Key Points
Question
Is subclinical right ventricular dysfunction associated with heart failure risk among elderly persons?
Findings
In this secondary analysis of a community-based cohort study, subclinical right ventricular dysfunction assessed by 3-dimensional echocardiography was present in nearly one-fifth of elderly persons, was associated with common heart failure risk factors, and increased in prevalence with more advanced American College of Cardiology/American Heart Association heart failure stage. When identified, asymptomatic right ventricular dysfunction identified individuals at heightened risk for the development of heart failure hospitalization or death independent of left ventricular ejection fraction and N-terminal pro b-type natriuretic.
Meaning
These findings suggest an important and underrecognized role of right ventricular dysfunction in the progression to heart failure and the potential utility of 3-dimensional echocardiography to more accurately assess right ventricular performance.
Abstract
Importance
Limited data exist on the prevalence and prognostic importance of right ventricular (RV) dysfunction for heart failure (HF) in the general population.
Objective
To assess the prevalence of RV dysfunction and its association with HF and mortality in a community-based elderly cohort.
Design, Setting, and Participants
Cross-sectional and time-to-event analysis of participants in the Atherosclerosis Risks in the Community (ARIC), a multicenter, population-based cohort study at the fifth study visit from 2011 to 2013, with a median follow-up of 4.1 years. This study included 1004 elderly participants in the ARIC study attending the fifth study visit who underwent both 3-dimensional and 2-dimensional RV echocardiography. Three-dimensional echocardiography data were analyzed between September 15, 2015, and July 24, 2016.
Exposures
Right ventricular ejection fraction (RVEF), RV–pulmonary artery (PA) coupling defined by the RVEF/PA systolic pressure (PASP) ratio, and RV longitudinal strain by 3-dimensional echocardiography.
Main Outcomes and Measures
For cross-sectional analysis, the prevalence of RV dysfunction across ACCF/AHA HF stages (0; A, at elevated risk for HF but without structural heart disease or clinical HF; B, structural heart disease but without clinical HF; and C, prevalent HF). For time-to-event analysis, a composite of incident HF hospitalization or all-cause death among participants free of HF at visit 5.
Results
Of the 1004 participants, mean (SD) age was 76 (5) years, 385 were men (38%), and 121 were black (12%). Mean (SD) RVEF was 53% (8%). Right ventricular EF, RVEF/PASP, and RV longitudinal strain were each progressively lower across advancing HF stages. Using reference limits from stage 0 participants, RVEF was abnormal in 103 asymptomatic persons with stage A HF (15%) and 27 with stage B HF (24%). Among participants free of HF at baseline, lower RVEF and worse RV-PA coupling (ie, lower RVEF/PASP ratio) both were associated with incident HF or death independent of LVEF and N-terminal pro b-type natriuretic peptide (hazard ratio, 1.20; 95% CI, 1.02-1.42 per 5% decrease in RVEF; P = .03; hazard ratio, 1.65, 95% CI, 1.15-2.37 per 0.5 unit decrease in RVEF/PASP ratio; P = .007).
Conclusions and Relevance
Right ventricular function and RV-PA coupling declined progressively across American College of Cardiology Foundation/American Heart Association HF stages. Among persons free of HF, lower RVEF was associated with incident HF or death independent of LVEF or N-terminal pro b-type natriuretic peptide.
This study assesses the prevalence of right ventricular dysfunction and its association with heart failure and mortality in a community-based elderly cohort of participants in the Atherosclerosis Risks in the Community (ARIC) Study.
Introduction
Right ventricular (RV) dysfunction is prognostic of adverse outcomes independent of left ventricular (LV) dysfunction across a broad spectrum of cardiovascular diseases.1,2,3,4,5,6,7,8,9 Limited data exist regarding the prevalence and predictive value of RV dysfunction in the general population, particularly among elderly individuals, who are at a heightened risk for HF. Furthermore, the RV is highly afterload sensitive. Recognized age-associated increases in LV stiffness, LV filling pressure, and pulmonary pressure suggest that elderly individuals may be at particular risk for RV dysfunction owing to impaired RV–pulmonary artery (RV-PA) coupling.10 The RV has a complex 3-dimensional (3-D) shape, which makes accurate quantification of RV size and function challenging by 2-dimensional (2-D) echocardiography. Alternative approaches include cardiac magnetic resonance imaging (CMR),1 radionuclide ventriculography,2 or right heart catheterization,3,4 but are limited by cost, duration, radiation exposure, and invasiveness. Therefore, only limited community-based data are available regarding correlates and prognostic value of RV structure and function and RV-PA coupling.
Three-dimensional echocardiography allows for the direct quantification of RV volumes and RV ejection fraction (RVEF) without relying on geometric assumptions regarding RV shape. Right ventricular ejection fraction demonstrates good accuracy relative to CMR, as do RV volumes, particularly when not significantly enlarged.11,12,13,14 Professional guidelines now recommend the use of 3-D echocardiography in the assessment of RV disorders.15 We used 3-D echocardiography to determine the alterations in RV function across American College of Cardiology Foundation/American Heart Association (ACCF/AHA) HF stages and its prognostic relevance for incident HF or death among elderly participants in the community-based Atherosclerosis Risk in Communities (ARIC) study.16
Methods
Study Setting
The ARIC is a population-based cohort study from 4 US communities (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland). The design and methods have been previously described.16 In brief, 15 792 middle-aged participants were enrolled between 1987 and 1989, and 6538 participants returned between 2011 and 2013 for a fifth study visit, at which time 2-D and 3-D echocardiography were performed.17 This study included the 1225 participants attending the fifth study visit who underwent 3-D and 2-D echocardiography with previously quantified 3-D LV and left arterial (LA) deformation.18,19 Of these participants, 1004 (82%) had optimal quality 3-D RV data (eFigure 1 in the Supplement). The study protocol was approved by institutional review boards at each field center, and additional approval was obtained by the ARIC steering committee. All participants provided written informed consent.
2-D Echocardiography
Design and methods of echocardiography in ARIC at visit 5 have been previously described in detail, including data on interobserver variability.17,19 Pulmonary artery systolic pressure (PASP) was calculated from the peak tricuspid regurgitation velocity by continuous wave Doppler as PASP = 4 × (peak velocityTR2) + 5.20,21,22 Mean pulmonary artery pressure (PAP) was calculated based on the tricuspid regurgitation velocity (TRV)–time integral as previously validated.22,23 Pulmonary vascular resistance (PVR) was estimated as 10 × TRV/VTIRVOT.24 Right ventricular stroke volume (SV) was calculated as RV end-diastolic volume (EDV) – RV end-systolic volume (ESV). Pulmonary capillary wedge pressure (PCWP) was estimated as PCWP = 11.96 + 0.596 × (E/e′septal).10 Right ventricular function was assessed based on the RV fractional area change, calculated as (RV end-diastolic area – RV end-systolic area)/RV end-diastolic area and tissue Doppler-based tricuspid annular peak systolic velocity.25
3-D Echocardiography
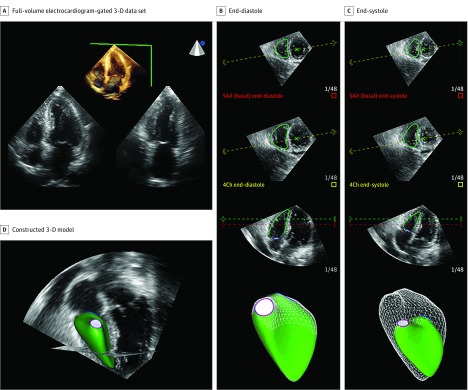
A full-volume echocardiography-gated 3-D data set was acquired from the apical position with a matrix-array 3-D transducer during breath hold with the highest frame rate achievable (20-26 Hz in our study), as previously described.19 This frame rate is in accordance with guideline recommendations,26 and higher than prior publications using this analysis software.11 Offline analysis was performed to measure RV volumes, RVEF, and RV freewall longitudinal strain (RVLS) using a semiautomatic algorithm (4-D RV-Function 2.0; TomTec Imaging Systems; Figure 1). The reproducibility and accuracy of this software when compared with CMR has been previously demonstrated.11
Figure 1. Representative Case From the Atherosclerosis Risk in Communities (ARIC) Study of Right Ventricular (RV) Quantification by 3-Dimensional (3-D) Echocardiography.
A single experienced cardiologist blinded to participant characteristics performed image quality assessment and subsequent analysis. Poor-quality data set was defined as significant acquisition artifacts (eg, stitch artifacts) or suboptimal endocardial definition. Quantitative measures were performed based on the recommendations of the American Society of Echocardiography.27 The RV-PA coupling was assessed as RVEF/PASP28,29 using PASP estimated by Doppler echocardiography and could be calculated in 728 participants owing to unmeasurable PASP in 276. Intraobserver variability of the 3-D echo-based RV measures was blindly assessed in 20 studies. The mean bias and coefficients of variation were RVEDV, −0.8 (95% CI, −4.2 to 2.6), 4.6%; RVESV, −1.38 (95% CI, −3.8 to 1.0), 8.2%; RVEF, 1.1 (95% CI, −1.5 to 3.7), 6.7%; and RVLS, −0.8 (−2.6 to 1.0), 8.0%.
Definitions of ACCF/AHA HF Stages
The ACCF/AHA HF stages were defined as follows: stage A (at elevated risk for HF but without structural heart disease or symptoms of HF), stage B (structural heart disease but without signs or symptoms of HF), and stage C (structural heart disease with earlier or current symptoms of HF).30,31,32 Stage 0 (low risk; free of HF risk factors or structural heart disease) was defined by the absence of criteria for stages A through C. Detailed definitions these stages in ARIC have been previously published30 and are provided in eTable 1 in the Supplement. Stage D was defined by therapy with an LV assist device or chronic intravenous inotropes. No participant was stage D.
Outcome
Incident HF after visit 5 was based on HF hospitalization or HF death according to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes (code 428 in any position) obtained by ARIC surveillance of hospital discharges.33 Deaths after visit 5 were ascertained by ARIC surveillance or the National Death Index.33
Statistical Methods
Clinical characteristics and 2-D/3-D echocardiographic measures were described by quartile of 3-D RVEF. Reference limits for 3-D measures were determined among stage 0 participants (low-risk; free of HF risk factors or structural heart disease) using quantile regression to define median, 10th, and 90th percentile limits with associated 95% confidence limits. Differences in 3-D RV measures and the prevalence of abnormal values between categories were assessed using multivariable linear and logistic regression models adjusting for age, sex, and race/ethnicity. The association between measures of RV function and a composite of HF hospitalization or all-cause death among participants free of prevalent HF at visit 5 was assessed using multivariable Cox regression models adjusted for age, sex, race/ethnicity, LVEF, and NT-proBNP. To assess the effect of potential selection bias related to unavailable 3-D RV measures owing to either unanalyzed or unanalyzable 3-D data or visit 5 nonattendance, we performed a sensitivity analysis using inverse probability of attrition weighting (IPAW) as detailed in eTables 2-5 in the Supplement.34,35 A 2-sided P value of less than .05 was considered statistically significant. All analyses were performed with Stata, version 15 (StataCorp).
Results
The mean (SD) age of the 1004 study participants was 76 (5) years, 619 were women (62%), and 121 were black (12%). Two-dimensional echocardiography-based measures of RV size and function did not differ appreciably between participants at visit 5 included vs not included in this 3-D analysis (eTable 2 in the Supplement). Mean (SD) values in 3-D RVEF and RVLS were 53.2% (8.2%) and −27.1% (8.2%), respectively (eFigure 2 in the Supplement). Worse 3-D RVEF was associated with male sex, higher body mass index, higher prevalence of cardiovascular diseases including coronary disease, stroke, and atrial fibrillation, and higher high-sensitivity troponin-T concentrations (Table). Right ventricular ejection fraction was significantly, but modestly, associated with 2-D echo-based measures of RV function including RV fractional area change (r = 0.23; P < .001) and tricuspid annular s′ (r = 0.14; P < .001).
Table. Clinical Characteristics by Quartile of RVEF.
| Characteristic | No. (%) | P Value for Trend | ||||
|---|---|---|---|---|---|---|
| Overall RVEF, 25.7%-76.5% (n = 1004) | Q1, 25.7%-48.1% (n = 251) | Q2, 48.1%-53.9% (n = 251) | Q3, 54.0%-58.7% (n = 251) | Q4, 58.7%-76.5% (n = 251) | ||
| Age, mean (SD), y | 76 (5) | 77 (5) | 76 (5) | 76 (5) | 76 (5) | .18 |
| Male | 385 (38) | 137 (55) | 104 (41) | 81 (32) | 63 (25) | <.001a |
| Black | 121 (12) | 35 (14) | 28 (11) | 30 (12) | 28 (11) | .75 |
| Field center | ||||||
| Forsyth County | 469 (47) | 121 (48) | 119 (47) | 114 (45) | 115 (46) | >.99 |
| Jackson | 88 (9) | 25 (10) | 21 (8) | 20 (8) | 22 (9) | |
| Minneapolis | 211 (21) | 49 (20) | 53 (21) | 56 (22) | 53 (21) | |
| Washington County | 236 (24) | 56 (22) | 58 (23) | 61 (24) | 61 (24) | |
| HF risk factors | ||||||
| Hypertension | 778 (78) | 205 (82) | 192 (77) | 188 (75) | 193 (77) | .30 |
| Diabetes | 316 (32) | 84 (34) | 76 (30) | 80 (32) | 76 (30) | .85 |
| Obesity | 235 (23) | 71 (28) | 52 (21) | 65 (26) | 47 (19) | .04a |
| Metabolic syndrome | 545 (55) | 151 (60) | 129 (51) | 140 (57) | 125 (50) | .07a |
| CKD | 221 (22) | 64 (26) | 55 (22) | 46 (19) | 56 (22) | .31 |
| Ever smoker | 617 (62) | 165 (66) | 150 (60) | 146 (58) | 156 (62) | .33 |
| Current smoking | 15 (6) | 17 (7) | 16 (7) | 11 (5) | 17 (7) | .62 |
| Prevalent CVD | ||||||
| CAD | 126 (13) | 48 (19) | 25 (10) | 24 (10) | 29 (12) | .003 |
| Prior MI | 66 (7) | 29 (12) | 13 (6) | 16 (7) | 8 (4) | .001a |
| PAD | 29 (4) | 7 (5) | 6 (3) | 8 (5) | 8 (5) | .93 |
| Stroke | 33 (3) | 15 (6) | 13 (5) | 3 (1) | 2 (1) | <.001a |
| Atrial fibrillation | 71 (7) | 31 (13) | 18 (7) | 9 (4) | 13 (5) | <.001a |
| Physical examination, mean (SD) | ||||||
| BMI | 27.1 (5.0) | 27.7 (4.4) | 27.0 (5.4) | 27.4 (5.5) | 26.4 (4.6) | .02a |
| Systolic BP, mm Hg | 131 (18) | 132 (18) | 130 (18) | 130 (18) | 133 (19) | .27 |
| Diastolic BP, mm Hg | 66 (11) | 67 (11) | 66 (10) | 66 (11) | 65 (11) | .59 |
| HR, bpm | 61 (10) | 61 (11) | 62 (9) | 61 (10) | 61 (9) | .75 |
| Laboratory values, mean (SD) | ||||||
| HbA1c, % | 5.9 (0.8) | 6.0 (0.9) | 5.8 (0.7) | 5.9 (0.9) | 5.8 (0.7) | .05 |
| eGFR, mL/min per 1.73 m2 | 69.0 (16.4) | 67.8 (17.4) | 69.6 (16.7) | 69.2 (14.8) | 69.5 (16.6) | .60 |
| LDL cholesterol, mg/dL | 104 (33) | 97 (31) | 106 (33) | 107 (34) | 107 (35) | .002 |
| HDL cholesterol, mg/dL | 53 (14) | 51 (14) | 54 (13) | 53 (14) | 56 (14) | <.001a |
| hsCRP | 1.9 (0.9 to 3.7) | 2.2 (1.1 to 4.1) | 1.8 (0.8 to 3.4) | 1.7 (0.8 to 3.4) | 1.7 (0.9 to 3.9) | .06 |
| Cardiac biomarkers | ||||||
| NT-proBNP, pg/mL | 135 (72 to 244) | 149 (70 to 293) | 122 (60 to 211) | 137 (78 to 219) | 134 (78 to 231) | .09 |
| hs-TnT, ng/mL | 0.009 (0.006 to 0.014) | 0.010 (0.007 to 0.018) | 0.009 (0.007 to 0.014) | 0.008 (0.006 to 0.013) | 0.009 (0.006 to 0.013) | <.001a |
| Left ventricular structure, mean (SD) | ||||||
| EDV, mL | 82.8 (24.2) | 90.0 (26.3) | 81.8 (22.8) | 81.2 (21.7) | 78.4 (24.5) | <.001 |
| ESV, mL | 28.3 (11.7) | 32.7 (14.0) | 28.0 (10.3) | 26.9 (9.5) | 25.6 (11.3) | <.001a,b |
| MWT, cm | 0.98 (0.13) | 1.02 (0.14) | 0.97 (0.12) | 0.97 (0.12) | 0.95 (0.12) | <.001a,b |
| LV mass, g | 144.2 (43.0) | 161.0 (48.3) | 142.5 (38.5) | 139.9 (37.6) | 133.5 (42.2) | <.001a,b |
| LV mass index, g/m2 | 79.3 (19.5) | 85.6 (21.5) | 78.2 (17.3) | 77.6 (18.4) | 75.6 (19.1) | <.001a,b |
| LV systolic function, mean (SD) | ||||||
| EF, % | 66.5 (5.8) | 64.5 (6.3) | 66.2 (5.7) | 67.2 (5.2) | 68.0 (5.2) | <.001a,b |
| GLS, % | −18.1 (2.4) | −17.3 (2.5) | −18.1 (2.3) | −18.3 (2.2) | −18.5 (2.4) | <.001a,b |
| Twist, degreec | 12.8 (4.0) | 12.3 (3.9) | 12.2 (3.8) | 13.1 (4.2) | 13.3 (4.0) | <.001a,b |
| Torsion, degree/cmc | 1.7 (0.6) | 1.6 (0.6) | 1.6 (0.6) | 1.8 (0.6) | 1.8 (0.6) | <.001a,b |
| LV diastolic function, mean (SD) | ||||||
| E wave | 70.2 (18.3) | 68.5 (20.4) | 69.5 (18.3) | 69.4 (17.8 | 73.6 (16.3) | .009a |
| e’ septal, cm/s | 5.8 (1.5) | 5.5 (1.5) | 5.8 (1.3) | 5.8 (1.5) | 5.9 (1.4) | .009a,b |
| E/e’ septal | 12.8 (4.5) | 13.2 (4.8) | 12.5 (4.6) | 12.6 (4.6) | 13.1 (3.9) | .27 |
| LA volume index, mL/m2 | 26.9 (8.8) | 29.5 (11.5) | 26.2 (7.1) | 26.2 (7.3) | 25.8 (8.0) | <.001a,b |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; bpm, beats per minute; CAD, coronary artery disease; CKD, chronic kidney disease; CVD, cardiovascular disease; e′, mitral early relaxation velocity; EDV, end-diastolic volume; E/e’, mitral inflow to mitral relaxation velocity ratio; EF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; ESV, end-systolic volume; GLS, global longitudinal strain; HbA1c, hemoglobin A1C; HDL, high-density lipoprotein; HF, heart failure; HR, heart rate; hsCRP, high-sensitivity C-reactive protein; hs-TnT, high-sensitivity troponin T; LA; left arterial, LDL, low-density lipoprotein; LV, left ventricular; MI, myocardial infraction; MWT, mean wall thickness; NT-proBNP, N-terminal pro b-type natriuretic peptide; PAD, peripheral artery disease; PVR pulmonary vascular resistance; Q, quartile; RVEF, right ventricular ejection fraction.
SI conversion factors: To convert HbA1C to proportion of total hemoglobin, multiply by 0.01; HDL cholesterol to millimoles per liter, multiply by 0.0259; hsCRP to nanomoles per liter, multiply by 9.524; hs-TnT to micrograms per liter, multiply by 1; LDL cholesterol to millimoles per liter, multiply by 0.0259; NT-proBNP to nanograms per liter, multiply by 1.
P < .05 from multivariable regression (linear or logistic as appropriate) after adjustment for age, sex, and race/ethnicity. P value for trend was tested with analysis of variance or Kruskal-Wallis as appropriate.
P < .05 for multivariable linear regression after adjustment for age, sex, race/ethnicity, and PVR.
Data from 3-dimensional echocardiography.
References limits for 3-D measures, determined based on the 10th percentile values from quantile regression in participants with stage 0 HF (n = 83; mean age, 74 years [range, 67-88 years], 60 women [72%], rate of death or HF, 0.3/100 person-year; 95% CI, 0.1-0.9) were as follows: RVEF, 45.4% (95% CI, 42.1% to 49.0%); RLVS, −21.9% (95% CI, −24.8% to −19.0%); RV end-diastolic volume, 50.0 mL/m2 (95% CI, 43.9 mL/m2 to 56.2 mL/m2); and RV end-systolic volume, 24.1 mL/m2 (20.1 mL/m2 to 28.2 mL/m2). Right ventricular ejection fraction was abnormal in 173 (17%) of the total study sample and RVLS was abnormal in 198 (20%) (eFigure 2 in the Supplement).
Physiologic Associations With RVEF
Mean (SD) PAP was 15.2 (3.4) mm Hg (>25 mm Hg in 0.4%) and mean (SD) PVR was 1.75 (0.41) WU (>2 WU in 15.4%). Higher PVR was significantly, although modestly, associated with lower RVEF, even after adjustment for PCWP (−3.18; 95% CI, −4.65 to −1.72; P < .001; eFigure 3A in the Supplement). No significant linear or nonlinear (data not shown) association was noted between RVEF and MPAP (eFigure 3B in the Supplement). The RVEF was directly associated with RV SV (R = 0.31, P < .001). Similar associations were noted for RVLS (eFigure 4 in the Supplement).
Lower RVEF was associated with greater LV mass, wall thickness, LA size, and lower TDI e′, even after adjusting for age, sex, race/ethnicity, and PVR (Table). Lower RVEF was also associated with larger LVESV and lower LVEF, longitudinal strain, twist, and torsion (Table). Right ventricular LS demonstrated similar associations with LV structure and function (Figure 2B; eFigure 4 in the Supplement). Concordant findings were observed in IPAW analysis (eTable 3 in the Supplement).
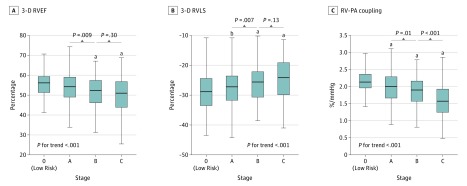
Figure 2. Mean Values of 3-Dimensional (3-D) Right Ventricular Ejection Fraction (RVEF), 3-D RV Longitudinal Strain (RVLS), and RVEF/Pulmonary Artery Systolic Pressure Ratio as a Measure of Right Ventricular–Pulmonary Artery (RV-PA) Coupling Across Heart Failure (HF) Stages.
Tests for trend across HF stages were performed using multivariable linear regression models adjusting for age, sex, and race/ethnicity.
aP <.05 for unadjusted and adjusted with age, sex and race/ethnicity (reference = stage 0).
bP <.05 for unadjusted.
RV Structure and Function Across HF Stages
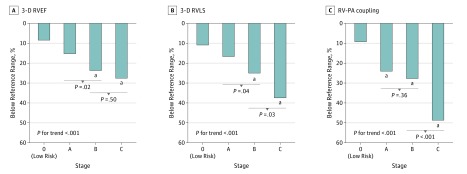
Eighty-three participants (8%) were classified as stage 0, 676 (67%) were stage A, 114 (11%) were stage B, and 131 (13%) were stage C. Across HF stages, significant trends were observed for higher RV volumes, lower RVEF, and worse RVLS (Figure 2; eFigure 5 in the Supplement), with progressive decrements noted in stage A and stage B HF. Right ventricular–PA coupling, assessed as the RVEF/PASP ratio, also declined across HF stages consistent with worse RV systolic function accounting for RV afterload. The highest prevalence of RV dysfunction was in stage C, among whom RVEF was abnormally low in 28 (25%) and RVLS was impaired in 49 (37%) (Figure 3). Similar findings were observed in sensitivity analyses incorporating inverse probability weights (eTable 4 in the Supplement).
Figure 3. Prevalence of Abnormal 3-Dimensional (3-D) Right Ventricular Ejection Fraction (RVEF), 3-D RV Longitudinal Strain (RVLS), and RVEF/Pulmonary Artery Systolic Pressure Ratio as a Measure of Right Ventricular–Pulmonary Artery (RV-PA) Coupling Across Heart Failure (HF) Stages.
Abnormal limits were based on the 90th or 10th percentile limits derived from the stage 0 participants. Test for trend of the proportion with abnormal measures across HF stages was performed using multivariable logistic regression models adjusting for age, sex, and race/ethnicity.
aP <.05 for unadjusted and adjusted with age, sex and race/ethnicity (reference = stage 0).
Association of RV Function With All-Cause Mortality or Incident HF
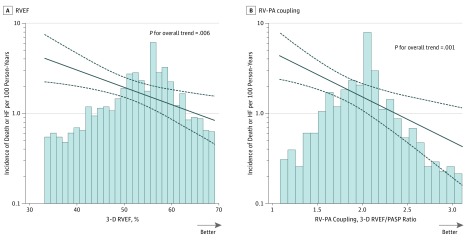
Of the 847 participants free of prevalent HF at visit 5, 59 (7.0%) experienced the composite outcome of incident HF hospitalization or all-cause mortality during a median follow-up of 4.1 years (25th and 75th percentile limits, 3.8-4.3 years). Lower RVEF at visit 5 was significantly and linearly associated with a higher event rate during follow-up (Figure 4A). After adjustment for age, sex, race/ethnicity, LVEF, and NT-proBNP, each 5% decrease in RVEF was associated with a 20% increase in the hazard for death or HF hospitalization (adjusted HR per 5% decrease in RVEF, 1.20; 95% CI, 1.02-1.42; P = .03; Figure 4A). This association remained significant after further adjusting for left atrial volume index (HR, 1.20; 95% CI, 1.02-1.42; P = .03) or E/e′ (HR, 1.18; 95% CI, 1.00-1.40; P = .05). Lower RVEF/PASP ratio, reflecting worse RV-PA coupling, was also associated with a higher risk of death or incident HF (demographic, LVEF, and NT-proBNP–adjusted HR per 0.5 unit decrease, 1.58; 95% CI, 1.10-2.28; P = .01; Figure 4B), which persisted after further adjustment for left atrial volume index (HR, 1.59; 95% CI, 1.10-2.30; P = .01) or E/e′ (HR, 1.58; 95% CI, 1.09-2.29; P = .02). Similar findings were observed in IPAW analyses (eTable 5 in the Supplement). Right ventricular LS was not significantly associated with risk of incident HF or death (HR per 1% decrease, 1.03; 95% CI, 0.99-1.07; P = .14 adjusting for age, sex, and race/ethnicity). Furthermore, no significant association with death or incident HF was noted for RV fractional area change (HR per 1% decrease, 1.02; 95% CI, 0.99-1.06; P = .22) or tricuspid annular s′ (HR per 1 cm/s decrease, 1.07; 95% CI, 0.96-1.19; P = .23) after adjustment for age, sex, race/ethnicity, and LVEF.
Figure 4. Association of Better Values for Right Ventricular Ejection Fraction (RVEF) and RV–Pulmonary Artery (PA) Coupling With Lower Incidence of All-Cause Mortality or Incident Heart Failure Hospitalization.
Solid line indicates incidence rate per 100 person-years at any given value of RVEF or RV-PA coupling. Dashed lines indicate the 95% confidence intervals for these incidence rates. Histograms show the population distribution of RVEF (A) and RV-PA coupling (B). The RV-PA coupling is assessed as the RVEF/PA systolic pressure ratio. After adjustment for age, sex, race/ethnicity, left ventricular ejection fraction, and N-terminal pro b-type natriuretic, the hazard ratio (HR) for all-cause mortality or incident heart failure per 5% decrease in RVEF was 1.20 (95% CI, 1.02-1.42; P = .03). The adjusted HR per 0.5 unit decrease in RVEF/PASP ratio was 1.58 (95% CI, 1.10-2.28; P = .01).
Discussion
In what is, to our knowledge, one of largest studies to quantify RV function (RVEF and RVLS) and RV-PA coupling (RVEF/PASP ratio) in a community-based sample, our study provides important, novel insights into the potential predictors of RV dysfunction and its association with HF risk. We report 3 novel findings: first, RV systolic function and RV-PA coupling decline progressively across ACCF/AHA HF stages, and RV function is impaired in nearly one-fourth of asymptomatic persons with stage B HF. Second, while RVEF is inversely associated with RV afterload, it is also associated directly with LV function independent of pulmonary vascular measures, suggesting parallel alterations in biventricular function. Third, among persons free of HF, both worse RVEF and worse RV-PA coupling are linearly associated with heighted risk of incident HF or death independent of LVEF, NT-proBNP, and measures of LV filling pressure. These associations were not observed for RV function assessed by RV fractional area change and tricuspid annular s′. These data suggest an important and underrecognized role of RV dysfunction in the progression to HF and suggest the potential utility of 3-D echocardiography to more accurately assess RV performance.
Previous studies of RV function have typically focused on patients with established cardiovascular disease, including HF,2,3,4,5,6 myocardial infarction,1 or pulmonary arterial hypertension.7,8,9 The relatively few studies to assess the RV in general population samples have generally used 2-D echocardiography,10,36 which is of limited accuracy and precision. Three-dimensional echocardiography uniquely allows for the acquisition of a 3-D volumetric data set from which RV volumes and EF can be quantified without geometric assumptions.11,12,13,14 However, 3-D echocardiographic RV assessment in previous general population studies has focused primarily on normal values for 3-D RVEF and volumes in younger healthy samples (typically aged 45-48 years),37,38 with scarce data on RV performance in elderly individuals and its association with HF risk. Among our large community-based elderly sample, we identified 83 participants free of cardiovascular disease or risk factors. Reference limits for RVEF (>45.5%) and RVLS (<21.9%) derived from this subgroup using quantile regression were consisted with a previous study in a healthy sample (n = 37, age >70 years)37 and with professional guidelines (RVEF <45%; GLS <20%).15
The RV is known to be particularly afterload sensitive, such that RVEF is inversely associated with MPAP or PVR in conditions such as pulmonary arterial hypertension or advanced HF with reduced LVEF.4,39 In our study, lower RVEF was associated with higher RV afterload as assessed by PVR and also with lower RV stroke volume, possibly explaining its lack of association with flow-dependent measures of afterload such as PA pressure. Lower RVEF and RVLS were also associated with worse LV systolic function (higher LV end-systolic volume, lower LVEF, and worse GLS) and LV diastolic function (larger LA size and lower e′). Importantly, these associations remained significant after accounting for pulmonary vascular measures, suggesting that greater RV afterload secondary to elevated LV filling pressure does not fully account for these associations. This is consistent with prior studies that identify RV dysfunction in early stage hypertension40 and obesity41 in absence of pulmonary hypertension, that demonstrate coupled impairments of RV and LV function independent of PAP in LV pressure overload42 and atrial fibrillation,43 and that suggest an important role for the interventricular septum40 and LV free wall44 in RV systolic function. Furthermore, we observed progressive decline in RV-PA coupling across HF stages, suggesting worsening RV contractility at any given afterload at greater HF stages, consistent with data suggesting higher afterload sensitivity of the RV in HFpEF.45 Together, these findings suggest parallel effects on biventricular function independent of the pulmonary vasculature.
The ACCF/AHA construct of HF stages was introduced to emphasize the progressive nature of HF, whereby risk factors promote detrimental alterations in cardiac structure and function, which in turn underlie clinical symptomatic HF. While the LV has been the primary focus for these cardiac alterations, we demonstrate that RV size increases and RV function, as reflected in RVEF, RVLS, and RV-PA coupling, decreases across HF stages. The prevalence of impaired RVEF increased by nearly 3-fold from stage 0 to stage B HF (asymptomatic stages) and was abnormal in one-fourth of stage B participants. We also demonstrate that reductions in RVEF in these asymptomatic stages (0 through B) are prognostically relevant, with each 5% decrease in RVEF associated with a 20% increase risk of death or HF hospitalization independent of demographics, LVEF, and NT-proBNP. Impaired RV-PA coupling also decreased with more advanced HF stage, was impaired in nearly one-half of stage C participants, and, among HF-free participants, was independently associated with incident HF or death. As PA pressure also increases across HF stages and is associated with incident HF, these findings support the importance of primary RV dysfunction, accounting for afterload, for HF.
By demonstrating the prognostic importance of alterations in RV function in a population-based sample, our findings extend on CMR-based data from the Multi-Ethnic Study of Atherosclerosis showing the association of greater RV mass with increased risk of HF or cardiovascular death.46 A 2017 study of 446 patients47 with prevalent cardiovascular disease (ischemic and valvular heart disease, cardiomyopathy, and pulmonary hypertension) demonstrated an association of lower RVEF by 3-D echocardiography with the composite of cardiac death, nonfatal myocardial infarction, ventricular fibrillation, and HF hospitalization.47 We now extend the prognostic relevance of RVEF to a community-based sample, largely free of prevalent cardiovascular disease, and focus on HF as a particularly relevant end point.
Limitations
Several limitations of this analysis should be noted. Of ARIC participants alive at the fifth visit, 6538 (64%) attended, which may have introduced ascertainment bias. Quantitative 3-D echocardiographic data on the RV were available in a relatively small proportion of participants attending visit 5, which may result in further selection bias. However, sensitivity analysis by IPAW provided consistent findings (eTable 2-5 in the Supplement), suggesting that the influence of bias on our findings is small, and among visit 5 attendees, RV size and function assessed by 2-D echocardiography did not differ clinically between those included vs not included in this 3-D analysis (eTable 2 in the Supplement). Second, we were not able to provide reference limits based on sex owing to the small number of men in stage 0. However, our use of indexed values for volumes should have minimized expected sex-based differences, and our reference values generally align with those of prior studies and guidelines.15 Third, M-mode of the tricuspid annular was not acquired in ARIC at visit 5, and therefore we were not able to evaluate the association of the tricuspid annular plane systolic excursion (TAPSE) or the TAPSE/PASP ratio with HF stages or incident HF or death. The TAPSE/PASP ratio is one of the most commonly used echocardiographic measures of RV-PA coupling, and we are unable to evaluate its performance relative to 3-D echocardiography-based RVEF/PASP ratio. However, TAPSE was initially validated against RVEF,48 and the RVEF/PASP ratio has previously been used as a measure of RV-PA coupling.28,29 Follow-up time after echocardiography at visit 5 was relatively short (median, 4.1 years) and the number of events relatively small (n = 59), which limited our power to assess the incremental prognostic value of 3-D echocardiographic measures of RV function over conventional echocardiographic measures. We were also unable to adjust for comorbidities, such as obesity, coronary disease, and atrial fibrillation, that were associated with RV functional measures and are established risk factors for HF. We therefore could not determine the prognostic relevance of RV dysfunction independent of these comorbidities, although RV dysfunction itself may be one means by which these comorbidities increase HF risk. Three-dimensional echocardiography-based RVLS did not demonstrate clear advantages vs 3-D RVEF in this study. However, the relatively low frame rates for 3-D images (20-26 Hz) compared with those for 2-D echocardiography (50-80 Hz) may have resulted in reduced sensitivity and/or precision of 3-D–based RVLS. While our data are not sufficient to demonstrate superiority of 3-D compared with 2-D echocardiography-based RV assessments to predict HF, they support further studies evaluating the utility of 3-D echocardiography to detect RV dysfunction and risk for HF.
Conclusions
In a large, community-based cohort of older persons, RV function and RV-PA coupling declined progressively across ACCF/AHA HF stages, with a nearly 3-fold increase in prevalence RV dysfunction from stage 0 to stage B HF, and is impaired in more than one-fourth of asymptomatic persons with stage B HF. Among persons free of HF, lower RVEF and lower RVEF/PASP ratio are linearly associated with a heighted risk of incident HF or death independent of LVEF and NT-proBNP. These data suggest an important and underrecognized role of RV dysfunction in the progression to HF and suggest the potential utility of 3-D echocardiography to more accurately assess RV performance.
eTable 1. Definitions of ACCF/AHA HF Stages and Criteria
eTable 2. Participant Characteristics at Visit 5 by Measurable 3-D RVEF
eTable 3. Inverse Probably Attrition Weighted Estimates (With 95% Confidence Intervals) of Measures of Cardiac Structure and Function by 3-D RVEF at ARIC Visit 5
eTable 4. Inverse Probably Attrition Weighted Estimates
eTable 5. Inverse Probably Attrition Weighted Estimates of Hazard Ratios of 3-D RVEF and RV-PA Coupling
eFigure 1. Study Diagram
eFigure 2. Histogram and Descriptive Statistics for 3-D RVEF and RVLS
eFigure 3. The Relationship of 3-D RVEF With PVR, Mean PA Pressure, LVEF, and LVGLS
eFigure 4. Association of RVLS With PVR, Mean PAP, LVEF and LVGLS
eFigure 5. Mean values of 3-D RV EDVI and ESVI and the Prevalence of Abnormal (based on the 90th or 10th Percentile Limits Derived From the Stage 0 overall) Across HF Stages
eReferences
Reference
- 1.Larose E, Ganz P, Reynolds HG, et al. Right ventricular dysfunction assessed by cardiovascular magnetic resonance imaging predicts poor prognosis late after myocardial infarction. J Am Coll Cardiol. 2007;49(8):855-862. [DOI] [PubMed] [Google Scholar]
- 2.Meyer P, Filippatos GS, Ahmed MI, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121(2):252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juillière Y, Barbier G, Feldmann L, Grentzinger A, Danchin N, Cherrier F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18(2):276-280. [DOI] [PubMed] [Google Scholar]
- 4.de Groote P, Millaire A, Foucher-Hossein C, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32(4):948-954. [DOI] [PubMed] [Google Scholar]
- 5.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35(48):3452-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammed SF, Hussain I, AbouEzzeddine OF, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130(25):2310-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Archer SL, Badesch DB, et al. ; ACCF/AHA . ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc, and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294. [DOI] [PubMed] [Google Scholar]
- 8.Eysmann SB, Palevsky HI, Reichek N, Hackney K, Douglas PS. Two-dimensional and Doppler-echocardiographic and cardiac catheterization correlates of survival in primary pulmonary hypertension. Circulation. 1989;80(2):353-360. [DOI] [PubMed] [Google Scholar]
- 9.Ghio S, Klersy C, Magrini G, et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140(3):272-278. [DOI] [PubMed] [Google Scholar]
- 10.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119(20):2663-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medvedofsky D, Addetia K, Patel AR, et al. Novel approach to three-dimensional echocardiographic quantification of right ventricular volumes and function from focused views. J Am Soc Echocardiogr. 2015;28(10):1222-1231. [DOI] [PubMed] [Google Scholar]
- 12.van der Zwaan HB, Geleijnse ML, McGhie JS, et al. Right ventricular quantification in clinical practice: two-dimensional vs. three-dimensional echocardiography compared with cardiac magnetic resonance imaging. Eur J Echocardiogr. 2011;12(9):656-664. [DOI] [PubMed] [Google Scholar]
- 13.Nesser HJ, Tkalec W, Patel AR, et al. Quantitation of right ventricular volumes and ejection fraction by three-dimensional echocardiography in patients: comparison with magnetic resonance imaging and radionuclide ventriculography. Echocardiography. 2006;23(8):666-680. [DOI] [PubMed] [Google Scholar]
- 14.Leibundgut G, Rohner A, Grize L, et al. Dynamic assessment of right ventricular volumes and function by real-time three-dimensional echocardiography: a comparison study with magnetic resonance imaging in 100 adult patients. J Am Soc Echocardiogr. 2010;23(2):116-126. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233-270. [DOI] [PubMed] [Google Scholar]
- 16.The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. [PubMed] [Google Scholar]
- 17.Shah AM, Cheng S, Skali H, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging. 2014;7(1):173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonçalves A, Hung CL, Claggett B, et al. Left atrial structure and function across the spectrum of cardiovascular risk in the elderly: the atherosclerosis risk in communities study. Circ Cardiovasc Imaging. 2016;9(2):e004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung C-L, Gonçalves A, Shah AM, Cheng S, Kitzman D, Solomon SD. Age- and sex-related influences on left ventricular mechanics in elderly individuals free of prevalent heart failure: the ARIC study (Atherosclerosis Risk in Communities). Circ Cardiovasc Imaging. 2017;10(1):e004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70(4):657-662. [DOI] [PubMed] [Google Scholar]
- 21.Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6(4):750-756. [DOI] [PubMed] [Google Scholar]
- 22.van Riel AC, Opotowsky AR, Santos M, et al. Accuracy of echocardiography to estimate pulmonary artery pressures with exercise: a simultaneous invasive-noninvasive comparison. Circ Cardiovasc Imaging. 2017;10(4):e005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aduen JF, Castello R, Lozano MM, et al. An alternative echocardiographic method to estimate mean pulmonary artery pressure: diagnostic and clinical implications. J Am Soc Echocardiogr. 2009;22(7):814-819. [DOI] [PubMed] [Google Scholar]
- 24.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41(6):1021-1027. [DOI] [PubMed] [Google Scholar]
- 25.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685-713. [DOI] [PubMed] [Google Scholar]
- 26.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14. [DOI] [PubMed] [Google Scholar]
- 27.Picard MH, Adams D, Bierig SM, et al. ; American Society of Echocardiography . American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr. 2011;24(1):1-10. [DOI] [PubMed] [Google Scholar]
- 28.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58(24):2511-2519. [DOI] [PubMed] [Google Scholar]
- 29.Kubba S, Davila CD, Forfia PR. Methods for evaluating right ventricular function and ventricular-arterial coupling. Prog Cardiovasc Dis. 2016;59(1):42-51. [DOI] [PubMed] [Google Scholar]
- 30.Shah AM, Claggett B, Loehr LR, et al. Heart failure stages among older adults in the community: the Atherosclerosis Risk in Communities Study. Circulation. 2017;135(3):224-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yancy CW, Jessup M, Bozkurt B, et al. ; WRITING COMMITTEE MEMBERS; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240-e327. [DOI] [PubMed] [Google Scholar]
- 32.Ponikowski P, Voors AA, Anker SD, et al. ; ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-2200. [DOI] [PubMed] [Google Scholar]
- 33.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101(7):1016-1022. [DOI] [PubMed] [Google Scholar]
- 34.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23(1):119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottesman RF, Rawlings AM, Sharrett AR, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol. 2014;179(8):956-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindqvist P, Waldenström A, Henein M, Mörner S, Kazzam E. Regional and global right ventricular function in healthy individuals aged 20-90 years: a pulsed Doppler tissue imaging study: Umeå General Population Heart Study. Echocardiography. 2005;22(4):305-314. [DOI] [PubMed] [Google Scholar]
- 37.Maffessanti F, Muraru D, Esposito R, et al. Age-, body size-, and sex-specific reference values for right ventricular volumes and ejection fraction by three-dimensional echocardiography: a multicenter echocardiographic study in 507 healthy volunteers. Circ Cardiovasc Imaging. 2013;6(5):700-710. [DOI] [PubMed] [Google Scholar]
- 38.Tamborini G, Marsan NA, Gripari P, et al. Reference values for right ventricular volumes and ejection fraction with real-time three-dimensional echocardiography: evaluation in a large series of normal subjects. J Am Soc Echocardiogr. 2010;23(2):109-115. [DOI] [PubMed] [Google Scholar]
- 39.Nienaber CA, Spielmann RP, Wasmus G, Montz R, Mathey DG, Bleifeld W. Right ventricular ejection fraction from equilibrium krypton-81m blood pool scans: a noninvasive predictor of pulmonary arterial hypertension. Eur Heart J. 1987;8(3):297-307. [DOI] [PubMed] [Google Scholar]
- 40.Pedrinelli R, Canale ML, Giannini C, et al. Right ventricular dysfunction in early systemic hypertension: a tissue Doppler imaging study in patients with high-normal and mildly increased arterial blood pressure. J Hypertens. 2010;28(3):615-621. [DOI] [PubMed] [Google Scholar]
- 41.McCabe C, Oliveira RKF, Rahaghi F, et al. Right ventriculo-arterial uncoupling and impaired contractile reserve in obese patients with unexplained exercise intolerance. Eur J Appl Physiol. 2018;118(7):1415-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottdiener JS, Gay JA, Maron BJ, Fletcher RD. Increased right ventricular wall thickness in left ventricular pressure overload: echocardiographic determination of hypertrophic response of the “nonstressed” ventricle. J Am Coll Cardiol. 1985;6(3):550-555. [DOI] [PubMed] [Google Scholar]
- 43.Cohen S, Gaddam S, Gemignani A, Wu WC, Sharma S, Choudhary G. Right ventricular function relates to functional capacity in men with atrial fibrillation and preserved left ventricular ejection fraction. Echocardiography. 2013;30(5):542-550. [DOI] [PubMed] [Google Scholar]
- 44.Li KS, Santamore WP. Contribution of each wall to biventricular function. Cardiovasc Res. 1993;27(5):792-800. [DOI] [PubMed] [Google Scholar]
- 45.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37(43):3293-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawut SM, Barr RG, Lima JA, et al. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi-Ethnic Study of Atherosclerosis (MESA)--right ventricle study. Circulation. 2012;126(14):1681-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagata Y, Wu VC, Kado Y, et al. Prognostic value of right ventricular ejection fraction assessed by transthoracic 3D echocardiography. Circ Cardiovasc Imaging. 2017;10(2):e005384. [DOI] [PubMed] [Google Scholar]
- 48.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107(3):526-531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definitions of ACCF/AHA HF Stages and Criteria
eTable 2. Participant Characteristics at Visit 5 by Measurable 3-D RVEF
eTable 3. Inverse Probably Attrition Weighted Estimates (With 95% Confidence Intervals) of Measures of Cardiac Structure and Function by 3-D RVEF at ARIC Visit 5
eTable 4. Inverse Probably Attrition Weighted Estimates
eTable 5. Inverse Probably Attrition Weighted Estimates of Hazard Ratios of 3-D RVEF and RV-PA Coupling
eFigure 1. Study Diagram
eFigure 2. Histogram and Descriptive Statistics for 3-D RVEF and RVLS
eFigure 3. The Relationship of 3-D RVEF With PVR, Mean PA Pressure, LVEF, and LVGLS
eFigure 4. Association of RVLS With PVR, Mean PAP, LVEF and LVGLS
eFigure 5. Mean values of 3-D RV EDVI and ESVI and the Prevalence of Abnormal (based on the 90th or 10th Percentile Limits Derived From the Stage 0 overall) Across HF Stages
eReferences