Abstract
Propolis is a natural product produced by honeybees. It has antioxidant effects as well as antimicrobial, antiseptic, antibacterial, anti-inflammatory and antimutagenic properties. Except these important healthy properties some cytotoxic effects causing allergies also have been reported. In this study have been evaluated changes of phenolic compounds including allergens molecules found in propolis. Before biotransformation, propolis samples were treated with different solvent (ethanol and polyethylene glycol) to facilitate solvation of solid samples. Biotransformation was done by three different strains of Lactobacillus plantarum (10, 8014, ATCC). Results demonstrated the importance of used solvent/treatment for extraction procedure and strains of L. plantrum. The lowest values of main allergens were determined as 321 ng/mL for BCAFE, 320 ng/mL for 1.1 DMAECAFE and 8.02 ng/mL for CAPE. The study is the first work deal with evaluation of bioconversion of propolis by different L. plantarum strains and their effects on phenolic profile.
Keywords: Propolis, Allergic molecules, Biotransformation, L. plantarum, Phenols
Introduction
Propolis is a natural resinous bee product which is being used to block holes and cracks, to repair combs, to make comb strength and to defense against microorganism (Santos et al., 2003; Valle, 2000). The honey bee produces propolis from plant-derived compounds and bee-released secretion compounds. Its content varies depending on the geographic origin and the plant sources (Seidel et al., 2008). Propolis constitutes mainly from resin, wax, essential oils, pollen and organic compounds called “biological active compounds” including polyphenols such as phenolic acids (benzoic acids and its derivatives, cinnamic acids and its derivatives, phenol alcohols), flavonoids, terpenes (sesquiterpene and triterpene hydrocarbons, terpene and sesquiterpene alcohols and their derivatives) and small amount of carotenoids, sugars and amino acids (Bankova et al., 2014; Wagh, 2013).
Propolis has been widely used as natural products for self-treat many diseases due to its antimicrobial, antifungal, antiviral properties for a long time (Bankskota et al., 2001; Ledón et al., 1997; Marcucci et al., 2001; Wagh, 2013). Nowadays, it has been presented in drug stores in a variety of forms including capsules, creams, powder and mouthwash solutions. It is still commonly used for cold syndromes, wound healing (Cardile et al., 2003). The potential health effects of propolis are strongly related to its polyphenol compounds which could be extracted by different solutions such as ethanol, water (Ivanovska et al., 1995; Krol et al., 1990; Menezes et al., 1999; Nagai et al., 2003; Nakajima et al., 2007; Song et al. 2002). The ethanol with high solubility nature is the most popular technique for the production of biologically active compounds of propolis, however usage of ethanolic extracts as therapeutic agent is limited due to some disadvantages such as flavor, limitations of application in ophthalmology, otorhinolaryngology and pediatrics (Heim et al., 2002; Kubiliene et al., 2015).
Although propolis has been used as an alternative medical compound due to its strong antioxidative effects, its sensitizer effects have not been known much. The allergic reactions against propolis have been reported for the first time in 1990’s and the prevalence of sensitizing patients raised from 1 to 15% since then (deGroot, 2013). It has been shown that the most important allergic molecules in propolis are caffeic acid esters (especially caffeic acid phenyl ester, 1,1 dimethyl allyl caffeic acid), cinnamic acids and its aromatic esters (benzyl cinnamate, benzyl ferulate, benzyl isoferulate, or cinnamyl cinnamate) having a cinnamoyl ester bond (Aliboni et al., 2010; Basista-Soltys, 2013; Gardana et al., 2012; Hausen, 2005). So the question was how these allergic molecules could be converted into non- reactive ones?
Changes in the phenolic compounds during biotransformation are directly related to the selected microorganism and its strains. As a result of biotransformation, the amount of free and volatile phenols is increased and at the same time different phenolic compounds are obtained. Biotransformation plays an important role in the evaluation of valuable wastes containing high phenolic compounds (Madiera Junior et al., 2015).
Lactic acid bacteria have been one of the preferred microorganisms during biotransformation with purpose to improve the functions of plant matrices (Curiel et al., 2015). Among them Lactobacillus plantarum is the most preferred lactic acid bacteria as taking place in metabolism of phenolic compounds during fermentation of different plant materials (Rodríguez et al., 2009). Ciafardini et al. demonstrated that oleuropein, the main phenolic component in olive, was first converted to aglycone form by L. plantarum, and then converted to hydroxytrasol and elenoic acid by the esterase enzyme that L. plantarum possesses (Ciafardini et al., 1994). In a study conducted with olive wastewater, it was reported that the concentrations of phenolic compounds such as p-hydroxyphenylacetic acid, vanillic acid, ferulic acid and tyrosol were obtained during biotransformation of larger phenolic compounds by L. plantarum strains (Kachouri and Hamdi, 2004).
Rodríguez et al. examined the ability of food phenolic acids to be metabolized by L. plantarum. It has been found that L. plantarum produced 4-vinyl phenol/4-ethyl phenol from p-coumaric acid, 4-vinyl catechol/4-ethyl catechol from caffeic acid, 4-vinyl guaiacol/4-ethyl guaiacol from ferulic acid, 3-(3-hydroxyphenyl) propionic acid from m-coumaric acid (Rodríguez et al., 2008).
L. plantarum have been used successfully used for pyrogallol production from gallic acid found in wine waste (Akcay and Yıldırım, 2013). (Thesis conducted by Yıldırım as supervisor).
Biotransformation studies related to using of lactic acid bacteria with purpose of changes in phenolic compounds are so promising. (Fritsch et al., 2016). However, reports on the use of L. plantarum in propolis biotransformation are limited. Related to the topic only the effect of L. helveticus on allergen molecules of propolis has been demonstrated (Gardana et al., 2012).
In this study were investigated the effects of different pretreatment before biotransformation and the effects of different L. plantarum strains during bioconversion on phenolic contents including allergenic molecules in propolis.
Materials and methods
Materials
The propolis samples were obtained from the local company found in Turkey. Raw solid samples were frozen, finely ground in a mill and passed through a 35 mesh sieve prior to beginning of biotransformation. Liquid (80% ethanol) and solid samples were stored at -20 °C prior all experiments.
Culture preparations
Strains of L. plantarum cultures (Lactobacillus plantarum ATCC, Lactobacillus plantarum 10; Lactobacillus plantarum 8014 from culture collection of Ege Univeristy Food Engineering Department) were activated by growing for 48–72 h at 30 °C in MRS agar. Stock cultures were stored at − 80 °C in 20% glycerol solutions.
Biotransformation
Propolis samples were treated with different solvents (10% ethanol and polyethylene glycol—PEG 40%) to facilitate salvation of solid propolis. The bioconversion was done at 30 °C/48 h under constant state conditions. Fermentations were performed by using different strains of L. plantarum with inoculation rate of 1.5%. As culture media was used propolis 1 g (solid state) and 1 mL (liquid sate).
Post-biotransformation treatment
After biotransformation, obtained bio-products were treated with 70 mL of ethyl acetate and incubated at room temperature for 10 min. The next step of phenolic fraction extraction was followed by centrifugation of mixture at 1500×g for 5 min. The solid residue was separated with ethyl acetate. Obtained solid extracts were dried and dissolved in 100 mL methanol. After last centrifugation at 4000×g for 1 min the supernatants were diluted in appropriate conditions for analyses.
Chemical analysis
UPLC MS/MS conditions
The stock solution (1 mg/mL) of chemical substances (caffeic acid, caffeic acid phenethyl ester, 1,1-dimethyl allyl ester caffeic acid, benzyl ester caffeic acid, ferulic acid, salicylic acid, gentisic acid, catechin, chlorogenic acid, vanillic acid and ethyl ferulate) were prepared using acetonitrile (ACN). These standard solutions were diluted with water/acetonitrile (50/50:v/v) mixture containing 0.1% formic acid with concentrations range of 1–10,000 ng/mL except salicylic acid diluted with concentrations range of 1–1000 pg/mL. These solutions were used for drawing the calibration curves of each chemical. The quantitative analyses were performed by using Waters® ACQUITY™ TQD tandem quadrupole UPLC-MS/MS system, with ACQUITY TQ detector in the electrospray ionization (ESI) and multiple reaction monitoring (MRM) mode (Waters, Milford, MA) option. This UPLC-MS/MS system was conducted by MassLynx™ 4.1 software. The MRM transitions, ion mode, cone voltage and collision energy of each substance were presented in Table 1.
Table 1.
UPLC/MSMS conditions for some phenolic compounds
| Compounds | MRM | Ion mode | Cone (V) | Collision energy (V) |
|---|---|---|---|---|
| CAFE | 181.05 > 89.03 | ES(+) | 30 | 27 |
| 181.05 > 135.09 | 30 | 19 | ||
| CAPE | 283.5 > 134.9 | ES(−) | 40 | 27 |
| 283.5 > 180.0 | 40 | 24 | ||
| 1,1-DMAECAFE | 247.2 > 133.1 | ES(−) | 40 | 40 |
| BCAFE | 269.22 > 134.13 | ES(−) | 40 | 19 |
| 269.22 > 178.11 | 40 | 19 | ||
| Ferulic Acid | 195.1 > 177.1 | ES(+) | 22 | 14 |
| Salicylic Acid | 137.1 > 93.1 | ES(−) | 30 | 19 |
| Gentisic Acid | 152.9 > 109.1 | ES(−) | 37 | 12 |
| Catechin | 289.1 > 245.2 | ES(−) | 34 | 16 |
| Chlorogenic Acid | 353.1 > 191.2 | ES(−) | 27 | 22 |
| Vanilic Acid | 167 > 108.1 | ES(−) | 27 | 17 |
| 167 > 152.1 | 27 | 17 | ||
| Ethyl Ferulate | 221.1 > 133.1 | ES(−) | 29 | 24 |
| 221.1 > 206.1 | 29 | 17 |
The samples were separated on a Waters Acquity UPLC BEH C18column (2.1 mm × 50 mm, 1.7 μm, Waters, Milford, MA, USA). Mobile phase A (0.2% formic acid in water [v/v]) and mobile phase B (0.1% formic acid in ACN) were operated with a gradient elution at 0.4 mL/min as follows: 75% A (0–0.5 min), 75% A → 2% A (0.5–2.1 min), 2% A → 75% A (2.1–2.7 min), 75% A → 75% A (2.7–4.0 min). The column temperature was set at 60 °C and the auto-sampler temperature was maintained at 10 °C. For MRM data collection, the capillary voltage was 3730 V and the source temperature was set to 150 °C. The desolvation temperature was chosen to be 150 °C, the cone gas flow was 40 L/h, and the desolvation gas flow was 600 L/h. The ion energy2 value was 0.5.
Chromatograms of standard solution of chemical substances that used for propolis profiling analysis were presented in Fig. 1.
Fig. 1.
Chromatograms of standard solution of chemical substances used for propolis profiling analysis
Statistical analysis
Significant differences between averages were obtained at the 95% significance level. By using a Post-Hoc test, the least significant differences (LSD) test was performed. Using multivariate exploratory techniques, principal component analysis (PCA) was performed. Principal component analysis permits the visualization of the original arrangement of wines in an n-dimensional space, by identifying the directions in which most of the information is retained. It is therefore possible to explain differences in the various samples by means of these factors obtained from the generalized correlation matrix of the data sets and at the same time to determine which variables contribute most to such differentiation.
Results and discussion
In most of the study done with propolis the phenolic contents have been evaluated on the base of raw materials (Castaldo and Capasso, 2002; Croci et al., 2009). In this study were investigated the phenolic compounds of propolis after its biotransformation by different strains of L. plantarum. The main phenolic components that were evaluated in the study were given in Table 1. Among them are found some special phenolics which are identified as allergic compounds namely CAPE (phenethyl caffeate) and BCAFE (benzyl caffeate) (Gardana et al., 2012).
The effect of preliminary treatment and relation among phenolics
Evaluations of phenolic compounds were done in both type of propolis form (solid and liquid). Since the solid form of propolis is sticky and insoluble in water, industrially it is resuspended partially by 80% ethanol. The liquid samples were obtained from local producer (treated with 80% of ethanol). The solid samples were resuspended by using of 10% ethanol or 40% polyethylene glycol (PEG). During study, dispersing of raw propolis by ethanol was used as controlled method since it is industrially used. Using of PEG 400 as resupended material was chosen due to the fact that it is considered as food grade additive approved by European Food Safety Authority (EFSA) having special properties as dissolving hydrophobic molecules (Gardana et al., 2012).
The least significant differences were determined for phenolic compounds concerning using of different solvents. They contents were affected by ethanol at each percentages and PEG used as a solvent (p < 0.005). The CAPE and 1,1 DMAECAFE compounds were influenced by PEG solvent (p < 0.005). Ferulic acid, catechin, chlorogenic acid and salicylic acid changed by ethanol at both percentages and PEG used as a solvents (p < 0.005). The relations demonstrated that among phenols found in propolis are found complex relations so that, used solvent could be a critical parameter for resuspension of some phenolic compounds with different values.
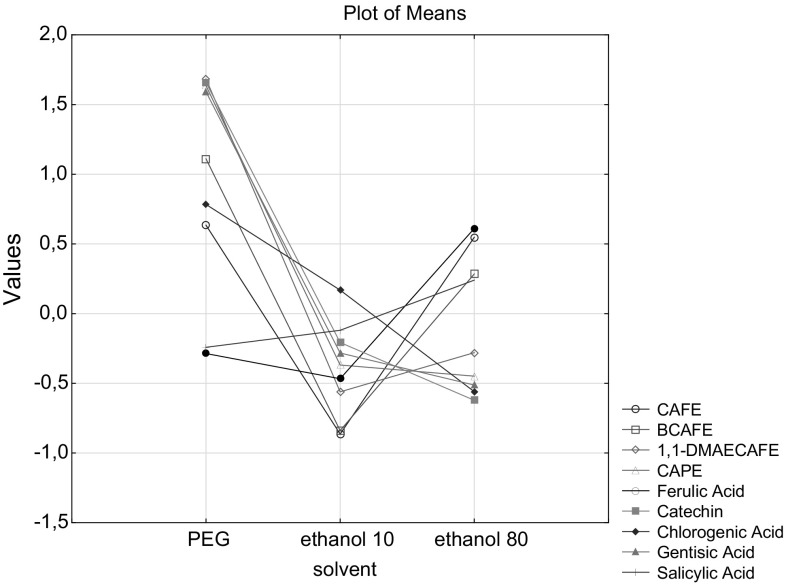
The standardized mean values of phenolic compounds found in propolis samples obtained by different extraction solutions and methods were seen from the Fig. 2. The order from the highest to the lowest values concerning using of PEG solvent was as: catechin > gentisic acid > CAPE > 1,1 DMAECAFE > BCAFE > chlorogenic acid > CAFÉ > salicylic acid > ferulic acid. In study done by Gardana et al. (Gardana et al., 2012), the best result for propolis extraction was obtained in samples treated with PEG solvent. Considering using of ethanol 80% the order was changed as: ferulic acid > CAFÉ > BCAFE > 1,1 DMAECAFE > CAPE > gentisic acid > catechin. Changing of ethanol percentage to 10%, changed the order as: chlorogenic acid > salicylic acid > catechin > gentisic acid > CAPE > ferulic acid > 1,1 DMAECAFE > BCAFE = CAFE. The lowest values BCAFE, 1,1 DMAECAFE and CAPE were determined as 321 ng/mL, 320 ng/mL and 8.02 ng/mL, respectively.
Fig. 2.
Standardized values of phenolic compounds including allergen molecules found in propolis samples after treatment with different solvents
The effect of different L. plantarum strains and relation among phenolics
Hausen (2005) demonstrated that the most sensitizers of propolis are as followed; phenyletyl caffeate > benzyl caffeate > 3-methyl 2 butenyl caffeate > geranyl caffeate > coumaric acid > ferulic acid. Gardana et al. (2012) stated that the allergens in propolis are mainly caffeic acid (CA) derivatives; especially 3-methyl-2-butenyl-CA (3M2B), 2-methyl-2-butenyl-CA (2M2B), 3-methyl-3-butenyl-CA (3M3B), and caffeic acid phenyl ethyl ester (CAFÉ). Based on this information, the main phenolic components which are identified as allergic compounds namely CAPE (caffeic acid phenyl ethyl ester; phenethyl caffeate) and 3M2B (1,1-dimethylallyl caffeate; 3-methyl-2-butenyl caffeate), benzyl caffeate and ferulic acid were determined.
Recently, Gardana et al. (2012) proposed a strategy to reduce allergenic molecules in propolis by L. helveticus. It was determined that L. helveticus significantly reduced caffeic acid esters of propolis. Lactic acid bacteria (LAB) as probiotic food have been used for food fermentation and preservation for a long time (Sánchez-Maldonado et al., 2011). Recent studies showed that Lactobacillus plantarum has the high metabolic activity towards phenolic acids. In our study, three different strains of L. plantarum have been used during biotransformation. The purpose was to evaluate propolis phenolics especially allergen molecules changes during biotransformation.
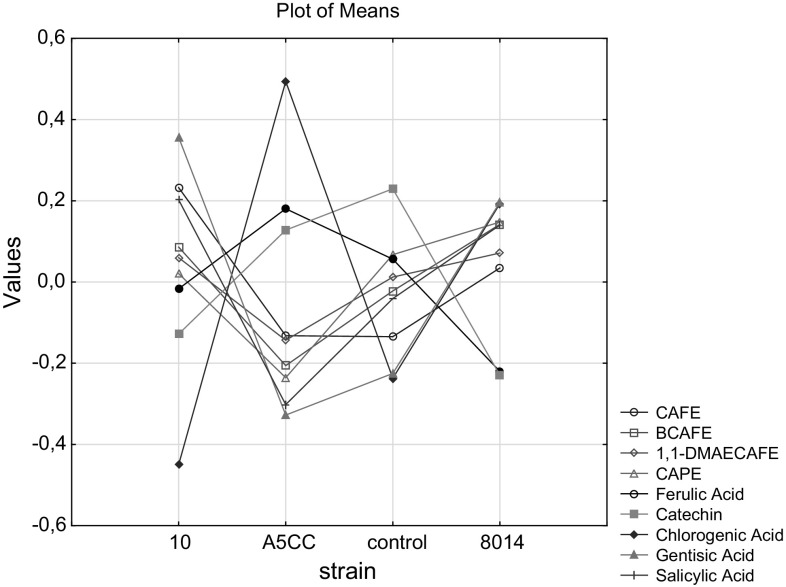
The highest phenolic yields were obtained by using PEG and ethanol (80%) as solvents which facilitate better salvation of solid propolis. As could be seen from the Fig. 3, L. plantarum ATCC strain significantly reduced allergen molecules (CAPE, 1,1 DMEA and BCAPE) of propolis samples obtained after treatment with 80% ethanol and PEG. Similarly, Gardana et al. (2012) demonstrated that L. helveticus had more effective in PEG solubilized propolis samples compared to those of 10% ethanol solubilized. Interestingly L. plantarum 8014 strain was more effective in reduction of other phenolic compounds. These results emphasized the possibilities of successful using of L. plantarum strains for bioconversion of propolis phenols in required manner.
Fig. 3.
Standardized values of phenolic compounds including allergen molecules in propolis samples inoculated with different L. plantarum strains
Overall evaluation of date by multivariate techniques
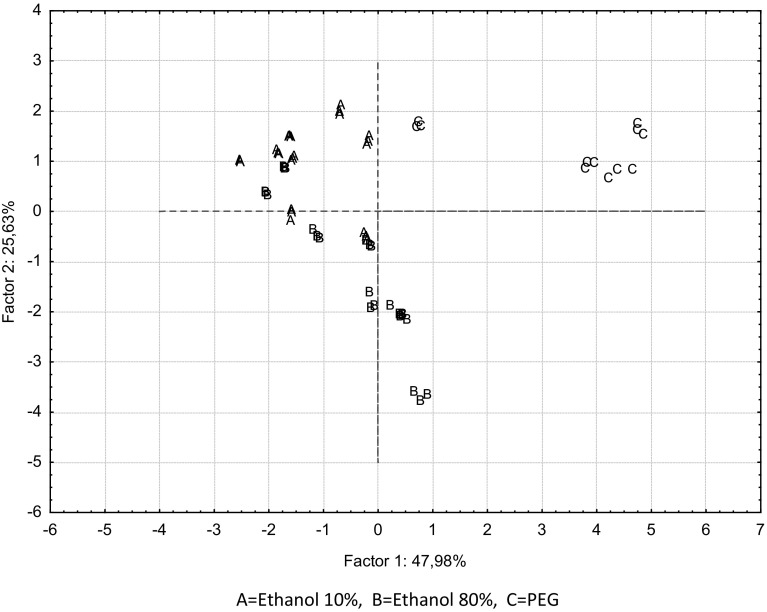
The eigenvalue number determined by PCA (principal component analysis) was found to be more than 60%, demonstrating the accuracy of performed analyses. As the distribution of analyzed parameters (Fig. 4), in samples produced by different solvents during treatment before biotransformation (Fig. 5) have the same x- and y-values (47.98 × 25.63) these figures were evaluated together to determine the relationship among parameters and samples. As a result there were determined close relations among analyzed parameters such as catechin, gentisic acid, CAPE, chlorogenic acid, 1,1 DMAECAFE and culture used for biotransformation by L. plantarum 8014. The second important result was determined concerning ferulic acid. There was found close relation between ferulic acid and L. plantarum ATCC culture used during biotransformation. The last important result was related to salicylic acid. There was demonstrated close relation between salicylic acid and L. plantarum 8014 culture used during biotransformation.
Fig. 4.
Distribution of analyses parameters (phenolic compounds including allergen molecules) by principal component analysis for propolis samples after biotransfomation
Fig. 5.
Distribution of propolis samples by principal component analysis according to used solvent during preliminary extraction treatment
Results of this work reveal, that types and percentage of solvents used for extraction and used L. plantarum strains have an important influence on phenolic profile of propolis composition including allergic molecules. Considering the specific effect of these parameters bioconversion of propolis by L. plantarum could be used for optimization of propolis extract on the base of required phenolic profile. This study demonstrated the possibilities of application of propolis bioconversion by L. plantarum strains. The study is the first work deal with evaluation of bioconversion of propolis by different L. plantarum strains and their effects on phenolic profile especially on reduction of allergic phenolic molecules. (Patent application No: 2015/16914, date: 25 December 2015, H. Kalkan Yıldırım and E.Y. Sözmen).
Acknowledgements
The authors gratefully acknowledge for the supports offered by “Aliye Uster Foundation” of Ege University and by “Ege University Research Foundation”.
References
- Akcay E, Yıldırım H.K., Production of pyrogallol by Lactobacillus plantarum strains, MS thesis, Ege University Graduate School of Natural and Applied Sciences, Izmir, Turkey (2013- unpublished)
- Aliboni A, D’Andrea A, Massanisso P. Propolis specimens from different locations of central Italy: Chemical profiling and gas chromatography–mass spectrometry (GC–MS) quantitative analysis of the allergenic esters benzyl cinnamate and benzyl salicylate. J. Agric. Food Chem. 2010;59:282–288. doi: 10.1021/jf1034866. [DOI] [PubMed] [Google Scholar]
- Bankova V, Popova M, Trusheva B. Propolis volatile compounds: chemical diversity and biological activity: a review. Chem. Cent. J. 2014;8:1–8. doi: 10.1186/1752-153X-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother. Res. 2001;15:561–571. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- Basista-Sołtys K. Allergy to Propolis in Beekeepers-A Literature Review. Occup. Med. Health Aff. 2013;1:1–3. [Google Scholar]
- Cardile V, Panico A, Gentile B, Borrelli F, Russo A. Effect of propolis on human. Life Sci. 2003;73:1027–1035. doi: 10.1016/S0024-3205(03)00381-3. [DOI] [PubMed] [Google Scholar]
- Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73:1–6. doi: 10.1016/S0367-326X(02)00185-5. [DOI] [PubMed] [Google Scholar]
- Ciafardini G, Marsilio V, Lanza B, Pozzi N. Hydrolysis of oleuropein by Lactobacillus plantarum strains associated with olive fermentation. Appl. Environ. Microbiol. 1994;60:4142–4147. doi: 10.1128/aem.60.11.4142-4147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci AN, Cioroiu B, Lazar D, Corciova A, Ivanescu B, Lazar MI. HPLC evaluation of phenolic and polyphenolic acids from propolis. Farmacia. 2009;57:52–57. [Google Scholar]
- Curiel JA, Pinto D, Marzani B, Filannino P, Farris GA, Gobbetti M, Rizzello CG. Lactic acid fermentation as a tool to enhance the antioxidant properties of Myrtus communis berries. Microb. Cell Fact. 2015;14:67. doi: 10.1186/s12934-015-0250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deGroot AC. Propolis: a review of properties applications chemical composition contact allergy and other adverse effects. Dermat. 2013;24:263–282. doi: 10.1097/DER.0000000000000011. [DOI] [PubMed] [Google Scholar]
- Fritsch C, Heinrich V, Vogel RF, Toelstede S. Phenolic acid degradation potential and growth behavior of lactic acid bacteria in sunflower substrates. Food Microbiol. 2016;57:178–186. doi: 10.1016/j.fm.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Gardana C, Barbieri A, Simonetti P, Guglielmetti S. Biotransformation strategy to reduce allergens in propolis. Appl. Environ. Microbiol. 2012;78:4654–4658. doi: 10.1128/AEM.00811-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausen BM. Evaluation of the main contact allergens in propolis (1995 to 2005) Dermat. 2005;16:127–129. [PubMed] [Google Scholar]
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J. Nut. Biochem. 2002;13:572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Ivanovska ND, Dimov VD, Bankova V, Popov S. Immunomodulatory action of propolis VI. Influence of a water soluble derivative on complement activity in vivo. J. Ethnopharmacol. 1995;47:145–147. doi: 10.1016/0378-8741(95)01272-F. [DOI] [PubMed] [Google Scholar]
- Kachouri F, Hamdi M. Enhancement of polyphenols in olive oil by contact with fermented olive mill wastewater by Lactobacillus plantarum. Process Biochem. 2004;39:841–845. doi: 10.1016/S0032-9592(03)00189-4. [DOI] [Google Scholar]
- Krol W, Czuba Z, Scheller S, Gabrys J, Grabiec S, Shani J. Anti-oxidant property of etanolic extract of propolis (Eep) evaluated by ınhibiting the chemilumnescence oxidation of luminol. Biochem. Int. 1990;21:593–597. [PubMed] [Google Scholar]
- Kubiliene L, Laugaliene V, Pavilonis A, Maruska A, Majiene D, Barcauskaite K, Kubilius R, Kasparaviciene G, Savickas A. Alternative preparation of propolis extracts: comparison of their composition and biological activities. BMC Complement. Altern. Med. 2015;15:156. doi: 10.1186/s12906-015-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledón N, Casacó A, González R, Merino N. Antipsoriatic, anti-inflammatory and analgesic effects of an extract of red propolis. Acta Pharmacol. Sin. 1997;18:274–276. [PubMed] [Google Scholar]
- Madeira Junior JV, Teixeira CB, Macedo GA. Biotransformation and bioconversion of phenolic compounds obtainment: an overview. Crit. Rev. Biotechnol. 2015;35:75–81. doi: 10.3109/07388551.2013.803020. [DOI] [PubMed] [Google Scholar]
- Marcucci MC, Ferreres F. García-Viguera C, Bankova VS, De Castro SL, Dantas AP, Valente PHM, Paulino N. Phenolic compounds from Brazilian propolis with pharmacological activities. J. Ethnopharmacol. 2001;74:105–112. doi: 10.1016/S0378-8741(00)00326-3. [DOI] [PubMed] [Google Scholar]
- Menezes H, Alvarez JM, Almeida EC. Mouse ear edema modulation by different propolis ethanol extracts. Arzneimittel-forschung. 1999;49:705–707. doi: 10.1055/s-0031-1300486. [DOI] [PubMed] [Google Scholar]
- Nagai T, Inoue R, Inoue H, Suzuki N. Preparation and antioxidant properties of water extract of propolis. Food Chem. 2003;80:29–33. doi: 10.1016/S0308-8146(02)00231-5. [DOI] [Google Scholar]
- Nakajima Y, Shimazawa M, Mishima S, Hara H. Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sci. 2007;80:370–377. doi: 10.1016/j.lfs.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Rodríguez H, Curiel JA. Landete JM, de las Rivas B, de Felipe FL., Gómez-Cordovés C, Muñoz R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009;132:79–90. doi: 10.1016/j.ijfoodmicro.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Rodríguez H. Landete JM, de las Rivas B, Muñoz R. Metabolism of food phenolic acids by Lactobacillus plantarum CECT 748T. Food Chem. 2008;107:1393–1398. doi: 10.1016/j.foodchem.2007.09.067. [DOI] [Google Scholar]
- Sánchez-Maldonado AF, Schieber A, Gänzle MG. Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011;111:1176–1184. doi: 10.1111/j.1365-2672.2011.05141.x. [DOI] [PubMed] [Google Scholar]
- Santos FA, Bastos EMAF, Maia ABRA, Uzeda M, Carvalho MAR, Farias LM. Brazilian propolis: physicochemical properties, plant origin and antibacterial activity on periodontopathogens. Phytother. Res. 2003;1:285–289. doi: 10.1002/ptr.1117. [DOI] [PubMed] [Google Scholar]
- Seidel V, Peyfoon E, Watson DG, Fearnley J. Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytother. Res. 2008;22:1256–1263. doi: 10.1002/ptr.2480. [DOI] [PubMed] [Google Scholar]
- Song YS, Jin C, Jung KJ, Park EH. Estrogenic effects of ethanol and ether extracts of propolis. J. Ethnopharmacol. 2002;82:89–95. doi: 10.1016/S0378-8741(02)00159-9. [DOI] [PubMed] [Google Scholar]
- Valle ML. Quantitative determination of antibacterian capacities of propolis. Apiacta. 2000;35:152–161. [Google Scholar]
- Wagh VD. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013;2013:1–11. doi: 10.1155/2013/308249. [DOI] [PMC free article] [PubMed] [Google Scholar]