Abstract
Purpose
The aim of our research was to study sex differences and the severity of inflammatory changes in target organs and the peculiarities of immunological disorders when low and high doses of lipopolysaccharide (LPS) were administered to rats.
Methods
Male and female 2- to 3-month-old Wistar rats (200–250 g) were injected intraperitoneally with Escherichia coli LPS in one of two doses: 1.5 or 15 mg/kg. In a day after the LPS injection, we studied endotoxin, corticosterone, sex steroids, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) activity levels in the serum; morphological disorders in the lung, liver, thymus, and spleen; ex vivo production of IL-2, IL-4, tumor necrosis factor (TNF), and interferon γ (IFNγ) by splenic cells activated by ConA; and relative amount of T- and B-lymphocytes in the peripheral blood.
Results
After the injection of low-dose LPS, the serum endotoxin level increased only in males and was combined with a more pronounced inflammatory response in the lungs and thymus and an increase in ALT and AST activity levels without any changes in corticosterone level. After the injection of high-dose LPS, the inflammatory and pathological changes in the target organs manifested as severe endotoxemia and sex differences of pathological changes in the lungs and liver were not revealed. The level of production of IL-2, IL-4, IFNγ, and TNF by splenic cells and the number of T-lymphocytes, including cytotoxic cells, in the peripheral blood, decreased in males, which is an evidence of a pronounced suppression of the immune response.
Conclusion
We have shown that the morphofunctional changes in the organs of the immune system in females and males, as well as the intensity of the sex differences of inflammation, depend on the severity of systemic inflammatory response, induced by different doses of LPS.
Keywords: endotoxinemia, SIRS, thymus, spleen, male rats, female rats
Introduction
Systemic inflammatory response syndrome (SIRS) and sepsis are severe complications of infectious and inflammatory diseases. In 26% of cases, SIRS leads to the development of sepsis, and in 18% to severe sepsis, which includes multiple organ failure, hypotension, and hypoperfusion. The morbidity of sepsis is very high and reaches 18–20 million cases per year worldwide, and its prevalence is increasing in most countries, including Russia. The treatment of sepsis in the USA costs up to $17 billion per year.1 Compared with gram-positive sepsis, gram-negative sepsis is developed more often (gram-negative: 62.2% and gram-positive: 46.8%), and gram-negative bacteremia is associated with a 2- to 3-fold higher mortality than gram-positive bacteremia.2 The most common bloodstream infection that can cause gram-negative sepsis is infection with Escherichia coli.2
According to the literature, lipopolysaccharide (LPS), a membrane component of gram-negative bacteria, is one of the main initiators of sepsis development.3 Injection of high doses of LPS leads to the development of system inflammatory response, which manifests itself via acute respiratory distress syndrome, vacuolar cell degeneration, necrosis in the liver, and endotoxemia.4 The key element of signal transmission in gram-negative infections is the activation of toll-like receptor (TLR)4 on the surface of mononuclear phagocytes, neutrophilic granulocytes, dendritic cells (DCs), endothelial cells, hepatocytes, and epithelial cells in the intestinal, respiratory, and urogenital tracts.5,6 The binding of LPS with TLR4 triggers an intracellular cascade, which leads to the activation of I-κB kinase, and further translocation of NF-κB through the nuclear membrane initiates the transcription of proinflammatory cytokine genes: IL-1, IL-6, IL-8, and tumor necrosis factor (TNF).7 Activation of NF-κB is necessary for the full expression of proinflammatory mediators, such as cytokines, adhesion molecules, and chemokines, and as a result, their hyperproduction leads to the development of multiple organ dysfunction associated with inflammation.8
Differences in the functions of cells, tissues, and the whole organism in males and females are determined by different levels of sex hormones and genes located on sex chromosomes. The sex chromosomes contribute to differences between the sexes at a molecular level, including sex-specific gene expression and sex-specific impact of genetic variation.9 From the zygote stage, females and males differ in their sex chromosome complement that might manifest on as sex differences from very early stages of the development and throughout the complete individuals’ life span.10 Polymorphism of X-linked genes and cellular mosaicism for X-linked parental alleles may offer additional advantages to women during host responses, in particular by providing a more adaptive and balanced cellular machinery during innate immune responses.11
Gonadal hormones exert specific effects on the male and female immunocompetence at both the cellular and the molecular levels.11 Estrogen receptors are expressed in most cells of the innate and adaptive immune system including T cells, B cells, neutrophils, macrophages, DCs, and natural killer (NK) cells.12 Androgen receptors were identified in T and B lymphocytes. Estrogens affect innate immune cells: estradiol increases the anti-inflammatory and decreases the chemotac-tic activity of neutrophils;13 long-term in vivo administration of estradiol leads to increased secretion of IL-1β, IL-6, and IL-12p40 after TLR-4 activation;14 high level of estradiol promotes the development of conventional IL-12-producing DCs and the expansion of IFNγ-producing killer DCs.12 In innate immune cells, testosterone decreases while estradiol increases the expression of TLR4.15,16 Both estrogens and androgens reduce the numbers of immature T lymphocytes, thus enhancing thymic involution during puberty and pregnancy.17,18 Also adaptive immunity differs between men and women: androgens stimulate the development of Th1 responses and activate CD8+ T cells, whereas estrogens stimulate Th2 responses and activate antibody production.12,19
Sex steroid hormones also modulate B-cell development and function.20 Estrogens and androgens suppress B lymphopoiesis in the bone marrow. Estradiol reduces apoptosis of immature B cells. However, estradiol also increases somatic hypermutation and class-switch recombination leading to high-affinity immunoglobulin (Ig)-producing cells. These effects might contribute to an improved humoral response in women and explain the increased susceptibility to autoimmune diseases.11
According to the results of experimental studies, survival rates of gram-negative sepsis are higher in females than in males;21,22 however, in clinical studies, the data are inconsistent and at times do not agree either with one another or with the results obtained in experiments.23–25 In a clinical trial, Nachtigall et al26 showed that sepsis develops more often in women; they have higher mortality rates (23%) than men (14%). Other authors, in contrast, have found higher survival rates for sepsis in women, which is associated with a high level of anti-inflammatory cytokine production.27 It has been shown that the risk of mortality from nosocomial infections in elderly women with acute sepsis is lower than that in men.28 According to Sakr et al29 in comparison with men, sepsis is less likely to develop in women, but mortality from sepsis is higher in women. Perhaps the inconsistency of the data obtained in clinical studies is linked with the varying severity of sepsis and the associated inflammatory and pathological processes. Besides in clinical research, scientists are faced with challenges, including differences in the medical history of septic patients, their subclinical status, social factors, and genetic predisposition, all of which mediate differences in the innate immune response.2 In comparison with men, in healthy women, the proinflammatory response to the administration of endotoxin is more pronounced.30 Other researchers have explained the inconsistencies in clinical studies data by the effect of sex hormones and genetic polymorphisms in the sex chromosomes.24,31 We assume that the presence and expression of sex differences can be determined by the severity of the course of systemic inflammation.
Inflammatory and pathological changes in systemic inflammatory response, which develops with the low dose of LPS introduction, are probably mediated by the activation of innate and adaptive immune reactions, which can be modulated by sex hormones. But the injection of sublethal dose of LPS leads to the development of endotoxin shock, primarily due to the direct damaging effect of endotoxin and the development of syndrome of disseminated intravascular coagulation, which is one of the causes of multiple organ dysfunction and mortality.
Therefore, we decided to determine the sex differences in the severity of inflammatory changes in target organs and the peculiarities of immunological disorders in the systemic inflammatory response induced by low-dose LPS, as well as in its more severe course caused by the administration of high-dose LPS (sublethal) to Wistar rats.
Materials and methods
Experimental animals
Male and female Wistar rats, 2–3 months old and weighing 200–250 g, were purchased from the animal breeding facility of the federal budget institution for science, the “Scientific Center for Biomedical Technology of the Federal Medical and Biological Agency”. The study received permission from the Bioethics Committee of the Science Research Institute of Human Morphology (Protocol No. 18a, December 22, 2016). All manipulations with animals were carried out according to the European convention for the protection of vertebrate animals used for experimental and other scientific purposes (ets no. 123), Strasbourg, 2006. Six rats per cages (18.5 × 60 × 38 cm) were housed in a temperature-regulated room at 12:12 hours light–dark cycle, relative humidity, between 55% and 65%, and unlimited access to water and food (“Char”, JSC “Range-Agro”, Russia). Male and female rats were housed separately in different rooms.
Determination of the phase of the estrous cycle
The phase of the estrous cycle in mature female Wistar rats was determined by vaginal smears stained by Romanowsky-Giemsa.32 SIRS was modeled in females with a stable 4-day cycle in the proestrus phase, in which the maximum levels of estradiol in the serum were observed.33
Modeling of SIRS
Males and females in the experimental groups were injected intraperitoneally with LPS from E. coli O26:B6 (Sigma-Aldrich Co., St Louis, MO, USA) in two doses: a low dose of 1.5 mg/kg, leading to pathological changes in target organs,34 and a high dose of 15 mg/kg, which is a sublethal dose for sexually mature rats and is model of endotoxin shock.35,36 The rats in the control groups received an intraperitoneal injection of physiological saline. The control groups of both male and female rats consisted of ten rats; the experimental group of females included eleven rats with low-dose LPS and 27 with high-dose LPS, and ten males were injected with low-dose LPS and 16 with a high dose. A higher quantity of animals in the groups receiving high-dose LPS is explained by the high rates of mortality.
Mortality of animals from SIRS
Within a day after the introduction of LPS, some animals died. Mortality rates of rats in response to the injection of low-dose LPS were two out of eleven (18%) in females and four out of ten (25%) in males, and after injection of high-dose LPS, 15 out of 27 (56%) in females and nine out of 16 (56%) in males. The high mortality rate caused by a high-dose LPS was associated with the development of endotoxin shock within 6 hours after the injection, and surviving animals showed severe manifestations of inflammation. The choice of high-dose LPS was considered and approved by the Bioethics Committee of the Science Research Institute of Human Morphology (Protocol No. 18a, December 22, 2016). All manipulations with animals were carried out according to the European convention for the protection of vertebrate animals used for experimental and other scientific purposes (ets no. 123), Strasbourg, 2006.
To withdraw the animals from the experiment, they were placed in a chamber with 80% CO2, which was used for anesthesia, and after taking blood from the jugular veins, the rats were decapitated.
Sample collection
Venous blood from jugular veins37 was centrifuged for 20 minutes at 200 ×g. The obtained serum was frozen at −70°C and stored for no more than 2 months. The liver, lungs, thymus, and spleen were fixed in Bouin’s solution (75 mL picric acid, 25 mL formalin, and 5 mL glacial acetic acid)38 for 24 hours, and organs were embedded in paraffin according to routine procedures. Histological sections of 4–5 µm thickness were produced and stained with hematoxylin and eosin (“BioVitrum”, Saint-Petersburg, Russia).
Morphological study
The histological slides were randomized and blinded. Using the light microscopy method, the number of neutrophils in the intra-alveolar septae of the lungs was counted in 10 high-power fields (25,000 µm2) per section, and the average number of neutrophils per slide was determined.39
The severity of pathological changes in the liver was estimated semiquantitatively by the double-blind method in points by two pathologists according to the following scale: 0 points – the absence of any vacuolar degeneration; 0.5 point – <30% of hepatocytes with vacuolar degeneration; 1 point – 31%–60% of hepatocytes with vacuolar degeneration; 2 points – >60% of hepatocytes with vacuolar degeneration; 3 points – 100% of hepatocytes with vacuolar degeneration; 4 points – hepatocytes with vacuolar degeneration and unit focal necrosis on the slice; and 5 points – hepatocytes with vacuolar degeneration and widespread necrosis (modified method).40
In the histological slices of the thymus and spleen, the volume fraction of the functional zones of immune organs was determined by the point-count method.41 The width of the subcapsular layer of the thymus was measured in micrometers at the original magnification 640×.
Isolation and cultivation of splenic cells
For isolation of splenic cells, a piece of spleen was aseptically removed from each rat, placed in Potter homogenizer containing the Roswell Park Memorial Institute (RPMI)-1640 medium, and single-cell suspensions were prepared. The red blood cells were lysed with distilled water. To activate cytokine synthesis and secretion, we cultivated 106/mL spleen cells in 1 mL of culture medium with concanavalin A (5 µg/mL) for 20 hours at 37°C and 5% CO2 in 24-well cultured plates. The culture medium consisted of RPMI-1640 (PanEco, Moscow, Russia), 5% inactivated FBS, 2 mM glutamine, and 50 µg/mL gentamicin.42 The cell viability was determined according to trypan blue exclusion.
ELISA
We estimated the concentration of corticosterone (IBL, Germany), total testosterone (DBC, Canada), estradiol (Cusa-bio, China), progesterone (DBC, Canada), and transforming growth factor-β (TGF-β; (eBioscience, USA) in the serum by ELISA. The endotoxin level in the serum was estimated by chromogenic LAL test (HBT, USA). In the culture fluid of splenic cells, we measured the concentrations of IL-2, IL-4, TNF, and IFNγ by ELISA test systems from eBioscience.
Biochemical analysis
To estimate the severity of liver damage, the activity of the indicator enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in rat serum was determined (DiaSys, Germany) using a blood biochemistry semi-automatic analyzer (Clima MC-15; RAL, Spain).
Apoptosis of thymic cells
To determine the number of apoptotic cells in the thymus, a suspension of cells at a concentration of 106/mL was stained with antibodies (anti-rat CD3 as marker of T lymphocytes) conjugated to phycoerythrin (PE). Then, the stained cell suspension was incubated with annexin V (Annexin V FITC Kit; Beckman Coulter, USA) conjugated to fluorescein isothiocyanate (FITC) and propidium iodide (PI). Flow cytometry evaluation of apoptotic cells (Annexin+PI−) was performed on a Cytomics FC 500 (Beckman Coulter).
Flow cytometry
Absolute and relative numbers of lymphocytes of various subpopulations in peripheral blood were counted using flow cytometry (Beckman Coulter). The following antibodies (eBioscience) were used for immune phenotypic analysis of the main subpopulations of lymphocytes: anti-rat CD3 (for T lymphocytes); anti-rat CD4 (for T helpers); anti-rat CD8a (for cytotoxic T cells); anti-rat CD45R (for B lymphocytes); anti-rat CD25 (for activated T cells); anti-mouse/rat Foxp3 (for regulatory T cells); and anti-rat CD314 (for NK cells). Erythrocytes were lysed with OptiLyse C solution (eBioscience).
Statistical analysis
Digital data were tested in Statistica 7.0. The nature of the distribution of data was determined by the Kolmogorov– Smirnov test. A comparison of the normally distributed data was made using one-way ANOVA on ranks. In case if data were non-normally distributed, a nonparametric test was used to establish the reliability of the differences between the indicators: Kruskal–Wallis method for multiple comparison and Conover’s method as the pair comparison test. The median (Me) and IQR (low–high) were calculated for values of the measured parameters. The differences were considered statistically significant when P<0.05. At least five observations were presented in each group. Data are represented graphically using box-and-whisker plots, which demonstrate the median, IQR, lower extreme (25%), and upper extreme (75%) of the data.
Results
Sex differences of inflammation induced by the injection of low-dose LPS
Serum endotoxin levels
After the administration of low-dose LPS, endotoxin levels increased significantly in the serum of males. In contrast, in females, the administration of low-dose LPS did not result in changes in the endotoxin concentration (Table 1).
Table 1.
The level of sex hormones, corticosterone, and endotoxin in the serum of mature female and male Wistar rats on the first day after the administration of low- or high-dose LPS
| Sex | Group | Estradiol, pmol/L | Progesterone, ng/mL | Total testosterone, ng/mL | Corticosterone, ng/mL | Endotoxin, eU/mL |
|---|---|---|---|---|---|---|
| Females | Control1 | 134.5 (108.6–170.4)n=5 | 9.5 (7.4–14.1)n=7 | 0.2 (0.2–0.3)n=6 | 58.6 (31.3–65.5)n=6 | 3.1 (2.9–3.2)n=10 |
| LPS 1.5 mg/kg2 | 84.1 (80.2–91.2)n=8 | 45 (40.5–48)n=6 | 1.3 (0.6–1.6)n=7 | 109.8 (107.3–140.3)n=6 | 3.5 (3.1–5.3)n=7 | |
| LPS 15 mg/kg3 | 132.5 (125.9–138.1)n=5 | 12.5 (8.6–31.5)n=6 | 0.5 (0.2–0.9)n=6 | 33.2 (21.8–41.6)n=6 | 22.1 (33.6–100)n=6 | |
| Males | Control4 | 67.4 (59.1–76.6)n=5 | 4.1 (3.50–4.94)n=7 | 3.4 (2.7–4.1)n=10 | 37.9 (34.8–41.0)n=10 | 3.3 (2.9–3.8)n=7 |
| LPS 1.5 mg/kg5 | 77.9 (72.7–78.3)n=5 | 6.1 (5.3–9.1)n=5 | 0.06 (0.04–0.09)n=6 | 26.0 (20.4–30.0)n=6 | 9.4 (7.9–10.8)n=6 | |
| LPS 15 mg/kg6 | 69.8 (56.4–70.4)n=5 | 3.8 (3.3–4.3)n=7 | 1.6 (0.4–3.1)n=7 | 18.8 (12.0–20.3)n=7 | 110 (105–125)n=7 | |
| Statistical significance of differences (P) | 0.011–2 0.0062–3 0.045–6 0.011–4 |
0.0081–2 0.0052–3 0.015–6 0.0031–4 0.0052–5 0.013–6 |
0.00061–2 0.00034–5 0.00025–6 0.00111–4 0.00092–5 |
0.041–2 0.0044–5 0.024–6 0.015–6 0.041–4 0.00022–5 0.013–6 |
0.0011–3 0.0012–3 0.0084–5 0.0044–6 0.0015–6 0.0052–5 0.0053–6 |
|
| F-value | 187 | 300 | 49 | 821 | 83 | |
Notes: Superscript 1–6 indicates the group number. Superscripts n=# is the number of observations in the group. Data shown as median (interquartile range).
Abbreviations: LPS, lipopolysaccharide; Me, median.
Serum corticosterone concentration
After the injection of low-dose LPS, an increase in serum corticosterone levels was found only in females, whereas in males, the concentration of this hormone did not change (Table 1). On the first day of SIRS development, the serum corticosterone concentration in females was significantly higher than that in males.
Sex steroid levels in the serum
After the administration of low-dose LPS, the serum levels of progesterone and total testosterone in females increased, whereas the concentration of estradiol was significantly lower than that in the control group (Table 1). After the injection of low-dose LPS in males, only the concentration of total testosterone decreased, but the level of the other studied steroid hormones did not change (Table 1).
Morphological changes in the lungs
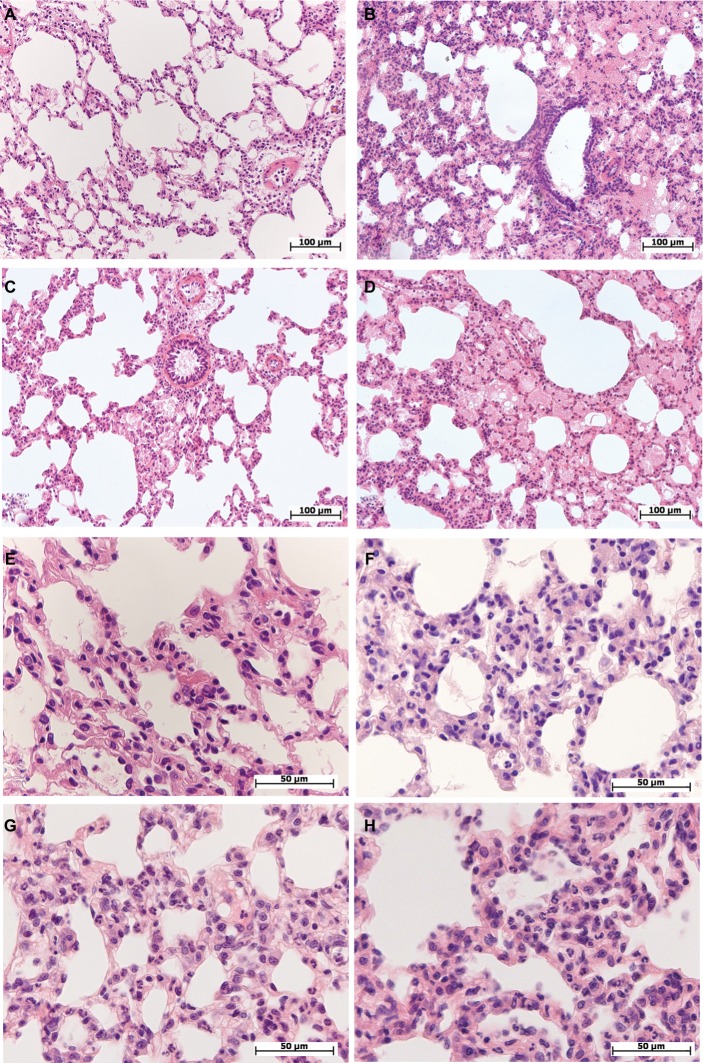
On the first day after the injection of low-dose LPS, 5–13 neutrophils were observed in the thickened alveolar septa of the lung in the field of vision at the original magnification, 400× (Figure 1E and F). In three out of seven females (43%) and in seven out of eight males (88%), alveolar edema of the lung was detected, which was characterized by the presence of homogeneous eosinophilic masses in the alveoli that partially or completely filled their lumens (Figure 1A and B). The number of neutrophils in the alveolar septa of male lungs was significantly higher than that in female lungs (Table 2).
Figure 1.
Morphological changes in the lung of female and male Wistar rats on the first day after LPS injection of one of two doses: 1.5 or 15 mg/kg.
Notes: Hematoxylin and eosin staining. (A) Female, 1.5 mg/kg LPS – intra-alveolar edema, enlarged perivascular lymphatic fissure, original magnification: 200×. (B) Male, 1.5 mg/kg LPS – pronounced intra-alveolar edema, foci with acute emphysema, original magnification: 200×. (C) Female, 15 mg/kg LPS – foci of intra-alveolar edema, bronchial lumen filled with eosinophilic masses, enlarged perivascular lymphatic fissures, original magnification: 200×. (D) Male, 15 mg/kg LPS – pronounced and widespread intra-alveolar edema, original magnification: 200×. (E) Female, 1.5 mg/kg LPS – thickened inter-alveolar septa with neutrophils, original magnification: 640×. (F) Male, 1.5 mg/kg LPS – neutrophil infiltration in inter-alveolar septa, original magnification: 640×. (G) Female, 15 mg/kg LPS – high number of neutrophils in inter-alveolar septa, edema, original magnification: 640×. (H) Male, 15 mg/kg LPS – pronounced neutrophil infiltration in inter-alveolar septa, original magnification: 640×.
Abbreviation: LPS, lipopolysaccharide.
Table 2.
The number of neutrophils in the inter-alveolar septa of the lung, semiquantitative evaluation of pathological changes in the liver, and ALT and AST enzyme activities in the serum of mature female and male Wistar rats on the first day after the administration of low- or high-dose LPS
| Sex | Group | Number of neutrophils in the inter-alveolar septa of the lung, 25,000 µm2 | Dystrophic and necrotic change index in hepatocytes, points | ALT, U/L | AST, U/L |
|---|---|---|---|---|---|
| Females | Control1 | 1.6 (1.4–1.9)n=10 | 1.5 (1.0–2.5)n=10 | 57 (54–92)n=7 | 143 (142–180)n=7 |
| LPS 1.5 mg/kg2 | 5.7 (4.7–7.6)n=9 | 3.0 (2.0–3.0)n=7 | 20 (16–31)n=9 | 39 (39–68)n=6 | |
| LPS 15 mg/kg3 | 23.6 (14.6–28.7)n=9 | 3.0 (3.0–4.0)n=6 | 736 (96–2141)n=5 | 771 (160–2652)n=5 | |
| Males | Control4 | 1.4 (1.4–2.0)n=8 | 1.0 (0.5–1.5)n=5 | 38 (30–41)n=5 | 72 (61–76)n=8 |
| LPS 1.5 mg/kg5 | 10.3 (8.1–13.9)n=6 | 2.0 (2.0–2.2)n=5 | 53 (46–69)n=6 | 93 (77–116)n=5 | |
| LPS 15 mg/kg6 | 27.8 (24.0–32.6)n=7 | 3.5 (2.0–4.0)n=7 | 297 (145–398)n=5 | 425 (188–604)n=5 | |
| Statistical significance of differences (P) | 0.000011–2 0.000011–3 0.0092–3 0.0034–5 0.00034–6 0.00075–6 0.0022–5 |
0.0031–2 0.0213 0.012–3 0.0054–5 0.014–6 0.015–6 |
0.021–2 0.011–3 0.0012–3 0.074–5 0.024–6 0.0015–6 |
0.021–2 0.011–3 0.0012–3 0.034–5 0.024–6 0.0015–6 |
|
| F-value | 170 | 300 | 6.5 | 11.4 | |
Notes: Superscript 1-6 indicates the group number. Superscripts n=# is the number of observations in the group. Data shown as median (interquartile range).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; LPS, lipopolysaccharide; Me, median.
Morphological changes in the liver
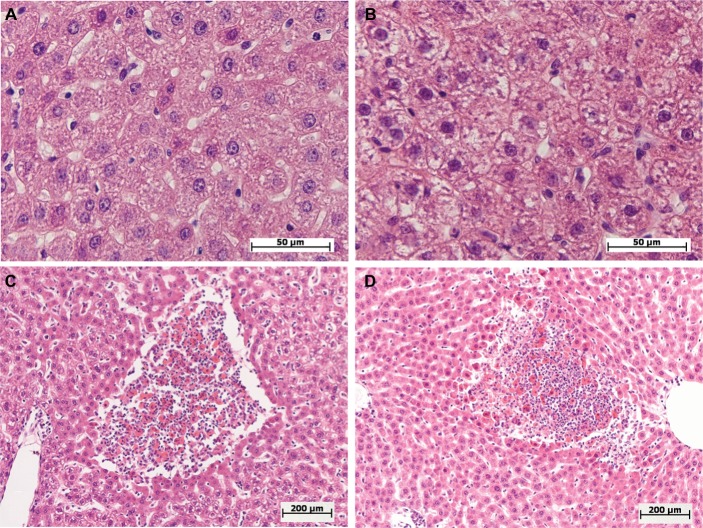
After the administration of low-dose LPS, pathological changes in hepatocytes as well as pronounced and widespread cell degeneration were found, especially at the periphery of the lobules, in the livers of female and male Wistar rats (Figure 2A and B). In the livers of one female and one male of seven rats in the group, foci of necrosis were found. On the first day after the administration of low-dose LPS, indicators of cell degeneration and necrotic liver changes in males and females did not differ significantly (Table 2).
Figure 2.
Morphological changes in the livers of female and male Wistar rats on the first day after the injection of one of two doses of LPS.
Notes: Hematoxylin and eosin staining. (A) Female, 1.5 mg/kg LPS – mild hepatocyte cell degeneration, original magnification: 640×. (B) Male, 1.5 mg/kg LPS – severe hepatocyte cell degenerations, original magnification: 640×. (C, D) Female and male, 15 mg/kg LPS – foci of necrosis, original magnification: 100×.
Abbreviation: LPS, lipopolysaccharide.
ALT and AST activity levels in the serum
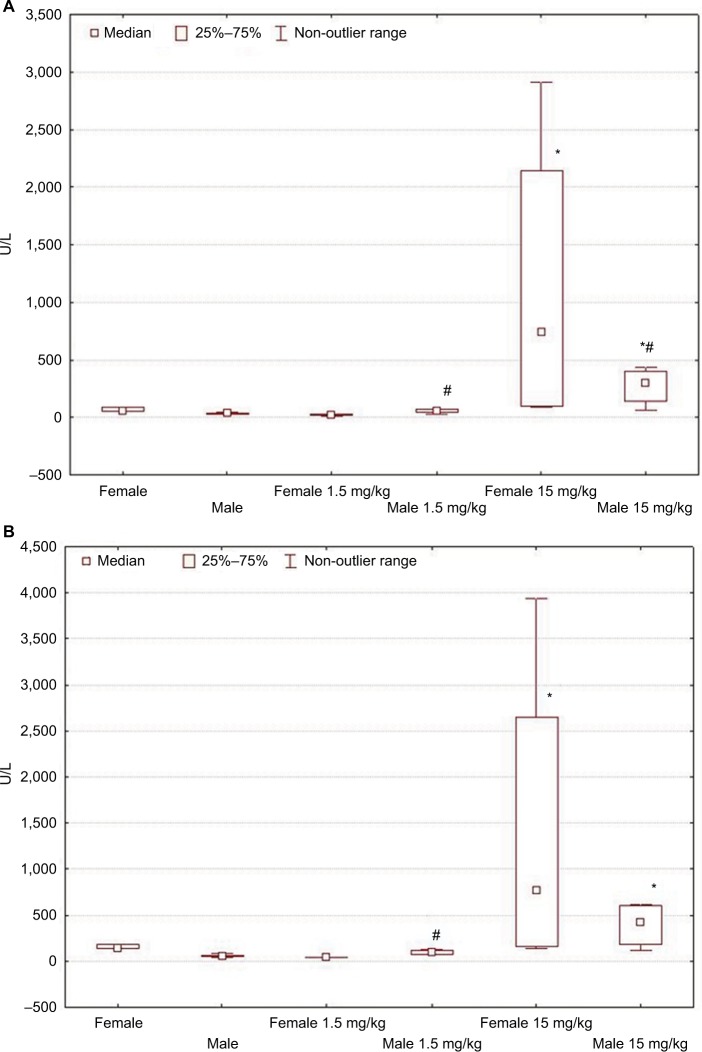
On the first day after the administration of low-dose LPS, the serum activity levels of ALT and AST in males increased in comparison with that of their control group, but in contrast, the activity levels decreased in the serum of females (Table 2; Figure 3A).
Figure 3.
Changes in ALT (A) and AST (B) activity levels in female and male Wistar rats on the first day after LPS injection of one of two doses: 1.5 or 15 mg/kg.
Notes: Statistically significant changes are indicated by: *in comparison with the control group and #in comparison with females of the appropriate experimental group.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; LPS, lipopolysaccharide.
Morphological changes in the thymus
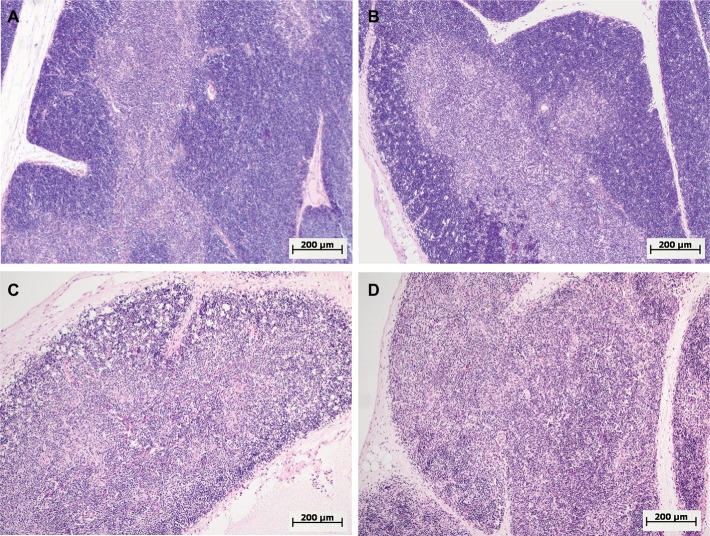
One day after the administration of low-dose LPS to female and male Wistar rats, signs of a mild involution of the thymus were found (Figure 4A and B). A morphometric evaluation of changes in the thymus showed broadening of the cortex in females on the first day of SIRS development. In the males, in contrast, there were no significant differences in the volume fraction of the cortex compared to that of the control group, and it was combined with the presence of macrophages and dead lymphocytes in four of ten males (Figure 4B; Table 3). In females, when low-dose LPS was administered, there were no pathological changes in the thymic cortex. The LPS-induced broadening of the thymic subcapsular zone, consisting of lymphoblasts, was observed in rats of both sexes (Table 3).
Figure 4.
Morphological changes in the thymus of female and male Wistar rats on the first day after LPS injection of one of two doses: 1.5 or 15 mg/kg.
Notes: Hematoxylin and eosin staining. Original magnification: 100×. (A) Female, 1.5 mg/kg LPS – hyperplasia of the thymus, a broad cortex densely populated with lymphocytes, distinct boundaries between the cortex and medulla. (B) Male, 1.5 mg/kg LPS – mild atrophy of the thymus. (C) Female, 15 mg/kg LPS – severe atrophy of the thymus, destruction of the cortex. (D) Male, 15 mg/kg LPS – severe atrophy of the thymus, more pronounced compared with that of females (C), inversion of cortex and medulla, indistinct boundaries between the cortex and medulla.
Abbreviation: LPS, lipopolysaccharide.
Table 3.
Morphometric characteristics of the thymus and spleen of mature female and male Wistar rats on the first day after the administration of low- or high-dose LPS
| Sex | Group | Thymus | Spleen, volume fraction, % | |||||
|---|---|---|---|---|---|---|---|---|
| Volume fraction of the cortex, % | Width of the subcapsular zone, µm | Percentage of apoptotic cells,106/mL | PALS | Lymphoid folliculi | Marginal zone | Germinal centers | ||
| Females | Control1 (n=10) | 49.6 (47.3–51.5) | 30.7 (28.4–31.7) | 1.3 (1.1–1.3) | 28.2 (27.5–29.1) | 15.5 (14.9–16.2) | 44.9 (43.8–46.2) | 22.4 (21.0–23.6) |
| LPS 1.5 mg/kg2 (n=7) | 57.0 (55.9–59.1) | 45.5 (40.9–49.4) | 4.2 (3.1–6.0) | 20.9 (18.8–23.2) | 14.7 (13.2–16.1) | 31.9 (29.1–33.7) | 46.1 (40.2–52.1) | |
| LPS 15 mg/kg3 (n=10) | 32.8 (29.4–41.1) | 0.0 (0.0–12.0) | 1.4 (0.4–1.6) | 29.6 (27.2–32.0) | 16.7 (13.4–23.4) | 22.9 (10.2–27.5) | 66.6 (60.9–69.3) | |
| Males | Control4 (n=9) | 55.0 (54.1–56.9) | 25.0 (19.5–29.7) | 0.4 (0.1–1.0) | 27.4 (25.8–29.3) | 14.8 (13.0–16.1) | 44.7 (42.5–46.6) | 22.2 (20.9–24.2) |
| LPS 1.5 mg/kg5 (n=6) | 55.1 (53.9–55.7) | 38.3 (35.6–41.2) | 3.6 (3.2–4.1) | 24.1 (23.1–24.9) | 17.0 (16.5–18.0) | 37.7 (37.1–38.5) | 30.7 (30.0–31.2) | |
| LPS 15 mg/kg6 (n=7) | 46.3 (39.4–53.3) | 0.0 (0.0–0.0) | 1.5 (0.9–2.3) | 27.0 (25.2–28.2) | 11.6 (9.8–16.1) | 12.8 (8.1–24.7) | 61.0 (51.0–75.1) | |
| Statistical significance of differences (P) | 0.0041–2 0.0031–3 0.0012–3 0.0024–6 0.045–6 0.011–4 |
0.0051–2 0.0011–3 0.0012–3 0.014–5 0.0014–6 0.0015–6 0.041–4 0.022–5 |
0.0041–2 0.0042–3 0.0034–5 0.044–6 0.0045–6 0.051–4 |
0.0081–2 0.0072–3 0.00084–5 0.045–6 |
0.035–6 0.023–6 |
0.0031–2 0.0011–3 0.022–3 0.00024–5 0.00044–6 0.00015–6 |
0.021–2 0.00061–3 0.0052–3 0.00014–5 0.00034–6 0.00015–6 0.012–5 |
|
| F-value | 4,813 | 1,631 | 74 | 2,639 | 1,440 | 1,130 | 712 | |
Notes: Superscript 1–6 indicates the group number. Superscripts n=# is the number of observations in the group. Data shown as median (interquartile range).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; LPS, lipopolysaccharide; PALS, periarterial lymphatic sheaths; Me, median.
Relative number of apoptotic thymic cells (%) and CD3+ T lymphocytes
After the administration of low-dose LPS, the relative number of apoptotic cells in the thymuses of females and males increased in comparison with those of their control groups (Table 3).
Morphological changes in the spleens
On the first day of SIRS development induced by the introduction of low-dose LPS, destruction of the white pulp due to a decrease in the proportion of the periarterial lymphatic sheaths (PALS) was observed in the spleens of female and male rats. In the B-zone of lymphatic folliculi, large germinal centers were found, and marginal zones of lymphatic folliculi were narrower than those of the control groups (Table 3).
Subpopulation of lymphocytes in the peripheral blood
On the first day after the administration of low-dose LPS, the relative number of NK cells in female and male Wistar rats increased (Table 4). The relative number of cytotoxic T lymphocytes decreased in both females and males (Table 4). The number of T helpers increased in females, whereas it did not change in males (Table 4).
Table 4.
The percentage of major lymphocyte subpopulations in the peripheral blood of mature female and male Wistar rats on the first day after the administration of low- or high-dose LPS
| Lymphocyte subpopulation, % | Females | Males | P-value | F-value | ||||
|---|---|---|---|---|---|---|---|---|
| Control (n=10) | LPS 1.5 mg/kg (n=9) | LPS 15 mg/kg (n=10) | Control (n=10) | LPS 1.5 mg/kg (n=6) | LPS 15 mg/kg (n=10) | |||
| T cells (CD3+) | 47.81 (42.3–56.4) | 42.52 (29.7–47.5) | 39.43 (30.7–52.7) | 53.01* (45.5–63.0) | 43.72 (38.1–53.2) | 23.23 (18.0–29.0) | 0.00083–3 | 503 |
| T-helper cells (CD3+ CD4+) | 47.14 (29.0–54.9) | 56.25 (47.9–59.1) | 65.26 (37.6–67.4) | 40.74 (33.7–45.0) | 44.75 (34.6–49.5) | 70.26 (60.5–73.0) | 0.055–5 0.026–6 | 692 |
| Cytotoxic T cells (CD3+ CD8a+) | 52.07 (44.0–70.8) | 40.88 (38.5–51.3) | 32.559 (26.8–46.3) | 59.97 (51.8–71.4) | 47.78 (46.5–63.6) | 28.69 (26.4–29.5) | 0.0039–9 | 396 |
| B cells (CD45+) | 15.310 (12.5–19.3) | 16.811 (12.5–23.3) | 18.212 (15.5–22.4) | 19.510 (15.5–21.7) | 25.811 (19.1–29.1) | 16.212 (13.0–24.8) | 346 | |
| NK cells (CD314+) | 4.913 (3.0–6.1) | 22.414 (18.9–27.5) | 26.315 (16.2–52.2) | 5.613 (2.9–9.6) | 30.814 (20.9–40.8) | 28.315 (21.0–38.7) | 165 | |
| Activated T-helper cells (CD4+ CD25+ Foxp3–) | 7.816 (5.0–11.2) | 6.417 (6.0–9.4) | 4.118 (2.8–5.0) | 3.316 (2.4–4.7) | 3.517 (2.0–4.6) | 2.618 (2.1–3.1) | 0.00216–16 0.00117–17 | 79 |
| Regulatory T cells (CD4+ CD25+ Foxp3+) | 3.818 (3.4–4.6) | 4.220 (2.3–4.3) | 2.221 (1.5–2.7) | 3.719 (3.6–4.8) | 4.220 (3.3–5.2) | 2.421 (1.4–3.2) | 370 | |
| Statistical significance of differences (P) | 0.034–5 0.027–8 |
0.000213–14 0.000213–15 0.00218–21 |
0.0420–21 |
0.051–3 0.0022–3 0.025–6 0.027–8 |
0.00057–9 0.018–9 0.000213–14 0.000113–15 |
0.0418–21 0.0317–18 0.0420–21 |
||
Notes: Superscript 1-21 indicates the group number. Superscripts n=# is the number of observations in the group. Data shown as median (interquartile range).
Abbreviations: LPS, lipopolysaccharide; Me, median; NK, natural killer.
Serum TGF-β and culture fluid cytokine concentrations of ConA-activated spleen cells
After the administration of low-dose LPS, the TGF-β concentration decreased in the serum of Wistar rats of both sexes (Table 5). The multidirectional changes in the level of Th1 cytokine IL-2 by spleen cells in females and males were revealed; in males, the level of IL-2 increased, and in females, it decreased (Table 5). The level of Th2 cytokine IL-4 in spleen cells from males also decreased (Table 5). In females, the level of IL-4 did not differ from the control values, and the secretion of TNF increased; however, no changes in the level of this proinflammatory cytokine were found in males (Table 5).
Table 5.
Serum TGF-β level and cytokine production by ConA-activated spleen cells in mature female and male Wistar rats on the first day after the administration of low- or high-dose LPS
| Sex | Group | TGF-β, ng/mL | IL-2, pg/mL | IL-4, pg/mL | IFNγ, pg/mL | TNF, pg/mL |
|---|---|---|---|---|---|---|
| Females | Control1 | 79.0 (63.2–81.9)n=5 | 4,980 (4,700–5,318)n=6 | 300 (284–374)n=7 | 378.9 (259.3–425,8)n=9 | 462.8 (354.8–568.0)n=10 |
| LPS 1.5 mg/kg2 | 23.0 (13.0–25.7)n=7 | 1,850 (1,295–2,220)n=5 | 250 (164–301)n=9 | 371.7 (190.2–501.4)n=5 | 850.2 (785.4–920.1)n=9 | |
| LPS 15 mg/kg3 | 37.2 (17.5–41.0)n=5 | 5,400 (5,100–5,400)n=5 | 86.8 (46.0–127.1)n=5 | 375.0 (320.3–421.9)n=6 | 172.7 (135.7–202.4)n=6 | |
| Males | Control4 | 50.8 (43.0–64.0)n=5 | 3,107 (2,216–5,953)n=7 | 252 (220–356)n=8 | 421.9 (359.4–1164)n=10 | 301.3 (237.8–385.6)n=8 |
| LPS 1.5 mg/kg5 | 20.5 (13.0–41.8)n=6 | 4,934 (4,361–6,950)n=6 | 46 (43–62.9)n=6 | 266.1 (228.1–382.4)n=6 | 209.0 (167.6–488.7)n=6 | |
| LPS 15 mg/kg6 | 20.2 (19.3–29.4)n=5 | 1,704 (1,257–2,682)n=5 | 17.8 (16.5–17.9)n=5 | 300.1 (206.5–351.6)n=6 | 100.8 (56.5–156.0)n=6 | |
| Statistical significance of differences (P) |
0.00051–2 0.0091–3 0.034–5 0.0094–6 |
0.0081–2 0.041–3 0.0052–3 0.044–5 0.044–6 0.015–6 0.012–5 0.0083–6 |
0.061–3 0.072–3 0.0054–5 0.044–6 0.00025–6 0.012–5 0.0073–6 |
0.074–5 0.044–6 0.045–6 0.021–4 |
0.021–2 0.021–3 0.012–3 0.024–6 0.025–6 0.021–4 0.022–5 |
|
| F-value | 198 | 299 | 151 | 24 | 63 | |
Notes: Superscript 1-6 indicates the group number. Superscripts n=# is the number of observations in the group. Data shown as median (interquartile range).
Abbreviations: IFNγ, interferon γ; LPS, lipopolysaccharide; TGF-β, transforming growth factor-β; Me, median.
Sex differences in inflammation induced by the injection of high-dose LPS
Serum endotoxin levels
After the injection of high-dose LPS into the blood of males and females, endotoxin levels were distinctly increased compared with those in the groups of animals administered low-dose LPS. The endotoxin level in males was >30-fold higher than that in the control group, and the level was approximately sevenfold higher in females than in the control group. The level of endotoxin in the serum of females with SIRS was lower than that in males (Table 1).
Serum corticosterone concentration
After the administration of high-dose LPS, the concentration of corticosterone in the serum of males decreased, while in females, its level did not differ from that of the control group (Table 1).
Sex steroid levels in the serum
Injection of high-dose LPS did not change the level of any studied sex steroid in either females or males (Table 1).
Morphological changes in the lungs
After the administration of high-dose LPS, alveolar edema was scarce and less widespread than after injection of low-dose LPS: it was detected in the lung of three females out of ten (30%) and nine males out of 14 (64%) (Figure 1C and D). Compared with the low dose, a day after the administration of high-dose LPS, the number of neutrophils in thickened alveolar septa was greater and amounted to 15–20 polymorphonuclear leukocytes (Figure 1G and H). After the injection of both low- or high-dose LPS, alveolar edema was more often detected in males. Inflammatory reactions, characterized by an increase in the number of neutrophils in the alveolar septa, were more pronounced after the administration of high-dose LPS, but sex differences in lung inflammation after high-dose LPS administration were not found (Table 2).
Morphological changes in the liver
After the injection of high-dose LPS, marked and widespread cell degeneration was revealed in the livers of females and males. Foci of necrosis were found in four females of ten (40%) and in seven males of 14 (50%) in the group (Figure 2C and D). Just as with the administration of a low dose, on a day after the injection of high-dose LPS, the indicators of cell degeneration and necrotic changes did not differ significantly between females and males (Table 2).
ALT and AST activity levels in the serum
When high-dose LPS was administered, the indicators of ALT and AST activities were significantly higher than the values in control groups in both males and females. Higher serum AST and ALT activities in rats of both sexes indicate more pronounced pathological changes in the liver when LPS was administered at a dose of 15 mg/kg than at a low dose (Table 2; Figure 3B).
Morphological changes in the thymus
One day after the administration of high-dose LPS in the thymus of both females and males, signs of a severe involution were found (Figure 4C and D), which were characterized by the contraction of the cortex and the appearance of macrophages phagocytosing the dying lymphocytes. The inversion of the cortex and medulla was observed in two males out of eight (25%) (Figure 4D). Using the morphometric evaluation of changes in the thymus, cortical contraction was found in both males and females. The subcapsular zone in most animals of both sexes was not detected (Table 3).
Relative number of apoptotic thymic cells (%) and CD3+ T lymphocytes
One day after the administration of high-dose LPS, the relative number of apoptotic cells increased only in the thymuses of males, whereas in females, there was no change (Table 3).
Morphological changes in the spleen
On the first day after the administration of high-dose LPS, no changes in the volume fraction of white and red pulp in the spleens of Wistar rats of both sexes were found (Table 3). However, compared with females, the volume fraction index of the white pulp of the spleen in males with SIRS was significantly lower. On the first day after the administration of high-dose LPS, the volume fraction of germinal centers of lymphatic folliculi in males and females was broader, and the marginal zones were narrower than those in the control groups (Table 3).
Subpopulation of lymphocytes in the peripheral blood
On the first day after the administration of high-dose LPS, the relative number of NK cells in the peripheral blood of female and male Wistar rats increased (Table 4). The number of regulatory T lymphocytes decreased in both females and males. Both low- and high-dose of LPS led to a decrease in the number of cytotoxic T lymphocytes in males (Table 4).
Serum TGF-β and culture fluid cytokine concentrations of ConA-activated spleen cells
In response to the administration of high-dose LPS, the TGF-β level decreased in the serum of female and male Wistar rats (Table 5). The level of ex vivo production of IL-4 and TNF decreased in females and males, but the change in the level of IL-4 in the culture supernatant of spleen cells from males was more pronounced than that in females. The concentration of IL-2 and IFNγ did not significantly change in females in response to the administration of high-dose LPS, whereas in males, a decrease in these cytokines was revealed (Table 5).
Discussion
According to the literature data, resistance to the development of infectious and inflammatory diseases, the severity of their clinical course, and survival and mortality depend on sex.43 Unfortunately, many researchers in the majority of both clinical and experimental studies do not take into account the features of inflammatory reactions depending on sex. However, a number of studies have shown that the incidence of SIRS and sepsis, as well as the mortality from these diseases, is higher in men than in women, which may be due to differences in the level of steroid sex hormones that have an immunomodulatory effect.43 In experimental studies, intraperitoneal injections of variable doses of E. coli LPS of different serotypes are frequently used for modeling sepsis and SIRS.44–46 However, in the literature, there are no studies devoted to the comparison of the features of the SIRS course depending on the dose of LPS and gender.
On the first day after the injection of low-dose LPS, the level of endotoxin in the serum increased in males, and this finding was combined with pronounced inflammation in the lungs and liver and severe involution in the thymus. According to the literature, high levels of serum endotoxin are an unfavorable prognostic criterion for septic shock development, and its therapeutic elimination increases survival, reduces the risk of multiple organ dysfunction, reduces serum C-reactive protein and procalcitonin levels, and increases blood pressure.47,48
The endotoxin level in the blood is determined by the quantitative and qualitative composition of the intestinal microbiota, the permeability of the epithelial barrier of the intestine, and the activity of antiendotoxin immunity (antibodies to LPS), which may differ between the sexes. The gut microbiota differs in men and women at the bacterial phyla level, the genus level, and the species level,49 and that gut microbiota may also differ between sexes in animal models.50 It was showed that the abundance for gram-negative species like the Bacteriodes–Prevotella group (together Bacteroidetes phylum) was higher in men.51 The ovarian hormones modulate gut permeability: estrogen level peak during the follicular phase correlated with improved epithelial barrier as shown in the ileum of proestrus rats.52 Decreased intestinal permeability in male rats was also found following estradiol supplementation. According to literature data, women at high estrogen levels commonly elicit a stronger humoral response.11 The data about sex differences of antibody titers to LPS are absent; however, it can be assumed that antiendotoxin immunity is also more active in women. Also it is known that compared with males, female resident macrophages express higher levels of pathogen-/injury-sensing TLRs and are more efficient at phagocytosis and bacterial killing. This increased capacity to detect and eliminate infectious stimuli like LPS is restrained by proportionally more CD4+ T lymphocytes, which limit the excessive cytokine production and recruitment of tissue-damaging neutrophils.53 Finally, the high activity of the immune system in females, the high density of intercellular contacts of the epithelial barrier of the intestine, and the smaller number of gram-negative bacteria colonizing the gut apparently determine the absence of changes in the endotoxin level after low-dose LPS injection.
The concentration of corticosterone in the serum decreases in males after the administration of LPS at a high dose, whereas a low dose does not affect the corticosterone level. But in females the injection of low-dose LPS, in contrast, causes increase in corticosterone. The absence of changes of corticosterone level and even its decrease in males appears to be due to irreversible damage to the adrenal cortical cells caused by high concentrations of endotoxin.54 A low concentration of the anti-inflammatory hormone corticosterone in the serum of males could be one of the reasons for more pronounced alterative changes in target organs after the administration of low- or high-dose LPS. Laboratory studies in rodents have demonstrated that basal corticosterone concentrations are higher in females and increase more rapidly in females than in males after exposure to stressors.55 Females are reported to exhibit higher adrenocorticotrophic hormone responses to stress, and increased baseline and stress-induced glucocorticoid release than males.56 In sepsis, high concentrations of corticosterone limit the hyperproduction of proinflammatory cytokines, reducing the level of kinase and NF-kB activation, and thereby reducing proinflammatory cytokine level.57 Thus, a decrease in the concentration of corticosterone a day after the administration of high-dose LPS could be one of the factors mediating the development of more pronounced alterative changes in target organs.
On the first day after the injection of low-dose LPS, mild atrophy of the thymus in males is more pronounced than that in females. In males, the morphological manifestations are more severe and are combined with a higher number of apoptotic thymocytes than those in females. It is known that LPS, along with corticosterone and TNF, has a direct nega tive effect on thymic cells, particularly on the double-positive lymphocytes (CD4+CD8+) inhabiting the cortex.58 The high level of endotoxin detected by us in the serum of male Wistar rats after the administration of low- and high-dose LPS could be one of the mechanisms causing a more pronounced thymic atrophy than that observed in females. The sex differences in pathological changes in the thymus after the administration of high-dose LPS appear to be primarily due to a higher level of endotoxin in the serum of males than due to the immunomodulatory action of sex steroids and corticosterone, whose concentrations do not change in response to the administration of high-dose LPS.
After the injection of low-dose LPS, the level of IL-2 in females was decreased, but TNF increased. In males, the level of IL-2, in contrast, was increased, and IL-4 was decreased. When high-dose LPS was administered, the males showed a marked decrease in the production of all cytokines, whereas in females, only the levels of IL-4 and TNF decreased. According to the literature, the spleen cells of people who die of sepsis produce low amounts of both proinflammatory and anti-inflammatory cytokines.59 Low concentrations of the cytokines measured by us in the culture fluid of spleen cells from males treated with a high dose of LPS indicate pronounced immunosuppression.
After the injection of either low- or high-dose LPS, a more pronounced decrease in the number of T lymphocytes, namely cytotoxic and regulatory T cells, in the peripheral blood of males is revealed in comparison with females. A decrease in the number of T lymphocytes in the peripheral blood of patients with sepsis and septic shock indicates immunosuppression.60 A more pronounced decrease in the number of T lymphocytes in the early stages of sepsis and septic shock is correlated with an unfavorable prognosis.61
Compared with rats injected with low-dose LPS, on the first day after the administration of high-dose LPS to males and females, the severity of inflammatory changes and immunological disorders are more pronounced, and the sex differences are less pronounced. High-dose LPS did not cause changes in the sex steroid levels in the serum in either males or females, whereas the administration of low-dose LPS resulted in a decrease in estradiol in females and testosterone in males. The main organ that metabolizes sex steroid hormones is the liver.62 With the introduction of high doses of LPS, extensive necrosis and pronounced hepatocyte dystrophy are revealed in the liver, which lead to disruption of the synthetic processes in liver cells and, correspondingly, to reduced steroid metabolism. The absence of sex differences in pathological changes in target organs after the administration of high-dose LPS could be associated with impaired synthesis and metabolism of sex steroids and, as a consequence, a decrease in the ability of sex steroids to modulate the development of the immune response after the introduction of LPS.
Conclusion
On the first day after the injection of low-dose LPS, the level of endotoxin in the serum increases only in males, and this finding is combined with a more pronounced inflammatory response in the lungs and an increase in ALT and AST activities. The introduction of low-dose LPS does not lead to changes in serum corticosterone in males, whereas in females, its concentration increases.
After the administration of low-dose LPS, a mild accidental involution was revealed in the thymuses of females, whereas in males, thymic atrophy was more pronounced. Morphological changes in the thymuses of females and males were accompanied by an increase in the apoptosis of thymocytes. In the spleens of both males and females, the white pulp, notably the PALS, was destroyed and the germinative centers of lymphoid folliculi were widened, but the marginal zones were narrowed. These splenic changes were more pronounced in females than in males. After the injection of low-dose LPS, production of the Th1-cytokine IL-2 by splenic cells from males was increased. In contrast, the production level of IL-2 was decreased in females. In the peripheral blood of both males and females with SIRS, the number of NK cells increased, and the number of cytotoxic T lymphocytes decreased. However, males also showed a decrease in the absolute number of T-regulatory lymphocytes and T helpers, whereas in females, these parameters remained unchanged.
One day after the injection of high-dose LPS, inflammatory and pathological changes in the target organs as a result of severe endotoxemia are more pronounced in males. In the thymus, in both sexes of rats, thymic involution is severe; the thymic cortex is destroyed by an increase in the number of apoptotic lymphocytes and phagocytic macrophages. However, more pronounced immunological disorders in the thymus were detected in males. On the first day after the administration of LPS, no morphological changes in T-dependent PALS were found in the spleen of either males or females, whereas the marginal zone of B-zone lymphoid folliculi was narrow, and their germinative centers were widened. These morphological changes were more pronounced in females. In females, SIRS production of IL-2 by splenic cells increased, and IL-4 and TNF decreased. In males, the concentrations of IL-2, IL-4, IFNγ, and TNF in the culture of spleen cells decreased. In the peripheral blood of males, the number of T lymphocytes, including cytotoxic cells, decreased compared to that in females, which is an evidence of more pronounced suppression of the immune response in males.
Thus, the injection of high-dose LPS in female and male Wistar rats results in the development of a more severe form of SIRS, which is primarily associated with an increase in the serum endotoxin levels in both male and female rats and the development of innate immune responses. However, after the administration of low-dose LPS, the level of endotoxin in the serum increases only in males, in combination with a more pronounced inflammatory response in the lungs and an increase in ALT and AST activities.
Footnotes
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torio CM, Andrews RM. HCUP Statistical Brief. Vol. 160. Agency for Healthcare Research and Quality; [Accessed October 15, 2018]. National inpatient hospital costs: the most expensive conditions by payer, 2011; p. 2013. Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb160.pdf. [PubMed] [Google Scholar]
- 2.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shukla P, Rao GM, Pandey G, et al. Therapeutic interventions in sepsis: current and anticipated pharmacological agents. Br J Pharmacol. 2014;171(22):5011–5031. doi: 10.1111/bph.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosyreva AM, Simonova EY, Makarova OV. Gender differences in pulmonary and immune response in acute experimental endotoxicosis. Bull Exp Biol Med. 2012;153(3):340–342. doi: 10.1007/s10517-012-1710-z. [DOI] [PubMed] [Google Scholar]
- 5.Zhang DM, Mao BL. Relationships between LPS-tolerance and TLR4 as well as its signaling pathway. Sheng Li Ke Xue Jin Zhan. 2003;34(3):277–279. [PubMed] [Google Scholar]
- 6.Nijland R, Hofland T, van Strijp JA. Recognition of LPS by TLR4: potential for anti-inflammatory therapies. Mar Drugs. 2014;12(7):4260–4273. doi: 10.3390/md12074260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solov’eva T, Davydova V, Krasikova I, Yermak I. Marine compounds with therapeutic potential in gram-negative sepsis. Mar Drugs. 2013;11(6):2216–2229. doi: 10.3390/md11062216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendrick SFW, Jones DEJ. Mechanisms of innate immunity in sepsis. In: Baudouin SV, editor. Sepsis. London: Springer-Verlag; 2008. pp. 5–10. [Google Scholar]
- 9.Kukurba KR, Parsana P, Balliu B, et al. Impact of the X Chromosome and sex on regulatory variation. Genome Res. 2016;26(6):768–777. doi: 10.1101/gr.197897.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchis-Segura C, Becker JB. Why we should consider sex (and study sex differences) in addiction research. Addict Biol. 2016;21(5):995–1006. doi: 10.1111/adb.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155(4):1137–1146. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calippe B, Douin-Echinard V, Laffargue M, et al. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol. 2008;180(12):7980–7988. doi: 10.4049/jimmunol.180.12.7980. [DOI] [PubMed] [Google Scholar]
- 15.Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78(3):432–437. doi: 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- 16.Rettew JA, Huet YM, Marriott I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology. 2009;150(8):3877–3884. doi: 10.1210/en.2009-0098. [DOI] [PubMed] [Google Scholar]
- 17.Tanriverdi F, Silveira LF, Maccoll GS, Bouloux PM. The hypothalamic-pituitary-gonadal axis: immune function and autoimmunity. J Endocrinol. 2003;176(3):293–304. doi: 10.1677/joe.0.1760293. [DOI] [PubMed] [Google Scholar]
- 18.Olsen NJ, Kovacs WJ. Evidence that androgens modulate human thymic T cell output. J Investig Med. 2011;59(1):32–35. doi: 10.2310/jim.0b013e318200dc98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González DA, Díaz BB, Rodríguez Pérez MC, Hernández AG, Chico BN, de León AC. Sex hormones and autoimmunity. Immunol Lett. 2010;133(1):6–13. doi: 10.1016/j.imlet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9(1):56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 21.Zellweger R, Wichmann MW, Ayala A, Stein S, Demaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25(1):106–110. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Drechsler S, Weixelbaumer K, Raeven P, et al. Relationship between age/gender-induced survival changes and the magnitude of inflammatory activation and organ dysfunction in post-traumatic sepsis. PLoS One. 2012;7(12):e51457. doi: 10.1371/journal.pone.0051457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg. 1999;134(12):1342–1347. doi: 10.1001/archsurg.134.12.1342. [DOI] [PubMed] [Google Scholar]
- 24.Hubacek JA, Stüber F, Fröhlich D, et al. Gene variants of the bactericidal/permeability increasing protein and lipopolysaccharide binding protein in sepsis patients: gender-specific genetic predisposition to sepsis. Crit Care Med. 2001;29(3):557–561. doi: 10.1097/00003246-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Combes A, Luyt CE, Trouillet JL, Nieszkowska A, Chastre J. Gender impact on the outcomes of critically ill patients with nosocomial infections. Crit Care Med. 2009;37(9):2506–2511. doi: 10.1097/CCM.0b013e3181a569df. [DOI] [PubMed] [Google Scholar]
- 26.Nachtigall I, Tafelski S, Rothbart A, et al. Gender-related outcome difference is related to course of sepsis on mixed ICUs: a prospective, observational clinical study. Crit Care. 2011;15(3):R151. doi: 10.1186/cc10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schröder J, Kahlke V, Staubach KH, Zabel P, Stüber F. Gender differences in human sepsis. Arch Surg. 1998;133(11):1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 28.Adrie C, Azoulay E, Francais A, et al. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132(6):1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 29.Sakr Y, Elia C, Mascia L, et al. The influence of gender on the epidemiology of and outcome from severe sepsis. Crit Care. 2013;17(2):R50. doi: 10.1186/cc12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med. 2007;35(6):1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- 31.Federman DD. The biology of human sex differences. N Engl J Med. 2006;354(14):1507–1514. doi: 10.1056/NEJMra052529. [DOI] [PubMed] [Google Scholar]
- 32.Long JA, Evans HM. The oestrus cycle in the rat and its associated phenomena. In: Leuschner AO, editor. Memoirs of the University of California. Berkeley: University of California Press; 1922. [Google Scholar]
- 33.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94(6):1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 34.Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Chapman MJ, Lesnik P. Enhanced dendritic cell survival attenuates lipopolysaccha-ride-induced immunosuppression and increases resistance to lethal endotoxic shock. J Immunol. 2008;180(10):6941–6946. doi: 10.4049/jimmunol.180.10.6941. [DOI] [PubMed] [Google Scholar]
- 35.Kosyreva AM, Makarova OV, Eyu O. [Age- and sex-related differences in the thymic morphological and functional changes in Wistar rats with systemic inflammatory response syndrome] Clin Experiment Morphol. 2016;1(17):18–26. [Google Scholar]
- 36.Huo R, Wang L, Wang X, et al. Removal of regulatory T cells prevents secondary chronic infection but increases the mortality of subsequent sub-acute infection in sepsis mice. Oncotarget. 2016;7(10):10962–10975. doi: 10.18632/oncotarget.7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1(2):87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilcox MH. Comparison of formalin and Bouin’s reagent for fixation of coagulase negative staphylococcal biofilm. J Clin Pathol. 1994;47(11):1044–1046. doi: 10.1136/jcp.47.11.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada M, Kubo H, Kobayashi S, et al. The increase in surface CXCR4 expression on lung extravascular neutrophils and its effects on neutrophils during endotoxin-induced lung injury. Cell Mol Immunol. 2011;8(4):305–314. doi: 10.1038/cmi.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1(5):431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 41.Weibel ER. Stereological methods. Vol 1: Practical methods for biological morphometry. 1st ed. London-New York-Toronto: Academic Press; 1979. [Google Scholar]
- 42.Lin K-H, Lin K-C, Lu W-J, Thomas P-A, Jayakumar T, Sheu J-R. Astaxanthin, a carotenoid, stimulates immune responses by enhancing IFN-γ and IL-2 secretion in primary cultured lymphocytes in vitro and ex vivo. Int J Mol Sci. 2016;17(1):44. doi: 10.3390/ijms17010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eshima N, Tokumaru O, Hara S, et al. Age-specific sex-related differences in infections: a statistical analysis of national surveillance data in Japan. PLoS One. 2012;7(7):e42261. doi: 10.1371/journal.pone.0042261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Wang Y, Zhang H, et al. Yohimbine enhances protection of berberine against LPS-induced mouse lethality through multiple mechanisms. PLoS One. 2012;7(12):e52863. doi: 10.1371/journal.pone.0052863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coldewey SM, Rogazzo M, Collino M, Patel NS, Thiemermann C. Inhibition of IκB kinase reduces the multiple organ dysfunction caused by sepsis in the mouse. Dis Model Mech. 2013;6(4):1031–1042. doi: 10.1242/dmm.012435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Ge QM, Bian F, Dong Y, Huang CM. Inhibition of TLR4 protects rat islets against lipopolysaccharide-induced dysfunction. Mol Med Rep. 2017;15(2):805–812. doi: 10.3892/mmr.2016.6097. [DOI] [PubMed] [Google Scholar]
- 47.Yaguchi A, Yuzawa J, Klein DJ, Takeda M, Harada T. Combining intermediate levels of the Endotoxin Activity Assay (EAA) with other biomarkers in the assessment of patients with sepsis: results of an observational study. Crit Care. 2012;16(3):R88. doi: 10.1186/cc11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adamik B, Smiechowicz J, Jakubczyk D, Kübler A. Elevated serum PCT in septic shock with endotoxemia is associated with a higher mortality rate. Medicine. 2015;94(27):e1085. doi: 10.1097/MD.0000000000001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, et al. Intestinal micro-biota is influenced by gender and body mass index. PLoS One. 2016;11(5):e0154090. doi: 10.1371/journal.pone.0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 51.Dominianni C, Sinha R, Goedert JJ, et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One. 2015;10(4):e0124599. doi: 10.1371/journal.pone.0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Homma H, Hoy E, Xu DZ, Lu Q, Feinman R, Deitch EA. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol. 2005;288(3):G466–G472. doi: 10.1152/ajpgi.00036.2004. [DOI] [PubMed] [Google Scholar]
- 53.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118(22):5918–5927. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanczkowski W, Alexaki VI, Tran N, et al. Hypothalamo-pituitary and immune-dependent adrenal regulation during systemic inflammation. Proc Natl Acad Sci U S A. 2013;110(36):14801–14806. doi: 10.1073/pnas.1313945110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205(5):807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- 56.Shanks N, McCormick CM, Meaney MJ. Sex differences in hypothalamic-pituitary-adrenal responding to endotoxin challenge in the neonate: reversal by gonadectomy. Brain Res Dev Brain Res. 1994;79(2):260–266. doi: 10.1016/0165-3806(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 57.Zacharowski K, Zacharowski PA, Koch A, et al. Toll-like receptor 4 plays a crucial role in the immune-adrenal response to systemic inflammatory response syndrome. Proc Natl Acad Sci U S A. 2006;103(16):6392–6397. doi: 10.1073/pnas.0601527103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsuji T, Asano Y, Handa T, Honma Y, Ichinose Y, Yokochi T. Induction of apoptosis in lymphoid tissues of mice after intramuscular injection of enterotoxigenic Escherichia coli enterotoxin. Immunobiology. 2000;201(3–4):377–390. doi: 10.1016/s0171-2985(00)80092-3. [DOI] [PubMed] [Google Scholar]
- 59.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pravda J. Metabolic theory of septic shock. World J Crit Care Med. 2014;3(2):45–54. doi: 10.5492/wjccm.v3.i2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rimmelé T, Payen D, Cantaluppi V, et al. Immune cell phenotype and function in sepsis. Shock. 2016;45(3):282–291. doi: 10.1097/SHK.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lucki NC, Sewer MB. Multiple roles for sphingolipids in steroid hormone biosynthesis. Subcell Biochem. 2008;49:387–412. doi: 10.1007/978-1-4020-8831-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]