Key Points
Question
Is it possible to identify brain functional connectivities that mediate the association of depressive symptoms with poor sleep quality and advance understanding of the differences in brain functional connectivity in individuals with higher scores on the Depressive Problems scale?
Findings
In 1017 participants in the Human Connectome Project, brain areas with increased functional connectivity associated with both sleep and Depressive Problems scores included the lateral orbitofrontal cortex, dorsolateral prefrontal cortex, anterior and posterior cingulate cortices, insula, parahippocampal gyrus, hippocampus, amygdala, temporal cortex, and precuneus. A mediation analysis showed that these functional connectivities underlie the association of depressive problem scores with poor sleep quality.
Meaning
In this study, the increased functional connectivity between these brain regions provides a neural basis for the association of depression with poor sleep quality; in this general population from the United States that was not selected for depression, Depressive Problems scores were correlated with functional connectivities in the brain, including the lateral orbitofrontal cortex, which has implications for the treatment of depression and poor sleep quality.
This study using Human Connectome Project cohort data examines brain areas that mediate the association of depression with poor sleep quality and advances understanding of the differences in brain connectivity in depression.
Abstract
Importance
Depression is associated with poor sleep quality. Understanding the neural connectivity that underlies both conditions and mediates the association between them is likely to lead to better-directed treatments for depression and associated sleep problems.
Objective
To identify the brain areas that mediate the association of depressive symptoms with poor sleep quality and advance understanding of the differences in brain connectivity in depression.
Design, Setting, and Participants
This study collected data from participants in the Human Connectome Project using the Adult Self-report of Depressive Problems portion of the Achenbach Adult Self-Report for Ages 18-59, a survey of self-reported sleep quality, and resting-state functional magnetic resonance imaging. Cross-validation of the sleep findings was conducted in 8718 participants from the UK Biobank.
Main Outcomes and Measures
Correlations between functional connectivity, scores on the Adult Self-Report of Depressive Problems, and sleep quality.
Results
A total of 1017 participants from the Human Connectome Project (of whom 546 [53.7%] were female; age range, 22 to 35 years) drawn from a general population in the United States were included. The Depressive Problems score was positively correlated with poor sleep quality (r = 0.371; P < .001). A total of 162 functional connectivity links involving areas associated with sleep, such as the precuneus, anterior cingulate cortex, and the lateral orbitofrontal cortex, were identified. Of these links, 39 were also associated with the Depressive Problems scores. The brain areas with increased functional connectivity associated with both sleep and Depressive Problems scores included the lateral orbitofrontal cortex, dorsolateral prefrontal cortex, anterior and posterior cingulate cortices, insula, parahippocampal gyrus, hippocampus, amygdala, temporal cortex, and precuneus. A mediation analysis showed that these functional connectivities underlie the association of the Depressive Problems score with poor sleep quality (β = 0.0139; P < .001).
Conclusions and Relevance
The implication of these findings is that the increased functional connectivity between these brain regions provides a neural basis for the association between depression and poor sleep quality. An important finding was that the Depressive Problems scores in this general population were correlated with functional connectivities between areas, including the lateral orbitofrontal cortex, cingulate cortex, precuneus, angular gyrus, and temporal cortex. The findings have implications for the treatment of depression and poor sleep quality.
Introduction
Many individuals with depression report poor sleep quality1 and disturbances of sleep, including insomnia and less slow-wave sleep.2 What is the association between depression and sleep? What are the brain systems associated with both depression and sleep quality? Understanding the answers to these questions may lead to better treatments for depression and may improve sleep quality.
Significant correlations between depression and poor sleep quality have been found in a meta-analysis.1 Poor sleep quality is typically measured with a high value on the Pittsburgh Sleep Quality Index (PSQI),3 which is closely correlated with insomnia.4 The widespread use of the PSQI has shown that subjective sleep disturbances are associated with depression.1,5,6,7 Moreover, there is a genetic component to the association between poor sleep quality and depression,2,7 and this makes it especially interesting to search for possible neural mechanisms that may mediate the association. Previously, it has been shown that sleep deprivation affects brain systems that are involved in emotion, such as the amygdala,8 and the anterior cingulate cortex has been described as a region in which genes that control circadian rhythms are dysregulated in depression9 and in which neurons increase their activity during sleep and disengagement from tasks.10
Depression is ranked by the World Health Organization as the leading cause of years of life lived with disability.11,12,13 In most countries, the percentage of people with depression during their lifetimes falls within an 8% to 12% range.11,13 There is a major personal burden to these individuals and their families of depression as well as a major economic burden.14 Thus, advances in our understanding of the neural bases of depression, and how they may be associated with poor sleep quality, are key areas for investigation.
In this study, we analyzed the neural association between depression and sleep quality by measuring the associations between functional connectivity between brain areas in the resting state and both poor sleep quality and depressive problems and used a mediating analysis to assess the underlying mechanisms. Resting-state functional connectivity between brain areas, which reflects correlations of activity, is a fundamental tool in augmenting understanding of the brain regions with altered connectivity and function in mental disorders.15
Methods
Participants and Data Preprocessing
Primary Data Set From the Human Connectome Project
The data set used for this investigation was selected from the March 2017 public data release from the Human Connectome Project (HCP; N = 1200) from the Washington University–University of Minnesota (WU-Minn HCP) Consortium. The participants included in this sample were scanned on a 3-T connectome-Skyra scanner (Siemens). The WU-Minn HCP Consortium obtained full informed consent from all participants, and research procedures and ethical guidelines were followed in accordance with Washington University institutional review board approval. The resting-state data were obtained and preprocessed by the HCP with its standardized method,18,19,20,21 with details provided in eFigure 1 in the Supplement. We emphasize that the participants in this investigation were from the Human Connectome Project and were not selected for their existing symptoms or diagnosis of depression; rather, measures of depressive feelings and behavior were available and were used in these analyses.
Construction of the Whole-Brain Functional Network
After preprocessing, the gray matter of the whole brain was parcellated into 250 regions of interest using the Shen atlas,22 which has been validated in resting-state functional magnetic resonance imaging (fMRI) studies23,24 as described in the eMethods in the Supplement. Then the time series were extracted in each region of interest by determining the mean of the signals of all voxels within that region. Pearson cross-correlations between all pairs of regional blood oxygen level–dependent signals were calculated for each participant, followed by z transformation to improve normality, and the whole-brain functional connectivity network (250 × 250 regions with 31 125 edges) was constructed using 2 scans, as described in the eMethods in the Supplement. Anatomical regions are listed in eTable 1 in the Supplement.
Sleep and Depression Phenotypes
The sleep phenotype of participants in the HCP was assessed by the self-reported PSQI total score, which combines 7 component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction in the past month (with further details in eFigure 1 in the Supplement), and which is considered to be a good measure of insomnia.4
The measure of depressive problems in the HCP, the Adult Self-report DSM-IV Depressive Problems raw score, is based on the Achenbach Adult Self-report for Ages 18-59.25 Specifically, the 123 items from Section VIII were administered (of which only 2 were on the topic of sleep quality). Then items associated with depressive symptoms were used to generate the raw score. In addition, this stage of data collection included obtaining information from participants on whether they had ever been diagnosed with depression via the question, “Has the participant experienced a diagnosed DSM-IV Major Depressive Episode over his/her lifetime?”
Statistical Analysis
Identifying Correlations of Functional Connectivity With Sleep Quality
To investigate the association between functional connectivity and sleep quality (PSQI total score, a very widely used measure of sleep quality that is a good measure of insomnia1,4,5,6,7), linear regression analysis was used, after removing 14 confounding variables: age, sex, years of education, race (categorized as white or other), handedness, head motion (mean framewise displacement), body mass index (calculated as weight in kilograms divided by height in meters squared), blood pressure (diastolic and systolic), total gray matter volume, total white matter volume, alcohol use, tobacco use, and marijuana use. To take into account the family relationships of the participants in the HCP dataset, the significance for all association analyses in this study was estimated by the permutation analysis of linear models technique26 in accordance with prior studies.20,27 To address the problem of multiple comparisons, the network-based statistic (NBS),28 a well-validated method for brain network association analysis that has previously been used widely in neuroimaging studies,28,29,30,31 was used to identify and ascribe significance to any connected subnetworks found in the set of significantly altered links found in the group with poor sleep quality (eTable 2 in the Supplement). We considered P < .05 (corrected for multiple comparisons) to be significant.
Correlation Between Sleep Quality and Depressive Score
We first investigated the association between the poor sleep quality measure and the depressive measure (ie, Adult Self-report DSM-IV Depressive Problems raw score) by partial correlation analysis after removing the 14 confounding variables described. The correlation was highly significant (r = 0.371; P < .001; Figure 1B). We note that only 2 of the 123 items in the Adult Self Report questionnaire, which are used to calculate the Depressive Problems Score, are on the topic of sleep quality, and so these are unlikely to account for the correlations in this study. Although participants of the HCP data set were not selected to have a psychiatric disorder and most are healthy control participants, the Depressive Problems raw score was a useful indicator of depression, in that 92 participants who reported having been diagnosed with a major depressive episode also had significantly higher scores on this measure.
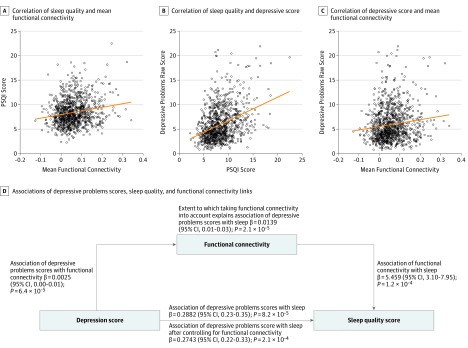
Figure 1. Association Between Depression, Sleep, and Functional Connectivity.
A, The correlation between the sleep quality and the mean strength of 162 significant functional connectivities. B, The correlation between the sleep quality and the depressive score. C, The correlation between the depressive score and the mean strength of 162 significant functional connectivities associated with sleep quality. D, The mediation implemented by functional connectivity from the depressive problems score on poor sleep quality (Pittsburgh Sleep Quality Index [PSQI] score; β = 0.0139; P < .001). The total assocation of the Depressive Problems score with the outcome shows that the regression coefficient32 (β) of the Depressive Problems score on the PSQI score was high when the functional connectivities were not taken into account. The direct association of the Depressive Problems score with the outcome (PSQI score), after controlling for the mediator (functional connectivity), shows some reduction in the regression coefficient when the association of the functional connectivities was removed.
Cross-Validation Using the UK Biobank Data set
We used a separate large data set (from the UK Biobank) to test whether the analyses of the HCP data are robust. This data set included 8718 participants.
Results
Demographics
The sample used in these analyses included 1017 participants (of whom 546 [53.7%] were female; age range, 22-35 years). Data on the participants, including their sleep and depression scores, are shown in Table 1. Further details are in eTable 2 of the Supplement.
Table 1. Demographic Characteristics of the 1017 Human Connectome Project Participantsa.
| Characteristic | No. (%) |
|---|---|
| Age, mean (SD), y | 28.8 (3.7) |
| Female | 546 (53.7) |
| Handednessb | 66.4 (43.5) |
| Race | |
| White | 767 (75.4) |
| Other | 250 (24.6) |
| Education, mean (SD), y | 15.0 (1.8) |
| BMI, mean (SD) | 26.4 (5.1) |
| Head motion, mean (SD), mm | 0.09 (0.03) |
| Blood pressure, mean (SD) | |
| Diastolic | 76.3 (10.6) |
| Systolic | 123.4 (13.8) |
| Total brain volume, mean (SD), mm3 | |
| Gray matter | 687195.0 (66512.9) |
| White matter | 445283.3 (56273.5) |
| Twin status | |
| Monozygotic | 264 (26.0) |
| Dizygotic | 154 (15.1) |
| Nontwin | 490 (48.2) |
| Substance use | |
| Marijuana use, mean (SD), incidents | 1.39 (1.67) |
| Frequency of alcohol use in the last 12 moc | 4.29 (1.54) |
| Drinks per drinking day | 2.27 (1.57) |
| Smoking history | |
| Never | 549 (54.0) |
| Smoked 1-19 cigarettes over lifetime | 87 (8.6) |
| Smoked ≥20 cigarettes over lifetime | 119 (11.7) |
| Regular smoker | 258 (25.4) |
| Pittsburgh Sleep Quality Index, mean (SD), pointsd | |
| Total score | 4.7 (2.8) |
| Subjective sleep quality | 0.88 (0.63) |
| Sleep latency | 0.96 (0.82) |
| Habitual sleep efficiency | 0.56 (0.82) |
| Sleep duratione | 0.43 (0.78) |
| Sleep disturbance | 1.08 (0.48) |
| Use of sleep medications | 0.23 (0.66) |
| Daytime dysfunction | 0.58 (0.64) |
| Amount of sleep, mean (SD), h | 6.83 (1.13) |
| Adult self-report DSM-IV Depressive Problems scale score, mean (SD) | |
| Raw score | 4.09 (3.46) |
| Sex-adjusted, age-adjusted T scoref | 53.80 (5.72) |
| No depressive symptoms | 1.29 (2.57) |
| Major depressive episode | |
| No | 925 (91.0) |
| Yes | 92 (9.0) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Further definitions are available at the Human Connectome Project Data Dictionary.33
Handedness of participant is measured from −100 to 100. Negative numbers indicate that a participant is more left-handed, while positive numbers indicate that a participant is more right-handed.
Frequency of any alcohol use in past 12 months was measured on an inverse scale of 1 to 7, where alcohol use on 4 to 7 days per week was scored 1 for male participants and 2 for female participants and 3 days per week was scored 2, 2 days per week was scored 3, 1 day per week was scored 4, 1 to 3 days per month was scored 5, 1 to 11 days per year was scored 6, and no use in the past 12 months was scored 7 for both sexes.
The Pittsburgh Sleep Quality Index was scored on a scale of 0 to 21, with each subscore on a scale of 0 to 3.
Amount was defined by the patient’s answer to the question, “During the past month, how many hours of actual sleep did you get at night?”
The T scores are calculated based on the distribution of the score for different ages and sexes.
Notably, 92 participants (9.0%) had been diagnosed with a major depressive episode (as defined by the DSM-IV) over their lifetimes. These participants had a significantly mean (SD) higher score (7.55 [4.83] points) than the other participants (3.70 [3.07] points; t982 = −10.8; P < .001) on the Depressive Problems scale.
Functional Connectivity Links Associated With Sleep Quality
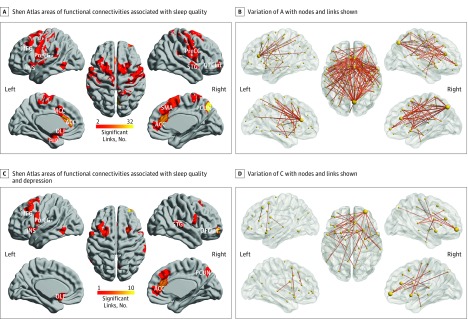
There were 162 significant links after NBS correction. Table 2 shows the 60 functional connectivity links that had associations of greatest significance with the PSQI score, including names, supporting statistics, and P values. All are significantly positively correlated with the poor sleep quality assessment. All links that are correlated with the PSQI score are shown in Figure 2. Many significantly correlated links involved the precuneus, the anterior cingulate cortex, the lateral orbitofrontal cortex, the hippocampus and parahippocampal cortex, and some motor areas (Figure 2; eFigure 2 in the Supplement). The correlation between the sleep quality and the mean strength of these significant functional connectivities is shown in Figure 1A (r = 0.181; P = 6.7 × 10−9).
Table 2. Top 60 Functional Connectivity Links Correlated With the Pittsburgh Sleep Quality Index Score.
| Functional Connectivity | r Valuea | P Value | |
|---|---|---|---|
| Region 1 | Region 2 | ||
| Right precuneus | Right postcentral gyrus | 0.123 | <.001 |
| Right middle frontal gyrus | Right calcarine fissure and surrounding cortex | 0.123 | <.001 |
| Right precuneus | Right insula | 0.124 | <.001 |
| Right olfactory cortex | Right caudate nucleus | 0.118 | <.001 |
| Lateral orbitofrontal cortex | Left middle frontal gyrus | 0.117 | <.001 |
| Right anterior cingulate and paracingulate gyri | Left postcentral gyrus | 0.121 | <.001 |
| Left postcentral gyrus | Left anterior cingulate and paracingulate gyri | 0.123 | <.001 |
| Right caudate nucleus | Left superior frontal gyrus, dorsolateral | 0.127 | <.001 |
| Lateral orbitofrontal cortex | Left precentral gyrus | 0.135 | <.001 |
| Right precuneus | Left precentral gyrus | 0.129 | <.001 |
| Left anterior cingulate and paracingulate gyri | Left precentral gyrus | 0.126 | <.001 |
| Left thalamus | Left precentral gyrus | 0.118 | <.001 |
| Right precuneus | Left insula | 0.117 | <.001 |
| Lateral orbitofrontal cortex | Left superior temporal gyrus | 0.121 | <.001 |
| Lateral orbitofrontal cortex | Left supplementary motor area | 0.120 | <.001 |
| Left olfactory cortex | Right middle frontal gyrus | 0.119 | <.001 |
| Right superior temporal gyrus | Right anterior cingulate and paracingulate gyri | 0.115 | <.001 |
| Right precentral gyrus | Right caudate nucleus | 0.118 | <.001 |
| Lateral orbitofrontal cortex | Right precentral gyrus | 0.115 | <.001 |
| Right precuneus | Right precentral gyrus | 0.122 | <.001 |
| Right supramarginal gyrus | Right precentral gyrus | 0.113 | <.001 |
| Right precuneus | Left middle frontal gyrus | 0.127 | <.001 |
| Right precuneus | Left postcentral gyrus | 0.116 | <.001 |
| Right anterior cingulate and paracingulate gyri | Left middle occipital gyrus | 0.119 | <.001 |
| Right precuneus | Left superior temporal gyrus | 0.121 | <.001 |
| Right precuneus | Left supramarginal gyrus | 0.131 | <.001 |
| Left hippocampus | Left caudate nucleus | 0.117 | <.001 |
| Left paracentral lobule | Left caudate nucleus | 0.114 | <.001 |
| Right precuneus | Left postcentral gyrus | 0.121 | <.001 |
| Right precentral gyrus | Right precuneus | 0.128 | <.001 |
| Right superior temporal gyrus | Right anterior cingulate and paracingulate gyri | 0.118 | <.001 |
| Right supplementary motor area | Right superior temporal gyrus | 0.119 | <.001 |
| Right precentral gyrus | Right putamen | 0.112 | <.001 |
| Right hippocampus | Right caudate nucleus | 0.107 | <.001 |
| Right supplementary motor area | Right precentral gyrus | 0.133 | <.001 |
| Lateral orbitofrontal cortex | Left inferior temporal gyrus | 0.110 | <.001 |
| Right middle frontal gyrus | Left precuneus | 0.108 | <.001 |
| Right precuneus | Left middle cingulate and paracingulate gyri | 0.130 | <.001 |
| Left thalamus | Left hippocampus | 0.115 | <.001 |
| Right anterior cingulate and paracingulate gyri | Left middle frontal gyrus | 0.115 | <.001 |
| Right precuneus | Right superior temporal gyrus | 0.124 | <.001 |
| Right postcentral gyrus | Right anterior cingulate and paracingulate gyri | 0.110 | <.001 |
| Right postcentral gyrus | Right supplementary motor area | 0.114 | <.001 |
| Right hippocampus | Right putamen | 0.113 | <.001 |
| Right precuneus | Right precentral gyrus | 0.116 | <.001 |
| Left olfactory cortex | Left superior frontal gyrus, dorsolateral | 0.113 | <.001 |
| Right anterior cingulate and paracingulate gyri | Left precentral gyrus | 0.121 | <.001 |
| Lateral orbitofrontal cortex | Left insula | 0.117 | <.001 |
| Right anterior cingulate and paracingulate gyri | Left middle frontal gyrus | 0.106 | <.001 |
| Lateral orbitofrontal cortex | Left middle occipital gyrus | 0.111 | <.001 |
| Lateral orbitofrontal cortex | Left insula | 0.115 | <.001 |
| Right precuneus | Left insula | 0.117 | <.001 |
| Right precuneus | Left supplementary motor area | 0.121 | <.001 |
| Left anterior cingulate and paracingulate gyri | Left postcentral gyrus | 0.122 | <.001 |
| Right precentral gyrus | Lateral orbitofrontal cortex | 0.109 | <.001 |
| Lateral orbitofrontal cortex | Right precuneus | 0.108 | <.001 |
| Lateral orbitofrontal cortex | Left middle cingulate and paracingulate gyri | 0.110 | <.001 |
| Right precentral gyrus | Left middle cingulate and paracingulate gyri | 0.121 | <.001 |
| Right anterior cingulate and paracingulate gyri | Left superior frontal gyrus, dorsolateral | 0.119 | <.001 |
| Right precentral gyrus | Left insula | 0.106 | <.001 |
All correlations are significant (defined as P < .05) with whole-brain network-based statistic correction.
Figure 2. The Shen Atlas Areas With Functional Connectivities.
Shen Atlas areas with 162 links associated with sleep quality that were significant after NBS correction shown in red (A), and with 162 links significant after NBS correction with nodes and links indicated (B). Shen Atlas areas with 30 functional connectivities associated with sleep quality and depressive problems are shown in red (C) and with links and nodes indicated (D). For the NBS, the primary threshold for each link-based P value was .05, and the number of permutations was 10 000. MFG indicates middle frontal gyrus; PreCG, precentral gyrus; INS, insula; MCC, midcingulate cortex; ACC, anterior cingulate cortex; OLF, olfactory tubercle; HIP, hippocampus; STG, superior temporal gyrus; OFClat, lateral orbitofrontal cortex; PCUN, precuneus; SMA, supplementary motor area.
Functional Connectivity Links Associated With Depressive Problems Scores and Sleep Quality
In the correlation analysis between functional connectivity, poor sleep quality, and depressive scores, we found that the PSQI score was positively correlated with the Depressive Problems raw score (r = 0.371; P < .001; Figure 1B). Then a permutation test was performed, which showed that the 162 significant links identified in the correlation analysis with the PSQI score were also significantly correlated with the Depressive Problems score (Table 2). Figure 2D shows 39 significant individual links identified in the sleep analysis that are also significantly correlated with Depressive Problems scores. The distribution of brain areas associated with these significant links included the lateral orbitofrontal cortex, the anterior cingulate cortex, and the precuneus, as shown in Figure 2C and eFigure 3 in the Supplement. Furthermore, we found that the mean strength of each of the 39 functional connectivities involved in both sleep quality and the Depressive Problems score (shown in Figure 2D) was also positively correlated with the Depressive Problems raw score (r = 0.133; P = 2.1 × 10−5; Figure 1C).
Functional Connectivity Links Associated With the Depressive Problems Score
eFigure 4 and eTable 2 in the Supplement show correlations between the functional connectivities across the whole brain and the Depressive Problems score. Key areas shown include the lateral orbitofrontal cortex, the dorsolateral prefrontal cortex, the anterior and posterior cingulate cortices, and the insula. If the 92 participants who had ever been diagnosed with depression were removed from this analysis, the correlations of some of these areas with the Depressive Problems score were still significant, including the anterior cingulate gyrus and middle frontal gyrus (middle frontal gyrus with right anterior cingulate cortex: r = 0.115; P = .001; left cuneus with left superior frontal gyrus, dorsolateral: r = −0.109; P = .002; right inferior parietal gyrus, excluding supramarginal and angular gyri with left middle cingulate and paracingulate gyri: r = 0.059; P = .01; right middle frontal gyrus with left anterior cingulate cortex: r = 0.059; P = .01; left postcentral gyrus with left inferior temporal lobe: r = 0.059; P = .01; eTable 2 in the Supplement). This provides evidence that some areas can be associated with depressive symptoms even in individuals who had never been diagnosed with depression. However, all of the links involving the lateral orbitofrontal cortex became nonsignificant if this subgroup of 92 individuals was removed (eTable 3 in the Supplement). This was supported by the finding of correlations between the depressive score and many functional connectivities involving the lateral orbitofrontal cortex when only this subgroup of 92 were included. In particular, in this subgroup of 92, correlations were found for the Depressive Problems score for 6 links involving the lateral orbitofrontal cortex (to the left middle frontal gyrus: r = 0.410; P < .001; left supramarginal gyrus: r = 0.377; P < .001; left middle cingulate and paracingulate gyri: r = 0.385; P < .001; left precuneus: r = 0.430; P < .001; left insula: r = 0.348; P < .001; and to 2 ROIs within the right middle cingulate and paracingulate gyri: r = 0.368 and r = 0.402; P < .001 for both), 7 links involving the anterior cingulate cortex (to the left supplementary motor area: r = 0.446; P < .001; right postcentral gyrus: r = 0.368; P < .001; temporal pole [middle temporal gyrus]: r = 0.338; P < .001; left inferior temporal lobe: r = 0.365; P < .001; left middle frontal gyrus: r = 0.321; P < .001; left middle occipital lobe: r = 0.329; P < .001; inferior frontal gyrus, opercular part: r = 0.373; P < .001), and 1 between the lateral orbitofrontal cortex and anterior cingulate cortex (r = 0.365; P < .001). The evidence from the HCP data set was thus that high lateral orbitofrontal cortex functional connectivities were especially associated with the Depressive Problems score, with this association being especially significant in people who at some point in the past had been diagnosed with depression. In the same 92 participants, other areas with high correlations (all with r values greater than 0.32 and corresponding P values less than .001) with the depressive problems score included the angular gyrus, anterior cingulate cortex, parahippocampal gyrus, and temporal cortex (eTable 3 in the Supplement).
Mediation Analysis
Given that the strengths of the functional connectivities were significantly associated with the PSQI score (β = 5.459 [95% CI, 3.10-7.95]; P = 1.2 × 10−4) and Depressive Problems score (β = 0.0025 [95% CI, 0.00-0.01]; P = 6.4 × 10−5), we assessed whether the functional connectivities significantly mediated32 this association. The mean strength of the 39 significant functional connectivities shown in Figure 2D involved in both poor sleep quality and Depressive Problems scores significantly mediated the relationship between the PSQI score and depressive problems score (Figure 1D) (4.8% of the total effect size; β = 0.0139 [95% CI, 0.01-0.03]; P = 2.1 × 10−5). We further found that all 39 links significantly mediated the association between the PSQI score and the Depressive Problems score (eTable 4 and eFigure 5 in the Supplement).
Cross-Validation Using the Biobank Data set
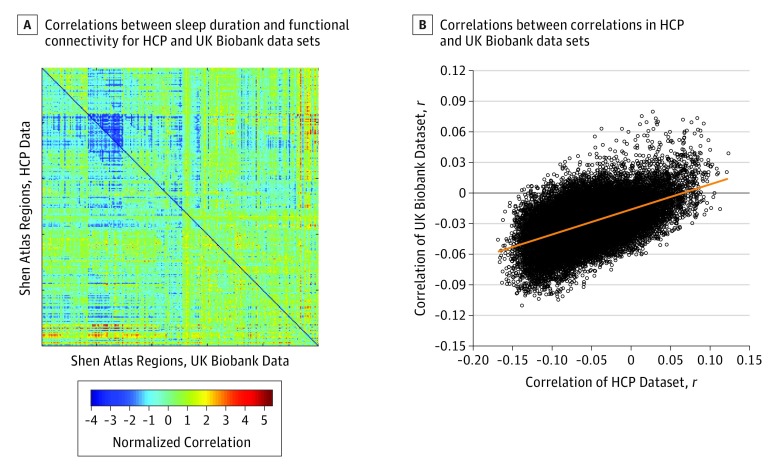
The association patterns between functional connectivities and sleep duration were very similar in both the HCP and the Biobank data sets (Figure 3A). Figure 3B shows a high positive correlation (r = 0.524; P < .001) between the r values for the correlation between the functional connectivity links for the whole brain and sleep duration in the HCP and Biobank data sets. More details are provided in the eTable 5 in the Supplement.
Figure 3. Cross-Validation Between the HCP and UK Biobank Data Sets for Functional Connectivity Correlations With Sleep Duration.
A, Correlations between the sleep duration and the functional connectivity for Human Connectome Project (HCP) data (top right) and for UK Biobank data (bottom left). Each axis shows reordered Shen atlas brain regions.22 The symmetry about the diagonal reflects the similarity of the sleep-associated connectivity in the HCP and UK Biobank data sets. B, Correlation between the r values between the functional connectivity links for the whole brain and sleep duration in the HCP and UK Biobank data sets.
Discussion
This is the first study, to our knowledge, to examine the neural mechanisms underlying the association of depression with sleep, a topic of great interest, with a large sample (n = 1017). The results show that both poor sleep quality and depressive problems are significantly positively correlated with functional connectivities involving the lateral orbitofrontal cortex, the dorsolateral prefrontal cortex, the cingulate cortex, and the precuneus. Further, a mediation analysis showed that the functional connectivity links between the brain areas identified played a significant role in the association of depressive problems with poor sleep quality. Much smaller associations were found in the reverse direction; that is, the associations of sleep quality with depressive problems mediated by these links were less significant. These findings provides a neural basis for understanding how depression is associated with poor sleep quality, and this in turn has implications for treatment because of the brain areas identified.
Areas in which functional connectivity was associated with depressive problems and their effects on sleep quality included the lateral orbitofrontal cortex, precuneus, and angular gyrus. There is increasing evidence that the lateral orbitofrontal cortex is a key brain region associated with depression. The theory is that it has increased sensitivity in depression of attractor networks activated by not receiving expected rewards (which can lead to sadness and depression).16,34 Consistently, the lateral orbitofrontal cortex has increased functional connectivity with a number of brain regions, including the precuneus.17 In depression, the precuneus itself also has increased functional connectivity with prefrontal cortex areas involved in short-term working memory (W. Cheng, PhD, written communication, June 21, 2018). The precuneus is implicated in representations of the sense of self and space and in autobiographical memory.35 These findings support the theory that the nonreward system in the lateral orbitofrontal cortex has increased influence on areas in which the self is represented, which results in low self-esteem, with the increased connectivity to the prefrontal cortex short-term memory system contributing to the rumination symptoms characteristic of depression.16,17
Part of the importance of the present investigation is that it provides strong support for a role of the lateral orbitofrontal cortex in depression (eFigure 4 and eTables 2 and 3 in the Supplement). The previous findings17 were in hundreds of patients with major depressive disorder and healthy control participants in China. The present findings were not on patients selected because of their existing depression but instead were on a general population in the United States in which a tendency to have depressive symptoms could be assessed. Interestingly, when the patients who had at some time been diagnosed with depression were removed, the links involving the lateral orbitofrontal cortex no longer exhibited the threshold level of significance. Moreover, if only the 92 participants who had had a diagnosis of depression were considered, then relatively high correlations with functional connectivities involving the lateral orbitofrontal cortex were found. This helps to cross-validate the findings in the data sets from China in which all these areas have been identified in a network of brain areas with different functional connectivity in depression.17,44 This important cross-validation with participants from the United States provides support for the theory that the lateral orbitofrontal cortex is a key brain area that might be targeted in the search for depression treatments.16,34,36 Consistent with this, repetitive transcranial magnetic stimulation and inhibition of the lateral orbitofrontal cortex may be useful in the treatment of depression.37
This study found that another brain region strongly associated with the effects of depressive problems on sleep quality is the precuneus. The precuneus is implicated in representations of the sense of self and space and in autobiographical memory.35,38,39 Acute depression is associated with impaired self-referential processing.40 On the bases of the findings of this study and others,16,17 we propose that the low self-esteem in depression is associated with increased functional connectivity between the precuneus (which is involved in the representation of self) and the lateral orbitofrontal cortex, which is involved in nonreward and therefore implicated in depression.
Another brain region strongly associated with the correlations between depressive problems and sleep quality is the anterior cingulate cortex. Interestingly, the supracallosal part of the anterior cingulate has especially increased functional connectivity with depressive problems and sleep quality and also poor sleep quality, because the supracallosal anterior cingulate has activations associated with punishment and negative emotional stimuli.41,42 Through separate analyses, we are assessing the implications that the supracallosal part of the anterior cingulate cortex may contribute to the negative and nonreward emotions in depression and their association with sleep quality and that the supracallosal anterior cingulate cortex has high functional connectivity with the convexity part of the lateral orbitofrontal cortex.
Strengths of the present investigation are the large number of participants (1017), leading to robust findings; cross-validation with an independent data set; the mediation analysis, which links the findings to recent advances in understanding brain mechanisms associated with depression16,17; and the inherent interest of the findings to a wide readership.
Limitations
Several limitations are considered. Most participants were healthy controls, and the depressive score was used as an indicator of depressive symptoms, not formal diagnosis; however, we did demonstrate a high correlation between the Depressive Problems score and whether a person had at some time been diagnosed with depression. We note that there is a strong association between depression and poor sleep, in that the correlation found in this study between the Depressive Problems score and PSQI score was high (r = 0.371, P < 1.0 × 10−10, Figure 1B); however, this does not address the directionality of the association, which could be in both directions. Indeed, we note that poor sleep quality also may have an effect on depression, as shown by the mediation analysis. We note that the causal relations between sleep and depression is an important topic that deserves much further investigation.2
Conclusions
Although previous studies have found changes in patients with depression in some of the regions of interest identified in this study, including the precuneus and angular gyrus,43 this is to our knowledge the first study to examine the neural mechanisms underlying the associations of depressive problems with sleep quality and to associate sleep quality with functional connectivity in a large sample of participants. A strength of this investigation is that the effects are likely to be very robust, given the large sample of participants with resting-state fMRI and the cross-validation with the UK Biobank data set with a sample of 8718 participants in one of its first uses of resting-state fMRI data. The understanding that we developed in this study is consistent with areas of the brain involved in short-term memory (the dorsolateral prefrontal cortex), the self (precuneus), and negative emotion (the lateral orbitofrontal cortex) being highly connected in depression, which results in increased ruminating thoughts that are at least part of the mechanism that impairs sleep quality.16,17,44
eMethods. Materials and Methods.
eFigure 1. The correlation between the PSQI total score and the other available sleep measures in the HCP dataset, based on the sample of 1017 individuals.
eFigure 2. The Shen atlas areas with functional connectivities related to sleep quality.
eFigure 3. Brain areas with functional connectivities related to both the sleep quality (PSQI) and the ASR DSM Depressive Problems scores from the HCP analysis.
eFigure 4. Brain areas with functional connectivities related to the ASR DSM Depressive Problems scores from the HCP analysis.
eFigure 5. Mediation analysis.
eTable 1. The anatomical regions defined in each hemisphere.
eTable 2. Functional connectivities related to the ASR DSM Depressive Problems scores from the HCP analysis using 1017 participants.
eTable 3. Functional connectivities correlated with the ASR DSM Depressive Problems scores from the HCP analysis based on the 92 participants who had at some time been diagnosed with depression.
eTable 4. The results of the mediation analysis performed for the 39 individual links that were correlated with sleep quality and the Depressive Problems score.
eTable 5. The demographic characteristics of participants of the Biobank dataset.
eReferences.
References
- 1.Becker NB, Jesus SN, João KADR, Viseu JN, Martins RIS. Depression and sleep quality in older adults: a meta-analysis. Psychol Health Med. 2017;22(8):889-895. doi: 10.1080/13548506.2016.1274042 [DOI] [PubMed] [Google Scholar]
- 2.Zaki NFW, Spence DW, BaHammam AS, Pandi-Perumal SR, Cardinali DP, Brown GM. Chronobiological theories of mood disorder. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):107-118. doi: 10.1007/s00406-017-0835-5 [DOI] [PubMed] [Google Scholar]
- 3.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 4.Dietch JR, Taylor DJ, Sethi K, Kelly K, Bramoweth AD, Roane BM. Psychometric evaluation of the PSQI in U.S. college students. J Clin Sleep Med. 2016;12(8):1121-1129. doi: 10.5664/jcsm.6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin JL, Fiorentino L, Jouldjian S, Josephson KR, Alessi CA. Sleep quality in residents of assisted living facilities: effect on quality of life, functional status, and depression. J Am Geriatr Soc. 2010;58(5):829-836. doi: 10.1111/j.1532-5415.2010.02815.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho HJ, Lavretsky H, Olmstead R, Levin MJ, Oxman MN, Irwin MR. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550. doi: 10.1176/appi.ajp.2008.07121882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory AM, Buysse DJ, Willis TA, et al. Associations between sleep quality and anxiety and depression symptoms in a sample of young adult twins and siblings. J Psychosom Res. 2011;71(4):250-255. doi: 10.1016/j.jpsychores.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 8.Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014;10:679-708. doi: 10.1146/annurev-clinpsy-032813-153716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunney BG, Li JZ, Walsh DM, et al. Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Mol Psychiatry. 2015;20(1):48-55. doi: 10.1038/mp.2014.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabbott PL, Rolls ET. Increased neuronal firing in resting and sleep in areas of the macaque medial prefrontal cortex. Eur J Neurosci. 2013;37(11):1737-1746. doi: 10.1111/ejn.12171 [DOI] [PubMed] [Google Scholar]
- 11.Drevets WC. Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci. 2007;1121:499-527. doi: 10.1196/annals.1401.029 [DOI] [PubMed] [Google Scholar]
- 12.Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiol Dis. 2013;52:4-11. doi: 10.1016/j.nbd.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotlib IH, Hammen CL, eds. Handbook of Depression. New York, NY: Guilford Press; 2009. [Google Scholar]
- 14.Matrix Executive Agency for Health and Consumers Economic analysis of workplace mental health promotion and mental disorder prevention programmes and of their potential contribution to EU health, social and economic policy objectives: specific request EAHC/2011/Health/19 for the implementation of framework contract EAHC/2010/Health/01 /Lot 2 2013. https://ec.europa.eu/health/sites/health/files/mental_health/docs/matrix_economic_analysis_mh_promotion_en.pdf. Published May 2013. Accessed June 11, 2018.
- 15.Deco G, Kringelbach ML. Great expectations: using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron. 2014;84(5):892-905. doi: 10.1016/j.neuron.2014.08.034 [DOI] [PubMed] [Google Scholar]
- 16.Rolls ET. A non-reward attractor theory of depression. Neurosci Biobehav Rev. 2016;68:47-58. doi: 10.1016/j.neubiorev.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 17.Cheng W, Rolls ET, Qiu J, et al. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain. 2016;139(pt 12):3296-3309. doi: 10.1093/brain/aww255 [DOI] [PubMed] [Google Scholar]
- 18.Colclough GL, Smith SM, Nichols TE, et al. The heritability of multi-modal connectivity in human brain activity. Elife. 2017;6:6. doi: 10.7554/eLife.20178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidaurre D, Abeysuriya R, Becker R, et al. Discovering dynamic brain networks from big data in rest and task [published online June 29, 2017]. Neuroimage. doi: 10.1016/j.neuroimage.2017.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SM, Nichols TE, Vidaurre D, et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18(11):1565-1567. doi: 10.1038/nn.4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro Schröder T, Haak KV, Zaragoza Jimenez NI, Beckmann CF, Doeller CF. Functional topography of the human entorhinal cortex. Elife. 2015;4:e06738. doi: 10.7554/eLife.06738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 2013;82:403-415. doi: 10.1016/j.neuroimage.2013.05.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg MD, Finn ES, Scheinost D, et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 2016;19(1):165-171. doi: 10.1038/nn.4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finn ES, Shen X, Scheinost D, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18(11):1664-1671. doi: 10.1038/nn.4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Achenbach System of Empirically Based Assessment Adult (Ages 18-59) Assessments. http://www.aseba.org/adults.html. Published 2018. Accessed June 14, 2018.
- 26.Winkler AM, Webster MA, Vidaurre D, Nichols TE, Smith SM. Multi-level block permutation. Neuroimage. 2015;123:253-268. doi: 10.1016/j.neuroimage.2015.05.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marquand AF, Haak KV, Beckmann CF. Functional corticostriatal connection topographies predict goal directed behaviour in humans. Nat Hum Behav. 2017;1(8):0146. doi: 10.1038/s41562-017-0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197-1207. doi: 10.1016/j.neuroimage.2010.06.041 [DOI] [PubMed] [Google Scholar]
- 29.Zalesky A, Fornito A, Seal ML, et al. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69(1):80-89. doi: 10.1016/j.biopsych.2010.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Wang J, Wu Q, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70(4):334-342. doi: 10.1016/j.biopsych.2011.05.018 [DOI] [PubMed] [Google Scholar]
- 31.Krienen FM, Yeo BT, Ge T, Buckner RL, Sherwood CC. Transcriptional profiles of supragranular-enriched genes associate with corticocortical network architecture in the human brain. Proc Natl Acad Sci U S A. 2016;113(4):E469-E478. doi: 10.1073/pnas.1510903113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preacher KJ, Kelley K. Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16(2):93-115. doi: 10.1037/a0022658 [DOI] [PubMed] [Google Scholar]
- 33.Elam J. Human Connectome Project. HCP Data Dictionary Public– Updated for the 1200 Subject Release. https://wiki.humanconnectome.org/display/PublicData/HCP+Data+Dictionary+Public-+Updated+for+the+1200+Subject+Release. Published May 15, 2018. Accessed June 14, 2018.
- 34.Rolls ET. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia. 2017;pii:S0028-3932(17)30347-0. doi: 10.1016/j.neuropsychologia.2017.1009.1021 [DOI] [PubMed] [Google Scholar]
- 35.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(pt 3):564-583. doi: 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- 36.Rolls ET. The roles of the orbitofrontal cortex via the habenula in non-reward and depression, and in the responses of serotonin and dopamine neurons. Neurosci Biobehav Rev. 2017;75:331-334. doi: 10.1016/j.neubiorev.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 37.Feffer K, Fettes P, Giacobbe P, Daskalakis ZJ, Blumberger DM, Downar J. 1Hz rTMS of the right orbitofrontal cortex for major depression: Safety, tolerability and clinical outcomes. Eur Neuropsychopharmacol. 2018;28(1):109-117. doi: 10.1016/j.euroneuro.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 38.Fossati P. Imaging autobiographical memory. Dialogues Clin Neurosci. 2013;15(4):487-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freton M, Lemogne C, Bergouignan L, Delaveau P, Lehéricy S, Fossati P. The eye of the self: precuneus volume and visual perspective during autobiographical memory retrieval. Brain Struct Funct. 2014;219(3):959-968. doi: 10.1007/s00429-013-0546-2 [DOI] [PubMed] [Google Scholar]
- 40.Delaveau P, Jabourian M, Lemogne C, et al. Antidepressant short-term and long-term brain effects during self-referential processing in major depression. Psychiatry Res Neuroimaging. 2016;247:17-24. doi: 10.1016/j.pscychresns.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 41.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15(2):56-67. doi: 10.1016/j.tics.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 42.Rolls ET. Emotion and Decision-Making Explained. Oxford, England: Oxford University Press; 2014. [Google Scholar]
- 43.Sundermann B, Olde Lütke Beverborg M, Pfleiderer B. Toward literature-based feature selection for diagnostic classification: a meta-analysis of resting-state fMRI in depression. Front Hum Neurosci. 2014;8:692. doi: 10.3389/fnhum.2014.00692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rolls ET. The Brain, Emotion, and Depression. Oxford, England: Oxford University Press; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Materials and Methods.
eFigure 1. The correlation between the PSQI total score and the other available sleep measures in the HCP dataset, based on the sample of 1017 individuals.
eFigure 2. The Shen atlas areas with functional connectivities related to sleep quality.
eFigure 3. Brain areas with functional connectivities related to both the sleep quality (PSQI) and the ASR DSM Depressive Problems scores from the HCP analysis.
eFigure 4. Brain areas with functional connectivities related to the ASR DSM Depressive Problems scores from the HCP analysis.
eFigure 5. Mediation analysis.
eTable 1. The anatomical regions defined in each hemisphere.
eTable 2. Functional connectivities related to the ASR DSM Depressive Problems scores from the HCP analysis using 1017 participants.
eTable 3. Functional connectivities correlated with the ASR DSM Depressive Problems scores from the HCP analysis based on the 92 participants who had at some time been diagnosed with depression.
eTable 4. The results of the mediation analysis performed for the 39 individual links that were correlated with sleep quality and the Depressive Problems score.
eTable 5. The demographic characteristics of participants of the Biobank dataset.
eReferences.