Abstract
Repetitive transcranial magnetic stimulation (rTMS) is an emerging therapy for the treatment of psychiatric disorders. However, the mechanisms underlying the therapeutic effects of rTMS are still unclear, limiting its optimisation. Lasting effects suggest changes in disease-related genes, so we conducted gene chip and qRT-PCR analyses of genes associated with psychiatric diseases in the mouse brain at various times following 1, 20, 30 or 40 days of rTMS. Many genes were differentially expressed in the rTMS-treated mouse brain compared to sham controls, including genes encoding neurotransmitter transporters (upregulation of EAAT4, GLAST, GLT-1, GAT2, GAT4, GLYT1 and GLYT2), and endoplasmic reticulum (ER)-stress proteins (downregulation of IRE1α, IRE1β, and XBP1, upregulation of ATF6 and GRP78/Bip). Expression changes in many of these genes were also observed 10 days after the last rTMS treatment. In PC12 cells, rTMS upregulated GRP78/Bip mRNA and enhanced resistance against H2O2 stress. These results suggest that rTMS differentially modulates multiple genes associated with psychiatric and neurodegenerative disorders. Sustained changes in the expression of these genes may underlie the therapeutic efficacy of chronic rTMS.
Abbreviations: GLT-1, glial glutamate transporter-1; GLYT, glycine transporter; GLAST, glutamate/aspartate transporter; EAAC1, excitatory amino acid carrier 1; EAAT4, excitatory amino acid transporter 4; GABA, γ-aminobutyric acid; GAT, GABA transporter; ER, endoplasmic reticulum; GRP78/Bip, glucose-regulated protein 78/immunoglobulin heavy chain-binding protein; ATF6, activating transcription factor 6
Keywords: rTMS, Glutamate transporter, GABA transporter, Glycine transporter, GRP78/Bip
Highlights
-
•
Gene expression changes in mouse brain were examined following rTMS.
-
•
rTMS altered expression of ER-stress and neurotransmitter transporter genes.
-
•
rTMS also induced ER-stress genes in PC12 cells and protected against H2O2 toxicity.
-
•
These expression changes may underlie the therapeutic benefits of rTMS.
1. Introduction
Repetitive transcranial magnetic stimulation (rTMS) is a novel non-invasive therapy for neurological and psychiatric diseases [1], [2], [3], [4]. Since Barker et al. first demonstrated that it is possible to activate both peripheral nerves and brain tissue using external magnetic stimulation [1], TMS has gained acceptance as a pain-free and non-invasive diagnostic tool in neurology, such as for evaluating peripheral neuropathies [5].
In addition, several studies have reported therapeutic benefits of TMS for patients with psychiatric disorders, such as depression, Parkinson's disease and schizophrenia [6], [7], [8]. These psychiatric disorders are associated with dysfunction in monoaminergic and glutamatergic neurotransmitter systems, suggesting that the benefits of rTMS arise from modulation of these neurotransmitter signalling pathways. For example, deceased expression of glutamate and GABA transporter has been reported in the post mortal brain of Schizophrenia patients [9], [10], [11]. Based on the NMDAR (N-methyl-D-aspartate receptor) hypofunction hypothesis in schizophrenia, we speculated that rTMS might have effects on glutamatergic, GABAergic and glycinergic systems, including NMDAR, non-NMDAR, metabotropic GluR (glutamate receptor), glutamate transporter, GABA transporter, and glycine transporter. The glycine transporter is expressed in glia surrounding glutamatergic synapses and regulates synaptic glycine concentrations influencing NMDA receptor-mediated neurotransmission. Conversely, increased expression of GluR1 is found in the post mortal brain of Schizophrenia patients [12]. Because GluR1 is essential for the proliferation and growth of melanoma [13], [14]; increased GluR1 might protect glutamatergic neurons. Because TMS is safe and relatively painlessness, it holds many possible applications as a therapeutic device for psychiatric disorders. However, the precise molecular mechanisms underlying the effects of TMS are unknown, which has impeded further optimisation for targeted regulation of processes involved in disease aetiology. Recent studies have demonstrated altered monoamine release after acute rTMS [15], [16]. In addition, we reported changes in the expression levels of monoamine transporters, dopamine receptor 2, HSP70 and circadian rhythm-related genes after acute and chronic rTMS [17], [18].
However, there have been few reports on changes in gene expression profiles following acute or chronic rTMS. This prompted us to evaluate gene expression changes in mouse brain following rTMS using gene chip technology. We demonstrate that rTMS induces lasting changes in the expression levels of multiple neurotransmitter transporter genes as well as several ER stress-related genes. Furthermore, we demonstrate that upregulation of the ER-stress gene GRP78/Bip in PC12 cells by rTMS enhances resistance against oxidative stress.
2. Materials and methods
2.1. Mice and rTMS conditions
Male C57Black mice (8 weeks old, 20–25 g) were chronically treated with rTMS for 20, 30 or 40 days (n = 50) or acutely for 1 day (n = 24). During treatment, the mice were housed in a light-controlled room (8:00 a.m. on, 8:00 p.m. off). A round coil (7.5 cm outer diameter) and a Nihon Kohden Rapid Rate Stimulator (Nihon Kohden, Japan) were used to perform the stimulation. For chronic rTMS, stimulation conditions were as follows: 20 Hz for 2 s, 20 times/day, inter-stimulus interval of 1 min and 30% machine output (representing about 0.75 T). The coil was placed over the head without touching the skull. Sham control mice were stimulated from a distance of more than 10 cm from the head. rTMS did not produce notable seizures or changes in behaviour, such as excessive struggling. Twenty-four hours after the last stimulation, the animals were sacrificed and their brains processed for further gene expression analysis. Mice subjected to acute rTMS (1 day using the same stimulus conditions) were sacrificed after 1, 4, 12 and 24 h for gene expression analysis. All the animal experiments were performed in compliance with institutional guidelines.
This study was approved by the Experimental Animal Committee of the RIKEN Institute and performed according to the guidelines for the care and use of experimental animals of RIKEN Institute (approval # H15-2B046).
2.2. RNA extraction
Whole mouse brain was divided at the midbrain into cerebrum and cerebellum with brain stem (CBS). Total RNA was isolated from cerebrum and CBS by acid–phenol extraction [19]. Poly(A)+ RNA was isolated from the samples using an mRNA purification kit (TaKaRa Bio, Japan) for expression analysis by TaqMan real-time RT-PCR. Primer Express Software (Applied Biosystems, Foster City, CA) was used to design the TaqMan primer and probe sets. Supplementary data 1 shows the nucleotide sequences of the primers. Contaminating genomic DNA was removed with RNase-free DNase I (TaKaRa Bio, Japan). Complementary DNAs were synthesised from 1 μg of mRNA per 100-μl reaction using MMLV Reverse Transcription Reagents (Invitrogen, Carlsbad, CA). The TaqMan PCR reaction mixture contained 15 μl of TaqMan Universal PCR Master Mix (Applied Biosystems) in a 30 μl reaction. Primers and probes were added in optimal concentrations. We used 1 μl of RT mix for each PCR. Each sample was amplified in duplicate and the experiment was repeated at least three times. PCR conditions were standard for the 7700 Sequence Detector System (Applied Biosystems): 2 min at 50 °C and 10 min at 95 °C, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The mRNA quantity for each gene of interest was normalised to the quantity of GAPDH mRNA within each sample.
2.3. Cell culture
PC12 cells were maintained in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal calf serum (FCS) at 37 °C under 5% CO2/95% air. Cells at subconfluence were harvested, diluted in culture medium and seeded in 24-well culture plates for cell viability assays or in 25 cm2 culture flasks for total RNA extraction. The cells were cultured with or without daily rTMS for 15 days. Then, the cells in 24-well plates were treated with H2O2 for 2 h. Viability was evaluated by MTT assay [20].
2.4. Data analysis
The data are presented as mean ± SE of at least three independent experiments, each performed in triplicate or duplicate. Means were compared by ANOVA (Fig. 1, Fig. 2) or Student's t-test as appropriate.
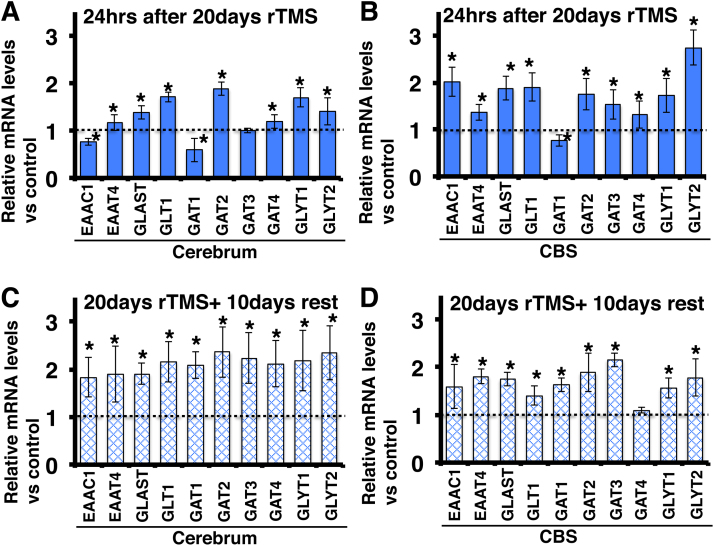
Fig. 1.
Effects of chronic rTMS on glutamate, GABA and glycine transporter gene expression in mouse brain. The mRNA levels were determined at 24 h (A, B) and 10 days after 20 days of rTMS stimulation (C, D). (A, C) Effects of chronic rTMS on gene expression in the cerebrum. (B, D) Effects of chronic rTMS on gene expression in the cerebellum plus brain stem (CBS). All mRNA levels are normalised to GAPDH expression within the same sample. Values presented as mean ± SEM of five independent experiments, each performed in triplicate. *Significantly different from control at P < 0.05.
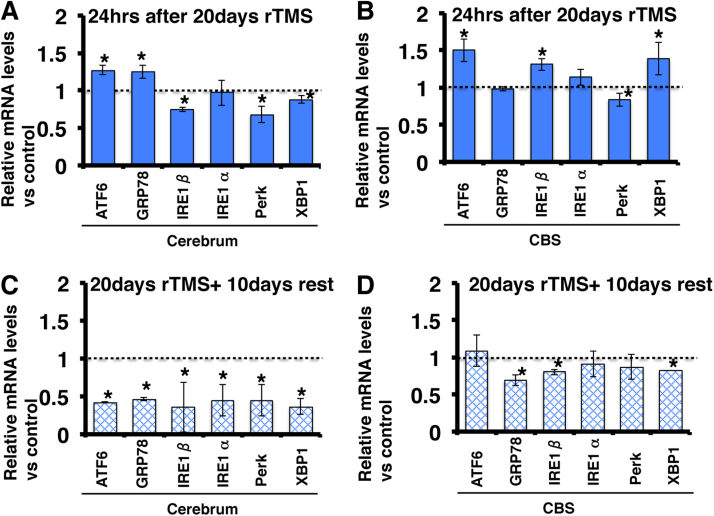
Fig. 2.
Effects of rTMS on ER stress-related gene expression in mouse brain. The mRNA levels were determined at 24 h (A, B) and 10 days after 20 days of rTMS stimulation (C, D). (A, C) Effects of chronic rTMS on gene expression in the cerebrum. (B, D) Effects of chronic rTMS on gene expression in cerebellum plus brain stem (CBS). All mRNA levels are normalised to GAPDH expression in the same sample. Values presented as mean ± SEM of five independent experiments, each performed in triplicate. *Significantly different from control at P < 0.05.
3. Results
3.1. Gene expression changes in mouse brain following rTMS
We stimulated the brains of 8-week-old C57 Black mice for 20, 30 or 40 days by rTMS and analysed the changes in gene expression using the Affymetrix GeneChip microarray. GeneChip analysis revealed altered expression levels of multiple mRNAs, including those encoding glutamate and glycine transporters as well as ER stress-related genes, in both cerebrum and CBS [21], [22]. In order to quantify these changes, we then measured mRNA levels by qRT-PCR (Fig. 1, Fig. 2, Table 1, Table 2, Table 3).
Table 1.
Summary of glutamate transporter gene expression changes following acute rTMS.
| 1 h | 4 h | 12 h | 24 h | |||
|---|---|---|---|---|---|---|
| EAAC1 | Cerebrum | Control | 1 ± 0.08 | 0.83 ± 0.06 | 1.04 ± 0.07 | 1.01 ± 0.13 |
| Mg+ | 1.04 ± 0.57 | 1.67 ± 0.27* | 0.91 ± 0.06* | 1.03 ± 0.08 | ||
| CBS | Control | 1 ± 0.28 | 1.72 ± 0.35 | 1.79 ± 0.26 | 2.09 ± 0.3 | |
| Mg+ | 0.93 ± 0.03 | 1.73 ± 1.28 | 1.69 ± 0.61 | 1.61 ± 0.28 | ||
| EAAT4 | Cerebrum | Control | 0.99 ± 0.13 | 0.82 ± 0.07 | 1.05 ± 0.09 | 0.99 ± 0.1 |
| Mg+ | 1.01 ± 0.13 | 1.7 ± 0.29* | 0.88 ± 0.07* | 1.05 ± 0.08 | ||
| CBS | Control | 1 ± 0.31 | 1.53 ± 0.38 | 1.74 ± 0.46 | 1.89 ± 0.15 | |
| Mg+ | 1.06 ± 0.05 | 1.56 ± 0.85 | 1.62 ± 0.7* | 1.57 ± 0.3 | ||
| GLAST | Cerebrum | Control | 1 ± 0.14 | 0.79 ± 0.05 | 0.97 ± 0.05 | 0.98 ± 0.14 |
| Mg+ | 0.99 ± 0.12 | 1.64 ± 0.33* | 0.87 ± 0.05* | 0.99 ± 0.07 | ||
| CBS | Control | 1 ± 0.02 | 1.6 ± 0.07 | 1.49 ± 0.29 | 1.98 ± 0.31 | |
| Mg+ | 0.81 ± 0.04* | 1.15 ± 0.09* | 1.38 ± 0.14 | 1.45 ± 0.05* | ||
| GLT1 | Cerebrum | Control | 1 ± 0.17 | 0.73 ± 0.04 | 0.86 ± 0.05 | 0.93 ± 0.11 |
| Mg+ | 0.96 ± 0.11 | 1.45 ± 0.31* | 0.76 ± 0.04* | 0.88 ± 0.09 | ||
| CBS | Control | 1 ± 0.34 | 1.51 ± 0.32 | 1.38 ± 0.33 | 2.39 ± 0.54 | |
| Mg+ | 0.76 ± 0.05 | 1.34 ± 0.9* | 1.56 ± 0.22* | 1.36 ± 0.26* | ||
| GAT1 | Cerebrum | Control | 1 ± 0.06 | 0.8 ± 0.05 | 0.97 ± 0.05 | 0.91 ± 0.11 |
| Mg+ | 0.99 ± 0.13 | 1.64 ± 0.32* | 0.83 ± 0.04* | 0.95 ± 0.08 | ||
| CBS | Control | 1 ± 0.38 | 1.78 ± 0.47 | 1.85 ± 0.45 | 1.97 ± 0.32 | |
| Mg+ | 0.92 ± 0.06 | 1.76 ± 1.05 | 1.73 ± 0.5 | 1.69 ± 0.41 | ||
| GAT2 | Cerebrum | Control | 0.99 ± 0.1 | 0.79 ± 0.07 | 0.95 ± 0.07 | 0.99 ± 0.19 |
| Mg+ | 0.87 ± 0.04 * | 1.52 ± 0.3* | 0.84 ± 0.04* | 0.89 ± 0.04 | ||
| CBS | Control | 1 ± 0.16 | 1.8 ± 0.14 | 1.82 ± 0.16 | 1.81 ± 0.09 | |
| Mg+ | 0.84 ± 0.03* | 2.11 ± 1.37* | 1.55 ± 0.06* | 1.51 ± 0.6* |
Values are mean ± SEM of three independent experiments, each performed in triplicate.
Significantly different from control at P < 0.05.
Table 2.
Summary of GABA and glycine transporter gene expression changes following rTMS.
| 1 h | 4 h | 12 h | 24 h | |||
|---|---|---|---|---|---|---|
| GAT3 | Cerebrum | Control | 0.99 ± 0.15 | 0.8 ± 0.07 | 0.94 ± 0.07 | 0.95 ± 0.15 |
| Mg+ | 0.89 ± 0.07 | 1.6 ± 0.31* | 0.88 ± 0.05 | 0.92 ± 0.08 | ||
| CBS | Control | 0.99 ± 0.02 | 1.47 ± 0.22 | 1.47 ± 0.35 | 1.55 ± 0.05 | |
| Mg+ | 1.12 ± 0.19 | 1.61 ± 0.98* | 1.18 ± 0.29 | 1.33 ± 0.24* | ||
| GAT4 | Cerebrum | Control | 0.99 ± 0.06 | 0.8 ± 0.03 | 0.99 ± 0.04 | 0.96 ± 0.11 |
| Mg+ | 0.99 ± 0.1 | 1.69 ± 0.32* | 0.85 ± 0.04* | 0.97 ± 0.08 | ||
| CBS | Control | 1 ± 0.04 | 2.12 ± 0.22 | 1.92 ± 0.29 | 2.42 ± 0.39 | |
| Mg+ | 1.32 ± 0.07* | 1.5 ± 0.46* | 2.14 ± 0.62 | 2.16 ± 0.47 | ||
| GLYT1 | Cerebrum | Control | 1 ± 0.12 | 0.81 ± 0.05 | 1.02 ± 0.06 | 1.04 ± 0.18 |
| Mg+ | 0.94 ± 0.12 | 1.6 ± 0.21* | 0.85 ± 0.04* | 1.03 ± 0.1 | ||
| CBS | Control | 1 ± 0.32 | 1.65 ± 0.42 | 1.63 ± 0.36 | 1.77 ± 0.21 | |
| Mg+ | 0.96 ± 0.03 | 1.55 ± 0.96 | 1.77 ± 0.44 | 1.74 ± 0.46 | ||
| GLYT2 | Cerebrum | Control | 1 ± 0.09 | 0.72 ± 0.07 | 1.02 ± 0.02 | 0.97 ± 0.49 |
| Mg+ | 0.87 ± 0.11* | 1.57 ± 0.17* | 0.77 ± 0.02* | 1.28 ± 0.4 | ||
| CBS | Control | 0.52 ± 0.3 | 1 ± 0.14 | 1.02 ± 0.19 | 1.18 ± 0.16 | |
| Mg+ | 0.37 ± 0.03 | 1.12 ± 0.79 | 1.07 ± 0.43 | 0.82 ± 0.16* |
Values are mean ± SEM of three independent experiments, each performed in triplicate.
Significantly different from control at P < 0.05.
Table 3.
Summary of ER stress-related gene expression changes following acute rTMS.
| 1 h | 4 h | 12 h | 24 h | |||
|---|---|---|---|---|---|---|
| ATF6 | Cerebrum | Control | 1 ± 0.01 | 1.04 ± 0.05 | 0.86 ± 0.06 | 1.24 ± 0.15 |
| Mg+ | 0.89 ± 0.08* | 0.87 ± 0.14* | 0.88 ± 0.05 | 0.8 ± 0* | ||
| CBS | Control | 1 ± 0.05 | 0.87 ± 0.07 | 1.02 ± 0.09 | 1.06 ± 0.17 | |
| Mg+ | 0.52 ± 0.11* | 1.05 ± 0.25 | 0.8 ± 0.01* | 0.41 ± 0.61* | ||
| GRP78/Bip | Cerebrum | Control | 1 ± 0.15 | 1.42 ± 0.31 | 0.81 ± 0.02 | 1.19 ± 0.19 |
| Mg+ | 1.38 ± 0.19* | 0.86 ± 0.08* | 1.14 ± 0.13* | 0.76 ± 0.05* | ||
| CBS | Control | 1 ± 0.02 | 0.95 ± 0.04 | 1.16 ± 0.13 | 2.19 ± 0.15 | |
| Mg+ | 1.4 ± 0.29* | 1.06 ± 0.05 | 0.74 ± 0.04* | 1.49 ± 0.45* | ||
| IRE1β | Cerebrum | Control | 1 ± 0.1 | 1.57 ± 0.18 | 1.27 ± 0.07 | 1.34 ± 0.05 |
| Mg+ | 1.03 ± 0.12 | 1.21 ± 0.16* | 0.96 ± 0.1* | 1.2 ± 0.08 | ||
| CBS | Control | 1 ± 0.18 | 1.39 ± 0.25 | 0.97 ± 0.44 | 1 ± 0.1 | |
| Mg+ | 1.15 ± 0.14* | 1.63 ± 0.02* | 0.99 ± 0.09* | 1.16 ± 0.13* | ||
| IRE1α | Cerebrum | Control | 1 ± 0.04 | 1.43 ± 0.07 | 1.05 ± 0.06 | 1.22 ± 0.09 |
| Mg+ | 0.86 ± 0.05* | 1.04 ± 0.19* | 1.02 ± 0.03 | 0.92 ± 0.04* | ||
| CBS | Control | 1 ± 0.11 | 1.04 ± 0.12 | 1 ± 0.12 | 1.34 ± 0.07 | |
| Mg+ | 0.56 ± 0.12* | 1.05 ± 0.25 | 1 ± 0.09 | 1.37 ± 0.04 | ||
| PERK | Cerebrum | Control | 1 ± 0.09 | 1.12 ± 0.16 | 0.91 ± 0.04 | 1.04 ± 0.1 |
| Mg+ | 0.82 ± 0* | 1.07 ± 0.11 | 0.91 ± 0.06 | 1.03 ± 0.05 | ||
| CBS | Control | 1 ± 0.11 | 0.92 ± 0.12 | 0.95 ± 0.12 | 1.05 ± 0.07 | |
| Mg+ | 0.79 ± 0.12* | 0.84 ± 0.25 | 0.73 ± 0.09* | 1.46 ± 0.04* | ||
| XBP1 | Cerebrum | Control | 1 ± 0.1 | 1 ± 0.03 | 0.83 ± 0.04 | 1 ± 0.02 |
| Mg+ | 1.14 ± 0.03* | 0.85 ± 0.09* | 0.97 ± 0.06* | 0.77 ± 0.05* | ||
| CBS | Control | 1.01 ± 0.01 | 1.08 ± 0.02 | 0.94 ± 0.01 | 1.94 ± 0.11 | |
| Mg+ | 1.3 ± 0.11* | 1.26 ± 0.01* | 0.79 ± 0.09* | 1.24 ± 0.46* |
Values are mean ± SEM of three independent experiments, each performed in triplicate.
Significantly different from control at P < 0.05.
3.2. Effects of acute and chronic rTMS on transporter genes in mouse brain
The GeneChip data showed that glutamate and glycine transporter mRNAs were altered after chronic rTMS. Thus, we examined the mRNA levels of the glutamate transporters EAAC1, EAAT4, GLAST and GLT1, the GABA transporters GAT1–4, and the glycine transporters GLYT1 and 2 both 24 h and 10 days after 20 days of rTMS. Twenty-four hours after the last rTMS application, EAAT4, GLAST, GLT1, GAT2, GAT4, GLYT1 and GLYT2 mRNA levels were upregulated in the cerebrum (Fig. 1A), and EAAC1, EAAT4, GLAST, GLT1, GAT2, GAT3, GAT4, GLYT1 and GLYT2 mRNA levels were upregulated in the CBS (Fig. 1B). Conversely, EAAC1 and GAT1 mRNA levels were downregulated in the cerebrum (Fig. 1A), while GAT1 mRNA level was downregulated in the CBS (Fig. 1B). Ten days after the last rTMS treatment, EAAC1, EAAT4, GLAST, GLT1, GAT1, GAT2, GAT3, GAT4, GLYT1 and GLYT2 mRNA levels were upregulated in both cerebrum and CBS (Fig. 1C, D), while GAT4 mRNA level was upregulated only in the cerebrum (Fig. 1D).
We next examined the acute effects of a single days’ rTMS administration after 1, 4, 12 and 24 h. These measurements revealed temporally complex and region-specific expression changes in transporter and ER stress-related genes. Expression changes in glutamate transporter genes are summarised in Table 1, GABA and glycine transporter genes in Table 2, and ER-stress genes in Table 3. After acute rTMS, EAAC1, GAT1 and GLYT1 mRNA levels were upregulated at 4 h and downregulated at 12 h in the cerebrum (Tables 1 and 2). EAAT4 mRNA levels were upregulated at 4 h and downregulated at 12 h in the cerebrum, while expression levels were unchanged in CBS at 4 h before decreasing at 12 h (Table 1). GLAST mRNA levels were upregulated at 4 h and downregulated at 12 h in the cerebrum. In contrast, expression was downregulated after 1, 4 and 24 h in CBS (Table 1). GAT2 mRNA levels were upregulated at 4 h and downregulated at 1 and 12 h in the cerebrum, but upregulated at 4 h and downregulated after 1, 4 and 24 h in CBS (Table 1). GAT3 mRNA levels were upregulated at 4 h in the cerebrum and CBS, but then downregulated only in the CBS at 24 h (Table 2). GAT4 mRNA levels were upregulated at 4 h and downregulated at 12 h in the cerebrum, but upregulated at 1 h and downregulated at 12 h in CBS (Table 2). GLYT2 mRNA levels were upregulated at 4 h and downregulated at 1 and 12 h in the cerebrum, but downregulated at 24 h in CBS (Table 2). In summary, rTMS appears to differentially regulate neurotransmitter transporter genes both dynamically following acute treatment and in a more sustained manner following chronic treatment.
3.3. Effects of acute and chronic rTMS on ER stress-related genes in mouse brain
GeneChip data revealed that multiple ER stress-related genes were also altered by rTMS [22], so examined the effects of acute and chronic rTMS on specific ER stress-related genes by qRT-PCR. Twenty-four hours after 20 days of rTMS, ATF6 and GRP78/Bip mRNA levels were upregulated in the cerebrum (Fig. 2A), and ATF6, IRE1β and XBP1 mRNA levels were upregulated in CBS (Fig. 2B). However, IRE1β, PERK and XBP1 mRNA levels were downregulated in the cerebrum (Fig. 2A) and PERK mRNA level was downregulated in CBS (Fig. 2B). Ten days after the last rTMS treatment, ATF6, GRP78/Bip, IRE1α, IRE1β, PERK and XBP1 mRNA levels were downregulated in the cerebrum (Fig. 2C), and GRP78/Bip, IRE1 β, PERK and XBP1 mRNA levels were downregulated in CBS (Fig. 2D). There were no changes in ATF6, IRE1α and PERK mRNA levels in CBS (Fig. 2D).
We then examined the acute effects of rTMS at 1, 4, 12 and 24 h (Table 3). ATF6 mRNA level was downregulated in the cerebrum at 1, 4 and 24 h, and in CBS at 1, 12 and 24 h after acute rTMS. GRP78/Bip mRNA level was upregulated at 1 and 12 h in the cerebrum and at 1 h in CBS, but was downregulated at 4 and 24 h in the cerebrum and at 12 and 24 h in CBS. IRE1β mRNA level was upregulated at 1, 4 and 24 h and downregulated at 12 h in CBS, but was downregulated at 4 and 12 h in the cerebrum. IRE1α mRNA level was downregulated at 1 h in the CBS, and downregulated at 1, 4 and 12 h in the cerebrum. PERK mRNA level was downregulated at 1 and 12 h in CBS and at 1 h in the cerebrum, but upregulated at 24 h in the CBS. XBP1 mRNA level was downregulated at 12 and 24 h in CBS and at 4 h in the cerebrum, but upregulated after 1 and 12 h in the cerebrum and at 1 and 4 h in CBS. Thus, similar to neurotransmitter transporter genes, rTMS induced temporally complex and region-specific expression changes in ER stress-related genes.
3.4. Effects of acute and chronic rTMS on ER stress-related genes in PC12 cells
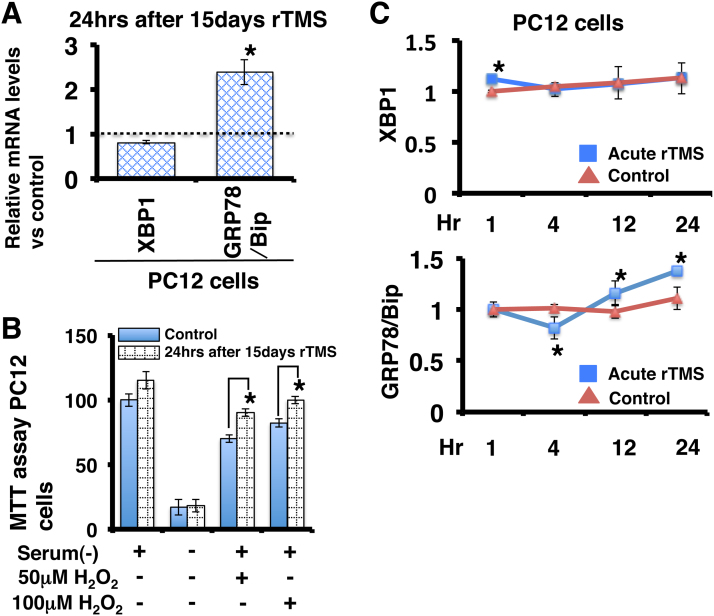
The effects of rTMS on ER stress-related genes were also examined in PC12 cells to investigate the functional significance. GRP78/Bip mRNA expression was upregulated after 15 days of rTMS in PC12 cells (Fig. 3A). After acute rTMS, XBP1 mRNA level was upregulated at 1 h, while GRP78/Bip mRNA level was downregulated at 4 h and upregulated at 12 and 24 h (Fig. 3C). ER stress-related genes are known to be induced by H2O2 via glutamate and glycine stimulation as a compensatory response to prevent cytotoxic protein misfolding, so we investigated whether rTMS increases PC12 cell resistance against H2O2. Indeed, 15 days of rTMS increased cell viability following 2 h of H2O2 exposure compared to controls (Fig. 3B).
Fig. 3.
Effects of chronic and acute rTMS on XBP1 and GRP78/Bip mRNA expression levels and H2O2 resistance in P12 cells. (A) Effects of chronic rTMS on XBP1 and GRP78/Bip mRNA levels in PC12 cells. (B) Viability of rTMS-treated and control PC12 cells exposed to H2O2 for 2 h. (C) Effects of acute rTMS on XBP1 and GRP78/Bip mRNA levels in PC12 cells at 1, 4, 12 and 24 h after stimulation. Values presented as mean ± SEM of three independent experiments, each performed in triplicate. *Significantly different from control at P < 0.05.
4. Discussion
Repetitive TMS is a promising treatment for psychiatric disorders. Persistent therapeutic effects suggest that rTMS induces lasting changes in the expression of genes involved in these disorders, such as genes associated with stress-responses and neurotransmission. To study such effects, we measured gene expression changes by gene chip arrays and qRT-PCR in mouse brain following an acute (1-day) or chronic (20-day) rTMS protocol. We have previously shown that acute and chronic rTMS alters monoamine transporter (MAT) mRNA expression, protein levels and function [17], dopamine receptor 2 mRNA expression, protein levels and function, circadian rhythm-related gene expression and both mRNA and protein expression levels of the stress response gene HSP70 [18]. Here we confirmed differential modulation of transporter and ER stress-related genes reported in previous studies [21], [22] and provide evidence for enhanced neuroprotection following rTMS.
In addition to changes in mouse cerebrum, we found that GRP78/Bip mRNA level was upregulated in PC12 cells by acute and chronic rTMS. Furthermore, 15 days of rTMS increased the resistance of PC12 cells against H2O2 toxicity. These results indicate that rTMS may also slow the progression of neurodegenerative disorder, such as Huntington's disease, through GRP78/Bip induction. Further investigations are needed on the effects of chronic rTMS because acute rTMS increased GRP78/Bip mRNA expression in the cerebrum 24 h after 20 days of rTMS, whereas other stress-associated genes such as ATF6, IRE1β, PERK and XBP1 were downregulated. Thus, the ER-stress response induced by rTMS appears highly specific.
Expression levels of EAAT4, GLAST, GLT1, GAT2, GAT4, GLYT1 and GLYT2 were upregulated in the cerebrum after chronic rTMS (Fig. 1A), while these same genes plus EAAC1 and GAT3 were upregulated in CBS (Fig. 1B). Furthermore, EAAC1, EAAT4, GLAST, GLT1, GAT1, GAT2, GAT3, GAT4, GLYT1 and GLYT2 mRNA levels were still upregulated in the cerebrum and CBS 10 days after the last rTMS administration (Fig. 1C, D). Thus, rTMS appears to induce widespread and sustained increases in glutamate, GABA and glycine transporters, possibly resulting in altered transmitter signalling. Indeed, the efficacy of NMDA receptor antagonists such as memantine for the treatment of dementia, obsessive-compulsive disorder (OCD), and certain schizophrenia symptoms suggests that chronic rTMS may result in symptom improvement by upregulating transporter-mediated glutamate uptake. Our results also suggest that ER stress-related genes may be involved in glutamate and glycine transporter expression changes induced by chronic rTMS.
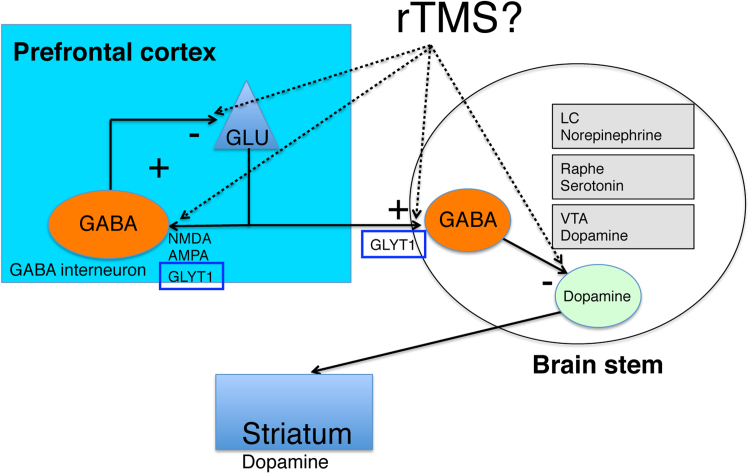
In conclusion, the therapeutic effects of chronic rTMS may depend on modulation of ER stress-related genes and glutamate, glycine and GABA transporter genes (Fig. 4). Further research is needed to identify the region-specific functional changes resulting from up- and downregulation of these genes following rTMS. Such information could facilitate the development of more effective rTMS protocols with fewer side effects.
Fig. 4.
Model for differential regulation of neurotransmitter transporters in mouse brain by rTMS. Chronic rTMS may alter glutamatergic, GABAergic and glycinergic neurotransmission by changing the rate of transmitter uptake from the synaptic cleft.
Acknowledgements
The authors would like to thank Mr Masaru Kurosawa and Dr Nobuyuki Nukina for their support for the experiment. The authors would like to thank Enago for the English language review.
Acknowledgments
Funding sources
This work was partly supported by a grant from the Japan Society for the Promotion of Science (JSPS). This work was also supported by Global COE Program ‘Center of Education and Research for the Advanced Genome-Based Medicine-For personalised medicine and the control of worldwide infectious disease’ of MEXT, Japan.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.10.015.
Appendix A. Transparency document
Supplementary material
References
- 1.Barker A.T., Freeston I.L., Jalinous R., Jarratt J.A. Magnetic stimulation of the human brain and peripheral nervous system: an introduction and the results of an initial clinical evaluation. Neurosurgery. 1987;20:100–109. doi: 10.1097/00006123-198701000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Post A., Keck M.E. Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms? J. Psychiatr. Res. 2001;35:193–215. doi: 10.1016/s0022-3956(01)00023-1. [DOI] [PubMed] [Google Scholar]
- 3.Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- 4.Roth B.J., Saypol J.M., Hallett M., Cohen L.G. A theoretical calculation of the electric field induced in the cortex during magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1991;81:47–56. doi: 10.1016/0168-5597(91)90103-5. [DOI] [PubMed] [Google Scholar]
- 5.George M.S., Wassermann E.M., Post R.M. Transcranial magnetic stimulation: a neuropsychiatric tool for the 21st century. J. Neuropsychiatry Clin. Neurosci. 1996;8:373–382. doi: 10.1176/jnp.8.4.373. [DOI] [PubMed] [Google Scholar]
- 6.Paus T., Barrett J. Transcranial magnetic stimulation (TMS) of the human frontal cortex: implications for repetitive TMS treatment of depression. J. Psychiatry Neurosci. 2004;29:268–279. [PMC free article] [PubMed] [Google Scholar]
- 7.Dragasevic N., Potrebic A., Damjanovic A., Stefanova E., Kostic V.S. Therapeutic efficacy of bilateral prefrontal slow repetitive transcranial magnetic stimulation in depressed patients with Parkinson's disease: an open study. Mov. Disord. 2002;17:528–532. doi: 10.1002/mds.10109. [DOI] [PubMed] [Google Scholar]
- 8.George M.S., Lisanby S.H., Sackeim H.A. Transcranial magnetic stimulation: applications in neuropsychiatry. Arch. Gen. Psychiatry. 1999;56:300–311. doi: 10.1001/archpsyc.56.4.300. [DOI] [PubMed] [Google Scholar]
- 9.Volk D., Austin M., Pierri J., Sampson A., Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am. J. Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- 10.Walsh T., McClellan J.M., McCarthy S.E., Addington A.M., Pierce S.B., Cooper G.M., Nord A.S., Kusenda M., Malhotra D., Bhandari A., Stray S.M., Rippey C.F., Roccanova P., Makarov V., Lakshmi B., Findling R.L., Sikich L., Stromberg T., Merriman B., Gogtay N., Butler P., Eckstrand K., Noory L., Gochman P., Long R., Chen Z., Davis S., Baker C., Eichler E.E., Meltzer P.S., Nelson S.F., Singleton A.B., Lee M.K., Rapoport J.L., King M.C., Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 11.Shan D., Lucas E.K., Drummond J.B., Haroutunian V., Meador-Woodruff J.H., McCullumsmith R.E. Abnormal expression of glutamate transporters in temporal lobe areas in elderly patients with schizophrenia. Schizophr. Res. 2013;144:1–8. doi: 10.1016/j.schres.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta D.S., McCullumsmith R.E., Beneyto M., Haroutunian V., Davis K.L., Meador-Woodruff J.H. Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse. 2005;57:123–131. doi: 10.1002/syn.20164. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Daim M., Funasaka Y., Komoto M., Nakagawa Y., Yanagita E., Nishigori C. Pharmacogenomics of metabotropic glutamate receptor subtype 1 and in vivo malignant melanoma formation. J. Dermatol. 2010;37:635–646. doi: 10.1111/j.1346-8138.2010.00833.x. [DOI] [PubMed] [Google Scholar]
- 14.Ohtani Y., Harada T., Funasaka Y., Nakao K., Takahara C., Abdel-Daim M., Sakai N., Saito N., Nishigori C., Aiba A. Metabotropic glutamate receptor subtype-1 is essential for in vivo growth of melanoma. Oncogene. 2008;27:7162–7170. doi: 10.1038/onc.2008.329. [DOI] [PubMed] [Google Scholar]
- 15.Kanno M., Matsumoto M., Togashi H., Yoshioka M., Mano Y. Effects of acute repetitive transcranial magnetic stimulation on dopamine release in the rat dorsolateral striatum. J. Neurol. Sci. 2004;217:73–81. doi: 10.1016/j.jns.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi T., Hayashi T., Okabe S., Nonaka I., Matsuda H., Iida H., Imabayashi E., Watabe H., Miyake Y., Ogawa M., Teramoto N., Ohta Y., Ejima N., Sawada T., Ugawa Y. Endogenous dopamine release induced by repetitive transcranial magnetic stimulation over the primary motor cortex: an [11C]raclopride positron emission tomography study in anesthetized macaque monkeys. Biol. Psychiatry. 2004;55:484–489. doi: 10.1016/j.biopsych.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda T., Kurosawa M., Uchikawa C., Kitayama S., Nukina N. Modulation of monoamine transporter expression and function by repetitive transcranial magnetic stimulation. Biochem. Biophys. Res. Commun. 2005;327:218–224. doi: 10.1016/j.bbrc.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda T., Kurosawa M., Morimoto C., Kitayama S., Nukina N. Multiple effects of repetitive transcranial magnetic stimulation on neuropsychiatric disorders. Biochem. Biophys. Res. Commun. 2013;436:121–127. doi: 10.1016/j.bbrc.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 20.Wang G.H., Mitsui K., Kotliarova S., Yamashita A., Nagao Y., Tokuhiro S., Iwatsubo T., Kanazawa I., Nukina N. Caspase activation during apoptotic cell death induced by expanded polyglutamine in N2a cells. Neuroreport. 1999;10:2435–2438. doi: 10.1097/00001756-199908200-00001. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda T., Kobayashi S., Morimoto C. Gene expression microarray data from mouse cerebrum treated by rTMS for 30 days. Data Brief. 2017;15:948–969. doi: 10.1016/j.dib.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda T., Kobayashi S., Morimoto C. Gene expression microarray data from mouse CBS treated by rTMS for 30 days, mouse cerebrum and CBS treated with rTMS for 40 days. Data Brief. 2018;17:1078–1081. doi: 10.1016/j.dib.2018.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material