Abstract
Pulmonary complications are a significant cause for morbidity and mortality in osteogenesis imperfecta (OI). However, to date, there have been few studies that have systematically evaluated pulmonary function in individuals with OI. We analyzed spirometry measurements, including forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1), in a large cohort of individuals with OI (n=217) enrolled in a multicenter, observational study. We show that individuals with the more severe form of the disease, OI type III, have significantly reduced FVC and FEV1 which do not follow the expected trends of the normal population. We also show that “normalization” of FVC and FEV1 using general population data to generate percent predicted values underestimates the pulmonary involvement in OI. Within each subtype of OI, we used linear mixed models to find potential correlations between FEV1 and FVC with the clinical variables including mobility, bisphosphonate use, and scoliosis. Our results are an important step in understanding the extent of pulmonary involvement in individuals with OI and for developing pulmonary endpoints for use in the routine patient care as well as in the investigation of new therapies.
Keywords: Osteogenesis imperfecta, pulmonary function, lung disease, spirometry
INTRODUCTION
Osteogenesis imperfecta (OI) refers to a phenotypically and genetically heterogeneous group of Mendelian disorders that typically manifest with increased bone fragility, recurrent fractures, bone deformities, short stature, hearing loss, and joint laxity1. OI can be caused by pathogenic variants in genes that encode: 1) proα1(I) and proα2(I) chains of type I collagen, 2) proteins required for the posttranslational modification, processing, and crosslinking of type I collagen, 3) components required for normal mineralization of bone, 4) transcription and signaling proteins required for the maturation and function of osteoblasts, and 5) genes whose functions are not completely understood to date1–4 More than 90% of OI occurs due to qualitative or quantitative abnormalities of type I procollagen4,5. Individuals with type I collagen- related OI are typically categorized by clinically severity into one of the four Sillence types: nondeforming (type I), perinatally lethal (type II), progressively deforming (type III), and common variable (type IV)2.
Type I collagen is widely expressed in the human body and is a component of the extracellular matrix of many tissues and organs. Unsurprisingly, individuals with OI can also manifest extraskeletal features that include pulmonary disease, muscle weakness, and cardiovascular abnormalities6–10. Pulmonary disease is a significant contributor to the mortality and morbidity in OI. In a register-based, nationwide cohort study from Denmark, Folkstead et al. reported that the subhazard ratio for deaths caused by respiratory diseases was 3 times higher in OI as compared to the reference population11,12. The burden of pulmonary disease in OI patients can be observed even during the neonatal period. Yimgang et al. assessed the at-birth health outcomes of 77 neonates with OI (60 with OI type I, 4 with OI type III, and 13 with OI type IV) and reported that 22% had respiratory complications in the neonatal period13. These data demonstrate that pulmonary disease is a major contributor to the morbidity and mortality in OI, especially in the more severe forms. To date, only 6 studies have systematically assessed pulmonary function using spirometry in individuals with OI8–10,14–16. Most of these studies were limited by small sample size. The largest study, to date, conducted by Wekre et al enrolled predominantly individuals with OI type I and had only 3 individuals with OI type III.
Here, we investigated the pulmonary functions in 217 individuals with OI by the use of spirometry. The participants were enrolled in a multicenter, observational, longitudinal study conducted by the OI Linked Clinical Research Centers (LCRC). The study population included children and adults with the mild, moderate, and severe forms of type I collagen-related OI. We present the observed and predicted values for forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1) in each subtype of OI. We show that the FVC and FEV1 in individuals with OI type III are significantly reduced and that the “normalization” method to calculate the percent predicted values may underestimate the pulmonary involvement in OI. Furthermore, we show potential correlations between FVC and FEV1 with clinical covariates like mobility, bisphosphonate use, and scoliosis and propose how these variables could be used to develop a OI-specific regression model to assess spirometry in OI.
MATERIALS AND METHODS
Study Population
The establishment of the LCRC and subjects enrolled in the Longitudinal Study of Osteogenesis Imperfecta have been previously described5,17. Participants were enrolled at one of the five clinical sites: Baylor College of Medicine (Houston, TX); Kennedy Krieger Institute (Baltimore, MD) and Nemours/Alfred I. DuPont Hospital for Children (Wilmington, DE); Oregon Health & Science University and Shriners Hospital for Children (Portland, OR); Shriners Hospital for Children (Chicago, IL); and Shriners Hospital for Children (Montreal, QC). The Collagen Diagnostic Laboratory at the University of Washington performed molecular and biochemical analyses. The study procedures were approved by the Institutional Review Boards of all participating clinical sites and informed consent was obtained from subjects or their legal guardians. The data collected from all of the sites were managed by the NIH Rare Disease Clinical Research Network’s (RDCRN) Data Management Coordinating Center at the College of Medicine, University of South Florida (Tampa, FL).
Overall of the 558 participants enrolled in the longitudinal study, spirometry data were available from 217 individuals (N=107 for OI type I, N=38 for OI type III, and N=55 for OI type IV). Spirometry data were also available on small numbers of individuals with rarer forms of OI, including OI type V (n=5), OI type VI (n=1), and OI type VII (n=2). The classification of type I collagen-related OI was made based on specific clinical characteristics that were outlined in the manual of operations (Supplementary Table 1) and whenever available, genotypic data were also used to determine the subtype of OI.
Data Collection
The following data were collected for analyses from the enrollment visit of the study: age, gender, OI subtype, race and ethnicity, family history of OI (yes or no), height in cm, weight in kg, arm span in cm, ambulatory status (wheelchair bound or not), and presence or absence of scoliosis. These data were collected in a standardized manner across all sites according to instructions outlined in the manual of operations and were reported using online case report forms5. Height, defined as the vertical distance between crown of head and soles of feet, was measured using a wall-mounted stadiometer and recorded to the nearest 0.1 cm. When participants could not stand, supine length was measured from the heels to the top of the head. Arm span was measured as the distance from one furthermost fingertip to the other furthermost fingertip when the participant’s arms were stretched out horizontally using a nonstretching long measuring tape to the nearest 0.1 cm. Arm span was measured in a single measure and was not a composite of multiple measurements. Arm span was also used to calculate height as previously described using the following formulae: 1) For Caucasian males, height = arm span/1.03, 2) for African American men, height = arm span/1.06, and 3) for women height = arm span/1.0118–20.
Spirometry was used to measure FVC and FEV1 in participants older than 6 years of age. FVC is the maximal volume of air that can be exhaled with maximal effort from a position of full inspiration and is typically reduced with airflow limitation or decreased lung capacity. FEV1 is the maximal volume of air that can be exhaled in the first second of a forced exhalation following a full inspiration and is the most important spirometric variable for assessment of the severity of airflow obstruction. For spirometry, the equipment preparation, calibration checks, participant preparation, and test administration were standardized across all sites (Supplemental information S1). For each participant, spirometry test was repeated until: three acceptable spirograms were obtained, a maximum of 8 tests had been performed, or the participant could no longer continue in the single session. Spirograms were deemed acceptable if they were free of artifacts, had good start volumes (volume <5% of FVC or 0.15 L, whichever was greater), and satisfactory exhalation (expiratory time duration of ≥6 seconds for individuals over 10 years of age OR ≥3 seconds for individuals under 10 years of age, OR a plateau in the volume-time curve with evidence of a continuously forced expiratory effort, where a plateau is defined as no change in volume for at least one second). Individual spirometry test sessions were concluded if the two largest values of FVC were within 0.15L of each other, and if the two largest values of FEV1 were within 0.15 L of each other21,22.
Statistical Analysis
The Z-scores for height for children (age < 20 years) were calculated using growth data from the Centers for Disease Control and Prevention (CDC)23. The height Z-scores for adults 20 years and older were calculated using the using the mean final adult height (SD) of 176.8 cm (6.7 cm) and 163.3 cm (6.1 cm) for males and females, respectively from the CDC growth curves23. To visualize the trends in spirometry measures, the observed values for FVC and FEV1 in L in OI types I, III and IV were plotted using scatter plots and non-parametric local regression (LOESS) smoothing curves were added for each OI type. The predicted values of FVC and FEV1 in L and FEV1/FVC ratio were calculated using reference population data generated by Hankinson et al24 The predicted FVC and FEV1 values in individuals with OI were compared with the predicted values for an age- and sex matched Caucasian population obtained from the NHANES database24,25. Percent predicted values for FEV1 and FVC were calculated by the formula (observed values/predicted value) × 100.
To elucidate the relationships between FVC and FEV1 with clinical covariates including age, sex, stature, mobility, scoliosis, and treatment with bisphosphonates, we developed separate linear models for OI types I, III, and IV using regression models (R)26. Hankinson et al. have previously shown that in the general population, FVC and FEV1 correlate with age, gender, height, age squared (age2), and height squared (height2). We developed a regression model using these covariates and further refined the model by the addition of independent variables specific to OI. This model included age, gender, height, age squared (age2), height squared (height2), wheelchair bound (yes or no), scoliosis (yes or no), and history of bisphosphonate use (yes or no) as well as interaction terms between gender and age, gender and age2, and gender and height2. The model was refined by step-wise elimination of each covariate until a combination of covariates reached the smallest AIC (Akaike Information Criterion) value by applying stepAIC() function in MASS library in R.
RESULTS
The characteristics of individuals enrolled in the longitudinal study with spirometry data available are outlined in Table 1. As would be expected, individuals with type I collagen-related OI, the most common form of the disorder, accounted for 92% of all participants. The number of individuals with the rarer forms of OI were limited (n=16) and thus, this population was excluded from statistical analyses.
Table 1:
Characteristics of individuals with OI enrolled in the study
| OI I | OI III | OI IV | Others | |
|---|---|---|---|---|
| Female n (%) | 55 (52.8) | 24 (64.8) | 34 (65.3) | 10 (62.5) |
| Mean age in years (range) | 27.2 (6.1–67.2) | 23.9 (7.8–53.9) | 25.0 (6.8–62.7) | 17.1 (6.2–48.0) |
| Black | 0 (0) | 3 (8.1) | 7 (13.5) | 2 (12.5) |
| Scoliosis n (%) | 28 (26.9) | 29 (78.4) | 22 (42.3) | 10 (62.5) |
| Wheelchair bound n (%) | 3 (2.9) | 19 (51.4) | 9 (17.3) | 9 (56.2) |
| History of bisphosphonate use n (%) | 49 (47.1) | 30 (81.1) | 34 (65.4) | 10 (62.5) |
Observed FEV1 and FVC in OI
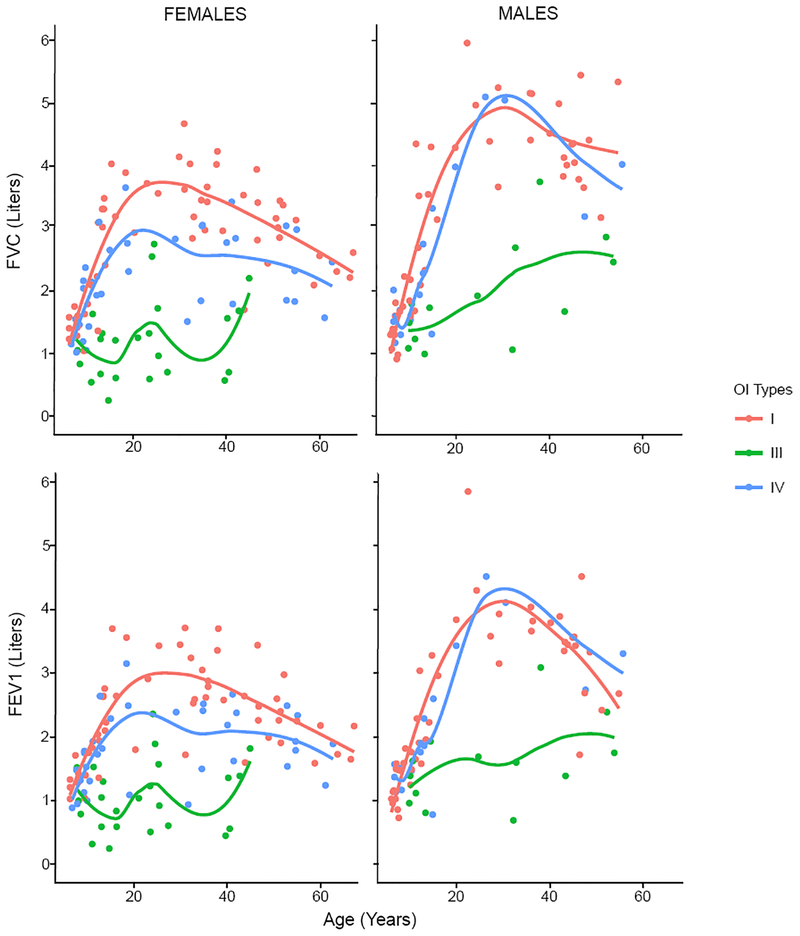
Across all ages, both males and females with OI type III had lower FVC and FEV1 than those with OI types I and IV (Figure 1). The differences were most apparent between the second and fourth decades of life. FVC and FEV1 were lower in females with OI type IV than those with OI type I, but a similar difference was not observed in males. FVC and FEV1 increased during the first three decades of life and gradually decreased thereafter in individuals with OI types I and IV. This is consistent with and comparable to the pattern in the general population24. The age-related increases were significantly blunted in OI type III. A FEV1 of less than 1.5 L is considered as a marker of moderate airway impairment in the general adult population irrespective of the predicted value27,28. Whereas no adults with OI type I and only two adults with OI type IV (9.5%) had FEV1 of less than 1.5 L, 65% (11 out of 17) of adults with OI type III had FEV1 less than 1.5 L (χ2 test, p<0.0001). As FEV1 and FVC were both decreased proportionately, the FEV1/FVC ratios were similar between all three subtypes of collagen I-related OI (Supplementary Figure 1).
Figure 1:
Observed FVC and FEV1 in type I collagen-related OI. Each dot represents observed FVC and FEV1 from the baseline visit of a single participant. The Lines represent the LOESS regression lines for each OI type. Whereas OI types I and IV demonstrate age-related increase in FVC and FEV1, such changes are absent in OI type III.
Predicted FEV1 and FVC in OI
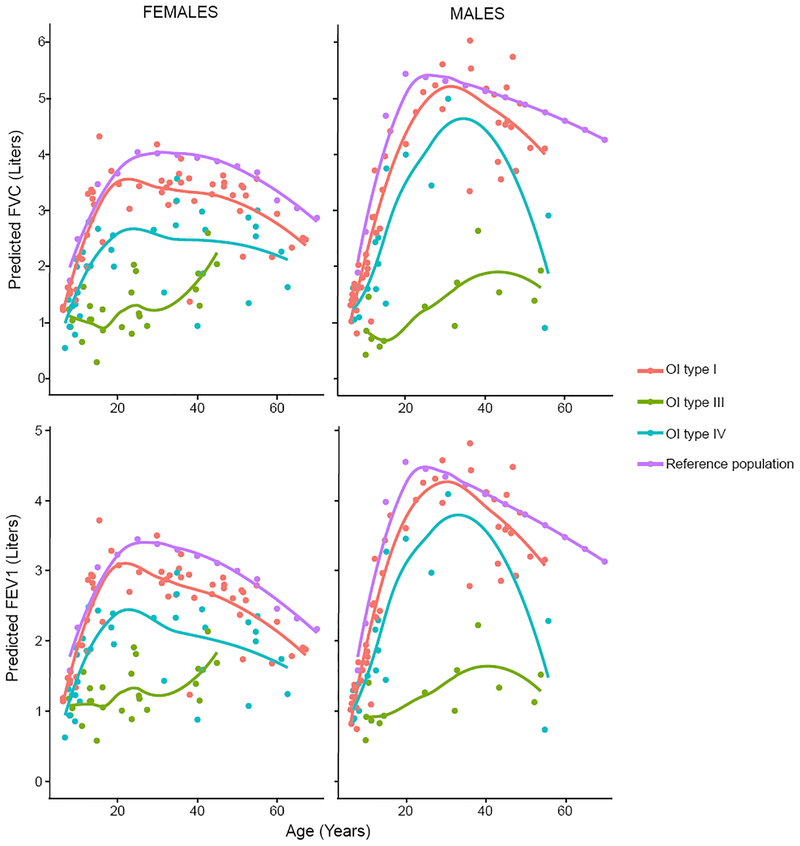
It is an accepted standard practice to “normalize” the observed FEV1 and FVC based on predictions for gender, race, age, and height of an individual21,22,25. We calculated the predicted FEV1 and FVC values based on regression for gender, race, age, and height from the general population data and subsequently calculated a percent predicted value for FVC and FEV1 in OI.24 Calculation of predicted volumes allowed for comparison of FVC and FEV1 in each OI type with the general population. As expected because of decreased height, the predicted FEV1 and FVC were lower in individuals with OI types III and IV compared to the general population and individuals with OI type l (Figure 2). The deviation from the predicted general population values was least for those with OI type I males. This is likely because predicted values correlate with height. The mean height Z-scores for OI type I was −1.2, whereas the Z-scores for OI types III and IV were −8.3 and −3.5, respectively (Supplementary Figure 2). Even though individuals with OI type III had lower observed FVC and FEV1, the “normalization” resulted in percent predicted values being no different from OI types I and IV (Supplementary figure 3).
Figure 2:
Predicted FVC and FEV1 values were calculated using reference population data generated by Hankinson et al. Each dot represents predicted FVC and FEV1 for a single participant. The lines represent the LOESS regression lines for control population and each OI type.
As the accurate measurement of height in type III and IV OI can be difficult due to bowing of lower limb bones, scoliosis, and inability to stand, we also calculated the height based on arm span18–20. The coefficient of correlations between the measured height and arm span calculated height were 0.89, 0.54, and 0.67 for OI types I, III, and IV, respectively; which suggested that measured height more closely correlated with arm span calculated height in milder forms of OI (Supplementary Figure 4). The predicted FEV1 and FVC were comparable when using either measured height or arm span calculated height in OI types I and IV, the predicted volumes changed significantly with the use of use of arm span imputed height in OI type III (Supplementary Figure 5).
Development of an OI-specific regression model for predicting FEV1 and FVC
One limitation of implementing the regression parameters developed by Hankinson et al. in individuals with OI is that the distribution of height in OI is different from the general population height. Additionally, the model does not account for limited mobility, scoliosis, and abnormal shape of the chest in OI. We attempted to develop linear models to refine calculating the predicted FEV1 and FVC in each subtype of type I collagen-related OI. A step-wise backward selection approach was used to select the covariates. The naïve model included age, height, gender, age2, height2, gender*age, gender*age2, gender*height2, mobility (wheel-chair bound or ambulatory), self-reported scoliosis (yes or no), history of bisphosphonate use (yes or no). As the vast majority of individuals in our cohort were white, race was omitted in the naïve model. After stepwise selection, the final models were generated and presented separately for males and females accounting for the interaction terms (Table 2).
Table 2:
Linear model to refine the predicted FVC and FEV1 in OI
| Intercept | Age (year) β1 | Age2 (year2) β2 | Height2 (cm) β3 | Mobility β4 | Scoliosis β5 | Bisphosphonate β6 | ||
|---|---|---|---|---|---|---|---|---|
| Female subjects | ||||||||
| OI type | ||||||||
| I | FEV1 | 0.06841 | 0.08875 | −0.00128 | 0.00005 | |||
| I | FVC | −0.28034 | 0.11847 | −0.00165 | 0.00007 | |||
| III | FEV1 | −0.93779 | 0.05093 | −0.00128 | 0.00014 | 0.31692 | ||
| III | FVC | −1.39049 | 0.10352 | −0.00214 | 0.00017 | −0.33132 | ||
| IV | FEV1 | 0.28869 | 0.04383 | −0.00066 | 0.00007 | −0.20545 | ||
| IV | FVC | −0.08906 | 0.07294 | −0.00107 | 0.00009 | |||
| Male subjects | ||||||||
| OI type | ||||||||
| I | FEV1 | −0.971 | 0.2153 | −0.00336 | 0.00005 | |||
| I | FVC | −0.97289 | 0.11847 | −0.00165 | 0.00012 | |||
| III | FEV1 | −0.82984 | 0.05093 | −0.00077 | 0.00014 | 0.31692 | ||
| III | FVC | −1.31875 | 0.10352 | −0.00133 | 0.00017 | −0.33132 | ||
| IV | FEV1 | −0.82372 | 0.16285 | −0.00218 | 0.00007 | −0.20545 | ||
| IV | FVC | −1.10702 | 0.18679 | −0.00251 | 0.00009 | |||
The linear model to evaluate correlation between FVC or FEV1 and other clinical covariates. FVC and FEV1 were calculated using the equation Intercept + β1 ∗ Age + β2 ∗ Age2 + β3 ∗ Height2 + β4 ∗ Mobility + β5 ∗ Scoliosis + β6 ∗ Bisphosphonate. The values in the table represent β coefficients. Cells without any numbers imply that the covariates were excluded in the stepwise selection used to refine the linear model. For age and height, nominal data were used. For mobility, wheel chair bound was represented as 0 and not wheel chair bound was represented as 1. For scoliosis, 0 represented no scoliosis and 1 represented presence of scoliosis. Similarly, history of ever exposure to bisphosphonates was represented as 0 and 1 represents history of treatment with BPN. R2 values for the model predicting variability in FVC and FEV1 across OI types were 0.70–0.79 and 0.63–0.72, respectively.
In OI type I, where 97% of individuals were ambulatory and only 27% reported scoliosis, we found that age, age2, and height2, correlated significantly with the observed FEV1 and FVC. This is similar to the regression results in the general population reported by Hankinson. In individuals with OI type III, not wheelchair bound was associated significantly with a higher FEV1 in both males and females, whereas presence of scoliosis showed a negative correlation with FVC. In OI type IV, a significant association between bisphosphonate use and FEV1 was observed.
DISCUSSION
To date, only 6 studies have systematically assessed pulmonary functions in OI8–10,14–16. In most of these studies, spirometry has been used as a primary measure of pulmonary function (Table 3). In one of these studies, LoMauro and colleagues performed a comprehensive analysis of lung functions in OI using standard spirometry, nocturnal oxygen saturation, radiographs, opto-electronic plethysmography and kinematic analysis and showed that in addition to abnormal spirometry volumes, individuals with OI type III had paradoxical inspiratory inward motion of the pulmonary rib cage and thoraco-abdominal asynchronies15. However, it is not possible to perform such sophisticated analyses in the clinical settings and most physicians use spirometry as the main measure of pulmonary function in routine patient care.
Table 3:
Studies that have evaluated pulmonary functions in Ol
| Study | Number enrolled | Age | Major Findings |
|---|---|---|---|
| Falvo et al 1973 | “severe disease” 4 “moderate disease” 6 “mild disease” 1 |
4–34 yrs |
|
| Widmann et al 1999 | “OI tarda” 8 “OI congenita” 7 |
21–45 yrs |
|
| Takken et al 2004 | OI I 17 | 8–21 yrs |
|
| Thiele et al 2012 | OI III 23 OI IV 23 |
4–20 yrs |
|
| LoMauro et al 2012 | OI III 7 OI IV 15 |
Mean (SD) OI III 26 (16) yrs Mean (SD) OI IV 16 (11) yrs |
|
| Wekre et al 2014 | OI I 60 OI III 3 OI IV 10 |
Mean (SD) 44 (12) |
|
In the current study, we measured FVC and FEV1 in 217 individuals with OI. The strengths of our study are the following: 1) to, date, this is the largest study to evaluate spirometry measures in OI, 2) the study population included both children and adults with varying degrees of severity, 3) the data were collected in a systematic manner from sites with extensive experience in the management of OI, 4) the sample size and other phenotypic characteristics allowed for robust statistical evaluations, and 5) patients were enrolled from across North America and thus our findings are more likely to be representative of the spirometry values from the OI population at large. We show that across all ranges, the FVC and FEV1 are significantly lower in individuals with OI type III when compared to individuals in the general population and those with OI types I and IV. Whereas the pattern of increase in FVC and FEV1 with age in individuals with OI types I and IV are consistent with the general population data albeit with lower volumes, there is significant blunting of the age-related increases in OI type III and two-thirds of adults with OI type III have FEV1 of less than 1.5 L.
A key question to be answered is whether these diminished volumes are “appropriate-for-size” in OI or whether they portend poor cardiopulmonary outcomes. In the NHANES I cohort, 4300 men and women (age range 25–74 years) were followed for an average of 13 years; a 1-liter decrement in FEV1 in the cohort, was associated with a 60% increase in mortality even when accounting for sex, race, age, serum cholesterol, systolic blood pressure, smoking status, alcohol consumption, and body mass index29,30. Similarly, in the Normative Aging Study of 1,956 men (age range 21–80 years) who were followed for 30 years, a 1-liter decrement in FEV1 at enrollment was associated with a relative risk of 1.67 for all-cause mortality (RR = 1.67, 95 percent CI 1.25–2.22) after adjusting for age, white cell count, serum cholesterol, systolic blood pressure, and smoking status31. In individuals with chronic obstructive lung disease (who by definition have decreased FEV1), lower FVC and FEV1 have not only been associated with increased overall mortality, but also outcomes during recovery from stressful situations such as acute exacerbations or cardiac surgery32,33. Whereas, the ability to predict the implications of low FVC and FEV1 in OI based on data from the general population and individuals with chronic obstructive pulmonary disease has significant limitations, it is possible that low pulmonary volumes in OI type III may interfere with the ability to respond to stressful situations like concomitant airway disease, pulmonary infections, and surgery.
In the general population, FVC and FEV1 are used to categorize the lung diseases into “restrictive” and “obstructive” categories. Restrictive lung physiology is characterized by symmetrically reduced lung volumes, typically defined by reduced FVC and FEV1 and thus a normal or increased FEV1/FVC ratio. Obstructive lung physiology characterized by resistance to expiratory airflow is defined by a FEV1/FVC ratio less than 0.7 (which is the lower limit of normal) and an FEV1 of less than 80 percent of predicted. A major limitation in the use of spirometric measures in OI is that the observed values have to be normalized based on age and stature of an individual. FVC and FEV1 measurements are typically compared to predicted values based on age, height, gender and ethnicity generated from the general population. We show that while this normalization process is appropriate for individuals with OI type I, it can lead to underestimation of the diminished FVC and FEV1 in individuals with OI type III. Thus, categorizing individuals with OI into “obstructive” and “restrictive” categories using the standard definitions is likely to be complicated. Using phenotypic data from the cohort, we tried to develop a regression model that uses age and height and incorporates mobility, scoliosis, and history of bisphosphonate use to predict FEV1 and FVC in OI types III and IV. These preliminary analyses showed that these covariates could be used to generate an OI-specific regression model.
There are limitations of the data set and the analyses that we have been able to perform. First, we have only conducted analyses on FVC and FEV1 from the enrollment visit. As only few individuals had repeated spirometric measures, we could not assess changes over time. Second, only FVC and FEV1 were systematically collected by electronic case report forms; maximal mid-expiratory flow rate (FEF25–75), forced expiratory volume in six seconds (FEV6), residual volume, total lung capacity, and diffusing capacity for carbon monoxide were not available. Third, treatment with bisphosphonates was used as a binary variable on an individual level; the dates of BPN treatment were not collected and thus analysis on the effects of long-term vs short-term treatment, remote vs concurrent treatment could not be performed. Fourth, scoliosis data was obtained by various methods including radiography, medical records, and self-reporting. The degree of scoliosis was not assessed systematically, and we could not correlate between varying degrees of scoliosis and lung functions. Fifth, due to clustering of the mobility data, effect of mobility could only be assessed using a dichotomous variable of whether individuals were mobile, or wheelchair bound.
Pulmonary disease in OI is multifactorial and can be a result of factors extrinsic to the lungs, as well as intrinsic lung abnormalities. Whereas the relevance of extrinsic factors, including short stature, immobility, scoliosis, recurrent rib fractures, muscle weakness, and chest wall abnormalities on pulmonary disease have been well documented10,14,15, the extent of intrinsic pulmonary abnormalities and the additional mechanisms that cause lung abnormalities in OI have been discovered recently using animal models of OI16,34–36. Thiele and colleagues studied Aga2/+ mice, a murine model of OI in which a single nucleotide change in the last intron introduces a new splice acceptor site which introduces a frame shift and extends the transcript beyond the usual stop site16,37. Mutations in humans that have the same effect of extending the transcript lead to stable mRNA but very unstable protein and an OI type I phenotype with marked intrafamilial variability38. In the Aga2/+ mice, Thiele and colleagues found diffuse pulmonary hemorrhage and inflammation that appeared to be independent of fracture16. Primary lung fibroblasts showed an altered gene expression profile consistent with altered markers of inflammation, hypoxia, and a generalized dysregulation of extracellular matrix. The subset of severely affected mice (Aga2severe) had decreased partial pressure of oxygen and increased partial pressure of carbon dioxide suggesting respiratory failure. It is to be noted that the Aga2severe mice also demonstrated right ventricular hypertrophy, abnormal cardiac collagen matrix, and decreased fractional shortening. abnormalities. In a different mouse model of severe form of type I collagen- related OI which harbors a splice site mutation in intron 9, Col1a1 (Col1a1Jrt/+), that results in skipping exon 9, Baglole and colleagues demonstrated alveolar airspace enlargement and that the number of fibers, thickness, and contractility of the diaphragm were reduced35. In a recessive murine model of OI (Crtap−/−) that recapitulates human OI type VIII, Baldridge and colleagues showed alveolar air space enlargement34,39. Subsequently, Grafe et al. showed that excessive transforming growth factor-β (TGF-β) signaling is a contributing mechanism for the pulmonary phenotype in Crtap−/− mice and that anti-TGFβ treatment, at least in part, rescues the phenotype of increased air space enlargement36. Whereas there are limited data regarding histological pulmonary abnormalities in humans with OI, decreased alveolar number and immature acinar development (similar to changes in the murine models), and pulmonary hypoplasia have reported in autopsies in the perinatal lethal form of OI40–43.
The findings in this study have implications for clinical care and identify areas where further research is needed. We recommend that clinicians assess pulmonary function in individuals with OI as part of the routine first evaluation. Spirometry should be considered in all individuals with OI, especially in those with OI types III and IV. Referral to a pulmonologist should be made based on symptomatology and spirometry results. While interpreting spirometry results, clinicians need to be aware of the limitations of calculating predicted and percent predicted values in OI. In individuals with no significant bone deformities or scoliosis, the predicted values may be calculated using measured height. In individuals with significant lower extremity bone deformities and scoliosis who do not have upper extremity long bone abnormalities, arm span calculated height may be more appropriate to calculate predicted values. In individuals with severe OI, who have multiple bone deformities, using either measured height or arm span calculated height is likely to underestimate the pulmonary involvement and, in such individuals, serial monitoring of observed FCV and FEV1 is probably the most reasonable way to assess pulmonary functions. In individuals who have low FEV1 or FVC, we recommend that periodic spirometry tests be done. In individuals with decreased pulmonary reserve, like adults with FEV1 less than 1.5 L, pneumococcal vaccination and annual influenza vaccination should be considered. Clinicians should also be aware that the dynamic stress on ribs caused during spirometry can increase the risk for rib fractures in individuals with severe forms of OI and appropriate precautions should be taken during performance of the test.
On the research front, we believe that this study is an important first step in developing OI-specific standards to predict FVC and FEV1 and in developing pulmonary endpoints for clinical research. Whereas FVC and FEV1 measurement remain the mainstays of evaluation of pulmonary physiology, more sophisticated measures may provide additional information. In assessing the contribution of abnormal chest wall architecture versus intrinsic lung abnormalities, the most direct, but somewhat invasive, method would be to measure intrapleural pressure using an esophageal balloon. Kyphoscoliosis, rib and vertebral fractures would lead to the generation of low negative intrapleural pressures during inhalation while restrictive lung disease due to connective tissue abnormalities would lead to the generation of very high negative intrapleural. Plethysmographic lung volumes, assessment of air flow and peak inspiratory and expiratory pressures, and the measurement of diffusing capacity and gas transfer will add to our understanding of the characteristics of the pulmonary physiology and risks in OI. Impulse oscillometry, a noninvasive, rapid, safe and validated technique that measures respiratory impedance is another modality that requires further study in OI.
In conclusion, we show that the normalization process used in spirometry analyses can underestimate the pulmonary involvement in severe forms of OI. We suggest that clinicians be aware of the limitations of spirometric measures when evaluating pulmonary function in OI.
Supplementary Material
Acknowledgements
The authors are very grateful to the participants and their families for their support. The study was developed and implemented by the support from the OI Foundation and Children’s Brittle Bone Foundation. This work was supported by the BBDC (1U54AR068069–0), a part of the NCATS’ RDCRN. BBDC is funded through a collaboration between the ORDR of NCATS, NIAMS, NICHD, and NIDCR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The BBDC is also supported by the OI Foundation. The work was supported by The Clinical Translational Core of BCM IDDRC (1U54HD083092) from the Eunice Kennedy Shriver NICHD. This work was also supported by Shriners of North America. AT was supported by T32GM07526–40. The authors would like to acknowledge the clinical research teams: M.Mullins, A.Tran, S.Carter (BCM); V.Vensel, J.Christie, A.Hata (OHSU); M.Durigova (Shriner’s, Montreal); and L.Davey (A.I. duPont Hospital).
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare for this work
REFERENCES
- 1.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363(9418):1377–1385. doi: 10.1016/S0140-6736(04)16051-0 [DOI] [PubMed] [Google Scholar]
- 2.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979; 16(2): 101–116. doi: 10.1136/jmg.16.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marini JC, Forlino A, Bächinger HP, et al. Osteogenesis imperfecta. Nat Rev Dis Prim. 2017;3:17052. [DOI] [PubMed] [Google Scholar]
- 4.Sykes B, Wordsworth P, Ogilvie D, Anderson J, Jones N. Osteogenesis imperfecta is linked to both type I collagen structural genes. Lancet. 1986;328(8498):69–72. doi: 10.1016/S0140-6736(86)91609-0 [DOI] [PubMed] [Google Scholar]
- 5.Patel RM, Nagamani SCS, Cuthbertson D, et al. A cross-sectional multicenter study of osteogenesis imperfecta in North America - results from the linked clinical research centers. Clin Genet. 2015;87(2):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folkestad L, Hald JD, Gram J, et al. Cardiovascular disease in patients with osteogenesis imperfecta — a nationwide, register-based cohort study. Int J Cardiol. 2016;225:250–257. [DOI] [PubMed] [Google Scholar]
- 7.Lamanna A, Fayers T, Clarke S, Parsonage W. Valvular and aortic diseases in osteogenesis imperfecta. Hear Lung Circ. 2013;22(10):801–810. [DOI] [PubMed] [Google Scholar]
- 8.Takken T, Terlingen HC, Helders PJM, Pruijs H, Van Der Ent CK, Engelbert RHH. Cardiopulmonary fitness and muscle strength in patients with osteogenesis imperfecta type I. J Pediatr. 2004;145(6):813–818. [DOI] [PubMed] [Google Scholar]
- 9.Wekre LL, Kjensli A, Aasand K, Falch JA, Eriksen EF. Spinal deformities and lung function in adults with osteogenesis imperfecta. Clin Respir J. 2014;8(4):437–443. [DOI] [PubMed] [Google Scholar]
- 10.Widmann RF, Bitan FD, Laplaza FJ, Burke SW, DiMaio MF, Schneider R. Spinal Deformity, Pulmonary Compromise, and Quality of Life in Osteogenesis Imperfecta. Spine (Phila Pa 1976). 1999;24(16):1673. [DOI] [PubMed] [Google Scholar]
- 11.Folkestad L, Hald JD, Canudas-Romo V, et al. Mortality and Causes of Death in Patients With Osteogenesis Imperfecta: A Register-Based Nationwide Cohort Study. J Bone Miner Res. 2016;31(12):2159–2166. [DOI] [PubMed] [Google Scholar]
- 12.Folkestad L. Mortality and morbidity in patients with osteogenesis imperfecta in Denmark. Dan Med J. 2018;65(4). [PubMed] [Google Scholar]
- 13.Yimgang DP, Brizola E, Shapiro JR. Health outcomes of neonates with osteogenesis imperfecta: a cross-sectional study. J Matern Neonatal Med. 2016;29(23):3889–3893. [DOI] [PubMed] [Google Scholar]
- 14.Falvo KA, Klain DB, Krauss AN, Root L, Auld PA. Pulmonary function studies in osteogenesis imperfecta. Am Rev Respir Dis. 1973;108(5):1258–1260. [DOI] [PubMed] [Google Scholar]
- 15.LoMauro A, Pochintesta S, Romei M, et al. Rib Cage Deformities Alter Respiratory Muscle Action and Chest Wall Function in Patients with Severe Osteogenesis Imperfecta. West J, ed. PLoS One. 2012;7(4):e35965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiele F, Cohrs CM, Flor A, et al. Cardiopulmonary dysfunction in the osteogenesis imperfecta mouse model Aga2 and human patients are caused by bone-independent mechanisms. Hum Mol Genet. 2012;21(16):3535–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellur S, Jain M, Cuthbertson D, et al. Cesarean delivery is not associated with decreased at-birth fracture rates in osteogenesis imperfecta. Genet Med. 2016; 18(6):570–576. doi: 10.1038/gim.2015.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker JM, Dillard TA, Phillips YY. Arm span-height relationships in patients referred for spirometry. Am J Respir Crit Care Med. 1996;154(2 Pt 1):533–536. [DOI] [PubMed] [Google Scholar]
- 19.Linderholm H, Lindgren U. Prediction of spirometric values in patients with scoliosis. Acta Orthop Scand. 1978;49(5):469–474. [DOI] [PubMed] [Google Scholar]
- 20.Hepper NGG, Black LF, Fowler WS. Relationships of lung volume to height and arm span in normal subjects and in patients with spinal deformity. Am Rev Respir Dis. 1965. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 23.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: Methods and Development. Vol 11; 2002. doi:12043359 [PubMed] [Google Scholar]
- 24.Hankinson IL, Odencrantz JR, Fedan KB. Spirometrie Reference Values from a Sample of the General U. S. Population. Am J Respir Crit Care Med. 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 25.Lung Function Testing: Selection of Reference Values and Interpretative Strategies. Am Rev Respir Dis. 1991;144(5):1202–1218. [DOI] [PubMed] [Google Scholar]
- 26.Ihaka R, Gentleman R. R: A Language for Data Analysis and Graphics. J Comput Graph Stat. 1996;5(3):299. [Google Scholar]
- 27.Petty TL, Enright PL. Simple Office Spirometry for Primary Care Practitioners. Olathe: National Lung Health Education Program; 2003. [Google Scholar]
- 28.Cooper BG. An update on contraindications for lung function testing. Thorax. 2011. [DOI] [PubMed] [Google Scholar]
- 29.Neas LM, Schwartz J. Pulmonary function levels as predictors of mortality in a national sample of US adults. Am J Epidemiol. 1998; 147(11):1011–1018. [DOI] [PubMed] [Google Scholar]
- 30.Cohen BB, Barbano HE, Cox CS, et al. Plan and operation of the NHANES I Epidemiologic Followup Study: 1982–84. Vital Health Stat 1. 1987;(22):1–142. [PubMed] [Google Scholar]
- 31.Weiss ST, Segal MR, Sparrow D, Wager C. Relation of FEV1 and peripheral blood leukocyte count to total mortality. The Normative Aging Study. Am J Epidemiol. 1995;142(5):493–8; discussion 499–503. [DOI] [PubMed] [Google Scholar]
- 32.McAllister DA, Wild SH, MacLay JD, et al. Forced expiratory volume in one second predicts length of stay and in-hospital mortality in patients undergoing cardiac surgery: a retrospective cohort study. Sun J, ed. PLoS One. 2013;8(5):e64565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(2):81–89. [DOI] [PubMed] [Google Scholar]
- 34.Baldridge D, Lennington J, Weis M, et al. Generalized connective tissue disease in Crtap−/− mouse. PLoS One. 2010;5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baglole CJ, Liang F, Traboulsi H, et al. Pulmonary and diaphragmatic pathology in collagen type I α1 mutant mice with osteogenesis imperfecta. PediatrRes. May 2018. [DOI] [PubMed] [Google Scholar]
- 36.Grafe I, Yang T, Alexander S, et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med. 2014;20(6):670–675. doi: 10.1038/nm.3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lisse TS, Thiele F, Fuchs H, et al. ER stress-mediated apoptosis in a new mouse model of Osteogenesis imperfecta. PLoS Genet. 2008. doi: 10.1371/journal.pgen.0040007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willing MC, Cohn DH, Byers PH. Frameshift mutation near the 3’ end of the COL1A1 gene of type I collagen predicts an elongated proα1(I) chain and results in osteogenesis imperfecta type I. J Clin Invest. 1990. doi: 10.1172/JCI114424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morello R, Bertin TK, Chen Y, et al. CRTAP Is Required for Prolyl 3- Hydroxylation and Mutations Cause Recessive Osteogenesis Imperfecta. Cell. 2006. doi: 10.1016/j.cell.2006.08.039 [DOI] [PubMed] [Google Scholar]
- 40.Thibeault DW, Pettett G, Mabry SM, Rezaiekhaligh MM. Osteogenesis imperfecta Type IIA and pulmonary hypoplasia with normal alveolar development. Pediatr Pulmonol. 1995;20(5):301–306. [DOI] [PubMed] [Google Scholar]
- 41.Shapiro JR, Burn VE, Chipman SD, et al. Pulmonary hypoplasia and osteogenesis imperfecta type II with defective synthesis of alpha I(1) procollagen. Bone. 1989; 10(3): 165–171. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez JI, Perera A, Regadera J, Collado F, Contreras F. [Lethal osteogenesis imperfecta. Anatomopathologic (optical and structural) study of 8 autopsy cases]. An Esp Pediatr. 1982; 17(1): 18–33. [PubMed] [Google Scholar]
- 43.Himakhun W, Rojnueangnit K, Prachukthum S. Perinatal lethal osteogenesis imperfecta in a Thai newborn: the autopsy and histopathogical findings. J Med Assoc Thai. 2012;95 Suppl 1:S190-4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.