Abstract
Parkinson’s disease, the second major neurodegenerative disease, has created a great impact on the elder people. Although the mechanisms underlying Parkinson’s disease are not fully understood, considerable evidence suggests that neuro-inflammation, oxidative stress, mitochondrial dysfunction, cell proliferation, differentiation and apoptosis are involved in the disease. p38MAPK, an important member of the mitogen-activated protein family, controls several important functions in the cell, suggesting a potential pathogenic role in PD. This review provides a brief description of the role and mechanism of p38MAPK in Parkinson’s disease.
Keywords: Parkinson’s disease, p38MAPK, neuro-inflammation, oxidative stress, mitochondrial dysfunction
1. Introduction
Parkinson’s disease (PD), the second most common neurodegenerative disease following Alzheimer’s disease, has created a great impact on the elderly, the family and society. The average age of onset is about 60 years old, and the prevalence of Parkinson’s disease in older people over 65 increases with age[1]. It is estimated that approximately 10 million individuals worldwide suffer from this disease, though many cases may go undiagnosed. With the growth of aging populations, the number will double over the next 25 years, which causes enormous social and economic problems.
The major pathological features of PD include progressive loss of dopaminergic (DA) neurons and formation of intracellular Lewy bodies (LBs) in the survival neurons of substantia nigra (SN). Its clinical manifestations include rest tremor, bradykinesia, muscle rigidity, posture gait abnormalities and other movement disorders, as well as cognitive disabilities, sleep disorders and other non-motor barrier [2, 3], some of the symptoms of non-motor symptoms can occur prior to motor symptoms, and neurodegenerative neuropathies are not limited to SNc but have a broader impact[4, 5]. The etiology and pathogenesis of PD are complex and not yet fully understood. More studies suggest that genetic mutations in proteins play major role for the development and progression of PD[3, 6]. Neuro-inflammatory [7], oxidative stress[8], mitochondrial dysfunction[9] and cell proliferation, differentiation, apoptosis involve in the pathogenesis of both familial and idiopathic PD [10].
The MAPK cascade is a major intracellular signaling system that transmits extracellular information to the nucleus and mediates various cell responses and plays an significant role in cell proliferation, differentiation and apoptosis[11], it is one of the important signal-regulated enzymes that connect the cell surface receptors with the decisive gene expression. p38 Mitogen-activated protein kinase (p38MAPK) is an important member of the mitogen-activated protein kinase family. The p38MAPK signaling cascade is a major signaling pathway for endogenous and endogenous stimulation (including growth factors, stress and cytokines) in respond to endothelial cell function and accordingly mediating a wide range of cellular effects, which provides cells with mechanisms to responding to external mitogenic signals[12, 13, 14]. p38MAPK play an important role in the pathogenesis of PD.
2. p38MAPK involves in neuro-inflammation in PD progression
The PD patients showed accumulation of pro-inflammatory cytokines in the brain and cerebrospinal fluid, which demonstrates that neuro-inflammation is occurring in the affected brain area[15, 16]. In vivo evidence of neuropathic inflammation in PD patients includes cytokines and other molecular mediators expression disorders[17, 18, 19], microglia activation[20], peripheral immune cell invasion and changes around the composition and performance in Substantia nigra pars compacta (SNpc) [21]. Neuro-inflammation is thought to be an prominent pathological factor that contributes towards the development and progression of PD[22].
The development of neuro-inflammation plays an important role in the immune system of the central nervous system, which includes microglia and astrocytes[23]. The neuro-inflammation process begins with the activation of glial cells, producing many neurotoxic components including reactive oxygen species (ROS), nitric oxide synthase (NOS), cytokines and other inflammatory mediators, all of which can lead to neurodegeneration[24, 25]. Inflammatory triggers such as Aβ, lipopolysaccharide (LPS) and MPTP can trigger inflammation and activate microglial cells. In addition to the generation of large amounts of free radicals after microglial activation, a large number of pro-inflammatory cytokines are released, such as IL-1β, TNF-α, TNF-γ.[26, 27]. These inflammatory mediators can damage neurons and further activate microglial cells resulting in a vicious circle that aggravates neuro-inflammation and degeneration[28].
Activated microglia is observed in various degenerative neurological conditions such as PD and amyotrophic lateral sclerosis (ALS). Activated microglia can also increase ROS such as NO, superoxide Etc. As a result, these reactive substances can pass directly through the dopaminergic neurons against the endogenous antioxidant system and eventually cause oxidative stress and degeneration of dopaminergic neurons[29]. In addition, a series of enzymes, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX) 1 and 2 can be produced, which can cause some damage to dopaminergic neurons[30, 31, 32].
p38MAPK plays an important role in neuro-inflammation and degeneration. Microglia reaction is the core of dopamine neuron degeneration, and recent studies have shown that p38MAPK signaling pathway plays a key role in microglial activation and response impact[33, 34]. Rotenone, dexmedetomidine and paraquat can all activate microglial cells by directly activating p38MAPK, which release large amounts of cytokines and thus damaging dopamine neurons[22, 35, 36]. These toxins can also induce NF-κB activation by directly activating p38MAPK, and iNOS expression is up-regulated. In glial cells, p38MAPK induces
iNOS to catalyze the production of nitric oxide (NO) in a large amount, excessive NO can cause lipid peroxidation and other nerve damage[37, 38], inhibiting the synthesis of DNA, leading to neuron death. It can also react with superoxide radicals to generate peroxynitrite and initiate a series of cytotoxicity, eventually leading to neuron loss [39].
In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced Parkinson’s disease model mice, MPTP can induce the activation of p38 MAPK in the midbrain substantia nigra[40, 41, 42]. The activation of p38 leads to the phosphorylation of p38 and the increase of p-p38 leads to the up-regulation of cyclooxygenase-2 (COX-2), and the up-regulation of COX-2 increases prostaglandin E2 (PGE2)[31, 40]. COX-2 overexpression, COX-2-mediated inflammatory response will further activate caspase-3, which results in dopaminergic neuron degeneration[43]. In addition, the high expression of COX-2 can induce inflammatory response, make reactive glial cell proliferation, increase the release of collagen damage[43, 44]. COX-2 overexpression and its mediated inflammatory response involve in the oxidative stress response in the substantia nigra and cause damage to dopaminergic neurons [45, 46].
Lipopolysaccharide (LPS) is a major component of gram-negative bacterial cell walls and is now known to be an effective stimulator of macrophages in the brain. In vitro and in vivo studies have shown that LPS induced the activation of microglial cells leading to ROS, NOS and pro-inflammatory factors such as IL-1β, IL-6, TNF-α,IFNs production[47, 48]. p38 signaling cascade contributes to immune-related cytotoxicity and neurodegenerative disease sequelae, in the LPS-induced PD model, LPS induces activation of the p38 and JNK pathways, which can increase IL-1β, TNF-α and the production of iNOS, which eventually leads to midbrain neuronal death. [24].
3. p38MAPK acts in oxidative stress in PD development
Oxygen is essential for all human life activities, and is crucial for all living cells. Oxidative stress exerts a causative role of in loss of dopamine neurons, which has been considered to be the pathological hallmark of PD. Genetic, environmental, drugs and other factors can induce oxidative stress response, triggering the body’s redox reaction imbalance, resulting in dopamine neuron loss[49, 50, 51].
Oxidative stress triggers the p38 MAPK pathway, activating mitochondria and other mitochondrial apoptotic pathways in dopamine neurons. Paraquat, rotenone and MPTP all can directly or indirectly activate the p38MAPK pathway, resulting in increased accumulation of ROS [35, 52]. On the other hand, activated p38MAPK can enhance the oxidative stress, making neurodegeneration[39].
Oxidative stress increases the steady-state levels of ROS and the ROS can regulate the activation of MAPKs in various stimuli-triggered apoptosis[53, 54], the production of ROS activates JNK and p38 MAPK[55], which can induce the production of ROS increased. Too much ROS can in turn affect the activation of p38MAPK, the formation of a feedback loop play an important role in the development of PD.
Fig 1.
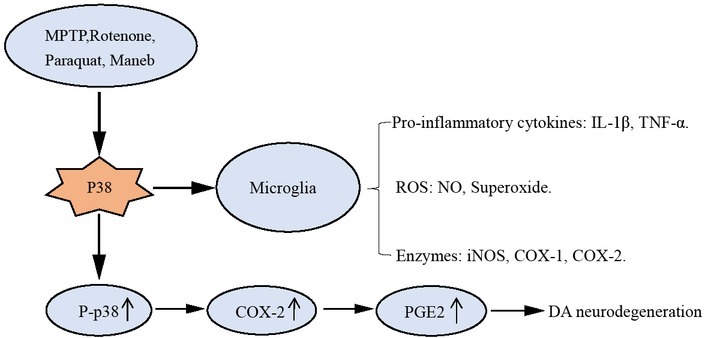
p38MAPK involves in neuro-inflammation in PD progression
4. p38MAPK makes an important role in mitochondrial dysfunction in PD occurrence
Mitochondria play a vital role in energy metabolism. They provide a large amount of available energy in the form of mitochondrial ATP for intracellular metabolic pathways[56, 57]. Mitochondria are highly dynamic, multifunctional organelles, in addition to their primary role in energy metabolism, they are also essential for many cellular processes including neurotransmission, synaptic maintenance, calcium homeostasis, cell death and neuronal survival[58, 59].
Mitochondrial dysfunction is a common feature of sporadic and familial PD. The main manifestations of mitochondrial dysfunction include ROS production, mitochondrial electron transport complex enzymatic activity defection, ATP depletion, caspase-3 release and mitochondrial DNA consumption[60, 61]. Inhibition of mitochondrial complex I or blockade of normal electron transfer may lead to ROS increase and ATP decrease[58], which may damage mitochondrial DNA, destroy respiratory chain and triggering a vicious cycle between mitochondrial damage and oxidation[4].
Energy failure, oxidative stress, genetic mutations and environmental toxicants in PD are closely linked to mitochondrial dysfunction[61]. Neurotoxins such as MPTP, rotenone and paraquat induce the death of dopamine neurons directly related to the mitochondrial complex I activity inhibition, which in turn may cause different mitochondrial disorders and subsequently neuronal degeneration[60, 62].
Mitochondria metabolism is the major sources for ROS that may contribute to intracellular oxidative stress, mitochondrial respiratory chain disorder, particularly complex I deficiency, and the increase of ROS may directly or indirectly lead to the production of sporadic PD[63, 64, 65]. Existing research shows that ROS can regulate intracellular signal cascades. Excessive ROS production can lead to intracellular stimulation and mitochondrial damage, eventually leading to apoptosis and necrosis.
MPTP, rotenone and paraquat can cause mitochondrial dysfunction, triggering other stimuli in neurons[42, 66]. MPTP is selectively toxic to dopaminergic neurons, it can cross the blood-brain barrier in minutes and is rapidly metabolized by monoamine oxidase B (MAOB) to the active metabolite MPP+ in the brain, which is selectively transported to dopaminergic neurons[67], then accumulates in the mitochondria[68, 69]. MPP+, an active metabolite in mitochondria, suppresses mitochondrial complex I in the electron transport chain, thereby disrupting the flow of electrons, leading to a decrease in ATP production and an increase in ROS production[68, 69, 70]. The expression of MAOB is regulated by the activation of p38 MAPK, and the activation of p38 MAPK is accompanied by astrocyte proliferation, and then causes astrocytes and neuron loss. The activation of MAOB can be prevented by inhibiting the p38 MAPK pathway[67, 71, 72].
Fig 2.
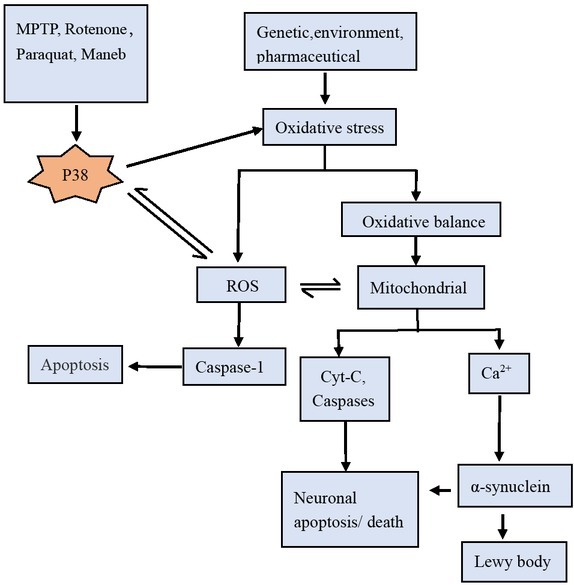
p38MAPK acts in oxidative stress in PD development
One possibility that cytoplasmic p38 affects mitochondria is that p38 activation induces the translocation of its substrate (p53) into mitochondria, which in turn eliminates unhealthy mitochondrial proteins and thereby protect mitochondrial dysfunction[73, 74].
On the other hand, activating the p38MAPK pathway may indirectly induce the mitochondrial pro-apoptotic protein Bax to produce CytC by activating p53, and CytC can activate caspase-3 and cause apoptosis of dopamine neurons[49, 58, 75]. In the study done by Fengsen Duan, ROS was found to regulate the expression of p38MAPK, eventually resulting in mitochondrial damage, which fed back each other and formed a vicious circle[55].
Mitochondrial dysfunction splays an important role in PD occurrence, progression and development. Currently there are many substances against mitochondrial damage used in PD treatment, such as antioxidant enzymes (SOD, CAT), α-lipoic acid, green tea polyphenols, melatonin, ginseng water extract, all showed an improved effect against PD[76, 77].
5. Conclusion
Parkinson’s disease affects approximately 1–2% of the population over 65 years of age, and up to 5% of the population by age 85. Though efforts have been made to elucidate the PD pathogenesis, the mechanisms are still not understood clearly. Neuro-inflammation, oxidative stress can accelerate the progress and development of Parkinson’s disease. Mitochondrial dysfunction plays an important role in the occurrence of PD[39].
p38, as a key member of the signal transduction pathways, plays a crucial role during the process of apoptosis. More and more evidence has shown that the activation of p38 MAPK signal pathway has a vital role in promoting the development of PD and the inhibitory effect of p38 can appropriately improve the therapeutic effect of PD, which may provide a new medicinal strategy for the treatment of PD, this pathway can be used as a breakthrough in the study of Parkinson’s disease, and then find effective control disease treatment. In vitro experimental studies showed that minocycline could prevent NO-induced phosphorylation of p38 and cell death associated with NO-induced toxicity, which was neuroprotective in many neurodegenerative models, such as the 1-methyl-4-phenyl-1, 2,3,6-hydrogen pyridine (MPTP) model of PD[78, 79].
Although there are many researches and some medicines have a therapeutic effect on Parkinson’s disease, none of them cure the disease fundamentally. Neuro-inflammation, oxidative stress and mitochondrial dysfunction in PD are closely liked to p38MAPK, it may be a target to PD. So for best understanding this signal pathway in PD occurrence progress and development is essential[80].
Fig 3.
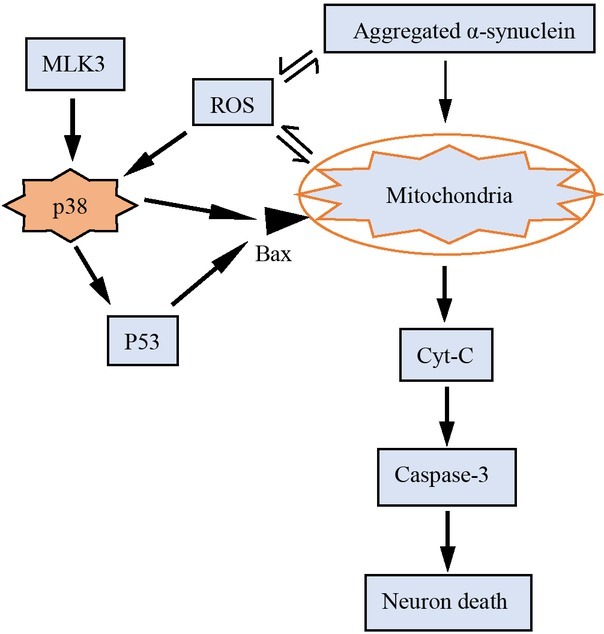
p38MAPK makes an important role in mitochondrial in PD occurrence
Acknowledgements
This work was supported by the fund of Yunnan key program of Science and Technology (2014FA031), National Natural Science Foundation of China (81460593) and Yunnan program of Science and Technology (2015FB006).
References
- [1].Rijk M C De, Tzourio C., Breteler M M, Dartigues J F, Amaducci L., Lopezpousa S., Manubensbertran J M, Alpérovitch A., Rocca W A. Prevalence of parkinsonism and Parkinson’s disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson’s disease[J], Journal of Neurology Neurosurgery & Psychiatry. 1997;62:10. doi: 10.1136/jnnp.62.1.10. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhangna Ke Kai Fu, Yihua Qiu, Yu-Ping Peng. Role of neuroinflammation in the pathogenesis of Parkinson’s disease[J], Transportation Medicine. 201256-58+62.
- [3].Xu Y., Deng Y., Qing H. The phosphorylation of α-synuclein: development and implication for the mechanism and therapy of the Parkinson’s disease[J] J. Neurochem. 2015;135:4. doi: 10.1111/jnc.13234. –. [DOI] [PubMed] [Google Scholar]
- [4].Nicole Exner, Lutz Anne Kathrin, Haass Christian, Winklhofer Konstanze F. Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences[J] EMBO J. 2012;31:3038. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Solla P., Cannas A., Floris G. L., Orofino G., Costantino E., Boi A., Serra C., Marrosu M. G., Marrosu F.. Behavioral, neuropsychiatric and cognitive disorders in Parkinson’s disease patients with and without motor complications[J], Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:1009. doi: 10.1016/j.pnpbp.2011.02.002. –. [DOI] [PubMed] [Google Scholar]
- [6].Zuo L., Motherwell M. S.. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease[J] Gene. 2013;532:18. doi: 10.1016/j.gene.2013.07.085. –. [DOI] [PubMed] [Google Scholar]
- [7].Whitton P. S.. Inflammation as a causative factor in the aetiology of Parkinson’s disease[J] Br. J. Pharmacol. 2007;150:963. doi: 10.1038/sj.bjp.0707167. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vera Dias, Junn Eunsung, Maral Mouradian M.. The Role of Oxidative Stress in Parkinson’s Disease[J] Journal of Parkinsons Disease. 2013;3:461. doi: 10.3233/JPD-130230. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wanglund C., Lynch D., Spathis R., Lum J. K., M R.. Evidence for mitochondrial dysfunction and its role in neurodegeneration in Guam ALS and PD[J] Amer. J. Hum. Biol. 2006;18:280. –. [Google Scholar]
- [10].Sun Sheng-Gang, Gang Li, Xuebing Cao, Calyx Tong. Research Progress on the Relationship between Parkinson ‘s Disease and Inflammatory Reaction[J] Chinese Journal of Neurology. 20034-6. [Google Scholar]
- [11].Zhen Junli, Weiping Wang, Liwei An, Ping Li Zhou, King Jia Li. Progress in research of p38 mitogen - activated protein kinase pathway and its related nervous system[J] Journal of Brain and Nervous Diseases. 2010:318. –. [Google Scholar]
- [12].Corre I, Paris F, Huot J. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells[J] Oncotarget. 2017;8:55684. doi: 10.18632/oncotarget.18264. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jin-Tao L. I., Wang Tin Hua, Li-Yan L. I. Role of P38,a Kind of Signaling Molecules in CNS Diseases[J] Journal of Kunming Medical University: 2012. [Google Scholar]
- [14].Coulthard Lydia R., White Danielle E., Jones Dominic L., Mcdermott Michael F., Burchill Susan A.. p38MAPK: stress responses from molecular mechanisms to therapeutics[J] Trends Mol. Med. 2009;15:369. doi: 10.1016/j.molmed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tiwari P. C., Pal R.. The potential role of neuroinflammation and transcription factors in Parkinson disease[J] Dialogues Clin. Neurosci. 2017;19:71. doi: 10.31887/DCNS.2017.19.1/rpal. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Przedborski Serge. Neuroinflammation and Parkinson’s disease[J], Handb. Clin. Neurol. 2007;83:535. doi: 10.1016/S0072-9752(07)83026-0. [DOI] [PubMed] [Google Scholar]
- [17].Makio Mogi, Harada Minoru, Kondo Tomoyshi, Riederer Peter, Inagaki Hirofumi, Minami Masayasu, Nagatsu Toshiharu. Interleukin-1β, interleukin-6, epidermal growth factor and transforming growth factor-α are elevated in the brain from parkinsonian patients[J] Neurosci. Lett. 1994;180:147. doi: 10.1016/0304-3940(94)90508-8. –. [DOI] [PubMed] [Google Scholar]
- [18].Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease[J] J. Neural Transm. Suppl. 2000;80:277. doi: 10.1007/978-3-7091-6301-6_19. –. [DOI] [PubMed] [Google Scholar]
- [19].Depino A. M., Earl C., Kaczmarczyk E., Ferrari C., Besedovsky H., Del A Rey, Pitossi F. J., Oertel W. H.. Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson’s disease[J] Eur. J. Neurosci. 2003;18:2731. doi: 10.1111/j.1460-9568.2003.03014.x. –. [DOI] [PubMed] [Google Scholar]
- [20].Mcgeer P. L., Itagaki S., Boyes B. E., G E.. Reactive microglia are positive for HLA‐DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains[J] Neurology. 1988;38:1285. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- [21].Mcgeer P. L., Itagaki S, Akiyama H, Mcgeer E. G.. Rate of cell death in parkinsonism indicates active neuropathological process[J] Ann. Neurol. 1988;24:574. doi: 10.1002/ana.410240415. –. [DOI] [PubMed] [Google Scholar]
- [22].Chao Y., Wong S. C., Tan E. K.. Evidence of inflammatory system involvement in Parkinson’s disease[J] Biomed Research International. 2014;2014:308654. doi: 10.1155/2014/308654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Skaper S. D.. The brain as a target for inflammatory processes and neuroprotective strategies[J] Ann. N. Y. Acad. Sci. 2007;1122:23. doi: 10.1196/annals.1403.002. –. [DOI] [PubMed] [Google Scholar]
- [24].Badshah H, Ali T, Rehman Shafiq Ur, Amin Faiz Ul, Ullah F, Kim T. H., Kim M. O.. Protective Effect of Lupeol Against Lipopolysaccharide-Induced Neuroinflammation via the p38/c-Jun N-Terminal Kinase Pathway in the Adult Mouse Brain[J] J. Neuroimmune Pharmacol. 2016;11:48. doi: 10.1007/s11481-015-9623-z. [DOI] [PubMed] [Google Scholar]
- [25].Hirsch E. C., Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson’s disease: a role in neurodegeneration?[J] Ann. Neurol. 1998;44:115. doi: 10.1002/ana.410440717. –. [DOI] [PubMed] [Google Scholar]
- [26].Susan Duty, Jenner Peter. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease[J] Br. J. Pharmacol. 2011;164:1357. doi: 10.1111/j.1476-5381.2011.01426.x. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee Mosley R., Benner Eric J., Kadiu Irena, Thomas Mark, Boska Michael D., Hasan Khader, Laurie Chad, Gendelman Howard E.. Neuroinflammation, Oxidative Stress and the Pathogenesis of Parkinson’s Disease[J] Clin. Neurosci. Res. 2006;6:261. doi: 10.1016/j.cnr.2006.09.006. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang X. H., Xie X., Luo X. G., Shang H., He Z. Y.. Inhibiting purinergic P2X7 receptors with the antagonist brilliant blue G is neuroprotective in an intranigral lipopolysaccharide animal model of Parkinson’s disease[J] Mol. Med. Report. 2017;15:768. doi: 10.3892/mmr.2016.6070. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Appel Stanley H., Beers David R., Henkel Jenny S.. T cell-microglial dialogue in Parkinson’s disease and amyotrophic lateral sclerosis: are we listening?[J] Trends Immunol. 2010;31:7. doi: 10.1016/j.it.2009.09.003. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Knott C, Stern G, Wilkin G. P.. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2[J] Molecular & Cellular Neuroscience. 2000;16:724. doi: 10.1006/mcne.2000.0914. –. [DOI] [PubMed] [Google Scholar]
- [31].Peter Teismann, Ferger Boris. Inhibition of the cyclooxygenase isoenzymes COX‐1 and COX‐2 provide neuroprotection in the MPTP‐ mouse model of Parkinson’s disease[J] Synapse. 2001;39:167. doi: 10.1002/1098-2396(200102)39:2<167::AID-SYN8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- [32].Li M., Dai F. R., Du X. P., Yang Q. D., Chen Y.. Neuroprotection by silencing iNOS expression in a 6-OHDA model of Parkinson’s disease[J] Journal of Molecular Neuroscience Mn. 2012;48:225. doi: 10.1007/s12031-012-9814-5. –. [DOI] [PubMed] [Google Scholar]
- [33].Li Gang, Rong Ma, Sheng-Gang Sun, Tong Tong Calyx. Role of p38 MAPK pathway in the degeneration of dopaminergic neurons mediated by microglial activation[J] Journal of Brain and Nervous Diseases. 2006;14:105. –. [Google Scholar]
- [34].Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF-kappa B-dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson’s disease[J] FASEB J. 2003;17:500. doi: 10.1096/fj.02-0314fje. [DOI] [PubMed] [Google Scholar]
- [35].Gao F., Chen D., Hu Q., Wang G.. Rotenone directly induces BV2 cell activation via the p38 MAPK pathway[J] PLoS One. 2013;8:e72046. doi: 10.1371/journal.pone.0072046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yinghao Pei, Cai Xiaomin, Jiao Chen, Sun Baodi, Sun Zhaorui, Xing Wang, Xiaomin Qian. The role of p38 MAPK in acute paraquat-induced lung injury in rats[J] Inhalation Toxicol. 2014;26:880. doi: 10.3109/08958378.2014.970784. –. [DOI] [PubMed] [Google Scholar]
- [37].Wang Qian, Hui Zhang, Name Liu, Zuofeng Zhang, Zifeng Wei, Sunna Mao Tong Yao, New Zhang Yu. P38 Signal Pathway Regulates NF-κB and iNOS Expression in Parkinson’s Disease Substantia Nigra[J] Journal of Southern Medical University. 2014;34:1176. –. [PubMed] [Google Scholar]
- [38].Wang Qian, Hui Zhang, Zuofeng Zhang, Zifeng Wei, Yongsheng Wang, Zhou Hongxia, New Zhang Yu. Regulation of P38MAPK on NF-κB and COX-2 in Parkinson’s Disease MPTP Model Mice[J] China Journal of Modern Medicine. 2012;22:15. –. [Google Scholar]
- [39].Jha S. K., Jha N. K., Kar R., Ambasta R. K., Kumar P.. p38 MAPK and PI3K/AKT Signalling Cascades inParkinson’s Disease[J] Int J Mol Cell Med. 2015;4:67. –. [PMC free article] [PubMed] [Google Scholar]
- [40].Wang Qian, Huan Zheng, Zuofeng Zhang, New Zhang Yu. Ginsenoside Rg1 Affects the Expression of COX-2 in the substantia nigra of MPTP Model of Parkinson’s Disease through P38 Signal Pathway[J] Journal of Southern Medical University. 2008;28:1594. –. [PubMed] [Google Scholar]
- [41].Hwang Chul Ju, Hee Pom Lee, Dong Young Choi, Heon Sang Jeong, Tae Hoon Kim, Tae Hyung Lee, Young Min Kim, Dae Bong Moon, Sung Sik Park, Young Kim Sun.. Inhibitory effect of thiacremonone on MPTP-induced dopaminergic neurodegeneration through inhibition of p38 activation[J] Oncotarget. 2016;7:46943. doi: 10.18632/oncotarget.10504. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ray A, Sehgal N, Karunakaran S, Rangarajan G, Ravindranath V. MPTP activates ASK1-p38 MAPK signaling pathway through TNF-dependent Trx1 oxidation in parkinsonism mouse model[J] Free Radic. Biol. Med. 2015;87:312. doi: 10.1016/j.freeradbiomed.2015.06.041. –. [DOI] [PubMed] [Google Scholar]
- [43].Stanley Fahn, David Sulzer.. Neurodegeneration and Neuroprotection in Parkinson Disease[J] NeuroRx. 2004;1:139. doi: 10.1602/neurorx.1.1.139. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Teacher Bright, Zhang Yu New, Zhang Zuofeng, Chen Hao.. Toxicity of COX-2 to dopaminergic neurons in the substantia nigra of Parkinson’s disease mice[J] Medical debate. 2006;27:1661. –. [Google Scholar]
- [45].Peter Teismann, Kim Tieu, Dongkug Choi, Duchu Wu, Ali Naini, Stéphane Hunot, Miquel Vila, Vernice Jacksonlewis, Serge Przedborski.. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration[J] Proc Natl Acad Sci U S A. 2003;100:5473. doi: 10.1073/pnas.0837397100. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson’s disease: is there a causal link?[J] Exp. Neurol. 2005;193:279. doi: 10.1016/j.expneurol.2005.01.013. –. [DOI] [PubMed] [Google Scholar]
- [47].Pålsson-Mcdermott E. M., O’neill L. A.. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4[J] Immunology. 2004;113:153. doi: 10.1111/j.1365-2567.2004.01976.x. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Haipeng Xaio, Banks W. A, Niehoff Michael L, Morley J. E. Effect of LPS on the permeability of the blood–brain barrier to insulin[J] Brain Res. 2001;896:36. doi: 10.1016/s0006-8993(00)03247-9. [DOI] [PubMed] [Google Scholar]
- [49].Kim E. K., Choi E. J.. Pathological roles of MAPK signaling pathways in human diseases[J] Biochim. Biophys. Acta. 2010;1802:396. doi: 10.1016/j.bbadis.2009.12.009. –. [DOI] [PubMed] [Google Scholar]
- [50].Peter Jenner, Olanow C. Warren. Oxidative stress and the pathogenesis of Parkinson’s disease[J] Neurology. 1996;47:161. doi: 10.1212/wnl.47.6_suppl_3.161s. –. [DOI] [PubMed] [Google Scholar]
- [51].Varçin M, Bentea E, Michotte Y, Sarre S. Oxidative Stress in Genetic Mouse Models of Parkinson’s Disease[J] Oxid. Med. Cell. Longev. 2012;2012:624925. doi: 10.1155/2012/624925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Karunakaran S, Ravindranath V. Activation of p38 MAPK in the substantia nigra leads to nuclear translocation of NF-kappaB in MPTP-treated mice: implication in Parkinson’s disease[J] J. Neurochem. 2009;109:1791. doi: 10.1111/j.1471-4159.2009.06112.x. –. [DOI] [PubMed] [Google Scholar]
- [53].Santabárbararuiz P, Lópezsantillán M, Martínezrodríguez I, Binaguicasas A, Pérez L, Milán M, Corominas M, Serras F. ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Drosophila Regeneration[J] PLoS Genet. 2015;11:e1005595. doi: 10.1371/journal.pgen.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Laura Dunn, Vanessa Fairfield, Shanay Daham, Juan P. Bolaños, Simon J. Heales. Pentose-phosphate pathway disruption in the pathogenesis of Parkinson’s disease[J] Transl. Neurosci. 2014;5:179. –. [Google Scholar]
- [55].Duan F., Yu Y., Guan R., Xu Z., Liang H., Hong L.. Vitamin K2 Induces Mitochondria-Related Apoptosis in Human Bladder Cancer Cells via ROS and JNK/p38 MAPK Signal Pathways[J] PLoS One. 2016;11:e0161886. doi: 10.1371/journal.pone.0161886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ju X., Wen Y., Metzger D, Jung M. The role of p38 in mitochondrial respiration in male and female mice[J] Neurosci. Lett. 2013;544:152. doi: 10.1016/j.neulet.2013.04.004. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yao J., Irwin R. W., Zhao L., Nilsen J, Hamilton R. T., Brinton R. D.. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease[J] Proc. Natl. Acad. Sci. U. S. A. 2009;106:14670. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Alzbeta Trancikova, Elpida Tsika, Moore Darren J.. Mitochondrial Dysfunction in Genetic Animal Models of Parkinson’s Disease[J] Antioxidants & Redox Signaling. 2012;16:896. doi: 10.1089/ars.2011.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Suzuki M, Motizuki N, Koya T, Kitabatake A. [Mitochondrial disorders] [J] J. Pediatr. Neurol. 2009;49:27. [PubMed] [Google Scholar]
- [60].Yu Luo, Alan Hoffer, Barry Hoffer, Xin Qi.. Mitochondria: A Therapeutic Target for Parkinson’s Disease?[J] Int. J. Mol. Sci. 2015;16:20704. doi: 10.3390/ijms160920704. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Helley Martin P., Jennifer Pinnell, Carolina Sportelli, Kim Tieu.. Mitochondria: A Common Target for Genetic Mutations and Environmental Toxicants in Parkinson’s Disease[J] Frontiers in Genetics. 2017;8:177. doi: 10.3389/fgene.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Votyakova Tatyana V., Reynolds Ian J. Mitochondrial Complex I Deficiency in Parkinson’s Disease: A Mechanism for Oxidant-Based Pathogenesis[M] 2007.
- [63].Onyango Isaac G.. Mitochondrial Dysfunction and Oxidative Stress in Parkinson’s Disease[J] Prog. Neurobiol. 2013;17:106. doi: 10.1016/j.pneurobio.2013.04.004. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zorov D. B., Juhaszova M, Sollott S. J.. Mitochondrial ROS-induced ROS release: an update and review[J] Biochim. Biophys. Acta. 2006;1757:509. doi: 10.1016/j.bbabio.2006.04.029. –. [DOI] [PubMed] [Google Scholar]
- [65].Jellinger Kurt A.. The role of α-synuclein in neurodegeneration — An update[J] Transl. Neurosci. 2012;3:75. –. [Google Scholar]
- [66].Langston J. William, Ian Irwin, Elizabeth B. Langston, Forno Lysia S.. 1-Methyl-4-phenylpyridinium ion (MPP + ): Identification of a metabolite of MPTP, a toxin selective to the substantia nigra[J] Neurosci. Lett. 1984;48:87. doi: 10.1016/0304-3940(84)90293-3. –. [DOI] [PubMed] [Google Scholar]
- [67].Hwang C. J., Young Choi D, Jung Y. Y., Lee Y. J., Yun J. S., Oh K. W., Han S. B., Oh S, Park M. H., Hong J. T.. Inhibition of p38 pathway-dependent MPTP-induced dopaminergic neurodegeneration in estrogen receptor alpha knockout mice[J] Horm. Behav. 2016;80:19. doi: 10.1016/j.yhbeh.2016.01.011. –. [DOI] [PubMed] [Google Scholar]
- [68].Nicklas W. J., Vyas I, Heikkila R. E.. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine[J] Life Sci. 1985;36:2503. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- [69].Hasegawa E, Takeshige K, Oishi T, Murai Y, Minakami S. 1-Methyl-4-phenylpyridinium (MPP+) induces NADH-dependent superoxide formation and enhances NADH-dependent lipid peroxidation in bovine heart submitochondrial particles[J] Biochem. Biophys. Res. Commun. 1990;170:1049. doi: 10.1016/0006-291x(90)90498-c. –. [DOI] [PubMed] [Google Scholar]
- [70].Chan P., Delanney L. E., Irwin I, Langston J. W., Di Monte D. Rapid ATP loss caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mouse brain[J] J. Neurochem. 2010;57:348. doi: 10.1111/j.1471-4159.1991.tb02134.x. –. [DOI] [PubMed] [Google Scholar]
- [71].Wong Wai K., Xiao Ming Out, Kevin Chen, Shih Jean C.. Activation of Human Monoamine Oxidase B Gene Expression by a Protein Kinase C MAPK Signal Transduction Pathway Involves c-Jun and Egr-1[J] J. Biol. Chem. 2002;277:22222. doi: 10.1074/jbc.M202844200. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Roy Choudhury G, Ryou M. G., Poteet E, Wen Y., R He, Sun F., Yuan F., Jin K., Yang S. H.. Involvement of p38 MAPK in reactive astrogliosis induced by ischemic stroke[J] Brain Res. 2014;1551:45. doi: 10.1016/j.brainres.2014.01.013. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kitamura N, Nakamura Y, Miyamoto Y, Miyamoto T, Kabu K, Yoshida M, Futamura M, Ichinose S, Arakawa H. Mieap, a p53-Inducible Protein, Controls Mitochondrial Quality by Repairing or Eliminating Unhealthy Mitochondria[J] PLoS One. 2011;6:e16060. doi: 10.1371/journal.pone.0016060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gomez-Lazaro M, Galindo Mf, Concannon Cg, Segura Mf, Fernandez-Gomez Fj, Llecha N, Comella Jx, Prehn Jh, Jordan J.. 6-Hydroxydopamine activates the mitochondrial apoptosis pathway through p38 MAPK-mediated, p53-independent activation of Bax and PUMA[J] J. Neurochem. 2008;104:1599. doi: 10.1111/j.1471-4159.2007.05115.x. –. [DOI] [PubMed] [Google Scholar]
- [75].Das N. R., Sharma S. S.. Cognitive Impairment Associated with Parkinson’s Disease: Role of Mitochondria[J] Curr. Neuropharmacol. 2016;14 doi: 10.2174/1570159X14666160104142349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].FernãNdez-Moriano C, Gonzã Lez-Burgos E. M. P. GãÂ3mez-Serranillos. Mitochondria-Targeted Protective Compounds in Parkinson’s and Alzheimer’s Diseases[J] Oxid. Med. Cell. Longev. 2015;2015:408927. doi: 10.1155/2015/408927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Absi E, Ayala A, Machado A, Parrado J. Protective effect of melatonin against the 1-methyl-4-phenylpyridinium-induced inhibition of complex I of the mitochondrial respiratory chain[J] J. Pineal Res. 2010;29:40. doi: 10.1034/j.1600-079x.2000.290106.x. –. [DOI] [PubMed] [Google Scholar]
- [78].Harper Sarah J, Neil Wilkie. MAPKs: new targets for neurodegeneration[J] Expert Opin. Ther. Targets. 2003;7:187. doi: 10.1517/14728222.7.2.187. [DOI] [PubMed] [Google Scholar]
- [79].Thomas M, Le W. D.. Minocycline: neuroprotective mechanisms in Parkinson’s disease[J] Curr. Pharm. Des. 2004;10:679. doi: 10.2174/1381612043453162. –. [DOI] [PubMed] [Google Scholar]
- [80].Koul Hari K., Pal Mintu, Koul Sweaty. Role of p38 MAP Kinase Signal Transduction in Solid Tumors[J] Genes Cancer. 2013;4:342. doi: 10.1177/1947601913507951. [DOI] [PMC free article] [PubMed] [Google Scholar]


