Abstract
Biodiversity affects the structure of ecological communities, but little is known about the interactive effects of diversity across multiple trophic levels. We used a large-scale forest diversity experiment to investigate the effects of tropical tree species richness on insectivorous birds, and the subsequent indirect effect on predation rates by birds. Diverse plots (four tree species) had higher bird abundance (61%), phylogenetic diversity (61%), and functional diversity (55%) than predicted based on single-species monocultures, which corresponded to higher attack rates on artificial caterpillars (65%). Tree diversity effects on attack rate were driven by complementarity among tree species, with increases in attack rate observed on all tree species in polycultures. Attack rates on artificial caterpillars were higher in plots with higher bird abundance and diversity, but the indirect effect of tree species richness was mediated by bird diversity, providing evidence that diversity can interact across trophic levels with consequences tied to ecosystem services and function.
Keywords: biodiversity, trophic interactions, complementarity, functional diversity, enemies hypothesis, insectivorous birds
1. Introduction
Biodiversity loss is one of the fundamental consequences of human-driven global change [1,2]. These losses are problematic not only for species conservation, but also because of the emergent, higher order effects of biodiversity on ecosystem function [3,4] and associated services [5,6]. Studies of biodiversity–ecosystem function (BEF) have reported extensively on the bottom-up effects of plant diversity on primary productivity [6,7] and, to a lesser extent, on the structure of associated animal communities [8–11]. In parallel, a number of BEF studies have addressed the top-down effects of predator diversity on lower trophic levels [12,13], collectively demonstrating that diversity can promote ecosystem productivity, stability, and resilience to disturbance [4,14]. However, most studies to date have approached these processes in terms of the top-down or bottom-up effects emanating from diversity within a single trophic level (i.e. plants or predators) without addressing the nonlinear dynamics of diversity across multiple trophic levels. As a result, the linkages between plant diversity and consumer (particularly predator) diversity are poorly understood.
Originally proposed to explain high rates of herbivory in simplified (non-diverse) agricultural systems, the Enemies Hypothesis (EH) conceptually links diversity effects across trophic levels [15]. According to Root [15], plant diversity increases predator abundance, and this in turn strengthens top-down control of herbivorous insects [15,16]. As such, the EH describes a compelling example of ecosystem services from biodiversity; high-diversity agricultural systems (i.e. polycultures) are on average associated with higher predator abundance (44%), herbivore mortality (54%), and reduced crop damage (30%) compared to low diversity (i.e. monoculture) systems (reviewed by Letourneau [16]). Accordingly, the EH guides agricultural practices aimed at maximizing biological control [17,18], although the importance of such dynamics to more complex, natural systems is unclear [19–23]. Vertebrate predators may be particularly notable in this regard given their prevalence in terrestrial ecosystems, sensitivity to anthropogenic impacts, and strong top-down effects on herbivores [24–26].
Despite the demonstrated importance of the EH, the underlying mechanisms driving plant mediation of predator effects are not well understood. The EH predicts plant diversity should increase predator abundance [15,16] through an increase in predator niche space [27]. While not formally proposed by the EH, this same mechanism can also increase predator diversity [9]. Both predator abundance and diversity have been demonstrated to affect top-down control of insects [12,28], however, the relative contributions of abundance versus diversity are rarely compared [16,29], and contingent on the identity and composition of species' traits [30,31]. Further, while past studies have documented the component effects of plant diversity on predation [20,23], or predator communities [32], these component pieces have largely been considered in isolation. As a result, a fundamental aspect of the EH mechanism—how predator communities mediate the indirect effects of tree diversity on herbivore suppression—is unknown.
Here, we quantitatively decompose the strength of EH effects operating via predator diversity versus predator abundance. Working within a tropical forest diversity experiment, we test for the effects of tree species richness directly on the composition of foraging insectivorous birds. We use clay model caterpillars to assess the indirect effects of tree species richness on predation rates and compare the extent to which these indirect effects of tree diversity were mediated by direct effects on bird abundance versus diversity. This study thus provides a novel test of the mechanisms underlying the EH in a natural ecosystem and, in so doing, demonstrates the importance of biodiversity across multiple trophic levels.
2. Material and methods
(a). Experimental design
We tested for tree diversity effects within the context of a large-scale forest diversity experiment (7.2 ha) in the Yucatan Peninsula (20°24′44″ N, 89°45′13″ W), ca 70 km southwest of Merida, Yucatan (Mexico) and found at the ‘Uxmal Experimental Site’ of the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). In 2011, we planted 74 replicate forest plots (21 × 21 m each) as polycultures of four tree species or single-species monocultures from a pool of six long-lived deciduous tree species (Swietenia macrophylla King (Meliaceae), Tabebuia rosea (Bertol.) DC. (Bignonaceae), Ceiba pentandra (L.) Gaertn. (Malvaceae), Enterolobium cyclocarpum (Jacq.) Griseb. (Fabaceae), Piscidia piscipula (L.) Sarg. (Fabaceae), and Cordia dodecandra A. DC. (Boraginaceae) (electronic supplementary material, figure S1; as described in [33]). At the time of sampling, the site was composed of ca 4 600 trees (4 780 originally planted) at a constant density of 64 plants per plot (3 m spacing between trees, 6 m aisles between plots). The average tree height was approximately 7–8 m when the experiment took place, with no measurable effect of tree species diversity on tree height [33].
(b). Bird community metrics
In July 2015, we assessed bird community composition within 32 of the total 74 plots, using two replicate monoculture plots for each of the six tree species (12 monocultures total) and 20 polyculture plots containing 14 unique four-species combinations of the same six tree species (electronic supplementary material, figure S1). To do so, two observers experienced with the local bird fauna conducted 10 min point count surveys at peak bird activity (06.00 to 09.30 on rain-free days), totalling 40 min of observation per plot, using 10 × 42 binoculars and field guides for identification [34]. During these observations birds were recorded if they were observed either perched or foraging within the focal plot, excluding individuals that were not observed to actively use the plot (e.g. passing through or flying high above canopy). The bird surveys were randomized under the constraints that each experimental plot was visited on four separate days during a two-week period, concurrent surveys were spaced by a minimum of 50 m, and neighbouring plots were not surveyed consecutively. The plot size (0.1 ha) and that of the larger experimental site are relatively small with respect to the territory sizes of some of the species observed. Accordingly, our data reflect the effects of tree species richness on the foraging movements of birds among plots rather than on the diversity of site overall. Of the total observed species (richness = 54), in our analysis we focus on the subset of species reported to feed partially or entirely on insects (richness = 44; based on [35]; electronic supplementary material, table S1).
We characterized the effects of tree species richness on three bird community metrics at the plot level: abundance, functional trait diversity (FD), and phylogenetic diversity (PD). Insectivorous bird abundance was measured as the total number of individuals per plot. We use FD and PD to quantify the ecological and phylogenetic dissimilarity among species, respectively, measured as the sum of all branches (phylogenetic or trait-based dendrogram) connecting species observed across all plot observations [36]. We calculate FD, the total trait diversity represented among observed species, based on available species-level traits hypothesized to be important for herbivore suppression: body mass, period of activity [diurnal or nocturnal], major diet type [vertebrate, invertebrate, fruit/nectar, plant/seed, omnivore], portion of diet by type [vertebrate, invertebrate, fruit, nectar, seeds, other plant], and relative time foraging in forest strata [ground, understory, mid-canopy, canopy, aerial] (compiled from [35], electronic supplementary material, table S1). Body mass was log transformed to reduce the influence of a few uncommon and larger-bodied species. All functional traits were rescaled (mean = 0, s.d. = 1) and weighted equally to calculate Gower's pairwise dissimilarity among species [37], from which we applied hierarchical clustering (UPGMA method) to construct a dendrogram reflecting bird species functional similarity. Here, we report on the subset of birds that consume any insect prey in their diet and traits based on a priori hypotheses. In addition, we quantify PD, the total evolutionary history shared among species, which can be used as a proxy for FD. To calculate PD, phylogenetic branch lengths were inferred from a 95% consensus tree containing mean branch lengths. This phylogeny was derived from 200 time-calibrated phylogenies of the Hackett-backbone, pruned to the species observed [38,39].
For each bird community metric (abundance, FD, and PD), we quantified the overall effect size of tree species richness as the grand mean of the log of the proportional difference (LR) between observed and expected bird communities for each polyculture plot, where the expected was calculated as the weighted mean of component tree species in monoculture. A positive LR where the 95% CI does not bracket zero indicates a significant positive effect of tree diversity, such that polyculture bird communities exceeded predictions under an additive scenario based on tree species monocultures.
(c). Assessing predation
Concurrent with bird surveys, we assessed predation in the same plots (all 12 monocultures and 17 of the 20 polycultures). Using established methods [40,41], we deployed and inspected artificial caterpillar models of light green plasticine clay (Lewis Newplast™) to record predation events. Tree species- and plot-level predation rates were calculated as the proportion of caterpillar models attacked per 24 h period. This method provides a highly consistent, unbiased assessment of predator effects on Lepidopteran larvae [20,40,41], one of the most abundant herbivore groups that feed on tropical trees. Using artificial models has the added benefit of controlling for variation in herbivore morphology, size, behaviour, and density that may occur among tree species and diversity treatments. Caterpillar models were exposed for two consecutive days on two opposing branches of 12 trees per plot (three trees of each species in polyculture) using superglue (Loctite™) to adhere them to the upper side of leaves. Caterpillar models were placed between 2 and 4 m in the canopy of interior trees, to exclude perimeter trees that bordered neighbouring plots (and potentially different tree species). After a 24 h period, caterpillar models were visually assessed for attack marks. Any attacked models were then replaced, and all models were again assessed after a second 24 h period. Caterpillar models were similar in size (4 × 25 mm) to generalist leaf-chewing herbivores found at the experimental site, and were sufficiently malleable that attack marks could be used to distinguish predation by birds from that caused by other taxonomic groups such as arthropods and mammals [40]. Caterpillar models attacked or lost after 24 h were replaced with intact models for a constant model density per plot (n = 24) in the following survey. Cases where the fate of the model was uncertain were excluded from predation rate calculations (n = 67; 4.05% of models). This experiment was executed twice over a three-week period in which we used the same 12 monoculture plots (but different trees) across iterations, and different polyculture plots between the first and second iterations (11 and 7 polyculture plots respectively). Tree- and plot-level attack rates were calculated from these data as the proportion of models attacked per 24 h period. There was no effect of experiment iteration on model attack rates, thus predation rates in monoculture plots are calculated as the mean proportion attacked across all four exposure days.
We calculated plot-level tree diversity effect on bird predation (LR) in the same manner used to assess diversity effects on bird community metrics. In addition, using tree-level predation data we calculated the net biodiversity effect on predation rates as the difference between observed predation in polyculture and the expected predation rate based on component tree species in monocultures. We also used tree-level predation data to compare attack rates between monoculture and polyculture separately for each tree species, and to decompose the net biodiversity effect on predation into two non-mutually exclusive mechanisms: selection effects and complementarity effects (following [42], electronic supplementary material, figure S2). Selection effects are driven by the increased probability of polycultures including species that have particularly strong effects on attack rates [42,43], such that the effect of tree species richness on predation is attributed to individual species' effects. In contrast, complementarity effects may arise due to interactions among tree species leading to non-additive increases in attack rates (e.g. niche differentiation, facilitation), where polycultures differ from additive predictions from monocultures due to multi-species processes [27,42].
(d). Comparing bird community metrics
We evaluated the pairwise relationships between bird community metrics and attack rate. We first used linear regression, comparing these models to all possible regression models (abundance, FD, and PD alone and in combination) using Akaike's information criterion (AIC). As bird abundance, FD, and PD were all positively correlated with plot-level attack rates, we used Structural Equation Modelling (SEM) to evaluate which of the observed bivariate relationships between the bird community metrics and attack rate mediated the indirect effects of tree species richness. Because of high covariance between bird abundance, PD, and FD (electronic supplementary material, figure S3), we only included FD based upon its stronger bivariate relationship with attack rate. Having dropped PD, we began with an initial SEM model that included indirect effects of tree species richness on attack rate via both FD and abundance; specifically, this model included the direct pathways from tree species richness to bird abundance and FD, the direct pathways from bird abundance and FD to attack rate, and the covariance between bird abundance and FD. Following the assessment of this initial hypothesized model, we then removed the non-significant pathways in a stepwise procedure. Both the initial model and this reduced model were then evaluated for their fit to the data using chi-square (p > 0.05 indicates valid model fit), and the significance of each pathway was evaluated with Z-statistics [44]. In addition, for both models we quantified the indirect effects of tree species richness as the product of the standardized regression coefficients of component direct pathways in the SEMs [44]. Indirect effects stemming from tree species richness were deemed significant when the two individual pathways making up the indirect effect were significant. The resulting indirect effects are visualized using curved grey arrows that encapsulate the underlying direct effects in the model, with non-significant pathways indicated by dashed lines. SEM was conducted using the ‘lavaan’ package in R [45,46].
3. Results
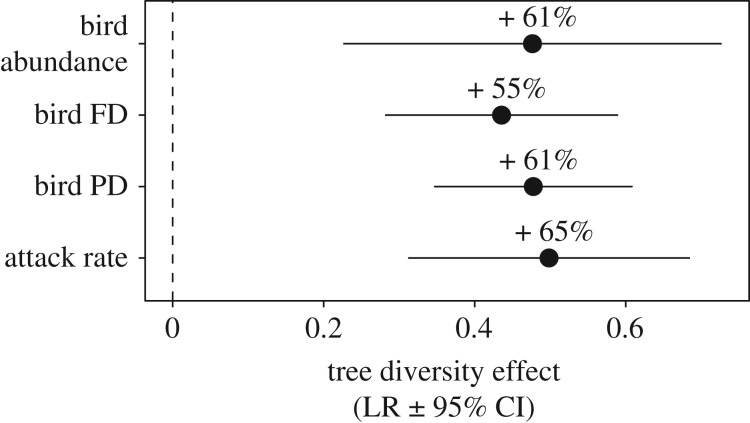
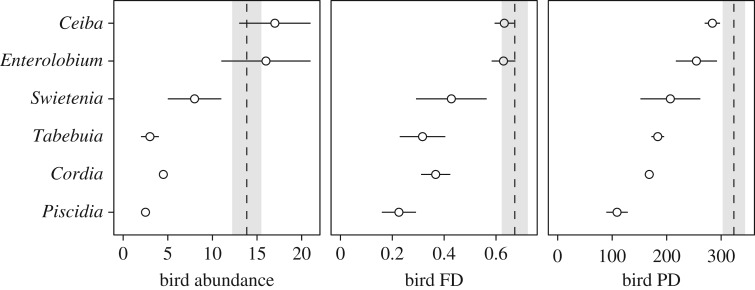
A total of 44 bird species (5.4 ± 0.5 species per plot; mean ± s.e.) and 381 individuals (11.9 ± 1.3 individuals per plot) that consume insects were observed in our surveys, with only 15 bird species observed in both monoculture and polyculture plots (three species observed exclusively in monocultures and 26 in polycultures). Tree species richness had a positive effect on all bird community metrics, resulting in higher bird abundance (61%), phylogenetic diversity (61%), and functional trait diversity (55%) in polyculture plots relative to expected values in monocultures (figure 1; LRabun = 0.48, 95% CI = 0.23 to 0.73; LRFD = 0.44, 95% CI = 0.28 to 0.59; LRPD = 0.47, 95% CI = 0.35 to 0.61). Mean bird abundance per plot increased from 8.5 (±1.99) to 13.9 individuals (±1.64) and species richness from 3.8 (±0.58) to 6.4 (±0.64) in monoculture and polyculture, respectively. In polycultures, mean bird PD exceeded that of the highest tree species in monoculture (i.e. transgressive overyielding; [47]) (figure 2). In contrast, mean bird abundance and FD in polyculture were not significantly different than observed for the highest tree species in monoculture (figure 2).
Figure 1.
Tree diversity effect (LR ± 95% CI) on attack rate (n = 17), bird abundance (n = 20), bird FD (n = 20), and bird PD (n = 20) at the plot level.
Figure 2.
Monoculture plot-level means (±s.e.; n = 2) of bird abundance (individuals per plot), FD (total Gower distance), and PD (millions of years) for each tree species (indicated by genus name). Dashed lines indicate the mean values observed in polyculture (shading indicating ± s.e.; n = 17), respectively.
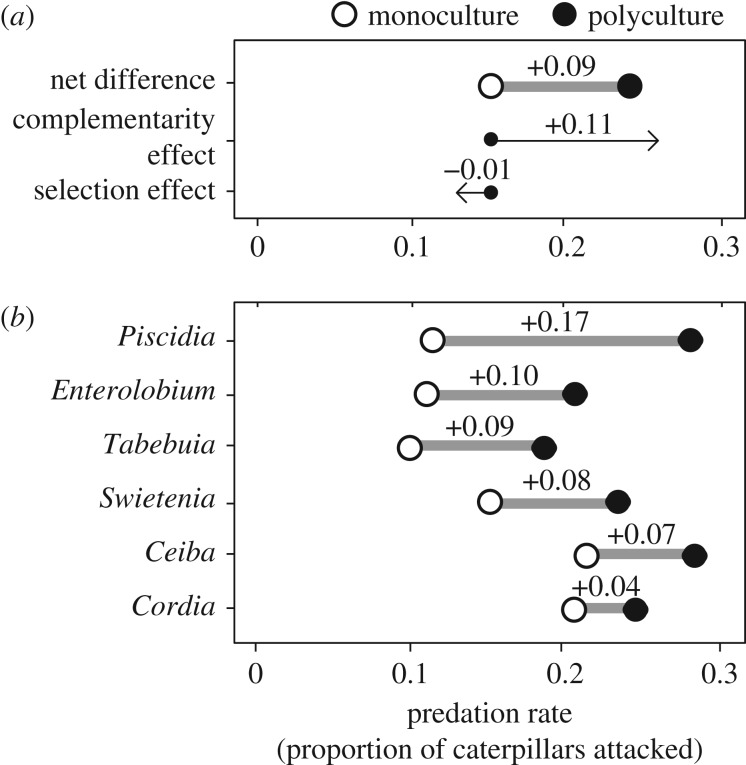
Across all experimental plots, birds were the dominant predators with a mean attack rate (proportion of caterpillar models attacked per 24 h) of 0.200 (±0.016 s.e.), more than threefold the attack rate by arthropods (0.057 ± 0.008 s.e.), while mammal and reptile predators were negligible (only two caterpillars attacked in total; 0.001 ± 0.0009 s.e.). In support of the EH, tree species richness increased bird attack rate, with polyculture plots (0.24 ± 0.022) exhibiting a significantly (64%) greater mean value than expected values based upon monocultures (0.15 ± 0.004) (figure 1). This corresponded to a mean increase in attack rate of 0.09 (±0.022) with tree diversity (figure 3), in which all but 3 (of 12) polyculture plots had stronger attack rates than the highest monoculture (electronic supplementary material, figure S2). Furthermore, the average attack rate across polyculture plots exceeded that of all tree species in monoculture plots (i.e. transgressive overyielding; [47]), due to increased attack rates for all tree species in polyculture plots compared to monoculture plots (figure 3). Accordingly, the net effect of tree diversity was attributed to a small, negative selection effect (−0.011 ± 0.004 s.e.; t-test, t16 = −2.78, p = 0.011) and a larger, positive effect of tree complementarity (0.105 ± 0.023; t-test, t16 = 4.52, p = 0.0003) (figure 3), indicating the positive effect of tree diversity on attack rate was due to non-additive effects among tree species.
Figure 3.
Differences in model attack rates between monoculture (open circles) and polyculture plots (filled circles). (a) Net plot-level effects depict mean expected polyculture values (weighted average of monocultures) and observed polycultures. This net effect is then decomposed into selection and complementarity effects. (b) Tree-level effects, comparing attack rate for each tree species when grown in monoculture versus tree-level attack rates when the species is included in polyculture.
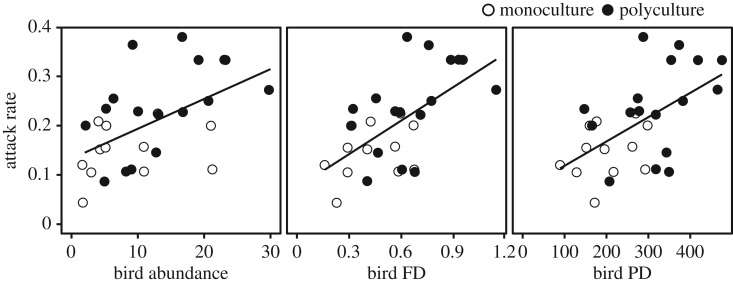
Differences in bird attack rate between monoculture and polyculture plots were mediated by the direct effects of tree species richness on bird communities; attack rate was positively correlated with bird abundance (R2 = 0.27, F1,27 = 10.37, p = 0.003), FD (R2 = 0.36, F1,27 = 15.55, p = 0.0005), and PD (R2 = 0.31, F1,27 = 12.36, p = 0.0016), with bird diversity (FD and PD) showing slightly stronger associations with attack rate than abundance (figure 4). When these bivariate models were compared with all possible multiple regression models using Akaike's information criteria, we found that models with bird FD and PD (alone) best explained variation in attack rate. Of the two top models (ΔAICc < 2), FD had the highest weight (0.437 compared to 0.180) but the relative effects of FD and PD were similar (βFD = 0.578, βPD = 0.534; table 1). In contrast, abundance was not included as a parameter in the top competing models and the bivariate model including abundance had weak support (weight = 0.053, ΔAICc = 4.21).
Figure 4.
Bivariate relationships between plot-level attack rates and bird abundance (R2 = 0.27, p = 0.003), FD (R2 = 0.36, p = 0.0005), and PD (R2 = 0.31, p = 0.001). Monoculture plots are indicated with open circles (n = 10) and polycultures are filled circles (n = 17). X-axis units are the same as in figure 2.
Table 1.
Comparing bird community metrics as predictors of model attack rates. Summary of AIC model selection results evaluating bird community metrics as predictors of model attack rates at the plot level (n = 27), ranked by model weight. For each model, parameter estimates for bird abundance (ABUN), functional diversity (FD), and phylogenetic diversity (PD) are provided as standardized beta coefficients (β) for comparison among bird community metrics.
| model | d.f. | logLik | AICc | ΔAICc | weight | βABUN | βFD | βPD |
|---|---|---|---|---|---|---|---|---|
| FD | 3 | 35.68 | −64.41 | 0.00 | 0.437 | 0.578 | ||
| PD | 3 | 34.80 | −62.64 | 1.77 | 0.180 | 0.534 | ||
| ABUN + FD | 4 | 35.84 | −62.02 | 2.38 | 0.132 | −0.189 | 0.749 | |
| FD + PD | 4 | 35.68 | −61.71 | 2.70 | 0.113 | 0.565 | 0.014 | |
| ABUN | 3 | 33.58 | −60.20 | 4.21 | 0.053 | 0.474 | ||
| ABUN + PD | 4 | 34.86 | −60.06 | 4.35 | 0.050 | 0.099 | 0.452 | |
| ABUN + FD + PD | 5 | 35.84 | −59.08 | 5.33 | 0.030 | −0.189 | 0.742 | 0.007 |
| (none) | 2 | 29.78 | −55.11 | 9.30 | 0.004 |
We used structural equation modelling to quantitatively compare the extent to which tree species richness indirectly affected attack rate via bird abundance versus diversity. Preliminary analyses indicated the multicollinearity between bird PD and FD (electronic supplementary material, figure S3; R2 = 0.87, p < 0.001) was too high to include both variables in our model, and suggested FD to be superior to PD in predicting variation in attack rates (table 1). Thus we compare bird FD and abundance as mediators of the indirect effect of tree species richness on attack rate (i.e. product of two partial beta coefficients for direct effects [44]). In our initial hypothesized model, the indirect effect of tree species richness mediated by bird FD had a positive effect on attack rate (indirect effect = 0.32, p = 0.043) while the bivariate relationship between bird abundance and attack rate was not significant (p = 0.82), suggesting that the indirect effect of tree species richness on attack rate was mediated largely, or entirely by bird diversity (figure 5a; SEM, X2 = 3.25, p = 0.071). When non-significant paths were removed from this initial model in a stepwise procedure, the resulting final model reflected a full mediation of tree diversity effects by bird FD (figure 5b; SEM, X2 = 3.25, p = 0.072).
Figure 5.
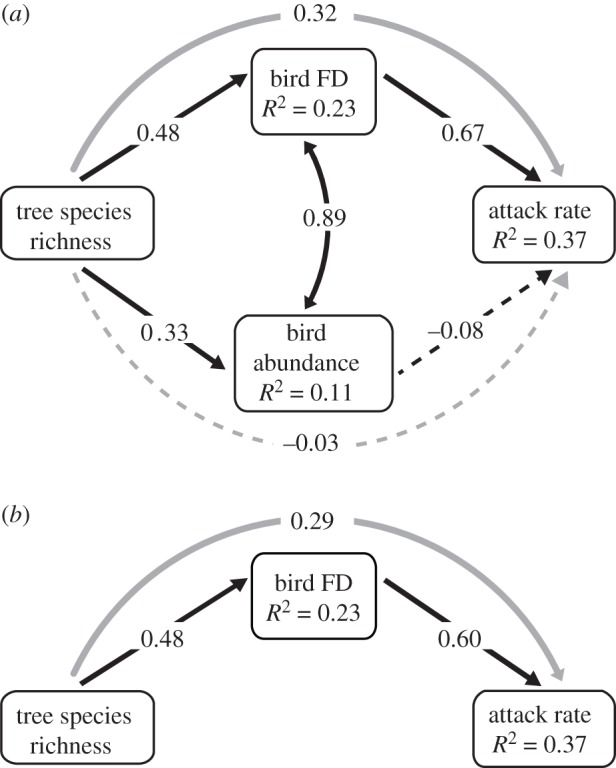
Initial (a; X2 = 3.25, p = 0.071) and final (b; X2 = 3.25, p = 0.072) structural equation models comparing the indirect effects of tree diversity on model attack rate, mediated by the bird community (curved grey arrows). Arrows represent causal relationships among variables, with direct effects in black and indirect effects indicated with grey. Dashed arrows indicate a non-significant path (p ≥ 0.05). For each path the magnitude of effect is provided as the standardized regression coefficient for direct effects, and the product of component standardized coefficients for indirect effects. R2 values of component models are provided in boxes with the response variable.
4. Discussion
Whereas past tests of the EH have documented the component relationships between plant diversity, predator communities, and their effects on herbivores [16,20,29], our findings demonstrate that the indirect effects of plant diversity on top-down control are primarily mediated by predator diversity in this tropical forest system. We link the positive effects of tree species richness on attack rate (65% higher) to tree species complementarity and increases in the functional diversity of foraging birds (55%), indicating that multi-species processes are responsible for the observed effects at multiple trophic levels. Decomposing EH effects, we highlight the importance of predator diversity over abundance in driving such dynamics.
Bird diversity may increase attack through several, non-mutually exclusive mechanisms. Predator diversity is most often presumed to increase prey attack due to predators foraging in separate microhabitats and thus eliminating enemy free space [48]. Our design likely underestimates such effects, as we placed caterpillar models in a uniform location across tree species (upper side of leaves on lower branches) and thus did not capture the breadth of foraging niches that span forest strata, microhabitats, and prey type. Alternatively, increased bird diversity may have reduced time spent on intra-specific interactions including territory defence or courtship [49,50], while abundance could increase competitive interactions. Consistent with our results, previous meta-analyses found that bird effects on arthropods in tropical agroforestry systems were correlated with bird diversity, but not abundance [28,51]. Together, these findings suggest a feedback linking diversity on multiple trophic levels to the top-down effects of predators.
Whereas other studies have found arthropod predators to be the dominant drivers of predation rates in forested systems and tropical regions [41,52], we found that birds were responsible for the majority of attacks on the artificial caterpillars and arthropod predation was very low in comparison. Our experimental system may differ because it lacks understory vegetation and is of relatively low diversity (even in high diversity plots) compared to typical forests at this latitude. Arthropod predators may be more sensitive to associated variation in fine-scale habitat structure compared to vertebrate predators, as well as lose enemy-free space from intraguild predators in this simplified system [53]. Further, the timing of experiments (spring versus summer) and corresponding differences in the phenology of arthropod and bird communities could have dramatic effects on predator foraging behaviour. Future research investigating predation pressure over time from complex predator communities will be valuable in understanding the mechanisms driving diversity effects.
Our work extends the mechanisms of the EH to forest ecosystems and vertebrate predators, both of which dominate terrestrial communities globally and provide valuable ecosystem services. While our focus is on plants and predators, it is likely that similar mechanisms influence other ecological processes contingent on species interactions. Birds in particular are exceptionally diverse and contribute to a variety of functions including pollination, nutrient cycling, and seed dispersal [54]. Accordingly, the consequences of biodiversity loss are likely more complex and far-reaching than is currently appreciated. This is exceedingly important given human dependence on forest services and products, and the rapid pace of environmental change [1].
Biodiversity effects are commonly studied with regard to a single trophic level, but the interactive effects of diversity across multiple trophic levels may affect ecosystem processes synergistically. Consequently, biodiversity loss at one trophic level has not only direct implications, but also indirect effects through the disruption of such synergies, that could result in negative feedbacks across trophic levels. As such, conservation strategies that consider multi-trophic biodiversity may support an array of community dynamics that are critical to ecological function.
Supplementary Material
Acknowledgements
We thank Teresa Quijano, Luis Díaz, and Christian Barajas for their assistance in the field and the staff at INIFAP for providing logistic support and accommodation. This manuscript is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1321846 award to C.S.N., as well as CONACYT (Mexico) grant CB 2015-250925 awarded to L.A.-R. and V.P.-T.
Ethics
This study was conducted in accordance with the laws, guidelines, and ethical standards of Mexico, where the experimental site is located.
Data accessibility
Data available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.2c448p6 [55]. A list of bird species observed and associated species-level traits used to calculate functional diversity are provided in electronic supplementary material, table S1.
Authors' contributions
All authors contributed to project conception; fieldwork was conducted by C.S.N. and L.A.-R.; C.S.N. analysed the data with substantial input from K.A.M.; C.S.N., L.A.-R., and K.A.M. wrote the manuscript. L.A.-R. and V.P.-T. contributed to the establishment of the forest diversity experiment.
Competing interests
The authors declare no competing interests.
References
- 1.Newbold T, et al. 2015. Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50. ( 10.1038/nature14324) [DOI] [PubMed] [Google Scholar]
- 2.Haddad NM, et al. 2015. Habitat fragmentation and its lasting impact on Earth's ecosystems. Sci. Adv. 1, e1500052 ( 10.1126/sciadv.1500052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooper DU, et al. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108. ( 10.1038/nature11118) [DOI] [PubMed] [Google Scholar]
- 4.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493. ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 5.Cardinale BJ, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486, 59–67. ( 10.1038/nature11148) [DOI] [PubMed] [Google Scholar]
- 6.Balvanera P, Pfisterer AB, Buchmann N, He J-S, Nakashizuka T, Raffaelli D, Schmid B. 2006. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 9, 1146–1156. ( 10.1111/j.1461-0248.2006.00963.x) [DOI] [PubMed] [Google Scholar]
- 7.Duffy JE, Godwin CM, Cardinale BJ. 2017. Biodiversity effects in the wild are common and as strong as key drivers of productivity. Nature 549, 261–264. ( 10.1038/nature23886) [DOI] [PubMed] [Google Scholar]
- 8.Haddad NM, Crutsinger GM, Gross K, Haarstad J, Knops JMH, Tilman D. 2009. Plant species loss decreases arthropod diversity and shifts trophic structure. Ecol. Lett. 12, 1029–1039. ( 10.1111/j.1461-0248.2009.01356.x) [DOI] [PubMed] [Google Scholar]
- 9.Dinnage R, Cadotte MW, Haddad NM, Crutsinger GM, Tilman D. 2012. Diversity of plant evolutionary lineages promotes arthropod diversity. Ecol. Lett. 15, 1308–1317. ( 10.1111/j.1461-0248.2012.01854.x) [DOI] [PubMed] [Google Scholar]
- 10.Scherber C, et al. 2010. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 468, 553–556. ( 10.1038/nature09492) [DOI] [PubMed] [Google Scholar]
- 11.Staab M, Blüthgen N, Klein A-M. 2015. Tree diversity alters the structure of a tri-trophic network in a biodiversity experiment. Oikos 124, 827–834. ( 10.1111/oik.01723) [DOI] [Google Scholar]
- 12.Griffin JN, Byrnes JEK, Cardinale BJ. 2013. Effects of predator richness on prey suppression: a meta-analysis. Ecology 94, 2180–2187. ( 10.1890/13-0179.1) [DOI] [PubMed] [Google Scholar]
- 13.Finke DL, Snyder WE. 2008. Niche partitioning increases resource exploitation by diverse communities. Science 321, 1488–1490. ( 10.1126/science.1160854) [DOI] [PubMed] [Google Scholar]
- 14.Haddad NM, Crutsinger GM, Gross K, Haarstad J, Tilman D. 2011. Plant diversity and the stability of foodwebs. Ecol. Lett. 14, 42–46. ( 10.1111/j.1461-0248.2010.01548.x) [DOI] [PubMed] [Google Scholar]
- 15.Root RB. 1973. Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecol. Monogr. 43, 95–124. ( 10.2307/1942161) [DOI] [Google Scholar]
- 16.Letourneau DK, et al. 2011. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 21, 9–21. ( 10.1890/09-2026.1) [DOI] [PubMed] [Google Scholar]
- 17.Crowder DW, Jabbour R. 2014. Relationships between biodiversity and biological control in agroecosystems: current status and future challenges. Biol. Control 75, 8–17. ( 10.1016/j.biocontrol.2013.10.010) [DOI] [Google Scholar]
- 18.Isbell F, et al. 2017. Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 105, 871–879. ( 10.1111/1365-2745.12789) [DOI] [Google Scholar]
- 19.Lemessa D, Hambäck PA, Hylander K. 2015. Arthropod but not bird predation in Ethiopian homegardens is higher in tree-poor than in tree-rich landscapes. PLoS ONE 10, e0126639 ( 10.1371/journal.pone.0126639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muiruri EW, Rainio K, Koricheva J. 2016. Do birds see the forest for the trees? Scale-dependent effects of tree diversity on avian predation of artificial larvae. Oecologia 180, 619–630. ( 10.1007/s00442-015-3391-6) [DOI] [PubMed] [Google Scholar]
- 21.Barbaro L, Rusch A, Muiruri EW, Gravellier B, Thiery D, Castagneyrol B. 2017. Avian pest control in vineyards is driven by interactions between bird functional diversity and landscape heterogeneity. J. Appl. Ecol. 54, 500–508. ( 10.1111/1365-2664.12740) [DOI] [Google Scholar]
- 22.Schuldt A, Both S, Bruelheide H, Härdtle W, Schmid B, Zhou H, Assmann T. 2011. Predator diversity and abundance provide little support for the enemies hypothesis in forests of high tree diversity. PLoS ONE 6, e22905 ( 10.1371/journal.pone.0022905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang B, Li B, He Y, Zhang L, Bruelheide H, Schuldt A. 2018. Tree diversity has contrasting effects on predation rates by birds and arthropods on three broadleaved, subtropical tree species. Ecol. Res. 33, 205–212. ( 10.1007/s11284-017-1531-7) [DOI] [Google Scholar]
- 24.Mooney KA, Gruner DS, Barber NA, Bael SAV, Philpott SM, Greenberg R. 2010. Interactions among predators and the cascading effects of vertebrate insectivores on arthropod communities and plants. Proc. Natl Acad. Sci. USA 107, 7335–7340. ( 10.1073/pnas.1001934107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallmann CA, Foppen RPB, van Turnhout CAM, de Kroon H, Jongejans E. 2014. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511, 341–343. ( 10.1038/nature13531) [DOI] [PubMed] [Google Scholar]
- 26.Chapman PM, Tobias JA, Edwards DP, Davies RG. 2018. Contrasting impacts of land-use change on phylogenetic and functional diversity of tropical forest birds. J. Appl. Ecol. 55, 1604–1614. ( 10.1111/1365-2664.13073) [DOI] [Google Scholar]
- 27.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 28.Philpott SM, Soong O, Lowenstein JH, Pulido AL, Lopez DT, Flynn DFB, DeClerck F. 2009. Functional richness and ecosystem services: bird predation on arthropods in tropical agroecosystems. Ecol. Appl. 19, 1858–1867. ( 10.1890/08-1928.1) [DOI] [PubMed] [Google Scholar]
- 29.Lichtenberg EM, et al. 2017. A global synthesis of the effects of diversified farming systems on arthropod diversity within fields and across agricultural landscapes. Glob. Change Biol. 23, 4946–4957. ( 10.1111/gcb.13714) [DOI] [PubMed] [Google Scholar]
- 30.Maas B, Tscharntke T, Saleh S, Putra DD, Clough Y. 2015. Avian species identity drives predation success in tropical cacao agroforestry. J. Appl. Ecol. 52, 735–743. ( 10.1111/1365-2664.12409) [DOI] [Google Scholar]
- 31.Maas B, et al. 2016. Bird and bat predation services in tropical forests and agroforestry landscapes. Biol. Rev. 91, 1081–1101. ( 10.1111/brv.12211) [DOI] [PubMed] [Google Scholar]
- 32.Charbonnier YM, Barbaro L, Barnagaud J-Y, Ampoorter E, Nezan J, Verheyen K, Jactel H. 2016. Bat and bird diversity along independent gradients of latitude and tree composition in European forests. Oecologia 182, 529–537. ( 10.1007/s00442-016-3671-9) [DOI] [PubMed] [Google Scholar]
- 33.Moreira X, Abdala-Roberts L, Parra-Tabla V, Mooney KA. 2014. Positive effects of plant genotypic and species diversity on anti-herbivore defenses in a tropical tree species. PLoS ONE 9, e105438 ( 10.1371/journal.pone.0105438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howell S, Webb S. 1995. A guide to the birds of Mexico and Northern Central America. New York, NY: Oxford University Press. [Google Scholar]
- 35.Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, Jetz W. 2014. EltonTraits 1.0: Species-level foraging attributes of the world's birds and mammals. Ecology 95, 2027 ( 10.1890/13-1917.1) [DOI] [Google Scholar]
- 36.Faith DP. 1992. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10. ( 10.1016/0006-3207(92)91201-3) [DOI] [Google Scholar]
- 37.Gower JC. 1971. A general coefficient of similarity and some of its properties. Biometrics 27, 857–871. ( 10.2307/2528823) [DOI] [Google Scholar]
- 38.Hackett SJ, et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768. ( 10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 39.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 40.Low PA, Sam K, McArthur C, Posa MRC, Hochuli DF. 2014. Determining predator identity from attack marks left in model caterpillars: guidelines for best practice. Entomol. Exp. Appl. 152, 120–126. ( 10.1111/eea.12207) [DOI] [Google Scholar]
- 41.Roslin T, et al. 2017. Higher predation risk for insect prey at low latitudes and elevations. Science 356, 742–744. ( 10.1126/science.aaj1631) [DOI] [PubMed] [Google Scholar]
- 42.Loreau M, Hector A. 2001. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76. ( 10.1038/35083573) [DOI] [PubMed] [Google Scholar]
- 43.Huston MA. 1997. Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia 110, 449–460. ( 10.1007/s004420050180) [DOI] [PubMed] [Google Scholar]
- 44.Grace JB. 2006. Structural equation modeling and natural systems. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 45.Rosseel Y. 2012. lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36. ( 10.18637/jss.v048.i02) [DOI] [Google Scholar]
- 46.R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org. [Google Scholar]
- 47.Schmid B, Hector A, Saha P, Loreau M. 2008. Biodiversity effects and transgressive overyielding. J. Plant Ecol. 1, 95–102. ( 10.1093/jpe/rtn011) [DOI] [Google Scholar]
- 48.Schmitz OJ. 2007. Predator diversity and trophic interactions. Ecology 88, 2415–2426. ( 10.1890/06-0937.1) [DOI] [PubMed] [Google Scholar]
- 49.Morse DH. 1970. Ecological aspects of some mixed-species foraging flocks of birds. Ecol. Monogr. 40, 119–168. ( 10.2307/1942443) [DOI] [Google Scholar]
- 50.Gómez JP, Bravo GA, Brumfield RT, Tello JG, Cadena CD. 2010. A phylogenetic approach to disentangling the role of competition and habitat filtering in community assembly of Neotropical forest birds. J. Anim. Ecol. 79, 1181–1192. ( 10.1111/j.1365-2656.2010.01725.x) [DOI] [PubMed] [Google Scholar]
- 51.Bael SAV, Philpott SM, Greenberg R, Bichier P, Barber NA, Mooney KA, Gruner DS. 2008. Birds as predators in tropical agroforestry systems. Ecology 89, 928–934. ( 10.1890/06-1976.1) [DOI] [PubMed] [Google Scholar]
- 52.Leles B, Xiao X, Pasion BO, Nakamura A, Tomlinson KW. 2017. Does plant diversity increase top-down control of herbivorous insects in tropical forest? Oikos 126, 1142–1149. ( 10.1111/oik.03562) [DOI] [Google Scholar]
- 53.Langellotto GA, Denno RF. 2004. Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. Oecologia 139, 1–10. ( 10.1007/s00442-004-1497-3) [DOI] [PubMed] [Google Scholar]
- 54.Whelan CJ, Wenny DG, Marquis RJ. 2008. Ecosystem services provided by birds. Ann. N. Y. Acad. Sci. 1134, 25–60. ( 10.1196/annals.1439.003) [DOI] [PubMed] [Google Scholar]
- 55.Nell CS, Abdala-Roberts L, Parra-Tabla V, Mooney KA. 2018. Data from: Tropical tree diversity mediates foraging and predatory effects of insectivorous birds Dryad Digital Repository. ( 10.5061/dryad.2c448p6) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Nell CS, Abdala-Roberts L, Parra-Tabla V, Mooney KA. 2018. Data from: Tropical tree diversity mediates foraging and predatory effects of insectivorous birds Dryad Digital Repository. ( 10.5061/dryad.2c448p6) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.2c448p6 [55]. A list of bird species observed and associated species-level traits used to calculate functional diversity are provided in electronic supplementary material, table S1.