critical for CaV2.2α1 subunit trafficking to the plasma membrane. We demonstrated that pretreatment of HEK-CaV2.2 cells with 6 decreased the cell-surface presentation of CaV2.2α1 proteins. Using patch-clamp studies, we also found that the compound significantly attenuated channel density at the cell membrane. Longer treatment time was necessary due to the slow kinetics of internalization and degradation of CaV2.2 channels containing the α2δ subunit (56). Collectively, these results confirm previous studies that the CaVα1⋅CaVβ interaction is responsible for channel trafficking. Subsequently, we explored whether the compounds affected cation currents through the channel during an acute application to exclude a direct effect on the CaV2.2α1 subunit. Patch-clamp analyses revealed that 6 had no acute effect on the amplitude of Ba2+ currents through the channel. This is reasonable, considering the latest cryo-electron microscopy structure of CaV1.1 (PDB ID code: 5GJV). Using this structure, we constructed a homology model of CaV2.2 (SI Appendix, Fig. S16). The model, which closely resembles that of CaV1.1, shows that the CaVα1⋅CaVβ interaction is located outside the channel pore. Hence, disruption of the CaVα1⋅Cavβ interaction may not affect cation flow through the channel.
We also demonstrated that 6 and 14 may modulate the voltage dependence of CaV2.2 activation. It is well established that the interaction of CaVβ3 and CaVα1 results in a shift of the half-maximum voltage of activation for CaV2.2 to more negative potentials (51, 52). A more negative half-activation potential for CaV2.2 means that the channel can be activated at more moderate membrane-depolarizing voltages. Conversely, disruption of the CaVα1⋅CaVβ protein–protein interaction is expected to shift the half-activation potential for CaV2.2 toward a more positive voltage, making it harder for the CaV2.2 channel to be activated at the moderate depolarizing voltages. Consistently, we found that 14, a membrane-permeable disruptor of the CaVα1⋅CaVβ protein–protein interaction, significantly shifted the G–V curves of CaV2.2 toward the positive potentials. The rightward shift of the mean half-activation potential was most pronounced after a 48-h pretreatment with 14. However, even following a brief 5-min treatment in patch-clamped HEK-CaV2.2 cells, the compound was capable of significantly shifting the G–V curves for CaV2.2 toward more positive potentials (although in a lesser degree than observed after 48-h pretreatments). Thus, these data indicate that stronger depolarizations are needed to activate CaV2.2 in the presence of 14.
N-type voltage-gated calcium channels (CaV2.2), largely localized to primary afferent terminals in laminae 1 and 2 of the dorsal horn, play an important role in pain signaling by contributing to the release of neurotransmitters such as glutamate, substance P, and calcitonin gene-related peptide (CGRP). Development of a highly efficacious, nonaddicting analgesic with a good side-effects profile has been challenging. Clinically approved blockers of this ion channel to date are encumbered by numerous off-target effects and exhibit narrow therapeutic windows. Novel small-molecule compounds that are nonaddictive and alter pain behavior following injury are sought for treatment of both subacute and chronic pain conditions. We tested whether 6 had an effect on rodent behavior in a neuropathic pain model. We found that 6 given systemically reversed TNI-hypersensitivity for 1 h but not 4 h, while the same dose of 2, which did not inhibit the in vitro CaVα1⋅CaVβ interaction, had no effect. Similar behavioral effects were observed for 14 for up to 24 h, which is suggestive of sustained pain relief. These observed effects may be due in part to a possible pharmacologic block of presynaptic neurotransmitter release of neuropeptides or changes in the excitability of nociceptive neurons present in animals with chronic pain conditions. Moreover, this effect may be attributed to a state-dependent affinity and enhanced high-frequency activation patterns in pain syndromes. That 6 was unable to alter the behavioral responses to acute thermal nociception may be attributed to lower-frequency firing patterns associated with normal nociception (57).
In sum, we identified small molecules that inhibit the protein–protein interaction between pore and auxiliary subunits of a voltage-gated ion channel. The compounds are α-helical mimetics. It is likely that another, similar protein–protein interaction exists in other ion channels that can be targeted with small molecules. The profound in vivo efficacy of 14 at a dose of 0.1 mg/kg suggests that this compound offers the potential to develop new treatments for various neurological conditions associated with aberrant trafficking and function of calcium channels.
Materials and Methods
Virtual Screening.
The crystal structure for CaVβ3 was obtained from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Databank (PDB ID code: 1VYT) (58). Structures were imported into SYBYL 8.0, a molecular modeling suite from Tripos, Inc., for predocking preparation. All binding partners, ions, and water molecules were removed. Hydrogen atoms were added, and the protonation states on polar residues were optimized with Reduce (version 3.03) (59). Structures were then loaded into AutoDockTools (60) for further processing. Further details about the docking and scoring of compounds are provided in SI Appendix.
Protein Expression and Purification.
The plasmid of the pET28a-CaV2.2β3 subunit was transformed into a competent Escherichia coli BL21(DE3) strain. The culture was grown in LB medium at 37 °C to an OD600 of ∼0.6 and then was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside at 16 °C for 16 h. Cells were collected by centrifugation and lysed by microfluidizer in lysis buffer (phosphate buffer, pH 7.6, 2 mM DTT). The His-CaVβ3 protein was purified at 4 °C using Ni-IMAC chromatography (His-Trap HP; GE Healthcare) and was eluted with 500 mM imidazole in lysis buffer with a gradient method. After the fractions consisting of His-CaVβ3 were combined and concentrated, the protein was further purified using size-exclusion chromatography (Superdex 200 prep grade; GE Healthcare) in PBS with 2 mM DTT.
FP and MST Analysis.
FP was used in a competition format to test compounds for inhibition of the CaVα⋅CaVβ3 protein–protein interaction. A fluorescently labeled peptide that contains the region of CaVα that binds to CaVβ3 was synthesized. The fluorescent fluorescein-AID peptide was found to bind to CaVβ3 with a Kd of 21 ± 2 nM. The high affinity of the CaVβ3 probe suggests that introduction of the fluorescent label to the peptide had no impact on its binding affinity, which is close to the value that Minor and coworkers (22) measured using isothermal titration calorimetry. MST analysis was carried out to confirm direct binding of small molecules to CaVβ3. Further details about the experimental setup are provided in SI Appendix.
HEK-CaV2.2 Cell Culture.
HEK293 cells stably expressing rat N-type CaV2.2α1B, rat CaVβ3, and rat α2δ-1 were obtained from Diane Lipscombe (Brown University, Providence, RI) (61). Cells were grown in DMEM supplemented with 10% FBS, 50 U penicillin, 50 μg/mL streptomycin, 5 μg/mL blasticidin, 5 μg/mL hygromycin, and 25 μg/mL zeocin at 37 °C in a humidified atmosphere of 95% air and 5% CO2. For patch-clamp experiments, HEK-CaV2.2 cells were plated on glass coverslips and were cultured for 24–48 h.
DRG Neuron Isolation.
All animal procedures were performed in accordance with NIH guidelines and were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. Young adult Sprague–Dawley rats were anesthetized (3% isoflurane) and then decapitated. The spinal column was removed and cut open, and DRGs were harvested from the lumbar L4–L6 vertebral levels. The ganglia were incubated in DMEM containing collagenase (1 mg/mL; Worthington; LS04194) and protease (1 mg/mL; Worthington; LS02104) for 30–60 min, and DRG neurons were dissociated by triturating in DMEM supplemented with 10% FBS. The isolated DRG neurons were plated on glass coverslips coated with Growth Factor Reduced Matrigel (Thermo Fisher Scientific) and were cultured in Eagle's minimum essential medium (Invitrogen) supplemented with 0.2% BSA and 20 ng/mL NGF-2.5S (BD Biosciences) overnight at 37 °C in a water-jacketed 5% CO2 incubator for the patch-clamp experiments.
Cell-Surface Protein Biotinylation and Western Blotting.
Cell-surface protein biotinylation and Western blotting were performed as previously described (62). Briefly, HEK-CaV2.2 cells were treated with 6 (BTT-266) or the vehicle (DMSO) for 48 h. The surface proteins were biotinylated with 5 mM biotin-X-NHS (EMD Millipore) in PBS for 30 min at 4 °C. After biotinylation, cells were quenched and washed with PBS containing 100 mM glycine. Then, cells were lysed in ice-cold RIPA buffer containing the Halt protease inhibitor mixture (Thermo Fisher Scientific). The lysate was cleared by centrifugation, the preabsorbed avidin-agarose beads were added, and the resulting suspension was rotated for 30 min at 4 °C. The avidin-agarose beads were spun down and then washed three times with the complete lysis buffer. Proteins were eluted from the beads by incubation with SDS gel loading buffer supplemented with 1 mM DTT for 10 min at 70 °C. The eluted proteins were separated using 10% SDS-PAGE, transferred onto PVDF (Immun-Blot; Bio-Rad), and probed with the anti-CaV2.2-α1B antibody from Alomone Labs (1:1,000 dilution; catalog no. AC002) and the Na+,K+-ATPase antibody (1:1,000 dilution; catalog no. 3010; Cell Signaling).
Whole-Cell Patch-Clamp Recordings in HEK-CaV2.2 Cells and DRG Neurons.
The whole-cell patch-clamp technique was used to record CaV2.2 currents in HEK-CaV2.2 cells and rat DRG neurons as described elsewhere (63). Details of the experimental procedures used to carry out the patch-clamp recordings reported in this work are provided in SI Appendix.
Animals.
Pathogen-free, adult, female Sprague–Dawley rats (weight at testing 150–200 g) (Harlan–Sprague–Dawley) were housed in a climate-controlled room on a 12-h light/dark cycle and were allowed to have food and water ad libitum. All procedures were approved by the Indiana University Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals (64) for the use of laboratory animals. All behavioral experiments were conducted by experimenters blinded to the treatment conditions and small-molecule candidate identity. The experiments were replicated a minimum of two times with independent cohorts of animals.
Drug Delivery.
For in vivo studies, small-molecule candidates 2, 6, and 14 were freshly prepared using saline (0.9%) on the day of the experiment. Morphine sulfate salt (Sigma-Aldrich) was freshly prepared on the day of the experiment in saline. All drugs were dissolved in 1 mL solution and administered by i.p. injection 1 h before all behavioral assays.
TNI.
TNI was performed to model nerve injury-induced neuropathic pain as described previously (53, 65). Using isoflurane anesthesia (4% induction and 2% maintenance), we isolated the right sciatic nerve under aseptic surgical conditions by blunt dissection of the femoral biceps muscle, and the tibial nerve was tightly ligated with 5–0 silk and transected distal to the ligation. An additional 2–3 mm of the distal nerve stump was removed to prevent reinnervation by the proximal nerve. The overlying muscle and skin was then sutured in two separate layers. Sham-injured animals were subjected to all preceding procedures with the exception of ligation and transection. All animals were returned to the housing facility and allowed to survive for 28 d.
Assessment of Tactile Hypersensitivity.
Mechanical stimuli were applied with seven filaments, each differing in the bending force delivered (10, 20, 40, 60, 80, 100, and 120 mN) and fitted with a flat tip and fixed diameter of 0.2 mm. The filaments were tested in the order of ascending force, with each filament delivered for 1 s. The withdrawal threshold stimulus was sequentially based on ascending force. A Hill equation was fitted to the function based on the percentage of specific responses to six stimulations to elicit a 50% withdrawal rate.
Foot Withdrawal to Thermal Stimulus.
To evaluate the PWT to thermal stimulation, we used the Hargreaves’ plantar test apparatus (Ugo Basile) (66). Measurements of the withdrawal latency of the paw began after the rats were habituated to the testing environment (infrared setting = 70). The measurements were repeated four times at 5-min intervals; the initial pair of measurements was not used. The averages of the three remaining pairs of measurements were employed as data.
Synthesis.
The synthesis route that was used to prepare compounds in this work and the structural characterization of the compounds are provided in SI Appendix.
Statistical Analysis.
Differences between means were compared by Student t tests. The biotinylation data were analyzed using an unpaired Student t test. The electrophysiological data were analyzed using an unpaired Student t test (Figs. 3 B and C and 4B) or a paired Student t test (Figs. 3D and 4A). Behavioral threshold values were statistically analyzed, and the significance of differences between the average of at least two preinjection tests and the mean obtained for each postinjection test was analyzed. In all tests, baseline data were obtained for the TNI-injured groups before drug or vehicle administration and were compared with the baseline values by repeated-measures ANOVA followed by the post hoc Tukey’s multiple-comparison test. A P value less than 0.05 was considered to indicate statistical significance between treatment and nontreatment groups.
Supplementary Material
Acknowledgments
The research was supported by a Clinical and Translational Sciences Institute Project Development Team grant (to S.O.M. and F.A.W.) and by the Indiana University Purdue University Indianapolis Research Support Fund (to S.O.M.). X.C. and A.G.O. were supported by NIH Grant R01HL115140. A.G.O., M.S.R., and F.A.W. were supported by Biomedical Laboratory Research and Development Merit Review Award I01BX002209 (from the US Department of Veterans Affairs) and the Indiana State Department of Health Spinal Cord and Brain Injury Fund. Computer time on the Big Red supercomputer at Indiana University is supported in part by Lilly Endowment, Inc., through its support for the Indiana University Pervasive Technology Institute, and in part by the Indiana Metabolomics and Cytomics (METACyt) Initiative.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813157115/-/DCSupplemental.
References
- 1.Atlas D. The voltage-gated calcium channel functions as the molecular switch of synaptic transmission. Annu Rev Biochem. 2013;82:607–635. doi: 10.1146/annurev-biochem-080411-121438. [DOI] [PubMed] [Google Scholar]
- 2.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 3.Catterall WA. Structure and modulation of Na+ and Ca2+ channels. Ann N Y Acad Sci. 1993;707:1–19. doi: 10.1111/j.1749-6632.1993.tb38038.x. [DOI] [PubMed] [Google Scholar]
- 4.Catterall WA. Molecular properties of sodium and calcium channels. J Bioenerg Biomembr. 1996;28:219–230. doi: 10.1007/BF02110697. [DOI] [PubMed] [Google Scholar]
- 5.Catterall WA. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24:307–323. doi: 10.1016/s0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann F, Biel M, Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann F, Lacinová L, Klugbauer N. Voltage-dependent calcium channels: From structure to function. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- 8.Dolphin AC. A short history of voltage-gated calcium channels. Br J Pharmacol. 2006;147:S56–S62. doi: 10.1038/sj.bjp.0706442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arikkath J, Campbell KP. Auxiliary subunits: Essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda T, et al. Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci. 2004;20:1–13. doi: 10.1111/j.1460-9568.2004.03434.x. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, et al. Structure of the voltage-gated calcium channel Ca(v)1.1 at 3.6 Å resolution. Nature. 2016;537:191–196. doi: 10.1038/nature19321. [DOI] [PubMed] [Google Scholar]
- 12.Altier C, et al. Differential role of N-type calcium channel splice isoforms in pain. J Neurosci. 2007;27:6363–6373. doi: 10.1523/JNEUROSCI.0307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourinet E, Zamponi GW. Voltage gated calcium channels as targets for analgesics. Curr Top Med Chem. 2005;5:539–546. doi: 10.2174/1568026054367610. [DOI] [PubMed] [Google Scholar]
- 14.Staats PS, Hekmat H, Staats AW. The psychological behaviorism theory of pain and the placebo: Its principles and results of research application. Adv Psychosom Med. 2004;25:28–40. doi: 10.1159/000079056. [DOI] [PubMed] [Google Scholar]
- 15.Bauer CS, et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Pinzon MA, Yenari MA, Sun GH, Kunis DM, Steinberg GK. SNX-111, a novel, presynaptic N-type calcium channel antagonist, is neuroprotective against focal cerebral ischemia in rabbits. J Neurol Sci. 1997;153:25–31. doi: 10.1016/s0022-510x(97)00196-2. [DOI] [PubMed] [Google Scholar]
- 18.Dolphin AC. Calcium channel diversity: Multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Richards MW, Butcher AJ, Dolphin AC. Ca2+ channel beta-subunits: Structural insights AID our understanding. Trends Pharmacol Sci. 2004;25:626–632. doi: 10.1016/j.tips.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Van Petegem F, Clark KA, Chatelain FC, Minor DL., Jr Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell DC, et al. Biophysical properties, pharmacology, and modulation of human, neuronal L-type (alpha(1D), Ca(V)1.3) voltage-dependent calcium currents. J Neurophysiol. 2001;85:816–827. doi: 10.1152/jn.2001.85.2.816. [DOI] [PubMed] [Google Scholar]
- 22.Van Petegem F, Duderstadt KE, Clark KA, Wang M, Minor DL., Jr Alanine-scanning mutagenesis defines a conserved energetic hotspot in the CaValpha1 AID-CaVbeta interaction site that is critical for channel modulation. Structure. 2008;16:280–294. doi: 10.1016/j.str.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butcher AJ, Leroy J, Richards MW, Pratt WS, Dolphin AC. The importance of occupancy rather than affinity of CaV(beta) subunits for the calcium channel I-II linker in relation to calcium channel function. J Physiol. 2006;574:387–398. doi: 10.1113/jphysiol.2006.109744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opatowsky Y, Chomsky-Hecht O, Kang MG, Campbell KP, Hirsch JA. The voltage-dependent calcium channel beta subunit contains two stable interacting domains. J Biol Chem. 2003;278:52323–52332. doi: 10.1074/jbc.M303564200. [DOI] [PubMed] [Google Scholar]
- 25.Cantí C, et al. Evidence for two concentration-dependent processes for beta-subunit effects on alpha1B calcium channels. Biophys J. 2001;81:1439–1451. doi: 10.1016/S0006-3495(01)75799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cukuroglu E, Engin HB, Gursoy A, Keskin O. Hot spots in protein-protein interfaces: Towards drug discovery. Prog Biophys Mol Biol. 2014;116:165–173. doi: 10.1016/j.pbiomolbio.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Kortemme T, Baker D. A simple physical model for binding energy hot spots in protein-protein complexes. Proc Natl Acad Sci USA. 2002;99:14116–14121. doi: 10.1073/pnas.202485799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clackson T, Wells JA. A hot spot of binding energy in a hormone-receptor interface. Science. 1995;267:383–386. doi: 10.1126/science.7529940. [DOI] [PubMed] [Google Scholar]
- 29.Keskin O, Ma B, Nussinov R. Hot regions in protein–Protein interactions: The organization and contribution of structurally conserved hot spot residues. J Mol Biol. 2005;345:1281–1294. doi: 10.1016/j.jmb.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 30.Bogan AA, Thorn KS. Anatomy of hot spots in protein interfaces. J Mol Biol. 1998;280:1–9. doi: 10.1006/jmbi.1998.1843. [DOI] [PubMed] [Google Scholar]
- 31.Gohlke H, Kiel C, Case DA. Insights into protein-protein binding by binding free energy calculation and free energy decomposition for the Ras-Raf and Ras-RalGDS complexes. J Mol Biol. 2003;330:891–913. doi: 10.1016/s0022-2836(03)00610-7. [DOI] [PubMed] [Google Scholar]
- 32.Metz A, et al. Hot spots and transient pockets: Predicting the determinants of small-molecule binding to a protein-protein interface. J Chem Inf Model. 2012;52:120–133. doi: 10.1021/ci200322s. [DOI] [PubMed] [Google Scholar]
- 33.Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 34.Pelay-Gimeno M, Glas A, Koch O, Grossmann TN. Structure-based design of inhibitors of protein-protein interactions: Mimicking peptide binding epitopes. Angew Chem Int Ed Engl. 2015;54:8896–8927. doi: 10.1002/anie.201412070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laraia L, McKenzie G, Spring DR, Venkitaraman AR, Huggins DJ. Overcoming chemical, biological, and computational challenges in the development of inhibitors targeting protein-protein interactions. Chem Biol. 2015;22:689–703. doi: 10.1016/j.chembiol.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arkin MR, Tang Y, Wells JA. Small-molecule inhibitors of protein-protein interactions: Progressing toward the reality. Chem Biol. 2014;21:1102–1114. doi: 10.1016/j.chembiol.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aeluri M, et al. Small molecule modulators of protein-protein interactions: Selected case studies. Chem Rev. 2014;114:4640–4694. doi: 10.1021/cr4004049. [DOI] [PubMed] [Google Scholar]
- 38.Thompson AD, Dugan A, Gestwicki JE, Mapp AK. Fine-tuning multiprotein complexes using small molecules. ACS Chem Biol. 2012;7:1311–1320. doi: 10.1021/cb300255p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watkins AM, Arora PS. Structure-based inhibition of protein-protein interactions. Eur J Med Chem. 2015;94:480–488. doi: 10.1016/j.ejmech.2014.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azzarito V, Long K, Murphy NS, Wilson AJ. Inhibition of α-helix-mediated protein-protein interactions using designed molecules. Nat Chem. 2013;5:161–173. doi: 10.1038/nchem.1568. [DOI] [PubMed] [Google Scholar]
- 41.Petros AM, et al. Discovery of a potent inhibitor of the antiapoptotic protein Bcl-xL from NMR and parallel synthesis. J Med Chem. 2006;49:656–663. doi: 10.1021/jm0507532. [DOI] [PubMed] [Google Scholar]
- 42.Wilson CG, Arkin MR. Small-molecule inhibitors of IL-2/IL-2R: Lessons learned and applied. Curr Top Microbiol Immunol. 2011;348:25–59. doi: 10.1007/82_2010_93. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Aguilar A, Bernard D, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction (MDM2 inhibitors) in clinical trials for cancer treatment. J Med Chem. 2015;58:1038–1052. doi: 10.1021/jm501092z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halgren T. New method for fast and accurate binding-site identification and analysis. Chem Biol Drug Des. 2007;69:146–148. doi: 10.1111/j.1747-0285.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- 45.Xu D, Jalal SI, Sledge GW, Meroueh SO. Small-molecule binding sites to explore protein-protein interactions in the cancer proteome. Mol Biosyst. 2016;12:3067–3087. doi: 10.1039/c6mb00231e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khanna M, et al. Targeting multiple conformations leads to small molecule inhibitors of the uPAR·uPA protein-protein interaction that block cancer cell invasion. ACS Chem Biol. 2011;6:1232–1243. doi: 10.1021/cb200180m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rullo AF, et al. Re-engineering the immune response to metastatic cancer: Antibody-recruiting small molecules targeting the urokinase receptor. Angew Chem Int Ed Engl. 2016;55:3642–3646. doi: 10.1002/anie.201510866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konopleva M, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Jerabek-Willemsen M, Wienken CJ, Braun D, Baaske P, Duhr S. Molecular interaction studies using microscale thermophoresis. Assay Drug Dev Technol. 2011;9:342–353. doi: 10.1089/adt.2011.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seidel SA, et al. Label-free microscale thermophoresis discriminates sites and affinity of protein-ligand binding. Angew Chem Int Ed Engl. 2012;51:10656–10659. doi: 10.1002/anie.201204268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasuda T, Lewis RJ, Adams DJ. Overexpressed Ca(v)beta3 inhibits N-type (Cav2.2) calcium channel currents through a hyperpolarizing shift of ultra-slow and closed-state inactivation. J Gen Physiol. 2004;123:401–416. doi: 10.1085/jgp.200308967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buraei Z, Yang J. Structure and function of the β subunit of voltage-gated Ca2+ channels. Biochim Biophys Acta. 2013;1828:1530–1540. doi: 10.1016/j.bbamem.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldman P, Due MR, Ripsch MS, Khanna R, White FA. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation. 2012;9:180. doi: 10.1186/1742-2094-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Due MR, et al. Carbamazepine potentiates the effectiveness of morphine in a rodent model of neuropathic pain. PLoS One. 2014;9:e107399. doi: 10.1371/journal.pone.0107399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heblich F, Tran Van Minh A, Hendrich J, Watschinger K, Dolphin AC. Time course and specificity of the pharmacological disruption of the trafficking of voltage-gated calcium channels by gabapentin. Channels (Austin) 2008;2:4–9. doi: 10.4161/chan.2.1.6045. [DOI] [PubMed] [Google Scholar]
- 56.Bernstein GM, Jones OT. Kinetics of internalization and degradation of N-type voltage-gated calcium channels: Role of the alpha2/delta subunit. Cell Calcium. 2007;41:27–40. doi: 10.1016/j.ceca.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Lai J, Porreca F, Hunter JC, Gold MS. Voltage-gated sodium channels and hyperalgesia. Annu Rev Pharmacol Toxicol. 2004;44:371–397. doi: 10.1146/annurev.pharmtox.44.101802.121627. [DOI] [PubMed] [Google Scholar]
- 58.Berman HM, et al. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Word JM, Lovell SC, Richardson JS, Richardson DC. Asparagine and glutamine: Using hydrogen atom contacts in the choice of side-chain amide orientation. J Mol Biol. 1999;285:1735–1747. doi: 10.1006/jmbi.1998.2401. [DOI] [PubMed] [Google Scholar]
- 60.Sanner MF. A component-based software environment for visualizing large macromolecular assemblies. Structure. 2005;13:447–462. doi: 10.1016/j.str.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Lin Y, McDonough SI, Lipscombe D. Alternative splicing in the voltage-sensing region of N-Type CaV2.2 channels modulates channel kinetics. J Neurophysiol. 2004;92:2820–2830. doi: 10.1152/jn.00048.2004. [DOI] [PubMed] [Google Scholar]
- 62.Chen X, et al. Molecular determinants of the sensitivity to Gq/11-phospholipase C-dependent gating, Gd3+ potentiation, and Ca2+ permeability in the transient receptor potential canonical type 5 (TRPC5) channel. J Biol Chem. 2017;292:898–911. doi: 10.1074/jbc.M116.755470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chakraborty S, et al. Bromoenol lactone inhibits voltage-gated Ca2+ and transient receptor potential canonical channels. J Pharmacol Exp Ther. 2011;339:329–340. doi: 10.1124/jpet.111.183673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
- 65.Xie JY, et al. Sustained relief of ongoing experimental neuropathic pain by a CRMP2 peptide aptamer with low abuse potential. Pain. 2016;157:2124–2140. doi: 10.1097/j.pain.0000000000000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhangoo S, et al. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: A mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.