Key Points
Questions
What is the association between apathy in older people without dementia and incident dementia?
Findings
In this systematic review and meta-analysis of 16 studies including 7365 patients, memory clinic patients with apathy had an approximately doubled risk of incident dementia, depending on age and cognitive function. Adjustment for apathy definition and duration of follow-up explained 95% of heterogeneity in patients with mild cognitive impairment; results seem generalizable to memory clinic populations.
Meaning
Apathy is a relevant, noninvasive, cheap, and easily implementable prognostic factor prodromal to dementia. It has important clinical significance because patients are vulnerable and tend to withdraw from care, requiring an active caregiving approach from clinicians.
Abstract
Importance
Fear of dementia is pervasive in older people with cognitive concerns. Much research is devoted to finding prognostic markers for dementia risk. Studies suggest apathy in older people may be prodromal to dementia and could be a relevant, easily measurable predictor of increased dementia risk. However, evidence is fragmented and methods vary greatly between studies.
Objective
To systematically review and quantitatively synthesize the evidence for an association between apathy in dementia-free older individuals and incident dementia.
Data Sources
Two reviewers conducted a systematic search of Medline, Embase, and PsychINFO databases.
Study Selection
Inclusion criteria were (1) prospective cohort studies, (2) in general populations or memory clinic patients without dementia, (3) with clear definitions of apathy and dementia, and (4) reporting on the association between apathy and incident dementia.
Data Extraction and Synthesis
PRISMA and MOOSE guidelines were followed. Data were extracted by 1 reviewer and checked by a second.
Main Outcomes and Measures
Main outcomes were pooled crude risk ratios, maximally adjusted reported hazard ratios (HR), and odds ratios (OR) using DerSimonian-Laird random effects models.
Results
The mean age of the study populations ranged from 69.2 to 81.9 years (median, 71.6 years) and the percentage of women ranged from 35% to 70% (median, 53%). After screening 2031 titles and abstracts, 16 studies comprising 7365 participants were included. Apathy status was available for 7299 participants. Studies included populations with subjective cognitive concerns (n = 2), mild cognitive impairment (n = 11), cognitive impairment no dementia (n = 1), or mixed cognitive and no cognitive impairment (n = 2). Apathy was present in 1470 of 7299 participants (20.1%). Follow-up ranged from 1.2 to 5.4 years. In studies using validated apathy definitions (n = 12), the combined risk ratio of dementia for patients with apathy was 1.81 (95% CI, 1.32-2.50; I2 = 76%; n = 12), the hazard ratio was 2.39 (95% CI, 1.27-4.51; I2 = 90%; n = 7), and the odds ratio was 17.14 (95% CI, 1.91-154.0; I2 = 60%; n = 2). Subgroup analyses, meta-regression, and individual study results suggested the association between apathy and dementia weakened with increasing follow-up time, age, and cognitive impairment. Meta-regression adjusting for apathy definition and follow-up time explained 95% of heterogeneity in mild cognitive impairment.
Conclusions and Relevance
Apathy was associated with an approximately 2-fold increased risk of dementia in memory clinic patients. Moderate publication bias may have inflated some of these estimates. Apathy deserves more attention as a relevant, cheap, noninvasive, and easily measureable marker of increased risk of incident dementia with high clinical relevance, particularly because these vulnerable patients may forgo health care.
This systematic review and meta-analysis investigates the association of apathy with incident dementia in older individuals enrolled in memory clinics.
Introduction
Fear of dementia is common in patients presenting to memory clinics with cognitive concerns.1 Although clinical evaluation can lead to a dementia diagnosis, patients often have milder conditions, including mild cognitive impairment (MCI) and isolated subjective cognitive concerns (SCC).2,3,4 The annual progression from MCI to dementia in clinical settings is about 5% to 15%, while 20% to 25% of patients revert to normal cognition and functioning.5 Patients with SCC have an increased risk (1.5- to 3-fold) of developing dementia compared with individuals without cognitive concerns, but most do not develop dementia in the near future.3 However, fear of dementia is pervasive in patients with SCC or MCI,1 and identifying those at increased risk is an important clinical concern.
Apart from memory loss and other cognitive disturbances, behavioral symptoms are common in most occurring forms of late-life dementia including Alzheimer disease (AD) and vascular dementia.6 One of the most prevalent behavioral symptoms is apathy, estimated to affect almost half of patients.7 Apathy is a disorder of motivation, manifesting itself as reduced interest, goal-directed cognition, and emotional expression.8 Apart from dementia, apathy also occurs in MCI9 and community-dwelling older people.10 It has high clinical relevance because patients with apathy tend to withdraw from care and may escape clinicians’ attention.11,12,13,14 Apathy has been associated with incident dementia and could be useful as an easily assessable, low-cost, noninvasive marker of increased risk, which is relatively common and specific for future cognitive decline compared with other neuropsychiatric symptoms.10,15,16,17 However, evidence is fragmented and apathy definitions vary greatly between studies.10 We aimed to systematically review and meta-analyze the evidence from longitudinal cohorts for the association between apathy in older people and the risk of incident dementia.
Methods
In this systematic review and meta-analysis following PRISMA and MOOSE guidelines,18 we collated longitudinal cohort studies assessing apathy and subsequent incident dementia. Study populations could involve the general community-dwelling population or memory clinic patients. Studies concerning participants selected for specific medical conditions (eg, Down syndrome or frailty) or patients from care settings were excluded because such conditions may modify the association between apathy and incident dementia. Authors could use any diagnostic criteria to define apathy and dementia, provided definitions were clearly specified. Given the difficulties of retrospectively assessing whether apathy symptoms preceded dementia, only prospective cohort studies that diagnosed apathy in individuals without dementia were included. Randomized clinical trials were excluded because interventions provided may influence the association between apathy and incident dementia. There were no restrictions on publication year, language, or length of follow-up. There was no registered predefined review protocol.
Medline, Embase, and PsychINFO databases were searched from inception to October 2, 2017, and deduplicated using the OVID platform.19 The full search is listed in eTable 1 in the Supplement. Search terms included apathy and commonly used apathy assessment instruments,20 cross referenced with dementia or AD and terms referring to risk, incident, or prediction. Two investigators (L.vW. and J.W.vD.) independently screened titles and abstracts for (1) prospective longitudinal studies published in peer-reviewed journals; (2) in unselected community-dwelling populations or nondemented populations with cognitive concerns with or without cognitive impairment; (3) that clearly defined apathy and dementia diagnoses; and (4) that reported data regarding the association between apathy and incident dementia. Conflicts regarding inclusion were resolved by consensus. Full texts and bibliographies of included studies and relevant reviews were hand searched for additional studies. Data were extracted by 1 reviewer (J.W.vD.) and checked by a second (L.vW.) using a piloted standardized extraction form (eTable 2 in the Supplement) and assessed for risk of bias using an adapted version of the Newcastle-Ottawa quality assessment scale for cohort studies.21
Risk ratios (RR) and 95% confidence intervals for incident dementia were calculated per study using the number of dementia cases in the apathy and nonapathy groups. If unavailable, authors were approached to supplement these data.22 If both AD and all-cause dementia were available,23,24 all-cause dementia was used. A sensitivity analysis used AD as preferred outcome. Risk ratios, reported odds ratios (OR), and reported hazard ratios (HR) were pooled separately across studies. Random-effects Der Simonian-Laird models were used because of the heterogeneous study characteristics.25 P values were 2-sided. For pooling reported effect sizes, maximally adjusted estimates were used. If these were overadjusted (<10 events per covariate), the most adjusted estimate without overadjustment was used. Studies using a validated recommended method to define apathy20 and those using custom measurements were analyzed separately because the apathy construct may differ greatly between these categories. Heterogeneity was assessed using I2 statistics.25 Leave-one-out analyses were performed, in which every study was consecutively excluded once to assess its influence on the overall estimate. Subgroup and sensitivity analyses, including their rationale and whether they were predefined, are listed in eTable 3 in the Supplement. Meta-analyses were conducted in R (R Programming), using the meta and metafor packages.26,27
Results
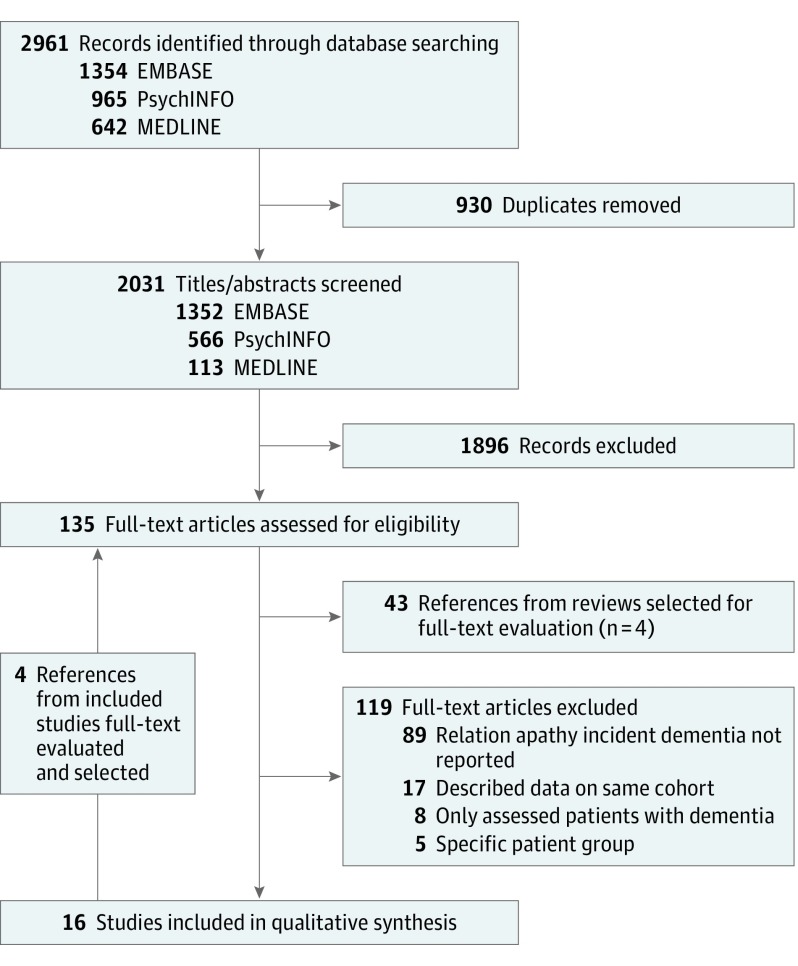
From 2031 titles and abstracts, 15 studies were selected (Figure 1). Hand searching selected study bibliographies yielded 1 additional study.28 Thus, 16 studies were included in the final synthesis.15,22,23,24,28,29,30,31,32,33,34,35,36,37,38,39
Figure 1. Flowchart of Search and Study Selection.
Table 1 provides an overview of the included studies. Fourteen concerned Western populations, one was a combined international population database,15 and one was from China.39 Four populations were derived from screened population-based cohorts23,35,38,39 and 12 were from memory clinics. Study populations included SCC (n = 2),29,30 MCI (n = 9),15,22,24,31,32,33,34,35,38 amnestic MCI (n = 2),28,36 cognitive impairment no dementia (n = 1),23 and mixed patients with no cognitive impairment (NCI) and patients with MCI (n = 2).37,39 The SCC studies excluded patients with abnormal neuropsychological test scores. Common exclusion criteria in MCI were psychiatric/somatic disorders possibly impairing cognition,15,24,31,33,36,38 cerebrovascular disease or magnetic resonance imaging/computed tomography lesions,24,28,31,33,36 and younger-onset MCI.24,28,31 The median population sample size was 245.5 (range, 51-1821),22,29,31 the median mean age was 71.6 years (range, 69.2-81.9 years),23,28,29 and the median percentage of women was 53% (range; 35%-70.0%).23,28,34
Table 1. Study Overviewa.
| Source | Cohort Nationality | Setting | Population | Exclusion Criteria | No. | Age, Mean (SD), y | Female, No. (%) | Apathy Measure | Apathy Cases, No. (%) |
|---|---|---|---|---|---|---|---|---|---|
| Bartolini et al,29 2005 | Italy | Memory clinic | SCC | Dementia, MCI, abnormal NP test scores | 222 | 69.2 (4.8) | 141 (64) | BDIb | 35 (15.8) |
| Burke et al,30 2016 | United States | Database of memory clinics | SCC | CDR >0, abnormal NP test scores | 1567 | 71.2 (10.9) | 988 (63) | NPI-Q | 297 (18.9) |
| Chan et al,39 2011 | China | CD sample and AR volunteers | NCI (35%); MCI (65%) | Age <60 y | 321 | 77.3 (8.3) | 225 (70) | NPI-i | 45 (14.0) |
| Brodaty et al,37 2012 | Australia | CD sample | NCI (63%); MCI (37%) | Inclusion, age 70-90 y; exclusion, MMSE<24, dementia, neurological disease, psychiatric/somatic disorders impairing cognition, developmental disability, malignancy, non-English, CI without SCC | 630 | 78.2 (4.6) | 346 (55) | NPI-i | 14 (2.2) |
| Van der Linde et al,38 2013 | United Kingdom | Population based | MCI (S) | Dementia, Parkinson disease, MMSE>26 | 879 | 56% ≥ 75c | 927/1416 (65) | GMS-AGECAT | 159 (18.1) |
| Pink et al,35 2015 | United States | Population based | MCI | None reported | 332 | Median (IQR): 82.1 (77.7-85.0) | 151 (45) | NPI-Q | 55 (16.6) |
| Teng et al,31 2007 | United States | Memory clinic | MCI | Age <50 y, neurological disease, CT/MRI lesions, psychiatric/somatic disorders impairing cognition | 51 | 72.8 (7.2) | 18 (35) | NPI-i | 13 (25.5) |
| Vicini Chilovi et al,32 2009 | Italy | Memory clinic | MCI | None reported | 124 | 71.3 (7.7) | 84 (68) | Clinical | 36 (29.0) |
| Ramakers et al,24 2010 | Netherlands | Memory clinic | MCI (GDS) | Age <56 y, cerebrovascular disease, brain trauma, psychiatric/somatic disorders impairing cognition | 263 | 66.9 (7.7) | 116 (44) | HAMDb | 171 (75.0) |
| Richard et al,15 2012 | International | Database of memory clinics | MCI (C) | Depression, MMSE<24, CDR not 0.5, neurological disease, psychiatric/somatic disorders impairing cognition, unstable medical condition | 397 | 74.8 (7.5) | 141 (36) | GDS-3Ab | 178 (44.8) |
| Somme et al,33 2013 | Spain | Memory clinic | MCI | Neurodegenerative/cerebrovascular disease, acute psychiatric illness, severe comorbidities | 132 | 69.8 (8.7) | 62 (47) | NPI-i >2 | 69 (52.3) |
| Rosenberg et al,23 2013 | United States | Database of memory clinics | MCI (C) | None reported | 1821 | 75.3 (9.3) | 920 (51) | NPI-Q | 298 (16.7) |
| Sobów et al,34 2014 | Poland | Memory clinic | MCI (NAW) | CDR not equal to 0.5 | 83 | 75.0 (1.9) | 58 (70) | NPI-i | 20 (24.1) |
| Robert et al,28 2008 | France | Health clinic | aMCI (C) | Age <58 y, <4 y of education, MMSE <25, depression, MRI brain lesions | 214 | 71.8 (5.4) | 90 (42) | AI | 47 (22.0) |
| Palmer et al,36 2010 | Italy | Memory clinic | aMCI | Cerebrovascular disease, depression/somatic disorders possibly impairing cognition, MRI brain lesions |
99 | 70.5 (6.6) | 37 (37) | Clinical | 12 (12.1) |
| Peters et al,23 2013 | United States | Population based | CIND | CDR >0.5 | 230 | 81.9 (5.1) | 115 (50) | NPI-i | 21 (9.2) |
Abbreviations: AI, Apathy Inventory; aMCI, amnestic mild cognitive impairment based on revised Peterson (Mayo Clinic) criteria; AR, actively responding; BDI, Beck Depression Inventory; CD, community dwelling; CDR, Clinical Dementia Rating; CIND, cognitive impairment no dementia; CT, computed tomography; GDS-3A, Geriatric Depression Scale 3 Apathy-related subitems; GMS-AGECAT, Geriatric Mental State Automated Geriatric Examination for Computer Assisted Taxonomy; HAMD, Hamilton rating scale for depression; MCI, mild cognitive impairment based on revised Peterson (Mayo Clinic) criteria; MCI (S), based on Stephan criteria; MCI (NAW), based on National Institute on Aging-Alzheimer Association workgroup; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; NCI, no coginitive impairment; NP, neuropsychological; NPI-i, Neuropsychiatric Inventory for informants; NPI-Q, Neuropsychiatric Inventory Questionnaire for clinical informants; SCC, subjective cognitive concerns.
Studies ordered by publication year within diagnostic group.
Apathy measure based on subitems on a depression scale.
Only a proportion of participants 75 years and older was available.
Twelve studies defined apathy using a validated rating scale recommended to measure apathy20 or a clinical diagnosis: 8 used any positive apathy score on the Neuropsychiatric Inventory informant (>0 of 12)23,31,34,37,39 or questionnaire version (>0 of 3),22,30,35 1 used Neuropsychiatric Inventory informant score of greater than 2 of 12,33 1 used the standard Apathy Inventory cutoff (>2 in any dimension),28 and 2 used standard clinical criteria.32,36 Four studies used a custom apathy definition based on a general neuropsychiatric assessment tool or a minimum number of motivational subitems on a depression scale.15,24,29,38 Overall, apathy at baseline was diagnosed in 1470 of 7299 participants (20.1%), prevalence ranging from 2.2% to 75% (median, 17.4%).15,28,37
Nine studies assessed AD as outcome (Table 2) using National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association40 criteria (n = 7)23,24,28,29,30,31,36 or a clinical diagnosis without further specification (n = 2).15,22 Three studies also reported all-cause dementia22,23,24: 1 using a clinical diagnosis without specification,22 1 using DSM-IV criteria,24,41 and 1 using DSM-III-revised criteria.23,42 Seven studies assessed all-cause dementia only: 4 using DSM-IV criteria,33,35,37,39 1 using standard criteria per subtype,32 1 using a clinical dementia rating of at least 1,34,43 and 1 using a comprehensive assessment score similar to a DSM-III diagnosis.38
Table 2. Dementia Incidence and Association With Apathy.
| Source | Dementia | Criteria | Follow-up, Mean (SD), y | Dementia Incidence | Reported Association Apathy-Dementia | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall, No. (%) | Apathy Group, No. (%) | No Apathy Group, No. (%) | Calculated RR (95% CI) | Crude (95% CI) | Adjusted (95% CI) | Adjustment | ||||
| Bartolini et al,29 2005 | AD | NINCDS | 1a | 33/222 (14.9) | 31/35 (88.6) | 2/187 (1.1) | 82.81 (20.76-330.37) | NA | OR: 3885 (154-97 902)b | Stepwise, depression (BDI), cognitive (TMT) |
| Burke et al,30 2016 | AD | NINCDS | 4.0 (0.6-8.8)c | 565/1441 (39.2) | 193/297 (65.0) | 372/1144 (32.5) | 2.00 (1.78-2.45) | HR: 6.99 (5.51-8.88) | HR: 9.51 (5.23-17.31) | Age, sex, race, Hispanic, ApE4, family history |
| Chan et al,39 2011 | Dementia | DSM-IV, TR | 2 | 51/321 (15.9) | 3/45 (6.7) | 48/276 (17.3) | 0.38 (0.13-1.18) | NA | OR: 0.31 (0.09-1.13) | Age, sex, education, baseline MMSE, depression, AbMB |
| Brodaty et al,37 2012 | Dementia | DSM-IV | 2 | 14/630 (2.2) | 0/14 (0.0) | 14/616 (2.2) | 1.42 (0.09-22.69) | NA | NA | NA |
| Van der Linde et al,38 2013 | Dementia | DSM-III | 2 | 128/879 (14.6) | 33/159 (20.8) | 95/720 (13.2) | 1.57 (1.10-2.49) | OR: 1.7 (1.1-2.7) | OR: 1.2 (0.7-1.9)d; OR: 1.0 (0.6-1.8)e | Age, sex, education, SOC, MMSE, SMC, OMC, ADL (BPS inst, emotional, stroke, MI, DM, SRH, smoking)e |
| Pink et al,35 2015 | Dementia | DSM-IV | 3.0 (2.5-5.3)f | 117/332 (35.2) | 25/55 (44.5) | 92/277 (33.2) | 1.34 (0.98-1.91) | NA | HR: 1.62 (1.03-2.54) | Age, sex, education, comorbidity |
| Teng et al,31 2007 | AD | NINCDS | 2.0 (1.2) | 12/51 (23.5) | 6/13 (46.2) | 6/38 (15.8) | 2.92 (1.14-7.48) | NA | NA | NA |
| Vicini Chilovi et al,32 2009 | Dementia | NINCDS,VaD, LBD | 2.0 (0.2) | 28/124 (22.6) | 13/36 (36.1) | 15/88 (17.0) | 2.12 (1.12-3.99) | NA | OR: 7.07 (1.9-25) | Age, depression (clinical diagnosis), ADL BI, Cognitive (ADAS) |
| Ramakers et al,24 2010 | Dementiab | NINCDS and DSM-IV | 5.4 | 90/225 (34.2) | 50/135 (37.0) | 40/90 (44.4) | 0.83 (0.61-1.15) | OR: 0.58 (0.35-0.96)b | OR: 0.67 (0.40-1.13)b | Age, sex, education |
| Richard et al,15 2012 | AD | Clinical | 2.7 (1.0) | 166/397 (41.8) | 82/178 (46.1) | 84/219 (38.4) | 1.20 (0.95-1.51) | HR: 1.43 (0.86-2.38)b | HR: 1.85 (1.09-3.15)b | Age, sex, education; cognitive (MMSE) |
| Somme et al,33 2013 | Dementia | DSM-IV | 3.5 (2.9) | 38/132 (28.0) | 21/69 (30.4) | 17/63 (27.0) | 1.13 (0.66-1.94) | NA | HR: 2.20 (1.003-4.82) | cognitive (MMSE) |
| Rosenberg et al,23 2013 | Dementiab | Clinical | 1.2 (0.3) | 527/1787 (29.5) | NA | NA | NA | NA | HR: 1.13 (1.00-1.28) | Age, sex, Hispanic race/ethnicity, cognitive (MMSE and CDR) |
| Sobów et al,34 2014 | Dementia | CDR = 1 | 2a | 27/83 (32.5) | 18/20 (90) | 9/63 (14.3) | 6.30 (3.38-11.74) | NA | OR: 70.7 (5.6-699) | Backward, sex, BMI, dBMI |
| Robert et al,28 2008 | AD | NINCDS | 3a | 59/215 (27.4) | 18/47 (38.3) | 41/168 (24.4) | 1.57 (1.00-2.46) | NA | HR: 2.48 (1.14-5.37) | Age, sex, education, cognitive (FCSRT) |
| Palmer et al,36 2010 | AD | NINCDS | 1.4 (8.4) | 15/99 (15.2) | 6/12 (50) | 9/87 (10.3) | 4.83 (2.09-11.18) | HR: 4.6 (1.3-16.2)d | HR: 6.9 (2.3-20.6)e | Age, sex, education, depression (clinical diagnosis), cognitive (MMSE)e |
| Peters et al,23 2013 | Dementiab | NINCDS and DSM-IIIR | 3.3 | 83/228 (36.4) | 7/21 (33.3) | 76/207 (36.7) | 0.91 (0.48-1.71) | NA | HR: 0.93 (0.43-2.02) | Age, education, ApE4, cognitive (3MS) |
Abbreviations: 3MS, Modified Mini-Mental State Examination; AbMB, aberrant motor behavior; AD, Alzheimer Disease; ADAS, Alzheimer Disease Assessment Scale; ADL BI, Activities of Daily Living, Barthel Index; ApE4, Apolipoprotein allele ε4; BDI, Beck Depression Inventory score; BL, baseline; BMI, body mass index; BPS, other behavioral and psychological symptoms; CDR, Clinical Dementia Rating; dBMI, change in BMI; DM, diabetes mellitus; FCSRT, Free and Cued Selective Reminding Test; HR, hazard ratio; LBD, Lewy Body Dementia Standard Criteria; MI, myocardial infarction; MMSE, Mini-Mental State Examination; NINCDS, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association; OMC, objective memory impairment; OR, odds ratio; RR, risk ratio; SMC, subjective memory concerns; SOC, social class; SRH, self rated health; TMT, Trail Making Test; VaD, Vascular Dementia Standard Criteria (NINDS-AIREN).
No mean follow-up but assessment for whole cohort at stated time.
Study assessed both AD and all-cause dementia.
Mean (minimum-maximum).
Most adjusted estimate not overadjusted.
Estimate considered overadjusted (<10 events per predictor).
Median (interquartile range).
Mean follow-up ranged from 1.2 to 5.4 years (median, 2.35).15,23,24,31,32 Instead of a mean follow-up time, 6 studies only reported a period until reassessment, ranging from 1 to 3 years.28,29,34 Overall, dementia occurred in 1953 of 7166 participants (27.3%) during follow-up, reported incidence ranging from 2.2% over 2 years to 41.8% over 1 year.15,37 All studies except 122 reported the number of dementia cases for apathy and nonapathy groups separately. Calculated RRs ranged from 0.38 to 82.81.24,29 Eight studies reported HRs,15,22,23,28,30,33,35,44 6 reported ORs,24,29,32,34,38,39 and 2 did not report any measure of association for apathy and dementia.31,37 Adjusted estimates ranged between 0.31 and 3885 for ORs24,29 and between 0.93 and 9.51 for HRs.23,30 Most studies adjusted for the main confounders10 of age (n = 11)15,22,23,24,28,30,32,35,36,38,39 and baseline cognition (n = 10).15,22,23,28,29,32,33,36 Four studies also reported unadjusted estimates,15,24,30,38 ranging from an OR of 0.58 to an HR of 6.99.24,30 Studies assessing both AD and all-cause dementia reported similar results for both outcomes (eTable 4 in the Supplement).22,23,24
eTable 5 in the Supplement lists study bias assessment scores and eTable 6 in the Supplement provides score motivations. The worst scoring categories were population representativeness, exposure assessment, and follow-up availability. Based on total Newcastle-Ottawa quality assessment scale score, 1 study had a relatively high bias risk31 and 6 scored worse than average (<7 of 9 points).29,31,33,34,37,38
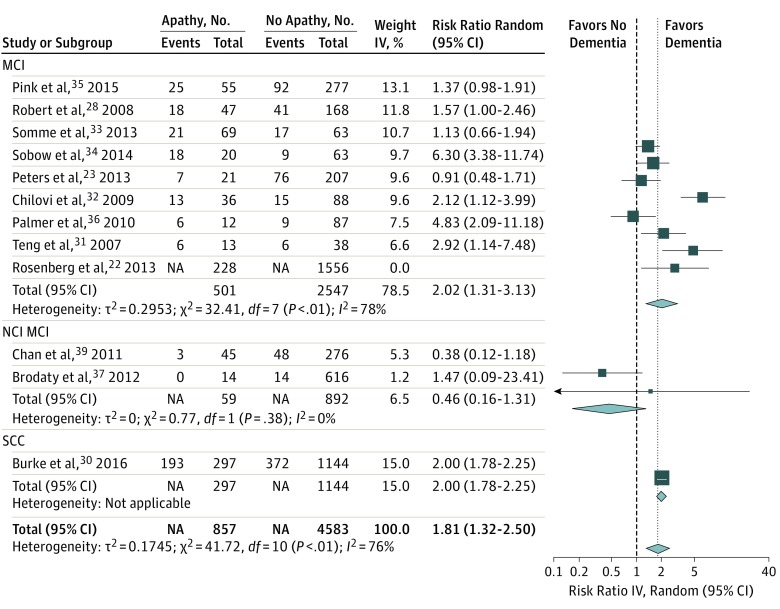
In studies using a recommended validated definition of apathy, the overall RR for developing dementia for patients with apathy (Figure 2) was 1.81 (95% CI, 1.32-2.50). Heterogeneity was high (I2 = 76%). The funnel plot suggested low risk of publication bias (eFigure 1 in the Supplement). Leave-one-out analyses results (eFigure 2 in the Supplement) ranged from 1.59 (95% CI, 1.19-2.12; I2 = 67%)34 to 1.96 (95% CI, 1.45-2.66; I2 = 73%).23 Analyzing AD as preferential outcome gave similar results (eFigure 3 in the Supplement). Figure 2 lists results within diagnostic subgroups (SCC vs MCI and NCI and MCI). Owing to the small number of studies in other diagnostic groups, subanalyses were restricted to patients with MCI. The overall RR in patients with MCI was 2.02 (95% CI, 1.31-3.13; I2 = 78%). Sensitivity analysis separating MCI, cognitive impairment no dementia, and anamnestic MCI subgroups gave similar results; therefore, they were analyzed as 1 diagnostic category. The funnel plot for RRs in patients with MCI suggests some publication bias (eFigure 4 in the Supplement). Leave-one-out analyses results ranged from 1.65 (95% CI, 1.18-2.31; I2 = 58%)34 to 2.22 (95% CI, 1.47-4.42; I2 = 79%).33
Figure 2. Forest Plot for Risk Ratio of Developing Dementia in Studies Using Recommended Validated Apathy Scales According to Subgroups Based on Diagnosis.
IV indicates inverse variance; MCI, mild cognitive impairment; NCI MCI, mixed normal cognition and MCI; SCC, subjective cognitive impairment.
Pooling maximally adjusted HRs over all studies (eFigure 5 in the Supplement) gave a combined HR of 2.39 (95% CI, 1.27-4.51), with considerable heterogeneity (I2 = 90%). Leave-one-out analyses results ranged from 1.74 (95% CI, 1.13-2.68),30 to 2.80 (95% CI, 1.35-5.79).23 In the MCI subgroup, the combined HR was 1.74 (95% CI, 1.13-2.68, I2 = 73%) (eFigure 5 in the Supplement). Leave-one-out analysis results ranged from 1.44 (95% CI, 1.03-1.99; I2 = 54%)36 to 2.02 (95% CI, 1.13-2.68; I2 = 59%).23 The funnel plot suggested some publication bias (eFigure 6 in the Supplement). Pooling maximally adjusted ORs (n = 3) gave an overall estimate of 4.60 (95% CI, 0.26-80.20; I2 = 89%). Estimates ranged from 1.48 (95% CI, 0.07-31.63; I2 = 91%) to 17.14 (95% CI, 1.91-153.98; I2 = 60%). The analyses pooling the ORs in MCI gave a combined OR of 17.14 (95% CI, 1.91-154.0; I2 = 60%). Because ORs in MCI were only available for 2 studies,32,34 no additional analyses were performed.
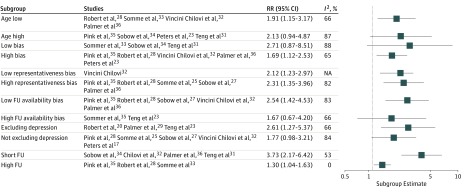
None of the subgroup pairs (Figure 3) were markedly different except for long follow-up (RR, 1.30; 95% CI, 1.04-1.63; I2 = 0%) vs short follow-up (RR, 3.73; 95% CI, 2.17-6.42; I2 = 53%). Meta-regression within MCI (eFigure 7 in the Supplement) showed a significant association between follow-up time and RR within studies (0.44 per year; 95% CI, 0.31-0.62; P < .001), accounting for an R2 of 95% of heterogeneity. There was no association between age and RR (0.94 per year; 95% CI, 0.31-0.62; R2 = 0%). Results of subgroup analyses for HRs in MCI (eFigure 8 in the Supplement) were comparable except for long vs short follow-up subgroup estimates being similar. Meta-regression showed no association between HRs and follow-up time (0.92 per year; 95% CI, 0.88-1.02; I2 = 70%; R2 = 0%) nor age (1.07 per year; 95% CI, 0.84-1.02; I2 = 76%; R2 = 0%). The meta-regression plot (eFigure 9 in the Supplement) suggested this disparity between RRs and HRs regarding follow-up time was largely owing to 1 study, unavailable for the RR analyses.22 Omitting this study, there was a significant association between follow-up time and HR (0.48 per year; 95% CI, 0.25-0.93), explaining R2 = 76% of heterogeneity (eFigure 10 in the Supplement).
Figure 3. Subgroup Analyses Within Mild Cognitive Impairment Patients Based on Risk Ratio .
Only studies using validated apathy scales are included. FU indicates follow-up; RR, risk ratio.
Including studies using custom apathy definitions (eFigure 11 in the Supplement) gave an RR of 1.82 overall (95% CI, 1.34-2.47; I2 = 87%) and of 1.66 within MCI (95% CI, 1.22-2.25; I2 = 80%) (eFigure 12 in the Supplement). Meta-regression within MCI suggested R2 = 97% of heterogeneity could be explained by follow-up time, definition type (recommended vs custom), and their interaction, with I2 = 10% heterogeneity remaining (eFigure 13 and 14 in the Supplement). The overall RR within studies using custom scales was 2.14 (95% CI, 1.04-4.41; I2 = 93.0%) and within MCI was 1.16 (95% CI, 0.84-1.60; I2 = 71%) (eFigure 15 in the Supplement). The small number of studies precluded subgroup analyses within this group.
Discussion
Apathy was consistently associated with an increased risk of incident dementia in patients with MCI and SCC in different settings and countries. However, heterogeneity was considerable. In MCI, the dementia risk was about double for patients with apathy and seemed higher for the short vs the long term, with meta-regression based on follow-up time explaining most heterogeneity. In SCC, the risk for patients with apathy may be as much as 2-fold to more than 7-fold higher, but data were too sparse for a reliable estimate. Results in mixed NCI-MCI populations are difficult to interpret owing to limited data on dementia development in both groups separately.
Subgroups of studies adjusting and not adjusting for age and cognition showed similar associations. This may be ecological fallacy. Within studies reporting both crude and adjusted estimates, adjustment for age and cognition increased the association of apathy with dementia, suggesting a stronger association in younger patients who are relatively cognitively intact. Concordantly, HRs seemed higher in SCC compared with MCI. Although usually considered an important confounder,10 subgroup analyses showed no marked difference between studies including or excluding patients with depression. Apathy HRs seemed higher in studies controlling for depression, suggesting stronger associations in patients without depression, but group sizes were insufficient to allow firm conclusions. Regression with apathy definition type and follow-up duration together explained more than 95% of heterogeneity. Studies using custom apathy definitions found lower estimates. This may be attributable to measurement error, diluting associations. The diminishing association between apathy and dementia with longer follow-up suggests apathy is predominantly prodromal to dementia rather than a causal risk factor10; the risk regressing to the mean over time. However, selective dropout of patients with apathy may also have weakened long-term associations. There were insufficient data to assess the influence of apathy severity, although some studies using the NPI used low (Neuropsychiatric Inventory informant >0) and higher (Neuropsychiatric Inventory questionnaire >0; Neuropsychiatric Inventory informant >2) apathy severity thresholds.
Results seem generalizable to memory clinic populations. Patient demographics and proportions of patients with MCI developing dementia correspond to the literature.3,5,45,46 The high dementia rate in SCC reported by Burke et al30 may reflect the long follow-up. Poor scores regarding representativeness bias mainly resulted from unclear exclusion percentages or custom MCI definitions. Small groups made representativeness bias subgroup analyses uninformative. Although some studies used many exclusion criteria, exclusion percentages were generally acceptable. Results seem applicable to all-cause and AD dementia: individual study reports and sensitivity analyses showing similar results for both outcomes. This is not surprising given the mixed pathologies underlying clinical AD diagnoses in old age.47 Because studies concerned memory clinic patients and/or individuals with cognitive symptoms, results may not be directly translatable to general community-dwelling older populations nor populations with comorbidity, preventing memory clinic consultation. Overall, validity seems high because most studies were conducted in clinical settings, using readily available and easily measureable apathy criteria and standard dementia definitions. However, because rating scales were often informant-based, patient-informant associations and informants’ expectations of normal behavior may have influenced apathy diagnoses, possibly mitigating the translatability of the results to the individual level.6,48,49,50 Generalizability to non-Western cultures may also be limited: all but 1 study concerned Western populations, and the construct of behaviors considered apathetic may vary between cultures.51
Limitations
Our review has some limitations. First, reviewing the published literature may have introduced publication bias, leading to overestimation of the association between apathy and dementia. Most studies used the NPI, which measures 12 separate neuropsychiatric symptoms. Whether associations between dementia and these individual symptoms are reported and/or published may depend on their effect size. The extreme estimate by Bartolini et al29 may exemplify the “winner’s curse,” ie, a newly discovered association often being inflated.52 The lack of studies in SCC between 2005 and 2016 could indicate absence of replication efforts during this time but also that replication was unsuccessful and not published. However, funnel plots suggested that, overall, publication bias was limited. Second, because apathy and incident dementia are associated with study dropout,13,53 the substantial attrition in some studies could have caused attrition bias, attenuating study estimates. Concordantly, studies with higher attrition bias risk seemed to report lower estimates. Third, ORs and RRs may give distorted results. Participants dropping out during follow-up should preferably be censored at the dropout time, not excluded or left in the denominator. With apathy being associated with dropout and mortality,13,54 noncensoring could introduce bias, attenuating the association between apathy and dementia. Hazard ratios were therefore the most appropriate summary measure. However, these were unavailable for half of the studies. The different effect measures and the considerable heterogeneity preclude exact estimates of the association between apathy and incident dementia. However, the estimates were overall consistent. Fourth, combining population-based and clinical studies requires some consideration because these populations differ in sample representativeness, prevalence rates, and disease context, potentially influencing associations between apathy and dementia and generalizability.55 Apathy prevalence and dementia risk estimates were similar in the 2 population-based MCI cohorts compared with memory clinic cohorts. However, inferences regarding mixed MCI-NCI populations are hampered by unclear generalizability and insufficient differentiated data, which may influence combined results. Finally, the value of the subgroup analyses is limited by the relatively small subgroups being easily dominated by single studies and the risk of type 1 error.
Conclusions
In conclusion, apathy was associated with an approximately 2-fold increased risk of dementia in memory clinic patients. The risk seems independent of concurrent depression, greater in the short term compared with the long term, and less strong with higher age and greater cognitive impairment. Withdrawal from activities and interests in older and/or more cognitively impaired individuals is less specific for underlying neuropathology, perhaps also reflecting changes in lifestyle and physical and mental ability. This suggests apathy is a particularly potent signal in relatively young and otherwise healthy individuals, in whom this behavior change is more easily noticed. Whether apathy, combined with other easily measureable clinical parameters, is a useful predictor on an individual level in clinical practice needs to be investigated in dedicated prognostic studies. The paucity of data on apathy in patients with SCC also warrants more research. Our results concur with findings that symptoms of apathy in community-dwelling older people increase the risk of cognitive decline and incident dementia.16,56 These findings support the concept of mild behavioral impairment as a prodromal syndrome to dementia, with apathy possibly being among its most pervasive manifestations, which is potentially relevant for dementia trials.57,58 However, many patients with apathy may not develop dementia, and the negative consequences of diagnoses without treatment options require consideration.59,60 Results suggest apathy in older people deserves more attention as a prognostic factor. It is clinically relevant because older people with apathy represent a medically highly vulnerable group that tends to withdraw from care and may require active engagement from clinicians.11,12,13,14,61 While much research is aimed at prognostic biomarkers based on advanced magnetic resonance imaging techniques or cerebrospinal fluid analyses, relatively simple measurement of neuropsychiatric symptoms merits consideration because it is less invasive, cheaper, and easier to implement on a broad scale.62,63,64 For population and health care systems under financial constraint, taking apathy as a marker should be explored as a possible alternative to invasive and relatively expensive investigations.
eTable 1. Full Search Strategy
eTable 2. Items on Data Extraction Form
eTable 3. Subanalyses and Rationale
eTable 4. Estimates for AD and Dementia Compared Within Studies
eTable 5. Bias Assessment Score Overview
eTable 6. Detailed Bias Assessment Table With Rationale for Scores
eFigure 1. Funnel Plot for the Overall Analysis
eFigure 2. Forest Plot of Leave-one-out Analysis for Risk Ratios
eFigure 3. Sensitivity Analysis With AD Instead of Dementia as Preferential Outcome
eFigure 4. Funnel Plot for the Risk Ratios Reported in Studies in Mild Cognitive Impairment
eFigure 5. Overall and Subgroup Analyses of HRs in Mild Cognitive Impairment
eFigure 6. Funnel Plot of Hazard Ratios Reported in Studies
eFigure 7. Meta-regression of Risk Ratios for Developing Dementia in Mild Cognitive Impairment
eFigure 8. Subgroup Analyses in MCI Based on Reported Maximally Adjusted Hazard Ratios
eFigure 9. Meta-regression of Reported Hazard Ratios for Mild Cognitive Impairment
eFigure 10. Meta-regression of Reported Hazard Ratios for Mild Cognitive Impairment Excluding 1 Study
eFigure 11. Forest plot of RR in Studies Using Validated and Custom Definitions Of Apathy
eFigure 12. Forest plot for Relative Risk of Developing Dementia Including Studies Using Validated and Custom Apathy According to Subgroups Based on Diagnosis
eFigure 13. Meta-regression of Log Risk Ratio of Dementia for Patients With Apathy in studies Using Recommended (Blue) and Custom (Grey) Definitions Over Follow-up Time
eFigure 14. Meta-regression Results of the Log Risk Ratio for Dementia in Participants With Apathy Predicted by FU-time, Apathy Definition Type and Their Interaction
eFigure 15. Overall and Subgroup Analyses of RR in MCI and SCC Patients for Studies Using Custom Definitions of Apathy
References
- 1.Commissaris CJAM, Verhey FRJ Jr, Ponds RWHM, Jolles J, Kok GJ. Public education about normal forgetfulness and dementia: importance and effects. Patient Educ Couns. 1994;24(2):109-115. doi: 10.1016/0738-3991(94)90004-3 [DOI] [PubMed] [Google Scholar]
- 2.Burmester B, Leathem J, Merrick P. Subjective cognitive complaints and objective cognitive function in aging: a systematic review and meta-analysis of recent cross-sectional findings. Neuropsychol Rev. 2016;26(4):376-393. doi: 10.1007/s11065-016-9332-2 [DOI] [PubMed] [Google Scholar]
- 3.Mendonça MD, Alves L, Bugalho P. From subjective cognitive complaints to dementia: who is at risk?: a systematic review. Am J Alzheimers Dis Other Demen. 2016;31(2):105-114. doi: 10.1177/1533317515592331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariani E, Monastero R, Mecocci P. Mild cognitive impairment: a systematic review. J Alzheimers Dis. 2007;12(1):23-35. doi: 10.3233/JAD-2007-12104 [DOI] [PubMed] [Google Scholar]
- 6.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350(7):h369. doi: 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Q-F, Tan L, Wang H-F, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271. doi: 10.1016/j.jad.2015.09.069 [DOI] [PubMed] [Google Scholar]
- 8.Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104. doi: 10.1016/j.eurpsy.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 9.Monastero R, Mangialasche F, Camarda C, Ercolani S, Camarda R. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J Alzheimers Dis. 2009;18(1):11-30. doi: 10.3233/JAD-2009-1120 [DOI] [PubMed] [Google Scholar]
- 10.Lanctôt KL, Agüera-Ortiz L, Brodaty H, et al. Apathy associated with neurocognitive disorders: recent progress and future directions. Alzheimers Dement. 2017;13(1):84-100. doi: 10.1016/j.jalz.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 11.Padala PR, Desouza CV, Almeida S, et al. The impact of apathy on glycemic control in diabetes: a cross-sectional study. Diabetes Res Clin Pract. 2008;79(1):37-41. doi: 10.1016/j.diabres.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 12.Hishikawa N, Fukui Y, Nakano Y, et al. Factors related to continuous and discontinuous attendance at memory clinics. Eur J Neurol. 2017;24(5):673-679. doi: 10.1111/ene.13268 [DOI] [PubMed] [Google Scholar]
- 13.Beishuizen CRL, Coley N, Moll van Charante EP, van Gool WA, Richard E, Andrieu S. Determinants of dropout and nonadherence in a dementia prevention randomized controlled trial: the prevention of dementia by intensive vascular care trial. J Am Geriatr Soc. 2017;65(7):1505-1513. doi: 10.1111/jgs.14834 [DOI] [PubMed] [Google Scholar]
- 14.Ellis JM, Doyle CJ, Selvarajah S. The relationship between apathy and participation in therapeutic activities in nursing home residents with dementia: evidence for an association and directions for further research. Dementia (London). 2016;15(4):494-509. doi: 10.1177/1471301214527300 [DOI] [PubMed] [Google Scholar]
- 15.Richard E, Schmand B, Eikelenboom P, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer’s disease in non-depressed subjects. Dement Geriatr Cogn Disord. 2012;33(2-3):204-209. doi: 10.1159/000338239 [DOI] [PubMed] [Google Scholar]
- 16.Geda YE, Roberts RO, Mielke MM, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171(5):572-581. doi: 10.1176/appi.ajp.2014.13060821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65(10):1193-1198. doi: 10.1001/archpsyc.65.10.1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolters Kluwer. Ovid: home. http://www.ovid.com/site/index.jsp. Accessed October 2, 2017.
- 20.Clarke DE, Ko JY, Kuhl EA, van Reekum R, Salvador R, Marin RS. Are the available apathy measures reliable and valid? a review of the psychometric evidence. J Psychosom Res. 2011;70(1):73-97. doi: 10.1016/j.jpsychores.2010.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells G, Shae BOD The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis (online). http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed June 8, 2018.
- 22.Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry. 2013;21(7):685-695. doi: 10.1016/j.jagp.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters ME, Rosenberg PB, Steinberg M, et al. ; Cache County Investigators . Neuropsychiatric symptoms as risk factors for progression from CIND to dementia: the Cache County Study. Am J Geriatr Psychiatry. 2013;21(11):1116-1124. doi: 10.1016/j.jagp.2013.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakers IH, Visser PJ, Aalten P, Kester A, Jolles J, Verhey FR. Affective symptoms as predictors of Alzheimer’s disease in subjects with mild cognitive impairment: a 10-year follow-up study. Psychol Med. 2010;40(7):1193-1201. doi: 10.1017/S0033291709991577 [DOI] [PubMed] [Google Scholar]
- 25.Higgins J, Green S Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collab. http://handbook.cochrane.org. Published 2011. Accessed June 8, 2018.
- 26.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 27.Schwarzer G, Carpenter JR, Rücker G. Meta-Analysis With R. Cham: Springer International Publishing; 2015, doi: 10.1007/978-3-319-21416-0 [DOI] [Google Scholar]
- 28.Robert PH, Berr C, Volteau M, et al. ; PréAL Study Group . Importance of lack of interest in patients with mild cognitive impairment. Am J Geriatr Psychiatry. 2008;16(9):770-776. doi: 10.1097/JGP.0b013e31817e73db [DOI] [PubMed] [Google Scholar]
- 29.Bartolini M, Coccia M, Luzzi S, Provinciali L, Ceravolo MG. Motivational symptoms of depression mask preclinical Alzheimer’s disease in elderly subjects. Dement Geriatr Cogn Disord. 2005;19(1):31-36. doi: 10.1159/000080968 [DOI] [PubMed] [Google Scholar]
- 30.Burke SL, Maramaldi P, Cadet T, Kukull W. Neuropsychiatric symptoms and Apolipoprotein E: associations with eventual Alzheimer’s disease development. Arch Gerontol Geriatr. 2016;65:231-238. doi: 10.1016/j.archger.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24(4):253-259. doi: 10.1159/000107100 [DOI] [PubMed] [Google Scholar]
- 32.Vicini Chilovi B, Conti M, Zanetti M, Mazzù I, Rozzini L, Padovani A. Differential impact of apathy and depression in the development of dementia in mild cognitive impairment patients. Dement Geriatr Cogn Disord. 2009;27(4):390-398. doi: 10.1159/000210045 [DOI] [PubMed] [Google Scholar]
- 33.Somme J, Fernández-Martínez M, Molano A, Zarranz JJ. Neuropsychiatric symptoms in amnestic mild cognitive impairment: increased risk and faster progression to dementia. Curr Alzheimer Res. 2013;10(1):86-94. doi: 10.1016/j.jalz.2011.05.428 [DOI] [PubMed] [Google Scholar]
- 34.Sobów T, Fendler W, Magierski R. Body mass index and mild cognitive impairment-to-dementia progression in 24 months: a prospective study. Eur J Clin Nutr. 2014;68(11):1216-1219. doi: 10.1038/ejcn.2014.167 [DOI] [PubMed] [Google Scholar]
- 35.Pink A, Stokin GB, Bartley MM, et al. Neuropsychiatric symptoms, APOE ε4, and the risk of incident dementia: a population-based study. Neurology. 2015;84(9):935-943. doi: 10.1212/WNL.0000000000001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis. 2010;20(1):175-183. doi: 10.3233/JAD-2010-1352 [DOI] [PubMed] [Google Scholar]
- 37.Brodaty H, Heffernan M, Draper B, et al. Neuropsychiatric symptoms in older people with and without cognitive impairment. J Alzheimers Dis. 2012;31(2):411-420. doi: 10.3233/JAD-2012-120169 [DOI] [PubMed] [Google Scholar]
- 38.van der Linde RM, Stephan BCM, Matthews FE, Brayne C, Savva GM; Medical Research Council Cognitive Function and Ageing Study . The presence of behavioural and psychological symptoms and progression to dementia in the cognitively impaired older population. Int J Geriatr Psychiatry. 2013;28(7):700-709. doi: 10.1002/gps.3873 [DOI] [PubMed] [Google Scholar]
- 39.Chan WC, Lam LCW, Tam CWC, et al. Neuropsychiatric symptoms are associated with increased risks of progression to dementia: a 2-year prospective study of 321 Chinese older persons with mild cognitive impairment. Age Ageing. 2011;40(1):30-35. doi: 10.1093/ageing/afq151 [DOI] [PubMed] [Google Scholar]
- 40.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 42.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 3rd ed Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 43.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. doi: 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 44.Palmer K, Lupo F, Perri R, et al. Predicting disease progression in Alzheimer’s disease: the role of neuropsychiatric syndromes on functional and cognitive decline. J Alzheimers Dis. 2011;24(1):35-45. doi: 10.3233/JAD-2010-101836 [DOI] [PubMed] [Google Scholar]
- 45.Cooper C, Sommerlad A, Lyketsos CG, Livingston G. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry. 2015;172(4):323-334. doi: 10.1176/appi.ajp.2014.14070878 [DOI] [PubMed] [Google Scholar]
- 46.Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130(6):439-451. doi: 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- 47.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200-208. doi: 10.1002/ana.21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfeifer L, Drobetz R, Fankhauser S, Mortby ME, Maercker A, Forstmeier S. Caregiver rating bias in mild cognitive impairment and mild Alzheimer’s disease: impact of caregiver burden and depression on dyadic rating discrepancy across domains. Int Psychogeriatr. 2013;25(8):1345-1355. doi: 10.1017/S1041610213000562 [DOI] [PubMed] [Google Scholar]
- 49.Sink KM, Covinsky KE, Barnes DE, Newcomer RJ, Yaffe K. Caregiver characteristics are associated with neuropsychiatric symptoms of dementia. J Am Geriatr Soc. 2006;54(5):796-803. doi: 10.1111/j.1532-5415.2006.00697.x [DOI] [PubMed] [Google Scholar]
- 50.Pfeifer L, Horn AB, Maercker A, Forstmeier S. Caregiver perception of apathy in persons with mild cognitive impairment or Alzheimer’s disease: a longitudinal study. Aging Ment Health. 2017;21(5):494-500. doi: 10.1080/13607863.2015.1118678 [DOI] [PubMed] [Google Scholar]
- 51.Kim G, DeCoster J, Huang C-H, Bryant AN. A meta-analysis of the factor structure of the Geriatric Depression Scale (GDS): the effects of language. Int Psychogeriatr. 2013;25(1):71-81. doi: 10.1017/S1041610212001421 [DOI] [PubMed] [Google Scholar]
- 52.Ioannidis JPA. Why most discovered true associations are inflated. Epidemiology. 2008;19(5):640-648. doi: 10.1097/EDE.0b013e31818131e7 [DOI] [PubMed] [Google Scholar]
- 53.Euser SM, Schram MT, Hofman A, Westendorp RGJ, Breteler MMB. Measuring cognitive function with age: the influence of selection by health and survival. Epidemiology. 2008;19(3):440-447. doi: 10.1097/EDE.0b013e31816a1d31 [DOI] [PubMed] [Google Scholar]
- 54.Eurelings LS, van Dalen JW, Ter Riet G, Moll van Charante EP, Richard E, van Gool WA; ICARA Study Group . Apathy and depressive symptoms in older people and incident myocardial infarction, stroke, and mortality: a systematic review and meta-analysis of individual participant data. Clin Epidemiol. 2018;10:363-379. doi: 10.2147/CLEP.S150915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ismail Z, Elbayoumi H, Fischer CE, et al. Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(1):58-67. doi: 10.1001/jamapsychiatry.2016.3162 [DOI] [PubMed] [Google Scholar]
- 56.van Dalen JW, Van Wanrooij LL, Moll van Charante EP, Richard E, van Gool WA. Apathy is associated with incident dementia in community-dwelling older people. Neurology. 2018;90(1):e82-e89. doi: 10.1212/WNL.0000000000004767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ismail Z, Smith EE, Geda Y, et al. ; ISTAART Neuropsychiatric Symptoms Professional Interest Area . Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195-202. doi: 10.1016/j.jalz.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mortby ME, Black SE, Gauthier S, et al. Dementia clinical trial implications of mild behavioral impairment. Int Psychogeriatr. 2018;30(2):171-175. doi: 10.1017/S1041610218000042 [DOI] [PubMed] [Google Scholar]
- 59.Canevelli M, Blasimme A, Vanacore N, Bruno G, Cesari M. Mild behavioral impairment: ethical, methodological and clinical reflections. Neurosci Biobehav Rev. 2016;69:402-403. doi: 10.1016/j.neubiorev.2016.08.025 [DOI] [PubMed] [Google Scholar]
- 60.Joosten-Weyn Banningh L, Vernooij-Dassen M, Rikkert MO, Teunisse JP. Mild cognitive impairment: coping with an uncertain label. Int J Geriatr Psychiatry. 2008;23(2):148-154. doi: 10.1002/gps.1855 [DOI] [PubMed] [Google Scholar]
- 61.Eurelings LS, Ligthart SA, van Dalen JW, Moll van Charante EP, van Gool WA, Richard E. Apathy is an independent risk factor for incident cardiovascular disease in the older individual: a population-based cohort study. Int J Geriatr Psychiatry. 2014;29(5):454-463. doi: 10.1002/gps.4026 [DOI] [PubMed] [Google Scholar]
- 62.Richard E, Schmand BA, Eikelenboom P, Van Gool WA; Alzheimer’s Disease Neuroimaging Initiative . MRI and cerebrospinal fluid biomarkers for predicting progression to Alzheimer’s disease in patients with mild cognitive impairment: a diagnostic accuracy study. BMJ Open. 2013;3(6):1-8. doi: 10.1136/bmjopen-2012-002541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stephan BCM, Tzourio C, Auriacombe S, et al. Usefulness of data from magnetic resonance imaging to improve prediction of dementia: population based cohort study. BMJ. 2015;350:h2863. doi: 10.1136/bmj.h2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alegret M, Cuberas-Borrós G, Espinosa A, et al. Cognitive, genetic, and brain perfusion factors associated with four year incidence of Alzheimer’s disease from mild cognitive impairment. J Alzheimers Dis. 2014;41(3):739-748. doi: 10.3233/JAD-132516 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Full Search Strategy
eTable 2. Items on Data Extraction Form
eTable 3. Subanalyses and Rationale
eTable 4. Estimates for AD and Dementia Compared Within Studies
eTable 5. Bias Assessment Score Overview
eTable 6. Detailed Bias Assessment Table With Rationale for Scores
eFigure 1. Funnel Plot for the Overall Analysis
eFigure 2. Forest Plot of Leave-one-out Analysis for Risk Ratios
eFigure 3. Sensitivity Analysis With AD Instead of Dementia as Preferential Outcome
eFigure 4. Funnel Plot for the Risk Ratios Reported in Studies in Mild Cognitive Impairment
eFigure 5. Overall and Subgroup Analyses of HRs in Mild Cognitive Impairment
eFigure 6. Funnel Plot of Hazard Ratios Reported in Studies
eFigure 7. Meta-regression of Risk Ratios for Developing Dementia in Mild Cognitive Impairment
eFigure 8. Subgroup Analyses in MCI Based on Reported Maximally Adjusted Hazard Ratios
eFigure 9. Meta-regression of Reported Hazard Ratios for Mild Cognitive Impairment
eFigure 10. Meta-regression of Reported Hazard Ratios for Mild Cognitive Impairment Excluding 1 Study
eFigure 11. Forest plot of RR in Studies Using Validated and Custom Definitions Of Apathy
eFigure 12. Forest plot for Relative Risk of Developing Dementia Including Studies Using Validated and Custom Apathy According to Subgroups Based on Diagnosis
eFigure 13. Meta-regression of Log Risk Ratio of Dementia for Patients With Apathy in studies Using Recommended (Blue) and Custom (Grey) Definitions Over Follow-up Time
eFigure 14. Meta-regression Results of the Log Risk Ratio for Dementia in Participants With Apathy Predicted by FU-time, Apathy Definition Type and Their Interaction
eFigure 15. Overall and Subgroup Analyses of RR in MCI and SCC Patients for Studies Using Custom Definitions of Apathy