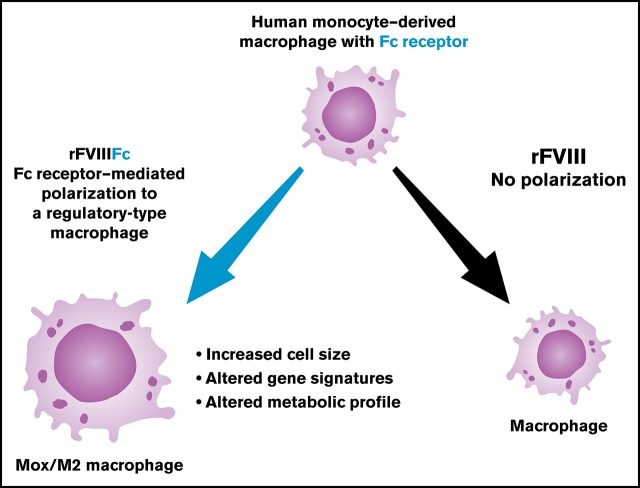
Key Points
rFVIIIFc can induce macrophages to polarize to an alternatively activated Mox/M2 phenotype with antioxidant characteristics.
This polarization is dependent on intact rFVIIIFc–FcR interactions.
Abstract
The main complication of replacement therapy with factor in hemophilia A (HemA) is the formation of inhibitors (neutralizing anti–factor VIII [FVIII] antibodies) in ∼30% of severe HemA patients. Because these inhibitors render replacement FVIII treatment essentially ineffective, preventing or eliminating them is of top priority in disease management. The extended half-life recombinant FVIII Fc fusion protein (rFVIIIFc) is an approved therapy for HemA patients. In addition, it has been reported that rFVIIIFc may induce tolerance to FVIII more readily than FVIII alone in HemA patients that have developed inhibitors. Given that the immunoglobulin G1 Fc region has the potential to interact with immune cells expressing Fc receptors (FcRs) and thereby affect the immune response to rFVIII, we investigated how human macrophages, expressing both FcRs and receptors reported to bind FVIII, respond to rFVIIIFc. We show herein that rFVIIIFc, but not rFVIII, uniquely skews macrophages toward an alternatively activated regulatory phenotype. rFVIIIFc initiates signaling events that result in morphological changes, as well as a specific gene expression and metabolic profile that is characteristic of the regulatory type Mox/M2-like macrophages. Further, these changes are dependent on rFVIIIFc-FcR interactions. Our findings elucidate mechanisms of potential immunomodulatory properties of rFVIIIFc.
Visual Abstract
Introduction
Hemophilia A (HemA) is characterized by the absence of functional endogenous coagulation factor VIII (FVIII), leading to impaired bleed control.1 Prophylactic factor replacement therapy is considered the optimal treatment for individuals with severe HemA.2,3 Although this treatment is successful in controlling bleeds and associated arthropathy in the majority of patients, ∼30% of individuals with severe hemophilia develop inhibitors, anti-FVIII neutralizing antibodies, which reduces treatment efficacy.4 To restore the ability to use replacement FVIII therapy, inhibitor-positive individuals undergo an immune tolerance induction (ITI) regimen. ITI treatment can restore normal rFVIII pharmacokinetics in ∼70% of these individuals with hemophilia.5,6
Recombinant FVIII Fc (rFVIIIFc) is an approved factor replacement therapy that is composed of a single molecule of rFVIII covalently fused to the Fc domain of immunoglobulin G1 (IgG1). This molecule has an extended half-life of ∼19 hours,7 compared with rFVIII molecules that do not have the Fc portion (8-12 hours).8 The prolonged half-life of rFVIIIFc is mediated by the interaction of the Fc portion of the molecule with neonatal Fc receptors (FcRns), protecting the fused rFVIII from lysosomal degradation.9,10 rFVIIIFc is also capable to interact with Fcγ receptors, expressed on multiple antigen-presenting cells (APCs) including B cells, thus it has a potential to influence the immune system. It has been reported that rFVIIIFc could reduce inhibitor titers in inhibitor-positive HemA patients in recent case reports.11,12 In addition, a retrospective chart review reported that rFVIIIFc achieved rapid time to tolerization in high-risk first-time ITI patients.13 In preclinical animal studies, decreased levels of inhibitor formation after rFVIIIFc treatment of HemA mice was reported, compared with rFVIII treatment. Reduced immunogenicity of rFVIIIFc in an animal model was attributed to the development of regulatory T cells and tolerogenic environment, potentially mediated by the interaction of the Fc domain of rFVIIIFc with the Fc receptors (FcRs) on APCs.14
Inhibitors are produced as result of a complex, T cell-dependent B cell-mediated action, implying that the administered FVIII molecule is presented by APCs in inhibitor-positive HemA patients. Macrophages are professional APCs able to adapt to their tissue environment by taking on a spectrum of phenotypes and functions. Under inflammatory circumstances, conventional, proinflammatory M1 macrophages rely on aerobic glycolysis to fulfill their bioenergetic needs for pathogen phagocytosis and killing, whereas alternatively activated, regulatory M2 macrophages rely on oxidative phosphorylation, including use of both glucose and lipids, to fuel their homeostatic functions,15 although this delineation is not absolute.16 The regulation of these metabolic changes is orchestrated by factors, such as the transcription factor peroxisome proliferator activating receptor γ (PPARγ), that has also been shown to regulate anti-inflammatory responses.17 One of the newly discovered functional classes of macrophages is the Mox macrophage, which is distinct from M1 or M2 macrophages.18 Mox macrophages play role in iron metabolism19 and sense oxidized lipoproteins to reprogram their metabolism toward redox-regulatory phenotype in mice.20 Mox macrophages are characterized by a nuclear factor (erythroid-derived 2)–like 2 (NRF2)–dependent antioxidant gene expression pattern, with heme oxygenase 1 (HO-1/HMOX1) being their hallmark molecule.21 In macrophages, NRF2 and HO-1 were shown to exhibit anti-inflammatory, antioxidant, and reparative effects,22-24 as demonstrated in HO-1 deficient mice25 and human cases of HMOX1 deficiency.26 HO-1 activity also interferes with the activation and maturation of APCs, downmodulating their capacity to prime T cells.27,28
Monocytes and macrophages have the unique potential to respond to rFVIIIFc. These cells express Fcγ receptors CD16 (FcγRIII), CD32 (FcγRII), and CD64 (FcγRI), as well as FcRn. Receptors implicated in FVIII uptake are also present on monocytes/macrophages.29 For example, LRP1/CD91, an endocytic receptor, has been shown to bind FVIII30 and was found to be upregulated in monocytes from HemA patients.31 As rFVIIIFc is administered IV, blood monocytes of HemA patients are the first innate immune cells to interact with the administered FVIII. In tissues, Kupffer cells, metallophilic macrophages, and marginal zone macrophages also accumulate FVIII.32-34
Taking the available data into consideration, this study aims to understand the mechanisms of rFVIIIFc recognition by human macrophages and its consequences on polarization and function. We report that in vitro treatment of macrophages with rFVIIIFc mediates functional polarization of the cells orientating them toward the alternatively activated Mox/M2 phenotype. These findings may help to elucidate the mechanisms of potential novel immunomodulatory properties of rFVIIIFc.
Materials and methods
Monocyte-derived macrophages, cell culture, and treatments
Human monocyte-derived macrophages were generated from CD14+ monocytes isolated from peripheral blood mononuclear cells of healthy human donors or inhibitor-negative HemA patients. The research was approved by the Western Institutional Review Board (WIRB #20162900 [healthy volunteers, n = 40] and #20100780 [HemA patients, n = 7]), and the blood donors gave written informed consent. Purified CD14+ monocytes were plated in RPMI 1640 Glutamax medium (Thermo Fisher Scientific) supplemented with penicillin, streptomycin, and 10% fetal bovine serum. Macrophage differentiation was achieved by plate adherence for 24 hours. Macrophages were treated with horse radish peroxidase immune complexes (HRP-ICs), human IgG1, B-domain deleted rFVIII, or rFVIIIFc (200 nM each) for 5 minutes to 24 hours. Treatment concentrations were determined in preliminary experiments (supplemental Figure 1). Mutant forms of rFVIIIFc molecules, that is, rFVIIIFc IHH (amino acid substitutions I253A, H310A, and H435A) and rFVIIIFc N297A (amino acid numbers correspond to the Fc domain numbering), unable to bind to the FcγRs or FcRns, respectively,14 were evaluated each at 200 nM concentration.
RNA sequencing and real-time quantitative reverse transcription polymerase chain reaction
Total RNA was isolated from macrophages using RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed using SuperScript III Vilo Kit (Thermo Fisher Scientific). High-throughput RNA sequencing was performed by New York Genome Center (supplemental Figure 2). Data were deposited into Gene Expression Omnibus database (accession number GSE118157). Quantitative real-time polymerase chain reaction (PCR) assays were performed using Taqman gene expression assays from Thermo Fisher Scientific and run on a 7500 Fast instrument. The comparative cycle threshold method was used to quantify transcripts relative to the endogenous control gene 36B4.
Metabolic profiling and validation assays
Metabolic screening of lysates from the treated macrophages (6 hours and 24 hours) was performed by Metabolon using the HD4 Global Metabolomics Technology. Mitochondrial transmembrane potential was measured using JC-1 (Thermo Fisher Scientific) and tetramethylrhodamine ethyl ester perchlorate (TMRE; Abcam) labeling. JC-1 (50 nM) or TMRE (200 nM) was added 15 minutes before the termination of the 24-hour treatment, and signal intensity was measured by flow cytometry. MitoTracker Green FM M7514 (Thermo Fisher) was used to determine mitochondrial mass. Glutathione production was measured using the reduced glutathione (GSH)/oxidized glutathione disulfide (GSSG)–Glo Assay kit (Promega); adenosine triphosphate (ATP) production was measured using the CellTiter-Glo MT Cell Viability Assay (Promega).
Statistical analysis
Statistical comparisons were made using paired Student t test, and level of significance was set to .05. Statistical calculations compared experimental samples to the IgG1-treated samples unless otherwise noted. For all experiments, the mean and standard deviation or standard error (SE) are reported for at least n = 3 (n = number of individual experiments). Significance: *P ≤ .05; **P ≤ .01; ***P ≤ .005. The Metabolon data set was analyzed using repeated measure analysis of variance. P ≤ .05 was considered as significant difference.
Additional information
Additional information can be found in the supplemental Materials and Methods.
Results
rFVIIIFc is recognized by macrophages via Fcγ receptors and initiates signaling without classical cell activation
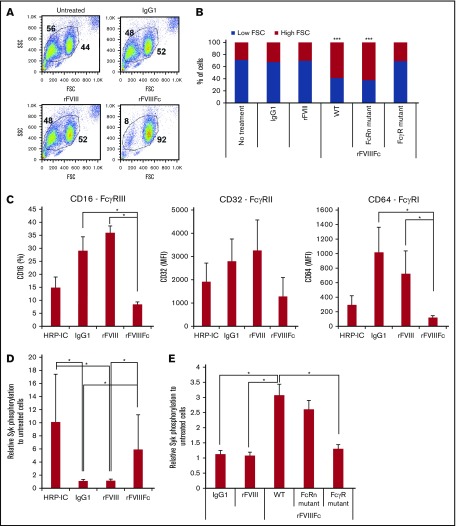
To investigate the effects of rFVIIIFc vs rFVIII on human monocyte-derived macrophages, the cells were treated with IgG1 as negative control, with rFVIII, or with rFVIIIFc for 24 hours (200 nM each). Cells treated with rFVIIIFc adhered strongly to the tissue culture plates, compared with IgG1- or rFVIII-treated cells (data not shown). Flow cytometry showed a distinct shift of the rFVIIIFc-treated macrophages in forward scatter, compared with untreated, IgG1-, or rFVIII-treated cells, indicating cell size changes (Figure 1A). These morphological effects were dependent on the specific interaction between rFVIIIFc and FcγRs, as these changes were not observed upon treatment with mutant rFVIIIFc-N297A (unable to bind to the FcγR), but were observed upon treatment with mutant rFVIIIFc-IHH (unable to bind to FcRn) (Figure 1B). These data suggested that rFVIIIFc actively influences macrophages via FcγR interactions. Next, HRP-IC as positive control for FcγR engagement and internalization, IgG1, rFVIII, or rFVIIIFc were used to treat macrophages, and expression of CD16, CD32, and CD64 was measured. HRP-IC or rFVIIIFc treatment, but not IgG1 or rFVIII treatment, decreased the cell surface expression of FcγRs (Figure 1C), indicating receptor engagement and internalization of rFVIIIFc by macrophages.
Figure 1.
rFVIIIFc induces Fcγ receptor internalization and signaling in an Fc-dependent fashion. (A) Forward scatter (FSC)/side scatter (SSC) pseudocolor plots of macrophages treated with IgG1, rFVIII, or rFVIIIFc (each at 200 nM) for 24 hours, illustrating the size change of the cells upon rFVIIIFc treatment. Numbers in FSC/SSC pseudocolor plots indicate the percentage of smaller and larger cell populations. One representative experiment is shown. (B) Graph shows cumulative data on macrophage size based on the percentages of low-FSC and high-FSC cell populations, observed by flow cytometry (n = 12). Significance indicated compared with untreated cells. (C) Macrophages (n = 4) were treated with HRP-ICs as positive control, IgG1 as negative control, rFVIII, or rFVIIIFc for 24 hours, and the cell surface expression of the FcγRs CD16, CD32, and CD64 was measured by flow cytometry. Downregulation of the FcγRs from the cell surface indicates internalization. (D) Macrophages (n = 10) were treated with HRP-IC, IgG1, rFVIII, or rFVIIIFc for 15 minutes, and the phosphorylation of Syk was measured using the Meso Scale Diagnostics (MSD) platform. Upregulation of pSyk levels shows direct FcγR engagement by HRP-IC and rFVIIIFc. (E) Mutant rFVIIIFc molecules unable to bind the FcRn (FcRn mutant) or the FcγRs (FcγR mutant) were used to treat macrophages (n = 4) along with the wild-type rFVIIIFc (WT), IgG1, and rFVIII for 30 minutes, and Syk phosphorylation was measured using the MSD platform. Downregulation of pSyk after FcRn and FcγR mutant treatment compared with WT rFVIIIFc shows the role of these receptors in signal transduction. Mean ± SE; *P ≤ .05, ***P ≤ .005.
To determine if rFVIIIFc engagement of FcγR results in signaling downstream of the FcγRs, Syk phosphorylation was monitored. After 15 minutes of treatment, phospho-Syk (pSyk) was clearly detected in HRP-IC- or rFVIIIFc-treated macrophages (Figure 1D), but not in IgG1- or rFVIII-treated cells. Syk phosphorylation induced by rFVIIIFc required FcγR binding, because treatment with the rFVIIIFc-N297A mutant did not generate pSyk, whereas the rFVIIIFc-IHH mutant was still able to induce pSyk (Figure 1E).
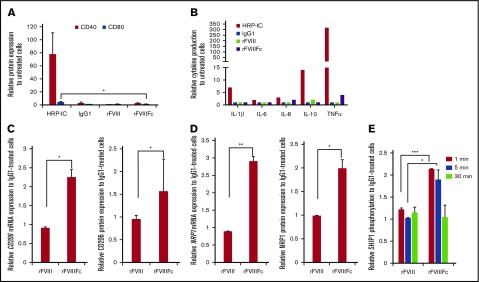
Although rFVIIIFc induced pSyk to levels comparable to those induced by HRP-IC, the signaling events generated by rFVIIIFc did not translate to immunogenic activation of macrophages. Cell surface expression of classical macrophage activation markers CD40 and CD80 were not upregulated (Figure 2A), nor were proinflammatory cytokines produced (Figure 2B), compared with HRP-IC treatment. Rather, rFVIIIFc-treated macrophages expressed higher levels of mannose receptor (CD206/MRC1) and neuropilin 1 (NRP1), compared with IgG1- or rFVIII-treated cells (Figure 2C-D). CD206/MRC1and NRP118 have been reported to be characteristic of alternatively activated M2 macrophages. In contrast, the positive control HRP-IC treatment resulted in production of a significant amount of proinflammatory cytokines (Figure 2B).
Figure 2.
rFVIIIFc treatment does not activate macrophages. (A) Macrophages (n = 3) were treated with IgG1, rFVIII, or rFVIIIFc for 24 hours, and the cell surface expression of the costimulatory/activation molecules CD40 and CD80 were measured by flow cytometry. The absence of significant molecule upregulation shows no classical activation of macrophages upon rFVIIIFc treatment. (B) Macrophages (n = 8) were treated with HRP-IC, IgG1, rFVIII, or rFVIIIFc for 24 hours, and the production of interleukin-1β (IL-1β), IL-6, IL-8, IL-10, and tumor necrosis factor α (TNFα) cytokines were measured from cell supernatants using multiplex enzyme-linked immunosorbent assay. Compared with the proinflammatory activation signal from HRP-IC, rFVIIIFc treatment of macrophages did not significantly upregulate proinflammatory cytokine production (error bars and significance not shown for graph clarity). RNA and protein levels of the alternatively activated macrophage markers mannose receptor CD206 (n = 7) (C) and NRP1 (n = 3) (D) were measured by quantitative PCR (qPCR) and flow cytometry, respectively. (E) Macrophages (n = 3) were treated with IgG1, rFVIII, or rFVIIIFc for 1, 5, or 30 minutes, and phosphorylated SHIP1 was measured using the MSD platform. Significant elevation of pSHIP1 levels after rFVIIIFc treatment compared with IgG1 or rFVIII treatments shows triggering of inhibitory signaling events. Mean ± SE; *P ≤ .05, **P ≤ .01, ***P ≤ .005.
To investigate whether inhibitory signaling molecules35 play a role in pathways triggered by rFVIIIFc, we measured the phosphorylation status of Src homology region 2 domain-containing phosphatase (SHP) and SH2-containing inositol phosphatase (SHIP) molecules after treatment with IgG1, rFVIII, or rFVIIIFc. Treatment with rFVIIIFc for 1 to 5 minutes resulted in the phosphorylation of SHIP1, compared with treatment with IgG1 or rFVIII (Figure 2E), whereas phosphorylation of SHP1, SHP2, and SHIP2 did not differ significantly between the different treatments (data not shown).
Taken together, these data indicate that although rFVIIIFc and HRP-IC engaged FcγRs, they triggered different signaling outcomes after Syk phosphorylation. The lack of classical, proinflammatory macrophage activation markers and the induction of SHIP1 phosphorylation, together with regulatory macrophage marker expression, suggest that rFVIIIFc does not induce an immunogenic response in the treated macrophages in vitro.
rFVIIIFc-treated macrophages exhibit specific gene expression pattern indicating a shift in phenotype
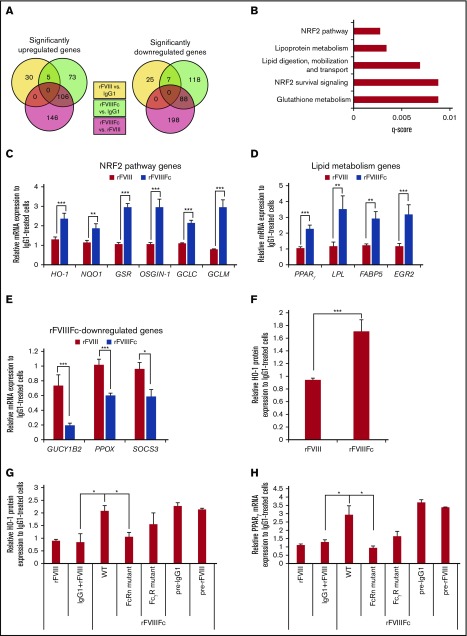
To further characterize the rFVIIIFc-treated macrophages, high-throughput RNA sequencing was performed on IgG1-, rFVIII-, or rFVIIIFc-treated cells. We found more than 200 genes that were significantly up- or downregulated in rFVIIIFc-treated macrophages, compared with IgG1- or rFVIII-treated cells (Figure 3A). Pathway analysis revealed the overrepresentation of NRF2-regulated antioxidant and the PPARγ-regulated lipid metabolism pathway genes (Figure 3B). qPCR validated significant changes in gene expression upon rFVIIIFc treatment, compared with rFVIII or IgG1 treatment. The key NRF2-regulated gene, HO-1, as well as genes involved in antioxidant responses and the production of GSH, were significantly upregulated upon rFVIIIFc treatment compared with rFVIII or IgG1 treatment (Figure 3C). Conversely, GUCY1B2, an NRF2 pathway inhibitor; PPOX, an inhibitor of HO-1; and SOCS3, a negative feedback regulator of proinflammatory cytokines, were downregulated in rFVIIIFc-treated cells (Figure 3E). PPARγ and related lipid metabolism genes were also elevated significantly upon rFVIIIFc treatment (Figure 3D). Protein expression of HO-1 was also significantly upregulated in rFVIIIFc-treated macrophages compared with either IgG1- or rFVIII-treated cells (Figure 3F). These genes/pathways have been used as markers of Mox and M2-like macrophages.17,21
Figure 3.
rFVIIIFc induces specific gene expression pattern in macrophages, resembling regulatory phenotype. Macrophages were treated with IgG1, rFVIII, or rFVIIIFc for 6 hours, and total RNA was isolated for high-throughput RNA sequencing. (A) Venn diagrams show significantly up- and downregulated genes between treatment groups (n = 3). (B) Pathway analysis of the first 200 significantly upregulated genes by rFVIIIFc treatment identifies NRF2 and lipid metabolism pathways as overrepresented processes affected by rFVIIIFc treatment. Validation of gene expression changes initiated by rFVIIIFc: NRF2 pathway–related genes (C), lipid metabolism–related genes (D), downregulated genes (E) (n = 6-15). (F) Macrophages (n = 12) were treated with IgG1, rFVIII, or rFVIIIFc for 24 hours, and the intracellular protein levels of HO-1 were measured by flow cytometry. Expression of HO-1 (G) and PPARγ (H) were followed, after treatment with IgG1, rFVIII, rFVIIIFc, modified rFVIIIFc unable to bind to FcRn (FcRn mutant), or modified rFVIIIFc unable to bind to FcγRs (FcγR mutant) for 6 hours (PPARγ gene expression) or 24 hours (HO-1 protein expression). Macrophages were also pretreated with IgG1 (pre-IgG1) or rFVIII (pre-rFVIII) for 1 hour before 24 hours rFVIIIFc treatment (n = 5). Mean ± SE; *P ≤ .05, **P ≤ .01, ***P ≤ .005.
Next, we investigated whether the rFVIIIFc-induced gene changes rely on the Fc part of the molecule, by following the expression of HO-1 and PPARγ. FcγR and FcRn binding mutants of rFVIIIFc were impaired in their ability to induce the expression of HO-1 protein and PPARγ transcript, suggesting the involvement of both receptors in mediating rFVIIIFc-induced alterations in macrophage phenotype (Figure 3G-H). Simultaneous administration of IgG1 and rFVIII did not elevate the expression of either HO-1 or PPARγ, and pretreatment with IgG1 or rFVIII did not impact the ability of rFVIIIFc to elevate the expression of these molecules (Figure 3G-H). These data suggest that the Fc must be linked to rFVIII to induce the alterations in macrophage phenotype and that the fusion molecule is recognized by macrophages in a manner distinct from recognition of rFVIII alone and IgG1 alone.
These data show that rFVIIIFc, compared with rFVIII, induces specific gene expression changes that suggest change in phenotype and metabolism in macrophages, and the Fc part of rFVIIIFc is instrumental in this process.
rFVIIIFc-treated macrophages exhibit a specific metabolism pattern resembling regulatory type cells
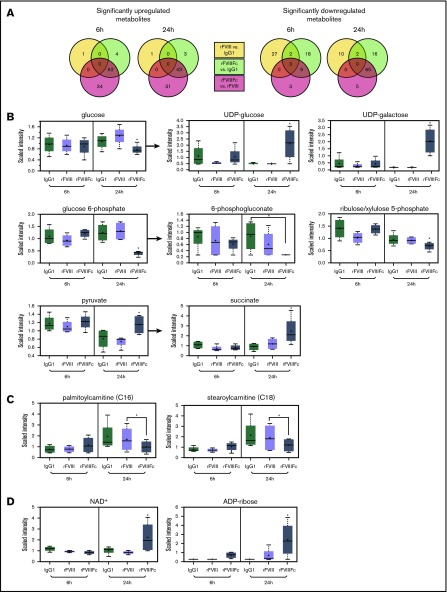
As the observed gene expression signature corresponded to regulation of metabolism, we investigated the metabolic profile of the rFVIIIFc-treated macrophages. Lysates of rFVIIIFc-treated cells showed a metabolite profile distinct from IgG1- or rFVIII-treated macrophages (Figure 4A; supplemental Figure 3). Of a total of 578 identified metabolites, 144 were changed significantly between the different treatments. A glucose utilization shift was seen in rFVIIIFc-treated cells compared with IgG1- or rFVIII-treated cells. First, glucose was decreased and glucose-6-phosphate was depleted at 24 hours (Figure 4B), with no change in lactate levels (data not shown). Second, most intermediates of the pentose phosphate pathway were significantly reduced at 24 hours in rFVIIIFc-treated macrophages (6-phosphogluconate and ribulose/xylulose 5-phosphate are shown in Figure 4B). Third, the pyruvate level was maintained at 24 hours in the rFVIIIFc-treated macrophages, while drastically decreased in IgG1- and rFVIII-treated cells (Figure 4B). The pyruvate appeared to feed acetyl-coenzyme A to the mitochondrial trichloroacetic acid (TCA) cycle resulting in elevated succinate levels (Figure 4B). The reduced nicotinamide adenine dinucleotide (NADH)+ generated by the TCA cycle may drive the oxidative phosphorylation, producing ATP for the bioenergetic needs of rFVIIIFc-treated macrophages. The data suggest that treatment with rFVIIIFc shuttles some glucose to produce UDP-glucose/UDP-galactose via 6-phosphogluconate (Figure 4B). In summary, rFVIIIFc seems to shift macrophage metabolism toward oxidative phosphorylation.
Figure 4.
rFVIIIFc-treated macrophages exhibit a characteristic metabolic signature, resembling the regulatory phenotype. Metabolite screening was performed on lysates of macrophages (n = 4) treated with IgG1, rFVIII, or rFVIIIFc for 6 hours or 24 hours. (A) Of a total of 578 identified metabolites, 144 were changed significantly between the different treatments. Selected individual metabolites are shown: glycolysis and related metabolism pathways (B), long chain fatty acids (C), and oxidative phosphorylation (D). Mean ± standard deviation; *P ≤ .05. UDP, uracil-diphosphate.
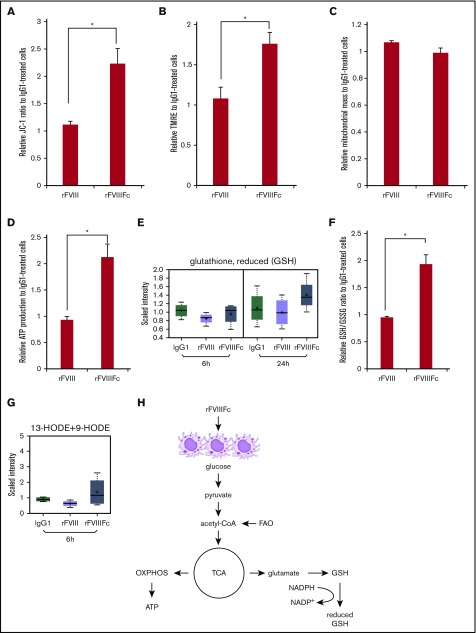
Changes in fatty acid metabolism were also noted. rFVIIIFc-treated macrophages showed upregulated β-oxidation of fatty acids, as indicated by a decrease in medium and long chain fatty acid levels (supplemental Figure 4; Figure 4C). These changes are consistent with the increased PPARγ gene expression (Figure 3D). Acetyl-coenzyme A from fatty acid metabolism can further feed the TCA cycle and oxidative phosphorylation. Elevated NAD+ and adenosine 5′-diphosphate–ribose levels found after rFVIIIFc treatment further suggest that macrophages are utilizing active oxidative phosphorylation (Figure 4D).
One other hallmark of oxidative phosphorylation is increased mitochondrial function, which can be assessed by measuring mitochondrial transmembrane potential (ΔϕM). We found significantly elevated mitochondrial functions in rFVIIIFc-treated macrophages, compared with rFVIII- or IgG1-treated cells, as measured by JC-1 ratio (Figure 5A), and by TMRE staining, which labels active mitochondria (Figure 5B). To confirm that the differences seen in mitochondrial activity did not result from the treatments affecting the mitochondrial mass, cells were labeled with MitoTracker. Figure 5C shows no treatment-associated alterations in mitochondrial mass. The significant increase of ATP levels measured in rFVIIIFc-treated cells proves active bioenergy production by mitochondria, compared with cells treated with rFVIII or IgG1 (Figure 5D).
Figure 5.
rFVIIIFc-treated macrophages exhibit elevated mitochondrial activity, bioenergy, and glutathione production. Macrophages were treated with IgG1, rFVIII, or rFVIIIFc for 24 hours. Significantly elevated levels of mitochondrial transmembrane potential (A) and active mitochondria staining (B) were measured from lysates of the rFVIIIFc-treated cells, compared with other treatments (n = 3). None of the treatments altered the total mitochondrial mass (C) indicating that rFVIIIFc-educated macrophages specifically upregulate their oxidative phosphorylation capacity (n = 3). Significantly elevated production of ATP (D, n = 5) and GSH (E, n = 3; F, n = 5) of rFVIIIFc-treated macrophages was measured compared with other treatments. (G) Endogenous PPARγ ligands 13-hydroxyoctadecadienoic acid (13-HODE) and 9-HODE were produced by rFVIIIFc-treated macrophages (n = 3). (H) Metabolism status of rFVIIIFc-treated macrophages is illustrated. Mean ± SE; *P ≤ .05.
Production of GSH is a strong indicator of antioxidant capacity of rFVIIIFc-treated macrophages, measured in the screening study (Figure 5E) and validated by determining the ratio between the reduced and oxidative forms of glutathione (Figure 5F). rFVIIIFc-treated macrophages also produced 9-HODE and 13-HODE, endogenous ligands for PPARγ (Figure 5G). These findings might explain how PPARγ is activated in rFVIIIFc-treated cells and subsequently executes its regulatory functions on lipid metabolism.
Mox macrophages are characterized by activation of NRF2-regulated antioxidant and regulatory activities, mainly through the enzymatic products of HO-1.36 This activity together with PPARγ-driven metabolic features, measured by gene expression and the metabolomics profiles, describe an M2-like metabolism with Mox-like features in rFVIIIFc-treated macrophages (summarized in Figure 5H).
Macrophages derived from HemA patients respond to rFVIIIFc treatment similarly to those from healthy individuals
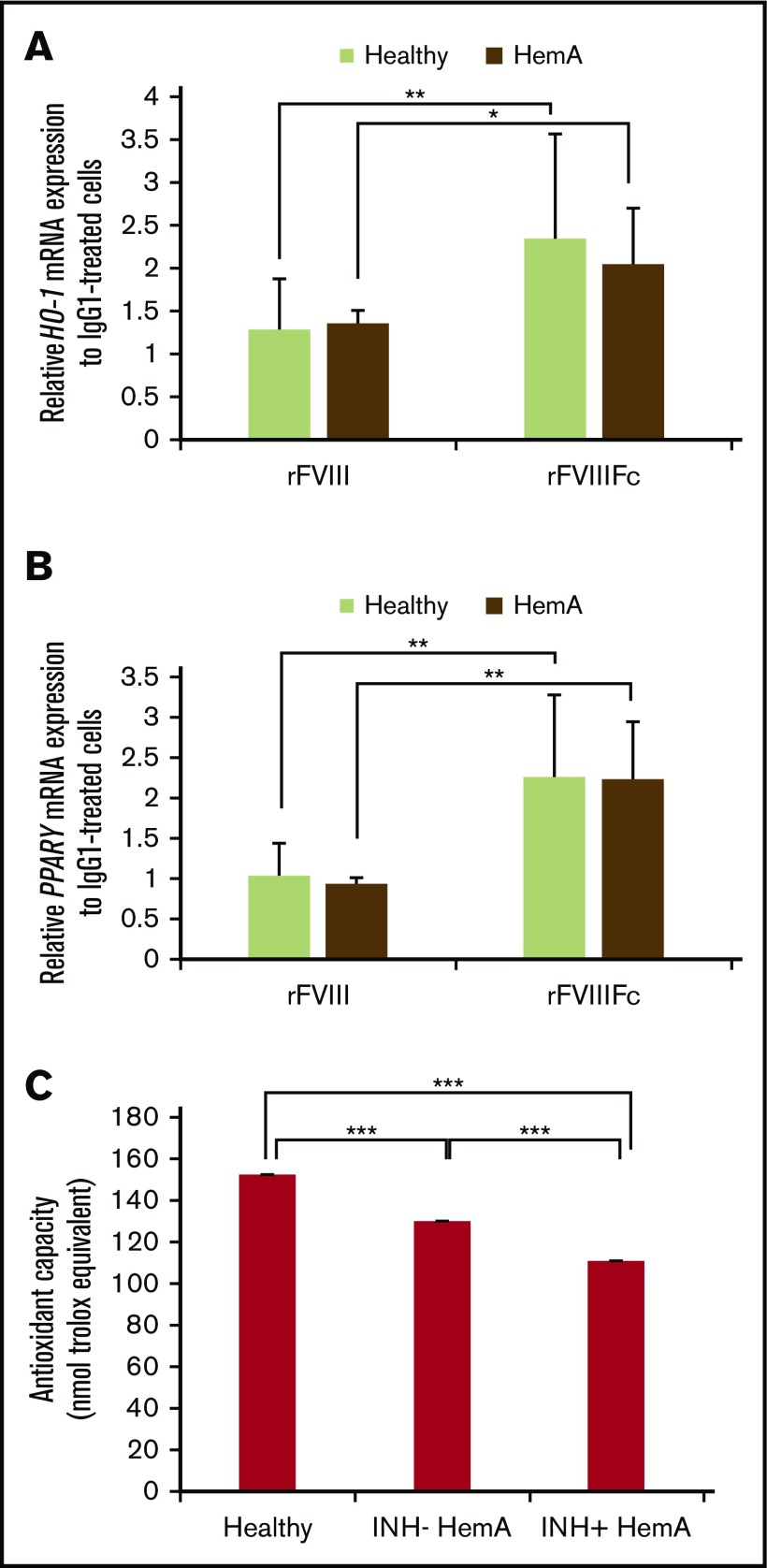
As the previous studies were conducted with cells isolated from healthy individuals, we wished to investigate whether macrophages from individuals with hemophilia respond similarly to normal macrophages when treated with rFVIIIFc in vitro. Monocytes were isolated from inhibitor-negative HemA patients, differentiated to macrophages, treated with IgG1, rFVIII, or rFVIIIFc, and messenger RNA expression of HO-1 and PPARγ were measured, alongside healthy macrophages. rFVIIIFc-treated hemophilia macrophages significantly upregulated the 2 signature genes, similarly to healthy macrophages (Figure 6A-B). Because cells were not available, we could not perform such gene expression experiments on inhibitor-positive individuals.
Figure 6.
HemA macrophages respond to rFVIIIFc treatment similarly to healthy individuals; plasma samples from individuals with hemophilia exhibit compromised antioxidant function. Macrophages from healthy donors and inhibitor-negative HemA patients were treated with IgG1, rFVIII, or rFVIIIFc for 6 hours, and RNA was isolated. Gene expression changes of HO-1 (n = 16 healthy donors and n = 7 HemA patients) (A) and PPARγ (n = 15 healthy donors and n = 7 HemA patients) (B) were measured by qPCR. (C) Antioxidative capacity of healthy vs inhibitor-negative vs inhibitor-positive HemA plasma samples (n = 27 each) shows significant impairment in hemophilia patients. Mean ± SE; *P ≤ .05, **P ≤ .01, ***P ≤ .005.
Plasma samples from hemophilia patients exhibit compromised antioxidant function
One of the main functions of Mox macrophages is the regulation of oxidative homeostasis as an adaptive cellular response against inflammatory responses and related oxidative injury.37 Progressive arthropathy accompanied by inflammation is triggered by recurrent joint bleeds in patients, especially in inhibitor-positive hemophilia patients.38 Inflammation-induced alterations in redox state might affect immune responses against FVIII in these patients. To investigate whether the oxidative state is different in healthy vs inhibitor-negative or inhibitor-positive HemA patients, plasma samples were analyzed for antioxidant capacity. Inhibitor-negative and inhibitor-positive plasma samples exhibited significantly decreased antioxidant capacity, but plasma samples from inhibitor-positive individuals were even more impaired than plasma from inhibitor-negatives, compared with plasma samples of nonhemophilia individuals (Figure 6C).
These data indicate that macrophages from inhibitor-negative HemA patients respond to rFVIIIFc with regulatory cell polarization. The rFVIIIFc-educated macrophages might have the capacity to contribute in restoring homeostatic antioxidative capacity in hemophilia patients.
Discussion
Among the various rFVIII molecules being used for HemA treatment,39 rFVIIIFc has an IgG1 Fc portion and therefore has the capacity to bind FcRns and FcγRs. The potential tolerogenic properties of Fc40-42 and its relevance to hemophilia treatment have been reviewed recently.43 However, the molecular mechanism behind the suggested immunomodulatory effects achieved by rFVIIIFc is not yet fully understood.
In this study, we aimed to investigate how rFVIIIFc engages the immune system. Based on our in vitro study, monocyte-derived human macrophages interact with rFVIIIFc through their FcRs, as shown by the downregulation of FcγRs from the surface of rFVIIIFc-treated cells. Moreover, rFVIIIFc induced phosphorylation of Syk, the proximal molecule of activator signaling, also suggesting functional engagement of FcγRs by rFVIIIFc. However, compared with the IC-induced proinflammatory activation of macrophages, rFVIIIFc did not activate the cells to become proinflammatory APCs. Rather, signaling events pointing toward immune regulation were detected.
Macrophages express both activating and inhibitory FcRs. Cross-linking of activating FcγR on APCs by antigen-antibody immune complexes enhances antigen uptake and maturation, expression of major histocompatibility complex II and costimulatory molecules, and secretion of proinflammatory cytokines, all of which substantially increase antigen presentation to T cells.44 The importance of Fc-FcγR interactions that inhibit or downregulate immune responses is also recognized.45 Molecular mechanisms involved in controlling the inhibitory signals from FcγRIIB/CD32B ligation on B cells have been reported.46,47 Moreover, presentation of peptides fused with IgG by B cells have been shown to induce tolerance.48,49 However, the activating and inhibitory roles for these FcRs are not entirely distinct.50 Under some conditions, inhibitory CD32B can mediate immune activation,51,52 or activating FcγRs can induce inhibitory signals.53 Because both activator (pSyk) and inhibitor (pSHIP1) signaling events were detected after rFVIIIFc treatment in macrophages, it is possible that rFVIIIFc acts both through activating and inhibitory FcγRs. Nonetheless, the net outcome of this engagement was not classical, proinflammatory M1 activation, but rather, rFVIIIFc seems to initiate a regulatory response in macrophages. It is also possible that FVIII receptors, simultaneously engaged by the same rFVIIIFc molecule on the same cell, have the capability to shape these FcR-initiated signaling events.
Macrophage polarization involves a coordinated metabolic and transcriptional rewiring. High-throughput RNA sequencing of the treated cells revealed an rFVIIIFc-specific gene expression pattern with overrepresentation of the repair/antioxidant NRF2 and the anti-inflammatory/lipid metabolism PPARγ pathways. These master regulator molecules and related genes are highly overlapping with the signature molecular pattern of the alternatively activated Mox and M2 macrophages. Metabolic profiling of rFVIIIFc-educated macrophages yielded a signature that is consistent with the metabolic description of alternatively activated macrophages. The metabolic profile first shows absence of increased glucose uptake, glucose-6-phosphate formation, and aerobic glycolysis associated with M1 activation.54 The profile also shows upregulated UDP-glucose/galactose production,55 lipid metabolism, β-oxidation, and mitochondrial activity, all pointing to an oxidative phosphorylation state characteristic of M2-like macrophages. Further, elevated GSH production is a hallmark signature of Mox macrophages.20 The above-mentioned findings would indicate that we have, for the first time, described the metabolic state of human Mox-like macrophages. In contrast to the rFVIIIFc-educated human macrophages, the oxidized phospholipid-induced metabolism in mouse Mox macrophages heavily relies on glucose metabolism and the pentose phosphate pathway to support GSH production and NRF2-dependent antioxidant gene expression, identified as a TLR2-Syk-ceramide-dependent redox homeostasis.20 The marked differences between these 2 types of antioxidant macrophage metabolism most likely come from the different initiation signals, one being an activator danger signal associated, whereas rFVIIIFc seems to activate macrophages in a tolerogenic, non–danger signal dependent way. Bone marrow–derived mouse macrophages treated with rFVIIIFc showed significant upregulation of Mox signature molecule HO-1 and M2 signature molecule arginase 1, as well as elevated GSH production, compared with IgG1- or rFVIII-treated cells (supplemental Figure 5). These experiments demonstrated that mouse macrophages are also capable of Mox/M2 polarization upon rFVIIIFc treatment in vitro.
The transcriptional upregulation and function of PPARγ has been shown to be IL-4 dependent.56 Here we describe an IL-4 independent, novel regulation of PPARγ expression. rFVIIIFc did not upregulate either IL-4 production or the expression of many of the “classical” IL-4-induced genes in macrophages (data not shown). The first-time demonstration of endogenous production of the PPARγ ligands 13-HODE and 9-HODE by rFVIIIFc-educated macrophages further suggests that these macrophages are equipped to execute tolerogenic functions. The high level of ATP production along with dynamic gene expression and phenotypic changes communicates that rFVIIIFc-treated macrophages actively respond to this treatment with robust cellular and molecular changes. The functional consequence of this active polarization is a characteristic macrophage population with immunomodulatory properties.
We further confirmed that the effects of rFVIIIFc involved Fc, as these changes were not observed in macrophages treated with rFVIII or with modified rFVIIIFc molecules, which were unable to bind either to FcRns or FcγRs. Combination treatment with IgG1 and rFVIII did not result in HO-1 or PPARγ upregulation showing that FcR engagement must occur with FVIII sensing, most probably coming from the same molecule. FcRn has been implicated in NRF2 activation through p62 activity during erythrophagocytosis, by acting as a protective mechanism to preserve macrophage homeostasis57; it is possible that a similar, noncanonical signaling pathway is activated in the absence of oxidative stress during rFVIIIFc phagocytosis, generating an FcRn-dependent antioxidant polarization in macrophages. These data support a hypothesis that FcRs and FVIII receptors might colocalize in the same lipid raft and collaborate to induce unique signaling events resulting in immunoregulatory polarization of macrophages.
Interestingly, induction of HO-1 in FVIII-deficient mice reduces immune response to therapeutic FVIII,25 and microsatellite polymorphisms in the HMOX1 promoter are associated with inhibitor development against therapeutic FVIII,26 indicating the importance of the immunomodulatory effects of the NRF2/HO-1 pathway in HemA. Macrophages from inhibitor-negative HemA patients showed similar induction of HO-1 and PPARγ upon rFVIIIFc treatment, suggesting the capacity to similarly polarize these cells. However, sufficient cell numbers from inhibitor-positive HemA individuals are not obtainable to test whether the polarization responses of those macrophages in response to rFVIIIFc might be different from inhibitor-negative individuals. Impaired antioxidant activity of plasma samples from HemA patients also suggest disturbances in the homeostatic state in these patients, possibly because of the ongoing immune activation and inflammation triggered by bleeds. By promoting the existence of regulatory/repair macrophages, rFVIIIFc might help to deal with this disturbed homeostasis in hemophilia patients.
Collectively, our data help to elucidate possible mechanisms by which the immune system responds to rFVIIIFc. We have shown for the first time that rFVIIIFc, in an FcR-dependent way, can induce macrophages to polarize to an Mox/M2 phenotype in vitro, without need for a danger signal or IL-4. This polarization results in an antioxidant state. Ultimately, this phenotypic change of macrophages might also contribute to the immune response in HemA patients. More data from controlled clinical studies will be needed to see the effects of rFVIIIFc on inhibitor development and ITI success.
Supplementary Material
The full-text version of this article contains a data supplement.
Footnotes
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE118157).
Authorship
Contribution: K.K.-T. designed the research, performed experiments, analyzed results, made the figures, and wrote the manuscript; G.M.R., A.S., and K.L.H. performed and analyzed experiments; J.D., R.T.P., and J.S. contributed in study design and discussions; and C.L. designed and discussed the research and wrote the manuscript.
Conflict-of-interest disclosure: K.K.-T., G.M.R., A.S., J.D., R.T.P., J.S., and C.L. are or were employees of Bioverativ, a Sanofi company. K.L.H. is an employee of Biogen.
Correspondence: Katalin Kis-Toth, Bioverativ, a Sanofi company, 225 Second Ave, Waltham, MA 02451; e-mail: katalin.kistoth@bioverativ.com.
REFERENCES
- 1.Franchini M, Mannucci PM. Past, present and future of hemophilia: a narrative review. Orphanet J Rare Dis. 2012;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. . Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535-544. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. ; Treatment Guidelines Working Group on Behalf of The World Federation of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1-e47. [DOI] [PubMed] [Google Scholar]
- 4.Gouw SC, van der Bom JG, Ljung R, et al. ; PedNet and RODIN Study Group. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med. 2013;368(3):231-239. [DOI] [PubMed] [Google Scholar]
- 5.Benson G, Auerswald G, Elezović I, et al. . Immune tolerance induction in patients with severe hemophilia with inhibitors: expert panel views and recommendations for clinical practice. Eur J Haematol. 2012;88(5):371-379. [DOI] [PubMed] [Google Scholar]
- 6.Colowick AB, Bohn RL, Avorn J, Ewenstein BM. Immune tolerance induction in hemophilia patients with inhibitors: costly can be cheaper. Blood. 2000;96(5):1698-1702. [PubMed] [Google Scholar]
- 7.Mahlangu J, Powell JS, Ragni MV, et al. ; A-LONG Investigators. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell JS, Josephson NC, Quon D, et al. . Safety and prolonged activity of recombinant factor VIII Fc fusion protein in hemophilia A patients. Blood. 2012;119(13):3031-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumont JA, Liu T, Low SC, et al. . Prolonged activity of a recombinant factor VIII-Fc fusion protein in hemophilia A mice and dogs. Blood. 2012;119(13):3024-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters RT, Toby G, Lu Q, et al. . Biochemical and functional characterization of a recombinant monomeric factor VIII-Fc fusion protein. J Thromb Haemost. 2013;11(1):132-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malec LM, Journeycake J, Ragni MV. Extended half-life factor VIII for immune tolerance induction in haemophilia. Haemophilia. 2016;22(6):e552-e554. [DOI] [PubMed] [Google Scholar]
- 12.Groomes CL, Gianferante DM, Crouch GD, Parekh DS, Scott DW, Lieuw K. Reduction of factor VIII inhibitor titers during immune tolerance induction with recombinant factor VIII-Fc fusion protein. Pediatr Blood Cancer. 2016;63(5):922-924. [DOI] [PubMed] [Google Scholar]
- 13.Carcao M, Shapiro A, Staber JM, et al. . Recombinant factor VIII Fc fusion protein for immune tolerance induction in patients with severe haemophilia A with inhibitors—a retrospective analysis. Haemophilia. 2018;24(2):245-252. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamoorthy S, Liu T, Drager D, et al. . Recombinant factor VIII Fc (rFVIIIFc) fusion protein reduces immunogenicity and induces tolerance in hemophilia A mice. Cell Immunol. 2016;301:30-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bories GFP, Leitinger N. Macrophage metabolism in atherosclerosis. FEBS Lett. 2017;591(19):3042-3060. [DOI] [PubMed] [Google Scholar]
- 16.Van den Bossche J, O’Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38(6):395-406. [DOI] [PubMed] [Google Scholar]
- 17.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. . Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marques L, Negre-Salvayre A, Costa L, Canonne-Hergaux F. Iron gene expression profile in atherogenic Mox macrophages. Biochim Biophys Acta. 2016;1862(6):1137-1146. [DOI] [PubMed] [Google Scholar]
- 20.Serbulea V, et al. . Macrophages sensing oxidized DAMPs reprogram their metabolism to support redox homeostasis and inflammation through a TLR2-Syk-ceramide dependent mechanism. Mol Metab. 2018;7:23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadl A, Meher AK, Sharma PR, et al. . Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107(6):737-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bretscher P, Egger J, Shamshiev A, et al. . Phospholipid oxidation generates potent anti-inflammatory lipid mediators that mimic structurally related pro-resolving eicosanoids by activating Nrf2. EMBO Mol Med. 2015;7(5):593-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi EH, Suzuki T, Funayama R, et al. . Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua S, Ek CJ, Mallard C, Johansson ME. Perinatal hypoxia-ischemia reduces α 7 nicotinic receptor expression and selective α 7 nicotinic receptor stimulation suppresses inflammation and promotes microglial Mox phenotype. BioMed Res Int. 2014;2014:718769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimitrov JD, Dasgupta S, Navarrete AM, et al. . Induction of heme oxygenase-1 in factor VIII-deficient mice reduces the immune response to therapeutic factor VIII. Blood. 2010;115(13):2682-2685. [DOI] [PubMed] [Google Scholar]
- 26.Repessé Y, Peyron I, Dimitrov JD, et al. ; ABIRISK consortium. Development of inhibitory antibodies to therapeutic factor VIII in severe hemophilia A is associated with microsatellite polymorphisms in the HMOX1 promoter. Haematologica. 2013;98(10):1650-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Huseini LM, Aw Yeang HX, Hamdam JM, et al. . Heme oxygenase-1 regulates dendritic cell function through modulation of p38 MAPK-CREB/ATF1 signaling. J Biol Chem. 2014;289(23):16442-16451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bunse CE, Fortmeier V, Tischer S, et al. . Modulation of heme oxygenase-1 by metalloporphyrins increases anti-viral T cell responses. Clin Exp Immunol. 2015;179(2):265-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartholt RB, van Velzen AS, Peyron I, Ten Brinke A, Fijnvandraat K, Voorberg J. To serve and protect: the modulatory role of von Willebrand factor on factor VIII immunogenicity. Blood Rev. 2017;31(5):339-347. [DOI] [PubMed] [Google Scholar]
- 30.Young PA, Migliorini M, Strickland DK. Evidence that factor VIII forms a bivalent complex with the low density lipoprotein (LDL) receptor-related protein 1 (LRP1): identification of cluster IV on LRP1 as the major binding site. J Biol Chem. 2016;291(50):26035-26044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franchini M, Urbani S, Amadei B, et al. . LRP1/CD91 is up-regulated in monocytes from patients with haemophilia A: a single-centre analysis. Haemophilia. 2013;19(3):e126-e132. [DOI] [PubMed] [Google Scholar]
- 32.Hartholt RB, Peyron I, Voorberg J. Hunting down factor VIII in the immunopeptidome. Cell Immunol. 2016;301:59-64. [DOI] [PubMed] [Google Scholar]
- 33.Lai JD, Cartier D, Hartholt RB, et al. . Early cellular interactions and immune transcriptome profiles in human factor VIII-exposed hemophilia A mice. J Thromb Haemost. 2018;16(3):533-545. [DOI] [PubMed] [Google Scholar]
- 34.van der Flier A, Liu Z, Tan S, et al. . FcRn rescues recombinant factor VIII Fc fusion protein from a VWF independent FVIII clearance pathway in mouse hepatocytes. PLoS One. 2015;10(4):e0124930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rauh MJ, Sly LM, Kalesnikoff J, et al. . The role of SHIP1 in macrophage programming and activation. Biochem Soc Trans. 2004;32(5):785-788. [DOI] [PubMed] [Google Scholar]
- 36.Chora AA, Fontoura P, Cunha A, et al. . Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest. 2007;117(2):438-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys. 2014;564:83-88. [DOI] [PubMed] [Google Scholar]
- 38.Oldenburg J, Kulkarni R, Srivastava A, et al. . Improved joint health in subjects with severe haemophilia A treated prophylactically with recombinant factor VIII Fc fusion protein. Haemophilia. 2018;24(1):77-84. [DOI] [PubMed] [Google Scholar]
- 39.Peters R, Harris T. Advances and innovations in haemophilia treatment. Nat Rev Drug Discov. 2018;17(7):493-508. [DOI] [PubMed] [Google Scholar]
- 40.Borel Y. Induction of immunological tolerance by a hapten (DNP) bound to a non-immunogenic protein carrier. Nat New Biol. 1971;230(14):180-182. [DOI] [PubMed] [Google Scholar]
- 41.Borel Y, Kilham L, Schwartz RS. Carrier-determined tolerance in various strains of mice: the role of isogenic IgG in the induction of hapten specific tolerance. Proc Soc Exp Biol Med. 1974;145(2):470-474. [DOI] [PubMed] [Google Scholar]
- 42.Borel Y, Golan DT, Kilham L, Borel H. Carrier determined tolerance with various subclasses of murine myeloma IgG. J Immunol. 1976;116(3):854-858. [PubMed] [Google Scholar]
- 43.Blumberg RS, Lillicrap D; IgG Fc Immune Tolerance Group. Tolerogenic properties of the Fc portion of IgG and its relevance to the treatment and management of hemophilia. Blood. 2018;131(20):2205-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcγ receptors in dendritic cells and macrophages [published correction appears in Nat Rev Immunol. 2014;14(5):349]. Nat Rev Immunol. 2014;14(2):94-108. [DOI] [PubMed] [Google Scholar]
- 45.Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications [published correction appears in Nat Rev Immunol. 2010;10(9):674]. Nat Rev Immunol. 2010;10(5):328-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolland S, Pearse RN, Kurosaki T, Ravetch JV. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 1998;8(4):509-516. [DOI] [PubMed] [Google Scholar]
- 47.Liu Q, Oliveira-Dos-Santos AJ, Mariathasan S, et al. . The inositol polyphosphate 5-phosphatase ship is a crucial negative regulator of B cell antigen receptor signaling. J Exp Med. 1998;188(7):1333-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Amine M, Melo M, Kang Y, Nguyen H, Qian J, Scott DW. Mechanisms of tolerance induction by a gene-transferred peptide-IgG fusion protein expressed in B lineage cells. J Immunol. 2000;165(10):5631-5636. [DOI] [PubMed] [Google Scholar]
- 49.Lei TC, Scott DW. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 2005;105(12):4865-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfirsch-Maisonnas S, Aloulou M, Xu T, et al. . Inhibitory ITAM signaling traps activating receptors with the phosphatase SHP-1 to form polarized “inhibisome” clusters. Sci Signal. 2011;4(169):ra24. [DOI] [PubMed] [Google Scholar]
- 51.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23(5):503-514. [DOI] [PubMed] [Google Scholar]
- 52.White AL, Chan HT, French RR, et al. . FcγRΙΙB controls the potency of agonistic anti-TNFR mAbs. Cancer Immunol Immunother. 2013;62(5):941-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blank U, Launay P, Benhamou M, Monteiro RC. Inhibitory ITAMs as novel regulators of immunity. Immunol Rev. 2009;232(1):59-71. [DOI] [PubMed] [Google Scholar]
- 54.Freemerman AJ, Johnson AR, Sacks GN, et al. . Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289(11):7884-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jha AK, Huang SC, Sergushichev A, et al. . Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419-430. [DOI] [PubMed] [Google Scholar]
- 56.Szanto A, Balint BL, Nagy ZS, et al. . STAT6 transcription factor is a facilitator of the nuclear receptor PPARγ-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33(5):699-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santarino IB, Viegas MS, Domingues NS, Ribeiro AM, Soares MP, Vieira OV. Involvement of the p62/NRF2 signal transduction pathway on erythrophagocytosis. Sci Rep. 2017;7:5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.