Abstract
Retinal degenerative diseases are a major cause of morbidity in modern society because visual impairment significantly decreases the quality of life of patients. A significant challenge in treating retinal degenerative diseases is their genetic and phenotypic heterogeneity. However, despite this diversity, many of these diseases share a common endpoint involving death of light-sensitive photoreceptors. Identifying common pathogenic mechanisms that contribute to photoreceptor death in these diverse diseases may lead to a unifying therapy for multiple retinal diseases that would be highly innovative and address a great clinical need. Because the retina and photoreceptors, in particular, have immense metabolic and energetic requirements, many investigators have hypothesized that metabolic dysfunction may be a common link unifying various retinal degenerative diseases. Here, we discuss a new area of research examining the role of NAD+ and sirtuins in regulating retinal metabolism and in the pathogenesis of retinal degenerative diseases. Indeed, the results of numerous studies suggest that NAD+ intermediates or small molecules that modulate sirtuin function could enhance retinal metabolism, reduce photoreceptor death, and improve vision. Although further research is necessary to translate these findings to the bedside, they have strong potential to truly transform the standard of care for patients with retinal degenerative diseases.
Keywords: retinal degeneration, neurodegeneration, mitochondria, metabolism, NAD+, sirtuins
1. Introduction
Vision is a central sense that is considered critical in modern society. Numerous studies have demonstrated that visual impairment is associated with significant morbidity and has a huge impact on one’s quality of life. For example, visual impairment is associated with clinically significant decreases in mobility and independence (Fenwick et al., 2016). Furthermore, in patients with the blinding disease retinitis pigmentosa (RP), there is a correlation between residual visual field and quality of life (Chaumet-Riffaud et al., 2017), suggesting that there is an association between the degree of vision loss and the extent of impairment in quality of life. These decreases in quality of life can contribute to poor mental health. In support, Heesterbeek and colleagues found in a prospective cohort of 540 older adults with vision impairment that these individuals exhibited twice the incidence of subthreshold depression and anxiety compared to older adults in general (Heesterbeek et al., 2017). Although these symptoms tended to fluctuate with time, having macular degeneration and problems with adaptation to vision loss were two of the risk factors identified for developing depressive symptoms (Heesterbeek et al., 2017). Therefore, vision loss and the associated sequelae have a significant impact on human beings individually and on society in general. As such, despite their challenges, preventing and reversing vision loss caused by diverse retinal diseases is of utmost priority.
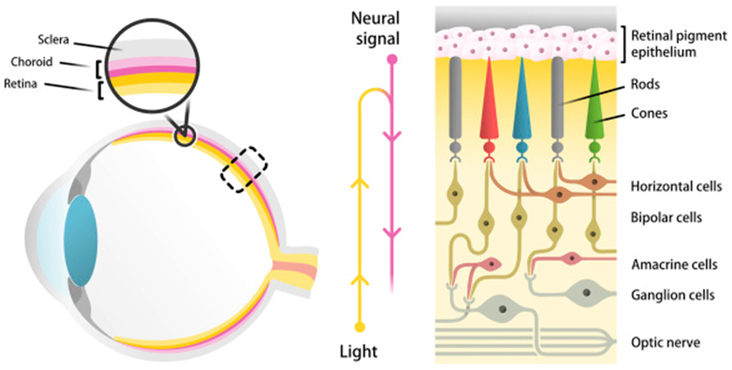
Retinal degenerative diseases make up a significant portion of the burden of blindness and are often untreatable. The retina is a complex, light-sensitive, neurovascular tissue with a highly organized structure that is essential to its function (Fig 1). Located at the posterior pole of each eye, the retina consists of numerous cell types, all of which must function in a coordinated manner to generate a neural signal to be transmitted to the occipital lobe of the brain via the optic nerve. Light photons entering the anterior surface of the eye first traverse across the retina before being sensed by the photoreceptors on the posterior aspect of the retina. Photoreceptors can be divided into two types: rod photoreceptors mediate dim, peripheral vision, whereas cone photoreceptors mediate central, color vision. The signal from the photoreceptors is then transmitted to secondary neurons known as bipolar cells before being transmitted to the retinal ganglion cells, whose axons coalesce to form the optic nerve. Horizontal and amacrine cells provide lateral modulation. As expected, conditions leading to the death of any of these subpopulations of retinal neurons can lead to visual impairment. In particular, photoreceptor death is a common cause of blindness in retinal degenerative diseases, as these light-sensitive neurons are responsible for the initial transduction of light.
Figure 1.
Schematic depicting the structural organization of the neurosensory retina and its location in the eye. The retina consists of numerous neuronal cell types, including rod and cone photoreceptors, bipolar cells, retinal ganglion cells, horizontal cells, and amacrine cells.
This review will first provide a brief clinical description of various examples of retinal degenerative diseases to frame a discussion regarding the limitations of their current treatment options. Next, this review will discuss an emerging focus on investigating the role of NAD+ and sirtuins in retinal neurodegeneration and speculate on how the findings of these studies may lead to novel pharmacologic approaches involving NAD+-based therapeutics. Although further research is necessary to more fully understand the underlying molecular and cellular pathways involved in these processes and, ultimately, to translate these findings from the bench to the bedside, these therapies have the potential to be highly innovative and may transform the standard of care for these patients by reducing the burden of blindness or preventing it altogether.
2. Retinal Degenerative Diseases
Retinal degenerative diseases are a heterogeneous family of multiple conditions all involving death or damage to cells of the retina. These diseases have a wide array of etiologies; some are acquired, some are a component of a broader systemic disease, and some are inherited. Although some of these conditions share phenotypic characteristics, most have different underlying pathogeneses. This diversity makes it rather challenging to develop unifying therapeutic strategies. Instead, the prevailing dogma has been to consider each disease as a separate entity with its own avenue of research, discovery, and translational pipeline. However, despite broad phenotypic and genotypic heterogeneity, what is common to these disorders is that many of them are associated with death of the light-sensitive photoreceptors, which is ultimately what leads to blindness. Other reviews focus on providing comprehensive overviews of the clinical characteristics and the pathophysiology of retinal degenerative diseases (Veleri et al., 2015). Instead, this review will present some examples of retinal degenerative diseases to illustrate the shortcomings of current therapeutic options, framing a discussion about the potential advantages of a unified therapeutic approach.
2.1. Age-related Macular Degeneration (AMD)
Age-related macular degeneration (AMD) is an acquired retinal degenerative disease that affects the central retina, called the macula. AMD is a leading cause of blindness in adults over the age of 50 years. AMD is projected to become an even larger problem over time and is predicted to affect as many as 288 million people by 2040 (Wong et al., 2014). Clinically, patients with early-stage AMD often complain about reduced light sensitivity, dark adaptation, and contrast sensitivity, which is believed to be related to early parafoveal rod photoreceptor degeneration (Curcio et al., 1996). On examination, these patients often present with the presence of lipid- and protein-rich deposits beneath the retinal pigment epithelium (RPE) known as drusen. Drusen themselves do not usually cause vision loss, but they are a significant risk factor for progression to advanced AMD that manifests either as advanced non-neovascular (dry) AMD or neovascular (wet) AMD. Both forms of advanced disease can lead to significant visual impairment related to secondary degeneration and death of macular photoreceptors, causing loss of central vision. In the case of advanced dry AMD, blindness is caused by death of the photoreceptors due to loss of the underlying RPE cells that are critical for photoreceptor survival and function. This stage of disease is called geographic atrophy (GA). In contrast, in wet AMD, pathological angiogenesis manifests as choroidal neovascularization (CNV), which can also cause photoreceptor death, sub-RPE and sub-retinal fibrosis, and, ultimately, blindness. It is often difficult for clinicians to counsel AMD patients regarding when progression to advanced disease may occur and what form it will take. The Age-Related Eye Disease Study (AREDS), sponsored by the National Eye Institute, was a large cohort study that provided key insights into the natural history of AMD. This study also identified risk factors associated with progression from early or intermediate AMD to advanced AMD, such as smoking and greater body mass index (BMI) (Clemons et al., 2005). Nonetheless, further research is necessary to determine the specific molecular and cellular mechanisms that underlie the pathogenesis of AMD.
Genome-wide association studies have provided some clues into potential pathogenic mechanisms underlying AMD by identifying that polymorphisms in complement factor H, hepatic lipase (LIPC), ATP-binding cassette transporter member 1 (ABCA1), and cholesterol ester transfer protein (CETP), are associated with early or advanced AMD (Hageman et al., 2005; Neale et al., 2010). These findings implicate altered regulation of inflammation and aberrant lipid homeostasis as potential contributors to the pathogenesis of AMD. In support, mouse models designed to mimic perturbations in inflammation or lipid homeostasis pathways in combination with environmental factors, such as a high-fat, Western diet, have been reported to recapitulate some features resembling AMD (Malek et al., 2005; Sene et al., 2013; Toomey et al., 2015). These findings confirm the relevance of these pathways in AMD and highlight the value of using mouse models for furthering our understanding of AMD.
Currently, approved therapies for wet AMD include drugs directed against vascular endothelial growth factor (VEGF). VEGF is a key driver of pathological angiogenesis, the hallmark of wet AMD. Although anti-VEGF therapies have revolutionized treatment for wet AMD, long-term studies have demonstrated that atrophic neurodegeneration with loss of photoreceptors proceeds despite treatment (Bhisitkul et al., 2015; Sene et al., 2015). Perhaps more importantly, some patients do not respond or are under-responsive to this therapy (Inoue et al., 2016; Kim et al., 2016; Sarwar et al., 2016; Sene et al., 2015), suggesting that VEGF-independent pathways may also contribute to AMD pathogenesis. More research is needed to identify alternate therapeutic strategies that may be able to provide clinical benefit for these patients and to prevent photoreceptor neurodegeneration in both forms of advanced AMD. Unfortunately, because the pathophysiology of dry AMD remains incompletely understood, there are currently no approved therapies for dry AMD. High-dose supplementation of vitamins C and E, beta carotene, and zinc has been suggested to modestly slow progression to advanced AMD (Age-Related Eye Disease Study Research, 2001), but the effect size is fairly small, and its mechanism is still unclear. Taken together, it is clear that there is a paucity of current treatment options for AMD patients beyond targeting VEGF in wet AMD.
2.2. Diabetic Retinopathy (DR)
Diabetes mellitus is a systemic metabolic disease characterized by deficits in blood glucose control. The pathophysiology of diabetes mellitus is complex and affects numerous organ systems, but one component of this systemic disease is diabetic retinopathy (DR). Clinically, DR consists of early microvascular damage, which is initially characterized by pericyte loss and microaneurysms in small-caliber vessels in the retina, followed by capillary wall damage, leakage, exudation, and retinal edema. In advanced disease, hypoxic pathologic neovascularization can cause vision loss secondary to hemorrhage and detachment of the neurosensory retina. In addition to this vascular phenotype, diabetic patients often exhibit changes on electroretinography (ERG), such as delayed implicit times (Satoh et al., 1994) and decreased oscillatory potential amplitudes (Coupland, 1987), which precede vascular changes. DR could therefore include two components – neuroretinal dysfunction and vascular dysfunction – both contributing to vision loss.
Studies in mouse models of DR support this possibility. For example, Rajagopal and colleagues identified that mice weaned to high-fat chow exhibit modest electrophysiological dysfunction in the form of increased latencies and decreased oscillatory potential amplitudes at 6 months that precede the vascular phenotype of microvascular disease that can be observed by 12 months (Rajagopal et al., 2016). These findings may have translational relevance since multifocal ERG implicit times in patients with diabetes but without retinopathy predict future development of DR (Harrison et al., 2011). Other groups have reported even more striking high-fat diet-induced retinal dysfunction in mice at 12 weeks in the form of reduced scotopic and photopic ERG amplitudes (Chang et al., 2015). Mechanistic studies suggest that this neuroretinal dysfunction may be due to the sensitivity of retinal neurons to systemic hyperglycemia. For example, in the streptozotocin-induced mouse model of diabetes, prolonged hyperglycemia leads to retinal oxidative stress (Du et al., 2013), which may contribute to photoreceptor death.
Currently, clinicians can treat only the vascular disease, including permeability-related macular edema and ischemic neovascularization, with anti-VEGF pharmacotherapy and intraocular steroids. Neuroprotective strategies that could mitigate neuroretinal dysfunction or prevent retinal neurodegeneration are highly attractive but are currently investigational. As in AMD, a molecular understanding of the pathophysiology of DR might improve our ability to develop more diverse therapeutic strategies to prevent vision loss in DR.
2.3. Inherited Retinal Degenerations (IRDs)
Inherited retinal degenerations (IRDs) are diverse diseases that are associated with progressive vision loss caused by mutations in over 250 genes. Some examples of IRDs include retinitis pigmentosa (RP), Leber congenital amaurosis (LCA), and cone-rod dystrophies. IRDs can be isolated or syndromic. For example, RP, one of the most common IRDs, can be caused by mutations in any of more than 100 genes or can be a clinical feature of Usher syndrome or Bardet-Biedl syndrome. Because of this genotypic diversity, RP has a complex and heterogeneous clinical presentation, often depending on the underlying mutation. In general, RP patients present with loss of night vision and decreased peripheral vision due to death of rod photoreceptors. As rod photoreceptor death progresses, cone photoreceptor death may follow as a secondary effect of losing rod photoreceptor-derived survival factors. Exome and targeted gene sequencing have made it possible to examine the genetic etiology of various retinal degenerative diseases, including RP.
Despite remarkable advances in our ability to identify the causative gene mutations associated with IRDs, including RP, our therapeutic strategies are still limited by lack of knowledge of the mechanisms by which these genes cause disease. One therapeutic approach has been gene therapy with the goal of replacing the defective copy or copies of the affected gene with a normal gene delivered by a carrier, usually a viral vector. For example, a Phase III trial recently evaluated the safety and efficacy of voretigene neparvovec (AAV2-hRPE65v2) in patients with retinal dystrophy caused by biallelic mutations in RPE65 (Russell et al., 2017). Using this approach, a wild-type copy of the RPE65 gene is delivered with an adenoviral vector to the sub-retinal space. In patients with viable retinal cells, normal RPE65 protein can restore the visual cycle and may lead to some vision improvement. Although still in its infancy compared to conventional gene therapy, CRISPR-Cas9 has also now made it possible to perform targeted gene editing to repair disease-causing mutations, which has been shown to be effective in restoring some vision in rodent models of retinal degenerative disease (Bakondi et al., 2016; Wu et al., 2016). However, one major challenge of both gene therapy and gene editing is that they would have to be optimized and targeted for each specific mutation, requiring the causative mutation to be identified in each individual patient. This requirement is a challenging proposition considering the diversity of mutations that have been identified, especially for RP, each affecting a fraction of the total patient population. Perhaps of additional concern, gene therapy is incredibly expensive in the present, making it difficult to use in widespread settings. Issues surrounding the durability of the effect, effect size, and scalability in more prevalent diseases further complicate the therapeutic landscape.
2.4. Limitations of Current Therapeutic Strategies
For many retinal degenerative diseases, our incomplete understanding of disease pathogenesis has led to a current strategy of addressing disease symptoms and endpoints rather than their underlying etiology. For example, in AMD and DR, patients receive anti-VEGF therapies to inhibit pathological angiogenesis. For IRDs, even if we have a sophisticated understanding of the underlying genetics, it is not always easy to deliver therapeutics in a clinical setting due to the challenges highlighted above. Therefore, an attractive option would be to identify a potential therapeutic strategy that could prevent photoreceptor death in multiple forms of retinal degenerative diseases. One example of such an approach is stem cell therapy. Previous mouse studies have shown that photoreceptor neuron transplantation is feasible and improves visual function (MacLaren et al., 2006; Pearson et al., 2012; Santos-Ferreira et al., 2015). These investigations have been the basis of ongoing human clinical trials using stem-cell based approaches in AMD (Mandai et al., 2017; Schwartz et al., 2015; Schwartz et al., 2016). Although these studies adopt the strategy of RPE transplantation rather than photoreceptor neuron transplantation since RPE dysfunction is known to contribute to photoreceptor degeneration in advanced dry AMD, they demonstrate that transplantation of cells into the eye is feasible, paving the way for future human clinical studies of photoreceptor transplantation.
There remain numerous challenges of using stem cells for photoreceptor transplantation, such as ensuring proper functional connectivity with the host retina, the time required to differentiate cells for transplantation, and potential tumorigenicity of transplanted cells. Recent studies also suggest that material transfer of proteins from transplanted photoreceptors to host cells may itself improve visual function, necessitating a closer look at the mechanism underlying rescue in transplantation studies (Pearson et al., 2016; Waldron et al., 2018). These are important issues that must be investigated thoroughly before stem cell therapy can be widely used in humans. The source of stem cells also poses a significant challenge. Although induced pluripotent stem cells (iPSCs) can be used to overcome the ethical concerns and regulatory challenges surrounding embryonic stem cells since iPSCs are reprogrammed from adult somatic cells, they have one major limitation. Specifically, since the source of iPSCs is often the patients themselves, the cells retain disease-causing mutations, requiring gene editing prior to transplantation. Although targeted gene editing can be achieved with CRISPR-Cas9 in mouse models (Burnight et al., 2017), successful application of this technology to treat retinal degenerative diseases in humans is still in its infancy.
2.5. What is on the Frontier?
Given the limitations of current therapeutic options, including those under investigation in clinical trials, there is a significant need for novel therapeutic approaches that target pathways involved in photoreceptor death that may be shared across multiple retinal degenerative diseases. Identifying such a common pathway contributing to photoreceptor death that is therapeutically modifiable may enable us to halt or delay photoreceptor death, especially in early phases of disease, and thereby preserve vision. One interesting characteristic of the retina and photoreceptors in particular is that they are highly metabolically active (Ames et al., 1992; Wong-Riley, 2010). In contrast with most other neurons, photoreceptors respond to stimuli by hyperpolarizing rather than depolarizing. In fact, at baseline in darkness, photoreceptors exist in a depolarized state, maintained by the presence of cyclic guanosine monophosphate (cGMP) binding to and keeping cyclic-nucleotide-gated (CNG) channels open and permitting influx of cations. Upon exposure to light, a signal transduction cascade is activated, leading to hydrolysis of cGMP to its non-cyclized form, GMP. This hydrolysis leads to dissociation of cGMP from CNG channels, channel closure, and loss of cationic influx, causing membrane potential hyperpolarization. The need for photoreceptors to maintain this constant state of depolarization, i.e., the dark current, has tremendous energy demands (Okawa et al., 2008).
In addition to having to maintain a ‘dark current,’ photoreceptors also have tremendous anabolic requirements, which further heighten energetic demands. Photoreceptors perform their primary function of phototransduction in a specialized subcellular region known as the outer segment. Photoreceptor outer segments consist of highly invaginated, membrane-rich structures that undergo diurnal turnover. This turnover leads to diurnal shedding of the photoreceptor membrane, which then must be phagocytosed by the RPE. To prevent shortening of the outer segments, photoreceptors have to regenerate this portion of the cellular membrane, necessitating anabolic processes to generate the biomolecules of cellular membranes, especially lipids. Recent studies have demonstrated that a shift towards aerobic glycolysis may facilitate anabolism and outer segment maintenance in photoreceptors (Chinchore et al., 2017). Evidently, photoreceptors must carefully coordinate their metabolism so that they have generate sufficient energy to maintain the ‘dark current’ while also generating the key biomolecules needed to maintain their specialized structure.
Many groups have hypothesized that these dual demands make photoreceptors particularly vulnerable to metabolic perturbations. In support, recent studies profiling retinal metabolism have highlighted that the retina operates at near-maximal respiratory capacity with limited reserve to handle additional energetic demands (Kooragayala et al., 2015). Moreover, there are examples of blinding diseases that are caused by mutations in metabolic enzymes. For example, a loss-of-function mutation in the beta subunit of isocitrate dehydrogenase 3 (IDH3B), a key enzyme in the Krebs cycle, has been reported to cause RP (Hartong et al., 2008). Although these patients harbored this IDH3B mutation in every cell of their body, they presented clinically with only a retinal phenotype. Although we cannot rule out the possibility that these patients have subclinical manifestations of disease in other organ systems, the fact that the most significant manifestation is in the eye supports the idea that photoreceptors are particularly sensitive to perturbations in metabolic pathways.
3. A Central Role for NAD+ in Retinal Degeneration
Nicotinamide adenine dinucleotide (NAD+) is a central coenzyme for the dehydrogenase enzymes that perform reduction-oxidation (redox) reactions, which connect the reactions of glycolysis and the citric acid cycle to oxidative phosphorylation via the electron transport chain. Although this is NAD+’s better-known role, NAD+ also has another important function as a co-substrate for NAD+-consuming enzymes, such as the sirtuin deacylases (SIRTs), poly(ADP-ribose) polymerases (PARPs), mono-ADP ribosyltransferases, and cyclic ADP-ribose hydrolase (CD38). These NAD+ consumers are distinct from the dehydrogenases with respect to how they utilize NAD+. While dehydrogenases use NAD+ as an electron acceptor, thereby reducing it to NADH, NAD+ consumers cleave a covalent bond within the NAD+ molecule and, in this process, generate nicotinamide. In the former case, NAD+ can be regenerated via a redox reaction; in the latter case, nicotinamide can be converted back to NAD+ through a salvage pathway to reattach the proper chemical moieties.
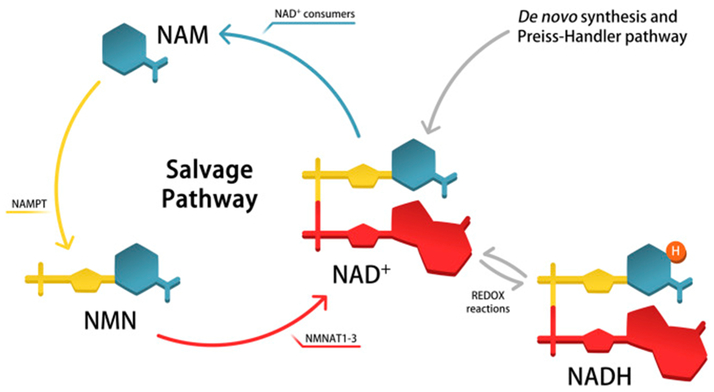
This salvage pathway of NAD+ biosynthesis beginning with nicotinamide is considered a dominant NAD+ biosynthetic pathway in mammals (Imai and Yoshino, 2013). In this pathway, nicotinamide, the product generated when NAD+ is used as a co-substrate by NAD+ consumers, is first converted to nicotinamide mononucleotide (NMN) by the enzyme nicotinamide phosphoribosyltransferase (NAMPT) before being adenylated by the NMN adenylyltransferses 1–3 (NMNAT1–3) to form NAD+. Other NAD+ biosynthetic pathways exist, including de novo synthesis from tryptophan. A schematic depicting these various pathways of NAD+ biosynthesis is presented in Figure 2. NAD+ has been reported to be essential for regulating metabolism, circadian rhythms, and aging (Garten et al., 2009; Mouchiroud et al., 2013; Nakahata et al., 2009; Ramsey et al., 2009). Given a central role of NAD+ biosynthesis in metabolism and the highly active metabolism of the retina and photoreceptors, many groups including ours hypothesized that NAD+ biosynthesis may also be important in the retina.
Figure 2.
Although nicotinamide adenine dinucleotide (NAD+) can be synthesized via numerous pathways, including de novo synthesis followed by the Preiss-Handler pathway, the pathway mediated by nicotinamide phosphoribosyltransferase (NAMPT) is the dominant mammalian pathway and has been shown to be essential in numerous cell types. NAMPT catalyzes the formation of nicotinamide mononucleotide (NMN) from nicotinamide (NAM), which is then converted to NAD+ by NMN adenylyltransferases 1–3 (NMNAT1–3).
The possibility that perturbations in NAD+ homeostasis may contribute to retinal neurodegeneration is also well supported by previous studies of neurodegeneration. Wallerian degeneration is a highly stereotyped process by which axonal injury via a cut or crush leads to degeneration of the axon distal to the injury. Serendipitously, investigators discovered a spontaneous mutant mouse line that had delayed Wallerian degeneration, calling these Wallerian degeneration slow (Wds) mice. In Wds mice, Wallerian degeneration in response to axonal injury is delayed due to a mutation that results in overexpression of Wlds, a chimeric protein that leads to increased NMNAT1 activity (Araki et al., 2004). More recent studies have supported that axonal NAD+ levels are a key regulator of the pace of axonal degeneration. In fact, other executioners that lead to NAD+ destruction via molecules such as SARM1 lead to accelerated axonal degeneration by promoting NAD+ destruction via its intrinsic NADase activity within the Toll/interleukin-1 receptor (TIR) domain (Essuman et al., 2017; Gerdts et al., 2015). These studies all point to a central role of NAD+ homeostasis for maintaining neuron survival and function.
3.1. NAD+ Biosynthesis in Photoreceptors
Photoreceptors are highly specialized neurons that may also rely on NAD+ homeostasis for their survival and function. This possibility is supported by clinical research identifying that LCA, a childhood blinding disease, can be caused by mutations in NMNAT1, a key enzyme necessary for NAD+ biosynthesis (Chiang et al., 2012; Falk et al., 2012; Koenekoop et al., 2012). This clinical phenotype has been replicated in mouse models. For example, mouse lines containing a p.v9M and a p.D243G mutation in the Nmnat1 gene generated through N-ethyl-N-nitrosourea mutagenesis recapitulate key aspects of human disease such as rapidly progressive photoreceptor degeneration, retinal vasculature attenuation, optic atrophy, and RPE loss (Greenwald et al., 2016). Given the severe anatomic perturbations, these mice also exhibit retinal dysfunction, confirming that NMNAT1 is important for retinal survival and function.
Other studies have confirmed that this NAMPT-mediated pathway is essential for retinal survival and function by perturbing the rate-limiting enzyme in this biosynthetic pathway, NAMPT. For example, inhibiting NAMPT with pharmacological inhibitors is toxic to the retina (Zabka et al., 2015). Moreover, deleting this enzyme specifically from rod photoreceptors using the Cre-lox system led to rapid retinal degeneration by six weeks of age at both the structural and functional level (Lin et al., 2016). Similarly, cone photoreceptor-specific Nampt deletion led to predominant cone dysfunction, confirming that NAMPT-mediated NAD+ biosynthesis is essential for photoreceptor survival and function in a predominately cell-autonomous manner. Of translational relevance, retinal NAD+ deficiency was a feature of multiple murine models of retinal degeneration, including light-induced retinal degeneration, streptozotocin-induced diabetic retinopathy, and in age-associated retinal decline, and preceded retinal neurodegeneration (Lin et al., 2016). Although the mechanism by which retinal NAD+ homeostasis becomes perturbed in these disparate disease models is unclear, these findings suggest that retinal NAD+ deficiency may be a therapeutic target for numerous blinding diseases. In support, long-term administration of NMN for 12 months in wild-type mice has been shown to reduce some of the physiological declines associated with aging, including modest improvement of age-associated declines in retinal function (Mills et al., 2016). Although we are not aware of any other known associations between mutations in NAD+ biosynthetic genes and retinal neurodegenerative disease, these strong phenotypes associated with murine mutant models suggest that these pathways are likely also important in context of human disease.
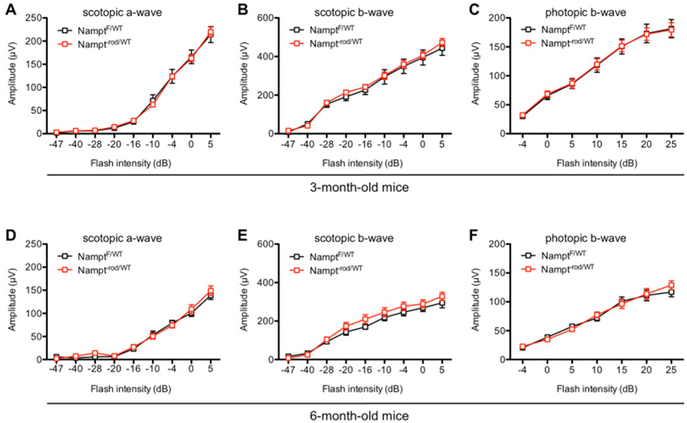
To better understand the role of NAD+ homeostasis in other contexts of retinal disease, which may have more modest retinal NAD+ deficiency, we tested whether deleting one copy of Nampt from rod photoreceptors was sufficient to cause retinal degeneration. Our results showed that mice lacking one copy of Nampt from rod photoreceptors and with approximately 30% reduction of Nampt expression from rod-enriched retinal isolates did not exhibit any baseline retinal degeneration at 6 weeks of age by electroretinography (ERG) (Lin et al., 2016). We also tested these mice further at 3 months and 6 months of age and did not identify any differences in either scotopic or photopic retinal function between Nampt−rod/WT and NamptF/WT mice (Figs 3A–F). These findings indicate that Nampt is haplosufficient at these time points. However, it remains possible that monoallelic Nampt deletion renders rod photoreceptors more vulnerable to other pathologic perturbations, such as prolonged aging (>12 months), or other disease states that may lead to retinal degeneration. These questions require further investigation.
Figure 3.
(A–C) At 3 months, mice with monoallelic Nampt deletion from rod photoreceptors (Nampt−rod/WT) did not exhibit any retinal dysfunction compared to NamptF/WT controls based on their scotopic a-wave, scotopic b-wave, or photopic b-wave amplitudes (N=6–7/group; 2-way mixed ANOVA). (D–F) Likewise, there was no significant difference in retinal function at 6 months of age (N=6–7/group; 2-way mixed ANOVA). Graphs depict mean ± S.E.M (A–F).
3.2. NAD+ Biosynthesis in Other Cell Types
NAD+ homeostasis has also been shown to be vitally important in the survival and function of other neurons in the retina. Glaucoma is another blinding disease that is multifactorial and highly complex, involving retinal ganglion cell degeneration. A recent report highlighted that glaucoma-prone mice exhibit retinal NAD+ deficiency with age, which leads to mitochondrial abnormalities and, ultimately, retinal neuronal dysfunction (Williams et al., 2017). This pathological process could be rescued with both supplementation of the NAD+ precursor nicotinamide (vitamin B3) and/or gene therapy to drive the expression of the NAD+ biosynthetic enzyme NMNAT1 (Williams et al., 2017). Of interest, recent studies have identified numerous cell types that depend exquisitely on NAMPT-mediated NAD+ biosynthesis, including projection neurons (Wang et al., 2017), hippocampal and cortical excitatory neurons (Stein et al., 2014), skeletal muscle (Frederick et al., 2016), and adipocytes (Stromsdorfer et al., 2016), among others. Cumulatively, these findings open up the possibility that NAD+-based interventions for retinal degenerative diseases may also have broad applicability to other diseases of other organ systems.
4. Sirtuins as Molecular Sensors of NAD+ Availability
Other lines of investigation have focused on understanding why maintaining NAD+ homeostasis is essential for neuron survival and function. One important role for NAD+ is to serve as co-substrates for sirtuins. Some sirtuins have dissociation constants near physiological concentrations and are therefore exquisitely sensitive to NAD+. Silencing information regulator 2 (Sir2) was first identified in yeast and shown to promote increased lifespan (Kaeberlein et al., 1999; Lin et al., 2000). Further studies have revealed that mammals possess seven Sir2 homologs, SIRT1 through SIRT7, which all have unique subcellular localization and unique deacylase functions. SIRT1 and SIRT2 are present in the cytoplasm and the nucleus; SIRT3, SIRT4, and SIRT5 are localized to the mitochondria; and SIRT6 and SIRT7 are exclusively nuclear. Studies in mouse models lacking various sirtuins have revealed that they play important roles in numerous cellular processes, including DNA damage, stress responses, metabolism, and aging (Verdin, 2015).
4.1. Sirtuins and Retinal Degeneration
Sirtuins have been reported to play important roles in the retina. All seven sirtuin homologs are highly expressed in the mouse retina at the mRNA level and generally have increased expression during the dark phase, except for SIRT6 (Ban et al., 2013). In aged rats demonstrating age-associated retinal dysfunction, SIRT1 expression was found to be reduced in the retina, while enhancing SIRT1 expression with the SIRT1 activator resveratrol, preserved youthful retinal function (Zeng and Yang, 2015). Moreover, SIRT1 has been shown to be involved in the pathogenesis of models of diabetic retinopathy (Duarte et al., 2015; Kowluru et al., 2014; Kubota et al., 2011; Mortuza et al., 2014; Zheng et al., 2012) and light-induced retinal degeneration (Kubota et al., 2010). Furthermore, SIRT1 has also been shown to play an important role in regulating vascular regeneration. In the mouse model of oxygen-induced ischemic retinopathy (OIR) animal model, conditional depletion of neuronal SIRT1 led to significantly reduced retinal vascular regeneration and increased hypoxia-induced pathologic vascular growth (Chen et al., 2013), although overexpression of neuronal SIRT1 did not demonstrate a protective effect, limiting therapeutic applicability (Michan et al., 2014). The mitochondrial sirtuins SIRT3 and SIRT5 have both also been shown to play important roles in regulating retinal homeostasis, as mice lacking SIRT3 alone or both SIRT3 and SIRT5 were more vulnerable to light-induced retinal neurodegeneration compared to control mice (Ban et al., 2017; Lin et al., 2016). Finally, SIRT6 is important for retinal function, as SIRT6 germline knockout mice exhibit retinal dysfunction (Silberman et al., 2014). More specifically, further studies have confirmed that SIRT6 is important for regulating glucose metabolism in the retina: SIRT6 inhibition shifts retinal metabolism towards a predominately glycolytic profile and thereby can attenuate retinal degeneration in mice (Zhang et al., 2016).
4.2. Sirtuins as a Therapeutic Target
Given these roles of sirtuins in retinal survival and function, sirtuin activation via pharmacological modulation may indeed be a therapeutic strategy for preventing retinal degenerative diseases. One approach for sirtuin activation is via the supply of NAD+ intermediates, such as NMN and nicotinamide riboside (NR). Supplementation with these NAD+ intermediates leads to increased intracellular NAD+ availability. Since sirtuins are so exquisitely sensitive to NAD+ levels, this increased NAD+ availability leads to enhanced sirtuin function, especially in contexts of cellular stress when NAD+ demands may be increased. An alternative approach is to directly activate sirtuins. Since the discovery of the first sirtuin-activating compounds (STACs) (Howitz et al., 2003), there has an explosion in efforts to identify novel STACs of various chemical classes with high-throughput screening and biochemical approaches (Bonkowski and Sinclair, 2016). STACs generally function by binding to a conserved N-terminal domain of sirtuins and lowering the Km of the substrate through an allosteric mechanism (Sinclair and Guarente, 2014). One advantage to this small-molecule approach is that it may be possible to target specific sirtuins, although further research is necessary since many of the current small molecules are designed to activate SIRT1. It also remains important not only to understand how distinct sirtuins interact with one another in various organ systems and disease contexts but also to devise strategies for cell- or tissue-specific delivery of these therapeutic agents. Given the diverse function of sirtuins and their unique intracellular functions, these future studies are important to ensure the development of novel therapeutic approaches that are both safe and efficacious.
5. A Possible Role for Poly(ADP)-Ribose Polymerase
Cumulatively, our results and those of other groups suggest that the retina and photoreceptors are particularly sensitive to perturbations in NAD+ availability. One important remaining question is why photoreceptors are so sensitive to perturbations in NAMPT-mediated NAD+ biosynthesis. The fact that photoreceptors rely on the NAMPT-mediated NAD+ biosynthetic pathway suggests that there may be baseline consumption of NAD+ by other NAD+-consuming enzymes to make this pathway so important for photoreceptor survival. One group has demonstrated that, systemically, the NAD+ consumer CD38 may play an important role in age-associated declines in NAD+ levels and thereby promote age-associated pathologies (Camacho-Pereira et al., 2016). A similar phenomenon may also occur in photoreceptors.
5.1. NAMPT deficiency in rod photoreceptors is associated with PARP activation
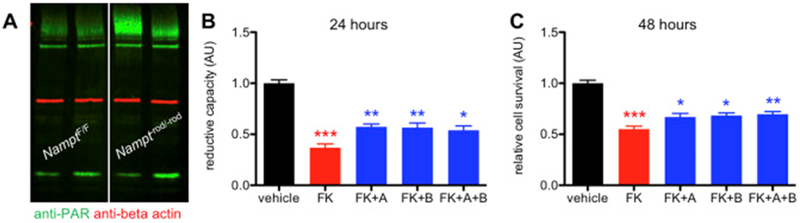
We hypothesized that, in photoreceptors, PARP activation may explain why photoreceptors are so dependent on NAMPT-mediated NAD+ biosynthesis. At baseline, photoreceptors face enormous challenges of having to handle light stimuli, which promote oxidative stress and DNA damage. Although photoreceptors may have the reserve to handle this stress at baseline, it is possible that metabolic perturbations, such as NAD+ deficiency, lead to further mitochondrial dysfunction and an inability to compensate, causing dysfunction and retinal degeneration. In support of this hypothesis, NAD+-deficient photoreceptors have impaired SIRT3 function (Lin et al., 2016). SIRT3 is known to regulate superoxide dismutase 2 (SOD2), a major mitochondrial antioxidant enzyme (Qiu et al., 2010). As a proof of concept, we examined whether there is PARP hyperactivation in the retinas of mice with retinal NAD+ deficiency by Western blot with anti-PAR antibody (clone: 10H; Enzo Life Sciences; Farmingdale, NY). Of interest, at three weeks of age, mice with Nampt deleted specifically from rod photoreceptors (Nampt−rod/-rod) exhibited significant increases in PARylated protein compared to Cre-negative controls, suggestive of PARP hyperactivation (Fig 4A). We have previously reported that Nampt−rod/-rod mice have no signs of retinal degeneration at 3 weeks by histology (Lin et al., 2016), suggesting that PARP activation may contribute to degeneration rather than being its consequence. These findings also suggest that PARP activation in the context of retinal NAD+ deficiency may indeed exacerbate NAD+ unavailability and thereby contribute to photoreceptors’ reliance on NAMPT.
Figure 4.
(A) Representative blot demonstrating that retinas from 3-week-old Nampt−rod/-rod mice displayed more PARylation compared to those from NamptF/F littermate controls. (B) PARP inhibition with either ABT-888 (+A), BYK 49187 (+B), or both (+A+B) in 661W photoreceptor cells treated with the NAMPT inhibitor FK866 (+FK) partially rescued reductive capacity at 24 hours (N=23–27/group; 1-way ANOVA with Tukey post-hoc test). (C) Likewise, PARP inhibition partially improved cell survival at 48 hours (N=24/group; 1-way ANOVA with Tukey post-hoc test). Graphs depict mean + S.E.M (B-C). (* P < .05; ** P < .01; *** P < .001; AU: arbitrary units; red asterisks indicate significant differences compared to the vehicle-treated group; blue asterisks indicate significant differences compared to the FK866-treated group).
5.2. PARP inhibition rescues photoreceptor redox potential and survival
To determine whether PARP inhibition may represent a potential therapeutic approach, we tested whether inhibition of PARP activation may improve NAD+ availability and thereby reduce photoreceptor death. We first tested this in a cell model in cone photoreceptor-like 661W cells (Tan et al., 2004). We treated photoreceptor cells with 20 μM FK866 (Santa Cruz Biotechnology; Dallas, TX) a small-molecule NAMPT inhibitor, with or without simultaneous co-treatment with either of the PARP inhibitors ABT-888 or BYK 49187 (Santa Cruz Biotechnology). As we have previously observed, FK866-treated photoreceptor cells exhibited significant reduction in cellular redox potential compared to vehicle-treated cells at 24 hours, as measured by the WST-1 assay (Sigma; St. Louis, MO) (Fig 4B). Of interest, photoreceptor cells co-treated with FK866 and either or both of the PARP inhibitors were partially protected from the loss of redox potential compared to photoreceptor cells treated with FK866 alone (Fig 4B).
Next, we wanted to determine whether preservation of redox potential also prevented photoreceptor cell death. Given the energetic demands of photoreceptors, loss of redox potential in FK866-treated photoreceptor cells rapidly caused cell death by 48 hours, which we quantified with calcein AM (Applied Biosystems; Foster City, CA) live cell staining (Fig 4C). Of interest, photoreceptor cells co-treated with FK866 and either or both PARP inhibitors were partially rescued from cell death compared to photoreceptor cells treated with FK866 alone (Fig 4C). Again, these findings support the notion that PARP activation is pathologic and that inhibiting PARP activation in the context of NAD+ deficiency promotes photoreceptor cell survival.
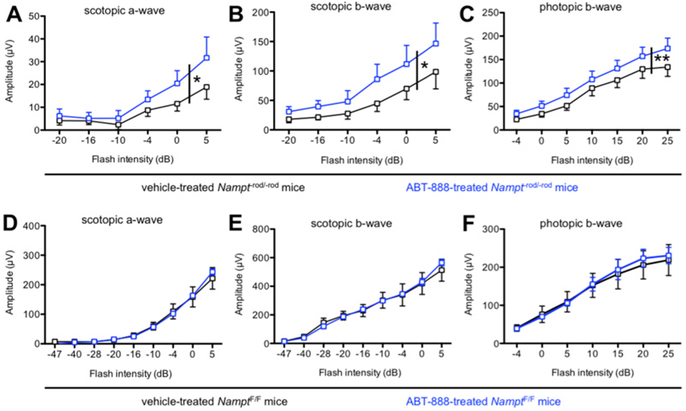
Finally, we wanted to test whether PARP inhibition also had an in vivo effect. We injected ABT-888 (20 mg/kg body weight) intraperitoneally into Nampt−rod/-rod mice daily beginning at postnatal day 5 until postnatal day 28. On the last day of injection, we performed ERG as described previously (Lin et al., 2016). Remarkably, ABT-888-treated Nampt−rod/-rod mice had significantly improved ERG responses under both scotopic and photopic conditions compared to vehicle-treated Nampt−rod/-rod mice (Figs 5A–C). Of interest, ABT-888 treatment had no effect on the ERG findings in NamptWT/WT littermates (Figs 5D–F). Taken together, these findings provide evidence that there is PARP activation in photoreceptors under conditions of NAD+ deficiency and that this PARP activation is pathologic and contributes to retinal degeneration, opening up a novel therapeutic approach. Of note, we observed only partial recovery upon PARP inhibition, suggesting that other pathways are also involved in NAD+ consumption. Further research is necessary to identify these pathways.
Figure 5.
(A-C) Intraperitoneal injections of the PARP inhibitor ABT-888 (20 mg/kg body weight) beginning at postnatal day 5 (P5) partially protected Nampt−rod/-rod mice from retinal dysfunction compared to vehicle-treated Nampt−rod/-rod mice based on scotopic a-wave, scotopic b-wave, and photopic b-wave amplitudes (N=8/group; 2-way mixed ANOVA). (D-F) ABT-888 had no significant effect on retinal function in NamptF/F mice based on scotopic a-wave, scotopic b-wave, and photopic b-wave amplitudes (N=3–4/group; 2-way mixed ANOVA). Graphs depict mean + or – S.E.M. (A-C) or mean ± S.E.M (D-F) (* P < .05; ** P < .01).
Our findings are consistent with the work of other groups showing that deletion of key proteins in the PARP pathway protect against retinal degeneration. For example, retinal explants from PARP1 germline knockout mice and PARG110 germline knockout mice exhibited less photoreceptor death in an ex vivo model of retinal degeneration compared to explants from wild-type mice (Sahaboglu et al., 2014; Sahaboglu et al., 2010). Despite some technical differences, these studies and ours confirm that PARP activation is pathologic in various mouse models of retinal degeneration. This PARP activation likely leads to increased NAD+ utilization, worsened NAD+ deficiency, more mitochondrial dysfunction, and, thus, further PARP activation, leading to a positive feedback loop that inevitably causes photoreceptor death. Although more studies are necessary to confirm this hypothesis, they provide a foundation for further examination of why photoreceptors are so sensitive to perturbations in NAD+ homeostasis.
6. Future Directions
Seminal work by our group and others have established the central importance of metabolism in retinal survival and function. Moreover, impaired retinal metabolism has been identified as a pathogenic feature of multiple forms of retinal degenerative diseases. These findings open up novel therapeutic avenues for treating retinal degenerative diseases. This approach would be highly innovative, as these therapies may have the potential to be effective for numerous diseases if they share common pathogenic mechanisms and thereby can overcome many of the limitations associated with current therapies that are compartmentalized to the disease or the mutation. Beyond the retina, these therapies focused on enhancing NAD+ availability and/or modulating sirtuin activity may also have efficacy for diseases of other organ systems, including not only neurodegenerative diseases but also diseases of other metabolically active cell types, such as muscular dystrophies. Although numerous studies have confirmed the importance of NAD+ and sirtuins in these various tissue types, more studies are necessary to fully unleash their translational potential.
6.1. Is the Subcellular Organization of NAD+ in the Retina Important?
Intracellular NAD+ partitions into subcellular pools in the nucleus, cytoplasm, and mitochondria. No study to our knowledge has determined which subcellular pool(s) of NAD+ are essential for photoreceptor function. The fact that mutations in NMNAT1, the NMNAT isoform with nuclear function, causes blindness suggests that the nuclear NAD+ pool is essential for vision. However, this possibility needs to be tested rigorously. Moreover, there may also be crosstalk and movement of NAD+ between these subcellular pools in the retina. Indeed, impaired NAD+ trafficking may also play pathogenic roles in disease. Further understanding of the subcellular organization of NAD+ in the retina is particularly important since sirtuins, molecular sensors of NAD+ availability, also have a distinct pattern of subcellular organization. Therefore, it is possible that restrictions in NAD+ availability in certain subcellular compartments but not others may cause impaired activity of a specific sirtuin, which are important principles to consider when developing novel therapeutic approaches.
6.2. What is the Role of an Extracellular Source of NAD+?
Moreover, recent studies suggest that there is also an extracellular source of NAD+, which may have its own important physiological role. In fact, NAMPT has two forms: intracellular NAMPT (iNAMPT) and extracellular NAMPT (eNAMPT). Whereas iNAMPT localizes intracellularly and likely contributes to NAD+ pools to be used within the cell, eNAMPT circulates in plasma and in other biofluids and can regulate physiology in a systemic manner. eNAMPT (also known as PBEF or visfatin) was previously thought to be a cytokine or a hormone based on its pleiotropic effects, but further investigation revealed that these effects are caused by its robust NAD+ biosynthetic function (Revollo et al., 2007). Recent studies have confirmed that eNAMPT’s ability to regulate systemic NAD+ biosynthesis allows it to regulate distant target cells, including pancreatic beta cells (Revollo et al., 2007) and hypothalamic neurons (Yoon et al., 2015). eNAMPT may also regulate retinal physiology.
6.3. The Challenge of Bioavailability
Numerous studies have tested whether NAD+ intermediates are therapeutic in animal models of disease (Lin et al., 2016; Mills et al., 2016; Williams et al., 2017). Human clinical trials have already begun to investigate whether NAD+ intermediates improve cardiometabolic health (NCT03151239), treat mitochondrial diseases/myopathies (NCT03432871), or improve cognition in patients with mild cognitive impairment (NCT02942888). Many other similar studies are currently underway to evaluate the therapeutic potential of NAD+ intermediates for diverse human diseases (see clinicaltrials.gov). However, there still remains some uncertainly regarding the in vivo pharmacokinetics of NAD+ intermediates. Although both NR and NMN have been shown to boost intracellular NAD+, how these metabolites enter the cell remains unclear. One possibility is that there is an NMN transporter that allows for rapid uptake of NMN directly into the cell for intracellular conversion into NAD+ (Yoshino et al., 2018). However, this hypothesis is challenged by another school of thought that contends that extracellular NMN is first converted to NR before entering the cell (Nikiforov et al., 2011; Ratajczak et al., 2016). Further studies are essential to clarify this discrepancy. Future randomized clinical trials designed to evaluate safety and efficacy of NMN and NR, especially those that compare them head to head, will be particularly informative in clarifying whether one of these NAD+ intermediates has superior bioavailability.
7. Summary and Conclusions
Retinal degenerative diseases are a major cause of morbidity in the modern world. Visual impairment significantly diminishes the quality of life of patients. A significant challenge in preventing blindness caused by retinal diseases is the genetic and phenotypic heterogeneity of the diseases and a variable understanding of disease pathogenesis. This limited understanding has led to either the widespread use of drugs that treat disease manifestations in relatively late phases of the natural history rather than the underlying cause or, in many instances, a complete lack of treatment options altogether. Indeed, more research is necessary to identify novel therapeutics for early and targeted intervention. Some strategies, such as gene therapy and stem cell-based therapeutic approaches, have been proposed, although they have limitations, such as the fact that gene therapy would have to be tailored for the causative mutation of each individual disease. The ability to identify a unifying therapy for diverse retinal diseases would be highly attractive and would address a great clinical need.
The immense metabolic and energetic requirements of the retina and photoreceptors have been the subject of research for many decades. However, in the past decade, a concept has emerged that metabolic dysfunction may be a common link unifying various forms of retinal degeneration. In this review, we surveyed the current state of a newly established area of investigation examining the role of NAD+ and sirtuins in regulating retinal metabolism and in the pathogenesis of retinal degenerative diseases. Indeed, these studies suggest that NAD+ plays an essential role in regulating retinal metabolism both through its dehydrogenase activity and through its vital function as a co-substrate for NAD+-dependent enzymes such as sirtuins. Based on these findings, NAD+ intermediates or small molecules that modulate sirtuin function may have the potential to enhance retinal metabolism and treat blinding diseases (Fig 6). Although further research is essential before these findings can be translated from the bench to the bedside, they have strong potential to truly transform the standard of care for retinal degenerative diseases.
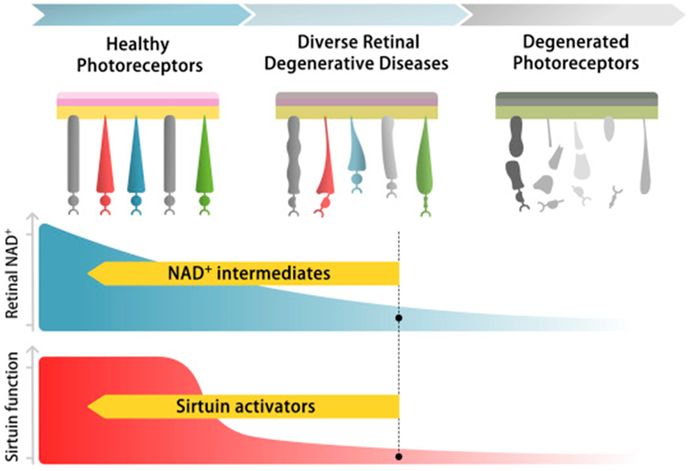
Figure 6.
We propose that decreased retinal NAD+ (middle panel) and consequent sirtuin dysfunction (bottom panel) contribute to photoreceptor dysfunction and death in multiple retinal degenerative diseases (top panel). Future therapies directed towards enhancing NAD+ availability (middle panel) and/or restoring optimal sirtuin function (bottom panel) may thus have efficacy for preventing blindness regardless of the underlying disease etiology.
Acknowledgements
This work was supported by the National Institutes of Health (R01 EY019287, P30 EY02687, T32 GM07200, UL1 TR002345, TL1 TR002344); the Starr Foundation (R.S.A.); the Carl Marshall Reeves and Mildred Almen Reeves Foundation (R.S.A.); the Bill and Emily Kuzma Family Gift for retinal research (R.S.A.); Research to Prevent Blindness (R.S.A.); the Jeffrey Fort Innovation Fund (R.S.A.); the Glenn Foundation (R.S.A.); and the Thome Foundation (R.S.A.). Additional funding comes from an unrestricted grant to the Department of Ophthalmology and Visual Sciences of Washington University School of Medicine from Research to Prevent Blindness. R.S.A. is a co-founder of Metro International, which is developing NMN-based therapeutics. The authors would like to thank Andrea Santeford and Teresa Chen for technical assistance and Danyel Cavazos for help with scientific illustrations.
8. References
- Age-Related Eye Disease Study Research, G., 2001. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation With Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. 8. Archives of ophthalmology 119, 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A 3rd, Li YY, Heher EC, Kimble CR, 1992. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. The Journal of neuroscience : the official journal of the Society for Neuroscience 12, 840–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J, 2004. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science (New York, N.Y.) 305, 1010–1013. [DOI] [PubMed] [Google Scholar]
- Bakondi B, Lv W, Lu B, Jones MK, Tsai Y, Kim KJ, Levy R, Akhtar AA, Breunig JJ, Svendsen CN, Wang S, 2016. In Vivo CRISPR/Cas9 Gene Editing Corrects Retinal Dystrophy in the S334ter-3 Rat Model of Autosomal Dominant Retinitis Pigmentosa. Molecular therapy : the journal of the American Society of Gene Therapy 24, 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban N, Ozawa Y, Inaba T, Miyake S, Watanabe M, Shinmura K, Tsubota K, 2013. Light-dark condition regulates sirtuin mRNA levels in the retina. Experimental gerontology 48, 1212–1217. [DOI] [PubMed] [Google Scholar]
- Ban N, Ozawa Y, Osada H, Lin JB, Toda E, Watanabe M, Yuki K, Kubota S, Apte RS, Tsubota K, 2017. Neuroprotective role of retinal SIRT3 against acute photo-stress. NPJ aging and mechanisms of disease 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhisitkul RB, Mendes TS, Rofagha S, Enanoria W, Boyer DS, Sadda SR, Zhang K, 2015. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. American journal of ophthalmology 159, 915–924. e912. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Sinclair DA, 2016. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nature reviews. Molecular cell biology 17, 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnight ER, Gupta M, Wiley LA, Anfinson KR, Tran A, Triboulet R, Hoffmann JM, Klaahsen DL, Andorf JL, Jiao C, Sohn EH, Adur MK, Ross JW, Mullins RF, Daley GQ, Schlaeger TM, Stone EM, Tucker BA, 2017. Using CRISPR-Cas9 to Generate Gene-Corrected Autologous iPSCs for the Treatment of Inherited Retinal Degeneration. Molecular therapy : the journal of the American Society of Gene Therapy 25, 1999–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Pereira J, Tarrago MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, Chini EN, 2016. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell metabolism 23, 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RC, Shi L, Huang CC, Kim AJ, Ko ML, Zhou B, Ko GY, 2015. High-Fat Diet-Induced Retinal Dysfunction. Investigative ophthalmology & visual science 56, 2367–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumet-Riffaud AE, Chaumet-Riffaud P, Cariou A, Devisme C, Audo I, Sahel JA, Mohand-Said S, 2017. Impact of Retinitis Pigmentosa on Quality of Life, Mental Health, and Employment Among Young Adults. American journal of ophthalmology 177, 169–174. [DOI] [PubMed] [Google Scholar]
- Chen J, Michan S, Juan AM, Hurst CG, Hatton CJ, Pei DT, Joyal JS, Evans LP, Cui Z, Stahl A, Sapieha P, Sinclair DA, Smith LE, 2013. Neuronal sirtuin1 mediates retinal vascular regeneration in oxygen-induced ischemic retinopathy. Angiogenesis 16, 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang PW, Wang J, Chen Y, Fu Q, Zhong J, Chen Y, Yi X, Wu R, Gan H, Shi Y, Chen Y, Barnett C, Wheaton D, Day M, Sutherland J, Heon E, Weleber RG, Gabriel LA, Cong P, Chuang K, Ye S, Sallum JM, Qi M, 2012. Exome sequencing identifies NMNAT1 mutations as a cause of Leber congenital amaurosis. Nature genetics 44, 972–974. [DOI] [PubMed] [Google Scholar]
- Chinchore Y, Begaj T, Wu D, Drokhlyansky E, Cepko CL, 2017. Glycolytic reliance promotes anabolism in photoreceptors. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL 3rd, 2005. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology 112, 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland SG, 1987. A comparison of oscillatory potential and pattern electroretinogram measures in diabetic retinopathy. Documenta ophthalmologica. Advances in ophthalmology 66, 207–218. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Medeiros NE, Millican CL, 1996. Photoreceptor loss in age-related macular degeneration. Investigative ophthalmology & visual science 37, 1236–1249. [PubMed] [Google Scholar]
- Du Y, Veenstra A, Palczewski K, Kern TS, 2013. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proceedings of the National Academy of Sciences of the United States of America 110, 16586–16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte DA, Rosales MA, Papadimitriou A, Silva KC, Amancio VH, Mendonca JN, Lopes NP, de Faria JB, de Faria JM, 2015. Polyphenol-enriched cocoa protects the diabetic retina from glial reaction through the sirtuin pathway. The Journal of nutritional biochemistry 26, 64–74. [DOI] [PubMed] [Google Scholar]
- Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J, 2017. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD+ Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron 93, 1334–1343. e1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MJ, Zhang Q, Nakamaru-Ogiso E, Kannabiran C, Fonseca-Kelly Z, Chakarova C, Audo I, Mackay DS, Zeitz C, Borman AD, Staniszewska M, Shukla R, Palavalli L, Mohand-Said S, Waseem NH, Jalali S, Perin JC, Place E, Ostrovsky J, Xiao R, Bhattacharya SS, Consugar M, Webster AR, Sahel JA, Moore AT, Berson EL, Liu Q, Gai X, Pierce EA, 2012. NMNAT1 mutations cause Leber congenital amaurosis. Nature genetics 44, 1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick EK, Ong PG, Man RE, Cheng CY, Sabanayagam C, Wong TY, Lamoureux EL, 2016. Association of Vision Impairment and Major Eye Diseases With Mobility and Independence in a Chinese Population. JAMA ophthalmology 134, 1087–1093. [DOI] [PubMed] [Google Scholar]
- Frederick DW, Loro E, Liu L, Davila A Jr., Chellappa K, Silverman IM, Quinn WJ 3rd, Gosai SJ, Tichy ED, Davis JG, Mourkioti F, Gregory BD, Dellinger RW, Redpath P, Migaud ME, Nakamaru-Ogiso E, Rabinowitz JD, Khurana TS, Baur JA, 2016. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell metabolism 24, 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Petzold S, Korner A, Imai S, Kiess W, 2009. Nampt: linking NAD biology, metabolism and cancer. Trends in endocrinology and metabolism: TEM 20, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J, 2015. SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science (New York, N.Y.) 348, 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald SH, Charette JR, Staniszewska M, Shi LY, Brown SD, Stone L, Liu Q, Hicks WL, Collin GB, Bowl MR, Krebs MP, Nishina PM, Pierce EA, 2016. Mouse Models of NMNAT1-Leber Congenital Amaurosis (LCA9) Recapitulate Key Features of the Human Disease. The American journal of pathology 186, 1925–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R, 2005. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America 102, 7227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison WW, Bearse MA Jr., Ng JS, Jewell NP, Barez S, Burger D, Schneck ME, Adams AJ, 2011. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investigative ophthalmology & visual science 52, 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Dange M, McGee TL, Berson EL, Dryja TP, Colman RF, 2008. Insights from retinitis pigmentosa into the roles of isocitrate dehydrogenases in the Krebs cycle. Nature genetics 40, 1230–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesterbeek TJ, van der Aa HPA, van Rens G, Twisk JWR, van Nispen RMA, 2017. The incidence and predictors of depressive and anxiety symptoms in older adults with vision impairment: a longitudinal prospective cohort study. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists) 37, 385–398. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA, 2003. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196. [DOI] [PubMed] [Google Scholar]
- Imai S, Yoshino J, 2013. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes, obesity & metabolism 15 Suppl 3, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Yamane S, Sato S, Sakamaki K, Arakawa A, Kadonosono K, 2016. Comparison of Time to Retreatment and Visual Function Between Ranibizumab and Aflibercept in Age-Related Macular Degeneration. American journal of ophthalmology 169, 95–103. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L, 1999. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes & development 13, 2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee DW, Chang YS, Kim JW, Kim CG, 2016. Twelve-month outcomes of treatment using ranibizumab or aflibercept for neovascular age-related macular degeneration: a comparative study. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 254, 2101–2109. [DOI] [PubMed] [Google Scholar]
- Koenekoop RK, Wang H, Majewski J, Wang X, Lopez I, Ren H, Chen Y, Li Y, Fishman GA, Genead M, Schwartzentruber J, Solanki N, Traboulsi EI, Cheng J, Logan CV, McKibbin M, Hayward BE, Parry DA, Johnson CA, Nageeb M, Poulter JA, Mohamed MD, Jafri H, Rashid Y, Taylor GR, Keser V, Mardon G, Xu H, Inglehearn CF, Fu Q, Toomes C, Chen R, 2012. Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nature genetics 44, 1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooragayala K, Gotoh N, Cogliati T, Nellissery J, Kaden TR, French S, Balaban R, Li W, Covian R, Swaroop A, 2015. Quantification of Oxygen Consumption in Retina Ex Vivo Demonstrates Limited Reserve Capacity of Photoreceptor Mitochondria. Investigative ophthalmology & visual science 56, 8428–8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Santos JM, Zhong Q, 2014. Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Investigative ophthalmology & visual science 55, 5653–5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Kurihara T, Ebinuma M, Kubota M, Yuki K, Sasaki M, Noda K, Ozawa Y, Oike Y, Ishida S, Tsubota K, 2010. Resveratrol prevents light-induced retinal degeneration via suppressing activator protein-1 activation. The American journal of pathology 177, 1725–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Ozawa Y, Kurihara T, Sasaki M, Yuki K, Miyake S, Noda K, Ishida S, Tsubota K, 2011. Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Investigative ophthalmology & visual science 52, 9142–9148. [DOI] [PubMed] [Google Scholar]
- Lin JB, Kubota S, Ban N, Yoshida M, Santeford A, Sene A, Nakamura R, Zapata N, Kubota M, Tsubota K, Yoshino J, Imai SI, Apte RS, 2016. NAMPT-Mediated NAD(+) Biosynthesis Is Essential for Vision In Mice. Cell reports 17, 69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L, 2000. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science (New York, N.Y.) 289, 2126–2128. [DOI] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR, 2006. Retinal repair by transplantation of photoreceptor precursors. Nature 444, 203–207. [DOI] [PubMed] [Google Scholar]
- Malek G, Johnson LV, Mace BE, Saloupis P, Schmechel DE, Rickman DW, Toth CA, Sullivan PM, Bowes Rickman C, 2005. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proceedings of the National Academy of Sciences of the United States of America 102, 11900–11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, Terada M, Nomiya Y, Tanishima S, Nakamura M, Kamao H, Sugita S, Onishi A, Ito T, Fujita K, Kawamata S, Go MJ, Shinohara C, Hata KI, Sawada M, Yamamoto M, Ohta S, Ohara Y, Yoshida K, Kuwahara J, Kitano Y, Amano N, Umekage M, Kitaoka F, Tanaka A, Okada C, Takasu N, Ogawa S, Yamanaka S, Takahashi M, 2017. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. The New England journal of medicine 376, 1038–1046. [DOI] [PubMed] [Google Scholar]
- Michan S, Juan AM, Hurst CG, Cui Z, Evans LP, Hatton CJ, Pei DT, Ju M, Sinclair DA, Smith LE, Chen J, 2014. Sirtuin1 over-expression does not impact retinal vascular and neuronal degeneration in a mouse model of oxygen-induced retinopathy. PloS one 9, e85031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, Yoshino J, Imai SI, 2016. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell metabolism 24, 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortuza R, Feng B, Chakrabarti S, 2014. miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia 57, 1037–1046. [DOI] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J, 2013. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 154, 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P, 2009. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science (New York, N.Y.) 324, 654–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, Tan PL, Oh EC, Merriam JE, Souied E, Bernstein PS, Li B, Frederick JM, Zhang K, Brantley MA Jr., Lee AY, Zack DJ, Campochiaro B, Campochiaro P, Ripke S, Smith RT, Barile GR, Katsanis N, Allikmets R, Daly MJ, Seddon JM, 2010. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proceedings of the National Academy of Sciences of the United States of America 107, 7395–7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov A, Dölle C, Niere M, Ziegler M, 2011. Pathways and Subcellular Compartmentation of NAD Biosynthesis in Human Cells: FROM ENTRY OF EXTRACELLULAR PRECURSORS TO MITOCHONDRIAL NAD GENERATION. The Journal of Biological Chemistry 286, 21767–21778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa H, Sampath AP, Laughlin SB, Fain GL, 2008. ATP consumption by mammalian rod photoreceptors in darkness and in light. Current biology : CB 18, 1917–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, Duran Y, Smith AJ, Chuang JZ, Azam SA, Luhmann UF, Benucci A, Sung CH, Bainbridge JW, Carandini M, Yau KW, Sowden JC, Ali RR, 2012. Restoration of vision after transplantation of photoreceptors. Nature 485, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Gonzalez-Cordero A, West EL, Ribeiro JR, Aghaizu N, Goh D, Sampson RD, Georgiadis A, Waldron PV, Duran Y, Naeem A, Kloc M, Cristante E, Kruczek K, Warre-Cornish K, Sowden JC, Smith AJ, Ali RR, 2016. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nature communications 7, 13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D, 2010. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell metabolism 12, 662–667. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Bligard GW, Zhang S, Yin L, Lukasiewicz P, Semenkovich CF, 2016. Functional Deficits Precede Structural Lesions in Mice With High-Fat Diet-Induced Diabetic Retinopathy. Diabetes 65, 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J, 2009. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science (New York, N.Y.) 324, 651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Joffraud M, Trammell SA, Ras R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud ME, Auwerx J, Yanes O, Brenner C, Canto C, 2016. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nature communications 7, 13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S, 2007. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell metabolism 6, 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, Wittes J, Pappas J, Elci O, McCague S, Cross D, Marshall KA, Walshire J, Kehoe TL, Reichert H, Davis M, Raffini L, George LA, Hudson FP, Dingfield L, Zhu X, Haller JA, Sohn EH, Mahajan VB, Pfeifer W, Weckmann M, Johnson C, Gewaily D, Drack A, Stone E, Wachtel K, Simonelli F, Leroy BP, Wright JF, High KA, Maguire AM, 2017. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet (London, England) 390, 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahaboglu A, Tanimoto N, Bolz S, Garrido MG, Ueffing M, Seeliger MW, Lowenheim H, Ekstrom P, Paquet-Durand F, 2014. Knockout of PARG110 confers resistance to cGMP-induced toxicity in mammalian photoreceptors. Cell death & disease 5, e1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahaboglu A, Tanimoto N, Kaur J, Sancho-Pelluz J, Huber G, Fahl E, Arango-Gonzalez B, Zrenner E, Ekstrom P, Lowenheim H, Seeliger M, Paquet-Durand F, 2010. PARP1 gene knock-out increases resistance to retinal degeneration without affecting retinal function. PloS one 5, e15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T, Postel K, Stutzki H, Kurth T, Zeck G, Ader M, 2015. Daylight vision repair by cell transplantation. Stem cells (Dayton, Ohio) 33, 79–90. [DOI] [PubMed] [Google Scholar]
- Sarwar S, Clearfield E, Soliman MK, Sadiq MA, Baldwin AJ, Hanout M, Agarwal A, Sepah YJ, Do DV, Nguyen QD, 2016. Aflibercept for neovascular age-related macular degeneration. The Cochrane database of systematic reviews 2, Cd011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Iijima H, Imai M, Abe K, Shibuya T, 1994. Photopic electroretinogram implicit time in diabetic retinopathy. Japanese journal of ophthalmology 38, 178–184. [PubMed] [Google Scholar]
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R, 2015. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet (London, England) 385, 509–516. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Tan G, Hosseini H, Nagiel A, 2016. Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Investigative ophthalmology & visual science 57, ORSFc1–9. [DOI] [PubMed] [Google Scholar]
- Sene A, Chin-Yee D, Apte RS, 2015. Seeing through VEGF: innate and adaptive immunity in pathological angiogenesis in the eye. Trends in molecular medicine 21, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S, Chowers I, Edwards PA, Baldan A, Parks JS, Ory DS, Apte RS, 2013. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell metabolism 17, 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman DM, Ross K, Sande PH, Kubota S, Ramaswamy S, Apte RS, Mostoslavsky R, 2014. SIRT6 is required for normal retinal function. PloS one 9, e98831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L, 2014. Small-molecule allosteric activators of sirtuins. Annual review of pharmacology and toxicology 54, 363–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Wozniak DF, Dearborn JT, Kubota S, Apte RS, Izumi Y, Zorumski CF, Imai S, 2014. Expression of Nampt in hippocampal and cortical excitatory neurons is critical for cognitive function. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 5800–5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromsdorfer KL, Yamaguchi S, Yoon MJ, Moseley AC, Franczyk MP, Kelly SC, Qi N, Imai S, Yoshino J, 2016. NAMPT-Mediated NAD(+) Biosynthesis in Adipocytes Regulates Adipose Tissue Function and Multi-organ Insulin Sensitivity in Mice. Cell reports 16, 1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR, 2004. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Investigative ophthalmology & visual science 45, 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey CB, Kelly U, Saban DR, Bowes Rickman C, 2015. Regulation of age-related macular degeneration-like pathology by complement factor H. Proceedings of the National Academy of Sciences of the United States of America 112, E3040–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veleri S, Lazar CH, Chang B, Sieving PA, Banin E, Swaroop A, 2015. Biology and therapy of inherited retinal degenerative disease: insights from mouse models. Disease models & mechanisms 8, 109–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E, 2015. NAD(+) in aging, metabolism, and neurodegeneration. Science (New York, N.Y.) 350, 1208–1213. [DOI] [PubMed] [Google Scholar]
- Waldron PV, Di Marco F, Kruczek K, Ribeiro J, Graca AB, Hippert C, Aghaizu ND, Kalargyrou AA, Barber AC, Grimaldi G, Duran Y, Blackford SJI, Kloc M, Goh D, Zabala Aldunate E, Sampson RD, Bainbridge JWB, Smith AJ, Gonzalez-Cordero A, Sowden JC, Ali RR, Pearson RA, 2018. Transplanted Donor- or Stem Cell-Derived Cone Photoreceptors Can Both Integrate and Undergo Material Transfer in an Environment-Dependent Manner. Stem cell reports 10, 406–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Q, Bao R, Zhang N, Wang Y, Polo-Parada L, Tarim A, Alemifar A, Han X, Wilkins HM, Swerdlow RH, Wang X, Ding S, 2017. Deletion of Nampt in Projection Neurons of Adult Mice Leads to Motor Dysfunction, Neurodegeneration, and Death. Cell reports 20, 2184–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, Smithies O, John SW, 2017. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science (New York, N.Y.) 355, 756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY, 2014. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet. Global health 2, e106–116. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, 2010. Energy metabolism of the visual system. Eye and brain 2, 99–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WH, Tsai YT, Justus S, Lee TT, Zhang L, Lin CS, Bassuk AG, Mahajan VB, Tsang SH, 2016. CRISPR Repair Reveals Causative Mutation in a Preclinical Model of Retinitis Pigmentosa. Molecular therapy : the journal of the American Society of Gene Therapy 24, 1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MJ, Yoshida M, Johnson S, Takikawa A, Usui I, Tobe K, Nakagawa T, Yoshino J, Imai S, 2015. SIRT1-Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell metabolism 21, 706–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Baur JA, Imai SI, 2018. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell metabolism 27, 513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka TS, Singh J, Dhawan P, Liederer BM, Oeh J, Kauss MA, Xiao Y, Zak M, Lin T, McCray B, La N, Nguyen T, Beyer J, Farman C, Uppal H, Dragovich PS, O’Brien T, Sampath D, Misner DL, 2015. Retinal toxicity, in vivo and in vitro, associated with inhibition of nicotinamide phosphoribosyltransferase. Toxicological sciences : an official journal of the Society of Toxicology 144, 163–172. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Yang K, 2015. Sirtuin 1 participates in the process of age-related retinal degeneration. Biochemical and biophysical research communications 468, 167–172. [DOI] [PubMed] [Google Scholar]
- Zhang L, Du J, Justus S, Hsu CW, Bonet-Ponce L, Wu WH, Tsai YT, Wu WP, Jia Y, Duong JK, Mahajan VB, Lin CS, Wang S, Hurley JB, Tsang SH, 2016. Reprogramming metabolism by targeting sirtuin 6 attenuates retinal degeneration. The Journal of clinical investigation 126, 4659–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Chen H, Li J, Li T, Zheng B, Zheng Y, Jin H, He Y, Gu Q, Xu X, 2012. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes 61, 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]