Abstract
Acute effects of oxidative damage induced by benzo[a]pyrene (B[a]P) on various organs are still not clear. In this study, we investigated oxidative stress and DNA damage in liver, lung, stomach, brain and kidney of ICR male mice induced by acute B[a]P treatment. B[a]P treatment led to a significant decrease at the different doses in body weight. For the variations of superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), glutathione (GSH) and GSH/GSSG, significant increases were observed at 24 h, then decreased till 72 h after B[a]P injection. The increase percent indicated in a dose- dependent decrease manner. However, glutathione peroxidase (GPx), GSSG and MDA were significantly increased in a time- and dose-dependent increase manner. DNA damage showed the significant and top levels at 24 h, and increased in proportion to the doses of B[a]P treatment. The total induction could be indicated by the variation of MDA at 24 h after B[a]P injection and showed the following order of predominance: lung > liver > kidney = stomach > brain. This was further certificated by histopathological changes in the examined organs. Additionally, the levels of serum glutamic-oxaloacetic transaminase (GOT), glutamic-pyruvic transaminase (GPT), and blood urea nitrogen (UN), creatinine were also significantly increased at 24 h after B[a]P injection. These findings suggested the disturbance of antioxidant responses and aggravation of DNA damages, and the different responses on various organs induced by acute B[a]P treatment in organism.
Keywords: Biochemistry, Cancer research, Toxicology
1. Introduction
As a ubiquitous environmental contaminant and a representative compound of polycyclic aromatic hydrocarbons (PAHs), benzo[a]pyrene (B[a]P) is mainly derived from anthropogenic activity during incomplete combustion of organic materials from various sources (Hattemer-Frey and Travis, 1991). B[a]P is a well-known genotoxic and carcinogenic compound. Generally, B[a]P and other PAHs are metabolized by both phase I and phase II biotransformation enzymes, including the cytochrome P450 (CYP) superfamily. B[a]P is metabolized to form phenols, epoxides, dihydrodiols, dihydrodiol epoxides, and the ultimate carcinogenic metabolite of anti-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro-B[a]P. Reactive oxygen species (ROS), such as superoxide anion (O2•−), hydroxyl radical (•OH) and hydrogen peroxide (H2O2), are simultaneously produced in this process (Palackal et al., 2001; Park et al., 2008).
It is known that ROS can induce lipid and protein oxidation, and deplete endogenous antioxidants by changing the oxidation-reduction (redox) state in an organism (Brucker et al., 2013; Ji et al., 2013). Antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), are to eliminate the excessive ROS in body (Pan et al., 2006). Simultaneously, ROS can be reduced by GPx at the expense of GSH to form glutathione disulfide (GSSG). Excessive ROS produced by B[a]P also alters the redox cycle which changes the redox status (GSH/GSSG ratio) in the system (Kiruthiga et al., 2010). Some B[a]P metabolites are further transformed by conjugation with glutathione which reaction is catalyzed by glutathione S-transferases (GST). It has been indicated that glutathione conjugation may be an important pathway for detoxification of B[a]P. In addition, GST is also involved in the prevention of lipid peroxidation (LPO). It has been found that B[a]P can induce oxidative stress and cause LPO in some researches (Vieira et al., 2008). When the ROS production rate is faster than elimination rate, LPO and DNA damage may occur and then lead the organism to suffer from oxidative damage (Pan et al., 2006; Liu et al., 2009).
Our previous study investigated the antioxidant enzymes, LPO and DNA damage under acute effects induced by B[a]P in cervix tissue (Gao et al., 2011a). However, the effects of environmental pollutants and the responses showed tissue different characters. Thus, the aim of this study is to investigate the acute effects of oxidative damage induced by B[a]P on liver, lung, kidney, stomach and brain, respectively. Antioxidant biomarkers, including SOD, CAT, GPx, GST, GSH, GSSG, GSH/GSSG ratio, LPO were measured after B[a]P treatment. Also, body weight, DNA damage and histopathological changes were analyzed after B[a]P treatment in mice. Additionally, the malfunction of liver and kidney biochemical parameters, including serum GPT, GOT, and blood urea nitrogen (UN), creatinine were analyzed in the experiment.
2. Materials and methods
2.1. Chemicals
B[a]P was purchased from Sigma (St. Louis, MO, USA). Collagenase 1A, PRMI-1640 medium and fetal calf serum (FCS) were purchased from Promega (Madison, USA). Normal melting point agarose and low melting point agarose were obtained from Bio-Rad (Hercules, CA, USA). All other chemicals were of analytical grade and were obtained from Sigma–Aldrich Chemical (Steinheim, Germany).
2.2. Animals and husbandry
Animal handling and experimental procedures were approved by the Animal Ethics Committee, and were applied to the Guidelines of Care and Use of Laboratory Animals of Xi'an Jiaotong University. Healthy ICR male mice, weighing 18–22 g (4–5 weeks), were purchased from the Experimental Animal Center of Xi'an Jiaotong University (Shaanxi Province, China). The animals were housed in a cross-ventilated room at 22 ± 2 °C of our departmental animal house. They were housed in a relative humidity of 50–60% and a 12 h light-dark cycle. The mice had free access to conventional laboratory feed and water. Before the experiment, they were housed in a one-week acclimatization period.
2.3. Experimental design
Fifteen groups (n = 10 mice per group) were used to investigate the B[a]P-induced acute effects on oxidative damage. B[a]P was dissolved shortly in sesame oil before injection. The first three groups served as vehicle control groups and were sampled 24, 48 and 72 h, after injection with an equal volume of sesame oil (injection volume in each case 1 ml/kg), respectively. B[a]P treated mice were divided into twelve groups (3 post-treatment sampling times × 4 doses of B[a]P). Based on the studies of an acute oxidative stress and DNA damage in mice (Jin et al., 2006; Amresh et al., 2007) as well as our previous reports (Gao et al., 2011a, Gao et al., 2011b, 2016), B[a]P was injected intraperitoneally with 100, 50, 25, 12.5 mg/kg, and each dose was further divided into three groups of 24, 48, 72 h after injection, respectively. All the animals were sacrificed by cervical decapitation under ether anesthesia at different exposure times. Livers, lungs, brains, stomachs and kidneys were excised immediately and collected. Half of the tissue samples were stored in ice and used for biochemical assays.
DNA damage was assayed by alkaline SCGE and 45 mice were used. Each post-treatment sampling time and per dose three animals as well as the three corresponding control groups were administrated as previously described. Alkaline SCGE combination with FPG was used to detect oxidative DNA damage, another 15 mice were analyzed after B[a]P injection 24 h.
Additionally, another 50 mice (10 mice per group, five groups) were used and were analyzed the serum glutamic-oxaloacetic transaminase (GOT, i.e. AST) and serum glutamic-pyruvic transaminase (GPT, i.e. ALT), blood urea nitrogen (UN) and creatinine to reflect the effect of B[a]P on liver and kidney after injection 24 h.
2.4. Biochemical analysis
The body weights of mice were measured when the experiment began (i.e., before B[a]P treatment) and end (i.e. B[a]P injection after 24, 48 or 72 h, respectively). Tissue homogenates were prepared in ice-cold 0.9% NaCl and centrifuged at 800 × g for 10 min. The supernatants were further centrifuged for 15 min at 12,000 × g to determine MDA and enzymatic assays (Livingstone, 1988). Total protein concentrations in the supernatants were assayed by Bradford method (Bradford, 1976) with bovine serum albumin used as a standard. SOD activity was measured by the method of Misra and Fridovich, (1972). CAT and GPx activities were examined according to the method of Aebi, (1984) and Hafeman (Hafeman et al., 1974), respectively. The activity of GST was examined in a substrate of 1-chloro-2, 4-dinitrobenzene, followed the formation of the conjugate with GSH at 340 nm by the method of Habig et al. (1974). A fluorometric assay was used to determine GSH contents as had been described by Hissin and Hilf (1976). GSSG levels were analyzed according to the method of Akerboom and Sies (1981). MDA levels were assayed as described by Ohkawa et al. (1979).
The blood and serum samples were analyzed for determination of the UN, creatinine, and GPT, GOT levels using a clinical chemistry automated analyzer (Mindray BS-220, Shenzhen, China) with recommended standard reagents (Roche, Basel, Switzerland) suitable for this analyzer, respectively.
2.5. Isolation of cells from liver, lung, stomach, brain, kidney
Collagenase 1A (2 ml, 0.1%) was used to prepare cell suspensions from liver, lung, stomach, brain, kidney which were incubated for 30 min at 37 °C. Then all the tissues were carefully cut free into a nylon (150 mesh) filter-funnel placed on a sterile tube. The cells were released after cutting open the capsule and 1 ml PRMI-1640 medium supplemented with 10% FCS was added. The cell suspensions were centrifuged at 40 × g for 3 min and were re-suspended in 1 ml PRMI-1640 medium. Cell viability was over 95% for all samples (Garry et al., 2003).
2.6. Alkaline single cell gel electrophoresis (SCGE) assay and modified SCGE assay
SCGE assay was used to evaluate the effects of B[a]P on DNA damage in liver, lung, stomach, brain and kidney. The procedure was carried out according to the method proposed by Singh et al. (1988) and Tice et al. (2000). In brief, cell pellets were suspended in 0.5% low melting point agarose (LMA) and were then applied to slides pre-coated with 1.5% normal-melting point agarose (NMA), and sealed with a coverslip. Then the slides were transformed to a cold plate for solidifying and the coverslips were removed. The slides were placed in lysis solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, 1% Triton X, 10% dimethyl sulfoxide, pH 10.0) for at least 1 h after the agarose gel solidified. All subsequent steps were controlled under red light to prevent possible DNA damage arising from cell manipulation. The slides were placed in alkaline electrophoresis buffer (300 mM NaOH, 1 mM Na2EDTA, pH ≥ 12.5) inside in a horizontal gel electrophoresis platform for 20 min. Electrophoresis was run at 25 V and 300 mA for 20 min after DNA unwinding period. Then the slides were washed two times with neutral buffer (0.4 M Trizma base, pH 7.5, 4 °C) for 8 min and air dried. The coded slides were stained with ethidium bromide (20 μg/ml) and reviewed under a fluorescence microscope (Nikon 027012; Nikon, Tokyo, Japan). An automated analysis system of Comet Assay Software Project (CASP) was used to score and analyze the slides. At least 100 cells were randomly scored from each slide and 3 slides were conducted per experimental group for the evaluation of DNA-damaged cells. The degree of DNA damage was scored by determining the percentage of DNA in the tail, tail DNA% = (tail DNA/(head DNA + tail DNA)) × 100. As previous description (Gao et al., 2011a, Gao et al., 2011b), each class of DNA damage in cell populations was defined according to the percentage of tail DNA which can be represented by image of comets: 0–20% (class 0), 20–40% (class 1), 40–60% (class 2), 60–80% (class 3) and >80% (class 4).
Additionally, a modified method was used to assay the effects of B[a]P induced oxidative DNA damage (Gao et al., 2011a, Gao et al., 2011b). Briefly, when the cells were lysed, the slides were washed with enzyme buffer (0.1M KCl, 40 mM HEPES, 0.5 mM Na2EDTA, 0.2 mg/mL bovine serum albumin, 4 °C, pH 8) for 2 × 8 min. Then 50 μL enzyme buffer contained 1.0 μg/mL of FPG were added to the agarose-embedded cells. Gels were sealed with a cover slip and incubated at 37 °C in the dark for 30 min. Following the slides were transferred into alkaline buffer for unwinding, electrophoresis was performed as the procedure in alkaline SCGE assay.
2.7. Pathological examination
Another half tissue samples were used to observe the pathological changes. The tissues were fixed with 10% phosphate-buffered neutral formalin. Then the samples were dehydrated in graded (50–100%) ethanol and embedded in paraffin. These paraffin masses were cut into 5-μm-thick slices and stained with hematoxylin and eosin (H&E) for the examination of pathological changes.
2.8. Statistical analysis
In this study, statistical calculations were performed using the SPSS (version 13.0) and P values were generated using unpaired Student's t-test. Results were expressed as mean ± SD for biochemical markers of oxidative stress measurement and DNA content of comet tail (%) measurement in SCGE assay. Statistical significance was defined as P < 0.05 throughout.
3. Results
3.1. B[a]P on body weight and other side effects
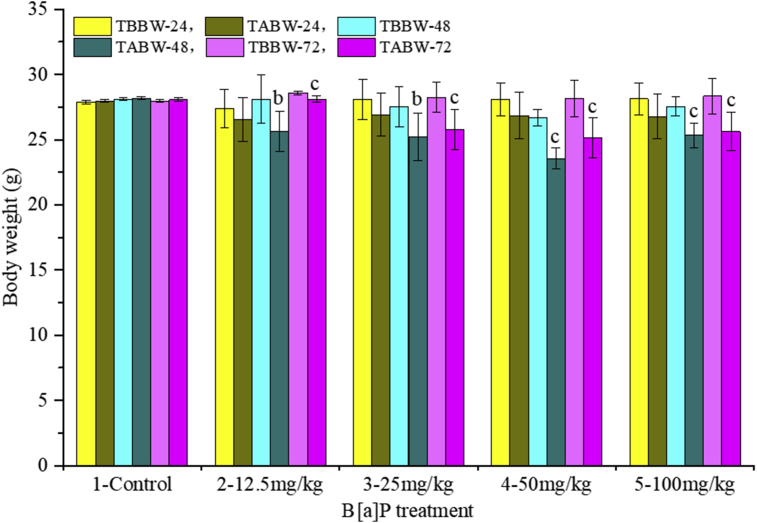
The variation of body weight was as shown in Fig. 1. Treatment after body weight (TABW) was decreased when compared with the corresponding treatment before body weight (TBBW) for all the doses of B[a]P injection after different exposure times. Especially, significant decreases were observed at 48 h, 72 h after different doses treatment (P < 0.01, P < 0.001, respectively). Also, other side effects, such as anorexia, fatigue and sleepiness, were observed in B[a]P treatment mice, especially at the doses of 50, 100 mg/kg groups.
Fig. 1.
Acute effects of B[a]P on body weight. Note: TBBW, treatment before body weight; TABW, treatment after body weight. Data were expressed as mean ± SD, n = 10; b represent P < 0.01 when compared with the corresponding TABW, c represent P < 0.001 when compared with the corresponding TABW.
3.2. Effects of B[a]P on antioxidants, MDA and DNA damage
Antioxidant enzymes activity, MDA and DNA damage were assayed in liver, lung, kidney, stomach and brain at 24, 48, 72 h time points after administration of 12.5, 25, 50, 100 mg/kg B[a]P, respectively. As shown in Table 1, SOD activity was significantly increased in stomach (P < 0.001), then in liver, kidney, brain (P < 0.01, P < 0.001, respectively), and in lung (P < 0.05, P < 0.01, P < 0.001, respectively) except that in lung at 72 h after 100 mg/kg.bw treatment when compared with the corresponding control groups. The CAT activity measured in liver were significantly increased (P < 0.01, P < 0.001, respectively). Significant increases were also observed in kidney, stomach and brain (P < 0.05, P < 0.01, P < 0.001, respectively) but not in kidney at 72 h after 100 mg/kg.bw, in stomach at 72 h after 50, 100 mg/kg.bw, in brain at 72 h after 25, 50, 100 mg/kg.bw, nor in lung at 24, 48, 72 h after 100 mg/kg.bw and at 72 h after 50 mg/kg.bw treatment (Table 2). An analysis of GPx activity showed significant increases (P < 0.05, P < 0.01, P < 0.001, respectively) in the stomachs and lungs except that at 24 h after 12.5 mg/kg.bw B[a]P-treated mice relative to the corresponding controls. GPx activity also showed significant increases (P < 0.001) in brain but not at 24, 48 h after 12.5 mg/kg.bw, at 24 h after 25 mg/kg.bw B[a]P treatment (Table 3). Also, GPx activity showed significant elevations at 72 h after the different doses of B[a]P, at 48 h after 100 mg/kg B[a]P treatment in kidney. Similar increases were observed in livers at 48 h after 50 mg/kg.bw, at different exposure times after 100 mg/kg.bw B[a]P treatment relative to the corresponding controls (Table 3). As indicated in Table 4, GST activity was significantly increased in kidney, stomach and brain (P < 0.05, P < 0.01, P < 0.001, respectively) except that in brain at 72 h after 100 mg/kg.bw treatment when compared with the corresponding control groups. Also, significant increases were observed (P < 0.05, P < 0.01, P < 0.001, respectively) in liver but not at 48, 72 h after 100 mg/kg.bw treatment. Similar significant increases were observed in lung but not at 72 h after 25, 50, 100 mg/kg.bw treatment. GSH levels increased significantly (P < 0.05, P < 0.01, P < 0.001, respectively) and relatively sensitive in stomach and liver. Similar results were observed in kidney and brain but not in kidney at 72 h after 100 mg/kg, nor in brain at 72 h after 50, 100 mg/kg B[a]P treatment. As for in lung, we observed significant increases at 24 h after 12.5, 25, 50 mg/kg.bw, at 48 h after 12.5, 25 mg/kg.bw B[a]P administration (Table 5). As for GSSG levels, significant elevations (P < 0.05, P < 0.01, P < 0.001, respectively) were observed in the examined organs. Relative lower GSSG levels were observed in brain (Table 6). We further analyzed the ratio of GSH/GSSG, significant differences (P < 0.05, P < 0.01, P < 0.001, respectively) were observed except those at 24 h after 50 mg/kg.bw B[a]P treatment in the examined organs and after 100 mg/kg in lung and stomach, at 48h after 12.5 mg/kg B[a]P treatment in lung and stomach, at 48 h after 25 mg/kg B[a]P treatment in liver, lung, stomach and kidney, at 72 h after 12.5 mg/kg B[a]P treatment in liver, lung and kidney, at 72 h after 25 mg/kg B[a]P treatment in kidney (Table 7). MDA levels increased significantly (P < 0.001) and relatively sensitive in kidney and brain (Table 8). Similar results were observed in lung and stomach except that in lung and stomach at 24 h after 12.5 mg/kg B[a]P treatment when compared with the corresponding controls. Significant increases were observed in liver (P < 0.05, P < 0.01, P < 0.001, respectively) but not at different exposure times after 12.5 mg/kg.bw B[a]P treatment, nor at 24, 48 h after 25 mg/kg B[a]P treatment relative to the controls. Additionally, SOD, CAT, GST activities, GSH levels and the ratio of GSH/GSSG was decreased in a time- and dose-dependent manner. However, GPx activities, MDA and GSSG levels were increased in a time- and dose-dependent manner.
Table 1.
Acute effects of benzo[a]pyrene on SOD activity (mean ± SD, n = 10).
| Group | Time | Liver | Lung | Kidney | Stomach | Brain |
|---|---|---|---|---|---|---|
| Control | 24 | 175.21 ± 11.42 | 160.56 ± 14.89 | 173.95 ± 12.69 | 166.38 ± 15.17 | 157.64 ± 13.92 |
| 12.5 mg/kg | 24 | 211.66 ± 11.68c | 187.73 ± 12.75c | 208.84 ± 11.18c | 240.47 ± 13.35c | 221.56 ± 12.56c |
| 25 mg/kg | 24 | 208.62 ± 11.25c | 183.22 ± 11.25b | 203.27 ± 11.15c | 233.07 ± 12.83c | 209.52 ± 13.46c |
| 50 mg/kg | 24 | 204.77 ± 14.18c | 180.88 ± 12.84b | 200.52 ± 11.63c | 221.75 ± 11.25c | 196.8 ± 11.91c |
| 100 mg/kg | 24 | 202.86 ± 11.22c | 175.56 ± 13.42a | 196.25 ± 12.32c | 215.53 ± 12.34c | 191.22 ± 11.35c |
| Control | 48 | 172.62 ± 11.54 | 158.42 ± 15.65 | 170.45 ± 13.65 | 165.62 ± 16.22 | 155.42 ± 12.87 |
| 12.5 mg/kg | 48 | 204.17 ± 11.95c | 186.91 ± 11.55c | 204.44 ± 12.84c | 236.11 ± 11.15c | 211.125 ± 12.21c |
| 25 mg/kg | 48 | 200.54 ± 12.81c | 180.97 ± 11.76b | 200.78 ± 11.92c | 227.17 ± 10.42c | 195.35 ± 13.34c |
| 50 mg/kg | 48 | 197.75 ± 13.17c | 176.16 ± 12.73a | 195.91 ± 11.53c | 213.35 ± 11.89c | 188.02 ± 12.32c |
| 100 mg/kg | 48 | 192.52 ± 12.75b | 173.62 ± 11.22a | 190.16 ± 11.16c | 206.32 ± 12.12c | 176.36 ± 12.56b |
| Control | 72 | 170.34 ± 10.85 | 157.78 ± 15.54 | 168.35 ± 11.78 | 165.22 ± 16.34 | 154.56 ± 12.67 |
| 12.5 mg/kg | 72 | 200.38 ± 9.45c | 182.26 ± 11.02c | 198.16 ± 11.98c | 228.91 ± 10.45c | 186.51 ± 10.87c |
| 25 mg/kg | 72 | 198.56 ± 12.42c | 178.46 ± 12.41b | 196.55 ± 11.65c | 219.14 ± 11.12c | 181.07 ± 11.71c |
| 50 mg/kg | 72 | 195.86 ± 11.43c | 172.46 ± 11.63a | 192.91 ± 11.36c | 210.42 ± 12.34c | 178.2 ± 12.78c |
| 100 mg/kg | 72 | 192.15 ± 12.37c | 168.63 ± 12.45 | 185.46 ± 12.37b | 198.85 ± 13.59c | 172.47 ± 13.12b |
SOD: unit mg−1 protein.
P < 0.05.
P < 0.01.
P < 0.001 between the corresponding control and B[a]P treatment groups, respectively.
Table 2.
Acute effects of benzo[a]pyrene on CAT activity (mean ± SD, n = 10).
| Group | Time | Liver | Lung | Kidney | Stomach | Brain |
|---|---|---|---|---|---|---|
| Control | 24 | 72.8 ± 8.10 | 14.36 ± 1.17 | 39.95 ± 4.62 | 12.83 ± 1.96 | 10.05 ± 1.12 |
| 12.5 mg/kg | 24 | 98.7 ± 9.73c | 20.46 ± 1.96c | 58.02 ± 12.65c | 21.32 ± 2.82c | 18.13 ± 1.71c |
| 25 mg/kg | 24 | 96.23 ± 4.54c | 18.17 ± 1.47c | 56.74 ± 13.86b | 19.45 ± 2.79c | 16.62 ± 1.21c |
| 50 mg/kg | 24 | 92.52 ± 5.21c | 16.08 ± 1.43b | 53.27 ± 8.01c | 16.28 ± 2.49b | 15.52 ± 1.86c |
| 100 mg/kg | 24 | 90.26 ± 6.19c | 15.12 ± 1.55 | 50.11 ± 12.13a | 15.53 ± 2.66a | 14.86 ± 1.23c |
| Control | 48 | 73.4 ± 8.25 | 13.68 ± 1.25 | 38.72 ± 4.23 | 12.26 ± 1.67 | 11.21 ± 1.16 |
| 12.5 mg/kg | 48 | 96.13 ± 8.65c | 17.85 ± 2.21c | 53.22 ± 13.24b | 19.62 ± 2.91c | 16.1 ± 1.21c |
| 25 mg/kg | 48 | 91.13 ± 7.26c | 16.70 ± 1.23c | 51.58 ± 14.80a | 17.48 ± 2.25c | 14.47 ± 1.76c |
| 50 mg/kg | 48 | 88.22 ± 6.65c | 15.13 ± 1.42a | 50.28 ± 12.01a | 15.36 ± 2.17b | 13.02 ± 1.20b |
| 100 mg/kg | 48 | 86.85 ± 7.36b | 14.63 ± 1.69 | 46.81 ± 11.14a | 15.09 ± 2.35b | 12.65 ± 1.25a |
| Control | 72 | 72.8 ± 8.16 | 12.35 ± 1.32 | 37.67 ± 4.55 | 11.87 ± 1.85 | 11.64 ± 1.28 |
| 12.5 mg/kg | 72 | 94.03 ± 6.13c | 14.62 ± 2.02b | 50.87 ± 14.91a | 16.01 ± 2.81b | 15.30 ± 1.20c |
| 25 mg/kg | 72 | 88.02 ± 5.63c | 13.84 ± 1.08a | 47.38 ± 7.98b | 14.38 ± 2.01b | 12.55 ± 1.69 |
| 50 mg/kg | 72 | 85.21 ± 7.73b | 13.11 ± 1.86 | 45.82 ± 7.44b | 13.15 ± 2.53 | 12.02 ± 1.53 |
| 100 mg/kg | 72 | 82.45 ± 6.16b | 12.66 ± 1.15 | 42.79 ± 9.55 | 12.68 ± 2.63 | 11.55 ± 1.32 |
P < 0.05.
P < 0.01.
P < 0.001 between the corresponding control and B[a]P treatment groups, respectively.
Table 3.
Acute effects of benzo[a]pyrene on GPx activity (mean ± SD, n = 10).
| Group | Time | Liver | Lung | Kidney | Stomach | Brain |
|---|---|---|---|---|---|---|
| Control | 24 | 80.33 ± 9.71 | 44.55 ± 5.63 | 56.74 ± 6.42 | 44.13 ± 4.76 | 44.22 ± 6.47 |
| 12.5 mg/kg | 24 | 83.06 ± 8.99 | 49.06 ± 5.08 | 61.58 ± 8.67 | 47.22 ± 4.57 | 46.69 ± 4.31 |
| 25 mg/kg | 24 | 85.06 ± 9.12 | 52.96 ± 5.13a | 62.92 ± 14.31 | 50.91 ± 4.14a | 50.03 ± 4.84 |
| 50 mg/kg | 24 | 88.97 ± 11.36 | 56.68 ± 7.28b | 63.52 ± 11.57 | 53.52 ± 6.07b | 62.28 ± 7.49c |
| 100 mg/kg | 24 | 92.48 ± 11.35a | 60.11 ± 6.32c | 65.41 ± 11.81 | 58.21 ± 6.86c | 65.72 ± 7.42c |
| Control | 48 | 82.11 ± 9.65 | 45.35 ± 5.52 | 57.65 ± 6.12 | 44.62 ± 4.75 | 44.75 ± 6.32 |
| 12.5 mg/kg | 48 | 87.44 ± 11.70 | 52.34 ± 5.54a | 63.44 ± 9.14 | 49.91 ± 4.72a | 49.81 ± 5.59 |
| 25 mg/kg | 48 | 90.44 ± 12.45 | 54.98 ± 6.56b | 65.42 ± 12.03 | 52.48 ± 5.43b | 56.67 ± 5.85c |
| 50 mg/kg | 48 | 91.90 ± 12.23 | 59.39 ± 6.74c | 66.81 ± 12.44 | 56.75 ± 6.52c | 65.54 ± 8.28c |
| 100 mg/kg | 48 | 95.83 ± 12.81a | 65.42 ± 7.11c | 68.2 ± 12.52a | 61.32 ± 7.11c | 68.37 ± 7.23c |
| Control | 72 | 84.25 ± 9.39 | 46.52 ± 5.19 | 59.17 ± 6.38 | 45.38 ± 4.53 | 45.37 ± 6.21 |
| 12.5 mg/kg | 72 | 90.12 ± 12.25 | 51.98 ± 4.75b | 65.63 ± 7.12a | 51.19 ± 5.93b | 55.03 ± 4.42c |
| 25 mg/kg | 72 | 93.12 ± 13.14 | 56.18 ± 7.36c | 67.45 ± 8.32a | 54.49 ± 5.59c | 63.56 ± 5.88c |
| 50 mg/kg | 72 | 96.62 ± 15.43a | 62.88 ± 7.33c | 69.43 ± 8.10b | 59.35 ± 6.61c | 67.30 ± 5.92c |
| 100 mg/kg | 72 | 98.92 ± 15.56a | 68.16 ± 5.63c | 71.28 ± 11.39b | 65.42 ± 6.82c | 76.33 ± 7.21c |
P < 0.05.
P < 0.01.
P < 0.001 between the corresponding control and B[a]P treatment groups, respectively.
Table 4.
Acute effects of benzo[a]pyrene on GST activity (mean ± SD, n = 10).
| Group | Time | Liver | Lung | Kidney | Stomach | Brain |
|---|---|---|---|---|---|---|
| Control | 24 | 78.23 ± 12.39 | 64.68 ± 9.12 | 54.75 ± 6.84 | 62.65 ± 9.51 | 56.89 ± 4.62 |
| 12.5 mg/kg | 24 | 98.49 ± 12.57b | 85.11 ± 8.26c | 75.72 ± 6.87c | 88.04 ± 9.33c | 76.13 ± 4.08c |
| 25 mg/kg | 24 | 95.16 ± 11.31b | 80.60 ± 5.95c | 72.65 ± 7.21c | 86.64 ± 8.19c | 72.74 ± 6.45c |
| 50 mg/kg | 24 | 92.88 ± 8.01b | 78.80 ± 7.26b | 68.33 ± 9.81b | 84.64 ± 8.29c | 66.36 ± 3.45c |
| 100 mg/kg | 24 | 89.17 ± 7.22a | 76.12 ± 7.33b | 65.56 ± 7.24b | 81.42 ± 7.32c | 64.62 ± 4.52b |
| Control | 48 | 77.52 ± 11.42 | 62.74 ± 9.24 | 52.25 ± 6.66 | 60.74 ± 9.67 | 55.81 ± 4.75 |
| 12.5 mg/kg | 48 | 94.53 ± 13.13b | 78.02 ± 8.26b | 73.97 ± 4.91c | 85.02 ± 9.34c | 72.29 ± 5.59c |
| 25 mg/kg | 48 | 92.61 ± 9.31b | 76.12 ± 5.59b | 70.45 ± 5.95c | 84.09 ± 8.66c | 68.81 ± 4.01c |
| 50 mg/kg | 48 | 90.35 ± 9.10a | 75.88 ± 6.19b | 65.80 ± 6.94c | 82.22 ± 5.77c | 62.78 ± 4.84b |
| 100 mg/kg | 48 | 85.56 ± 9.04 | 74.76 ± 7.02b | 62.25 ± 7.14b | 80.06 ± 6.13c | 60.74 ± 4.96a |
| Control | 72 | 76.34 ± 11.63 | 62.12 ± 9.36 | 50.85 ± 6.81 | 60.13 ± 9.74 | 55.12 ± 4.55 |
| 12.5 mg/kg | 72 | 91.77 ± 11.23b | 71.10 ± 6.63a | 70.98 ± 6.44c | 80.41 ± 8.89c | 66.34 ± 5.73c |
| 25 mg/kg | 72 | 89.95 ± 11.88a | 69.44 ± 6.05 | 68.76 ± 7.18c | 81.93 ± 8.75c | 64.04 ± 4.71c |
| 50 mg/kg | 72 | 87.74 ± 7.76a | 68.85 ± 6.46 | 62.21 ± 8.59b | 81.39 ± 5.14c | 60.43 ± 3.95a |
| 100 mg/kg | 72 | 81.28 ± 7.86 | 65.52 ± 7.12 | 58.11 ± 7.52a | 78.24 ± 6.29c | 58.36 ± 4.58 |
P < 0.05.
P < 0.01.
P < 0.001 between the corresponding control and B[a]P treatment groups, respectively.
Table 5.
Acute effects of benzo[a]pyrene on GSH level (mean ± SD, n = 10).
| Group | Time | Liver | Lung | Kidney | Stomach | Brain |
|---|---|---|---|---|---|---|
| Control | 24 | 40.23 ± 5.64 | 49.32 ± 7.38 | 48.83 ± 6.71 | 47.93 ± 4.11 | 19.02 ± 4.73 |
| 12.5 mg/kg | 24 | 58.96 ± 5.81c | 62.12 ± 4.09c | 68.07 ± 6.88c | 68.84 ± 5.44c | 35.71 ± 5.21c |
| 25 mg/kg | 24 | 56.42 ± 5.39c | 60.73 ± 5.45c | 64.26 ± 8.79c | 66.44 ± 4.99c | 32.60 ± 6.01c |
| 50 mg/kg | 24 | 52.94 ± 4.60c | 57.78 ± 4.16b | 60.25 ± 4.77c | 63.39 ± 3.77c | 27.76 ± 5.11c |
| 100 mg/kg | 24 | 50.47 ± 5.12c | 53.52 ± 5.21 | 57.25 ± 6.23a | 61.42 ± 3.13c | 25.63 ± 4.11b |
| Control | 48 | 39.62 ± 5.45 | 48.55 ± 7.42 | 47.25 ± 6.52 | 46.63 ± 4.52 | 18.23 ± 4.68 |
| 12.5 mg/kg | 48 | 56.29 ± 6.95c | 57.70 ± 5.80b | 63.26 ± 7.09c | 63.99 ± 4.56c | 32.43 ± 5.57c |
| 25 mg/kg | 48 | 53.43 ± 5.85c | 56.89 ± 5.58a | 62.80 ± 6.41c | 62.94 ± 4.95c | 30.23 ± 6.62c |
| 50 mg/kg | 48 | 50.37 ± 5.57c | 53.18 ± 4.16 | 58.21 ± 4.33c | 60.28 ± 3.32c | 25.08 ± 4.64b |
| 100 mg/kg | 48 | 47.75 ± 5.24b | 51.42 ± 4.85 | 53.21 ± 5.11a | 58.24 ± 4.45c | 23.86 ± 4.42a |
| Control | 72 | 39.12 ± 5.66 | 47.67 ± 7.51 | 46.68 ± 6.34 | 45.14 ± 4.33 | 18.14 ± 4.58 |
| 12.5 mg/kg | 72 | 52.45 ± 6.96c | 52.47 ± 4.35 | 60.73 ± 9.94b | 58.69 ± 5.05c | 30.83 ± 5.78c |
| 25 mg/kg | 72 | 51.14 ± 6.06c | 52.12 ± 6.57 | 57.31 ± 8.48b | 58.53 ± 3.03c | 26.54 ± 5.72b |
| 50 mg/kg | 72 | 47.25 ± 6.02b | 51.36 ± 4.62 | 54.03 ± 6.67a | 57.22 ± 3.72c | 22.13 ± 4.14 |
| 100 mg/kg | 72 | 45.56 ± 5.31a | 50.63 ± 5.14 | 50.03 ± 5.74 | 57.14 ± 4.31c | 21.81 ± 4.06 |
P < 0.05.
P < 0.01.
P < 0.001 between the corresponding control and B[a]P treatment groups, respectively.
Table 6.
Acute effects of benzo[a]pyrene on GSSG level of various organs (mean ± SD, n = 10).
| Group | Time | Liver | Lung | Kidney | Stomach | Brain |
|---|---|---|---|---|---|---|
| Control | 24 | 6.22 ± 0.41 | 7.63 ± 0.55 | 7.56 ± 0.63 | 7.42 ± 0.65 | 2.94 ± 0.32 |
| 12.5 mg/kg | 24 | 7.65 ± 0.55c | 8.43 ± 0.74a | 8.24 ± 0.74a | 9.34 ± 0.78c | 3.87 ± 0.36c |
| 25 mg/kg | 24 | 7.91 ± 0.63c | 8.55 ± 0.75b | 8.86 ± 0.82c | 9.35 ± 0.72c | 3.92 ± 0.38c |
| 50 mg/kg | 24 | 8.51 ± 0.71c | 8.62 ± 0.71b | 9.37 ± 0.92c | 9.56 ± 0.85c | 4.61 ± 0.41c |
| 100 mg/kg | 24 | 8.72 ± 0.73c | 8.66 ± 0.78b | 9.9 ± 0.96c | 9.58 ± 0.86c | 5.27 ± 0.51c |
| Control | 48 | 6.13 ± 0.62 | 7.51 ± 0.68 | 7.31 ± 0.65 | 7.22 ± 0.71 | 2.82 ± 0.32 |
| 12.5 mg/kg | 48 | 7.79 ± 0.75c | 8.36 ± 0.82a | 8.63 ± 0.82c | 9.41 ± 0.86c | 4.09 ± 0.36c |
| 25 mg/kg | 48 | 8.29 ± 0.81c | 8.66 ± 0.85b | 9.41 ± 0.78c | 9.73 ± 0.87c | 4.28 ± 0.35c |
| 50 mg/kg | 48 | 8.77 ± 0.85c | 9.2 ± 0.84c | 10.12 ± 0.96c | 10.14 ± 0.92c | 5.28 ± 0.45c |
| 100 mg/kg | 48 | 9.21 ± 0.88c | 9.43 ± 0.88c | 10.19 ± 0.92c | 10.31 ± 0.96c | 5.42 ± 0.52c |
| Control | 72 | 6.05 ± 0.68 | 7.38 ± 0.71 | 7.22 ± 0.77 | 6.98 ± 0.65 | 2.65 ± 0.23 |
| 12.5 mg/kg | 72 | 8.21 ± 0.83c | 8.58 ± 0.85b | 8.96 ± 0.84c | 9.8 ± 0.82c | 4.26 ± 0.41c |
| 25 mg/kg | 72 | 8.59 ± 0.83c | 9.24 ± 0.92c | 9.63 ± 0.86c | 10.11 ± 0.92c | 4.59 ± 0.45c |
| 50 mg/kg | 72 | 9.03 ± 0.86c | 9.7 ± 0.95c | 10.25 ± 0.86c | 10.58 ± 0.93c | 5.34 ± 0.53c |
| 100 mg/kg | 72 | 9.53 ± 0.92c | 9.91 ± 0.86c | 10.83 ± 0.96c | 10.95 ± 0.96c | 5.83 ± 0.55c |
P < 0.05.
P < 0.01.
P < 0.001 between the corresponding control and B[a]P treatment groups, respectively.
Table 7.
Acute effects of benzo[a]pyrene on GSH/GSSG ratio of various organs (mean ± SD, n = 10).
| Group | Time | Liver | Lung | Kidney | Stomach | Brain |
|---|---|---|---|---|---|---|
| Control | 24 | 6.47 ± 0.32 | 6.46 ± 0.36 | 6.46 ± 0.41 | 6.45 ± 0.35 | 6.47 ± 0.36 |
| 12.5 mg/kg | 24 | 7.71 ± 0.36c | 7.36 ± 0.41a | 8.26 ± 0.45c | 7.37 ± 0.45c | 9.23 ± 0.56c |
| 25 mg/kg | 24 | 7.13 ± 0.42b | 7.1 ± 0.45b | 7.25 ± 0.38c | 7.1 ± 0.52b | 8.31 ± 0.71c |
| 50 mg/kg | 24 | 6.22 ± 0.45 | 6.7 ± 0.52 | 6.43 ± 0.36 | 6.63 ± 0.51 | 6.02 ± 0.62 |
| 100 mg/kg | 24 | 5.78 ± 0.52b | 6.18 ± 0.55 | 5.78 ± 0.42b | 6.41 ± 0.48 | 4.86 ± 0.52c |
| Control | 48 | 6.46 ± 0.55 | 6.46 ± 0.56 | 6.46 ± 0.45 | 6.45 ± 0.53 | 6.46 ± 0.39 |
| 12.5 mg/kg | 48 | 7.22 ± 0.56c | 6.9 ± 0.61 | 7.33 ± 0.46c | 6.8 ± 0.55 | 7.92 ± 0.63c |
| 25 mg/kg | 48 | 6.44 ± 0.71 | 6.56 ± 0.55 | 6.67 ± 0.45 | 6.46 ± 0.46 | 7.06 ± 0.66a |
| 50 mg/kg | 48 | 5.74 ± 0.51b | 5.78 ± 0.46b | 5.75 ± 0.51b | 5.94 ± 0.38a | 4.75 ± 0.42c |
| 100 mg/kg | 48 | 5.18 ± 0.45c | 5.45 ± 0.46c | 5.22 ± 0.52c | 5.64 ± 0.42b | 4.4 ± 0.41c |
| Control | 72 | 6.46 ± 0.52 | 6.45 ± 0.48 | 6.46 ± 0.61 | 6.46 ± 0.45 | 6.46 ± 0.51 |
| 12.5 mg/kg | 72 | 6.38 ± 0.51 | 6.11 ± 0.52 | 6.78 ± 0.58 | 5.99 ± 0.55a | 7.23 ± 0.63b |
| 25 mg/kg | 72 | 5.95 ± 0.53a | 5.64 ± 0.62b | 5.95 ± 0.55 | 5.78 ± 0.46b | 5.78 ± 0.55a |
| 50 mg/kg | 72 | 5.17 ± 0.55c | 5.29 ± 0.58c | 5.27 ± 0.48c | 5.4 ± 0.49c | 4.14 ± 0.43c |
| 100 mg/kg | 72 | 4.78 ± 0.46c | 5.1 ± 0.47c | 4.62 ± 0.41c | 5.21 ± 0.42c | 3.74 ± 0.32c |
P < 0.05.
P < 0.01.
P < 0.001 between the corresponding control and B[a]P treatment groups, respectively.
Table 8.
Acute effects of benzo[a]pyrene on MDA level (mean ± SD, n = 10).
| Group | Time | Liver | Lung | Kidney | Stomach | Brain |
|---|---|---|---|---|---|---|
| Control | 24 | 6.05 ± 1.55 | 5.58 ± 1.26 | 3.98 ± 0.17 | 1.43 ± 0.15 | 1.49 ± 0.08 |
| 12.5 mg/kg | 24 | 7.59 ± 1.19 | 7.09 ± 1.23 | 4.77 ± 0.67c | 1.62 ± 0.12 | 1.63 ± 0.25c |
| 25 mg/kg | 24 | 7.95 ± 1.40 | 7.41 ± 1.73a | 5.17 ± 1.08c | 1.77 ± 0.19c | 1.75 ± 0.19c |
| 50 mg/kg | 24 | 8.85 ± 2.11a | 8.84 ± 1.82c | 5.81 ± 1.24c | 2.09 ± 0.12c | 1.85 ± 0.29c |
| 100 mg/kg | 24 | 9.57 ± 2.38a | 9.23 ± 1.32c | 6.22 ± 1.26c | 2.15 ± 0.16c | 2.05 ± 0.21c |
| Control | 48 | 6.12 ± 1.46 | 5.66 ± 1.21 | 4.00 ± 0.18 | 1.45 ± 0.15 | 1.51 ± 0.09 |
| 12.5 mg/kg | 48 | 7.96 ± 1.76 | 7.36 ± 1.41b | 4.86 ± 0.31c | 1.76 ± 0.16c | 1.73 ± 0.23c |
| 25 mg/kg | 48 | 8.16 ± 1.06 | 7.80 ± 1.16c | 5.55 ± 0.82c | 1.84 ± 0.18c | 1.96 ± 0.25c |
| 50 mg/kg | 48 | 9.58 ± 2.14b | 8.92 ± 2.16c | 5.93 ± 1.13c | 1.99 ± 0.15c | 1.99 ± 0.22c |
| 100 mg/kg | 48 | 11.63 ± 2.47c | 9.35 ± 2.72c | 6.33 ± 1.06c | 2.18 ± 0.18c | 2.39 ± 0.26c |
| Control | 72 | 6.35 ± 1.35 | 5.72 ± 1.24 | 4.02 ± 0.18 | 1.86 ± 0.13 | 1.52 ± 0.11 |
| 12.5 mg/kg | 72 | 8.61 ± 1.84 | 7.49 ± 1.49c | 4.94 ± 0.84c | 1.95 ± 0.12c | 1.76 ± 0.24c |
| 25 mg/kg | 72 | 9.22 ± 1.46b | 8.54 ± 2.62c | 6.91 ± 0.96c | 2.06 ± 0.13c | 2.14 ± 0.32c |
| 50 mg/kg | 72 | 11.93 ± 2.19c | 11.54 ± 2.62c | 7.21 ± 1.38c | 2.14 ± 0.19c | 2.21 ± 0.27c |
| 100 mg/kg | 72 | 14.03 ± 2.35c | 13.12 ± 3.16c | 7.96 ± 1.45c | 2.36 ± 0.21c | 2.58 ± 0.31c |
P < 0.05.
P < 0.01.
P < 0.001 between the corresponding control and B[a]P treatment groups, respectively.
DNA damage was indicated as comet tail DNA content (%) and was shown in Table 9. Similarly, DNA damage increased significantly (P < 0.001) accompanied by the doses of B[a]P elevation, and decreased with the B[a]P exposure time prolonged. Additionally, SCGE combination with FPG resulted in more obvious increases in DNA content (%) at 24 h after B[a]P treatment.
Table 9.
Acute effects of benzo[a]pyrene on DNA damage (DNA content of comet tail, %) of various organs (mean ± SD, n = 100 cells).
| Group | Time | Liver | Lung | Kidney | Stomach | Brain |
|---|---|---|---|---|---|---|
| Control | 24 | 0.48 ± 0.19 | 0.41 ± 0.11 | 0.56 ± 0.09 | 0.79 ± 0.17 | 0.51 ± 0.26 |
| 12.5 mg/kg | 24 | 5.72 ± 1.72c | 3.43 ± 0.34c | 6.21 ± 1.61c | 2.96 ± 0.18c | 4.34 ± 1.22c |
| 25 mg/kg | 24 | 6.54 ± 2.04c | 5.65 ± 1.12c | 8.33 ± 1.55c | 4.35 ± 0.53c | 6.28 ± 0.89c |
| 50 mg/kg | 24 | 11.95 ± 1.55c | 9.75 ± 2.10c | 11.89 ± 4.83c | 6.23 ± 0.34c | 9.14 ± 5.09c |
| 100 mg/kg | 24 | 12.13 ± 1.34c | 10.55 ± 1.32c | 12.32 ± 2.57c | 9.02 ± 0.54c | 10.23 ± 1.52c |
| Control | 48 | 0.45 ± 0.16 | 0.38 ± 0.12 | 0.53 ± 0.07 | 0.77 ± 0.18c | 0.48 ± 0.24c |
| 12.5 mg/kg | 48 | 4.43 ± 2.90c | 3.37 ± 1.95c | 4.22 ± 2.44c | 2.42 ± 0.23c | 3.56 ± 1.17c |
| 25 mg/kg | 48 | 5.94 ± 3.31c | 4.05 ± 0.16c | 8.01 ± 2.19c | 3.85 ± 0.32c | 5.80 ± 1.72c |
| 50 mg/kg | 48 | 9.12 ± 1.52c | 7.93 ± 2.77c | 7.16 ± 3.49c | 4.85 ± 0.37c | 8.45 ± 1.45c |
| 100 mg/kg | 48 | 10.31 ± 1.85c | 9.65 ± 1.25c | 10.22 ± 2.52c | 7.21 ± 0.62c | 8.85 ± 1.36c |
| Control | 72 | 0.43 ± 0.17 | 0.37 ± 0.12 | 0.50 ± 0.07 | 0.76 ± 0.17c | 0.47 ± 0.22 |
| 12.5 mg/kg | 72 | 3.14 ± 1.50c | 2.83 ± 1.62c | 3.13 ± 1.35c | 1.21 ± 0.18c | 3.14 ± 1.76c |
| 25 mg/kg | 72 | 7.38 ± 1.26c | 3.24 ± 0.11c | 3.98 ± 1.82c | 2.14 ± 0.23c | 4.78 ± 2.12c |
| 50 mg/kg | 72 | 7.68 ± 2.64c | 6.93 ± 2.67c | 5.70 ± 2.32c | 3.51 ± 0.26c | 7.35 ± 2.07c |
| 100 mg/kg | 72 | 8.00 ± 4.50c | 8.49 ± 2.44c | 6.23 ± 2.12c | 5.27 ± 0.36c | 7.78 ± 1.23c |
| Control FPG | 24 | 0.52 ± 0.12 | 0.44 ± 0.09 | 0.58 ± 0.10 | 0.83 ± 0.16 | 0.54 ± 0.21 |
| 12.5 mg/kg FPG | 24 | 8.06 ± 1.83c | 5.14 ± 0.42c | 9.16 ± 1.83c | 4.48 ± 0.35c | 6.35 ± 1.27c |
| 25 mg/kg FPG | 24 | 9.56 ± 2.13c | 7.35 ± 1.26c | 11.73 ± 2.14c | 6.24 ± 0.75c | 9.11 ± 0.96c |
| 50 mg/kg FPG | 24 | 14.82 ± 1.67c | 11.63 ± 2.26c | 13.75 ± 3.21c | 7.32 ± 0.46c | 11.21 ± 3.12c |
| 100 mg/kg FPG | 24 | 16.15 ± 1.42c | 12.62 ± 1.51c | 16.35 ± 2.64c | 10.91 ± 0.82c | 12.11 ± 1.83c |
P < 0.001 between the corresponding control and B[a]P treatment groups.
3.3. Effects of B[a]P stimulation in antioxidant enzymes activity, MDA and DNA damage levels at 24 h
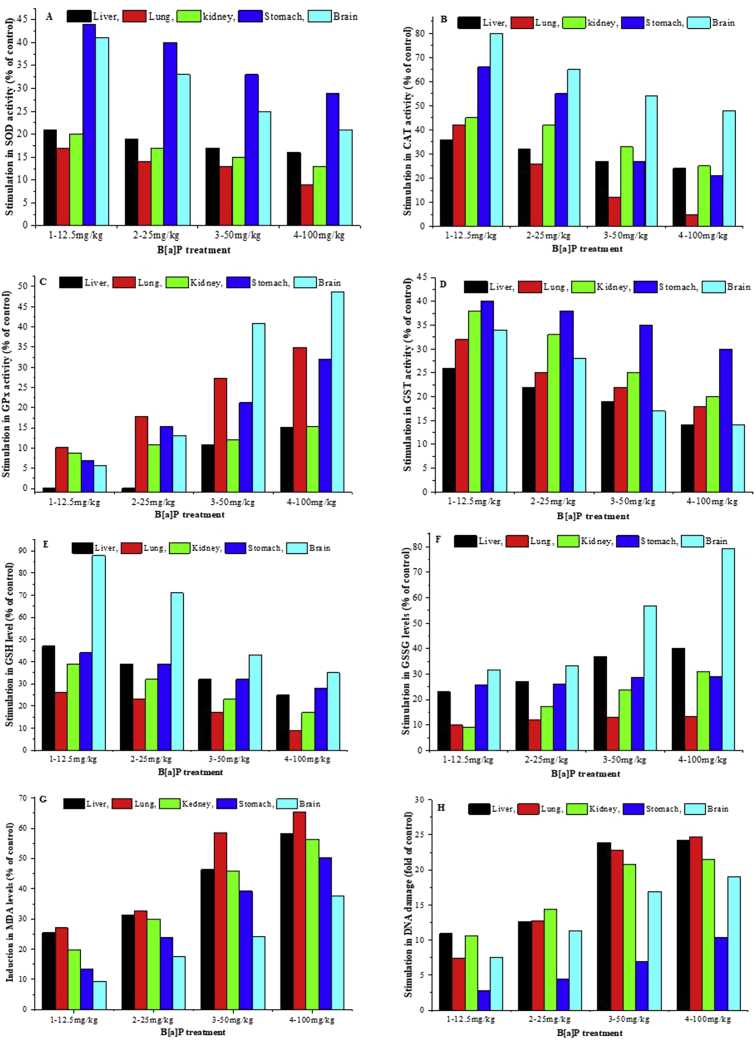
We further assayed the stimulation in antioxidant enzymes activity, MDA and DNA damage levels in liver, lung, kidney, stomach and brain at 24 h after B[a]P injection. As shown in Fig. 2A, B[a]P produced a 16% to 21%, 9% to 17%, 13% to 20%, 29% to 44%, 21% to 41% stimulation in SOD activity in liver, lung, kidney, stomach and brain, respectively, relative to the controls at 24 h. Increments in CAT activity ranging from 24% to 36% in liver, 5% to 42% in lung, 25% to 45% kidney, 21% to 66% in stomach, and 48% to 80% in brain were detected when compared with the controls at 24 h (Fig. 2B). For GPx activity (Fig. 2C), there was 3%–15.13% increase in liver, 10.13% to 34.93% increase in lung, 8.53% to 15.28% increase in kidney, 7.00% to 31.91% increase in stomach, 5.58% to 48.62% increase in brain of B[a]P treatment groups when compared with the corresponding controls. GST activity was also higher by 14%–26%, 18%–32%, 20%–38%, 30%–40%, and 14%–34% (Fig. 2D) in B[a]P exposed groups relative to the corresponding controls at 24 h in liver, lung, kidney, stomach and brain, respectively. Increases in GSH level ranging from 25% to 47% in liver, 9% to 26% in lung, 17% to 39% in kidney, 28% to 44% in stomach, and 35% to 88% in brain (Fig. 2E) were observed relative to the controls at 24 h. B[a]P produced a 23% to 40%, 10% to 13.5%, 9% to 31%, 26% to 29%, 31% to 79% stimulation in GSSG level in liver, lung, kidney, stomach and brain, respectively, relative to the controls at 24 h (Fig. 2F). There was an increase in MDA ranging between 25.45% and 58.18% in liver, 27.06 and 65.41% in lung, 19.85% and 56.28% in kidney, 13.18% and 50.34% in stomach, 9.39% and 37.58% in brain (Fig. 2G) when compared with the corresponding controls at 24 h, respectively. Great elevations from 10.92 to 24.27 fold in liver, 7.36 to 24.73 fold in lung, 10.58 to 21.50 fold in kidney, 2.75 to 10.42 fold in stomach, 7.51 to 19.05 fold in brain (Fig. 2H) were noted in DNA damage. So, for antioxidant enzymes, the increase rates relative to the corresponding control were higher in brain, stomach, then in kidney, lung and liver. However, the high increased rates in liver, lung, followed by kidney, brain and stomach were noted in MDA and DNA damage.
Fig. 2.
B[a]P stimulation in antioxidant enzymes activity, GSSG, MDA and DNA damage at 24 h. Values represent the percent of: [(meanB[a]P treatment−meancontrol)/meancontrol].
3.4. Effects of B[a]P on serum GPT, GOT and blood UN, creatinine at 24 h
We additionally the levels of serum GPT, GOT and blood UN, creatine to indicate the effects of B[a]P at 24 h after injection on the function of liver and kidney. As shown in Table 10, the levels of GPT, GOT were significantly increased (P < 0.001) at 24 h after B[a]P treatment. Similarly, the levels of blood UN and creatinine were also significantly increased (P < 0.05, P < 0.001) except that at 24 h after 12.5 mg/kg B[a]P injection.
Table 10.
Acute effects of benzo[a]pyrene on the levels of GPT, GOT and UN, creatinine (mean ± SD, n = 10).
| Group | Time | GPT (IU/L) | GOT (IU/L) | UN (mg/dl) | Creatinine (mg/dl) |
|---|---|---|---|---|---|
| Control | 24 | 35.3 ± 1.82 | 62.4 ± 3.12 | 13.23 ± 1.12 | 0.07 ± 0.01 |
| 12.5 mg/kg | 24 | 52.5 ± 2.67b | 66.3 ± 3.23a | 15.32 ± 1.22b | 0.075 ± 0.01 |
| 25 mg/kg | 24 | 64.1 ± 3.52b | 69.5 ± 3.45b | 16.31 ± 1.23b | 0.08 ± 0.011a |
| 50 mg/kg | 24 | 76.7 ± 3.72b | 76.6 ± 3.72b | 17.4 ± 1.52b | 0.082 ± 0.012a |
| 100 mg/kg | 24 | 121.1 ± 7.23b | 84.9 ± 4.56b | 23.6 ± 2.12b | 0.11 ± 0.013b |
P < 0.05.
P < 0.001 between the corresponding control and B[a]P treatment groups, respectively.
3.5. Effects of B[a]P in pathological changes of liver, lung, kidney, stomach and brain
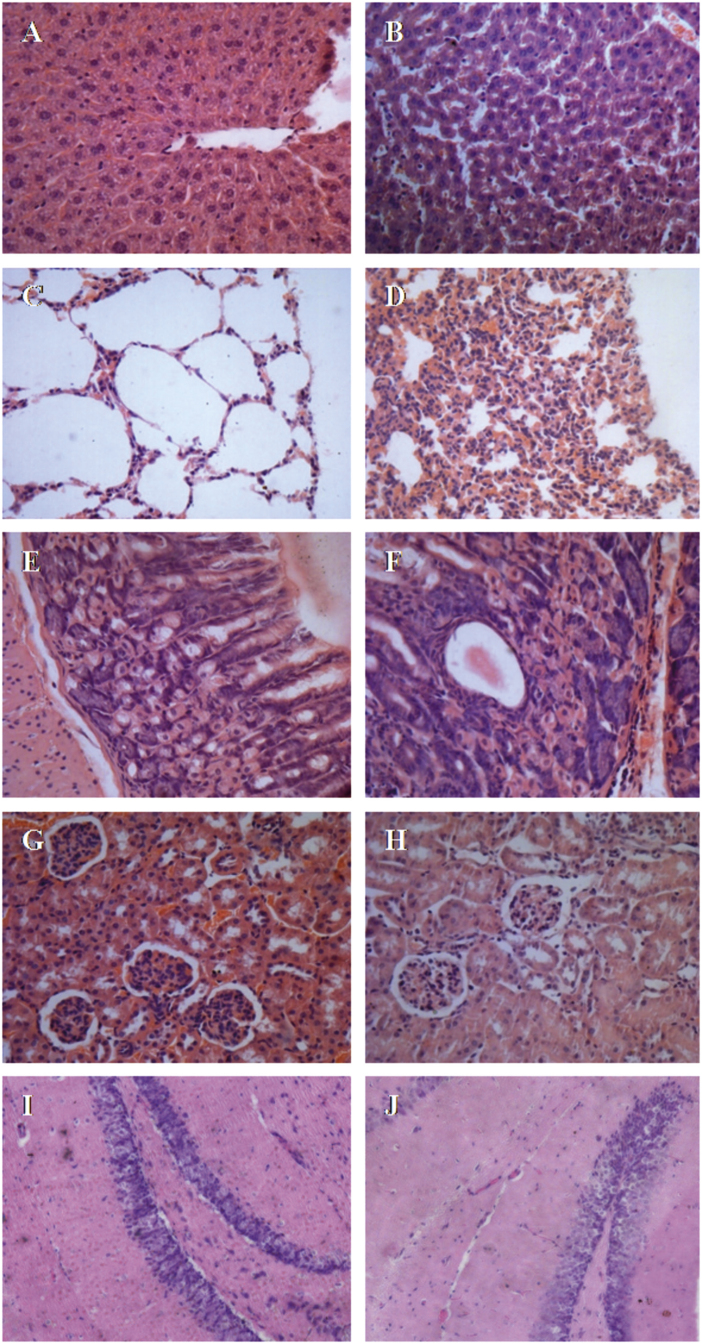
In the examined organs, acute B[a]P treatment resulted in some pathological changes. Here we showed the representative micrographs of sections from 50 mg/kg B[a]P treated mice and the control animals (Fig. 3). Liver histology remained almost normal when compared with the control (Fig. 3A) except obvious expansion of hepatic sinusoid between hepatic cords (Fig. 3B). Normal alveolar structure indicated by a large alveoli and thin alveolar walls were observed in lung tissue in the control group (Fig. 3C). There was the thickening alveolar wall, compressed alveolar and severe inflammatory cells infiltration phenomenon in B[a]P treated mice (Fig. 3D). The normal structure of stomach indicated by the stomach mucosa, primary cells and B cells, gastric pit and the clear smooth muscle layer in the control group was noted (Fig. 3E). There was a small amount of inflammatory cell infiltration between the bottom of the stomach mucosa and muscle layer in B[a]P treated mice (Fig. 3F). Kidney tissue showed almost normal as indicated by glomerulus, a collection of small tubes, tubular and other organizational structures (Fig. 3H) in B[a]P induced mice as in control (Fig. 3G). Slighter staining in nucleus of dentate gyrus (DG) cells of hippocampus (Fig. 3J) in B[a]P treated mice brain was observed when compared with the control (Fig. 3I).
Fig. 3.
Representative histopathological changes of liver, lung, kidney, stomach and brain tissues induced by 50 mg/kg treatment B[a]P in mice. A-B for Liver, C-D for Lung, E-F for stomach, G-H for Kidney and I-J for Brain (HE staining, Magnification: A-H, ×400; I-J, ×200).
4. Discussion
A growing number of studies have indicated that high levels of air pollutants are associated with numerous adverse health outcomes, such as cardiovascular and respiratory problems, even increased mortality dates (Friesen et al., 2010; Sancini et al., 2014; He et al., 2010). However, the mechanisms responsible for such an association have not been fully identified. As in mentioned above, the primary aim of this study was to find out the acute effects of oxidative damage induced by B[a]P in the multiple organs of liver, lung, kidney, stomach, brain, and to find out the differences between their responses. In this paper, we report a transient increase in oxidative stress with acute B[a]P treatment in mice.
The body and organ weights of rodents are important considerations in medical research and animal science (Tanaka et al., 2009). In the present study, B[a]P treatment induced a significant decrease in body weight from the doses of 12.5 mg/kg to 100 mg/kg. Our results are in line with the studies of Sumedha et al. (2006) and Rajendran et al. (2008) which have indicated the diminish in body weight in experimental animals after B[a]P administration. Weight loss and tissue wasting are generally observed in cancer animal models or patients (Paul et al., 2011). For a typical carcinogenic compound, the results may be due to the fact that the cancer cachexia was induced by B[a]P through suppressing the growth in body weight.
B[a]P induced oxidative damage was determined. Significant increases were observed for antioxidants, including SOD, CAT, GST and GSH levels in B[a]P treated mice. The results implied the transient induction of antioxidants in acute B[a]P exposure. Similar results were also found in other published studies. For example, Pan et al. (2006) reported that the antioxidant enzyme activities turned to be higher or to be restrained in PAHs acute exposure in Chlamys Ferrari. There was also information that showed the acute toxicity of B[a]P and anthracene increasing CAT activity. Besides, isolated PAHs also increased SOD, GST and GPx activities, GSH levels in goby Pomatoschistus microps (Vieira et al., 2008). This induction may be due to the oxidative stress induced by B[a]P through the excessive ROS production, especially considerable amount of O•2− and hydrogen peroxide (H2O2) which needs to be converted by SOD and CAT. Simultaneously, the induction of GST and GSH implied the more production of electrophilic substances which need to be detoxified during acute B[a]P treatment (Pan et al., 2006; Vieira et al., 2008; Wu et al., 2007; Amresh et al., 2007). In addition, the GSH levels and CAT, GST, SOD activities were highest at 24 h, then decreased till 72 h after B[a]P treatment. The findings are in line with the studies reported by Pan et al. (2006) and Kim et al. (2000), demonstrating that the antioxidant enzymes, such as SOD and CAT, were inhibited at 24 h after B[a]P treatment, and O•2−, H2O2 was accumulated soon in the organism. In the present study, for GSSG levels, significant increases were induced and increased in a dose- and time-dependent manner. The results suggested the enhanced oxidative stress, especially resulted from excessive ROS (Kim et al., 2008). This is corresponded to the increased activity of GPx till 72 h after B[a]P treatment which may be adaptable response to the accumulation of ROS (Mates, 2000; Pi et al., 2002). However, the enhanced GPx activity has not counteracted the accumulation of ROS. The variation of GSH/GSSG suggested that B[a]P treatment altered the redox cycle in the system. The decrease in the ratio of GSH/GSSG would be more sensitive to B[a]P with the elevated doses and prolonged treatment times (Kiruthiga et al., 2010). Therefore, MDA levels was increased till 72 h after B[a]P treatment and this increase was enhanced with the doses elevation.
As a classical method of DNA damage detection, the SCGE assay is usually used in experimental and epidemiological studies (Garry et al., 2003). In the present study, we used the SCGE assay to detect DNA damage induced by B[a]P. The results showed that different doses of B[a]P treatment significantly induced DNA damage in liver, lung, kidney, stomach and brain tissues. This demonstrated that the cytochrome P-450 isoenzyme, especially CYP1A1, existed in these tissues, could catalyze B[a]P into ROS and electrophilic metabolites such as BPDE to induce DNA damage (Albrecht et al., 2001) and to produce genotoxic activity (Yang et al., 2000). The results of SCGE combination FPG further demonstrated that B[a]P induced oxidative DNA damage (Gao et al., 2011a, Gao et al., 2011b). Also, the results indicated that the highest DNA damage was induced at 24 h after B[a]P treatment, and then decreased till 72 h. This may be due to the fact that the DNA damage induced by B[a]P was partially repaired by the nucleotide excision repair system (Garry et al., 2004; Cemeli et al., 2009). However, DNA damage remained significantly higher when compared with the corresponding control animals. The DNA damage was enhanced with the increase of B[a]P dose and similar to our previous study in cervix tissue (Gao et al., 2011a, Gao et al., 2011b).
Based on the previous variations of antioxidants and DNA damage with prolonged time after B[a]P treatment, we further analyzed the stimulation in antioxidants, MDA and DNA damage levels at 24 h in these organs. As for the variation of SOD, CAT and GSH among these organs, the relative high stimulation was in stomach and brain, the relative low stimulation appeared in lung and liver (Fig. 2). As for GPx variation, the highest stimulation was observed in brain, then in lung, followed in stomach, liver and kidney. Thus, the highest stimulation GSSG was also found in brain, then in liver, kidney and stomach, last in lung. However, the relative higher stimulation of GST activity was observed in stomach and kidney, followed by lung and liver, last in brain. This may be the previous relative high stimulation of SOD, CAT, GSH and GPx which cleared more excessive ROS, then further need relative low activity of GST to detoxify the toxic effects in brain (Pan et al., 2006; Gao et al., 2011a, Gao et al., 2011b; Kim et al., 2000). Herein, the induction of MDA on these tissues was as the following order of predominance: lung > liver > kidney > stomach > brain (Fig. 2F). The results suggested that B[a]P induced relative high oxidative stress in liver and lung, lower oxidative stress in stomach and brain (Dasgupta et al., 2004). Further, the induction of DNA damage on these tissues was as the following order of predominance: liver > lung > kidney > brain > stomach (Fig. 2G). Liver is the most important organs of xenobiotic metabolism and is primarily involved in the metabolism of B[a]P (Saunders et al., 2006). B[a]P is one of the main carcinogens in cigarette smoke which plays a major role in the induction of lung carcinogenesis (Hecht et al., 2002). Our results are in line with these previous publications and show the relative high oxidative stress and DNA damage in liver and lung. Numerous studies have indicated that renal changes are induced by B[a]P treatment in rodents (Alejandro et al., 2000; Knuckles et al., 2001; Nanez et al., 2005). As a result, the findings showed oxidative stress and DNA damages in kidney induced by B[a]P. The stomach is also exposed to exogenous carcinogens such as B[a]P which is used to induce tumors in the lung, skin, and forestomach of mice (Lee et al., 2005). Experimental data have demonstrated that B[a]P easily crosses the blood-brain barrier and induces acute neurobehavioral toxicity through oxidative stress (Murawska-Ciałowicz et al., 2011). Thus, the results also indicated oxidative stress and DNA damage in stomach and brain. However, the results of induction in MDA and DNA damage are not consistent with each other in stomach and brain. This may be due to the more sensitive DNA in brain than that in stomach. This need to be further studied to elucidate the exact molecular mechanism in the future. Additionally, the variations of serum GPT, GOT and blood UN, creatinine were determined. The findings indicated that B[a]P was a toxic agent for the liver and kidney (Valentovic et al., 2006; Gao et al., 2011b; Aktay et al., 2011).
The stimulation of antioxidants and induction of MDA and DNA damage were further demonstrated in the analysis of HE examination in liver, lung, kidney, stomach and brain tissues. HE examination in tissue pathology was with the following order of predominance: lung > liver > kidney = stomach > brain (Fig. 3). As acute effects on these organs, the highest injury was observed in lung, including the alveolar wall thickening, alveolar compression, and a large number of inflammatory cell infiltrations. Then significant expansion of hepatic sinusoid between hepatic cord in liver and a small amount of inflammatory cell infiltration in stomach were observed. As for other organs, the relative less injury was observed than that in lung. This is associated with the important role of B[a]P in lung carcinogenesis (Hecht et al., 2002). The results of HE examination were consistent with the induction of MDA in these organs. This implied that LPO may indicate more effects on these organs. Actually, the level of LPO was an important indicator in evaluating the oxidative stress in organism (Ji et al., 2013; Pan et al., 2006). Oxidative stress plays an important role in the pathogenesis of cancer. The abnormal histological tissue may further progress into precancerous lesion, even to cancer in organisms. In summary, in acute toxic effects, B[a]P induced a decrease in body weight. B[a]P modified antioxidants through temporally improving SOD, CAT, GST, GSH, GPx levels, and increasing GSSG and MDA levels in the experiment. The redox cycle was altered by B[a]P through the indicative variation of GSH/GSSG. This stimulation indicated time- and dose- dependent decreases for SOD, CAT, GST, GSH levels and GSH/GSSG. However, this induction showed time- and dose-dependent increases for GPx activity, GSSG and MDA levels. As for DNA damage, the induction indicated time-dependent decrease and dose-dependent increase manner. B[a]P also induced the significant elevation of serum GPT, GOT and blood UN, creatinine. The total induction may be represented by the variation of MDA at 24 h after B[a]P treatment and showed by the following order of predominance: lung > liver > kidney = stomach > brain, which was also demonstrated by histopathological variation. Further studies are necessary to examine the specific mechanism for the enhanced toxicity in the examined organs associated with B[a]P treatment.
Declarations
Author contribution statement
Chun Deng, Jianghong Gao and Hongyan Zhao: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Shunyan Qi: Conceived and designed the experiments; Performed the experiments.
Meili Gao: Performed the experiments; Wrote the paper.
Funding statement
This work was supported by the Fundamental Research Funds for the Central Universities (1191320021) of China and the General Research & Development Plan of Shaanxi Province (No. 2017SF-117).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aebi H. Catalase in vivo. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Albrecht C., Adolf B., Weishaupt C., Höhr D., Zeitträger I., Friemann J., Borm P.J. Clara-cell hyperplasia after quartz and coal-dust instillation in rat lung. Inhal. Toxicol. 2001;13:191–205. doi: 10.1080/08958370150502430. [DOI] [PubMed] [Google Scholar]
- Alejandro N.F., Parrish A.R., Bowes R.C., Burghardt R.C., Ramos K.S. Phenotypic profiles of cultured glomerular cells following repeated cycles of hydrocarbon injury. Kidney Int. 2000;57:1571–1580. doi: 10.1046/j.1523-1755.2000.01001.x. [DOI] [PubMed] [Google Scholar]
- Amresh G., Rao C.V., Singh P.N. Antioxidant activity of Cissampelos pareirao benzo(a)pyrene-induced mucosal injury in mice. Nutr. Res. 2007;27:625–632. [Google Scholar]
- Aktay G., Emre M.H., Polat A. Influence of dihydropyridine calcium antagonist nitrendipine on benzo(a)pyrene-induced oxidative stress. Arch Pharm Res. 2011;34:1171–1175. doi: 10.1007/s12272-011-0715-x. [DOI] [PubMed] [Google Scholar]
- Akerboom T.P., Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brucker N., Moro A.M., Charão M.F., Durgante J., Freitas F., Baierle M., Nascimento S., Gauer B., Bulcão R.P., Bubols G.B., Ferrari P.D., Thiesen F.V., Gioda A., Duarte M.M., de Castro I., Saldiva P.H., Garcia S.C. Biomarkers of occupational exposure to air pollution, inflammation and oxidative damage in taxi drivers. Sci. Total Environ. 2013;463–464:884–893. doi: 10.1016/j.scitotenv.2013.06.098. [DOI] [PubMed] [Google Scholar]
- Cemeli E., Baumgartner A., Anderson D. Antioxidants and the comet assay. Mutat. Res. 2009;681:51–67. doi: 10.1016/j.mrrev.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Dasgupta T., Banerjee S., Yadava P.K., Rao A.R. Chemopreventive potential of Azadirachta indica (Neem) leaf extract in murine carcinogenesis model systems. J. Ethnopharmacol. 2004;92:23–36. doi: 10.1016/j.jep.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Friesen M.C., Demers P.A., Spinelli J.J., Eisen E.A., Lorenzi M.F., Le N.D. Chronic and acute effects of coal tar pitch exposure and cardiopulmonary mortality among aluminum smelter workers. Am. J. Epidemiol. 2010;172:790–799. doi: 10.1093/aje/kwq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Li Y., Long J., Shah W., Fu L., Lai B., Wang Y. Induction of oxidative stress and DNA damage in cervix in acute treatment with benzo[a]pyrene. Mutat. Res. 2011;719:52–59. doi: 10.1016/j.mrgentox.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Gao M., Li Y., Sun Y., Shah W., Yang S., Wang Y., Long J. Benzo[a]pyrene exposure increases toxic biomarkers and morphological disorders in mouse cervix. Basic Clin. Pharmacol. Toxicol. 2011;109:398–406. doi: 10.1111/j.1742-7843.2011.00755.x. [DOI] [PubMed] [Google Scholar]
- Gao M., Li Y., Ji X., Xue X., Chen L., Feng G., Zhang H., Wang H., Shah W., Hou Z., Kong Y. Disturbance of Bcl-2, Bax, Caspase-3, Ki-67 and C-myc expression in acute and subchronic exposure to benzo(a)pyrene in cervix. Acta Histochem. 2016;118:63–73. doi: 10.1016/j.acthis.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Garry S., Nesslany F., Aliouat E., Haguenoer J.M., Marzin D. Assessment of genotoxic effect of benzo[a]pyrene in endotracheally treated rat using the comet assay. Mutat. Res. 2003;534:33–43. doi: 10.1016/s1383-5718(02)00252-8. [DOI] [PubMed] [Google Scholar]
- Garry S., Nesslany F., Aliouat E., Haguenoer J.M., Marzin D. Hematite (Fe2O3 ) acts by oxydative stress and potentiates benzo[a]pyrene genotoxicity. Mutat. Res. 2004;563:117–129. doi: 10.1016/j.mrgentox.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Hissin P.J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Habig W.M., Pabst M.J., Jakoby W.B. Glutathione-S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. PMID: 4436300. [PubMed] [Google Scholar]
- Hafeman D.G., Sunde R.A., Hoekstra W.G. Effect of dietary selenium and erythrocyte and liver glutathione peroxidase in the rat. Nutrition. 1974;104:580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- He F., Shaffer M.L., Rodriguez-Colon S., Bixler E.O., Vgontzas A.N., Williams R.W., Wu R., Cascio W.E., Liao D. Acute effects of fine particulate air pollution on ST segment height: a longitudinal study. Environ. Health. 2010;8:68. doi: 10.1186/1476-069X-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattemer-Frey H.A., Travis C.C. Benzo-a-pyrene: environmental partitioning and human exposure. Toxicol. Ind. Health. 1991;17:141–157. doi: 10.1177/074823379100700303. [DOI] [PubMed] [Google Scholar]
- Hecht S.S., Upadhyaya P., Wang M., Bliss R.L., McIntee E.J., Kenney P.M. Inhibition of lung tumorigenesis in A/J mice by Nacetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine and myo-inositol, individually and in combination. Carcinogenesis. 2002;23:1455–1461. doi: 10.1093/carcin/23.9.1455. [DOI] [PubMed] [Google Scholar]
- Ji K., Xing C., Jiang F., Wang X., Guo H., Nan J., Qian L., Yang P., Lin J., Li M., Li J., Liao L., Tang J. Benzo[a]pyrene induces oxidative stress and endothelial progenitor cell dysfunction via the activation of the NF-κB pathway. Int. J. Mol. Med. 2013;31:922–930. doi: 10.3892/ijmm.2013.1288. [DOI] [PubMed] [Google Scholar]
- Jin N.Z., Zhu Y.P., Zhou J.W., Mao L., Zhao R.C., Fang T.H., Wang X.R. Preventive effects of quercetin against benzo[a]pyrene-induced DNA damages and pulmonary precancerous pathologic changes in mice. Basic Clin. Pharmacol. Toxicol. 2006;98:593–598. doi: 10.1111/j.1742-7843.2006.pto_382.x. [DOI] [PubMed] [Google Scholar]
- Knuckles M.E., Inyang F., Ramesh A. Acute and subchronic oral toxicities of benzo(α)pyrene in f-344 rats. Toxicol. Sci. 2001;61:382–388. doi: 10.1093/toxsci/61.2.382. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Kwack S.J., Lee B.M. Lipid peroxidation, antioxidant enzymes, and benzo[a]pyrene-quinones in the blood of rats treated with benzo[a]pyrene. Chem. Biol. Interact. 2000;127:139–150. doi: 10.1016/s0009-2797(00)00177-0. [DOI] [PubMed] [Google Scholar]
- Kiruthiga P.V., Karutha Pandian S., Pandima Devi K. Silymarin protects PBMC against B(a)P induced toxicity by replenishing redox status and modulating glutathione metabolizing enzymes—an in vitro study. Toxicol. Appl. Pharmacol. 2010;247:116–128. doi: 10.1016/j.taap.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Yu M.H., Lee I.S. Inhibitory effects of methanol extract of plum (Prunus salicina L., cv. ‘Soldam’) fruits against benzo(a)pyrene-induced toxicity in mice. Food Chem. Toxicol. 2008;46:3407–3413. doi: 10.1016/j.fct.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Livingstone D.R. Responses of microsomal NADPH-cytochrome c reductase activity and cytochrome P450 in digestive glands ofMytilus edulisandLittorea to enviromental and experimental exposure to pollutants. Mar. Ecol. Prog. Ser. 1988;46:37–43. [Google Scholar]
- Liu H., David W., Ye Y., Cui B., Huang Y., Adán C., Wang Z. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci. 2009;176:375–382. [Google Scholar]
- Lee B.M., Kwack S.J., Kim H.S. Age-related changes in oxidative DNA damage and benzo(a)pyrene diolepoxide-I (BPDE-I)–DNA adduct levels in human stomach. J. Toxicol. Environ. Health A. 2005;68:1599–1610. doi: 10.1080/15287390500182818. [DOI] [PubMed] [Google Scholar]
- Mates J.M. Effect of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- Misra H.P., Fridovich I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. Biol. Chem. 1972;247:3170–3175. PMID: 4623845. [PubMed] [Google Scholar]
- Murawska-Ciałowicz E., Jethon Z., Magdalan J., Januszewska L., Podhorska-Okołów M., Zawadzki M., Sozański T., Dzięgiel P. Effects of melatonin on lipid peroxidation and antioxidative enzyme activities in the liver, kidneys and brain of rats administered with benzo(a)pyrene. Exp. Toxicol. Pathol. 2011;63:97–103. doi: 10.1016/j.etp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Nanez A.1., Alejandro N.F., Falahatpisheh M.H., Kerzee J.K., Roths J.B., Ramos K.S. Disruption of glomerular cell–cell and cell–matrix interactions in hydrocarbon nephropathy. Am. J. Physiol. Renal. Physiol. 2005;289:F1291–F1303. doi: 10.1152/ajprenal.00107.2005. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Palackal N.T., Burczynski M.E., Harvey R.G., Penning T.M. Metabolic activation of polycyclic aromatic hydrocarbon trans-dihydrodiols by ubiquitously expressed aldehyde reductase (AKR1A1) Chem. Biol. Interact. 2001;130–132:815–824. doi: 10.1016/s0009-2797(00)00237-4. [DOI] [PubMed] [Google Scholar]
- Paul S., Bhattacharyya S.S., Samaddar A., Boujedaini N., Khuda-Bukhsh A.R. Anticancer potentials of root extract of Polygala senega against benzo[a]pyrene-induced lung cancer in mice. Zhong Xi Yi Jie He Xue Bao. 2011;9:320–327. doi: 10.3736/jcim20110314. [DOI] [PubMed] [Google Scholar]
- Park J.H., Mangal D., Tacka K.A., Quinn A.M., Harvey R.G., Blair I.A., Penning T.M. Evidence for the aldo-keto reductase pathway of polycyclic aromatic trans-dihydrodiol activation in human lung A549 cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6846–6851. doi: 10.1073/pnas.0802776105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L.Q., Ren J., Liu J. Responses of antioxidant systems and LPO level to benzo(a)pyrene and benzo(k)fluoranthene in the haemolymph of the scallop Chlamys Ferrari. Environ. Pollut. 2006;141:443–451. doi: 10.1016/j.envpol.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Pi J., Yamauchi H., Kumagai Y., Sun G., Yoshida Y., Aikawa H., Hopenhayn Rich C., Shimojo N. Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water. Environ. Health Perspect. 2002;110:331–336. doi: 10.1289/ehp.02110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P., Ekambaram G., Sakthisekaran D. Protective role of mangiferin against benzo(a)pyrene induced lung carcinogenesis in experimental animals. Biol. Pharm. Bull. 2008;31:1053–1058. doi: 10.1248/bpb.31.1053. [DOI] [PubMed] [Google Scholar]
- Saunders C.R., Das S.K., Ramesh A., Shockley D.C., Mukherjee S., Shyamali M. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J. Appl. Toxicol. 2006;26:427–438. doi: 10.1002/jat.1157. [DOI] [PubMed] [Google Scholar]
- Sancini G., Farina F., Battaglia C., Cifola I., Mangano E., Mantecca P., Camatini M., Palestini P. Health risk assessment for air pollutants: alterations inlung and cardiac gene expression in mice exposed to milano winter fine Partic ulate Matter (PM2.5) PLoS One. 2014;9 doi: 10.1371/journal.pone.0109685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumedha S.A., Marjorie E.A., Abhaya S.B. Effect of cigarette smoke on body weight, food intake and reproductive organs in adults albino rats. Indian J. Exp. Biol. 2006;44:562–565. PMID: 16872045. [PubMed] [Google Scholar]
- Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Tice R.R., Agurell E., Anderson D., Burlinson B., Hartmann A., Kobayashi H., Miyamae Y., Rojas E., Ryu J.C., Sasaki Y.F. The single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Mizorogi T., Nishijima K., Kuwahara S., Tsujio M., Aoyama H., Taguchi C., Kobayashi M., Horio F., Ohno T. Body and major organ weights of A/J-Chr 11SM consomic mice. Exp. Anim. 2009;58:357–361. doi: 10.1538/expanim.58.357. [DOI] [PubMed] [Google Scholar]
- Valentovic M.A., Alejandro N., Betts Carpenter A., Brown P.I., Ramos K. Streptozotocin (STZ) diabetes enhances benzo(alpha)pyrene induced renal injury in Sprague Dawley rats. Toxicol. Lett. 2006;164:214–220. doi: 10.1016/j.toxlet.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Vieira L.R., Sousa A., Frasco M.F., Lima I., Morgado F., Guilhermino L. Acute effects of Benzo[a]pyrene, anthracene and a fuel oil on biomarkers of the common goby Pomatoschistus microps (Teleostei, Gobiidae) Sci. Total Environ. 2008;395:87–100. doi: 10.1016/j.scitotenv.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Wu Y.Q., Wang C.G., Wang Y., Zhao Y., Chen Y.X., Zuo Z.H. Antioxidant responses to benzo[a]pyrene, tributyltin and their mixture in the spleen of Sebasticus marmoratus. J. Environ. Sci. (China) 2007;19:1129–1135. doi: 10.1016/s1001-0742(07)60184-3. [DOI] [PubMed] [Google Scholar]
- Yan Y., Griffiths W.J., Nordling M., Nygren J., Möller L., Bergman J., Liepinsh E., Otting G., Gustafsson J.A., Rafter J., Sjövall J. Ring opening of benzo[a]pyrene in the germ-free rat is a novel pathway for formation of potentially genotoxic metabolites. Biochemistry. 2000;39:15585–15591. doi: 10.1021/bi001148y. [DOI] [PubMed] [Google Scholar]