Abstract
Diabetes is a metabolic condition that is exponentially increasing worldwide. Current monitoring methods for diabetes are invasive, painful, and expensive. Herein, we present the first multipatient clinical trial that demonstrates clearly that tear fluid may be a valuable marker for systemic glucose measurements. The NovioSense Glucose Sensor, worn under the lower eye lid (inferior conjunctival fornix), is reported to continuously measure glucose levels in the basal tear fluid with good correlation to blood glucose values, showing clear clinical feasibility in both animals and humans. Furthermore, the polysaccharide coated device previously reported by our laboratory when worn, does not induce pain or irritation. In a phase II clinical trial, six patients with type 1 Diabetes Mellitus were enrolled and the capability of the device to measure glucose in the tear fluid was evaluated. The NovioSense Glucose Sensor gives a stable signal and the results correlate well to blood glucose values obtained from finger-prick measurements determined by consensus error grid analysis.
Introduction
Diabetes Mellitus is a chronic condition that when uncontrolled results in prolonged elevated levels of blood glucose that cause long-term medical complications, such as micro- and macrovascular damage.1 Cardiovascular complications are the most frequent cause of death in diabetic patients.1,2 Diabetes is a significant and growing problem affecting all parts of the globe, especially in rapidly developing countries.3 There are many systematic reviews on the global spread of diabetes.4−6 Cho et al. presented data reporting that in 2017 there were 451 million people (age between 18–99 years) with diagnosed diabetes worldwide. This number is expected to increase to 693 million by 2045.4 The global prevalence of diabetes and its impact on social, financial, and health systems is dramatic, with the complications of diabetes often hiding the true cost of the condition. There is a clear need for improved modalities of day-to-day diabetes management, with such methods needing to address lower cost and increased testing compliance especially in the insulin treated population.
Over the past 50 years, enzymatic biosensing technology based on finger-prick glucose testing has remained the method of choice for the majority of diabetics.7,8 Its mechanism of action relies on the enzymatic electrochemical detection of glucose and involves sampling of capillary blood from a finger to be analyzed with the use of test strips and a glucometer. The effectiveness of finger-prick glucose is dependent on patient compliance to performing multiple finger pricks during the day. Its frequency is often severely reduced by pain, social barriers, or time constraints, which are associated with the measurement.9 Moreover, finger-prick glucose gives only a single snapshot in time; thus, periods of hyper- or hypoglycemia can be missed, which may have serious or fatal consequences. To reduce inconveniences during blood sampling, several possibilities of measuring glucose levels with the use of other body fluids have been reported.8,10 Currently, commercial solutions, such as those provided by Medtronic, Dexcom, or Abbott offer a number of different continuous glucose monitoring devices that utilize interstitial fluid for their measurements (CGM). However, they are still invasive and costly in comparison to finger pricking, and for most devices, multiple finger pricking is required for calibration.
Tear fluid is a rich source of biomarkers, thus, an interesting matrix to explore for diagnostic purposes.11,12 A number of proof-of concept enzymatic prototype devices have attempted to demonstrate the potential to be used as tear glucose sensors but with little success. Contact lenses are the examples of wearable electronics which sparked attention of many scientists, although so far no prototype has been successfully reported in a clinical setting.13−16 Appropriate localization of the sensor to effectively measure glucose levels in tears is crucial, and requires analysis and consideration of the dynamic nature of tears in the eye. Tears are produced in a complex interplay between the lacrimal, accessory lacrimal glands and the goblet cells of the conjunctiva in response to differing environmental or emotional stimuli.17 The volume of the tear film is approximately 6–8 μL and the rate of flow across the surface of the eye is between 1 and 2 μL per minute. Tears flow into the lacrimal canaliculi and then pass into the lacrimal sac between the upper and lower eyelids (Figure 1A). Consequently, the pocket of the conjunctival fornix acts as a flow cell to create an ideal location to place a sensor, since it is immersed in a high volume of tear fluid in proximity to the meibomian glands. Several reports are available in the literature demonstrating the use of sensors on the outer surface of the eye or by external sampling techniques to measure glucose in tear fluid; however, none of them have demonstrated a link between tear to blood glucose in a clinical setting.14,18,19
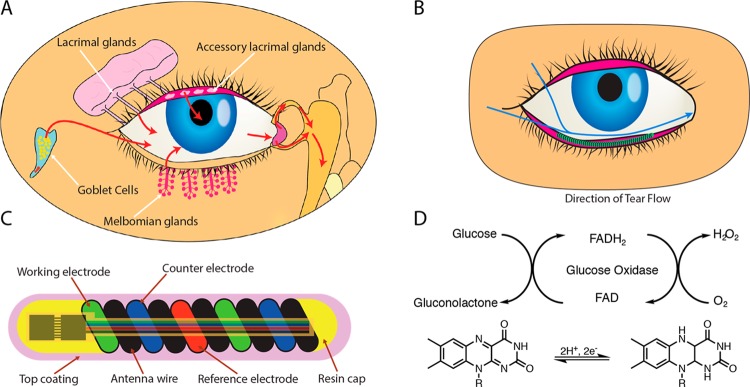
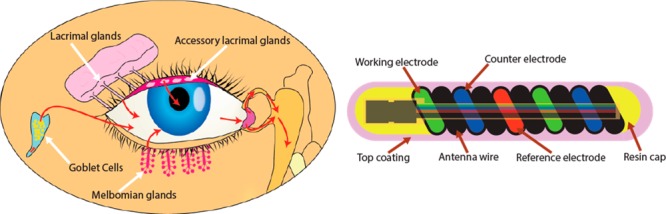
Figure 1.
Schematic illustration and properties of NovioSense minimally-invasive tear glucose sensor. (A) Illustration showing the tear fluid production. (B) Direction of the tear flow and anatomical position of the NovioSense device. (C) Design and structure of the final product showing electronic components. (D) Mechanism of glucose detection where glucose oxidase is used as the enzymatic sensing element.
Soft, structural biomacromolecules and their synthetic analogues that self-associate into two- and three-dimensional structures such as membranes and hydrogels forming cellular structures20,21 and interstitial fluid components, respectively,22−24 have been the focus of our research for a number of years. More recently, they have been applied as coatings and delivery agents for therapeutic25 and device applications.26,27 NovioSense BV has developed a unique patented sensor platform utilizing a microelectrochemical cell, coated with a polysaccharide-based hydrogel coating, as has been previously reported in this journal and in the patent literature.28 The coating may be tailored for a range of applications to be deployed in environments where the degree of biointegration can be tuned. For example, in blood contact applications, such as those reported previously from our laboratory, the degree of antifouling nature and oxygen permeability are both critical to functionality, whereas an intersitial application requires a certain degree of biointegration; this is achieved by tuning both the base material properties and selecting the appropriate peripheral functionalities from a library of monomers.24,28 The polysaccharide material employed in this report not only forms a hydrogel-like network encapsulating the sensor that acts as a barrier between the metal surface and the soft tissue of the eye, but also acts to stabilize and prevent enzyme migration out of the coating, while allowing for free diffusion of the analyte into the coating.27−29 The use of a biomacromolecule bound enzyme rather than direct grafting to the surface or nanoparticle bound enzymatic systems allows for scalable production of the devices using standard dip, spray, or spin-coating techniques from aqueous media. Herein, we report the successful outcome of a six-patient clinical trial, demonstrating the utility of a glucose sensor to measure glucose in tear fluid, located in the lower eyelid, and subsequent derivation of blood glucose levels. Preclinical studies have been performed in an appropriate animal model and in human volunteers to confirm the tolerability and electrochemical characteristics of the sensor device when placed in the lower conjunctival fornix. The NovioSense Glucose Sensor device is a flexible, spring-shaped coil, which fits in lower eyelid and conforms to the surface contours of the eye. The sensor has a classical generation 1 glucose detection design. Its anatomical position makes this coiled sensor not noticeable while wearing which is more advantageous than existing CGM technologies. Moreover, the device reported herein can be deployed in either continuous or single point modes, the latter of which offers a system with unlimited painless finger-prick equivalent measurements at a significantly lower cost than existing devices, thus, addressing the significant unmet needs.
The NovioSense sensor is placed in the fornix of the lower eyelid and bathed in tear fluid.27 The location of the device provides an ideal environment for measurement during basal tear flow (see Figure 1B). The device consists of a flexible coil made of multiple wires (electrodes) coiled in parallel to form an amperometric cell with the shape of a spring of 15 mm long and 1.3 mm in diameter (see Figure 1C). The wires are made from uncoated platinum/iridium (Pt/Ir, 90/10), and stainless steel coated with 10 μm of polyesterimide, which serves as an isolator and a spacer. The coil represents a device of high flexibility while presenting an ideal platform to act as an antenna for wireless data and power transfer. In the final design, a pair of application specific silicon microchips will be incorporated to control the sensor and the wireless data transfer. The sensor will not contain any power source and the readings are triggered with an external device by telemetry. To increase comfort while wearing, the device is protected with a hydrophilic and biocompatible polysaccharide based hydrogel coating that gives a smooth transition between the surface of the eye and the metal spring. The hydrophilic polysaccharide based coating additionally contains immobilized glucose oxidase as the enzymatic sensing element (patent EP2699690B1).29 The sensor utilizes a well-established, robust enzymatic detection of glucose (Figure 1D). The enzyme converts glucose and oxygen into gluconolactone and hydrogen peroxide. In the course of this reaction, hydrogen peroxide is oxidized and detected on a working electrode using a chronoamperometric measurement (see Figure 1D). Various reports are available in the open literature on the concentration of glucose in tears. For the purpose of this trial, the linear range of the sensor was optimized with the highest linearity in the range of 0.1–1 mM (r2 = 0.999).
Experimental Section
Materials and Methods
Assembly of the Tear Glucose Sensor
Pt/Ir coils (POLYFIL AG, Switzerland) were used for RE, CE, and WE. The manufacturing process has been outsourced to the external company EPflex GmbH, Dettingen. The electrodes were separated by three spacer wires (stainless steel wires (POLYFIL AG, Switzerland). All six wires were assembled in an alternating fashion. The sharp ends of the device were deburred with laser. Afterward, three thin Pt/Ir wires (60 μm in diameter, Lake Region Medical, U.S.A.), coated with biocompatible parylene (Curtiss Wright, U.S.A.), were welded on the RE, WE, and CE electrodes as extension wires. UV curable glue (DYMAX 1128-A-M) was applied on the top of the welds to ensure fixation. Heat-shrink tubes were applied to the external connection. Also, UV curable glue was applied over the welded 60 μm Pt/Ir wire. The device assembly was carried out at EPflex GmbH, Dettingen.
Functionalization of the Sensor
The procedure for the formation of biocompatible coating and functionalization of the tear glucose sensor with addition of glucose oxidase (BBI Enzymes) is described in detail described in Patent (EP2699690B1).29
Sensing Measurement
The electrochemical measurement was performed using potentiostat Eco Chemie potentiostat PGSTAT204 (Metrohm BV, Netherlands). The chronoamperometry technique was used for the measurements where the current was measured as a function of time. The constant potential of +0.5 V was applied between the working and reference electrodes.
Calibration of the Device
The NovioSense Glucose Sensor devices were calibrated after the trial in PBS (1×) solution, using 0–16 mM glucose (Sigma-Aldrich) concentration. Calibration plots were obtained by increasing glucose concentration incrementally see Supporting Information, Figure SF1.
Sterilization of the Clinical Devices
The clinical devices were packed in a clean room and sterilized with Ethylene Oxide (EtO) by Medicoplast GmbH. The device is sterilized using optimized cycle MP6. The treatment with EtO has not induced any cytotoxic effect as tested by Toxikon according to ISO 10993 part 5 and no ethylene oxide residuals were detected as tested by Toxikon according to ISO 10993-7.
In Vitro Biocompatibility Validation Using XTT Assay
Cytotoxicity tests were outsourced and conducted by Toxikon Europe NV, Belgium according to ISO 10993-5, 2009, Biological Evaluation of Medical Devices; Part 5: Tests in vitro cytotoxicity and ISO 10993-12, 2012, Biological Evaluation of Medical Devices; Part 12: Sample Preparation and Reference Materials. The study was conducted in accordance with Good Laboratory Practice (GLP). The test was based on the measurement of viability of L929 mouse fibroblasts (105 cells/mL) in response to an extract of the NovioSense Glucose Sensor functionalized with GOx dummy device via XTT assay (Roche). The NovioSense Glucose Sensor devices were extracted in 0.9% sodium chloride at a ratio of 60 cm2/20 mL. The NovioSense Glucose Sensor device extract was finally diluted in serum-supplemented Minimum Essential Medium (MEM-complete) at a ratio of 1/4. The L929 cells were exposed in quadruplicate at this 1/4 dilution. MEM-complete contained following: MEM (LONZA:BE12-125F), penicillin/streptomycin (LONZA: DE17-602E), fetal bovine serum (Greiner-bio-one FBSEU500), l-glutamine (LONZA:BE17-605E), Hepes buffer (LONZA: BE17–737E).
Animal Trials on New Zealand White Rabbits
The irritation studies were outsourced and conducted by Toxikon Europe NV, Belgium. The study was conducted in compliance with the current U.S. Food and Drug Administration 21 CRF, Part 58 Good Laboratory Practices for Nonclincal Laboratory Studies. The animal species, number and route of the test articles administration are recommended by the ISO 10993–10 guidelines. The test article was extracted and administrated in vivo through a medium compatible with the test system, as indicated on the GLP Test Requisition Form. The NovioSense Glucose Sensor device was combined with 9 mL of vehicle following ISO 10993-12 ratio of 6 cm2 per 1 mL. The NovioSense Glucose Sensor device was extracted in sodium chloride and CSO at 70 °C for 24 h. After which the solutions were cooled down to the room temperature and agitated vigorously prior to administration. Animals selected for the study were weighted and examined to ensure that their skin was free from irritation, trauma, and disease. Each animal was weighed and clipped free of fur on the dorsal side prior to injection. A volume of 0.2 mL per site of one extract was injected intracutaneously at one side of each of three rabbits, five sites for the test article extract, and five posterior sites for the control. Similarly, at the other side of each rabbit, the other extract was injected. The maximum injections per rabbit was limited to two test articles and two corresponding control articles. The injection sites of each animal were observed for signs of erythema and edema immediately following the injection and at 24, 48, and 72 h after. Observations were scored according to the Classification System for Scoring Skin Reactions. At the end of the study, the animals were weighed and returned to the general colony.
Animal Trials on Sheep Model
Experiments conducted on sheep have been carried out by a Contract Research Organization (CRO) at Medanex Clinic located in Belgium. Medanex Clinic, a licence holder for performing preclinical trials with a protocol approved by Ethic Committee. Documentation was carried out in line with GLP; however, the laboratories where the experiments were carried was in the process of obtaining GLP and AAALAC accreditation at the time of the experiments.
Clinical Subject Recruitment
The clinical trial was approved by Medisch Ethische Toetsingscommissie (METC) Zuid-West Holland, ID: NL61532.098.17 and Dutch Health Care Inspectorate (IGZ), ID: VGR2002461. The study was carried out in accordance with Good Clinical Practice (GCP). Subjects were recruited for the study from diabetes outpatient clinic of Haaglanden Medical Centre. The subjects were informed at least 3 days prior to the actual device positioning. A signed informed consent form (ICF) was collected from each patient before any study related activities were started.
Clinical Study Design
The subjects reported on the day of the trial fasted, on their basal insulin only and were asked to wear the device throughout the trial (for up to 5 h) in which the glucose measurements in the tear fluid were taken. After insertion of the device into the inferior conjunctival fornix by the attending ophthalmologist, the basal evaluation of the eye and glucose measurements were performed for a remaining 4.5 h. Capillary blood samples to measure glucose values during the experiment time points were taken with a Point-Of-Care system (ACCU-CHEK Inform II, Roche) and served as a reference. Additionally, patients wore a commercially available CGM (continuous glucose monitor) sensor to serve as a supporting control to the acquired data. Throughout the experiment the subjects received a standard eye examination against irritation and eye damage caused by the presence/function of the device.
Statistical Analysis
The median absolute relative difference (MedARD) was calculated. MedARD was used to assess the accuracy of the acquired data of the sensor in respect to the reference measurement and calculated from equation below:
where YRBG is the reference blood glucose concentration in capillary blood and subcutaneous fluid and YCGM is the glucose concentration measured at the same point in time by the CGM NovioSense sensor. Since in real-life, patients measure glucose in capillary blood, the glucose data from both the subcutaneous as well as the eye sensor were compared with capillary glucose measurements with a Point-Of-Care measurement, which is the standard for capillary glucose measurement in the clinic.
Results and Discussion
To determine the ability of the NovioSense device to measure glucose and to benchmark, its performance against existing CGM products, a series of calibrations were carried out using both the NovioSense sensor and Dexcom G4 sensor. Dexcom sensors were prepared by removing the elements and connecting them to a laboratory electrochemical set up (Materials and Methods). Two key criteria were addressed: linearity and interference. The response of the Dexcom device from a standard electrochemical measurement to a standard glucose solution up to 20 mM was plotted against the NovioSense Glucose Sensor device (Figure S1). The Dexcom sensor shows good linearity in the low region however deviates at levels above 10 mM. The NovioSense sensor shows excellent linearity across the concentration regime from 0–20 mM despite being optimized for a low concentration accuracy. In addition to the glucose response, the tolerance of the measurement to common interferences was investigated. Interference is anything other than glucose that also induces the sensor to respond. To determine the relative response to physiological interferences both sensors, NovioSense Glucose Sensor and Dexcom G4, were tested (Figure 2A and B). The NovioSense sensor responds specifically to glucose even with the presence of six different interferers (ascorbic acid, acetaminophen, lactic acid, urea, citric acid, and pyruvic acid) at physiological concentrations (Figure 2B).
Figure 2.
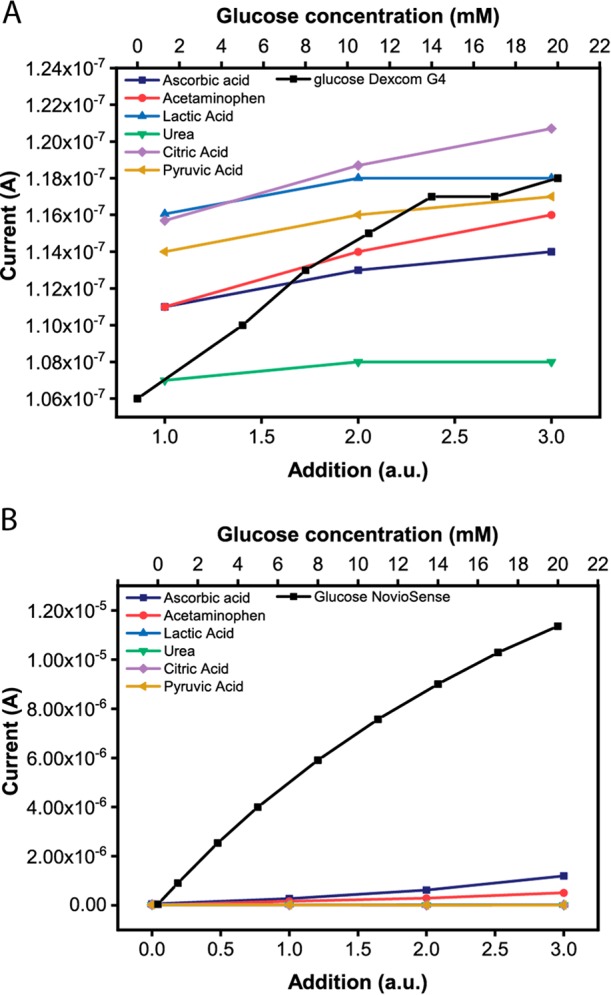
Electrochemical performance of NovioSense minimal-invasive tear glucose sensor. (A) Response of the Dexcom G4 device to interferences and glucose. (B) The NovioSense device response to interferences and glucose.
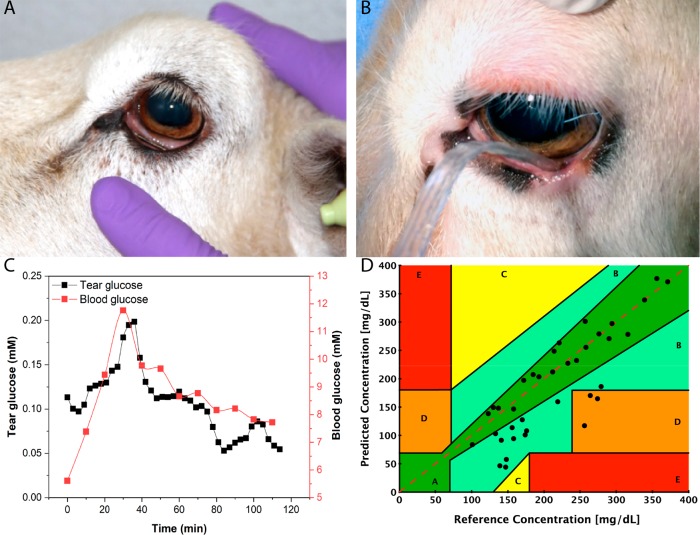
In vitro and in vivo tests were performed to evaluate the biocompatibility of the NovioSense Glucose Sensor device in accordance with ISO 10993: parts 5 and 12. Cellular biocompatibility has been assessed using XTT assay with L929 mouse fibroblast cells. The cells were incubated with extracts from coated NovioSense devices functionalized with glucose oxidase (see Materials and Methods) for 2 days. After the experiment, 94% of the cells remained viable, thus, confirming that the sensor does not produce any acute cytotoxic effect. In vivo screening tests were designed to evaluate the potential of the device and its extracts to cause irritation. Extracts from the sensor were prepared in a polar (saline) and a nonpolar (cotton seed oil) solution. A primary irritation index is determined based on the evaluation criteria from ISO 10993-10 guidelines (see details in Materials and Methods). The sensor was evaluated for its potential to produce irritation after intracutaneous injection of the extracts in six New Zealand White rabbits for 72 h. No biological reactions, such as erythema, edema, or necrosis, were detected. To validate the concept of tolerance and measurement in the eye, a series of further animal tests were carried out by the Medanex Clinic, a license holder to perform preclinical trials, with protocols approved by the relevant Ethics Committee. A sheep model was identified as the most appropriate ophthalmic model because of the geometry of both the eye and the eye socket in comparison to human eyes. Initial tolerance trials were carried out placing a dummy device of the same form and coating as the functional device into the eye of the sheep (see Figure 3A). The device was positioned under the lower eyelid using sterile tweezers and kept in place for 2 h. The animal did not shake its head to perturb the device from its position while wearing the device. After the 2 h, ophthalmologic evaluation found no symptoms of irritation or any abnormalities to the eye. Prolonged placement of the device was challenging due to the presence of the third eye lid in the sheep model causing dislocation. This is a common issue and is present in many large mammals that are suitable as a model for the human eye. In a separate trial, functional prototypes were tested to validate the measurement cycle, correlation of glucose to blood glucose, and initial accuracy of the device. A wired sensor prototype was used with the polysaccharide sensor coating to prevent damage to the ocular surface (see Figure 3B). The sheep received two venous access ports for glucose infusion and direct blood sampling. Blood samples were analyzed using hospital laboratory glucose measurement and in parallel glucose test strips, respectively. In total, three sheep received the functional device in the eye. The evaluation time was 5 h and a clear correlation between tears and blood glucose was observed (Figure 3C). From the obtained animal data, a Clarke Error grid was plotted to determine the accuracy of the sensor to measure blood glucose values. Values obtained from the sensors were plotted against the hospital laboratory derived value for the same sample. The data were collected and the combined animal trial data shown in Figure 3D. It shows that 92% of data within the A and B regions were comparable to existing marketed technologies at the time of testing. A number of outlying data points seen in the D region where the tear sensor is underestimating glucose concentration were observed; this was seen to correlate with the administration of a higher dose of anesthetic and concomitant reduction in muscle action around the eye. Modification of the anesthetic dose by the attending veterinarian eliminated this effect. Despite the concentration of glucose found in tears being significantly lower in comparison to blood, its fluctuations recorded over time resembled the same pattern.
Figure 3.
Preclinical evaluation using sheep as a model. (A) Picture of a sheep wearing a dummy device to determine tolerability and irritation for 2 h (tests performed with N = 2). (B) Functional tests with the use of the NovioSense prototype where the extension wires of the device were connected to a measurement unit (potentiostat; tests performed with N = 3). The data were acquired for 5 h. (C) A graph of glucose concentration vs time of the trial, where a clear correlation and glucose fluctuation pattern are visible. (D) Clarke Error Grid showing accuracy of the NovioSense device vs reference, where 92% of acquired data fall within the A and B regions, giving sufficient accuracy to proceed to the next phase of development.
Clinical devices were designed to investigate the ability of the sensor to collect data in a human eye and to evaluate factors such as signal-to-noise level, stability, repeatability, and accuracy. To conduct the trial, a prototype device was manufactured, where three ultrathin and flexible Pt/Ir (90/10, diameter of 60 μm) extension wires were attached to the NovioSense coil devices. This solution enabled the sensor to be attached to a standard electrochemical measurement device (Materials and Methods). The extension wires were isolated with biocompatible parylene coating for safety and comfort. A bespoke magnetic interconnect was used to attach the wires to the commercial potentiostat. Six patients were enrolled in the study with diagnosed type 1 Diabetes Mellitus according to the research protocol, approved by the Regional Ethics Committee (METC Zuidwest Holland) and Competent Authority (Healthcare Inspectorate, IGZ). All subjects were included in the tolerability and effectiveness analysis. Five out of six patients wore an Abbott FreeStyle Libre, CGM device to measure glucose levels in interstitial fluid, used in the study as a reference to the NovioSense device. Additionally, a glucose value from capillary blood was measured by finger prick with a hospital point of care glucose meter (Roche). Before the trial began, every clinical device had undergone checks in sterile water where stable blank signals were recorded. Each subject wore the NS glucose sensor in the right eye for 4.5 h and glucose from blood and interstitial fluid and tear glucose was tested every 15 min. Figure 4A shows one of the six clinical subjects enrolled in the study where stable signals from the tear fluid of the eye were recorded up to 4.5 h.
Figure 4.
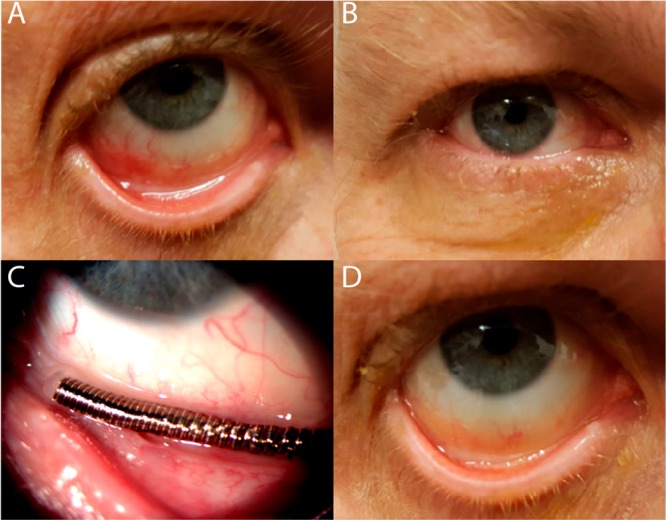
Clinical trial-phase II with tear glucose sensor in the eye. Pictures of the eyes taken at three points of the trial. (A) Baseline evaluation. Temporary redness developed, caused by mechanical rubbing of the eye by the subject prior to sensor insertion. (B) Middle of the trial. The subject is wearing the NovioSense prototype device with ultrathin (d = 60 μm) extension Pt/Ir wires, protected with parylene coating. The wires are connected to a bespoke magnetic connector immobilized on the arm and further connected to the potentiostat enabling conducting the measurement. (C) Picture showing the position of the sensor in inferior conjunctival fornix. (D) After the trial. No redness or damage to the subject eye were seen. The glucose sensor was coated with the standard NovioSense coating.
Detailed microscopic examination of the inferior conjunctival fornix was performed at various points along the course of the study. Additionally, a baseline photograph was taken to show the degree of vascularization and to locate any inflammation already existing in the vicinity (Figure 4B). This served as a reference to determine any acute response of NovioSense sensor during the investigation. In the course of the trial no adverse events have been observed. Patients did not report any discomfort during the trial or during insertion and removal.
Glucose values in the tear fluid were found to be significantly lower than in the blood and were consistent with previous reports and the animal data reported above. Sensors were calibrated prior to insertion into the eye against standard glucose solutions. The glucose data were corrected for concentration using a simple multiplication factor dependent on the calibration algorithm.12
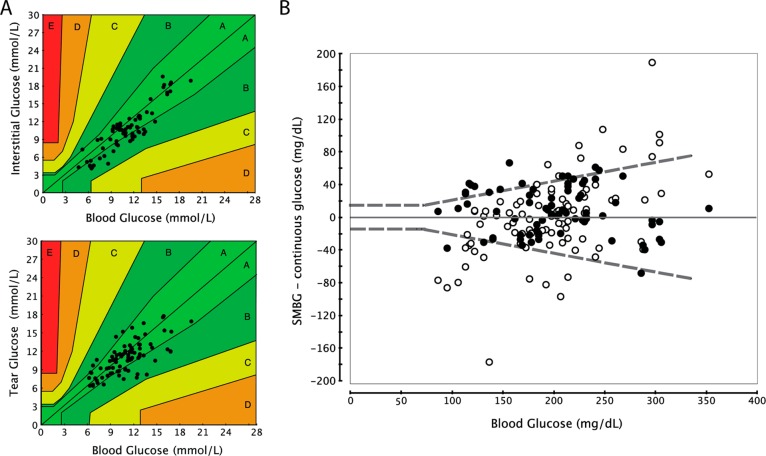
The error grid analysis showed that 95% of data for NovioSense and 100% of data from the Abbott device was found in the A+B regions. For the NovioSense Glucose Sensor device 70% of the data was found to be in the A region compared to 71% for the Abbott FreeStyle Libre (Figure 5A). The tabulated data were analyzed to determine the average relative differences (ARD) and the median ARDs (MedARD) were calculated. The median ARD value weights the ARD data toward the number-average rather than the weight-average value. The calculated MedARD for the NovioSense device is 12.5 which appears to be in line with the Abbott FreeStyle Libre data (MedARD 12.0). In addition to this, a superimposed plot of the NovioSense data versus Abbott Freestyle Libre data published by Abbott Laboratories in the open literature is supplied in Supporting Information, Figure S2.30 In order to determine the suitability for use as a blood glucose meter for diabetic patients’ blood, glucose meters should conform to ISO 15197:2013 (Figure 5B). The standard requires 95% of blood glucose results should reach within ±0.83 mmol/L of laboratory results at concentrations under 5.6 mmol/L and within ±20% of laboratory results at concentrations of 5.6 mmol/L or more. Although this study was not designed to test compliance to ISO 15197:2013 in terms of protocol and sample size it is a valuable measure of the NovioSense device’s potential. The NovioSense device could measure 70% of the data points within the 20% accuracy criteria, with the Abbott device scoring 78% of data within 20% accuracy. As demonstrated in the accuracy analysis, the NovioSense tear sensor device performs on parity with the Abbott FreeStyle Libre device (Figure 5).
Figure 5.
Clinical comparison of the data acquired from clinical subjects wearing the NovioSense tear glucose sensor vs the FreeStyle Libre, Abbott. (A) Consensus Grid acquired from six clinical subjects wearing both the NovioSense and FreeStyle Libre GCM device for 4.5 h. (B) Analysis of difference of NovioSense and Abbott Data vs blood glucose data in accordance with ISO 15197:2013, where black dots represent data from the Abbott Sensor and white dots represent NovioSense data.
Conclusions
Herein we demonstrate the use of a non-invasive biosensor to measure glucose in the tear fluid of patients with type 1 diabetes and, for the first time, demonstrate that a non-blood-derived biofluid-based sensor coated with a polymer matrix can be used to accurately record glucose levels in a clinical setting. The design of a flexible coil that adjusts to the shape of the eye and the polysaccharide coating acts as a soft interface that makes it comfortable while wearing and invisible to the outside world. In vitro and in vivo tolerability tests have demonstrated that the sensor does not induce any immune response. The device in its entirety has been assessed using the ISO standard testing for biocompatibility considering acute responses in the time frame of the clinical trial. No adverse effects were reported. The NovioSense Glucose Sensor device is based on the same amperometric measurement technique used by current existing GCM devices on the market, such as Medtronic and Dexcom, but employing a proprietary polysaccharide based coating technology rather than a complex multidomain membrane coating such as that used in the Dexcom sensor.31 Unlike current commercial solutions, the biopolymer-based hydrophilic coating, not only provides a smooth interface between the sensor and surrounded tissue of the eye but also contains the immobilized glucose oxidase bioreceptor.29 When compared to the Dexcom sensor that employs a mixed membrane consisting of hydrophilic and hydrophobic domains the NovioSense sensor and its homogeneous polysaccharide coating was shown to measure glucose in sub mmol concentrations that could be accurately related to blood glucose levels as well as a high tolerance to interferences. The NovioSense sensor performed on par with that of the Abbott Freestyle Libre. Further studies in a larger cohort are currently being undertaken. Fluctuations in blood glucose levels will be investigated to assess the ability to detect hypoglycemic events which are currently challenging in interstitial glucose measurement.
Acknowledgments
C.J.W. and I.B. gratefully acknowledge the funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Grant Agreement No. 642687. C.J.W. gratefully acknowledges Mr. Hans Hanssen, Professor Leo Koole, and Dr. Erik van der Veen for support and discussion in conducting the in vivo sheep testing.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biomac.8b01429.
Sensor response of NovioSense vs Dexcom sensor, comparison of accuracy of NovioSense sensor and Abbott literature data (PDF).
Author Contributions
# These authors (A.K. and D.V.) contributed equally to this work. The manuscript was written through contributions of all authors. The preclinical experiments were conceived, designed, conducted, and the data analyzed by J.L., N.A., J.T., C.W., I.B., and D.V. The clinical protocol was conceived and designed by N.G.D., M.J., C.W., D.A.W., D.V., and A.K. N.G.D. and M.J. performed the clinical trials. Clinical data analysis was carried out by A.K., D.V., and C.W. All authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): Dr. Wilson is a shareholder of NovioSense BV.
Supplementary Material
References
- Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32 ( (1), ), S62–S67. 10.2337/dc09-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruder T. D.; Schweitzer W.; Ampanozi G.; Gascho D.; Flach P. M.; Thali M. J.; Hatch G. M. Imaging Findings of Diabetes on Post-Mortem CT. J. Forensic Radiol. Imaging 2018, 13, 27–41. 10.1016/j.jofri.2018.05.002. [DOI] [Google Scholar]
- Koye D. N.; Magliano D. J.; Nelson R. G.; Pavkov M. E. The Global Epidemiology of Diabetes and Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25 (2), 121–132. 10.1053/j.ackd.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N. H.; Shaw J. E.; Karuranga S.; Huang Y.; da Rocha Fernandes J. D.; Ohlrogge A. W.; Malanda B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Ogurtsova K.; da Rocha Fernandes J. D.; Huang Y.; Linnenkamp U.; Guariguata L.; Cho N. H.; Cavan D.; Shaw J. E.; Makaroff L. E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- da Rocha Fernandes J.; Ogurtsova K.; Linnenkamp U.; Guariguata L.; Seuring T.; Zhang P.; Cavan D.; Makaroff L. E. IDF Diabetes Atlas Estimates of 2014 Global Health Expenditures on Diabetes. Diabetes Res. Clin. Pract. 2016, 117, 48–54. 10.1016/j.diabres.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Olczuk D.; Priefer R. A History of Continuous Glucose Monitors (CGMs) in Self-Monitoring of Diabetes Mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12 (2), 181–187. 10.1016/j.dsx.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Bruen D.; Delaney C.; Florea L.; Diamond D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17 (8), 1–21. 10.3390/s17081866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. C.; Smith E.; Chubb B.; Wolden M. L. Frequency of Blood Glucose Testing among Insulin-Treated Diabetes Mellitus Patients in the United Kingdom. J. Med. Econ. 2014, 17 (3), 167–175. 10.3111/13696998.2013.873722. [DOI] [PubMed] [Google Scholar]
- Svechtarova M.; Buzzacchera I.; Toebes J. B.; Lauko J.; Anton N.; Wilson C. J. Sensor Devices Inspired by the Five Senses: A Review. Electroanalysis 2016, 28 (6), 1201–1241. 10.1002/elan.201600047. [DOI] [Google Scholar]
- Lane J. D.; Krumholz D. M.; Sack R. A.; Morris C. Tear Glucose Dynamics in Diabetes Mellitus. Curr. Eye Res. 2006, 31 (11), 895–901. 10.1080/02713680600976552. [DOI] [PubMed] [Google Scholar]
- Baca J. T.; Finegold D. N.; Asher S. A. Tear Glucose Analysis for the Noninvasive Detection and Monitoring of Diabetes Mellitus. Ocul. Surf. 2007, 5 (4), 280–293. 10.1016/S1542-0124(12)70094-0. [DOI] [PubMed] [Google Scholar]
- Chu M. X.; Miyajima K.; Takahashi D.; Arakawa T.; Sano K.; Sawada S.; Kudo H.; Iwasaki Y.; Akiyoshi K.; Mochizuki M.; Mitsubayashi K. Soft Contact Lens Biosensor for in Situ Monitoring of Tear Glucose as Non-Invasive Blood Sugar Assessment. Talanta 2011, 83 (3), 960–965. 10.1016/j.talanta.2010.10.055. [DOI] [PubMed] [Google Scholar]
- Kim J.; Kim M.; Lee M. S.; Kim K.; Ji S.; Kim Y. T.; Park J.; Na K.; Bae K. H.; Kim H. K.; Bien F.; Lee C. Y.; Park J.-U. Wearable Smart Sensor Systems Integrated on Soft Contact Lenses for Wireless Ocular Diagnostics. Nat. Commun. 2017, 8, 1–8. 10.1038/ncomms14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.; Kim J.; Kim S.-Y.; Cheong W. H.; Jang J.; Park Y.-G.; Na K.; Kim Y.-T.; Heo J. H.; Lee C. Y.; Lee J. H.; Bien F.; Park J.-U. Soft, Smart Contact Lenses with Integrations of Wireless Circuits, Glucose Sensors, and Displays. Science Advances 2018, 4 (1), 1–12. 10.1126/sciadv.aap9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parviz B. A.; Yao H.; Liao Y.; Lingley A. R.; Afanasiev A.; Lahdesmaki I.; Otis B. P. A Contact Lens with Integrated Telecommunication Circuit and Sensors for Wireless and Continuous Tear Glucose Monitoring. J. Micromech. Microeng. 2012, 22 (7), 75007. 10.1088/0960-1317/22/7/075007. [DOI] [Google Scholar]
- Tomlinson A.; Trees G.; Occhipinti J. Tear Production and Evaporation in the Normal Eye. Ophthalmic Physiol. Opt. 1991, 11 (1), 44–47. 10.1111/j.1475-1313.1991.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Yao H.; Shum A. J.; Cowan M.; Lähdesmäki I.; Parviz B. A. A Contact Lens with Embedded Sensor for Monitoring Tear Glucose Level. Biosens. Bioelectron. 2011, 26 (7), 3290–3296. 10.1016/j.bios.2010.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan X.; Yoon H. S.; Park J. Y. A Wearable Electrochemical Glucose Sensor Based on Simple and Low-Cost Fabrication Supported Micro-Patterned Reduced Graphene Oxide Nanocomposite Electrode on Flexible Substrate. Biosens. Bioelectron. 2018, 109, 75–82. 10.1016/j.bios.2018.02.054. [DOI] [PubMed] [Google Scholar]
- Percec V.; Wilson D. A.; Leowanawat P.; Wilson C. J.; Hughes A. D.; Kaucher M. S.; Hammer D. A.; Levine D. H.; Kim A. J.; Bates F. S.; Davis K. P.; Lodge T. P.; Klein M. L.; DeVane R. H.; Aqad E.; Rosen B. M.; Argintaru A. O.; Sienkowska M. J.; Rissanen K.; Numellin S.; Ropponen J. Self-Assembly of Janus Dendrimers into Uniform Dendrimersomes and Other Complex Architectures. Science 2010, 328 (5981), 1009–1014. 10.1126/science.1185547. [DOI] [PubMed] [Google Scholar]
- Xiao Q.; Ludwig A.-K.; Romanò C.; Buzzacchera I.; Sherman S. E.; Vetro M.; Vértesy S.; Kaltner H.; Reed E. H.; Möller M.; Wilson C. J.; Hammer D. H.; Oscarson; Klein M. L.; Gabius H.-J.; Percec V. Exploring Functional Pairing between Surface Glycoconjugates and Human Galectins Using Programmable Glycodendrimersomes. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (11), E2509–E2518. 10.1073/pnas.1720055115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A. E.; EKSTEEN A. Z. H.; Wilson C.; Geutjes P. J.; FEITZ W. F. j; Oosterwijk E.; Universiteit S. K.. Polymer Suitable for Use in Cell Culture. EP3021872B1, 2017.
- Kouwer P. H. J.; Koepf M.; Le Sage V. a a; Jaspers M.; van Buul A. M.; Eksteen-Akeroyd Z. H.; Woltinge T.; Schwartz E.; Kitto H. J.; Hoogenboom R.; Picken S. J.; Nolte R. J. M.; Mendes E.; Rowan A. E. Responsive Biomimetic Networks from Polyisocyanopeptide Hydrogels. Nature 2013, 493 (7434), 651–655. 10.1038/nature11839. [DOI] [PubMed] [Google Scholar]
- Zimoch J.; Padial J. S.; Klar A. S.; Vallmajo-Martin Q.; Meuli M.; Biedermann T.; Wilson C. J.; Rowan A.; Reichmann E. Polyisocyanopeptide Hydrogels: A Novel Thermo-Responsive Hydrogel Supporting Pre-Vascularization and the Development of Organotypic Structures. Acta Biomater. 2018, 70, 129–139. 10.1016/j.actbio.2018.01.042. [DOI] [PubMed] [Google Scholar]
- Mandal S.; Eksteen-Akeroyd Z. H.; Jacobs M. J.; Hammink R.; Koepf M.; Lambeck A. J. A.; van Hest J. C. M.; Wilson C. J.; Blank K.; Figdor C. G.; Rowan A. E. Therapeutic Nanoworms: Towards Novel Synthetic Dendritic Cells for Immunotherapy. Chem. Sci. 2013, 4 (11), 4168–4174. 10.1039/c3sc51399h. [DOI] [Google Scholar]
- Celikkin N.; Padial J. S.; Costantini M.; Hendrikse H.; Cohn R.; Wilson C. J.; Rowan A. E.; Świȩszkowski W. 3D Printing of Thermoresponsive Polyisocyanide (PIC) Hydrogels as Bioink and Fugitive Material for Tissue Engineering. Polymers (Basel, Switz.) 2018, 10 (5), 555. 10.3390/polym10050555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig A.; Lauko J.; Grabmaier A.; Wilson C. Wireless Tear Glucose Sensor. Procedia Eng. 2014, 87, 66–69. 10.1016/j.proeng.2014.11.267. [DOI] [Google Scholar]
- Buzzacchera I.; Vorobii M.; Kostina N. Y.; De Los Santos Pereira A.; Riedel T.; Bruns M.; Ogieglo W.; Möller M.; Wilson C. J.; Rodriguez-Emmenegger C. Polymer Brush-Functionalized Chitosan Hydrogels as Antifouling Implant Coatings. Biomacromolecules 2017, 18 (6), 1983–1992. 10.1021/acs.biomac.7b00516. [DOI] [PubMed] [Google Scholar]
- Hanssen H. J.; Tweehuysen R.. Electrochemical Biosensor Based on Hollow Coils, Method for Making and Use of the Sensor and a Medical Device Comprising the Sensor. EP2699690B1, 2014.
- Bailey T.; Bode B. W.; Christiansen M. P.; Klaff L. J.; Alva S. The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol. Ther. 2015, 17 (11), 787–794. 10.1089/dia.2014.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarraugh G. The Chemistry of Commercial Continuous Glucose Monitors. Diabetes Technol. Ther. 2009, 11 (1), S17–S24. 10.1089/dia.2008.0133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.