Abstract
The fact that more than 78 million adults in the US are considered overweight or obese highlights the need to develop new, effective strategies to treat obesity and its associated complications, including type 2 diabetes, kidney disease and cardiovascular disease. While the neurohypophyseal peptide oxytocin (OT) is well recognized for its peripheral effects to stimulate uterine contraction during parturition and milk ejection during lactation, release of OT within the brain is implicated in prosocial behaviors and in the regulation of energy balance. Previous findings indicate that chronic administration of OT decreases food intake and weight gain or elicits weight loss in diet-induced obese (DIO) mice and rats. Furthermore, chronic systemic treatment with OT largely reproduces the effects of central administration to reduce weight gain in DIO and genetically obese rodents at doses that do not appear to result in tolerance. These findings have now been recently extended to more translational models of obesity showing that chronic subcutaneous or intranasal OT treatment is sufficient to elicit body weight loss in DIO nonhuman primates and pre-diabetic obese humans. This review assesses the potential use of OT as a therapeutic strategy for treatment of obesity in rodents, nonhuman primates, and humans, and identifies potential mechanisms that mediate this effect.
Keywords: Obesity, Food intake, Energy expenditure, Oxytocin
1. Source and functions of oxytocin
The obesity epidemic and its associated complications [1–3] have become major health concerns [4]. Health care costs to treat obesity were projected to be nearly 147 billion dollars in 2008 [5]. Obesity affects approximately 78 million adults and 12.5 million children and adolescents in the US alone [6]. These estimates are attributed, in part, to increased dietary intake of high fructose corn syrup and sugar [7–9] which is linked with the development of metabolic abnormalities characteristic of metabolic syndrome (e.g. dyslipidemia, weight gain, and visceral fat) in humans [7–12] and diet-induced obese (DIO) nonhuman primates following long-term exposure to high fructose diet [13]. The resulting defects in the secretion of [14] and/or response [15–17] to peripheral satiety signals following prolonged maintenance on high fat or high fructose diets are hypothesized as underlying factors in the increased rise of obesity. However, current weight loss therapies are largely unsuccessful and there is an increased need for more effective treatments for this growing epidemic and associated complications.
While the neurohypophyseal peptide oxytocin (OT) is well recognized for its role in lactation [18], uterine contraction [19], osmoregulation [20], and prosocial behavior [21,22], it has attracted interest as a therapeutic strategy to treat schizophrenia [21,23], autism spectrum disorder [21,22,24,25], and obesity [26–35]. Currently, almost 400 completed, ongoing, or future funded investigations in humans list OT in pre-clinical trials to reduce caloric intake, gastric emptying, and obesity in addition to other conditions (ClinicalTrials.gov registry, National Institutes of Health). Taking into consideration the increased prevalence of obesity combined with the relatively modest effects of existing weight loss therapies, this review focuses on timely findings that assess efficacy of OT treatment for decreasing food intake and body weight in diet-induced [27,28,30–32] and genetically obese rodents [28,30,36,37] as well as in DIO nonhuman primates [26] and obese humans [33,34]. We also discuss the potential mechanisms involved in mediating these effects.
OT is synthesized predominantly by neuronal cell bodies that are located in the parvocellular division of the paraventricular nucleus (PVN) as well as the magnocellular neurons of both the PVN and the supraoptic nucleus (SON) in rodents [38–42], nonhuman primates [43–46] and humans [47–51] (for review see [52,53]). OT is also expressed to a lesser degree in a few other forebrain areas (e.g. bed nucleus of the stria terminalis medial preoptic area (MPA), anterior hypothalamus, medial amygdala, and subzona incerta) [54,55]. OT is released from somatodentrites within the SON and PVN, as well as distally at synaptic terminals on axons that originate from magnocellular PVN and SON projections to the neurohypophysis, as well as parvocellular PVN projections to CNS areas which include the arcuate nucleus (ARC) [56], ventral tegmental area (VTA) [57], nucleus of the solitary tract (NTS) [38,39], and spinal cord [39]. Recent evidence suggests that magnocellular PVN and SON OT neurons also project to the ARC [56] (see Fig. 1). OT is also synthesized in the periphery [58], including the gastrointestinal (GI) tract in rodents and humans [59,60]. Previous findings reveal that the GI tract [61] and nodose ganglion [60] in rodents as well as adipocytes in both rodents and humans [62–68] express oxytocin receptors (OTRs). The potential role(s) of OTR signaling through adipocytes, the GI tract, or vagal sensory afferent nerves in the control of energy balance is discussed in Sections 7 and 8.
Fig. 1.
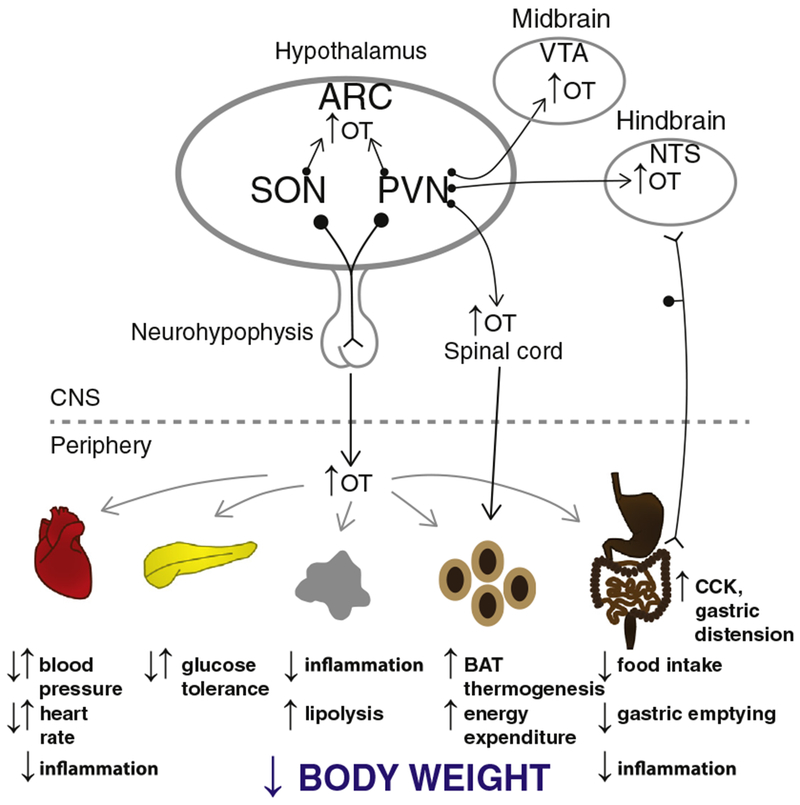
A schematic of circuitry that potentially contributes to the effectiveness of OT on energy homeostasis. OT release within the CNS, spinal cord, and from the neurohypophysis into the circulation (shown in black arrows) may impact metabolic processes (shown in gray) that result in the reduction of bodyweight. Dotted arrow represents implicated pathways from sympathetic preganglionic neurons in the spinal cord to WAT [149,150]. Reported opposing effects denoted by arrows pointed in opposite directions to one another. Abbreviation: ARC, arcuate nucleus; CCK, cholecystokinin; BAT, brown adipose tissue; NTS, nucleus of the solitary tract; PVN, paraventricular nucleus; SON, supraoptic nucleus; and VTA, ventral tegmental area. This figure has been modified from a previous manuscript [Endocrinology Coming full circle: contributions of central and peripheral oxytocin actions to energy balance 154(2):589–96, 2013; http://www.ncbi.nlm.nih.gov/pubmed/23270805].
2. OTR distribution in CNS areas linked to energy balance in rodents, nonhuman primates and humans
There is a wide distribution of OTRs in areas linked to the control of energy balance in rodents, including the basal ganglia (e.g. nucleus accumbens and central amygdala), hypothalamus [e.g. ARC, MPA, PVN, SON, and ventromedial hypothalamus (VMH)], midbrain (e.g. VTA), hindbrain [e.g. dorsal motor nucleus of the vagus (DMV), NTS or area postrema (AP)] [52,69–71], and spinal cord [72]. In contrast to rodents, there is a more restricted distribution of OTRs in areas linked to energy balance in nonhuman primates (e.g. nucleus accumbens, preoptic area, VMH, DMV, and spinal cord) [73–75] and humans (e.g. central amygdala, anterior hypothalamus, MPA, PVN, VMH, AP, NTS, and spinal cord) [76–78]. Limitations with respect to specificity of pharmacological tools and/or antibodies may preclude drawing definitive conclusions about species differences. However, the similarity in patterns of OTR expression implicates important roles of discrete nuclei within the basal ganglia, hypothalamus, hindbrain and spinal cord in regulating body weight that are well conserved across species.
3. Role of OT signaling in energy balance
Existing data indicate that OT plays a critical role in the control of energy homeostasis [26–28,30–32,34–36,56,62,79–89]. For example, global deficiencies in either OT [90] or OTRs [91] are associated with adult-onset obesity in mice. Furthermore, copy number variations linked with the OTR gene (OXTR) are associated with an extreme early-onset obesity phenotype in humans [92]. Moreover, DIO mice exhibit defects in OT release within the hypothalamic PVN [31] which may, in turn, contribute to the corresponding reductions in circulating OT displayed by these animals [31], Zucker rats [64], db/db mice [93]), as well as obese humans and individuals with type 2 diabetes [94]. However, the reductions in circulating OT are not necessarily observed across all rodent models of obesity, for reasons that are not entirely clear, as circulating levels in DIO rats and ob/ob mice are, in fact, not decreased relative to their respective lean counterparts [30,62]. In some cases, OT levels are increased in certain obese rat models [95–98] as well as in obese humans [99] but are reduced following chronic pair-feeding in rats [97] or gastric banding-associated weight loss in humans [99]. In addition, other apparent inconsistencies arise in certain pathological states of energy deficit in humans where it has been shown that nocturnal levels of OT are reduced in both anorexia nervosa [100] and amenorrhea [101]. Whether these are considered adaptive or contributing factors in the development of anorexia nervosa remains to be determined [100]. OT levels are, however, positively correlated with body weight, body fat, and resting energy expenditure in amenorrheic athletes which may implicate a potential role of OT in the regulation of energy expenditure in certain states of energy deficit [102]. However, the pathogenesis of Prader-Willi syndrome, a genetic disorder that afflicts up to 350,000–400,000 humans in the world [103] is characterized by hyperphagia and severe obesity and linked to a reduced number and size of PVN OT neurons [104], suggesting that a reduction in OT secretion within the CNS and/or periphery, in some circumstances, may be pathogenic and contribute to obesity. Existing data show that both acute administration and chronic administration of OT are sufficient to circumvent the effects of impaired or defective leptin signaling to decrease body weight gain in DIO [27,28,30–32] and genetically obese rodent models [29,30,36,62,93,105] as well as in DIO nonhuman primates [26] and obese humans [33]. Collectively, these findings suggest that OT plays a principal role in energy balance, although the mechanisms contributing to these effects have not been fully established.
4. Intact response to OT despite impaired leptin signaling
OT-elicited decreases in food intake likely contribute to its effectiveness in reducing body weight (or attenuating weight gain) in rodents and nonhuman primates [26]. The finding that OT dose-dependently reduces food intake, whether administered systemically, intranasally or directly into the central nervous system (CNS), is well documented [27–30,36,56,62,79,80,83,84,86,88,105–109]. While many such investigations have targeted the lateral, third, and fourth ventricles, the data show that OT reduces food intake following administration directly into the ARC [56], VMH [108], VTA [107] and NTS [88]. All of these sites express OTRs and many also receive OT projections from the PVN or SON (see Fig. 1). Moreover, central and/or systemic administration of OT inhibits food intake in mice and rats with impaired (e.g. DIO models) [27,28,30–32] or defective leptin signaling (e.g. obese Zucker rat [29], Koletsky rat [30], ob/ob mice [62], and db/db mice [105]), as well as in obese mice with Sim1 haploinsufficiency [36]. Furthermore, central or systemic administration of OT reduces food intake without increasing pica consumption (marker of visceral illness) or eliciting a conditioned taste aversion in mice [31,105] or rats [108]. Collectively, these findings suggest that OT signaling is potentially an attractive therapeutic target in both DIO and genetic models of obesity.
In addition to suppressing consumption of low fat/high carbohydrate diets, including standard rodent [27–32,36,56,62,79,80,83,84,88,106,109,110] and monkey chow [26], acute or chronic central or systemic administration of OT also reduces consumption of sucrose [107], fructose-sweetened beverages [26], intraoral glucose [86], as well as high fat diets (HFDs) in mice, rats, or nonhuman primates [27,28,30–32]. These findings may also translate to humans as intranasal OT appears to reduce consumption of fat in a limited number of subjects although statistical significance was lost after adjusting for multiple comparisons [34]. It is worth noting that the HFDs used in some of these studies (D12331, D12451 or D12492; Research Diets Inc., New Brunswick, NJ or HFD32; CLEA Japan, Inc., Tokyo, Japan) contained between 6.7 and 17% kcal from sucrose [27–28,30–32], which may have contributed to the effectiveness of OT to reduce food consumption on the HFDs. Conflicting with previous pharmacological studies, however, is a report that incorporated the Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) technology in which Cre recombinase-dependent adeno-associated virus (AAV)-hM3Dq was administered bilaterally into the PVN of mice. These investigators administered clozapine-N-oxide (CNO) to activate PVN OT neurons in oxytocin-Ires-Cre mice [89], but this intervention failed to result in decreased consumption of chow relative to vehicle treatment. These findings raise important questions as to whether a) CNO-elicited activation of PVN OT neurons results in an anorexigenic response when administered during the light cycle at a time that coincides with the diurnal rhythmicity of circulating [32] or cerebrospinal fluid levels of OT [111] and b) the response varies with respect to energy status (fasted vs. ad libitum fed), or diet (chow, high fat or high fat/high sucrose diet).
While PVN OT neurons are downstream targets of melanocortin 4 receptor (MC4R) signaling in rats [110,112] and mice [113], the extent to which MC4R signaling directly impacts PVN OT neurons controlling food intake is unclear. Optogenetic stimulation of PVN OT neurons suppresses the feeding response evoked by photostimulation of orexigenic agouti-related protein (AGRP) neurons, but photostimulation of PVN OT neurons alone fails to inhibit refeeding following a 24-h fast in mice [114]. While it will be important to determine if optogenetic activation of PVN OT neurons is sufficient to elicit release of OT from long axonal projections to the hindbrain [115,116], these findings are, however, consistent with what appears to be a relatively minor role of PVN OT neurons to suppress the refeeding response following a prolonged fast in rats [117]. Shah and colleagues demonstrated that reexpression of MC4Rs in PVN OT neurons in oxytocin-Ires-Cre;Mc4RloxTB/loxTB mice failed to attenuate the obesity phenotype in MC4R null MC4RloxTB/loxTB mice [118]. These findings suggest that direct MC4R signaling through PVN OT neurons may not be required to regulate body weight in mice. Alternatively, MC4R-expressing interneurons that in turn project to PVN OT neurons may be more important in regulating body weight in mice.
Impairments of OT signaling have been associated with increased consumption of carbohydrate-rich chow [31,32,36,79,80,82,106,119,120], sucrose [107,121–124], fructose [122], and glucose [86,122], as well as fat [31,32] in mice or rats, implicating a physiological role of endogenous OT to reduce intake of fat and sugar. While consistent with studies showing that ingestion of sucrose and intralipid stimulates Fos (marker of neuronal activation) in PVN OT neurons [123], disruptions in OT signaling, however, do not consistently lead to increased consumption of chow, sucrose, and HFDs. For example, OTR null mice and mice without OTRs in specific forebrain areas (e.g. lateral septum, ventral pallidum, and hippocampus) do not consume more sucrose relative to wild-type mice [125]. However, since the extent to which OTRs were deleted from CNS areas linked to the control of body weight (e.g. hypothalamus) was unknown, it is not clear whether compensatory up-regulation of OTRs in extra-hypothalamic areas such as the hindbrain and periphery may have contributed to these effects. It is also possible that these effects may be attributed to up-regulation of the structurally similar peptide, vasopressin, or vasopressin 1a receptor, both of which are linked to the control of body weight [126–129]. Furthermore, OTR and OT null mice fail to consume more chow relative to wild-type or littermate control mice [90,91,121]. Studies indicate that OTR null mice still consume larger meals during the dark cycle [130] despite showing no change in overall chow intake. In addition, removal of approximately 95% of PVN OT neurons following Cre-mediated diphtheria toxin administration in adult oxytocin-Ires-Cre mice failed to impact intake of chow or HFDs [87]. However, caution should be exercised when interpreting these latter findings as they could have been influenced by loss of other neurotransmitters or neuromodulators expressed by PVN OT neurons. Additional work needs to address the potential influence of gliosis in OT projection sites as contributing to these effects [131]. Moreover, variable experimental outcomes on food intake maybe related to different genetic strain backgrounds [123] or site specific developmental changes in OT [132] or OTR expression [52,132,133] that may be associated with length of diet exposure [68] or age [52,68,132,133] in these mice at the time when potential impairment in PVN OT signaling was initiated.
5. Effects of OT on energy expenditure
In addition to suppressing food intake, OT may also elicit weight loss in rodents and nonhuman primates by stimulating energy expenditure and lipolysis. Indirect evidence from pair-feeding studies in mice and rats, where the amount of weight loss following OT exceeds that of pair-fed control animals [27,30,62], implicates increased energy expenditure and possibly lipolysis as contributing to negative energy balance and weight loss (see Fig. 1).
Decreases in OT signaling have been shown to be associated with obesity and reductions in energy expenditure [32,87,90,91,134], including defects in sympathetic nervous system activity, brown adipose tissue (BAT) thermogenesis [90,91,134], and oxygen consumption [32,87], in the absence of hyperphagia in mice [87,90,91,121,130]. In contrast, direct injections of OT into the CNS increased energy expenditure [31,32,108], body temperature [71], and heart rate [71,135] in mice or rats. In addition, chronic subcutaneous administration of OT increased energy expenditure in DIO nonhuman primates [26] but failed to stimulate energy expenditure when given acutely by intranasal administration into humans [34,35]. It may be that, in humans, acute administration of OT impacts food intake at doses subthreshold for eliciting changes in energy expenditure. Future studies should determine whether chronic intranasal administration of OT recapitulates the effects of chronic subcutaneous administration to stimulate energy expenditure in DIO nonhuman primates [26] and whether these effects translate to obese humans. Sutton and colleagues demonstrated that acute CNO-induced activation of PVN OT neurons in oxytocin-Ires-Cre mice increased Fos in thoracic sympathetic preganglionic neurons in the spinal cord [89]. OT also activated thoracic sympathetic preganglionic neurons in the cat [136] as well as the stellate ganglia in the dog [137]. The well-defined polysynaptic projections from the OT neurons in the PVN to interscapular BAT (IBAT) [138] and stellate ganglia [139] as well as both polysynaptic [138] and monosynaptic projections to spinal cord [39] in rats, strongly suggest the possibility that OT may have a physiological role in regulating sympathetic nervous system activity. In addition to a role of PVN OT neurons, it is likely that OT neurons located outside the PVN are important in stimulating BAT. OT is also expressed in the subzona incerta in Siberian hamsters [55], a CNS region with robust sympathetic innervation of IBAT [140]. It will be exciting to determine the extent to which stimulation of subzona incerta OT neurons increases BAT thermogenesis in a mouse model.
Thus, the ability of OT to stimulate energy expenditure appears to contribute to its effectiveness in eliciting weight loss and reductions in weight gain, although the location of OTR populations that mediate these effects remains to be determined. Acute CNO-elicited activation of PVN OT neurons in oxytocin-Ires-Cre mice increased energy expenditure as well as activity and also tended to increase IBAT temperature (P = 0.13) relative to vehicle [89], which may implicate OTR populations in the PVN, VTA, NTS, or spinal cord. OT injections into the median raphe increase both heart rate and body temperature [71] supporting a role of OTRs in this region in the regulation of energy expenditure. In addition, direct VMH injections of OT also stimulate energy expenditure in rats [108]. In addition, adeno-associated viral expression of OTRs into the VMH/dorsomedial hypothalamus (DMH) of OTR null mice corrected defects in β3- and α2-adrenceptor mRNA expression in IBAT and rescued deficits in cold-induced thermogenesis [134], providing additional evidence that OTRs in the DMH/VMH are associated with the regulation of energy expenditure. In addition to potential functions in either the VMH or DMH, OT may also stimulate sympathetic nervous system activity via polysynaptic projections from OTR-expressing premotor NTS neurons to IBAT [39,138,139]. It will be important to address the extent to which ablation of OTR populations in the hypothalamus, midbrain, hindbrain, and spinal cord reduces energy expenditure and predisposes animals to diet-induced obesity.
Several reports have shown that persistent reductions in food intake and body weight result in reductions in energy expenditure in both animal models [141] and humans [142–144]. However, despite the persistent weight loss and reductions in food intake, chronic CNS [27] or systemic OT treatment [28,30] is not associated with a reduction in energy expenditure in DIO mice or rats, observations that we have confirmed. While a control group that lost a comparable amount of body weight to that of an OT-treated group was not examined in these studies in mice or rats [27,28,30], these findings raise the possibility that, in rodent models, sustained OT treatment may also prevent the reduction in energy expenditure associated with weight loss.
Existing data support a potential role of OT to stimulate the transformation of white adipose tissue (WAT) to the more metabolically active “brite” or “beige” adipose tissue, a process termed “browning” [145]. For example, chronic subcutaneous administration of OT appeared to increase the number of uncoupling protein-1 (+) cells in the subcutaneous and visceral fat of obese db/db mice [93]. However, the data were not quantified in this study and it is not clear to what extent these findings may translate to rodent models of DIO. In addition, Lawson and colleagues demonstrated that OT secretion in amenorrheic athletes is positively correlated with resting energy expenditure as well as fibroblast growth-factor 21 (FGF-21) and irisin [102], two factors that induce the conversion of WAT to the “brite” or “beige” adipose tissue [146–148]. OT could potentially elicit “browning” through a direct effect on adipocytes [65–67], where OTRs are expressed in both inguinal [63] and epididymal fat [62–65] as well as indirectly through outgoing polysynaptic sympathetic nervous system projections from the PVN to inguinal [149] and epididymal fat [149,150]. These findings support an important role for OT in regulating energy expenditure and raise the question as to what extent weight loss attributed to chronic increases in CNS OT is associated with “browning” of WAT.
6. Gender specific effects of OT on regulation of body weight?
The majority of published data has focused on the role of OT on energy homeostasis in male animal models. Thus, it is not clear if OT has gender specific effects on the regulation of body weight. OT reduces food intake and weight gain in obese female Sim1 haploinsufficient mice [36] and elicits weight loss in obese men and women [33]. There are also mixed results from the limited number of studies that have examined the impact of global loss of either OT or OTRs on body weight. Both male and female OT null mice develop obesity to a similar extent [90] whereas only male but not female OTR null mice develop obesity [91]. Similarly, selective ablation of PVN OT neurons results in predisposition to HFD-elicited obesity in male but not female mice [87]. Thus far, existing data from genetic loss of function studies have done little to clarify potential gender specific effects of OT on regulation of energy homeostasis. If OT is to be considered for anti-obesity therapy, future studies should address the gender specific effects of chronic OT on weight loss in male and female animal models, including nonhuman primates and humans.
7. Effects of OT on lipolysis and impact on fat mass
OT increases free fatty acids and glycerol and/or reduces triglycerides in cultured 3T3-L1 adipocytes [27,68], rats [27] and DIO nonhuman primates [26]. These findings suggest that OT may reduce body weight by increasing lipolysis, perhaps through a direct action on adipocytes [65–67], which express OTRs [62–68], or from an indirect action via outgoing polysynaptic sympathetic nervous system circuits from the PVN to both inguinal WAT [149] and epididymal WAT [149,150]. OT also increases epididymal WAT expression of hormone sensitive lipase (an enzyme linked with lipolysis) [27,62] and reduces expression of fatty acid synthase (an enzyme linked to lipogenesis) in rats [62], the latter suggesting that OT may also inhibit lipogenesis. Triglycerides also appeared to be lower following chronic intranasal OT in a limited number of pre-diabetic obese humans but these effects were not statistically significant [33]. Furthermore, chronic OT central or systemic infusions in vivo result in decreases in fat mass in mice and rats [27,28,30,32,62,93], including the adipocyte area from mesenteric [28], epididymal [28,62,151], subcutaneous [93], perirenal [93] and epicardiac [93] fat depots. Importantly, chronic central or systemic administration of OT reduced fat mass in lean or DIO rats [27,30], DIO C57Bl/6 mice [32], db/db mice [93] and ob/ob mice [62] without reducing lean mass [27,32,62,93]. In contrast, one study reported that chronic systemic OT reduced lean mass in lean C57Bl6/J mice, although this was unexpected [62] and the mechanisms underlying these effects are unclear. Chronic central or systemic OT infusions are also associated with reductions in the respiratory quotient in DIO mice [28] and rats [27] relative to either vehicle treatment [27,28] or pair-fed control animals [27]. Recent findings indicate that intranasal OT also reduces respiratory quotient in lean and obese men [34]. Overall, these data suggest that OT treatment is also associated with increased lipolysis as well as lipid utilization or oxidation.
Consistent with a potential physiological role of OT to stimulate lipolysis, in vivo data from OT or OTR null mice report increases of abdominal fat deposition [90,91] and increases of perirenal, mesenteric, and epididymal fat depot weights in relation to wild-type littermate control mice [91]. Furthermore, selective lentiviral reduction of PVN OT mRNA [32] or ablation of PVN or SON OT neurons in mice [87] is associated with increases of body fat while not impacting lean mass. Interestingly, mice exposed to HFDs for 7 weeks [68], ob/ob mice [62] and obese Zucker rats [63,64] also showed increased OTR mRNA expression in epididymal [62–64,68], mesenteric [68], perirenal [68], or subcutaneous WAT [68], raising the possibility that reductions in endogenous OT signaling in WAT may impact lipolysis and fat mass in certain rodent models of obesity. These findings unveil potential mechanisms whereby OT could decrease body adiposity via separate or combined effects of attenuation of food intake, and stimulation of both lipolysis and energy expenditure.
8. Potential role of peripheral OTRs in the nodose ganglia or GI tract?
In addition to the presence of OTRs in adipocytes, the existence of OTRs elsewhere in the periphery, specifically the GI tract [59–61,152–154], the enteric nervous system [60,152], smooth muscle cells [61,152] and the vagus nerve [60], raises the possibility that circulating OT, in addition to actions at central OTRs [106,155,156], may also act in the periphery to reduce food intake. Systemic treatment with OTR antagonists that penetrate the blood brain barrier (BBB) increases food intake in mice and rats [32,123], effects that could possibly be mediated through both central and peripheral OTRs. Studies indicate that systemic administration of the non-penetrant antagonist L-371,257 stimulates food intake and weight gain in rats [106]. Furthermore, systemic administration of OT in mice reduces food intake and elicits Fos in the hindbrain, in part, through activation of vagal afferent nerves [105]. These findings are consistent with a potential physiological role of peripheral OTRs in the regulation of food intake and body weight.
The peripheral effects of circulating OT to reduce food intake may be attributed to a direct action of circulating OT to suppress gastric emptying. These data are mixed, however, as peripheral treatment with OT has been reported to have no effect on gastric emptying rate in humans [157] and rats [158] or a stimulatory effect on gastric motility in rabbits, possibly due to species differences [159]. However, systemic OT reduces gastric emptying in both mice [160] and rats [153,154]. Furthermore, these effects are also attenuated by pretreatment with an OTR antagonist, Atosiban [153,154], suggesting that these effects are mediated by OTRs.
The extent to which circulating OT enters the CNS is still controversial [106,155,156,161–163]. Flanagan, Rogers, and colleagues have shown that central administration of OT inhibits gastric motility [164,165] and these effects appear to be mediated by OTRs in the dorsal vagal complex [165]. While circulating OT may inhibit gastric motility through a central mechanism these effects may also be mediated through stimulation of OTRs that are expressed in the enteric nervous system [60,152], smooth muscle cells [61,152] or nodose ganglion [60].
OT may suppress gastric emptying through an indirect effect mediated through the release of the meal-related satiety peptide, cholecystokinin (CCK-8), and CCK-elicited activation stimulates the release of CCK-8 [153,154], both of which occur within the time period whereby peripheral administration of OT reduces food intake [106]. Furthermore, these effects of systemic OT to suppress gastric emptying are blocked by the CCK1 receptor antagonist, devazepide [153,154]. Iwasaki and colleagues demonstrated that both systemic OT and CCK-8 activate single vagal afferent neurons [105] raising the possibility of both a direct as well as an indirect action mediated through the release of CCK-8 [153]. Determining the impact of peripheral administration of OT to suppress feeding in rats with gastric cannulas that remain open (sham feeding; no gastric distension) or closed (real feeding) would help identify whether decreases in gastric emptying are required to elicit the anorexigenic response to OT.
Another potential mechanism whereby systemic OT may inhibit food intake is through the suppression of ghrelin. Previous findings indicate that systemic OT reduces circulating levels of ghrelin in men [166] within a time frame that coincides with the hypophagic effects of OT on rodents [106]. In contrast, intranasal administration of OT, at a dose that reduced total caloric intake [34] and cookie consumption [35] and increased circulating levels of OT in other studies [167,168], failed to reduce plasma ghrelin [34,35], suggesting that this mechanism is not likely to contribute to the anorexigenic response to circulating OT.
9. Does cholesterol contribute to OT’s effects in the setting of HFDs?
Existing studies indicate that OT treatment appears to be more effective at reducing food intake [28,30] and weight gain [27,30,31] in DIO mice and rats maintained on the HFDs relative to low fat diets, including chow. Similarly, impairments of OT signaling result in increased weight gain and decreased energy expenditure in mice fed a HFD but not in chow-fed control mice [87]. This raises an intriguing question of whether higher levels of cholesterol, or sucrose (as previously discussed), may explain the more pronounced effects seen in animals maintained on the HFDs. While the amount of cholesterol in the HFDs used in many existing studies is variable, in some studies it was higher than levels found in rodent chow (« 60–200 mg/kg) and thus may have contributed to increased effectiveness of OT reported in this model.
OTRs are observed in two affinity states (both high and low), and cholesterol is required for high-affinity OT binding [52]. The presence of cholesterol in media that contain solubilized OTRs can potentially enhance high-affinity OT binding and stabilize the OTR in a high-affinity state [93,169]. The potential role of cholesterol levels is a possible complicating factor in interpreting the results of studies on the impact of OT on HFD-fed animals. Additional studies to examine the contributions of cholesterol to OTR binding in HFD-fed DIO animals relative to chow-fed control animals will be helpful in addressing the extent to which increased OTR binding may contribute to the enhanced sensitivity to OT.
10. What contributes to OT’s prolonged effects to reduce body weight gain?
Chronic administration of OT into either the CNS or periphery over periods between 7 and 84 days is sufficient to decrease either body weight or body weight gain in DIO mice and rats [27,28,30–32] and genetically obese mice [62,93]. Interestingly, these effects of OT are maintained despite reports of reduced OTR binding in many areas of the CNS following chronic CNS infusions [170,171]. Moreover, in translational studies in DIO nonhuman primates, body weight loss was found to be less than that of vehicle-treated animals out to 7 weeks during the washout period. In addition, these animals did not begin to regain significant body weight until week 3 of the washout period. These sustained carryover effects appear to be mediated by decreases in food intake that continued for the initial 2 weeks into the washout phase [26] and are similar to other findings in obese nonhuman primates that received fibroblast growth factor-21 [172,173], adipotide [174], or a melanocortin 4 agonist, BIM-22493 [175]. Moreover, Maejima and colleagues showed that reductions in body weight gain persisted for 9 days following termination of OT treatment in DIO mice [28]. Furthermore, the effects of OT on exploratory and anti-aggressive behavior continued for at least 1 week following the end of treatment in male rats [176]. Similarly, the effects of chronic intracerebroventricular (ICV) or subcutaneous administration of OT to reduce blood pressure persisted for nearly 10 days after OT treatment ended in male and female rats [177]. Acute systemic administration of OT is also capable of activating PVN OT neurons [178,179] and stimulating the release of OT in the PVN in a rodent model [32]. These self-stimulatory properties [27] in addition to its prolonged bioavailability of OT in the CNS are thought to contribute to its favorable effects on prosocial behavior [180] and may also explain its sustained effects on body weight loss following stoppage of treatment [28].
The extent to which chronic CNS OT suppresses key orexigenic and energy conservation mechanisms in the CNS to restore energy reserves following weight loss and leptin deficiency has not been examined. In addition, the degree to which any OT-elicited reductions in orexigenic gene expression persist following cessation of treatment is also unclear. In lean and DIO animals, chronic food restriction increases gene expression of orexigenic peptides neuropeptide Y (NPY) [171,181–183] and AGRP [183] in the ARC and decreases gene expression of the anorexigenic peptide precursor proopiomelanocortin (POMC) in the ARC [171,182]. However, no changes in mediobasal hypothalamic expression of NPY, AGRP or POMC were observed in wild-type or ob/ob mice following 14-day subcutaneous infusions of OT [62]. Future studies should include gene profiling and RNA sequencing at the beginning and end of the washout period in the mediobasal hypothalamus. In addition, it would be informative to determine the impact of chronic OT signaling on the expression of downstream targets in the hindbrain (e.g. glucagon-like peptide-1 [109], POMC [29], and catecholamines [106]) and other CNS areas linked to energy homeostasis.
11. Does OT reduce inflammation in animal models of obesity or hyperlipidemia?
It is well established that obesity is associated with macrophage infiltration (reviewed in [184]) and increased inflammation in the vasculature as well as peripheral tissues, including liver, muscle and fat [185]. Previous findings indicate that inflammatory responses within the mediobasal hypothalamus, particularly in ARC POMC neurons [186], occur much sooner than in peripheral tissues and precede the majority of weight gain during the course of diet-induced obesity [185,186]. Emerging evidence suggests that systemic OT has anti-inflammatory properties in peripheral tissues across a variety of obese animal models. For example, chronic systemic OT reduces macrophage infiltration in epididymal WAT of ob/ob mice without impacting macrophage infiltration in wild-type mice [62]. In addition, chronic systemic OT also reduces the expression of pro-inflammatory markers, such as NF-κB, interleukin 1 beta (IL1-β), and interleukin 6 (IL-6) from heart tissue of db/db mice [93] as well as in rats following myocardial infarction [170]. Interestingly, chronic systemic OT also reduces IL-6 secretion from epididymal fat in hyperlipidemic apolipoprotein E (ApoE) deficient mice [187] and C-reactive protein in Watanabe Heritable Hyperlipidemic rabbits [188]. OTR deficient mice also display increased levels of IL1-β, IL-6 and tumor necrosis factor alpha (TNF-α) mRNA expression in the colons following experimental colitis, findings that suggest a possible protective anti-inflammatory effect [160]. These findings support an anti-inflammatory role of OT in peripheral tissues in genetically obese and hyperlipidemic animals.
Despite these existing data it is still unclear if OT has similar anti-inflammatory properties in the brain as observed in peripheral tissues. Future research needs to address the questions of whether increased OT signaling is sufficient to prevent the mediobasal hypothalamic inflammatory responses that occur before notable weight gain in animals maintained on a HFD [186], and also whether this aids in the prevention of diet-induced obesity. Maejima and colleagues identified an OT projection from the PVN to the ARC in mice [56] (see Fig. 1) as well as the existence of OTRs in POMC neurons in the ARC of a reporter knock-in mouse line (OxtrVenusΔNeo/+) where the OTR gene was replaced with Venus cDNA (an enhanced yellow fluorescent protein) [71]. Moreover, OTRs are also expressed in hypothalamic astrocytes [189] and glial cells in the subfornical organ, cerebellar cortex, and organum vasculosum of the lamina terminalis [71], although it is uncertain if this is true in the ARC. In addition, both central administration and systemic administration of OT also induce Fos within the ARC in mice [28,56] where OT also acts to reduce chow intake [56]. These effects may also be mediated through a direct action of OT on POMC neurons in mice [56]. Together, these findings raise questions as to whether a) impairments in OT release and in the vicinity of ARC astrocytes or POMC neurons that express OTRs may contribute to the pro-inflammatory responses following consumption of HFDs and b) increased OT signaling within ARC astrocytes or POMC neurons may prevent or ameliorate the anti-inflammatory effects associated with exposure to HFDs.
12. Translational potential
One of the key challenges with regard to OT’s use as a therapeutic strategy to effectively treat obesity and take advantage of actions at both central and peripheral OTRs (as discussed in Sections 6 and 7) will be how to circumvent its relatively short half-life in the circulation (T1/2 = 6 min; [190]) relative to the CNS (T1/2 = 19 min; [161]). Some studies have aimed at modifying its activity and plasma stability through refinements in the disulfide bond [191], which is essential for OT’s biological activity [63,192,193]. Other studies report that several longer acting OT analogues have been engineered to extend its half-life. Limited studies thus far have shown promise of longer acting OT analogues in the regulation of body weight. One such example is carbetocin, an OT analogue which has a half-life of 17.2 min in horses [93] and 41 min in non-pregnant women [194]. Its increased stabilization is thought to be attributed to N-terminal desamination as well as replacement of the 1–6 disulfide bridge with a methylene group [195]. Altirriba and colleagues reported that carbetocin reduced body weight gain over 10 days in genetically obese ob/ob mice [62]. Zhang and colleagues also reported that twice daily 3V administration of the commercially available OT analogues, [Ser4, Ile8]-OT (isotocin) and [Asu1,6]-OT, improved glucose tolerance and reduced fasting blood insulin levels independent of changes in body weight in pre-diabetic C57B1/6J mice maintained on HFDs for 2 months as well as in mice with streptozotocin-elicited diabetes [33]. Furthermore, improvements in glucose tolerance independent of changes in body weight were also obtained following peripheral administration of [Ser4, Ile8]-OT [33]. While these findings are consistent with other reports of OT improving glucose tolerance in lean mice [83], DIO mice [28,32], DIO rats [27], and possibly normal-weight [35] and obese humans [33], recent studies, however, indicate that chronic infusions of OT actually worsen glucose tolerance in ob/ob mice [62]. Thus, additional studies are needed to examine these parameters in pre-clinical trials in obese humans.
While it is evident that improvements continue to be made in engineering OT analogues, it is unclear if multiple injections/day or injections in the early part of the light cycle to coincide with diurnal peaks of circulating OT [32] will be required to maximize the ability of OT to reduce body weight in nonhuman primates [26] or humans [33]. Future investigations should examine if intermittent or pulsatile delivery [83,196–201] of OT will extend its effectiveness and circumvent reductions in OTR binding known to occur in the CNS following chronic CNS infusions [170,171]. In addition, studies should examine if inhibition of oxytocinase activity[64], PEGylation [202–204] or conjugation to albumin [205] in combination with oral delivery [206] will produce prolonged effects and eliminate the need for multiple injections.
Intranasal delivery enables relatively rapid uptake into the CSF of several neuropeptides, including the melanocyte-stimulating hormone/adrenocorticotrophic hormone (4–10), vasopressin, and insulin within 30 min in humans [207]. Intranasal delivery appears to effectively enable OT to enter the CNS in mice, rats, nonhuman primates and humans within 30–35 min post-treatment [162,208,209]. However, the extent to which this effect is due to elevations in exogenous or endogenous OT is being debated [162,210]. OT is one of the few hormones that is capable of stimulating its own release through magnocellular SON [211,212] and PVN [180] OT autoreceptors following a central or systemic treatment. Systemic OT is capable of activating vagal afferents [105] where OTRs are expressed [60], inducing Fos within PVN OT neurons [179,213] and eliciting release of OT within the CNS [32] and likely back into the peripheral circulation. In addition, central infusions of OT up-regulate hypothalamic OT mRNA and increase circulating OT levels [27]. Given that circulating levels of OT are elevated by 40 or 60 min following intranasal administration in nonhuman primates [214] and humans [167,168,215] it is possible that intranasal OT is entering the circulation directly or indirectly following the release of endogenous OT into the CNS and peripheral circulation. Future studies that examine CNS levels of radiolabelled OT following intranasal administration into OT deficient mice with intact or severed vagal afferent signaling to the hindbrain will be helpful in addressing the extent to which OT enters the CNS without impacting release of endogenous OT within the CNS.
Agents that stimulate the sympathetic nervous system, may adversely impact cardiac function in rodents [216,217] and in humans [218], which is of particular concern for the large proportion of overweight and obese individuals with hypertension [219]. Current data, however, suggest that the intranasal route of administration is not associated with unwanted side effects [220], including adverse effects on blood pressure or heart rate relative to vehicle treatment in rats [210], healthy adults [34,167,215] and pre-diabetic obese men and women [33]. In addition, chronic subcutaneous infusions of OT failed to impact systolic blood pressure [28] or heart rate [28,93] in DIO [28], db/db [28,93] or hyperlipidemic ApoE deficient mice [187]. In rats, chronic subcutaneous or ICV injections reduced blood pressure without impacting heart rate [177]. In contrast, acute administration of OT into the CNS or periphery is associated with increases, decreases, or no changes in heart rate [71,210,221,222], blood pressure [177,210,223], and body temperature [71,221] in mice or rats (for review see [224,225]). Preliminary data in our laboratory indicate that subcutaneous administration of OT does not significantly alter heart rate, blood pressure or body temperature at 90-min post-treatment in a limited number of sedated nonhuman primates (unpublished observations). Given these variable outcomes on blood pressure and heart rate following acute vs. chronic delivery and various routes of administration of OT it will be important to monitor potential adverse cardiovascular effects throughout the course of treatment in future pre-clinical trials.
In an effort to avoid unwanted peripheral side effects, some investigations have administered OT intranasally by nebulizer to allow for more targeted entry into the CNS without increasing circulating levels [214], but the data are mixed [226]. Regardless, it is encouraging that the intranasal route of administration has yielded positive prosocial effects in nonhuman primates [208], male and female individuals with schizophrenia [227,228], and in men and women who experience social rejection [229]. Furthermore, chronic subcutaneous injections of OT at doses that reduced food intake and body weight and increased energy expenditure in DIO nonhuman primates were also not associated with nausea or diarrhea [26]. The added benefit of potentially ameliorating obesity and its related complications without eliciting adverse side effects only adds to the excitement surrounding OT as a potential therapeutic strategy in humans.
Given the demand for new successful and noninvasive therapeutic strategies to treat obesity it is somewhat perplexing why there has yet to be a single clinical study performed to systematically examine the ability of chronic OT treatment to elicit weight loss in obese humans. While one preliminary report by Zhang and colleagues found that chronic intranasal OT evoked weight loss over an 8-week period in prediabetic obese men and women [33], they did not identify whether these effects may be due to decreases in food intake as well as elevations in energy expenditure and/or lipolysis. Recent findings indicate that acute intranasal administration of OT reduced total caloric intake [34] and cookie consumption [35] in humans as well as chow intake in mice [83] suggesting that reductions in food intake may contribute to the anti-obesogenic effects following this route of administration. Recently we provided the first key evidence that chronic subcutaneous administration of OT is a potential treatment strategy to produce prolonged weight loss in DIO nonhuman primates by a mechanism of reducing energy intake and increasing both energy expenditure and lipolysis. While the percent weight loss achieved in this study was below that reported in longer term (≥ 1 year) trials in humans treated with FDA-approved drugs [e.g. Qsymia (phentermine + topiramate)], it was similar in magnitude to that achieved with either orlistat or lorcaserin [230,231]. These findings collectively provide key pre-clinical data to justify future more extensive investigations that determine the impact of chronic intranasal OT treatment on weight loss, preference towards macronutrients and suppression of CNS feeding reward circuitry in both male and female obese nonhuman primates and humans.
13. Conclusions
It is evident that impairments in OT signaling contribute to obese phenotypes potentially through increases in food intake as well as reductions in energy expenditure and possibly lipolysis. The extent to which reductions in OT signaling are essential to the etiology of altered energy homeostasis or of maintaining the obese phenotype in the case of diet-induced obesity is unclear. While there is much enthusiasm over the potential use of OT as a therapeutic strategy to treat eating disorders and obesity, we await the results of ongoing pre-clinical trials in obese humans for additional confirmation of its feasibility as a long-term weight loss strategy in the absence of adverse side effects. It remains to be determined if chronic systemic administration of OT is associated with unwanted peripheral side effects such as uterine cramping in women. However, pre-labor uterine sensitivity to OT stems from a more than 150-fold up-regulation in myometrial OTRs [232], and this increase in OTR expression appears to be primed by high estrogen levels just prior to term [233] suggesting that it is unlikely to elicit uterine cramping in non-pregnant women. One potential concern is whether chronic OT, at doses that elicit weight loss, may also interact with vasopressin 2 receptors (V2Rs) in sufficient concentrations to cause hyponatremia (serum sodium < 135 mmol/L) [234]. This complication is not likely, however, given that OT has nearly a 90-fold higher affinity for the OTRs than the V2Rs [235]. While combination therapies that include V2R antagonists are effective in correcting hyponatremia in humans [236,237], further studies will be important to determine whether hyponatremia is a potential side effect during chronic OT treatment.
It is clear that there are several means by which OT, given centrally or peripherally, can influence body weight, namely through reductions in food intake as well as increases in energy expenditure and/or lipolysis. Based on the finding that circulating OT may act through central [106] or peripheral OTRs to reduce food intake [105,106] and the observation that peripheral administration can stimulate the release of CNS OT [32], further studies are needed to address the relevance of peripheral OTR signaling in the setting of diet-induced obesity [60,105,106,153,154,160] and whether activation of CNS OT circuits is, in fact, necessary in order to maximize its therapeutic potential when given systemically. While rodent and the small number of nonhuman primate and human studies are promising thus far, OTRs may alter the function of other G protein-coupled receptors, including the beta-2 adrenergic receptor [238]. Therefore, multi-drug treatments and/or intermittent delivery [83,196–199] may need to be utilized to achieve the greatest benefits of targeting the OT signaling pathway [239]. Caution should be exercised before administering to children and young adults as one study suggested that behavioral impairments occur following chronic intranasal treatment in developing prairie voles [240]. While no major deleterious effects on social behavior were reported following chronic intranasal administration of OT (at doses being used in clinical trials) in wild-type mice and a mouse model of autism spectrum disorder [241], additional studies should address the long-term neuroendocrine and behavioral effects following multiple doses of OT in developing animals and nonhuman primates [24,240].
HIGHLIGHTS.
Release of oxytocin within the CNS is implicated in the control of energy balance.
Oxytocin elicits weight loss in obese rodents, nonhuman primates and humans.
We assess potential mechanisms that may contribute to its anti-obesity effects.
Acknowledgments
This review is based on work presented in the symposium titled, “OT in Energy Balance Control” during the XXIInd Annual Meeting of the Society for the Study of Ingestive Behavior, July 29–August 2, 2014, in Seattle, WA. This material is based upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (VA). The research in our laboratory has been supported by the California National Primate Research Center (CNPRC) Pilot Award (core grant #0D011107) and the Department of VA Merit Review Research Program. DGB is the recipient of a VA Senior Research Career Scientist award. We also acknowledge the contributions of Dr. Christian Roth and Dr. Karen Bales for their review of this manuscript. In addition, we acknowledge Zachary Roberts for his technical assistance with Fig. 1.
References
- [1].Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. , The metabolic syndrome, Endocr. Rev 29 (2008) 777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Eckel RH, Grundy SM, Zimmet PZ, The metabolic syndrome, Lancet 365 (2005) 1415–1428. [DOI] [PubMed] [Google Scholar]
- [3].Grundy SM, Metabolic syndrome pandemic, Arterioscler. Thromb. Vasc. Biol 28 (2008) 629–636. [DOI] [PubMed] [Google Scholar]
- [4].Smyth S, Heron A, Diabetes and obesity: the twin epidemics, Nat. Med 12 (2006) 75–80. [DOI] [PubMed] [Google Scholar]
- [5].Finkelstein EA, Trogdon JG, Cohen JW, Dietz W, Annual medical spending attributable to obesity: payer-and service-specific estimates, Health Aff 28 (2009) w822–w831. [DOI] [PubMed] [Google Scholar]
- [6].Ogden CL, Carroll MD, Kit BK, Flegal KM, Prevalence of obesity in the United States, 2009–2010, NCHS Data Brief (2012). [PubMed] [Google Scholar]
- [7].Bray GA, Nielsen SJ, Popkin BM, Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity, The American Journal of Clinical Nutrition 79 (2004) 537–543. [DOI] [PubMed] [Google Scholar]
- [8].Havel PJ, Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism, Nutr. Rev 63 (2005) 133–157. [DOI] [PubMed] [Google Scholar]
- [9].Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB, Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk, Circulation 121 (2010) 1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Malik VS, Schulze MB, Hu FB, Intake of sugar-sweetened beverages and weight gain: a systematic review, The American Journal of Clinical Nutrition 84 (2006) 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stanhope KL, Havel PJ, Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance, Curr. Opin. Lipidol 19 (2008) 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. , Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans, J. Clin. Invest 119 (2009) 1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, et al. , Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes, Clinical and Translational Science 4 (2011) 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, et al. , Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways, JAMA: The Journal of the American Medical Association 309 (2013) 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Covasa M, Grahn J, Ritter RC, High fat maintenance diet attenuates hindbrain neuronal response to CCK, Regul. Pept 86 (2000) 83–88. [DOI] [PubMed] [Google Scholar]
- [16].Covasa M, Ritter RC, Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin, Peptides 19 (1998) 1407–1415. [DOI] [PubMed] [Google Scholar]
- [17].Covasa M, Ritter RC, Reduced sensitivity to the satiation effect of intestinal oleate in rats adapted to high-fat diet, Am. J. Phys 277 (1999) R279–R285. [DOI] [PubMed] [Google Scholar]
- [18].Braude R, Mitchell KG, Observations on the relationship between oxytocin and adrenaline in milk ejection in the sow, The Journal of Endocrinology 8 (1952) 238–241. [DOI] [PubMed] [Google Scholar]
- [19].den Hertog CE, de Groot AN, van Dongen PW, History and use of oxytocics, Eur. J. Obstet. Gynecol. Reprod. Biol 94 (2001) 8–12. [DOI] [PubMed] [Google Scholar]
- [20].Verbalis JG, Blackburn RE, Hoffman GE, Stricker EM, Establishing behavioral and physiological functions of central oxytocin: insights from studies of oxytocin and ingestive behaviors, Adv. Exp. Med. Biol 395 (1995) 209–225. [PubMed] [Google Scholar]
- [21].Striepens N, Kendrick KM, Maier W, Hurlemann R, Prosocial effects of oxytocin and clinical evidence for its therapeutic potential, Front. Neuroendocrinol 32 (2011)426–450. [DOI] [PubMed] [Google Scholar]
- [22].Yamasue H, Yee JR, Hurlemann R, Rilling JK, Chen FS, Meyer-Lindenberg A, et al. , Integrative approaches utilizing oxytocin to enhance prosocial behavior: from animal and human social behavior to autistic social dysfunction, J Neurosci. 32 (2012) 14109–14117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Montag C, Brockmann EM, Bayerl M, Rujescu D, Muller DJ, Gallinat J, Oxytocin and oxytocin receptor gene polymorphisms and risk for schizophrenia: a case-control study, The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry (2012). [DOI] [PubMed] [Google Scholar]
- [24].Miller G, Neuroscience. The promise and perils of oxytocin, Science 339 (2013) 267–269. [DOI] [PubMed] [Google Scholar]
- [25].Young LJ, Barrett CE, Neuroscience. Can oxytocin treat autism? Science 347 (2015) 825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Blevins JE, Graham JL, Morton GJ, Bales KL, Schwartz MW, Baskin DG, et al. , Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys, Am J Physiol-Reg I (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Deblon N, Veyrat-Durebex C, Bourgoin L, Caillon A, Bussier AL, Petrosino S, et al. , Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats, PLoS One 6 (2011) e25565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T, Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass, Aging 3 (2011) 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, et al. , Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway, Cell Metab. 10 (2009) 355–365. [DOI] [PubMed] [Google Scholar]
- [30].Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, et al. , Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats, Am J Physiol-Endoc M. 302 (2012) E134–E144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, et al. , Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance, Neuron 69 (2011) 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang G, Cai D, Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment, Am J Physiol Endocrinol Metab. 301 (2011) E1004–E1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J, et al. , Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models, PLoS One 8 (2013) e61477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ, Oxytocin reduces caloric intake in men, Obesity (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, et al. , Oxytocin reduces reward-driven food intake in humans, Diabetes 62 (2013) 3418–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR, Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice, Mol. Endocrinol 22 (2008) 1723–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tung YC, Ma M, Piper S, Coll A, O’Rahilly S, Yeo GS, Novel leptin-regulated genes revealed by transcriptional profiling of the hypothalamic paraventricular nucleus, J Neurosci. 28 (2008) 12419–12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rinaman L, Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats, J. Comp. Neurol 399 (1998) 101–109. [DOI] [PubMed] [Google Scholar]
- [39].Sawchenko PE, Swanson LW, Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat, J. Comp. Neurol 205 (1982) 260–272. [DOI] [PubMed] [Google Scholar]
- [40].Swaab DF, Nijveldt F, Pool CW, Distribution of oxytocin and vasopressin in the rat supraoptic and paraventricular nucleus, The Journal of Endocrinology 67 (1975) 461–462. [DOI] [PubMed] [Google Scholar]
- [41].Swaab DF, Pool CW, Nijveldt F, Immunofluorescence of vasopressin and oxytocin in the rat hypothalamo-neurohypophypopseal system, J. Neural Transm 36 (1975) 195–215. [DOI] [PubMed] [Google Scholar]
- [42].Sawchenko PE, Swanson LW, Vale WW, Corticotropin-releasing factor: co-expression within distinct subsets of oxytocin-, vasopressin-, and neurotensin-immunoreactive neurons in the hypothalamus of the male rat, J Neurosci. 4 (1984) 1118–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kawata M, Sano Y, Immunohistochemical identification of the oxytocin and vasopressin neurons in the hypothalamus of the monkey (Macaca fuscata), Anat. Embryol 165 (1982) 151–167. [DOI] [PubMed] [Google Scholar]
- [44].Antunes JL, Zimmerman EA, The hypothalamic magnocellular system of the rhesus monkey: an immunocytochemical study, J. Comp. Neurol 181 (1978) 539–565. [DOI] [PubMed] [Google Scholar]
- [45].Ginsberg SD, Hof PR, Young WG, Morrison JH, Noradrenergic innervation of vasopressin- and oxytocin-containing neurons in the hypothalamic paraventricular nucleus of the macaque monkey: quantitative analysis using double-label immunohistochemistry and confocal laser microscopy, J. Comp. Neurol 341 (1994) 476–491. [DOI] [PubMed] [Google Scholar]
- [46].Sofroniew MV, Weindl A, Schrell U, Wetzstein R, Immunohistochemistry of vasopressin, oxytocin and neurophysin in the hypothalamus and extrahypothalamic regions of the human and primate brain, Acta Histochem. Suppl 24 (1981) 79–95. [PubMed] [Google Scholar]
- [47].Sukhov RR, Walker LC, Rance NE, Price DL, Young WS 3rd., Vasopressin and oxytocin gene expression in the human hypothalamus, J. Comp. Neurol 337 (1993) 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dierickx K, Vandesande F, Immunocytochemical localization of the vasopressinergic and the oxytocinergic neurons in the human hypothalamus, Cell Tissue Res. 184 (1977) 15–27. [DOI] [PubMed] [Google Scholar]
- [49].Paulin C, Dubois PM, Czernichow P, Dubois MP, Immunocytological evidence for oxytocin neurons in the human fetal hypothalamus, Cell Tissue Res. 188 (1978) 259–264. [DOI] [PubMed] [Google Scholar]
- [50].George JM, Immunoreactive vasopressin and oxytocin: concentration in individual human hypothalamic nuclei, Science 200 (1978) 342–343. [DOI] [PubMed] [Google Scholar]
- [51].Koutcherov Y, Mai JK, Ashwell KW, Paxinos G, Organization of the human paraventricular hypothalamic nucleus, J. Comp. Neurol 423 (2000) 299–318. [PubMed] [Google Scholar]
- [52].Gimpl G, Fahrenholz F, The oxytocin receptor system: structure, function, and regulation, Physiol. Rev 81 (2001) 629–683. [DOI] [PubMed] [Google Scholar]
- [53].Ragen BJ, Bales KL, Oxytocin and vasopressin in non-human primates, in: Choleris E, Pfaff DW, Kavaliers M (Eds.), Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior, Cambridge University Press, Cambridge, United Kingdom: 2013, pp. 288–306. [Google Scholar]
- [54].Rosen GJ, de Vries GJ, Goldman SL, Goldman BD, Forger NG, Distribution of oxytocin in the brain of a eusocial rodent, Neuroscience 155 (2008) 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vaughan CH, Shrestha YB, Bartness TJ, Characterization of a novel melanocortin receptor-containing node in the SNS outflow circuitry to brown adipose tissue involved in thermogenesis, Brain Res. 1411 (2011) 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Maejima Y, Sakuma K, Santoso P, Gantulga D, Katsurada K, Ueta Y, et al. , Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus, FEBS Lett. 588 (2014) 4404–4412. [DOI] [PubMed] [Google Scholar]
- [57].Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ, Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat, Endocrinology 151 (2010) 2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jankowski M, Hajjar F, Kawas SA, Mukaddam-Daher S, Hoffman G, McCann SM, et al. , Rat heart: a site of oxytocin production and action, Proc. Natl. Acad. Sci. U. S. A 95 (1998) 14558–14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ohlsson B, Truedsson M, Djerf P, Sundler F, Oxytocin is expressed throughout the human gastrointestinal tract, Regul. Pept 135 (2006) 7–11. [DOI] [PubMed] [Google Scholar]
- [60].Welch MG, Tamir H, Gross KJ, Chen J, Anwar M, Gershon MD, Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium, J. Comp. Neurol 512 (2009)256–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Qin J, Feng M, Wang C, Ye Y, Wang PS, Liu C, Oxytocin receptor expressed on the smooth muscle mediates the excitatory effect of oxytocin on gastric motility in rats, Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 21 (2009) 430–438. [DOI] [PubMed] [Google Scholar]
- [62].Altirriba J, Poher AL, Caillon A, Arsenijevic D, Veyrat-Durebex C, Lyautey J, et al. , Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes, Endocrinology (2014) en20141466. [DOI] [PubMed] [Google Scholar]
- [63].Gajdosechova L, Krskova K, Olszanecki R, Zorad S, Differential regulation of oxytocin receptor in various adipose tissue depots and skeletal muscle types in obese Zucker rats, Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme (2015). [DOI] [PubMed] [Google Scholar]
- [64].Gajdosechova L, Krskova K, Segarra AB, Spolcova A, Suski M, Olszanecki R, et al. , Hypooxytocinaemia in obese Zucker rats relates to oxytocin degradation in liver and adipose tissue, The Journal of Endocrinology 220 (2014) 333–343. [DOI] [PubMed] [Google Scholar]
- [65].Muchmore DB, Little SA, de Haen C, A dual mechanism of action of ocytocin in rat epididymal fat cells, The Journal of Biological Chemistry 256 (1981) 365–372. [PubMed] [Google Scholar]
- [66].Schaffler A, Binart N, Scholmerich J, Buchler C, Hypothesis paper: brain talks with fat—evidence for a hypothalamic-pituitary-adipose axis? Neuropeptides 39 (2005) 363–367. [DOI] [PubMed] [Google Scholar]
- [67].Tsuda T, Ueno Y, Yoshikawa T, Kojo H, Osawa T, Microarray profiling of gene expression in human adipocytes in response to anthocyanins, Biochem. Pharmacol 71 (2006) 1184–1197. [DOI] [PubMed] [Google Scholar]
- [68].Yi K, So K, Hata Y, Suzuki Y, Kato D, Watanabe K, et al. , The regulation of oxytocin receptor gene expression during adipogenesis, J. Neuroendocrinol (2015). [DOI] [PubMed] [Google Scholar]
- [69].Gould BR, Zingg HH, Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor–LacZ reporter mouse, Neuroscience 122 (2003) 155–167. [DOI] [PubMed] [Google Scholar]
- [70].Verbalis JG, The brain oxytocin receptor(s)? Front. Neuroendocrinol 20 (1999) 146–156. [DOI] [PubMed] [Google Scholar]
- [71].Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, et al. , Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice, J Neurosci. 29 (2009) 2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Barberis C, Jard S, Oxytocin receptors in the central nervous system. Distribution, development, and species differences, Ann. N. Y. Acad. Sci 652 (1992) 29–38. [DOI] [PubMed] [Google Scholar]
- [73].Boccia ML, Panicker AK, Pedersen C, Petrusz P, Oxytocin receptors in nonhuman primate brain visualized with monoclonal antibody, Neuroreport 12 (2001) 1723–1726. [DOI] [PubMed] [Google Scholar]
- [74].Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ, The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta), Psychoneuroendocrinology 45 (2014) 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Schorscher-Petcu A, Dupre A, Tribollet E, Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset, Neurosci. Lett 461 (2009) 217–222. [DOI] [PubMed] [Google Scholar]
- [76].Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA, Immunohistochemical localization of oxytocin receptors in human brain, Neuroscience 253 (2013) 155–164. [DOI] [PubMed] [Google Scholar]
- [77].Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss JJ, Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study, Brain Res. 500 (1989) 223–230. [DOI] [PubMed] [Google Scholar]
- [78].Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study, Brain Res. 555 (1991) 220–232. [DOI] [PubMed] [Google Scholar]
- [79].Arletti R, Benelli A, Bertolini A, Influence of oxytocin on feeding behavior in the rat, Peptides 10 (1989) 89–93. [DOI] [PubMed] [Google Scholar]
- [80].Arletti R, Benelli A, Bertolini A, Oxytocin inhibits food and fluid intake in rats, Physiol. Behav 48 (1990) 825–830. [DOI] [PubMed] [Google Scholar]
- [81].Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG, Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin, Brain Res. 993 (2003)30–41. [DOI] [PubMed] [Google Scholar]
- [82].Blevins JE, Schwartz MW, Baskin DG, Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size, Am J Physiol Regul Integr Comp Physiol. 287 (2004) R87–R96. [DOI] [PubMed] [Google Scholar]
- [83].Maejima Y, Rita RS, Santoso P, Aoyama M, Hiraoka Y, Nishimori K, et al. , Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-Fos induction in limited brain areas, Neuroendocrinology (2015). [DOI] [PubMed] [Google Scholar]
- [84].Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG, Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats, Peptides 1991 (1991) 113–118. [DOI] [PubMed] [Google Scholar]
- [85].Olson BR, Drutarosky MD, Stricker EM, Verbalis JG, Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake, Endocrinology 129 (1991) 785–791. [DOI] [PubMed] [Google Scholar]
- [86].Lokrantz CM, Uvnas-Moberg K, Kaplan JM, Effects of central oxytocin administration on intraoral intake of glucose in deprived and nondeprived rats, Physiol. Behav 62 (1997) 347–352. [DOI] [PubMed] [Google Scholar]
- [87].Wu Z, Xu Y, Zhu Y, Sutton AK, Zhao R, Lowell BB, et al. , An obligate role of oxytocin neurons in diet induced energy expenditure, PLoS One 7 (2012) e45167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ong ZY, Alhadeff AL, Grill HJ, Medial nucleus tractus solitarius oxytocin receptor signaling and food intake control: the role of gastrointestinal satiation signal processing, Am J Physiol Regul Integr Comp Physiol. (2015), 10.1152/ajpregu.00534.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sutton AK, Pei H, Burnett KH, Myers MG Jr., Rhodes CJ, Olson DP, Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus, J Neurosci. 34 (2014) 15306–15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Camerino C, Low sympathetic tone and obese phenotype in oxytocin-deficient mice, Obesity 17 (2009) 980–984. [DOI] [PubMed] [Google Scholar]
- [91].Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K, Oxytocin receptor-deficient mice developed late-onset obesity, Neuroreport 19 (2008) 951–955. [DOI] [PubMed] [Google Scholar]
- [92].Wheeler E, Huang N, Bochukova EG, Keogh JM, Lindsay S, Garg S, et al. , Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity, Nat. Genet (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Plante E, Menaouar A, Danalache BA, Yip D, Broderick TL, Chiasson J, et al. , Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic Male db/db mice, Endocrinology (2015), 10.1210/en.2014-1718. [DOI] [PubMed] [Google Scholar]
- [94].Qian W, Zhu T, Tang B, Yu S, Hu H, Sun W, et al. , Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients, J. Clin. Endocrinol. Metab 99 (2014) 4683–4689. [DOI] [PubMed] [Google Scholar]
- [95].Northway MG, Morris M, Geisinger KR, MacLean DB, Effects of a gastric implant on body weight and gastrointestinal hormones in cafeteria diet obese rats, Physiol. Behav 45 (1989) 331–335. [DOI] [PubMed] [Google Scholar]
- [96].Schroeder M, Gelber V, Moran TH, Weller A, Long-term obesity levels in female OLETF rats following time-specific post-weaning food restriction, Horm. Behav 58 (2010) 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Schroeder M, Moran TH, Weller A, Attenuation of obesity by early-life food restriction in genetically hyperphagic male OLETF rats: peripheral mechanisms, Horm. Behav 57 (2010) 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Schroeder M, Zagoory-Sharon O, Shbiro L, Marco A, Hyun J, Moran TH, et al. , Development of obesity in the Otsuka Long-Evans Tokushima Fatty rat, Am J Physiol Regul Integr Comp Physiol. 297 (2009) R1749–R1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Stock S, Granstrom L, Backman L, Matthiesen AS, Uvnas-Moberg K, Elevated plasma levels of oxytocin in obese subjects before and after gastric banding, Int. J. Obes 13 (1989) 213–222. [PubMed] [Google Scholar]
- [100].Lawson EA, Donoho DA, Blum JI, Meenaghan EM, Misra M, Herzog DB, et al. , Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass, The Journal of Clinical Psychiatry 72 (2011) 1546–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lawson EA, Ackerman KE, Estella NM, Guereca G, Pierce L, Sluss PM, et al. , Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters, European journal of endocrinology/European Federation of Endocrine Societies 168 (2013) 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lawson EA, Ackerman KE, Slattery M, Marengi DA, Clarke H, Misra M, Oxytocin secretion is related to measures of energy homeostasis in young amenorrheic athletes, J. Clin. Endocrinol. Metab 99 (2014) E881–E885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Butler MG, Prader-Willi syndrome: obesity due to genomic imprinting, Current Genomics 12 (2011) 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Swaab DF, Purba JS, Hofman MA, Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases, J. Clin. Endocrinol. Metab 80 (1995) 573–579. [DOI] [PubMed] [Google Scholar]
- [105].Iwasaki Y, Maejima Y, Suyama S, Yoshida M, Arai T, Katsurada K, et al. , Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity, Am J Physiol Regul Integr Comp Physiol. (2014), 10.1152/ajpregu.00344.2014. [DOI] [PubMed] [Google Scholar]
- [106].Ho JM, Anekonda VT, Thompson BW, Zhu M, Curry RW, Hwang BH, et al. , Hindbrain oxytocin receptors contribute to the effects of circulating oxytocin on food intake in male rats, Endocrinology (2014), 10.1210/en.2014-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mullis K, Kay K, Williams DL, Oxytocin action in the ventral tegmental area affects sucrose intake, Brain Res. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Noble EE, Billington CJ, Kotz CM, Wang C, Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure, Am J Physiol Regul Integr Comp Physiol. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Rinaman L, Rothe EE, GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats, Am J Physiol Regul Integr Comp Physiol. 283 (2002) R99–106. [DOI] [PubMed] [Google Scholar]
- [110].Yosten GL, Samson WK, The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist, Am J Physiol Regul Integr Comp Physiol. 298 (2010) R1642–R1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Perlow MJ, Reppert SM, Artman HA, Fisher DA, Self SM, Robinson AG, Oxytocin, vasopressin, and estrogen-stimulated neurophysin: daily patterns of concentration in cerebrospinal fluid, Science 216 (1982) 1416–1418. [DOI] [PubMed] [Google Scholar]
- [112].Olszewski PK, Wirth MM, Shaw TJ, Grace MK, Billington CJ, Giraudo SQ, et al. , Role of alpha-MSH in the regulation of consummatory behavior: immunohistochemical evidence, Am J Physiol Regul Integr Comp Physiol. 281 (2001) R673–R680. [DOI] [PubMed] [Google Scholar]
- [113].Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, et al. , Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter, J Neurosci. 23 (2003) 7143–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Atasoy D, Betley JN, Su HH, Sternson SM, Deconstruction of a neural circuit for hunger, Nature 488 (2012) 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Pinol RA, Bateman R, Mendelowitz D, Optogenetic approaches to characterize the long-range synaptic pathways from the hypothalamus to brain stem autonomic nuclei, J. Neurosci. Methods 210 (2012) 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Pinol RA, Jameson H, Popratiloff A, Lee NH, Mendelowitz D, Visualization of oxytocin release that mediates paired pulse facilitation in hypothalamic pathways to brainstem autonomic neurons, PLoS One 9 (2014) e112138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Singru PS, Wittmann G, Farkas E, Zseli G, Fekete C, Lechan RM, Refeeding-activated glutamatergic neurons in the hypothalamic paraventricular nucleus (PVN) mediate effects of melanocortin signaling in the nucleus tractus solitarius (NTS), Endocrinology 153 (2012) 3804–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Shah BP, Vong L, Olson DP, Koda S, Krashes MJ, Ye C, et al. , MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 13193–13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Baskin DG, Kim F, Gelling RW, Russell BJ, Schwartz MW, Morton GJ, et al. , A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain, Endocrinology 151 (2010) 4207–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Blouet C, Jo YH, Li X, Schwartz GJ, Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit, J Neurosci. 29 (2009) 8302–8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L, Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion, Am J Physiol Regul Integr Comp Physiol. 289 (2005) R1798–R1806. [DOI] [PubMed] [Google Scholar]
- [122].Herisson FM, Brooks LL, Waas JR, Levine AS, Olszewski PK, Functional relationship between oxytocin and appetite for carbohydrates versus saccharin, Neuroreport 25 (2014) 909–914. [DOI] [PubMed] [Google Scholar]
- [123].Olszewski PK, Klockars A, Olszewska AM, Fredriksson R, Schioth HB, Levine AS, Molecular, immunohistochemical, and pharmacological evidence of oxytocin’s role as inhibitor of carbohydrate but not fat intake, Endocrinology 151 (2010) 4736–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Olszewski PK, Allen K, Levine AS, Effect of oxytocin receptor blockade on appetite for sugar is modified by social context, Appetite 86 (2015) 81–87. [DOI] [PubMed] [Google Scholar]
- [125].Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS 3rd., A conditional knockout mouse line of the oxytocin receptor, Endocrinology 149 (2008) 3256–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Aoyagi T, Birumachi J, Hiroyama M, Fujiwara Y, Sanbe A, Yamauchi J, et al. , Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice, Endocrinology 148 (2007) 2075–2084. [DOI] [PubMed] [Google Scholar]
- [127].Enhorning S, Leosdottir M, Wallstrom P, Gullberg B, Berglund G, Wirfalt E, et al. , Relation between human vasopressin 1a gene variance, fat intake, and diabetes, The American Journal of Clinical Nutrition 89 (2009) 400–406. [DOI] [PubMed] [Google Scholar]
- [128].Pei H, Sutton AK, Burnett KH, Fuller PM, Olson DP, AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding, Molecular Metabolism 3 (2014) 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Langhans W, Delprete E, Scharrer E, Mechanisms of vasopressin’s anorectic effect, Physiol. Behav 49 (1991) 169–176. [DOI] [PubMed] [Google Scholar]
- [130].Yamashita M, Takayanagi Y, Yoshida M, Nishimori K, Kusama M, Onaka T, Involvement of prolactin-releasing peptide in the activation of oxytocin neurons in response to food intake, J. Neuroendocrinol 25 (2013) 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wu Q, Howell MP, Palmiter RD, Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions, J Neurosci. 28 (2008) 9218–9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Matsuzaki T, Iwasa T, Munkhzaya M, Tungalagsuvd A, Kawami T, Murakami M, et al. , Developmental changes in hypothalamic oxytocin and oxytocin receptor mRNA expression and their sensitivity to fasting in male and female rats, International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 41C (2015) 105–109. [DOI] [PubMed] [Google Scholar]
- [133].Lukas M, Bredewold R, Neumann ID, Veenema AH, Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats, Neuropharmacology 58 (2010) 78–87. [DOI] [PubMed] [Google Scholar]
- [134].Kasahara Y, Sato K, Takayanagi Y, Mizukami H, Ozawa K, Hidema S, et al. , Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice, Endocrinology 154 (2013) 4305–4315. [DOI] [PubMed] [Google Scholar]
- [135].Higa KT, Mori E, Viana FF, Morris M, Michelini LC, Baroreflex control of heart rate by oxytocin in the solitary-vagal complex, Am J Physiol Regul Integr Comp Physiol. 282 (2002) R537–R545. [DOI] [PubMed] [Google Scholar]
- [136].Backman SB, Henry JL, Effects of oxytocin and vasopressin on thoracic sympathetic preganglionic neurons in the cat, Brain Res. Bull 13 (1984) 679–684. [DOI] [PubMed] [Google Scholar]
- [137].Armour JA, Klassen GA, Oxytocin modulation of intrathoracic sympathetic ganglionic neurons regulating the canine heart, Peptides 11 (1990) 533–537. [DOI] [PubMed] [Google Scholar]
- [138].Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ, The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat, Neuroscience 110 (2002) 515–526. [DOI] [PubMed] [Google Scholar]
- [139].Jansen AS, Wessendorf MW, Loewy AD, Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion, Brain Res. 683 (1995) 1–24. [DOI] [PubMed] [Google Scholar]
- [140].Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ, Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence, Am J Physiol Regul Integr Comp Physiol. 295 (2008) R417–R428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Fosgerau K, Raun K, Nilsson C, Dahl K, Wulff BS, Novel alpha-MSH analog causes weight loss in obese rats and minipigs and improves insulin sensitivity, The Journal of Endocrinology 220 (2014) 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Rosenbaum M, Kissileff HR, Mayer LE, Hirsch J, Leibel RL, Energy intake in weight-reduced humans, Brain Res. 1350 (2010) 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL, Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones, J. Clin. Endocrinol. Metab 87 (2002) 2391–2394. [DOI] [PubMed] [Google Scholar]
- [144].Schwartz A, Doucet E, Relative changes in resting energy expenditure during weight loss: a systematic review, Obesity reviews: an official journal of the International Association for the Study of Obesity 11 (2010) 531–547. [DOI] [PubMed] [Google Scholar]
- [145].Nedergaard J, Cannon B, The browning of white adipose tissue: some burning issues, Cell Metab. 20 (2014) 396–407. [DOI] [PubMed] [Google Scholar]
- [146].Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. , FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis, Genes Dev. 26 (2012) 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. , Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human, Cell 150 (2012) 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. , A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis, Nature 481 (2012) 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Shi H, Bartness TJ, Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat, Brain Res. Bull 54 (2001) 375–385. [DOI] [PubMed] [Google Scholar]
- [150].Stanley S, Pinto S, Segal J, Perez CA, Viale A, DeFalco J, et al. , Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 7024–7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Eckertova M, Ondrejcakova M, Krskova K, Zorad S, Jezova D, Subchronic treatment of rats with oxytocin results in improved adipocyte differentiation and increased gene expression of factors involved in adipogenesis, Br. J. Pharmacol 162 (2011) 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Monstein HJ, Grahn N, Truedsson M, Ohlsson B, Oxytocin and oxytocin-receptor mRNA expression in the human gastrointestinal tract: a polymerase chain reaction study, Regul. Pept 119 (2004) 39–44. [DOI] [PubMed] [Google Scholar]
- [153].Wu CL, Doong ML, Wang PS, Involvement of cholecystokinin receptor in the inhibition of gastrointestinal motility by oxytocin in ovariectomized rats, Eur. J. Pharmacol 580 (2008) 407–415. [DOI] [PubMed] [Google Scholar]
- [154].Wu CL, Hung CR, Chang FY, Pau KY, Wang PS, Pharmacological effects of oxytocin on gastric emptying and intestinal transit of a non-nutritive liquid meal in female rats, Naunyn Schmiedeberg’s Arch. Pharmacol 367 (2003)406–413. [DOI] [PubMed] [Google Scholar]
- [155].Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, et al. , Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications, Psychopharmacology 185 (2006) 218–225. [DOI] [PubMed] [Google Scholar]
- [156].Ring RH, Schechter LE, Leonard SK, Dwyer JM, Platt BJ, Graf R, et al. , Receptor and behavioral pharmacology of WAY-267464, a non-peptide oxytocin receptor agonist, Neuropharmacology 58 (2010) 69–77. [DOI] [PubMed] [Google Scholar]
- [157].Borg J, Simren M, Ohlsson B Oxytocin reduces satiety scores without affecting the volume of nutrient intake or gastric emptying rate in healthy subjects. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 2011, 23:56–61, e5. [DOI] [PubMed] [Google Scholar]
- [158].McCann MJ, Verbalis JG, Stricker EM, LiCl and CCK inhibit gastric emptying and feeding and stimulate OT secretion in rats, Am. J. Phys 256 (1989) R463–R468. [DOI] [PubMed] [Google Scholar]
- [159].Li L, Kong X, Liu H, Liu C, Systemic oxytocin and vasopressin excite gastrointestinal motility through oxytocin receptor in rabbits, Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 19 (2007) 839–844. [DOI] [PubMed] [Google Scholar]
- [160].Welch MG, Margolis KG, Li Z, Gershon MD, Oxytocin regulates gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance in mice, Am. J. Physiol. Gastrointest. Liver Physiol 307 (2014) G848–G862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Mens WB, Witter A, van Wimersma Greidanus TB, Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF, Brain Res. 262 (1983) 143–149. [DOI] [PubMed] [Google Scholar]
- [162].Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R, Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice, Psychoneuroendocrinology 38 (2013) 1985–1993. [DOI] [PubMed] [Google Scholar]
- [163].Kendrick KM, Keverne EB, Baldwin BA, Sharman DF, Cerebrospinal fluid levels of acetylcholinesterase, monoamines and oxytocin during labour, parturition, vaginocervical stimulation, lamb separation and suckling in sheep, Neuroendocrinology 44 (1986) 149–156. [DOI] [PubMed] [Google Scholar]
- [164].Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM, Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally, Brain Res. 578 (1992) 256–260. [DOI] [PubMed] [Google Scholar]
- [165].Rogers RC, Hermann GE, Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility, Peptides 8 (1987) 505–513. [DOI] [PubMed] [Google Scholar]
- [166].Vila G, Riedl M, Resl M, van der Lely AJ, Hofland LJ, Clodi M, et al. , Systemic administration of oxytocin reduces basal and lipopolysaccharide-induced ghrelin levels in healthy men, The Journal of Endocrinology 203 (2009) 175–179. [DOI] [PubMed] [Google Scholar]
- [167].Burri A, Heinrichs M, Schedlowski M, Kruger TH, The acute effects of intranasal oxytocin administration on endocrine and sexual function in males, Psychoneuroendocrinology 33 (2008) 591–600. [DOI] [PubMed] [Google Scholar]
- [168].Striepens N, Kendrick KM, Hanking V, Landgraf R, Wullner U, Maier W, et al. , Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans, Sci. Rep 3 (2013) 3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Gimpl G, Klein U, Reilander H, Fahrenholz F, Expression of the human oxytocin receptor in baculovirus-infected insect cells: high-affinity binding is induced by a cholesterol-cyclodextrin complex, Biochemistry 34 (1995) 13794–13801. [DOI] [PubMed] [Google Scholar]
- [170].Insel TR, Winslow JT, Witt DM, Homologous regulation of brain oxytocin receptors, Endocrinology 130 (1992) 2602–2608. [DOI] [PubMed] [Google Scholar]
- [171].Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID, Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice, Psychoneuroendocrinology 42 (2014) 225–236. [DOI] [PubMed] [Google Scholar]
- [172].Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, et al. , The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21, Endocrinology 148 (2007) 774–781. [DOI] [PubMed] [Google Scholar]
- [173].Veniant MM, Komorowski R, Chen P, Stanislaus S, Winters K, Hager T, et al. , Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys, Endocrinology 153 (2012) 4192–4203. [DOI] [PubMed] [Google Scholar]
- [174].Barnhart KF, Christianson DR, Hanley PW, Driessen WH, Bernacky BJ, Baze WB, et al. , A peptidomimetic targeting white fat causes weight loss and improved insulin resistance in obese monkeys, Sci. Transl. Med 3 (108) (2011) ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, et al. , Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques, Diabetes 62 (2013) 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Calcagnoli F, Meyer N, de Boer SF, Althaus M, Koolhaas JM, Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats, Horm. Behav 65 (2014) 427–433. [DOI] [PubMed] [Google Scholar]
- [177].Petersson M, Alster P, Lundeberg T, Uvnas-Moberg K, Oxytocin causes a long-term decrease of blood pressure in female and male rats, Physiol. Behav 60 (1996) 1311–1315. [DOI] [PubMed] [Google Scholar]
- [178].Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, et al. , Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus, Addict. Biol 15 (2010) 448–463. [DOI] [PubMed] [Google Scholar]
- [179].Hicks C, Jorgensen W, Brown C, Fardell J, Koehbach J, Gruber CW, et al. , The nonpeptide oxytocin receptor agonist WAY 267,464: receptor-binding profile, prosocial effects and distribution of c-Fos expression in adolescent rats, J. Neuroendocrinol 24 (2012) 1012–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Kendrick KM, Oxytocin, motherhood and bonding, Exp. Physiol 85 (2000) Spec No: 111S–24S. [DOI] [PubMed] [Google Scholar]
- [181].Banks WA, During MJ, Niehoff ML, Brain uptake of the glucagon-like peptide-1 antagonist exendin(9–39) after intranasal administration, The Journal of Pharmacology and Experimental Therapeutics 309 (2004) 469–475. [DOI] [PubMed] [Google Scholar]
- [182].Levin BE, Dunn-Meynell AA, Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats, Am J Physiol Regul Integr Comp Physiol. 286 (2004) R771–R778. [DOI] [PubMed] [Google Scholar]
- [183].Yu Y, Deng C, Huang XF, Obese reversal by a chronic energy restricted diet leaves an increased Arc NPY/AgRP, but no alteration in POMC/CART, mRNA expression in diet-induced obese mice, Behav. Brain Res 205 (2009) 50–56. [DOI] [PubMed] [Google Scholar]
- [184].Tateya S, Kim F, Tamori Y, Recent advances in obesity-induced inflammation and insulin resistance, Front. Endocrinol 4 (2013) 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, et al. , Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance, Arterioscler. Thromb. Vasc. Biol 28 (2008) 1982–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. , Obesity is associated with hypothalamic injury in rodents and humans, J. Clin. Invest 122 (2012) 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Nation DA, Szeto A, Mendez AJ, Brooks LG, Zaias J, Herderick EE, et al. , Oxytocin attenuates atherosclerosis and adipose tissue inflammation in socially isolated ApoE−/− mice, Psychosom. Med 72 (2010) 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Szeto A, Rossetti MA, Mendez AJ, Noller CM, Herderick EE, Gonzales JA, et al. , Oxytocin administration attenuates atherosclerosis and inflammation in Watanabe Heritable Hyperlipidemic rabbits, Psychoneuroendocrinology 38 (2013) 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Parent AS, Rasier G, Matagne V, Lomniczi A, Lebrethon MC, Gerard A, et al. , Oxytocin facilitates female sexual maturation through a glia-to-neuron signaling pathway, Endocrinology 149 (2008) 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Vankrieken L, Godart A, Thomas K, Oxytocin determination by radioimmunoassay, Gynecol. Obstet. Investig 16 (1983) 180–185. [DOI] [PubMed] [Google Scholar]
- [191].Muttenthaler M, Andersson A, de Araujo AD, Dekan Z, Lewis RJ, Alewood PF, Modulating oxytocin activity and plasma stability by disulfide bond engineering, J. Med. Chem 53 (2010) 8585–8596. [DOI] [PubMed] [Google Scholar]
- [192].Stymiest JL, Mitchell BF, Wong S, Vederas JC, Synthesis of oxytocin analogues with replacement of sulfur by carbon gives potent antagonists with increased stability, The Journal of Organic Chemistry 70 (2005) 7799–7809. [DOI] [PubMed] [Google Scholar]
- [193].Wood SP, Tickle IJ, Treharne AM, Pitts JE, Mascarenhas Y, Li JY, et al. , Crystal structure analysis of deamino-oxytocin: conformational flexibility and receptor binding, Science 232 (1986) 633–636. [DOI] [PubMed] [Google Scholar]
- [194].Sweeney G, Holbrook AM, Levine M, Yip M, Alfredsson K, Cappi S, et al. , Pharmacokinetics of carbetocin, a long-acting oxytocin analogue, in non pregnant women, Curr. Ther. Res. Clin. Exper 47 (1990) 528–539. [Google Scholar]
- [195].Hunter DJ, Schulz P, Wassenaar W, Effect of carbetocin, a long-acting oxytocin analog on the postpartum uterus, Clin. Pharmacol. Ther 52 (1992) 60–67. [DOI] [PubMed] [Google Scholar]
- [196].Chelikani PK, Haver AC, Reeve JR Jr., Keire DA, Reidelberger RD, Daily, intermittent intravenous infusion of peptide YY(3–36) reduces daily food intake and adiposity in rats, Am J Physiol Regul Integr Comp Physiol. 290 (2006) R298–R305. [DOI] [PubMed] [Google Scholar]
- [197].Chelikani PK, Haver AC, Reidelberger RD, Effects of intermittent intraperitoneal infusion of salmon calcitonin on food intake and adiposity in obese rats, Am J Physiol Regul Integr Comp Physiol. 293 (2007) R1798–R1808. [DOI] [PubMed] [Google Scholar]
- [198].Contreras GA, Lee YH, Mottillo EP, Granneman JG, Inducible brown adipocytes in subcutaneous inguinal white fat: the role of continuous sympathetic stimulation, Am J Physiol Endocrinol Metab. 307 (2014) E793–E799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Henry KE, Elfers CT, Burke RM, Chepurny OG, Holz GG, Blevins JE, et al. Vitamin B conjugation of peptide-YY decreases food intake compared to native peptide-YY upon subcutaneous administration in male rats. Endocrinology. 2015: en20141825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Bray GA, Popkin BM, Dietary fat intake does affect obesity! The American Journal of Clinical Nutrition 68 (1998) 1157–1173. [DOI] [PubMed] [Google Scholar]
- [201].Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, et al. , Prosocial effects of oxytocin in two mouse models of autism spectrum disorders, Neuropharmacology 72 (2013) 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Dirkx E, van Eys GJ, Schwenk RW, Steinbusch LK, Hoebers N, Coumans WA, et al. , Protein kinase-D1 overexpression prevents lipid-induced cardiac insulin resistance, J. Mol. Cell. Cardiol 76 (2014) 208–217. [DOI] [PubMed] [Google Scholar]
- [203].Gault VA, Bhat VK Irwin N Flatt PR, A novel glucagon-like peptide-1 (GLP-1)/glucagon hybrid peptide with triple-acting agonist activity at glucose-dependent insulinotropic polypeptide, GLP-1, and glucagon receptors and therapeutic potential in high fat-fed mice, The Journal of Biological Chemistry 288 (2013) 35581–35591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Irwin N, Frizelle P, O’Harte FP, Flatt PR, (pGlu-Gln)-CCK-8[mPEG]: a novel, long-acting, mini-PEGylated cholecystokinin (CCK) agonist that improves metabolic status in dietary-induced diabetes, Biochim. Biophys. Acta 1830 (2013) 4009–4016. [DOI] [PubMed] [Google Scholar]
- [205].Kriegeskorte A, Block D, Drescher M, Windmuller N, Mellmann A, Baum C, et al. , Inactivation of thyA in Staphylococcus aureus attenuates virulence and has a strong impact on metabolism and virulence gene expression, MBio. 5 (2014) e01447–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].van Blitterswijk M, Mullen B, Wojtas A, Heckman MG, Diehl NN, Baker MC, et al. , Genetic modifiers in carriers of repeat expansions in the C9ORF72 gene, Mol. Neurodegener 9 (2014) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL, Sniffing neuropeptides: a transnasal approach to the human brain, Nat. Neurosci 5 (2002) 514–516. [DOI] [PubMed] [Google Scholar]
- [208].Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML, Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatto), Proc. Natl. Acad. Sci. U. S. A 109 (2012) 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Gossen A, Hahn A, Westphal L, Prinz S, Schultz RT, Grunder G, et al. , Oxytocin plasma concentrations after single intranasal oxytocin administration — a study in healthy men, Neuropeptides 46 (2012) 211–215. [DOI] [PubMed] [Google Scholar]
- [210].Ludwig M, Tobin VA, Callahan MF, Papadaki E, Becker A, Engelmann M, et al. , Intranasal application of vasopressin fails to elicit changes in brain immediate early gene expression, neural activity and behavioural performance of rats, J. Neuroendocrinol 25 (2013) 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Moos F, Richard P, Paraventricular and supraoptic bursting oxytocin cells in rat are locally regulated by oxytocin and functionally related, J. Physiol 408 (1989) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Yamashita H, Okuya S, Inenaga K, Kasai M, Uesugi S, Kannan H, et al. , Oxytocin predominantly excites putative oxytocin neurons in the rat supraoptic nucleus in vitro, Brain Res. 416 (1987) 364–368. [DOI] [PubMed] [Google Scholar]
- [213].Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS, Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats, Neuropharmacology 58 (2010) 38–43. [DOI] [PubMed] [Google Scholar]
- [214].Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB, CSF and blood oxytocin concentration changes following intranasal delivery in macaque, PLoS One 9 (2014) e103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Kirkpatrick MG, Francis SM, Lee R, de Wit H, Jacob S, Plasma oxytocin concentrations following MDMA or intranasal oxytocin in humans, Psychoneuroendocrinology 46 (2014) 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Kuo JJ, Silva AA, Hall JE, Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function, Hypertension 41 (2003) 768–774. [DOI] [PubMed] [Google Scholar]
- [217].Skibicka KP, Grill HJ, Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins, Endocrinology 150 (2009) 5351–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, et al. , Modulation of blood pressure by central melanocortinergic pathways, N. Engl. J. Med 360 (2009) 44–52. [DOI] [PubMed] [Google Scholar]
- [219].Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS, Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004, J. Am. Coll. Surg 207 (2008) 928–934. [DOI] [PubMed] [Google Scholar]
- [220].MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ, A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research, Psychoneuroendocrinology 36 (2011) 1114–1126. [DOI] [PubMed] [Google Scholar]
- [221].Hicks C, Ramos L, Reekie T, Misagh GH, Narlawar R, Kassiou M, et al. , Body temperature and cardiac changes induced by peripherally administered oxytocin, vasopressin and the non-peptide oxytocin receptor agonist WAY 267,464: a biotelemetry study in rats, Br. J. Pharmacol 171 (2014) 2868–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Yang Z, Han D, Coote JH, Cardiac sympatho-excitatory action of PVN-spinal oxytocin neurons, Autonomic Neuroscience: Basic & Clinical 147 (2009) 80–85. [DOI] [PubMed] [Google Scholar]
- [223].Yosten GL, Samson WK, Neural circuitry underlying the central hypertensive action of nesfatin-1: melanocortins, corticotropin-releasing hormone, and oxytocin, Am J Physiol Regul Integr Comp Physiol. 306 (2014) R722–R727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Gutkowska J, Jankowski M, Oxytocin revisited: its role in cardiovascular regulation, J. Neuroendocrinol 24 (2012) 599–608. [DOI] [PubMed] [Google Scholar]
- [225].Petersson M, Cardiovascular effects of oxytocin, Prog. Brain Res 139 (2002) 281–288. [DOI] [PubMed] [Google Scholar]
- [226].Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA, Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques, Psychoneuroendocrinology 45 (2014) 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Goldman MB, Gomes AM, Carter CS, Lee R, Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia, Psychopharmacology 216 (2011) 101–110. [DOI] [PubMed] [Google Scholar]
- [228].Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM, Peripheral oxytocin is associated with reduced symptom severity in schizophrenia, Schizophr. Res 124 (2010) 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Alvares GA, Hickie IB, Guastella AJ, Acute effects of intranasal oxytocin on subjective and behavioral responses to social rejection, Exp. Clin. Psychopharmacol 18 (2010) 316–321. [DOI] [PubMed] [Google Scholar]
- [230].Chan EW, He Y, Chui CS, Wong AY, Lau WC, Wong IC, Efficacy and safety of lorcaserin in obese adults: a meta-analysis of 1-year randomized controlled trials (RCTs) and narrative review on short-term RCTs, Obesity reviews: an official journal of the International Association for the Study of Obesity 14 (2013) 383–392. [DOI] [PubMed] [Google Scholar]
- [231].Yanovski SZ, Yanovski JA, Long-term drug treatment for obesity: a systematic and clinical review, JAMA: the journal of the American Medical Association 311 (2014) 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Fuchs AR, Fuchs F, Husslein P, Soloff MS, Fernstrom MJ, Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor, Science 215 (1982) 1396–1398. [DOI] [PubMed] [Google Scholar]
- [233].Pound LD, Kievit P, Grove KL, The nonhuman primate as a model for type 2 diabetes, Current Opinion in Endocrinology, Diabetes, and Obesity 21 (2014) 89–94. [DOI] [PubMed] [Google Scholar]
- [234].Salazar M, Hu BB, Vazquez J, Wintz RL, Varon J, Exogenous vasopressin-induced hyponatremia in patients with vasodilatory shock: two case reports and literature review, J. Intensive Care Med (2013). [DOI] [PubMed] [Google Scholar]
- [235].Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, et al. , Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics, J. Neuroendocrinol 24 (2012) 609–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Palm C, Reimann D, Gross P, The role of V2 vasopressin antagonists in hyponatremia, Cardiovasc. Res 51 (2001) 403–408. [DOI] [PubMed] [Google Scholar]
- [237].Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, et al. , Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia, N. Engl. J. Med 355 (2006) 2099–2112. [DOI] [PubMed] [Google Scholar]
- [238].Devost D, Zingg HH, Homo- and hetero-dimeric complex formations of the human oxytocin receptor, J. Neuroendocrinol 16 (2004) 372–377. [DOI] [PubMed] [Google Scholar]
- [239].Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, et al. , REVIEW: oxytocin: crossing the bridge between basic science and pharmacotherapy, CNS Neurosci. Ther 16 (2010) e138–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, et al. , Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles, Biol. Psychiatry 4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, et al. , Longterm exposure to intranasal oxytocin in a mouse autism model, Translational Psychiatry 4 (2014) e480. [DOI] [PMC free article] [PubMed] [Google Scholar]


