Key Points
Question
Does polymyxin B hemoperfusion improve survival in patients with septic shock and high levels of endotoxin in the blood?
Findings
In this multicenter, randomized, clinical trial that included 450 adults with septic shock and high circulating endotoxin activity, polymyxin B hemoperfusion compared with sham hemoperfusion did not significantly decrease 28-day mortality, 37.7% vs 34.5%, respectively.
Meaning
Polymyxin B hemoperfusion was not effective in reducing mortality in septic shock.
Abstract
Importance
Polymyxin B hemoperfusion reduces blood endotoxin levels in sepsis. Endotoxin activity can be measured in blood with a rapid assay. Treating patients with septic shock and elevated endotoxin activity using polymyxin B hemoperfusion may improve clinical outcomes.
Objective
To test whether adding polymyxin B hemoperfusion to conventional medical therapy improves survival compared with conventional therapy alone among patients with septic shock and high endotoxin activity.
Design, Setting, and Participants
Multicenter, randomized clinical trial involving 450 adult critically ill patients with septic shock and an endotoxin activity assay level of 0.60 or higher enrolled between September 2010 and June 2016 at 55 tertiary hospitals in North America. Last follow-up was June 2017.
Interventions
Two polymyxin B hemoperfusion treatments (90-120 minutes) plus standard therapy completed within 24 hours of enrollment (n = 224 patients) or sham hemoperfusion plus standard therapy (n = 226 patients).
Main Outcomes and Measures
The primary outcome was mortality at 28 days among all patients randomized (all participants) and among patients randomized with a multiple organ dysfunction score (MODS) of more than 9.
Results
Among 450 eligible enrolled patients (mean age, 59.8 years; 177 [39.3%] women; mean APACHE II score 29.4 [range, 0-71 with higher scores indicating greater severity), 449 (99.8%) completed the study. Polymyxin B hemoperfusion was not associated with a significant difference in mortality at 28 days among all participants (treatment group, 84 of 223 [37.7%] vs sham group 78 of 226 [34.5%]; risk difference [RD], 3.2%; 95% CI, −5.7% to 12.0%; relative risk [RR], 1.09; 95% CI, 0.85-1.39; P = .49) or in the population with a MODS of more than 9 (treatment group, 65 of 146 [44.5%] vs sham, 65 of 148 [43.9%]; RD, 0.6%; 95% CI, −10.8% to 11.9%; RR, 1.01; 95% CI, 0.78-1.31; P = .92). Overall, 264 serious adverse events were reported (65.1% treatment group vs 57.3% sham group). The most frequent serious adverse events were worsening of sepsis (10.8% treatment group vs 9.1% sham group) and worsening of septic shock (6.6% treatment group vs 7.7% sham group).
Conclusions and Relevance
Among patients with septic shock and high endotoxin activity, polymyxin B hemoperfusion treatment plus conventional medical therapy compared with sham treatment plus conventional medical therapy did not reduce mortality at 28 days.
Trial Registration
ClinicalTrials.gov Identifier: NCT01046669
This clinical trial involving patients with septic shock and high endotoxin activity compares the effect of treatment with polymyxin B hemoperfusion vs sham therapy and usual care on mortality.
Introduction
Endotoxin activates the host inflammatory response and mediates the clinical syndrome of sepsis. Elevated endotoxin levels occur independently of the causative organism isolated from a primary infection site. Evidence suggests that the gastrointestinal tract can act as a reservoir of endotoxin in septic shock.1 Endotoxin activity can be reliably measured.2 High endotoxin activity is associated with multiple organ failure and mortality in sepsis.3
Pharmacological therapies to neutralize endotoxin activity and improve clinical outcomes have yielded negative results in clinical trials. Failed interventions include monoclonal human and mouse antibody directed against endotoxin, recombinant bacterial permeability–increasing protein, phospholipid emulsion, and an antagonist of the TLR4 receptor.4,5,6,7,8 An alternative strategy is to remove endotoxin from the blood through selective adsorption using high-affinity polymyxin B hemoperfusion.9 Previous clinical trials targeting endotoxin in patients with septic shock aimed to enroll those with a clinical suspicion of a gram-negative source of infection, rather than patients with documented high levels of endotoxin activity.
The EUPHRATES (Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized Controlled trial of Adults Treated for Endotoxemia and Septic Shock) trial was designed to enroll only patients with documented elevated levels of endotoxin activity (defined as an endotoxin activity assay ≥0.60), reasoning that patients with documented elevated levels of endotoxin activity would be most likely to respond to polymyxin B hemoperfusion therapy. The hypothesis was that the treatment would improve survival in patients with septic shock and elevated endotoxin activity levels.
Methods
Trial Design and Setting
In this multicenter, randomized, blinded, sham-controlled trial enrollment occurred at 55 tertiary hospitals in the United States and Canada and was designed by the steering committee in collaboration with the sponsor. Five sites were dropped for inactive screening.
The study was approved by the institutional review boards for all study sites. All enrolled patients or legally authorized representatives provided written informed consent. The protocol and its amendments are available in Supplement 1; the statistical analysis plan, Supplement 2. Detailed methods have been published.10
The trial received an Investigational Device Exemption from the US Food and Drug Administration (FDA). Study participants were enrolled between September 2010 and June 2016 at 55 tertiary hospitals in North America with last follow-up in June 2017.
Participants
Adults aged 18 years or older with septic shock and high endotoxin-activity assay levels in the blood were enrolled. Race/ethnicity was measured to define characteristics of enrolled patients and was obtained from medical records classification, which used fixed categories. Patients were eligible to participate if they met the following criteria: (1) arterial hypotension requiring vasopressor therapy equal to or greater than an equivalent of norepinephrine 0.05 μg/kg/min for at least 2 consecutive hours and for no more than 30 hours prior to randomization; (2) received intravenous antibiotics for a documented or suspected infection; (3) received at least 30 mL/kg of intravenous crystalloid fluid (or equivalent) in the preceding 24 hours; and (4) had at least 1 additional new organ dysfunction due to the acute illness. A detailed list of eligibility criteria appears in the protocol (Supplement 1). Potentially eligible patients fulfilling these criteria had a blood endotoxin activity assay (Spectral Medical, Toronto, Canada) level measured; and were eligible for randomization if the endotoxin activity assay level was 0.60 or higher, which is considered the threshold for high endotoxin activity and is associated with higher intensive care unit (ICU) mortality.11
A description of the endotoxin activity assay measurement, technique, and clinical significance are available elsewhere.12 Patients were excluded if there were (1) documented treatment limitations in the medical record, such as limits in treatment that prohibited further escalation in the intensity or scope of organ support or initiation of renal replacement therapy or (2) a terminal disease state that would have precluded short-term survival. A do not resuscitate order was not an exclusion.
After the second interim analysis, the data and safety monitoring board (DSMB) recommended restricting subsequent enrollment to patients with a greater mortality risk. Therefore, an additional exclusion criterion was introduced for patients with a Multiple Organ Dysfunction Score (MODS) of 9 or less (MODS range, 0-24 with 24 being worst possible score). The MODS greater than 9 group became a primary analysis of interest for the FDA and the statistical analysis plan (SAP) was changed on August 27, 2014.
Treatment Allocation
Patient eligibility prior to randomization was confirmed by a clinical coordinating center (Cooper Clinical Coordinating Center, Camden, New Jersey). Eligible patients were stratified by site and randomly assigned in a 1:1 ratio to either polymyxin B hemoperfusion plus conventional medical therapy or sham hemoperfusion plus conventional medical therapy. Random allocation was concealed by a centralized web-based program using block sizes of 2 and 4.
Study Interventions
The investigational treatment used a selective endotoxin adsorption cartridge consisting of the antibiotic polymyxin B covalently bound to polystyrene-polypropylene fibers. An electrochemical interaction between polymyxin B and endotoxin results in irreversible binding of circulating blood endotoxin to the cartridge.9 Hemoperfusion treatments were performed using a standard hemodialysis machine via centrally inserted standard dialysis catheters at a prescribed blood flow rate of 100 mL/min (range, 80-120 mL/min). Each patient received 2 hemoperfusion treatments within 24 hours with a target duration for each treatment of 2 hours (minimum of 90 minutes). Anticoagulation was recommended with intravenous unfractionated heparin to maintain circuit patency during each treatment. Citrate anticoagulation was not permitted.
All sites agreed, when feasible, to follow the tenets of the Surviving Sepsis Campaign clinical practice guidelines for management of sepsis.13
Blinding
All hemoperfusion treatments were concealed at the bedside. For patients allocated to polymyxin B hemoperfusion, hemoperfusion was performed, whereas patients allocated to the control group received sham hemoperfusion. For sham treatments, a hemodialysis machine was primed at the bedside with a standard bloodline and dialyzer, a dialysis catheter was cut to simulate insertion and taped to the skin beneath an opaque dressing, and 0.9% saline was recirculated in the circuit for 2 hours to simulate a hemoperfusion treatment. The site-specific principal investigator, coinvestigators, and members of the clinical care team responsible for making clinical decisions for patient care (ie, intensive care physician, fellows, and residents) were blinded to the treatment assignment. The research study nurse, clinical trial pharmacist, bedside nurse, physician designated for placement of the dialysis catheter, and nephrologist performing the treatments were unblinded to the treatment assignment. Site-specific study coordinators designated in each patient case report form whether or not blinding remained through day 28.
Study Outcomes
The primary efficacy end point at the start of the trial was 28-day mortality for all participants. Following the second interim analysis and recommendations of the DSMB and discussion with the FDA, on April 9, 2014, the MODS population (MODS >9) was designated as the population in which the primary end point would be evaluated and subsequently were the only patients enrolled in the trial. Outcomes for all participants and for patients with MODS of more than 9 were reported separately.
A hierarchical testing strategy was used for testing end points. With this strategy, secondary and exploratory end points are collectively tested only if the primary end point achieves statistical significance. In that circumstance, each secondary end point would be tested in a prespecified hierarchical order until statistical significance is not achieved. Secondary outcome variables and their order were survival time from baseline to death within 28 days and change in MODS, mean arterial pressure (MAP), urine output, and creatinine concentration from baseline to 72 hours. A per-protocol 28-day mortality analysis (patients receiving 2 treatments in either group with no major protocol violations) was to be performed for all participants and for the MODS group if there was a 5% difference in the number of participants randomized and the number of per-protocol participants.
Sample Size Estimation
Based on the EUPHAS trial, a sample size of 360 participants was estimated to provide 80% power to detect an absolute risk reduction of 15% for the primary end point of 28-day mortality at a significance level of .05 (2-sided).14 It was anticipated that the event rate for mortality at 28 days in the control group would be 35% based on prior clinical trials and the Surviving Sepsis Campaign database.15 Two interim analyses were performed. The first interim analysis was performed for safety only and the second was performed to evaluate efficacy. The sample size was recalculated following the second interim analysis based on the patients enrolled in the control group using the O’Brien-Fleming approach to adjust the type I error. Using this approach, the planned sample size was adjusted to 450 patients, with subsequent enrollment restricted to patients with a MODS of more than 9. This protocol modification was based upon recommendations by the DSMB and approved by the FDA. Full details of this sample size adjustment are included in the study protocol (Supplement 1).
Statistical Analysis
Statistical analyses were performed using SAS (SAS Institute Inc) software for Windows version 9.3. Descriptive analyses were performed on baseline variables using mean (SD), median (interquartile range [IQR]), or proportions and percentages as appropriate.
The primary efficacy analysis for 28-day mortality between the 2 groups included (1) all participants randomized and (2) participants in the MODS group, using a χ2 test and reported as a risk difference (RD) and risk ratio (RR) with a 95% CI. Patients with unknown survival status at 28 days were excluded from the primary efficacy analysis and sensitivity analysis was performed using best-case (all survived at 28 days) and worst-case (all deceased at 28 days) scenarios. A group-sequential design according to the approach recommended by O’Brien-Fleming for efficacy, including 1 interim analysis, was performed, using a 2-tailed test and the significance levels of .05 and .048 for the interim and final analyses, respectively.
Survival analysis, with censoring at 28 days, was performed using a Kaplan-Meier curve and the log-rank test. Assessment of change in endotoxin activity assay levels was performed using generalized estimating equations. A general linear mixed-effects model was used to compare the mean change in endotoxin activity assay at day 2 and day 3 from the mean baseline level. To assess the effect of polymyxin B hemoperfusion treatments on endotoxin activity assay activity, only patients who received 2 of either treatment were included in these analyses. For analyses of adverse and serious adverse events, the population used was all participants randomized who had treatment initiated.
Results
Participant Characteristics
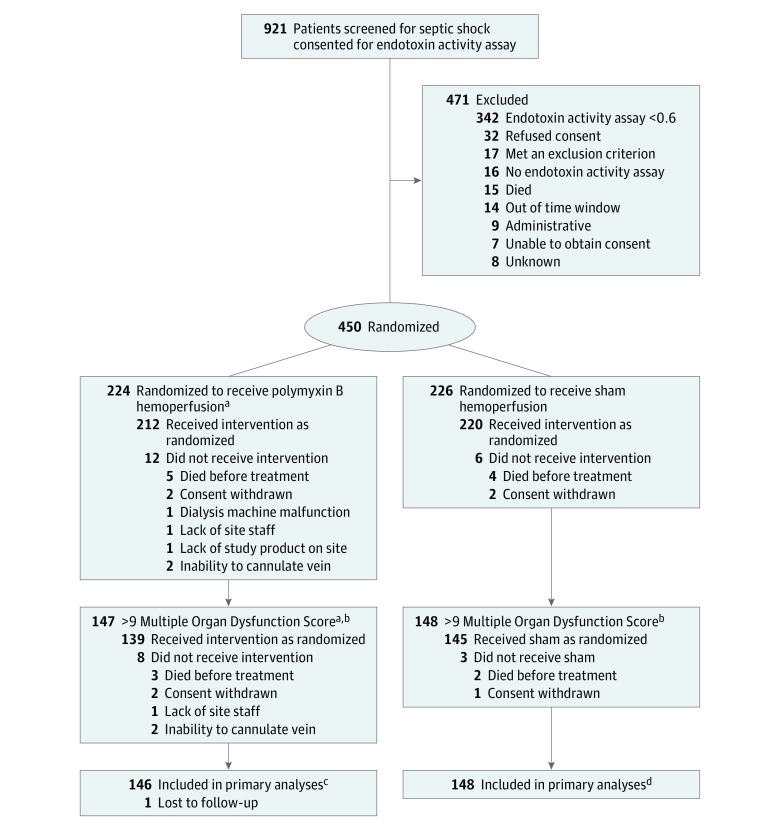
Among 921 patients with septic shock who were eligible for endotoxin activity assay testing, 450 fulfilled eligibility and consented to participate. Of the 450 enrolled patients, 224 received polymyxin B hemoperfusion (mean age, 60.9 years; women, 84 [37.5%]; mean Acute Physiology and Chronic Health Evaluation [APACHE II], score 29.4) and 226 received sham treatment (mean age, 58.8 years; women, 93 [41.2%]; mean APACHE II score, 28.1) (Figure and Table 1). Of these, 12 of 224 patients in the polymyxin B hemoperfusion group and 6 of 226 patients in the sham group did not receive treatment. There were 295 patients with a MODS of more than 9, of whom 147 received polymyxin B hemoperfusion (mean MODS score, 11.9 [SD, 2.0]) and 148 received sham treatment (mean MODS score, 11.9 [SD, 1.8]). The groups were similar on baseline characteristics, case-mix, and organ support (Table 1). Patients were severely ill as evidenced by high APACHE II scores, receipt of mechanical ventilation, and vasopressor support (Table 1). The mean time between randomization and receipt of intervention for all participants was 3:30 hours (95% CI, 3:15-3:45 hours). Comparisons by treatment groups are found in eTable 1 in Supplement 3.
Figure. Patient Recruitment, Randomization, and Flow of the Study.
aOne patient was lost to follow-up after randomization.
bProtocol adjusted following interim analysis to enroll only patients with very high severity of illness based on a Multiple Organ Dysfunction Score of more than 9.
cPrimary analyses also included all participants randomized in the study (223 in the polymyxin B hemoperfusion group).
dPrimary analyses also included all participants randomized in the study (226 in the sham hemoperfusion group).
Table 1. Baseline Characteristics of All Participants and Those With a MODS of More Than 9a.
| Variables | All Participants, No. (%) | MODS >9 Population, No. (%) | ||
|---|---|---|---|---|
| Polymyxin-B Hemoperfusion (n = 224) |
Sham (n = 226) |
Polymyxin-B Hemoperfusion (n = 147) |
Sham (n = 148) |
|
| Age, mean (SD), y | 60.9 (15.1) | 58.8 (14.7) | 59.5 (15.1) | 59.2 (14.0) |
| Sex | ||||
| Women | 84 (37.5) | 93 (41.2) | 51 (34.7) | 57 (38.5) |
| Men | 140 (62.5) | 133 (58.8) | 93 (65.3) | 91 (61.5) |
| Race/ethnicity | ||||
| White | 183 (81.7) | 187 (82.7) | 119 (81.0) | 112 (75.7) |
| Black | 22 (9.8) | 13 (5.8) | 13 (8.8) | 13 (8.8) |
| Hispanic | 10 (4.5) | 12 (5.3) | 7 (4.8) | 10 (6.8) |
| Asian | 3 (1.3) | 9 (4.0) | 2 (1.4) | 8 (5.4) |
| Otherb | 6 (2.7) | 5 (2.2) | 6 (4.1) | 5 (3.4) |
| Arterial pressure, mean (SD), mm Hg | 71.8 (9.9) | 73.3 (10.5) | 71.0 (9.7) | 72.9 (10.6) |
| APACHE II score, mean (SD)c | 29.4 (9.0) | 28.1 (8.5) | 32.0 (8.8) | 30.5 (8.1) |
| MODS score, mean (SD) | 10.0 (3.3) | 10.0 (3.3) | 11.9 (2.0) | 11.9 (1.8) |
| Mechanical ventilation | 208 (93.0) | 217 (96.0) | 142 (97.9) | 147 (99.3) |
| Microorganismsd | ||||
| No growth | 73 (32.9) | 78 (34.7) | 49 (33.3) | 44 (29.7) |
| Gram negative | 53 (23.9) | 30 (13.3) | 36 (24.5) | 21 (14.2) |
| Gram positive | 49 (22.1) | 51 (22.7) | 31 (21.1) | 41 (27.7) |
| Other | 15 (6.8) | 15 (6.7) | 10 (6.8) | 9 (6.1) |
| Mixed | 32 (14.4) | 51 (22.7) | 21 (14.3) | 33 (22.3) |
| Bacteremiae | 72 (33.0) | 62 (28.1) | 48 (33.1) | 45 (30.8) |
| Site of infection | ||||
| Intra-abdominal | 71 (32.4) | 80 (35.7) | 48 (33.6) | 56 (37.8) |
| Lung | 75 (34.3) | 87 (38.8) | 50 (35.0) | 56 (37.8) |
| Mixed | 10 (4.6) | 13 (5.8) | 7 (4.9) | 7 (4.7) |
| Otherf | 63 (28.8) | 44 (19.6) | 38 (26.6) | 29 (19.6) |
| Cumulative vasopressor index, mean (SD)g | ||||
| 0 to ≤5 | 102 (45.7) | 89 (39.4) | 55 (37.7) | 49 (33.1) |
| 6 to ≤10 | 86 (38.6) | 109 (48.2) | 63 (43.2) | 77 (52.0) |
| 11 to ≤15 | 33 (14.8) | 25 (11.1) | 27 (18.5) | 19 (12.8) |
| 16 to ≤20 | 2 (0.9) | 3 (1.3) | 1 (0.7) | 3 (2.0) |
| Norepinephrine dose, mean (SD), μg/kg/min | ||||
| 0 to ≤0.05 | 18 (8.0) | 11 (4.9) | 8 (5.4) | 4 (2.7) |
| 0.05 to ≤0.1 | 34 (15.2) | 27 (12.0) | 22 (15.0) | 15 (10.1) |
| >0.1 | 159 (71.0) | 177 (78.3) | 112 (76.2) | 122 (82.4) |
| Missing or not applicable | 13 (5.8) | 11 (4.9) | 5 (3.4) | 7 (4.7) |
| AKIN AKI stageh | ||||
| No AKI | 59 (26.3) | 59 (26.1) | 26 (17.7) | 27 (18.2) |
| Stage 1 | 28 (12.5) | 31 (13.7) | 18 (12.2) | 19 (12.8) |
| Stage 2 | 33 (14.7) | 27 (12.0) | 19 (12.9) | 18 (12.2) |
| Stage 3 | 104 (46.4) | 109 (48.2) | 84 (57.1) | 84 (56.8) |
| Renal replacement therapy | 47 (21.0) | 62 (27.4) | 37 (25.2) | 45 (30.4) |
| Endotoxin activity assay levels, mean (SD) | 0.77 (0.1) | 0.77 (0.1) | 0.80 (0.2) | 0.80 (0.2) |
| Range, No. (%) | ||||
| 0.60 to 0.69 | 70 (31.3) | 78 (34.5) | 49 (33.3) | 49 (33.1) |
| 0.70 to 0.79 | 54 (24.1) | 63 (27.9) | 28 (19.1) | 39 (26.4) |
| 0.80 to 0.89 | 57 (25.5) | 49 (21.7) | 36 (24.5) | 31 (21.0) |
| 0.90 to 0.99 | 23 (10.3) | 22 (9.7) | 17 (11.6) | 18 (12.2) |
| ≥1.00 | 20 (8.9) | 14 (6.2) | 17 (11.6) | 11 (7.4) |
Abbreviations: AKIN AKI, Acute Kidney Injury Network Acute Kidney Injury; APACHE, Acute Physiology and Chronic Health Evaluation; MODS, Multiple Organ Dysfunction Score.
MODS score measures altered organ function in acutely ill patients using 6 organ systems with weighted scores (range of score, 0 for normal to 4 for the most severe) of each organ system (MODS range, 0-24). A higher score is associated with greater burden of organ dysfunction. A MODS of 9 to 12 points has a hospital mortality of approximately 50%. Prior to the protocol amendment, the MODS score was calculated at baseline. After the amendment, MODS > 9 was included in the screening process and required to obtain EAA.
Race not coded as white, black, Hispanic, or Asian.
APACHE II score severity of disease classification is based on physiologic measures, age, and comorbid conditions. A higher score is associated with greater illness acuity and higher risk of death (APACHE II score range, 0-71). An APACHE II score of 30-34 is associated with a 70% hospital mortality. APACHE II score was obtained at baseline (time of randomization to the initiation of study treatment) using the worst physiologic variable during that period.
Microorganisms were determined by culture from blood, respiratory, urine, fluid, or tissue.
Bacteremia is defined as bacteria in the bloodstream from microbiology reports. No adjudication was performed.
Genitourinary, dermatologic, cardiovascular, or neurological.
CVI score includes cumulative points for equivalent doses of vasopressor support at a point in time. Dose points range from 1-4 for each vasopressor (dopamine, epinephrine, norepinephrine, phenylephrine, and vasopressin). Total CVI score range, 1-20; CVI score 16-20 = 5-6 high-dose vasopressors). CVI score was obtained at baseline with a single score calculated for the vasopressor dose at the time of the assessment.
The AKIN AKI classification scheme for acute kidney injury using changes in baseline serum creatinine and urine output. Range: no AKI to stage 3 AKI. Stage 1 AKI defined as an increase in serum creatinine (SCr) ≥ 0.3 mg/dL over 48 hours or ≥1.5x baseline over 7 days or an episode of urine output (UO) <0.5 mL/kg/h for ≥ 6 hours. Stage 2 AKI defined as an increase in SCr ≥2x baseline or UO <0.5 mL/kg/h for ≥ 12 hours. Stage 3 AKI defined as an increase in SCr ≥3x baseline or UO < 0.3 ml/kg/h for ≥24 hours or anuria for ≥12 hours. Stage 2 and 3 are associated with increased mortality. Among patients with AKI and sepsis, mortality is approximately 30%. AKIN AKI stage was obtained at baseline.
Primary Outcomes
One patient in the polymyxin B hemoperfusion group and none in the sham group were lost to follow-up. Consistent with the SAP, missing outcome data for survival status were excluded from the primary analysis. Among all participants, the mortality at 28 days in the polymyxin B hemoperfusion group was 37.7% (84 of 223) compared with 34.5% (78 of 226) in the sham group (risk difference [RD], 3.15; 95% CI, −5.73 to 12.04; risk ratio [RR], 1.09; 95% CI, 0.85-1.39; P = .49) (Table 2). In the MODS population, 28-day mortality was 44.5% (65 of 146) in the polymyxin B hemoperfusion group and 43.9% (65 of 148) in the sham group (RD, 0.60; 95% CI, −10.75 to 11.97; RR, 1.01; 95% CI, 0.78-1.31; P = .92) (Table 2).
Table 2. Summary of the Primary End Point of 28-Day Mortality for All Participants and for Patients With MODS of More Than 9.
| No./Total (%) | (95% CI) | P Valuea | |||
|---|---|---|---|---|---|
| Polymyxin-B Hemoperfusion | Sham | Risk Difference | Risk Ratio | ||
| All Participants | 84/223 (37.7) | 78/226 (34.5) | 3.15 (−5.73 to 12.04) | 1.09 (0.85 to 1.39) | .49 |
| >9 MODSb | 65/146 (44.5) | 65/148 (43.9) | 0.60 (−10.75 to 11.97) | 1.01 (0.78 to 1.31) | .92 |
P values were calculated by χ2 and were unadjusted.
Multiple Organ Dysfunction Score (MODS)–measure of altered organ function in acutely ill patients using 6 organ systems with weighted scores (0, normal; 4, severe) of each organ system (MODS range, 0-24). A higher score is associated greater burden of organ dysfunction. A MODS of 9 to 12 points has a hospital mortality of approximately 50%. Prior to the protocol amendment, the MODS score was calculated at baseline (time of randomization to the initiation of the study treatment). After the amendment, MODS of more than 9 was included at the time of screening, prior to randomization.
Because the primary outcome did not achieve statistical significance, secondary and exploratory end point analyses are not reported herein. They are available in supplemental materials (eTable 2 and eFigures 1A and 1B in Supplement 3). A per-protocol analysis of 28-day mortality was performed because the difference between randomized and per-protocol patients was greater than 5% in both the all participants and the MODS groups. These results also showed no difference in mortality (Table 3).
Table 3. Per-Protocol (Each Group Received 2 Treatments) 28-Day Mortality.
| Population | No./Total (%) | Difference, % (95% CI) | P Valuea | |
|---|---|---|---|---|
| Polymyxin-B Hemoperfusion | Sham | |||
| All participants | 50/173 (28.9) | 59/202 (29.2) | −0.3 (−9.5 to 8.9) | .94 |
| >9 MODS | 38/115 (33.0) | 47/129 (36.4) | −3.1 (−15.2 to 9.0) | .58 |
Abbreviation: MODS, Multiple Organ Dysfunction Score.
P values calculated using χ2.
Endotoxin Activity Assay Comparison Over Time
For patients who received 2 treatments, there was no significant difference in mean change of endotoxin activity assay between baseline and day 2 or 3 between treatment groups in all participants or among those in the MODS >9 group (eTables 3 and 4 in Supplement 3).
Adverse Events
Overall, 264 serious adverse events were reported, 65.1% polymyxin B hemoperfusion and 57.3% sham groups (eTable 5 in Supplement). The most frequent serious adverse events were worsening of sepsis (10.8% polymyxin B hemoperfusion vs 9.1% sham) and worsening of septic shock (6.6% polymyxin B hemoperfusion vs 7.7% sham). There were 2 serious adverse events adjudicated as probably related to polymyxin B hemoperfusion; both related to the dialysis catheter (deep venous thrombosis; venous air embolism). There were no other serious adverse events related to the polymyxin B hemoperfusion cartridge. There were 11 adverse events related to the device or its components in the polymyxin B hemoperfusion group and 5 in the sham group among randomized patients who had treatment initiated (eTable 6 in Supplement 3). These data do not include circuit clotting because circuit clotting could not occur in the sham group. Among patients in the polymyxin B hemoperfusion group, circuit clotting occurred in 17 of 212 participants (8%), resulting in 4 patients discontinuing treatment.
Blinding
Partial blinding (treatment team) was reported as being maintained for 97.5% of patients randomized.
Discussion
In this multicenter randomized clinical trial involving 450 adults with septic shock and high circulating endotoxin activity, polymyxin B hemoperfusion compared with sham hemoperfusion did not significantly decrease 28-day mortality among all randomized patients or among randomized patients with more severe illness, based on a MODS of more than 9. The findings suggest that polymyxin B hemoperfusion should not be used with the goal of improving survival in critically ill patients with septic shock.
Over the past several decades, numerous clinical trials have evaluated a spectrum of novel therapeutics to treat patients with sepsis and septic shock.15 To date, none of these has improved patient outcomes. One plausible explanation for this apparent failure is heterogeneity across patients fulfilling the diagnostic criteria for sepsis. Sepsis trials have generally included a wide continuum of patients based on the presence of a clinical syndrome and have typically not focused on a specific therapeutic target.16 An emerging concept in sepsis research has been to adopt a theragnostic approach, in which a diagnostic test is used to identify a pathophysiological phenomenon (ie, endotoxemia) that is treated with a directed therapy against the specific pathophysiology. This trial was designed to identify patients with septic shock and endotoxemia at high risk of death and apply a directed treatment to remove circulating endotoxin.
Two recent randomized trials compared polymyxin B hemoperfusion with standard therapy among critically ill patients with abdominal sepsis and had contradictory results. The EUPHAS trial was an Italian multicenter randomized comparison of early postoperative polymyxin B hemoperfusion (2 treatments) compared with standard care among 64 patients with abdominal sepsis or septic shock.14 EUPHAS found that treatment with polymyxin B hemoperfusion produced an increase in mean arterial pressure, a decrease in vasopressor requirements and reduced organ dysfunction in the 72 hours following surgery. EUPHAS also suggested polymyxin B hemoperfusion was associated with improved survival at 28 days, a secondary end point, following adjustment for the Sequential Organ Failure Assessment score. A French multicenter randomized trial (ABDOMIX) similarly compared polymyxin B hemoperfusion (2 treatments) with standard care among 243 patients with peritonitis and septic shock undergoing emergency surgery.17 The ABDOMIX trial found no significant difference in 28-day mortality (primary end point) among patients treated with polymyxin B hemoperfusion compared with standard care and no significant differences in key secondary end points, including 90-day mortality or change in organ dysfunction in the week following treatment.
Results reported herein, to our knowledge, represent the largest trial involving polymyxin B hemoperfusion therapy performed to date. This study has a number of strengths. First, the trial was blinded with sham hemoperfusion treatments. Second, the trial successfully enrolled a wide spectrum of patients with septic shock, high acuity, and higher risk of death than patients enrolled in prior trials.14,17 Third, the trial specifically treated patients more likely to benefit from polymyxin B hemoperfusion by enrolling only patients with high endotoxin activity. No prior trial used a strategy to enrich the design by measuring endotoxin activity and selecting those most likely to derive benefit.
There are multiple possible reasons polymyxin B hemoperfusion failed to improve survival. First, there may be no beneficial effect of polymyxin B hemoperfusion if therapy is initiated after the onset of septic shock and multiorgan dysfunction. In a post hoc analysis from the ABDOMIX study, polymyxin B hemoperfusion treatments failed to reduce several proinflammatory and anti-inflammatory mediators when compared with standard care.17 Second, although prior data support both the ability of the polymyxin B hemoperfusion cartridge to remove circulating endotoxin and the ability of the endotoxin activity assay to measure endotoxin activity,12,18 the relative timing, dose, and duration of polymyxin B hemoperfusion, as applied in this trial, may have been insufficient to significantly reduce the endotoxin burden, modify the clinical course, or both. This concept is supported by failure to observe significant reductions in endotoxin activity assay levels following polymyxin B hemoperfusion treatments. This may be particularly evident for patients with high endotoxin activity assay levels at baseline (ie, greater endotoxin burden). In a series of in vivo experiments, Romaschin et al19 found that by converting endotoxin activity assay units into a concentration of endotoxin, patients with endotoxin activity assay levels exceeding 0.90 might have an endotoxin burden of more than 50 μg/mL. This would far exceed the adsorptive capacity of the polymyxin B hemoperfusion cartridge dose used in this trial. Third, the whole blood assay may insufficiently reflect the endotoxin burden, when considering the compartmentalization of endotoxin and the complexity of endotoxin protein binding.20
Limitations
This study has several limitations. First, blinding can be challenging in device trials with inadvertent introduction of treatment bias if blinding is not maintained. However, this trial attempted to conceal treatment allocation from the clinical care team and blinding appeared well preserved. Second, this study may have lacked statistical power to detect small differences in the primary end point between treatment groups. Third, the SAP did not allow assigning importance to secondary or exploratory analyses because the primary end point analysis was negative.
Conclusions
Among patients with septic shock and high endotoxin activity, polymyxin B hemoperfusion treatment plus conventional medical therapy compared with sham treatment plus conventional medical therapy did not reduce mortality at 28 days.
Study Protocol
Statistical Analysis Plan
eFigure 1. Probability of Death
eTable 1. Time from randomization to receipt of intervention
eTable 2. Secondary outcomes for the All participants and MODS >9 populations
eTable 3. EAA values for all participants, per-protocol (two PMX-HP or two Sham treatments)
eTable 4. EAA values for participants with MODS >9, per-protocol (two PMX-HP or two Sham treatments)
eTable 5. Serious adverse events among patients randomized and had treatment initiated. Reported for an occurrence of 5 events or more
eTable 6. Adverse events associated with the device (PMX-HP cartridge), its components, heparin and central venous catheter among patients randomized, and had treatment initiated
Data sharing
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Opal SM, Scannon PJ, Vincent JL, et al. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999;180(5):1584-1589. doi: 10.1086/315093 [DOI] [PubMed] [Google Scholar]
- 2.Romaschin AD, Harris DM, Ribeiro MB, et al. A rapid assay of endotoxin in whole blood using autologous neutrophil dependent chemiluminescence. J Immunol Methods. 1998;212(2):169-185. doi: 10.1016/S0022-1759(98)00003-9 [DOI] [PubMed] [Google Scholar]
- 3.Klein DJ, Derzko A, Foster D, et al. Daily variation in endotoxin levels is associated with increased organ failure in critically ill patients. Shock. 2007;28(5):524-529. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Birmingham MC, Balk RA, et al. ; E5 Study Investigators . E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. JAMA. 2000;283(13):1723-1730. doi: 10.1001/jama.283.13.1723 [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Tomayko JF, Angus DC, et al. ; Lipid Infusion and Patient Outcomes in Sepsis (LIPOS) Investigators . Efficacy and safety of a phospholipid emulsion (GR270773) in gram-negative severe sepsis: results of a phase II multicenter, randomized, placebo-controlled, dose-finding clinical trial. Crit Care Med. 2009;37(11):2929-2938. doi: 10.1097/CCM.0b013e3181b0266c [DOI] [PubMed] [Google Scholar]
- 6.Levin M, Quint PA, Goldstein B, et al. ; rBPI21 Meningococcal Sepsis Study Group . Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. Lancet. 2000;356(9234):961-967. doi: 10.1016/S0140-6736(00)02712-4 [DOI] [PubMed] [Google Scholar]
- 7.McCloskey RV, Straube RC, Sanders C, Smith SM, Smith CR; CHESS Trial Study Group . Treatment of septic shock with human monoclonal antibody HA-1A: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1994;121(1):1-5. doi: 10.7326/0003-4819-121-1-199407010-00001 [DOI] [PubMed] [Google Scholar]
- 8.Opal SM, Laterre PF, Francois B, et al. ; ACCESS Study Group . Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309(11):1154-1162. doi: 10.1001/jama.2013.2194 [DOI] [PubMed] [Google Scholar]
- 9.Shoji H. Extracorporeal endotoxin removal for the treatment of sepsis: endotoxin adsorption cartridge (Toraymyxin). Ther Apher Dial. 2003;7(1):108-114. doi: 10.1046/j.1526-0968.2003.00005.x [DOI] [PubMed] [Google Scholar]
- 10.Klein DJ, Foster D, Schorr CA, Kazempour K, Walker PM, Dellinger RP. The EUPHRATES trial (Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized controlled trial of Adults Treated for Endotoxemia and Septic shock): study protocol for a randomized controlled trial. Trials. 2014;15:218. doi: 10.1186/1745-6215-15-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall JC, Foster D, Vincent JL, et al. ; MEDIC study . Diagnostic and prognostic implications of endotoxemia in critical illness: results of the MEDIC study. J Infect Dis. 2004;190(3):527-534. doi: 10.1086/422254 [DOI] [PubMed] [Google Scholar]
- 12.Romaschin AD, Klein DJ, Marshall JC. Bench-to-bedside review: clinical experience with the endotoxin activity assay. Crit Care. 2012;16(6):248. doi: 10.1186/cc11495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Rhodes A, et al. ; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup . Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580-637. doi: 10.1097/CCM.0b013e31827e83af [DOI] [PubMed] [Google Scholar]
- 14.Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301(23):2445-2452. doi: 10.1001/jama.2009.856 [DOI] [PubMed] [Google Scholar]
- 15.Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014;40(11):1623-1633. doi: 10.1007/s00134-014-3496-0 [DOI] [PubMed] [Google Scholar]
- 16.Opal SM, Dellinger RP, Vincent JL, Masur H, Angus DC. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C?*. Crit Care Med. 2014;42(7):1714-1721. doi: 10.1097/CCM.0000000000000325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Payen DM, Guilhot J, Launey Y, et al. ; ABDOMIX Group . Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 2015;41(6):975-984. doi: 10.1007/s00134-015-3751-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novelli G, Feretti G, Ruberto F, Morabito V, Pugliese F. Early management of endotoxemia using the endotoxin activity assay and polymyxin-B based hemoperfusion: endotoxin removal in septic shock in clinical settings In: Ronco C, Piccinni P, Rosner MH, eds. Endotoxin and Endotoxin Shock: Disease Diagnosis and Therapy. Vol 167 Basel, Switzerland: Karger; 2010:91-101. [DOI] [PubMed] [Google Scholar]
- 19.Romaschin AD, Obiezu-Forster CV, Shoji H, Klein DJ. Novel Insights into the direct removal of endotoxin by polymyxin B hemoperfusion. Blood Purif. 2017;44(3):193-197. doi: 10.1159/000475982 [DOI] [PubMed] [Google Scholar]
- 20.Pais de Barros JP, Gautier T, Sali W, et al. Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the limulus amebocyte lysate assay. J Lipid Res. 2015;56(7):1363-1369. doi: 10.1194/jlr.D059725 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
Statistical Analysis Plan
eFigure 1. Probability of Death
eTable 1. Time from randomization to receipt of intervention
eTable 2. Secondary outcomes for the All participants and MODS >9 populations
eTable 3. EAA values for all participants, per-protocol (two PMX-HP or two Sham treatments)
eTable 4. EAA values for participants with MODS >9, per-protocol (two PMX-HP or two Sham treatments)
eTable 5. Serious adverse events among patients randomized and had treatment initiated. Reported for an occurrence of 5 events or more
eTable 6. Adverse events associated with the device (PMX-HP cartridge), its components, heparin and central venous catheter among patients randomized, and had treatment initiated
Data sharing