Abstract
The aim of this study was to identify sleep-promoting substance from Polygonatum sibiricum rhizome extract (PSE) with the regulation of sleep architecture. PSE showed a decrease in sleep latency time and an increase in the sleeping time. In the electroencephalography analysis of rats, PSE (150 mg/kg) showed an increase of non-rapid eye movement by 38% and a decrease of rapid eye movement by 31% compared to the control. This sleep-promoting activity was found to be involved in the GABAA-BDZ receptor. The chemical structure of the pure compound was determined by the 1H and 13C nuclear magnetic resonance spectroscopy and gas chromatography mass spectrometry analysis; active compound was glyceryl-1-monolinoleate. The commercial standard glyceryl-1-monolinoleate showed a similar inhibitory concentration on [3H]-flumazenil binding to GABAA-BDZ receptors with final active fraction of PSE. The results indicate that glyceryl-1-monolinoleate is a major active compound responsible for the PSE-derived sleep promotion.
Keywords: Glyceryl monolinoleate, Polygonatum sibiricum, Insomnia, Sleep, GABA type A-benzodiazepine receptor
Introduction
Insomnia is one of the most common sleep disorder with symptoms such as difficulties in initiation and continuation of sleep and may lead to mental and physical impairments (Sivertsen et al., 2009). In particular, continued deprivation of sleep may cause increased heart rate, high blood pressure, and lethargic activity (Kato et al., 2000). While several pharmacological agents including benzodiazepines, barbiturates, and antidepressants could alleviate insomnia (Brattstrom, 2007), continuous administration of such medications accompany side effects such as drug dependence, daytime sedation, and development of tolerance (Fang et al., 2010). Hence, alternative therapies using dietary supplements and natural substances are gaining recognition. Herbal medicine is one of the most frequently used interventions for the treatment of insomnia for thousands of years (Shi et al., 2014). For instance, valerian (Valeriana officinalis) roots, alone or in combination with hops (Humulus lupulus L.), chamomile (Matricaria chamomilla), and passion flower (Passiflora), has been used as calming and sleep-promoting herbs (Abourashed et al., 2004; Srivastava et al., 2010; Villet et al., 2016). These herbal treatments have been reported to induce sedative effects via γ-aminobutyric acid-ergic (GABAergic) or serotonergic neurotransmission regulation in animals and humans (Attele et al., 2000).
Polygonatum sibiricum, commonly known as Hwang Jeong (a Solomon’s seal), belongs to the family Liliaceae and has been used as a traditional medicinal herb in East Asia., The roots of these medicinal plants are also widely used as tea particularly in Korea (Debnath et al., 2013b). The extract of P. sibiricum has been reported to exhibit hypoglycemic, cardiotonic, antioxidant, and anti-inflammatory activities and is known to reduce lipid levels, fight aging, and strengthen immune system (Debnath et al., 2013b; Kato and Miura, 1994; Zheng et al., 1998). Previous studies have shown that P. sibiricum rhizome contains polysaccharides (Liu et al., 2007), steroidal saponins (Son et al., 1990), flavones (Chopin et al., 1977), lignins (Sun and Li, 2001), and alkaloids (Sun et al., 2005). Nonetheless, the effect of P. sibiricum on sleep promotion and its active compound(s) has not been studied.
In this study, we measured the sleep promotion effect of P. sibiricum through pentobarbital-induced sleep test in mice. Furthermore, P. sibiricum-derived sleep promoting component was isolated through bioassay-guided fractionation and open column chromatography followed by elucidation of the chemical structure by gas chromatography mass spectrometry (GC–MS) and 1H and 13C nuclear magnetic resonance (NMR) spectroscopy.
Materials and methods
Materials
Pentobarbital and isoflurane were purchased from Hanlim Pharm. Co. Ltd. (Seoul, Korea) and Troikaa Pharmaceutical Ltd. (Gujarat, India), respectively. For the GABAA-BDZ receptor-binding assay, a radioligand, flumazenil (Ro 15-1788), was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA, USA). All other chemicals and reagents were of the highest grade available.
Plant material and preparation of extracts
The rhizome of P. sibiricum was provided by the Korea Oriental Medicine Industry Association (Seoul, Korea) and subjected to extraction with distilled water at 80 °C for 2 h, followed by another extraction with fresh distilled water for 1 h. Both batches of aqueous extracts were pooled, filtered, and concentrated by evaporation at 60 °C in a rotary evaporator (R-100, Büchi Labortechnik AG, Flawil, Switzerland). The concentrate was lyophilized to obtain P. sibiricum extract (PSE), then stored at 4 °C until further analysis (Debnath et al., 2013a).
Animals
Male ICR mice (4 weeks old, 18–22 g) and Sprague–Dawley rats (8 weeks old, 180–200 g) were purchased from Orient Bio Inc. (Seongnam, Korea). All animals were housed in cages at 24 °C and 55% relative humidity in a 12 h light/dark cycle and acclimated for 1 week while food and water were freely available. All animal experiments were approved by the Korea University Animal Care Committee (KUIACUC-2017-49, Seoul, Korea).
The pentobarbital-induced sleep test
The mice were fasted for 24 h before the experiment and the experiment was conducted between 1:00 and 5:00 pm. For oral administration, benzodiazepine alprazolam (BDZ, 2.5 mg/kg) and PSE (50, 100, and 150 mg/kg) were resuspended in saline and glyceryl-1-monolinoleate (5, 10, and 20 mg/kg) was resuspended in 4% (v/v) dimethyl sulfoxide. Pentobarbital (35 and 42 mg/kg) was intraperitoneally injected 45 min after the oral administration of samples. After injection, the mice were placed in individual cages and subjected to measurements of sleep latency and duration. Sleep latency was defined as the period between pentobarbital injection and sleep onset, and sleep duration denotes the time elapsed between the righting reflex loss and recovery. Mice that failed to fall asleep within 10 min after pentobarbital injection were excluded from the experiments (Yang et al., 2013).
Electroencephalography (EEG) recordings and analysis
Rats were anesthetized with 2% (v/v) isoflurane for electrode insertion surgery, and anesthesia was maintained with 1% (v/v) isoflurane in an oxygen/air mixture using a gas anesthesia mask on a stereotaxic instrument frame. The scalp hair of rats was shaved, disinfected with 70% (v/v) ethanol, and the skin was opened to expose the skull. Four holes were drilled into the skull and small screws with electrode were placed on striatum, cortex and hippocampus, then fixed with dental cement. All rats were injected with antibiotics and individually housed in a cage at a temperature-controlled facility with free access to water and food. After 7 days for recovery, the rats were randomly subdivided into control and treatment groups. The experiments were conducted in the daytime between 10 am and 5 pm for 4 days. BDZ (2.5 mg/kg) and PSE (100, 150 mg/kg) were administered orally 1 h before the experimental analysis; EEG signals were amplified, filtered (0.5–30.0 Hz), recorded, and stored using Iox2 (version 2.8.0.13, emka Technologies, Paris, France). EEG spectra were analyzed in 1 Hz frequency bins and in standard frequency bands (β: 12–30 Hz; α: 9–12 Hz; theta (θ): 4–9 Hz; δ 0.5–4 Hz). After each recording, fast Fourier transform (FFT) was performed every 2 s, and the FFT data were averaged in the range of 0–30 Hz for 10 s intervals to calculate the wake and sleep time in the ecgAUTO3 software (version 3.3.0.20, emka Technologies).
Gas chromatography
The chromatographic separation was performed using GC-14B (Shimadzu, Tokyo, Japan) equipped with a flame ionization detector and a DB-5 column (0.25 μm film thickness × 0.53 mm diameter × 30 m length). Helium was used as a carrier gas at a flow rate of 12.4 mL/min. The oven temperature was maintained at 80 °C for 2 min and increased to 100 °C at 2 °C/min and from 100 °C to 200 °C at 20 °C/min (holding time, 3 min). The samples (3 μL) were injected at a split ratio of 3:1 at an injector temperature of 250 °C. The identification of glyceryl monolinoleate was performed by GC–MS using library.
Isolation and purification of sleep-promoting compound
The powdered P. sibiricum rhizome (800 g) was partitioned with ethyl acetate (EtOAc; 3 L × 2), n-butanol (3 L), and H2O (3 L). The extract of EtOAc (30 g) was added to silica gel column chromatography (SiO2 cc, 8 × 15 cm) and eluted with n-hexane–EtOAc (5:1 → 1:1, 5.0 L of each) to produce 12 fractions (SIL-1 to SIL-12); elution was monitored by TLC. Chromatographic separations were performed by a thin-layer chromatography (TLC) (Merck Co., Darmstadt, Germany) using glass plates precoated with silica and visualized under UV at 254 and 366 nm wavelengths by spraying 10% (v/v) sulfuric acid (H2SO4) staining reagent. Fractions SIL-7 and SIL-8 (Ve/Vt 0.49–0.65, 34 mg) were subjected to ODS cc and eluted with methanol (MeOH)–H2O (8:1, 0.9 L) to give 10 fractions (ODS-1 to ODS-10) to yield compound (ODS-7, 4.2 mg, Ve/Vt 0.45–0.82, ODS TLC Rf 0.31, MeOH–H2O = 10:1). The data for 1H and 13C NMR were acquired using a Bruker AVANCE II 400 (1H NMR at 400 MHz, 13C NMR at 100 MHz) spectrometer (Bruker, Germany) in pyridine-d5 with tetramethylsilane (TMS).
γ-Aminobutyric acid type A-benzodiazepine receptor-binding assay
The assay for evaluating the binding to GABAA-BDZ receptor was performed with some modifications in the previously described method (Risa et al., 2004). The cerebral cortex samples isolated from four male SD rats were homogenized with cold Tris–HCl buffer (30 mM, pH 7.4). The suspension was centrifuged at 27,000×g and 4 °C for 15 min. The pellet was washed with Tris–HCl buffer, resuspended in Tris–HCl buffer, and incubated at 37 °C for 30 min. Following incubation, the suspension was centrifuged at 27,000×g for 10 min. The final concentration of the suspension was adjusted to 33.3 µg protein in 100 μL binding buffer. The membrane suspension was mixed with test sample solution and [3H]-flumazenil and adjusted to 0.8 nM with binding buffer as the final buffer solution. The binding reaction was carried out on ice for 40 min and the unbound [3H]-flumazenil was separated using Whatman GF/B glass fiber filter (Brandel Inc., Gaithersburg, MD, USA) using harvester equipment. The filter was immersed in the scintillation cocktail solution and the radioligand-bound membrane was counted and calculated by liquid scintillation counter (Hidex, Finland). The evaluation of the total binding capacity and nonspecific binding capacity of the radioligand was performed by the following equation:
where DPM, disintegrations per minute; TB, total binding; and NSB, nonspecific binding.
This study was approved by the Korea University Animal Care Committee (Protocol # KUIACUC-2017-49, Seoul, Korea).
Statistical analyses
In the binding assay, EC50 values were calculated with Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA) and displacement binding curves were fitted to a one-site competition binding model. Statistical analyzes were performed using the Statistical Package for Social Sciences version 12.0 (SPSS Inc., Chicago, IL, USA). Differences among groups were evaluated by one-way analysis of variance (ANOVA) and Dunnett’s and Tukey’s multiple comparison test. All data are expressed as mean ± standard deviation (SD) and values of p < 0.05 were considered statistically significant.
Results and discussion
Sleep-promoting effect of P. sibiricum extract
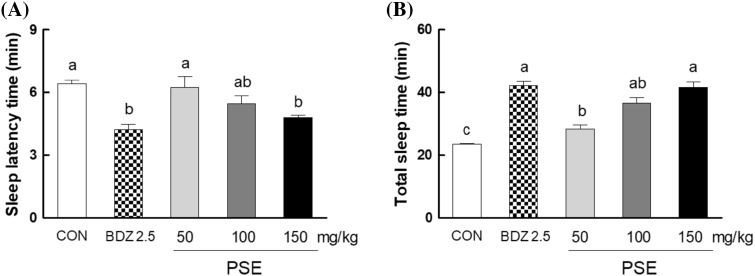
Figure 1 shows the effects of PSE (50, 100, and 150 mg/kg) on sleep latency and duration in pentobarbital (35 mg/kg)-induced sleep model. Oral administration of PSE significantly increased sleep duration in a dose dependent manner; PSE of 100 and 150 mg/kg increased total sleep time by 72 and 86%, respectively, compared with normal control (Fig. 1A). The low dose of PSE (50 mg/kg) showed a similar level of sleep duration to the normal control (Fig. 1A). In contrast, pentobarbital-induced sleep latency was shortened by PSE treatment. The administration of PSE (150 mg/kg) reduced sleep latency by 25% compared to the control group (Fig. 1B). This result showed that PSE enhances sleep with the decrease of sleep latency in pentobarbital-induced model.
Fig. 1.
Effects of P. sibiricum extract (at 50, 100, and 150 mg/kg) on sleep latency time (A) and total sleep time (B) in mice that received of pentobarbital (35 mg/kg, i.p.). CON: 0.9% NaCl (physiological saline) group (normal control), BDZ: benzodiazepine treatment (positive control). Data are expressed as mean ± standard error of the mean (SEM) for each group, n = 12. Different letters indicate significant differences at p < 0.05 by Tukey’s test
Sleep disorder or insomnia has been a serious problem to the people in recent years with the increase of insomoniac, resulting in a negative impact on mental and physical health (Lalley-Chareczko et al., 2017). Many attempts have been made to treat insomnia or sleep disorders using synthetic medicines, but drug-based treatment has not been free from side effects, deteriorating the quality of life (Gooneratne, 2008; Zhang et al., 2010). Thus, in recent years there has been increasing interest in effective and less toxic medicinal products and dietary supplements, and there is a growing need for alternative therapies such as natural product based therapies. In many studies, natural products were shown to exert an alleviating effect for sleep disorders. Valerian (Valeriana officinalis) roots administration have been known to shorten sleep onset in human (Balderer and Borbely, 1985). Lettuce (Lactuca sativa) extract was reported to increase seep duration in animal model (Ghorbani et al., 2013).
Effect of P. sibiricum extract on sleep pattern
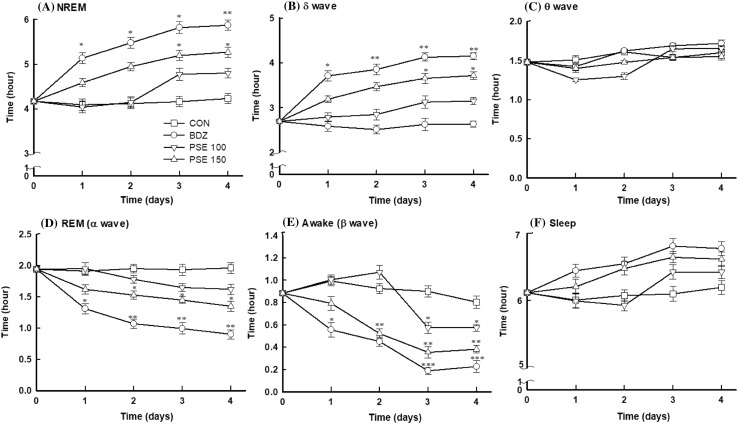
To determine the change of sleep pattern after PSE administration for 4 days, brain waves of PSE–treated rats was analyzed during sleep. The non-rapid eye movement (NREM) duration in rats administered with PSE was significantly increased in the time course (Fig. 2A). The rats treated with PSE low dose (100 mg/kg) showed a small increase in NREM from day 3, but until day 2, NREM time was not significantly changed (Fig. 2A). High dose (150 mg/kg) of PSE consistently increased NREM in which NREM was increased by 26% (p < 0.05) after 4 days compared to before administration (0 day), and the administration of benzodiazepine (BDZ, 2.5 mg/kg), a positive control, showed an increase of NREM by 41% compared with the normal control (p < 0.01) (Fig. 2A). In Delta waves, which represent deep sleep, PSE and BDZ treatments showed a consistent increase for 4 days, similar to those of NREM (Fig. 2B). In particular, PSE (150 mg/kg) increased the duration of delta wave by about 1.4 times (~ 3.7 h) compared with normal control, However, the theta wave, which means relatively shallower than delta wave, showed a slight increase in PSE treatments, compared to the control but there was no significant difference (Fig. 2C). This result showed that PSE improves the sleep quality by increasing deep sleep. In contrast, PSE significantly reduced rapid eye movement (REM) during sleep; REM was reduced by 31% in rats treated with PSE high dose (150 mg/kg) (Fig. 2D). Beta (β) wave, which refer to the awakening, was also significantly decreased with the PSE treatments (Fig. 2E). High dose of PSE (150 mg/kg) reduced awake time by 57% compared with the control (Fig. 2E). This PSE-induced regulation of NREM and REM results in the increase of total sleep time in PSE-treated animals (Fig. 2F). High dose of PSE (150 mg/kg) increased total sleep time by 8%. This result indicated that the PSE modulates the NREM and REM to improve sleep duration and quality.
Fig. 2.
Effects of P. sibiricum extract on sleep quantity and quality. The EEG analyses were conducted for 4 days, and P. sibiricum extract was administered orally 1 h before the experiments. CON: 0.9% NaCl (physiological saline) group (normal control), BDZ: benzodiazepine treatment (positive control), CON: control; PSE 100 or PSE 150: P. sibiricum extract at 100 or 150 mg/kg. Values are presented as mean ± standard error of the mean (SEM) for each group, n = 8. Asterisks indicate significant differences: *p < 0.05, **p < 0.01, and ***p < 0.001 by one-way ANOVA followed by Dunnett’s test
Our study shows that PSE significantly increases NREM during sleep while decreasing REM (Fig. 2A, D). These results show that PSE contributes to the improvement of total sleep time and with a positive change in the sleep architecture of NREM and REM. Thus, PSE has been shown to improve total sleep time and quality by increasing NREM, and in particular, increase the time that delta wave occur in NREM (Fig. 2B). The NREM consists of three stages, the first stage is the theta wave, and is in a comfortable wake state. Eye movements occur in two stages, and the third stage is characterized by slow delta waves (McKenna et al., 2017). In general, the body restores tissue and strengthens the immune system during this slow sleep (McKenna et al., 2017). Thus, we have found that PSE can promote a change in healthy sleep architecture.
Binding affinity of fractions
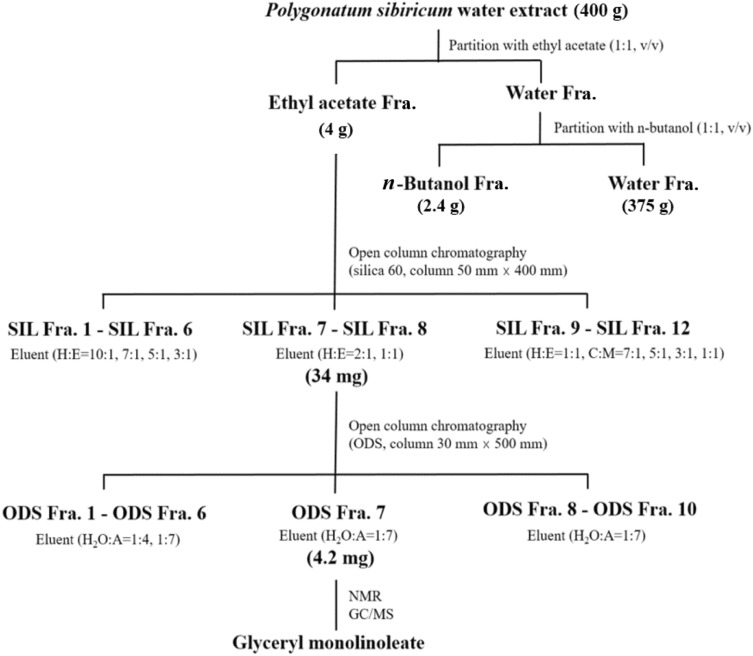
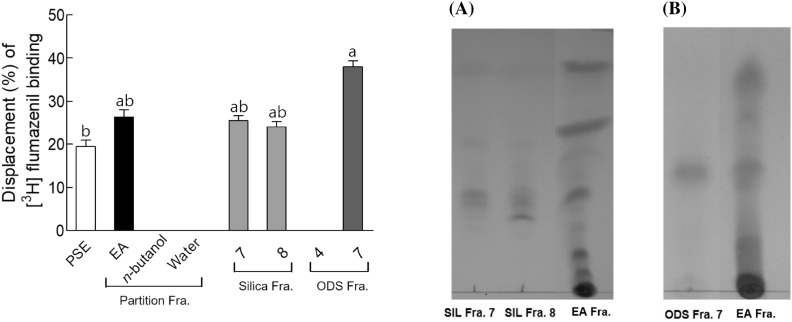
The basic scheme for the isolation of sleep-promoting component from PSE was illustrated in Fig. 3. The PSE was fractionated by solvent-partitioned extraction to yield three fractions: ethyl acetate, n-butanol, and remaining aqueous layer, which were evaluated for their sleep-promoting ability using GABAA-BDZ receptor assay. Of these, the ethyl acetate layer showed the most effective binding displacement with around 26% inhibition on antagonist (3H flumazenil) binding (Fig. 4). Therefore, the ethyl acetate fraction obtained from PSE was subjected to open column chromatography. Co-TLC (silica, chloroform/methanol, 10-1) profile of silica fractions was evaluated to elucidate the constituents from 12 different fractions (Fig. 4A). Among these fractions, fractions 7 (25.54%) and 8 (24.12%) showed the maximum binding displacement as compared with PSE (19.5%). Therefore, silica fractions 7 and 8 were further purified with ODS open column chromatography. Among the final 10 fractions obtained, ODS fraction 7 showed a single band when analyzed by TLC (ODS, acetone/acetonitrile, 1-10) (Fig. 4B) and showed the most effective binding displacement with 38.12% (Fig. 4). ODS fraction 7 was analyzed for the structure of the active component.
Fig. 3.
The schematic diagram of the isolation process for the sleep-inducing compound from Polygonatum sibiricum water extract. H:E (a mixture of hexane and ethyl acetate), C:M (a mixture of chloroform and methanol), H2O:A (a mixture of water and acetonitrile)
Fig. 4.
[3H]-flumazenil binding displacement (%) of fractions from Polygonatum sibiricum water extract to GABAA receptor and TLC patterns of SIL Fra. 7, 8 (A) and ODS Fra. 7 (B) with ethyl acetate fraction (EA) separated from Polygonatum sibiricum water extract. The concentration of samples was 0.5 mg/mL. PSE: P. sibiricum extract, EA: PSE ethyl acetate fraction, SIL: fractions of isolated with silica gel of PSE ethyl acetate fraction, ODS: fractions isolated by ODS after isolation using silica gel. Data are expressed as mean ± standard deviation (n = 3). Different letters indicate significant differences at p < 0.05 by Tukey’s test
During this purifying process, competitive binding assays on the GABA receptor was executed every steps of purification using isotopic antagonist ([3H]-flumazenil) of GABA receptor, because GABA receptor is a main machinery for sleep regulation (Agosto et al., 2008; Eban-Rothschild et al., 2018).
Identification of active compound
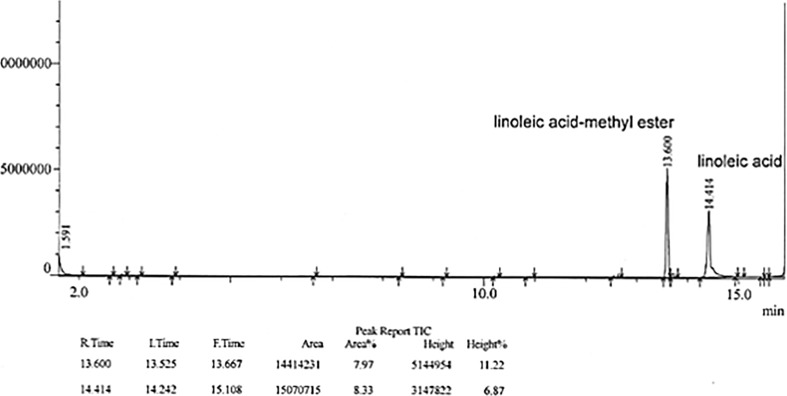
Gas chromatography mass spectrometry analysis of methyl ester derivatives of PSE revealed the presence of glyceryl monolinoleate (Fig. 5). Glyceryl monolinoleate was obtained as an amorphous powder.
Fig. 5.
GC-MS analysis of glyceryl monolinoleate isolated from P. sibiricum water extract
The analysis of compound showed a brown color on the silica gel TLC upon heating following treatment with 10% (v/v) aqueous H2SO4, indicating that compound may be a glyceride (Fig. 4B). Signals in 1H- and 13C-NMR (400 MHz, CD3OD-d4, δH) spectra of compound confirmed the presence of glycerol and fatty acid. Compound (glyceryl monolinoleate), a colorless oil, exhibited a value of [α]25D: + 5.0 (c 0.3, MeOH), EI-MS m/z: 354[M]+, and characteristic fragment ions of m/z 262 [M-2], followed by loss of glycerol in EI MS data. 1H NMR (400 MHz, CD3OD) δ5.35 (4H, m, olefine of linolenoyl), 4.04 (1H, dd, J = 11.2, 4.4 Hz, CH2OH of glyceryl, sn-1a), 3.96 (1H, dd, J = 11.2, 6.4 Hz, CH2OH of glyceryl, sn-1b), 3.71 (1H, dd, m, CHOH of glyceryl, sn-2), 3.44 (2H, dd, J = 11.2, 6.4 Hz, CH2OH of glyceryl, sn-3), 2.67 (2H, t, J = 6.0 Hz, double ally-methylene of linolenoyl, H-11′), 2.25 (2H, m, ally-methylene of linolenoyl, H-2′), 1.96 (4H, m, ally-methylene of linolenoyl, H-8′, 17′), 1.52 (2H, m, ally-methylene of linolenoyl, H-14′), 1.22 (24H, m, methylene of linolenoyl), and 0.80 (3H, t, J = 6.8, terminal methyl). 13C NMR (CD3OD) 175.4 (C-1′), 130.9 (C-9′), 130.8 (C- 10′), 129.1 (C-12′), 129.0 (C-13′), 71.1 (sn–C-2), 66.4 (sn–C-1), 64.0 (sn–C-3), 34.9–23.6 (C-methylene of linolenoyl), and 14.4 (C-18). The fatty acid type was determined using GC/MS analysis. Methylated fatty acid was obtained following alkaline hydrolysis and esterification of compound. The qualitative analysis of the fatty acid was conducted by comparing its molecular ion peaks ([M]+) and fragmentation ion peaks with those of Wily Library in GC/MS experiment. Compound was identified to be glyceryl monolinoleate. These results were in accordance with the spectroscopic data of glyceryl monolinoleate reported in a previous study (Qadir et al., 2017). The chemical structures of 1- and 2-glyceryl monolinoleate are shown in Fig. 6A.
Fig. 6.
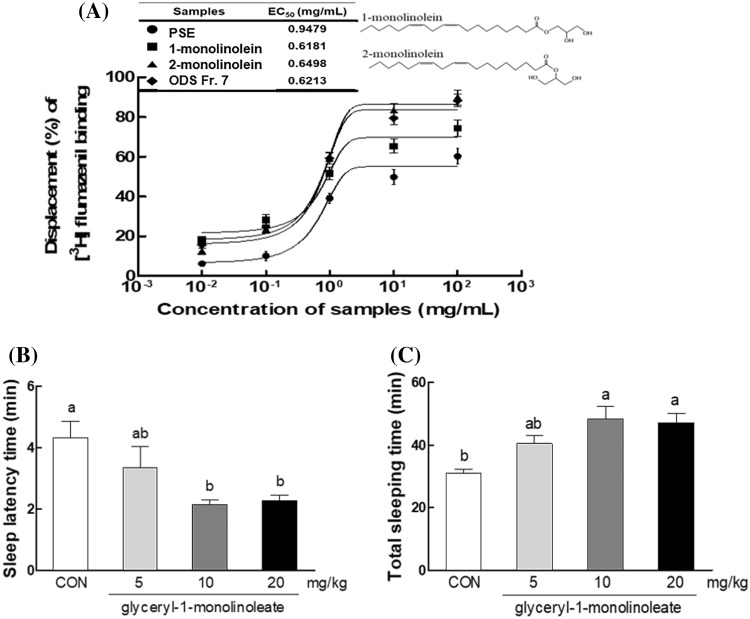
Displacement (%) of [3H]-flumazenil binding induced by glyceryl monolinoleate in GABAA receptor-binding assay (A), effect of glyceryl-1-monolinoleate (at 5, 10, 20 mg/kg) on sleep latency time (B), and total sleep time (C) in mice that received of pentobarbital (42 mg/kg, i.p.). CON: 0.9% NaCl (physiological saline) group (normal control). Data are expressed as mean ± standard error of the mean (SEM) for each group, n = 12. Different letters indicate significant differences at p < 0.05 by Tukey’s test
The present study showed, for the first time, that PSE-derived active component is glyceryl monolinoleate (Fig. 6). This lipid molecule was shown to be responsible for the PSE-derived sleep promotion based on the GABA receptor binding ability (Fig. 6A). Many studies have reported the changes in the central GABAergic neurotransmission in animals and humans administered with the extract of some sedative herbal plants (Choi et al., 2017; Johnston, 2005). For instance, kava–kava (Piper methysticum) exerts sedative and hypnotic effects based on positive allosteric modulation of GABAA receptors (Johnston, 2005). Valerian extract and its compound, valerenic acid have been known to have a strong affinity to GABAA receptor to exhibit sleep promoting effect (Choi et al., 2017). However, no study has been performed on the molecular targets of PSE for sleep regulation. PSE-mediated sleep promotion is also expected to be with the GABAergic action. However, detailed analysis on sleep-related neurotransmitters would be supported to confirm GABAergic mechanism of PSE in the future. Several studies have reported the isolation and biological effect of glyceryl monolinoleate from various plants (Okuyama et al., 2001; Yan et al., 2011). Glyceryl monolinoleate has been shown to inhibit writhing in mice (Okuyama et al., 2001). However, no study has been reported on the sleep-promoting effect of glyceryl monolinoleate.
Binding activity and sleep-promoting effect of P. sibiricum active compound
The EC50 value of PSE on [3H]-flumazenil binding to the GABAA-BDZ receptor was 0.9479 mg/mL, and ODS Fr. 7 had an EC50 value of 0.6213 mg/mL which lower than PSE. EC50 values of 1-monolinolein and 2-monolinolein, which are two isomers of glyceryl monolinoleate, on the [3H]-flumazenil binding to the GABAA-BDZ receptor was 0.6181 mg/mL, 0.6498 mg/mL, respectively (Fig. 6A). This result showed that 1-monolinolein is the active ingredient responsible for the sleep-promotion via the GABAA receptor binding.
In this study, glyceryl monolinoleate was tested for its sleep-promoting effect by using binding displacement activity with GABAA-BDZ receptor (Fig. 6A). Monoglycerides were shown to bind to GABAA receptor, and of two isoforms, glyceryl-1-monolinoleate displayed slightly higher activity than glyceryl-2-monolinoleate (Fig. 6A). The results on the relative activities of two glyceryl monolinoleate suggest that GABAA receptor-ligand binding affinity was slightly higher in the acyl ester at the primary position than that at the secondary position of glyceryl monolinoleate. This result indicated that glyceryl-1-monolinoleate could be a main component for sleep promotion from PSE. However, co-existence of two monolinoleate types in the final active fraction of PSE could not be ruled out.
Thus, the effects of glyceryl-1-monolinoleate (5, 10, and 20 mg/kg) on sleep latency and duration in pentobarbital (42 mg/kg) induced sleep model were evaluated. As a result, glyceryl-1-monolimoleate at 10 and 20 mg/kg significantly increased sleep duration (p < 0.05) compared to control group (Fig. 6C), and significantly decreased pentobarbital-induced sleep latency (p < 0.05) (Fig. 6B). This result showed that glyceryl-1-monolinoleate, which is an active compound of PSE, acts as a sleep active compound through the reduction of sleep latency and the promotion of sleep in the pentobarbital induced model.
Monoglyceride such as glyceryl monolinoleate has been known to be prevalent in natural resources, but its content is in a small level in animal or plants (Wang et al., 2017). The amount of glyceryl monolinoleate from PSE in current study is also very low (0.02%). This low yields of glyceryl monolinoleate from PSE is shown to be due to the its lowered endogenous level, in addition, water extraction process in this work may contribute to the its low yield. Thus, extraction using solvent such as alcohol may enhance this active compound from P. sibiricum. Many studies have been performed on the lipid molecules related to the sleep (Cravatt et al., 1995, Krueger et al., 1986). One of the most widely known lipid molecules is oleamide (McKinney and Cravatt, 2005; Yang et al., 1999). Oleamide is an amide form of fatty acid oleic acid, interestingly, similar to the glyceryl monolinoleate. It is endogenous lipid amid exist in brain or plasma in animal. In particular, it has been known to be accumulated in the cerebrospinal fluid during sleep deprivation to induce sleep in animals (Cravatt et al., 1995; McKinney and Cravatt, 2005). Furthermore, oleamide has been recognized to associated with the regulation of multiple neurotransmitters including GABA, 5-HT, ionotropic, canabinoid signaling (Coyne et al., 2002; Mendelson and Basile, 1999; Thomas et al., 1998; Yehuda et al., 1998; Yost et al., 1998). In addition, connection between fatty acid and sleep has been reported, although it is controversial (Cravatt et al., 1995; Irmisch et al., 2007). Therefore, structural similarity of PSE-derive glyceryl monolinoleate to oleamide is shown to be associated with the sleep promotion.
Besides GABAergic system, serotonin (5-hydroxytryptamine, 5-HT) is another neurotransmitter that is associated with the physiology of the sleep cycle in both vertebrates and invertebrates (Portas et al., 2000; Yuan et al., 2006). Since oleamide, a brain lipid derivative, also has been known to regulate serotonergic system to induce sleep, PSE-derived glyceryl monolinoleate may interact or regulate 5-HT receptor to regulate sleep behavior. Sleep-related neurotransmitters including serotonergic signaling would be examined on glyceryl monolinoleate in the next work.
We also found that glyceryl-1-monolinoleate, a sleep active compound isolated from PSE, has a sleep-promoting activity through pentobarbital-induced sleep testing, a common method for evaluating sleep enhancement in animals. However, attempts to isolate a single active compound from some plant extract may be meaningless. Because biological effects are often seen as synergistic effects and interactions between the various components of plant extract (Williamson, 2001). In some cases it may be possible to isolate a one active ingredient from a plant extract, but in general the plant extract contains a variety of psychoactive components and can thus exhibit a synergistic effect through the interaction of these ingredients (Heinrich et al., 2004). Therefore, we should not exclude that glyceryl-1-monolinoleate, which is a sleep active compound of P. sibiricum extract, in this study may have synergistic effects with various components contained in the extract.
In conclusion, current study showed two main results. One is that PSE has a sleep promoting effect via pentobarbital-induced condition and analysis of sleep architecture, in which PSE mediated sleep promotion by increasing NREM and delta wave. Second PSE-derived sleep promoting substance is glyceryl monolinoleate as evidenced by GC–MS and NMR analysis. To the best of our knowledge, this is the first study to identify and confirm glyceryl monolinoleate as a sleep-promoting compound from P. sibiricum. This study suggests that glyceryl monolinoleate isolated from PSE may exhibit potential role as a sleep-promoting phytochemical. Further research will be needed to examine the sleep-inducing effect of glyceryl-1-monolinoleate in animals and humans.
Acknowledgements
This research was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through the High Value-Added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (116004-02-2-HD020).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Abourashed EA, Koetter U, Brattstrom A. In vitro binding experiments with a valerian, hops and their fixed combination extract (Ze91019) to selected central nervous system receptors. Phytomedicine. 2004;11:633–638. doi: 10.1016/j.phymed.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABA A receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat. Neurosci. 2008;11:354–359. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attele AS, Xie JT, Yuan CS. Treatment of insomnia: an alternative approach. Altern. Med. Rev. 2000;5:249–259. [PubMed] [Google Scholar]
- Balderer G, Borbely AA. Effect of valerian on human sleep. Psychopharmacology (Berl.) 1985;87:406–409. doi: 10.1007/BF00432503. [DOI] [PubMed] [Google Scholar]
- Brattstrom A. Scientific evidence for a fixed extract combination (Ze 91019) from valerian and hops traditionally used as a sleep-inducing aid. Wien. Med. Wochenschr. 2007;157:367–370. doi: 10.1007/s10354-007-0442-6. [DOI] [PubMed] [Google Scholar]
- Choi HS, Ko BS, Kim HD, Hong KB, Suh HJ. Effect of valerian/hop mixture on sleep-related behaviors in Drosophila melanogaster. Biol. Pharm. Bull. 2017;40:1101–1110. doi: 10.1248/bpb.b17-00262. [DOI] [PubMed] [Google Scholar]
- Chopin J, Dellamonica G, Besson E, Skrzypczakowa L, Budzianowski J, Mabry TJ. C-Galactosylflavones from Polygonatum-multiflorum. Phytochemistry. 1977;16:1999–2001. doi: 10.1016/0031-9422(77)80112-X. [DOI] [Google Scholar]
- Coyne L, Lees G, Nicholson RA, Zheng J, Neufield KD. The sleep hormone oleamide modulates inhibitory ionotropic receptors in mammalian CNS in vitro. Br. J. Pharmacol. 2002;135:1977–1987. doi: 10.1038/sj.bjp.0704651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- Debnath T, Park DK, Lee BR, Jin HL, Lee SY, Samad NB, Lim BO. Antioxidant activity of inonotus obliquus grown on germinated brown rice extracts. J. Food Biochem. 2013;37:456–464. doi: 10.1111/j.1745-4514.2012.00658.x. [DOI] [Google Scholar]
- Debnath T, Park SR, Jo JE, Lim BO. Antioxidant and anti-inflammatory activity of Polygonatum sibiricum rhizome extracts. Asian Pac. J. Trop. Dis. 2013;3:308–313. doi: 10.1016/S2222-1808(13)60074-2. [DOI] [Google Scholar]
- Eban-Rothschild A, Appelbaum L, de Lecea L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. 2018;43:937–952. doi: 10.1038/npp.2017.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Hao JF, Zhou HY, Zhu LX, Wang JH, Song FQ. Pharmacological studies on the sedative-hypnotic effect of semen Ziziphi spinosae (Suanzaoren) and radix et rhizoma Salviae miltiorrhizae (Danshen) extracts and the synergistic effect of their combinations. Phytomedicine. 2010;17:75–80. doi: 10.1016/j.phymed.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Ghorbani A, Rakhshandeh H, Sadeghnia HR. Potentiating effects of Lactuca sativa on pentobarbital-induced sleep. Iran J. Pharm. Res. 2013;12:401–406. [PMC free article] [PubMed] [Google Scholar]
- Gooneratne NS. Complementary and alternative medicine for sleep disturbances in older adults. Clin. Geriatr. Med. 2008;24:121–138. doi: 10.1016/j.cger.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich M, Barnes J, Gibbons S, Williamson E. Fundamentals of pharmacognosy and phytotherapy. Churchill Livingstone: Elsevier Science Ltd., UK; 2004. [Google Scholar]
- Irmisch G, Schlafke D, Gierow W, Herpertz S, Richter J. Fatty acids and sleep in depressed inpatients. Prostaglandins Leukot. Essent. Fatty Acids. 2007;76:1–7. doi: 10.1016/j.plefa.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Johnston GA. GABAA receptor channel pharmacology. Curr. Pharm. Des. 2005;11:1867–1885. doi: 10.2174/1381612054021024. [DOI] [PubMed] [Google Scholar]
- Kato A, Miura T. Hypoglycemic action of the rhizomes of Polygonatum officinale in normal and diabetic mice. Planta Med. 1994;60:201–203. doi: 10.1055/s-2006-959458. [DOI] [PubMed] [Google Scholar]
- Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–1175. doi: 10.1161/01.HYP.35.5.1173. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Kubillus S, Shai S, Davenne D. Enhancement of slow-wave sleep by endotoxin and lipid A. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1986;20:7. doi: 10.1152/ajpregu.1986.251.3.R591. [DOI] [PubMed] [Google Scholar]
- Lalley-Chareczko L, Segal A, Perlis ML, Nowakowski S, Tal JZ, Grandner MA. Sleep disturbance partially mediates the relationship between intimate partner violence and physical/mental health in women and men. J. Interpers. Violence. 2017;32:2471–2495. doi: 10.1177/0886260515592651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Dong Q, Dong XT, Fang JN, Ding K. Structural investigation of two neutral polysaccharides isolated from rhizome of Polygonatum sibiricum. Carbohyd. Polym. 2007;70:304–309. doi: 10.1016/j.carbpol.2007.04.012. [DOI] [Google Scholar]
- McKenna JT, Zielinski MR, McCarley RW. Neurobiology of REM sleep, NREM sleep homeostasis, gamma band oscillations, Springer, New York, NY. pp 55–77 (2017)
- McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu. Rev. Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Basile AS. The hypnotic actions of oleamide are blocked by a camabinoid receptor antagonist. Neuroreport. 1999;10:3237–3239. doi: 10.1097/00001756-199910190-00021. [DOI] [PubMed] [Google Scholar]
- Okuyama E, Hasegawa T, Matsushita T, Fujimoto H, Ishibashi M, Yamazaki M. Analgesic components of saposhnikovia root (Saposhnikovia divaricata) Chem. Pharm. Bull. 2001;49:154–160. doi: 10.1248/cpb.49.154. [DOI] [PubMed] [Google Scholar]
- Portas CM, Bjorvatn B, Ursin R. Serotonin and the sleep/wake cycle: special emphasis on microdialysis studies. Prog. Neurobiol. 2000;60:13–35. doi: 10.1016/S0301-0082(98)00097-5. [DOI] [PubMed] [Google Scholar]
- Qadir A, Singh SP, Akhtar J, Ali A, Arif M. Phytochemical and GC-MS analysis of Saudi Arabian Ajwa variety of date seed oil and extracts obtained by the slow pyrolysis method. Orient. Pharm. Exp. Med. 2017;17:81–87. doi: 10.1007/s13596-017-0257-y. [DOI] [Google Scholar]
- Risa J, Risa A, Adsersen A, Gauguin B, Stafford GI, van Staden J, Jager AK. Screening of plants used in southern Africa for epilepsy and convulsions in the GABA(A)-benzodiazepine receptor assay. J. Ethnopharmacol. 2004;93:177–182. doi: 10.1016/j.jep.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Shi Y, Dong J-W, Zhao J-H, Tang L-N, Zhang J-J. Herbal insomnia medications that target GABAergic systems: a review of the psychopharmacological evidence. Curr. Neuropharmacol. 2014;12:289–302. doi: 10.2174/1570159X11666131227001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivertsen B, Krokstad S, Overland S, Mykletun A. The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J. Psychosom. Res. 2009;67:109–116. doi: 10.1016/j.jpsychores.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Son KH, Do JC, Kang SS. Steroidal saponins from the rhizomes of Polygonatum sibiricum. J. Nat. Prod. 1990;53:333–339. doi: 10.1021/np50068a010. [DOI] [PubMed] [Google Scholar]
- Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol. Med. Report. 2010;3:895–901. doi: 10.3892/mmr.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Li X. Studies on chemical constituents of Polygonatum sibiricum (II) Zhongcaoyao. 2001;32:586–588. [Google Scholar]
- Sun LR, Li X, Wang SX. Two new alkaloids from the rhizome of Polygonatum sibiricum. J. Asian Natural Prod. Res. 2005;7:127–130. doi: 10.1080/10286020310001625157. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Carson MJ, Sutcliffe JG. Oleamide-induced modulation of 5-hydroxytryptamine receptor-mediated signaling. Ann. N. Y. Acad. Sci. 1998;861:183–189. doi: 10.1111/j.1749-6632.1998.tb10190.x. [DOI] [PubMed] [Google Scholar]
- Villet S, Vacher V, Colas A, Danno K, Masson J-L, Marijnen P, Bordet M-F. Open-label observational study of the homeopathic medicine Passiflora compose for anxiety and sleep disorders. Homeopathy. 2016;105:84–91. doi: 10.1016/j.homp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Wang YM, Zhao JQ, Yang JL, Tao YD, Mei LJ, Shi YP. Chemical constituents from Ligularia purdomii (Turrill) Chittenden. Biochem. Syst. Ecol. 2017;72:8–11. doi: 10.1016/j.bse.2017.03.007. [DOI] [Google Scholar]
- Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8:401–409. doi: 10.1078/0944-7113-00060. [DOI] [PubMed] [Google Scholar]
- Yan L, Cheng XR, Zeng Q, Qin JJ, Zhang WD, Jin HZ. Phytane and neoclerodane diterpenes from the aerial parts of Inula nervosa wall. Biochem. Syst. Ecol. 2011;39:700–703. doi: 10.1016/j.bse.2011.06.001. [DOI] [Google Scholar]
- Yang H, Lee YC, Han KS, Singh H, Yoon M, Park JH, Cho CW, Cho S. Green and gold kiwifruit peel ethanol extracts potentiate pentobarbital-induced sleep in mice via a GABAergic mechanism. Food Chem. 2013;136:160–163. doi: 10.1016/j.foodchem.2012.07.111. [DOI] [PubMed] [Google Scholar]
- Yang JY, Wu CF, Song HR. Studies on the sedative and hypnotic effects of oleamide in mice. Arzneimittelforschung. 1999;49:663–667. doi: 10.1055/s-0031-1300479. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids and sleep: mini-review and hypothesis. Med. Hypotheses. 1998;50:139–145. doi: 10.1016/S0306-9877(98)90200-6. [DOI] [PubMed] [Google Scholar]
- Yost CS, Hampson AJ, Leonoudakis D, Koblin DD, Bornheim LM, Gray AT. Oleamide potentiates benzodiazepine-sensitive gamma-aminobutyric acid receptor activity but does not alter minimum alveolar anesthetic concentration. Anesth. Analg. 1998;86:1294–1300. doi: 10.1097/00000539-199806000-00031. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr. Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Zhang DY, Tashiro M, Shibuya K, Okamura N, Funaki Y, Yoshikawa T, Kato M, Yanai K. Next-day residual sedative effect after nighttime administration of an over-the-counter antihistamine sleep aid, diphenhydramine, measured by positron emission tomography. J. Clin. Psychopharmacol. 2010;30:694–701. doi: 10.1097/JCP.0b013e3181fa8526. [DOI] [PubMed] [Google Scholar]
- Zheng H, Dong Z, She J. Modernization of traditional chinese medicine and application. Beijing, China: Xueyuan Press; 1998. pp. 4071–4074. [Google Scholar]