Abstract
Permethrin is a pyrethroid insecticide that acts thru membrane depolarization and is known to disrupt calcium levels in neurons. Disrupted calcium homeostasis is linked to oxidative stress as well as many other cellular mis-functions and permethrin has been reported to disrupt lipid and glucose metabolism in animals and mammalian cell models. It is not known, however, if permethrin influences calcium levels and its associated cellular mechanisms in liver cells. Thus, the goal of the current study was to investigate the mechanisms of permethrin on calcium-mediated cellular signaling pathway, particularly on activation of extracellular signal-related kinase (ERK1/2 or p42/p44) using human hepatocytes, HepG2. The current results showed that permethrin treatment induced oxidative stress and phosphorylation of ERK1/2, which were dependent upon voltage-sensitive sodium channels (VSSC). It was further determined that permethrin-induced ERK1/2 activation was mediated by the metabotropic glutamate receptors (mGluRs)-phosphoinositide phospholipase C (PLC)-protein kinase C (PKC) pathway, but not by changes of intracellular calcium or ER stress-mediated mechanisms.
Keywords: Permethrin, ERK1/2, Membrane depolarization, mGluR-PLC-PKC Pathway
1. Introduction
Pyrethroid insecticides are widely used on crops, lawns, textiles, and as insect repellants, which accounted for approximately 17% of the global pesticides use in 2013 (Soderlund, 2015; Sparks, 2013). Permethrin is the most widely used pyrethroid and acts as an agonist on neuronal voltage-sensitive sodium channels (VSSC) where it causes membrane depolarization, resulting in death of insects (Vais et al., 2001). Along with reports of various environmental pollutants involved in the development of metabolic diseases, such as obesity and diabetes (Cox et al., 2007; Rezg et al., 2010; Starling et al., 2014; Wang et al., 2011), previous in vitro studies have likewise reported that permethrin induced adipogenesis and insulin resistance (Kim et al., 2014; Xiao et al., 2017c). In animal studies, permethrin promoted high fat diet-induced insulin resistance in both male and female mice (Xiao et al., 2017b; Xiao et al., 2018), potentiated high fat diet-induced weight gain, inhibited hepatic fatty acid oxidation and promoted gluconeogenesis in male mice (Xiao et al., 2018). How permethrin causes these effects, as well as its effect on the liver function, are not completely understood.
Extracellular signal-related kinase 1/2 (ERK1/2) is a member of mitogen-activated protein kinases (MAPKs) family, which acts as a signaling transducer from cellular receptors to nuclear transcriptional factors that senses cellular stress. ERK1/2 is involved in several intracellular stress responses, such as inflammation, apoptosis (Sawatzky et al., 2006) and cell death (Cagnol and Chambard, 2010). It is also associated with obesity and insulin resistance (Bost et al., 2005; Zheng et al., 2009) and the activation of hepatic ERK1/2 was observed in high fat diet-fed animals (Jiao et al., 2013) and may be related to lipotoxicity induced by fatty acid overload during non-alcoholic fatty liver disease (NAFLD) (Kohjima et al., 2007).
The neurotoxicity of permethrin is caused by delaying the closure of insect VSSC, which then lead to membrane depolarization, hyperexcitation, and cell death, resulting in death of insects (Vais et al., 2001). Pyrethroids are also known to activate VSSC in mammal neurons (Ghiasuddin and Soderlund, 1985). There is limited information, however, on the effect of permethrin on VSSC in non-neural tissues such as human hepatocytes, as well as the relationship between membrane depolarization and ERK1/2 activation as possible causes of increased adipogenesis and insulin resistance. Thus, the aim of this study was to investigate the mechanisms of permethrin-induced activation of ERK1/2, including involvement of membrane depolarization, Ca2+ signaling pathways, endoplasmic reticulum (ER) stress, and the metabotropic glutamate receptors (mGluRs)-phosphoinositide phospholipase C (PLC)-protein kinase C (PKC) pathways using HepG2 hepatocytes.
2. Materials and methods
2.1. Materials and chemicals
Permethrin (analytical grade, 98.1% purity, a mixture of 38.7% cis and 59.4% trans isomers) was purchased from Sigma-Aldrich (St. Louis, MO). HepG2 human hepatoma cells were purchased from American Type Culture Collection (Manassas, VA). Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS) and penicillin/streptomycin were purchased from Thermo Fisher Scientific (Waltham, MA), Sigma-Aldrich (St. Louis, MO) or GE Healthcare (Marlborough, MA). Rabbit antibodies against human ERK1/2, p-ERK1/2, inositol-requiring enzyme 1 α (IRE1α), binding immunoglobulin protein (BiP), protein kinase RNA-like endoplasmic reticulum kinase (PERK), phosphorylated-PERK, and mouse antibodies against CCAAT-enhancer-binding protein homologous protein (CHOP) were obtained from Cell Signaling Technology (Danvers, MA). Rabbit antibodies against eukaryotic initiation factor 2α (eIF2α), phosphorylated eIF2α, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and secondary antibodies including horseradish peroxidase (HRP)-conjugated anti-rabbit IgG and HRP-conjugated anti-mouse IgG were obtained from Santa Cruz Biotechnology (Dallas, TX). Rabbit antibodies against phosphorylated-IRE1α were purchased from Abcam (Cambridge, UK). All other chemicals were obtained with either analytical grade or cell-culture grade from Fisher Scientific (Waltham, MA).
2.2. Cell culture and treatment
HepG2 human hepatoma cells were maintained in DMEM containing 10% heat-inactivated FBS, 10,000 U/mL penicillin and 10 mg/mL streptomycin at 37 °C with 5% CO2 and 95% air. Cells were subcultured every 48–72 h at a confluency of 80–90%. The effect of permethrin on HepG2 cell viabilities was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described (Mosmann, 1983). In brief, HepG2 cells were seeded and treated with various concentrations of permethrin for 24 h and were then subject to MTT assay. Cell viabilities were then determined by the formation of formazan from MTT by measuring the absorbance at 570 nm (SpectraMax i3, Molecular Devices LLC, San Jose, CA). Dimethyl sulfoxide (DMSO) was used as a vehicle for delivering permethrin into culture media at a final concentration of 0.1% v/v in all treatment groups as previously described (Sun et al., 2017b). Up to 50 µM of permethrin did not show significant change in cell viabilities after 24 h (Suppl. Fig. S1). Thus, 10 and 50 µM of permethrin were used in further experiments. As previously reported, the serum level of permethrin recorded without clinical neurotoxicity was 2.2 µM in an oral intoxication case (Gotoh et al., 1998). Additionally, the levels of cypermethrin (a type II pyrethroid) in the blood from pesticide factory workers were measured at about 500 nM (Khan et al., 2010). In comparison, the concentrations of permethrin used in this study were significantly higher. However, lipophilic insecticides accumulate in lipid-rich cellular compartments, such as intracellular lipid droplets (Jaga and Dharmani, 2003). Thus, the concentration of permethrin tested, although high when compared to serum levels, may still be biologically relevant in the hepatocyte model system used.
Antagonists used, including tetrodotoxin, bisindolylmaleimide I, U-73122, and nimodipine on ERK1/2 activation did not activate ERK1/2 (Suppl. Fig. S2). Aminoethoxydiphenyl borate and PHCCC were reported not to influence ERK1/2 activation in previous studies (Choe and Wang, 2001; Wu et al., 2016).
2.3. Membrane depolarization assay
The levels of membrane depolarization were determined as previously described (Miao and Joyner, 1994) using the voltage sensitive dye, bis-(1,3-dibutylbarbituric Acid) trimethine oxonol (DiBAC(4)3). DiBAC(4)3 fluorescence dye was first dissolved in DMSO as stock solution at 250µM and the working solution was freshly made in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (20 mmol/L HEPES, 115 mmol/L NaCl, 5.4 mmol/L KCl, 1.8 mmol/L CaCl2, 0.8 mmol/L MgCl2, 13.8 mmol/L glucose, pH 7.4) with a final DiBAC(4)3 concentration of 250 nM. Treatment compounds were diluted with fresh DiBAC(4)3 working solutions and fluorescence signal was recorded by kinetic reading for 40 min with 1 min interval at excitation 485 nm and emission 516 nm (SpectraMax i3, Molecular Devices LLC, San Jose, CA).
2.4. Immunoblotting
Protein expression levels were performed by western blotting with modification as previously described (Kim et al., 2014). In brief, proteins were extracted from cell lysate and then separated by SDS-polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane. Target proteins were then detected by incubating corresponding primary antibodies and followed by HRP-conjugated secondary antibodies. The protein-antibody complexes were treated with enhanced chemiluminescence substrate and exposed by Image Station 4000MM (Kodak, Rochester, NY) for visualization and quantification.
2.5. Measurement of intracellular [Ca2+]
Intracellular calcium levels were measured by cell permeate fluorescence dye, Fura-2-acetoxymethyl ester (Fura-2 AM), using the ratiometric method (Roe et al., 1990). The conformation of fluorescent dye changes when chelated with Ca2+, resulting in change of excitation wavelength from 380 nm to 340 nm, but did not influence the emission wavelength and 510 nm. Fura-2 AM was diluted in HEPES buffer containing 0.01% pluronic acid. Before permethrin treatment, cells were pre-incubated with 2µM Fura-2 AM for 60 min at 37 °C. Dye was then removed and cells were incubated with HEPES buffer for another 30 min at 37 °C for ester hydrolysis and further compartmentation of Fura-2 AM into intracellular organelles, including endoplasmic reticulum (ER). ER stores most of the intracellular calcium, where the concentration of calcium is around 1000x higher compared to the cytosol (Samtleben et al., 2013), which suggest that intracellular Ca2+ data obtained in this study represents Ca2+ from the ER . Cells were then treated with permethrin and intracellular calcium levels were estimated by the ratio of fluorescence intensities detected at emission 510 nm with excitation wavelengths of 340 nm and 380 nm (510 nm/340 nm vs. 510 nm/380 nm) (SpectraMax i3, Molecular Devices LLC, San Jose, CA).
2.6. Measurement of intracellular reactive oxygen species (ROS)
Intracellular ROS were measured by fluorescence dye 2',7'-dichlorofluorescin diacetate (DCFDA) assay as described (Lebel et al., 1992) with modifications. DCFDA was first dissolved in DMSO and 25µM DCFDA solution was freshly prepared in HEPES buffer. Cells were pre-incubated with DCFDA for 45 min at 37 °C. DCFDA was then replaced by permethrin treatment and fluorescence was measured at excitation 485 nm and emission 535 nm at 3, 24, and 48 h after treatment (SpectraMax i3, Molecular Devices LLC, San Jose, CA).
2.7. Statistical Analysis
Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test using Graphpad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA). Comparisons of treatments with a P-value of less than 0.05 were considered significantly different.
3. Results
3.1. Permethrin and membrane depolarization
It is known that permethrin disrupts the closure of neuronal voltage-sensitive sodium channels (VSSC) that can lead to increased influx of Na+ into the cytosol (Casida et al., 1983). The excessive influx of Na+ makes the inside of the cell more positive causing depolarization. A similar action of permethrin, however, has not been reported in hepatocytes. Data in Fig. 1 indicated that 10 and 50 µM permethrin caused membrane depolarization in a dose-dependent manner in HepG2 cells at 40 min following permethrin treatment. In order to further confirm that permethrin-induced membrane depolarization in this model is due to permethrin action on VSSC, 10 µM of a VSSC specific antagonist, tetrodotoxin (TTX), was co-treated with permethrin. Depolarizing effects of 10 and 50 µM permethrin were significantly diminished by TTX (10% inhibition, P = 0.0466 and 58% inhibition, P < 0.0001 when compared to 10 and 50 µM permethrin, respectively). This result suggests that permethrin induces membrane depolarization of HepG2 cells through its action on non-neural VSSC. Additionally, permethrin rapidly depolarized cells over the first minute following treatment and the depolarization was persistent for up to 40 minutes in a kinetic assessment of membrane potential (Suppl. Fig. S3). The depolarization by permethrin was also inhibited by TTX throughout the tested 40-minute period.
Figure 1. Permethrin-induced membrane depolarization via voltage sensitive sodium channel (VSSC).
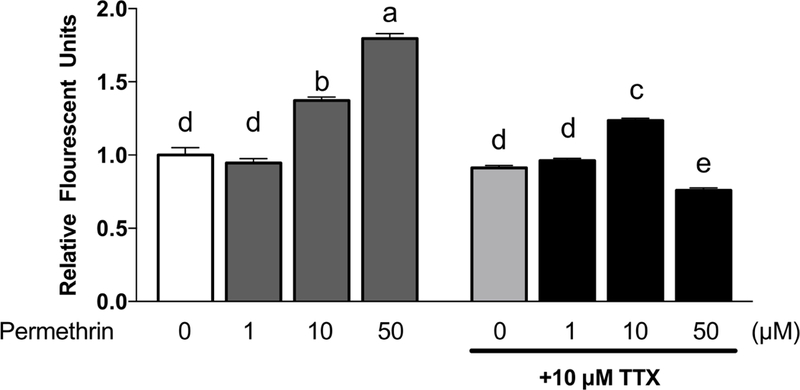
HepG2 cells were incubated with 250 nM DiBAC(4)3 membrane potential probe and 1, 10 and 50 µM of permethrin with or without 10 µM TTX. Fluorescence was determined following excitation at 485 nm as the emission 516 nm at 40 min after treatment. Numbers are mean ± S.E.M. n=6. Means with different letters are statistically different at P < 0.05. TTX: tetrodotoxin; DiBAC(4)3: bis-(1,3-dibutylbarbituric acid) trimethine oxonol.
3.2. Permethrin induced ERK1/2 activation via VSSC
Previous studies reported that permethrin altered hepatic energy metabolism (Xiao et al., 2018), and may contribute to its effects on the development of metabolic diseases. Since the activation of ERK1/2 can be related to obesity (Jiao et al., 2013) and insulin resistance (Zheng et al., 2009), we determined the phosphorylation (activation) of ERK1/2 after the treatment of permethrin in HepG2 cells as a potential mediator of permethrin-mediated metabolic alteration. Treatments of 10 and 50 µM of permethrin significantly increased the level of phospho-ERK1/2 a 30 min exposure; 117% and 130% increases compared to the control, respectively (P < 0.0001 for both) (Fig. 2A). 10 µM of permethrin treatment also caused the activation of ERK at 48 h after treatment, resulting in a 250% increase over the control (P < 0.0001, Fig. 2B). The phosphorylation of ERK1/2 by permethrin was significantly reduced when TTX was added in a dose-dependent manner, which suggested that the permethrin-induced activation of ERK1/2 was due, in part, to its agonistic effect on VSSC (Fig. 2C).
Figure 2. Permethrin induced ERK1/2 activation through voltage-sensitive sodium channels (VSSC).
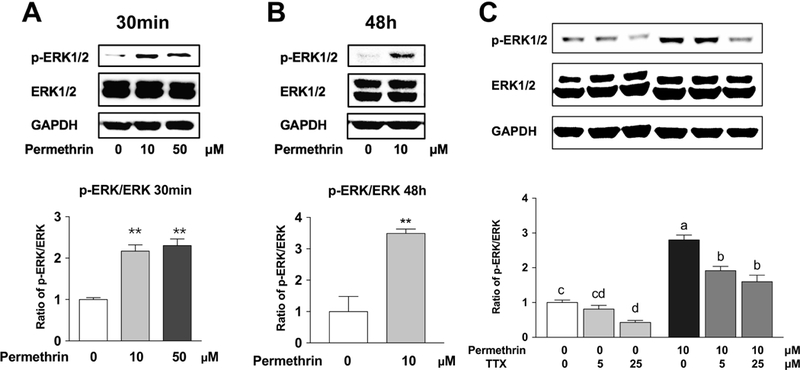
(A) Permethrin-induced phosphorylation (activation) of ERK1/2 (30 min). (B) Permethrin-induced phosphorylation of ERK1/2 (48 h). (C) TTX diminished permethrin-induced activation of ERK1/2. HepG2 cells were treated with permethrin for 30 min (A) and 48 h (B). HepG2 cells were pre-treated with 5 and 25 µM TTX for 1 h and 10 µM permethrin was added for another 30 minutes (C). The ratio of p-ERK/ERK was measured by western blot. Numbers are mean ± S.E.M. (n=3–4 for A & B and n=5 for C). **Statistically different from the control group at P < 0.01. Means with different letters are statistically different at P < 0.05. ERK1/2: Extracellular signal-related kinase 1/2; TTX: tetrodotoxin.
3.3. ERK1/2 activation and ER calcium release
Next, we investigated how permethrin led to ERK1/2 activation. Intracellular calcium homeostasis maintains several normal cellular functions, which can be disrupted by changes of membrane potential (Locknar et al., 2004). Changes of membrane potential can stimulate the opening of membrane voltage-gated Ca2+ channels, leading to calcium influx into the cell. Increased cytosolic Ca2+ can activate inositol 1,4,5-trisphosphate (IP3)-dependent Ca2+ channel in ER, known as the Ca2+-induced Ca2+ release, resulting in ER stress (Locknar et al., 2004; Verkhratsky and Shmigol, 1996). Results in Fig. 3A showed that 10 and 50µM of permethrin stimulation significantly decreased the ER calcium level after 30 minutes (22%, P = 0.0016 and 24%, P = 0.0006 compared to the control, respectively). The effects of 50µM permethrin last for 3 h (30% reduction compared to the respective control, P < 0.0001), with the ER calcium level returning to control levels after 24 h.
Figure 3. Intracellular calcium and permethrin-induced ERK1/2 activation in HepG2 cells.
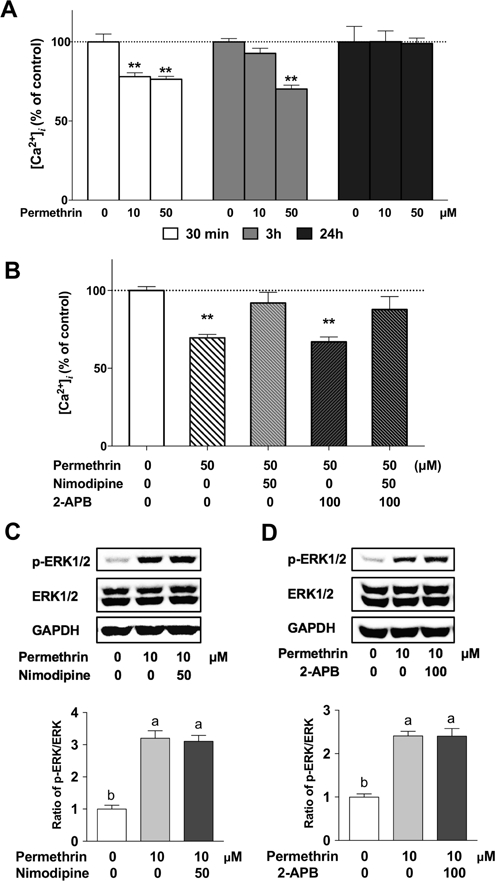
(A) Permethrin decreased intracellular calcium level. (B) L-type calcium channel blocker nimodipine but not 2-APB, IP3-dependent calcium channel blocker inhibited permethrin-induced calcium decrease. (C) L-type calcium channel blocker nimodipine and ERK1/2 activation. (D) 2-APB and ERK1/2 activation. HepG2 cells were pre-treated with 2 µM Fura-2 AM as and treated with permethrin to measure the calcium level in HepG2 cells (A & B). Calcium channel blockers, nimodipine (50µM) and 2-APB (100µM) were treated with or without permethrin and intracellular calcium was measured after 30 minutes (B). HepG2 cells were pre-treated with 50µM nimodipine or 100µM 2-APB for 1h and followed by a 30-minute co-treatment with 10µM permethrin and calcium channel blockers and p-ERK/ERK were analyzed by western blotting (C & D). Numbers are mean ± S.E.M. n=5–6. **Statistically different from the control group at P < 0.01. Means with different letters are statistically different at P < 0.05. 2-APB: aminoethoxydiphenyl borate; ERK1/2: Extracellular signal-related kinase 1/2.
Most intracellular calcium is stored in the endoplasmic reticulum (ER) (Coe and Michalak, 2009). There are two types of calcium channels that are involved in intracellular Ca2+ transport including non-neuronal cells; the L-type calcium channel and inositol triphosphate (IP3) dependent calcium channel (Berridge et al., 2000). In Fig. 3B, the release of Ca2+ from ER caused by permethrin was blocked by nimodipine, a L-type calcium channel blocker, but not by the IP3 receptor blocker, aminoethoxydiphenyl borate (2-APB). These findings suggest that the L-type calcium channel may be responsible for the permethrin-induced ER calcium release. Nimodipine or 2-APB were then co-applied with permethrin to understand if the manipulation of calcium efflux from the ER can alter the activation of ERK1/2 caused by permethrin. However, the results showed that permethrin-induced ERK1/2 activation was not influenced by either nimodipine (Fig. 3C) or 2-APB (Fig. 3D). These findings suggest that permethrin activates ERK1/2 independent to the calcium-mediated mechanism in HepG2 cells.
3.4. Intracellular ROS level and ER stress pathways
Oxidative stress, caused by reactive oxygen species (ROS), can result in cell damages and cellular dysfunctions, including ER stress (Malhotra and Kaufman, 2007). ER stress, also called unfolded protein response (UPR), results from accumulation of the unfold or misfolded proteins (Hampton, 2000). It has been reported that disrupted calcium homeostasis (Krebs et al., 2015) can result in ER stress, which then leads to ERK1/2 activation (Hu et al., 2004). In addition, permethrin was reported to induce ER stress in nerve cells (Hossain and Richardson, 2011) and adipocytes (Xiao et al., 2017c). Thus, we determined the effects of permethrin on the production of ROS and ER stress. Treatments of 10 and 50µM of permethrin significantly increased intracellular ROS level by 10% (P < 0.0001) and 12% (P < 0.0001) compared to the control, respectively, at 3h, 24, and 48 h following permethrin treatment (Fig. 4A). The ER stress pathways share a common activator BiP and downstream marker CHOP, which can be activated via the PERK-eIF2α pathway or IRE1α pathway. Permethrin treatment, however, did not result in any significant effects on markers of ER stress tested in this model (Fig. 4B-G).
Figure 4. Effects of permethrin on oxidative stress and ER stress.
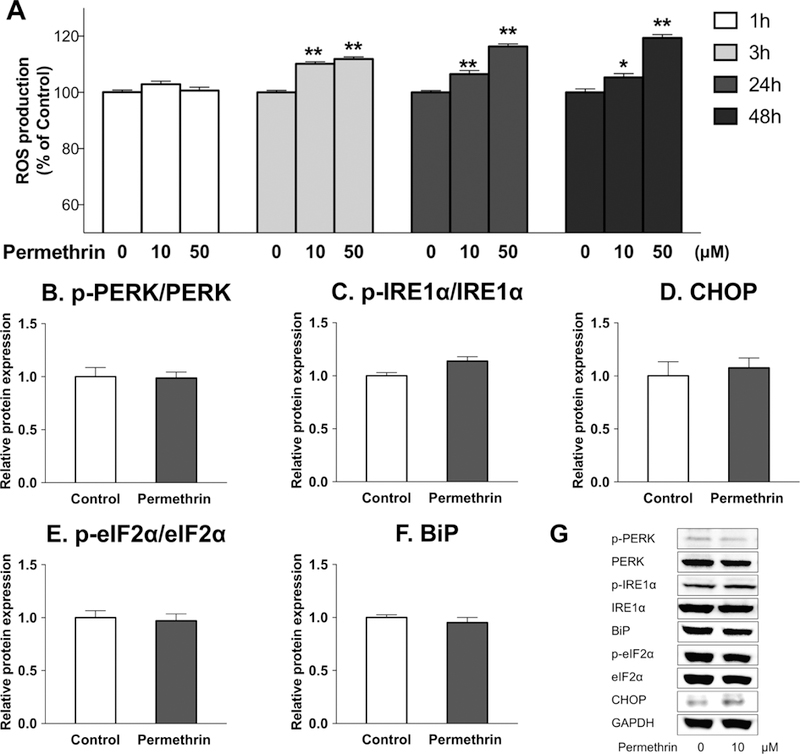
(A) Permethrin induced ROS production. (B-G) Effects of permethrin on ER stress: (B) ratio p-PERK/PERK; (C) ratio of p-IRE1α/IRE1α; (D) CHOP; (F) ratio of p-eIF2α/eIF2α; (G) BiP; (H) Representative pictures of ER stress pathways. HepG2 cells were pre-incubated with 25 µM DCFDA as ROS probe and treated with 10 and 50 µM permethrin and ROS levels (A) and were measured at 1, 3, 24 and 48 h. HepG2 cells were treated with 10µM permethrin for 48 h and ER stress-related markers ER stress-related markers (B-G) were analyzed. Numbers are mean ± S.E.M. n=8–10 for A and n=5 for B-G. *Statistically different from the respective control at P < 0.05, ** P < 0.01. PERK: protein kinase RNA-like endoplasmic reticulum kinase; IRE1α: inositol-requiring enzyme 1 α; CHOP: CCAAT-enhancer-binding protein homologous protein; eIF2α: eukaryotic initiation factor 2 α; BiP: binding immunoglobulin protein; DCFDA: 2',7'-dichlorodihydrofluorescein diacetate; ROS: reactive oxygen species.
3.5. ERK1/2 activation via mGluR-PLC-PKC pathway
There is an alternative pathway that may be associated with the permethrin-induced phosphorylation of ERK1/2 besides the ER stress mediated mechanism, the mGluR-PLC-PKC pathway (Monick et al., 2000; Ohana et al., 2006). In order to determine if this pathway may be involved in the activation of ERK1/2 by permethrin, different antagonists were used to evaluate their impacts on phosphorylation of ERK1/2. The PKC antagonist, bisindolylmaleimide I (BIM) is known to inhibit PKC isoforms (α, β1, β2, γ, δ, and ε), which all can be activated by DAG, the product from the activation of its upstream regulator, PLC (Toullec et al., 1991). The activation of ERK1/2 by permethrin was inhibited by 1µM of BIM (48% inhibition, P < 0.0001 compared to 10 µM permethrin, Fig. 5A). In addition, the PLC inhibitor, U-73122, is known to inhibit PLC isoform β and γ that can be activated by group I mGLuR (Heemskerk et al., 1997). Treatment of 2µM of U73122 also diminished permethrin-induced ERK1/2 phosphorylation (47% inhibition, P = 0.0016 compared to 10 µM permethrin, Fig. 5B). The group I mGluR is a G protein-coupled receptor that responses to membrane potential and regulates PLC. Treatment of 25µM PHCCC, the antagonist of group I mGluR, inhibited activation of ERK1/2 induced by permethrin (44% inhibition, P = 0.0011 compared to 10 µM permethrin, Fig. 5C). These results together suggest that the mGluR-PLC-PKC pathway is important for permethrin-induced phosphorylation of ERK1/2 in this model. To confirm if activation of group l mGluR itself would be enough to activate ERK1/2, cells were treated with a group l mGluR agonist, (S)-3,5-DHPG, which significantly activated ERK1/2 at both concentrations tested (64% increase with 10µM (P = 0.0050) and 116% increase with 50µM (P < 0.0001) compared to the control, Fig. 5D). These findings indicating that permethrin activated ERK1/2 via the mGluR-PLC-PKC-dependent pathway.
Figure 5. Permethrin induced phosphorylation (activation) of ERK1/2 via mGluR-PLC-PKC dependent pathway.
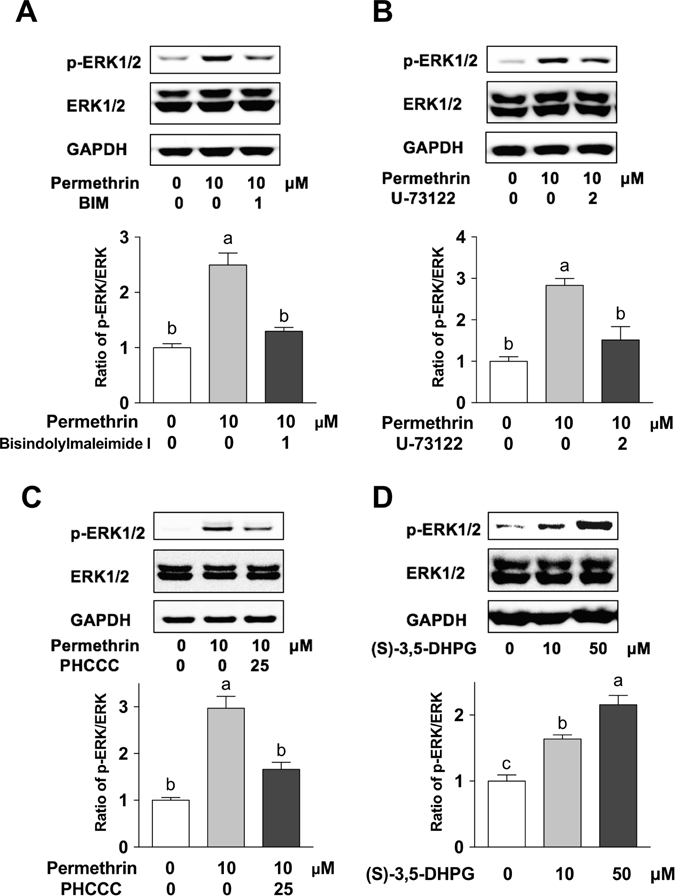
Effects of bisindolylmaleimide I (a PKC antagonist, A), U-73122 (a PLC antagonist, B), and PHCCC (a group I mGluR antagonist, C) on permethrin’s effect on ERK1/2 activation. (D) Effect of group I mGluR agonists on ERK1/2 activation. Cells were treated with or without antagonist for 1 h before permethrin treatment, then co-incubated with permethrin for 30 min (A-C). HepG2 cells were treated with 10 and 50µM of group I mGluR agonist (S)-3,5-DHPG for 30 min (D). Numbers are mean ± S.E.M. n=5. Means with different letters are statistically different at P < 0.05. ERK1/2: Extracellular signal-related kinase 1/2; mGluR: metabotropic glutamate receptor; PKC: protein kinase C; PLC: phosphoinositide phospholipase C; (S)-3,5-DHPG: (S)-3,5-Dihydroxyphenylglycine.
4. Discussions
In this study, we showed that permethrin induced phosphorylation of ERK1/2 in HepG2 cells through VSSC. It was also found that permethrin may have triggered ER calcium release as a result of membrane depolarization caused by VSSC, but calcium signaling did not involve in permethrin-induced ERK1/2 activation. The current results also showed that permethrin increased ROS, but not ER stress. Furthermore, it was suggested that permethrin-induced ERK1/2 activation was, in part, mediated by the mGluR-PLC-PKC pathway.
There is emerging evidence reporting the correlation between the exposure to insecticides and metabolic diseases, such as diabetes and obesity (Rezg et al., 2010; Sun et al., 2017a; Swaminathan, 2013; Xiao et al., 2017a). Previous research has revealed that permethrin promoted adipogenesis in 3T3-L1 adipocytes and impaired insulin-signaling pathway in C2C12 myotubes (Kim et al., 2014; Sun et al., 2017b; Xiao et al., 2017c). In addition, we reported that treatment permethrin promoted fat accumulation in HepG2 cells (Yang et al., 2018). Moreover, the subchronic exposure to a relatively low level of permethrin potentiated high fat diet-induced insulin resistance in both male and female mice and promoted high fat diet-induced weight gain in male mice by inhibited hepatic fatty acid oxidation and promoted gluconeogenesis (Xiao et al., 2017b; Xiao et al., 2018). The current results further support the role of permethrin in metabolic alteration, particularly in hepatocytes. Indeed, this study is the first to report the role of permethrin in metabolic dysfunction in the hepatocytes, which is, in part, associated with its role on non-neural VSSC. As the disruption of hepatic physiological functions is often associated with metabolic diseases, the current findings suggest the potential role of permethrin exposure and alteration of hepatic functions.
Most insecticides target the insect nervous system and it is well established that permethrin targets neuronal VSSC, resulting in mortality. The study of VSSC in hepatocyte, however, is limited and the functions of VSSC in hepatocytes are still largely unknown. Along with the previous in vivo reports that permethrin has effects on non-neural tissues, including the liver, the results from the current study support the contention that permethrin acts on non-neural tissues via VSSC-mediated mechanisms. As other types of insecticides with different targets in insect neurons also potentiated adipogenesis and altered insulin responsiveness that are similar to permethrin (Kim et al., 2013; Kim et al., 2016; Park et al., 2013; Shen et al., 2017; Sun et al., 2016), it is possible that these insecticides may cause metabolic dysfunction via their target sites in non-neural cells. This needs to be further confirmed to determine the role of insecticides in metabolic diseases.
Pyrethroid insecticides, including permethrin, have been reported to induce oxidative stress in the liver (Atessahin et al., 2005; Chargui et al., 2012; Giray et al., 2001; Mossa et al., 2013; Tuzmen et al., 2008; Wang et al., 2016), which is consistent with the induction of oxidative stress by permethrin found in this study. Increased intracellular ROS is known to be associated with metabolic diseases (Roberts and Sindhu, 2009). Oxidative stress can result from ER stress, however, the correlation between oxidative stress and ER stress was not observed in the current study. Although oxidative stress can be one pathway leading to ERK1/2 activation (Son et al., 2013), the current results have revealed that permethrin induced ERK1/2 activation (30 min) prior to ROS induction (3 h). Therefore, we speculate that permethrin-induced oxidative stress was independent of its effect on ERK1/2 activation at 30 min. ERK1/2 activation and oxidative stress were both observed with relatively long-term permethrin treatment (48 h), although we did not measure time-course study for phosphorylated ERK1/2 between 30 min and 48 h. Thus, we cannot exclude the possibility that the activation of ERK1/2 transiently fluctuates during this ime. Therefore, further detailed time-course investigation will be needed to determine the correlation between permethrin-induced ERK1/2 activation and oxidative stress.
In mammalian cells, the ER is a major Ca2+ store and the highest concentration of intracellular Ca2+ can be found there (Görlach et al., 2006). Disrupt ion of ER Ca2+ level may result in ER stress as high calcium levels are required for certain ER chaperones allowing proper protein folding (Mekahli et al., 2011). It is known that ERK1/2 activation may be calcium signal dependent (Schmitt et al., 2004) and result from ER stress (Hetz, 2012; Hu et al., 2004). Calcium disruption can also lead to ER stress (Timmins et al., 2009). In previous research, permethrin induced ER stress via calcium-mediated-pathway in adipocytes (Xiao et al., 2017c). However, alteration of intracellular calcium and ER stress were not correlated with permethrin-induced activation of ERK in the current study. Hepatocytes are known to have abundant ER due to their intensive protein production and protein folding capabilities as well as for anabolic and catabolic (including detoxification) metabolic functions (Lamond, 2002). Therefore, it is possible that the liver cells may be less sensitive to the induction of calcium-mediated ER stress following the loss of ER calcium caused by permethrin. In addition, compared with the immediate membrane depolarization and short-term (30 min) response of calcium flux following permethrin treatment, induction of ER stress usually take hours even with targeted pharmaceutical inducers (Oslowski and Urano, 2011). Therefore, after the longer treatment (>3 h), stabilized membrane potential and intracellular calcium levels would likely not have a significant effect on ER stress.
In other studies, pyrethroid insecticides, including permethrin and cypermethrin, have been reported to activate ERK1/2 in myotubes and macrophages (Huang et al., 2016; Sun et al., 2017b). It was also previously reported that the depolarization of nerve cells results in the activation of group I mGluRs and subsequent activation of PLC, PKC and ERK1/2 (Ohno-Shosaku et al., 2002; Thandi et al., 2002). In this conical pathway, activation of mGluR releases the beta/gamma subunit of the associated G-protein, which activates PLC to cleave phosphatidylinositol 4,5-bisphosphate into IP3 and diacylglycerol (DAG). IP3 binds to the IP3R and releases ER calcium, and in the presence of elevated calcium and DAG activates PKC, leading to ERK1/2 phosphorylation. It has been shown, however, that DAG can bind PKC directly causing ERK1/2 phosphorylation itself (Griner and Kazanietz, 2007; Huang, 1989). The current results suggested that permethrin-induced phosphorylation of ERK via PKC activation is through the DAG-activated PKC pathway, but without IP3 mediated calcium release. Nonetheless, our results cannot eliminate the possibility that calcium released via L-type calcium channel plays a role in PKC activation. Lastly, a number of other reports have suggested that the PKC-ERK1/2 pathway may contribute to the effects of insecticides on the development of metabolic diseases, such as diabetes (Haneda et al., 2001; Koya et al., 2000; Noh and King, 2007; Soetikno et al., 2011). To confirm our hypothesis that permethrin treatment-induced ERK1/2 is related to metabolic disease beyond HepG2 model used in the current study, activation of hepatic ERK1/2 in mice was evaluated by analyzing liver samples from the previous in vivo study (Xiao et al., 2018). As shown in Sup. Fig S4, male mice administrated with 50, 500, and 5000 mg/kg body weight of permethrin showed significant activation of hepatic ERK1/2 activation compared to the control. In the same study, it was reported that permethrin altered hepatic lipid metabolism and caused insulin resistance (Xiao et al., 2018).
The HepG2 human hepatoma cells provide a convenient in vitro platform to study the mechanism of how permethrin activates ERK1/2 via VSSC. By monitoring membrane potential and intracellular calcium level, we were able to investigate the response of the hepatocytes exposed to pesticides over a relatively short time period, which have not been fully studied before. The HepG2 cells were reported to display similar genotypes of normal liver cells (Thrift et al., 1986), as well as liver functions, such as lipid metabolism (Sassa et al., 1987). However, several differences are still present between HepG2 cells and normal hepatocytes, such as the activity of drug metabolism (Gerets et al., 2012). Moreover, it was reported previously that the interaction of dietary fat and permethrin treatment play critical roles in permethrin-induced metabolic disruptions (Xiao et al., 2017b; Xiao et al., 2018). Therefore, further studies are needed to strengthen the significance of the current findings including determination of the role of ERK1/2 activation in permethrin-induced metabolic disruptions.
5. Conclusions
Based on the results of this study, we found that HepG2 cells express functional VSSC that respond to permethrin. Permethrin increased the phosphorylation of ERK1/2, in part, through the mGluR-PLC-PKC pathway, independent of intracellular calcium homeostasis and ER stress. The current study suggests a role for ERK1/2 activation as a potential underlying mechanism of permethrin-induced hepatic metabolic disruptions.
Supplementary Material
Permethrin (PER) depolarized HepG2 cells via voltage-sensitive sodium channels.
PER activated extracellular signal-related kinase 1/2 (ERK1/2).
PER induced reactive oxygen species production.
PER acts via metabotropic glutamate receptors-phospholipase C-protein kinase C.
Acknowledgement
This project was in part supported by NIH R21ES023676.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References:
- Atessahin A, Yilmaz S, Karahan I, Pirincci I, Tasdemir B, 2005. The effects of vitamin E and selenium on cypermethrin-induced oxidative stress in rats. Turk J Vet Anim Sci 29, 385–391. [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD, 2000. The versatility and universality of calcium signalling. Nat Rev Mol Cell Bio 1, 11–21. [DOI] [PubMed] [Google Scholar]
- Bost F, Aouadi M, Caron L, Binetruy B, 2005. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 87, 51–56. [DOI] [PubMed] [Google Scholar]
- Cagnol S, Chambard JC, 2010. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. The FEBS Journal 277, 2–21. [DOI] [PubMed] [Google Scholar]
- Casida JE, Gammon DW, Glickman AH, Lawrence LJ, 1983. Mechanisms of selective action of pyrethroid insecticides. Annual review of pharmacology and toxicology 23, 413–438. [DOI] [PubMed] [Google Scholar]
- Chargui I, Grissa I, Bensassi F, Hrira MY, Haouem S, Haouas Z, Bencheikh H, 2012. Oxidative Stress, Biochemical and Histopathological Alterations in the Liver and Kidney of Female Rats Exposed to Low Doses of Deltamethrin (DM): A Molecular Assessment. Biomed Environ Sci 25, 672–683. [DOI] [PubMed] [Google Scholar]
- Choe ES, Wang JQ, 2001. Group I metabotropic glutamate receptor activation increases phosphorylation of cAMP response element-binding protein, Elk-1, and extracellular signal-regulated kinases in rat dorsal striatum. Mol Brain Res 94, 75–84. [DOI] [PubMed] [Google Scholar]
- Coe H, Michalak M, 2009. Calcium binding chaperones of the endoplasmic reticulum. Gen Physiol Biophys 28, F96–F103. [PubMed] [Google Scholar]
- Cox S, Niskar AS, Narayan KMV, Marcus M, 2007. Prevalence of self-reported diabetes and exposure to organochlorine pesticides among Mexican Americans: hispanic health and nutrition examination survey, 1982–1984. Environmental health perspectives 115, 1747–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerets H, Tilmant K, Gerin B, Chanteux H, Depelchin B, Dhalluin S, Atienzar F, 2012. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell biology and toxicology 28, 69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasuddin SM, Soderlund DM, 1985. Pyrethroid insecticides: potent, stereospecific enhancers of mouse brain sodium channel activation. Pesticide Biochemistry and Physiology 24, 200–206. [Google Scholar]
- Giray B, Gurbay A, Hincal F, 2001. Cypermethrin-induced oxidative stress in rat brain and liver is prevented by Vitamin E or allopurinol. Toxicology letters 118, 139–146. [DOI] [PubMed] [Google Scholar]
- Görlach A, Klappa P, Kietzmann DT, 2006. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Sign 8, 1391–1418. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Kawakami M, Matsumoto N, Okada Y, 1998. Permethrin emulsion ingestion: clinical manifestations and clearance of isomers. Journal of Toxicology: Clinical Toxicology 36, 57–61. [DOI] [PubMed] [Google Scholar]
- Griner EM, Kazanietz MG, 2007. Protein kinase C and other diacylglycerol effectors in cancer. Nature Reviews Cancer 7, 281. [DOI] [PubMed] [Google Scholar]
- Hampton RY, 2000. ER stress response: getting the UPR hand on misfolded proteins. Current Biology 10, R518–R521. [DOI] [PubMed] [Google Scholar]
- Haneda M, Koya D, Kikkawa R, 2001. Cellular mechanisms in the development and progression of diabetic nephropathy: activation of the DAG-PKC-ERK pathway. Am J Kidney Dis 38, S178–181. [DOI] [PubMed] [Google Scholar]
- Heemskerk JWM, Farndale RW, Sage SO, 1997. Effects of U73122 and U73343 on human platelet calcium signalling and protein tyrosine phosphorylation. Bba-Mol Cell Res 1355, 81–88. [DOI] [PubMed] [Google Scholar]
- Hetz C, 2012. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Bio 13, 89. [DOI] [PubMed] [Google Scholar]
- Hossain MM, Richardson JR, 2011. Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicological sciences : an official journal of the Society of Toxicology 122, 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Exton JH, 2004. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. Journal of Biological Chemistry 279, 49420–49429. [DOI] [PubMed] [Google Scholar]
- Huang F, Liu QY, Xie SJ, Xu J, Huang B, Wu YH, Xia DJ, 2016. Cypermethrin Induces Macrophages Death through Cell Cycle Arrest and Oxidative Stress-Mediated JNK/ERK Signaling Regulated Apoptosis. International Journal of Molecular Sciences 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K-P, 1989. The mechanism of protein kinase C activation. Trends in neurosciences 12, 425–432. [DOI] [PubMed] [Google Scholar]
- Jaga K, Dharmani C, 2003. Global surveillance of DDT and DDE levels in human tissues. International Journal of Occupational Medicine and Environmental Health 16, 7–20. [PubMed] [Google Scholar]
- Jiao P, Feng B, Li YJ, He Q, Xu HY, 2013. Hepatic ERK activity plays a role in energy metabolism. Mol Cell Endocrinol 375, 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan DA, Hashmi I, Mahjabeen W, Naqvi TA, 2010. Monitoring health implications of pesticide exposure in factory workers in Pakistan. Environ Monit Assess 168, 231–240. [DOI] [PubMed] [Google Scholar]
- Kim J, Park Y, Yoon KS, Clark JM, Park Y, 2013. Imidacloprid, a neonicotinoid insecticide, induces insulin resistance. J Toxicol Sci 38, 655–660. [DOI] [PubMed] [Google Scholar]
- Kim J, Park Y, Yoon KS, Clark JM, Park Y, 2014. Permethrin Alters Adipogenesis in 3T3-L1 Adipocytes and Causes Insulin Resistance in C2C12 Myotubes. Journal of Biochemical and Molecular Toxicology 28, 418–424. [DOI] [PubMed] [Google Scholar]
- Kim J, Sun QC, Yue YR, Yoon KS, Whang KY, Clark JM, Park Y, 2016. 4,4 ‘-Dichlorodiphenyltrichloroethane (DDT) and 4,4 ‘-dichlorodiphenyldichloroethylene (DDE) promote adipogenesis in 3T3-L1 adipocyte cell culture. Pesticide Biochemistry and Physiology 131, 40–45. [DOI] [PubMed] [Google Scholar]
- Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimo T, Fujino T, Yada M, Yada R, Harada N, Takayanagi R, Nakamuta M, 2007. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med 20, 351–358. [PubMed] [Google Scholar]
- Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R, 2000. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. Faseb J 14, 439–447. [DOI] [PubMed] [Google Scholar]
- Krebs J, Agellon LB, Michalak M, 2015. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem Bioph Res Co 460, 114–121. [DOI] [PubMed] [Google Scholar]
- Lamond AI, 2002. Molecular biology of the cell, 4th edition. Nature 417, 383–383. [Google Scholar]
- Lebel CP, Ischiropoulos H, Bondy SC, 1992. Evaluation of the Probe 2',7'-Dichlorofluorescin as an Indicator of Reactive Oxygen Species Formation and Oxidative Stress. Chemical research in toxicology 5, 227–231. [DOI] [PubMed] [Google Scholar]
- Locknar SA, Barstow KL, Tompkins JD, Merriam LA, Parsons RL, 2004. Calcium-induced calcium release regulates action potential generation in guinea-pig sympathetic neurones. J Physiol-London 555, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ, 2007. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Sign 9, 2277–2294. [DOI] [PubMed] [Google Scholar]
- Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L, 2011. Endoplasmic-Reticulum Calcium Depletion and Disease. Csh Perspect Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao K, Joyner WL, 1994. In-Situ Study of the Membrane-Potential in Microvascular Endothelial-Cells Using a Fluorescent-Probe. Microvasc Res 48, 135–142. [DOI] [PubMed] [Google Scholar]
- Monick MM, Carter AB, Flaherty DM, Peterson MW, Hunninghake GW, 2000. Protein kinase C zeta plays a central role in activation of the p42/44 mitogen-activated protein kinase by endotoxin in alveolar macrophages. J Immunol 165, 4632–4639. [DOI] [PubMed] [Google Scholar]
- Mosmann T, 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods 65, 55–63. [DOI] [PubMed] [Google Scholar]
- Mossa ATH, Refaie AA, Ramadan A, Bouajila J, 2013. Amelioration of Prallethrin-Induced Oxidative Stress and Hepatotoxicity in Rat by the Administration of Origanum majorana Essential Oil. BioMed research international 2013, 859085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh H, King GL, 2007. The role of protein kinase C activation in diabetic nephropathy. Kidney Int Suppl, S49–53. [DOI] [PubMed] [Google Scholar]
- Ohana L, Barchad O, Parnas I, Parnas H, 2006. The metabotropic glutamate G-protein-coupled receptors mGluR3 and mGluR1a are voltage-sensitive. Journal of Biological Chemistry 281, 24204–24215. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M, 2002. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci 15, 953–961. [DOI] [PubMed] [Google Scholar]
- Oslowski CM, Urano F, 2011. Measuring Er Stress and the Unfolded Protein Response Using Mammalian Tissue Culture System. Method Enzymol 490, 71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Kim Y, Kim J, Yoon KS, Clark J, Lee J, 2013. Imidacloprid, a neonicotinoid insecticide, potentiates adipogenesis in 3T3-L1 adipocytes. Journal of agricultural and food chemistry 61, 255–259. [DOI] [PubMed] [Google Scholar]
- Rezg R, Mornagui B, El-Fazaa S, Gharbi N, 2010. Organophosphorus pesticides as food chain contaminants and type 2 diabetes: a review. Trends in Food Science & Technology 21, 345–357. [Google Scholar]
- Roberts CK, Sindhu KK, 2009. Oxidative stress and metabolic syndrome. Life sciences 84, 705–712. [DOI] [PubMed] [Google Scholar]
- Roe M, Lemasters J, Herman B, 1990. Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium 11, 63–73. [DOI] [PubMed] [Google Scholar]
- Samtleben S, Jaepel J, Fecher C, Andreska T, Rehberg M, Blum R, 2013. Direct imaging of ER calcium with targeted-esterase induced dye loading (TED). Journal of visualized experiments: JoVE 7, e50317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S, Sugita O, Galbraith RA, Kappas A, 1987. Drug-Metabolism by the Human Hepatoma-Cell, Hep G2. Biochem Bioph Res Co 143, 52–57. [DOI] [PubMed] [Google Scholar]
- Sawatzky DA, Willoughby DA, Colville-Nash PR, Rossi AG, 2006. The involvement of the apoptosis-modulating proteins ERK 1/2, Bcl-X-L and Bax in the resolution of acute inflammation in vivo. Am J Pathol 168, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JM, Wayman GA, Nozaki N, Soderling TR, 2004. Calcium activation of ERK mediated by calmodulin kinase I. J Biol Chem 279, 24064–24072. [DOI] [PubMed] [Google Scholar]
- Shen PY, Hsieh TH, Yue YR, Sun QC, Clark JM, Park Y, 2017. Deltamethrin increases the fat accumulation in 3T3-L1 adipocytes and Caenorhabditis elegans. Food and Chemical Toxicology 101, 149–156. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, 2015. Resmethrin, the first modern pyrethroid insecticide. Pest Manag Sci 71, 801–807. [DOI] [PubMed] [Google Scholar]
- Soetikno V, Watanabe K, Sari FR, Harima M, Thandavarayan RA, Veeraveedu PT, Arozal W, Sukumaran V, Lakshmanan AP, Arumugam S, Suzuki K, 2011. Curcumin attenuates diabetic nephropathy by inhibiting PKC-alpha and PKC-beta1 activity in streptozotocin-induced type I diabetic rats. Mol Nutr Food Res 55, 1655–1665. [DOI] [PubMed] [Google Scholar]
- Son Y, Kim S, Chung HT, Pae HO, 2013. Reactive Oxygen Species in the Activation of MAP Kinases. Hydrogen Peroxide and Cell Signaling, Pt C 528, 27–48. [DOI] [PubMed] [Google Scholar]
- Sparks TC, 2013. Insecticide discovery: An evaluation and analysis. Pesticide Biochemistry and Physiology 107, 8–17. [DOI] [PubMed] [Google Scholar]
- Starling AP, Umbach DM, Kamel F, Long S, Sandler DP, Hoppin JA, 2014. Pesticide use and incident diabetes among wives of farmers in the Agricultural Health Study. Occup Environ Med 71, 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Qi W, Yang JJ, Yoon KS, Clark JM, Park Y, 2016. Fipronil promotes adipogenesis via AMPKalpha-mediated pathway in 3T3-L1 adipocytes. Food and Chemical Toxicology 92, 217–223. [DOI] [PubMed] [Google Scholar]
- Sun QC, Clark JM, Park Y, 2017a. Environmental pollutants and type 2 diabetes: a review of human studies. Toxicol Environ Chem 99, 1283–1303. [Google Scholar]
- Sun QC, Peng Y, Qi WP, Kim Y, Clark JM, Kim D, Park Y, 2017b. Permethrin decreased insulin-stimulated AKT phosphorylation dependent on extracellular signal-regulated kinase-1 (ERK), but not AMP-activated protein kinase alpha (AMPK alpha), in C2C12 myotubes. Food and Chemical Toxicology 109, 95–101. [DOI] [PubMed] [Google Scholar]
- Swaminathan K, 2013. Pesticides and human diabetes: a link worth exploring? Diabetic Med 30, 1268–1271. [DOI] [PubMed] [Google Scholar]
- Thandi S, Blank JL, Challiss RA, 2002. Group-I metabotropic glutamate receptors, mGlu1 and mGlu5a, couple to extracellular signal-regulated kinase (ERK) activation via distinct, but overlapping, signalling pathways. J Neurochem 83, 1139–1153. [DOI] [PubMed] [Google Scholar]
- Thrift RN, Forte TM, Cahoon BE, Shore VG, 1986. Characterization of Lipoproteins Produced by the Human-Liver Cell-Line, Hep-G2, under Defined Conditions. Journal of Lipid Research 27, 236–250. [PubMed] [Google Scholar]
- Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, 2009. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. The Journal of clinical investigation 119, 2925–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grandperret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J, 1991. The Bisindolylmaleimide Gf-109203x Is a Potent and Selective Inhibitor of Protein-Kinase-C. Journal of Biological Chemistry 266, 15771–15781. [PubMed] [Google Scholar]
- Tuzmen N, Candan N, Kaya E, Demiryas N, 2008. Biochemical effects of chlorpyrifos and deltamethrin on altered antioxidative defense mechanisms and lipid peroxidation in rat liver. Cell Biochem Funct 26, 119–124. [DOI] [PubMed] [Google Scholar]
- Vais H, Williamson MS, Devonshire AL, Usherwood PNR, 2001. The molecular interactions of pyrethroid insecticides with insect and mammalian sodium channels. Pest Manag Sci 57, 877–888. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Shmigol A, 1996. Calcium-induced calcium release in neurones. Cell Calcium 19, 1–14. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhu Y, Cai X, Yu J, Yang X, Cheng J, 2011. Abnormal glucose regulation in pyrethroid pesticide factory workers. Chemosphere 82, 1080–1082. [DOI] [PubMed] [Google Scholar]
- Wang X, Martinez MA, Dai MH, Chen DM, Ares I, Romero A, Castellano V, Martinez M, Rodriguez JL, Martinez-Larranaga MR, Anadon A, Yuan ZH, 2016. Permethrin-induced oxidative stress and toxicity and metabolism. A review. Environmental Research 149, 86–104. [DOI] [PubMed] [Google Scholar]
- Wu C-Y, Hsu W-L, Wang C-H, Liang J-L, Tsai M-H, Yen C-J, Li H-W, Chiu S-J, Chang C-H, Huang Y-B, 2016. A Novel Strategy for TNF-Alpha Production by 2-APB Induced Downregulated SOCE and Upregulated HSP70 in O. tsutsugamushi-Infected Human Macrophages. PloS one 11, e0159299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Clark JM, Park Y, 2017a. Potential contribution of insecticide exposure and development of obesity and type 2 diabetes. Food and Chemical Toxicology 105, 456–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Kim Y, Kim D, Yoon KS, Clark JM, Park Y, 2017b. Permethrin alters glucose metabolism in conjunction with high fat diet by potentiating insulin resistance and decreases voluntary activities in female C57BL/6J mice. Food and Chemical Toxicology 108, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Qi WP, Clark JM, Park Y, 2017c. Permethrin potentiates adipogenesis via intracellular calcium and endoplasmic reticulum stress-mediated mechanisms in 3T3-L1 adipocytes. Food and Chemical Toxicology 109, 123–129. [DOI] [PubMed] [Google Scholar]
- Xiao X, Sun Q, Kim Y, Yang SH, Qi W, Kim D, Yoon KS, Clark JM, Park Y, 2018. Exposure to permethrin promotes high fat diet-induced weight gain and insulin resistance in male C57BL/6J mice. Food and Chemical Toxicology 111, 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Qi W, Choi S, Clark JM, Park Y, 2018. Permethrin and ivermectin modulate lipid metabolism in steatosis-induced HepG2 hepatocytes 255th American chemical society national meeting. New Orleans, LA AGFD; 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Zhang W, Pendleton E, Leng S, Wu J, Chen R, Sun XJ, 2009. Improved insulin sensitivity by calorie restriction is associated with reduction of ERK and p70S6K activities in the liver of obese Zucker rats. J Endocrinol 203, 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


