Visual Abstract
Key Words: autonomic imbalance, pulmonary arterial hypertension, pulmonary vascular remodeling, vagal nerve stimulation
Abbreviations and Acronyms: BNP, brain natriuretic peptide; eNOS, endothelial nitric oxide synthase; HF, high-frequency; HRV, heart rate variability; IL, interleukin; MCP, monocyte chemotactic protein; mRNA, messenger ribonucleic acid; NE, norepinephrine; NO, nitric oxide; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PAP, pulmonary arterial pressure; PVR, pulmonary vascular resistance; RV, right ventricular; RVEDP, right ventricular end-diastolic pressure; SS, sham-stimulated; VNS, vagal nerve stimulation
Highlights
-
•
Autonomic imbalance has been documented in patients with PAH.
-
•
Electrical VNS is known to restore autonomic balance and improve heart failure.
-
•
This study aimed to elucidate the therapeutic effects of VNS on severe PAH in a rat model.
-
•
VNS significantly restored autonomic balance, decreased mean pulmonary arterial pressure, attenuated pulmonary vascular remodeling, and preserved right ventricular function. In addition, VNS markedly improved the survival of rats with PAH.
-
•
Our findings may contribute greatly to the development of device therapy for PAH and widen the clinical applicability of VNS.
Summary
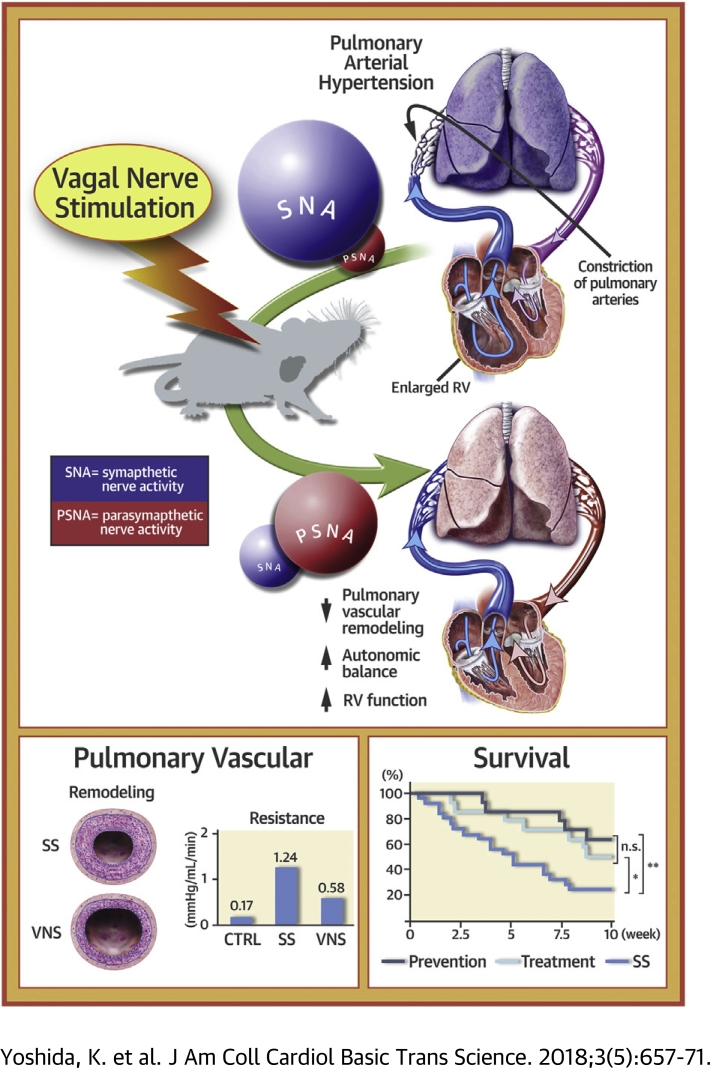
This study aimed to elucidate the therapeutic effects of electrical vagal nerve stimulation (VNS) on severe pulmonary arterial hypertension in a rat model. In a pathophysiological study, VNS significantly restored autonomic balance, decreased mean pulmonary arterial pressure, attenuated pulmonary vascular remodeling, and preserved right ventricular function. In a survival study, VNS significantly improved the survival rate in both the prevention (VNS from 0 to 5 weeks after a SU5416 injection) and treatment (VNS from 5 to 10 weeks) protocols. Thus, VNS may serve as a novel therapeutic strategy for pulmonary arterial hypertension.
Pulmonary arterial hypertension (PAH) is a fatal disease characterized by progressive vascular remodeling and increased pulmonary vascular resistance (PVR). The increase in right ventricular (RV) afterload often leads to RV decompensation (1) and premature death. Despite recent advances in the treatment of PAH (2), the prognosis remains poor, especially in patients with severe RV failure (3). Thus, novel treatments must be developed to improve its outcome.
PAH disrupts autonomic balance by deactivating the parasympathetic nervous system (4) and activating the sympathetic nervous system (5). Previous reports indicated that autonomic imbalance predicts a high mortality of PAH (6). Furthermore, Carver et al. (7) reported that vagotomy of the pulmonary region in rats leads to fibrosis in both pulmonary arteries (PAs) and airways. Therefore, autonomic function may play an important role in structural homeostasis in PAs. Recently, sympatho-inhibitory therapies such as PA denervation 8, 9 and renal denervation (10) have been shown to reduce pulmonary vascular remodeling and PVR. In addition, a growing body of evidence is accumulating both on the benefits 11, 12 and disadvantages (13) of beta-blocker therapy for PAH. However, it remains unknown how direct activation of the parasympathetic nervous system affects PAH.
Vagal nerve stimulation (VNS) is a device therapy that directly and electrically activates the parasympathetic nerve. In animal experiments, VNS has shown significant therapeutic benefits for various cardiovascular diseases, including myocardial infarction (14), fatal arrhythmia (15), and chronic heart failure (16). VNS has been used in patients with heart failure (17) and epilepsy (18). Although clinical trials have shown the safety of VNS, the efficacy of VNS for heart failure remains controversial (19).
In the present study, we examined how VNS affects autonomic function, hemodynamic variables, pulmonary histopathology, and survival in the SU5416/hypoxia/normoxia model of PAH using Fischer 344 rats, a model that reproduces essential histopathological features of severe PAH, including catastrophic prognosis in humans 20, 21.
Methods
The Institutional Animal Care and Use Committee of Kyushu University, Fukuoka, Japan, approved all experimental procedures. We conducted animal experimentation and care in strict accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health.
We used 10-week-old male Fischer 344 rats (Japan SLC, Hamamatsu, Japan) (n = 102). All rats were housed in a room maintained at constant temperature (25 ± 2°C) and 12-h light/dark cycle, and given water ad libitum during the entire experiment. To induce PAH, rats weighing 180 to 200 g were given a subcutaneous injection of SU5416 (204005-46-9, Tocris Bioscience, Bristol, United Kingdom; 20 mg/kg) followed by exposure to hypoxia (10% oxygen) for 3 weeks and then returned to normoxic conditions, according to previous reports 20, 21.
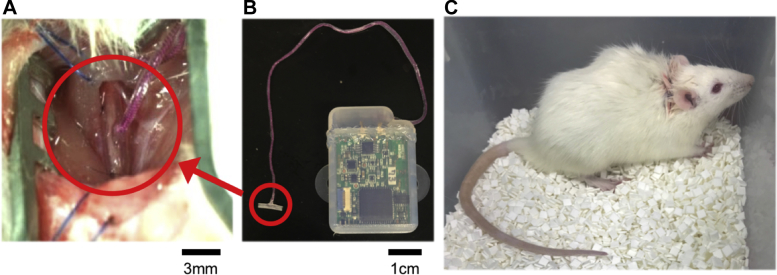
First, the effects of chronic VNS on PAH in free-moving rats were evaluated. We then analyzed the direct effects of acute VNS on advanced PAH in anesthetized rats. To administer chronic VNS, we attached a pair of electrodes to the right cervical vagal nerve and implanted a neurostimulator (ANRE-210i, ANPEX Co., Ltd., Tokyo, Japan) subcutaneously in the back of the rat 3 days (to allow time for recovery from the invasive implantation procedure) before the SU5416 injection (Figure 1). The vagal nerve was stimulated at 20 Hz and 180-μs pulse width. Initiation of stimulation and titration of amplitude were operated from the exterior. The current intensity of VNS was adjusted at just below the symptom threshold (97 ± 6 μA). Chronic VNS did not directly affect heart rate or arterial pressure (AP) under a conscious state, as we previously reported (22). There was no implantation-related death in this study, but 11 rats were excluded from the protocol because of mechanical failure of the VNS system. To administer acute VNS, an external stimulation device (SEN-3401, Nihon Kohden, Tokyo, Japan) was used for electrical stimulation in rats with advanced PAH under anesthesia. The electrodes and stimulation parameters were the same as in chronic VNS.
Figure 1.
Photographic Images of Vagal Nerve Stimulation in Rats
Electrodes are (A) attached to the right cervical vagal nerve and (B) connected to a neurostimulator (C) implanted subcutaneously in the back of a rat.
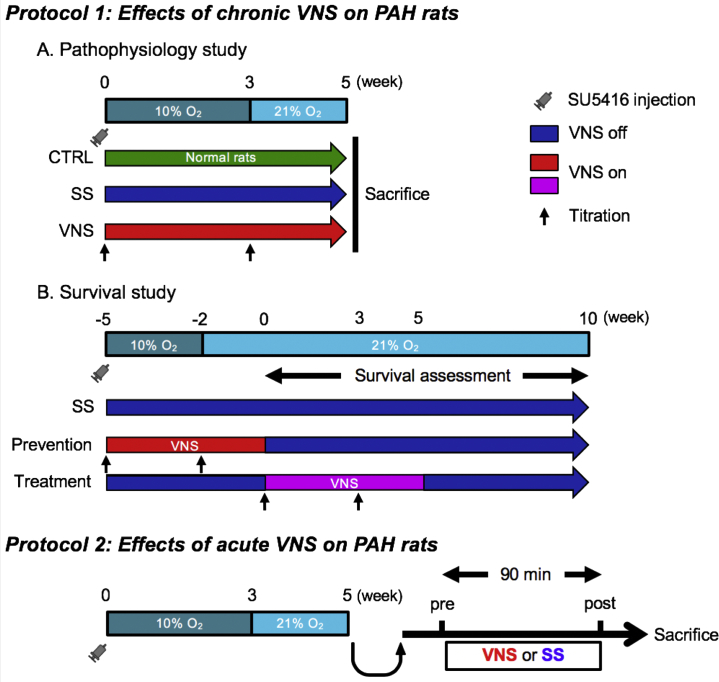
Experimental protocols
Protocol 1: Effects of chronic VNS on PAH in free-moving rats
Pathophysiological study
A pathophysiological study was conducted in rats (n = 28) that had undergone 5-week VNS from 0 week after SU5416 injection (VNS, n = 10), rats that were sham-stimulated (SS; n = 9), and age-matched control rats (n = 9) (Figure 2). We assessed autonomic function, hemodynamic variables, histology of lung and right ventricle, and inflammatory responses, and then compared these findings among the 3 groups.
Figure 2.
Protocols
Protocol 1 assesses the therapeutic effects of 5-week chronic vagal nerve stimulation (VNS) delivered by an implantable neurostimulator in free-moving rats with pulmonary arterial hypertension (PAH). Protocol 2 assesses the effects of 90-min acute VNS delivered by an external stimulation device in anesthetized rats with advanced PAH. CTRL = control; O2 = oxygen; SS = sham stimulation.
Survival study
The effects of chronic VNS on survival of PAH rats (n = 54) were examined. Rats that underwent 5-week VNS 0 week (–5 week in Figure 2) after SU5416 injection (prevention group, n = 14) were compared with SS rats (SS group, n = 26). We also examined the survival of rats that underwent 5-week VNS from 5 weeks (0 week in Figure 2) after SU5416 injection (treatment group, n = 14). The intensity of VNS was titrated at 0 and 3 weeks after VNS initiation in both the prevention and treatment protocols. We observed survival of all rats for 10 weeks from 5 weeks (0 week in Figure 2) after SU5416 injection. Rats in protocol 1B were different from those in protocol 1A.
Protocol 2: Effects of acute VNS on established PAH in anesthetized rats
To examine the direct effects of VNS on pulmonary vascular properties and to elucidate the beneficial mechanisms of action of VNS on PAH, the effects of acute VNS was evaluated in rats with advanced PAH. Rats with PAH 5 weeks after SU5416 injection (n = 9) were anesthetized and administered acute VNS via an external device (n = 5) or SS (n = 4) for 90 min. We simultaneously measured hemodynamic variables during VNS and sampled lung tissues at the end of the experiment for gene and protein assays.
Echocardiography
Echocardiography was performed under general anesthesia (isoflurane: 1.5%) in protocol 1A. In the 2-dimensional parasternal short-axis view, we measured RV diastolic dimensions, systolic dimensions, and wall thickness at the level of the papillary muscles. We also estimated pulmonary artery acceleration time by Doppler and normalized it by cardiac cycle length (12).
Heart rate variability
In protocol 1A, heart rate variability (HRV) was evaluated under conscious state at 5 weeks after SU5416 injection by using an electrocardiographic telemetry system (TA11ETA-F10 Implant, Data Sciences International, St. Paul, Minnesota). Power spectral density was used to quantify the HRV (see the Supplemental Materials for details).
Hemodynamic assessment and RV hypertrophy
Hemodynamic variables in protocols 1A and 2 were assessed by measuring RV pressure, pulmonary arterial pressure (PAP), left ventricular pressure, and AP. We used 2-F Mikro-Tip pressure catheters (SPR-320, Millar Instruments, Houston, Texas) for RV pressure, PAP and left ventricular pressure, and a fluid-filled transducer system (DX-300, Nihon Kohden) for AP. A flow probe (2.5PS, Transonic Systems, Ithaca, New York) was placed in the aortic root for measurements of cardiac output and cardiac index (cardiac output normalized by body weight) (see the Supplemental Materials for details). After catheterization, the right ventricle was dissected from the left ventricle and interventricular septum. The Fulton index (a weight ratio of the right ventricle to the left ventricle plus septum) was calculated for assessment of RV hypertrophy (21).
Plasma norepinephrine and brain natriuretic peptide
Blood samples were collected from the carotid artery after hemodynamic studies were completed in protocol 1A. Plasma norepinephrine (NE) levels were measured as an index of sympathetic activation by using high-performance liquid chromatography (SRL, Tokyo, Japan) 6, 22. Plasma brain natriuretic peptide (BNP), which is an important prognostic factor of PAH (23), were assayed by using a BNP 45 Rat ELISA Kit (ab108816, Abcam, Cambridge, United Kingdom).
Histopathological and immunohistochemical analyses
Histopathological and immunohistochemical analyses were performed in protocol 1A. We assessed PA luminal occlusive lesions, migration of CD68-positive perivascular macrophages, cell proliferation, and apoptosis. We also assessed fibrotic area, capillary density, and the apoptotic ratio in the right ventricle (see the Supplemental Materials for details).
Multiple cytokine biomarkers
In protocol 1A, inflammatory cytokines in the lung were evaluated by using the Bio-Plex Pro Rat Cytokine 23-Plex Assay (#12005641, Bio-Rad, Hercules, California) (24). We used the standard sample in quantitative analysis. Interleukin (IL)-1β, IL-6, tumor necrosis factor-α, monocyte chemotactic protein-1 (MCP-1), and IL-10 levels were also evaluated.
Immunoblot analysis of the expression of endothelial nitric oxide synthase and phosphorylated endothelial nitric oxide synthase
In protocol 2, protein expressions of endothelial nitric oxide synthase (eNOS) and phosphorylated eNOS were evaluated after acute VNS by using Western blot analysis (see the Supplemental Materials for details).
Reverse transcription polymerase chain reaction analysis
In protocol 2, messenger ribonucleic acid (mRNA) levels of pro-inflammatory cytokines (Il1b, Il6, Tnfa, Mcp1), anti-inflammatory cytokine (Il10), and alpha-7 nicotinic acetylcholine receptor (Alpha7nachr) were evaluated by using reverse transcription polymerase chain reaction (see Supplemental Materials).
Statistical analysis
In protocol 1A, differences among the 3 groups were tested by using 1-way analysis of variance, followed by post hoc Tukey-Kramer tests. Post hoc comparisons were conducted if a significant result was obtained in the overall analysis of variance. In protocol 1B, survival was estimated by using the Kaplan-Meier method and analyzed by using the log-rank test among the SS-prevention, SS-treatment, and prevention-treatment groups. In protocol 2, differences between pre-stimulation and post-stimulation data were tested by using paired Student’s t-tests, and differences in gene and protein assays between SS and VNS were tested by using Student’s t-test. Statistical analyses were performed by using JMP Pro 11 (SAS Institute, Inc., Cary, North Carolina). Values are given as mean ± SEM. Differences were considered significant when p values were <0.05.
Results
Effects of chronic VNS on autonomic function
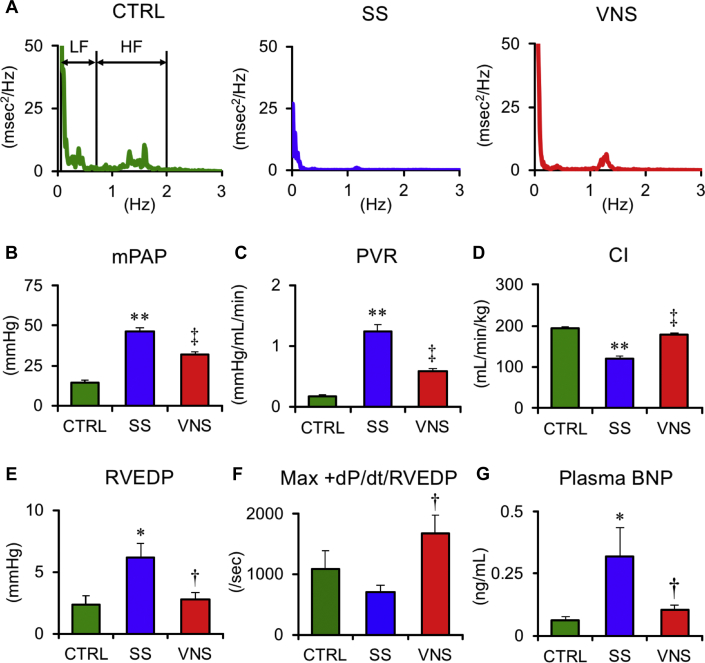
All rats survived until 5 weeks after SU5416 injection in protocol 1A. Representative traces of power spectral density of HRV are shown in Figure 3A. HRV and plasma NE data are summarized in Table 1. Compared with SS, VNS increased the high-frequency component and tended to decrease the low-frequency/high-frequency ratio, indicating improvement of autonomic balance. This outcome is supported by the finding that VNS markedly decreased plasma NE levels, indicating the suppression of sympatho-excitation.
Figure 3.
Effects of Chronic VNS on Power Spectral Density of Heart Rate, Hemodynamic Variables, and Plasma BNP
Values are mean ± SEM. Differences were tested by using 1-way analysis of variance, followed by post hoc Tukey-Kramer tests. ∗p < 0.05 and ∗∗p < 0.01 vs. CTRL. †p < 0.05 and ‡p < 0.01 vs. SS. (A) Representative traces of power spectral density of heart rate in CTRL, SS, and VNS in rats at 5 weeks after SU5416 injection. The frequency range of 0.04–0.73 Hz was defined as low frequency (LF) and 0.73–2.0 Hz as high frequency (HF). The HF of HRV that represents parasympathetic nerve activity is lower in SS than CTRL but is higher in VNS than in SS. (B to G) Mean pulmonary arterial pressure (mPAP), pulmonary vascular resistance (PVR), cardiac index (CI), right ventricular end-diastolic pressure (RVEDP), and maximum first derivation of right ventricular pressure normalized by RVEDP (Max +dP/dt/RVEDP) are shown. VNS significantly reduces mPAP and improves right ventricular function. CTRL, n = 5; SS, n = 6; and VNS, n = 6. BNP = brain natriuretic peptide; other abbreviations as in Figure 2.
Table 1.
HRV and Plasma NE Concentrations
| Control | SS | VNS | p Value | |
|---|---|---|---|---|
| HRV | ||||
| n | 4 | 5 | 4 | |
| Heart rate, beats/min | 337 ± 5 | 367 ± 20 | 351 ± 12 | 0.381 |
| HF, ms2 | 2.0 ± 0.3 | 0.3 ± 0.1∗ | 1.1 ± 0.2† | <0.001 |
| LF, ms2 | 8.0 ± 1.8 | 1.4 ± 0.4∗ | 2.8 ± 0.3 | 0.002 |
| LF/HF ratio | 4.3 ± 1.4 | 6.8 ± 1.5 | 2.8 ± 0.6 | 0.127 |
| SDNN, ms | 8.6 ± 2.1 | 4.3 ± 0.8 | 5.0 ± 0.5 | 0.077 |
| Sympathetic activation | ||||
| n | 9 | 9 | 10 | |
| Plasma NE concentration, pg/ml | 378 ± 58 | 7800 ± 2992‡ | 978 ± 306† | 0.019 |
Values are mean ± SEM. Differences were tested by using 1-way analysis of variance, followed by post hoc Tukey-Kramer test.
HF = high-frequency component of heart rate variability (HRV) (0.04 to 0.73 Hz); LF = low-frequency component of HRV (0.73–2.0 Hz); NE = norepinephrine; SDNN = SD of the normal to normal intervals; VNS = vagal nerve stimulation.
p < 0.05 and *p < 0.01 vs. control.
p < 0.05 vs. sham stimulation (SS).
Effects of chronic VNS on heart weight and echocardiographic findings
As shown in Table 2, body weight did not differ among the 3 groups. Compared with SS, VNS significantly decreased RV hypertrophy, RV diastolic dimensions, RV systolic dimensions, and RV wall thickness but increased pulmonary artery acceleration time normalized to cycle length, reflecting lowered RV systolic pressure.
Table 2.
Body and Heart Weights and Echocardiographic Data
| Control | SS | VNS | p Value | |
|---|---|---|---|---|
| Body and heart weights | ||||
| n | 5 | 6 | 6 | |
| Body weight, g | 299 ± 8 | 278 ± 6∗ | 274 ± 5 | 0.049 |
| RV weight, mg | 136 ± 4 | 353 ± 16† | 241 ± 15‡ | <0.001 |
| LV+S weight, mg | 546 ± 9 | 584 ± 19 | 515 ± 17§ | 0.023 |
| RVH | 0.25 ± 0.01 | 0.61 ± 0.02† | 0.47 ± 0.02‡ | <0.001 |
| Echocardiography | ||||
| n | 4 | 5 | 5 | |
| RVDd, mm | 1.9 ± 0.1 | 3.4 ± 0.3† | 2.5 ± 0.1§ | <0.001 |
| RVDs, mm | 1.2 ± 0.1 | 2.7 ± 0.3† | 1.7 ± 0.1§ | <0.001 |
| RVWT, mm | 0.83 ± 0.03 | 1.28 ± 0.04† | 1.05 ± 0.05§ | <0.001 |
| PAAT/CL, % | 20.8 ± 1.8 | 8.8 ± 0.6† | 14.6 ± 1.9‡ | <0.001 |
| Heart rate, beats/min | 354 ± 7 | 311 ± 14 | 329 ± 14 | 0.099 |
Values are mean ± SEM. Differences were tested by using one-way analysis of variance, followed by post hoc Tukey-Kramer test.
LV + S = left ventricle plus septum; RV = right ventricular; RVH = ratio of right ventricle/LV + S; RVDd = RV diastolic dimensions; RVDs = RV systolic dimensions; PAAT/CL = pulmonary artery acceleration time normalized to cycle length; RVWT = RV wall thickness; other abbreviations as in Table 1.
p < 0.05 and †p < 0.01 vs. control.
p < 0.05 and ‡p < 0.01 vs. SS.
Effects of chronic VNS on hemodynamic variables and plasma BNP
Figures 3B to 3G display hemodynamic variables and plasma BNP levels in protocol 1A. Compared with SS, VNS significantly decreased mean PAP (VNS 32.0 ± 1.7 vs. SS 46.4 ± 2.2 mm Hg; p < 0.01) and PVR (VNS 0.58 ± 0.05 vs. SS 1.24 ± 0.11 mm Hg/ml/min; p < 0.01), and increased cardiac index (VNS 186 ± 18 vs. SS 121 ± 8 ml/min/kg; p < 0.01). VNS also decreased right ventricular end-diastolic pressure (RVEDP) (VNS 2.4 ± 0.3 vs. SS 6.2 ± 1.1 mm Hg; p < 0.05), increased the maximum first derivation of RV pressure normalized by RVEDP (Max +dP/dt/RVEDP) (VNS 1,617 ± 298/s vs. SS 695 ± 128/s; p < 0.05) and decreased plasma BNP (VNS 0.09 ± 0.02 vs. SS 0.36 ± 0.11 ng/ml; p < 0.05). VNS did not affect systemic hemodynamic variables (Supplemental Figure 1).
Effects of chronic VNS on PA histopathology
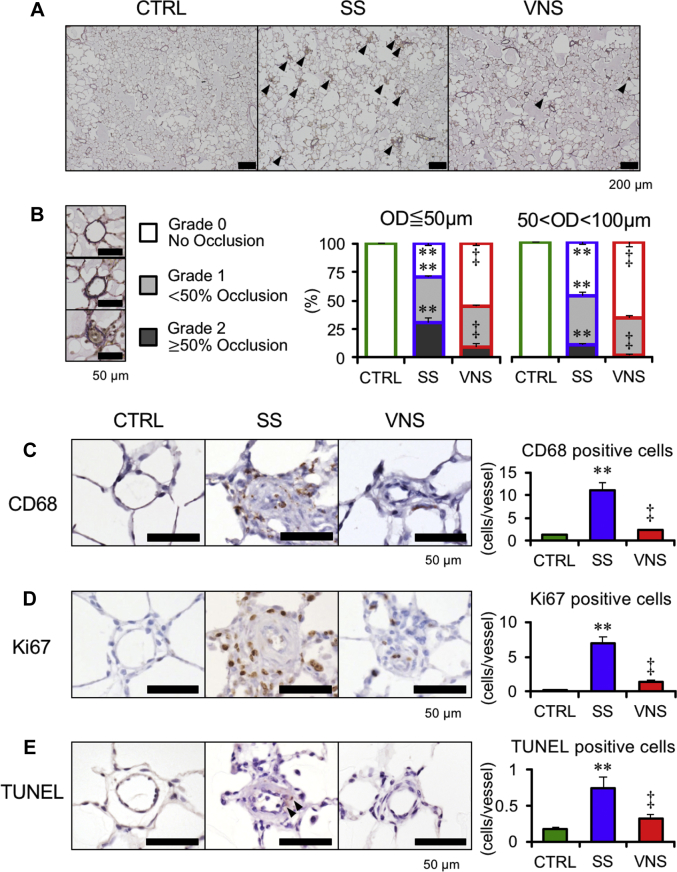
Figure 4 shows the histopathological analyses of PAs in protocol 1A. We assessed PA occlusive lesions by using Verhoeff–van Gieson staining. Compared with SS, VNS significantly increased grade 0 lesions and decreased grade 2 lesions irrespective of vascular size (Figures 4A and 4B), indicating that VNS attenuated PA occlusive lesions. VNS markedly reduced the number of perivascular macrophages (CD68-positive), proliferative cells (Ki67-positive), and apoptotic cells (terminal deoxynucleotidyl transferase dUTP nick end labeling–positive) (Figures 4C to 4E).
Figure 4.
Effects of Chronic VNS on Histology of PA
Values are mean ± SEM. Differences were tested by using 1-way analysis of variance, followed by post hoc Tukey-Kramer test. ∗∗p < 0.01 vs. CTRL. ‡p < 0.01 vs. SS. (A) Representative photomicrographs of Verhoeff–van Gieson staining. Arrowheads indicate occlusive pulmonary artery (PA). (B) Pulmonary arterial occlusions are graded as grade 0 (no luminal occlusion; white), grade 1 (< 50% occlusion; light gray), and grade 2 (≧50% occlusion; dark gray). Percentage of occlusive PAs with outer diameter (OD) ≦50 μm (left) and 50 < OD <100 μm (right). Representative photomicrographs of (C) immunostained CD68-positive cells, (D) Ki67-positive cells, and (E) terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells are shown (n = 4 in each group). Arrowheads in (E) indicate TUNEL-positive cells. Other abbreviations as in Figure 2.
Effects of chronic VNS on RV histology
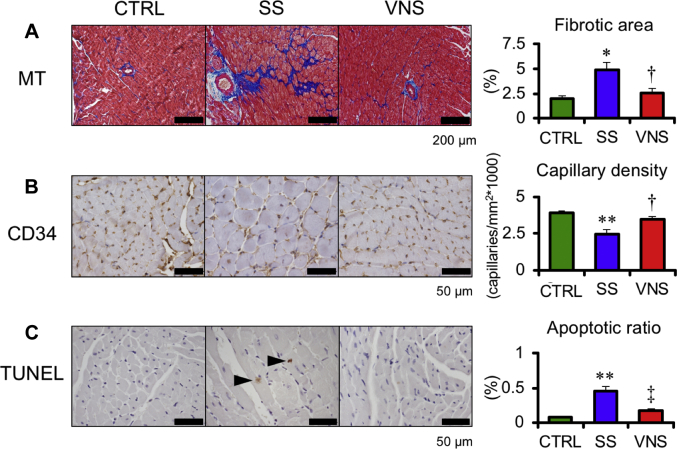
RV histology is shown in Figure 5. At 5 weeks after SU5416 injection, VNS decreased fibrosis (VNS 2.6 ± 0.5% vs. SS 4.9 ± 0.8%; p < 0.05), increased the capillary density (VNS 3.5 ± 0.2 vs. SS 2.5 ± 0.3 capillaries/mm2∗1,000; p < 0.05), and attenuated the apoptotic ratio (terminal deoxynucleotidyl transferase dUTP nick end labeling–positive myocytes divided by the total number of cardiomyocytes per field, VNS 0.17 ± 0.03% vs. SS 0.46 ± 0.06%; p < 0.01).
Figure 5.
Effects of Chronic VNS on Histology of the Right Ventricle
Values are mean ± SEM. Differences were tested by using 1-way analysis of variance, followed by post hoc Tukey-Kramer test. ∗p < 0.05 and ∗∗p < 0.01 vs. CTRL. †p < 0.05 and ‡p < 0.01 vs. SS. Representative photomicrographs of (A) Masson trichrome staining (MT), (B) immunostained CD34-positive cells, and (C) terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells are shown (n = 4 in each group). Arrowhead in (C) indicates TUNEL-positive cell. Other abbreviations as in Figure 2.
Effects of chronic VNS on inflammatory cytokines in lung
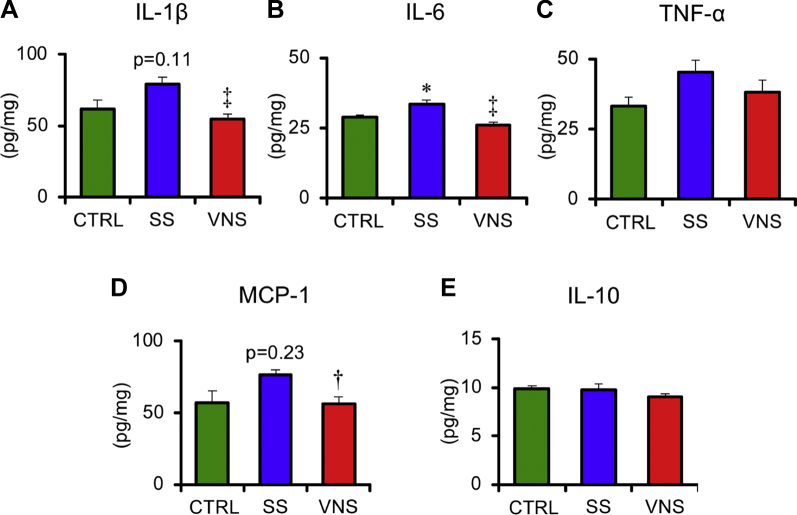
As shown in Figure 6, VNS significantly reduced the protein levels of IL-1β (VNS 55 ± 4 vs. SS 79 ± 5 pg/mg; p < 0.01), IL-6 (VNS 26 ± 1 vs. SS 34 ± 1 pg/mg; p < 0.01), and MCP-1 (VNS 57 ± 5 vs. SS 76 ± 4 pg/mg; p < 0.05). VNS did not affect tumor necrosis factor-α or IL-10.
Figure 6.
Effects of Chronic VNS on Inflammation of Lung
Values are mean ± SEM. Differences were tested by using 1-way analysis of variance, followed by post hoc Tukey-Kramer test. ∗p < 0.05 vs. CTRL, †p < 0.05 and ‡p < 0.01 vs. SS. Quantitative analysis of cytokines and chemokine in CTRL (n = 5), SS (n = 6), and VNS (n = 6) rats at 5 weeks after SU5416 injection. Samples were obtained from the lung. The levels of protein expression of (A) interleukin (IL)-1β, (B) IL-6, (C) tumor necrosis factor (TNF)-α, (D) monocyte chemotactic protein (MCP)-1, and (E) IL-10 are shown. Other abbreviations as in Figure 2.
Effects of chronic VNS on survival
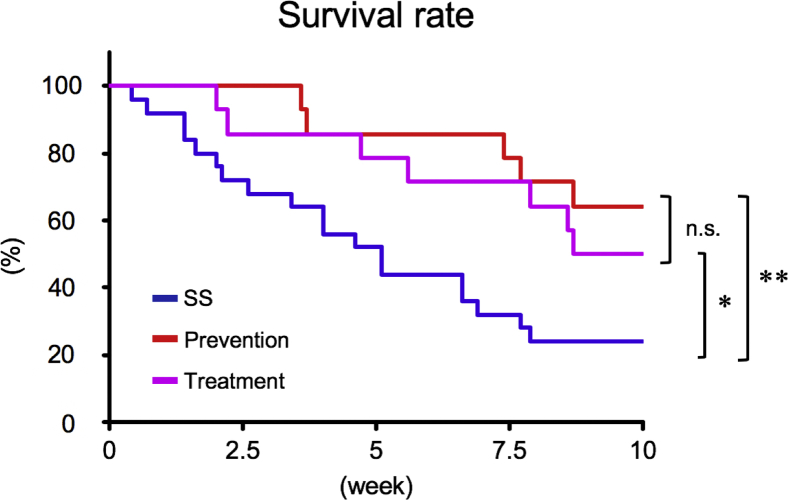
Ten-week survival in rats with PAH is shown in Figure 7. At 5 weeks after SU5416 injection, 1 rat in the SS group died and was excluded from the survival protocol. Five-week VNS markedly improved the survival rate both in the prevention (64.2%; p < 0.01) and treatment (50.0%; p < 0.05) groups compared with the SS group (24.0%). Risk reduction relative to the SS group was 53.0% in the prevention group and 34.2% in the treatment group. The survival rate at the end of observation (10 weeks from 5 weeks after SU5416 injection) did not differ significantly between the prevention and treatment groups (p = 0.45).
Figure 7.
Effects of Chronic VNS on Survival
Five-week VNS markedly improved survival both in the prevention (red, n = 14; p < 0.01) and treatment (purple, n = 14; p < 0.05) groups compared with the SS (blue, n = 25) group, with relative risk reductions of 53.0% and 34.2%, respectively. The survival assessment was started from 5 weeks after SU5416 injection. Prevention, 5-week VNS initiated from 0 weeks after SU5416 injection; treatment, 5-week VNS initiated from 5 weeks after SU5416 injection (0 weeks in Figure 7). Differences were tested by using the Kaplan-Meier method with log-rank testing among the SS-prevention, SS-treatment, and prevention-treatment groups. *p < 0.05 and **p < 0.01 vs. SS. n.s. = not significant; other abbreviations as in Figure 2.
Effects of acute VNS on hemodynamics, nitric oxide synthesis, and inflammation
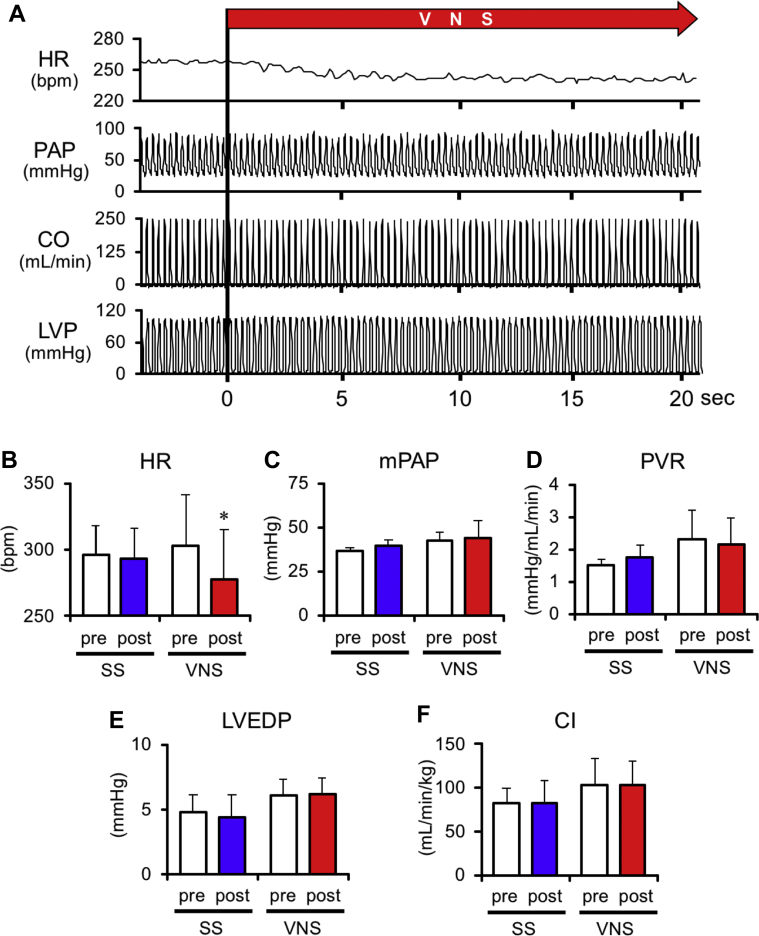
Figure 8A presents the hemodynamic response to acute VNS under anesthesia; the pooled data are summarized in Figures 8B to 8F. Acute VNS significantly reduced heart rate by 8.6 ± 2.6%. However, VNS did not change mean PAP, PVR, or cardiac index. These results indicate that VNS did not acutely change pulmonary vascular mechanical properties.
Figure 8.
Effects of Acute VNS on Hemodynamic Variables
Values are mean ± SEM. Differences were tested by using a paired Student's t-test. ∗p < 0.05 vs. pre-stimulation. (A) Representative time series of hemodynamic variables under acute VNS in a rat at 5 weeks after SU5416 injection. Heart rate (HR) decreases soon after initiation of VNS, whereas no significant changes in pulmonary arterial pressure (PAP), cardiac output (CO), and left ventricular pressure (LVP) are observed. (B–F) Effects of acute VNS on hemodynamic variables. The white columns represent pre-stimulation, and the blue and red columns represent post SS (n = 5) and VNS (n = 4), respectively. CI = cardiac index; LVEDP = left ventricular end-diastolic pressure; other abbreviations as in Figures 2 and 3.
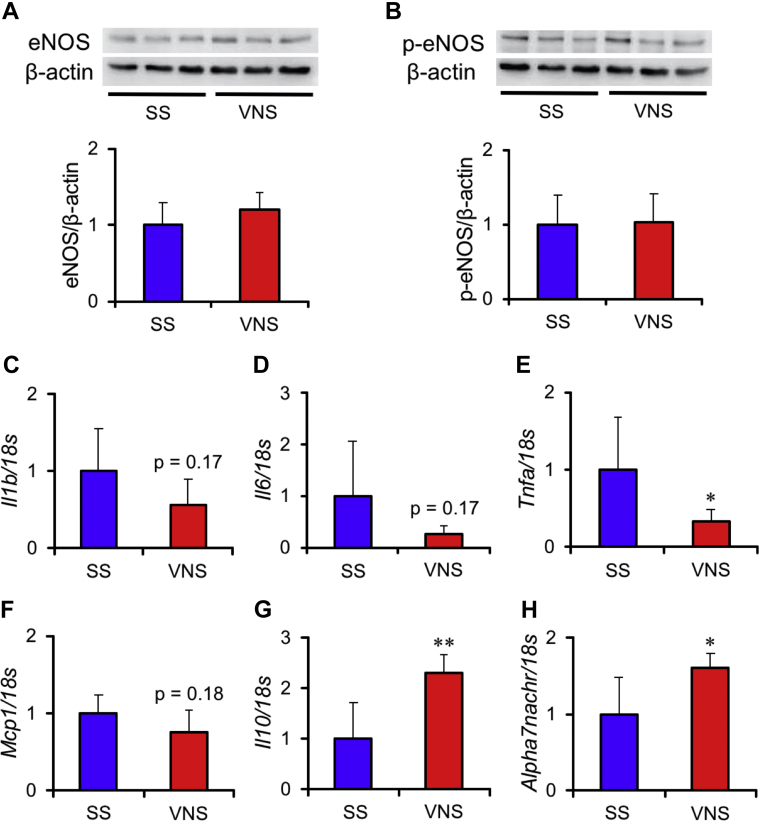
Compared with SS, VNS did not alter the protein expression of eNOS (Figure 9A) or phosphorylated eNOS (Figure 9B) in the lung. In mRNA expression levels of cytokines, VNS down-regulated Tnfa (p < 0.05) (Figure 9E), and up-regulated the anti-inflammatory cytokine Il10 (p < 0.01) (Figure 9G). While, VNS did not alter Il1β (Figure 9C), Il6 (Figure 9D), and Mcp1 (Figure 9F). In addition, VNS up-regulated Alpha7nachr, which is known as a receptor that modulates anti-inflammatory responses in VNS (p < 0.05) (Figure 9H).
Figure 9.
Effects of Acute VNS on NO Synthesis and Inflammation in the Lung
Values are mean ± SEM. Differences were tested by using Student’s t-test. ∗p < 0.05 and ∗∗p < 0.01 vs. SS. Effects of acute VNS (n = 3) or SS (n = 3) on nitric oxide (NO) synthesis in the lung of rats 5 weeks after SU5416 injection. Representative Western blotting of (A) endothelial nitric oxide synthase (eNOS) and (B) phosphorylated eNOS (p-eNOS) are shown in the upper panels. Graphs indicate semi-quantitative analysis of Western blots of eNOS and p-eNOS normalized by β-actin. In addition, effects of acute VNS (n = 4) or SS (n = 5) on messenger ribonucleic acid expressions of (C)Il1b, (D)Il6, (E)Tnfa, (F)Mcp1, (G)Il10, and (H)Alpha7nachr are shown; 18s was used as the loading control. Each value was normalized by the averaged value in SS. Other abbreviations as in Figure 2.
Discussion
The goal of the present study was to investigate the therapeutic effects of VNS in rats with PAH and to explore novel therapeutic strategies for PAH. We showed that chronic VNS significantly improved survival in rats with PAH, restored autonomic balance, and ameliorated pulmonary vascular remodeling while preserving RV function. Furthermore, acute VNS did not change nitric oxide (NO) synthesis or pulmonary vascular mechanics, despite acute as well as chronic VNS exhibiting significant inflammatory suppression in rats with PAH.
Effect of chronic VNS on autonomic balance
Autonomic imbalance in PAH 4, 5 predicts a high mortality (6). As shown in Table 1, chronic VNS increased the high-frequency component of HRV and decreased plasma NE levels in rats with PAH. These data indicate that autonomic balance in the rats with SU5416/hypoxia/normoxia model–induced PAH was restored by VNS.
VNS directly activates the parasympathetic nervous system. In the present study, chronic VNS significantly increased the high-frequency component of HRV, indicating restoration of normal parasympathetic activity. VNS also reduced plasma NE levels. We have previously reported that VNS inhibits sympathetic nerve activity by stimulating the afferent pathway (25). However, we cannot directly extrapolate our previous finding to the present study because we stimulated the vagal nerve at a low intensity that did not reduce either heart rate or AP. Furthermore, chronic VNS in this study significantly lowered PAP and improved RV function. Because sympathetic activation in PAH results from a complex interplay of mechanical, neurohumoral, and inflammatory stresses, the VNS-induced circulatory improvement could have reduced such complex stresses and evoked the sympatho-inhibitory effect.
Therapeutic effect of chronic VNS
Prognosis of PAH remains poor despite marked advances in pharmacological therapies 2, 3. Development of alternative therapeutic targets or more effective therapeutic modalities is therefore needed.
There are several reports showing the effect of sympatho-inhibition in PAH. Chen et al. (9) found that PA denervation significantly reduced PAP and PVR, indicating the contribution of sympathetic activation to pulmonary vascular constriction and PAP increase. Da Silva Gonçalves Bos et al. (10) showed that renal denervation reduced pulmonary vascular remodeling and PAP and concluded that the central beneficial mechanism of renal denervation results from suppression of the renin-angiotensin-aldosterone system. In contrast, Bogaard et al. (11) and de Man et al. (12) reported that beta-blocker therapy did not ameliorate pulmonary vascular remodeling or reduce PAP. Therefore, whether sympatho-inhibition improves PAH remains controversial.
In the present study, chronic VNS significantly lowered PAP and PVR (Figure 3) with a reduction of PA occlusive lesions, indicating regression of pulmonary vascular remodeling (Figure 4). Da Silva Gonçalves Bos et al. (26) reported that pyridostigmine, an oral acetylcholinesterase inhibitor, reduced pulmonary vascular remodeling through parasympathetic activation. However, they concluded that other pharmacological effects of pyridostigmine independent of parasympathetic activation might be involved in its beneficial mechanism for PAH. Because we directly and electrically stimulated the vagal nerve, our results showed the pure effect of parasympathetic activation on PAH.
RV function is tightly linked to poor prognosis of PAH (3). In this study, chronic VNS significantly increased Max +dP/dt/RVEDP and decreased RVEDP, while increasing the cardiac index (Figure 3), indicating the improvement of RV function. We also showed that VNS attenuated fibrosis, decreased apoptosis of cardiomyocytes, and increased capillary density in the right ventricle (Figure 5). Li et al. (16) showed that VNS improved left ventricular function and survival in rats with post–myocardial infarction heart failure. Following their study, several investigators reported that VNS exerts anti-apoptotic and anti-fibrotic effects 27, 28. In addition, it is well established that sympatho-inhibition by beta-blocker therapy reduces fibrosis and apoptosis, and increases capillary density in the right ventricle via anti-inflammatory effects 11, 12. On the basis of these previous studies, our results suggest that VNS may have a direct beneficial effect on RV function in rats with severe PAH. Conversely, reduction of PAP could account for the improvement in RV function (29). In this study, we also showed that VNS reduced PVR and PAP. Thus, we cannot differentiate between direct and indirect beneficial effects of VNS on RV function.
The impact of chronic VNS on survival in this study is the most critical finding for clinical translation. As shown in Figure 7, VNS markedly improved survival both in the prevention and the treatment protocols. In the treatment protocol, despite initiating VNS after PAH was established, VNS markedly improved survival. This result indicates that VNS may be effective even when applied to the established stage of PAH in clinical settings.
Beneficial mechanisms of VNS on PA remodeling
In patients with severe PAH, perivascular macrophages are increased in PA (30). The activation of macrophage releases inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor-α and produces T-cell–derived cytokines, which further facilitate the inflammatory process of PAH. The cytokines and chemokines subsequently evoke further inflammatory cascades and remodel the pulmonary vasculature either directly or indirectly through the production of growth factors.
Dysregulation of cell proliferation and apoptosis in pulmonary vascular smooth muscle lead to medial hypertrophy and result in lumen obstruction (31). Pro-inflammatory cytokines, including IL-6 and IL-10, play a crucial role in PA cell proliferation 32, 33. Pharmacological inhibition of nuclear factor–kappaB, a transcription factor of pro-inflammatory cytokines, attenuates pulmonary vascular remodeling by decreasing cell proliferation and apoptosis (34). Furthermore, reactive oxygen species and metabolic abnormality also enhance cell proliferation in PA and lead to pulmonary vascular remodeling (2).
Pavlov and Tracey et al. (35) have reported that VNS inhibits the release of pro-inflammatory cytokines originating from splenic macrophages through activation of α7nAChR. They named this circuit the “cholinergic anti-inflammatory pathway.” Kong et al. (27) have shown that VNS up-regulates α7nAChR and limits infarct size via anti-inflammatory effects in a rat model of myocardial infarction.
In the present study, VNS significantly reduced perivascular macrophage infiltration (Figure 4C) and down-regulated pro-inflammatory cytokines IL-6, IL-1β, and MCP-1 in the lung (Figure 6). In addition, acute VNS down-regulated mRNA expression of Tnfa and up-regulated Alpha7nachr (Figure 9). Combining our current data with previous findings, we speculate that chronic VNS induces general suppression of inflammatory responses, improves PA cell proliferation/apoptosis imbalance, and attenuates pulmonary vascular remodeling.
It is well known that VNS releases acetylcholine and induces local NO production in heart and systemic arteries 28, 36 and that the increase in NO lowers PVR in PAH (37). However, acute VNS did not change eNOS and phosphorylated eNOS protein levels or pulmonary vascular characteristics (Figure 9). Although we did not measure endogenous NO production, these data suggest that acute VNS exerts no direct effect on pulmonary vasoconstriction in rats with PAH, at least in the present stimulation settings.
Study Limitations
This study is the first report, to the best of our knowledge, to show the major therapeutic effects of electrical VNS on PAH model rats. Because an implantable device for VNS has already been applied clinically, and its safety and feasibility confirmed, our findings that VNS improves severe PAH in rats may contribute greatly to the development of device therapy for PAH and widen the clinical applicability of VNS.
However, we cannot directly translate our findings into clinical PAH therapy because several critical issues must be addressed. First, it is well known that none of the PAH animal models can fully represent the complex pathophysiologies of clinical PAH. To establish the clinical feasibility of VNS therapy for PAH, we must examine the effects of VNS on different PAH or right heart failure models, such as a monocrotaline-PAH model and PA banding–induced right heart failure model. Second, we titrated VNS at an intensity just below the symptom threshold (respiratory twitching) (22). Although we assumed that this stimulation setting is clinically applicable, further optimizations of stimulation parameters are needed for clinical application. Third, because of the limitation of battery life, we examined the effects of 5-week VNS in rats with PAH in this study. Technological development of the VNS device is also essential to evaluate how lifelong VNS affects established PAH. Fourth, it remains unclear whether VNS is beneficial in addition to treatment with multiple standard drugs for PAH. Further preclinical studies are needed to examine the clinical benefit of VNS. Solving those issues may enhance the clinical translational value of VNS from a therapeutic standpoint.
Conclusions
Chronic VNS restored autonomic balance, ameliorated pulmonary vascular remodeling while preserving RV function, and markedly improved the survival rate in rats with PAH. VNS-induced anti-inflammatory response may be an important mechanism contributing to the benefits. VNS may be a potential therapeutic strategy for PAH.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Severe PAH with right heart failure remains a disease with poor prognosis despite the advances in multiple drug therapy. Autonomic imbalance has been documented in patients with PAH, and much interest has been focused on interventions targeting the autonomic imbalance in PAH. This study is the first to show the enormous therapeutic effect of electrical VNS on PAH. VNS ameliorated pulmonary vascular remodeling, preserved RV function, and markedly improved survival in rats with PAH. Thus, VNS has the potential for development as a novel strategy for treating PAH.
TRANSLATIONAL OUTLOOK: An implantable device for electrical VNS has already been applied clinically. Our finding that VNS improves severe PAH in rats may contribute greatly to the development of device therapy for PAH and widening of the potential of VNS. Further investigations that clarify patient selection, treatment timing and period, and stimulation parameters of VNS are required to maximize the impact of VNS on PAH.
Acknowledgments
The authors thank Takuya Akashi, Takako Takehara, and the staff of the Center for Disruptive Cardiovascular Medicine, Kyushu University, the Department of Cardiovascular Medicine, Kyushu University and the Center for Clinical and Translational Research of Kyushu University Hospital for technical support.
Footnotes
This work was supported by the Japan Agency for Medical Research and Development (18he1102003h0004, 18hm0102041h0003, and 18he1902003h0001), Actelion Academia Prize 2015, and the Japan Society for the Promotion of Science (18K15893). Drs. Saku and Kishi work in a department endowed by Omron Healthcare Co. Dr. Sunagawa works in a department endowed by Omron Healthcare Co. and Actelion Pharmaceuticals Japan. Dr. Tsutsui has received honoraria from Daiichi Sankyo, Inc., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Boehringer Ingelheim Japan, Inc., Novartis Pharma K.K., Bayer Yakuhin, Ltd., Bristol-Myers Squibb KK, and Astellas Pharma Inc.; and research funding from Actelion Pharmaceuticals Japan, Daiichi Sankyo, Inc., and Astellas Pharma Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Vonk Noordegraaf A., Westerhof B.E., Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 2.Lai Y.C., Potoka K.C., Champion H.C., Mora A.L., Gladwin M.T. Pulmonary arterial hypertension: the clinical syndrome. Circ Res. 2014;115:115–130. doi: 10.1161/CIRCRESAHA.115.301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van De Veerdonk M.C., Kind T., Marcus J.T. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Wensel R., Jilek C., Dörr M. Impaired cardiac autonomic control relates to disease severity in pulmonary hypertension. Eur Respir J. 2009;34:895–901. doi: 10.1183/09031936.00145708. [DOI] [PubMed] [Google Scholar]
- 5.Velez-Roa S., Ciarka A., Najem B., Vachiery J.L., Naeije R., van de Borne P. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation. 2004;110:1308–1312. doi: 10.1161/01.CIR.0000140724.90898.D3. [DOI] [PubMed] [Google Scholar]
- 6.Nootens M., Kaufmann E., Rector T. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol. 1995;26:1581–1585. doi: 10.1016/0735-1097(95)00399-1. [DOI] [PubMed] [Google Scholar]
- 7.Carver T.W., Srinathan S.K., Velloff C.R., Perez Fontan J.J. Increased type I procollagen mRNA in airways and pulmonary vessels after vagal denervation in rats. Am J Respir Cell Mol Biol. 1997;17:691–701. doi: 10.1165/ajrcmb.17.6.2830. [DOI] [PubMed] [Google Scholar]
- 8.Rothman A.M., Arnold N.D., Chang W. Pulmonary artery denervation reduces pulmonary artery pressure and induces histological changes in an acute porcine model of pulmonary hypertension. Circ Cardiovasc Interv. 2015;8(11):e002569. doi: 10.1161/CIRCINTERVENTIONS.115.002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S.L., Zhang H., Xie D.J. Hemodynamic, functional, and clinical responses to pulmonary artery denervation in patients with pulmonary arterial hypertension of different causes. Circ Cardiovasc Interv. 2015;8(11):e002837. doi: 10.1161/CIRCINTERVENTIONS.115.002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silva Gonçalves Bos D., Happé C., Schalij I. Renal denervation reduces pulmonary vascular remodeling and right ventricular diastolic stiffness in experimental pulmonary hypertension. J Am Coll Cardiol Basic Trans Science. 2017;2:22–35. doi: 10.1016/j.jacbts.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogaard H.J., Natarajan R., Mizuno S. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med. 2010;182:652–660. doi: 10.1164/rccm.201003-0335OC. [DOI] [PubMed] [Google Scholar]
- 12.De Man F.S., Handoko M.L., Van Ballegoij J.J.M. Bisoprolol delays progression towards right heart failure in experimental pulmonary hypertension. Circ Heart Fail. 2012;5:97–105. doi: 10.1161/CIRCHEARTFAILURE.111.964494. [DOI] [PubMed] [Google Scholar]
- 13.Provencher S., Herve P., Jais X. Deleterious effects of β-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology. 2006;130:120–126. doi: 10.1053/j.gastro.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Arimura T., Saku K., Kakino T. Intravenous electrical vagal nerve stimulation prior to coronary reperfusion in a canine ischemia-reperfusion model markedly reduces infarct size and prevents subsequent heart failure. Int J Cardiol. 2017;227:704–710. doi: 10.1016/j.ijcard.2016.10.074. [DOI] [PubMed] [Google Scholar]
- 15.Vanoli E., De Ferrari G.M., Stramba-Badiale M., Hull S.S., Foreman R.D., Schwartz P.J. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 16.Li M., Zheng C., Sato T., Kawada T., Sugimachi M., Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 17.Zannad F., De Ferrari G.M., Tuinenburg A.E. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the neural cardiac therapy for heart failure (NECTAR-HF) randomized controlled trial. Eur Heart J. 2015;36:425–433. doi: 10.1093/eurheartj/ehu345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penry J.K., Dean J.C. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31:S40–S43. doi: 10.1111/j.1528-1157.1990.tb05848.x. [DOI] [PubMed] [Google Scholar]
- 19.Chaterjee N.A., Singh J.P. Novel interventional therapies to modulate the autonomic tone in heart failure. J Am Coll Cardiol HF. 2015;3:786–802. doi: 10.1016/j.jchf.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Alzoubi A., Almalouf P., Toba M. TRPC4 inactivation confers a survival benefit in severe pulmonary arterial hypertension. Am J Pathol. 2013;183:1779–1788. doi: 10.1016/j.ajpath.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang B., Deng Y., Suen C. Marked strain-specific differences in the SU5416 rat model of severe pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2016;54:461–468. doi: 10.1165/rcmb.2014-0488OC. [DOI] [PubMed] [Google Scholar]
- 22.Nishizaki A., Sakamoto K., Saku K. Optimal titration is important to maximize the beneficial effects of vagal nerve stimulation in chronic heart failure. J Card Fail. 2016;22:631–638. doi: 10.1016/j.cardfail.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa A., Ejiri K., Matsubara H. Long-term patient survival with idiopathic/heritable pulmonary arterial hypertension treated at a single center in Japan. Life Sci. 2014;118:414–419. doi: 10.1016/j.lfs.2014.01.077. [DOI] [PubMed] [Google Scholar]
- 24.Harwani S.C., Ratcliff J., Sutterwala F.S. Nicotine mediates CD161a+ renal macrophage infiltration and premature hypertension in the spontaneously hypertensive rat. Circ Res. 2016;119:1101–1115. doi: 10.1161/CIRCRESAHA.116.309402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saku K., Kishi T., Sakamoto K. Afferent vagal nerve stimulation resets baroreflex neural arc and inhibits sympathetic nerve activity. Physiol Rep. 2014;2:e12136. doi: 10.14814/phy2.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva Gonçalves Bos D., Van Der Bruggen C.E., Kurakula K. Contribution of impaired parasympathetic activity to right ventricular dysfunction and pulmonary vascular remodeling in pulmonary arterial hypertension. Circulation. 2018;137:910–924. doi: 10.1161/CIRCULATIONAHA.117.027451. [DOI] [PubMed] [Google Scholar]
- 27.Kong S.S., Liu J.J., Hwang T.C., Yu X.J., Lu Y., Zang W.J. Tumour necrosis factor-alpha and its receptors in the beneficial effects of vagal stimulation after myocardial infarction in rats. Clin Exp Pharmacol Physiol. 2011;38:300–306. doi: 10.1111/j.1440-1681.2011.05505.x. [DOI] [PubMed] [Google Scholar]
- 28.Hamann J.J., Ruble S.B., Stolen C. Vagus nerve stimulation improves left ventricular function in a canine model of chronic heart failure. Eur J Heart Fail. 2013;15:1319–1326. doi: 10.1093/eurjhf/hft118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmboe S., Andersen A., Johnsen J. Inotropic effects of prostacyclins on the right ventricle are abolished in isolated rat hearts with right-ventricular hypertrophy and failure. J Cardiovasc Pharmacol. 2017;69:1–12. doi: 10.1097/FJC.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 30.Rabinovitch M., Guignabert C., Humbert M., Nicolls M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhary K.R., Taha M., Cadete V.J., Godoy R.S., Stewart D.J. Proliferative versus degenerative paradigms in pulmonary arterial hypertension. Circ Res. 2017;120:1237–1239. doi: 10.1161/CIRCRESAHA.116.310097. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto-Kataoka T., Hosen N., Sonobe T. Interleukin-6/interleukin-21 signaling axis is critical in the pathogenesis of pulmonary arterial hypertension. Proc Natl Acad Sci U S A. 2015;112:E2677–E2686. doi: 10.1073/pnas.1424774112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito T., Okada T., Miyashita H. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res. 2007;101:734–741. doi: 10.1161/CIRCRESAHA.107.153023. [DOI] [PubMed] [Google Scholar]
- 34.Farkas D., Alhussaini A.A., Kraskauskas D. Nuclear factor kappaB inhibition reduces lung vascular lumen obliteration in severe pulmonary hypertension in rats. Am J Respir Cell Mol Biol. 2014;51:413–425. doi: 10.1165/rcmb.2013-0355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavlov V.A., Tracey K.J. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci. 2017;20:156–166. doi: 10.1038/nn.4477. [DOI] [PubMed] [Google Scholar]
- 36.Chapleau M.W., Rotella D.L., Reho J.J., Rahmouni K., Stauss H.M. Chronic vagal nerve stimulation prevents high-salt diet-induced endothelial dysfunction and aortic stiffening in stroke-prone spontaneously hypertensive rats. Am J Physiol Hear Circ Physiol. 2016;311:H276–H285. doi: 10.1152/ajpheart.00043.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Channick R.N., Newhart J.W., Johnson F.W. Pulsed delivery of inhaled nitric oxide to patients with primary pulmonary hypertension: an ambulatory delivery system and initial clinical tests. Chest. 1996;109:1545–1549. doi: 10.1378/chest.109.6.1545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.